User login
PRESsed for time
A 36‐year‐old woman was admitted after new‐onset Hseizures. She had been diagnosed with breast cancer 5 years prior to admission. At that time, she underwent left radical mastectomy and lymph node dissection. Lymph nodes were positive for metastatic disease with negative HER‐2‐Neu and positive estrogen and progesterone receptors. She was treated with docetaxel and tamoxifen but subsequently developed metastatic left hip lesions and was treated with letrozole and anastrozole. Three years later, scans revealed further metastatic disease to the liver, lung, and vertebral column. She was subsequently treated with capecitabine, until further disease progression led to the use of carboplatin and paclitaxel. Seven months prior to admission, her cancer was progressing and she was switched to doxorubicin, gemcitabine, and bevacizumab. Six weeks prior to admission, both positron emission tomography (PET) and computed tomography (CT) scan of her whole body and magnetic resonance imaging (MRI) of the brain illustrated significant improvement. Her last dose of bevacizumab was given 3 weeks prior to her admission.
Two weeks prior to admission, patient reported new‐onset daily headache. These were often localized in the occipital region. She reported some associated nausea and occasional emesis. Subsequently, she developed photophobia and phonophobia. On seeking outpatient treatment for her headache, it was noted that her systolic blood pressure had increased from a baseline of 100 mm Hg to 170 mm Hg. On the day prior to admission, she reported severe headache and several episodes of emesis and later that evening had a witnessed tonic‐clonic seizure.
The patient presented to an outside hospital and had an unremarkable noncontrast CT scan of her brain. An examination of her cerebrospinal fluid revealed negative gram stain, and a normal white blood cell count and protein level. She was treated with lorazepam, phenytoin, and decadron. On becoming more alert, she insisted on going home, where she later developed recurrent headache and presented to our emergency room.
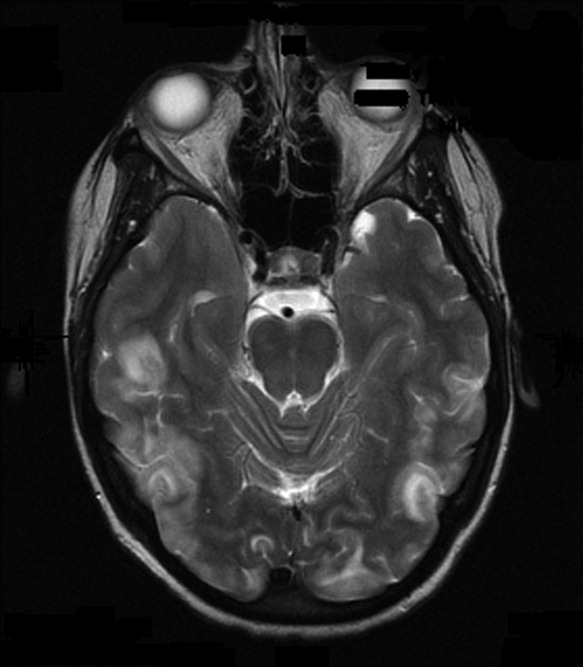
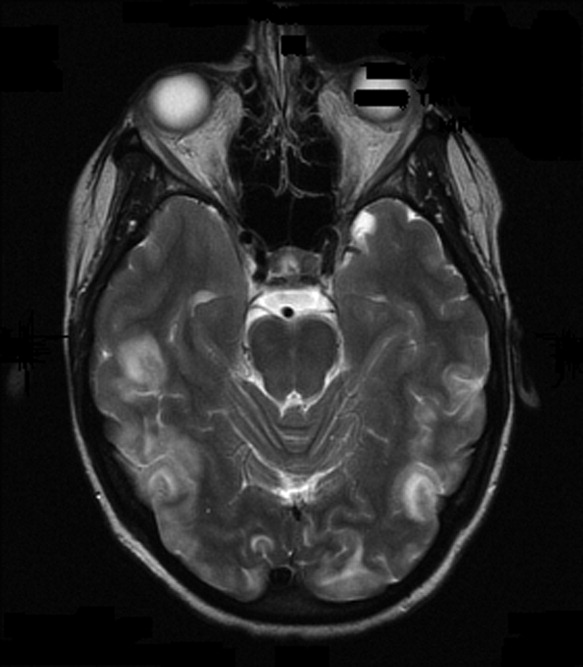
On admission to our service, she was noted to be confused and irritable, and unable to provide any history. Her exam revealed a blood pressure of 143/102 mmHg. No localizing neurologic signs were noted and her laboratory values were normal. After sedation, MRI of the brain was obtained (Figure 1). This revealed diffuse and patchy gyriform hyperintensity of the white matter, most consistent with posterior reversible encephalopathy syndrome (PRES).
