User login
Antidepressants for chronic pain
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
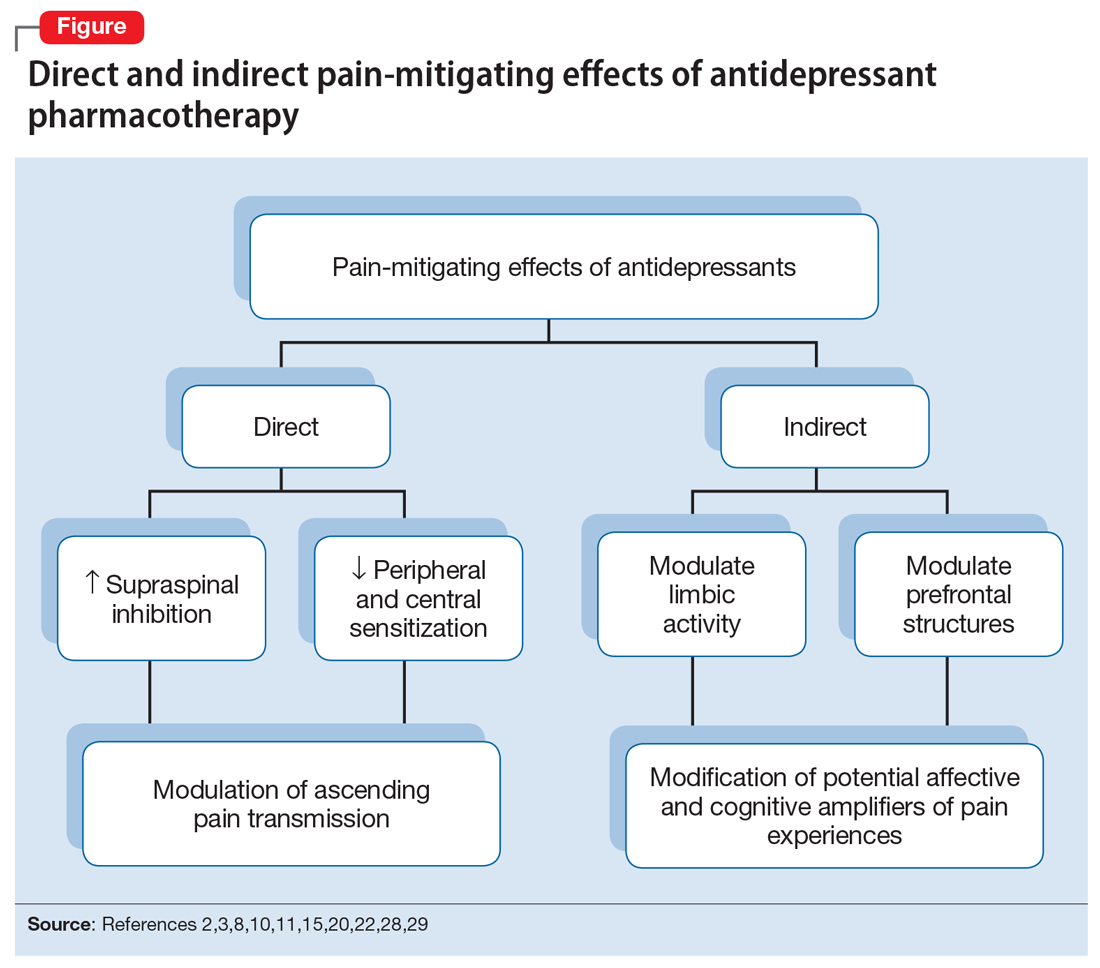
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.
Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
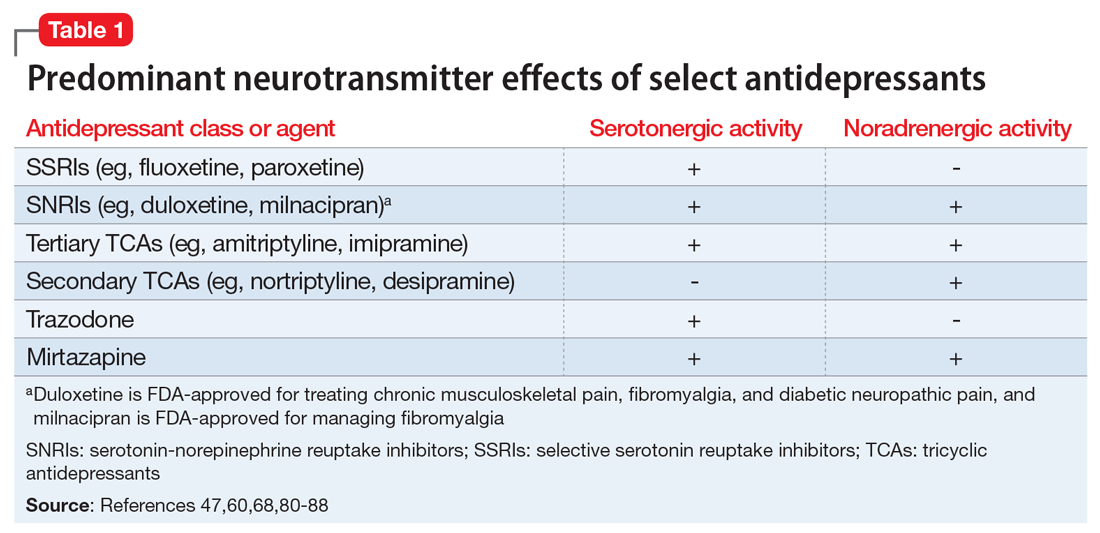
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
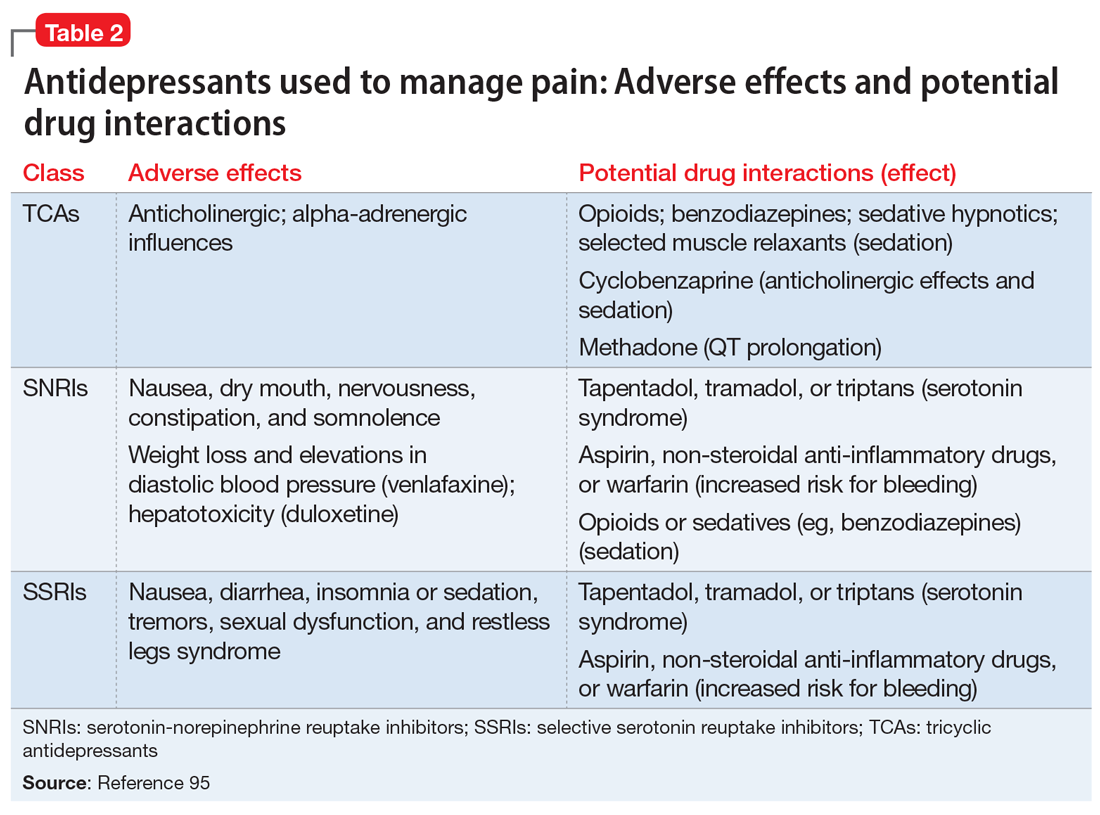
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.

Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
Approximately 55 years ago, tricyclic antidepressants (TCAs) began to be used to treat neuropathic pain.1 Eventually, clinical trials emerged suggesting the utility of TCAs for other chronic pain conditions, such as fibromyalgia (FM) and migraine prophylaxis. However, despite TCAs’ effectiveness in mitigating painful conditions, their adverse effects limited their use.
Pharmacologic advancements have led to the development of other antidepressant classes, including selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), and the use of these agents has come to eclipse that of TCAs. In the realm of pain management, such developments have raised the hope of possible alternative co-analgesic agents that could avoid the adverse effects associated with TCAs. Some of these agents have demonstrated efficacy for managing chronic pain states, while others have demonstrated only limited utility.
This article provides a synopsis of systematic reviews and meta-analyses examining the role of antidepressant therapy for managing several chronic pain conditions, including pain associated with neuropathy, FM, headache, and irritable bowel syndrome (IBS). Because the literature base is rapidly evolving, it is necessary to revisit the information gleaned from clinical data with respect to treatment effectiveness, and to clarify how antidepressants might be positioned in the management of chronic pain.
The effectiveness of antidepressants for pain
The pathophysiologic processes that precipitate and maintain chronic pain conditions are complex (Box 12-10). The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects and indirect effects (Box 22,3,8,10,11-33).
Box 1
The pathophysiologic processes precipitating and maintaining chronic pain conditions are complex. Persistent and chronic pain results from changes in sensitivity within both ascending pathways (relaying pain information from the periphery to the spinal cord and brain) and descending pain pathways (functioning to modulate ascending pain information).2,3 Tissue damage or peripheral nerve injury can lead to a cascade of neuroplastic changes within the CNS, resulting in hyperexcitability within the ascending pain pathways.
