User login
Work With a Hospital Without Working for It
PHILADELPHIA – With hospitals buying up physician practices, many doctors are tempted to take the bait, but Alice G. Gosfield, a lawyer who specializes in physician practice ownership strategies, called this the “employment delusion” and the “acquisition fantasy.”
Many physicians don't recognize that “the common law term for the employer-employee relationship is 'master-servant,'” she said. The master “gets to tell you who, what, where, when, and why and how, and if you think that a contract can prevent that from happening, you would be wrong.”
Ms. Gosfield gave the meeting audience a real-world dose of how even the best-laid plans go awry when physicians sell out to hospital groups. She also shared strategies on how doctors can avoid selling their practices to hospitals but still affiliate with hospital groups.
She debunked myths about how selling out to a hospital group can guarantee financial security. “The hospital is getting paid under the same stupid reimbursement formula that you are,” she said. “The only way that revenue stream ends up being more than what you're getting in your practice is if they are paying you for doing other things besides clinical work.”
Another delusion is that the contract is a safeguard. “A contract is only as good as the will of the parties to abide by it,” Ms. Gosfield said. Not infrequently, one party will break the contract with little recourse outside the courts, “and litigation is a really, really bad way of solving business problems,” she added.
She singled out two strategies for selling a practice to a hospital: the sale of physical assets, including diagnostic “toys and weapons” but not the practice per se; and noncompete covenants. “It has to be fair-market value under the Stark regulations,” she said of the latter, “and somebody – not a lawyer – has to do a valuation.”
But hospitals will not pay for good will. “They're not going to make you whole for what it took you to build your practice,” she said. “I don't care how long it took, what your sweat equity was, what all the pains were – you are not going to get that back from a hospital in terms of an acquisition or lease or other kind of arrangement.”
For self-preservation, she implored physicians to adopt the quality improvement measures that will provide the basis for Medicare reimbursement in 2012. “Now is the time to change your clinical and administrative processes,” she said. “Don't wait until the conditions they're going to be focused on are published. We all know what the conditions are.” That information is already available from the National Quality Forum, she said.
Among alternatives to selling, Ms. Gosfield suggested leasing the practice to the hospital, entering into comanagement contracts, having the hospital place a new physician in the practice, gainsharing, giving the hospital the right of first refusal if another entity offers to buy the practice, having the hospital provide CME for practice physicians and ancillary staff, leasing practice staff to the hospital, and having the practice provide contract services to the hospital.
“Your group stays as your group,” she said in describing the leasing process.” you reassign your right to get paid to the hospital. They pay you a salary. They will require productivity measures, but they can pay you irrespective of whether they get paid, which is not how your system works when you're in private practice.”
A comanagement contract involves the physician's providing on-call services or advising the hospital on its care delivery systems. This could include performance bonuses when the hospital achieves specified results, she said, but she advised against getting paid an hourly fee. “Swapping an hour in your office for an hour of their time – you can't make it up,” she said.
Having the hospital place a physician in the practice should be carefully structured, Ms. Gosfield said. Her preferred arrangement would have the hospital subsidize the up-front costs with a loan, then forgive the loan for each month the doctor stays in the community after the subsidy ends. One problem with this approach, she pointed out, is that “you can't then have a restricted covenant which prohibits this young doctor that you brought in and introduced to your patients from opening up next door,” she said.
Physicians can benefit from the right collaboration with a hospital, she said. “The things that unite you with the hospital are more than the things that divide you,” Ms. Gosfield noted. “You will do better holding hands crossing the dangerous street of health care in the future with the hospital, but you need to maintain your own identity.”
Ms. Gosfield reported no disclosures.
PHILADELPHIA – With hospitals buying up physician practices, many doctors are tempted to take the bait, but Alice G. Gosfield, a lawyer who specializes in physician practice ownership strategies, called this the “employment delusion” and the “acquisition fantasy.”
Many physicians don't recognize that “the common law term for the employer-employee relationship is 'master-servant,'” she said. The master “gets to tell you who, what, where, when, and why and how, and if you think that a contract can prevent that from happening, you would be wrong.”
Ms. Gosfield gave the meeting audience a real-world dose of how even the best-laid plans go awry when physicians sell out to hospital groups. She also shared strategies on how doctors can avoid selling their practices to hospitals but still affiliate with hospital groups.
She debunked myths about how selling out to a hospital group can guarantee financial security. “The hospital is getting paid under the same stupid reimbursement formula that you are,” she said. “The only way that revenue stream ends up being more than what you're getting in your practice is if they are paying you for doing other things besides clinical work.”
Another delusion is that the contract is a safeguard. “A contract is only as good as the will of the parties to abide by it,” Ms. Gosfield said. Not infrequently, one party will break the contract with little recourse outside the courts, “and litigation is a really, really bad way of solving business problems,” she added.
She singled out two strategies for selling a practice to a hospital: the sale of physical assets, including diagnostic “toys and weapons” but not the practice per se; and noncompete covenants. “It has to be fair-market value under the Stark regulations,” she said of the latter, “and somebody – not a lawyer – has to do a valuation.”
But hospitals will not pay for good will. “They're not going to make you whole for what it took you to build your practice,” she said. “I don't care how long it took, what your sweat equity was, what all the pains were – you are not going to get that back from a hospital in terms of an acquisition or lease or other kind of arrangement.”
For self-preservation, she implored physicians to adopt the quality improvement measures that will provide the basis for Medicare reimbursement in 2012. “Now is the time to change your clinical and administrative processes,” she said. “Don't wait until the conditions they're going to be focused on are published. We all know what the conditions are.” That information is already available from the National Quality Forum, she said.
Among alternatives to selling, Ms. Gosfield suggested leasing the practice to the hospital, entering into comanagement contracts, having the hospital place a new physician in the practice, gainsharing, giving the hospital the right of first refusal if another entity offers to buy the practice, having the hospital provide CME for practice physicians and ancillary staff, leasing practice staff to the hospital, and having the practice provide contract services to the hospital.
“Your group stays as your group,” she said in describing the leasing process.” you reassign your right to get paid to the hospital. They pay you a salary. They will require productivity measures, but they can pay you irrespective of whether they get paid, which is not how your system works when you're in private practice.”
A comanagement contract involves the physician's providing on-call services or advising the hospital on its care delivery systems. This could include performance bonuses when the hospital achieves specified results, she said, but she advised against getting paid an hourly fee. “Swapping an hour in your office for an hour of their time – you can't make it up,” she said.
Having the hospital place a physician in the practice should be carefully structured, Ms. Gosfield said. Her preferred arrangement would have the hospital subsidize the up-front costs with a loan, then forgive the loan for each month the doctor stays in the community after the subsidy ends. One problem with this approach, she pointed out, is that “you can't then have a restricted covenant which prohibits this young doctor that you brought in and introduced to your patients from opening up next door,” she said.
Physicians can benefit from the right collaboration with a hospital, she said. “The things that unite you with the hospital are more than the things that divide you,” Ms. Gosfield noted. “You will do better holding hands crossing the dangerous street of health care in the future with the hospital, but you need to maintain your own identity.”
Ms. Gosfield reported no disclosures.
PHILADELPHIA – With hospitals buying up physician practices, many doctors are tempted to take the bait, but Alice G. Gosfield, a lawyer who specializes in physician practice ownership strategies, called this the “employment delusion” and the “acquisition fantasy.”
Many physicians don't recognize that “the common law term for the employer-employee relationship is 'master-servant,'” she said. The master “gets to tell you who, what, where, when, and why and how, and if you think that a contract can prevent that from happening, you would be wrong.”
Ms. Gosfield gave the meeting audience a real-world dose of how even the best-laid plans go awry when physicians sell out to hospital groups. She also shared strategies on how doctors can avoid selling their practices to hospitals but still affiliate with hospital groups.
She debunked myths about how selling out to a hospital group can guarantee financial security. “The hospital is getting paid under the same stupid reimbursement formula that you are,” she said. “The only way that revenue stream ends up being more than what you're getting in your practice is if they are paying you for doing other things besides clinical work.”
Another delusion is that the contract is a safeguard. “A contract is only as good as the will of the parties to abide by it,” Ms. Gosfield said. Not infrequently, one party will break the contract with little recourse outside the courts, “and litigation is a really, really bad way of solving business problems,” she added.
She singled out two strategies for selling a practice to a hospital: the sale of physical assets, including diagnostic “toys and weapons” but not the practice per se; and noncompete covenants. “It has to be fair-market value under the Stark regulations,” she said of the latter, “and somebody – not a lawyer – has to do a valuation.”
But hospitals will not pay for good will. “They're not going to make you whole for what it took you to build your practice,” she said. “I don't care how long it took, what your sweat equity was, what all the pains were – you are not going to get that back from a hospital in terms of an acquisition or lease or other kind of arrangement.”
For self-preservation, she implored physicians to adopt the quality improvement measures that will provide the basis for Medicare reimbursement in 2012. “Now is the time to change your clinical and administrative processes,” she said. “Don't wait until the conditions they're going to be focused on are published. We all know what the conditions are.” That information is already available from the National Quality Forum, she said.
Among alternatives to selling, Ms. Gosfield suggested leasing the practice to the hospital, entering into comanagement contracts, having the hospital place a new physician in the practice, gainsharing, giving the hospital the right of first refusal if another entity offers to buy the practice, having the hospital provide CME for practice physicians and ancillary staff, leasing practice staff to the hospital, and having the practice provide contract services to the hospital.
“Your group stays as your group,” she said in describing the leasing process.” you reassign your right to get paid to the hospital. They pay you a salary. They will require productivity measures, but they can pay you irrespective of whether they get paid, which is not how your system works when you're in private practice.”
A comanagement contract involves the physician's providing on-call services or advising the hospital on its care delivery systems. This could include performance bonuses when the hospital achieves specified results, she said, but she advised against getting paid an hourly fee. “Swapping an hour in your office for an hour of their time – you can't make it up,” she said.
Having the hospital place a physician in the practice should be carefully structured, Ms. Gosfield said. Her preferred arrangement would have the hospital subsidize the up-front costs with a loan, then forgive the loan for each month the doctor stays in the community after the subsidy ends. One problem with this approach, she pointed out, is that “you can't then have a restricted covenant which prohibits this young doctor that you brought in and introduced to your patients from opening up next door,” she said.
Physicians can benefit from the right collaboration with a hospital, she said. “The things that unite you with the hospital are more than the things that divide you,” Ms. Gosfield noted. “You will do better holding hands crossing the dangerous street of health care in the future with the hospital, but you need to maintain your own identity.”
Ms. Gosfield reported no disclosures.
Reform Brings Tighter Rules on Utilization Rates, Self-Referrals
PHILADELPHIA — Health care reform, in the guise of the Patient Protection and Affordable Care Act signed into law by President Obama last spring, will require physicians to deal with new regulations on utilization of imaging equipment and self-referrals.
Despite rumblings of overturning the law, Dr. Kim Williams said at the meeting that health care reform is here to stay. “It would be very difficult, though not impossible – but very difficult to flip a house of Congress and to repeal this.” Regulations will be phased in over the next 4 years, he said.
The equipment utilization rate that Medicare uses to establish reimbursements is due in 2011 for an adjustment for three types of imaging – MRI, CT, and PET – but excludes single-photon emission computed tomography (SPECT). Dr. Williams of Wayne State University in Detroit, described the equipment utilization rate as “a mechanism to actually decrease reimbursement.” Medicare actually rolled back the rate for MRI, CT, and PET from 90% to 50% in 2010, but will bump it back up to 75% in 2011, he said.
Meanwhile, the Affordable Care Act tightens requirements on self-referrals. Dr. Williams raised a hypothetical situation. “Running an EKG – is that self -eferral? Yes,” he said, “but it hasn't come onto anyone's radar screen because it isn't a lot of money.” He cited other “elements of the house of medicine” with accusing cardiology and other specialties of inappropriate self-referral.
