User login
Painful Nonhealing Vulvar and Perianal Erosions
The Diagnosis: Cutaneous Crohn Disease
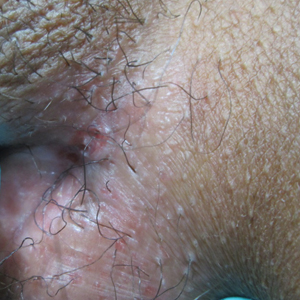
A punch biopsy of the vulvar skin revealed epidermal hyperplasia with moderate spongiosis and exocytosis of lymphocytes and neutrophils in the epidermis. A brisk mixed inflammatory infiltrate of epithelioid histiocytes, multinucleate foreign body-type giant cells, lymphocytes, plasma cells, neutrophils, and eosinophils in a granulomatous pattern also were present in the dermis (Figure). Periodic acid-Schiff and acid-fast bacillus stains were negative. Given the history of Crohn disease (CD) and the characteristic dermal noncaseating granulomas on histology, the patient was diagnosed with cutaneous CD.
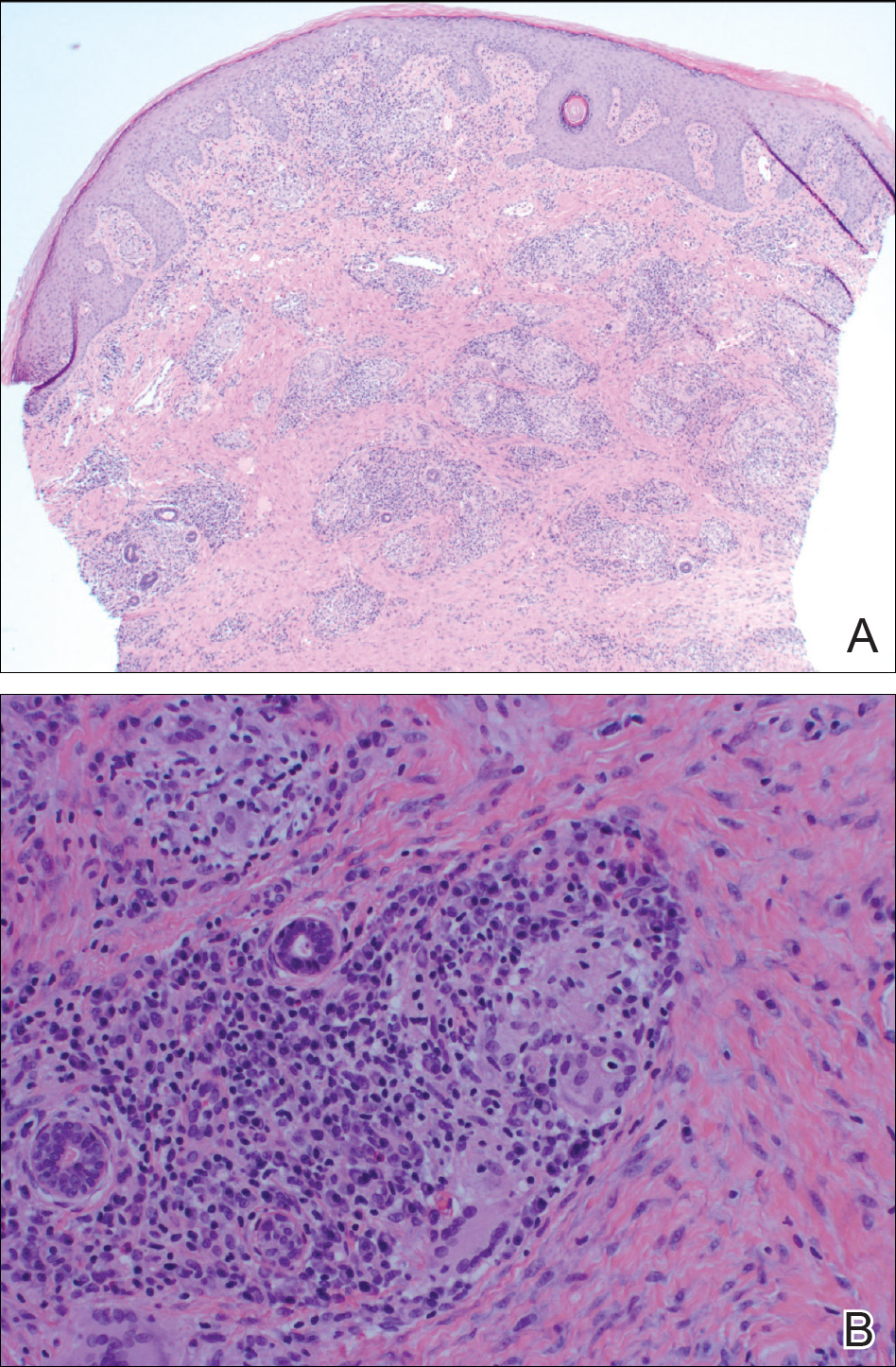
Although the patient was offered a topical corticosteroid, she deferred topical therapy. Given the lack of response to adalimumab, the gastroenterology department switched the patient to a treatment of infliximab 5 mg/kg every 8 weeks. Azathioprine was discontinued and the patient was switched to intramuscular methotrexate 25 mg/mL weekly. Slow reepithelialization of the vulvar and perianal erosions occurred on this regimen.