The descending pain pathways consist of the midbrain periaqueductal gray area (PGA), the rostroventral medulla (RVM), and the dorsolateral pontomesencephalic tegmentum (DLPT). The axons of the RVM (the outflow of which is serotonergic) and DLPT (the outflow of which is noradrenergic) terminate in the dorsal horn of the spinal cord,4 and thereby dampen pain signals arising from the periphery. Diminished output from descending pain pathways can heighten the pain experience. Input from the cortex, hypothalamus, and amygdala (among other structures) converges upon the PGA, RVM and DLPT, and can influence the degree of pain modulation emerging from descending pathways. In this way, thoughts, appraisals, and mood are believed to comprise cognitive and affective modifiers of pain experiences.
Devising effective chronic pain treatment becomes challenging; multimodal treatment approaches often are advocated, including pharmacologic treatment with analgesics in combination with co-analgesic medications such as antidepressants. Although a description of multimodal treatment is beyond the scope of this article, such treatment also would encompass physical therapy, occupational therapy, and psychotherapeutic interventions to augment rehabilitative efforts and the functional capabilities of patients who struggle with persisting pain.
Although the direct pain-mitigating effects of antidepressants are not fully understood, it is believed that augmentation of monoamine neurotransmission from supraspinal nuclei (ie, the RVM and DLPT) modulate pain transmission from the periphery.5,6 In addition, there is evidence that some effects of tricyclic antidepressants can modulate several other functions that impact peripheral and central sensitization.7-10
During the last several decades, antidepressants have been used to address—and have demonstrated clinical utility for—a variety of chronic pain states. However, antidepressants are not a panacea; some chronic pain conditions are more responsive to antidepressants than are others. The chronic painful states most amenable to antidepressants are those that result primarily from a process of neural sensitization, as opposed to acute somatic or visceral nociception. Hence, several meta-analyses and evidence-based reviews have long suggested the usefulness of antidepressants for mitigating pain associated with neuropathy,34,35 FM,36,37 headache,38 and IBS.39,40
Box 2
The pain-mitigating effects of antidepressants can be thought of in terms of direct analgesic effects (impacting neurotransmission of descending pathways independent of influences on mood) and indirect effects (presumably impacting cortical and limbic output to the periaqueductal gray area, the rostroventral medulla, and the dorsolateral pontomesencephalic tegmentum brought about by improvement in mood and/or cognitive appraisals) (Figure2,3,8,10,11,15,20,22,28,29). Support for the direct analgesic effects has been garnered from initial empirical work that demonstrated pain relief among patients with pain who are not depressed. Additionally, among patients who have depression and experience pain, analgesia reportedly often occurs within 2 weeks, which is before antidepressant effects are appreciated,11-15 and, at least for some antidepressants, occurs at doses far lower than those required to produce mood-elevating effects.11,12,16
On the other hand, it is well established that significant comorbidities exist between chronic pain states and psychiatric disorders (eg, depression and somatic symptom and related disorders).17-21 There may be common physiological substrates underlying chronic pain and depression.20,22 There are bidirectional influences of limbic (affective) systems and those CNS structures involved in pain processing and integration. The effects of pain and depression are reciprocal; the presence of one makes the management of the other more challenging.23-27 Mood disturbances can, therefore, impact pain processing by acting as affective and cognitive amplifiers of pain by leading to catastrophizing, pain severity augmentation, poor coping with pain-related stress, etc.28,29 It is plausible that the mood-elevating effects of antidepressants can improve pain by indirect effects, by modulating limbic activity, which in turn, impacts coping, cognitive appraisals of pain, etc.
Patients with somatoform disorders (using pre-DSM-5 terminology) frequently present with chronic pain, often in multiple sites.19 Such patients are characterized by hypervigilance for, and a predisposition to focus on, physical sensations and to appraise these sensations as reflecting a pathological state.30 Neuroimaging studies have begun to identify those neural circuits involved in somatoform disorders, many of which act as cognitive and affective amplifiers of visceral-somatic sensory processing. Many of these neural circuits overlap, and interact with, those involved in pain processing.31 Antidepressants can mitigate the severity of unexplained physical complaints, including pain, among patients who somatize32,33; however, due to the heterogeneity of studies upon which this claim is based, the quality of the evidence is reportedly low.33 There is uncertainty whether, or to what extent, antidepressant benefits among patients who somatize are due to a direct impact on pain modulation, or indirect effects on mood or cognitive appraisals/perceptions.
Despite the uncertainties about the exact mechanisms through which antidepressants exert analgesic effects, antidepressants can be appropriately used to treat patients with selected chronic pain syndromes, regardless of whether or not the patient has a psychiatric comorbidity. For those patients with pain and psychiatric comorbidities, the benefits may be brought about via direct mechanisms, indirect mechanisms, or a combination of both.

Continue to: Neuropathic pain
Neuropathic pain
Several treatment guidelines advocate for the use of antidepressants for neuropathic pain.41-44 For decades, TCAs have been employed off-label to successfully treat many patients with neuropathic pain states. Early investigations suggested that TCAs were robustly efficacious in managing patients with neuropathy.45-48 Calculated number-needed-to-treat (NNT) values for TCAs were quite low (ie, reflecting that few patients would need to be treated to yield a positive response in one patient compared with placebo), and were comparable to, if not slightly better than, the NNTs generated for anticonvulsants and α2-δ ligands, such as gabapentin or pregabalin.45-48
Unfortunately, early studies involving TCAs conducted many years ago do not meet contemporary standards of methodological rigor; they featured relatively small samples of patients assessed for brief post-treatment intervals with variable outcome measures. Thus, the NNT values obtained in meta-analyses based on these studies may overestimate treatment benefits.49 Further, NNT values derived from meta-analyses tended to combine all drugs within a particular antidepressant class (eg, amitriptyline, nortriptyline, desipramine, and imipramine among the TCAs) employed at diverse doses. Taken together, these limitations raise questions about the results of those meta-analyses.
Subsequent meta-analyses, which employed strict criteria to eliminate data from studies with potential sources of bias and used a primary outcome of frequencies of patients reporting at least 30% pain reduction compared with a placebo-controlled sample, suggest that the effectiveness of TCAs as a class for treating neuropathic pain is not as compelling as once was thought. Meta-analyses of studies employing specific TCAs revealed that there was little evidence to support the use of desipramine,50 imipramine,51 or nortriptyline52 in managing diabetic neuropathy or postherpetic neuralgia. Studies evaluating amitriptyline (dose range 12.5 to 150 mg/d), found low-level evidence of effectiveness; the benefit was expected to be present for a small subset (approximately 25%) of patients with neuropathic pain.53
There is moderate-quality evidence that duloxetine (60 to 120 mg/d) can produce a ≥50% improvement in pain severity ratings among patients with diabetic peripheral neuropathy.54 Although head-to-head studies with other antidepressants are limited, it appears that duloxetine and amitriptyline have comparable efficacy, even though the NNTs for amitriptyline were derived from lower-quality studies than those for duloxetine. Duloxetine is the only antidepressant to receive FDA approval for managing diabetic neuropathy. By contrast, studies assessing the utility of venlafaxine in neuropathic pain comprised small samples for brief durations, which limits the ability to draw clear (unbiased) support for its usefulness.55
Given the diversity of pathophysiologic processes underlying the disturbances that cause neuropathic pain disorders, it is unsurprising that the effectiveness of amitriptyline and duloxetine were not generalizable to all neuropathic pain states. Although amitriptyline produced pain-mitigating effects in patients with diabetic neuropathy and post-herpetic neuralgia, and duloxetine mitigated pain among patients with diabetic neuropathy, there was no evidence to suggest their effectiveness in phantom limb pain or human immunodeficiency virus-related and spinal cord-related neuropathies.35,53,54,56-58
Continue to: Fibromyalgia
Fibromyalgia
As with the issues encountered in interpreting the effectiveness of antidepressants in neuropathic pain, interpreting results gleaned from clinical trials of antidepressants for treating FM are fraught with similar difficulties. Although amitriptyline has been a first-line treatment for FM for many years, the evidence upon which such recommendations were based consisted of low-level studies that had a significant potential for bias.59 Large randomized trials would offer more compelling data regarding the efficacy of amitriptyline, but the prohibitive costs of such studies makes it unlikely they will be conducted. Amitriptyline (25 to 50 mg/d) was effective in mitigating FM-related pain in a small percentage of patients studied, with an estimated NNT of 4.59 Adverse effects, often contributing to treatment discontinuation, were encountered more frequently among patients who received amitriptyline compared with placebo.
Selective serotonin reuptake inhibitors failed to demonstrate significant pain relief (estimated NNT of 10), or improvement in fatigue or sleep problems, even though the studies upon which such conclusions were based were low-level studies with a high potential for bias.60 Although SSRIs have limited utility for mitigating pain, they are still quite useful for reducing depression among patients with FM.60
By contrast, the SNRIs duloxetine and milnacipran provided clinically relevant benefit over placebo in the frequency of patients reporting pain relief of ≥30%, as well as patients’ global impression of change.61 These agents, however, failed to provide clinically relevant benefit over placebo in improving health-related quality of life, reducing sleep problems, or improving fatigue. Nonetheless, duloxetine and milnacipran are FDA-approved for managing pain in FM. Studies assessing the efficacy of venlafaxine in the treatment of FM to date have been limited by small sample sizes, inconsistent dosing, lack of a placebo control, and lack of blinding, which limits the ability to clearly delineate the role of venlafaxine in managing FM.62
Mirtazapine (15 to 45 mg/d) showed a clinically relevant benefit compared with placebo for participant-reported pain relief of ≥30% and sleep disturbances. There was no benefit in terms of participant-reported improvement of quality of life, fatigue, or negative mood.63 The evidence was considered to be of low quality overall.
