User login
Identification and Management of Middle East Respiratory Syndrome
Human coronaviruses (CoVs) were first identified in the mid-1960s. Coronaviruses are a large family of viruses that cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS).1
In 2003, SARS caused one of the most devastating global epidemics known to the developed world. The important lesson learned from the SARS epidemic was that CoVs can cause severe and rapidly spreading infection. Since then, 2 human CoVs, HCoV-HKU1 and HCoV-NL63, have been identified as common causes of human respiratory tract infections.2,3 In September 2012, a novel CoV was recognized to cause a fatal human infection. This virus has become known as the Middle East respiratory syndrome CoV (MERS-CoV).4
Related: Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Similar to SARS-CoV, MERS-CoV human infection has a high fatality rate and the ability to spread from person to person.5,6 Person-to-person transmission has resulted in secondary cases among close contacts, including health care providers (HCPs) who should, therefore, be cognizant of this infection. Federal HCPs in particular may be more likely to become involved in the care of patients with this disease, because many military personnel are returning from deployment in the Middle East.
History of MERS-CoV
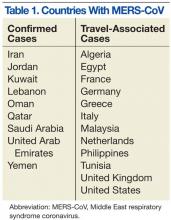
MERS-CoV was first identified as an infectious disease in humans in Saudi Arabia. In June 2012, the index case was hospitalized with pneumonia and acute renal injury.7 Since then, MERS-CoV human infections and clusters of infection have been identified in multiple countries in the Arabian Peninsula (Table 1).8 There have also been cases of MERS-CoV infection in other countries involving travelers who had visited the Arabian Peninsula and, in some instances, after returning home, their close contacts (Table 1).8
On May 2, 2014, the first confirmed U.S. case was reported in Indiana in a HCP who had recently been to Saudi Arabia.9 A second case of a HCP traveling from Saudi Arabia was identified on May 13, 2014, in Orlando, Florida.9 As of June 11, 2014, 699 laboratory‐confirmed cases of MERS-CoV infection had been reported to the World Health Organization in 20 countries, resulting in 209 deaths. All cases to date have originated in 6 countries of the Arabian Peninsula.5,8
The Organism
Coronaviruses are enveloped RNA viruses named for the crownlike spikes on their surface. They are common viruses known to cause respiratory infections in humans.1 It is thought that most people are infected with these viruses during their lifetime. These viruses generally cause mild-to-moderate upper respiratory tract illnesses, otherwise known as the common cold. On occasion, CoVs can cause lower respiratory tract infections in elderly patients, neonates, and immune-compromised individuals.1
Related: Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Coronaviruses are also known to infect animals. Most known CoVs cause disease in only 1 animal species or, at most, in a small number of closely related species. However, SARS-CoV was noted to infect people and various animals, including monkeys, civets, raccoon dogs, cats, dogs, and rodents. The origin and natural reservoir of SARS-CoV was ultimately determined to be bats.10
Genetic sequencing has determined that the MERS-CoV is different from any other known human CoV. MERS-CoV is a beta-CoV and, like the SARS-CoV, is closely related to bat CoVs.11-15 The origin of the MERS-CoV is not known, but an animal reservoir is suspected. Because MERS-CoV is similar to SARS-CoV, bats are considered a possible animal reservoir. Dromedary camels may act as intermediate hosts by spreading the virus to humans.16-18 However, there is no consensus on the animal reservoir for MERS-CoV. It is also not known how the virus has spread from animals to humans.
Case Definition
In order to aid in the rapid recognition of MERS, the CDC has established case definitions.8
A patient under investigation is an individual with fever (> 38oC, > 100.4oF) and pneumonia or acute respiratory distress syndrome (ARDS); and either:
- history of travel from countries in or near the Arabian Peninsula within 14 days before the onset of symptoms; or
- close contact with a symptomatic traveler who developed fever and ARDS within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
A confirmed case is a patient with laboratory confirmation of MERS-CoV infection. A probable case is a patient under investigation with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact of a laboratory- confirmed MERS-CoV case.
Transmission
MERS-CoV is thought to be of animal origin, but the mode of transmission from the animal reservoir is not known. It seems likely that some of the infections have occurred via intermittent zoonotic transmission, possibly by an environmental source.19 The presence of case clusters, however, suggests that human-to-human transmission also can occur. Human-to-human transmission has occurred in individuals living with an infected person and in HCPs caring for infected patients.20-24 The human-to-human transmission through close contact so far has been nonsustained.
It has been estimated that 75% of the reported cases are secondary, meaning that the patient acquired the MERS-CoV infection from another infected person. There is no evidence of sustained spread of MERS-CoV in community settings. The mode of human-to-human transmission has not been determined. Possible modes of transmission include droplet and contact transmission. The number of contacts infected by individuals with confirmed infections seems limited; the transmissibility, therefore, currently seems to be low.25,26 The results of a study of the transmissibility and epidemic potential for MERS-CoV suggest that it does not yet have pandemic potential.27
Bats may serve as a reservoir for MERS-CoV. However, because human contact with bats is uncommon, they are viewed as unlikely candidates for an immediate source of infection in most humans. It is possible that another animal or vector serves as an intermediate host. Camels have been proposed as a possible intermediate host, but this remains unproven. Interestingly, the MERS-CoV index patient had been caring for several ill camels in his herd; the camels had signs of respiratory illness, including nasal discharge.11,28 MERS-CoV sequences were subsequently isolated from a juvenile camel belonging to the index patient.
