User login
Fever, rash, and leukopenia in a 32-year-old man • Dx?
THE CASE
A 32-year-old man was admitted to our hospital with fever, chills, malaise, leukopenia, and a rash. About 3 weeks earlier, he’d had oral maxillofacial surgery and started a 10-day course of prophylactic amoxicillin/clavulanic acid. Fifteen days after the surgery, he developed a fever (temperature, 103˚ F), chills, arthralgia, myalgia, cough, diarrhea, and malaise. He was seen by his physician, who obtained a chest x-ray showing a lingular infiltrate. The physician diagnosed influenza and pneumonia in this patient, and prescribed oseltamivir, azithromycin, and an additional course of amoxicillin/clavulanic acid.
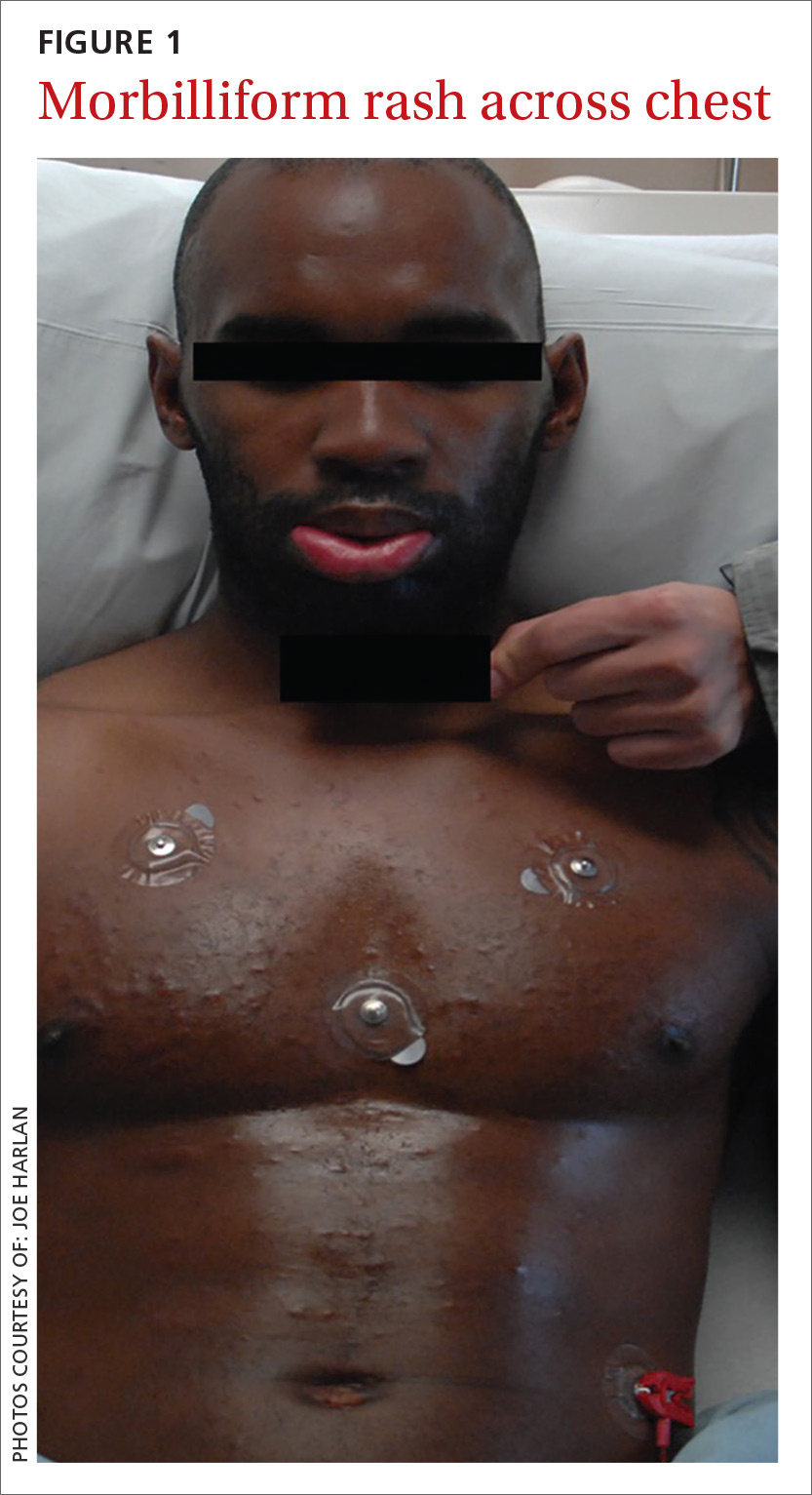
Upon admission to the hospital, laboratory tests revealed a white blood cell count (WBC) of 3.1 k/mcL (normal: 3.2-10.8 k/mcL). The patient’s physical examination was notable for lip edema, white mucous membrane plaques, submandibular and inguinal lymphadenopathy, and a morbilliform rash across his chest (FIGURE 1). Broad-spectrum antibiotics were initiated for presumed sepsis.
On hospital day (HD) 1, tests revealed a WBC count of 1.8 k/mcL, an erythrocyte sedimentation rate of 53 mm/hr (normal: 20-30 mm/hr for women, 15-20 mm/hr for men), and a C-reactive protein level of 6.7 mg/dL (normal: <0.5 mg/dL). A repeat chest x-ray and orofacial computerized tomography scan were normal.
By HD 3, all bacterial cultures were negative, but the patient was positive for human herpesvirus (HHV)-6 on viral cultures. His leukopenia persisted and he had elevated levels of alanine transaminase ranging from 40 to 73 U/L (normal: 6-43 U/L) and aspartate aminotransferase ranging from 66 to 108 U/L (normal range: 10-40 U/L), both downtrending during his hospitalization. He also had elevated levels of antinuclear antibodies (ANAs) and anti-Smith (Sm) antibody titers.
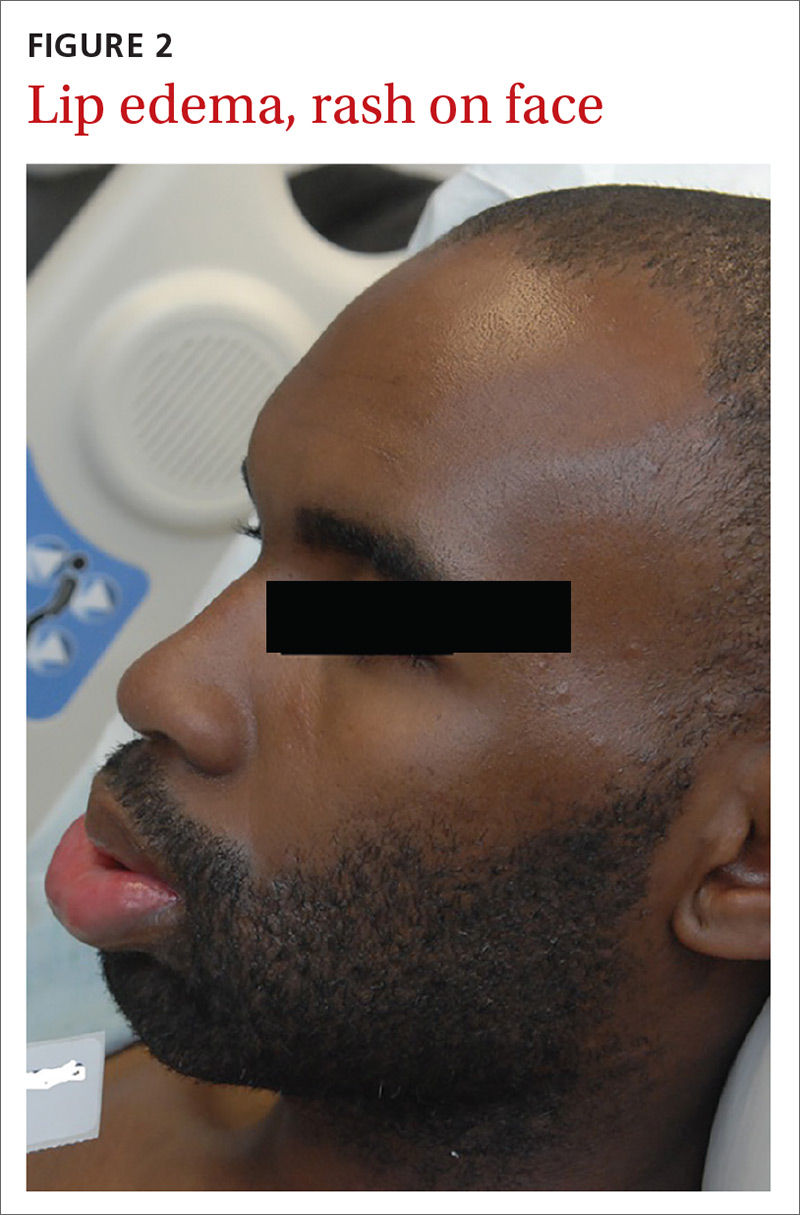
A posterior-auricular biopsy was consistent with lymphocytic perivasculitis. The rash continued to progress, involving his chest, abdomen, and face (FIGURE 2). Bacterial and viral cultures remained negative and on HD 4, broad-spectrum antibiotics were discontinued.
THE DIAGNOSIS
We diagnosed the patient with DRESS (drug reaction with eosinophilia and systemic symptoms) based on persistent fever, onset of cutaneous manifestations (facial edema and morbilliform eruption), lymphadenopathy, increased liver function tests, and recent exposure to an offending drug. The patient did not have eosinophilia; however, atypical lymphocytes were present on his peripheral smear.
DISCUSSION
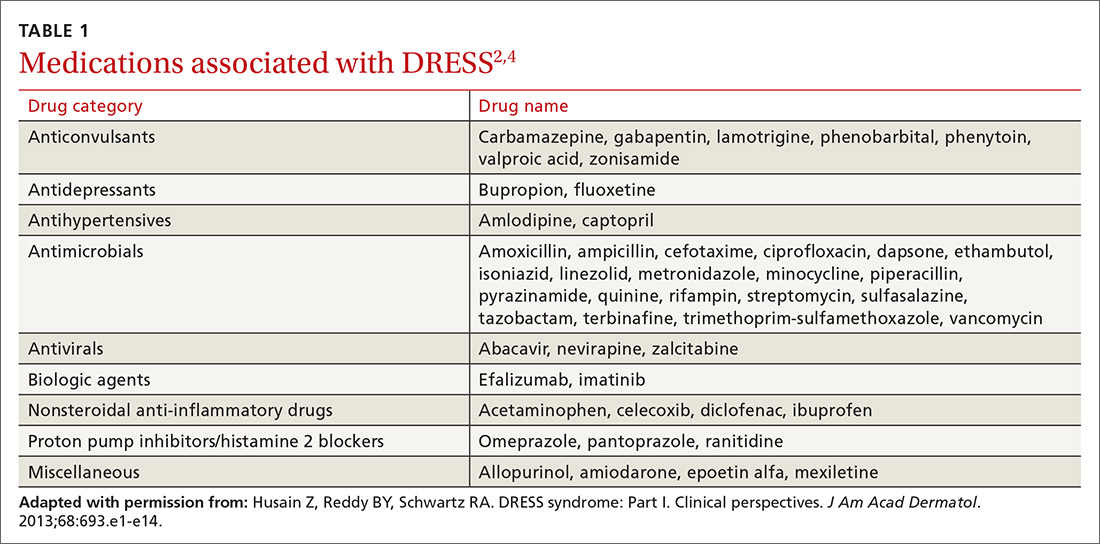
DRESS is typically characterized by fever, rash, eosinophilia, atypical lymphocytes, lymphadenopathy, and organ involvement (primarily liver, but multiple organ systems can be affected).1 Patients with severe symptoms have renal involvement, anemia, respiratory and cardiac symptoms (chest pain, tachycardia, and myocarditis), and transaminase levels up to 5 times greater than normal.1-3 Anticonvulsants and antibiotics are the most common offending classes among the medications that are associated with DRESS (TABLE 1).2,4
The reported incidence of DRESS is between one in 1000 and one in 10,000 drug exposures.1 Due to the broad presentation and a lack of established diagnostic criteria associated with DRESS, this number may be even higher. DRESS has a 10% mortality rate,1 and hepatic necrosis is the most common cause of death.2
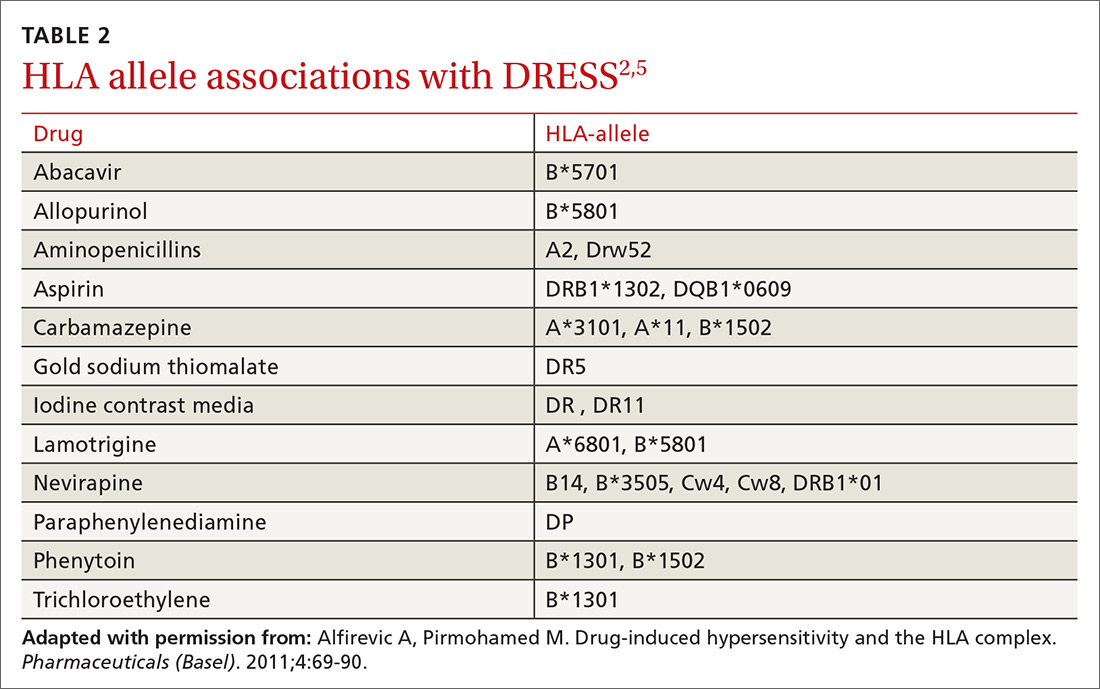
Certain people may be more prone to DRESS. People with certain gene mutations that code for drug detoxification enzymes have shown a greater incidence of DRESS.5 Viral reactivation, commonly of HHV-6, has also been shown to have an effect on the pathogenesis of DRESS. Additionally, genetic predisposition involving specific human leukocyte antigens (HLAs) makes certain people more prone to the development of DRESS (TABLE 2).2,5
Case reports have demonstrated a link between certain autoimmune syndromes and DRESS, specifically Grave’s disease and type 1 diabetes mellitus.2
A unique finding of this case was the presence of elevated ANA and anti-Sm antibody titers at initial presentation, with spontaneous negative seroconversion 2 months later. Because of these 2 findings, as well as the patient’s leukopenia and rash, he briefly met 4 of the 11 criteria set forth by the American College of Rheumatology for a diagnosis of systemic lupus erythematosus (SLE).6 It is unclear whether the transiently elevated anti-Sm antibody titers were an acute phase reactant due to DRESS, a viral illness, or an evolving autoimmune process.
The false-positive rate for anti-Sm antibodies in association with DRESS has not been previously reported.
MAKING THE DIAGNOSIS
Distinguishing DRESS from other life-threatening cutaneous drug reactions, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, can be difficult. Likewise, acute bacterial/viral infections, autoimmune syndromes, vasculitis, and hematologic diseases can mimic DRESS.7 Exposure to an offending drug 2 to 6 weeks prior to the onset of symptoms is supportive of DRESS.
This scoring system can help. The RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) has developed a scoring system to aid in the accurate diagnosis of DRESS.1,8 The scoring consists of 8 categories: fever, eosinophilia, enlarged lymph nodes, atypical lymphocytes, skin involvement, organ involvement, time of resolution, and the evaluation of other potential causes.1 Each category is graded a number from -1 (not supportive of DRESS) to 2 (highly supportive of DRESS) based on the patient’s presentation. The total score grades potential cases as “no,” “possible,” “probable,” or “definite.”1,8 In one review, cases classified as “probable” or “definite” by the RegiSCAR scoring system constituted 88% of the cases reported in the literature.1
Two tests that can also aid in the diagnosis of DRESS include patch testing (exposing the skin to a diluted version of the suspected offending drug and observing for a local reaction) and lymphocyte transformation tests. The latter are a better method of diagnosing drug-induced DRESS, with a sensitivity of 60% to 70%, and a specificity of 85%.9 However, this testing is not readily available.
Once DRESS is diagnosed, the offending drug should be immediately discontinued. For mild cases, supportive treatment is recommended. For more severe cases, the use of corticosteroids tapered over several months is the treatment of choice.10 Further studies are needed to determine the optimal type of corticosteroids, as well as the dose, route, and duration of therapy. Immunotherapy, plasmapheresis, and antivirals have been used with mixed results.10,11
Our patient was started on topical and systemic oral corticosteroids. Within 24 hours, his fever resolved and his rash improved. By HD 7, his laboratory values were normal and he was discharged.
The patient was advised that in the future, he should avoid exposure to the penicillin class of medication.
THE TAKEAWAY
The presence of rash, fever, lymphadenopathy, eosinophilia, atypical lymphocytes, liver involvement, and HHV-6 reactivation in the absence of sepsis should raise suspicion for DRESS. Early diagnosis, discontinuation of the culprit drug, and timely treatment are imperative in the management of the condition. Due to a potential genetic predisposition to DRESS, clinicians should use caution when treating first-degree family members with the same class of medication that was problematic for their relative. Long-term sequelae, such as Grave’s disease and diabetes mellitus, have been reported following DRESS. Therefore, long-term monitoring with appropriate testing is recommended.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
3. Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequelae of DRESS-2 cases. J Am Acad Dermatol. 2011;65:889-890.
4. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1-21.
5. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity and the HLA complex. Pharmaceuticals (Basel). 2011;4:69-90.
6. American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at: https://www.rheumatology.org/Portals/0/Files/1982%20SLE%20Classification_Excerpt.pdf. Accessed August 30, 2017.
7. Descamps V, Ben Saïd B, Sassolas B, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2010;137:703-708.
8. Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
9. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820.
10. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome part II: management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
11. Funck-Brentano E, Duong TA, Bouvresses S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252.
THE CASE
A 32-year-old man was admitted to our hospital with fever, chills, malaise, leukopenia, and a rash. About 3 weeks earlier, he’d had oral maxillofacial surgery and started a 10-day course of prophylactic amoxicillin/clavulanic acid. Fifteen days after the surgery, he developed a fever (temperature, 103˚ F), chills, arthralgia, myalgia, cough, diarrhea, and malaise. He was seen by his physician, who obtained a chest x-ray showing a lingular infiltrate. The physician diagnosed influenza and pneumonia in this patient, and prescribed oseltamivir, azithromycin, and an additional course of amoxicillin/clavulanic acid.
Upon admission to the hospital, laboratory tests revealed a white blood cell count (WBC) of 3.1 k/mcL (normal: 3.2-10.8 k/mcL). The patient’s physical examination was notable for lip edema, white mucous membrane plaques, submandibular and inguinal lymphadenopathy, and a morbilliform rash across his chest (FIGURE 1). Broad-spectrum antibiotics were initiated for presumed sepsis.
On hospital day (HD) 1, tests revealed a WBC count of 1.8 k/mcL, an erythrocyte sedimentation rate of 53 mm/hr (normal: 20-30 mm/hr for women, 15-20 mm/hr for men), and a C-reactive protein level of 6.7 mg/dL (normal: <0.5 mg/dL). A repeat chest x-ray and orofacial computerized tomography scan were normal.
By HD 3, all bacterial cultures were negative, but the patient was positive for human herpesvirus (HHV)-6 on viral cultures. His leukopenia persisted and he had elevated levels of alanine transaminase ranging from 40 to 73 U/L (normal: 6-43 U/L) and aspartate aminotransferase ranging from 66 to 108 U/L (normal range: 10-40 U/L), both downtrending during his hospitalization. He also had elevated levels of antinuclear antibodies (ANAs) and anti-Smith (Sm) antibody titers.
A posterior-auricular biopsy was consistent with lymphocytic perivasculitis. The rash continued to progress, involving his chest, abdomen, and face (FIGURE 2). Bacterial and viral cultures remained negative and on HD 4, broad-spectrum antibiotics were discontinued.
THE DIAGNOSIS
We diagnosed the patient with DRESS (drug reaction with eosinophilia and systemic symptoms) based on persistent fever, onset of cutaneous manifestations (facial edema and morbilliform eruption), lymphadenopathy, increased liver function tests, and recent exposure to an offending drug. The patient did not have eosinophilia; however, atypical lymphocytes were present on his peripheral smear.
DISCUSSION
DRESS is typically characterized by fever, rash, eosinophilia, atypical lymphocytes, lymphadenopathy, and organ involvement (primarily liver, but multiple organ systems can be affected).1 Patients with severe symptoms have renal involvement, anemia, respiratory and cardiac symptoms (chest pain, tachycardia, and myocarditis), and transaminase levels up to 5 times greater than normal.1-3 Anticonvulsants and antibiotics are the most common offending classes among the medications that are associated with DRESS (TABLE 1).2,4
The reported incidence of DRESS is between one in 1000 and one in 10,000 drug exposures.1 Due to the broad presentation and a lack of established diagnostic criteria associated with DRESS, this number may be even higher. DRESS has a 10% mortality rate,1 and hepatic necrosis is the most common cause of death.2
Certain people may be more prone to DRESS. People with certain gene mutations that code for drug detoxification enzymes have shown a greater incidence of DRESS.5 Viral reactivation, commonly of HHV-6, has also been shown to have an effect on the pathogenesis of DRESS. Additionally, genetic predisposition involving specific human leukocyte antigens (HLAs) makes certain people more prone to the development of DRESS (TABLE 2).2,5
Case reports have demonstrated a link between certain autoimmune syndromes and DRESS, specifically Grave’s disease and type 1 diabetes mellitus.2
A unique finding of this case was the presence of elevated ANA and anti-Sm antibody titers at initial presentation, with spontaneous negative seroconversion 2 months later. Because of these 2 findings, as well as the patient’s leukopenia and rash, he briefly met 4 of the 11 criteria set forth by the American College of Rheumatology for a diagnosis of systemic lupus erythematosus (SLE).6 It is unclear whether the transiently elevated anti-Sm antibody titers were an acute phase reactant due to DRESS, a viral illness, or an evolving autoimmune process.
The false-positive rate for anti-Sm antibodies in association with DRESS has not been previously reported.
MAKING THE DIAGNOSIS
Distinguishing DRESS from other life-threatening cutaneous drug reactions, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, can be difficult. Likewise, acute bacterial/viral infections, autoimmune syndromes, vasculitis, and hematologic diseases can mimic DRESS.7 Exposure to an offending drug 2 to 6 weeks prior to the onset of symptoms is supportive of DRESS.
This scoring system can help. The RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) has developed a scoring system to aid in the accurate diagnosis of DRESS.1,8 The scoring consists of 8 categories: fever, eosinophilia, enlarged lymph nodes, atypical lymphocytes, skin involvement, organ involvement, time of resolution, and the evaluation of other potential causes.1 Each category is graded a number from -1 (not supportive of DRESS) to 2 (highly supportive of DRESS) based on the patient’s presentation. The total score grades potential cases as “no,” “possible,” “probable,” or “definite.”1,8 In one review, cases classified as “probable” or “definite” by the RegiSCAR scoring system constituted 88% of the cases reported in the literature.1
Two tests that can also aid in the diagnosis of DRESS include patch testing (exposing the skin to a diluted version of the suspected offending drug and observing for a local reaction) and lymphocyte transformation tests. The latter are a better method of diagnosing drug-induced DRESS, with a sensitivity of 60% to 70%, and a specificity of 85%.9 However, this testing is not readily available.
