User login
In reply: Kidney stones
In Reply: I thank Dr. Keller for his kind letter.
With respect to expulsive therapy, Dellabella et al1 randomly assigned 210 patients to receive nifedipine, tamsulosin, or phloroglucinol. All the patients also received a corticosteroid. The most effective therapy was tamsulosin, though this was not a placebo-controlled study. In a separate study, Borghi et al2 compared methylprednisolone plus nifedipine and methylprednisolone plus placebo. The nifedipine-methylpednisolone combination seemed to result in more prompt stone passage.
With respect to calcium supplements in calcium kidney stone disease, Curhan et al3 prospectively examined stone risk associated with dietary calcium as well as calcium supplements. This seemed to show that with calcium supplements there was no increased risk, and there may have even been some benefit. In another study by Borghi et al,4 normal dietary calcium intake was shown to be associated with lower stone risk than a low calcium intake. Further, the study by Curhan et al3 seemed to indicate the same.
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 2005; 174:167–172.
- Borghi L, Meschi T, Amato F, et al. Nifedipine and methylprednisolone
in facilitating ureteral stone passage: a randomized, double blind, placebo-controlled study. J Urol 1994; 152:1095–1098. - Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164:885–891.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
In Reply: I thank Dr. Keller for his kind letter.
With respect to expulsive therapy, Dellabella et al1 randomly assigned 210 patients to receive nifedipine, tamsulosin, or phloroglucinol. All the patients also received a corticosteroid. The most effective therapy was tamsulosin, though this was not a placebo-controlled study. In a separate study, Borghi et al2 compared methylprednisolone plus nifedipine and methylprednisolone plus placebo. The nifedipine-methylpednisolone combination seemed to result in more prompt stone passage.
With respect to calcium supplements in calcium kidney stone disease, Curhan et al3 prospectively examined stone risk associated with dietary calcium as well as calcium supplements. This seemed to show that with calcium supplements there was no increased risk, and there may have even been some benefit. In another study by Borghi et al,4 normal dietary calcium intake was shown to be associated with lower stone risk than a low calcium intake. Further, the study by Curhan et al3 seemed to indicate the same.
In Reply: I thank Dr. Keller for his kind letter.
With respect to expulsive therapy, Dellabella et al1 randomly assigned 210 patients to receive nifedipine, tamsulosin, or phloroglucinol. All the patients also received a corticosteroid. The most effective therapy was tamsulosin, though this was not a placebo-controlled study. In a separate study, Borghi et al2 compared methylprednisolone plus nifedipine and methylprednisolone plus placebo. The nifedipine-methylpednisolone combination seemed to result in more prompt stone passage.
With respect to calcium supplements in calcium kidney stone disease, Curhan et al3 prospectively examined stone risk associated with dietary calcium as well as calcium supplements. This seemed to show that with calcium supplements there was no increased risk, and there may have even been some benefit. In another study by Borghi et al,4 normal dietary calcium intake was shown to be associated with lower stone risk than a low calcium intake. Further, the study by Curhan et al3 seemed to indicate the same.
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 2005; 174:167–172.
- Borghi L, Meschi T, Amato F, et al. Nifedipine and methylprednisolone
in facilitating ureteral stone passage: a randomized, double blind, placebo-controlled study. J Urol 1994; 152:1095–1098. - Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164:885–891.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 2005; 174:167–172.
- Borghi L, Meschi T, Amato F, et al. Nifedipine and methylprednisolone
in facilitating ureteral stone passage: a randomized, double blind, placebo-controlled study. J Urol 1994; 152:1095–1098. - Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164:885–891.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
Nephrolithiasis: Treatment, causes, and prevention
COMMON AND ON THE INCREASE
Nephrolithiasis is common, with a lifetime prevalence of 10% in men and 5% in women.1,2 Studies have shown that the prevalence is increasing in the United States. In the second National Health and Nutrition Examination Survey (1988–1994), the prevalence in adults ages 20 to 74 was greater than in the 1976–1980 survey (5.2% vs 3.2%).3 The increase was observed in whites but not in African Americans or Mexican Americans, was greater in men than in women, and was greater with age in each time period.
In addition, stones often recur, and each stone event can be associated with significant metabolic and intervention-related morbidity.
PRESENTATION: SEVERE COLIC
Most patients present with moderate to severe colic, caused by the stone entering the ureter. Stones in the proximal (upper) ureter cause pain in the flank or anterior upper abdomen. When the stone reaches the distal third of the ureter, pain is noted in the ipsilateral testicle or labia. A stone at the junction of the ureter and the bladder often causes dysuria, urgency, and frequency and may be mistaken for a lower urinary tract infection.
Less often, patients present with silent ureteral obstruction, unexplained persistent urinary infection, or painless hematuria. However, even in patients with symptoms, the absence of hematuria does not exclude urolithiasis. In a study of 397 patients presenting with acute symptomatic urolithiasis, 9% did not have hematuria.4
The differential diagnosis in a patient with symptoms suggesting renal colic includes:
- Musculoskeletal pain
- Herpes zoster
- Diverticulitis
- Duodenal ulcer
- Cholecystitis
- Pyelonephritis
- Renal infarct
- Renal hemorrhage
- Gynecologic disorders
- Ureteral obstruction from renal papillary necrosis with sloughed papillae, a blood clot, or a ureteral stricture.
HELICAL CT WITHOUT CONTRAST IS THE PREFERED IMAGING STUDY
The diagnosis can be confirmed by computed tomography (CT), renal ultrasonography, or intravenous pyelography.
Helical CT without contrast is the preferred imaging study in patients with suspected nephrolithiasis. It has several advantages over other imaging studies: it requires no radiocontrast material; it shows the distal ureters; it can detect radiolucent stones (ie, uric acid stones), radio-opaque stones, and stones as small as 1 to 2 mm; and it can detect hydronephrosis and intra-abdominal and renal disorders other than stones that could be causing the patient’s symptoms.
In a study in 100 consecutive patients presenting to an emergency department with flank pain, helical CT had a sensitivity of 98%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 97% for the diagnosis of ureteral stones.5 In a study of 1,000 consecutive patients with suspected stones, helical CT identified significant, additional, or alternative reasons for the patient’s symptoms in 10% of cases.6
Ultrasonography has the advantage of not using radiation, but it is less sensitive for detecting stones and can image only the kidney and the proximal ureter. A retrospective study in 123 patients found that, compared with helical CT as the gold standard, ultrasonography had a sensitivity of 24% and a specificity of 90%.7 Ultrasonography may also miss stones smaller than 3 mm in diameter.
Conventional radiography (kidney-ureter-bladder view) is inadequate for diagnosis as it may miss stones in the kidney or ureter (even small radio-opaque stones) and provides no information about possible obstruction.
Intravenous pyelography has few advantages in renal lithiasis, exposes the patient to the risk of radiocontrast infusion and contrast-mediated acute renal injury, and gives less information than noncontrast CT.
MEDICAL MANAGEMENT OF ACUTE STONE EVENTS
Most stones are smaller than 5 mm and readily pass without interventions such as lithotripsy, ureteroscopy, or percutaneous nephrolithotomy. (For more information on these interventions, see the review by Samplaski and colleagues in this issue of the Cleveland Clinic Journal of Medicine.8)
Even if the stone is as large as 1 cm, I would let the patient try to pass it spontaneously if it is in the distal ureter, and I would allow up to 4 weeks for this to happen.
For most patients, pain management is paramount. Randomized controlled trials suggest that parenteral nonsteroidal anti-inflammatory drugs (NSAIDs) are as effective as narcotics for controlling the pain of renal colic.9 Diclofenac (Voltaren) has been used in several studies.
To hasten stone passage, some recommend inducing high urine flow with oral intake of at least 2 to 3 L of fluids per 24 hours to ensure a urine output of at least 2 L per day.
Drugs may also help the stone to pass. A recent study in 210 patients with ureteral stones averaging 6 mm in diameter showed that tamsulosin (Flomax) increased the likelihood of spontaneous stone passage.10 A meta-analysis of 693 patients in nine randomized trials concluded that alpha-blockers and calcium channel blockers increased the likelihood of stone passage compared with no treatment.11 Borghi et al,12 in a randomized, double-blind study in 86 patients with unilateral ureteral stones, reported a higher rate of stone passage in patients treated with methylprednisolone (Medrol) 16 mg/day plus nifedipine (Procardia) 40 mg/day than in those given methylprednisolone alone.
PREVENTING RECURRENT STONES: PRINCIPLES AND SPECIFICS
Urinary stone disease recurs in 30% to 50% of patients within 5 years.1,13,14
In preventing recurrent stones, some principles apply to all patients and some are specific to the type of stone the patient had.
Stones form when the urine is supersaturated
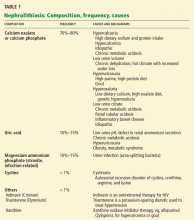
Nephrolithiasis occurs when the concentration of stone-forming salts such as calcium oxalate, calcium phosphate, or uric acid is high. When the concentration is high enough to allow crystals to form or preformed crystals to grow, the urine is said to be supersaturated.
Several facors are the major determinants of whether the urine is supersaturated by different salts:
- Calcium oxalate—low urine volume and high concentrations of calcium and oxalate
- Calcium phosphate—a high urine calcium concentration and alkaline urine
- Uric acid—acidic urine
- Cystine—a high urinary cystine concentration and acidic urine.
Increasing daily fluid intake
Since the urinary concentration of stone-forming salts is strongly affected by the daily urine volume, it follows that increasing daily fluid intake is important in preventing recurrent stone disease.
In one study,15 199 patients with a first calcium stone were randomized to a program of high oral fluid intake or no intervention. Five years later, 12 (12%) of the 99 patients in the high-fluid intake group had had a second stone, compared with 27 (27%) in the untreated group (P = .008). Of interest, the baseline 24-hour urine volumes were significantly lower in patients with stones than in 101 normal controls (P = .001), suggesting that habitual low daily fluid intake is a risk factor for calcium stone disease.13
PREVENTING CALCIUM STONES
Most stones are composed of calcium oxalate or calcium phosphate. Calcium stone disease occurs most often in the 3rd to 5th decades of life.