Although CD has numerous cutaneous features, cutaneous CD, also known as metastatic CD, is the rarest cutaneous manifestation of CD.1 This disease process is characterized by noncaseating granulomatous cutaneous lesions that are not contiguous with the affected gastrointestinal tract.2 The pathogenesis of cutaneous CD is unknown. Young adults tend to be more predisposed to developing cutaneous CD, likely due to the age distribution of CD.3
Cutaneous CD commonly presents in patients with a well-established history of gastrointestinal CD but occasionally can be the presenting sign of CD.1 The most common sites of involvement are the legs, vulva, penis, trunk, face, and intertriginous areas. Cutaneous CD findings can be divided into 2 subgroups: genital and nongenital lesions. Genital findings involve ulceration, erythema, edema, and fissuring of the vulva, labia, clitoris, scrotum, penis, and perineum. Nongenital cutaneous manifestations include ulcers; erythematous papules, plaques, and nodules; abscesslike lesions; and lichenoid papules.4,5 The severity of cutaneous lesions does not correlate to the severity of gastrointestinal disease; however, colon involvement is more common in patients with cutaneous CD.6
Histologically, cutaneous CD presents as noncaseating granulomatous inflammation in the papillary and reticular dermis. These granulomas consist of epithelioid histiocytes and multinucleated giant cells with a lymphocytic infiltrate.5
Given the rarity of cutaneous CD, treatment approach is based on anecdotal evidence from case reports and case series. For a single lesion or localized disease, topical superpotent or intralesional steroids are recommended for initial therapy.3 Oral metronidazole also is an effective treatment and can be combined with topical or intralesional steroids.7 For disseminated disease, systemic corticosteroids have shown efficacy.3 Other reported treatment options include oral corticosteroids, sulfasalazine, azathioprine, 6-mercaptopurine, infliximab, and adalimumab. If monotherapy fails, combination therapy may be needed. Surgical debridement may be attempted if medical therapy fails but is complicated by wound dehiscence and disease recurrence.3
Although genital ulcers can be a presentation of Behçet disease and genital herpes infection, genital nodules and plaques are not typical for these 2 diseases. Also, the patient did not have oral ulcers, which is a common feature of Behçet disease. Genital sarcoidosis is extremely rare, and cutaneous CD was more likely given the patient's medical history. Finally, Jacquet dermatitis is more common in children, and patients with this condition typically have history of fecal and urinary incontinence.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Stingeni L, Neve D, Bassotti G, et al. Cutaneous Crohn's disease successfully treated with adalimumab [published online Sep 15, 2015]. J Eur Acad Dermatol Venerol. 2016;30:E72-E74.
- Kurtzman DJ, Jones T, Fangru L, et al. Metastatic Crohn's disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813.
- Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33:417-431.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review [published online June 19, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease, part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
The Diagnosis: Cutaneous Crohn Disease
A punch biopsy of the vulvar skin revealed epidermal hyperplasia with moderate spongiosis and exocytosis of lymphocytes and neutrophils in the epidermis. A brisk mixed inflammatory infiltrate of epithelioid histiocytes, multinucleate foreign body-type giant cells, lymphocytes, plasma cells, neutrophils, and eosinophils in a granulomatous pattern also were present in the dermis (Figure). Periodic acid-Schiff and acid-fast bacillus stains were negative. Given the history of Crohn disease (CD) and the characteristic dermal noncaseating granulomas on histology, the patient was diagnosed with cutaneous CD.

Although the patient was offered a topical corticosteroid, she deferred topical therapy. Given the lack of response to adalimumab, the gastroenterology department switched the patient to a treatment of infliximab 5 mg/kg every 8 weeks. Azathioprine was discontinued and the patient was switched to intramuscular methotrexate 25 mg/mL weekly. Slow reepithelialization of the vulvar and perianal erosions occurred on this regimen.
Although CD has numerous cutaneous features, cutaneous CD, also known as metastatic CD, is the rarest cutaneous manifestation of CD.1 This disease process is characterized by noncaseating granulomatous cutaneous lesions that are not contiguous with the affected gastrointestinal tract.2 The pathogenesis of cutaneous CD is unknown. Young adults tend to be more predisposed to developing cutaneous CD, likely due to the age distribution of CD.3
Cutaneous CD commonly presents in patients with a well-established history of gastrointestinal CD but occasionally can be the presenting sign of CD.1 The most common sites of involvement are the legs, vulva, penis, trunk, face, and intertriginous areas. Cutaneous CD findings can be divided into 2 subgroups: genital and nongenital lesions. Genital findings involve ulceration, erythema, edema, and fissuring of the vulva, labia, clitoris, scrotum, penis, and perineum. Nongenital cutaneous manifestations include ulcers; erythematous papules, plaques, and nodules; abscesslike lesions; and lichenoid papules.4,5 The severity of cutaneous lesions does not correlate to the severity of gastrointestinal disease; however, colon involvement is more common in patients with cutaneous CD.6
Histologically, cutaneous CD presents as noncaseating granulomatous inflammation in the papillary and reticular dermis. These granulomas consist of epithelioid histiocytes and multinucleated giant cells with a lymphocytic infiltrate.5
Given the rarity of cutaneous CD, treatment approach is based on anecdotal evidence from case reports and case series. For a single lesion or localized disease, topical superpotent or intralesional steroids are recommended for initial therapy.3 Oral metronidazole also is an effective treatment and can be combined with topical or intralesional steroids.7 For disseminated disease, systemic corticosteroids have shown efficacy.3 Other reported treatment options include oral corticosteroids, sulfasalazine, azathioprine, 6-mercaptopurine, infliximab, and adalimumab. If monotherapy fails, combination therapy may be needed. Surgical debridement may be attempted if medical therapy fails but is complicated by wound dehiscence and disease recurrence.3
Although genital ulcers can be a presentation of Behçet disease and genital herpes infection, genital nodules and plaques are not typical for these 2 diseases. Also, the patient did not have oral ulcers, which is a common feature of Behçet disease. Genital sarcoidosis is extremely rare, and cutaneous CD was more likely given the patient's medical history. Finally, Jacquet dermatitis is more common in children, and patients with this condition typically have history of fecal and urinary incontinence.
The Diagnosis: Cutaneous Crohn Disease
A punch biopsy of the vulvar skin revealed epidermal hyperplasia with moderate spongiosis and exocytosis of lymphocytes and neutrophils in the epidermis. A brisk mixed inflammatory infiltrate of epithelioid histiocytes, multinucleate foreign body-type giant cells, lymphocytes, plasma cells, neutrophils, and eosinophils in a granulomatous pattern also were present in the dermis (Figure). Periodic acid-Schiff and acid-fast bacillus stains were negative. Given the history of Crohn disease (CD) and the characteristic dermal noncaseating granulomas on histology, the patient was diagnosed with cutaneous CD.