Once DRESS is diagnosed, the offending drug should be immediately discontinued. For mild cases, supportive treatment is recommended. For more severe cases, the use of corticosteroids tapered over several months is the treatment of choice.10 Further studies are needed to determine the optimal type of corticosteroids, as well as the dose, route, and duration of therapy. Immunotherapy, plasmapheresis, and antivirals have been used with mixed results.10,11
Our patient was started on topical and systemic oral corticosteroids. Within 24 hours, his fever resolved and his rash improved. By HD 7, his laboratory values were normal and he was discharged.
The patient was advised that in the future, he should avoid exposure to the penicillin class of medication.
THE TAKEAWAY
The presence of rash, fever, lymphadenopathy, eosinophilia, atypical lymphocytes, liver involvement, and HHV-6 reactivation in the absence of sepsis should raise suspicion for DRESS. Early diagnosis, discontinuation of the culprit drug, and timely treatment are imperative in the management of the condition. Due to a potential genetic predisposition to DRESS, clinicians should use caution when treating first-degree family members with the same class of medication that was problematic for their relative. Long-term sequelae, such as Grave’s disease and diabetes mellitus, have been reported following DRESS. Therefore, long-term monitoring with appropriate testing is recommended.
THE CASE
A 32-year-old man was admitted to our hospital with fever, chills, malaise, leukopenia, and a rash. About 3 weeks earlier, he’d had oral maxillofacial surgery and started a 10-day course of prophylactic amoxicillin/clavulanic acid. Fifteen days after the surgery, he developed a fever (temperature, 103˚ F), chills, arthralgia, myalgia, cough, diarrhea, and malaise. He was seen by his physician, who obtained a chest x-ray showing a lingular infiltrate. The physician diagnosed influenza and pneumonia in this patient, and prescribed oseltamivir, azithromycin, and an additional course of amoxicillin/clavulanic acid.
Upon admission to the hospital, laboratory tests revealed a white blood cell count (WBC) of 3.1 k/mcL (normal: 3.2-10.8 k/mcL). The patient’s physical examination was notable for lip edema, white mucous membrane plaques, submandibular and inguinal lymphadenopathy, and a morbilliform rash across his chest (FIGURE 1). Broad-spectrum antibiotics were initiated for presumed sepsis.
On hospital day (HD) 1, tests revealed a WBC count of 1.8 k/mcL, an erythrocyte sedimentation rate of 53 mm/hr (normal: 20-30 mm/hr for women, 15-20 mm/hr for men), and a C-reactive protein level of 6.7 mg/dL (normal: <0.5 mg/dL). A repeat chest x-ray and orofacial computerized tomography scan were normal.
By HD 3, all bacterial cultures were negative, but the patient was positive for human herpesvirus (HHV)-6 on viral cultures. His leukopenia persisted and he had elevated levels of alanine transaminase ranging from 40 to 73 U/L (normal: 6-43 U/L) and aspartate aminotransferase ranging from 66 to 108 U/L (normal range: 10-40 U/L), both downtrending during his hospitalization. He also had elevated levels of antinuclear antibodies (ANAs) and anti-Smith (Sm) antibody titers.
A posterior-auricular biopsy was consistent with lymphocytic perivasculitis. The rash continued to progress, involving his chest, abdomen, and face (FIGURE 2). Bacterial and viral cultures remained negative and on HD 4, broad-spectrum antibiotics were discontinued.
THE DIAGNOSIS
We diagnosed the patient with DRESS (drug reaction with eosinophilia and systemic symptoms) based on persistent fever, onset of cutaneous manifestations (facial edema and morbilliform eruption), lymphadenopathy, increased liver function tests, and recent exposure to an offending drug. The patient did not have eosinophilia; however, atypical lymphocytes were present on his peripheral smear.
DISCUSSION
DRESS is typically characterized by fever, rash, eosinophilia, atypical lymphocytes, lymphadenopathy, and organ involvement (primarily liver, but multiple organ systems can be affected).1 Patients with severe symptoms have renal involvement, anemia, respiratory and cardiac symptoms (chest pain, tachycardia, and myocarditis), and transaminase levels up to 5 times greater than normal.1-3 Anticonvulsants and antibiotics are the most common offending classes among the medications that are associated with DRESS (TABLE 1).2,4
The reported incidence of DRESS is between one in 1000 and one in 10,000 drug exposures.1 Due to the broad presentation and a lack of established diagnostic criteria associated with DRESS, this number may be even higher. DRESS has a 10% mortality rate,1 and hepatic necrosis is the most common cause of death.2
Certain people may be more prone to DRESS. People with certain gene mutations that code for drug detoxification enzymes have shown a greater incidence of DRESS.5 Viral reactivation, commonly of HHV-6, has also been shown to have an effect on the pathogenesis of DRESS. Additionally, genetic predisposition involving specific human leukocyte antigens (HLAs) makes certain people more prone to the development of DRESS (TABLE 2).2,5
Case reports have demonstrated a link between certain autoimmune syndromes and DRESS, specifically Grave’s disease and type 1 diabetes mellitus.2
A unique finding of this case was the presence of elevated ANA and anti-Sm antibody titers at initial presentation, with spontaneous negative seroconversion 2 months later. Because of these 2 findings, as well as the patient’s leukopenia and rash, he briefly met 4 of the 11 criteria set forth by the American College of Rheumatology for a diagnosis of systemic lupus erythematosus (SLE).6 It is unclear whether the transiently elevated anti-Sm antibody titers were an acute phase reactant due to DRESS, a viral illness, or an evolving autoimmune process.
The false-positive rate for anti-Sm antibodies in association with DRESS has not been previously reported.
MAKING THE DIAGNOSIS
Distinguishing DRESS from other life-threatening cutaneous drug reactions, particularly Stevens-Johnson syndrome and toxic epidermal necrolysis, can be difficult. Likewise, acute bacterial/viral infections, autoimmune syndromes, vasculitis, and hematologic diseases can mimic DRESS.7 Exposure to an offending drug 2 to 6 weeks prior to the onset of symptoms is supportive of DRESS.
This scoring system can help. The RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) has developed a scoring system to aid in the accurate diagnosis of DRESS.1,8 The scoring consists of 8 categories: fever, eosinophilia, enlarged lymph nodes, atypical lymphocytes, skin involvement, organ involvement, time of resolution, and the evaluation of other potential causes.1 Each category is graded a number from -1 (not supportive of DRESS) to 2 (highly supportive of DRESS) based on the patient’s presentation. The total score grades potential cases as “no,” “possible,” “probable,” or “definite.”1,8 In one review, cases classified as “probable” or “definite” by the RegiSCAR scoring system constituted 88% of the cases reported in the literature.1
Two tests that can also aid in the diagnosis of DRESS include patch testing (exposing the skin to a diluted version of the suspected offending drug and observing for a local reaction) and lymphocyte transformation tests. The latter are a better method of diagnosing drug-induced DRESS, with a sensitivity of 60% to 70%, and a specificity of 85%.9 However, this testing is not readily available.
Once DRESS is diagnosed, the offending drug should be immediately discontinued. For mild cases, supportive treatment is recommended. For more severe cases, the use of corticosteroids tapered over several months is the treatment of choice.10 Further studies are needed to determine the optimal type of corticosteroids, as well as the dose, route, and duration of therapy. Immunotherapy, plasmapheresis, and antivirals have been used with mixed results.10,11
Our patient was started on topical and systemic oral corticosteroids. Within 24 hours, his fever resolved and his rash improved. By HD 7, his laboratory values were normal and he was discharged.
The patient was advised that in the future, he should avoid exposure to the penicillin class of medication.
THE TAKEAWAY
The presence of rash, fever, lymphadenopathy, eosinophilia, atypical lymphocytes, liver involvement, and HHV-6 reactivation in the absence of sepsis should raise suspicion for DRESS. Early diagnosis, discontinuation of the culprit drug, and timely treatment are imperative in the management of the condition. Due to a potential genetic predisposition to DRESS, clinicians should use caution when treating first-degree family members with the same class of medication that was problematic for their relative. Long-term sequelae, such as Grave’s disease and diabetes mellitus, have been reported following DRESS. Therefore, long-term monitoring with appropriate testing is recommended.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
3. Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequelae of DRESS-2 cases. J Am Acad Dermatol. 2011;65:889-890.
4. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1-21.
5. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity and the HLA complex. Pharmaceuticals (Basel). 2011;4:69-90.
6. American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at: https://www.rheumatology.org/Portals/0/Files/1982%20SLE%20Classification_Excerpt.pdf. Accessed August 30, 2017.
7. Descamps V, Ben Saïd B, Sassolas B, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2010;137:703-708.
8. Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
9. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820.
10. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome part II: management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
11. Funck-Brentano E, Duong TA, Bouvresses S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252.
1. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588-597.
2. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
3. Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequelae of DRESS-2 cases. J Am Acad Dermatol. 2011;65:889-890.
4. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18:1-21.
5. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity and the HLA complex. Pharmaceuticals (Basel). 2011;4:69-90.
6. American College of Rheumatology. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. Available at: https://www.rheumatology.org/Portals/0/Files/1982%20SLE%20Classification_Excerpt.pdf. Accessed August 30, 2017.
7. Descamps V, Ben Saïd B, Sassolas B, et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2010;137:703-708.
8. Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
9. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809-820.
10. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome part II: management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
11. Funck-Brentano E, Duong TA, Bouvresses S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252.
Pediatric hypertension: Often missed and mismanaged
› Screen for hypertension in all children over the age of 3 at every visit. C
› Order laboratory evaluation, echocardiography, and renovascular imaging for all children given a diagnosis of hypertension. C
› Advise parents that children with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics, but those with stage 2 hypertension, target-organ damage, or symptomatic hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling) until BP is well controlled. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood hypertension is on the rise: Recent data from the National Health and Nutrition Survey suggest 10% of children and adolescents have prehypertension and 4% have hypertension.1-4 Unfortunately, the condition often is missed. In a study of 14,187 children and adolescents who had at least 3 well-child visits at an outpatient academic medical center, 507 patients met the criteria for hypertension, yet only 131 (26%) had this diagnosis documented in their electronic health record.5
In a survey of 89 pediatricians, >50% of respondents said they were not familiar with the most current published recommendations for diagnosing and treating pediatric hypertension.6 Respondents also indicated that the most common reason for not initiating pharmacotherapy for children with hypertension was a lack of familiarity with appropriate antihypertensive agents (54%), followed by concern for adverse medication effects. Delayed diagnosis, evaluation, and treatment of hypertension in young patients can increase the likelihood of serious consequences, including target-organ damage such as left ventricular hypertrophy (LVH). In this review, we’ll describe the factors that put children and adolescents at risk for hypertension, and offer an evidence-based approach to diagnosis and treatment.
Obesity is a key risk factor
An estimated 17% of children aged 2 to 19 are obese.7 Obesity increases a child’s risk for hypertension by approximately 3- to 5-fold, and body mass index (BMI) is greater in children with primary hypertension compared with those with secondary hypertension.8 Hypertension is more common among Hispanic and non-Hispanic black male children and adolescents compared with their white counterparts; these ethnic disparities are not found in females.9,10 Poor diets and physical inactivity further contribute to obesity and hypertension risk. Children who were born preterm or had a very low birth weight also are at increased risk.11
Unchecked hypertension can lead to cardiac, vascular damage
Some children and adolescents with undiagnosed and untreated hypertension have evidence of target-organ damage, including cardiac dysfunction and pathologic vascular abnormalities. LVH is present in 20% to 41% of children and adolescents with hypertension.12,13 Carotid intima-media thickness, an established surrogate marker for atherosclerosis, is abnormally increased in children with hypertension, even after adjusting for BMI.14 Other target organ effects include impaired cognitive function, reduced glomerular filtration rate, microalbuminuria, and retinal arteriolar narrowing.15-17
Pediatric hypertension may persist into adulthood. A meta-analysis of more than 50 studies found that elevated blood pressure (BP) in childhood increases the risk for hypertension as an adult.18
NHLBI recommendations call for a BP check at every visit
The National Heart, Lung, and Blood Institute (NHLBI) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (“the 4th Report”) recommends measuring BP in all children over age 3 during every health care visit.12 Children under age 3 should have their BP checked in certain circumstances, including preterm delivery, congenital heart disease, recurrent urinary tract infections, renal/urologic disease, organ transplantation, malignancy, and systemic illnesses associated with hypertension.12 The 4th Report is endorsed by the American Academy of Pediatrics (AAP); however, the American Academy of Family Physicians and the US Preventive Services Task Force have concluded that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease (CVD).19,20
Does the child have hypertension? That depends on several factors
Determining whether a child has hypertension requires that you consult national BP standards to determine if he or she is within the normal range. Normal BP standards for children and adolescents are based on gender, age, and height percentile, and provide a precise classification based on body size.12 These tables are available from the NHLBI Web site at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. Height percentiles in these tables correspond with the Centers for Disease Control and Prevention (CDC) growth charts published in 2000.21 The Baylor College of Medicine Children’s Nutrition Research Center has a web-based calculator to help physicians determine BP percentiles in children and adolescents; it is available at http://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. The International Pediatric Hypertension Association also offers BP charts and calculators at http://www.iphapediatrichypertension.org.
The diagnostic parameters for pediatric hypertension are listed in TABLE 1.12 The higher systolic or diastolic BP percentile value is used to determine a child’s overall BP category. A child is considered normotensive if the BP is <90th percentile. Hypertension is an average systolic or diastolic BP that is ≥95th percentile on at least 3 separate occasions. Stage 1 hypertension is BP levels ranging from the 95th percentile to 5 mm Hg above the 99th percentile, and stage 2 hypertension is BP levels greater than 5 mm Hg above the 99th percentile.
For example, assume you are evaluating a 12-year-old boy who is 61 inches tall and has a BP of 129/87 mm Hg. According to the CDC growth charts, his height puts him in the 75th percentile for his age. Using the NHLBI chart, you determine that he falls in the 95th-99th percentile for BP, and thus, using the categories in TABLE 1, is given a diagnosis of Stage 1 hypertension.
Accurate BP measurement requires using an appropriate cuff size that covers 80% of the child’s upper arm. When the child is between cuff sizes, use the larger cuff because small cuffs overestimate BP readings. BP readings should be taken on the right arm with the arm supported at heart level after the child has been sitting quietly for at least 5 minutes.12 One study showed that the initial BP readings taken in the triage area were significantly higher—often by >10 mm Hg—compared with follow-up measurements in the examination room.22
The preferred method of BP measurement is auscultation; however, oscillometric devices also are acceptable. These devices are easier to use, help eliminate digit bias, and minimize observer variation, but they typically read approximately 6 to 9 mm Hg higher than auscultation.23 For any BP measurement obtained by oscillometry that is >90th percentile, repeat the measurement by auscultation at least twice during the same office visit, and use an average of the repeated measurements.12 Obtain measurements of a lower extremity when you suspect congenital heart disease (eg, aortic coarctation). For any patient in whom you confirm a BP measurement >95th percentile, repeat the measurement within 2 weeks; for BPs >99th percentile, reevaluation should occur within one week.
Ambulatory BP monitoring (ABPM). Because BP measurements have a circadian pattern (higher during the day and reduced by 10% during sleep24) an ABPM device that provides 50 to 60 readings over 24 hours can be useful when evaluating children and adolescents for white-coat hypertension (elevated clinic BP with normal ambulatory BP), masked hypertension (normal clinic BP with elevated ambulatory BP), prehypertension and secondary hypertension (BP generally does not follow circadian patterns).25 ABPM is more accurate than BP self-measurement, but usually is limited to children older than age 5
Steps to take for clinical evaluation
Start by conducting a thorough history and physical examination, looking for information that can help you select the most appropriate tests for the next phase of evaluation.8,12 Calculate the patient’s BMI to screen for obesity, ask about a family history of hypertension or CVD, and determine if the patient is taking any medications that might cause hypertension, such as amphetamines, corticosteroids, or cyclosporine.8 Assess for signs and symptoms that suggest an underlying disease, such as renal disease (hematuria, edema, fatigue) or heart disease (chest pain, exertional dyspnea, palpitations).12
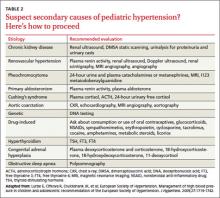
All children diagnosed with hypertension should be screened for secondary causes (TABLE 2). The recommended evaluation is to obtain a renal function panel, electrolytes, urinalysis, urine culture, complete blood count, renovascular imaging, and echocardiogram.12 The most common etiologies for secondary hypertension are renal parenchymal disease (68%), renovascular abnormalities (10%), and endocrinopathies (10%).26 Other causes, such as aortic coarctation, obstructive sleep apnea, iatrogenic factors (eg, toxins, medications, drugs of abuse), and genitourinary abnormalities, account for only a small percentage of cases and should be investigated as clinically indicated.26
Renovascular assessment depends on facility expertise. Imaging options include renal ultrasound (with or without Doppler), computed tomography angiography, renal flow scan, and magnetic resonance angiography. These studies have similar sensitivities and specificities.27 For patients in whom you strongly suspect renovascular disease, renal arteriography (digital subtraction angiography) provides the best images, although it is the most invasive study.27
Refer children and adolescents who are found to have significant abnormalities during the initial evaluation to the appropriate specialist. BP measurements often improve when secondary causes are treated.
Which drugs for which patients?
Pharmacologic management is indicated for pediatric patients with stage 1 or stage 2 hypertension, secondary hypertension, and those with evidence of target-organ damage.12 The goal of therapy is to reduce BP to <95th percentile. In patients with target organ damage, renal disease, or diabetes mellitus, the goal is <90th percentile.12,15,28 Intensive management of BP (≤50th percentile) in children with chronic kidney disease has been shown to delay progression to renal failure,29 but it is uncertain if lower BP goals can slow or prevent additional subclinical target organ damage.
Pharmacotherapy for hypertensive children or adolescents can be challenging because recommendations of which medication to use are based upon expert opinion and extrapolation from randomized trials of adults. The length of therapy (often lifelong), potential adverse effects, and unproven direct mortality benefit complicate this decision. Medication choice usually is based on physician preference or experience.12 The most common antihypertensive drugs prescribed are angiotensin-converting enzyme (ACE) inhibitors (26%), followed by diuretics (20%), and beta-blockers (17%).30,31 The starting doses and other details of medications commonly used to treat pediatric hypertension are listed in TABLE 3.28,32-34
One approach to choosing an antihypertensive drug for children is to measure the patient’s ambulatory plasma renin activity (PRA) level before initiating therapy. Those with high PRA levels (>0.65 ng/mL/h), presumably due to peripheral vasoconstriction, may benefit more from ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-adrenergic antagonists.35 Individuals with low PRA levels (<0.65 ng/mL/h) maintain higher volume/sodium excess and may benefit more from diuretics or calcium channel blockers.35
Ethnicity also may guide medication selection. African American adults do not respond well to ACE inhibitor monotherapy due to decreased PRA and increased salt hypersensitivity.36 One meta-analysis found that African American children and adolescents had inadequate BP response to 6 individual ACE inhibitors, even at higher doses compared with white children and those of other ethnicities, who showed significant improvement in BP.37 ARBs may be a more effective alternative for this population.
Most experts recommend initiating a single agent at a low dose.12 A systematic review found that except for African American children, pediatric patients experienced comparable reductions in BP with ACE inhibitors (10.7/8.1 mm Hg), ARBs (10.5/6.9 mm Hg), and calcium channel blockers (9.3/7.2 mm Hg).38 In addition, ACE inhibitors and ARBs significantly reduced proteinuria by 49% and 59%, respectively.38
Schedule follow-up visits for 2 to 4 weeks (or sooner for patients with stage 2 hypertension) after initiating pharmacotherapy. If BP response is suboptimal, consider increasing the dose before adding a second agent. If the patient experiences significant adverse effects or has an inadequate BP response, changing to a drug from a different class is recommended.39 Patients who do not adequately respond to these approaches may require combination therapy; in such cases, strongly consider consultation with pediatric nephrologist or cardiologist.39 Medication compliance should be verified (eg, by pill counting, parental supervision) in patients who do not respond to therapy. Once BP control has been achieved, visits every 3 to 4 months are appropriate, with periodic laboratory monitoring, especially for children taking diuretics, ACE inhibitors, or ARBs or who have underlying renal disease.
Recommend exercise, but carefully monitor athlete's BP
Encourage obese and overweight children and adolescents to lose weight to maintain a BMI <95th percentile. Current guidelines based on expert opinion recommend that children and adolescents should engage in 60 minutes of daily physical activity.12 A meta-analysis found physical activity led to a 1% and 3% reduction in systolic and diastolic BP, respectively, although these results were not statistically significant.40
Be aware, however, that children and adolescents with hypertension who engage in certain competitive sports can significantly increase their BP and may be at risk for complications.41 According to the AAP guidelines, patients with stage 2 hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling, boxing, cycling, decathlon, triathlon) until BP is well controlled.41 Patients with target-organ damage, uncontrolled hypertension, or symptomatic hypertension should not participate until BP is well controlled. Patients with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics. Reassess BP every 6 months in patients who are prehypertensive and every one to 2 weeks for those with stage 1 hypertension. When the patient’s BP remains <90th percentile, routine surveillance every 3 to 6 months is recommended.
What about sodium? Encourage parents of pediatric patients with hypertension to limit their child’s salt intake to 1.2 g/d for those age 4 to 8 and 1.5 g/d for older children.42 A meta-analysis found salt reduction decreased systolic BP by 1.2 mm Hg and diastolic BP by 1.3 mm Hg.43 Though these benefits are small, reducing sodium intake can be one of several lifestyle modifications, such as increased activity and quitting smoking, that can reduce young patients’ risk of hypertension and related cardiovascular sequelae.
CORRESPONDENCE
Robert Gauer, MD, Womack Army Medical Center, 2817 Reilly Road, Fort Bragg NC 28310; robert.l.gauer2.civ@mail.mil
1. McNiece KL, Poffenbarger TS, Tuner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-644.e1.
2. Moore WE, Eichner JE, Cohn EM, et al. Blood pressure screening of school children in a multiracial school district: the Healthy Kids Project. Am J Hypertens. 2009;22:351-356.
3. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437-1439.
4. Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151-161.
5. Hansen ML, Gunn PW, Kaelber DC. Under diagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
6. Boneparth A, Flynn JT. Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila). 2009;48:44-49.
7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490.
8. Feld LG, Corey H. Hypertension in childhood. Pediatr Rev. 2007;28:283-298.
9. Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502-508.
10. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
11. de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226-234.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576.
13. Ramaswamy P, Lytrivi ID, Paul C, et al. Regression of left ventricular hypertrophy in children with antihypertensive therapy. Pediatr Nephrol. 2007;22:141-143.
14. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40-44.
15. Flynn JT. Pediatric hypertension update. Curr Opin Nephrol Hypertens. 2010;19:292-297.
16. Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156-1162.
17. Kupferman JC, Lande MB, Adams HR, et al. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401-408.
18. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review of meta-regression analysis. Circulation. 2008;117:3171-3180.
19. Hypertension. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 7, 2014.
20. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed October 10, 2012.
21. Growth Charts. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/growthcharts. Accessed July 31, 2012.
22. Podoll A, Grenier M, Croix B, et al. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538-e543.
23. Flynn JT, Pierce CB, Miller ER 3rd, et al; Chronic Kidney Disease in Children Study Group. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434-440.e.1.