Naturally occurring inhibitors of calcium crystal formation in the urine include citrate, nephrocalcin, uropontin, and magnesium. Of these, only citrate and magnesium levels are routinely measured; low levels of citrate are treated as a cause of calcium stone disease. It follows that the risk of calcium nephrolithiasis is the result of the interplay between the supersaturated state and the level of urinary inhibitors.16
Hypercalciuria and calcium oxalate stones
Calcium oxalate stones begin as crystals that form on the surface of the renal papillae over collections of suburothelial calcium phosphate particles called Randall plaque.17 The driving force for calcium oxalate overgrowth on plaque is calcium oxalate supersaturation, which is strongly linked to high urinary calcium excretion. The fraction of papillary surface covered by plaque in patients with idiopathic calcium oxalate stones correlates directly with the urine calcium level and inversely with urine volume and pH.18
Most patients with calcium oxalate stones have hypercalciuria (defined as 24-hour urinary calcium excretion > 300 mg in men, > 250 mg in women, or > 4 mg/kg in men or women).
Hypercalciuria can be idiopathic
Hypercalciuria can occur in primary hyperparathyroidism, sarcoidosis, vitamin D excess, corticosteroid treatment, renal tubular acidosis, hyperthyroidism, and malignant neoplasms. If none of these conditions is present, elevated urinary calcium excretion is considered idiopathic.
Some patients with idiopathic hypercalciuria have a strong family history of hypercalciuria and, likely, a genetic basis for the disease. This condition has been categorized by the presumed site of the primary abnormality:
Absorptive hypercalciuria. Most patients with idiopathic hypercalciuria absorb too much calcium from the intestine. In many of them, 1,25 dihydroxyvitamin D levels are slightly high and serum phosphorous levels are slightly low; the hypothesis is that they produce more 1,25 dihydroxyvitamin D or are more sensitive to it.19 However, Breslau et al20 showed that not all patients with idiopathic hypercalciuria have absorptive hypercalciuria mediated by 1,25 dihydroxyvitamin D, which suggests that the intestinal hyperabsorption of calcium has other mechanisms.
Resorptive hypercalciuria occurs if increased bone turnover leads to urinary loss of bone calcium.
Renal leak is due to a primary defect in renal tubular transport that causes loss of calcium into the urine and a secondary increase in intestinal calcium absorption or mobilization from bone.
This categorization is based on measuring fasting and 24-hour urine calcium, urinary calcium responses to a low-calcium diet, and responses to an oral calcium load.21 However, these studies are difficult to do and have been shown to have minimal clinical value.
To reduce calcium in the urine, limit sodium, give thiazides
Idiopathic hypercalciuria is worsened by a diet high in sodium22,23 and animal protein.24 Thiazide diuretics lower urinary calcium excretion and promote mineral retention.25 Therefore, treatment of idiopathic hypercalciuria consists of high fluid intake, dietary sodium restriction, and thiazide diuretics.
Calcium restriction is not advised
For several reasons, a calcium-restricted diet is not advised for patients with idiopathic hypercalciuria. 26 Dietary calcium restriction can put the patient into negative calcium balance. Further, it is thought that with less calcium to bind to dietary oxalate, more unbound oxalate can be absorbed in the colon and eventually excreted in the urine. This increase in urinary oxalate can be to the point of supersaturation, even though urinary calcium levels remain unchanged.25,27,28 This, in turn, increases the likelihood of stone formation.
Several studies showed that a higher intake of dietary calcium is actually associated with fewer calcium stone events in both men and women.25,27,28
Further, a study in 120 Italian patients with hypercalciuric calcium oxalate stones concluded that a diet that is normal in calcium, low in sodium, and low in animal protein was associated with a lower frequency of calcium stones than a low-calcium diet.29 Although both diets were associated with a reduction in urinary calcium concentrations, urinary oxalate excretion rose in those on a low-calcium diet and fell in those on a normal-calcium diet. The reduction in urinary oxalate excretion in patients on a normal-calcium diet was attributed to intestinal binding of dietary oxalate by dietary calcium, thus lessening the amount of free oxalate available for absorption. Although calcium oxalate excretion fell in both groups, it fell more in those on a normal calcium intake. Compared with those on a low-calcium diet, the patients on the normal-calcium, low-sodium, low-protein diet had a 50% lower risk of stones at 5 years.
Hyperparathyroidism
Primary hyperparathyroidism can cause hypercalciuria and nephrolithiasis. In one series,30 56 (4.9%) of 1,132 consecutive patients with nephrolithiasis had a confirmed diagnosis of hyperparathyroidism. Parathyroidectomy prevented subsequent stone disease in 48 patients.
However, only 17% to 24% of patients with primary hyperparathyroidism have urinary stones composed of calcium oxalate or calcium phosphate.31,32 In many studies, it was difficult to determine why a minority of these patients develop stones, but two studies shed some light on this.
Parks et al30 found that, compared with nephrolithiasis patients with idiopathic hypercalciuria, those with primary hyperparathyroidism have elevated serum calcium levels (but usually < 11.5 mg/dL), greater degrees of hypercalciuria (352 mg/day vs 252 mg/day, P < .001), and lower serum phosphate levels (2.45 vs 3.10 mg/dL, P < .001).
Odvina et al33 found, in a study of 131 patients with proven primary hyperparathyroidism, that 78 had nephrolithiasis and 53 did not. Those with stones excreted more calcium (343 mg/day) than those without stones (273 mg/day), had a higher urinary saturation of calcium oxalate and brushite, and excreted twice as much calcium following a 1-g oral calcium load.
These studies suggest that in patients with primary hyperparathyroidism, the risk of forming stones is related to the degree of hypercalciuria, and in particular to the increased intestinal absorption of dietary calcium.
Renal tubular acidosis
Features of distal renal tubular acidosis are systemic metabolic acidosis, alkaline urine, hypokalemia, hypercalciuria, hypocitraturia, and nephrolithiasis. The chronic metabolic acidosis results in loss of bone calcium, contributes to hypercalciuria, and is responsible for the hypocitraturia.34 Stone formation is the result of excessive urinary calcium excretion, the deficiency of the urinary crystal inhibitor citrate, and persistently alkaline urine.
Treatment with sodium bicarbonate or potassium citrate corrects the metabolic acidosis, reduces the loss of calcium from bone, corrects hypokalemia, and increases urinary citrate.
Too much uric acid in the urine
Elevated urinary uric acid excretion (> 800 mg/day in men, > 750 mg/day in women) is associated with formation of calcium oxalate stones35 and, in conjunction with low urine pH, with uric acid stones. An increase in dissolved uric acid salts induces heterogeneous calcium oxalate nucleation.36 In one randomized clinical trial,37 giving allopurinol (Zyloprim) lowered urinary uric acid excretion and was associated with a lower rate of calcium stone disease.
Too much oxalate in the urine
The 95th percentile for urinary oxalate excretion is 45 mg/day in women and 55 mg/day in men.38 Hyperoxaluria increases calcium oxalate supersaturation and contributes to calcium stone formation.
Normally, 90% of dietary oxalate binds to dietary calcium in the small intestine and passes into the stool as calcium oxalate; 10% of dietary oxalate remains free and is absorbed in the colon and subsequently excreted in the urine.
Hyperoxaluria may simply be a result of high dietary oxalate intake. However, increased enteric absorption of dietary oxalate can occur in those on a low-calcium diet (in which less calcium is available to bind to dietary oxalate, as described above) and may partially explain why a low-calcium diet has been associated with increased frequency of calcium stone disease.
Patients with enteric malabsorption of fat (eg, due to inflammatory bowel disease or intestinal bypass surgery for obesity) may also develop hyperoxaluria. This occurs because the excess enteric fat binds dietary calcium and allows free oxalate to be more readily absorbed in the colon.39
Rarely, hyperoxaluria is caused by one of several recessively inherited disorders of oxalate metabolism.40
The growing number of people with obesity has resulted in an upsurge in gastric bypass surgery. Although the current procedures do not pose the same metabolic risks as were noted in the 1970s when a different type of bypass was performed, the incidence of kidney stones does appear to be higher after these procedures. A recent analysis of 1,436 patients undergoing Roux-en-Y gastric bypass surgery found that 60 of them developed calcium stones afterward. Of these, 31 who underwent metabolic studies were found to have higher oxalate and lower citrate levels at 12 months of follow-up.41
Not enough citrate, a stone inhibitor
Hypocitraturia is defined as a daily urine citrate excretion less than 500 mg in women and 434 mg in men.42 As already mentioned, citrate plays an important role in inhibiting calcium crystal formation and preventing stone formation.
Urinary citrate excretion is mainly determined by tubular reabsorption, which is increased by acid loads and decreased by alkali loads.43 Low urine citrate levels are often seen in conditions that cause chronic metabolic acidosis, such as inflammatory bowel disease, intestinal malabsorption, and renal tubular acidosis—all of which are associated with increased occurrence of nephrolithiasis. However, in most nephrolithiasis patients with hypocitraturia, the cause is not apparent, and the mechanism of the hypocitraturia cannot be determined.44
In recent years, high-protein, low-carbohydrate diets have become popular for weight reduction, but they also have metabolic effects that increase the risk of stones.45 The metabolism of a diet high in animal protein produces more hydrogen ions that are buffered by bone, releasing calcium from bone and increasing urinary calcium excretion. These diets also cause intracellular acidosis, resulting in decreased urinary excretion of citrate. As a result of these effects, the stone-forming propensity of the urine is increased.
STRUVITE STONES MUST BE REMOVED
Struvite stones are the result of chronic upper urinary infection with urease-producing bacteria (Proteus sp, Haemophilus sp, Klebsiella sp, and Ureaplasma urealyticum).46,47 The hydrolysis of urea yields ammonium and hydroxyl ions and a persistently alkaline urine, and this scenario promotes the formation of stones composed of magnesium ammonium phosphate, ie, struvite.
Struvite stones, which are often branched (“staghorn” stones), occur more often in women and in patients who have chronic urinary obstruction or a neurologic disorder that impairs normal emptying of the bladder.
Treatment requires eradicating the infection with antibiotics and removing the bacteria-laden stones by one of several interventional techniques. Acetohydroxamic acid inhibits urease and has been used to treat struvite stone disease, but it has frequent and serious adverse effects.48
URIC ACID STONES FORM IN VERY ACIDIC URINE
Uric acid stones occur especially in patients with unusually low urine pH and hyperuricosuria. In some patients, this very low urine pH is the result of a defect in renal ammonia secretion, which results in less buffering of secreted hydrogen ions.49
The tendency to form uric acid stones is reported to be increasing in obese people with the metabolic syndrome. Some studies have shown that the defect in ammonia production by the kidney may be the result of insulin resistance.50
Urate stones are radiolucent but can be seen on ultrasonography and helical CT. On helical CT, they can be distinguished from calcium stones by their lower density.51
Since uric acid is much more soluble in an alkaline solution, both prevention and treatment should consist of alkalinization of urine to a pH of more than 6.0 with oral sodium bicarbonate or citrate solution and hydration. This treatment may actually dissolve uric acid stones. If hyperuricemia or hyperuricosuria is present, allopurinol can be prescribed.