Although the patient was offered a topical corticosteroid, she deferred topical therapy. Given the lack of response to adalimumab, the gastroenterology department switched the patient to a treatment of infliximab 5 mg/kg every 8 weeks. Azathioprine was discontinued and the patient was switched to intramuscular methotrexate 25 mg/mL weekly. Slow reepithelialization of the vulvar and perianal erosions occurred on this regimen.
Although CD has numerous cutaneous features, cutaneous CD, also known as metastatic CD, is the rarest cutaneous manifestation of CD.1 This disease process is characterized by noncaseating granulomatous cutaneous lesions that are not contiguous with the affected gastrointestinal tract.2 The pathogenesis of cutaneous CD is unknown. Young adults tend to be more predisposed to developing cutaneous CD, likely due to the age distribution of CD.3
Cutaneous CD commonly presents in patients with a well-established history of gastrointestinal CD but occasionally can be the presenting sign of CD.1 The most common sites of involvement are the legs, vulva, penis, trunk, face, and intertriginous areas. Cutaneous CD findings can be divided into 2 subgroups: genital and nongenital lesions. Genital findings involve ulceration, erythema, edema, and fissuring of the vulva, labia, clitoris, scrotum, penis, and perineum. Nongenital cutaneous manifestations include ulcers; erythematous papules, plaques, and nodules; abscesslike lesions; and lichenoid papules.4,5 The severity of cutaneous lesions does not correlate to the severity of gastrointestinal disease; however, colon involvement is more common in patients with cutaneous CD.6
Histologically, cutaneous CD presents as noncaseating granulomatous inflammation in the papillary and reticular dermis. These granulomas consist of epithelioid histiocytes and multinucleated giant cells with a lymphocytic infiltrate.5
Given the rarity of cutaneous CD, treatment approach is based on anecdotal evidence from case reports and case series. For a single lesion or localized disease, topical superpotent or intralesional steroids are recommended for initial therapy.3 Oral metronidazole also is an effective treatment and can be combined with topical or intralesional steroids.7 For disseminated disease, systemic corticosteroids have shown efficacy.3 Other reported treatment options include oral corticosteroids, sulfasalazine, azathioprine, 6-mercaptopurine, infliximab, and adalimumab. If monotherapy fails, combination therapy may be needed. Surgical debridement may be attempted if medical therapy fails but is complicated by wound dehiscence and disease recurrence.3
Although genital ulcers can be a presentation of Behçet disease and genital herpes infection, genital nodules and plaques are not typical for these 2 diseases. Also, the patient did not have oral ulcers, which is a common feature of Behçet disease. Genital sarcoidosis is extremely rare, and cutaneous CD was more likely given the patient's medical history. Finally, Jacquet dermatitis is more common in children, and patients with this condition typically have history of fecal and urinary incontinence.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Stingeni L, Neve D, Bassotti G, et al. Cutaneous Crohn's disease successfully treated with adalimumab [published online Sep 15, 2015]. J Eur Acad Dermatol Venerol. 2016;30:E72-E74.
- Kurtzman DJ, Jones T, Fangru L, et al. Metastatic Crohn's disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813.
- Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33:417-431.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review [published online June 19, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease, part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Stingeni L, Neve D, Bassotti G, et al. Cutaneous Crohn's disease successfully treated with adalimumab [published online Sep 15, 2015]. J Eur Acad Dermatol Venerol. 2016;30:E72-E74.
- Kurtzman DJ, Jones T, Fangru L, et al. Metastatic Crohn's disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813.
- Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33:417-431.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review [published online June 19, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease, part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
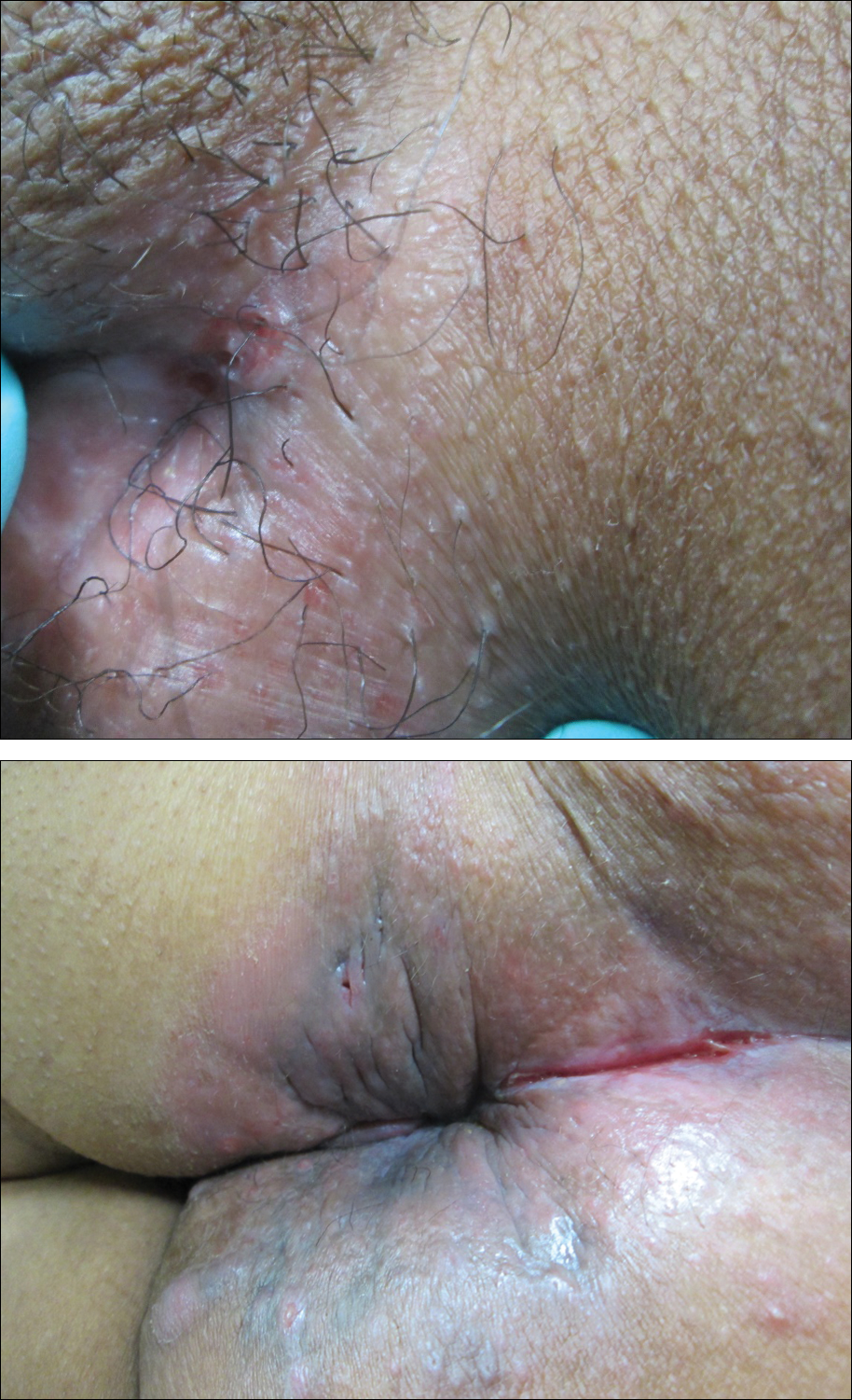
