User login
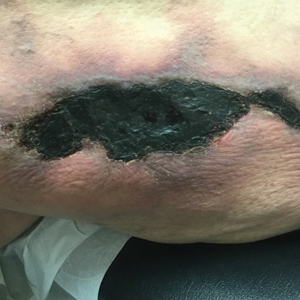
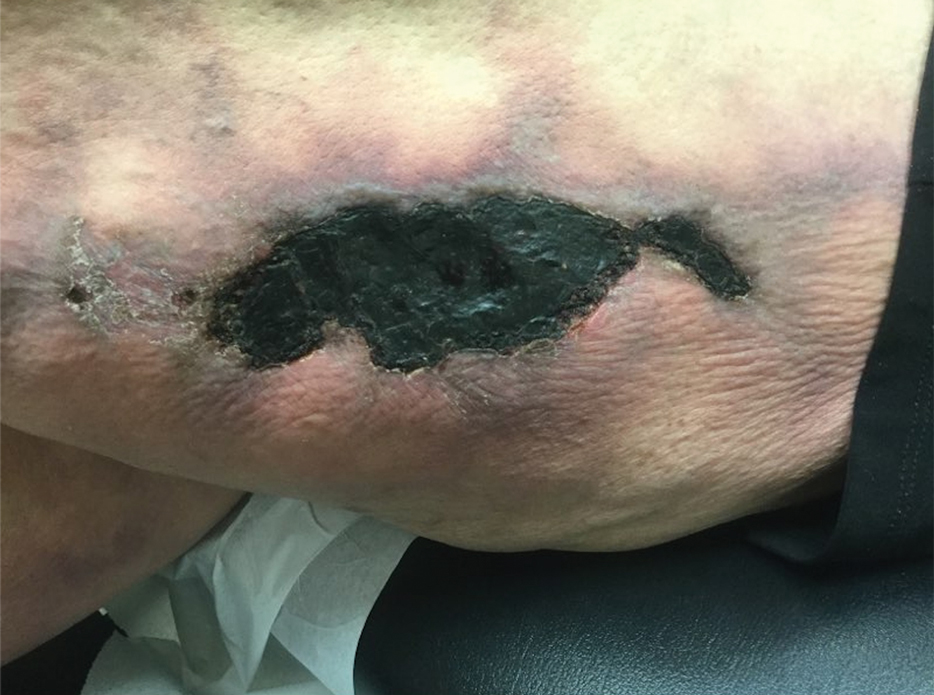
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.
Physicians Must Encourage HPV Vaccine
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
Physician Advocacy for Zoster Vaccination
Herpes zoster (HZ) infection occurs when the varicella-zoster virus (VZV) is reactivated due to waning cellular immunity associated with age or immunosuppression. It results in a painful blistering cutaneous eruption.1 The incidence and rate of complications from HZ infection increase with age.2 The most common complication of HZ infection is postherpetic neuralgia (PHN), which can be extremely debilitating.1
In 2006 the US Food and Drug Administration approved a live attenuated HZ vaccine that boosts VZV cell-mediated immunity and largely reduces HZ disease burden. The HZ vaccine contains the same strain of VZV as the varicella vaccine but contains 14 times more virus particles.3 In a study of the efficacy and safety of the HZ vaccine, HZ vaccination was associated with a 51% reduction in HZ incidence, a 61% reduction in HZ disease burden, and a 67% reduction in PHN incidence at 3-year follow-up.4 In adults aged 60 to 69 years, the benefit of the HZ vaccine resulted from the reduction in HZ incidence.5 However, in adults 70 years and older, the benefit resulted from the reduction in PHN incidence and severity. Overall, the absolute benefit of the HZ vaccine was greatest in the older age group, as the severity and incidence of HZ and PHN are highest in these patients.5 Although efficacy declines with time, a long-term persistence substudy demonstrated that the HZ vaccine still reduced the incidence and severity of HZ.6
The HZ vaccine currently is approved for adults aged 50 years or older.3 Antivirals that are active against VZV (eg, acyclovir, valacyclovir, famciclovir) should not be administered 24 hours before or 14 days after vaccination.1 Concurrent administration of the HZ vaccine and the pneumococcal vaccine is not recommended due to risk for reduced immunogenicity of the zoster vaccine.5 Because it is a live vaccine, the HZ vaccine is not recommended in immunocompromised patients. However, the HZ vaccine can be safely given to moderately immunosuppressed patients. The HZ vaccine also is well tolerated and stimulates a strong cell-mediated immune response in adults who have had prior HZ infections. Herpes zoster vaccination is recommended in patients with a history of shingles, though there are no published data showing that it reduces the already low rate of recurrent HZ infections.7
Despite strong efficacy data and established guidelines, a low vaccination rate has been reported8 due to doubts about its long-term efficacy, failure of both physicians and patients to recognize the burden of disease imposed by HZ infection and PHN, and concerns about reimbursement and out-of-pocket costs for the patient.5 Furthermore, many patients who are eligible to receive the HZ vaccine may not do so because they do not remember having chickenpox and therefore do not feel they are at risk for developing shingles.