Like the equipment utilization rate, the disclosure provisions on self-referral cover MRI, CT, and PET but not SPECT, at least not yet, Dr. Williams said. “Most of us look at that [from the viewpoint that] a patient expects a self-respecting practice to own its equipment, so it isn't that onerous,” he said. “But the devil is in the details.”
Among those details he outlined: “One will have to inform patients in writing at the time of the referral that they can obtain services from someone other than the referring physician or someone in the referring physician's practice.” That takes the form of a list of at least 10 other providers within a 25-mile radius, including phone numbers and distance. The final regulation should go into effect on January 1.
The Affordable Care Act also empowers the Medicare Payment Advisory Commission (MedPAC) to make nonbinding recommendations to Congress on payment revisions. One problematic area MedPAC is looking at is developing payment tools that take into account providers' utilization rates, Dr. Williams said. “If your utilization is high, you get less reimbursement,” he said. “The problem with that is that nobody mentioned risk adjustment.”
Dr. Williams said that he had no relevant disclosures.
PHILADELPHIA — Health care reform, in the guise of the Patient Protection and Affordable Care Act signed into law by President Obama last spring, will require physicians to deal with new regulations on utilization of imaging equipment and self-referrals.
Despite rumblings of overturning the law, Dr. Kim Williams said at the meeting that health care reform is here to stay. “It would be very difficult, though not impossible – but very difficult to flip a house of Congress and to repeal this.” Regulations will be phased in over the next 4 years, he said.
The equipment utilization rate that Medicare uses to establish reimbursements is due in 2011 for an adjustment for three types of imaging – MRI, CT, and PET – but excludes single-photon emission computed tomography (SPECT). Dr. Williams of Wayne State University in Detroit, described the equipment utilization rate as “a mechanism to actually decrease reimbursement.” Medicare actually rolled back the rate for MRI, CT, and PET from 90% to 50% in 2010, but will bump it back up to 75% in 2011, he said.
Meanwhile, the Affordable Care Act tightens requirements on self-referrals. Dr. Williams raised a hypothetical situation. “Running an EKG – is that self -eferral? Yes,” he said, “but it hasn't come onto anyone's radar screen because it isn't a lot of money.” He cited other “elements of the house of medicine” with accusing cardiology and other specialties of inappropriate self-referral.
Like the equipment utilization rate, the disclosure provisions on self-referral cover MRI, CT, and PET but not SPECT, at least not yet, Dr. Williams said. “Most of us look at that [from the viewpoint that] a patient expects a self-respecting practice to own its equipment, so it isn't that onerous,” he said. “But the devil is in the details.”
Among those details he outlined: “One will have to inform patients in writing at the time of the referral that they can obtain services from someone other than the referring physician or someone in the referring physician's practice.” That takes the form of a list of at least 10 other providers within a 25-mile radius, including phone numbers and distance. The final regulation should go into effect on January 1.
The Affordable Care Act also empowers the Medicare Payment Advisory Commission (MedPAC) to make nonbinding recommendations to Congress on payment revisions. One problematic area MedPAC is looking at is developing payment tools that take into account providers' utilization rates, Dr. Williams said. “If your utilization is high, you get less reimbursement,” he said. “The problem with that is that nobody mentioned risk adjustment.”
Dr. Williams said that he had no relevant disclosures.
PHILADELPHIA — Health care reform, in the guise of the Patient Protection and Affordable Care Act signed into law by President Obama last spring, will require physicians to deal with new regulations on utilization of imaging equipment and self-referrals.
Despite rumblings of overturning the law, Dr. Kim Williams said at the meeting that health care reform is here to stay. “It would be very difficult, though not impossible – but very difficult to flip a house of Congress and to repeal this.” Regulations will be phased in over the next 4 years, he said.
The equipment utilization rate that Medicare uses to establish reimbursements is due in 2011 for an adjustment for three types of imaging – MRI, CT, and PET – but excludes single-photon emission computed tomography (SPECT). Dr. Williams of Wayne State University in Detroit, described the equipment utilization rate as “a mechanism to actually decrease reimbursement.” Medicare actually rolled back the rate for MRI, CT, and PET from 90% to 50% in 2010, but will bump it back up to 75% in 2011, he said.
Meanwhile, the Affordable Care Act tightens requirements on self-referrals. Dr. Williams raised a hypothetical situation. “Running an EKG – is that self -eferral? Yes,” he said, “but it hasn't come onto anyone's radar screen because it isn't a lot of money.” He cited other “elements of the house of medicine” with accusing cardiology and other specialties of inappropriate self-referral.
Like the equipment utilization rate, the disclosure provisions on self-referral cover MRI, CT, and PET but not SPECT, at least not yet, Dr. Williams said. “Most of us look at that [from the viewpoint that] a patient expects a self-respecting practice to own its equipment, so it isn't that onerous,” he said. “But the devil is in the details.”
Among those details he outlined: “One will have to inform patients in writing at the time of the referral that they can obtain services from someone other than the referring physician or someone in the referring physician's practice.” That takes the form of a list of at least 10 other providers within a 25-mile radius, including phone numbers and distance. The final regulation should go into effect on January 1.
The Affordable Care Act also empowers the Medicare Payment Advisory Commission (MedPAC) to make nonbinding recommendations to Congress on payment revisions. One problematic area MedPAC is looking at is developing payment tools that take into account providers' utilization rates, Dr. Williams said. “If your utilization is high, you get less reimbursement,” he said. “The problem with that is that nobody mentioned risk adjustment.”
Dr. Williams said that he had no relevant disclosures.
Study Validates Alternative Stress Agent in Asthma, COPD
PHILADELPHIA – Individuals with asthma or chronic obstructive pulmonary disease can tolerate the imaging agent regadenoson well if they need to undergo cardiac stress testing, a study has shown.
Dr. Bruce Prenner, a San Diego allergist, reported on findings from a multicenter trial involving 999 patients who received either regadenoson or a placebo. “Regadenoson has a greater affinity for the A2B receptors and the other types of receptors, and thus the risk of bronchospasm and bronchoreactive events should be quite low,” he said at the meeting.
The risks of adenosine inducing breathing problems in individuals with asthma and COPD have been well documented. This study set out to determine how regadenoson affected forced expiratory volume in 1 second (FEV1) in 999 study subjects, 532 with asthma and 467 with COPD. About half of the patients received the placebo. The primary end point was a greater than 15% decrease in forced expiratory function from baseline within 2 hours of the dose being administered, Dr. Prenner said.
“In the asthma group, 1.1% of [regadenoson] patients had an FEV1 decrease greater than 15%, and 2.9% of patients on placebo,” had such a reduction, he said. Among patients with COPD, 4.2% receiving regadenoson and 5.4% on placebo met the primary end point, he said.
Respiratory problems such as wheezing, dyspnea, obstructive airwaves disorder and tachypnea were more common with regadenoson than placebo: 13% vs. 2%, respectively, in the asthma group; and 19% vs. 4% in the COPD patients. “The asthma patients had less frequency in terms of previous studies,” Dr. Prenner said. He said the variation between regadenoson and placebo was driven by dyspnea, a known side effect of A2A agonists.
However, within 1 day of injection, use of short-acting bronchodilators was similar for those who received both regadenoson and placebo, Dr. Prenner reported. In subjects with asthma, 1.4% of the regadenoson group and 1.1% of the placebo group used the inhalers. Among those with COPD, inhaler the rates were 1.6% and 1.3%, respectively, for the regadenoson and placebo cohorts.
The study showed no clinically meaningful differences between treatments in pulmonary function tests in either group, Dr. Prenner said. While the incidence of adverse events was higher with regadenoson, the adverse event profile was similar to that in previous regadenoson trials in nonasthmatic COPD patients. Of six serious adverse events with regadenoson, three were considered treatment related.
“This information should be very useful in considering the selection of regadenoson as a bottom-line stress agent for myocardial perfusion imaging in these types of patient populations,” Dr. Prenner said.
Dr. Prenner is a scientific adviser to Astellas, the manufacturer of regadenoson, and serves on its speakers bureau.
PHILADELPHIA – Individuals with asthma or chronic obstructive pulmonary disease can tolerate the imaging agent regadenoson well if they need to undergo cardiac stress testing, a study has shown.
Dr. Bruce Prenner, a San Diego allergist, reported on findings from a multicenter trial involving 999 patients who received either regadenoson or a placebo. “Regadenoson has a greater affinity for the A2B receptors and the other types of receptors, and thus the risk of bronchospasm and bronchoreactive events should be quite low,” he said at the meeting.
The risks of adenosine inducing breathing problems in individuals with asthma and COPD have been well documented. This study set out to determine how regadenoson affected forced expiratory volume in 1 second (FEV1) in 999 study subjects, 532 with asthma and 467 with COPD. About half of the patients received the placebo. The primary end point was a greater than 15% decrease in forced expiratory function from baseline within 2 hours of the dose being administered, Dr. Prenner said.
“In the asthma group, 1.1% of [regadenoson] patients had an FEV1 decrease greater than 15%, and 2.9% of patients on placebo,” had such a reduction, he said. Among patients with COPD, 4.2% receiving regadenoson and 5.4% on placebo met the primary end point, he said.
Respiratory problems such as wheezing, dyspnea, obstructive airwaves disorder and tachypnea were more common with regadenoson than placebo: 13% vs. 2%, respectively, in the asthma group; and 19% vs. 4% in the COPD patients. “The asthma patients had less frequency in terms of previous studies,” Dr. Prenner said. He said the variation between regadenoson and placebo was driven by dyspnea, a known side effect of A2A agonists.
However, within 1 day of injection, use of short-acting bronchodilators was similar for those who received both regadenoson and placebo, Dr. Prenner reported. In subjects with asthma, 1.4% of the regadenoson group and 1.1% of the placebo group used the inhalers. Among those with COPD, inhaler the rates were 1.6% and 1.3%, respectively, for the regadenoson and placebo cohorts.
The study showed no clinically meaningful differences between treatments in pulmonary function tests in either group, Dr. Prenner said. While the incidence of adverse events was higher with regadenoson, the adverse event profile was similar to that in previous regadenoson trials in nonasthmatic COPD patients. Of six serious adverse events with regadenoson, three were considered treatment related.
“This information should be very useful in considering the selection of regadenoson as a bottom-line stress agent for myocardial perfusion imaging in these types of patient populations,” Dr. Prenner said.
Dr. Prenner is a scientific adviser to Astellas, the manufacturer of regadenoson, and serves on its speakers bureau.
PHILADELPHIA – Individuals with asthma or chronic obstructive pulmonary disease can tolerate the imaging agent regadenoson well if they need to undergo cardiac stress testing, a study has shown.
Dr. Bruce Prenner, a San Diego allergist, reported on findings from a multicenter trial involving 999 patients who received either regadenoson or a placebo. “Regadenoson has a greater affinity for the A2B receptors and the other types of receptors, and thus the risk of bronchospasm and bronchoreactive events should be quite low,” he said at the meeting.
The risks of adenosine inducing breathing problems in individuals with asthma and COPD have been well documented. This study set out to determine how regadenoson affected forced expiratory volume in 1 second (FEV1) in 999 study subjects, 532 with asthma and 467 with COPD. About half of the patients received the placebo. The primary end point was a greater than 15% decrease in forced expiratory function from baseline within 2 hours of the dose being administered, Dr. Prenner said.
“In the asthma group, 1.1% of [regadenoson] patients had an FEV1 decrease greater than 15%, and 2.9% of patients on placebo,” had such a reduction, he said. Among patients with COPD, 4.2% receiving regadenoson and 5.4% on placebo met the primary end point, he said.
Respiratory problems such as wheezing, dyspnea, obstructive airwaves disorder and tachypnea were more common with regadenoson than placebo: 13% vs. 2%, respectively, in the asthma group; and 19% vs. 4% in the COPD patients. “The asthma patients had less frequency in terms of previous studies,” Dr. Prenner said. He said the variation between regadenoson and placebo was driven by dyspnea, a known side effect of A2A agonists.