24. Villar VA, Liu T, Jose PA. Recent trends in pediatric hypertension research. J Med Liban. 2010;58:179-184.
25. Swartz SJ, Srivaths PR, Croix B, et al. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177-1181.
26. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
27. Tullus K, Roebuck DJ, McLaren CA, et al. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25:1049-1056.
28. Flynn JT. Management of hypertension in the young: role of antihypertensive medications. J Cardiovasc Pharmacol. 2011;58:111-120.
29. ESCAPE Trial Group; Wühl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
30. Yoon EY, Cohn L, Rocchini A, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1-e8.
31. Blowey DL. Update on the pharmacologic treatment of hypertension in pediatrics. J Clin Hypertens (Greenwich). 2012;14:383-387.
32. Welch WP, Yang W, Taylor-Zapata P, et al. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens. 2012;14:388-395.
33. Lexicomp Pharmaceutical Reference, Version 1.8.3(155). Lexi-Comp Web site. Available at: http://online.lexi.com/crlsql/servlet/crlonline. Accessed July 31, 2012.
34. Robinson RF, Nahata MC, Batisky DL, et al. Pharmacologic treatment of chronic pediatric hypertension. Pediatr Drugs. 2005;7:27-40.
35. Hanevold CD. Concepts guiding therapy for hypertension in children. Expert Rev Cardiovasc Ther. 2009;7:647-657.
36. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614-627.
37. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319.
38. Simonetti GD, Rizzi M, Donadini R, et al. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25:2370-2376.
39. Lurbe E, Álvarez J, Redon J. Diagnosis and treatment of hypertension in children. Curr Hypertens Rep. 2010;12:480-486.
40. Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8-16.
41. McCambridge TM, Benjamin HJ, Breener JS, et al; Council on Sports Medicine and Fitness. Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 2010;125:1287-1294.
42. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services Web site. Available at: http://www.health.gov/PAguidelines/guidelines/default.aspx. Updated March 11, 2013. Accessed February 7, 2014.
43. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
› Screen for hypertension in all children over the age of 3 at every visit. C
› Order laboratory evaluation, echocardiography, and renovascular imaging for all children given a diagnosis of hypertension. C
› Advise parents that children with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics, but those with stage 2 hypertension, target-organ damage, or symptomatic hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling) until BP is well controlled. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood hypertension is on the rise: Recent data from the National Health and Nutrition Survey suggest 10% of children and adolescents have prehypertension and 4% have hypertension.1-4 Unfortunately, the condition often is missed. In a study of 14,187 children and adolescents who had at least 3 well-child visits at an outpatient academic medical center, 507 patients met the criteria for hypertension, yet only 131 (26%) had this diagnosis documented in their electronic health record.5
In a survey of 89 pediatricians, >50% of respondents said they were not familiar with the most current published recommendations for diagnosing and treating pediatric hypertension.6 Respondents also indicated that the most common reason for not initiating pharmacotherapy for children with hypertension was a lack of familiarity with appropriate antihypertensive agents (54%), followed by concern for adverse medication effects. Delayed diagnosis, evaluation, and treatment of hypertension in young patients can increase the likelihood of serious consequences, including target-organ damage such as left ventricular hypertrophy (LVH). In this review, we’ll describe the factors that put children and adolescents at risk for hypertension, and offer an evidence-based approach to diagnosis and treatment.
Obesity is a key risk factor
An estimated 17% of children aged 2 to 19 are obese.7 Obesity increases a child’s risk for hypertension by approximately 3- to 5-fold, and body mass index (BMI) is greater in children with primary hypertension compared with those with secondary hypertension.8 Hypertension is more common among Hispanic and non-Hispanic black male children and adolescents compared with their white counterparts; these ethnic disparities are not found in females.9,10 Poor diets and physical inactivity further contribute to obesity and hypertension risk. Children who were born preterm or had a very low birth weight also are at increased risk.11
Unchecked hypertension can lead to cardiac, vascular damage
Some children and adolescents with undiagnosed and untreated hypertension have evidence of target-organ damage, including cardiac dysfunction and pathologic vascular abnormalities. LVH is present in 20% to 41% of children and adolescents with hypertension.12,13 Carotid intima-media thickness, an established surrogate marker for atherosclerosis, is abnormally increased in children with hypertension, even after adjusting for BMI.14 Other target organ effects include impaired cognitive function, reduced glomerular filtration rate, microalbuminuria, and retinal arteriolar narrowing.15-17
Pediatric hypertension may persist into adulthood. A meta-analysis of more than 50 studies found that elevated blood pressure (BP) in childhood increases the risk for hypertension as an adult.18
NHLBI recommendations call for a BP check at every visit
The National Heart, Lung, and Blood Institute (NHLBI) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (“the 4th Report”) recommends measuring BP in all children over age 3 during every health care visit.12 Children under age 3 should have their BP checked in certain circumstances, including preterm delivery, congenital heart disease, recurrent urinary tract infections, renal/urologic disease, organ transplantation, malignancy, and systemic illnesses associated with hypertension.12 The 4th Report is endorsed by the American Academy of Pediatrics (AAP); however, the American Academy of Family Physicians and the US Preventive Services Task Force have concluded that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease (CVD).19,20
Does the child have hypertension? That depends on several factors
Determining whether a child has hypertension requires that you consult national BP standards to determine if he or she is within the normal range. Normal BP standards for children and adolescents are based on gender, age, and height percentile, and provide a precise classification based on body size.12 These tables are available from the NHLBI Web site at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. Height percentiles in these tables correspond with the Centers for Disease Control and Prevention (CDC) growth charts published in 2000.21 The Baylor College of Medicine Children’s Nutrition Research Center has a web-based calculator to help physicians determine BP percentiles in children and adolescents; it is available at http://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. The International Pediatric Hypertension Association also offers BP charts and calculators at http://www.iphapediatrichypertension.org.
The diagnostic parameters for pediatric hypertension are listed in TABLE 1.12 The higher systolic or diastolic BP percentile value is used to determine a child’s overall BP category. A child is considered normotensive if the BP is <90th percentile. Hypertension is an average systolic or diastolic BP that is ≥95th percentile on at least 3 separate occasions. Stage 1 hypertension is BP levels ranging from the 95th percentile to 5 mm Hg above the 99th percentile, and stage 2 hypertension is BP levels greater than 5 mm Hg above the 99th percentile.
For example, assume you are evaluating a 12-year-old boy who is 61 inches tall and has a BP of 129/87 mm Hg. According to the CDC growth charts, his height puts him in the 75th percentile for his age. Using the NHLBI chart, you determine that he falls in the 95th-99th percentile for BP, and thus, using the categories in TABLE 1, is given a diagnosis of Stage 1 hypertension.
Accurate BP measurement requires using an appropriate cuff size that covers 80% of the child’s upper arm. When the child is between cuff sizes, use the larger cuff because small cuffs overestimate BP readings. BP readings should be taken on the right arm with the arm supported at heart level after the child has been sitting quietly for at least 5 minutes.12 One study showed that the initial BP readings taken in the triage area were significantly higher—often by >10 mm Hg—compared with follow-up measurements in the examination room.22
The preferred method of BP measurement is auscultation; however, oscillometric devices also are acceptable. These devices are easier to use, help eliminate digit bias, and minimize observer variation, but they typically read approximately 6 to 9 mm Hg higher than auscultation.23 For any BP measurement obtained by oscillometry that is >90th percentile, repeat the measurement by auscultation at least twice during the same office visit, and use an average of the repeated measurements.12 Obtain measurements of a lower extremity when you suspect congenital heart disease (eg, aortic coarctation). For any patient in whom you confirm a BP measurement >95th percentile, repeat the measurement within 2 weeks; for BPs >99th percentile, reevaluation should occur within one week.
Ambulatory BP monitoring (ABPM). Because BP measurements have a circadian pattern (higher during the day and reduced by 10% during sleep24) an ABPM device that provides 50 to 60 readings over 24 hours can be useful when evaluating children and adolescents for white-coat hypertension (elevated clinic BP with normal ambulatory BP), masked hypertension (normal clinic BP with elevated ambulatory BP), prehypertension and secondary hypertension (BP generally does not follow circadian patterns).25 ABPM is more accurate than BP self-measurement, but usually is limited to children older than age 5
Steps to take for clinical evaluation
Start by conducting a thorough history and physical examination, looking for information that can help you select the most appropriate tests for the next phase of evaluation.8,12 Calculate the patient’s BMI to screen for obesity, ask about a family history of hypertension or CVD, and determine if the patient is taking any medications that might cause hypertension, such as amphetamines, corticosteroids, or cyclosporine.8 Assess for signs and symptoms that suggest an underlying disease, such as renal disease (hematuria, edema, fatigue) or heart disease (chest pain, exertional dyspnea, palpitations).12
All children diagnosed with hypertension should be screened for secondary causes (TABLE 2). The recommended evaluation is to obtain a renal function panel, electrolytes, urinalysis, urine culture, complete blood count, renovascular imaging, and echocardiogram.12 The most common etiologies for secondary hypertension are renal parenchymal disease (68%), renovascular abnormalities (10%), and endocrinopathies (10%).26 Other causes, such as aortic coarctation, obstructive sleep apnea, iatrogenic factors (eg, toxins, medications, drugs of abuse), and genitourinary abnormalities, account for only a small percentage of cases and should be investigated as clinically indicated.26
Renovascular assessment depends on facility expertise. Imaging options include renal ultrasound (with or without Doppler), computed tomography angiography, renal flow scan, and magnetic resonance angiography. These studies have similar sensitivities and specificities.27 For patients in whom you strongly suspect renovascular disease, renal arteriography (digital subtraction angiography) provides the best images, although it is the most invasive study.27
Refer children and adolescents who are found to have significant abnormalities during the initial evaluation to the appropriate specialist. BP measurements often improve when secondary causes are treated.
Which drugs for which patients?
Pharmacologic management is indicated for pediatric patients with stage 1 or stage 2 hypertension, secondary hypertension, and those with evidence of target-organ damage.12 The goal of therapy is to reduce BP to <95th percentile. In patients with target organ damage, renal disease, or diabetes mellitus, the goal is <90th percentile.12,15,28 Intensive management of BP (≤50th percentile) in children with chronic kidney disease has been shown to delay progression to renal failure,29 but it is uncertain if lower BP goals can slow or prevent additional subclinical target organ damage.
Pharmacotherapy for hypertensive children or adolescents can be challenging because recommendations of which medication to use are based upon expert opinion and extrapolation from randomized trials of adults. The length of therapy (often lifelong), potential adverse effects, and unproven direct mortality benefit complicate this decision. Medication choice usually is based on physician preference or experience.12 The most common antihypertensive drugs prescribed are angiotensin-converting enzyme (ACE) inhibitors (26%), followed by diuretics (20%), and beta-blockers (17%).30,31 The starting doses and other details of medications commonly used to treat pediatric hypertension are listed in TABLE 3.28,32-34
One approach to choosing an antihypertensive drug for children is to measure the patient’s ambulatory plasma renin activity (PRA) level before initiating therapy. Those with high PRA levels (>0.65 ng/mL/h), presumably due to peripheral vasoconstriction, may benefit more from ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-adrenergic antagonists.35 Individuals with low PRA levels (<0.65 ng/mL/h) maintain higher volume/sodium excess and may benefit more from diuretics or calcium channel blockers.35
Ethnicity also may guide medication selection. African American adults do not respond well to ACE inhibitor monotherapy due to decreased PRA and increased salt hypersensitivity.36 One meta-analysis found that African American children and adolescents had inadequate BP response to 6 individual ACE inhibitors, even at higher doses compared with white children and those of other ethnicities, who showed significant improvement in BP.37 ARBs may be a more effective alternative for this population.
Most experts recommend initiating a single agent at a low dose.12 A systematic review found that except for African American children, pediatric patients experienced comparable reductions in BP with ACE inhibitors (10.7/8.1 mm Hg), ARBs (10.5/6.9 mm Hg), and calcium channel blockers (9.3/7.2 mm Hg).38 In addition, ACE inhibitors and ARBs significantly reduced proteinuria by 49% and 59%, respectively.38
Schedule follow-up visits for 2 to 4 weeks (or sooner for patients with stage 2 hypertension) after initiating pharmacotherapy. If BP response is suboptimal, consider increasing the dose before adding a second agent. If the patient experiences significant adverse effects or has an inadequate BP response, changing to a drug from a different class is recommended.39 Patients who do not adequately respond to these approaches may require combination therapy; in such cases, strongly consider consultation with pediatric nephrologist or cardiologist.39 Medication compliance should be verified (eg, by pill counting, parental supervision) in patients who do not respond to therapy. Once BP control has been achieved, visits every 3 to 4 months are appropriate, with periodic laboratory monitoring, especially for children taking diuretics, ACE inhibitors, or ARBs or who have underlying renal disease.
Recommend exercise, but carefully monitor athlete's BP
Encourage obese and overweight children and adolescents to lose weight to maintain a BMI <95th percentile. Current guidelines based on expert opinion recommend that children and adolescents should engage in 60 minutes of daily physical activity.12 A meta-analysis found physical activity led to a 1% and 3% reduction in systolic and diastolic BP, respectively, although these results were not statistically significant.40
Be aware, however, that children and adolescents with hypertension who engage in certain competitive sports can significantly increase their BP and may be at risk for complications.41 According to the AAP guidelines, patients with stage 2 hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling, boxing, cycling, decathlon, triathlon) until BP is well controlled.41 Patients with target-organ damage, uncontrolled hypertension, or symptomatic hypertension should not participate until BP is well controlled. Patients with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics. Reassess BP every 6 months in patients who are prehypertensive and every one to 2 weeks for those with stage 1 hypertension. When the patient’s BP remains <90th percentile, routine surveillance every 3 to 6 months is recommended.
What about sodium? Encourage parents of pediatric patients with hypertension to limit their child’s salt intake to 1.2 g/d for those age 4 to 8 and 1.5 g/d for older children.42 A meta-analysis found salt reduction decreased systolic BP by 1.2 mm Hg and diastolic BP by 1.3 mm Hg.43 Though these benefits are small, reducing sodium intake can be one of several lifestyle modifications, such as increased activity and quitting smoking, that can reduce young patients’ risk of hypertension and related cardiovascular sequelae.
CORRESPONDENCE
Robert Gauer, MD, Womack Army Medical Center, 2817 Reilly Road, Fort Bragg NC 28310; robert.l.gauer2.civ@mail.mil
› Screen for hypertension in all children over the age of 3 at every visit. C
› Order laboratory evaluation, echocardiography, and renovascular imaging for all children given a diagnosis of hypertension. C
› Advise parents that children with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics, but those with stage 2 hypertension, target-organ damage, or symptomatic hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling) until BP is well controlled. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood hypertension is on the rise: Recent data from the National Health and Nutrition Survey suggest 10% of children and adolescents have prehypertension and 4% have hypertension.1-4 Unfortunately, the condition often is missed. In a study of 14,187 children and adolescents who had at least 3 well-child visits at an outpatient academic medical center, 507 patients met the criteria for hypertension, yet only 131 (26%) had this diagnosis documented in their electronic health record.5
In a survey of 89 pediatricians, >50% of respondents said they were not familiar with the most current published recommendations for diagnosing and treating pediatric hypertension.6 Respondents also indicated that the most common reason for not initiating pharmacotherapy for children with hypertension was a lack of familiarity with appropriate antihypertensive agents (54%), followed by concern for adverse medication effects. Delayed diagnosis, evaluation, and treatment of hypertension in young patients can increase the likelihood of serious consequences, including target-organ damage such as left ventricular hypertrophy (LVH). In this review, we’ll describe the factors that put children and adolescents at risk for hypertension, and offer an evidence-based approach to diagnosis and treatment.
Obesity is a key risk factor
An estimated 17% of children aged 2 to 19 are obese.7 Obesity increases a child’s risk for hypertension by approximately 3- to 5-fold, and body mass index (BMI) is greater in children with primary hypertension compared with those with secondary hypertension.8 Hypertension is more common among Hispanic and non-Hispanic black male children and adolescents compared with their white counterparts; these ethnic disparities are not found in females.9,10 Poor diets and physical inactivity further contribute to obesity and hypertension risk. Children who were born preterm or had a very low birth weight also are at increased risk.11
Unchecked hypertension can lead to cardiac, vascular damage
Some children and adolescents with undiagnosed and untreated hypertension have evidence of target-organ damage, including cardiac dysfunction and pathologic vascular abnormalities. LVH is present in 20% to 41% of children and adolescents with hypertension.12,13 Carotid intima-media thickness, an established surrogate marker for atherosclerosis, is abnormally increased in children with hypertension, even after adjusting for BMI.14 Other target organ effects include impaired cognitive function, reduced glomerular filtration rate, microalbuminuria, and retinal arteriolar narrowing.15-17
Pediatric hypertension may persist into adulthood. A meta-analysis of more than 50 studies found that elevated blood pressure (BP) in childhood increases the risk for hypertension as an adult.18
NHLBI recommendations call for a BP check at every visit
The National Heart, Lung, and Blood Institute (NHLBI) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (“the 4th Report”) recommends measuring BP in all children over age 3 during every health care visit.12 Children under age 3 should have their BP checked in certain circumstances, including preterm delivery, congenital heart disease, recurrent urinary tract infections, renal/urologic disease, organ transplantation, malignancy, and systemic illnesses associated with hypertension.12 The 4th Report is endorsed by the American Academy of Pediatrics (AAP); however, the American Academy of Family Physicians and the US Preventive Services Task Force have concluded that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease (CVD).19,20
Does the child have hypertension? That depends on several factors
Determining whether a child has hypertension requires that you consult national BP standards to determine if he or she is within the normal range. Normal BP standards for children and adolescents are based on gender, age, and height percentile, and provide a precise classification based on body size.12 These tables are available from the NHLBI Web site at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. Height percentiles in these tables correspond with the Centers for Disease Control and Prevention (CDC) growth charts published in 2000.21 The Baylor College of Medicine Children’s Nutrition Research Center has a web-based calculator to help physicians determine BP percentiles in children and adolescents; it is available at http://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. The International Pediatric Hypertension Association also offers BP charts and calculators at http://www.iphapediatrichypertension.org.
The diagnostic parameters for pediatric hypertension are listed in TABLE 1.12 The higher systolic or diastolic BP percentile value is used to determine a child’s overall BP category. A child is considered normotensive if the BP is <90th percentile. Hypertension is an average systolic or diastolic BP that is ≥95th percentile on at least 3 separate occasions. Stage 1 hypertension is BP levels ranging from the 95th percentile to 5 mm Hg above the 99th percentile, and stage 2 hypertension is BP levels greater than 5 mm Hg above the 99th percentile.
For example, assume you are evaluating a 12-year-old boy who is 61 inches tall and has a BP of 129/87 mm Hg. According to the CDC growth charts, his height puts him in the 75th percentile for his age. Using the NHLBI chart, you determine that he falls in the 95th-99th percentile for BP, and thus, using the categories in TABLE 1, is given a diagnosis of Stage 1 hypertension.
Accurate BP measurement requires using an appropriate cuff size that covers 80% of the child’s upper arm. When the child is between cuff sizes, use the larger cuff because small cuffs overestimate BP readings. BP readings should be taken on the right arm with the arm supported at heart level after the child has been sitting quietly for at least 5 minutes.12 One study showed that the initial BP readings taken in the triage area were significantly higher—often by >10 mm Hg—compared with follow-up measurements in the examination room.22
The preferred method of BP measurement is auscultation; however, oscillometric devices also are acceptable. These devices are easier to use, help eliminate digit bias, and minimize observer variation, but they typically read approximately 6 to 9 mm Hg higher than auscultation.23 For any BP measurement obtained by oscillometry that is >90th percentile, repeat the measurement by auscultation at least twice during the same office visit, and use an average of the repeated measurements.12 Obtain measurements of a lower extremity when you suspect congenital heart disease (eg, aortic coarctation). For any patient in whom you confirm a BP measurement >95th percentile, repeat the measurement within 2 weeks; for BPs >99th percentile, reevaluation should occur within one week.
Ambulatory BP monitoring (ABPM). Because BP measurements have a circadian pattern (higher during the day and reduced by 10% during sleep24) an ABPM device that provides 50 to 60 readings over 24 hours can be useful when evaluating children and adolescents for white-coat hypertension (elevated clinic BP with normal ambulatory BP), masked hypertension (normal clinic BP with elevated ambulatory BP), prehypertension and secondary hypertension (BP generally does not follow circadian patterns).25 ABPM is more accurate than BP self-measurement, but usually is limited to children older than age 5
Steps to take for clinical evaluation
Start by conducting a thorough history and physical examination, looking for information that can help you select the most appropriate tests for the next phase of evaluation.8,12 Calculate the patient’s BMI to screen for obesity, ask about a family history of hypertension or CVD, and determine if the patient is taking any medications that might cause hypertension, such as amphetamines, corticosteroids, or cyclosporine.8 Assess for signs and symptoms that suggest an underlying disease, such as renal disease (hematuria, edema, fatigue) or heart disease (chest pain, exertional dyspnea, palpitations).12
All children diagnosed with hypertension should be screened for secondary causes (TABLE 2). The recommended evaluation is to obtain a renal function panel, electrolytes, urinalysis, urine culture, complete blood count, renovascular imaging, and echocardiogram.12 The most common etiologies for secondary hypertension are renal parenchymal disease (68%), renovascular abnormalities (10%), and endocrinopathies (10%).26 Other causes, such as aortic coarctation, obstructive sleep apnea, iatrogenic factors (eg, toxins, medications, drugs of abuse), and genitourinary abnormalities, account for only a small percentage of cases and should be investigated as clinically indicated.26
Renovascular assessment depends on facility expertise. Imaging options include renal ultrasound (with or without Doppler), computed tomography angiography, renal flow scan, and magnetic resonance angiography. These studies have similar sensitivities and specificities.27 For patients in whom you strongly suspect renovascular disease, renal arteriography (digital subtraction angiography) provides the best images, although it is the most invasive study.27
Refer children and adolescents who are found to have significant abnormalities during the initial evaluation to the appropriate specialist. BP measurements often improve when secondary causes are treated.
Which drugs for which patients?
Pharmacologic management is indicated for pediatric patients with stage 1 or stage 2 hypertension, secondary hypertension, and those with evidence of target-organ damage.12 The goal of therapy is to reduce BP to <95th percentile. In patients with target organ damage, renal disease, or diabetes mellitus, the goal is <90th percentile.12,15,28 Intensive management of BP (≤50th percentile) in children with chronic kidney disease has been shown to delay progression to renal failure,29 but it is uncertain if lower BP goals can slow or prevent additional subclinical target organ damage.
Pharmacotherapy for hypertensive children or adolescents can be challenging because recommendations of which medication to use are based upon expert opinion and extrapolation from randomized trials of adults. The length of therapy (often lifelong), potential adverse effects, and unproven direct mortality benefit complicate this decision. Medication choice usually is based on physician preference or experience.12 The most common antihypertensive drugs prescribed are angiotensin-converting enzyme (ACE) inhibitors (26%), followed by diuretics (20%), and beta-blockers (17%).30,31 The starting doses and other details of medications commonly used to treat pediatric hypertension are listed in TABLE 3.28,32-34
One approach to choosing an antihypertensive drug for children is to measure the patient’s ambulatory plasma renin activity (PRA) level before initiating therapy. Those with high PRA levels (>0.65 ng/mL/h), presumably due to peripheral vasoconstriction, may benefit more from ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-adrenergic antagonists.35 Individuals with low PRA levels (<0.65 ng/mL/h) maintain higher volume/sodium excess and may benefit more from diuretics or calcium channel blockers.35
Ethnicity also may guide medication selection. African American adults do not respond well to ACE inhibitor monotherapy due to decreased PRA and increased salt hypersensitivity.36 One meta-analysis found that African American children and adolescents had inadequate BP response to 6 individual ACE inhibitors, even at higher doses compared with white children and those of other ethnicities, who showed significant improvement in BP.37 ARBs may be a more effective alternative for this population.