Headache
Amitriptyline has been employed off-label to address headache prophylaxis since 1964.64 Compared with placebo, it is efficacious in ameliorating migraine frequency and intensity as well as the frequency of tension headache.65,66 However, SSRIs and SNRIs (venlafaxine) failed to produce significant reductions in migraine frequency or severity or the frequencies of tension headache when compared with placebo.67,68
Continue to: Irritable bowel syndrome
Irritable bowel syndrome
Early studies addressing antidepressant efficacy in IBS reveal inconsistencies. For example, whereas some suggest that TCAs are effective in mitigating chronic, severe abdominal pain,39,40 others concluded that TCAs failed to demonstrate a significant analgesic benefit.69 A recent meta-analysis that restricted analysis of efficacy to randomized controlled trials (RCTs) with more rigorous methodological adherence found that pain relief in IBS is possible with both TCAs as well as SSRIs. However, adverse effects were more commonly encountered with TCAs than with SSRIs. Some of the inconsistencies in treatment efficacy reported in early studies may be due to variations in responsiveness of subsets of IBS patients. Specifically, the utility of TCAs appears to be best among patients with diarrheal-type (as opposed to constipation-type) IBS, presumably due to TCAs’ anticholinergic effects, whereas SSRIs may provide more of a benefit for patients with predominantly constipation-type IBS.40,70
Other chronic pain conditions
Antidepressants have been used to assist in the management of several other pain conditions, including oral-facial pain, interstitial cystitis, non-cardiac chest pain, and others. The role of antidepressants for such conditions remains unclear due to limitations in the prevailing empirical work, such as few trials, small sample sizes, variations in outcome measures, and insufficient randomization and blinding.71-76 The interpretation of results from systematic reviews and meta-analyses is limited because of these shortcomings.77 Hence, it has not always been possible to determine whether, and to what extent, patients with such conditions may benefit from antidepressants.
Neuromodulatory effects and efficacy for pain
The interplay of norepinephrine (NE) and serotonin (5-HT) neurotransmitter systems and cellular mechanisms involved in the descending modulation of pain pathways is complex. Experimental animal models of pain modulation suggest that 5-HT can both inhibit as well as promote pain perception by different physiological mechanisms, in contrast to NE, which is predominately inhibitory. While 5-HT in the descending modulating system can inhibit pain transmission ascending to the brain from the periphery, it appears that an intact noradrenergic system is necessary for the inhibitory influences of the serotonergic system to be appreciated.16,78,79 Deficiencies in one or both of these neurotransmitter systems may contribute to hyperactive pain processing, and thereby precipitate or maintain chronic pain.
Pain mitigation may be achieved best by enhancing both neurotransmitters simultaneously, less so by enhancing NE alone, and least by enhancing 5-HT alone.6 The ability to impact pain modulation would, therefore, depend on the degree to which an antidepressant capitalizes on both noradrenergic and serotonergic neurotransmission. Antidepressants commonly employed to manage pain are presented in Table 147,60,68,80-88 according to their primary neurotransmitter effects. Thus, the literature summarized above suggests that antidepressants that influence both NE and 5-HT transmission have greater analgesic effects than antidepressants with more specific effects, such as influencing 5-HT reuptake alone.80-85 It is unsurprising, therefore, that the SSRIs have not been demonstrated to be as consistently analgesic.47,60,68,80,86-88
Similarly, pharmacodynamic and pharmacokinetic differences within antidepressant classes may influence analgesic effectiveness. Simultaneous effects on NE and 5-HT are achieved at low doses with duloxetine and milnacipran. By contrast, 5-HT effects predominate at low doses for venlafaxine. To achieve pain-mitigating effects, higher doses of venlafaxine generally are required.89 Therefore, inconsistencies across studies regarding the analgesic benefits of venlafaxine may be attributable to variability in dosing; patients treated with lower doses may not have experienced sufficient NE effects to garner positive results.
Continue to: The differences in analgesic efficacy...
The differences in analgesic efficacy among specific TCAs may be understood in a similar fashion. Specifically, tertiary TCAs (imipramine and amitriptyline) inhibit both 5-HT and NE reuptake.6,90 Secondary amines (desipramine and nortriptyline) predominantly impact NE reuptake, possibly accounting for the lesser pain-mitigating benefit achieved with these agents, such as for treating neuropathic pain. Further, in vivo imipramine and amitriptyline are rapidly metabolized to secondary amines that are potent and selective NE reuptake inhibitors. In this way, the secondary amines may substantially lose the ability to modulate pain transmission because of the loss of concurrent 5-HT influences.90
Clinical pearls
The following practical points can help guide clinicians regarding the usefulness of antidepressants for pain management:
- Antidepressants can alleviate symptoms of depression and pain. The pain-mitigating effects of antidepressants are possible even among chronic pain patients who are not depressed. Antidepressants may confer benefits for chronic pain patients with depression and other comorbid conditions, such as somatic symptom and related disorders.
- Antidepressants are useful for select chronic pain states. Although the noradrenergic and serotonergic antidepressants (SNRIs and, to some extent, amitriptyline) appear to have efficacy for neuropathic pain and FM, the benefits of SSRIs appear to be less robust. On the other hand, SSRIs and TCAs may have potential benefit for patients with IBS. However, the results of meta-analyses are limited in the ability to provide information about which patients will best respond to which specific antidepressant or how well. Future research directed at identifying characteristics that can predict which patients are likely to benefit from one antidepressant vs another would help inform how best to tailor treatment to individual needs.
- The pain-mitigating effects of antidepressants often emerge early in the course of treatment (often before mood-elevating effects are observed). For example, in the case of amitriptyline, pain relief may be possible for some patients at doses generally lower than those required for mood-elevating effects. To date, there is limited information in the literature to determine what constitutes a sufficient duration of treatment, or when treatment should be modified.
- Failure to reduce pain should raise questions about whether the dose should be increased, an alternative agent should be tried, or combinations with other analgesic agents should be considered. Failure to achieve pain-mitigating effects with one antidepressant does not mean failure with others. Hence, failure to achieve desired effects with one agent might warrant an empirical trial with another agent. Presently, too few double-blind RCTs have been conducted to assess the pain-mitigating effects of other antidepressants (eg, bupropion and newer SNRIs such as desvenlafaxine and levomilnacipran). Meta-analysis of the analgesic effectiveness of these agents or comparisons to the efficacy of other antidepressant classes is, therefore, impossible at this time.
Because many chronic pain states are complex, patients will seldom experience clinically relevant benefit from any one intervention.53 The bigger implication for clinical research is to determine whether there is a sequence or combination of medication use that will provide overall better clinical effectiveness.53 Only limited data are available exploring the utility of combining pharmacologic approaches to address pain.91 For example, preliminary evidence suggests that combinations of complementary strategies, such as duloxetine combined with pregabalin, may result in significantly greater numbers of FM patients achieving ≥30% pain reduction compared with monotherapy with either agent alone or placebo.92
- Antidepressant selection may need to be based on medication-related adverse effect profiles and the potential for drug interactions. These factors are useful to consider in delineating multimodal treatment regimens for chronic pain in light of patients’ comorbidities and co-medication regimen. For example, the adverse effects of TCAs (anticholinergic and alpha-adrenergic influences) limit their utility for treating pain. Some of these effects can be more problematic in select populations, such as older adults or those with orthostatic difficulties, among others. TCAs are contraindicated in patients with closed-angle glaucoma, recent myocardial infarction, cardiac arrhythmias, poorly controlled seizures, or severe benign prostatic hypertrophy. Although the pain-mitigating effects of SNRIs have not been demonstrated to significantly exceed those of TCAs,68,93,94 SNRIs would offer an advantage of greater tolerability of adverse effects and relative safety in patients with comorbid medical conditions that would otherwise preclude TCA use. The adverse effects and common drug interactions associated with antidepressants are summarized in Table 295.
Conclusion
Chronic, nonmalignant pain conditions afflict many patients and significantly impair their ability to function. Because of heightened concerns related to the appropriateness of, and restricting inordinate access to, long-term opioid analgesics, clinicians need to explore the usefulness of co-analgesic agents, such as antidepressants. Significant comorbidities exist between psychiatric disorders and chronic pain, and psychiatrists are uniquely positioned to diagnose and treat psychiatric comorbidities, as well as pain, among their patients, especially since they understand the kinetics and dynamics of antidepressants.
Bottom Line
Antidepressants can alleviate symptoms of depression and pain. Noradrenergic and serotonergic antidepressants appear to have efficacy for pain associated with neuropathy and fibromyalgia, while selective serotonin reuptake inhibitors and tricyclic antidepressants may have benefit for patients with irritable bowel syndrome. However, evidence regarding which patients will best respond to which specific antidepressant is limited.
Continue to: Related Resources
Related Resources
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
- Maletic V, Demuri B. Chronic pain and depression: treatment of 2 culprits in common. Current Psychiatry. 2016;15(3):41,47-50,52.
Drug Brand Names
Amitriptyline • Elavil, Endep
Bupropion • Wellbutrin, Zyban
Carisoprodol • Rela, Soma
Cyclobenzaprine • Amrix, Flexeril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Fluoxetine • Prozac
Gabapentin • Horizant, Neurontin
Imipramine • Tofranil
Levomilnacipran • Fetzima
Methadone • Dolophine, Methadose
Milnacipran • Savella
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Pregabalin • Lyrica, Lyrica CR
Tapentadol • Nucynta
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Warfarin • Coumadin, Jantoven
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
1. Paoli F, Darcourt G, Cossa P. Preliminary note on the action of imipramine in painful states [in French]. Rev Neurol (Paris). 1960;102:503-504.
2. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219-245.
3. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2(2):83-91.
4. Lamont LA, Tranquilli WJ, Grimm KA. Physiology of pain. Vet Clin North Am Small Anim Pract. 2000;30(4):703-728, v.
5. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack’s Textbook of Pain. 5th ed. Burlington, MA: Elsevier Health Sciences; 2005:125-142.
6. Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7(4):331-336.
7. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473-484.
8. Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454-458.
9. Kawasaki Y, Kumamoto E, Furue H, et al. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682-689.
10. McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22(2):139-156.
11. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry. 2000;7(5):257-277.
12. Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262-270.
13. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305-316.
14. Fishbain DA, Detke MJ, Wernicke J, et al. The relationship between antidepressant and analgesic responses: findings from six placebo-controlled trials assessing the efficacy of duloxetine in patients with major depressive disorder. Curr Med Res Opin. 2008;24(11):3105-3115.
15. Harada E, Tokuoka H, Fujikoshi S, et al. Is duloxetine’s effect on painful physical symptoms in depression an indirect result of improvement of depressive symptoms? Pooled analyses of three randomized controlled trials. Pain. 2016;157(3):577-584.
16. Kehoe WA. Antidepressants for chronic pain: selection and dosing considerations. Am J Pain Med. 1993;3(4):161-165.
17. Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(2):1070-1078.