However, within 1 day of injection, use of short-acting bronchodilators was similar for those who received both regadenoson and placebo, Dr. Prenner reported. In subjects with asthma, 1.4% of the regadenoson group and 1.1% of the placebo group used the inhalers. Among those with COPD, inhaler the rates were 1.6% and 1.3%, respectively, for the regadenoson and placebo cohorts.
The study showed no clinically meaningful differences between treatments in pulmonary function tests in either group, Dr. Prenner said. While the incidence of adverse events was higher with regadenoson, the adverse event profile was similar to that in previous regadenoson trials in nonasthmatic COPD patients. Of six serious adverse events with regadenoson, three were considered treatment related.
“This information should be very useful in considering the selection of regadenoson as a bottom-line stress agent for myocardial perfusion imaging in these types of patient populations,” Dr. Prenner said.
Dr. Prenner is a scientific adviser to Astellas, the manufacturer of regadenoson, and serves on its speakers bureau.
Radiation Dose Reduction Methods Gain Traction
PHILADELPHIA – Population exposure to medical radiation increased by 700% between 1980 and 2006, and nuclear cardiologists are feeling the pressure to reduce patients' radiation exposure, according to Dr. Milena J. Henzlova of Mt. Sinai School of Medicine in New York.
“This is the first time in history that exposure to medical radiation exceeds natural radiation in the population,” she reported at the meeting.
Reasons for this increased radiation exposure are varied and range from the availability of improved technology and deteriorating health of the general population, to the economic interests of manufacturers and providers, Dr. Henzlova said. In addition, “more noncardiologists are also referring [patients] for diagnostic studies, which is unusual in other medical subspecialties”
Dr. Henzlova outlined ways in which nuclear cardiologists could reduce radiation dosing in their patient populations.
One method is to follow existing American Society of Nuclear Cardiology guidelines and appropriateness criteria and the ALARA – as low as reasonably achievable – principle, she advised.
She also encouraged physicians to focus dose reduction on younger patients, though “if the patient is in the ninth decade of life, maybe this principle becomes irrelevant.”
More broadly, more regulation of the imaging equipment might help rein in the dosing. Furthermore, physicians and patients should become better educated in the hazards of medical radiation and the benefits of dose reduction, she said.
Dose-reduction methods nuclear cardiologists can use immediately include what she called “stress-first testing” rather than full stress-rest testing.
“At least 50% of our stress-rest studies are normal, and when we looked at huge sets of data, we found that 60%–70% were normal,” Dr. Henzlova said. “If there is a reason for the rest imaging, it's to find the reversibility of a defect; but in more than half of patients there is no defect to start with.”
Her group studied results of more than 10,000 stress-rest tests and found that outcomes over 5 years were almost identical between the stress-only and image study groups. “We concluded that a normal stress study has the same 1-year prognosis as a full stress-rest study,” she said. “This is an attractive alternative to the stress-rest study in appropriately selected patients. Ultimately, time is saved, radiation is saved, and cost is decreased.”
Nuclear cardiologists could also opt for a 2-day rest study with lower doses of radiation, she said, referring to U.S. and European protocols. In a cohort at the Mt. Sinai School of Medicine, total microcuries (mCi) ranged from 48 to 72, compared with 33.8 to 47.3 in a European report on 2-day studies.
Dr. Benjamin Chow, of the University of Ottawa Heart Institute in Canada, also reported on dose reduction methods for cardiac CT. The methods he described included minimizing tube current (the number of electrons used) and tube voltage (the energy level of the electrons).
“The bottom line is that if you maintain adequate signal dose and adequate initial quality with lower tube current, in general that lowers radiation exposure,” he said.
The dose-reduction methods Dr. Chow reviewed included maintaining the tube current as the CT projects across the chest but reducing it across smaller body areas, turning off the scanner when the patient changes position, using bowtie filters, and activating the padding function, which can also reduce costs by up to 82%. He cautioned, however, that there would be some loss of image quality with padding.
Tube modulation is another method of maximizing imaging during the patient's diastasis and minimizing it during systole, he said, but this also has its drawbacks, because although it restricts the amount of time the patient is exposed to radiation, “you may lose the ability to read different phases of the study.”
Dr. Chow said that his center routinely uses breast shields for women undergoing cardiac CT, which have been shown to reduce radiation exposure to the breast and lung, by 30% and 15%, respectively.
Neither Dr. Henzlova nor Chow had any relevant disclosures.
Vitals
PHILADELPHIA – Population exposure to medical radiation increased by 700% between 1980 and 2006, and nuclear cardiologists are feeling the pressure to reduce patients' radiation exposure, according to Dr. Milena J. Henzlova of Mt. Sinai School of Medicine in New York.
“This is the first time in history that exposure to medical radiation exceeds natural radiation in the population,” she reported at the meeting.
Reasons for this increased radiation exposure are varied and range from the availability of improved technology and deteriorating health of the general population, to the economic interests of manufacturers and providers, Dr. Henzlova said. In addition, “more noncardiologists are also referring [patients] for diagnostic studies, which is unusual in other medical subspecialties”
Dr. Henzlova outlined ways in which nuclear cardiologists could reduce radiation dosing in their patient populations.
One method is to follow existing American Society of Nuclear Cardiology guidelines and appropriateness criteria and the ALARA – as low as reasonably achievable – principle, she advised.
She also encouraged physicians to focus dose reduction on younger patients, though “if the patient is in the ninth decade of life, maybe this principle becomes irrelevant.”
More broadly, more regulation of the imaging equipment might help rein in the dosing. Furthermore, physicians and patients should become better educated in the hazards of medical radiation and the benefits of dose reduction, she said.
Dose-reduction methods nuclear cardiologists can use immediately include what she called “stress-first testing” rather than full stress-rest testing.
“At least 50% of our stress-rest studies are normal, and when we looked at huge sets of data, we found that 60%–70% were normal,” Dr. Henzlova said. “If there is a reason for the rest imaging, it's to find the reversibility of a defect; but in more than half of patients there is no defect to start with.”
Her group studied results of more than 10,000 stress-rest tests and found that outcomes over 5 years were almost identical between the stress-only and image study groups. “We concluded that a normal stress study has the same 1-year prognosis as a full stress-rest study,” she said. “This is an attractive alternative to the stress-rest study in appropriately selected patients. Ultimately, time is saved, radiation is saved, and cost is decreased.”
Nuclear cardiologists could also opt for a 2-day rest study with lower doses of radiation, she said, referring to U.S. and European protocols. In a cohort at the Mt. Sinai School of Medicine, total microcuries (mCi) ranged from 48 to 72, compared with 33.8 to 47.3 in a European report on 2-day studies.
Dr. Benjamin Chow, of the University of Ottawa Heart Institute in Canada, also reported on dose reduction methods for cardiac CT. The methods he described included minimizing tube current (the number of electrons used) and tube voltage (the energy level of the electrons).
“The bottom line is that if you maintain adequate signal dose and adequate initial quality with lower tube current, in general that lowers radiation exposure,” he said.
The dose-reduction methods Dr. Chow reviewed included maintaining the tube current as the CT projects across the chest but reducing it across smaller body areas, turning off the scanner when the patient changes position, using bowtie filters, and activating the padding function, which can also reduce costs by up to 82%. He cautioned, however, that there would be some loss of image quality with padding.
Tube modulation is another method of maximizing imaging during the patient's diastasis and minimizing it during systole, he said, but this also has its drawbacks, because although it restricts the amount of time the patient is exposed to radiation, “you may lose the ability to read different phases of the study.”
Dr. Chow said that his center routinely uses breast shields for women undergoing cardiac CT, which have been shown to reduce radiation exposure to the breast and lung, by 30% and 15%, respectively.
Neither Dr. Henzlova nor Chow had any relevant disclosures.
Vitals
PHILADELPHIA – Population exposure to medical radiation increased by 700% between 1980 and 2006, and nuclear cardiologists are feeling the pressure to reduce patients' radiation exposure, according to Dr. Milena J. Henzlova of Mt. Sinai School of Medicine in New York.
“This is the first time in history that exposure to medical radiation exceeds natural radiation in the population,” she reported at the meeting.
Reasons for this increased radiation exposure are varied and range from the availability of improved technology and deteriorating health of the general population, to the economic interests of manufacturers and providers, Dr. Henzlova said. In addition, “more noncardiologists are also referring [patients] for diagnostic studies, which is unusual in other medical subspecialties”
Dr. Henzlova outlined ways in which nuclear cardiologists could reduce radiation dosing in their patient populations.
One method is to follow existing American Society of Nuclear Cardiology guidelines and appropriateness criteria and the ALARA – as low as reasonably achievable – principle, she advised.
She also encouraged physicians to focus dose reduction on younger patients, though “if the patient is in the ninth decade of life, maybe this principle becomes irrelevant.”
More broadly, more regulation of the imaging equipment might help rein in the dosing. Furthermore, physicians and patients should become better educated in the hazards of medical radiation and the benefits of dose reduction, she said.
Dose-reduction methods nuclear cardiologists can use immediately include what she called “stress-first testing” rather than full stress-rest testing.
“At least 50% of our stress-rest studies are normal, and when we looked at huge sets of data, we found that 60%–70% were normal,” Dr. Henzlova said. “If there is a reason for the rest imaging, it's to find the reversibility of a defect; but in more than half of patients there is no defect to start with.”
Her group studied results of more than 10,000 stress-rest tests and found that outcomes over 5 years were almost identical between the stress-only and image study groups. “We concluded that a normal stress study has the same 1-year prognosis as a full stress-rest study,” she said. “This is an attractive alternative to the stress-rest study in appropriately selected patients. Ultimately, time is saved, radiation is saved, and cost is decreased.”
Nuclear cardiologists could also opt for a 2-day rest study with lower doses of radiation, she said, referring to U.S. and European protocols. In a cohort at the Mt. Sinai School of Medicine, total microcuries (mCi) ranged from 48 to 72, compared with 33.8 to 47.3 in a European report on 2-day studies.
Dr. Benjamin Chow, of the University of Ottawa Heart Institute in Canada, also reported on dose reduction methods for cardiac CT. The methods he described included minimizing tube current (the number of electrons used) and tube voltage (the energy level of the electrons).
“The bottom line is that if you maintain adequate signal dose and adequate initial quality with lower tube current, in general that lowers radiation exposure,” he said.
The dose-reduction methods Dr. Chow reviewed included maintaining the tube current as the CT projects across the chest but reducing it across smaller body areas, turning off the scanner when the patient changes position, using bowtie filters, and activating the padding function, which can also reduce costs by up to 82%. He cautioned, however, that there would be some loss of image quality with padding.
Tube modulation is another method of maximizing imaging during the patient's diastasis and minimizing it during systole, he said, but this also has its drawbacks, because although it restricts the amount of time the patient is exposed to radiation, “you may lose the ability to read different phases of the study.”
Dr. Chow said that his center routinely uses breast shields for women undergoing cardiac CT, which have been shown to reduce radiation exposure to the breast and lung, by 30% and 15%, respectively.
Neither Dr. Henzlova nor Chow had any relevant disclosures.
Vitals
Protocol Helps Identify Candidates for CRT
PHILADELPHIA – A protocol for using serial gated single-photon emission computed tomography with a single injection radiotracer helped in patient selection for cardiac resynchronization therapy and guided left ventricle lead placement, investigators at the University of Pittsburgh reported.
The phased analysis protocol involved leaving the cardiac resynchronization therapy (CRT) device inactive at the time of implantation, injecting a single dose of radiotracer, and acquiring a resting gated single-photon emission computed tomography (SPECT); then activating the CRT and acquiring another gated SPECT, said Dr. Mati Friehling. The study was selected as winner of the ASNC's Young Investigator Award Competition.
“There is great value of phase analysis which suggests a new technique to evaluate LV synchrony,” Dr. Friehling said. “There's a linear relationship between the count changes throughout the cardiac cycle and myocardial wall thickening.”
The count changes are key to determining the precise mechanical contraction for CRT, he said.