Most experts recommend initiating a single agent at a low dose.12 A systematic review found that except for African American children, pediatric patients experienced comparable reductions in BP with ACE inhibitors (10.7/8.1 mm Hg), ARBs (10.5/6.9 mm Hg), and calcium channel blockers (9.3/7.2 mm Hg).38 In addition, ACE inhibitors and ARBs significantly reduced proteinuria by 49% and 59%, respectively.38
Schedule follow-up visits for 2 to 4 weeks (or sooner for patients with stage 2 hypertension) after initiating pharmacotherapy. If BP response is suboptimal, consider increasing the dose before adding a second agent. If the patient experiences significant adverse effects or has an inadequate BP response, changing to a drug from a different class is recommended.39 Patients who do not adequately respond to these approaches may require combination therapy; in such cases, strongly consider consultation with pediatric nephrologist or cardiologist.39 Medication compliance should be verified (eg, by pill counting, parental supervision) in patients who do not respond to therapy. Once BP control has been achieved, visits every 3 to 4 months are appropriate, with periodic laboratory monitoring, especially for children taking diuretics, ACE inhibitors, or ARBs or who have underlying renal disease.
Recommend exercise, but carefully monitor athlete's BP
Encourage obese and overweight children and adolescents to lose weight to maintain a BMI <95th percentile. Current guidelines based on expert opinion recommend that children and adolescents should engage in 60 minutes of daily physical activity.12 A meta-analysis found physical activity led to a 1% and 3% reduction in systolic and diastolic BP, respectively, although these results were not statistically significant.40
Be aware, however, that children and adolescents with hypertension who engage in certain competitive sports can significantly increase their BP and may be at risk for complications.41 According to the AAP guidelines, patients with stage 2 hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling, boxing, cycling, decathlon, triathlon) until BP is well controlled.41 Patients with target-organ damage, uncontrolled hypertension, or symptomatic hypertension should not participate until BP is well controlled. Patients with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics. Reassess BP every 6 months in patients who are prehypertensive and every one to 2 weeks for those with stage 1 hypertension. When the patient’s BP remains <90th percentile, routine surveillance every 3 to 6 months is recommended.
What about sodium? Encourage parents of pediatric patients with hypertension to limit their child’s salt intake to 1.2 g/d for those age 4 to 8 and 1.5 g/d for older children.42 A meta-analysis found salt reduction decreased systolic BP by 1.2 mm Hg and diastolic BP by 1.3 mm Hg.43 Though these benefits are small, reducing sodium intake can be one of several lifestyle modifications, such as increased activity and quitting smoking, that can reduce young patients’ risk of hypertension and related cardiovascular sequelae.
CORRESPONDENCE
Robert Gauer, MD, Womack Army Medical Center, 2817 Reilly Road, Fort Bragg NC 28310; robert.l.gauer2.civ@mail.mil
1. McNiece KL, Poffenbarger TS, Tuner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-644.e1.
2. Moore WE, Eichner JE, Cohn EM, et al. Blood pressure screening of school children in a multiracial school district: the Healthy Kids Project. Am J Hypertens. 2009;22:351-356.
3. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437-1439.
4. Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151-161.
5. Hansen ML, Gunn PW, Kaelber DC. Under diagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
6. Boneparth A, Flynn JT. Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila). 2009;48:44-49.
7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490.
8. Feld LG, Corey H. Hypertension in childhood. Pediatr Rev. 2007;28:283-298.
9. Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502-508.
10. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
11. de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226-234.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576.
13. Ramaswamy P, Lytrivi ID, Paul C, et al. Regression of left ventricular hypertrophy in children with antihypertensive therapy. Pediatr Nephrol. 2007;22:141-143.
14. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40-44.
15. Flynn JT. Pediatric hypertension update. Curr Opin Nephrol Hypertens. 2010;19:292-297.
16. Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156-1162.
17. Kupferman JC, Lande MB, Adams HR, et al. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401-408.
18. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review of meta-regression analysis. Circulation. 2008;117:3171-3180.
19. Hypertension. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 7, 2014.
20. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed October 10, 2012.
21. Growth Charts. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/growthcharts. Accessed July 31, 2012.
22. Podoll A, Grenier M, Croix B, et al. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538-e543.
23. Flynn JT, Pierce CB, Miller ER 3rd, et al; Chronic Kidney Disease in Children Study Group. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434-440.e.1.
24. Villar VA, Liu T, Jose PA. Recent trends in pediatric hypertension research. J Med Liban. 2010;58:179-184.
25. Swartz SJ, Srivaths PR, Croix B, et al. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177-1181.
26. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
27. Tullus K, Roebuck DJ, McLaren CA, et al. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25:1049-1056.
28. Flynn JT. Management of hypertension in the young: role of antihypertensive medications. J Cardiovasc Pharmacol. 2011;58:111-120.
29. ESCAPE Trial Group; Wühl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
30. Yoon EY, Cohn L, Rocchini A, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1-e8.
31. Blowey DL. Update on the pharmacologic treatment of hypertension in pediatrics. J Clin Hypertens (Greenwich). 2012;14:383-387.
32. Welch WP, Yang W, Taylor-Zapata P, et al. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens. 2012;14:388-395.
33. Lexicomp Pharmaceutical Reference, Version 1.8.3(155). Lexi-Comp Web site. Available at: http://online.lexi.com/crlsql/servlet/crlonline. Accessed July 31, 2012.
34. Robinson RF, Nahata MC, Batisky DL, et al. Pharmacologic treatment of chronic pediatric hypertension. Pediatr Drugs. 2005;7:27-40.
35. Hanevold CD. Concepts guiding therapy for hypertension in children. Expert Rev Cardiovasc Ther. 2009;7:647-657.
36. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614-627.
37. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319.
38. Simonetti GD, Rizzi M, Donadini R, et al. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25:2370-2376.
39. Lurbe E, Álvarez J, Redon J. Diagnosis and treatment of hypertension in children. Curr Hypertens Rep. 2010;12:480-486.
40. Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8-16.
41. McCambridge TM, Benjamin HJ, Breener JS, et al; Council on Sports Medicine and Fitness. Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 2010;125:1287-1294.
42. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services Web site. Available at: http://www.health.gov/PAguidelines/guidelines/default.aspx. Updated March 11, 2013. Accessed February 7, 2014.
43. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
1. McNiece KL, Poffenbarger TS, Tuner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-644.e1.
2. Moore WE, Eichner JE, Cohn EM, et al. Blood pressure screening of school children in a multiracial school district: the Healthy Kids Project. Am J Hypertens. 2009;22:351-356.
3. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437-1439.
4. Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151-161.
5. Hansen ML, Gunn PW, Kaelber DC. Under diagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
6. Boneparth A, Flynn JT. Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila). 2009;48:44-49.
7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490.
8. Feld LG, Corey H. Hypertension in childhood. Pediatr Rev. 2007;28:283-298.
9. Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502-508.
10. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
11. de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226-234.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576.
13. Ramaswamy P, Lytrivi ID, Paul C, et al. Regression of left ventricular hypertrophy in children with antihypertensive therapy. Pediatr Nephrol. 2007;22:141-143.
14. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40-44.
15. Flynn JT. Pediatric hypertension update. Curr Opin Nephrol Hypertens. 2010;19:292-297.
16. Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156-1162.
17. Kupferman JC, Lande MB, Adams HR, et al. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401-408.
18. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review of meta-regression analysis. Circulation. 2008;117:3171-3180.
19. Hypertension. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 7, 2014.
20. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed October 10, 2012.
21. Growth Charts. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/growthcharts. Accessed July 31, 2012.
22. Podoll A, Grenier M, Croix B, et al. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538-e543.
23. Flynn JT, Pierce CB, Miller ER 3rd, et al; Chronic Kidney Disease in Children Study Group. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434-440.e.1.
24. Villar VA, Liu T, Jose PA. Recent trends in pediatric hypertension research. J Med Liban. 2010;58:179-184.
25. Swartz SJ, Srivaths PR, Croix B, et al. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177-1181.
26. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
27. Tullus K, Roebuck DJ, McLaren CA, et al. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25:1049-1056.
28. Flynn JT. Management of hypertension in the young: role of antihypertensive medications. J Cardiovasc Pharmacol. 2011;58:111-120.
29. ESCAPE Trial Group; Wühl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
30. Yoon EY, Cohn L, Rocchini A, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1-e8.
31. Blowey DL. Update on the pharmacologic treatment of hypertension in pediatrics. J Clin Hypertens (Greenwich). 2012;14:383-387.
32. Welch WP, Yang W, Taylor-Zapata P, et al. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens. 2012;14:388-395.
33. Lexicomp Pharmaceutical Reference, Version 1.8.3(155). Lexi-Comp Web site. Available at: http://online.lexi.com/crlsql/servlet/crlonline. Accessed July 31, 2012.
34. Robinson RF, Nahata MC, Batisky DL, et al. Pharmacologic treatment of chronic pediatric hypertension. Pediatr Drugs. 2005;7:27-40.
35. Hanevold CD. Concepts guiding therapy for hypertension in children. Expert Rev Cardiovasc Ther. 2009;7:647-657.
36. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614-627.
37. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319.
38. Simonetti GD, Rizzi M, Donadini R, et al. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25:2370-2376.
39. Lurbe E, Álvarez J, Redon J. Diagnosis and treatment of hypertension in children. Curr Hypertens Rep. 2010;12:480-486.
40. Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8-16.
41. McCambridge TM, Benjamin HJ, Breener JS, et al; Council on Sports Medicine and Fitness. Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 2010;125:1287-1294.
42. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services Web site. Available at: http://www.health.gov/PAguidelines/guidelines/default.aspx. Updated March 11, 2013. Accessed February 7, 2014.
43. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
Elevated troponin but no CVD: What’s the prognosis?
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
Patients with elevated troponin levels and chronic renal disease, pulmonary hypertension, pulmonary embolism, chronic obstructive pulmonary disease, sepsis, or acute ischemic stroke have a 2- to 5-fold increased risk of death, even in the absence of known cardiovascular disease (TABLE)1-6 (strength of recommendation: B, meta-analysis, multiple prospective and retrospective observational studies.)
EVIDENCE SUMMARY
To investigate the prognostic value of troponin on overall mortality, a multicenter prospective study followed 847 patients 18 years and older (mean age 59 years) with end-stage renal disease whose troponin T levels were measured 3 months from the start of peritoneal dialysis or hemodialysis until transplantation or death.1 At enrollment, 566 patients had a troponin level of ≤0.04 ng/dL, 188 had a value between 0.05 and 0.10 ng/dL, and 93 had a level of more than 0.10 ng/dL.
Using Cox regression, patients whose troponin levels were more than 0.10 ng/dL had an increased hazard ratio (HR) for all-cause mortality of 2.2 (95% confidence interval [CI], 1.7-2.8) compared with patients who had levels ≤0.04 ng/dL. Cardiovascular mortality also was higher (HR=1.9; 95% CI, 0.9-3.7) with troponin elevations, but didn’t reach statistical significance. Investigators found no significant differences in mortality risk between patients on peritoneal or hemodialysis, patients with or without a history of acute myocardial infarction, or patients who suffered cerebrovascular accidents.
Elevated troponin raises risk of death 5-fold in pulmonary embolism patients
A meta-analysis of 20 trials with a total of 1985 patients assessed the prognostic value of troponin for short-term mortality in patients admitted with acute pulmonary embolism.2 Sixteen studies (1527 patients) were prospective trials and the remainder (458 patients) were retrospective trials. Investigators obtained troponin levels for all patients at admission. They used several different troponin assays (both I and T), but most of the studies used the assay manufacturers’ cutoff points (exceeding the 99th percentile).
High troponin levels were associated with a 5-fold increased risk of short-term death, defined as in-hospital death up to 30 days after discharge (19.7% with elevated troponin vs 3.7% with normal troponin; odds ratio [OR]=5.24; 95% CI, 3.3-8.4).
Increased risk of death among those with pulmonary hypertension, COPD A prospective single-center study of 56 patients with chronic pulmonary hypertension found that the 14% of those whose troponin T was elevated (≥0.01 ng/mL) had a lower survival rate than the other patients. Patients who either had a positive troponin on initial assessment or developed troponin elevation within the 2-year follow-up period had a cumulative 24-month survival rate of 29%, compared with 81% for their troponin T-negative counterparts (P=.001).3
Patients with elevated troponin levels and certain conditions have a 2- to 5-fold increased risk of death, even without known cardiovascular disease.
Elevated troponin I is an independent predictor of mortality in severe sepsis
A double-blind, placebo-controlled, phase 3 trial evaluated the effect of drotrecogin alfa (activated)—withdrawn from the market in 2011—on survival of patients with severe sepsis.5 Investigators used positive troponin I levels (≥0.06 ng/mL) as a prognostic indicator of mortality. Patients who were troponin-positive had a 28-day mortality rate of 32%, compared with 14% in the troponin-negative group (P<.0001).
A bias of this study is that the patients with positive troponin levels tended to be older and more critically ill. However, in a multivariate model, troponin I still remained an independent predictor of mortality.
Elevated troponin predicts increased death risk in up to 20% of stroke patients
A systematic review of 15 trials with a total of 2901 patients evaluated the relationship between troponin levels and stroke.6 Investigators assessed the prevalence of elevated troponin in acute stroke patients, the association of elevated troponin levels with electrocardiographic changes, and the overall morbidity and mortality associated with troponin levels. Thirteen of the 15 studies used a troponin T or I level obtained within 72 hours of admission and a cut-off level of 0.1 ng/mL. The remaining 2 studies used troponin I cut-off levels >0.2 and 0.4 ng/mL.
Overall, 18% of acute stroke patients had elevated troponin levels. Studies that excluded patients with known cardiac disease had a lower prevalence of elevated levels (10% vs 22%). Patients with elevated troponin levels had an associated overall increased risk of death (OR=2.9; 95% CI, 1.7-4.8) and were 3 times more likely to have ischemic changes on electrocardiogram (OR=3.0; 95% CI, 1.5-6.2). Investigators concluded that elevated troponin levels occur in as many as one in 5 patients and are associated with an increased risk of death.
Troponin elevations may be observed in congestive heart failure, chest wall trauma, cardioversion/defibrillator shocks, rhabdomyolysis, and ultra-endurance activities.7 However, this analysis didn’t address prognostic implications of elevated troponins.
RECOMMENDATIONS
No recommendation exists for biochemical testing of troponins in various medical conditions except in the presence of signs and symptoms consistent with acute coronary syndrome. The American College of Cardiology and American Heart Association recommend routine testing of cardiac troponins in patients hospitalized for worsening congestive heart failure symptoms.8
The European Society of Cardiology recommends measuring troponin levels to further stratify risk in non-high-risk patients with confirmed pulmonary embolus.9
The National Academy of Clinical Biochemistry recommends using cardiac troponins to help define mortality risk in end-stage renal disease and critically ill patients.10
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
1. Havekes B, van Manen J, Krediet R, et al. Serum troponin T concentration as a predictor of mortality in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2006;47:823-829.
2. Becattini C, Vedovati MC, Agnelli G. Prognostic value of tropo- nins in acute pulmonary embolism. Circulation. 2007;116:427- 433.
3. Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848.
4. Brekke PH, Omland T, Holmedal SH, et al. Troponin T eleva- tion and long-term mortality after chronic obstructive pulmo- nary disease exacerbation. Eur Respir J. 2008;31:563-570.
5. John J, Woodward DB, Wang Y, et al. Troponin I as a prog- nosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270-275.
6. Kerr G, Ray G, Wu O, et al. Elevated troponin after stroke: a sys- tematic review. Cerebrovasc Dis. 2009;28:220-226.
7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of el- evated troponins. Heart. 2006;92:987-993.
8. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diag- nosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines devel- oped in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
9. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. Eur Heart J. 2008;29:2276-2315.
10. Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086-2096.
Evidence-based answers from the Family Physicians Inquiries Network
Does blood pressure screening benefit children?
SCREENING MAY NOT SHOW BENEFITS in childhood but could pay off for adults. Although major professional organizations recommend measuring blood pressure (BP) at every clinic visit for all children older than 3 years (strength of recommendation [SOR]: C, expert opinion), scant evidence links earlier detection and treatment of childhood hypertension with improved patient-oriented outcomes.
However, detecting childhood hypertension may help identify adults who would benefit from earlier treatment. Children with elevated BP have a more than 60% chance of being hypertensive as young adults (SOR: B, prospective cohort study). Children with systolic BP above the 95th percentile had a more than 4-fold increase in coronary artery disease as adults compared with children below the 95th percentile (SOR: B, retrospective study).
Identifying hypertension in children is associated with a 15-fold greater likelihood of hypertension in their parents (SOR: B, case series).
Evidence summary
The US Preventive Services Task Force considers screening adults for hypertension a grade A recommendation because it’s known to improve patient outcomes through early diagnosis, treatment, and prevention of serious cardiovascular complications.1
The Fourth Task Force of the National High Blood Pressure Education Program Working Group, endorsed by the American Academy of Pediatrics, states that maintaining a large national database of BP values throughout childhood allows physicians to recognize children and adolescents with elevated BP.2 Data indicate that, in this population, the prevalence of prehypertension is 10% and the prevalence of hypertension is 4%.3
The Task Force suggests that detecting and treating childhood hypertension should be important because of increasing childhood obesity, the risk of developing left ventricular hypertrophy, and other intermediate cardiovascular effects in undiagnosed and untreated children. The Task Force acknowledges, however, that prospective longitudinal outcome studies in untreated children and adolescents are lacking.
Hypertensive children often grow up to be hypertensive adults
A prospective cohort study showed that children with elevated BP had a greater likelihood of adult hypertension than children with normal BP. Investigators followed 2445 children 7 to 18 years of age to determine whether elevated BP in childhood correlated with increased BP in adulthood.
Investigators obtained BP, height, and weight measurements biennially during the children’s school years and when they were young adults between 20 and 30 years of age. Twelve to 13 years later, 24% of children with BP above the 90th percentile still had BP above the 90th percentile (relative risk [RR]=2.4; P<.001) and 39% had BP above the 80th percentile (RR=1.9; P<.001). Ninety-four percent of children with more than 3 normal readings during the study were normotensive as young adults. Children with one or 2 abnormal readings had a 17% and 24% chance, respectively, of having hypertension as adults (P<.001).4
High childhood systolic BP may predict CAD in adulthood
A retrospective study evaluated 126 children 10 to 17 years of age who were admitted to the hospital for an elective surgical procedure between 1950 and 1967. Children with documented BP readings at admission were eligible for the study; children with preexisting cardiac and renal disease were excluded. Investigators reassessed patients as adults (age range 42-68 years); the mean follow-up period was 42 years.
Mean BP was 125/80 mm Hg at admission and 133/75 mm Hg at follow-up. Univariate logistic regression analysis showed a significant association between systolic BP in childhood and coronary artery disease at follow-up (odds ratio [OR]=1.052; 95% confidence interval [CI], 1.005-1.101; P=.027). Children with systolic BP at or above the 95th percentile had a 4-fold increase in coronary artery disease at follow-up compared with children whose systolic BP was below the 95th percentile (29% vs 7%, P=.03). Investigators also found an association between elevated BP in childhood and a diagnosis of hypertension at follow-up (P=.007).
Limitations of the study included small sample size, selection bias, changes in the definition of hypertension during the 4 decades since the study began, and limited childhood BP data (a single measurement at admission for surgery).5
Parents of hypertensive children are likely to be hypertensive themselves
Screening BP in children has the potential to identify families at increased risk for cardiovascular disease. A case series found a high incidence of hypertension among the parents of children with elevated BP. Investigators measured several risk factors, including BP in 141 children (mean age 10.5±3.4 years) and 108 parents (at least one a biological parent, mean age 38.5±7.5 years). They obtained 2 BP readings 15 to 30 minutes apart.
Parents of children with BPs at or above the 95th percentile had a 15-fold greater likelihood of hypertension themselves (OR=14.7; 95% CI, 3.02-71.56; P=.009, positive predictive value=75%; negative predictive value=81%).6 Limitations of the study included small sample size, high prevalence of obesity and black ethnicity in the study population (a population with a greater incidence of hypertension), and only 2 BP measurements in the same day, which isn’t diagnostic for hypertension.
Recommendations
The American College of Obstetricians and Gynecologists recommends screening girls for hypertension between 13 and 15 years of age.7
The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease.8
The European Society of Hypertension and European Society of Cardiology recommend that children older than 3 years have auscultatory BP measurements at each clinic visit.9
1. US Preventive Services Task Force. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed June 19, 2012.
2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl):555-576.
3. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
4. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633-641.
5. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323-328.
6. Reis EC, Kip KE, Marroquin OC, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789-e1797.
7. American Congress of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 452: Primary and preventive care: periodic assessments. Obstet Gynecol. 2009;114:1444-1451.
8. American Academy of Family Physicians. Hypertension. Available at http://www.aafp.org/online/en/home/clinical/exam/hypertension.html. Accessed April 16, 2012.
9. Lurbe E, Cifkova R, Cruickshank J, et al. Management of high blood pressure in children and adolescents: recommendation of the European Society of Hypertension. J Hypertens. 2009;27:1719-1742.
SCREENING MAY NOT SHOW BENEFITS in childhood but could pay off for adults. Although major professional organizations recommend measuring blood pressure (BP) at every clinic visit for all children older than 3 years (strength of recommendation [SOR]: C, expert opinion), scant evidence links earlier detection and treatment of childhood hypertension with improved patient-oriented outcomes.
However, detecting childhood hypertension may help identify adults who would benefit from earlier treatment. Children with elevated BP have a more than 60% chance of being hypertensive as young adults (SOR: B, prospective cohort study). Children with systolic BP above the 95th percentile had a more than 4-fold increase in coronary artery disease as adults compared with children below the 95th percentile (SOR: B, retrospective study).
Identifying hypertension in children is associated with a 15-fold greater likelihood of hypertension in their parents (SOR: B, case series).
Evidence summary
The US Preventive Services Task Force considers screening adults for hypertension a grade A recommendation because it’s known to improve patient outcomes through early diagnosis, treatment, and prevention of serious cardiovascular complications.1
The Fourth Task Force of the National High Blood Pressure Education Program Working Group, endorsed by the American Academy of Pediatrics, states that maintaining a large national database of BP values throughout childhood allows physicians to recognize children and adolescents with elevated BP.2 Data indicate that, in this population, the prevalence of prehypertension is 10% and the prevalence of hypertension is 4%.3
The Task Force suggests that detecting and treating childhood hypertension should be important because of increasing childhood obesity, the risk of developing left ventricular hypertrophy, and other intermediate cardiovascular effects in undiagnosed and untreated children. The Task Force acknowledges, however, that prospective longitudinal outcome studies in untreated children and adolescents are lacking.
Hypertensive children often grow up to be hypertensive adults
A prospective cohort study showed that children with elevated BP had a greater likelihood of adult hypertension than children with normal BP. Investigators followed 2445 children 7 to 18 years of age to determine whether elevated BP in childhood correlated with increased BP in adulthood.
Investigators obtained BP, height, and weight measurements biennially during the children’s school years and when they were young adults between 20 and 30 years of age. Twelve to 13 years later, 24% of children with BP above the 90th percentile still had BP above the 90th percentile (relative risk [RR]=2.4; P<.001) and 39% had BP above the 80th percentile (RR=1.9; P<.001). Ninety-four percent of children with more than 3 normal readings during the study were normotensive as young adults. Children with one or 2 abnormal readings had a 17% and 24% chance, respectively, of having hypertension as adults (P<.001).4
High childhood systolic BP may predict CAD in adulthood
A retrospective study evaluated 126 children 10 to 17 years of age who were admitted to the hospital for an elective surgical procedure between 1950 and 1967. Children with documented BP readings at admission were eligible for the study; children with preexisting cardiac and renal disease were excluded. Investigators reassessed patients as adults (age range 42-68 years); the mean follow-up period was 42 years.