18. DeVeaugh-Geiss AM, West SL, Miller WC, et al. The adverse effects of comorbid pain on depression outcomes in primary care patients: results from the ARTIST trial. Pain Medicine. 2010;11(5):732-741.
19. Egloff N, Cámara RJ, von Känel R, et al. Hypersensitivity and hyperalgesia in somatoform pain disorders. Gen Hosp Psychiatry. 2014;36(3):284-290.
20. Goesling J, Clauw DW, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psych Reports. 2013;15(12):421.
21. Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-Month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
22. Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7(5):403-412.
23. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
24. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
25. Kroenke K, Shen J, Oxman TE, et al. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134(1-2):209-215.
26. Mavandadi S, Ten Have TR, Katz IR, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. J Am Geriatr Soc. 2007;55(2):202-211.
27. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15(8):699-707.
28. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262-268.
29. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: Prevalence, work loss, and help seeking. J Affect Disord. 2006;92(2-3):185-193.
30. Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:17.
31. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27(1):e40-e50.
32. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med. 1998;60(4):503-509.
33. Kleinstäuber M, Witthöft M, Steffanowski A, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;(11):CD010628.
34. Collins SL, Moore RA, McQuay HJ, et al. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449-458.
35. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81(12):1372-1373.
36. Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104-113.
37. O’Malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666.
38. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med. 2001;111(1):54-63.
39. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65-72.
40. Lesbros-Pantoflickova D, Michetti P, Fried M et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11-12):1253-1269.
41. Centre for Clinical Practice at NICE (UK). Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London, UK: National Institute for Health and Care Excellence, (UK); 2013.
42. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(suppl 10):S22-S32.
43. Moulin D, Boulanger A, Clark AJ, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328-35.
44. Mu A, Weinberg E, Moulin DE, et al. Pharmacologic management of chronic neuropathic pain: Review of the Canadian Pain Society consensus statement. Can Fam Physician. 2017;63(11):844-852.
45. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305.
46. Hempenstall K, Nurmikko TJ, Johnson RW, et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164.
47. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389-400.
48. Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain. 2008;9(suppl 1):S19-S30.
49. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
50. Hearn L, Moore RA, Derry S, et al. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003.
51. Hearn L, Derry S, Phillips T, et al. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(5):CD010769.
52. Derry S, Wiffen PJ, Aldington D, et al. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209.
53. Moore R, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242.
54. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
55. Gallagher HC, Gallagher RM, Butler M, et al. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(8):CD011091.
56. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
57. Dinat N, Marinda E, Moch S, et al. Randomized, Double-Blind, Crossover Trial of Amitriptyline for Analgesia in Painful HIV-Associated Sensory Neuropathy. PLoS One. 2015;10(5):e0126297. doi: 10.1371/journal.pone.0126297.eCollection 2015.
58. Mehta S, McIntyre A, Janzen S, et al; Spinal Cord Injury Rehabilitation Evidence Team. Systematic review of pharmacologic treatments of pain after spinal cord injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381-1391.e1.
59. Moore RA, Derry S, Aldington D, et al. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;(12):CD008242..
60. Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;(6):CD011735.
61. Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev. 2018;(2):CD010292.
62. VanderWeide LA, Smith SM, Trinkley KE. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J Clin Pharm Ther. 2015;40(1):1-6.
63. Welsch P, Bernardy K, Derry S, et al. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(8):CD012708.
64. Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;283(7345):1236-1239.
65. Jackson JL, William S, Laura S, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: https://doi.org/10.1136/bmj.c5222
66. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine. 2017;96(22):e6989. doi: 10.1097/MD.0000000000006989.
67. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev. 2015;(4):CD002919.
68. Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;(5):CD011681.
69. Quartero AO, Meineche-Schmidt V, Muris J, et al. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD003460.
70. Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367-378.
71. Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol. 2014;12(8):1224-1245.
72. Kelada E, Jones A. Interstitial cystitis. Arch Gynecol Obstet. 2007;275(4):223-229.
73. Leo RJ, Dewani S. A systematic review of the utility of antidepressant pharmacotherapy in the treatment of vulvodynia pain. J Sex Med. 2013;10(10):2497-2505.
74. McMillan R, Forssell H, Buchanan JA, et al. Interventions for treating burning mouth syndrome. Cochrane Database Syst Rev. 2016;11:CD002779.
75. Patel DN. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent. 2013;14(2):55-56.
76. Wang W, Sun YH, Wang YY, et al. Treatment of functional chest pain with antidepressants: a meta-analysis. Pain Physician. 2012;15(2):E131-E142.
77. Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141.
78. Sorkin L. Nociceptive transmission within the spinal cord. Mt Sinai J Med. 1991;58(3):208-216.
79. Yokogawa F, Kiuchi Y, Ishikawa Y, et al. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95(1):163-168, table of contents.
80. Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26(1):30-36.
81. Max MB. Treatment of post-herpetic neuralgia: antidepressants. Ann Neurol. 1994;35(suppl):S50-S53.
82. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
83. McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68(2-3):217-227.
84. Mochizucki D. Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol Clin Exp. 2004;19(suppl 1):15-19.
85. Sussman N. SNRIs versus SSRIs: mechanisms of action in treating depression and painful physical symptoms. Primary Care Companion J Clin Psychiatry. 2003;5(suppl 7):19-26.
86. Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother. 2014;48(6):777-784.
87. Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384-389.
88. Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One. 2015;10(8):e0127815. doi: 10.1371/journal.pone.0127815. eCollection 2015.
89. Zijlstra TR , Barendregt PJ , van de Laar MA. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2002;46(suppl 9):S105.
90. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880.
91. Thorpe J, Shum B, Moore RA, et al. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev. 2018;(2):CD010585.
92. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532-1540.
93. Häuser W, Petzke F, Üçeyler N, et al. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford). 2011;50(3):532-543.
94. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy: a systematic review. Clin J Pain. 2016;32(11):1005-1010.
95. Riediger C, Schuster T, Barlinn K, et al. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307.
Self-mutilation after recent-onset psychosis
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
The delirious substance abuser
CASE: Hurt and confused
Emergency medical services (EMS) are called to Ms. K’s apartment after her roommate found her lying on the floor moaning. The roommate tells EMS that Ms. K, age 29, appeared confused and was slurring her words, and reports that this change in her awareness progressed rapidly over a few hours. EMS personnel find that Ms. K has multiple contusions on her arms and face, which they presume to be self-inflicted. A marijuana pipe is discovered at Ms. K’s apartment.
In the emergency room (ER), Ms. K is inattentive and has difficulty following simple commands. Her speech is mumbled and her thoughts are disorganized. She displays psychomotor restlessness in the form of combativeness. Ms. K cannot provide meaningful historical data and is disoriented to place and time. The ER staff requests a psychiatric consultation.
Family members reveal that Ms. K has no preexisting medical conditions, is not taking prescription medications, but has a history of substance abuse (sporadic cocaine and cannabis use). Her family is unaware of recent substance use.
Physical examination reveals tachycardia (heart rate 110 to 120 beats per minute), hypotension (blood pressure 78/49 mm Hg), hypothermia (temperature 88ºF), and peripheral pulse oximetry of 84%. Her pupils are dilated and reactive to light; no conjunctival injection is noted. Her lung fields are clear on auscultation, but she is noted to have a rapid, irregular heartbeat. The abdomen is positive for bowel sounds, soft on palpation, and without any repositioning or notable overt signs of tenderness. Ms. K’s toes show purple discoloration with poor capillary refill. The dorsalis pedis pulses are reported to be 1+ bilaterally; however, the remainder of the arterial pulse examination is normal.
Her sodium, potassium, and chloride values are normal, but she has an abnormal anion gap (28.1 mEq/L), blood urea nitrogen (53 mg/dL), creatinine (2.9 mg/dL), creatine kinase (10,857 U/L), creatine kinase MB (432.6 ng/mL), and hyperglycemia (glucose 425 mg/dL). Arterial blood gas reveals hypoxia (Po2 of 55 mm Hg), with metabolic acidosis (sodium bicarbonate 10 with compensatory Pco2 of 33 mm Hg). Her urine is cloudy, positive for protein, ketones, hemoglobin, and glucose. She is thought to have a high anion gap acidosis related to dehydration, lactic acidosis (lactic acid 20 mEq/L), and hyperglycemia. Urine toxicology is positive for cannabinoids; ethylene glycol and methanol screen negatively, which rules these out as potential contributors to her high anion gap acidosis.
Ms. K is intubated and IV fluids are initiated for rhabdomyolysis and acute renal failure. Dialysis is implemented on a short-term basis. Her mental state improves gradually over 3 days.
The authors’ observations
Based on the abrupt onset of inattention and confusion, disorganized speech, memory impairments, and psychomotor agitation, we made an initial diagnosis of delirium; however, the precise etiology remained unclear. DSM-IV-TR diagnostic criteria for delirium are described in Table 1. Although delirium due to multiple etiologies does not have a DSM-IV-TR coding designation, we speculated that multiple causes contributed to Ms. K’s presentation. Acute renal failure secondary to dehydration as well as rhabdomyolysis, hypoxia, and hyperglycemia were implicated as general medical conditions etiologically linked to delirium. Because Ms. K has no preexisting medical conditions and her roommate and family stated she had a history of substance abuse, we also considered a presumptive diagnosis of substance-induced delirium. The medical team speculated that, based on information provided by her family, Ms. K may have had a seizure or may have fallen, which would account for her multiple contusions, and could have led to muscle injury and breakdown and the resultant rhabdomyolysis.
The possibility of cannabinoid-induced delirium has been reported, albeit rarely.1-3 However, Ms. K’s presentation—hypothermia, variable heart rate, lack of dry mucous membranes—was not consistent with significant anticholinergic toxicity or cannabinoid intoxication (Table 2).
By contrast, cocaine-induced delirium has been reported and initially appeared to be a plausible cause of Ms. K’s symptoms (Table 2). Delirium related to excess ingestion of cocaine may be related to the drug’s secondary effects resulting in rhabdomyolysis and renal dysfunction.4-6 Although several mechanisms underlying this relationship have been proposed, no single specific mechanism has been identified. The basis for cocaine ingestion and the resultant metabolic and renal effects, as observed in Ms. K’s case, likely are multifactorial. Mechanisms of the rhabdomyolysis might include:
- blockade of synaptic catecholamine reuptake and induction of adrenergic agonism, resulting in vasoconstriction and ischemia and leading to muscle damage
- cocaine-induced seizures and/or prolonged unconsciousness, leading to muscle compression and breakdown of muscle tissue
- a period of exertion induced by cocaine, precipitating an excited delirium and associated rhabdomyolysis
- a surge in dopamine concentrations, similar to neuroleptic malignant syndrome, precipitates hyperthermia, muscle rigidity, and psychomotor agitation, disrupting neuromuscular homeostasis and leading to rhabdomyolysis.