“One question is, why do we care about the acute response?” Dr. Friehling said. “When we put a CRT device in, based on conventional criteria, we assume that acute resynchronization occurs, and this will give us a long-term benefit, but not always.
“We decided to use a gated SPECT-based approach because it gives us a congregant evaluation of the patient, including function and scar location and scar extent, which may be helpful for actual LV lead position.”
The single-dose radiotracer was devised to limit radiation exposure, he said.
The study analyzed a total of 44 patients after CRT device implantation, 18 of whom had improvement of dyssynchrony, 11 of whom had no change, and 15 of whom actually had deterioration of dyssynchrony, Dr. Friehling said.
The leads were concordant in 22 patients, only 1 of whom actually worsened. In the remaining 22 with discordant leads, 8 had improvement or were unchanged, and 14 saw their dyssynchrony worsen, according to Dr. Friehling.
“There's a very high specificity and positive predictive value for an improving or unchanged synchrony,” said Dr. Friehling.
“The responses are based on small changes in LV volume injection fraction according to echocardiography, which can be highly variable. We used harder end points such as death, CHF hospitalizations, ICD shocks, and viability of patients secondary to HF failure symptoms,” he noted.
Five deaths were reported among the 29 patients in the study group who had improved or unchanged dyssynchrony, Dr. Friehling said, while among the 15 patients who had deterioration of dyssynchrony, 8 had a cardiac event.
“Serial imaging based on conventional criteria can result in acute worsening of synchrony in some patients,” Dr. Friehling said.
“The acute change in synchrony does appear to be associated with long-term outcome, and we may be able to use a baseline SPECT study to guide LV lead placement and to predict the acute response. Therefore SPECT may be a valuable tool for selecting patients for CRT.”
Dr. Friehling had no disclosures, but two of his coinvestigators disclosed relationships with Emory Cardiac Toolbox.
PHILADELPHIA – A protocol for using serial gated single-photon emission computed tomography with a single injection radiotracer helped in patient selection for cardiac resynchronization therapy and guided left ventricle lead placement, investigators at the University of Pittsburgh reported.
The phased analysis protocol involved leaving the cardiac resynchronization therapy (CRT) device inactive at the time of implantation, injecting a single dose of radiotracer, and acquiring a resting gated single-photon emission computed tomography (SPECT); then activating the CRT and acquiring another gated SPECT, said Dr. Mati Friehling. The study was selected as winner of the ASNC's Young Investigator Award Competition.
“There is great value of phase analysis which suggests a new technique to evaluate LV synchrony,” Dr. Friehling said. “There's a linear relationship between the count changes throughout the cardiac cycle and myocardial wall thickening.”
The count changes are key to determining the precise mechanical contraction for CRT, he said.
“One question is, why do we care about the acute response?” Dr. Friehling said. “When we put a CRT device in, based on conventional criteria, we assume that acute resynchronization occurs, and this will give us a long-term benefit, but not always.
“We decided to use a gated SPECT-based approach because it gives us a congregant evaluation of the patient, including function and scar location and scar extent, which may be helpful for actual LV lead position.”
The single-dose radiotracer was devised to limit radiation exposure, he said.
The study analyzed a total of 44 patients after CRT device implantation, 18 of whom had improvement of dyssynchrony, 11 of whom had no change, and 15 of whom actually had deterioration of dyssynchrony, Dr. Friehling said.
The leads were concordant in 22 patients, only 1 of whom actually worsened. In the remaining 22 with discordant leads, 8 had improvement or were unchanged, and 14 saw their dyssynchrony worsen, according to Dr. Friehling.
“There's a very high specificity and positive predictive value for an improving or unchanged synchrony,” said Dr. Friehling.
“The responses are based on small changes in LV volume injection fraction according to echocardiography, which can be highly variable. We used harder end points such as death, CHF hospitalizations, ICD shocks, and viability of patients secondary to HF failure symptoms,” he noted.
Five deaths were reported among the 29 patients in the study group who had improved or unchanged dyssynchrony, Dr. Friehling said, while among the 15 patients who had deterioration of dyssynchrony, 8 had a cardiac event.
“Serial imaging based on conventional criteria can result in acute worsening of synchrony in some patients,” Dr. Friehling said.
“The acute change in synchrony does appear to be associated with long-term outcome, and we may be able to use a baseline SPECT study to guide LV lead placement and to predict the acute response. Therefore SPECT may be a valuable tool for selecting patients for CRT.”
Dr. Friehling had no disclosures, but two of his coinvestigators disclosed relationships with Emory Cardiac Toolbox.
PHILADELPHIA – A protocol for using serial gated single-photon emission computed tomography with a single injection radiotracer helped in patient selection for cardiac resynchronization therapy and guided left ventricle lead placement, investigators at the University of Pittsburgh reported.
The phased analysis protocol involved leaving the cardiac resynchronization therapy (CRT) device inactive at the time of implantation, injecting a single dose of radiotracer, and acquiring a resting gated single-photon emission computed tomography (SPECT); then activating the CRT and acquiring another gated SPECT, said Dr. Mati Friehling. The study was selected as winner of the ASNC's Young Investigator Award Competition.
“There is great value of phase analysis which suggests a new technique to evaluate LV synchrony,” Dr. Friehling said. “There's a linear relationship between the count changes throughout the cardiac cycle and myocardial wall thickening.”
The count changes are key to determining the precise mechanical contraction for CRT, he said.
“One question is, why do we care about the acute response?” Dr. Friehling said. “When we put a CRT device in, based on conventional criteria, we assume that acute resynchronization occurs, and this will give us a long-term benefit, but not always.
“We decided to use a gated SPECT-based approach because it gives us a congregant evaluation of the patient, including function and scar location and scar extent, which may be helpful for actual LV lead position.”
The single-dose radiotracer was devised to limit radiation exposure, he said.
The study analyzed a total of 44 patients after CRT device implantation, 18 of whom had improvement of dyssynchrony, 11 of whom had no change, and 15 of whom actually had deterioration of dyssynchrony, Dr. Friehling said.
The leads were concordant in 22 patients, only 1 of whom actually worsened. In the remaining 22 with discordant leads, 8 had improvement or were unchanged, and 14 saw their dyssynchrony worsen, according to Dr. Friehling.
“There's a very high specificity and positive predictive value for an improving or unchanged synchrony,” said Dr. Friehling.
“The responses are based on small changes in LV volume injection fraction according to echocardiography, which can be highly variable. We used harder end points such as death, CHF hospitalizations, ICD shocks, and viability of patients secondary to HF failure symptoms,” he noted.
Five deaths were reported among the 29 patients in the study group who had improved or unchanged dyssynchrony, Dr. Friehling said, while among the 15 patients who had deterioration of dyssynchrony, 8 had a cardiac event.
“Serial imaging based on conventional criteria can result in acute worsening of synchrony in some patients,” Dr. Friehling said.
“The acute change in synchrony does appear to be associated with long-term outcome, and we may be able to use a baseline SPECT study to guide LV lead placement and to predict the acute response. Therefore SPECT may be a valuable tool for selecting patients for CRT.”
Dr. Friehling had no disclosures, but two of his coinvestigators disclosed relationships with Emory Cardiac Toolbox.
Device Choice May Influence Embolization Rates in Leg Revascularization
NEW YORK – Choice of procedure for lower-leg revascularization, whether angioplasty, atherectomy, or laser treatment, may influence distal embolization rates and the need for embolic protection devices, according to a study presented at the annual meeting of the Eastern Vascular Society.
The study from Columbia University Medical Center in New York found that distal embolization occurred in fewer than 2% of all revascularization procedures studied, but that newer atherectomy devices may be linked to higher complication rates, Dr. Gauram Shrikhande reported. The study reviewed runoff in 2,137 lesions in approximately 1,000 patients treated from 2004 to 2009.
"On angiography, significant embolization occurs at a lower rate during percutaneous low-extremity interventions, and although rare, it does not affect patency and limb salvage rates if runoff can be reestablished using salvage techniques at the time of the procedure," Dr. Shrikhande said.
However, the analysis showed higher rates of distal embolization with newer atherectomy devices, he said. "Distal embolization during the time of percutaneous lower-extremity intervention is a major concern due to ischemic consequences," Dr. Shrikhande said. "As percutaneous lower-extremity arterial interventions become commonplace and devices are rapidly introduced, it is necessary that we better define rates of distal embolization."
The study compared outcomes among five types of interventions: angioplasty alone; angioplasty with stent; the SilverHawk plaque excision system (FoxHollow Technologies); two newer atherectomy devices – the Diamondback 360 (CSI) and the Jet Stream G2 device (Pathway Medical Technologies Inc.); and an excimer laser (Spectranetics Corp.).
In the study, distal embolization rates ranged from less than 1.0% with both angioplasty procedures and 1.9% for the SilverHawk device to 3.6 % for the excimer laser and 22% for the newer atherectomy devices, he said. "Embolic protection may be considered for certain atherectomy devices, in TASC C and D lesions, and for chronic total occlusions and in-stent restenosis," Dr. Shrikhande said.
The average age of the patients was 71 years; 57% were male, 57% had diabetes mellitus, and 54% had a history of smoking. Indications for intervention were claudication in 44%, tissue loss in 42%, and leg pain at rest in 14%.
The lesions were characterized as stenotic (62.4%), chronic total occlusions (28.8%), and in-stent restenosis (8.8%), according to study results. "Total occlusions and in-stent restenosis lesions had higher rates of embolization than native stenotic lesions," Dr. Shrikhande said. The average length of treated lesions was 10 cm, and 30% were located in the femoral artery.
One of the problems with the study was the relatively low number of patients treated with the newer atherectomy devices, Dr. Shrikhande acknowledged. "This is an ongoing collection of data, and we hope to continue to collect data and update these results," he said.
The results provide cause for rethinking the management of specific lesions, Dr. Shrikhande said. "For in-stent restenosis, I would be more cautions using the newer atherectomy devices, and I would heavily consider using a distal embolic protection device at the outset the procedure," he said.
Dr. Linda Harris of Buffalo, N.Y., raised an issue of cost with atherectomy. "You’ve shown that all the atherectomy devices have a higher rate of peripheral embolization," she said. "They already cost more than the balloons we use for angioplasty and/or stents, now you’re adding potentially embolic protection devices and/or catheters to withdraw the clot that you’ve now embolized." She questioned the utility and cost-benefit of any atherectomy device.
The Columbia study did not include a cost analysis, Dr. Shrikhande said. "I do still feel, however, that the atherectomy devices do have an important role in certain lesions – peripheral lesions, popliteal lesions, and osteal-tibial lesions," he said. "I would continue to use them in selected situations, with the caveats of potential embolization risks."
Dr. Shrikhande had no disclosures relevant to the study.
NEW YORK – Choice of procedure for lower-leg revascularization, whether angioplasty, atherectomy, or laser treatment, may influence distal embolization rates and the need for embolic protection devices, according to a study presented at the annual meeting of the Eastern Vascular Society.
The study from Columbia University Medical Center in New York found that distal embolization occurred in fewer than 2% of all revascularization procedures studied, but that newer atherectomy devices may be linked to higher complication rates, Dr. Gauram Shrikhande reported. The study reviewed runoff in 2,137 lesions in approximately 1,000 patients treated from 2004 to 2009.
"On angiography, significant embolization occurs at a lower rate during percutaneous low-extremity interventions, and although rare, it does not affect patency and limb salvage rates if runoff can be reestablished using salvage techniques at the time of the procedure," Dr. Shrikhande said.
However, the analysis showed higher rates of distal embolization with newer atherectomy devices, he said. "Distal embolization during the time of percutaneous lower-extremity intervention is a major concern due to ischemic consequences," Dr. Shrikhande said. "As percutaneous lower-extremity arterial interventions become commonplace and devices are rapidly introduced, it is necessary that we better define rates of distal embolization."
The study compared outcomes among five types of interventions: angioplasty alone; angioplasty with stent; the SilverHawk plaque excision system (FoxHollow Technologies); two newer atherectomy devices – the Diamondback 360 (CSI) and the Jet Stream G2 device (Pathway Medical Technologies Inc.); and an excimer laser (Spectranetics Corp.).