A 38-year-old woman with a history of Crohn disease presented with painful nonhealing vulvar and perianal erosions of 6 months' duration. The erosions developed 4 months after discontinuing adalimumab for a planned surgery. During this time, the patient also had an exacerbation of Crohn colitis and developed an anal fistula. Prior to this break in adalimumab, the patient's Crohn disease was well controlled on adalimumab 40 mg every 2 weeks, azathioprine 100 mg daily, and mesalamine 4.8 g daily. Despite restarting adalimumab and therapy with multiple antibiotics (ie, metronidazole, ciprofloxacin), the erosions persisted. On physical examination erythematous plaques and nodules were present at the vulvar (top) and perianal (bottom) skin. In addition, well-demarcated erosions measuring 20 mm and 80 mm were present on the vulvar and perianal skin, respectively. Human immunodeficiency virus screening and rapid plasma reagin were negative.
Physician Advocacy for Zoster Vaccination
Herpes zoster (HZ) infection occurs when the varicella-zoster virus (VZV) is reactivated due to waning cellular immunity associated with age or immunosuppression. It results in a painful blistering cutaneous eruption.1 The incidence and rate of complications from HZ infection increase with age.2 The most common complication of HZ infection is postherpetic neuralgia (PHN), which can be extremely debilitating.1
In 2006 the US Food and Drug Administration approved a live attenuated HZ vaccine that boosts VZV cell-mediated immunity and largely reduces HZ disease burden. The HZ vaccine contains the same strain of VZV as the varicella vaccine but contains 14 times more virus particles.3 In a study of the efficacy and safety of the HZ vaccine, HZ vaccination was associated with a 51% reduction in HZ incidence, a 61% reduction in HZ disease burden, and a 67% reduction in PHN incidence at 3-year follow-up.4 In adults aged 60 to 69 years, the benefit of the HZ vaccine resulted from the reduction in HZ incidence.5 However, in adults 70 years and older, the benefit resulted from the reduction in PHN incidence and severity. Overall, the absolute benefit of the HZ vaccine was greatest in the older age group, as the severity and incidence of HZ and PHN are highest in these patients.5 Although efficacy declines with time, a long-term persistence substudy demonstrated that the HZ vaccine still reduced the incidence and severity of HZ.6
The HZ vaccine currently is approved for adults aged 50 years or older.3 Antivirals that are active against VZV (eg, acyclovir, valacyclovir, famciclovir) should not be administered 24 hours before or 14 days after vaccination.1 Concurrent administration of the HZ vaccine and the pneumococcal vaccine is not recommended due to risk for reduced immunogenicity of the zoster vaccine.5 Because it is a live vaccine, the HZ vaccine is not recommended in immunocompromised patients. However, the HZ vaccine can be safely given to moderately immunosuppressed patients. The HZ vaccine also is well tolerated and stimulates a strong cell-mediated immune response in adults who have had prior HZ infections. Herpes zoster vaccination is recommended in patients with a history of shingles, though there are no published data showing that it reduces the already low rate of recurrent HZ infections.7
Despite strong efficacy data and established guidelines, a low vaccination rate has been reported8 due to doubts about its long-term efficacy, failure of both physicians and patients to recognize the burden of disease imposed by HZ infection and PHN, and concerns about reimbursement and out-of-pocket costs for the patient.5 Furthermore, many patients who are eligible to receive the HZ vaccine may not do so because they do not remember having chickenpox and therefore do not feel they are at risk for developing shingles.
The HZ vaccine is an important factor in public health prevention strategy, as HZ infection and PHN are common, incurable, and incapacitating. The HZ vaccine is the most efficacious agent currently available on the market for prevention. It is important for dermatologists to educate our patients and encourage them to receive the HZ vaccine to safeguard their long-term health.
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
2. Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. J Intern Med. 2011;269:496-506.
3. Javed S, Javed SA, Trying SK. Varicella vaccines. Curr Open Infect Dis. 2012;25:135-140.
4. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;353:2271-2284.
5. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197-213.
6. Keating GM. Shingles (herpes zoster) vaccine (Zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs. 2013;73:1227-1244.
7. Herpes zoster vaccination. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/vpd-vac/shingles/hcp-vaccination.htm. Updated March 12, 2015. Accessed April 21, 2015.