We were uncertain about the plausibility that acute cocaine intoxication caused Ms. K’s medical sequelae, in light of her toxicology findings. If cocaine use was the inciting event, and because the delirium reportedly had developed over several hours, we would expect cocaine to be detected in the toxicology screen. However, it was not detected. Cocaine can remain detectable in urine for 2 to 4 days,7 which raised our speculation that remote cocaine abuse could account for Ms. K’s current presentation and the timeline the roommate initially relayed to EMS personnel was inaccurate. We needed to clarify the timeline and progression of Ms. K’s symptoms with the roommate. In addition, we suggested to the medical team that alternative substances of abuse could be causing Ms. K’s symptoms and the roommate might be the only person who could unveil this possibility.
Table 1
DSM-IV-TR criteria for delirium due to multiple etiologies
| A. Disturbance of consciousness (ie, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention |
| B. A change in cognition (such as memory deficit, disorientation, language disturbances) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia |
| C. The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day |
| D. There is evidence from the history, physical examination, or laboratory findings that the delirium has >1 etiology (eg, >1 etiological general medical condition, a general medical condition plus substance intoxication or medication side effect) |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Table 2
Diagnostic criteria for cannabis and cocaine intoxication
| Diagnostic criteria | Cannabis intoxication | Cocaine intoxication |
|---|---|---|
| Recurrent use | + | + |
| Symptom onset | During or shortly after use | During or shortly after use |
| Behavioral changes | Impaired motor coordination | Hypervigilance, stereotyped behaviors |
| Psychological changes | Euphoria, anxiety, sensation of slowed time, social withdrawal, impaired judgment | Euphoria, anxiety, tension, anger, changes in sociability, interpersonal sensitivity, impaired social or occupational functioning |
| Associated criteria (≥2) | Conjunctival injection, increased appetite, dry mouth, tachycardia | Tachycardia or bradycardia, papillary dilation, elevated or lowered blood pressure, chills/perspiration, nausea/vomiting, evidence of weight loss, psychomotor changes, muscular weakness, chest pain, cardiac arrhythmias, seizure, dyskinesia, dystonia, delirium, coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | ||
HISTORY: Unknown substance
Ms. K’s roommate is contacted for supplemental history. The roommate reports that recently he observed Ms. K “snorting” a brown/tan-colored substance. He had not seen her use this substance previously, and when he asked her what it was, she reportedly said that it was “PeeVee” (also called “bath salts”) purchased over the Internet.
The authors’ observations
MDPV is a novel chemical compound that is used as a recreational drug (Table 3).8 It commonly is acquired from Internet sources and sold as “bath salts.” Its use first emerged in approximately 2004, and its popularity has been increasing because of its easy availability and relatively low cost.9 The American Association of Poison Control Centers received 302 calls related to MDPV toxicity in 2010 and 5,625 calls related to MDPV use between January 1 and October 31, 2011.10,11
MDPV has psychoactive properties, with stimulant effects acting as a norepinephrine-dopamine reuptake inhibitor.8,9,12 When snorted, ingested orally, or inserted rectally, the agent produces effects comparable to cocaine or psychostimulants such as methylphenidate or dextroamphetamine.
Acute effects of MDPV include heightened alertness, diminished need for sleep, hyperarousal, and euphoria.8,9 These symptoms often are accompanied by increases in heart rate and blood pressure, sweating, and peripheral vasoconstriction. Individuals may abuse MDPV to acquire sustained attention, reduce their need for sleep, or for aphrodisiac effects. In many cases, anxiety and irritability can accompany the desired euphoric effects. For some, the euphoric effects can be superseded by anxiety or agitation. Mood and attention effects are estimated to last 3 to 4 hours; however, tachycardia and hypertension can persist for 6 to 8 hours.
MDPV use can trigger cravings and lead to binging. Euphoric stimulation with MDPV can become dysphoric as the dose and duration of use increase. Extended use has been associated with agitation, irritability, aggression, panic and marked anxiety, psychosis, and delirium.8,9 Anxiety can range from mild dysphoric stimulation to extreme panic-like states. In moderate forms, a state of sympathetic discharge can occur, producing physiologic effects resembling panic attacks, including hypertension, tachycardia, sweating, and peripheral vasoconstriction. In more severe cases, users may experience a feeling of impending doom, marked distress, and frank psychosis. Patients may experience disorientation and unsystematized paranoid delusions. Case reports of intoxication have described self-injurious behaviors, such as cutting, which may account for the contusions observed on Ms. K’s face and arms. Increasingly, MDPV use has resulted in ER presentations with patients manifesting abrupt onset confusion, anxiety, and self-injurious behaviors.
The mechanisms underlying MDPV-induced delirium have not been definitively identified. Given the similarities in mechanism of action between MDPV and cocaine, causes for delirium related to MDPV are similarly presumed to be multifactorial. The course of delirium associated with MDPV intoxication is self-limited and requires supportive measures.8,9
Suspect MDPV abuse in patients who present with signs or symptoms of stimulant intoxication but have a negative toxicology screen for cocaine and other psychostimulants. MDPV is not detected on routine toxicology assessments; however, it can be identified through laboratories with gas chromatography/mass spectroscopy capabilities. However, the time needed to obtain the results may exceed the clinical course of the patient’s delirium. One of the limitations in Ms. K’s case was the lack of gas chromatography/mass spectroscopy to confirm MDPV ingestion. Ms. K’s roommate could not locate any unused brown powder within their apartment to bring in for laboratory investigations. Recently, screening assessments for MDPV have become commercially available (see Related Resources).
Table 3
Overview of MDPV features
| Chemical name | 3,4-methylenedioxypyrovalerone |
| Popular names | MDPV, PV, PeeVee, Super coke, Magic |
| Sources | Sold as “bath salts” by Internet sources, “head shops,” and gas stations |
| Mode of use | Oral, snorting, smoking, rectal insertion, intravenous |
| Acute effects | Increased energy, perception of heightened alertness/attention, aphrodisiac properties, increased sociability |
| Adverse psychological effects | Anxiety (panic attacks), irritability, agitation, confusion, suicidal ideations, visual distortions |
| Adverse physical effects | Insomnia/overstimulation, bruxism, muscle twitching, pupil dilation/blurred vision, anorexia, headache, nausea/vomiting, hyperthermia, irregular heart beat, tachycardia, dyspnea, fatigue |
| Effects of protracted use | Dysphoria, depression, anhedonia |
| LD50 | Unknown |
| LD50: lethal dose; MDPV: methylenedioxypyrovalerone Source: Reference 8 | |
OUTCOME: Referral to treatment
Dialysis is discontinued within 1 day of hospitalization. Ms. K’s peripheral arterial perfusion improves, as does her thermoregulatory status. Her mental status improvements coincide with improvements in her physical and metabolic status.
Ms. K is able to sustain attention when speaking with interviewers. She is aware of her surroundings and is no longer distracted by extraneous stimuli. Her speech is articulate and her thoughts are linear. There is no evidence of any residual thought disorganization, delusions, or hallucinations.
Initially, Ms. K is reluctant to acknowledge her substance use, but eventually, she concedes to acquiring a stimulant from an Internet source and abusing it in undetermined amounts. She had no experience with using MDPV and did not know how to avoid ingesting dangerous amounts. We educate Ms. K about the dangers she faced during this hospitalization and the potential life-threatening outcomes. She is amenable to pursuing outpatient substance abuse treatment. Her roommate is enlisted to facilitate her follow-up with this treatment.
The authors’ observations
Managing MDPV toxicity presents a diagnostic dilemma for medical personnel and psychiatrists when evaluating and managing acute delirium. MDPV ingestion may go unrecognized in clinical settings because toxicology assessments for it are not readily available and patients’ historical information may be unreliable.
Because of the seriousness of sequelae associated with MDPV use, state and federal agencies have intervened. Until recently, bath salts did not have a controlled substance designation. In October 2011, the US Drug Enforcement Administration (DEA) ruled to make MDPV a controlled substance for 1 year, with the possibility of a 6-month extension.13 Although this ruling is temporary, it makes possession, sale, or distribution of these chemicals, or the products that contain them, illegal in the United States. In the interim, the DEA and the US Department of Health and Human Services will determine whether MDPV should remain a controlled substance.
- American Screening Corp. (MDPV screening). www.americanscreeningcorp.com.
- U.S. Drug Enforcement Administration. 3, 4-Methylenedioxypyrovalerone (MDPV). www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones [published online ahead of print November 23, 2011]. J Med Toxicol. doi: 10.1007/s13181-011-0193-z.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. André C, Jaber-Filho JA, Bento RM, et al. Delirium following ingestion of marijuana in chocolate cookies. CNS Spectr. 2006;11(4):262-264.
2. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1-20.
3. Meyer ME. Psychiatric consequences of marijuana use: the state of the evidence. In: Tinklenberg JR ed. Marijuana and health hazards: methodologic issues in current research. New York, NY: Academic Press; 1975:33–152.
4. Ruttenber AJ, Lawler-Heavner J, Yin M, et al. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Forensic Sci. 1997;42(1):25-31.
5. Ruttenber AJ, McAnally HB, Wetli CV. Cocaine-associated rhabdomyolysis and excited delirium: different stages of the same syndrome. Am J Forensic Med Pathol. 1999;20(2):120-127.
6. Singhal PC, Rubin RB, Peters A, et al. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28(3):321-330.
7. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
8. Psychonaut WebMapping Research Group. MDPV report. London United Kingdom: Institute of Psychiatry, King’s College. http://www.psychonautproject.eu/documents/reports/MDPV.pdf. Accessed November 23, 2011.
9. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
10. American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/dnn/Portals/0/Bath%20Salts%20Data%20for%20Website%2011.03.2011.pdf. Updated November 3 2011. Accessed November 23, 2011.
11. Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan November 13, 2010-March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624-627.
