User login
Prodromal symptoms of schizophrenia: What to look for
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
Schizophrenia is characterized by psychotic symptoms that typically follow a prodromal period of premonitory signs and symptoms that appear before the manifestation of the full-blown syndrome. Signs and symptoms during the prodromal phase are subsyndromal, which implies a lower degree of intensity, duration, or frequency than observed when the patient meets the full criteria for the syndrome. Early detection of prodromal symptoms can improve prognosis, but these subtle symptoms may go unrecognized.
In schizophrenia, a patient may exhibit prodromal signs and symptoms before the appearance of pathognomonic symptoms, such as delusions, hallucinations, and disorganization. The schizophrenia prodrome can be conceptualized as a period of prepsychotic disturbances depicting an alteration in the individual’s behavior and perception. Prodromal symptoms can last from weeks to years before the psychotic illness clinically manifests.1 The prodromal symptom cluster typically becomes evident during adolescence and young adulthood.2
In the mid-1990s, investigators tried to identify a “putative prodrome” for psychosis. The term “at-risk mental state” (ARMS) for psychosis is based on retrospective reports of prodromal symptoms in first-episode psychosis. Over the next 2 decades, scales such as the Comprehensive Assessment of ARMS (CAARMS)3 and the Structured Interview for Prodromal Syndrome4 were designed to enhance the objectivity and diagnostic accuracy of the ARMS. These scales have reasonable interrater reliability.5
Researchers also have attempted to stage the severity of ARMS.6 Key symptom group predictors were studied to determine which individual symptoms or cluster of symptoms are most associated with poor outcomes and progression to psychosis. Raballo et al7 found the severity of the CAARMS disorganization dimension was the strongest predictor of transition to frank psychosis. Other research suggests that approximately one-third of ARMS patients transition to psychosis within 3 years, another one-third have persistent attenuated psychotic symptoms, and the remaining one-third experience symptom remission.8,9
Despite multiple studies and meta-analyses, current scales and clinical predictors continue to be imperfect.8 Efforts to identify specific biological markers and predictors of transition to clinical psychosis have not been successful for ARMS.10,11 The Table8,9,12,13 summarizes diagnostic criteria that have been developed to more clearly identify which ARMS patients face the highest imminent risk for transition to psychosis; these have been referred to as ultra high-risk (UHR) criteria.14 These UHR criteria depict 3 categories of clinical presentation believed to confer risk of transition to psychosis: attenuated psychotic symptoms, transient psychotic symptoms, and genetic predisposition. Subsequent research found that certain additional symptom variables, as well as combinations of specific symptom clusters, conferred increased risk and improved the positive predictive sensitivity to as high as 83%.15 In addition to the UHR criteria, the Table8,9,12,13 also lists these additional variables shown to confer a high positive predictive value (PPV) of transition, alone or in combination with the UHR criterion. Thompson et al16 provide more detailed information on these later variables and their relative PPV.

What about treatment?
While discussion of the optimal treatment options for patients with prodromal symptoms of schizophrenia is beyond the scope of this article, early interventions can focus on preventing the biological, psychological, and social disruption that results from such symptoms. Establishing a therapeutic alliance with the patient while they retain insight and engaging supportive family members is a key starting point. Case management, cognitive-behavioral or supportive therapy, and treatment of comorbid mood, anxiety, or substance use disorders are helpful. There is no clear consensus on the utility of pharmacotherapy in the prodromal stage of psychosis. While scales and structured interviews can guide assessment, clinical judgment is the key driver of the appropriateness of initiating pharmacologic treatment to address symptoms. Because up to two-thirds of patients who satisfy UHR criteria do not go on to develop schizophrenia,16 clinicians should be thoughtful about the risks and benefits of antipsychotics.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
1. George M, Maheshwari S, Chandran S, et al. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59(4):505-509.
2. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370.
3. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39(11-12):964-971.
4. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703-715.
5. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal Questionnaire--brief version (PQ-B). Schizophr Res. 2011;129(1):42-46.
6. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2(9):825-834.
7. Raballo A, Nelson B, Thompson A, et al. The comprehensive assessment of at-risk mental states: from mapping the onset to mapping the structure. Schizophr Res. 2011;127(1-3):107-114.
8. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229.
9. Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756.
10. Castle DJ. Is it appropriate to treat people at high-risk of psychosis before first onset? - no. Med J Aust. 2012;196(9):557.
11. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 2013;58(1):13-18.
12. Nelson B, Yung AR. Can clinicians predict psychosis in an ultra high risk group? Aust N Z J Psychiatry. 2010;44(7):625-630.
13. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405-416.
14. Yung AR, Phillips LJ, Yuen HP, et al. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2-3):131-142.
15. Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251.
16. Thompson A, Marwaha S, Broome MR. At-risk mental state for psychosis: identification and current treatment approaches. BJPsych Advances. 2016;22(3):186-193.
Lithium for bipolar disorder: Which patients will respond?
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
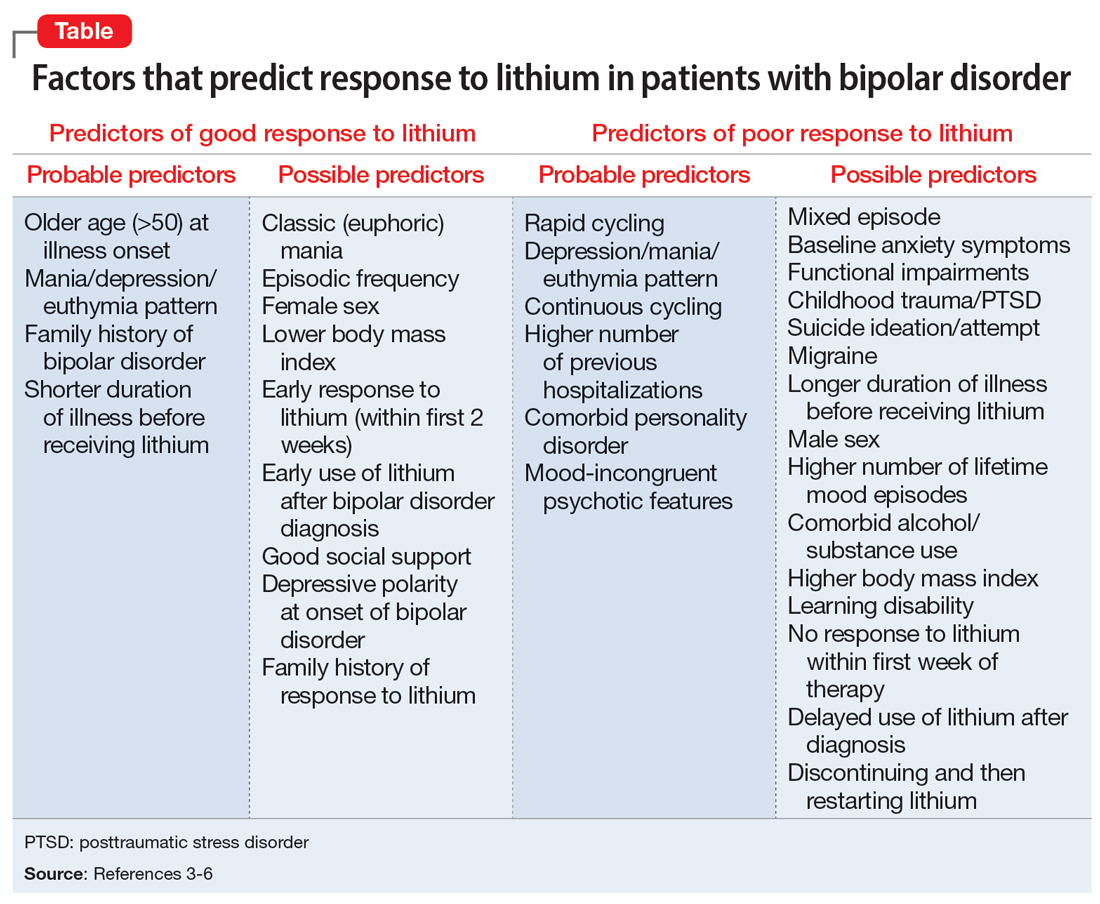
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.
Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Switching antipsychotics: A guide to dose equivalents
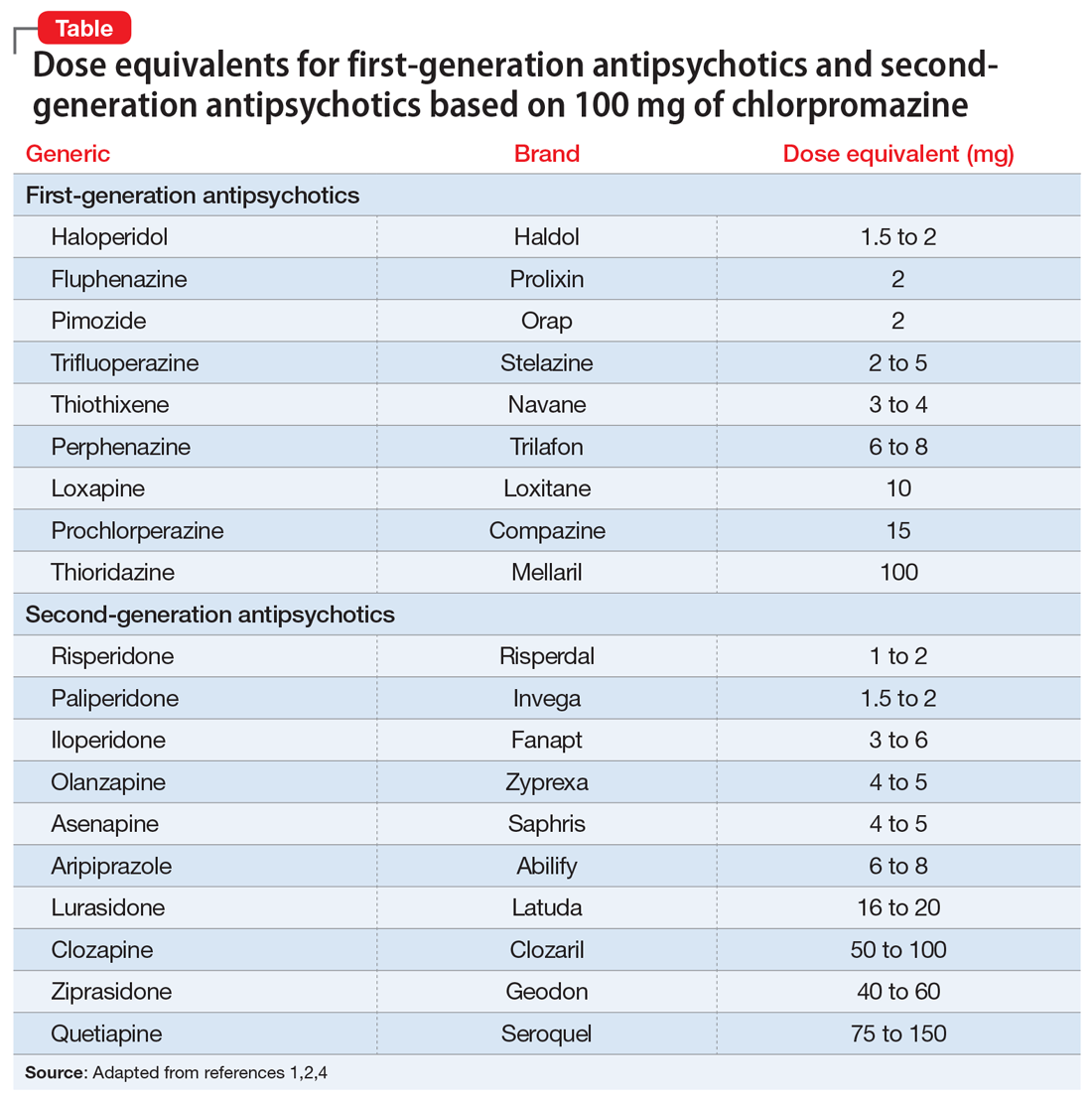
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
Chlorpromazine (CPZ), a low-potency first-generation antipsychotic (FGA), was the first medication approved for the management of schizophrenia. Since its approval, some psychiatrists have prescribed subsequent antipsychotics based on CPZ’s efficacy and dosing. Comparing dosages of newer antipsychotics using a CPZ equivalent as a baseline remains a relevant method of determining which agent to prescribe, and at what dose.1,2
Psychiatrists frequently care for patients who are treatment-refractory or older adults with poor medication tolerance and age-related medical illness. Quick access to the comparative potency of different antipsychotics can help guide titration to the approximate equivalent dose of CPZ when initiating a medication, switching from 1 antipsychotic to another, or augmenting or combining antipsychotics. Fortunately, many authors, such as Woods2and Davis,3 have codified the dosing ratio equivalences of FGAs and second-generation antipsychotics (SGAs) using CPZ, 100 mg. To help psychiatrists use CPZ dosages as a point of comparison for prescribing other antipsychotics, the Table1,2,4 (page 14) lists dose equivalents for oral FGAs and SGAs based on CPZ, 100 mg. (For information on dose equivalents for injectable antipsychotics, see “Second-generation long-acting injectable antipsychotics: A practical guide,”
While this information cannot replace a psychiatrist’s clinical judgment, it can serve as a clinically useful prescribing tool. In addition to providing this Table, we discuss what you should consider when using these equivalents to switch antipsychotics and estimate the ultimate dose target for effective management of psychotic disorders.
A few caveats