In the study, distal embolization rates ranged from less than 1.0% with both angioplasty procedures and 1.9% for the SilverHawk device to 3.6 % for the excimer laser and 22% for the newer atherectomy devices, he said. "Embolic protection may be considered for certain atherectomy devices, in TASC C and D lesions, and for chronic total occlusions and in-stent restenosis," Dr. Shrikhande said.
The average age of the patients was 71 years; 57% were male, 57% had diabetes mellitus, and 54% had a history of smoking. Indications for intervention were claudication in 44%, tissue loss in 42%, and leg pain at rest in 14%.
The lesions were characterized as stenotic (62.4%), chronic total occlusions (28.8%), and in-stent restenosis (8.8%), according to study results. "Total occlusions and in-stent restenosis lesions had higher rates of embolization than native stenotic lesions," Dr. Shrikhande said. The average length of treated lesions was 10 cm, and 30% were located in the femoral artery.
One of the problems with the study was the relatively low number of patients treated with the newer atherectomy devices, Dr. Shrikhande acknowledged. "This is an ongoing collection of data, and we hope to continue to collect data and update these results," he said.
The results provide cause for rethinking the management of specific lesions, Dr. Shrikhande said. "For in-stent restenosis, I would be more cautions using the newer atherectomy devices, and I would heavily consider using a distal embolic protection device at the outset the procedure," he said.
Dr. Linda Harris of Buffalo, N.Y., raised an issue of cost with atherectomy. "You’ve shown that all the atherectomy devices have a higher rate of peripheral embolization," she said. "They already cost more than the balloons we use for angioplasty and/or stents, now you’re adding potentially embolic protection devices and/or catheters to withdraw the clot that you’ve now embolized." She questioned the utility and cost-benefit of any atherectomy device.
The Columbia study did not include a cost analysis, Dr. Shrikhande said. "I do still feel, however, that the atherectomy devices do have an important role in certain lesions – peripheral lesions, popliteal lesions, and osteal-tibial lesions," he said. "I would continue to use them in selected situations, with the caveats of potential embolization risks."
Dr. Shrikhande had no disclosures relevant to the study.
NEW YORK – Choice of procedure for lower-leg revascularization, whether angioplasty, atherectomy, or laser treatment, may influence distal embolization rates and the need for embolic protection devices, according to a study presented at the annual meeting of the Eastern Vascular Society.
The study from Columbia University Medical Center in New York found that distal embolization occurred in fewer than 2% of all revascularization procedures studied, but that newer atherectomy devices may be linked to higher complication rates, Dr. Gauram Shrikhande reported. The study reviewed runoff in 2,137 lesions in approximately 1,000 patients treated from 2004 to 2009.
"On angiography, significant embolization occurs at a lower rate during percutaneous low-extremity interventions, and although rare, it does not affect patency and limb salvage rates if runoff can be reestablished using salvage techniques at the time of the procedure," Dr. Shrikhande said.
However, the analysis showed higher rates of distal embolization with newer atherectomy devices, he said. "Distal embolization during the time of percutaneous lower-extremity intervention is a major concern due to ischemic consequences," Dr. Shrikhande said. "As percutaneous lower-extremity arterial interventions become commonplace and devices are rapidly introduced, it is necessary that we better define rates of distal embolization."
The study compared outcomes among five types of interventions: angioplasty alone; angioplasty with stent; the SilverHawk plaque excision system (FoxHollow Technologies); two newer atherectomy devices – the Diamondback 360 (CSI) and the Jet Stream G2 device (Pathway Medical Technologies Inc.); and an excimer laser (Spectranetics Corp.).
In the study, distal embolization rates ranged from less than 1.0% with both angioplasty procedures and 1.9% for the SilverHawk device to 3.6 % for the excimer laser and 22% for the newer atherectomy devices, he said. "Embolic protection may be considered for certain atherectomy devices, in TASC C and D lesions, and for chronic total occlusions and in-stent restenosis," Dr. Shrikhande said.
The average age of the patients was 71 years; 57% were male, 57% had diabetes mellitus, and 54% had a history of smoking. Indications for intervention were claudication in 44%, tissue loss in 42%, and leg pain at rest in 14%.
The lesions were characterized as stenotic (62.4%), chronic total occlusions (28.8%), and in-stent restenosis (8.8%), according to study results. "Total occlusions and in-stent restenosis lesions had higher rates of embolization than native stenotic lesions," Dr. Shrikhande said. The average length of treated lesions was 10 cm, and 30% were located in the femoral artery.
One of the problems with the study was the relatively low number of patients treated with the newer atherectomy devices, Dr. Shrikhande acknowledged. "This is an ongoing collection of data, and we hope to continue to collect data and update these results," he said.
The results provide cause for rethinking the management of specific lesions, Dr. Shrikhande said. "For in-stent restenosis, I would be more cautions using the newer atherectomy devices, and I would heavily consider using a distal embolic protection device at the outset the procedure," he said.
Dr. Linda Harris of Buffalo, N.Y., raised an issue of cost with atherectomy. "You’ve shown that all the atherectomy devices have a higher rate of peripheral embolization," she said. "They already cost more than the balloons we use for angioplasty and/or stents, now you’re adding potentially embolic protection devices and/or catheters to withdraw the clot that you’ve now embolized." She questioned the utility and cost-benefit of any atherectomy device.
The Columbia study did not include a cost analysis, Dr. Shrikhande said. "I do still feel, however, that the atherectomy devices do have an important role in certain lesions – peripheral lesions, popliteal lesions, and osteal-tibial lesions," he said. "I would continue to use them in selected situations, with the caveats of potential embolization risks."
Dr. Shrikhande had no disclosures relevant to the study.
FROM THE ANNUAL MEETING OF THE EASTERN VASCULAR SOCIETY
Device Choice May Influence Embolization Rates in Leg Revascularization
NEW YORK – Choice of procedure for lower-leg revascularization, whether angioplasty, atherectomy, or laser treatment, may influence distal embolization rates and the need for embolic protection devices, according to a study presented at the annual meeting of the Eastern Vascular Society.
The study from Columbia University Medical Center in New York found that distal embolization occurred in fewer than 2% of all revascularization procedures studied, but that newer atherectomy devices may be linked to higher complication rates, Dr. Gauram Shrikhande reported. The study reviewed runoff in 2,137 lesions in approximately 1,000 patients treated from 2004 to 2009.
"On angiography, significant embolization occurs at a lower rate during percutaneous low-extremity interventions, and although rare, it does not affect patency and limb salvage rates if runoff can be reestablished using salvage techniques at the time of the procedure," Dr. Shrikhande said.
However, the analysis showed higher rates of distal embolization with newer atherectomy devices, he said. "Distal embolization during the time of percutaneous lower-extremity intervention is a major concern due to ischemic consequences," Dr. Shrikhande said. "As percutaneous lower-extremity arterial interventions become commonplace and devices are rapidly introduced, it is necessary that we better define rates of distal embolization."
The study compared outcomes among five types of interventions: angioplasty alone; angioplasty with stent; the SilverHawk plaque excision system (FoxHollow Technologies); two newer atherectomy devices – the Diamondback 360 (CSI) and the Jet Stream G2 device (Pathway Medical Technologies Inc.); and an excimer laser (Spectranetics Corp.).
In the study, distal embolization rates ranged from less than 1.0% with both angioplasty procedures and 1.9% for the SilverHawk device to 3.6 % for the excimer laser and 22% for the newer atherectomy devices, he said. "Embolic protection may be considered for certain atherectomy devices, in TASC C and D lesions, and for chronic total occlusions and in-stent restenosis," Dr. Shrikhande said.
The average age of the patients was 71 years; 57% were male, 57% had diabetes mellitus, and 54% had a history of smoking. Indications for intervention were claudication in 44%, tissue loss in 42%, and leg pain at rest in 14%.
The lesions were characterized as stenotic (62.4%), chronic total occlusions (28.8%), and in-stent restenosis (8.8%), according to study results. "Total occlusions and in-stent restenosis lesions had higher rates of embolization than native stenotic lesions," Dr. Shrikhande said. The average length of treated lesions was 10 cm, and 30% were located in the femoral artery.
One of the problems with the study was the relatively low number of patients treated with the newer atherectomy devices, Dr. Shrikhande acknowledged. "This is an ongoing collection of data, and we hope to continue to collect data and update these results," he said.
The results provide cause for rethinking the management of specific lesions, Dr. Shrikhande said. "For in-stent restenosis, I would be more cautions using the newer atherectomy devices, and I would heavily consider using a distal embolic protection device at the outset the procedure," he said.
Dr. Linda Harris of Buffalo, N.Y., raised an issue of cost with atherectomy. "You’ve shown that all the atherectomy devices have a higher rate of peripheral embolization," she said. "They already cost more than the balloons we use for angioplasty and/or stents, now you’re adding potentially embolic protection devices and/or catheters to withdraw the clot that you’ve now embolized." She questioned the utility and cost-benefit of any atherectomy device.
The Columbia study did not include a cost analysis, Dr. Shrikhande said. "I do still feel, however, that the atherectomy devices do have an important role in certain lesions – peripheral lesions, popliteal lesions, and osteal-tibial lesions," he said. "I would continue to use them in selected situations, with the caveats of potential embolization risks."
Dr. Shrikhande had no disclosures relevant to the study.
NEW YORK – Choice of procedure for lower-leg revascularization, whether angioplasty, atherectomy, or laser treatment, may influence distal embolization rates and the need for embolic protection devices, according to a study presented at the annual meeting of the Eastern Vascular Society.
The study from Columbia University Medical Center in New York found that distal embolization occurred in fewer than 2% of all revascularization procedures studied, but that newer atherectomy devices may be linked to higher complication rates, Dr. Gauram Shrikhande reported. The study reviewed runoff in 2,137 lesions in approximately 1,000 patients treated from 2004 to 2009.
"On angiography, significant embolization occurs at a lower rate during percutaneous low-extremity interventions, and although rare, it does not affect patency and limb salvage rates if runoff can be reestablished using salvage techniques at the time of the procedure," Dr. Shrikhande said.
However, the analysis showed higher rates of distal embolization with newer atherectomy devices, he said. "Distal embolization during the time of percutaneous lower-extremity intervention is a major concern due to ischemic consequences," Dr. Shrikhande said. "As percutaneous lower-extremity arterial interventions become commonplace and devices are rapidly introduced, it is necessary that we better define rates of distal embolization."
The study compared outcomes among five types of interventions: angioplasty alone; angioplasty with stent; the SilverHawk plaque excision system (FoxHollow Technologies); two newer atherectomy devices – the Diamondback 360 (CSI) and the Jet Stream G2 device (Pathway Medical Technologies Inc.); and an excimer laser (Spectranetics Corp.).
In the study, distal embolization rates ranged from less than 1.0% with both angioplasty procedures and 1.9% for the SilverHawk device to 3.6 % for the excimer laser and 22% for the newer atherectomy devices, he said. "Embolic protection may be considered for certain atherectomy devices, in TASC C and D lesions, and for chronic total occlusions and in-stent restenosis," Dr. Shrikhande said.
The average age of the patients was 71 years; 57% were male, 57% had diabetes mellitus, and 54% had a history of smoking. Indications for intervention were claudication in 44%, tissue loss in 42%, and leg pain at rest in 14%.
The lesions were characterized as stenotic (62.4%), chronic total occlusions (28.8%), and in-stent restenosis (8.8%), according to study results. "Total occlusions and in-stent restenosis lesions had higher rates of embolization than native stenotic lesions," Dr. Shrikhande said. The average length of treated lesions was 10 cm, and 30% were located in the femoral artery.
One of the problems with the study was the relatively low number of patients treated with the newer atherectomy devices, Dr. Shrikhande acknowledged. "This is an ongoing collection of data, and we hope to continue to collect data and update these results," he said.