12. Westphal F, Junge T, Rösner P, et al. Mass and NMR spectroscopic characterization of 3, 4-methylenedioxypyrovalerone: a designer drug with α-pyrrolidinophenone structure. Forensic Sci Int. 2009;190(1-3):1-8.
13. U.S. Drug Enforcement Administration. Chemicals used in “bath salts” now under federal control and regulation. http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Accessed November 23, 2011.
CASE: Hurt and confused
Emergency medical services (EMS) are called to Ms. K’s apartment after her roommate found her lying on the floor moaning. The roommate tells EMS that Ms. K, age 29, appeared confused and was slurring her words, and reports that this change in her awareness progressed rapidly over a few hours. EMS personnel find that Ms. K has multiple contusions on her arms and face, which they presume to be self-inflicted. A marijuana pipe is discovered at Ms. K’s apartment.
In the emergency room (ER), Ms. K is inattentive and has difficulty following simple commands. Her speech is mumbled and her thoughts are disorganized. She displays psychomotor restlessness in the form of combativeness. Ms. K cannot provide meaningful historical data and is disoriented to place and time. The ER staff requests a psychiatric consultation.
Family members reveal that Ms. K has no preexisting medical conditions, is not taking prescription medications, but has a history of substance abuse (sporadic cocaine and cannabis use). Her family is unaware of recent substance use.
Physical examination reveals tachycardia (heart rate 110 to 120 beats per minute), hypotension (blood pressure 78/49 mm Hg), hypothermia (temperature 88ºF), and peripheral pulse oximetry of 84%. Her pupils are dilated and reactive to light; no conjunctival injection is noted. Her lung fields are clear on auscultation, but she is noted to have a rapid, irregular heartbeat. The abdomen is positive for bowel sounds, soft on palpation, and without any repositioning or notable overt signs of tenderness. Ms. K’s toes show purple discoloration with poor capillary refill. The dorsalis pedis pulses are reported to be 1+ bilaterally; however, the remainder of the arterial pulse examination is normal.
Her sodium, potassium, and chloride values are normal, but she has an abnormal anion gap (28.1 mEq/L), blood urea nitrogen (53 mg/dL), creatinine (2.9 mg/dL), creatine kinase (10,857 U/L), creatine kinase MB (432.6 ng/mL), and hyperglycemia (glucose 425 mg/dL). Arterial blood gas reveals hypoxia (Po2 of 55 mm Hg), with metabolic acidosis (sodium bicarbonate 10 with compensatory Pco2 of 33 mm Hg). Her urine is cloudy, positive for protein, ketones, hemoglobin, and glucose. She is thought to have a high anion gap acidosis related to dehydration, lactic acidosis (lactic acid 20 mEq/L), and hyperglycemia. Urine toxicology is positive for cannabinoids; ethylene glycol and methanol screen negatively, which rules these out as potential contributors to her high anion gap acidosis.
Ms. K is intubated and IV fluids are initiated for rhabdomyolysis and acute renal failure. Dialysis is implemented on a short-term basis. Her mental state improves gradually over 3 days.
The authors’ observations
Based on the abrupt onset of inattention and confusion, disorganized speech, memory impairments, and psychomotor agitation, we made an initial diagnosis of delirium; however, the precise etiology remained unclear. DSM-IV-TR diagnostic criteria for delirium are described in Table 1. Although delirium due to multiple etiologies does not have a DSM-IV-TR coding designation, we speculated that multiple causes contributed to Ms. K’s presentation. Acute renal failure secondary to dehydration as well as rhabdomyolysis, hypoxia, and hyperglycemia were implicated as general medical conditions etiologically linked to delirium. Because Ms. K has no preexisting medical conditions and her roommate and family stated she had a history of substance abuse, we also considered a presumptive diagnosis of substance-induced delirium. The medical team speculated that, based on information provided by her family, Ms. K may have had a seizure or may have fallen, which would account for her multiple contusions, and could have led to muscle injury and breakdown and the resultant rhabdomyolysis.
The possibility of cannabinoid-induced delirium has been reported, albeit rarely.1-3 However, Ms. K’s presentation—hypothermia, variable heart rate, lack of dry mucous membranes—was not consistent with significant anticholinergic toxicity or cannabinoid intoxication (Table 2).
By contrast, cocaine-induced delirium has been reported and initially appeared to be a plausible cause of Ms. K’s symptoms (Table 2). Delirium related to excess ingestion of cocaine may be related to the drug’s secondary effects resulting in rhabdomyolysis and renal dysfunction.4-6 Although several mechanisms underlying this relationship have been proposed, no single specific mechanism has been identified. The basis for cocaine ingestion and the resultant metabolic and renal effects, as observed in Ms. K’s case, likely are multifactorial. Mechanisms of the rhabdomyolysis might include:
- blockade of synaptic catecholamine reuptake and induction of adrenergic agonism, resulting in vasoconstriction and ischemia and leading to muscle damage
- cocaine-induced seizures and/or prolonged unconsciousness, leading to muscle compression and breakdown of muscle tissue
- a period of exertion induced by cocaine, precipitating an excited delirium and associated rhabdomyolysis
- a surge in dopamine concentrations, similar to neuroleptic malignant syndrome, precipitates hyperthermia, muscle rigidity, and psychomotor agitation, disrupting neuromuscular homeostasis and leading to rhabdomyolysis.
We were uncertain about the plausibility that acute cocaine intoxication caused Ms. K’s medical sequelae, in light of her toxicology findings. If cocaine use was the inciting event, and because the delirium reportedly had developed over several hours, we would expect cocaine to be detected in the toxicology screen. However, it was not detected. Cocaine can remain detectable in urine for 2 to 4 days,7 which raised our speculation that remote cocaine abuse could account for Ms. K’s current presentation and the timeline the roommate initially relayed to EMS personnel was inaccurate. We needed to clarify the timeline and progression of Ms. K’s symptoms with the roommate. In addition, we suggested to the medical team that alternative substances of abuse could be causing Ms. K’s symptoms and the roommate might be the only person who could unveil this possibility.
Table 1
DSM-IV-TR criteria for delirium due to multiple etiologies
| A. Disturbance of consciousness (ie, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention |
| B. A change in cognition (such as memory deficit, disorientation, language disturbances) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia |
| C. The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day |
| D. There is evidence from the history, physical examination, or laboratory findings that the delirium has >1 etiology (eg, >1 etiological general medical condition, a general medical condition plus substance intoxication or medication side effect) |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Table 2
Diagnostic criteria for cannabis and cocaine intoxication
| Diagnostic criteria | Cannabis intoxication | Cocaine intoxication |
|---|---|---|
| Recurrent use | + | + |
| Symptom onset | During or shortly after use | During or shortly after use |
| Behavioral changes | Impaired motor coordination | Hypervigilance, stereotyped behaviors |
| Psychological changes | Euphoria, anxiety, sensation of slowed time, social withdrawal, impaired judgment | Euphoria, anxiety, tension, anger, changes in sociability, interpersonal sensitivity, impaired social or occupational functioning |
| Associated criteria (≥2) | Conjunctival injection, increased appetite, dry mouth, tachycardia | Tachycardia or bradycardia, papillary dilation, elevated or lowered blood pressure, chills/perspiration, nausea/vomiting, evidence of weight loss, psychomotor changes, muscular weakness, chest pain, cardiac arrhythmias, seizure, dyskinesia, dystonia, delirium, coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | ||
HISTORY: Unknown substance
Ms. K’s roommate is contacted for supplemental history. The roommate reports that recently he observed Ms. K “snorting” a brown/tan-colored substance. He had not seen her use this substance previously, and when he asked her what it was, she reportedly said that it was “PeeVee” (also called “bath salts”) purchased over the Internet.
The authors’ observations
MDPV is a novel chemical compound that is used as a recreational drug (Table 3).8 It commonly is acquired from Internet sources and sold as “bath salts.” Its use first emerged in approximately 2004, and its popularity has been increasing because of its easy availability and relatively low cost.9 The American Association of Poison Control Centers received 302 calls related to MDPV toxicity in 2010 and 5,625 calls related to MDPV use between January 1 and October 31, 2011.10,11
MDPV has psychoactive properties, with stimulant effects acting as a norepinephrine-dopamine reuptake inhibitor.8,9,12 When snorted, ingested orally, or inserted rectally, the agent produces effects comparable to cocaine or psychostimulants such as methylphenidate or dextroamphetamine.
Acute effects of MDPV include heightened alertness, diminished need for sleep, hyperarousal, and euphoria.8,9 These symptoms often are accompanied by increases in heart rate and blood pressure, sweating, and peripheral vasoconstriction. Individuals may abuse MDPV to acquire sustained attention, reduce their need for sleep, or for aphrodisiac effects. In many cases, anxiety and irritability can accompany the desired euphoric effects. For some, the euphoric effects can be superseded by anxiety or agitation. Mood and attention effects are estimated to last 3 to 4 hours; however, tachycardia and hypertension can persist for 6 to 8 hours.
MDPV use can trigger cravings and lead to binging. Euphoric stimulation with MDPV can become dysphoric as the dose and duration of use increase. Extended use has been associated with agitation, irritability, aggression, panic and marked anxiety, psychosis, and delirium.8,9 Anxiety can range from mild dysphoric stimulation to extreme panic-like states. In moderate forms, a state of sympathetic discharge can occur, producing physiologic effects resembling panic attacks, including hypertension, tachycardia, sweating, and peripheral vasoconstriction. In more severe cases, users may experience a feeling of impending doom, marked distress, and frank psychosis. Patients may experience disorientation and unsystematized paranoid delusions. Case reports of intoxication have described self-injurious behaviors, such as cutting, which may account for the contusions observed on Ms. K’s face and arms. Increasingly, MDPV use has resulted in ER presentations with patients manifesting abrupt onset confusion, anxiety, and self-injurious behaviors.
The mechanisms underlying MDPV-induced delirium have not been definitively identified. Given the similarities in mechanism of action between MDPV and cocaine, causes for delirium related to MDPV are similarly presumed to be multifactorial. The course of delirium associated with MDPV intoxication is self-limited and requires supportive measures.8,9
Suspect MDPV abuse in patients who present with signs or symptoms of stimulant intoxication but have a negative toxicology screen for cocaine and other psychostimulants. MDPV is not detected on routine toxicology assessments; however, it can be identified through laboratories with gas chromatography/mass spectroscopy capabilities. However, the time needed to obtain the results may exceed the clinical course of the patient’s delirium. One of the limitations in Ms. K’s case was the lack of gas chromatography/mass spectroscopy to confirm MDPV ingestion. Ms. K’s roommate could not locate any unused brown powder within their apartment to bring in for laboratory investigations. Recently, screening assessments for MDPV have become commercially available (see Related Resources).