Upon reflection, the patient had new onset hypertension that coincided with the initiation and dosing of bevacizumab. Bevacizumab, an antineoplastic agent, is a recombinant humanized monoclonal antibody that binds to and neutralizes vascular endothelial growth factor, thereby preventing angiogenesis.1 It is known to cause grade 3 hypertension in a minority of patients. Therefore, it was postulated that the patient's persistent blood pressure elevation resulted in vasogenic brain edema, precipitating her seizure. Subsequent to the diagnosis, her blood pressure was aggressively controlled with oral enalapril, metoprolol, triamterene/hydrochlorothiazide, and hydralazine. By hospital day 7, her headache had subsided and her altered mental status had resolved. She had no further episodes of seizures and bevacizumab was discontinued.
PRES has a distinct constellation of clinical symptoms and radiologic findings. The name PRES is a misnomer, as this syndrome is not always reversible, nor is it restricted to the white matter or to the posterior areas of the brain.2 It is hypothesized that a sudden rise in blood pressure leads to elevations in intracranial pressure, which exceeds the brain's autoregulatory mechanisms. This subsequently leads to transudation of fluid into the brain parenchyma. Interestingly, it appears that it is not the absolute level of systolic blood pressure that is critical in the development of PRES, but the rate of change in blood pressure. Hence, patients with chronic hypertension have developed adaptive vascular changes that protect them from this type of parenchymal damage.
PRES has gained increasing recognition due to the use of immunosuppressive and chemotherapeutic medications in organ transplant and oncology patients. Drugs such as cyclosporine, tacrolimus, fludarabine, vincristine, cisplatin, cytarabine, interferon‐alpha, interleukin, antiretroviral therapy, erythropoietin, granulocyte stimulating factor, and intravenous immunoglobulin have all been implicated.3 In addition to increasing blood pressure, these agents likely cause direct toxic injury to the brain, disrupting the blood‐brain barrier and resulting in subsequent edema. Other conditions associated with PRES include renal disease, vasculitis, endocrine disorders, porphyria, cocaine or amphetamine abuse, and stimulant abuse.
Clinically, PRES can present as headache, altered mental status, confusion, drowsiness progressing to stupor, emesis, abnormal visual perceptions, visual neglect, cortical blindness, difficulty with memory and concentration, brisk deep tendon reflexes, weakness, ataxia, and seizure activity. PRES has a characteristic appearance on neuroimaging that differentiates it from other forms of hypertensive encephalopathy. Edema of the white or gray matter in the posterior cerebral hemispheres, particularly the bilateral parietooccipital regions, is seen. PRES can also diffusely involve the brain stem, cerebellum, basal ganglia, and the frontal lobes. Abnormalities on neuroimaging are often symmetric but clinical manifestations can be asymmetric. MRI and CT scans can both be utilized for characterization of PRES.4
There are currently no published guidelines for the management of PRES. Expert opinion suggests removing the underlying cause and aggressively treating the hypertension.5 Furthermore, initiation and duration of antiepileptics remains controversial. After aggressive blood pressure control, resolution of findings on neuroimaging studies are expected anywhere from 8 days to 17 months.
Timely recognition of PRES is critical for prevention of further neurologic compromise. Immediate discontinuation of offending agents, as well as aggressive treatment of blood pressure, is the cornerstone treatment for PRES. In the future, a better understanding of the pathophysiology of PRES can lead to improved diagnostic and management options.
- ,,.Reversible posterior leukoencephalopathy syndrome and bevacizumab.N Engl J Med.2006;354(9):980–982.