8. Langan SM, Smeeth L, Margolis D, et al. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.
Herpes zoster (HZ) infection occurs when the varicella-zoster virus (VZV) is reactivated due to waning cellular immunity associated with age or immunosuppression. It results in a painful blistering cutaneous eruption.1 The incidence and rate of complications from HZ infection increase with age.2 The most common complication of HZ infection is postherpetic neuralgia (PHN), which can be extremely debilitating.1
In 2006 the US Food and Drug Administration approved a live attenuated HZ vaccine that boosts VZV cell-mediated immunity and largely reduces HZ disease burden. The HZ vaccine contains the same strain of VZV as the varicella vaccine but contains 14 times more virus particles.3 In a study of the efficacy and safety of the HZ vaccine, HZ vaccination was associated with a 51% reduction in HZ incidence, a 61% reduction in HZ disease burden, and a 67% reduction in PHN incidence at 3-year follow-up.4 In adults aged 60 to 69 years, the benefit of the HZ vaccine resulted from the reduction in HZ incidence.5 However, in adults 70 years and older, the benefit resulted from the reduction in PHN incidence and severity. Overall, the absolute benefit of the HZ vaccine was greatest in the older age group, as the severity and incidence of HZ and PHN are highest in these patients.5 Although efficacy declines with time, a long-term persistence substudy demonstrated that the HZ vaccine still reduced the incidence and severity of HZ.6
The HZ vaccine currently is approved for adults aged 50 years or older.3 Antivirals that are active against VZV (eg, acyclovir, valacyclovir, famciclovir) should not be administered 24 hours before or 14 days after vaccination.1 Concurrent administration of the HZ vaccine and the pneumococcal vaccine is not recommended due to risk for reduced immunogenicity of the zoster vaccine.5 Because it is a live vaccine, the HZ vaccine is not recommended in immunocompromised patients. However, the HZ vaccine can be safely given to moderately immunosuppressed patients. The HZ vaccine also is well tolerated and stimulates a strong cell-mediated immune response in adults who have had prior HZ infections. Herpes zoster vaccination is recommended in patients with a history of shingles, though there are no published data showing that it reduces the already low rate of recurrent HZ infections.7
Despite strong efficacy data and established guidelines, a low vaccination rate has been reported8 due to doubts about its long-term efficacy, failure of both physicians and patients to recognize the burden of disease imposed by HZ infection and PHN, and concerns about reimbursement and out-of-pocket costs for the patient.5 Furthermore, many patients who are eligible to receive the HZ vaccine may not do so because they do not remember having chickenpox and therefore do not feel they are at risk for developing shingles.
The HZ vaccine is an important factor in public health prevention strategy, as HZ infection and PHN are common, incurable, and incapacitating. The HZ vaccine is the most efficacious agent currently available on the market for prevention. It is important for dermatologists to educate our patients and encourage them to receive the HZ vaccine to safeguard their long-term health.
Herpes zoster (HZ) infection occurs when the varicella-zoster virus (VZV) is reactivated due to waning cellular immunity associated with age or immunosuppression. It results in a painful blistering cutaneous eruption.1 The incidence and rate of complications from HZ infection increase with age.2 The most common complication of HZ infection is postherpetic neuralgia (PHN), which can be extremely debilitating.1
In 2006 the US Food and Drug Administration approved a live attenuated HZ vaccine that boosts VZV cell-mediated immunity and largely reduces HZ disease burden. The HZ vaccine contains the same strain of VZV as the varicella vaccine but contains 14 times more virus particles.3 In a study of the efficacy and safety of the HZ vaccine, HZ vaccination was associated with a 51% reduction in HZ incidence, a 61% reduction in HZ disease burden, and a 67% reduction in PHN incidence at 3-year follow-up.4 In adults aged 60 to 69 years, the benefit of the HZ vaccine resulted from the reduction in HZ incidence.5 However, in adults 70 years and older, the benefit resulted from the reduction in PHN incidence and severity. Overall, the absolute benefit of the HZ vaccine was greatest in the older age group, as the severity and incidence of HZ and PHN are highest in these patients.5 Although efficacy declines with time, a long-term persistence substudy demonstrated that the HZ vaccine still reduced the incidence and severity of HZ.6
The HZ vaccine currently is approved for adults aged 50 years or older.3 Antivirals that are active against VZV (eg, acyclovir, valacyclovir, famciclovir) should not be administered 24 hours before or 14 days after vaccination.1 Concurrent administration of the HZ vaccine and the pneumococcal vaccine is not recommended due to risk for reduced immunogenicity of the zoster vaccine.5 Because it is a live vaccine, the HZ vaccine is not recommended in immunocompromised patients. However, the HZ vaccine can be safely given to moderately immunosuppressed patients. The HZ vaccine also is well tolerated and stimulates a strong cell-mediated immune response in adults who have had prior HZ infections. Herpes zoster vaccination is recommended in patients with a history of shingles, though there are no published data showing that it reduces the already low rate of recurrent HZ infections.7
Despite strong efficacy data and established guidelines, a low vaccination rate has been reported8 due to doubts about its long-term efficacy, failure of both physicians and patients to recognize the burden of disease imposed by HZ infection and PHN, and concerns about reimbursement and out-of-pocket costs for the patient.5 Furthermore, many patients who are eligible to receive the HZ vaccine may not do so because they do not remember having chickenpox and therefore do not feel they are at risk for developing shingles.
The HZ vaccine is an important factor in public health prevention strategy, as HZ infection and PHN are common, incurable, and incapacitating. The HZ vaccine is the most efficacious agent currently available on the market for prevention. It is important for dermatologists to educate our patients and encourage them to receive the HZ vaccine to safeguard their long-term health.
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
2. Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. J Intern Med. 2011;269:496-506.
3. Javed S, Javed SA, Trying SK. Varicella vaccines. Curr Open Infect Dis. 2012;25:135-140.
4. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;353:2271-2284.
5. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197-213.
6. Keating GM. Shingles (herpes zoster) vaccine (Zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs. 2013;73:1227-1244.
7. Herpes zoster vaccination. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/vpd-vac/shingles/hcp-vaccination.htm. Updated March 12, 2015. Accessed April 21, 2015.
8. Langan SM, Smeeth L, Margolis D, et al. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
2. Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. J Intern Med. 2011;269:496-506.
3. Javed S, Javed SA, Trying SK. Varicella vaccines. Curr Open Infect Dis. 2012;25:135-140.
4. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;353:2271-2284.
5. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197-213.
6. Keating GM. Shingles (herpes zoster) vaccine (Zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs. 2013;73:1227-1244.
7. Herpes zoster vaccination. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/vpd-vac/shingles/hcp-vaccination.htm. Updated March 12, 2015. Accessed April 21, 2015.