The HZ vaccine is an important factor in public health prevention strategy, as HZ infection and PHN are common, incurable, and incapacitating. The HZ vaccine is the most efficacious agent currently available on the market for prevention. It is important for dermatologists to educate our patients and encourage them to receive the HZ vaccine to safeguard their long-term health.
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
2. Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. J Intern Med. 2011;269:496-506.
3. Javed S, Javed SA, Trying SK. Varicella vaccines. Curr Open Infect Dis. 2012;25:135-140.
4. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;353:2271-2284.
5. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197-213.
6. Keating GM. Shingles (herpes zoster) vaccine (Zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs. 2013;73:1227-1244.
7. Herpes zoster vaccination. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/vpd-vac/shingles/hcp-vaccination.htm. Updated March 12, 2015. Accessed April 21, 2015.
8. Langan SM, Smeeth L, Margolis D, et al. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.
Herpes zoster (HZ) infection occurs when the varicella-zoster virus (VZV) is reactivated due to waning cellular immunity associated with age or immunosuppression. It results in a painful blistering cutaneous eruption.1 The incidence and rate of complications from HZ infection increase with age.2 The most common complication of HZ infection is postherpetic neuralgia (PHN), which can be extremely debilitating.1
In 2006 the US Food and Drug Administration approved a live attenuated HZ vaccine that boosts VZV cell-mediated immunity and largely reduces HZ disease burden. The HZ vaccine contains the same strain of VZV as the varicella vaccine but contains 14 times more virus particles.3 In a study of the efficacy and safety of the HZ vaccine, HZ vaccination was associated with a 51% reduction in HZ incidence, a 61% reduction in HZ disease burden, and a 67% reduction in PHN incidence at 3-year follow-up.4 In adults aged 60 to 69 years, the benefit of the HZ vaccine resulted from the reduction in HZ incidence.5 However, in adults 70 years and older, the benefit resulted from the reduction in PHN incidence and severity. Overall, the absolute benefit of the HZ vaccine was greatest in the older age group, as the severity and incidence of HZ and PHN are highest in these patients.5 Although efficacy declines with time, a long-term persistence substudy demonstrated that the HZ vaccine still reduced the incidence and severity of HZ.6
The HZ vaccine currently is approved for adults aged 50 years or older.3 Antivirals that are active against VZV (eg, acyclovir, valacyclovir, famciclovir) should not be administered 24 hours before or 14 days after vaccination.1 Concurrent administration of the HZ vaccine and the pneumococcal vaccine is not recommended due to risk for reduced immunogenicity of the zoster vaccine.5 Because it is a live vaccine, the HZ vaccine is not recommended in immunocompromised patients. However, the HZ vaccine can be safely given to moderately immunosuppressed patients. The HZ vaccine also is well tolerated and stimulates a strong cell-mediated immune response in adults who have had prior HZ infections. Herpes zoster vaccination is recommended in patients with a history of shingles, though there are no published data showing that it reduces the already low rate of recurrent HZ infections.7
Despite strong efficacy data and established guidelines, a low vaccination rate has been reported8 due to doubts about its long-term efficacy, failure of both physicians and patients to recognize the burden of disease imposed by HZ infection and PHN, and concerns about reimbursement and out-of-pocket costs for the patient.5 Furthermore, many patients who are eligible to receive the HZ vaccine may not do so because they do not remember having chickenpox and therefore do not feel they are at risk for developing shingles.