- ,,, et al.A reversible posterior leukoencephalopathy syndrome.N Engl J Med.1996;334(8):494–500.
- ,,,,,.Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies.Am J Hematol.2004;77(1):72–76.
- ,,, et al.Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI.Neurology.1998;51(5):1369–1376.
- .Posterior leukoencephalopathy syndrome.Postgrad Med J.2001;77(903):24–28.
A 36‐year‐old woman was admitted after new‐onset Hseizures. She had been diagnosed with breast cancer 5 years prior to admission. At that time, she underwent left radical mastectomy and lymph node dissection. Lymph nodes were positive for metastatic disease with negative HER‐2‐Neu and positive estrogen and progesterone receptors. She was treated with docetaxel and tamoxifen but subsequently developed metastatic left hip lesions and was treated with letrozole and anastrozole. Three years later, scans revealed further metastatic disease to the liver, lung, and vertebral column. She was subsequently treated with capecitabine, until further disease progression led to the use of carboplatin and paclitaxel. Seven months prior to admission, her cancer was progressing and she was switched to doxorubicin, gemcitabine, and bevacizumab. Six weeks prior to admission, both positron emission tomography (PET) and computed tomography (CT) scan of her whole body and magnetic resonance imaging (MRI) of the brain illustrated significant improvement. Her last dose of bevacizumab was given 3 weeks prior to her admission.
Two weeks prior to admission, patient reported new‐onset daily headache. These were often localized in the occipital region. She reported some associated nausea and occasional emesis. Subsequently, she developed photophobia and phonophobia. On seeking outpatient treatment for her headache, it was noted that her systolic blood pressure had increased from a baseline of 100 mm Hg to 170 mm Hg. On the day prior to admission, she reported severe headache and several episodes of emesis and later that evening had a witnessed tonic‐clonic seizure.
The patient presented to an outside hospital and had an unremarkable noncontrast CT scan of her brain. An examination of her cerebrospinal fluid revealed negative gram stain, and a normal white blood cell count and protein level. She was treated with lorazepam, phenytoin, and decadron. On becoming more alert, she insisted on going home, where she later developed recurrent headache and presented to our emergency room.
On admission to our service, she was noted to be confused and irritable, and unable to provide any history. Her exam revealed a blood pressure of 143/102 mmHg. No localizing neurologic signs were noted and her laboratory values were normal. After sedation, MRI of the brain was obtained (Figure 1). This revealed diffuse and patchy gyriform hyperintensity of the white matter, most consistent with posterior reversible encephalopathy syndrome (PRES).

Upon reflection, the patient had new onset hypertension that coincided with the initiation and dosing of bevacizumab. Bevacizumab, an antineoplastic agent, is a recombinant humanized monoclonal antibody that binds to and neutralizes vascular endothelial growth factor, thereby preventing angiogenesis.1 It is known to cause grade 3 hypertension in a minority of patients. Therefore, it was postulated that the patient's persistent blood pressure elevation resulted in vasogenic brain edema, precipitating her seizure. Subsequent to the diagnosis, her blood pressure was aggressively controlled with oral enalapril, metoprolol, triamterene/hydrochlorothiazide, and hydralazine. By hospital day 7, her headache had subsided and her altered mental status had resolved. She had no further episodes of seizures and bevacizumab was discontinued.
PRES has a distinct constellation of clinical symptoms and radiologic findings. The name PRES is a misnomer, as this syndrome is not always reversible, nor is it restricted to the white matter or to the posterior areas of the brain.2 It is hypothesized that a sudden rise in blood pressure leads to elevations in intracranial pressure, which exceeds the brain's autoregulatory mechanisms. This subsequently leads to transudation of fluid into the brain parenchyma. Interestingly, it appears that it is not the absolute level of systolic blood pressure that is critical in the development of PRES, but the rate of change in blood pressure. Hence, patients with chronic hypertension have developed adaptive vascular changes that protect them from this type of parenchymal damage.