8. Langan SM, Smeeth L, Margolis D, et al. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.
Palmoplantar Pustular Psoriasis Following Initiation of a Beta-blocker: Disease Control With Low-Dose Methotrexate
Psoriasis affects 1% to 2% of individuals in the United States, typically within the third decade of life.1,2 Psoriasis lesions may be persistent or relapsing plaques or pustules. The epidermal thickening that often is noted in psoriasis is secondary to the elongation of rete ridges. Parakeratosis, which also is often noted in psoriasis, is the accumulation of cells with retained nuclei within the cornified layer. Localized pustular psoriasis is a variant of psoriasis that displays scaling erythematous plaques studded with pustules. The pustules are most frequently observed on the palms, soles, and nails of affected individuals.1 Palmoplantar pustular psoriasis is most commonly seen in women in their fifth and sixth decades of life.3 One agent commonly used in the treatment of psoriasis is methotrexate, a prodrug that is converted to polyglutamyl derivatives and acts as a dihydrofolate reductase inhibitor.4,5 We report a case of palmoplantar pustular psoriasis that was triggered by initiation of a beta-blocker. The patient’s condition was controlled with a low-dose methotrexate regimen.
Case Report
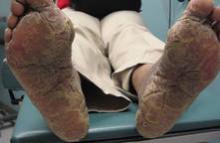
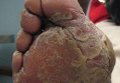
A 76-year-old woman with a history of hypertension, hyperlipidemia, and hypothyroidism presented with erythema and pustules on the bilateral palms and soles 6 weeks following initiation of a beta-blocker. On discontinuation of the beta-blocker, the lesions showed minimal improvement without resolution. The patient then was started on fluocinonide ointment 0.05% and acitretin 25 mg 3 times weekly. Improvement (25%) was noted over the course of 9 months; acitretin then was increased to 25 mg 4 times weekly, but no change was noted (Figure). Acitretin then was discontinued and she was started on methotrexate 2.5 mg weekly, followed by improvement of the lesions on the palms and soles. This regimen was continued and the patient was stable at 2-year follow-up with moderate hyperpigmentation of the palms and minimal hyperpigmentation of the soles, both without erythema or exudates.
Comment
Palmoplantar pustular psoriasis is a rare form of psoriasis; it may, however, be induced by a variety of medications.6 A causal relationship to psoriasis has been documented with beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, and synthetic antimalarials. Other drugs linked to psoriasis are angiotensin-converting enzyme inhibitors, interferons, and terbinafine.7 Anti–tumor necrosis factor a agents such as in-fliximab and etanercept also have been reported to induce pustular psoriasis.6 These drugs have been reported to aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.8 The pathogenesis of psoriasis triggered by beta-blockers is thought to be due to decreased intraepidermal cyclic adenosine monophosphate, leading to an increase in epidermal cell turnover.7
Palmoplantar pustular psoriasis is a debilitating chronic illness that can span decades.9 Not only can it be socially stigmatizing, but it also interferes with patients’ quality of life.10 Various therapies are used to treat this condition including coal tar, topical corticosteroids with or without polythene occlusion, photochemotherapy, tetracyclines, systemic retinoids, cyclosporine, biologics, and methotrexate.9 There currently is no therapeutic standard for controlling this disease, as treatment often is fraught with medication resistance and intolerance as well as frequent relapses. Many medications also are used without firm evidence proving they are beneficial.11
Despite the advent of biologics, methotrexate remains commonly used in the treatment of psoriasis as monotherapy or in combination with other drugs. In comparison to biologics, methotrexate is less expensive, has established efficacy data, and can be administered orally.12 Although it was previously believed that the antiproliferative action of methotrexate via antifolate metabolism led to improvement of psoriatic lesions, in vitro data point to the anti-inflammatory activity of methotrexate playing the more dominant role in disease improvement. Methotrexate also inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to the buildup of adeno-sine in tissue and consequently contributing to its anti-inflammatory properties.12
In psoriasis patients, methotrexate is commonly used in dosages up to 30 mg weekly.5 Our patient demonstrates a rare case of palmoplantar pustular psoriasis that was well controlled using low-dose methotrexate (2.5 mg weekly). Some cases report low doses of 15 to 20 mg for long-term control in psoriasis.13 However, the successful use of doses as low as 2.5 mg for control of any variant of psoriasis is rare.
Conclusion
Although it has been shown to be effective in the treatment of psoriasis, the use of methotrexate is not benign; it has been associated with hepatotoxicity and bone marrow toxicity.12 It is important for dermatologists to recognize that pustular psoriasis can be treated with low-dose methotrexate to avoid potentially toxic effects of higher doses of methotrexate, which is especially true in cases of drug-induced disease, as seen in our patient.
1. Timothy H. Diseases of the skin. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York City, NY: McGraw-Hill Professional; 2009:183-208.
2. Chlapek BH. Dermatologic emergencies. In: Stone CK, Humphries RL, eds. Current Diagnosis & Treatment: Emergency Medicine. 6th ed. New York City, NY: McGraw-Hill Companies; 2007:270-284.
3. Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2009;35:219-222.
4. Imboden JB, Donald FA, Stone JH, et al. Medications. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Rheumatology Diagnosis & Treatment. 2nd ed. New York City, NY: Lange Medical Books/McGraw-Hill; 2004:355-383.
5. Warren RB, Chalmers RJG, Griffiths EM, et al. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33:551-554.
6. Park J, Lee S. A case of tumor necrosis factor-alpha inhibitors-induced pustular psoriasis. Ann Dermatol. 2010;22:212-215.
7. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Am J Clin Dermatol. 2000;1:159-165.
8. Basavaraj K, Ashok N, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351-1361.
9. Chalmers R, Hollis S, Leonardi-Bee J, et al. Interventions for chronic palmoplantar pustulosis (review). Cochrane Database Syst Rev. 2009;1:1-51.
10. Spuls P, Hadi S, Rivera L, et al. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14:21-25.
11. Mrowietz U, van de Kerkhof PCM. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164:942-946.
12. Kanwar A, Yanav S, Dogra S. Psoriasis: what is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol. 2010;76:622-633.
13. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-388.