CYSTINE STONES ALSO FORM IN ACIDIC URINE
Cystine stone disease occurs in people who have inherited an autosomally recessive gastrointestinal and renal tubular transport disorder of four amino acids, ie, cystine, ornithine, arginine, and lysine.52 Of these, cystine is the most insoluble in normally acidic urine and thus precipitates into stones. The onset is at a younger age than in calcium stone disease; the stones are radio-opaque.
Cystine solubility is about 243 mg/L in normal urine and rises with pH. Some patients can excrete as much as 1,000 mg per day.
Treatment53,54 consists of:
- Hydration, to achieve daily urine volumes of 3 to 3.5 L
- Alkalinization of the urine to a pH higher than 6.5 with potassium alkali (potassium citrate) or sodium bicarbonate
- Reduction of protein and sodium intake to reduce cystine excretion.
If these measures fail, D-penicillamine (Depen), tiopronin (Thiola), or captopril (Capoten)55–57 can be given to convert the cystine to a more soluble disulfide cysteine-drug complex. Captopril has only a modest effect at best and is usually given with another disulfide-complexing drug; it also has the disadvantage of producing hypotension. Adverse effects of D-penicillamine and tiopronin include abdominal pain, loss of taste, fever, proteinuria, and, in rare cases, nephrotic syndrome.
WORKUP AND MANAGEMENT OF NEPHROLITHIASIS
Anyone under age 20 with an initial stone deserves a more extensive evaluation, including screening for renal tubular acidosis, cystinuria, and hyperoxaluria. A more extensive workup is also warranted in patients with a history of chronic diarrhea, sarcoidosis, or a condition associated with renal tubular acidosis (eg, Sjögren syndrome), in patients with a family history of kidney stones, in patients with high-protein weight-loss diets, and in those undergoing gastric bypass surgery for obesity. In these high-risk patients, the evaluation should include 24-hour urine studies to measure calcium, oxalate, citrate, uric acid, creatinine, sodium, and volume.
Other diagnostic clues are often helpful in the decision to do a more comprehensive evaluation.
- Nephrocalcinosis on roentgenography suggests hyperparathyroidism, medullary sponge kidney, or renal tubular acidosis.
- Hypercalcemia that develops after treatment of hypercalciuria with a thiazide diuretic suggests latent hyperparathyroidism.
- A history of recurrent urinary tract infections or of anatomic abnormalities in the urinary tract should lead to an evaluation for struvite stone disease.
- Uric acid stones should be suspected in a patient with metabolic syndrome or a history of gout and are usually accompanied by a urine pH lower than 5.5.
- A urinalysis showing cystine crystals always indicates cystinuria, which should be confirmed by 24-hour urine cystine determination.
- A family history of renal stones is more common in idiopathic hypercalciuria, cystinuria, primary hyperoxaluria, and renal tubular acidosis.
- Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int 1979; 16:624–631.
- Hiatt RA, Dales LG, Friedman GD, Hunkeler EM. Frequency of urolithiasis in a prepaid medical care program. Am J Epidemiol 1982; 115:255–265.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823.
- Li J, Kennedy D, Levine M, Kumar A, Mullen J. Absent hematuria and expensive computerized tomography: case characteristics of emergency urolithiasis. J Urol 2001; 165:782–784.
- Fielding JR, Steele G, Fox LA, Heller H, Loughlin KR. Spiral computerized tomography in the evaluation of acute flank pain: a replacement for excretory urography. J Urol 1997; 157:2071–2073.
- Katz DS, Scheer M, Lumerman JH, Mellinger BC, Stillman CA, Lane MJ. Alternative or additional diagnoses on unenhanced helical computed tomography for suspected renal colic: experience with 1,000 consecutive examinations. Urology 2000; 56:53–57.
- Fowler KAB, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 2002; 222:109–113.
- Samplaski MK, Irwin BH, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med 2009; 76:592–598.
- Labrecque M, Dostaler LP, Rousselle R, Nguyen T, Poirier S. Efficacy of nonsteroidal anti-inflammatory drugs in the treatment of acute renal colic. A meta-analysis. Arch Intern Med 1994; 154:1381–1387.
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine, and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 2005; 174:167–172.
- Hollingsworth JM, Togers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet 2006; 368:1171–1179.
- Borghi L, Meschi T, Amato F, et al. Nifedipine and methylprednisolone in facilitating ureteral stone passage: a randomized, double blind, placebo-controlled study. J Urol 1994; 152:1095–1098.
- Williams RE. Long-term survey of 538 patients with upper urinary tract stone. Br J Urol 1963; 35:416–437.
- Coe FL, Keck J, Norton ER. The natural history of urolithiasis. JAMA 1977; 238:1519–1523.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water, and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843.
- Robertson WG, Peacock M, Marshall RW, Marshall DH, Nordin BE. Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N Engl J Med 1976; 294:249–252.
- Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003; 111:607–616.
- Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int 2003; 64:2150–2154.
- Broadus AE, Horst RL, Lang R, Littledike ET, Rasmussen H. The importance of circulating 1,25-dihydroxyvitamin D in the pathogenesis of hypercalciuria and renal-stone formation in primary hyperparathyroidism. N Engl J Med 1980; 302:421–426.
- Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab 1992; 75:1446–1452.
- Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med 1995; 98:50–59.
- Breslau NA, Sakhaee K, Pak CY. Impaired adaptation to saltinduced urinary calcium losses in postmenopausal osteoporosis. Trans Assoc Am Physicians 1985; 98:107–115.
- Burtis W, Gay L, Insogna K, Ellison A, Broadus A. Dietary hypercalciuria in patients with calcium oxalate kidney stones. Am J Clin Nutr 1994; 60:424–429.
- Hess B, Ackermann D, Essig M, Takkinen R, Jaeger P. Renal mass and serum calcitriol in male idiopathic calcium renal stone formers: role of protein intake. J Clin Endocrinol Metab 1995; 80:1916–1921.
- Coe FL, Parks JH, Bushinsky DA, Langman CB, Favus MJ. Chlorthalidone promotes mineral retention in patients with idiopathic hypercalciuria. Kidney Int 1988; 33:1140–1146.
- Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation, and diagnostic criteria. Am J Med 1980; 69:19–30.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126:497–504.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Parks JH, Coe FL, Evan AP, Worcester EM. Clinical and laboratory characteristics of calcium stone-formers with and without primary hyperparathyroidism. Br J Urol 2008; 103:670–678.
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 1999; 341:1249–1255.
- Mollerup CL, Vestergaard P, Frokjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 2002; 325:807.
- Odvina CV, Sakhaee K, Heller HJ, et al. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res 2007; 35:123–128.
- Lemann J, Adams ND, Gray RW. Urinary calcium excretion in human beings. N Engl J Med 1979; 301:535–541.
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 1977; 87:404–410.
- Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implication for calcium oxalate stone genesis. Chem Biol 2003; 10:271–278.
- Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386–1389.
- Coe FL, Parks JH. Pathogenesis and treatment of nephrolithiasis. In:The Kidney. Philadelphia: Lippincott Williams & Wilkins, 2000:1841–1867.
- Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63:255–265.
- Danpure CJ, Rumsby G. Molecular aetiology of primary hyperoxaluria and its implications for clinical management. Expert Rev Mol Med 2004; 6:1–16.
- Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 2007; 72:100–107.
- Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int 1986; 30:85–90.
- Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 1988; 255:F301–F306.
- Sakhaee K, Williams RH, Oh MS, et al. Alkali absorption and citrate excretion in calcium nephrolithiasis. J Bone Miner Res 1993; 8:789–794.
- Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate, high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 2002; 40:265–274.
- Griffith DP. Struvite stones. Kidney Int 1978; 13:372–382.
- Jennis FS, Lavan JN, Neale FC, Posen S. Staghorn calculi of the kidney: clinical, bacteriological and biochemical features. Br J Urol 1970; 42:511–518.
- Griffith DP, Gibson JR, Clinton CW, Musher DM. Acetohydroxamic acid: clinical studies of a urease inhibitor in patients with staghorn renal calculi. J Urol 1978; 119:9–15.
- Kamel KS, Cheema-Dhadli S, Halperin ML. Studies on the pathophysiology of the low urine pH in patients with uric acid stones. Kidney Int 2002; 61:988–994.
- Abate N, Chandalia M, Cabo-Chan AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int 2004; 65:386–392.
- Zarse CA, McAteer JA, Tann M, et al. Helical computed tomography accurately reports urinary stone composition using attenuation values: in vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology 2004; 63:828–833.
- Palacin M. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005; 20:112–124.
- Sakhaee K. Pathogenesis and medical management of cystinuria. Semin Nephrol 1996; 16:435–447.
- Shekarriz B, Stoller ML. Cystinuria and other noncalcareous calculi. Endocrinol Metab Clin North Am 2002; 31:951–977.
- Streem SB, Hall P. Effect of captopril on urinary cystine excretion in homozygous cystinuria. J Urol 1989; 142:1522–1524.
- Perazella MA, Buller GK. Successful treatment of cystinuria with captopril. Am J Kidney Dis 1993; 21:504–507.
- Sloand JA, Izzo JL. Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 1987; 147:1409–1412.
COMMON AND ON THE INCREASE
Nephrolithiasis is common, with a lifetime prevalence of 10% in men and 5% in women.1,2 Studies have shown that the prevalence is increasing in the United States. In the second National Health and Nutrition Examination Survey (1988–1994), the prevalence in adults ages 20 to 74 was greater than in the 1976–1980 survey (5.2% vs 3.2%).3 The increase was observed in whites but not in African Americans or Mexican Americans, was greater in men than in women, and was greater with age in each time period.
In addition, stones often recur, and each stone event can be associated with significant metabolic and intervention-related morbidity.
PRESENTATION: SEVERE COLIC
Most patients present with moderate to severe colic, caused by the stone entering the ureter. Stones in the proximal (upper) ureter cause pain in the flank or anterior upper abdomen. When the stone reaches the distal third of the ureter, pain is noted in the ipsilateral testicle or labia. A stone at the junction of the ureter and the bladder often causes dysuria, urgency, and frequency and may be mistaken for a lower urinary tract infection.