PRES has gained increasing recognition due to the use of immunosuppressive and chemotherapeutic medications in organ transplant and oncology patients. Drugs such as cyclosporine, tacrolimus, fludarabine, vincristine, cisplatin, cytarabine, interferon‐alpha, interleukin, antiretroviral therapy, erythropoietin, granulocyte stimulating factor, and intravenous immunoglobulin have all been implicated.3 In addition to increasing blood pressure, these agents likely cause direct toxic injury to the brain, disrupting the blood‐brain barrier and resulting in subsequent edema. Other conditions associated with PRES include renal disease, vasculitis, endocrine disorders, porphyria, cocaine or amphetamine abuse, and stimulant abuse.
Clinically, PRES can present as headache, altered mental status, confusion, drowsiness progressing to stupor, emesis, abnormal visual perceptions, visual neglect, cortical blindness, difficulty with memory and concentration, brisk deep tendon reflexes, weakness, ataxia, and seizure activity. PRES has a characteristic appearance on neuroimaging that differentiates it from other forms of hypertensive encephalopathy. Edema of the white or gray matter in the posterior cerebral hemispheres, particularly the bilateral parietooccipital regions, is seen. PRES can also diffusely involve the brain stem, cerebellum, basal ganglia, and the frontal lobes. Abnormalities on neuroimaging are often symmetric but clinical manifestations can be asymmetric. MRI and CT scans can both be utilized for characterization of PRES.4
There are currently no published guidelines for the management of PRES. Expert opinion suggests removing the underlying cause and aggressively treating the hypertension.5 Furthermore, initiation and duration of antiepileptics remains controversial. After aggressive blood pressure control, resolution of findings on neuroimaging studies are expected anywhere from 8 days to 17 months.
Timely recognition of PRES is critical for prevention of further neurologic compromise. Immediate discontinuation of offending agents, as well as aggressive treatment of blood pressure, is the cornerstone treatment for PRES. In the future, a better understanding of the pathophysiology of PRES can lead to improved diagnostic and management options.
A 36‐year‐old woman was admitted after new‐onset Hseizures. She had been diagnosed with breast cancer 5 years prior to admission. At that time, she underwent left radical mastectomy and lymph node dissection. Lymph nodes were positive for metastatic disease with negative HER‐2‐Neu and positive estrogen and progesterone receptors. She was treated with docetaxel and tamoxifen but subsequently developed metastatic left hip lesions and was treated with letrozole and anastrozole. Three years later, scans revealed further metastatic disease to the liver, lung, and vertebral column. She was subsequently treated with capecitabine, until further disease progression led to the use of carboplatin and paclitaxel. Seven months prior to admission, her cancer was progressing and she was switched to doxorubicin, gemcitabine, and bevacizumab. Six weeks prior to admission, both positron emission tomography (PET) and computed tomography (CT) scan of her whole body and magnetic resonance imaging (MRI) of the brain illustrated significant improvement. Her last dose of bevacizumab was given 3 weeks prior to her admission.
Two weeks prior to admission, patient reported new‐onset daily headache. These were often localized in the occipital region. She reported some associated nausea and occasional emesis. Subsequently, she developed photophobia and phonophobia. On seeking outpatient treatment for her headache, it was noted that her systolic blood pressure had increased from a baseline of 100 mm Hg to 170 mm Hg. On the day prior to admission, she reported severe headache and several episodes of emesis and later that evening had a witnessed tonic‐clonic seizure.
The patient presented to an outside hospital and had an unremarkable noncontrast CT scan of her brain. An examination of her cerebrospinal fluid revealed negative gram stain, and a normal white blood cell count and protein level. She was treated with lorazepam, phenytoin, and decadron. On becoming more alert, she insisted on going home, where she later developed recurrent headache and presented to our emergency room.
On admission to our service, she was noted to be confused and irritable, and unable to provide any history. Her exam revealed a blood pressure of 143/102 mmHg. No localizing neurologic signs were noted and her laboratory values were normal. After sedation, MRI of the brain was obtained (Figure 1). This revealed diffuse and patchy gyriform hyperintensity of the white matter, most consistent with posterior reversible encephalopathy syndrome (PRES).