Symptoms
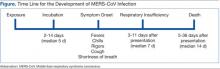
The incubation time after exposure to symptom onset ranges from 1.9 to 14.7 days (Figure). The median incubation period is 5.2 days.21 Patients are not believed to be contagious during the incubation period. Early symptoms of human infection with MERS-CoV include fever, chills or rigors, cough, and shortness of breath. Less frequently encountered symptoms include hemoptysis, sore throat, myalgias, diarrhea, vomiting, and abdominal pain (Table 2).20,21,23,26,27,29 Many patients infected with MERS-CoV develop a severe lower respiratory tract illness. The patient may progress to ARDS and require intubation and mechanical ventilator support. Mechanical ventilation has been required in 72% of patients.29 The median time from presentation for medical care to respiratory failure is 7 days, ranging from 3 to 11 days (Figure).
Physical Examination
The patients with MERS-CoV infection have been predominantly male and middle aged with an average age of 52 years. The clinical features depend on the severity of the illness. Some infected individuals have remained asymptomatic.27 Other patients have experienced mild lower respiratory illness and have not required hospitalization. However, about 40% of patients have experienced severe illness with pneumonia, respiratory insufficiency, multi-organ failure, and death. The percentage of severe illness is likely an overestimation, because patients with less severe symptoms probably are not tested for MERS-CoV. Most of the patients who have experienced a severe illness and/or death also had underlying comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, and chronic renal disease.23,29
Laboratory Data
As with SARS-CoV, lymphopenia has been reported in patients infected with MERS-CoV.29 Other complete blood cell count abnormalities include leukopenia, lymphocytosis, thrombocytopenia, and anemia (Table 3).23,24,26,30 Blood chemistry profiles have identified elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels.29 Some patients have experienced progressive renal failure signaled by rising serum creatinine and blood urea nitrogen levels.11,23,24,26 Testing for disseminated intravascular coagulation and hemolysis has been positive in some patients.20,30 Oxyhemoglobin desaturation develops in patients with severe pneumonia.
Radiographic Imaging
Chest radiographs have been abnormal in the majority of patients with MERS-CoV. The radiographic findings may be minimal to extensive, depending on the severity of illness. The reported radiographic abnormalities include increased bronchovesicular markings, airspace opacities, patchy infiltrates, interstitial changes, confluent consolidations, nodular opacities, reticular infiltrate, pleural effusion, and total opacification of lung segments and lobes. These radiographic findings may be unilateral or bilateral.29
Specific Testing for MERS-CoV
The CDC recommends that lower respiratory tract specimens be collected for testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). The FDA has issued an emergency use authorization of the rRT-PCR assay developed by the CDC. The CDC recommends that multiple specimens from different sites in the lower respiratory system be collected at different times to increase the likelihood of detecting MERS-CoV. Acute and convalescent serum samples also should be obtained for serologic testing. Lower respiratory specimens (sputum, tracheal aspirates, and bronchoalveolar lavage fluid) are more sensitive than are upper respiratory tract samples (nasopharyngeal throat swabs and nasopharyngeal aspirates). Respiratory specimens should be collected as soon as possible after symptom onset. If negative testing is obtained from a patient with a high index of suspicion for MERS-CoV infection, then repeat testing should be performed.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneumonia
Several serology assays have been developed for the detection of MERS-CoV. An immunofluorescence assay should be confirmed with a neutralization test. In certain cases, the diagnosis should be confirmed by nucleic acid sequencing. The CDC has developed MERS-CoV testing kits, which have been provided to state health departments. Any case of suspected or proven MERS-CoV in the U.S. should be reported to the state and local health departments. Visit the CDC website for more information about collecting, handling, and testing clinical specimens from patients under investigation for MERS: http://www.cdc.gov/coronavirus/mers/guidelines -clinical-specimens.html.
Prognosis
Complications from the MERS- CoV infection include severe pneumonia and ARDS requiring mechanical ventilation, multi-organ failure, renal failure requiring dialysis, consumptive coagulopathy, and pericarditis.20,21,23,26,27,29 About 30% of people with MERS-CoV have died. SARS-CoV was the first CoV to cause severe lower respiratory disease and death in otherwise healthy humans; MERS-CoV is now the second.6 Death occurs a median of 14 days after presentation with a range of 5 to 36 days.20,21,23,26,27,29
Treatment
There is no available specific therapy recommended for MERS-CoV infection; therefore, the management of patients is supportive. As with other CoVs, there is no antiviral agent treatment for MERS-CoV. In experimental settings, combination therapy with interferon-alpha-2b and ribavirin seems promising.31 However, critically ill patients with MERS-CoV did not seem to respond favorably when treated with this regimen.32
Vaccine
There is no licensed vaccine for MERS-CoV, although experimental vaccines are being developed. Vaccines have successfully prevented CoV infection in animal models. The development of an effective vaccine for humans against MERS-CoV may, therefore, be a realistic possibility. Unfortunately, a vaccine is likely years away from approval.
