User login
Update on Tinea Capitis Diagnosis and Treatment
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
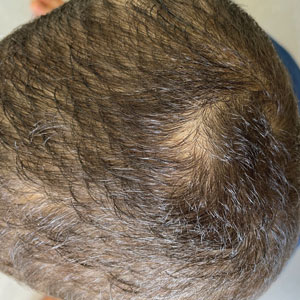
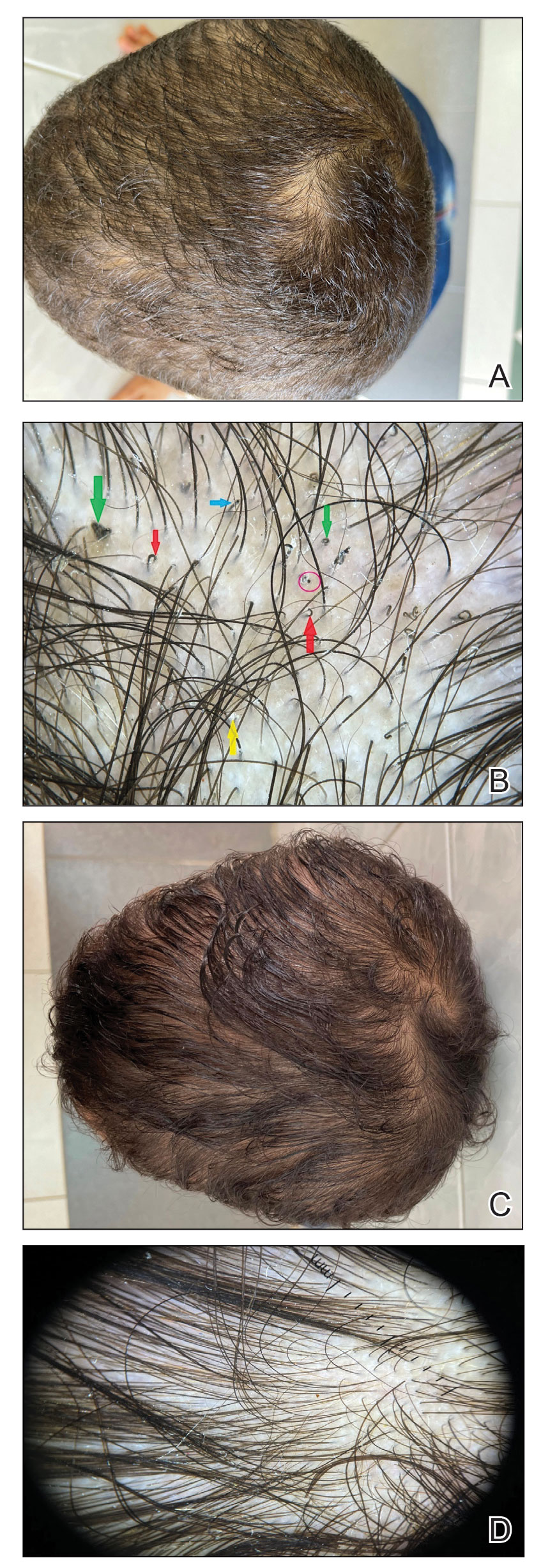
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4
Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
Herpes Zoster May Be a Marker for COVID-19 Infection During Pregnancy
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
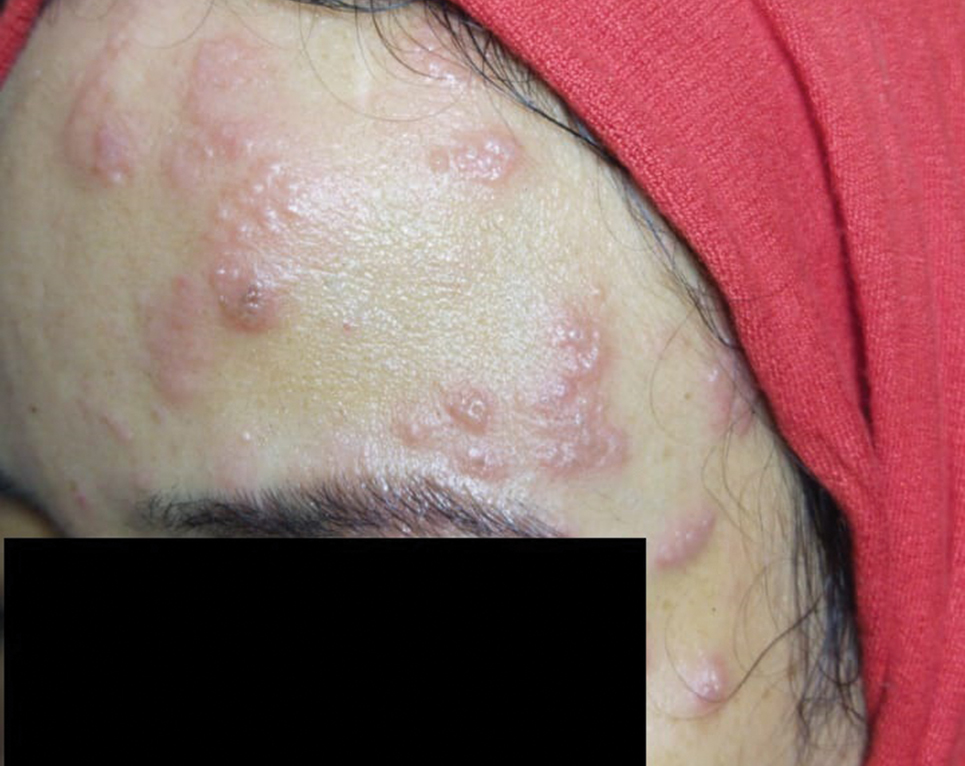
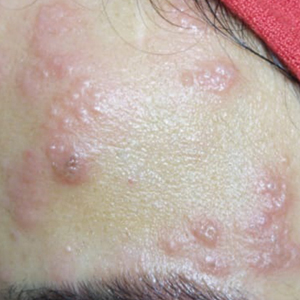
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.
Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.
Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.
Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Practice Points
- The vesicular rash of coronavirus disease 2019 (COVID-19) has been reported to have different forms of presentation.
- Pregnant women appear to be at increased risk for complications from COVID-19 infection.
- The clinical presentation of herpes zoster should be carefully monitored and reported for further assessment, especially if associated with other signs of COVID-19 infection.