Upon reflection, the patient had new onset hypertension that coincided with the initiation and dosing of bevacizumab. Bevacizumab, an antineoplastic agent, is a recombinant humanized monoclonal antibody that binds to and neutralizes vascular endothelial growth factor, thereby preventing angiogenesis.1 It is known to cause grade 3 hypertension in a minority of patients. Therefore, it was postulated that the patient's persistent blood pressure elevation resulted in vasogenic brain edema, precipitating her seizure. Subsequent to the diagnosis, her blood pressure was aggressively controlled with oral enalapril, metoprolol, triamterene/hydrochlorothiazide, and hydralazine. By hospital day 7, her headache had subsided and her altered mental status had resolved. She had no further episodes of seizures and bevacizumab was discontinued.
PRES has a distinct constellation of clinical symptoms and radiologic findings. The name PRES is a misnomer, as this syndrome is not always reversible, nor is it restricted to the white matter or to the posterior areas of the brain.2 It is hypothesized that a sudden rise in blood pressure leads to elevations in intracranial pressure, which exceeds the brain's autoregulatory mechanisms. This subsequently leads to transudation of fluid into the brain parenchyma. Interestingly, it appears that it is not the absolute level of systolic blood pressure that is critical in the development of PRES, but the rate of change in blood pressure. Hence, patients with chronic hypertension have developed adaptive vascular changes that protect them from this type of parenchymal damage.
PRES has gained increasing recognition due to the use of immunosuppressive and chemotherapeutic medications in organ transplant and oncology patients. Drugs such as cyclosporine, tacrolimus, fludarabine, vincristine, cisplatin, cytarabine, interferon‐alpha, interleukin, antiretroviral therapy, erythropoietin, granulocyte stimulating factor, and intravenous immunoglobulin have all been implicated.3 In addition to increasing blood pressure, these agents likely cause direct toxic injury to the brain, disrupting the blood‐brain barrier and resulting in subsequent edema. Other conditions associated with PRES include renal disease, vasculitis, endocrine disorders, porphyria, cocaine or amphetamine abuse, and stimulant abuse.
Clinically, PRES can present as headache, altered mental status, confusion, drowsiness progressing to stupor, emesis, abnormal visual perceptions, visual neglect, cortical blindness, difficulty with memory and concentration, brisk deep tendon reflexes, weakness, ataxia, and seizure activity. PRES has a characteristic appearance on neuroimaging that differentiates it from other forms of hypertensive encephalopathy. Edema of the white or gray matter in the posterior cerebral hemispheres, particularly the bilateral parietooccipital regions, is seen. PRES can also diffusely involve the brain stem, cerebellum, basal ganglia, and the frontal lobes. Abnormalities on neuroimaging are often symmetric but clinical manifestations can be asymmetric. MRI and CT scans can both be utilized for characterization of PRES.4
There are currently no published guidelines for the management of PRES. Expert opinion suggests removing the underlying cause and aggressively treating the hypertension.5 Furthermore, initiation and duration of antiepileptics remains controversial. After aggressive blood pressure control, resolution of findings on neuroimaging studies are expected anywhere from 8 days to 17 months.
Timely recognition of PRES is critical for prevention of further neurologic compromise. Immediate discontinuation of offending agents, as well as aggressive treatment of blood pressure, is the cornerstone treatment for PRES. In the future, a better understanding of the pathophysiology of PRES can lead to improved diagnostic and management options.
- ,,.Reversible posterior leukoencephalopathy syndrome and bevacizumab.N Engl J Med.2006;354(9):980–982.
- ,,, et al.A reversible posterior leukoencephalopathy syndrome.N Engl J Med.1996;334(8):494–500.
- ,,,,,.Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies.Am J Hematol.2004;77(1):72–76.
- ,,, et al.Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI.Neurology.1998;51(5):1369–1376.
- .Posterior leukoencephalopathy syndrome.Postgrad Med J.2001;77(903):24–28.
- ,,.Reversible posterior leukoencephalopathy syndrome and bevacizumab.N Engl J Med.2006;354(9):980–982.
- ,,, et al.A reversible posterior leukoencephalopathy syndrome.N Engl J Med.1996;334(8):494–500.
- ,,,,,.Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies.Am J Hematol.2004;77(1):72–76.
- ,,, et al.Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI.Neurology.1998;51(5):1369–1376.
- .Posterior leukoencephalopathy syndrome.Postgrad Med J.2001;77(903):24–28.