Less often, patients present with silent ureteral obstruction, unexplained persistent urinary infection, or painless hematuria. However, even in patients with symptoms, the absence of hematuria does not exclude urolithiasis. In a study of 397 patients presenting with acute symptomatic urolithiasis, 9% did not have hematuria.4
The differential diagnosis in a patient with symptoms suggesting renal colic includes:
- Musculoskeletal pain
- Herpes zoster
- Diverticulitis
- Duodenal ulcer
- Cholecystitis
- Pyelonephritis
- Renal infarct
- Renal hemorrhage
- Gynecologic disorders
- Ureteral obstruction from renal papillary necrosis with sloughed papillae, a blood clot, or a ureteral stricture.
HELICAL CT WITHOUT CONTRAST IS THE PREFERED IMAGING STUDY
The diagnosis can be confirmed by computed tomography (CT), renal ultrasonography, or intravenous pyelography.
Helical CT without contrast is the preferred imaging study in patients with suspected nephrolithiasis. It has several advantages over other imaging studies: it requires no radiocontrast material; it shows the distal ureters; it can detect radiolucent stones (ie, uric acid stones), radio-opaque stones, and stones as small as 1 to 2 mm; and it can detect hydronephrosis and intra-abdominal and renal disorders other than stones that could be causing the patient’s symptoms.
In a study in 100 consecutive patients presenting to an emergency department with flank pain, helical CT had a sensitivity of 98%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 97% for the diagnosis of ureteral stones.5 In a study of 1,000 consecutive patients with suspected stones, helical CT identified significant, additional, or alternative reasons for the patient’s symptoms in 10% of cases.6
Ultrasonography has the advantage of not using radiation, but it is less sensitive for detecting stones and can image only the kidney and the proximal ureter. A retrospective study in 123 patients found that, compared with helical CT as the gold standard, ultrasonography had a sensitivity of 24% and a specificity of 90%.7 Ultrasonography may also miss stones smaller than 3 mm in diameter.
Conventional radiography (kidney-ureter-bladder view) is inadequate for diagnosis as it may miss stones in the kidney or ureter (even small radio-opaque stones) and provides no information about possible obstruction.
Intravenous pyelography has few advantages in renal lithiasis, exposes the patient to the risk of radiocontrast infusion and contrast-mediated acute renal injury, and gives less information than noncontrast CT.
MEDICAL MANAGEMENT OF ACUTE STONE EVENTS
Most stones are smaller than 5 mm and readily pass without interventions such as lithotripsy, ureteroscopy, or percutaneous nephrolithotomy. (For more information on these interventions, see the review by Samplaski and colleagues in this issue of the Cleveland Clinic Journal of Medicine.8)
Even if the stone is as large as 1 cm, I would let the patient try to pass it spontaneously if it is in the distal ureter, and I would allow up to 4 weeks for this to happen.
For most patients, pain management is paramount. Randomized controlled trials suggest that parenteral nonsteroidal anti-inflammatory drugs (NSAIDs) are as effective as narcotics for controlling the pain of renal colic.9 Diclofenac (Voltaren) has been used in several studies.
To hasten stone passage, some recommend inducing high urine flow with oral intake of at least 2 to 3 L of fluids per 24 hours to ensure a urine output of at least 2 L per day.
Drugs may also help the stone to pass. A recent study in 210 patients with ureteral stones averaging 6 mm in diameter showed that tamsulosin (Flomax) increased the likelihood of spontaneous stone passage.10 A meta-analysis of 693 patients in nine randomized trials concluded that alpha-blockers and calcium channel blockers increased the likelihood of stone passage compared with no treatment.11 Borghi et al,12 in a randomized, double-blind study in 86 patients with unilateral ureteral stones, reported a higher rate of stone passage in patients treated with methylprednisolone (Medrol) 16 mg/day plus nifedipine (Procardia) 40 mg/day than in those given methylprednisolone alone.
PREVENTING RECURRENT STONES: PRINCIPLES AND SPECIFICS
Urinary stone disease recurs in 30% to 50% of patients within 5 years.1,13,14
In preventing recurrent stones, some principles apply to all patients and some are specific to the type of stone the patient had.
Stones form when the urine is supersaturated
Nephrolithiasis occurs when the concentration of stone-forming salts such as calcium oxalate, calcium phosphate, or uric acid is high. When the concentration is high enough to allow crystals to form or preformed crystals to grow, the urine is said to be supersaturated.
Several facors are the major determinants of whether the urine is supersaturated by different salts:
- Calcium oxalate—low urine volume and high concentrations of calcium and oxalate
- Calcium phosphate—a high urine calcium concentration and alkaline urine
- Uric acid—acidic urine
- Cystine—a high urinary cystine concentration and acidic urine.
Increasing daily fluid intake
Since the urinary concentration of stone-forming salts is strongly affected by the daily urine volume, it follows that increasing daily fluid intake is important in preventing recurrent stone disease.
In one study,15 199 patients with a first calcium stone were randomized to a program of high oral fluid intake or no intervention. Five years later, 12 (12%) of the 99 patients in the high-fluid intake group had had a second stone, compared with 27 (27%) in the untreated group (P = .008). Of interest, the baseline 24-hour urine volumes were significantly lower in patients with stones than in 101 normal controls (P = .001), suggesting that habitual low daily fluid intake is a risk factor for calcium stone disease.13
PREVENTING CALCIUM STONES
Most stones are composed of calcium oxalate or calcium phosphate. Calcium stone disease occurs most often in the 3rd to 5th decades of life.
Naturally occurring inhibitors of calcium crystal formation in the urine include citrate, nephrocalcin, uropontin, and magnesium. Of these, only citrate and magnesium levels are routinely measured; low levels of citrate are treated as a cause of calcium stone disease. It follows that the risk of calcium nephrolithiasis is the result of the interplay between the supersaturated state and the level of urinary inhibitors.16
Hypercalciuria and calcium oxalate stones
Calcium oxalate stones begin as crystals that form on the surface of the renal papillae over collections of suburothelial calcium phosphate particles called Randall plaque.17 The driving force for calcium oxalate overgrowth on plaque is calcium oxalate supersaturation, which is strongly linked to high urinary calcium excretion. The fraction of papillary surface covered by plaque in patients with idiopathic calcium oxalate stones correlates directly with the urine calcium level and inversely with urine volume and pH.18
Most patients with calcium oxalate stones have hypercalciuria (defined as 24-hour urinary calcium excretion > 300 mg in men, > 250 mg in women, or > 4 mg/kg in men or women).
Hypercalciuria can be idiopathic
Hypercalciuria can occur in primary hyperparathyroidism, sarcoidosis, vitamin D excess, corticosteroid treatment, renal tubular acidosis, hyperthyroidism, and malignant neoplasms. If none of these conditions is present, elevated urinary calcium excretion is considered idiopathic.
Some patients with idiopathic hypercalciuria have a strong family history of hypercalciuria and, likely, a genetic basis for the disease. This condition has been categorized by the presumed site of the primary abnormality:
Absorptive hypercalciuria. Most patients with idiopathic hypercalciuria absorb too much calcium from the intestine. In many of them, 1,25 dihydroxyvitamin D levels are slightly high and serum phosphorous levels are slightly low; the hypothesis is that they produce more 1,25 dihydroxyvitamin D or are more sensitive to it.19 However, Breslau et al20 showed that not all patients with idiopathic hypercalciuria have absorptive hypercalciuria mediated by 1,25 dihydroxyvitamin D, which suggests that the intestinal hyperabsorption of calcium has other mechanisms.
Resorptive hypercalciuria occurs if increased bone turnover leads to urinary loss of bone calcium.
Renal leak is due to a primary defect in renal tubular transport that causes loss of calcium into the urine and a secondary increase in intestinal calcium absorption or mobilization from bone.
This categorization is based on measuring fasting and 24-hour urine calcium, urinary calcium responses to a low-calcium diet, and responses to an oral calcium load.21 However, these studies are difficult to do and have been shown to have minimal clinical value.
To reduce calcium in the urine, limit sodium, give thiazides
Idiopathic hypercalciuria is worsened by a diet high in sodium22,23 and animal protein.24 Thiazide diuretics lower urinary calcium excretion and promote mineral retention.25 Therefore, treatment of idiopathic hypercalciuria consists of high fluid intake, dietary sodium restriction, and thiazide diuretics.
Calcium restriction is not advised
For several reasons, a calcium-restricted diet is not advised for patients with idiopathic hypercalciuria. 26 Dietary calcium restriction can put the patient into negative calcium balance. Further, it is thought that with less calcium to bind to dietary oxalate, more unbound oxalate can be absorbed in the colon and eventually excreted in the urine. This increase in urinary oxalate can be to the point of supersaturation, even though urinary calcium levels remain unchanged.25,27,28 This, in turn, increases the likelihood of stone formation.
Several studies showed that a higher intake of dietary calcium is actually associated with fewer calcium stone events in both men and women.25,27,28
Further, a study in 120 Italian patients with hypercalciuric calcium oxalate stones concluded that a diet that is normal in calcium, low in sodium, and low in animal protein was associated with a lower frequency of calcium stones than a low-calcium diet.29 Although both diets were associated with a reduction in urinary calcium concentrations, urinary oxalate excretion rose in those on a low-calcium diet and fell in those on a normal-calcium diet. The reduction in urinary oxalate excretion in patients on a normal-calcium diet was attributed to intestinal binding of dietary oxalate by dietary calcium, thus lessening the amount of free oxalate available for absorption. Although calcium oxalate excretion fell in both groups, it fell more in those on a normal calcium intake. Compared with those on a low-calcium diet, the patients on the normal-calcium, low-sodium, low-protein diet had a 50% lower risk of stones at 5 years.
Hyperparathyroidism
Primary hyperparathyroidism can cause hypercalciuria and nephrolithiasis. In one series,30 56 (4.9%) of 1,132 consecutive patients with nephrolithiasis had a confirmed diagnosis of hyperparathyroidism. Parathyroidectomy prevented subsequent stone disease in 48 patients.
However, only 17% to 24% of patients with primary hyperparathyroidism have urinary stones composed of calcium oxalate or calcium phosphate.31,32 In many studies, it was difficult to determine why a minority of these patients develop stones, but two studies shed some light on this.
Parks et al30 found that, compared with nephrolithiasis patients with idiopathic hypercalciuria, those with primary hyperparathyroidism have elevated serum calcium levels (but usually < 11.5 mg/dL), greater degrees of hypercalciuria (352 mg/day vs 252 mg/day, P < .001), and lower serum phosphate levels (2.45 vs 3.10 mg/dL, P < .001).
Odvina et al33 found, in a study of 131 patients with proven primary hyperparathyroidism, that 78 had nephrolithiasis and 53 did not. Those with stones excreted more calcium (343 mg/day) than those without stones (273 mg/day), had a higher urinary saturation of calcium oxalate and brushite, and excreted twice as much calcium following a 1-g oral calcium load.