Bioactive equivalent dosages should be targeted as a rough guide when switching from one FGA or SGA to another. Common indications for switching antipsychotics include an inadequate therapeutic response after a medication trial of an adequate dose and duration; relapse of psychosis despite medication adherence; intolerable adverse effects; cost; a new-onset, contraindicating medical illness; and lapses in medication compliance that necessitate a change to IM formulations.5 Keep in mind that medication changes should be tailored to the patient’s specific clinical characteristics.
Several other clinical and pharmacologic variabilities should be kept in mind when switching antipsychotics using CPZ dosage equivalents5,6:
- The therapeutic CPZ equivalent doses may be less precise for SGAs than for FGAs because the equivalents are largely based on dopaminergic blockade instead of cholinergic, serotonergic, or histaminergic systems
- For some antipsychotics, the relationship between dose and potency is nonlinear. For example, as the dosage of haloperidol increases, its relative antipsychotic potency decreases
- Differences in half-lives between 2 agents can add complexity to calculating the dosage equivalent
- Regardless of comparative dosing, before initiating a new antipsychotic, psychiatrists should read the dosing instructions in the FDA-approved package insert, and exercise caution before titrating a new medication to the maximum recommended dose.
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how
1. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207-208.
2. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663-667.
3. Davis JM. Dose equivalence of the anti-psychotic drugs. J Psych Res. 1974;11:65-69.
4. Psychiatric pharmacy essentials: antipsychotic dose equivalents. College of Psychiatric and Neurologic Pharmacists. Accessed February 2, 2021. https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents
5. Guidelines for antipsychotic medication switches. Humber NHS. Last Reviewed September 2012. Accessed February 2, 2021. https://www.psychdb.com/_media/meds/antipsychotics/nhs_guidelines_antipsychotic_switch.pdf
6. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric Times. Published March 14, 2013. Accessed February 2, 2021. https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how