The results provide cause for rethinking the management of specific lesions, Dr. Shrikhande said. "For in-stent restenosis, I would be more cautions using the newer atherectomy devices, and I would heavily consider using a distal embolic protection device at the outset the procedure," he said.
Dr. Linda Harris of Buffalo, N.Y., raised an issue of cost with atherectomy. "You’ve shown that all the atherectomy devices have a higher rate of peripheral embolization," she said. "They already cost more than the balloons we use for angioplasty and/or stents, now you’re adding potentially embolic protection devices and/or catheters to withdraw the clot that you’ve now embolized." She questioned the utility and cost-benefit of any atherectomy device.
The Columbia study did not include a cost analysis, Dr. Shrikhande said. "I do still feel, however, that the atherectomy devices do have an important role in certain lesions – peripheral lesions, popliteal lesions, and osteal-tibial lesions," he said. "I would continue to use them in selected situations, with the caveats of potential embolization risks."
Dr. Shrikhande had no disclosures relevant to the study.
NEW YORK – Choice of procedure for lower-leg revascularization, whether angioplasty, atherectomy, or laser treatment, may influence distal embolization rates and the need for embolic protection devices, according to a study presented at the annual meeting of the Eastern Vascular Society.
The study from Columbia University Medical Center in New York found that distal embolization occurred in fewer than 2% of all revascularization procedures studied, but that newer atherectomy devices may be linked to higher complication rates, Dr. Gauram Shrikhande reported. The study reviewed runoff in 2,137 lesions in approximately 1,000 patients treated from 2004 to 2009.
"On angiography, significant embolization occurs at a lower rate during percutaneous low-extremity interventions, and although rare, it does not affect patency and limb salvage rates if runoff can be reestablished using salvage techniques at the time of the procedure," Dr. Shrikhande said.
However, the analysis showed higher rates of distal embolization with newer atherectomy devices, he said. "Distal embolization during the time of percutaneous lower-extremity intervention is a major concern due to ischemic consequences," Dr. Shrikhande said. "As percutaneous lower-extremity arterial interventions become commonplace and devices are rapidly introduced, it is necessary that we better define rates of distal embolization."
The study compared outcomes among five types of interventions: angioplasty alone; angioplasty with stent; the SilverHawk plaque excision system (FoxHollow Technologies); two newer atherectomy devices – the Diamondback 360 (CSI) and the Jet Stream G2 device (Pathway Medical Technologies Inc.); and an excimer laser (Spectranetics Corp.).
In the study, distal embolization rates ranged from less than 1.0% with both angioplasty procedures and 1.9% for the SilverHawk device to 3.6 % for the excimer laser and 22% for the newer atherectomy devices, he said. "Embolic protection may be considered for certain atherectomy devices, in TASC C and D lesions, and for chronic total occlusions and in-stent restenosis," Dr. Shrikhande said.
The average age of the patients was 71 years; 57% were male, 57% had diabetes mellitus, and 54% had a history of smoking. Indications for intervention were claudication in 44%, tissue loss in 42%, and leg pain at rest in 14%.
The lesions were characterized as stenotic (62.4%), chronic total occlusions (28.8%), and in-stent restenosis (8.8%), according to study results. "Total occlusions and in-stent restenosis lesions had higher rates of embolization than native stenotic lesions," Dr. Shrikhande said. The average length of treated lesions was 10 cm, and 30% were located in the femoral artery.
One of the problems with the study was the relatively low number of patients treated with the newer atherectomy devices, Dr. Shrikhande acknowledged. "This is an ongoing collection of data, and we hope to continue to collect data and update these results," he said.
The results provide cause for rethinking the management of specific lesions, Dr. Shrikhande said. "For in-stent restenosis, I would be more cautions using the newer atherectomy devices, and I would heavily consider using a distal embolic protection device at the outset the procedure," he said.
Dr. Linda Harris of Buffalo, N.Y., raised an issue of cost with atherectomy. "You’ve shown that all the atherectomy devices have a higher rate of peripheral embolization," she said. "They already cost more than the balloons we use for angioplasty and/or stents, now you’re adding potentially embolic protection devices and/or catheters to withdraw the clot that you’ve now embolized." She questioned the utility and cost-benefit of any atherectomy device.
The Columbia study did not include a cost analysis, Dr. Shrikhande said. "I do still feel, however, that the atherectomy devices do have an important role in certain lesions – peripheral lesions, popliteal lesions, and osteal-tibial lesions," he said. "I would continue to use them in selected situations, with the caveats of potential embolization risks."
Dr. Shrikhande had no disclosures relevant to the study.
FROM THE ANNUAL MEETING OF THE EASTERN VASCULAR SOCIETY
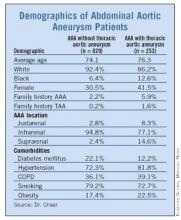
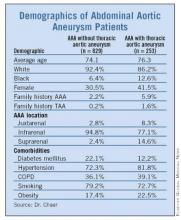
Incidence, Risks for Thoracic Aneurysm in AAA Defined
NEW YORK – About one in four patients with abdominal aortic aneurysm may be at risk for thoracic aneurysm, judging by results of a single-center retrospective study of more than 1,000 patients.
Dr. Rabih Chaer, a vascular surgeon at the University of Pittsburgh, and his colleagues found that, among 1,082 patients diagnosed with abdominal aortic aneurysms (AAA) who had chest CT at follow-up, 23.4% had some sort of thoracic aneurysm afterward.
“Despite the clinical associations that have been observed between AAAs and peripheral aneurysms and thoracic aneurysms, screening for other common aneurysms continues to be controversial,” Dr. Chaer said at the annual meeting of the Eastern Vascular Society.
Therefore, they conducted the study to quantify the risk for thoracic aneurysm in these patients and to identify risk factors that could provide screening parameters, he said. The researchers defined an aneurysm as a greater than 50% increase in the adjacent aorta diameter or a 3 cm or larger increase in the setting of AAA, Dr. Chaer said. Thoracic aneurysms were categorized by two subgroups: synchronous (occurring within 2 years of initial AAA diagnosis) and metachronous (occurring 2 years or more after diagnosis). About 11% of patients had the former, and 12.6% the latter, Dr. Chaer said. The average time to diagnosis was 2.3 years, he said.
In all, the researchers considered 2,196 patients diagnosed with AAA between 2000 and 2008, but only 49% (1,082) had chest CT that qualified them for further analysis, Dr. Chaer noted. The chest studies were conducted for suspected pulmonary disease in 74% of patients, for chest screening in 15%, and for miscellaneous reasons in 11%.
One predisposing factor for thoracic aneurysm was the type of AAA, Dr. Chaer explained. “Those patients who had a thoracic aneurysm component were more likely to have a suprarenal or juxtarenal aortic aneurysm, and those patients who did not have any thoracic aneurysm were more likely to have had an infrarenal aneurysm,” he said.
The median age of patients who had a thoracic aneurysm vs. those who did not was 76 years vs. 74 years, he said.
Other predictors for thoracic aortic aneurysm included African American race, family history of thoracic aneurysm, personal history of obesity hypertension, and an AAA diameter more than 5 cm on presentation, he said. Factors that conferred a protective effect were a diagnosis of diabetes mellitus, infrarenal AAA location, and – “counterintuitively” – a history of smoking.
“We propose that routine or targeted screening with chest CT at the time of aortic aneurysm diagnosis may be indicated, not only to really define the natural history of disease, but more importantly to try to prevent late aortic events,” Dr. Chaer said.
But Dr. James Black, of Johns Hopkins University in Baltimore, questioned the cost effectiveness of routine screening. At his institution, chest CT would add about $3,000 per patient, he said. “If you took a chest CT at diagnosis of AAA for 100 patients, 90% of the scans would be negative for thoracic aneurysm, at a rough cost in our institution of about $300,000 a year,” he said.
Cost of routine chest CT is an issue, Dr. Chaer acknowledged, although the chest CT could be done in the same scan as the abdominal CT.
“It would be nice to have a surrogate marker for thoracic aneurysm,” Dr. Chaer said. “Although we found that a thoracic aneurysm was more common in patients who had a juxtarenal aneurysm, those numbers were not hard enough to confidently say that the juxtarenal component is always predictive of a surrogate marker of thoracic aneurysm development. It is something that could be the subject of future studies.”
In addition, there is a need to identify risk factors. “We are trying to identify a high-risk group of patients in whom it would be more cost effective to screen,” he said. “That would bring down the number significantly and therefore the cost.”
Dr. Chaer noted that the heterogeneous population and the retrospective nature were limitations of the study. He reported no disclosures relevant to the presentation.
Demographics of Abdominal Aortic Aneurysm Patients
NEW YORK – About one in four patients with abdominal aortic aneurysm may be at risk for thoracic aneurysm, judging by results of a single-center retrospective study of more than 1,000 patients.
Dr. Rabih Chaer, a vascular surgeon at the University of Pittsburgh, and his colleagues found that, among 1,082 patients diagnosed with abdominal aortic aneurysms (AAA) who had chest CT at follow-up, 23.4% had some sort of thoracic aneurysm afterward.
“Despite the clinical associations that have been observed between AAAs and peripheral aneurysms and thoracic aneurysms, screening for other common aneurysms continues to be controversial,” Dr. Chaer said at the annual meeting of the Eastern Vascular Society.
Therefore, they conducted the study to quantify the risk for thoracic aneurysm in these patients and to identify risk factors that could provide screening parameters, he said. The researchers defined an aneurysm as a greater than 50% increase in the adjacent aorta diameter or a 3 cm or larger increase in the setting of AAA, Dr. Chaer said. Thoracic aneurysms were categorized by two subgroups: synchronous (occurring within 2 years of initial AAA diagnosis) and metachronous (occurring 2 years or more after diagnosis). About 11% of patients had the former, and 12.6% the latter, Dr. Chaer said. The average time to diagnosis was 2.3 years, he said.
In all, the researchers considered 2,196 patients diagnosed with AAA between 2000 and 2008, but only 49% (1,082) had chest CT that qualified them for further analysis, Dr. Chaer noted. The chest studies were conducted for suspected pulmonary disease in 74% of patients, for chest screening in 15%, and for miscellaneous reasons in 11%.
One predisposing factor for thoracic aneurysm was the type of AAA, Dr. Chaer explained. “Those patients who had a thoracic aneurysm component were more likely to have a suprarenal or juxtarenal aortic aneurysm, and those patients who did not have any thoracic aneurysm were more likely to have had an infrarenal aneurysm,” he said.
The median age of patients who had a thoracic aneurysm vs. those who did not was 76 years vs. 74 years, he said.
Other predictors for thoracic aortic aneurysm included African American race, family history of thoracic aneurysm, personal history of obesity hypertension, and an AAA diameter more than 5 cm on presentation, he said. Factors that conferred a protective effect were a diagnosis of diabetes mellitus, infrarenal AAA location, and – “counterintuitively” – a history of smoking.
“We propose that routine or targeted screening with chest CT at the time of aortic aneurysm diagnosis may be indicated, not only to really define the natural history of disease, but more importantly to try to prevent late aortic events,” Dr. Chaer said.
But Dr. James Black, of Johns Hopkins University in Baltimore, questioned the cost effectiveness of routine screening. At his institution, chest CT would add about $3,000 per patient, he said. “If you took a chest CT at diagnosis of AAA for 100 patients, 90% of the scans would be negative for thoracic aneurysm, at a rough cost in our institution of about $300,000 a year,” he said.
Cost of routine chest CT is an issue, Dr. Chaer acknowledged, although the chest CT could be done in the same scan as the abdominal CT.
“It would be nice to have a surrogate marker for thoracic aneurysm,” Dr. Chaer said. “Although we found that a thoracic aneurysm was more common in patients who had a juxtarenal aneurysm, those numbers were not hard enough to confidently say that the juxtarenal component is always predictive of a surrogate marker of thoracic aneurysm development. It is something that could be the subject of future studies.”
In addition, there is a need to identify risk factors. “We are trying to identify a high-risk group of patients in whom it would be more cost effective to screen,” he said. “That would bring down the number significantly and therefore the cost.”