Infection Control Measures
Careful attention to infection control precautions is critical to the containment of MERS-CoV. Patients should be encouraged to inform HCPs about symptoms and potential exposure risks, in particular travel to and/or exposure to travelers from the Arabian Peninsula. This practice should help to limit the transmission of MERS-CoV to HCPs. Standard contact and airborne precautions should be followed in patients with suspected or proven MERS-CoV infection.
Infection control measures should include hand hygiene; avoiding close contact with people who are sick; avoiding touching the eyes, nose, and/or mouth with unwashed hands; and disinfecting frequently touched surfaces. Patients with suspected or proven MERS-CoV should be admitted to single occupancy rooms to diminish the possibility of viral transmission to other patients. All persons entering the room of a patient with suspected or proven MERS-CoV should wear fitted N-95 filtering respirators. Until the mode of transmission is better defined, protective eyewear should be worn during all patient contacts. With implementation of these measures, there has been no institution that has experienced an outbreak of MERS-CoV infection. Unfortunately, the duration of viral shedding is not yet known.
Travel Restrictions
At this time the CDC has not recommended MERS-related travel restrictions. Because the spread of MERS-CoV has occurred in health care institutions, the CDC advises HCPs traveling to the Arabian Peninsula to follow recommendations for infection control of confirmed or suspected cases of MERS-CoV and to monitor their own health closely. Travelers who are going to the Arabian Peninsula for other reasons are advised to follow standard infection control precautions, such as hand washing and avoiding contact with ill people. Visit the CDC website for updated information of travel restrictions: http://www.cdc.gov/coronavirus/mers/travel.html.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103-S108.
2. Woo PC, Lau SK, Chu CM, et al. Characteristics and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884-895.
3. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368-373.
4. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790-7792.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update–as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf. Accessed February 3, 2015.
6. Byrd RP Jr, Roy TM. Severe acute respiratory syndrome. Fed Pract. 2003;20(9):62-71.
7. International Society for Infectious Diseases. Novel coronavirus–Saudi Arabia: Human isolate; Archive number: 20120920.1302733. ProMED-mail Website. http://www.promedmail.org/direct .php?id=20120920.1302733. Published September 20, 2012. Accessed January 23, 2015.
8. Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers. Updated July 31, 2014. Accessed January 23, 2015.
9. Centers for Disease Control and Prevention. MERS in the U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers/US.html. Updated December 9, 2014. Accessed January 23, 2015.
10. Lau SK, Li KS, Huang Y, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6): 2808-2819.
11. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
12. Cotten M, Lam TT, Watson SJ, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736-742B.
13. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456-459.
14. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697-1699.
15. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819-1823.
16. Chu DK, Poon LL, Gomaa MM, et al. MERS coronavirus in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049-1053.
17. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis. 2013;13(10):859-866.
18. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis. 2014;14(2):140-145.
19. Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch Virol. 2014;159(7):1575-1584.
20. World Health Organization. Global Alert and Response. MERS-CoV summary and literature update—as of 31 May 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130531 /en. Accessed January 23, 2015.
21. Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416.
22. Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114.
23. Guery B, Poissy J, el Mansouf L, et al; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381(9885):2265-2272.
24. Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487-2494.
25. Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20(4):620-625.
26. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397.
27. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369(9):884-886.
28. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012-1015.
29. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13(9):752-761.
30. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745-751.
31. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313-1317.
32. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis. 2014;20:42-46.
Human coronaviruses (CoVs) were first identified in the mid-1960s. Coronaviruses are a large family of viruses that cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS).1
In 2003, SARS caused one of the most devastating global epidemics known to the developed world. The important lesson learned from the SARS epidemic was that CoVs can cause severe and rapidly spreading infection. Since then, 2 human CoVs, HCoV-HKU1 and HCoV-NL63, have been identified as common causes of human respiratory tract infections.2,3 In September 2012, a novel CoV was recognized to cause a fatal human infection. This virus has become known as the Middle East respiratory syndrome CoV (MERS-CoV).4
Related: Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Similar to SARS-CoV, MERS-CoV human infection has a high fatality rate and the ability to spread from person to person.5,6 Person-to-person transmission has resulted in secondary cases among close contacts, including health care providers (HCPs) who should, therefore, be cognizant of this infection. Federal HCPs in particular may be more likely to become involved in the care of patients with this disease, because many military personnel are returning from deployment in the Middle East.