The HZ vaccine is an important factor in public health prevention strategy, as HZ infection and PHN are common, incurable, and incapacitating. The HZ vaccine is the most efficacious agent currently available on the market for prevention. It is important for dermatologists to educate our patients and encourage them to receive the HZ vaccine to safeguard their long-term health.
Herpes zoster (HZ) infection occurs when the varicella-zoster virus (VZV) is reactivated due to waning cellular immunity associated with age or immunosuppression. It results in a painful blistering cutaneous eruption.1 The incidence and rate of complications from HZ infection increase with age.2 The most common complication of HZ infection is postherpetic neuralgia (PHN), which can be extremely debilitating.1
In 2006 the US Food and Drug Administration approved a live attenuated HZ vaccine that boosts VZV cell-mediated immunity and largely reduces HZ disease burden. The HZ vaccine contains the same strain of VZV as the varicella vaccine but contains 14 times more virus particles.3 In a study of the efficacy and safety of the HZ vaccine, HZ vaccination was associated with a 51% reduction in HZ incidence, a 61% reduction in HZ disease burden, and a 67% reduction in PHN incidence at 3-year follow-up.4 In adults aged 60 to 69 years, the benefit of the HZ vaccine resulted from the reduction in HZ incidence.5 However, in adults 70 years and older, the benefit resulted from the reduction in PHN incidence and severity. Overall, the absolute benefit of the HZ vaccine was greatest in the older age group, as the severity and incidence of HZ and PHN are highest in these patients.5 Although efficacy declines with time, a long-term persistence substudy demonstrated that the HZ vaccine still reduced the incidence and severity of HZ.6
The HZ vaccine currently is approved for adults aged 50 years or older.3 Antivirals that are active against VZV (eg, acyclovir, valacyclovir, famciclovir) should not be administered 24 hours before or 14 days after vaccination.1 Concurrent administration of the HZ vaccine and the pneumococcal vaccine is not recommended due to risk for reduced immunogenicity of the zoster vaccine.5 Because it is a live vaccine, the HZ vaccine is not recommended in immunocompromised patients. However, the HZ vaccine can be safely given to moderately immunosuppressed patients. The HZ vaccine also is well tolerated and stimulates a strong cell-mediated immune response in adults who have had prior HZ infections. Herpes zoster vaccination is recommended in patients with a history of shingles, though there are no published data showing that it reduces the already low rate of recurrent HZ infections.7
Despite strong efficacy data and established guidelines, a low vaccination rate has been reported8 due to doubts about its long-term efficacy, failure of both physicians and patients to recognize the burden of disease imposed by HZ infection and PHN, and concerns about reimbursement and out-of-pocket costs for the patient.5 Furthermore, many patients who are eligible to receive the HZ vaccine may not do so because they do not remember having chickenpox and therefore do not feel they are at risk for developing shingles.
The HZ vaccine is an important factor in public health prevention strategy, as HZ infection and PHN are common, incurable, and incapacitating. The HZ vaccine is the most efficacious agent currently available on the market for prevention. It is important for dermatologists to educate our patients and encourage them to receive the HZ vaccine to safeguard their long-term health.
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
2. Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. J Intern Med. 2011;269:496-506.
3. Javed S, Javed SA, Trying SK. Varicella vaccines. Curr Open Infect Dis. 2012;25:135-140.
4. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;353:2271-2284.
5. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197-213.
6. Keating GM. Shingles (herpes zoster) vaccine (Zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs. 2013;73:1227-1244.
7. Herpes zoster vaccination. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/vpd-vac/shingles/hcp-vaccination.htm. Updated March 12, 2015. Accessed April 21, 2015.
8. Langan SM, Smeeth L, Margolis D, et al. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.
1. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
2. Gilden D. Efficacy of live zoster vaccine in preventing zoster and postherpetic neuralgia. J Intern Med. 2011;269:496-506.
3. Javed S, Javed SA, Trying SK. Varicella vaccines. Curr Open Infect Dis. 2012;25:135-140.
4. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;353:2271-2284.
5. Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197-213.
6. Keating GM. Shingles (herpes zoster) vaccine (Zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs. 2013;73:1227-1244.
7. Herpes zoster vaccination. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vaccines/vpd-vac/shingles/hcp-vaccination.htm. Updated March 12, 2015. Accessed April 21, 2015.
8. Langan SM, Smeeth L, Margolis D, et al. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.