Mean BP was 125/80 mm Hg at admission and 133/75 mm Hg at follow-up. Univariate logistic regression analysis showed a significant association between systolic BP in childhood and coronary artery disease at follow-up (odds ratio [OR]=1.052; 95% confidence interval [CI], 1.005-1.101; P=.027). Children with systolic BP at or above the 95th percentile had a 4-fold increase in coronary artery disease at follow-up compared with children whose systolic BP was below the 95th percentile (29% vs 7%, P=.03). Investigators also found an association between elevated BP in childhood and a diagnosis of hypertension at follow-up (P=.007).
Limitations of the study included small sample size, selection bias, changes in the definition of hypertension during the 4 decades since the study began, and limited childhood BP data (a single measurement at admission for surgery).5
Parents of hypertensive children are likely to be hypertensive themselves
Screening BP in children has the potential to identify families at increased risk for cardiovascular disease. A case series found a high incidence of hypertension among the parents of children with elevated BP. Investigators measured several risk factors, including BP in 141 children (mean age 10.5±3.4 years) and 108 parents (at least one a biological parent, mean age 38.5±7.5 years). They obtained 2 BP readings 15 to 30 minutes apart.
Parents of children with BPs at or above the 95th percentile had a 15-fold greater likelihood of hypertension themselves (OR=14.7; 95% CI, 3.02-71.56; P=.009, positive predictive value=75%; negative predictive value=81%).6 Limitations of the study included small sample size, high prevalence of obesity and black ethnicity in the study population (a population with a greater incidence of hypertension), and only 2 BP measurements in the same day, which isn’t diagnostic for hypertension.
Recommendations
The American College of Obstetricians and Gynecologists recommends screening girls for hypertension between 13 and 15 years of age.7
The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease.8
The European Society of Hypertension and European Society of Cardiology recommend that children older than 3 years have auscultatory BP measurements at each clinic visit.9
SCREENING MAY NOT SHOW BENEFITS in childhood but could pay off for adults. Although major professional organizations recommend measuring blood pressure (BP) at every clinic visit for all children older than 3 years (strength of recommendation [SOR]: C, expert opinion), scant evidence links earlier detection and treatment of childhood hypertension with improved patient-oriented outcomes.
However, detecting childhood hypertension may help identify adults who would benefit from earlier treatment. Children with elevated BP have a more than 60% chance of being hypertensive as young adults (SOR: B, prospective cohort study). Children with systolic BP above the 95th percentile had a more than 4-fold increase in coronary artery disease as adults compared with children below the 95th percentile (SOR: B, retrospective study).
Identifying hypertension in children is associated with a 15-fold greater likelihood of hypertension in their parents (SOR: B, case series).
Evidence summary
The US Preventive Services Task Force considers screening adults for hypertension a grade A recommendation because it’s known to improve patient outcomes through early diagnosis, treatment, and prevention of serious cardiovascular complications.1
The Fourth Task Force of the National High Blood Pressure Education Program Working Group, endorsed by the American Academy of Pediatrics, states that maintaining a large national database of BP values throughout childhood allows physicians to recognize children and adolescents with elevated BP.2 Data indicate that, in this population, the prevalence of prehypertension is 10% and the prevalence of hypertension is 4%.3
The Task Force suggests that detecting and treating childhood hypertension should be important because of increasing childhood obesity, the risk of developing left ventricular hypertrophy, and other intermediate cardiovascular effects in undiagnosed and untreated children. The Task Force acknowledges, however, that prospective longitudinal outcome studies in untreated children and adolescents are lacking.
Hypertensive children often grow up to be hypertensive adults
A prospective cohort study showed that children with elevated BP had a greater likelihood of adult hypertension than children with normal BP. Investigators followed 2445 children 7 to 18 years of age to determine whether elevated BP in childhood correlated with increased BP in adulthood.
Investigators obtained BP, height, and weight measurements biennially during the children’s school years and when they were young adults between 20 and 30 years of age. Twelve to 13 years later, 24% of children with BP above the 90th percentile still had BP above the 90th percentile (relative risk [RR]=2.4; P<.001) and 39% had BP above the 80th percentile (RR=1.9; P<.001). Ninety-four percent of children with more than 3 normal readings during the study were normotensive as young adults. Children with one or 2 abnormal readings had a 17% and 24% chance, respectively, of having hypertension as adults (P<.001).4
High childhood systolic BP may predict CAD in adulthood
A retrospective study evaluated 126 children 10 to 17 years of age who were admitted to the hospital for an elective surgical procedure between 1950 and 1967. Children with documented BP readings at admission were eligible for the study; children with preexisting cardiac and renal disease were excluded. Investigators reassessed patients as adults (age range 42-68 years); the mean follow-up period was 42 years.
Mean BP was 125/80 mm Hg at admission and 133/75 mm Hg at follow-up. Univariate logistic regression analysis showed a significant association between systolic BP in childhood and coronary artery disease at follow-up (odds ratio [OR]=1.052; 95% confidence interval [CI], 1.005-1.101; P=.027). Children with systolic BP at or above the 95th percentile had a 4-fold increase in coronary artery disease at follow-up compared with children whose systolic BP was below the 95th percentile (29% vs 7%, P=.03). Investigators also found an association between elevated BP in childhood and a diagnosis of hypertension at follow-up (P=.007).
Limitations of the study included small sample size, selection bias, changes in the definition of hypertension during the 4 decades since the study began, and limited childhood BP data (a single measurement at admission for surgery).5
Parents of hypertensive children are likely to be hypertensive themselves
Screening BP in children has the potential to identify families at increased risk for cardiovascular disease. A case series found a high incidence of hypertension among the parents of children with elevated BP. Investigators measured several risk factors, including BP in 141 children (mean age 10.5±3.4 years) and 108 parents (at least one a biological parent, mean age 38.5±7.5 years). They obtained 2 BP readings 15 to 30 minutes apart.
Parents of children with BPs at or above the 95th percentile had a 15-fold greater likelihood of hypertension themselves (OR=14.7; 95% CI, 3.02-71.56; P=.009, positive predictive value=75%; negative predictive value=81%).6 Limitations of the study included small sample size, high prevalence of obesity and black ethnicity in the study population (a population with a greater incidence of hypertension), and only 2 BP measurements in the same day, which isn’t diagnostic for hypertension.
Recommendations
The American College of Obstetricians and Gynecologists recommends screening girls for hypertension between 13 and 15 years of age.7
The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease.8
The European Society of Hypertension and European Society of Cardiology recommend that children older than 3 years have auscultatory BP measurements at each clinic visit.9
1. US Preventive Services Task Force. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed June 19, 2012.
2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl):555-576.
3. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
4. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633-641.
5. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323-328.
6. Reis EC, Kip KE, Marroquin OC, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789-e1797.
7. American Congress of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 452: Primary and preventive care: periodic assessments. Obstet Gynecol. 2009;114:1444-1451.
8. American Academy of Family Physicians. Hypertension. Available at http://www.aafp.org/online/en/home/clinical/exam/hypertension.html. Accessed April 16, 2012.
9. Lurbe E, Cifkova R, Cruickshank J, et al. Management of high blood pressure in children and adolescents: recommendation of the European Society of Hypertension. J Hypertens. 2009;27:1719-1742.
1. US Preventive Services Task Force. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed June 19, 2012.
2. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl):555-576.
3. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
4. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633-641.
5. Erlingsdottir A, Indridason OS, Thorvaldsson O, et al. Blood pressure in children and target-organ damage later in life. Pediatr Nephrol. 2010;25:323-328.
6. Reis EC, Kip KE, Marroquin OC, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789-e1797.
7. American Congress of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 452: Primary and preventive care: periodic assessments. Obstet Gynecol. 2009;114:1444-1451.
8. American Academy of Family Physicians. Hypertension. Available at http://www.aafp.org/online/en/home/clinical/exam/hypertension.html. Accessed April 16, 2012.
9. Lurbe E, Cifkova R, Cruickshank J, et al. Management of high blood pressure in children and adolescents: recommendation of the European Society of Hypertension. J Hypertens. 2009;27:1719-1742.
Evidence-based answers from the Family Physicians Inquiries Network
When a fetus survives methotrexate exposure
CASE A 28-year-old woman, gravida 4, para 3, requested medical termination of her pregnancy at approximately 7 weeks’ gestation. She was given intramuscular methotrexate 50 mg/m2 and oral misoprostol 400 mcg. Nine weeks later, she presented at our primary care clinic complaining of mild pelvic pain. Given her history, we ordered transvaginal ultrasound, which showed a viable pregnancy with an average ultrasound age of 16 weeks’ gestation and grossly normal fetal anatomy. We counseled the patient regarding the risks associated with maintaining the pregnancy after exposure to methotrexate, and she and her husband elected to proceed with the pregnancy.
Throughout the pregnancy, a perinatologist conducted serial 2-dimensional ultrasounds. At 30 weeks’ gestation, ultrasound revealed mild hydrocephalus but yielded poor visualization of the kidneys, heart, and spine. A repeat ultrasound at 34 weeks demonstrated unchanged hydrocephalus and normal fetal anatomy with appropriate interval growth. A fetal echocardiogram in utero showed no cardiac anomalies. At 39 weeks, the patient underwent induction of labor due to severe oligohydramnios. A viable female infant weighing 2456 g was delivered by spontaneous vaginal delivery, with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively.
The infant was small for her gestational age. Her hands had 4 digits each, including the thumb (FIGURE 1A). The upper extremities were shorter than expected in relation to the infant’s torso and were locked in 90 degrees of flexion at the elbow (FIGURE 1B). Her lower extremities were both normal in length, but her left ankle joint was everted with considerable laxity at the tibiotalar joint. The infant’s mandible deviated to the right. An ultrasound of her head showed dilation of the lateral ventricle, consistent with nonobstructive hydrocephalus. A skeletal survey revealed bilateral radial-humeral synostosis at approximately 90 degrees of flexion. The second day after birth, the infant was transferred to a tertiary medical facility for further evaluation by pediatric subspecialists.
A pediatric orthopedic surgeon noted bilateral hip dysplasia and fitted the infant for a Pavlik harness. Ultrasound of the spine identified a dysmorphic sacrum with a thickened conus medullaris ending at the level of L2-L3, with increased risk for a tethered spinal cord. To correct the hydrocephalus, a neurosurgeon recommended intervention in the neonatal period. The parents were counseled to expect some degree of developmental disability, and karyotyping was performed to rule out potential genetically linked syndromes.
FIGURE 1
Methotrexate exposure led to these congenital anomalies
Methotrexate exposure at 7 weeks’ gestation resulted in this child having 4 digits on each hand (A), shortened arms locked in 90 degrees of flexion at the elbow, and an everted left ankle joint (B).
Risks associated with methotrexate
In the 1960s, methotrexate was commonly used as an abortifacient.1 Its use for that purpose became rare, however, after multiple reports from the 1950s to the 1970s of congenital malformations in infants exposed to the drug in utero, either inadvertently or after attempted abortion.1 In the 1990s, its use increased again in conjunction with misoprostol, primarily for medical management of suspected ectopic pregnancies and less often for elective terminations. Use of the combination resulted in fewer reports of congenital anomalies.1
The failure rate of medical abortion using methotrexate varies. In 1999, one study reported an 8% failure rate when methotrexate was used with misoprostol.2 In 2004, methotrexate alone led to a failure rate of 31% in medical termination of early pregnancy.3 In 2005, another study of methotrexate and misoprostol used in combination for elective termination reported a failure rate of 2% to 10%.4
Risk is not always foreseen
Methotrexate is widely used to treat such conditions as neoplastic disease and autoimmune disorders. Unintended exposure of a fetus to methotrexate is a very real possibility when the drug is used to treat a mother’s rheumatoid arthritis, psoriasis, or systemic lupus erythematosus. Methotrexate is a folic acid antagonist that produces its most teratogenic effects between 6 and 8 weeks postconception.4 Anomalies associated with methotrexate exposure include skull defects, central nervous system abnormalities, limb defects, gastrointestinal and cardiopulmonary defects, developmental delay, and cognitive impairment.4 Even at low doses and with short-term exposure, methotrexate can cause substantial fetal anomalies. In 2002, a woman who was unknowingly 3.5 weeks pregnant used oral methotrexate 7.5 mg/d for 2 days to treat her psoriasis.5 During a fetal anatomy sonogram at 18 weeks’ gestation, multiple anomalies were noted and later confirmed at fetopsy.
A meta-analysis on the safety of methotrexate in treating rheumatoid arthritis concluded that, for doses typical in this setting, data were lacking regarding the safety and risks of the drug during conception, pregnancy, and lactation.6 The review said that rheumatologists should discourage patients from continuing methotrexate if they wish to become pregnant, and that any continuing pregnancy should be closely monitored.6
The exact malformation rate after in utero exposure to methotrexate is unknown.7 Kozlowski et al reported 10 pregnancies in which the fetus was exposed to low-dose methotrexate (5 mg orally every week) for the treatment of rheumatoid arthritis.8 Five of the pregnancies were carried to term and the newborns exhibited no abnormalities, thus illustrating the drug’s variability for teratogenicity. The risk is real, however, and methotrexate can remain in human tissue for up to 8 months, thereby putting a fetus at risk for exposure even after a mother has discontinued the drug.2
The importance of primary care counsel
Incidental exposure. Inform any pregnant patient who has used methotrexate of the potential for congenital anomalies. The capacity to make educated decisions about elective termination of pregnancy requires a full disclosure of risks. In particular, ultrasound may not identify teratogenic effects from methotrexate exposure, and antenatal diagnosis of congenital anomalies is uncommon.9 Diagnosis is usually made at delivery. Thorough counseling on this point is imperative to prevent a false reassurance of having a normal fetus.
Did medical termination fail? Despite methotrexate’s widespread use for pregnancy termination, insufficient published data exist to guide the counseling of patients who have experienced a failed termination. Nevertheless, primary care physicians are often called on to counsel such patients.
Only about half of women who undergo a medical termination procedure attend follow-up visits with the abortion provider.10 One reason is the distance some patients travel and the associated costs. A 2000 report showed that 87% of counties in the United States lack even a single abortion provider, and that approximately 25% of women travel 50 miles or more for their abortions.11
Financial hardship leads some women to opt for continuing a pregnancy after a failed elective termination.1 That was the case with our patient. When she began experiencing pelvic pain after the termination procedure, she did not return to the abortion clinic, but instead sought guidance from her primary care physician at our medical center. After learning that she was 16 weeks pregnant, she opted to proceed with the pregnancy because she couldn’t afford a second elective termination.
Primary care involvement makes sense for other reasons as well. Protocols requiring in-person follow-up appointments after elective termination may not make the best use of the medical system.10 The high proportion of “no shows” can lead to scheduling difficulties and reduce a provider’s availability to perform abortions. This in turn would lead to a loss of income for the provider and could possibly increase the total cost of medical care.
One proposed solution has been to teach women how to recognize the signs and symptoms of a successful abortion or possible complications. However, a study of methotrexate-misoprostol abortion in the United States showed that women were often unable to assess whether they had successfully aborted.10 Of 50 women, 28 thought they had aborted by day 9, and 13 of those (46%) were still pregnant.10 A patient’s overestimation of her ability to make such judgments is thought to be another reason for the low follow-up rates post termination.
When termination is performed—regardless of the modality used—it is imperative to confirm that it was successful. Primary care providers, who are usually accessible and offer cost-effective care, can provide such confirmation. In addition, primary care physicians may need to address the psychological stress caused by elective termination.
1. Wheeler M, O’Meara P, Stanford M. Fetal methotrexate and misoprostol exposure: the past revisited. Teratology. 2002;66:73-76.
2. Carbonell Esteve JL, Varela L, Velazco A, et al. 25 mg or 50 mg of oral methotrexate followed by vaginal misoprostol 7 days after for early abortion: a randomized trial. Gynecol Obstet Invest. 1999;47:182-187.
3. Addar MH. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: case report. J Obstet Gynecol Can. 2004;26:1001-1003.
4. Yedlinsky NT, Morgan FC, Whitecar PW. Anomalies associated with failed methotrexate and misoprostol termination. Obstet Gynecol. 2005;105:1203-1205.
5. Nguyen C, Duhl AJ, Escallon CS, et al. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet Gynecol. 2002;99:599-602.
6. Martinez Lopez JA, Loza E, Carmona L. Systemic review of the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy and breastfeeding). Clin Exp Rheumatol. 2009;27:678-684.
7. Goffman D, Cole DS, Bobby P, et al. Failed methotrexate termination of pregnancy: a case report. J Perinatol. 2006;26:645-647.
8. Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first trimester exposure to low dose methotrexate in eight patients with rheumatic disease. Am J Med. 1990;88:589-592.
9. Chapa JB, Hibbard JU, Weber EM, et al. Prenatal diagnosis of methotrexate embryopathy. Obstet Gynecol. 2003;101:1104-1107.
10. Grossman D, Ellertson C, Grimes DA, et al. Routine follow-up visits after first trimester induced abortion. Obstet Gynecol. 2004;103:738-745.
11. Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6-15.
CORRESPONDENCE Tammy Donoway, DO, Family Medicine, Womack Army Medical Center, 4-2817 Reilly Road, Fort Bragg, NC 28310; tammy.donoway@us.army.mil
CASE A 28-year-old woman, gravida 4, para 3, requested medical termination of her pregnancy at approximately 7 weeks’ gestation. She was given intramuscular methotrexate 50 mg/m2 and oral misoprostol 400 mcg. Nine weeks later, she presented at our primary care clinic complaining of mild pelvic pain. Given her history, we ordered transvaginal ultrasound, which showed a viable pregnancy with an average ultrasound age of 16 weeks’ gestation and grossly normal fetal anatomy. We counseled the patient regarding the risks associated with maintaining the pregnancy after exposure to methotrexate, and she and her husband elected to proceed with the pregnancy.
Throughout the pregnancy, a perinatologist conducted serial 2-dimensional ultrasounds. At 30 weeks’ gestation, ultrasound revealed mild hydrocephalus but yielded poor visualization of the kidneys, heart, and spine. A repeat ultrasound at 34 weeks demonstrated unchanged hydrocephalus and normal fetal anatomy with appropriate interval growth. A fetal echocardiogram in utero showed no cardiac anomalies. At 39 weeks, the patient underwent induction of labor due to severe oligohydramnios. A viable female infant weighing 2456 g was delivered by spontaneous vaginal delivery, with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively.
The infant was small for her gestational age. Her hands had 4 digits each, including the thumb (FIGURE 1A). The upper extremities were shorter than expected in relation to the infant’s torso and were locked in 90 degrees of flexion at the elbow (FIGURE 1B). Her lower extremities were both normal in length, but her left ankle joint was everted with considerable laxity at the tibiotalar joint. The infant’s mandible deviated to the right. An ultrasound of her head showed dilation of the lateral ventricle, consistent with nonobstructive hydrocephalus. A skeletal survey revealed bilateral radial-humeral synostosis at approximately 90 degrees of flexion. The second day after birth, the infant was transferred to a tertiary medical facility for further evaluation by pediatric subspecialists.
A pediatric orthopedic surgeon noted bilateral hip dysplasia and fitted the infant for a Pavlik harness. Ultrasound of the spine identified a dysmorphic sacrum with a thickened conus medullaris ending at the level of L2-L3, with increased risk for a tethered spinal cord. To correct the hydrocephalus, a neurosurgeon recommended intervention in the neonatal period. The parents were counseled to expect some degree of developmental disability, and karyotyping was performed to rule out potential genetically linked syndromes.
FIGURE 1

Methotrexate exposure led to these congenital anomalies
Methotrexate exposure at 7 weeks’ gestation resulted in this child having 4 digits on each hand (A), shortened arms locked in 90 degrees of flexion at the elbow, and an everted left ankle joint (B).
Risks associated with methotrexate
In the 1960s, methotrexate was commonly used as an abortifacient.1 Its use for that purpose became rare, however, after multiple reports from the 1950s to the 1970s of congenital malformations in infants exposed to the drug in utero, either inadvertently or after attempted abortion.1 In the 1990s, its use increased again in conjunction with misoprostol, primarily for medical management of suspected ectopic pregnancies and less often for elective terminations. Use of the combination resulted in fewer reports of congenital anomalies.1
The failure rate of medical abortion using methotrexate varies. In 1999, one study reported an 8% failure rate when methotrexate was used with misoprostol.2 In 2004, methotrexate alone led to a failure rate of 31% in medical termination of early pregnancy.3 In 2005, another study of methotrexate and misoprostol used in combination for elective termination reported a failure rate of 2% to 10%.4
Risk is not always foreseen
Methotrexate is widely used to treat such conditions as neoplastic disease and autoimmune disorders. Unintended exposure of a fetus to methotrexate is a very real possibility when the drug is used to treat a mother’s rheumatoid arthritis, psoriasis, or systemic lupus erythematosus. Methotrexate is a folic acid antagonist that produces its most teratogenic effects between 6 and 8 weeks postconception.4 Anomalies associated with methotrexate exposure include skull defects, central nervous system abnormalities, limb defects, gastrointestinal and cardiopulmonary defects, developmental delay, and cognitive impairment.4 Even at low doses and with short-term exposure, methotrexate can cause substantial fetal anomalies. In 2002, a woman who was unknowingly 3.5 weeks pregnant used oral methotrexate 7.5 mg/d for 2 days to treat her psoriasis.5 During a fetal anatomy sonogram at 18 weeks’ gestation, multiple anomalies were noted and later confirmed at fetopsy.
A meta-analysis on the safety of methotrexate in treating rheumatoid arthritis concluded that, for doses typical in this setting, data were lacking regarding the safety and risks of the drug during conception, pregnancy, and lactation.6 The review said that rheumatologists should discourage patients from continuing methotrexate if they wish to become pregnant, and that any continuing pregnancy should be closely monitored.6
The exact malformation rate after in utero exposure to methotrexate is unknown.7 Kozlowski et al reported 10 pregnancies in which the fetus was exposed to low-dose methotrexate (5 mg orally every week) for the treatment of rheumatoid arthritis.8 Five of the pregnancies were carried to term and the newborns exhibited no abnormalities, thus illustrating the drug’s variability for teratogenicity. The risk is real, however, and methotrexate can remain in human tissue for up to 8 months, thereby putting a fetus at risk for exposure even after a mother has discontinued the drug.2
The importance of primary care counsel
Incidental exposure. Inform any pregnant patient who has used methotrexate of the potential for congenital anomalies. The capacity to make educated decisions about elective termination of pregnancy requires a full disclosure of risks. In particular, ultrasound may not identify teratogenic effects from methotrexate exposure, and antenatal diagnosis of congenital anomalies is uncommon.9 Diagnosis is usually made at delivery. Thorough counseling on this point is imperative to prevent a false reassurance of having a normal fetus.
Did medical termination fail? Despite methotrexate’s widespread use for pregnancy termination, insufficient published data exist to guide the counseling of patients who have experienced a failed termination. Nevertheless, primary care physicians are often called on to counsel such patients.
Only about half of women who undergo a medical termination procedure attend follow-up visits with the abortion provider.10 One reason is the distance some patients travel and the associated costs. A 2000 report showed that 87% of counties in the United States lack even a single abortion provider, and that approximately 25% of women travel 50 miles or more for their abortions.11
Financial hardship leads some women to opt for continuing a pregnancy after a failed elective termination.1 That was the case with our patient. When she began experiencing pelvic pain after the termination procedure, she did not return to the abortion clinic, but instead sought guidance from her primary care physician at our medical center. After learning that she was 16 weeks pregnant, she opted to proceed with the pregnancy because she couldn’t afford a second elective termination.
Primary care involvement makes sense for other reasons as well. Protocols requiring in-person follow-up appointments after elective termination may not make the best use of the medical system.10 The high proportion of “no shows” can lead to scheduling difficulties and reduce a provider’s availability to perform abortions. This in turn would lead to a loss of income for the provider and could possibly increase the total cost of medical care.