History of MERS-CoV
MERS-CoV was first identified as an infectious disease in humans in Saudi Arabia. In June 2012, the index case was hospitalized with pneumonia and acute renal injury.7 Since then, MERS-CoV human infections and clusters of infection have been identified in multiple countries in the Arabian Peninsula (Table 1).8 There have also been cases of MERS-CoV infection in other countries involving travelers who had visited the Arabian Peninsula and, in some instances, after returning home, their close contacts (Table 1).8
On May 2, 2014, the first confirmed U.S. case was reported in Indiana in a HCP who had recently been to Saudi Arabia.9 A second case of a HCP traveling from Saudi Arabia was identified on May 13, 2014, in Orlando, Florida.9 As of June 11, 2014, 699 laboratory‐confirmed cases of MERS-CoV infection had been reported to the World Health Organization in 20 countries, resulting in 209 deaths. All cases to date have originated in 6 countries of the Arabian Peninsula.5,8
The Organism
Coronaviruses are enveloped RNA viruses named for the crownlike spikes on their surface. They are common viruses known to cause respiratory infections in humans.1 It is thought that most people are infected with these viruses during their lifetime. These viruses generally cause mild-to-moderate upper respiratory tract illnesses, otherwise known as the common cold. On occasion, CoVs can cause lower respiratory tract infections in elderly patients, neonates, and immune-compromised individuals.1
Related: Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Coronaviruses are also known to infect animals. Most known CoVs cause disease in only 1 animal species or, at most, in a small number of closely related species. However, SARS-CoV was noted to infect people and various animals, including monkeys, civets, raccoon dogs, cats, dogs, and rodents. The origin and natural reservoir of SARS-CoV was ultimately determined to be bats.10
Genetic sequencing has determined that the MERS-CoV is different from any other known human CoV. MERS-CoV is a beta-CoV and, like the SARS-CoV, is closely related to bat CoVs.11-15 The origin of the MERS-CoV is not known, but an animal reservoir is suspected. Because MERS-CoV is similar to SARS-CoV, bats are considered a possible animal reservoir. Dromedary camels may act as intermediate hosts by spreading the virus to humans.16-18 However, there is no consensus on the animal reservoir for MERS-CoV. It is also not known how the virus has spread from animals to humans.
Case Definition
In order to aid in the rapid recognition of MERS, the CDC has established case definitions.8
A patient under investigation is an individual with fever (> 38oC, > 100.4oF) and pneumonia or acute respiratory distress syndrome (ARDS); and either:
- history of travel from countries in or near the Arabian Peninsula within 14 days before the onset of symptoms; or
- close contact with a symptomatic traveler who developed fever and ARDS within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
A confirmed case is a patient with laboratory confirmation of MERS-CoV infection. A probable case is a patient under investigation with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact of a laboratory- confirmed MERS-CoV case.
Transmission
MERS-CoV is thought to be of animal origin, but the mode of transmission from the animal reservoir is not known. It seems likely that some of the infections have occurred via intermittent zoonotic transmission, possibly by an environmental source.19 The presence of case clusters, however, suggests that human-to-human transmission also can occur. Human-to-human transmission has occurred in individuals living with an infected person and in HCPs caring for infected patients.20-24 The human-to-human transmission through close contact so far has been nonsustained.
It has been estimated that 75% of the reported cases are secondary, meaning that the patient acquired the MERS-CoV infection from another infected person. There is no evidence of sustained spread of MERS-CoV in community settings. The mode of human-to-human transmission has not been determined. Possible modes of transmission include droplet and contact transmission. The number of contacts infected by individuals with confirmed infections seems limited; the transmissibility, therefore, currently seems to be low.25,26 The results of a study of the transmissibility and epidemic potential for MERS-CoV suggest that it does not yet have pandemic potential.27
Bats may serve as a reservoir for MERS-CoV. However, because human contact with bats is uncommon, they are viewed as unlikely candidates for an immediate source of infection in most humans. It is possible that another animal or vector serves as an intermediate host. Camels have been proposed as a possible intermediate host, but this remains unproven. Interestingly, the MERS-CoV index patient had been caring for several ill camels in his herd; the camels had signs of respiratory illness, including nasal discharge.11,28 MERS-CoV sequences were subsequently isolated from a juvenile camel belonging to the index patient.
Symptoms
The incubation time after exposure to symptom onset ranges from 1.9 to 14.7 days (Figure). The median incubation period is 5.2 days.21 Patients are not believed to be contagious during the incubation period. Early symptoms of human infection with MERS-CoV include fever, chills or rigors, cough, and shortness of breath. Less frequently encountered symptoms include hemoptysis, sore throat, myalgias, diarrhea, vomiting, and abdominal pain (Table 2).20,21,23,26,27,29 Many patients infected with MERS-CoV develop a severe lower respiratory tract illness. The patient may progress to ARDS and require intubation and mechanical ventilator support. Mechanical ventilation has been required in 72% of patients.29 The median time from presentation for medical care to respiratory failure is 7 days, ranging from 3 to 11 days (Figure).
Physical Examination
The patients with MERS-CoV infection have been predominantly male and middle aged with an average age of 52 years. The clinical features depend on the severity of the illness. Some infected individuals have remained asymptomatic.27 Other patients have experienced mild lower respiratory illness and have not required hospitalization. However, about 40% of patients have experienced severe illness with pneumonia, respiratory insufficiency, multi-organ failure, and death. The percentage of severe illness is likely an overestimation, because patients with less severe symptoms probably are not tested for MERS-CoV. Most of the patients who have experienced a severe illness and/or death also had underlying comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, and chronic renal disease.23,29
Laboratory Data
As with SARS-CoV, lymphopenia has been reported in patients infected with MERS-CoV.29 Other complete blood cell count abnormalities include leukopenia, lymphocytosis, thrombocytopenia, and anemia (Table 3).23,24,26,30 Blood chemistry profiles have identified elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels.29 Some patients have experienced progressive renal failure signaled by rising serum creatinine and blood urea nitrogen levels.11,23,24,26 Testing for disseminated intravascular coagulation and hemolysis has been positive in some patients.20,30 Oxyhemoglobin desaturation develops in patients with severe pneumonia.