These studies suggest that in patients with primary hyperparathyroidism, the risk of forming stones is related to the degree of hypercalciuria, and in particular to the increased intestinal absorption of dietary calcium.
Renal tubular acidosis
Features of distal renal tubular acidosis are systemic metabolic acidosis, alkaline urine, hypokalemia, hypercalciuria, hypocitraturia, and nephrolithiasis. The chronic metabolic acidosis results in loss of bone calcium, contributes to hypercalciuria, and is responsible for the hypocitraturia.34 Stone formation is the result of excessive urinary calcium excretion, the deficiency of the urinary crystal inhibitor citrate, and persistently alkaline urine.
Treatment with sodium bicarbonate or potassium citrate corrects the metabolic acidosis, reduces the loss of calcium from bone, corrects hypokalemia, and increases urinary citrate.
Too much uric acid in the urine
Elevated urinary uric acid excretion (> 800 mg/day in men, > 750 mg/day in women) is associated with formation of calcium oxalate stones35 and, in conjunction with low urine pH, with uric acid stones. An increase in dissolved uric acid salts induces heterogeneous calcium oxalate nucleation.36 In one randomized clinical trial,37 giving allopurinol (Zyloprim) lowered urinary uric acid excretion and was associated with a lower rate of calcium stone disease.
Too much oxalate in the urine
The 95th percentile for urinary oxalate excretion is 45 mg/day in women and 55 mg/day in men.38 Hyperoxaluria increases calcium oxalate supersaturation and contributes to calcium stone formation.
Normally, 90% of dietary oxalate binds to dietary calcium in the small intestine and passes into the stool as calcium oxalate; 10% of dietary oxalate remains free and is absorbed in the colon and subsequently excreted in the urine.
Hyperoxaluria may simply be a result of high dietary oxalate intake. However, increased enteric absorption of dietary oxalate can occur in those on a low-calcium diet (in which less calcium is available to bind to dietary oxalate, as described above) and may partially explain why a low-calcium diet has been associated with increased frequency of calcium stone disease.
Patients with enteric malabsorption of fat (eg, due to inflammatory bowel disease or intestinal bypass surgery for obesity) may also develop hyperoxaluria. This occurs because the excess enteric fat binds dietary calcium and allows free oxalate to be more readily absorbed in the colon.39
Rarely, hyperoxaluria is caused by one of several recessively inherited disorders of oxalate metabolism.40
The growing number of people with obesity has resulted in an upsurge in gastric bypass surgery. Although the current procedures do not pose the same metabolic risks as were noted in the 1970s when a different type of bypass was performed, the incidence of kidney stones does appear to be higher after these procedures. A recent analysis of 1,436 patients undergoing Roux-en-Y gastric bypass surgery found that 60 of them developed calcium stones afterward. Of these, 31 who underwent metabolic studies were found to have higher oxalate and lower citrate levels at 12 months of follow-up.41
Not enough citrate, a stone inhibitor
Hypocitraturia is defined as a daily urine citrate excretion less than 500 mg in women and 434 mg in men.42 As already mentioned, citrate plays an important role in inhibiting calcium crystal formation and preventing stone formation.
Urinary citrate excretion is mainly determined by tubular reabsorption, which is increased by acid loads and decreased by alkali loads.43 Low urine citrate levels are often seen in conditions that cause chronic metabolic acidosis, such as inflammatory bowel disease, intestinal malabsorption, and renal tubular acidosis—all of which are associated with increased occurrence of nephrolithiasis. However, in most nephrolithiasis patients with hypocitraturia, the cause is not apparent, and the mechanism of the hypocitraturia cannot be determined.44
In recent years, high-protein, low-carbohydrate diets have become popular for weight reduction, but they also have metabolic effects that increase the risk of stones.45 The metabolism of a diet high in animal protein produces more hydrogen ions that are buffered by bone, releasing calcium from bone and increasing urinary calcium excretion. These diets also cause intracellular acidosis, resulting in decreased urinary excretion of citrate. As a result of these effects, the stone-forming propensity of the urine is increased.
STRUVITE STONES MUST BE REMOVED
Struvite stones are the result of chronic upper urinary infection with urease-producing bacteria (Proteus sp, Haemophilus sp, Klebsiella sp, and Ureaplasma urealyticum).46,47 The hydrolysis of urea yields ammonium and hydroxyl ions and a persistently alkaline urine, and this scenario promotes the formation of stones composed of magnesium ammonium phosphate, ie, struvite.
Struvite stones, which are often branched (“staghorn” stones), occur more often in women and in patients who have chronic urinary obstruction or a neurologic disorder that impairs normal emptying of the bladder.
Treatment requires eradicating the infection with antibiotics and removing the bacteria-laden stones by one of several interventional techniques. Acetohydroxamic acid inhibits urease and has been used to treat struvite stone disease, but it has frequent and serious adverse effects.48
URIC ACID STONES FORM IN VERY ACIDIC URINE
Uric acid stones occur especially in patients with unusually low urine pH and hyperuricosuria. In some patients, this very low urine pH is the result of a defect in renal ammonia secretion, which results in less buffering of secreted hydrogen ions.49
The tendency to form uric acid stones is reported to be increasing in obese people with the metabolic syndrome. Some studies have shown that the defect in ammonia production by the kidney may be the result of insulin resistance.50
Urate stones are radiolucent but can be seen on ultrasonography and helical CT. On helical CT, they can be distinguished from calcium stones by their lower density.51
Since uric acid is much more soluble in an alkaline solution, both prevention and treatment should consist of alkalinization of urine to a pH of more than 6.0 with oral sodium bicarbonate or citrate solution and hydration. This treatment may actually dissolve uric acid stones. If hyperuricemia or hyperuricosuria is present, allopurinol can be prescribed.
CYSTINE STONES ALSO FORM IN ACIDIC URINE
Cystine stone disease occurs in people who have inherited an autosomally recessive gastrointestinal and renal tubular transport disorder of four amino acids, ie, cystine, ornithine, arginine, and lysine.52 Of these, cystine is the most insoluble in normally acidic urine and thus precipitates into stones. The onset is at a younger age than in calcium stone disease; the stones are radio-opaque.
Cystine solubility is about 243 mg/L in normal urine and rises with pH. Some patients can excrete as much as 1,000 mg per day.
Treatment53,54 consists of:
- Hydration, to achieve daily urine volumes of 3 to 3.5 L
- Alkalinization of the urine to a pH higher than 6.5 with potassium alkali (potassium citrate) or sodium bicarbonate
- Reduction of protein and sodium intake to reduce cystine excretion.
If these measures fail, D-penicillamine (Depen), tiopronin (Thiola), or captopril (Capoten)55–57 can be given to convert the cystine to a more soluble disulfide cysteine-drug complex. Captopril has only a modest effect at best and is usually given with another disulfide-complexing drug; it also has the disadvantage of producing hypotension. Adverse effects of D-penicillamine and tiopronin include abdominal pain, loss of taste, fever, proteinuria, and, in rare cases, nephrotic syndrome.
WORKUP AND MANAGEMENT OF NEPHROLITHIASIS
Anyone under age 20 with an initial stone deserves a more extensive evaluation, including screening for renal tubular acidosis, cystinuria, and hyperoxaluria. A more extensive workup is also warranted in patients with a history of chronic diarrhea, sarcoidosis, or a condition associated with renal tubular acidosis (eg, Sjögren syndrome), in patients with a family history of kidney stones, in patients with high-protein weight-loss diets, and in those undergoing gastric bypass surgery for obesity. In these high-risk patients, the evaluation should include 24-hour urine studies to measure calcium, oxalate, citrate, uric acid, creatinine, sodium, and volume.
Other diagnostic clues are often helpful in the decision to do a more comprehensive evaluation.
- Nephrocalcinosis on roentgenography suggests hyperparathyroidism, medullary sponge kidney, or renal tubular acidosis.
- Hypercalcemia that develops after treatment of hypercalciuria with a thiazide diuretic suggests latent hyperparathyroidism.
- A history of recurrent urinary tract infections or of anatomic abnormalities in the urinary tract should lead to an evaluation for struvite stone disease.
- Uric acid stones should be suspected in a patient with metabolic syndrome or a history of gout and are usually accompanied by a urine pH lower than 5.5.
- A urinalysis showing cystine crystals always indicates cystinuria, which should be confirmed by 24-hour urine cystine determination.
- A family history of renal stones is more common in idiopathic hypercalciuria, cystinuria, primary hyperoxaluria, and renal tubular acidosis.
COMMON AND ON THE INCREASE
Nephrolithiasis is common, with a lifetime prevalence of 10% in men and 5% in women.1,2 Studies have shown that the prevalence is increasing in the United States. In the second National Health and Nutrition Examination Survey (1988–1994), the prevalence in adults ages 20 to 74 was greater than in the 1976–1980 survey (5.2% vs 3.2%).3 The increase was observed in whites but not in African Americans or Mexican Americans, was greater in men than in women, and was greater with age in each time period.
In addition, stones often recur, and each stone event can be associated with significant metabolic and intervention-related morbidity.
PRESENTATION: SEVERE COLIC
Most patients present with moderate to severe colic, caused by the stone entering the ureter. Stones in the proximal (upper) ureter cause pain in the flank or anterior upper abdomen. When the stone reaches the distal third of the ureter, pain is noted in the ipsilateral testicle or labia. A stone at the junction of the ureter and the bladder often causes dysuria, urgency, and frequency and may be mistaken for a lower urinary tract infection.
Less often, patients present with silent ureteral obstruction, unexplained persistent urinary infection, or painless hematuria. However, even in patients with symptoms, the absence of hematuria does not exclude urolithiasis. In a study of 397 patients presenting with acute symptomatic urolithiasis, 9% did not have hematuria.4
The differential diagnosis in a patient with symptoms suggesting renal colic includes:
- Musculoskeletal pain
- Herpes zoster
- Diverticulitis
- Duodenal ulcer
- Cholecystitis
- Pyelonephritis
- Renal infarct
- Renal hemorrhage
- Gynecologic disorders
- Ureteral obstruction from renal papillary necrosis with sloughed papillae, a blood clot, or a ureteral stricture.
HELICAL CT WITHOUT CONTRAST IS THE PREFERED IMAGING STUDY
The diagnosis can be confirmed by computed tomography (CT), renal ultrasonography, or intravenous pyelography.