One proposed solution has been to teach women how to recognize the signs and symptoms of a successful abortion or possible complications. However, a study of methotrexate-misoprostol abortion in the United States showed that women were often unable to assess whether they had successfully aborted.10 Of 50 women, 28 thought they had aborted by day 9, and 13 of those (46%) were still pregnant.10 A patient’s overestimation of her ability to make such judgments is thought to be another reason for the low follow-up rates post termination.
When termination is performed—regardless of the modality used—it is imperative to confirm that it was successful. Primary care providers, who are usually accessible and offer cost-effective care, can provide such confirmation. In addition, primary care physicians may need to address the psychological stress caused by elective termination.
CASE A 28-year-old woman, gravida 4, para 3, requested medical termination of her pregnancy at approximately 7 weeks’ gestation. She was given intramuscular methotrexate 50 mg/m2 and oral misoprostol 400 mcg. Nine weeks later, she presented at our primary care clinic complaining of mild pelvic pain. Given her history, we ordered transvaginal ultrasound, which showed a viable pregnancy with an average ultrasound age of 16 weeks’ gestation and grossly normal fetal anatomy. We counseled the patient regarding the risks associated with maintaining the pregnancy after exposure to methotrexate, and she and her husband elected to proceed with the pregnancy.
Throughout the pregnancy, a perinatologist conducted serial 2-dimensional ultrasounds. At 30 weeks’ gestation, ultrasound revealed mild hydrocephalus but yielded poor visualization of the kidneys, heart, and spine. A repeat ultrasound at 34 weeks demonstrated unchanged hydrocephalus and normal fetal anatomy with appropriate interval growth. A fetal echocardiogram in utero showed no cardiac anomalies. At 39 weeks, the patient underwent induction of labor due to severe oligohydramnios. A viable female infant weighing 2456 g was delivered by spontaneous vaginal delivery, with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively.
The infant was small for her gestational age. Her hands had 4 digits each, including the thumb (FIGURE 1A). The upper extremities were shorter than expected in relation to the infant’s torso and were locked in 90 degrees of flexion at the elbow (FIGURE 1B). Her lower extremities were both normal in length, but her left ankle joint was everted with considerable laxity at the tibiotalar joint. The infant’s mandible deviated to the right. An ultrasound of her head showed dilation of the lateral ventricle, consistent with nonobstructive hydrocephalus. A skeletal survey revealed bilateral radial-humeral synostosis at approximately 90 degrees of flexion. The second day after birth, the infant was transferred to a tertiary medical facility for further evaluation by pediatric subspecialists.
A pediatric orthopedic surgeon noted bilateral hip dysplasia and fitted the infant for a Pavlik harness. Ultrasound of the spine identified a dysmorphic sacrum with a thickened conus medullaris ending at the level of L2-L3, with increased risk for a tethered spinal cord. To correct the hydrocephalus, a neurosurgeon recommended intervention in the neonatal period. The parents were counseled to expect some degree of developmental disability, and karyotyping was performed to rule out potential genetically linked syndromes.
FIGURE 1

Methotrexate exposure led to these congenital anomalies
Methotrexate exposure at 7 weeks’ gestation resulted in this child having 4 digits on each hand (A), shortened arms locked in 90 degrees of flexion at the elbow, and an everted left ankle joint (B).
Risks associated with methotrexate
In the 1960s, methotrexate was commonly used as an abortifacient.1 Its use for that purpose became rare, however, after multiple reports from the 1950s to the 1970s of congenital malformations in infants exposed to the drug in utero, either inadvertently or after attempted abortion.1 In the 1990s, its use increased again in conjunction with misoprostol, primarily for medical management of suspected ectopic pregnancies and less often for elective terminations. Use of the combination resulted in fewer reports of congenital anomalies.1
The failure rate of medical abortion using methotrexate varies. In 1999, one study reported an 8% failure rate when methotrexate was used with misoprostol.2 In 2004, methotrexate alone led to a failure rate of 31% in medical termination of early pregnancy.3 In 2005, another study of methotrexate and misoprostol used in combination for elective termination reported a failure rate of 2% to 10%.4
Risk is not always foreseen
Methotrexate is widely used to treat such conditions as neoplastic disease and autoimmune disorders. Unintended exposure of a fetus to methotrexate is a very real possibility when the drug is used to treat a mother’s rheumatoid arthritis, psoriasis, or systemic lupus erythematosus. Methotrexate is a folic acid antagonist that produces its most teratogenic effects between 6 and 8 weeks postconception.4 Anomalies associated with methotrexate exposure include skull defects, central nervous system abnormalities, limb defects, gastrointestinal and cardiopulmonary defects, developmental delay, and cognitive impairment.4 Even at low doses and with short-term exposure, methotrexate can cause substantial fetal anomalies. In 2002, a woman who was unknowingly 3.5 weeks pregnant used oral methotrexate 7.5 mg/d for 2 days to treat her psoriasis.5 During a fetal anatomy sonogram at 18 weeks’ gestation, multiple anomalies were noted and later confirmed at fetopsy.
A meta-analysis on the safety of methotrexate in treating rheumatoid arthritis concluded that, for doses typical in this setting, data were lacking regarding the safety and risks of the drug during conception, pregnancy, and lactation.6 The review said that rheumatologists should discourage patients from continuing methotrexate if they wish to become pregnant, and that any continuing pregnancy should be closely monitored.6
The exact malformation rate after in utero exposure to methotrexate is unknown.7 Kozlowski et al reported 10 pregnancies in which the fetus was exposed to low-dose methotrexate (5 mg orally every week) for the treatment of rheumatoid arthritis.8 Five of the pregnancies were carried to term and the newborns exhibited no abnormalities, thus illustrating the drug’s variability for teratogenicity. The risk is real, however, and methotrexate can remain in human tissue for up to 8 months, thereby putting a fetus at risk for exposure even after a mother has discontinued the drug.2
The importance of primary care counsel
Incidental exposure. Inform any pregnant patient who has used methotrexate of the potential for congenital anomalies. The capacity to make educated decisions about elective termination of pregnancy requires a full disclosure of risks. In particular, ultrasound may not identify teratogenic effects from methotrexate exposure, and antenatal diagnosis of congenital anomalies is uncommon.9 Diagnosis is usually made at delivery. Thorough counseling on this point is imperative to prevent a false reassurance of having a normal fetus.
Did medical termination fail? Despite methotrexate’s widespread use for pregnancy termination, insufficient published data exist to guide the counseling of patients who have experienced a failed termination. Nevertheless, primary care physicians are often called on to counsel such patients.
Only about half of women who undergo a medical termination procedure attend follow-up visits with the abortion provider.10 One reason is the distance some patients travel and the associated costs. A 2000 report showed that 87% of counties in the United States lack even a single abortion provider, and that approximately 25% of women travel 50 miles or more for their abortions.11
Financial hardship leads some women to opt for continuing a pregnancy after a failed elective termination.1 That was the case with our patient. When she began experiencing pelvic pain after the termination procedure, she did not return to the abortion clinic, but instead sought guidance from her primary care physician at our medical center. After learning that she was 16 weeks pregnant, she opted to proceed with the pregnancy because she couldn’t afford a second elective termination.
Primary care involvement makes sense for other reasons as well. Protocols requiring in-person follow-up appointments after elective termination may not make the best use of the medical system.10 The high proportion of “no shows” can lead to scheduling difficulties and reduce a provider’s availability to perform abortions. This in turn would lead to a loss of income for the provider and could possibly increase the total cost of medical care.
One proposed solution has been to teach women how to recognize the signs and symptoms of a successful abortion or possible complications. However, a study of methotrexate-misoprostol abortion in the United States showed that women were often unable to assess whether they had successfully aborted.10 Of 50 women, 28 thought they had aborted by day 9, and 13 of those (46%) were still pregnant.10 A patient’s overestimation of her ability to make such judgments is thought to be another reason for the low follow-up rates post termination.
When termination is performed—regardless of the modality used—it is imperative to confirm that it was successful. Primary care providers, who are usually accessible and offer cost-effective care, can provide such confirmation. In addition, primary care physicians may need to address the psychological stress caused by elective termination.
1. Wheeler M, O’Meara P, Stanford M. Fetal methotrexate and misoprostol exposure: the past revisited. Teratology. 2002;66:73-76.
2. Carbonell Esteve JL, Varela L, Velazco A, et al. 25 mg or 50 mg of oral methotrexate followed by vaginal misoprostol 7 days after for early abortion: a randomized trial. Gynecol Obstet Invest. 1999;47:182-187.
3. Addar MH. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: case report. J Obstet Gynecol Can. 2004;26:1001-1003.
4. Yedlinsky NT, Morgan FC, Whitecar PW. Anomalies associated with failed methotrexate and misoprostol termination. Obstet Gynecol. 2005;105:1203-1205.
5. Nguyen C, Duhl AJ, Escallon CS, et al. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet Gynecol. 2002;99:599-602.
6. Martinez Lopez JA, Loza E, Carmona L. Systemic review of the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy and breastfeeding). Clin Exp Rheumatol. 2009;27:678-684.
7. Goffman D, Cole DS, Bobby P, et al. Failed methotrexate termination of pregnancy: a case report. J Perinatol. 2006;26:645-647.
8. Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first trimester exposure to low dose methotrexate in eight patients with rheumatic disease. Am J Med. 1990;88:589-592.
9. Chapa JB, Hibbard JU, Weber EM, et al. Prenatal diagnosis of methotrexate embryopathy. Obstet Gynecol. 2003;101:1104-1107.
10. Grossman D, Ellertson C, Grimes DA, et al. Routine follow-up visits after first trimester induced abortion. Obstet Gynecol. 2004;103:738-745.
11. Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6-15.
CORRESPONDENCE Tammy Donoway, DO, Family Medicine, Womack Army Medical Center, 4-2817 Reilly Road, Fort Bragg, NC 28310; tammy.donoway@us.army.mil
1. Wheeler M, O’Meara P, Stanford M. Fetal methotrexate and misoprostol exposure: the past revisited. Teratology. 2002;66:73-76.
2. Carbonell Esteve JL, Varela L, Velazco A, et al. 25 mg or 50 mg of oral methotrexate followed by vaginal misoprostol 7 days after for early abortion: a randomized trial. Gynecol Obstet Invest. 1999;47:182-187.
3. Addar MH. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: case report. J Obstet Gynecol Can. 2004;26:1001-1003.
4. Yedlinsky NT, Morgan FC, Whitecar PW. Anomalies associated with failed methotrexate and misoprostol termination. Obstet Gynecol. 2005;105:1203-1205.
5. Nguyen C, Duhl AJ, Escallon CS, et al. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet Gynecol. 2002;99:599-602.
6. Martinez Lopez JA, Loza E, Carmona L. Systemic review of the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy and breastfeeding). Clin Exp Rheumatol. 2009;27:678-684.
7. Goffman D, Cole DS, Bobby P, et al. Failed methotrexate termination of pregnancy: a case report. J Perinatol. 2006;26:645-647.
8. Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first trimester exposure to low dose methotrexate in eight patients with rheumatic disease. Am J Med. 1990;88:589-592.
9. Chapa JB, Hibbard JU, Weber EM, et al. Prenatal diagnosis of methotrexate embryopathy. Obstet Gynecol. 2003;101:1104-1107.
10. Grossman D, Ellertson C, Grimes DA, et al. Routine follow-up visits after first trimester induced abortion. Obstet Gynecol. 2004;103:738-745.
11. Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6-15.
CORRESPONDENCE Tammy Donoway, DO, Family Medicine, Womack Army Medical Center, 4-2817 Reilly Road, Fort Bragg, NC 28310; tammy.donoway@us.army.mil
Does cervical membrane stripping in women with group B Streptococcus put the fetus at risk?
NO DIRECT EVIDENCE points to fetal harm from cervical membrane stripping (CMS) to induce labor in term pregnancies complicated by group B Streptococcus (GBS) colonization (strength of recommendation [SOR]: B, a Cochrane systematic review).
Evidence summary
A Cochrane review of 22 trials (N=2797) comparing CMS with no CMS in uncomplicated term deliveries demonstrated no significant differences in fetal outcomes.1 The groups showed similar rates of maternal infection and fever (relative risk [RR]=1.05; 95% confidence interval [CI], 0.68-1.65), neonatal infection (RR=0.92; 95% CI, 0.30-2.82), and Apgar scores <7 at 5 minutes (RR=1.13; 95% CI, 0.53-2.43). Two perinatal deaths were reported in each group. The review was limited by relatively small trials and heterogeneity between trial results, suggesting the possibility of publication bias.
Most of the studies included in the meta-analysis didn’t specifically include or exclude women with GBS colonization, nor did the review subanalyze patients into a GBS-positive and GBS-negative arm. Considering that GBS colonization was reported in 19% to 26% of pregnancies, it’s likely that GBS colonization was present in both CMS and control groups in the review.2,3
Study shows no CMS effects, but may be underpowered
A randomized prospective study (N=98) included in the Cochrane review specifically considered the effects of CMS and maternal GBS colonization.4 Colonization rates for the study were 17% (9/44 in the study group, 8/54 in the control group). Women in the study group underwent weekly CMS beginning at 38 weeks of gestation; the control group didn’t undergo CMS. Repeat GBS testing was performed at 40 weeks for all patients with initial GBS-negative cultures.
Three patients were GBS-positive on repeat testing (one in the study group, 2 in the control group). No admissions to the neonatal intensive care unit or neonatal infections occurred in either group. The study may have been underpowered to detect any effect, however.4
Recommendations
The American College of Obstetricians and Gynecologists’ 2009 Practice Bulletin on induction of labor states that the data are insufficient to either recommend or discourage CMS to induce labor in women who are GBS-positive.5
The 2009 Department of Veterans Affairs/Department of Defense Clinical Practice Guideline for Pregnancy Management also cites insufficient data to support or oppose CMS in GBS-positive term pregnant women.6
1. Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labor. Cochrane Database Syst Rev. 2005;(1):CD000451.-
2. Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol. 1991;77:604-610.
3. Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
4. Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus? Am J Obstet Gynecol. 2002;187:S221.-[Abstract.]
5. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Induction of labor. Obstet Gynecol. 2009;114:386-397.
6. United States Department of Veterans Affairs and US Department of Defense, Pregnancy Management Working Group. VA/DoD clinical practice guideline for pregnancy management, 2009. Available at: www.healthquality.va.gov/up/mpg_v2_1_full.pdf. Accessed April 16, 2010.
NO DIRECT EVIDENCE points to fetal harm from cervical membrane stripping (CMS) to induce labor in term pregnancies complicated by group B Streptococcus (GBS) colonization (strength of recommendation [SOR]: B, a Cochrane systematic review).
Evidence summary
A Cochrane review of 22 trials (N=2797) comparing CMS with no CMS in uncomplicated term deliveries demonstrated no significant differences in fetal outcomes.1 The groups showed similar rates of maternal infection and fever (relative risk [RR]=1.05; 95% confidence interval [CI], 0.68-1.65), neonatal infection (RR=0.92; 95% CI, 0.30-2.82), and Apgar scores <7 at 5 minutes (RR=1.13; 95% CI, 0.53-2.43). Two perinatal deaths were reported in each group. The review was limited by relatively small trials and heterogeneity between trial results, suggesting the possibility of publication bias.
Most of the studies included in the meta-analysis didn’t specifically include or exclude women with GBS colonization, nor did the review subanalyze patients into a GBS-positive and GBS-negative arm. Considering that GBS colonization was reported in 19% to 26% of pregnancies, it’s likely that GBS colonization was present in both CMS and control groups in the review.2,3
Study shows no CMS effects, but may be underpowered
A randomized prospective study (N=98) included in the Cochrane review specifically considered the effects of CMS and maternal GBS colonization.4 Colonization rates for the study were 17% (9/44 in the study group, 8/54 in the control group). Women in the study group underwent weekly CMS beginning at 38 weeks of gestation; the control group didn’t undergo CMS. Repeat GBS testing was performed at 40 weeks for all patients with initial GBS-negative cultures.
Three patients were GBS-positive on repeat testing (one in the study group, 2 in the control group). No admissions to the neonatal intensive care unit or neonatal infections occurred in either group. The study may have been underpowered to detect any effect, however.4
Recommendations
The American College of Obstetricians and Gynecologists’ 2009 Practice Bulletin on induction of labor states that the data are insufficient to either recommend or discourage CMS to induce labor in women who are GBS-positive.5
The 2009 Department of Veterans Affairs/Department of Defense Clinical Practice Guideline for Pregnancy Management also cites insufficient data to support or oppose CMS in GBS-positive term pregnant women.6
NO DIRECT EVIDENCE points to fetal harm from cervical membrane stripping (CMS) to induce labor in term pregnancies complicated by group B Streptococcus (GBS) colonization (strength of recommendation [SOR]: B, a Cochrane systematic review).
Evidence summary
A Cochrane review of 22 trials (N=2797) comparing CMS with no CMS in uncomplicated term deliveries demonstrated no significant differences in fetal outcomes.1 The groups showed similar rates of maternal infection and fever (relative risk [RR]=1.05; 95% confidence interval [CI], 0.68-1.65), neonatal infection (RR=0.92; 95% CI, 0.30-2.82), and Apgar scores <7 at 5 minutes (RR=1.13; 95% CI, 0.53-2.43). Two perinatal deaths were reported in each group. The review was limited by relatively small trials and heterogeneity between trial results, suggesting the possibility of publication bias.
Most of the studies included in the meta-analysis didn’t specifically include or exclude women with GBS colonization, nor did the review subanalyze patients into a GBS-positive and GBS-negative arm. Considering that GBS colonization was reported in 19% to 26% of pregnancies, it’s likely that GBS colonization was present in both CMS and control groups in the review.2,3
Study shows no CMS effects, but may be underpowered
A randomized prospective study (N=98) included in the Cochrane review specifically considered the effects of CMS and maternal GBS colonization.4 Colonization rates for the study were 17% (9/44 in the study group, 8/54 in the control group). Women in the study group underwent weekly CMS beginning at 38 weeks of gestation; the control group didn’t undergo CMS. Repeat GBS testing was performed at 40 weeks for all patients with initial GBS-negative cultures.
Three patients were GBS-positive on repeat testing (one in the study group, 2 in the control group). No admissions to the neonatal intensive care unit or neonatal infections occurred in either group. The study may have been underpowered to detect any effect, however.4
Recommendations
The American College of Obstetricians and Gynecologists’ 2009 Practice Bulletin on induction of labor states that the data are insufficient to either recommend or discourage CMS to induce labor in women who are GBS-positive.5
The 2009 Department of Veterans Affairs/Department of Defense Clinical Practice Guideline for Pregnancy Management also cites insufficient data to support or oppose CMS in GBS-positive term pregnant women.6
1. Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labor. Cochrane Database Syst Rev. 2005;(1):CD000451.-
2. Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol. 1991;77:604-610.
3. Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
4. Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus? Am J Obstet Gynecol. 2002;187:S221.-[Abstract.]
5. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Induction of labor. Obstet Gynecol. 2009;114:386-397.
6. United States Department of Veterans Affairs and US Department of Defense, Pregnancy Management Working Group. VA/DoD clinical practice guideline for pregnancy management, 2009. Available at: www.healthquality.va.gov/up/mpg_v2_1_full.pdf. Accessed April 16, 2010.
1. Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labor. Cochrane Database Syst Rev. 2005;(1):CD000451.-
2. Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol. 1991;77:604-610.
3. Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
4. Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus? Am J Obstet Gynecol. 2002;187:S221.-[Abstract.]
5. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Induction of labor. Obstet Gynecol. 2009;114:386-397.
6. United States Department of Veterans Affairs and US Department of Defense, Pregnancy Management Working Group. VA/DoD clinical practice guideline for pregnancy management, 2009. Available at: www.healthquality.va.gov/up/mpg_v2_1_full.pdf. Accessed April 16, 2010.
Evidence-based answers from the Family Physicians Inquiries Network
Ureteral calculi: What should you consider before intervening?
THE SIZE OF THE CALCULI, their location, and complicating factors such as infection should all be considered.
Most ureteral calculi smaller than 5 mm pass spontaneously, as do approximately half of calculi between 5 and 10 mm. Calculi larger than 10 mm are unlikely to pass without intervention. Distal calculi are more likely to pass spontaneously than calculi in mid- or proximal ureteral locations; most spontaneous passage occurs within 4 to 6 weeks (strength of recommendation [SOR]: A, prospective cohort studies).
All patients with calculi complicated by such factors as obstruction, infection, renal injury, or a single kidney require surgical consultation (SOR: C, expert opinion).
Medical expulsion therapy with alpha-blockers (usually tamsulosin) and nifedipine improves passage rates, including for some calculi larger than 10 mm (SOR: A, meta-analysis of prospective cohort studies).
Evidence summary
A meta-analysis of 5 prospective cohort studies evaluated the rate of spontaneous passage of ureteral calculi according to size. Calculi smaller than 5 mm passed spontaneously in 68% of patients (5 studies, N=224). Calculi between 5 and 10 mm passed spontaneously in 47% of patients (3 studies, N=104).1
A prospective cohort study evaluated spontaneous passage rates of ureteral calculi by size in 172 patients who were diagnosed by unenhanced helical computed tomography.2 Investigators found spontaneous passage rates of 87% for 1-mm calculi, 76% for 2- to 4-mm calculi, 60% for 5- to 7-mm calculi, 48% for 7- to 9-mm calculi, and 25% for calculi larger than 9 mm.
Spontaneous passage rates differed significantly for calculi 1 to 4 mm in size compared with calculi 5 to 7 mm in size (P<.001) and for calculi 5 to 7 mm in size compared with calculi 8 mm or larger (P<.001). Calculi in either the distal ureter or ureterovesicular junction were more likely to pass that those in the mid- or proximal ureter (75% to 79% vs 48% to 60%; P<.001).
Most smaller calculi pass in 4 to 6 weeks
Another prospective cohort study (N=75) found that most calculi pass spontaneously within 4 to 6 weeks. In 95% of patients, calculi passed within 31 days (2 mm or smaller), 40 days (2-4 mm), or 39 days (4-6 mm).3
Some cases require prompt surgery
The American Urological Association (AUA) expert panel recommends early surgical intervention, regardless of calculus size, under the following circumstances: obstruction with high-grade hydronephrosis, infection, impending renal deterioration, intractable pain, nausea and vomiting, or obstruction in a solitary or transplanted kidney.1
Medical expulsion therapy trumps waiting for distal calculi to pass
A meta-analysis comparing rates of calculus passage found that medical expulsion therapy was more effective than expectant management for patients with distal ureteral calculi. Sixteen RCTs (N=1235) evaluated alpha-antagonists (mostly tamsulosin), and 9 RCTs (N=686) evaluated nifedipine. Treat ment periods for medical expulsion therapy ranged from 30 to 60 days.
Alpha-antagonists increased expulsion rates over expectant management for calculi ranging in size from 3 to 18 mm with a mean diameter greater than 5 mm (relative risk [RR]=1.59; 95% confidence interval [CI], 1.44-1.75; number needed to treat [NNT]=3). The mean time until passage ranged from 2.7 to 14.2 days. Nifedipine also increased expulsion rates for calculi with a mean diameter larger than 5 mm, ranging in size from 3.9 to 12.8 mm (RR=1.50; 95% CI, 1.34-1.68; NNT=4).4
Recommendations
The Joint European Association of Urology/ AUA Nephrolithiasis Guideline Panel recommends observation with periodic evaluation for patients newly diagnosed with ureteral calculi smaller than 10 mm.1 Patients may be offered medical expulsion therapy to facilitate calculus passage. Surveillance should be maintained until calculi pass; intervention should be considered if calculi don’t pass spontaneously within about 30 days.
The Panel states that patients with ureteral calculi larger than 10 mm could be observed (with or without medical expulsion therapy); however, most cases will require surgical intervention.1
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Medical Department of the United States Army or the US Army Service at large.
1. European Association of Urology/American Urology Association Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. Available at: www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=uc. Accessed August 16, 2010.
2. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to calculus size and location as revealed by unenhanced helical CT. Am J Roentgenol. 2002;178:101-103.