Radiographic Imaging
Chest radiographs have been abnormal in the majority of patients with MERS-CoV. The radiographic findings may be minimal to extensive, depending on the severity of illness. The reported radiographic abnormalities include increased bronchovesicular markings, airspace opacities, patchy infiltrates, interstitial changes, confluent consolidations, nodular opacities, reticular infiltrate, pleural effusion, and total opacification of lung segments and lobes. These radiographic findings may be unilateral or bilateral.29
Specific Testing for MERS-CoV
The CDC recommends that lower respiratory tract specimens be collected for testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). The FDA has issued an emergency use authorization of the rRT-PCR assay developed by the CDC. The CDC recommends that multiple specimens from different sites in the lower respiratory system be collected at different times to increase the likelihood of detecting MERS-CoV. Acute and convalescent serum samples also should be obtained for serologic testing. Lower respiratory specimens (sputum, tracheal aspirates, and bronchoalveolar lavage fluid) are more sensitive than are upper respiratory tract samples (nasopharyngeal throat swabs and nasopharyngeal aspirates). Respiratory specimens should be collected as soon as possible after symptom onset. If negative testing is obtained from a patient with a high index of suspicion for MERS-CoV infection, then repeat testing should be performed.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneumonia
Several serology assays have been developed for the detection of MERS-CoV. An immunofluorescence assay should be confirmed with a neutralization test. In certain cases, the diagnosis should be confirmed by nucleic acid sequencing. The CDC has developed MERS-CoV testing kits, which have been provided to state health departments. Any case of suspected or proven MERS-CoV in the U.S. should be reported to the state and local health departments. Visit the CDC website for more information about collecting, handling, and testing clinical specimens from patients under investigation for MERS: http://www.cdc.gov/coronavirus/mers/guidelines -clinical-specimens.html.
Prognosis
Complications from the MERS- CoV infection include severe pneumonia and ARDS requiring mechanical ventilation, multi-organ failure, renal failure requiring dialysis, consumptive coagulopathy, and pericarditis.20,21,23,26,27,29 About 30% of people with MERS-CoV have died. SARS-CoV was the first CoV to cause severe lower respiratory disease and death in otherwise healthy humans; MERS-CoV is now the second.6 Death occurs a median of 14 days after presentation with a range of 5 to 36 days.20,21,23,26,27,29
Treatment
There is no available specific therapy recommended for MERS-CoV infection; therefore, the management of patients is supportive. As with other CoVs, there is no antiviral agent treatment for MERS-CoV. In experimental settings, combination therapy with interferon-alpha-2b and ribavirin seems promising.31 However, critically ill patients with MERS-CoV did not seem to respond favorably when treated with this regimen.32
Vaccine
There is no licensed vaccine for MERS-CoV, although experimental vaccines are being developed. Vaccines have successfully prevented CoV infection in animal models. The development of an effective vaccine for humans against MERS-CoV may, therefore, be a realistic possibility. Unfortunately, a vaccine is likely years away from approval.
Infection Control Measures
Careful attention to infection control precautions is critical to the containment of MERS-CoV. Patients should be encouraged to inform HCPs about symptoms and potential exposure risks, in particular travel to and/or exposure to travelers from the Arabian Peninsula. This practice should help to limit the transmission of MERS-CoV to HCPs. Standard contact and airborne precautions should be followed in patients with suspected or proven MERS-CoV infection.
Infection control measures should include hand hygiene; avoiding close contact with people who are sick; avoiding touching the eyes, nose, and/or mouth with unwashed hands; and disinfecting frequently touched surfaces. Patients with suspected or proven MERS-CoV should be admitted to single occupancy rooms to diminish the possibility of viral transmission to other patients. All persons entering the room of a patient with suspected or proven MERS-CoV should wear fitted N-95 filtering respirators. Until the mode of transmission is better defined, protective eyewear should be worn during all patient contacts. With implementation of these measures, there has been no institution that has experienced an outbreak of MERS-CoV infection. Unfortunately, the duration of viral shedding is not yet known.
Travel Restrictions
At this time the CDC has not recommended MERS-related travel restrictions. Because the spread of MERS-CoV has occurred in health care institutions, the CDC advises HCPs traveling to the Arabian Peninsula to follow recommendations for infection control of confirmed or suspected cases of MERS-CoV and to monitor their own health closely. Travelers who are going to the Arabian Peninsula for other reasons are advised to follow standard infection control precautions, such as hand washing and avoiding contact with ill people. Visit the CDC website for updated information of travel restrictions: http://www.cdc.gov/coronavirus/mers/travel.html.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Human coronaviruses (CoVs) were first identified in the mid-1960s. Coronaviruses are a large family of viruses that cause a range of illnesses in humans, from the common cold to severe acute respiratory syndrome (SARS).1
In 2003, SARS caused one of the most devastating global epidemics known to the developed world. The important lesson learned from the SARS epidemic was that CoVs can cause severe and rapidly spreading infection. Since then, 2 human CoVs, HCoV-HKU1 and HCoV-NL63, have been identified as common causes of human respiratory tract infections.2,3 In September 2012, a novel CoV was recognized to cause a fatal human infection. This virus has become known as the Middle East respiratory syndrome CoV (MERS-CoV).4
Related: Health Care Use Among Iraq and Afghanistan Veterans With Infectious Diseases
Similar to SARS-CoV, MERS-CoV human infection has a high fatality rate and the ability to spread from person to person.5,6 Person-to-person transmission has resulted in secondary cases among close contacts, including health care providers (HCPs) who should, therefore, be cognizant of this infection. Federal HCPs in particular may be more likely to become involved in the care of patients with this disease, because many military personnel are returning from deployment in the Middle East.