Helical CT without contrast is the preferred imaging study in patients with suspected nephrolithiasis. It has several advantages over other imaging studies: it requires no radiocontrast material; it shows the distal ureters; it can detect radiolucent stones (ie, uric acid stones), radio-opaque stones, and stones as small as 1 to 2 mm; and it can detect hydronephrosis and intra-abdominal and renal disorders other than stones that could be causing the patient’s symptoms.
In a study in 100 consecutive patients presenting to an emergency department with flank pain, helical CT had a sensitivity of 98%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 97% for the diagnosis of ureteral stones.5 In a study of 1,000 consecutive patients with suspected stones, helical CT identified significant, additional, or alternative reasons for the patient’s symptoms in 10% of cases.6
Ultrasonography has the advantage of not using radiation, but it is less sensitive for detecting stones and can image only the kidney and the proximal ureter. A retrospective study in 123 patients found that, compared with helical CT as the gold standard, ultrasonography had a sensitivity of 24% and a specificity of 90%.7 Ultrasonography may also miss stones smaller than 3 mm in diameter.
Conventional radiography (kidney-ureter-bladder view) is inadequate for diagnosis as it may miss stones in the kidney or ureter (even small radio-opaque stones) and provides no information about possible obstruction.
Intravenous pyelography has few advantages in renal lithiasis, exposes the patient to the risk of radiocontrast infusion and contrast-mediated acute renal injury, and gives less information than noncontrast CT.
MEDICAL MANAGEMENT OF ACUTE STONE EVENTS
Most stones are smaller than 5 mm and readily pass without interventions such as lithotripsy, ureteroscopy, or percutaneous nephrolithotomy. (For more information on these interventions, see the review by Samplaski and colleagues in this issue of the Cleveland Clinic Journal of Medicine.8)
Even if the stone is as large as 1 cm, I would let the patient try to pass it spontaneously if it is in the distal ureter, and I would allow up to 4 weeks for this to happen.
For most patients, pain management is paramount. Randomized controlled trials suggest that parenteral nonsteroidal anti-inflammatory drugs (NSAIDs) are as effective as narcotics for controlling the pain of renal colic.9 Diclofenac (Voltaren) has been used in several studies.
To hasten stone passage, some recommend inducing high urine flow with oral intake of at least 2 to 3 L of fluids per 24 hours to ensure a urine output of at least 2 L per day.
Drugs may also help the stone to pass. A recent study in 210 patients with ureteral stones averaging 6 mm in diameter showed that tamsulosin (Flomax) increased the likelihood of spontaneous stone passage.10 A meta-analysis of 693 patients in nine randomized trials concluded that alpha-blockers and calcium channel blockers increased the likelihood of stone passage compared with no treatment.11 Borghi et al,12 in a randomized, double-blind study in 86 patients with unilateral ureteral stones, reported a higher rate of stone passage in patients treated with methylprednisolone (Medrol) 16 mg/day plus nifedipine (Procardia) 40 mg/day than in those given methylprednisolone alone.
PREVENTING RECURRENT STONES: PRINCIPLES AND SPECIFICS
Urinary stone disease recurs in 30% to 50% of patients within 5 years.1,13,14
In preventing recurrent stones, some principles apply to all patients and some are specific to the type of stone the patient had.
Stones form when the urine is supersaturated
Nephrolithiasis occurs when the concentration of stone-forming salts such as calcium oxalate, calcium phosphate, or uric acid is high. When the concentration is high enough to allow crystals to form or preformed crystals to grow, the urine is said to be supersaturated.
Several facors are the major determinants of whether the urine is supersaturated by different salts:
- Calcium oxalate—low urine volume and high concentrations of calcium and oxalate
- Calcium phosphate—a high urine calcium concentration and alkaline urine
- Uric acid—acidic urine
- Cystine—a high urinary cystine concentration and acidic urine.
Increasing daily fluid intake
Since the urinary concentration of stone-forming salts is strongly affected by the daily urine volume, it follows that increasing daily fluid intake is important in preventing recurrent stone disease.
In one study,15 199 patients with a first calcium stone were randomized to a program of high oral fluid intake or no intervention. Five years later, 12 (12%) of the 99 patients in the high-fluid intake group had had a second stone, compared with 27 (27%) in the untreated group (P = .008). Of interest, the baseline 24-hour urine volumes were significantly lower in patients with stones than in 101 normal controls (P = .001), suggesting that habitual low daily fluid intake is a risk factor for calcium stone disease.13
PREVENTING CALCIUM STONES
Most stones are composed of calcium oxalate or calcium phosphate. Calcium stone disease occurs most often in the 3rd to 5th decades of life.
Naturally occurring inhibitors of calcium crystal formation in the urine include citrate, nephrocalcin, uropontin, and magnesium. Of these, only citrate and magnesium levels are routinely measured; low levels of citrate are treated as a cause of calcium stone disease. It follows that the risk of calcium nephrolithiasis is the result of the interplay between the supersaturated state and the level of urinary inhibitors.16
Hypercalciuria and calcium oxalate stones
Calcium oxalate stones begin as crystals that form on the surface of the renal papillae over collections of suburothelial calcium phosphate particles called Randall plaque.17 The driving force for calcium oxalate overgrowth on plaque is calcium oxalate supersaturation, which is strongly linked to high urinary calcium excretion. The fraction of papillary surface covered by plaque in patients with idiopathic calcium oxalate stones correlates directly with the urine calcium level and inversely with urine volume and pH.18
Most patients with calcium oxalate stones have hypercalciuria (defined as 24-hour urinary calcium excretion > 300 mg in men, > 250 mg in women, or > 4 mg/kg in men or women).
Hypercalciuria can be idiopathic
Hypercalciuria can occur in primary hyperparathyroidism, sarcoidosis, vitamin D excess, corticosteroid treatment, renal tubular acidosis, hyperthyroidism, and malignant neoplasms. If none of these conditions is present, elevated urinary calcium excretion is considered idiopathic.
Some patients with idiopathic hypercalciuria have a strong family history of hypercalciuria and, likely, a genetic basis for the disease. This condition has been categorized by the presumed site of the primary abnormality:
Absorptive hypercalciuria. Most patients with idiopathic hypercalciuria absorb too much calcium from the intestine. In many of them, 1,25 dihydroxyvitamin D levels are slightly high and serum phosphorous levels are slightly low; the hypothesis is that they produce more 1,25 dihydroxyvitamin D or are more sensitive to it.19 However, Breslau et al20 showed that not all patients with idiopathic hypercalciuria have absorptive hypercalciuria mediated by 1,25 dihydroxyvitamin D, which suggests that the intestinal hyperabsorption of calcium has other mechanisms.
Resorptive hypercalciuria occurs if increased bone turnover leads to urinary loss of bone calcium.
Renal leak is due to a primary defect in renal tubular transport that causes loss of calcium into the urine and a secondary increase in intestinal calcium absorption or mobilization from bone.
This categorization is based on measuring fasting and 24-hour urine calcium, urinary calcium responses to a low-calcium diet, and responses to an oral calcium load.21 However, these studies are difficult to do and have been shown to have minimal clinical value.
To reduce calcium in the urine, limit sodium, give thiazides
Idiopathic hypercalciuria is worsened by a diet high in sodium22,23 and animal protein.24 Thiazide diuretics lower urinary calcium excretion and promote mineral retention.25 Therefore, treatment of idiopathic hypercalciuria consists of high fluid intake, dietary sodium restriction, and thiazide diuretics.
Calcium restriction is not advised
For several reasons, a calcium-restricted diet is not advised for patients with idiopathic hypercalciuria. 26 Dietary calcium restriction can put the patient into negative calcium balance. Further, it is thought that with less calcium to bind to dietary oxalate, more unbound oxalate can be absorbed in the colon and eventually excreted in the urine. This increase in urinary oxalate can be to the point of supersaturation, even though urinary calcium levels remain unchanged.25,27,28 This, in turn, increases the likelihood of stone formation.
Several studies showed that a higher intake of dietary calcium is actually associated with fewer calcium stone events in both men and women.25,27,28
Further, a study in 120 Italian patients with hypercalciuric calcium oxalate stones concluded that a diet that is normal in calcium, low in sodium, and low in animal protein was associated with a lower frequency of calcium stones than a low-calcium diet.29 Although both diets were associated with a reduction in urinary calcium concentrations, urinary oxalate excretion rose in those on a low-calcium diet and fell in those on a normal-calcium diet. The reduction in urinary oxalate excretion in patients on a normal-calcium diet was attributed to intestinal binding of dietary oxalate by dietary calcium, thus lessening the amount of free oxalate available for absorption. Although calcium oxalate excretion fell in both groups, it fell more in those on a normal calcium intake. Compared with those on a low-calcium diet, the patients on the normal-calcium, low-sodium, low-protein diet had a 50% lower risk of stones at 5 years.
Hyperparathyroidism
Primary hyperparathyroidism can cause hypercalciuria and nephrolithiasis. In one series,30 56 (4.9%) of 1,132 consecutive patients with nephrolithiasis had a confirmed diagnosis of hyperparathyroidism. Parathyroidectomy prevented subsequent stone disease in 48 patients.
However, only 17% to 24% of patients with primary hyperparathyroidism have urinary stones composed of calcium oxalate or calcium phosphate.31,32 In many studies, it was difficult to determine why a minority of these patients develop stones, but two studies shed some light on this.
Parks et al30 found that, compared with nephrolithiasis patients with idiopathic hypercalciuria, those with primary hyperparathyroidism have elevated serum calcium levels (but usually < 11.5 mg/dL), greater degrees of hypercalciuria (352 mg/day vs 252 mg/day, P < .001), and lower serum phosphate levels (2.45 vs 3.10 mg/dL, P < .001).
Odvina et al33 found, in a study of 131 patients with proven primary hyperparathyroidism, that 78 had nephrolithiasis and 53 did not. Those with stones excreted more calcium (343 mg/day) than those without stones (273 mg/day), had a higher urinary saturation of calcium oxalate and brushite, and excreted twice as much calcium following a 1-g oral calcium load.
These studies suggest that in patients with primary hyperparathyroidism, the risk of forming stones is related to the degree of hypercalciuria, and in particular to the increased intestinal absorption of dietary calcium.
Renal tubular acidosis
Features of distal renal tubular acidosis are systemic metabolic acidosis, alkaline urine, hypokalemia, hypercalciuria, hypocitraturia, and nephrolithiasis. The chronic metabolic acidosis results in loss of bone calcium, contributes to hypercalciuria, and is responsible for the hypocitraturia.34 Stone formation is the result of excessive urinary calcium excretion, the deficiency of the urinary crystal inhibitor citrate, and persistently alkaline urine.