3. Miller OF, Kane CJ. Time to calculus passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688-691.
4. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
THE SIZE OF THE CALCULI, their location, and complicating factors such as infection should all be considered.
Most ureteral calculi smaller than 5 mm pass spontaneously, as do approximately half of calculi between 5 and 10 mm. Calculi larger than 10 mm are unlikely to pass without intervention. Distal calculi are more likely to pass spontaneously than calculi in mid- or proximal ureteral locations; most spontaneous passage occurs within 4 to 6 weeks (strength of recommendation [SOR]: A, prospective cohort studies).
All patients with calculi complicated by such factors as obstruction, infection, renal injury, or a single kidney require surgical consultation (SOR: C, expert opinion).
Medical expulsion therapy with alpha-blockers (usually tamsulosin) and nifedipine improves passage rates, including for some calculi larger than 10 mm (SOR: A, meta-analysis of prospective cohort studies).
Evidence summary
A meta-analysis of 5 prospective cohort studies evaluated the rate of spontaneous passage of ureteral calculi according to size. Calculi smaller than 5 mm passed spontaneously in 68% of patients (5 studies, N=224). Calculi between 5 and 10 mm passed spontaneously in 47% of patients (3 studies, N=104).1
A prospective cohort study evaluated spontaneous passage rates of ureteral calculi by size in 172 patients who were diagnosed by unenhanced helical computed tomography.2 Investigators found spontaneous passage rates of 87% for 1-mm calculi, 76% for 2- to 4-mm calculi, 60% for 5- to 7-mm calculi, 48% for 7- to 9-mm calculi, and 25% for calculi larger than 9 mm.
Spontaneous passage rates differed significantly for calculi 1 to 4 mm in size compared with calculi 5 to 7 mm in size (P<.001) and for calculi 5 to 7 mm in size compared with calculi 8 mm or larger (P<.001). Calculi in either the distal ureter or ureterovesicular junction were more likely to pass that those in the mid- or proximal ureter (75% to 79% vs 48% to 60%; P<.001).
Most smaller calculi pass in 4 to 6 weeks
Another prospective cohort study (N=75) found that most calculi pass spontaneously within 4 to 6 weeks. In 95% of patients, calculi passed within 31 days (2 mm or smaller), 40 days (2-4 mm), or 39 days (4-6 mm).3
Some cases require prompt surgery
The American Urological Association (AUA) expert panel recommends early surgical intervention, regardless of calculus size, under the following circumstances: obstruction with high-grade hydronephrosis, infection, impending renal deterioration, intractable pain, nausea and vomiting, or obstruction in a solitary or transplanted kidney.1
Medical expulsion therapy trumps waiting for distal calculi to pass
A meta-analysis comparing rates of calculus passage found that medical expulsion therapy was more effective than expectant management for patients with distal ureteral calculi. Sixteen RCTs (N=1235) evaluated alpha-antagonists (mostly tamsulosin), and 9 RCTs (N=686) evaluated nifedipine. Treat ment periods for medical expulsion therapy ranged from 30 to 60 days.
Alpha-antagonists increased expulsion rates over expectant management for calculi ranging in size from 3 to 18 mm with a mean diameter greater than 5 mm (relative risk [RR]=1.59; 95% confidence interval [CI], 1.44-1.75; number needed to treat [NNT]=3). The mean time until passage ranged from 2.7 to 14.2 days. Nifedipine also increased expulsion rates for calculi with a mean diameter larger than 5 mm, ranging in size from 3.9 to 12.8 mm (RR=1.50; 95% CI, 1.34-1.68; NNT=4).4
Recommendations
The Joint European Association of Urology/ AUA Nephrolithiasis Guideline Panel recommends observation with periodic evaluation for patients newly diagnosed with ureteral calculi smaller than 10 mm.1 Patients may be offered medical expulsion therapy to facilitate calculus passage. Surveillance should be maintained until calculi pass; intervention should be considered if calculi don’t pass spontaneously within about 30 days.
The Panel states that patients with ureteral calculi larger than 10 mm could be observed (with or without medical expulsion therapy); however, most cases will require surgical intervention.1
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Medical Department of the United States Army or the US Army Service at large.
THE SIZE OF THE CALCULI, their location, and complicating factors such as infection should all be considered.
Most ureteral calculi smaller than 5 mm pass spontaneously, as do approximately half of calculi between 5 and 10 mm. Calculi larger than 10 mm are unlikely to pass without intervention. Distal calculi are more likely to pass spontaneously than calculi in mid- or proximal ureteral locations; most spontaneous passage occurs within 4 to 6 weeks (strength of recommendation [SOR]: A, prospective cohort studies).
All patients with calculi complicated by such factors as obstruction, infection, renal injury, or a single kidney require surgical consultation (SOR: C, expert opinion).
Medical expulsion therapy with alpha-blockers (usually tamsulosin) and nifedipine improves passage rates, including for some calculi larger than 10 mm (SOR: A, meta-analysis of prospective cohort studies).
Evidence summary
A meta-analysis of 5 prospective cohort studies evaluated the rate of spontaneous passage of ureteral calculi according to size. Calculi smaller than 5 mm passed spontaneously in 68% of patients (5 studies, N=224). Calculi between 5 and 10 mm passed spontaneously in 47% of patients (3 studies, N=104).1
A prospective cohort study evaluated spontaneous passage rates of ureteral calculi by size in 172 patients who were diagnosed by unenhanced helical computed tomography.2 Investigators found spontaneous passage rates of 87% for 1-mm calculi, 76% for 2- to 4-mm calculi, 60% for 5- to 7-mm calculi, 48% for 7- to 9-mm calculi, and 25% for calculi larger than 9 mm.
Spontaneous passage rates differed significantly for calculi 1 to 4 mm in size compared with calculi 5 to 7 mm in size (P<.001) and for calculi 5 to 7 mm in size compared with calculi 8 mm or larger (P<.001). Calculi in either the distal ureter or ureterovesicular junction were more likely to pass that those in the mid- or proximal ureter (75% to 79% vs 48% to 60%; P<.001).
Most smaller calculi pass in 4 to 6 weeks
Another prospective cohort study (N=75) found that most calculi pass spontaneously within 4 to 6 weeks. In 95% of patients, calculi passed within 31 days (2 mm or smaller), 40 days (2-4 mm), or 39 days (4-6 mm).3
Some cases require prompt surgery
The American Urological Association (AUA) expert panel recommends early surgical intervention, regardless of calculus size, under the following circumstances: obstruction with high-grade hydronephrosis, infection, impending renal deterioration, intractable pain, nausea and vomiting, or obstruction in a solitary or transplanted kidney.1
Medical expulsion therapy trumps waiting for distal calculi to pass
A meta-analysis comparing rates of calculus passage found that medical expulsion therapy was more effective than expectant management for patients with distal ureteral calculi. Sixteen RCTs (N=1235) evaluated alpha-antagonists (mostly tamsulosin), and 9 RCTs (N=686) evaluated nifedipine. Treat ment periods for medical expulsion therapy ranged from 30 to 60 days.
Alpha-antagonists increased expulsion rates over expectant management for calculi ranging in size from 3 to 18 mm with a mean diameter greater than 5 mm (relative risk [RR]=1.59; 95% confidence interval [CI], 1.44-1.75; number needed to treat [NNT]=3). The mean time until passage ranged from 2.7 to 14.2 days. Nifedipine also increased expulsion rates for calculi with a mean diameter larger than 5 mm, ranging in size from 3.9 to 12.8 mm (RR=1.50; 95% CI, 1.34-1.68; NNT=4).4
Recommendations
The Joint European Association of Urology/ AUA Nephrolithiasis Guideline Panel recommends observation with periodic evaluation for patients newly diagnosed with ureteral calculi smaller than 10 mm.1 Patients may be offered medical expulsion therapy to facilitate calculus passage. Surveillance should be maintained until calculi pass; intervention should be considered if calculi don’t pass spontaneously within about 30 days.
The Panel states that patients with ureteral calculi larger than 10 mm could be observed (with or without medical expulsion therapy); however, most cases will require surgical intervention.1
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Medical Department of the United States Army or the US Army Service at large.
1. European Association of Urology/American Urology Association Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. Available at: www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=uc. Accessed August 16, 2010.
2. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to calculus size and location as revealed by unenhanced helical CT. Am J Roentgenol. 2002;178:101-103.
3. Miller OF, Kane CJ. Time to calculus passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688-691.
4. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
1. European Association of Urology/American Urology Association Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. Available at: www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=uc. Accessed August 16, 2010.
2. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to calculus size and location as revealed by unenhanced helical CT. Am J Roentgenol. 2002;178:101-103.
3. Miller OF, Kane CJ. Time to calculus passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688-691.
4. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
Evidence-based answers from the Family Physicians Inquiries Network
How does electronic fetal heart rate monitoring affect labor and delivery outcomes?
CONTINUOUS ELECTRONIC FETAL MONITORING (EFM) REDUCES THE RISK OF NEONATAL SEIZURE BY 50% compared with intermittent auscultation (IA) (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
EFM increases the incidence of cesarean section by 66% and the incidence of operative vaginal delivery by 16% (SOR: A, systematic review of RCTs). It has no effect on the rates of cerebral palsy or neonatal mortality (SOR: A, systematic review of RCTs).
An estimate from a Cochrane meta-analysis suggests that a cohort of 628 women receiving EFM could expect to experience 1 less neonatal seizure and 11 more cesarean sections compared with IA controls.
Evidence summary
Continuous EFM is designed to detect early fetal hypoxia and thereby decrease neonatal morbidity and mortality compared with IA. IA is defined as auscultation of the fetal heart rate for at least 60 seconds every 15 minutes during the first stage of labor and every 5 minutes during the second stage of labor.
A decrease in seizures, but not deaths or cerebral palsy
A 2006 Cochrane systematic review examined 12 RCTs (with >37,000 women) that compared continuous EFM with IA.1 Continuous EFM reduced the risk of neonatal seizure by 50% (relative risk [RR]=0.50; 95% confidence interval [CI], 0.31-0.80), but had no effect on the rate of neonatal death (RR=0.85; 95% CI, 0.59-1.23) or development of cerebral palsy (RR=1.74; 95% CI, 0.97-3.11).
Reduction of seizures was consistent across all trials. However, a subgroup analysis of high-risk pregnancies (advanced maternal age, diabetes mellitus, chronic hypertension, renal disease, preeclampsia, cardiac disease, renal disease, previous delivery of a low-birth-weight infant) didn’t find a statistically significant decrease in seizures.
Cesarean deliveries rise, regardless of patient risk status
Continuous EFM raised the rates of cesarean delivery (RR=1.66; 95% CI, 1.30-2.13) and instrumental vaginal deliveries (RR=1.16; 95% CI, 1.01-1.32). The increased rate of cesarean section in the EFM group was consistent regardless of clinical risk status (low- vs high-risk women). One additional cesarean section was performed for every 58 women monitored continuously. For “high-risk” women, 1 additional cesarean section was performed for every 12 women monitored continuously.1
Cesarean section rates varied widely among the individual trials (2.3%-35%). Analysis suggested that studies with higher baseline rates showed the greatest increases with continuous EFM. The rate for all studies combined was just 4.3%; 69% of patients included in the meta-analysis were contributed by the Dublin trial, which had an average cesarean rate of 2.3%.1 By comparison, the US Division of Vital Statistics reported a cesarean rate of 32.3% in 2008.2
EFM reduces death from fetal hypoxia
A 1995 meta-analysis, including 9 of the Cochrane review studies with a total of 18,561 women, evaluated the additional outcome of death resulting from fetal hypoxia.3 Compared with IA, EFM was associated with a 59% reduction in death from fetal hypoxia (RR=0.41; 95% CI, 0.17-0.98). Continuous EFM prevented 1 perinatal death per 1000 births. The reduction in perinatal mortality was offset by a 53% increase in cesarean deliveries and a 23% increase in operative vaginal deliveries.3
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) doesn’t recommend for or against continuous fetal heart rate monitoring in uncomplicated labor, recognizing either EFM or IA as acceptable in uncomplicated patients.4 ACOG does recommend continuous EFM for women with high-risk conditions (suspected fetal growth restriction, preeclampsia, and type 1 diabetes mellitus).
The US Preventive Services Task Force doesn’t support routine intrapartum EFM for low-risk woman. The Task Force found insufficient evidence for using EFM in high-risk pregnancies.5
The Royal College of Obstetricians and Gynaecologists and the Royal Australian and New Zealand College of Obstetricians and Gynecologists both recommend continuous EFM for high-risk women and IA for low-risk patients.6,7
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army Medical Department or the US Army at large.
1. Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2006;(3):CD006066.-
2. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2008. Natl Vital Stat Rep. 2010;58(16):1-18.
3. Vintzileos AM, Nochimson DJ, Guzman ER, et al. Intrapartum electronic heart rate monitoring versus intermittent auscultation: a meta-analysis. Obstet Gynecol. 1995;85:149-155.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists, Number 106, July 2009. Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192-202.
5. US Preventive Services Task Force. Screening for intrapartum electronic fetal monitoring. Rockville, MD: Agency for Healthcare Research and Quality; 1996. Available at: www.ahrq.gov/clinic/uspstf/uspsiefm.htm. Accessed March 7, 2010.
6. National Institute for Health and Clinical Excellence (NICE). Intrapartum Care: Management and Delivery of Care to Women in Labour. London: NICE; 2007.
7. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Clinical Guidelines. Intrapartum Fetal Surveillance Guidelines. May 2006. Available at: www.ranzcog.edu.au/publications/womenshealth.shtml. Accessed December 9, 2008.
CONTINUOUS ELECTRONIC FETAL MONITORING (EFM) REDUCES THE RISK OF NEONATAL SEIZURE BY 50% compared with intermittent auscultation (IA) (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
EFM increases the incidence of cesarean section by 66% and the incidence of operative vaginal delivery by 16% (SOR: A, systematic review of RCTs). It has no effect on the rates of cerebral palsy or neonatal mortality (SOR: A, systematic review of RCTs).
An estimate from a Cochrane meta-analysis suggests that a cohort of 628 women receiving EFM could expect to experience 1 less neonatal seizure and 11 more cesarean sections compared with IA controls.
Evidence summary
Continuous EFM is designed to detect early fetal hypoxia and thereby decrease neonatal morbidity and mortality compared with IA. IA is defined as auscultation of the fetal heart rate for at least 60 seconds every 15 minutes during the first stage of labor and every 5 minutes during the second stage of labor.
A decrease in seizures, but not deaths or cerebral palsy
A 2006 Cochrane systematic review examined 12 RCTs (with >37,000 women) that compared continuous EFM with IA.1 Continuous EFM reduced the risk of neonatal seizure by 50% (relative risk [RR]=0.50; 95% confidence interval [CI], 0.31-0.80), but had no effect on the rate of neonatal death (RR=0.85; 95% CI, 0.59-1.23) or development of cerebral palsy (RR=1.74; 95% CI, 0.97-3.11).
Reduction of seizures was consistent across all trials. However, a subgroup analysis of high-risk pregnancies (advanced maternal age, diabetes mellitus, chronic hypertension, renal disease, preeclampsia, cardiac disease, renal disease, previous delivery of a low-birth-weight infant) didn’t find a statistically significant decrease in seizures.
Cesarean deliveries rise, regardless of patient risk status
Continuous EFM raised the rates of cesarean delivery (RR=1.66; 95% CI, 1.30-2.13) and instrumental vaginal deliveries (RR=1.16; 95% CI, 1.01-1.32). The increased rate of cesarean section in the EFM group was consistent regardless of clinical risk status (low- vs high-risk women). One additional cesarean section was performed for every 58 women monitored continuously. For “high-risk” women, 1 additional cesarean section was performed for every 12 women monitored continuously.1
Cesarean section rates varied widely among the individual trials (2.3%-35%). Analysis suggested that studies with higher baseline rates showed the greatest increases with continuous EFM. The rate for all studies combined was just 4.3%; 69% of patients included in the meta-analysis were contributed by the Dublin trial, which had an average cesarean rate of 2.3%.1 By comparison, the US Division of Vital Statistics reported a cesarean rate of 32.3% in 2008.2
EFM reduces death from fetal hypoxia
A 1995 meta-analysis, including 9 of the Cochrane review studies with a total of 18,561 women, evaluated the additional outcome of death resulting from fetal hypoxia.3 Compared with IA, EFM was associated with a 59% reduction in death from fetal hypoxia (RR=0.41; 95% CI, 0.17-0.98). Continuous EFM prevented 1 perinatal death per 1000 births. The reduction in perinatal mortality was offset by a 53% increase in cesarean deliveries and a 23% increase in operative vaginal deliveries.3
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) doesn’t recommend for or against continuous fetal heart rate monitoring in uncomplicated labor, recognizing either EFM or IA as acceptable in uncomplicated patients.4 ACOG does recommend continuous EFM for women with high-risk conditions (suspected fetal growth restriction, preeclampsia, and type 1 diabetes mellitus).
The US Preventive Services Task Force doesn’t support routine intrapartum EFM for low-risk woman. The Task Force found insufficient evidence for using EFM in high-risk pregnancies.5
The Royal College of Obstetricians and Gynaecologists and the Royal Australian and New Zealand College of Obstetricians and Gynecologists both recommend continuous EFM for high-risk women and IA for low-risk patients.6,7
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army Medical Department or the US Army at large.
CONTINUOUS ELECTRONIC FETAL MONITORING (EFM) REDUCES THE RISK OF NEONATAL SEIZURE BY 50% compared with intermittent auscultation (IA) (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
EFM increases the incidence of cesarean section by 66% and the incidence of operative vaginal delivery by 16% (SOR: A, systematic review of RCTs). It has no effect on the rates of cerebral palsy or neonatal mortality (SOR: A, systematic review of RCTs).
An estimate from a Cochrane meta-analysis suggests that a cohort of 628 women receiving EFM could expect to experience 1 less neonatal seizure and 11 more cesarean sections compared with IA controls.
Evidence summary
Continuous EFM is designed to detect early fetal hypoxia and thereby decrease neonatal morbidity and mortality compared with IA. IA is defined as auscultation of the fetal heart rate for at least 60 seconds every 15 minutes during the first stage of labor and every 5 minutes during the second stage of labor.
A decrease in seizures, but not deaths or cerebral palsy
A 2006 Cochrane systematic review examined 12 RCTs (with >37,000 women) that compared continuous EFM with IA.1 Continuous EFM reduced the risk of neonatal seizure by 50% (relative risk [RR]=0.50; 95% confidence interval [CI], 0.31-0.80), but had no effect on the rate of neonatal death (RR=0.85; 95% CI, 0.59-1.23) or development of cerebral palsy (RR=1.74; 95% CI, 0.97-3.11).
Reduction of seizures was consistent across all trials. However, a subgroup analysis of high-risk pregnancies (advanced maternal age, diabetes mellitus, chronic hypertension, renal disease, preeclampsia, cardiac disease, renal disease, previous delivery of a low-birth-weight infant) didn’t find a statistically significant decrease in seizures.
Cesarean deliveries rise, regardless of patient risk status
Continuous EFM raised the rates of cesarean delivery (RR=1.66; 95% CI, 1.30-2.13) and instrumental vaginal deliveries (RR=1.16; 95% CI, 1.01-1.32). The increased rate of cesarean section in the EFM group was consistent regardless of clinical risk status (low- vs high-risk women). One additional cesarean section was performed for every 58 women monitored continuously. For “high-risk” women, 1 additional cesarean section was performed for every 12 women monitored continuously.1
Cesarean section rates varied widely among the individual trials (2.3%-35%). Analysis suggested that studies with higher baseline rates showed the greatest increases with continuous EFM. The rate for all studies combined was just 4.3%; 69% of patients included in the meta-analysis were contributed by the Dublin trial, which had an average cesarean rate of 2.3%.1 By comparison, the US Division of Vital Statistics reported a cesarean rate of 32.3% in 2008.2
EFM reduces death from fetal hypoxia
A 1995 meta-analysis, including 9 of the Cochrane review studies with a total of 18,561 women, evaluated the additional outcome of death resulting from fetal hypoxia.3 Compared with IA, EFM was associated with a 59% reduction in death from fetal hypoxia (RR=0.41; 95% CI, 0.17-0.98). Continuous EFM prevented 1 perinatal death per 1000 births. The reduction in perinatal mortality was offset by a 53% increase in cesarean deliveries and a 23% increase in operative vaginal deliveries.3
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) doesn’t recommend for or against continuous fetal heart rate monitoring in uncomplicated labor, recognizing either EFM or IA as acceptable in uncomplicated patients.4 ACOG does recommend continuous EFM for women with high-risk conditions (suspected fetal growth restriction, preeclampsia, and type 1 diabetes mellitus).
The US Preventive Services Task Force doesn’t support routine intrapartum EFM for low-risk woman. The Task Force found insufficient evidence for using EFM in high-risk pregnancies.5
The Royal College of Obstetricians and Gynaecologists and the Royal Australian and New Zealand College of Obstetricians and Gynecologists both recommend continuous EFM for high-risk women and IA for low-risk patients.6,7
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army Medical Department or the US Army at large.
1. Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2006;(3):CD006066.-
2. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2008. Natl Vital Stat Rep. 2010;58(16):1-18.
3. Vintzileos AM, Nochimson DJ, Guzman ER, et al. Intrapartum electronic heart rate monitoring versus intermittent auscultation: a meta-analysis. Obstet Gynecol. 1995;85:149-155.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists, Number 106, July 2009. Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192-202.
5. US Preventive Services Task Force. Screening for intrapartum electronic fetal monitoring. Rockville, MD: Agency for Healthcare Research and Quality; 1996. Available at: www.ahrq.gov/clinic/uspstf/uspsiefm.htm. Accessed March 7, 2010.
6. National Institute for Health and Clinical Excellence (NICE). Intrapartum Care: Management and Delivery of Care to Women in Labour. London: NICE; 2007.
7. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Clinical Guidelines. Intrapartum Fetal Surveillance Guidelines. May 2006. Available at: www.ranzcog.edu.au/publications/womenshealth.shtml. Accessed December 9, 2008.
1. Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2006;(3):CD006066.-
2. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2008. Natl Vital Stat Rep. 2010;58(16):1-18.
3. Vintzileos AM, Nochimson DJ, Guzman ER, et al. Intrapartum electronic heart rate monitoring versus intermittent auscultation: a meta-analysis. Obstet Gynecol. 1995;85:149-155.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists, Number 106, July 2009. Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192-202.
5. US Preventive Services Task Force. Screening for intrapartum electronic fetal monitoring. Rockville, MD: Agency for Healthcare Research and Quality; 1996. Available at: www.ahrq.gov/clinic/uspstf/uspsiefm.htm. Accessed March 7, 2010.
6. National Institute for Health and Clinical Excellence (NICE). Intrapartum Care: Management and Delivery of Care to Women in Labour. London: NICE; 2007.
7. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Clinical Guidelines. Intrapartum Fetal Surveillance Guidelines. May 2006. Available at: www.ranzcog.edu.au/publications/womenshealth.shtml. Accessed December 9, 2008.
Evidence-based answers from the Family Physicians Inquiries Network
Does low-dose aspirin reduce preeclampsia and other maternal-fetal complications?
Yes. The use of low-dose aspirin during pregnancy decreases the risk of preeclampsia for women considered at increased risk. The effect is smaller for women without risk factors (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs] and systematic reviews [SRs] of RCTs).
Rates of preterm delivery, perinatal death, and incidence of small-for-gestational age infants are decreased for women treated with low-dose aspirin (SOR: A, based on SRs and RCTs). A meta-analysis of RCTs has found no increased rates of harm from low-dose aspirin therapy, including placental abruption or other antepartum bleeding complications (SOR: A, based on SRs and RCTs).
I prescribe 81 mg/day of aspirin for women with previous severe preeclampsia
John Hill, DO
Department of Family Medicine, University of Colorado, Denver
Confused about when to use aspirin in pregnancy? You’re not alone. Over my 20 years of practice, I have reacted to disparate guidelines ranging from “never use aspirin in pregnancy” to “always use low-dose aspirin.” This review helps simplify my clinical practice.