History of MERS-CoV
MERS-CoV was first identified as an infectious disease in humans in Saudi Arabia. In June 2012, the index case was hospitalized with pneumonia and acute renal injury.7 Since then, MERS-CoV human infections and clusters of infection have been identified in multiple countries in the Arabian Peninsula (Table 1).8 There have also been cases of MERS-CoV infection in other countries involving travelers who had visited the Arabian Peninsula and, in some instances, after returning home, their close contacts (Table 1).8
On May 2, 2014, the first confirmed U.S. case was reported in Indiana in a HCP who had recently been to Saudi Arabia.9 A second case of a HCP traveling from Saudi Arabia was identified on May 13, 2014, in Orlando, Florida.9 As of June 11, 2014, 699 laboratory‐confirmed cases of MERS-CoV infection had been reported to the World Health Organization in 20 countries, resulting in 209 deaths. All cases to date have originated in 6 countries of the Arabian Peninsula.5,8
The Organism
Coronaviruses are enveloped RNA viruses named for the crownlike spikes on their surface. They are common viruses known to cause respiratory infections in humans.1 It is thought that most people are infected with these viruses during their lifetime. These viruses generally cause mild-to-moderate upper respiratory tract illnesses, otherwise known as the common cold. On occasion, CoVs can cause lower respiratory tract infections in elderly patients, neonates, and immune-compromised individuals.1
Related: Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Coronaviruses are also known to infect animals. Most known CoVs cause disease in only 1 animal species or, at most, in a small number of closely related species. However, SARS-CoV was noted to infect people and various animals, including monkeys, civets, raccoon dogs, cats, dogs, and rodents. The origin and natural reservoir of SARS-CoV was ultimately determined to be bats.10
Genetic sequencing has determined that the MERS-CoV is different from any other known human CoV. MERS-CoV is a beta-CoV and, like the SARS-CoV, is closely related to bat CoVs.11-15 The origin of the MERS-CoV is not known, but an animal reservoir is suspected. Because MERS-CoV is similar to SARS-CoV, bats are considered a possible animal reservoir. Dromedary camels may act as intermediate hosts by spreading the virus to humans.16-18 However, there is no consensus on the animal reservoir for MERS-CoV. It is also not known how the virus has spread from animals to humans.
Case Definition
In order to aid in the rapid recognition of MERS, the CDC has established case definitions.8
A patient under investigation is an individual with fever (> 38oC, > 100.4oF) and pneumonia or acute respiratory distress syndrome (ARDS); and either:
- history of travel from countries in or near the Arabian Peninsula within 14 days before the onset of symptoms; or
- close contact with a symptomatic traveler who developed fever and ARDS within 14 days after traveling from countries in or near the Arabian Peninsula; or
- is a member of a cluster of patients with severe acute respiratory illness of unknown etiology in which MERS-CoV is being evaluated, in consultation with state and local health departments.
A confirmed case is a patient with laboratory confirmation of MERS-CoV infection. A probable case is a patient under investigation with absent or inconclusive laboratory results for MERS-CoV infection who is a close contact of a laboratory- confirmed MERS-CoV case.
Transmission
MERS-CoV is thought to be of animal origin, but the mode of transmission from the animal reservoir is not known. It seems likely that some of the infections have occurred via intermittent zoonotic transmission, possibly by an environmental source.19 The presence of case clusters, however, suggests that human-to-human transmission also can occur. Human-to-human transmission has occurred in individuals living with an infected person and in HCPs caring for infected patients.20-24 The human-to-human transmission through close contact so far has been nonsustained.
It has been estimated that 75% of the reported cases are secondary, meaning that the patient acquired the MERS-CoV infection from another infected person. There is no evidence of sustained spread of MERS-CoV in community settings. The mode of human-to-human transmission has not been determined. Possible modes of transmission include droplet and contact transmission. The number of contacts infected by individuals with confirmed infections seems limited; the transmissibility, therefore, currently seems to be low.25,26 The results of a study of the transmissibility and epidemic potential for MERS-CoV suggest that it does not yet have pandemic potential.27
Bats may serve as a reservoir for MERS-CoV. However, because human contact with bats is uncommon, they are viewed as unlikely candidates for an immediate source of infection in most humans. It is possible that another animal or vector serves as an intermediate host. Camels have been proposed as a possible intermediate host, but this remains unproven. Interestingly, the MERS-CoV index patient had been caring for several ill camels in his herd; the camels had signs of respiratory illness, including nasal discharge.11,28 MERS-CoV sequences were subsequently isolated from a juvenile camel belonging to the index patient.