Table 3
Overview of MDPV features
| Chemical name | 3,4-methylenedioxypyrovalerone |
| Popular names | MDPV, PV, PeeVee, Super coke, Magic |
| Sources | Sold as “bath salts” by Internet sources, “head shops,” and gas stations |
| Mode of use | Oral, snorting, smoking, rectal insertion, intravenous |
| Acute effects | Increased energy, perception of heightened alertness/attention, aphrodisiac properties, increased sociability |
| Adverse psychological effects | Anxiety (panic attacks), irritability, agitation, confusion, suicidal ideations, visual distortions |
| Adverse physical effects | Insomnia/overstimulation, bruxism, muscle twitching, pupil dilation/blurred vision, anorexia, headache, nausea/vomiting, hyperthermia, irregular heart beat, tachycardia, dyspnea, fatigue |
| Effects of protracted use | Dysphoria, depression, anhedonia |
| LD50 | Unknown |
| LD50: lethal dose; MDPV: methylenedioxypyrovalerone Source: Reference 8 | |
OUTCOME: Referral to treatment
Dialysis is discontinued within 1 day of hospitalization. Ms. K’s peripheral arterial perfusion improves, as does her thermoregulatory status. Her mental status improvements coincide with improvements in her physical and metabolic status.
Ms. K is able to sustain attention when speaking with interviewers. She is aware of her surroundings and is no longer distracted by extraneous stimuli. Her speech is articulate and her thoughts are linear. There is no evidence of any residual thought disorganization, delusions, or hallucinations.
Initially, Ms. K is reluctant to acknowledge her substance use, but eventually, she concedes to acquiring a stimulant from an Internet source and abusing it in undetermined amounts. She had no experience with using MDPV and did not know how to avoid ingesting dangerous amounts. We educate Ms. K about the dangers she faced during this hospitalization and the potential life-threatening outcomes. She is amenable to pursuing outpatient substance abuse treatment. Her roommate is enlisted to facilitate her follow-up with this treatment.
The authors’ observations
Managing MDPV toxicity presents a diagnostic dilemma for medical personnel and psychiatrists when evaluating and managing acute delirium. MDPV ingestion may go unrecognized in clinical settings because toxicology assessments for it are not readily available and patients’ historical information may be unreliable.
Because of the seriousness of sequelae associated with MDPV use, state and federal agencies have intervened. Until recently, bath salts did not have a controlled substance designation. In October 2011, the US Drug Enforcement Administration (DEA) ruled to make MDPV a controlled substance for 1 year, with the possibility of a 6-month extension.13 Although this ruling is temporary, it makes possession, sale, or distribution of these chemicals, or the products that contain them, illegal in the United States. In the interim, the DEA and the US Department of Health and Human Services will determine whether MDPV should remain a controlled substance.
- American Screening Corp. (MDPV screening). www.americanscreeningcorp.com.
- U.S. Drug Enforcement Administration. 3, 4-Methylenedioxypyrovalerone (MDPV). www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones [published online ahead of print November 23, 2011]. J Med Toxicol. doi: 10.1007/s13181-011-0193-z.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Hurt and confused
Emergency medical services (EMS) are called to Ms. K’s apartment after her roommate found her lying on the floor moaning. The roommate tells EMS that Ms. K, age 29, appeared confused and was slurring her words, and reports that this change in her awareness progressed rapidly over a few hours. EMS personnel find that Ms. K has multiple contusions on her arms and face, which they presume to be self-inflicted. A marijuana pipe is discovered at Ms. K’s apartment.
In the emergency room (ER), Ms. K is inattentive and has difficulty following simple commands. Her speech is mumbled and her thoughts are disorganized. She displays psychomotor restlessness in the form of combativeness. Ms. K cannot provide meaningful historical data and is disoriented to place and time. The ER staff requests a psychiatric consultation.
Family members reveal that Ms. K has no preexisting medical conditions, is not taking prescription medications, but has a history of substance abuse (sporadic cocaine and cannabis use). Her family is unaware of recent substance use.
Physical examination reveals tachycardia (heart rate 110 to 120 beats per minute), hypotension (blood pressure 78/49 mm Hg), hypothermia (temperature 88ºF), and peripheral pulse oximetry of 84%. Her pupils are dilated and reactive to light; no conjunctival injection is noted. Her lung fields are clear on auscultation, but she is noted to have a rapid, irregular heartbeat. The abdomen is positive for bowel sounds, soft on palpation, and without any repositioning or notable overt signs of tenderness. Ms. K’s toes show purple discoloration with poor capillary refill. The dorsalis pedis pulses are reported to be 1+ bilaterally; however, the remainder of the arterial pulse examination is normal.
Her sodium, potassium, and chloride values are normal, but she has an abnormal anion gap (28.1 mEq/L), blood urea nitrogen (53 mg/dL), creatinine (2.9 mg/dL), creatine kinase (10,857 U/L), creatine kinase MB (432.6 ng/mL), and hyperglycemia (glucose 425 mg/dL). Arterial blood gas reveals hypoxia (Po2 of 55 mm Hg), with metabolic acidosis (sodium bicarbonate 10 with compensatory Pco2 of 33 mm Hg). Her urine is cloudy, positive for protein, ketones, hemoglobin, and glucose. She is thought to have a high anion gap acidosis related to dehydration, lactic acidosis (lactic acid 20 mEq/L), and hyperglycemia. Urine toxicology is positive for cannabinoids; ethylene glycol and methanol screen negatively, which rules these out as potential contributors to her high anion gap acidosis.
Ms. K is intubated and IV fluids are initiated for rhabdomyolysis and acute renal failure. Dialysis is implemented on a short-term basis. Her mental state improves gradually over 3 days.
The authors’ observations
Based on the abrupt onset of inattention and confusion, disorganized speech, memory impairments, and psychomotor agitation, we made an initial diagnosis of delirium; however, the precise etiology remained unclear. DSM-IV-TR diagnostic criteria for delirium are described in Table 1. Although delirium due to multiple etiologies does not have a DSM-IV-TR coding designation, we speculated that multiple causes contributed to Ms. K’s presentation. Acute renal failure secondary to dehydration as well as rhabdomyolysis, hypoxia, and hyperglycemia were implicated as general medical conditions etiologically linked to delirium. Because Ms. K has no preexisting medical conditions and her roommate and family stated she had a history of substance abuse, we also considered a presumptive diagnosis of substance-induced delirium. The medical team speculated that, based on information provided by her family, Ms. K may have had a seizure or may have fallen, which would account for her multiple contusions, and could have led to muscle injury and breakdown and the resultant rhabdomyolysis.
The possibility of cannabinoid-induced delirium has been reported, albeit rarely.1-3 However, Ms. K’s presentation—hypothermia, variable heart rate, lack of dry mucous membranes—was not consistent with significant anticholinergic toxicity or cannabinoid intoxication (Table 2).
By contrast, cocaine-induced delirium has been reported and initially appeared to be a plausible cause of Ms. K’s symptoms (Table 2). Delirium related to excess ingestion of cocaine may be related to the drug’s secondary effects resulting in rhabdomyolysis and renal dysfunction.4-6 Although several mechanisms underlying this relationship have been proposed, no single specific mechanism has been identified. The basis for cocaine ingestion and the resultant metabolic and renal effects, as observed in Ms. K’s case, likely are multifactorial. Mechanisms of the rhabdomyolysis might include:
- blockade of synaptic catecholamine reuptake and induction of adrenergic agonism, resulting in vasoconstriction and ischemia and leading to muscle damage
- cocaine-induced seizures and/or prolonged unconsciousness, leading to muscle compression and breakdown of muscle tissue
- a period of exertion induced by cocaine, precipitating an excited delirium and associated rhabdomyolysis
- a surge in dopamine concentrations, similar to neuroleptic malignant syndrome, precipitates hyperthermia, muscle rigidity, and psychomotor agitation, disrupting neuromuscular homeostasis and leading to rhabdomyolysis.
We were uncertain about the plausibility that acute cocaine intoxication caused Ms. K’s medical sequelae, in light of her toxicology findings. If cocaine use was the inciting event, and because the delirium reportedly had developed over several hours, we would expect cocaine to be detected in the toxicology screen. However, it was not detected. Cocaine can remain detectable in urine for 2 to 4 days,7 which raised our speculation that remote cocaine abuse could account for Ms. K’s current presentation and the timeline the roommate initially relayed to EMS personnel was inaccurate. We needed to clarify the timeline and progression of Ms. K’s symptoms with the roommate. In addition, we suggested to the medical team that alternative substances of abuse could be causing Ms. K’s symptoms and the roommate might be the only person who could unveil this possibility.
Table 1
DSM-IV-TR criteria for delirium due to multiple etiologies
| A. Disturbance of consciousness (ie, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention |
| B. A change in cognition (such as memory deficit, disorientation, language disturbances) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia |
| C. The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day |
| D. There is evidence from the history, physical examination, or laboratory findings that the delirium has >1 etiology (eg, >1 etiological general medical condition, a general medical condition plus substance intoxication or medication side effect) |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
Table 2
Diagnostic criteria for cannabis and cocaine intoxication
| Diagnostic criteria | Cannabis intoxication | Cocaine intoxication |
|---|---|---|
| Recurrent use | + | + |
| Symptom onset | During or shortly after use | During or shortly after use |
| Behavioral changes | Impaired motor coordination | Hypervigilance, stereotyped behaviors |
| Psychological changes | Euphoria, anxiety, sensation of slowed time, social withdrawal, impaired judgment | Euphoria, anxiety, tension, anger, changes in sociability, interpersonal sensitivity, impaired social or occupational functioning |
| Associated criteria (≥2) | Conjunctival injection, increased appetite, dry mouth, tachycardia | Tachycardia or bradycardia, papillary dilation, elevated or lowered blood pressure, chills/perspiration, nausea/vomiting, evidence of weight loss, psychomotor changes, muscular weakness, chest pain, cardiac arrhythmias, seizure, dyskinesia, dystonia, delirium, coma |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | ||
HISTORY: Unknown substance
Ms. K’s roommate is contacted for supplemental history. The roommate reports that recently he observed Ms. K “snorting” a brown/tan-colored substance. He had not seen her use this substance previously, and when he asked her what it was, she reportedly said that it was “PeeVee” (also called “bath salts”) purchased over the Internet.