Treatment with sodium bicarbonate or potassium citrate corrects the metabolic acidosis, reduces the loss of calcium from bone, corrects hypokalemia, and increases urinary citrate.
Too much uric acid in the urine
Elevated urinary uric acid excretion (> 800 mg/day in men, > 750 mg/day in women) is associated with formation of calcium oxalate stones35 and, in conjunction with low urine pH, with uric acid stones. An increase in dissolved uric acid salts induces heterogeneous calcium oxalate nucleation.36 In one randomized clinical trial,37 giving allopurinol (Zyloprim) lowered urinary uric acid excretion and was associated with a lower rate of calcium stone disease.
Too much oxalate in the urine
The 95th percentile for urinary oxalate excretion is 45 mg/day in women and 55 mg/day in men.38 Hyperoxaluria increases calcium oxalate supersaturation and contributes to calcium stone formation.
Normally, 90% of dietary oxalate binds to dietary calcium in the small intestine and passes into the stool as calcium oxalate; 10% of dietary oxalate remains free and is absorbed in the colon and subsequently excreted in the urine.
Hyperoxaluria may simply be a result of high dietary oxalate intake. However, increased enteric absorption of dietary oxalate can occur in those on a low-calcium diet (in which less calcium is available to bind to dietary oxalate, as described above) and may partially explain why a low-calcium diet has been associated with increased frequency of calcium stone disease.
Patients with enteric malabsorption of fat (eg, due to inflammatory bowel disease or intestinal bypass surgery for obesity) may also develop hyperoxaluria. This occurs because the excess enteric fat binds dietary calcium and allows free oxalate to be more readily absorbed in the colon.39
Rarely, hyperoxaluria is caused by one of several recessively inherited disorders of oxalate metabolism.40
The growing number of people with obesity has resulted in an upsurge in gastric bypass surgery. Although the current procedures do not pose the same metabolic risks as were noted in the 1970s when a different type of bypass was performed, the incidence of kidney stones does appear to be higher after these procedures. A recent analysis of 1,436 patients undergoing Roux-en-Y gastric bypass surgery found that 60 of them developed calcium stones afterward. Of these, 31 who underwent metabolic studies were found to have higher oxalate and lower citrate levels at 12 months of follow-up.41
Not enough citrate, a stone inhibitor
Hypocitraturia is defined as a daily urine citrate excretion less than 500 mg in women and 434 mg in men.42 As already mentioned, citrate plays an important role in inhibiting calcium crystal formation and preventing stone formation.
Urinary citrate excretion is mainly determined by tubular reabsorption, which is increased by acid loads and decreased by alkali loads.43 Low urine citrate levels are often seen in conditions that cause chronic metabolic acidosis, such as inflammatory bowel disease, intestinal malabsorption, and renal tubular acidosis—all of which are associated with increased occurrence of nephrolithiasis. However, in most nephrolithiasis patients with hypocitraturia, the cause is not apparent, and the mechanism of the hypocitraturia cannot be determined.44
In recent years, high-protein, low-carbohydrate diets have become popular for weight reduction, but they also have metabolic effects that increase the risk of stones.45 The metabolism of a diet high in animal protein produces more hydrogen ions that are buffered by bone, releasing calcium from bone and increasing urinary calcium excretion. These diets also cause intracellular acidosis, resulting in decreased urinary excretion of citrate. As a result of these effects, the stone-forming propensity of the urine is increased.
STRUVITE STONES MUST BE REMOVED
Struvite stones are the result of chronic upper urinary infection with urease-producing bacteria (Proteus sp, Haemophilus sp, Klebsiella sp, and Ureaplasma urealyticum).46,47 The hydrolysis of urea yields ammonium and hydroxyl ions and a persistently alkaline urine, and this scenario promotes the formation of stones composed of magnesium ammonium phosphate, ie, struvite.
Struvite stones, which are often branched (“staghorn” stones), occur more often in women and in patients who have chronic urinary obstruction or a neurologic disorder that impairs normal emptying of the bladder.
Treatment requires eradicating the infection with antibiotics and removing the bacteria-laden stones by one of several interventional techniques. Acetohydroxamic acid inhibits urease and has been used to treat struvite stone disease, but it has frequent and serious adverse effects.48
URIC ACID STONES FORM IN VERY ACIDIC URINE
Uric acid stones occur especially in patients with unusually low urine pH and hyperuricosuria. In some patients, this very low urine pH is the result of a defect in renal ammonia secretion, which results in less buffering of secreted hydrogen ions.49
The tendency to form uric acid stones is reported to be increasing in obese people with the metabolic syndrome. Some studies have shown that the defect in ammonia production by the kidney may be the result of insulin resistance.50
Urate stones are radiolucent but can be seen on ultrasonography and helical CT. On helical CT, they can be distinguished from calcium stones by their lower density.51
Since uric acid is much more soluble in an alkaline solution, both prevention and treatment should consist of alkalinization of urine to a pH of more than 6.0 with oral sodium bicarbonate or citrate solution and hydration. This treatment may actually dissolve uric acid stones. If hyperuricemia or hyperuricosuria is present, allopurinol can be prescribed.
CYSTINE STONES ALSO FORM IN ACIDIC URINE
Cystine stone disease occurs in people who have inherited an autosomally recessive gastrointestinal and renal tubular transport disorder of four amino acids, ie, cystine, ornithine, arginine, and lysine.52 Of these, cystine is the most insoluble in normally acidic urine and thus precipitates into stones. The onset is at a younger age than in calcium stone disease; the stones are radio-opaque.
Cystine solubility is about 243 mg/L in normal urine and rises with pH. Some patients can excrete as much as 1,000 mg per day.
Treatment53,54 consists of:
- Hydration, to achieve daily urine volumes of 3 to 3.5 L
- Alkalinization of the urine to a pH higher than 6.5 with potassium alkali (potassium citrate) or sodium bicarbonate
- Reduction of protein and sodium intake to reduce cystine excretion.
If these measures fail, D-penicillamine (Depen), tiopronin (Thiola), or captopril (Capoten)55–57 can be given to convert the cystine to a more soluble disulfide cysteine-drug complex. Captopril has only a modest effect at best and is usually given with another disulfide-complexing drug; it also has the disadvantage of producing hypotension. Adverse effects of D-penicillamine and tiopronin include abdominal pain, loss of taste, fever, proteinuria, and, in rare cases, nephrotic syndrome.
WORKUP AND MANAGEMENT OF NEPHROLITHIASIS
Anyone under age 20 with an initial stone deserves a more extensive evaluation, including screening for renal tubular acidosis, cystinuria, and hyperoxaluria. A more extensive workup is also warranted in patients with a history of chronic diarrhea, sarcoidosis, or a condition associated with renal tubular acidosis (eg, Sjögren syndrome), in patients with a family history of kidney stones, in patients with high-protein weight-loss diets, and in those undergoing gastric bypass surgery for obesity. In these high-risk patients, the evaluation should include 24-hour urine studies to measure calcium, oxalate, citrate, uric acid, creatinine, sodium, and volume.
Other diagnostic clues are often helpful in the decision to do a more comprehensive evaluation.
- Nephrocalcinosis on roentgenography suggests hyperparathyroidism, medullary sponge kidney, or renal tubular acidosis.
- Hypercalcemia that develops after treatment of hypercalciuria with a thiazide diuretic suggests latent hyperparathyroidism.
- A history of recurrent urinary tract infections or of anatomic abnormalities in the urinary tract should lead to an evaluation for struvite stone disease.
- Uric acid stones should be suspected in a patient with metabolic syndrome or a history of gout and are usually accompanied by a urine pH lower than 5.5.
- A urinalysis showing cystine crystals always indicates cystinuria, which should be confirmed by 24-hour urine cystine determination.
- A family history of renal stones is more common in idiopathic hypercalciuria, cystinuria, primary hyperoxaluria, and renal tubular acidosis.
- Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int 1979; 16:624–631.
- Hiatt RA, Dales LG, Friedman GD, Hunkeler EM. Frequency of urolithiasis in a prepaid medical care program. Am J Epidemiol 1982; 115:255–265.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823.
- Li J, Kennedy D, Levine M, Kumar A, Mullen J. Absent hematuria and expensive computerized tomography: case characteristics of emergency urolithiasis. J Urol 2001; 165:782–784.
- Fielding JR, Steele G, Fox LA, Heller H, Loughlin KR. Spiral computerized tomography in the evaluation of acute flank pain: a replacement for excretory urography. J Urol 1997; 157:2071–2073.
- Katz DS, Scheer M, Lumerman JH, Mellinger BC, Stillman CA, Lane MJ. Alternative or additional diagnoses on unenhanced helical computed tomography for suspected renal colic: experience with 1,000 consecutive examinations. Urology 2000; 56:53–57.
- Fowler KAB, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 2002; 222:109–113.
- Samplaski MK, Irwin BH, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med 2009; 76:592–598.
- Labrecque M, Dostaler LP, Rousselle R, Nguyen T, Poirier S. Efficacy of nonsteroidal anti-inflammatory drugs in the treatment of acute renal colic. A meta-analysis. Arch Intern Med 1994; 154:1381–1387.
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine, and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 2005; 174:167–172.
- Hollingsworth JM, Togers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet 2006; 368:1171–1179.
- Borghi L, Meschi T, Amato F, et al. Nifedipine and methylprednisolone in facilitating ureteral stone passage: a randomized, double blind, placebo-controlled study. J Urol 1994; 152:1095–1098.
- Williams RE. Long-term survey of 538 patients with upper urinary tract stone. Br J Urol 1963; 35:416–437.
- Coe FL, Keck J, Norton ER. The natural history of urolithiasis. JAMA 1977; 238:1519–1523.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water, and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843.
- Robertson WG, Peacock M, Marshall RW, Marshall DH, Nordin BE. Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N Engl J Med 1976; 294:249–252.
- Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003; 111:607–616.
- Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int 2003; 64:2150–2154.
- Broadus AE, Horst RL, Lang R, Littledike ET, Rasmussen H. The importance of circulating 1,25-dihydroxyvitamin D in the pathogenesis of hypercalciuria and renal-stone formation in primary hyperparathyroidism. N Engl J Med 1980; 302:421–426.
- Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab 1992; 75:1446–1452.
- Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med 1995; 98:50–59.
- Breslau NA, Sakhaee K, Pak CY. Impaired adaptation to saltinduced urinary calcium losses in postmenopausal osteoporosis. Trans Assoc Am Physicians 1985; 98:107–115.