Symptoms
The incubation time after exposure to symptom onset ranges from 1.9 to 14.7 days (Figure). The median incubation period is 5.2 days.21 Patients are not believed to be contagious during the incubation period. Early symptoms of human infection with MERS-CoV include fever, chills or rigors, cough, and shortness of breath. Less frequently encountered symptoms include hemoptysis, sore throat, myalgias, diarrhea, vomiting, and abdominal pain (Table 2).20,21,23,26,27,29 Many patients infected with MERS-CoV develop a severe lower respiratory tract illness. The patient may progress to ARDS and require intubation and mechanical ventilator support. Mechanical ventilation has been required in 72% of patients.29 The median time from presentation for medical care to respiratory failure is 7 days, ranging from 3 to 11 days (Figure).
Physical Examination
The patients with MERS-CoV infection have been predominantly male and middle aged with an average age of 52 years. The clinical features depend on the severity of the illness. Some infected individuals have remained asymptomatic.27 Other patients have experienced mild lower respiratory illness and have not required hospitalization. However, about 40% of patients have experienced severe illness with pneumonia, respiratory insufficiency, multi-organ failure, and death. The percentage of severe illness is likely an overestimation, because patients with less severe symptoms probably are not tested for MERS-CoV. Most of the patients who have experienced a severe illness and/or death also had underlying comorbid conditions, such as diabetes mellitus, hypertension, chronic heart disease, and chronic renal disease.23,29
Laboratory Data
As with SARS-CoV, lymphopenia has been reported in patients infected with MERS-CoV.29 Other complete blood cell count abnormalities include leukopenia, lymphocytosis, thrombocytopenia, and anemia (Table 3).23,24,26,30 Blood chemistry profiles have identified elevated aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels.29 Some patients have experienced progressive renal failure signaled by rising serum creatinine and blood urea nitrogen levels.11,23,24,26 Testing for disseminated intravascular coagulation and hemolysis has been positive in some patients.20,30 Oxyhemoglobin desaturation develops in patients with severe pneumonia.
Radiographic Imaging
Chest radiographs have been abnormal in the majority of patients with MERS-CoV. The radiographic findings may be minimal to extensive, depending on the severity of illness. The reported radiographic abnormalities include increased bronchovesicular markings, airspace opacities, patchy infiltrates, interstitial changes, confluent consolidations, nodular opacities, reticular infiltrate, pleural effusion, and total opacification of lung segments and lobes. These radiographic findings may be unilateral or bilateral.29
Specific Testing for MERS-CoV
The CDC recommends that lower respiratory tract specimens be collected for testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). The FDA has issued an emergency use authorization of the rRT-PCR assay developed by the CDC. The CDC recommends that multiple specimens from different sites in the lower respiratory system be collected at different times to increase the likelihood of detecting MERS-CoV. Acute and convalescent serum samples also should be obtained for serologic testing. Lower respiratory specimens (sputum, tracheal aspirates, and bronchoalveolar lavage fluid) are more sensitive than are upper respiratory tract samples (nasopharyngeal throat swabs and nasopharyngeal aspirates). Respiratory specimens should be collected as soon as possible after symptom onset. If negative testing is obtained from a patient with a high index of suspicion for MERS-CoV infection, then repeat testing should be performed.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneumonia
Several serology assays have been developed for the detection of MERS-CoV. An immunofluorescence assay should be confirmed with a neutralization test. In certain cases, the diagnosis should be confirmed by nucleic acid sequencing. The CDC has developed MERS-CoV testing kits, which have been provided to state health departments. Any case of suspected or proven MERS-CoV in the U.S. should be reported to the state and local health departments. Visit the CDC website for more information about collecting, handling, and testing clinical specimens from patients under investigation for MERS: http://www.cdc.gov/coronavirus/mers/guidelines -clinical-specimens.html.
Prognosis
Complications from the MERS- CoV infection include severe pneumonia and ARDS requiring mechanical ventilation, multi-organ failure, renal failure requiring dialysis, consumptive coagulopathy, and pericarditis.20,21,23,26,27,29 About 30% of people with MERS-CoV have died. SARS-CoV was the first CoV to cause severe lower respiratory disease and death in otherwise healthy humans; MERS-CoV is now the second.6 Death occurs a median of 14 days after presentation with a range of 5 to 36 days.20,21,23,26,27,29
Treatment
There is no available specific therapy recommended for MERS-CoV infection; therefore, the management of patients is supportive. As with other CoVs, there is no antiviral agent treatment for MERS-CoV. In experimental settings, combination therapy with interferon-alpha-2b and ribavirin seems promising.31 However, critically ill patients with MERS-CoV did not seem to respond favorably when treated with this regimen.32
Vaccine
There is no licensed vaccine for MERS-CoV, although experimental vaccines are being developed. Vaccines have successfully prevented CoV infection in animal models. The development of an effective vaccine for humans against MERS-CoV may, therefore, be a realistic possibility. Unfortunately, a vaccine is likely years away from approval.
Infection Control Measures
Careful attention to infection control precautions is critical to the containment of MERS-CoV. Patients should be encouraged to inform HCPs about symptoms and potential exposure risks, in particular travel to and/or exposure to travelers from the Arabian Peninsula. This practice should help to limit the transmission of MERS-CoV to HCPs. Standard contact and airborne precautions should be followed in patients with suspected or proven MERS-CoV infection.