The authors’ observations
MDPV is a novel chemical compound that is used as a recreational drug (Table 3).8 It commonly is acquired from Internet sources and sold as “bath salts.” Its use first emerged in approximately 2004, and its popularity has been increasing because of its easy availability and relatively low cost.9 The American Association of Poison Control Centers received 302 calls related to MDPV toxicity in 2010 and 5,625 calls related to MDPV use between January 1 and October 31, 2011.10,11
MDPV has psychoactive properties, with stimulant effects acting as a norepinephrine-dopamine reuptake inhibitor.8,9,12 When snorted, ingested orally, or inserted rectally, the agent produces effects comparable to cocaine or psychostimulants such as methylphenidate or dextroamphetamine.
Acute effects of MDPV include heightened alertness, diminished need for sleep, hyperarousal, and euphoria.8,9 These symptoms often are accompanied by increases in heart rate and blood pressure, sweating, and peripheral vasoconstriction. Individuals may abuse MDPV to acquire sustained attention, reduce their need for sleep, or for aphrodisiac effects. In many cases, anxiety and irritability can accompany the desired euphoric effects. For some, the euphoric effects can be superseded by anxiety or agitation. Mood and attention effects are estimated to last 3 to 4 hours; however, tachycardia and hypertension can persist for 6 to 8 hours.
MDPV use can trigger cravings and lead to binging. Euphoric stimulation with MDPV can become dysphoric as the dose and duration of use increase. Extended use has been associated with agitation, irritability, aggression, panic and marked anxiety, psychosis, and delirium.8,9 Anxiety can range from mild dysphoric stimulation to extreme panic-like states. In moderate forms, a state of sympathetic discharge can occur, producing physiologic effects resembling panic attacks, including hypertension, tachycardia, sweating, and peripheral vasoconstriction. In more severe cases, users may experience a feeling of impending doom, marked distress, and frank psychosis. Patients may experience disorientation and unsystematized paranoid delusions. Case reports of intoxication have described self-injurious behaviors, such as cutting, which may account for the contusions observed on Ms. K’s face and arms. Increasingly, MDPV use has resulted in ER presentations with patients manifesting abrupt onset confusion, anxiety, and self-injurious behaviors.
The mechanisms underlying MDPV-induced delirium have not been definitively identified. Given the similarities in mechanism of action between MDPV and cocaine, causes for delirium related to MDPV are similarly presumed to be multifactorial. The course of delirium associated with MDPV intoxication is self-limited and requires supportive measures.8,9
Suspect MDPV abuse in patients who present with signs or symptoms of stimulant intoxication but have a negative toxicology screen for cocaine and other psychostimulants. MDPV is not detected on routine toxicology assessments; however, it can be identified through laboratories with gas chromatography/mass spectroscopy capabilities. However, the time needed to obtain the results may exceed the clinical course of the patient’s delirium. One of the limitations in Ms. K’s case was the lack of gas chromatography/mass spectroscopy to confirm MDPV ingestion. Ms. K’s roommate could not locate any unused brown powder within their apartment to bring in for laboratory investigations. Recently, screening assessments for MDPV have become commercially available (see Related Resources).
Table 3
Overview of MDPV features
| Chemical name | 3,4-methylenedioxypyrovalerone |
| Popular names | MDPV, PV, PeeVee, Super coke, Magic |
| Sources | Sold as “bath salts” by Internet sources, “head shops,” and gas stations |
| Mode of use | Oral, snorting, smoking, rectal insertion, intravenous |
| Acute effects | Increased energy, perception of heightened alertness/attention, aphrodisiac properties, increased sociability |
| Adverse psychological effects | Anxiety (panic attacks), irritability, agitation, confusion, suicidal ideations, visual distortions |
| Adverse physical effects | Insomnia/overstimulation, bruxism, muscle twitching, pupil dilation/blurred vision, anorexia, headache, nausea/vomiting, hyperthermia, irregular heart beat, tachycardia, dyspnea, fatigue |
| Effects of protracted use | Dysphoria, depression, anhedonia |
| LD50 | Unknown |
| LD50: lethal dose; MDPV: methylenedioxypyrovalerone Source: Reference 8 | |
OUTCOME: Referral to treatment
Dialysis is discontinued within 1 day of hospitalization. Ms. K’s peripheral arterial perfusion improves, as does her thermoregulatory status. Her mental status improvements coincide with improvements in her physical and metabolic status.
Ms. K is able to sustain attention when speaking with interviewers. She is aware of her surroundings and is no longer distracted by extraneous stimuli. Her speech is articulate and her thoughts are linear. There is no evidence of any residual thought disorganization, delusions, or hallucinations.
Initially, Ms. K is reluctant to acknowledge her substance use, but eventually, she concedes to acquiring a stimulant from an Internet source and abusing it in undetermined amounts. She had no experience with using MDPV and did not know how to avoid ingesting dangerous amounts. We educate Ms. K about the dangers she faced during this hospitalization and the potential life-threatening outcomes. She is amenable to pursuing outpatient substance abuse treatment. Her roommate is enlisted to facilitate her follow-up with this treatment.
The authors’ observations
Managing MDPV toxicity presents a diagnostic dilemma for medical personnel and psychiatrists when evaluating and managing acute delirium. MDPV ingestion may go unrecognized in clinical settings because toxicology assessments for it are not readily available and patients’ historical information may be unreliable.
Because of the seriousness of sequelae associated with MDPV use, state and federal agencies have intervened. Until recently, bath salts did not have a controlled substance designation. In October 2011, the US Drug Enforcement Administration (DEA) ruled to make MDPV a controlled substance for 1 year, with the possibility of a 6-month extension.13 Although this ruling is temporary, it makes possession, sale, or distribution of these chemicals, or the products that contain them, illegal in the United States. In the interim, the DEA and the US Department of Health and Human Services will determine whether MDPV should remain a controlled substance.
- American Screening Corp. (MDPV screening). www.americanscreeningcorp.com.
- U.S. Drug Enforcement Administration. 3, 4-Methylenedioxypyrovalerone (MDPV). www.deadiversion.usdoj.gov/drugs_concern/mdpv.pdf.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones [published online ahead of print November 23, 2011]. J Med Toxicol. doi: 10.1007/s13181-011-0193-z.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. André C, Jaber-Filho JA, Bento RM, et al. Delirium following ingestion of marijuana in chocolate cookies. CNS Spectr. 2006;11(4):262-264.
2. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1-20.
3. Meyer ME. Psychiatric consequences of marijuana use: the state of the evidence. In: Tinklenberg JR ed. Marijuana and health hazards: methodologic issues in current research. New York, NY: Academic Press; 1975:33–152.
4. Ruttenber AJ, Lawler-Heavner J, Yin M, et al. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Forensic Sci. 1997;42(1):25-31.
5. Ruttenber AJ, McAnally HB, Wetli CV. Cocaine-associated rhabdomyolysis and excited delirium: different stages of the same syndrome. Am J Forensic Med Pathol. 1999;20(2):120-127.
6. Singhal PC, Rubin RB, Peters A, et al. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28(3):321-330.
7. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
8. Psychonaut WebMapping Research Group. MDPV report. London United Kingdom: Institute of Psychiatry, King’s College. http://www.psychonautproject.eu/documents/reports/MDPV.pdf. Accessed November 23, 2011.
9. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
10. American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/dnn/Portals/0/Bath%20Salts%20Data%20for%20Website%2011.03.2011.pdf. Updated November 3 2011. Accessed November 23, 2011.
11. Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan November 13, 2010-March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624-627.
12. Westphal F, Junge T, Rösner P, et al. Mass and NMR spectroscopic characterization of 3, 4-methylenedioxypyrovalerone: a designer drug with α-pyrrolidinophenone structure. Forensic Sci Int. 2009;190(1-3):1-8.
13. U.S. Drug Enforcement Administration. Chemicals used in “bath salts” now under federal control and regulation. http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Accessed November 23, 2011.
1. André C, Jaber-Filho JA, Bento RM, et al. Delirium following ingestion of marijuana in chocolate cookies. CNS Spectr. 2006;11(4):262-264.
2. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1-20.
3. Meyer ME. Psychiatric consequences of marijuana use: the state of the evidence. In: Tinklenberg JR ed. Marijuana and health hazards: methodologic issues in current research. New York, NY: Academic Press; 1975:33–152.
4. Ruttenber AJ, Lawler-Heavner J, Yin M, et al. Fatal excited delirium following cocaine use: epidemiologic findings provide new evidence for mechanisms of cocaine toxicity. J Forensic Sci. 1997;42(1):25-31.
5. Ruttenber AJ, McAnally HB, Wetli CV. Cocaine-associated rhabdomyolysis and excited delirium: different stages of the same syndrome. Am J Forensic Med Pathol. 1999;20(2):120-127.
6. Singhal PC, Rubin RB, Peters A, et al. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28(3):321-330.
7. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
8. Psychonaut WebMapping Research Group. MDPV report. London United Kingdom: Institute of Psychiatry, King’s College. http://www.psychonautproject.eu/documents/reports/MDPV.pdf. Accessed November 23, 2011.
9. Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365(10):967-968.
10. American Association of Poison Control Centers. Bath salts data. http://www.aapcc.org/dnn/Portals/0/Bath%20Salts%20Data%20for%20Website%2011.03.2011.pdf. Updated November 3 2011. Accessed November 23, 2011.
11. Centers for Disease Control and Prevention. Emergency department visits after use of a drug sold as “bath salts”—Michigan November 13, 2010-March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(19):624-627.
12. Westphal F, Junge T, Rösner P, et al. Mass and NMR spectroscopic characterization of 3, 4-methylenedioxypyrovalerone: a designer drug with α-pyrrolidinophenone structure. Forensic Sci Int. 2009;190(1-3):1-8.
13. U.S. Drug Enforcement Administration. Chemicals used in “bath salts” now under federal control and regulation. http://www.justice.gov/dea/pubs/pressrel/pr102111.html. Accessed November 23, 2011.