Dr. Chaer noted that the heterogeneous population and the retrospective nature were limitations of the study. He reported no disclosures relevant to the presentation.
Demographics of Abdominal Aortic Aneurysm Patients
NEW YORK – About one in four patients with abdominal aortic aneurysm may be at risk for thoracic aneurysm, judging by results of a single-center retrospective study of more than 1,000 patients.
Dr. Rabih Chaer, a vascular surgeon at the University of Pittsburgh, and his colleagues found that, among 1,082 patients diagnosed with abdominal aortic aneurysms (AAA) who had chest CT at follow-up, 23.4% had some sort of thoracic aneurysm afterward.
“Despite the clinical associations that have been observed between AAAs and peripheral aneurysms and thoracic aneurysms, screening for other common aneurysms continues to be controversial,” Dr. Chaer said at the annual meeting of the Eastern Vascular Society.
Therefore, they conducted the study to quantify the risk for thoracic aneurysm in these patients and to identify risk factors that could provide screening parameters, he said. The researchers defined an aneurysm as a greater than 50% increase in the adjacent aorta diameter or a 3 cm or larger increase in the setting of AAA, Dr. Chaer said. Thoracic aneurysms were categorized by two subgroups: synchronous (occurring within 2 years of initial AAA diagnosis) and metachronous (occurring 2 years or more after diagnosis). About 11% of patients had the former, and 12.6% the latter, Dr. Chaer said. The average time to diagnosis was 2.3 years, he said.
In all, the researchers considered 2,196 patients diagnosed with AAA between 2000 and 2008, but only 49% (1,082) had chest CT that qualified them for further analysis, Dr. Chaer noted. The chest studies were conducted for suspected pulmonary disease in 74% of patients, for chest screening in 15%, and for miscellaneous reasons in 11%.
One predisposing factor for thoracic aneurysm was the type of AAA, Dr. Chaer explained. “Those patients who had a thoracic aneurysm component were more likely to have a suprarenal or juxtarenal aortic aneurysm, and those patients who did not have any thoracic aneurysm were more likely to have had an infrarenal aneurysm,” he said.
The median age of patients who had a thoracic aneurysm vs. those who did not was 76 years vs. 74 years, he said.
Other predictors for thoracic aortic aneurysm included African American race, family history of thoracic aneurysm, personal history of obesity hypertension, and an AAA diameter more than 5 cm on presentation, he said. Factors that conferred a protective effect were a diagnosis of diabetes mellitus, infrarenal AAA location, and – “counterintuitively” – a history of smoking.
“We propose that routine or targeted screening with chest CT at the time of aortic aneurysm diagnosis may be indicated, not only to really define the natural history of disease, but more importantly to try to prevent late aortic events,” Dr. Chaer said.
But Dr. James Black, of Johns Hopkins University in Baltimore, questioned the cost effectiveness of routine screening. At his institution, chest CT would add about $3,000 per patient, he said. “If you took a chest CT at diagnosis of AAA for 100 patients, 90% of the scans would be negative for thoracic aneurysm, at a rough cost in our institution of about $300,000 a year,” he said.
Cost of routine chest CT is an issue, Dr. Chaer acknowledged, although the chest CT could be done in the same scan as the abdominal CT.
“It would be nice to have a surrogate marker for thoracic aneurysm,” Dr. Chaer said. “Although we found that a thoracic aneurysm was more common in patients who had a juxtarenal aneurysm, those numbers were not hard enough to confidently say that the juxtarenal component is always predictive of a surrogate marker of thoracic aneurysm development. It is something that could be the subject of future studies.”
In addition, there is a need to identify risk factors. “We are trying to identify a high-risk group of patients in whom it would be more cost effective to screen,” he said. “That would bring down the number significantly and therefore the cost.”
Dr. Chaer noted that the heterogeneous population and the retrospective nature were limitations of the study. He reported no disclosures relevant to the presentation.
Demographics of Abdominal Aortic Aneurysm Patients
FROM THE ANNUAL MEETING OF THE EASTERN VASCULAR SOCIETY
Incidence, Risks for Thoracic Aneurysm in AAA Defined
NEW YORK – About one in four patients with abdominal aortic aneurysm may be at risk for thoracic aneurysm, judging by results of a single-center retrospective study of more than 1,000 patients.
Dr. Rabih Chaer, a vascular surgeon at the University of Pittsburgh, and his colleagues found that, among 1,082 patients diagnosed with abdominal aortic aneurysms (AAA) who had chest CT at follow-up, 23.4% had some sort of thoracic aneurysm afterward.
“Despite the clinical associations that have been observed between AAAs and peripheral aneurysms and thoracic aneurysms, screening for other common aneurysms continues to be controversial,” Dr. Chaer said at the annual meeting of the Eastern Vascular Society.
Therefore, they conducted the study to quantify the risk for thoracic aneurysm in these patients and to identify risk factors that could provide screening parameters, he said. The researchers defined an aneurysm as a greater than 50% increase in the adjacent aorta diameter or a 3 cm or larger increase in the setting of AAA, Dr. Chaer said. Thoracic aneurysms were categorized by two subgroups: synchronous (occurring within 2 years of initial AAA diagnosis) and metachronous (occurring 2 years or more after diagnosis). About 11% of patients had the former, and 12.6% the latter, Dr. Chaer said. The average time to diagnosis was 2.3 years, he said.
In all, the researchers considered 2,196 patients diagnosed with AAA between 2000 and 2008, but only 49% (1,082) had chest CT that qualified them for further analysis, Dr. Chaer noted. The chest studies were conducted for suspected pulmonary disease in 74% of patients, for chest screening in 15%, and for miscellaneous reasons in 11%.
One predisposing factor for thoracic aneurysm was the type of AAA, Dr. Chaer explained. “Those patients who had a thoracic aneurysm component were more likely to have a suprarenal or juxtarenal aortic aneurysm, and those patients who did not have any thoracic aneurysm were more likely to have had an infrarenal aneurysm,” he said.
The median age of patients who had a thoracic aneurysm vs. those who did not was 76 years vs. 74 years, he said.
Other predictors for thoracic aortic aneurysm included African American race, family history of thoracic aneurysm, personal history of obesity hypertension, and an AAA diameter more than 5 cm on presentation, he said. Factors that conferred a protective effect were a diagnosis of diabetes mellitus, infrarenal AAA location, and – “counterintuitively” – a history of smoking.
“We propose that routine or targeted screening with chest CT at the time of aortic aneurysm diagnosis may be indicated, not only to really define the natural history of disease, but more importantly to try to prevent late aortic events,” Dr. Chaer said.
But Dr. James Black, of Johns Hopkins University in Baltimore, questioned the cost effectiveness of routine screening. At his institution, chest CT would add about $3,000 per patient, he said. “If you took a chest CT at diagnosis of AAA for 100 patients, 90% of the scans would be negative for thoracic aneurysm, at a rough cost in our institution of about $300,000 a year,” he said.
Cost of routine chest CT is an issue, Dr. Chaer acknowledged, although the chest CT could be done in the same scan as the abdominal CT.
“It would be nice to have a surrogate marker for thoracic aneurysm,” Dr. Chaer said. “Although we found that a thoracic aneurysm was more common in patients who had a juxtarenal aneurysm, those numbers were not hard enough to confidently say that the juxtarenal component is always predictive of a surrogate marker of thoracic aneurysm development. It is something that could be the subject of future studies.”
In addition, there is a need to identify risk factors. “We are trying to identify a high-risk group of patients in whom it would be more cost effective to screen,” he said. “That would bring down the number significantly and therefore the cost.”
Dr. Chaer noted that the heterogeneous population and the retrospective nature were limitations of the study. He reported no disclosures relevant to the presentation.
Demographics of Abdominal Aortic Aneurysm Patients
NEW YORK – About one in four patients with abdominal aortic aneurysm may be at risk for thoracic aneurysm, judging by results of a single-center retrospective study of more than 1,000 patients.
Dr. Rabih Chaer, a vascular surgeon at the University of Pittsburgh, and his colleagues found that, among 1,082 patients diagnosed with abdominal aortic aneurysms (AAA) who had chest CT at follow-up, 23.4% had some sort of thoracic aneurysm afterward.
“Despite the clinical associations that have been observed between AAAs and peripheral aneurysms and thoracic aneurysms, screening for other common aneurysms continues to be controversial,” Dr. Chaer said at the annual meeting of the Eastern Vascular Society.
Therefore, they conducted the study to quantify the risk for thoracic aneurysm in these patients and to identify risk factors that could provide screening parameters, he said. The researchers defined an aneurysm as a greater than 50% increase in the adjacent aorta diameter or a 3 cm or larger increase in the setting of AAA, Dr. Chaer said. Thoracic aneurysms were categorized by two subgroups: synchronous (occurring within 2 years of initial AAA diagnosis) and metachronous (occurring 2 years or more after diagnosis). About 11% of patients had the former, and 12.6% the latter, Dr. Chaer said. The average time to diagnosis was 2.3 years, he said.
In all, the researchers considered 2,196 patients diagnosed with AAA between 2000 and 2008, but only 49% (1,082) had chest CT that qualified them for further analysis, Dr. Chaer noted. The chest studies were conducted for suspected pulmonary disease in 74% of patients, for chest screening in 15%, and for miscellaneous reasons in 11%.
One predisposing factor for thoracic aneurysm was the type of AAA, Dr. Chaer explained. “Those patients who had a thoracic aneurysm component were more likely to have a suprarenal or juxtarenal aortic aneurysm, and those patients who did not have any thoracic aneurysm were more likely to have had an infrarenal aneurysm,” he said.
The median age of patients who had a thoracic aneurysm vs. those who did not was 76 years vs. 74 years, he said.
Other predictors for thoracic aortic aneurysm included African American race, family history of thoracic aneurysm, personal history of obesity hypertension, and an AAA diameter more than 5 cm on presentation, he said. Factors that conferred a protective effect were a diagnosis of diabetes mellitus, infrarenal AAA location, and – “counterintuitively” – a history of smoking.
“We propose that routine or targeted screening with chest CT at the time of aortic aneurysm diagnosis may be indicated, not only to really define the natural history of disease, but more importantly to try to prevent late aortic events,” Dr. Chaer said.
But Dr. James Black, of Johns Hopkins University in Baltimore, questioned the cost effectiveness of routine screening. At his institution, chest CT would add about $3,000 per patient, he said. “If you took a chest CT at diagnosis of AAA for 100 patients, 90% of the scans would be negative for thoracic aneurysm, at a rough cost in our institution of about $300,000 a year,” he said.
Cost of routine chest CT is an issue, Dr. Chaer acknowledged, although the chest CT could be done in the same scan as the abdominal CT.
“It would be nice to have a surrogate marker for thoracic aneurysm,” Dr. Chaer said. “Although we found that a thoracic aneurysm was more common in patients who had a juxtarenal aneurysm, those numbers were not hard enough to confidently say that the juxtarenal component is always predictive of a surrogate marker of thoracic aneurysm development. It is something that could be the subject of future studies.”
In addition, there is a need to identify risk factors. “We are trying to identify a high-risk group of patients in whom it would be more cost effective to screen,” he said. “That would bring down the number significantly and therefore the cost.”
Dr. Chaer noted that the heterogeneous population and the retrospective nature were limitations of the study. He reported no disclosures relevant to the presentation.
Demographics of Abdominal Aortic Aneurysm Patients
NEW YORK – About one in four patients with abdominal aortic aneurysm may be at risk for thoracic aneurysm, judging by results of a single-center retrospective study of more than 1,000 patients.
Dr. Rabih Chaer, a vascular surgeon at the University of Pittsburgh, and his colleagues found that, among 1,082 patients diagnosed with abdominal aortic aneurysms (AAA) who had chest CT at follow-up, 23.4% had some sort of thoracic aneurysm afterward.