Psoriasis affects 1% to 2% of individuals in the United States, typically within the third decade of life.1,2 Psoriasis lesions may be persistent or relapsing plaques or pustules. The epidermal thickening that often is noted in psoriasis is secondary to the elongation of rete ridges. Parakeratosis, which also is often noted in psoriasis, is the accumulation of cells with retained nuclei within the cornified layer. Localized pustular psoriasis is a variant of psoriasis that displays scaling erythematous plaques studded with pustules. The pustules are most frequently observed on the palms, soles, and nails of affected individuals.1 Palmoplantar pustular psoriasis is most commonly seen in women in their fifth and sixth decades of life.3 One agent commonly used in the treatment of psoriasis is methotrexate, a prodrug that is converted to polyglutamyl derivatives and acts as a dihydrofolate reductase inhibitor.4,5 We report a case of palmoplantar pustular psoriasis that was triggered by initiation of a beta-blocker. The patient’s condition was controlled with a low-dose methotrexate regimen.
Case Report
A 76-year-old woman with a history of hypertension, hyperlipidemia, and hypothyroidism presented with erythema and pustules on the bilateral palms and soles 6 weeks following initiation of a beta-blocker. On discontinuation of the beta-blocker, the lesions showed minimal improvement without resolution. The patient then was started on fluocinonide ointment 0.05% and acitretin 25 mg 3 times weekly. Improvement (25%) was noted over the course of 9 months; acitretin then was increased to 25 mg 4 times weekly, but no change was noted (Figure). Acitretin then was discontinued and she was started on methotrexate 2.5 mg weekly, followed by improvement of the lesions on the palms and soles. This regimen was continued and the patient was stable at 2-year follow-up with moderate hyperpigmentation of the palms and minimal hyperpigmentation of the soles, both without erythema or exudates.
Comment
Palmoplantar pustular psoriasis is a rare form of psoriasis; it may, however, be induced by a variety of medications.6 A causal relationship to psoriasis has been documented with beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, and synthetic antimalarials. Other drugs linked to psoriasis are angiotensin-converting enzyme inhibitors, interferons, and terbinafine.7 Anti–tumor necrosis factor a agents such as in-fliximab and etanercept also have been reported to induce pustular psoriasis.6 These drugs have been reported to aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.8 The pathogenesis of psoriasis triggered by beta-blockers is thought to be due to decreased intraepidermal cyclic adenosine monophosphate, leading to an increase in epidermal cell turnover.7
Palmoplantar pustular psoriasis is a debilitating chronic illness that can span decades.9 Not only can it be socially stigmatizing, but it also interferes with patients’ quality of life.10 Various therapies are used to treat this condition including coal tar, topical corticosteroids with or without polythene occlusion, photochemotherapy, tetracyclines, systemic retinoids, cyclosporine, biologics, and methotrexate.9 There currently is no therapeutic standard for controlling this disease, as treatment often is fraught with medication resistance and intolerance as well as frequent relapses. Many medications also are used without firm evidence proving they are beneficial.11
Despite the advent of biologics, methotrexate remains commonly used in the treatment of psoriasis as monotherapy or in combination with other drugs. In comparison to biologics, methotrexate is less expensive, has established efficacy data, and can be administered orally.12 Although it was previously believed that the antiproliferative action of methotrexate via antifolate metabolism led to improvement of psoriatic lesions, in vitro data point to the anti-inflammatory activity of methotrexate playing the more dominant role in disease improvement. Methotrexate also inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to the buildup of adeno-sine in tissue and consequently contributing to its anti-inflammatory properties.12
In psoriasis patients, methotrexate is commonly used in dosages up to 30 mg weekly.5 Our patient demonstrates a rare case of palmoplantar pustular psoriasis that was well controlled using low-dose methotrexate (2.5 mg weekly). Some cases report low doses of 15 to 20 mg for long-term control in psoriasis.13 However, the successful use of doses as low as 2.5 mg for control of any variant of psoriasis is rare.
Conclusion
Although it has been shown to be effective in the treatment of psoriasis, the use of methotrexate is not benign; it has been associated with hepatotoxicity and bone marrow toxicity.12 It is important for dermatologists to recognize that pustular psoriasis can be treated with low-dose methotrexate to avoid potentially toxic effects of higher doses of methotrexate, which is especially true in cases of drug-induced disease, as seen in our patient.
Psoriasis affects 1% to 2% of individuals in the United States, typically within the third decade of life.1,2 Psoriasis lesions may be persistent or relapsing plaques or pustules. The epidermal thickening that often is noted in psoriasis is secondary to the elongation of rete ridges. Parakeratosis, which also is often noted in psoriasis, is the accumulation of cells with retained nuclei within the cornified layer. Localized pustular psoriasis is a variant of psoriasis that displays scaling erythematous plaques studded with pustules. The pustules are most frequently observed on the palms, soles, and nails of affected individuals.1 Palmoplantar pustular psoriasis is most commonly seen in women in their fifth and sixth decades of life.3 One agent commonly used in the treatment of psoriasis is methotrexate, a prodrug that is converted to polyglutamyl derivatives and acts as a dihydrofolate reductase inhibitor.4,5 We report a case of palmoplantar pustular psoriasis that was triggered by initiation of a beta-blocker. The patient’s condition was controlled with a low-dose methotrexate regimen.
Case Report
A 76-year-old woman with a history of hypertension, hyperlipidemia, and hypothyroidism presented with erythema and pustules on the bilateral palms and soles 6 weeks following initiation of a beta-blocker. On discontinuation of the beta-blocker, the lesions showed minimal improvement without resolution. The patient then was started on fluocinonide ointment 0.05% and acitretin 25 mg 3 times weekly. Improvement (25%) was noted over the course of 9 months; acitretin then was increased to 25 mg 4 times weekly, but no change was noted (Figure). Acitretin then was discontinued and she was started on methotrexate 2.5 mg weekly, followed by improvement of the lesions on the palms and soles. This regimen was continued and the patient was stable at 2-year follow-up with moderate hyperpigmentation of the palms and minimal hyperpigmentation of the soles, both without erythema or exudates.