- Burtis W, Gay L, Insogna K, Ellison A, Broadus A. Dietary hypercalciuria in patients with calcium oxalate kidney stones. Am J Clin Nutr 1994; 60:424–429.
- Hess B, Ackermann D, Essig M, Takkinen R, Jaeger P. Renal mass and serum calcitriol in male idiopathic calcium renal stone formers: role of protein intake. J Clin Endocrinol Metab 1995; 80:1916–1921.
- Coe FL, Parks JH, Bushinsky DA, Langman CB, Favus MJ. Chlorthalidone promotes mineral retention in patients with idiopathic hypercalciuria. Kidney Int 1988; 33:1140–1146.
- Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation, and diagnostic criteria. Am J Med 1980; 69:19–30.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126:497–504.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Parks JH, Coe FL, Evan AP, Worcester EM. Clinical and laboratory characteristics of calcium stone-formers with and without primary hyperparathyroidism. Br J Urol 2008; 103:670–678.
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 1999; 341:1249–1255.
- Mollerup CL, Vestergaard P, Frokjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 2002; 325:807.
- Odvina CV, Sakhaee K, Heller HJ, et al. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res 2007; 35:123–128.
- Lemann J, Adams ND, Gray RW. Urinary calcium excretion in human beings. N Engl J Med 1979; 301:535–541.
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 1977; 87:404–410.
- Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implication for calcium oxalate stone genesis. Chem Biol 2003; 10:271–278.
- Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386–1389.
- Coe FL, Parks JH. Pathogenesis and treatment of nephrolithiasis. In:The Kidney. Philadelphia: Lippincott Williams & Wilkins, 2000:1841–1867.
- Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63:255–265.
- Danpure CJ, Rumsby G. Molecular aetiology of primary hyperoxaluria and its implications for clinical management. Expert Rev Mol Med 2004; 6:1–16.
- Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 2007; 72:100–107.
- Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int 1986; 30:85–90.
- Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 1988; 255:F301–F306.
- Sakhaee K, Williams RH, Oh MS, et al. Alkali absorption and citrate excretion in calcium nephrolithiasis. J Bone Miner Res 1993; 8:789–794.
- Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate, high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 2002; 40:265–274.
- Griffith DP. Struvite stones. Kidney Int 1978; 13:372–382.
- Jennis FS, Lavan JN, Neale FC, Posen S. Staghorn calculi of the kidney: clinical, bacteriological and biochemical features. Br J Urol 1970; 42:511–518.
- Griffith DP, Gibson JR, Clinton CW, Musher DM. Acetohydroxamic acid: clinical studies of a urease inhibitor in patients with staghorn renal calculi. J Urol 1978; 119:9–15.
- Kamel KS, Cheema-Dhadli S, Halperin ML. Studies on the pathophysiology of the low urine pH in patients with uric acid stones. Kidney Int 2002; 61:988–994.
- Abate N, Chandalia M, Cabo-Chan AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int 2004; 65:386–392.
- Zarse CA, McAteer JA, Tann M, et al. Helical computed tomography accurately reports urinary stone composition using attenuation values: in vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology 2004; 63:828–833.
- Palacin M. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005; 20:112–124.
- Sakhaee K. Pathogenesis and medical management of cystinuria. Semin Nephrol 1996; 16:435–447.
- Shekarriz B, Stoller ML. Cystinuria and other noncalcareous calculi. Endocrinol Metab Clin North Am 2002; 31:951–977.
- Streem SB, Hall P. Effect of captopril on urinary cystine excretion in homozygous cystinuria. J Urol 1989; 142:1522–1524.
- Perazella MA, Buller GK. Successful treatment of cystinuria with captopril. Am J Kidney Dis 1993; 21:504–507.
- Sloand JA, Izzo JL. Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 1987; 147:1409–1412.
- Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int 1979; 16:624–631.
- Hiatt RA, Dales LG, Friedman GD, Hunkeler EM. Frequency of urolithiasis in a prepaid medical care program. Am J Epidemiol 1982; 115:255–265.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823.
- Li J, Kennedy D, Levine M, Kumar A, Mullen J. Absent hematuria and expensive computerized tomography: case characteristics of emergency urolithiasis. J Urol 2001; 165:782–784.
- Fielding JR, Steele G, Fox LA, Heller H, Loughlin KR. Spiral computerized tomography in the evaluation of acute flank pain: a replacement for excretory urography. J Urol 1997; 157:2071–2073.
- Katz DS, Scheer M, Lumerman JH, Mellinger BC, Stillman CA, Lane MJ. Alternative or additional diagnoses on unenhanced helical computed tomography for suspected renal colic: experience with 1,000 consecutive examinations. Urology 2000; 56:53–57.
- Fowler KAB, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 2002; 222:109–113.
- Samplaski MK, Irwin BH, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med 2009; 76:592–598.
- Labrecque M, Dostaler LP, Rousselle R, Nguyen T, Poirier S. Efficacy of nonsteroidal anti-inflammatory drugs in the treatment of acute renal colic. A meta-analysis. Arch Intern Med 1994; 154:1381–1387.
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine, and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol 2005; 174:167–172.
- Hollingsworth JM, Togers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet 2006; 368:1171–1179.
- Borghi L, Meschi T, Amato F, et al. Nifedipine and methylprednisolone in facilitating ureteral stone passage: a randomized, double blind, placebo-controlled study. J Urol 1994; 152:1095–1098.
- Williams RE. Long-term survey of 538 patients with upper urinary tract stone. Br J Urol 1963; 35:416–437.
- Coe FL, Keck J, Norton ER. The natural history of urolithiasis. JAMA 1977; 238:1519–1523.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water, and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843.
- Robertson WG, Peacock M, Marshall RW, Marshall DH, Nordin BE. Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N Engl J Med 1976; 294:249–252.
- Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003; 111:607–616.
- Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int 2003; 64:2150–2154.
- Broadus AE, Horst RL, Lang R, Littledike ET, Rasmussen H. The importance of circulating 1,25-dihydroxyvitamin D in the pathogenesis of hypercalciuria and renal-stone formation in primary hyperparathyroidism. N Engl J Med 1980; 302:421–426.
- Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab 1992; 75:1446–1452.
- Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med 1995; 98:50–59.
- Breslau NA, Sakhaee K, Pak CY. Impaired adaptation to saltinduced urinary calcium losses in postmenopausal osteoporosis. Trans Assoc Am Physicians 1985; 98:107–115.
- Burtis W, Gay L, Insogna K, Ellison A, Broadus A. Dietary hypercalciuria in patients with calcium oxalate kidney stones. Am J Clin Nutr 1994; 60:424–429.
- Hess B, Ackermann D, Essig M, Takkinen R, Jaeger P. Renal mass and serum calcitriol in male idiopathic calcium renal stone formers: role of protein intake. J Clin Endocrinol Metab 1995; 80:1916–1921.
- Coe FL, Parks JH, Bushinsky DA, Langman CB, Favus MJ. Chlorthalidone promotes mineral retention in patients with idiopathic hypercalciuria. Kidney Int 1988; 33:1140–1146.
- Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation, and diagnostic criteria. Am J Med 1980; 69:19–30.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126:497–504.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Parks JH, Coe FL, Evan AP, Worcester EM. Clinical and laboratory characteristics of calcium stone-formers with and without primary hyperparathyroidism. Br J Urol 2008; 103:670–678.
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 1999; 341:1249–1255.
- Mollerup CL, Vestergaard P, Frokjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 2002; 325:807.
- Odvina CV, Sakhaee K, Heller HJ, et al. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res 2007; 35:123–128.
- Lemann J, Adams ND, Gray RW. Urinary calcium excretion in human beings. N Engl J Med 1979; 301:535–541.
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 1977; 87:404–410.
- Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implication for calcium oxalate stone genesis. Chem Biol 2003; 10:271–278.
- Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386–1389.
- Coe FL, Parks JH. Pathogenesis and treatment of nephrolithiasis. In:The Kidney. Philadelphia: Lippincott Williams & Wilkins, 2000:1841–1867.
- Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63:255–265.
- Danpure CJ, Rumsby G. Molecular aetiology of primary hyperoxaluria and its implications for clinical management. Expert Rev Mol Med 2004; 6:1–16.
- Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 2007; 72:100–107.
- Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int 1986; 30:85–90.
- Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 1988; 255:F301–F306.
- Sakhaee K, Williams RH, Oh MS, et al. Alkali absorption and citrate excretion in calcium nephrolithiasis. J Bone Miner Res 1993; 8:789–794.
- Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate, high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 2002; 40:265–274.
- Griffith DP. Struvite stones. Kidney Int 1978; 13:372–382.
- Jennis FS, Lavan JN, Neale FC, Posen S. Staghorn calculi of the kidney: clinical, bacteriological and biochemical features. Br J Urol 1970; 42:511–518.
- Griffith DP, Gibson JR, Clinton CW, Musher DM. Acetohydroxamic acid: clinical studies of a urease inhibitor in patients with staghorn renal calculi. J Urol 1978; 119:9–15.
- Kamel KS, Cheema-Dhadli S, Halperin ML. Studies on the pathophysiology of the low urine pH in patients with uric acid stones. Kidney Int 2002; 61:988–994.
- Abate N, Chandalia M, Cabo-Chan AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int 2004; 65:386–392.
- Zarse CA, McAteer JA, Tann M, et al. Helical computed tomography accurately reports urinary stone composition using attenuation values: in vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology 2004; 63:828–833.
- Palacin M. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005; 20:112–124.
- Sakhaee K. Pathogenesis and medical management of cystinuria. Semin Nephrol 1996; 16:435–447.
- Shekarriz B, Stoller ML. Cystinuria and other noncalcareous calculi. Endocrinol Metab Clin North Am 2002; 31:951–977.
- Streem SB, Hall P. Effect of captopril on urinary cystine excretion in homozygous cystinuria. J Urol 1989; 142:1522–1524.
- Perazella MA, Buller GK. Successful treatment of cystinuria with captopril. Am J Kidney Dis 1993; 21:504–507.
- Sloand JA, Izzo JL. Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 1987; 147:1409–1412.
KEY POINTS
- During an acute stone event, medical management focuses on pain control. Hydration and certain drugs may help the stone to pass.
- Most stones are composed of calcium oxalate or calcium phosphate. Less common are uric acid, magnesium ammonium phosphate, and cystine stones.
- To prevent stones from recurring, patients who have had any type of stone should maintain an adequate fluid intake to keep the urine dilute.
- Paradoxically, calcium restriction is not warranted for patients who have had calcium stones, and may even be harmful.
- Alkalinization of the urine may help prevent recurrent uric acid stones and cystine stones.