“Despite the clinical associations that have been observed between AAAs and peripheral aneurysms and thoracic aneurysms, screening for other common aneurysms continues to be controversial,” Dr. Chaer said at the annual meeting of the Eastern Vascular Society.
Therefore, they conducted the study to quantify the risk for thoracic aneurysm in these patients and to identify risk factors that could provide screening parameters, he said. The researchers defined an aneurysm as a greater than 50% increase in the adjacent aorta diameter or a 3 cm or larger increase in the setting of AAA, Dr. Chaer said. Thoracic aneurysms were categorized by two subgroups: synchronous (occurring within 2 years of initial AAA diagnosis) and metachronous (occurring 2 years or more after diagnosis). About 11% of patients had the former, and 12.6% the latter, Dr. Chaer said. The average time to diagnosis was 2.3 years, he said.
In all, the researchers considered 2,196 patients diagnosed with AAA between 2000 and 2008, but only 49% (1,082) had chest CT that qualified them for further analysis, Dr. Chaer noted. The chest studies were conducted for suspected pulmonary disease in 74% of patients, for chest screening in 15%, and for miscellaneous reasons in 11%.
One predisposing factor for thoracic aneurysm was the type of AAA, Dr. Chaer explained. “Those patients who had a thoracic aneurysm component were more likely to have a suprarenal or juxtarenal aortic aneurysm, and those patients who did not have any thoracic aneurysm were more likely to have had an infrarenal aneurysm,” he said.
The median age of patients who had a thoracic aneurysm vs. those who did not was 76 years vs. 74 years, he said.
Other predictors for thoracic aortic aneurysm included African American race, family history of thoracic aneurysm, personal history of obesity hypertension, and an AAA diameter more than 5 cm on presentation, he said. Factors that conferred a protective effect were a diagnosis of diabetes mellitus, infrarenal AAA location, and – “counterintuitively” – a history of smoking.
“We propose that routine or targeted screening with chest CT at the time of aortic aneurysm diagnosis may be indicated, not only to really define the natural history of disease, but more importantly to try to prevent late aortic events,” Dr. Chaer said.
But Dr. James Black, of Johns Hopkins University in Baltimore, questioned the cost effectiveness of routine screening. At his institution, chest CT would add about $3,000 per patient, he said. “If you took a chest CT at diagnosis of AAA for 100 patients, 90% of the scans would be negative for thoracic aneurysm, at a rough cost in our institution of about $300,000 a year,” he said.
Cost of routine chest CT is an issue, Dr. Chaer acknowledged, although the chest CT could be done in the same scan as the abdominal CT.
“It would be nice to have a surrogate marker for thoracic aneurysm,” Dr. Chaer said. “Although we found that a thoracic aneurysm was more common in patients who had a juxtarenal aneurysm, those numbers were not hard enough to confidently say that the juxtarenal component is always predictive of a surrogate marker of thoracic aneurysm development. It is something that could be the subject of future studies.”
In addition, there is a need to identify risk factors. “We are trying to identify a high-risk group of patients in whom it would be more cost effective to screen,” he said. “That would bring down the number significantly and therefore the cost.”
Dr. Chaer noted that the heterogeneous population and the retrospective nature were limitations of the study. He reported no disclosures relevant to the presentation.
Demographics of Abdominal Aortic Aneurysm Patients
FROM THE ANNUAL MEETING OF THE EASTERN VASCULAR SOCIETY
Clopidogrel Deemed Safe in Vascular Surgery
NEW YORK – Considerable controversy surrounds the use of the anticoagulant clopidogrel in patients before, during, and after surgery for peripheral artery disease, but in a study of more than 10,000 patients, rates of postoperative bleeding complications were similar between those who continued the drugs before surgery and those who did not.
Dr. David H. Stone presented the results of the study, which involved 10,406 patients and 12 centers from the Vascular Study Group of New England, at the annual meeting of the Eastern Vascular Society. Four previously published single-center studies of the use of clopidogrel (Plavix, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership) in vascular surgery patients accounted for fewer than 200 patients, he noted.
The patients in the New England study had carotid endarterectomy (CEA), lower extremity bypass, or endovascular or open abdominal aortic aneurysm (AAA) repair over a 6-year period. The study looked at reoperation for bleeding across all procedures and at blood transfusions in all procedures except CEA. Across all measures, the variations between the clopidogrel and non-clopidogrel groups were not statistically significant, said Dr. Stone of the Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
The largest variations were seen in the blood transfusion rates in endovascular AAA repair (16% in the clopidogrel group vs. 11% in the non-clopidogrel group), and in open AAA repair (43% vs. 38%, respectively).
The rates of reoperation for rebleeding, however, were almost identical in CEA, lower extremity bypass, and endovascular AAA repair. In open AAA repair, the clopidogrel group actually had a lower rate than did the non-clopidogrel group (1.6% vs. 2.4%). “Based on our sample size, these conclusions are most robust for carotid endarterectomy and open AAA repair,” Dr. Stone said.
Dr. John J. Ricotta of Washington (D.C.) Hospital Center questioned the strength of using the blood transfusion rate as an outcome measure, because standards for using transfusion can vary among centers. “With the exception of open aneurysm repair, you picked operations that are relevant but are not highly associated with bleeding problems,” he said.
“I would agree with that analysis, but surprisingly in our own region and apparently in Europe the majority of surgeons – just looking at carotid endarterectomy – still hold Plavix prior to surgery for obviously a perception of increased bleeding,” Dr. Stone said.
In lower extremity bypass, Dr. Ricotta said, more precise predictors of bleeding problems are reoperation, obesity, and long incisions. “Did you look at these variables?” he asked.
Dr. Stone said that the study did not include an analysis of body mass index specifically, but that the pending CASPAR (Clopidogrel and Acetyl Salicylic Acid in Bypass Surgery for Peripheral Arterial Disease) trial would show no disparity between groups undergoing lower extremity bypass.
“Based on our analysis of over 10,000 patients, perioperative antiplatelet regimens, including Plavix, appear safe among those undergoing commonly performed vascular operations,” Dr. Stone said. “Accordingly, we believe that Plavix can be safely continued in patients with important indications for its use, such as symptomatic carotid disease or recent drug-eluting cardiac stent placement.”
Dr. Stone said that he had no relationships to disclose.
NEW YORK – Considerable controversy surrounds the use of the anticoagulant clopidogrel in patients before, during, and after surgery for peripheral artery disease, but in a study of more than 10,000 patients, rates of postoperative bleeding complications were similar between those who continued the drugs before surgery and those who did not.
Dr. David H. Stone presented the results of the study, which involved 10,406 patients and 12 centers from the Vascular Study Group of New England, at the annual meeting of the Eastern Vascular Society. Four previously published single-center studies of the use of clopidogrel (Plavix, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership) in vascular surgery patients accounted for fewer than 200 patients, he noted.
The patients in the New England study had carotid endarterectomy (CEA), lower extremity bypass, or endovascular or open abdominal aortic aneurysm (AAA) repair over a 6-year period. The study looked at reoperation for bleeding across all procedures and at blood transfusions in all procedures except CEA. Across all measures, the variations between the clopidogrel and non-clopidogrel groups were not statistically significant, said Dr. Stone of the Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
The largest variations were seen in the blood transfusion rates in endovascular AAA repair (16% in the clopidogrel group vs. 11% in the non-clopidogrel group), and in open AAA repair (43% vs. 38%, respectively).
The rates of reoperation for rebleeding, however, were almost identical in CEA, lower extremity bypass, and endovascular AAA repair. In open AAA repair, the clopidogrel group actually had a lower rate than did the non-clopidogrel group (1.6% vs. 2.4%). “Based on our sample size, these conclusions are most robust for carotid endarterectomy and open AAA repair,” Dr. Stone said.
Dr. John J. Ricotta of Washington (D.C.) Hospital Center questioned the strength of using the blood transfusion rate as an outcome measure, because standards for using transfusion can vary among centers. “With the exception of open aneurysm repair, you picked operations that are relevant but are not highly associated with bleeding problems,” he said.
“I would agree with that analysis, but surprisingly in our own region and apparently in Europe the majority of surgeons – just looking at carotid endarterectomy – still hold Plavix prior to surgery for obviously a perception of increased bleeding,” Dr. Stone said.
In lower extremity bypass, Dr. Ricotta said, more precise predictors of bleeding problems are reoperation, obesity, and long incisions. “Did you look at these variables?” he asked.
Dr. Stone said that the study did not include an analysis of body mass index specifically, but that the pending CASPAR (Clopidogrel and Acetyl Salicylic Acid in Bypass Surgery for Peripheral Arterial Disease) trial would show no disparity between groups undergoing lower extremity bypass.
“Based on our analysis of over 10,000 patients, perioperative antiplatelet regimens, including Plavix, appear safe among those undergoing commonly performed vascular operations,” Dr. Stone said. “Accordingly, we believe that Plavix can be safely continued in patients with important indications for its use, such as symptomatic carotid disease or recent drug-eluting cardiac stent placement.”
Dr. Stone said that he had no relationships to disclose.
NEW YORK – Considerable controversy surrounds the use of the anticoagulant clopidogrel in patients before, during, and after surgery for peripheral artery disease, but in a study of more than 10,000 patients, rates of postoperative bleeding complications were similar between those who continued the drugs before surgery and those who did not.
Dr. David H. Stone presented the results of the study, which involved 10,406 patients and 12 centers from the Vascular Study Group of New England, at the annual meeting of the Eastern Vascular Society. Four previously published single-center studies of the use of clopidogrel (Plavix, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership) in vascular surgery patients accounted for fewer than 200 patients, he noted.
The patients in the New England study had carotid endarterectomy (CEA), lower extremity bypass, or endovascular or open abdominal aortic aneurysm (AAA) repair over a 6-year period. The study looked at reoperation for bleeding across all procedures and at blood transfusions in all procedures except CEA. Across all measures, the variations between the clopidogrel and non-clopidogrel groups were not statistically significant, said Dr. Stone of the Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
The largest variations were seen in the blood transfusion rates in endovascular AAA repair (16% in the clopidogrel group vs. 11% in the non-clopidogrel group), and in open AAA repair (43% vs. 38%, respectively).
The rates of reoperation for rebleeding, however, were almost identical in CEA, lower extremity bypass, and endovascular AAA repair. In open AAA repair, the clopidogrel group actually had a lower rate than did the non-clopidogrel group (1.6% vs. 2.4%). “Based on our sample size, these conclusions are most robust for carotid endarterectomy and open AAA repair,” Dr. Stone said.
Dr. John J. Ricotta of Washington (D.C.) Hospital Center questioned the strength of using the blood transfusion rate as an outcome measure, because standards for using transfusion can vary among centers. “With the exception of open aneurysm repair, you picked operations that are relevant but are not highly associated with bleeding problems,” he said.
“I would agree with that analysis, but surprisingly in our own region and apparently in Europe the majority of surgeons – just looking at carotid endarterectomy – still hold Plavix prior to surgery for obviously a perception of increased bleeding,” Dr. Stone said.
In lower extremity bypass, Dr. Ricotta said, more precise predictors of bleeding problems are reoperation, obesity, and long incisions. “Did you look at these variables?” he asked.
Dr. Stone said that the study did not include an analysis of body mass index specifically, but that the pending CASPAR (Clopidogrel and Acetyl Salicylic Acid in Bypass Surgery for Peripheral Arterial Disease) trial would show no disparity between groups undergoing lower extremity bypass.
“Based on our analysis of over 10,000 patients, perioperative antiplatelet regimens, including Plavix, appear safe among those undergoing commonly performed vascular operations,” Dr. Stone said. “Accordingly, we believe that Plavix can be safely continued in patients with important indications for its use, such as symptomatic carotid disease or recent drug-eluting cardiac stent placement.”
Dr. Stone said that he had no relationships to disclose.
Major Finding: The rates of reoperation for rebleeding after three different vascular procedures were almost identical, regardless of whether patients used clopidogrel preoperatively or not.
Data Source: Multicenter study of 10,406 patients.
Disclosures: Dr. Stone said he had no relationships to disclose.