Infection control measures should include hand hygiene; avoiding close contact with people who are sick; avoiding touching the eyes, nose, and/or mouth with unwashed hands; and disinfecting frequently touched surfaces. Patients with suspected or proven MERS-CoV should be admitted to single occupancy rooms to diminish the possibility of viral transmission to other patients. All persons entering the room of a patient with suspected or proven MERS-CoV should wear fitted N-95 filtering respirators. Until the mode of transmission is better defined, protective eyewear should be worn during all patient contacts. With implementation of these measures, there has been no institution that has experienced an outbreak of MERS-CoV infection. Unfortunately, the duration of viral shedding is not yet known.
Travel Restrictions
At this time the CDC has not recommended MERS-related travel restrictions. Because the spread of MERS-CoV has occurred in health care institutions, the CDC advises HCPs traveling to the Arabian Peninsula to follow recommendations for infection control of confirmed or suspected cases of MERS-CoV and to monitor their own health closely. Travelers who are going to the Arabian Peninsula for other reasons are advised to follow standard infection control precautions, such as hand washing and avoiding contact with ill people. Visit the CDC website for updated information of travel restrictions: http://www.cdc.gov/coronavirus/mers/travel.html.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103-S108.
2. Woo PC, Lau SK, Chu CM, et al. Characteristics and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884-895.
3. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368-373.
4. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790-7792.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update–as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf. Accessed February 3, 2015.
6. Byrd RP Jr, Roy TM. Severe acute respiratory syndrome. Fed Pract. 2003;20(9):62-71.
7. International Society for Infectious Diseases. Novel coronavirus–Saudi Arabia: Human isolate; Archive number: 20120920.1302733. ProMED-mail Website. http://www.promedmail.org/direct .php?id=20120920.1302733. Published September 20, 2012. Accessed January 23, 2015.
8. Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers. Updated July 31, 2014. Accessed January 23, 2015.
9. Centers for Disease Control and Prevention. MERS in the U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers/US.html. Updated December 9, 2014. Accessed January 23, 2015.
10. Lau SK, Li KS, Huang Y, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6): 2808-2819.
11. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
12. Cotten M, Lam TT, Watson SJ, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736-742B.
13. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456-459.
14. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697-1699.
15. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819-1823.
16. Chu DK, Poon LL, Gomaa MM, et al. MERS coronavirus in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049-1053.
17. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis. 2013;13(10):859-866.
18. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis. 2014;14(2):140-145.
19. Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch Virol. 2014;159(7):1575-1584.
20. World Health Organization. Global Alert and Response. MERS-CoV summary and literature update—as of 31 May 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130531 /en. Accessed January 23, 2015.
21. Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416.
22. Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114.
23. Guery B, Poissy J, el Mansouf L, et al; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381(9885):2265-2272.
24. Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487-2494.
25. Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20(4):620-625.
26. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397.
27. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369(9):884-886.
28. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012-1015.
29. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13(9):752-761.
30. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745-751.
31. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313-1317.
32. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis. 2014;20:42-46.
1. To KK, Hung IF, Chan JF, Yuen KY. From SARS coronavirus to novel animal and human coronaviruses. J Thorac Dis. 2013;5(suppl 2):S103-S108.
2. Woo PC, Lau SK, Chu CM, et al. Characteristics and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884-895.
3. van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10(4):368-373.
4. de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790-7792.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update–as of 11 June 2014. http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf. Accessed February 3, 2015.
6. Byrd RP Jr, Roy TM. Severe acute respiratory syndrome. Fed Pract. 2003;20(9):62-71.
7. International Society for Infectious Diseases. Novel coronavirus–Saudi Arabia: Human isolate; Archive number: 20120920.1302733. ProMED-mail Website. http://www.promedmail.org/direct .php?id=20120920.1302733. Published September 20, 2012. Accessed January 23, 2015.
8. Centers for Disease Control and Prevention. Middle East respiratory syndrome (MERS). Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers. Updated July 31, 2014. Accessed January 23, 2015.
9. Centers for Disease Control and Prevention. MERS in the U.S. Centers for Disease Control and Prevention Website. http://www.cdc.gov/coronavirus/mers/US.html. Updated December 9, 2014. Accessed January 23, 2015.
10. Lau SK, Li KS, Huang Y, et al. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J Virol. 2010;84(6): 2808-2819.
11. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814-1820.
12. Cotten M, Lam TT, Watson SJ, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736-742B.
13. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19(3):456-459.
14. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19(10):1697-1699.
15. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819-1823.
16. Chu DK, Poon LL, Gomaa MM, et al. MERS coronavirus in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(6):1049-1053.
17. Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. Lancet Infect Dis. 2013;13(10):859-866.
18. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis. 2014;14(2):140-145.
19. Abdel-Moneim AS. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Arch Virol. 2014;159(7):1575-1584.
20. World Health Organization. Global Alert and Response. MERS-CoV summary and literature update—as of 31 May 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130531 /en. Accessed January 23, 2015.
21. Assiri A, McGeer A, Perl TM, et al; KSA MERS-CoV Investigation Team. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416.
22. Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114.
23. Guery B, Poissy J, el Mansouf L, et al; MERS-CoV study group. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381(9885):2265-2272.
24. Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487-2494.
25. Reuss A, Litterst A, Drosten C, et al. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20(4):620-625.
26. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397.
27. Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369(9):884-886.
28. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(6):1012-1015.
29. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13(9):752-761.
30. Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(9):745-751.
31. Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313-1317.
32. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: An observational study. Int J Infect Dis. 2014;20:42-46.