Comment
Palmoplantar pustular psoriasis is a rare form of psoriasis; it may, however, be induced by a variety of medications.6 A causal relationship to psoriasis has been documented with beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, and synthetic antimalarials. Other drugs linked to psoriasis are angiotensin-converting enzyme inhibitors, interferons, and terbinafine.7 Anti–tumor necrosis factor a agents such as in-fliximab and etanercept also have been reported to induce pustular psoriasis.6 These drugs have been reported to aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.8 The pathogenesis of psoriasis triggered by beta-blockers is thought to be due to decreased intraepidermal cyclic adenosine monophosphate, leading to an increase in epidermal cell turnover.7
Palmoplantar pustular psoriasis is a debilitating chronic illness that can span decades.9 Not only can it be socially stigmatizing, but it also interferes with patients’ quality of life.10 Various therapies are used to treat this condition including coal tar, topical corticosteroids with or without polythene occlusion, photochemotherapy, tetracyclines, systemic retinoids, cyclosporine, biologics, and methotrexate.9 There currently is no therapeutic standard for controlling this disease, as treatment often is fraught with medication resistance and intolerance as well as frequent relapses. Many medications also are used without firm evidence proving they are beneficial.11
Despite the advent of biologics, methotrexate remains commonly used in the treatment of psoriasis as monotherapy or in combination with other drugs. In comparison to biologics, methotrexate is less expensive, has established efficacy data, and can be administered orally.12 Although it was previously believed that the antiproliferative action of methotrexate via antifolate metabolism led to improvement of psoriatic lesions, in vitro data point to the anti-inflammatory activity of methotrexate playing the more dominant role in disease improvement. Methotrexate also inhibits 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to the buildup of adeno-sine in tissue and consequently contributing to its anti-inflammatory properties.12
In psoriasis patients, methotrexate is commonly used in dosages up to 30 mg weekly.5 Our patient demonstrates a rare case of palmoplantar pustular psoriasis that was well controlled using low-dose methotrexate (2.5 mg weekly). Some cases report low doses of 15 to 20 mg for long-term control in psoriasis.13 However, the successful use of doses as low as 2.5 mg for control of any variant of psoriasis is rare.
Conclusion
Although it has been shown to be effective in the treatment of psoriasis, the use of methotrexate is not benign; it has been associated with hepatotoxicity and bone marrow toxicity.12 It is important for dermatologists to recognize that pustular psoriasis can be treated with low-dose methotrexate to avoid potentially toxic effects of higher doses of methotrexate, which is especially true in cases of drug-induced disease, as seen in our patient.
1. Timothy H. Diseases of the skin. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York City, NY: McGraw-Hill Professional; 2009:183-208.
2. Chlapek BH. Dermatologic emergencies. In: Stone CK, Humphries RL, eds. Current Diagnosis & Treatment: Emergency Medicine. 6th ed. New York City, NY: McGraw-Hill Companies; 2007:270-284.
3. Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2009;35:219-222.
4. Imboden JB, Donald FA, Stone JH, et al. Medications. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Rheumatology Diagnosis & Treatment. 2nd ed. New York City, NY: Lange Medical Books/McGraw-Hill; 2004:355-383.
5. Warren RB, Chalmers RJG, Griffiths EM, et al. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33:551-554.
6. Park J, Lee S. A case of tumor necrosis factor-alpha inhibitors-induced pustular psoriasis. Ann Dermatol. 2010;22:212-215.
7. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Am J Clin Dermatol. 2000;1:159-165.
8. Basavaraj K, Ashok N, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351-1361.
9. Chalmers R, Hollis S, Leonardi-Bee J, et al. Interventions for chronic palmoplantar pustulosis (review). Cochrane Database Syst Rev. 2009;1:1-51.
10. Spuls P, Hadi S, Rivera L, et al. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14:21-25.
11. Mrowietz U, van de Kerkhof PCM. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164:942-946.
12. Kanwar A, Yanav S, Dogra S. Psoriasis: what is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol. 2010;76:622-633.
13. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-388.
1. Timothy H. Diseases of the skin. In: McPhee SJ, Hammer GD, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 6th ed. New York City, NY: McGraw-Hill Professional; 2009:183-208.
2. Chlapek BH. Dermatologic emergencies. In: Stone CK, Humphries RL, eds. Current Diagnosis & Treatment: Emergency Medicine. 6th ed. New York City, NY: McGraw-Hill Companies; 2007:270-284.
3. Adişen E, Gürer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2009;35:219-222.
4. Imboden JB, Donald FA, Stone JH, et al. Medications. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Rheumatology Diagnosis & Treatment. 2nd ed. New York City, NY: Lange Medical Books/McGraw-Hill; 2004:355-383.
5. Warren RB, Chalmers RJG, Griffiths EM, et al. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33:551-554.
6. Park J, Lee S. A case of tumor necrosis factor-alpha inhibitors-induced pustular psoriasis. Ann Dermatol. 2010;22:212-215.
7. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Am J Clin Dermatol. 2000;1:159-165.
8. Basavaraj K, Ashok N, Rashmi R, et al. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351-1361.
9. Chalmers R, Hollis S, Leonardi-Bee J, et al. Interventions for chronic palmoplantar pustulosis (review). Cochrane Database Syst Rev. 2009;1:1-51.
10. Spuls P, Hadi S, Rivera L, et al. Retrospective analysis of the treatment of psoriasis of the palms and soles. J Dermatolog Treat. 2003;14:21-25.
11. Mrowietz U, van de Kerkhof PCM. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164:942-946.
12. Kanwar A, Yanav S, Dogra S. Psoriasis: what is new in nonbiologic systemic therapy in the era of biologics? Indian J Dermatol. 2010;76:622-633.
13. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382-388.
- Beta-blockers, lithium, tetracyclines, nonsteroidal anti-inflammatory drugs, adalimumab, synthetic antimalarials, angiotensin-converting enzyme inhibitors, interferons, terbinafine, infliximab, and etanercept can aggravate preexisting psoriasis, provoke lesions in uninvolved skin in individuals with psoriasis, and induce psoriasis in patients without a personal or family history of psoriasis.
- Methotrexate can be effective and safe in treating palmoplantar pustular psoriasis when prescribed at a low dose.