With the benefit of evidence from multiple RCTs over the past 7 years, I now personally use 81 mg of aspirin each day in 2 groups of women: those who had severe preeclampsia in a prior pregnancy, and those who develop signs of preeclampsia or strong risk factors for it before the third trimester in their current pregnancy.
Evidence summary
Systematic reviews show aspirin lowers rates of preeclampsia
Four SRs published between 2001 and 20071-4 and a Cochrane Review updated in 20065 have demonstrated that low-dose aspirin helps to prevent preeclampsia, reduction in preterm delivery rates, and decreased perinatal mortality.
The 2001 SR by Duley1 included 39 trials and 30,563 patients. Patients were classified either as high-risk (previous severe preeclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease) or moderate-risk (remainder of subjects). Four individual studies (with a combined weight of 27%) did not support aspirin therapy. The largest trial not supporting aspirin therapy included 6275 subjects and had a relative risk of 1.14 (95% CI, 0.94–1.38).
Most studies in this review compared aspirin alone with placebo (28,802 subjects). However, 4 studies either compared combination therapy with aspirin or other thromboprophylaxis therapy (dipyridamole, heparin, or ozagrel). Although there were differences in risk stratification, variable doses of aspirin, and varied gestational age at trial entry, all studies reported an overall 15% reduction of preeclampsia (RR=0.85; 95% CI, 0.78–0.92).
The 2003 SR by Coomarasamy2 included 14 trials and 12,416 patients. The study exclusively evaluated high-risk pregnancies: women with history (or family history) of preeclampsia, chronic hypertension, gestational diabetes, or renal disease. The overall reduction in preeclampsia was 14% (relative risk [RR]=0.86; 95% confidence interval [CI], 0.76–0.96). Results were consistent across RCTs, and only 2 of the 14 studies (with a combined weight of 7.1%) did not support aspirin therapy.
TABLE
Low-dose aspirin reduces risk of preeclampsia, but how does it affect other maternal and fetal outcomes?
| STUDY (YEAR) | DEVELOPMENT OF PREECLAMPSIA | PRETERM DELIVERY | NEONATAL DEATH | SGA OR LOW BIRTH WEIGHT | RISK OF ABRUPTION & BLEEDING |
|---|---|---|---|---|---|
| Duley (2001)1 | Moderate-risk patients: 15% reduction High-risk patients: 15% reduction NNT=100 | 8% reduction NNT=72 | 14% reduction NNT= 250 | 8% reduction* | Not reported |
| Coomarasamy (2003)2 | 14% reduction | 14% reduction | 21% reduction | 215-g weight gain in aspirin group | No significant clinical difference in risk RR=0.98) |
| Ruano (2005)3 | Low-risk patients: no significant reduction High-risk patients: 13% reduction | Not reported | |||
| Askie (2007)4 | 10% reduction | 10% reduction | 9% reduction | 10% reduction | No significant clinical difference in risk (RR=0.90–1.15) |
| Cochrane (2007)5 | 19% reduction NNT=69 (overall), 118 (moderate risk), 18 (high-risk) | 7% reduction NNT=83 | 16% reduction NNT=227 | 8% reduction* | No significant clinical difference in risk (RR=1.06) |
| * Borderline for statistical significance (RR=0.92). | |||||
| SGA, small for gestational age; NNT, number needed to treat; RR, relative risk. | |||||
Ruano’s 2005 SR3 included 22 trials with 33,598 subjects and specifically compared low-risk vs high-risk patients. The authors concluded that there was no significant reduction in preeclampsia with the use of low-dose aspirin in the low-risk arm (RR=0.95; 95% CI, 0.81–1.11), and a 13% reduction among high-risk subjects (RR=0.87; 95% CI, 0.79–0.96).3
A 2007 meta-analysis by Askie4 included 31 trials with 32,217 women and their 32,819 infants. Main outcomes (regardless of initial maternal risks) were 1) onset of preeclampsia, 2) neonatal death, 3) preterm birth at <34 weeks gestation, 4) infant small for gestational age, and 5) pregnancy with serious adverse outcome. Results of these outcome measures consistently showed a relative risk reduction of 10% for subjects taking low-dose aspirin, except for neonatal deaths, which had a 9% reduction. This study also suggested that multiparous women and women with a history of hypertensive disorder of pregnancy may derive a larger benefit from low-dose aspirin.
A Cochrane Review5 updated in 2007 demonstrated that low-dose aspirin provided a moderate (19%) reduction in the overall risk of developing preeclampsia. New stratified analysis of the data indicates that in moderate-risk women, antiplatelet therapy is associated with a 15% reduction, and that high-risk women have a 27% reduction in the risk of developing preeclampsia. The effect on small-for-gestational-age infants revealed no overall clinically significant differences.
Aspirin dosing: One study recommends >75 mg/day
Studies varied in the aspirin dosage they used and duration of treatment. In all RCTs, the dose of aspirin ranged from 50 mg/day to 150 mg/day. Earlier trials used lower doses of aspirin (50–75 mg/day), while recent trials used 100 mg or more per day.
Early RCTs revealed no correlation between the dose of aspirin and the prevention of preeclampsia. However, Villar et al6 showed a greater effect among women treated with doses greater than 75 mg/day of aspirin (RR=0.49; 95% CI, 0.38–0.63).6
No evidence of harm from aspirin
There is no evidence of harm from low-dose aspirin therapy—including placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other neonatal bleeding complications, admission to neonatal care unit, induction of labor, or caesarean delivery—regardless of initial risk stratification.7
Recommendations from others
The 2002 American College of Obstetricians and Gynecologists Practice Bulletin states that low-dose aspirin in women at low risk has not been shown to prevent preeclampsia and therefore is not recommended. They make no specific statement regarding the use of low-dose aspirin in moderate- to high-risk pregnancies.8
The Australasian Society for the Study of Hypertension in Pregnancy conclude that low-dose aspirin for prevention of preeclampsia is reasonable for the following conditions: 1) prior fetal loss after first trimester due to placental insufficiency or severe fetal growth retardation, and 2) women with severe early onset preeclampsia in previous pregnancy necessitating delivery ≤32 weeks gestation. Despite difficulties in predicting who will deliver preterm, consider women who have had severe early-onset preeclampsia in a previous pregnancy for low-dose aspirin therapy.9
The Canadian Hypertension Society Consensus Panel concludes low-dose aspirin therapy is effective in decreasing the incidence of preterm delivery and early-onset preeclampsia among women at risk of developing the syndrome.10
1. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ 2001;322:329-333.
2. Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of pre-eclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003;101:1319-1332.
3. Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin—a systematic review and meta-analysis of the main randomized controlled trials. Clinics 2005;60:407-414.
4. Askie LM, Duley L, Henderson-Stewart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-1798.
5. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complication (review). Cochrane Database Syst Rev 2003;(4):CD004659.-
6. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from randomized controlled trials. Semin Nephrol 2004;24:607-615.
7. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent pre-eclampsia: a framework for clinical decision making. BJOG 2003;110:882-888.
8. ACOG Committee on Obstetric Practice. Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin, no 33. Int J Gynaecol Obstet 2002;77:67-75.
9. Brown MA, Brennecke SP, Crowther CA, et al. Aspirin and prevention of pre-eclampsia. Aust NZ J Obstet Gynaecol 1995;35:38-41.
10. Moutquin JM, Garner PR, Burrows RF, Rey E, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;57:907-919.
Yes. The use of low-dose aspirin during pregnancy decreases the risk of preeclampsia for women considered at increased risk. The effect is smaller for women without risk factors (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs] and systematic reviews [SRs] of RCTs).
Rates of preterm delivery, perinatal death, and incidence of small-for-gestational age infants are decreased for women treated with low-dose aspirin (SOR: A, based on SRs and RCTs). A meta-analysis of RCTs has found no increased rates of harm from low-dose aspirin therapy, including placental abruption or other antepartum bleeding complications (SOR: A, based on SRs and RCTs).
I prescribe 81 mg/day of aspirin for women with previous severe preeclampsia
John Hill, DO
Department of Family Medicine, University of Colorado, Denver
Confused about when to use aspirin in pregnancy? You’re not alone. Over my 20 years of practice, I have reacted to disparate guidelines ranging from “never use aspirin in pregnancy” to “always use low-dose aspirin.” This review helps simplify my clinical practice.
With the benefit of evidence from multiple RCTs over the past 7 years, I now personally use 81 mg of aspirin each day in 2 groups of women: those who had severe preeclampsia in a prior pregnancy, and those who develop signs of preeclampsia or strong risk factors for it before the third trimester in their current pregnancy.
Evidence summary
Systematic reviews show aspirin lowers rates of preeclampsia
Four SRs published between 2001 and 20071-4 and a Cochrane Review updated in 20065 have demonstrated that low-dose aspirin helps to prevent preeclampsia, reduction in preterm delivery rates, and decreased perinatal mortality.
The 2001 SR by Duley1 included 39 trials and 30,563 patients. Patients were classified either as high-risk (previous severe preeclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease) or moderate-risk (remainder of subjects). Four individual studies (with a combined weight of 27%) did not support aspirin therapy. The largest trial not supporting aspirin therapy included 6275 subjects and had a relative risk of 1.14 (95% CI, 0.94–1.38).
Most studies in this review compared aspirin alone with placebo (28,802 subjects). However, 4 studies either compared combination therapy with aspirin or other thromboprophylaxis therapy (dipyridamole, heparin, or ozagrel). Although there were differences in risk stratification, variable doses of aspirin, and varied gestational age at trial entry, all studies reported an overall 15% reduction of preeclampsia (RR=0.85; 95% CI, 0.78–0.92).
The 2003 SR by Coomarasamy2 included 14 trials and 12,416 patients. The study exclusively evaluated high-risk pregnancies: women with history (or family history) of preeclampsia, chronic hypertension, gestational diabetes, or renal disease. The overall reduction in preeclampsia was 14% (relative risk [RR]=0.86; 95% confidence interval [CI], 0.76–0.96). Results were consistent across RCTs, and only 2 of the 14 studies (with a combined weight of 7.1%) did not support aspirin therapy.
TABLE
Low-dose aspirin reduces risk of preeclampsia, but how does it affect other maternal and fetal outcomes?
| STUDY (YEAR) | DEVELOPMENT OF PREECLAMPSIA | PRETERM DELIVERY | NEONATAL DEATH | SGA OR LOW BIRTH WEIGHT | RISK OF ABRUPTION & BLEEDING |
|---|---|---|---|---|---|
| Duley (2001)1 | Moderate-risk patients: 15% reduction High-risk patients: 15% reduction NNT=100 | 8% reduction NNT=72 | 14% reduction NNT= 250 | 8% reduction* | Not reported |
| Coomarasamy (2003)2 | 14% reduction | 14% reduction | 21% reduction | 215-g weight gain in aspirin group | No significant clinical difference in risk RR=0.98) |
| Ruano (2005)3 | Low-risk patients: no significant reduction High-risk patients: 13% reduction | Not reported | |||
| Askie (2007)4 | 10% reduction | 10% reduction | 9% reduction | 10% reduction | No significant clinical difference in risk (RR=0.90–1.15) |
| Cochrane (2007)5 | 19% reduction NNT=69 (overall), 118 (moderate risk), 18 (high-risk) | 7% reduction NNT=83 | 16% reduction NNT=227 | 8% reduction* | No significant clinical difference in risk (RR=1.06) |
| * Borderline for statistical significance (RR=0.92). | |||||
| SGA, small for gestational age; NNT, number needed to treat; RR, relative risk. | |||||
Ruano’s 2005 SR3 included 22 trials with 33,598 subjects and specifically compared low-risk vs high-risk patients. The authors concluded that there was no significant reduction in preeclampsia with the use of low-dose aspirin in the low-risk arm (RR=0.95; 95% CI, 0.81–1.11), and a 13% reduction among high-risk subjects (RR=0.87; 95% CI, 0.79–0.96).3
A 2007 meta-analysis by Askie4 included 31 trials with 32,217 women and their 32,819 infants. Main outcomes (regardless of initial maternal risks) were 1) onset of preeclampsia, 2) neonatal death, 3) preterm birth at <34 weeks gestation, 4) infant small for gestational age, and 5) pregnancy with serious adverse outcome. Results of these outcome measures consistently showed a relative risk reduction of 10% for subjects taking low-dose aspirin, except for neonatal deaths, which had a 9% reduction. This study also suggested that multiparous women and women with a history of hypertensive disorder of pregnancy may derive a larger benefit from low-dose aspirin.
A Cochrane Review5 updated in 2007 demonstrated that low-dose aspirin provided a moderate (19%) reduction in the overall risk of developing preeclampsia. New stratified analysis of the data indicates that in moderate-risk women, antiplatelet therapy is associated with a 15% reduction, and that high-risk women have a 27% reduction in the risk of developing preeclampsia. The effect on small-for-gestational-age infants revealed no overall clinically significant differences.
Aspirin dosing: One study recommends >75 mg/day
Studies varied in the aspirin dosage they used and duration of treatment. In all RCTs, the dose of aspirin ranged from 50 mg/day to 150 mg/day. Earlier trials used lower doses of aspirin (50–75 mg/day), while recent trials used 100 mg or more per day.
Early RCTs revealed no correlation between the dose of aspirin and the prevention of preeclampsia. However, Villar et al6 showed a greater effect among women treated with doses greater than 75 mg/day of aspirin (RR=0.49; 95% CI, 0.38–0.63).6
No evidence of harm from aspirin
There is no evidence of harm from low-dose aspirin therapy—including placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other neonatal bleeding complications, admission to neonatal care unit, induction of labor, or caesarean delivery—regardless of initial risk stratification.7
Recommendations from others
The 2002 American College of Obstetricians and Gynecologists Practice Bulletin states that low-dose aspirin in women at low risk has not been shown to prevent preeclampsia and therefore is not recommended. They make no specific statement regarding the use of low-dose aspirin in moderate- to high-risk pregnancies.8
The Australasian Society for the Study of Hypertension in Pregnancy conclude that low-dose aspirin for prevention of preeclampsia is reasonable for the following conditions: 1) prior fetal loss after first trimester due to placental insufficiency or severe fetal growth retardation, and 2) women with severe early onset preeclampsia in previous pregnancy necessitating delivery ≤32 weeks gestation. Despite difficulties in predicting who will deliver preterm, consider women who have had severe early-onset preeclampsia in a previous pregnancy for low-dose aspirin therapy.9
The Canadian Hypertension Society Consensus Panel concludes low-dose aspirin therapy is effective in decreasing the incidence of preterm delivery and early-onset preeclampsia among women at risk of developing the syndrome.10
Yes. The use of low-dose aspirin during pregnancy decreases the risk of preeclampsia for women considered at increased risk. The effect is smaller for women without risk factors (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs] and systematic reviews [SRs] of RCTs).
Rates of preterm delivery, perinatal death, and incidence of small-for-gestational age infants are decreased for women treated with low-dose aspirin (SOR: A, based on SRs and RCTs). A meta-analysis of RCTs has found no increased rates of harm from low-dose aspirin therapy, including placental abruption or other antepartum bleeding complications (SOR: A, based on SRs and RCTs).
I prescribe 81 mg/day of aspirin for women with previous severe preeclampsia
John Hill, DO
Department of Family Medicine, University of Colorado, Denver
Confused about when to use aspirin in pregnancy? You’re not alone. Over my 20 years of practice, I have reacted to disparate guidelines ranging from “never use aspirin in pregnancy” to “always use low-dose aspirin.” This review helps simplify my clinical practice.
With the benefit of evidence from multiple RCTs over the past 7 years, I now personally use 81 mg of aspirin each day in 2 groups of women: those who had severe preeclampsia in a prior pregnancy, and those who develop signs of preeclampsia or strong risk factors for it before the third trimester in their current pregnancy.
Evidence summary
Systematic reviews show aspirin lowers rates of preeclampsia
Four SRs published between 2001 and 20071-4 and a Cochrane Review updated in 20065 have demonstrated that low-dose aspirin helps to prevent preeclampsia, reduction in preterm delivery rates, and decreased perinatal mortality.
The 2001 SR by Duley1 included 39 trials and 30,563 patients. Patients were classified either as high-risk (previous severe preeclampsia, diabetes, chronic hypertension, renal disease, or autoimmune disease) or moderate-risk (remainder of subjects). Four individual studies (with a combined weight of 27%) did not support aspirin therapy. The largest trial not supporting aspirin therapy included 6275 subjects and had a relative risk of 1.14 (95% CI, 0.94–1.38).
Most studies in this review compared aspirin alone with placebo (28,802 subjects). However, 4 studies either compared combination therapy with aspirin or other thromboprophylaxis therapy (dipyridamole, heparin, or ozagrel). Although there were differences in risk stratification, variable doses of aspirin, and varied gestational age at trial entry, all studies reported an overall 15% reduction of preeclampsia (RR=0.85; 95% CI, 0.78–0.92).
The 2003 SR by Coomarasamy2 included 14 trials and 12,416 patients. The study exclusively evaluated high-risk pregnancies: women with history (or family history) of preeclampsia, chronic hypertension, gestational diabetes, or renal disease. The overall reduction in preeclampsia was 14% (relative risk [RR]=0.86; 95% confidence interval [CI], 0.76–0.96). Results were consistent across RCTs, and only 2 of the 14 studies (with a combined weight of 7.1%) did not support aspirin therapy.
TABLE
Low-dose aspirin reduces risk of preeclampsia, but how does it affect other maternal and fetal outcomes?
| STUDY (YEAR) | DEVELOPMENT OF PREECLAMPSIA | PRETERM DELIVERY | NEONATAL DEATH | SGA OR LOW BIRTH WEIGHT | RISK OF ABRUPTION & BLEEDING |
|---|---|---|---|---|---|
| Duley (2001)1 | Moderate-risk patients: 15% reduction High-risk patients: 15% reduction NNT=100 | 8% reduction NNT=72 | 14% reduction NNT= 250 | 8% reduction* | Not reported |
| Coomarasamy (2003)2 | 14% reduction | 14% reduction | 21% reduction | 215-g weight gain in aspirin group | No significant clinical difference in risk RR=0.98) |
| Ruano (2005)3 | Low-risk patients: no significant reduction High-risk patients: 13% reduction | Not reported | |||
| Askie (2007)4 | 10% reduction | 10% reduction | 9% reduction | 10% reduction | No significant clinical difference in risk (RR=0.90–1.15) |
| Cochrane (2007)5 | 19% reduction NNT=69 (overall), 118 (moderate risk), 18 (high-risk) | 7% reduction NNT=83 | 16% reduction NNT=227 | 8% reduction* | No significant clinical difference in risk (RR=1.06) |
| * Borderline for statistical significance (RR=0.92). | |||||
| SGA, small for gestational age; NNT, number needed to treat; RR, relative risk. | |||||
Ruano’s 2005 SR3 included 22 trials with 33,598 subjects and specifically compared low-risk vs high-risk patients. The authors concluded that there was no significant reduction in preeclampsia with the use of low-dose aspirin in the low-risk arm (RR=0.95; 95% CI, 0.81–1.11), and a 13% reduction among high-risk subjects (RR=0.87; 95% CI, 0.79–0.96).3
A 2007 meta-analysis by Askie4 included 31 trials with 32,217 women and their 32,819 infants. Main outcomes (regardless of initial maternal risks) were 1) onset of preeclampsia, 2) neonatal death, 3) preterm birth at <34 weeks gestation, 4) infant small for gestational age, and 5) pregnancy with serious adverse outcome. Results of these outcome measures consistently showed a relative risk reduction of 10% for subjects taking low-dose aspirin, except for neonatal deaths, which had a 9% reduction. This study also suggested that multiparous women and women with a history of hypertensive disorder of pregnancy may derive a larger benefit from low-dose aspirin.
A Cochrane Review5 updated in 2007 demonstrated that low-dose aspirin provided a moderate (19%) reduction in the overall risk of developing preeclampsia. New stratified analysis of the data indicates that in moderate-risk women, antiplatelet therapy is associated with a 15% reduction, and that high-risk women have a 27% reduction in the risk of developing preeclampsia. The effect on small-for-gestational-age infants revealed no overall clinically significant differences.
Aspirin dosing: One study recommends >75 mg/day
Studies varied in the aspirin dosage they used and duration of treatment. In all RCTs, the dose of aspirin ranged from 50 mg/day to 150 mg/day. Earlier trials used lower doses of aspirin (50–75 mg/day), while recent trials used 100 mg or more per day.
Early RCTs revealed no correlation between the dose of aspirin and the prevention of preeclampsia. However, Villar et al6 showed a greater effect among women treated with doses greater than 75 mg/day of aspirin (RR=0.49; 95% CI, 0.38–0.63).6
No evidence of harm from aspirin
There is no evidence of harm from low-dose aspirin therapy—including placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other neonatal bleeding complications, admission to neonatal care unit, induction of labor, or caesarean delivery—regardless of initial risk stratification.7
Recommendations from others
The 2002 American College of Obstetricians and Gynecologists Practice Bulletin states that low-dose aspirin in women at low risk has not been shown to prevent preeclampsia and therefore is not recommended. They make no specific statement regarding the use of low-dose aspirin in moderate- to high-risk pregnancies.8
The Australasian Society for the Study of Hypertension in Pregnancy conclude that low-dose aspirin for prevention of preeclampsia is reasonable for the following conditions: 1) prior fetal loss after first trimester due to placental insufficiency or severe fetal growth retardation, and 2) women with severe early onset preeclampsia in previous pregnancy necessitating delivery ≤32 weeks gestation. Despite difficulties in predicting who will deliver preterm, consider women who have had severe early-onset preeclampsia in a previous pregnancy for low-dose aspirin therapy.9
The Canadian Hypertension Society Consensus Panel concludes low-dose aspirin therapy is effective in decreasing the incidence of preterm delivery and early-onset preeclampsia among women at risk of developing the syndrome.10
1. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ 2001;322:329-333.
2. Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of pre-eclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003;101:1319-1332.
3. Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin—a systematic review and meta-analysis of the main randomized controlled trials. Clinics 2005;60:407-414.
4. Askie LM, Duley L, Henderson-Stewart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-1798.
5. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complication (review). Cochrane Database Syst Rev 2003;(4):CD004659.-
6. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from randomized controlled trials. Semin Nephrol 2004;24:607-615.
7. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent pre-eclampsia: a framework for clinical decision making. BJOG 2003;110:882-888.
8. ACOG Committee on Obstetric Practice. Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin, no 33. Int J Gynaecol Obstet 2002;77:67-75.
9. Brown MA, Brennecke SP, Crowther CA, et al. Aspirin and prevention of pre-eclampsia. Aust NZ J Obstet Gynaecol 1995;35:38-41.
10. Moutquin JM, Garner PR, Burrows RF, Rey E, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;57:907-919.
1. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ 2001;322:329-333.
2. Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of pre-eclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003;101:1319-1332.
3. Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin—a systematic review and meta-analysis of the main randomized controlled trials. Clinics 2005;60:407-414.
4. Askie LM, Duley L, Henderson-Stewart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-1798.
5. Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complication (review). Cochrane Database Syst Rev 2003;(4):CD004659.-
6. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from randomized controlled trials. Semin Nephrol 2004;24:607-615.
7. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent pre-eclampsia: a framework for clinical decision making. BJOG 2003;110:882-888.
8. ACOG Committee on Obstetric Practice. Diagnosis and management of pre-eclampsia and eclampsia. ACOG Practice Bulletin, no 33. Int J Gynaecol Obstet 2002;77:67-75.
9. Brown MA, Brennecke SP, Crowther CA, et al. Aspirin and prevention of pre-eclampsia. Aust NZ J Obstet Gynaecol 1995;35:38-41.
10. Moutquin JM, Garner PR, Burrows RF, Rey E, et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. Can Med Assoc J 1997;57:907-919.
Evidence-based answers from the Family Physicians Inquiries Network