User login
As the Story Unfolds
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
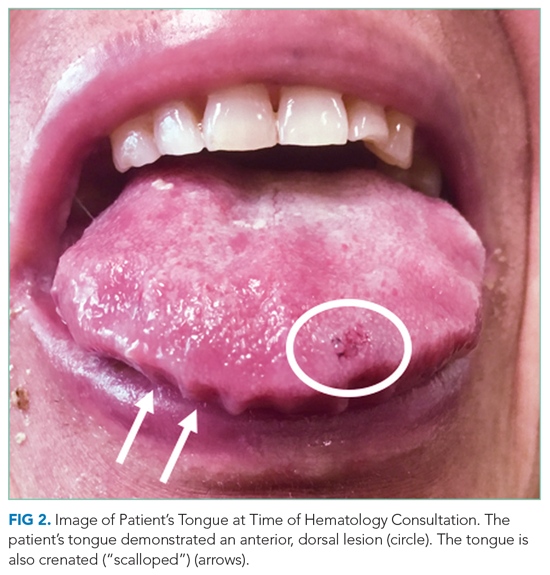
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).
Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
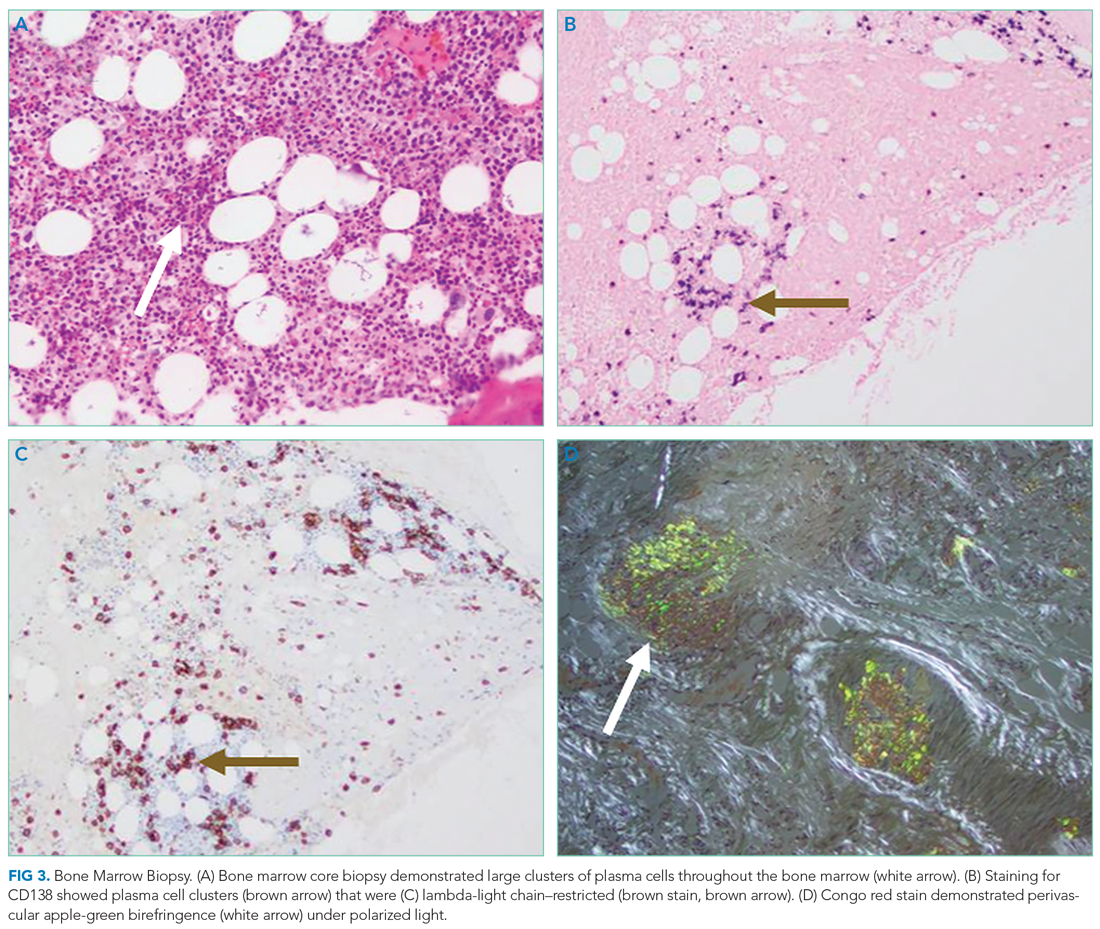
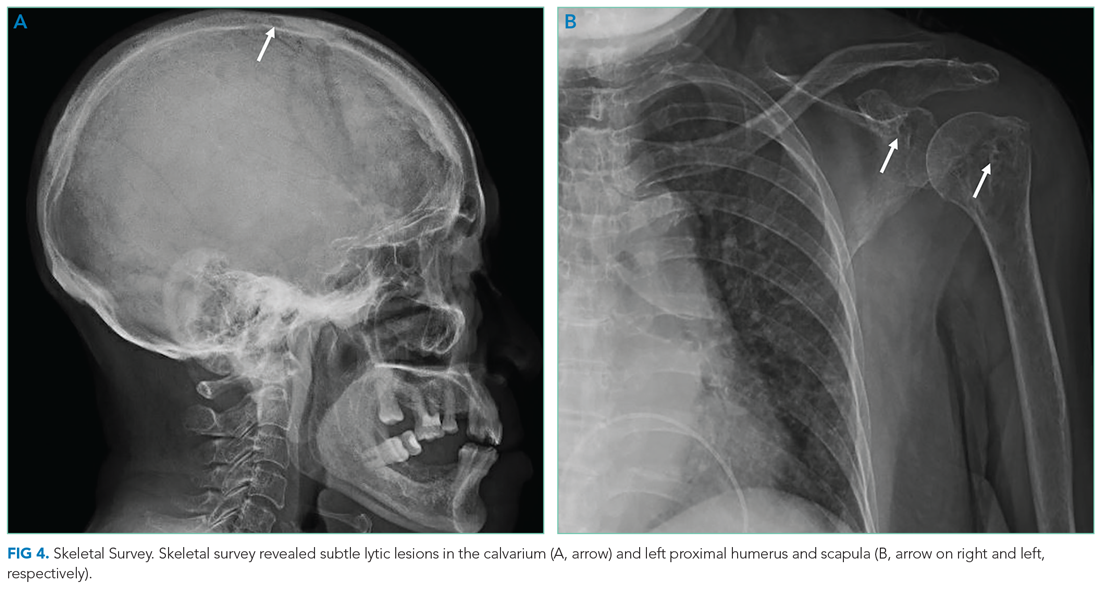
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1
After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.
DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
© 2021 Society of Hospital Medicine
Strategies of Female Teaching Attending Physicians to Navigate Gender-Based Challenges: An Exploratory Qualitative Study
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).
The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.
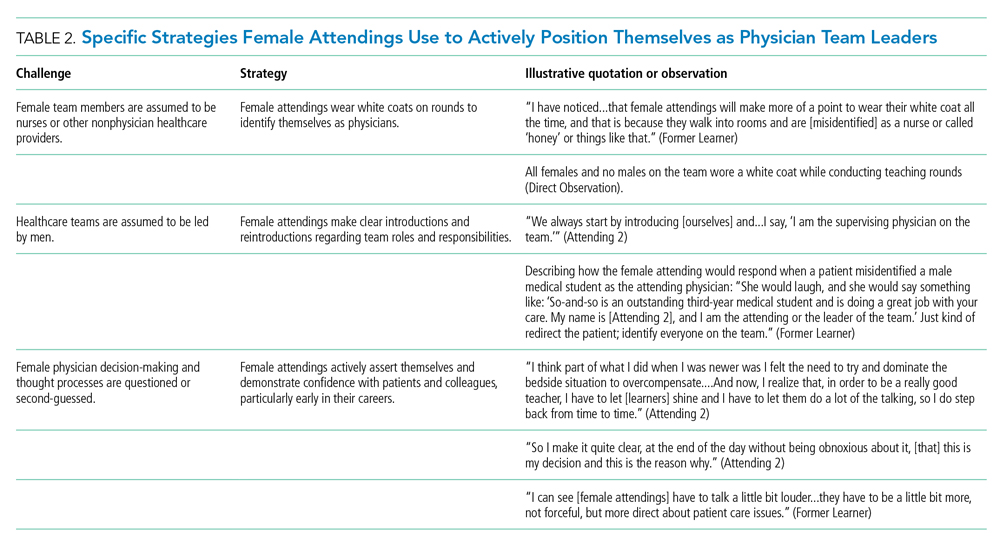
We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.
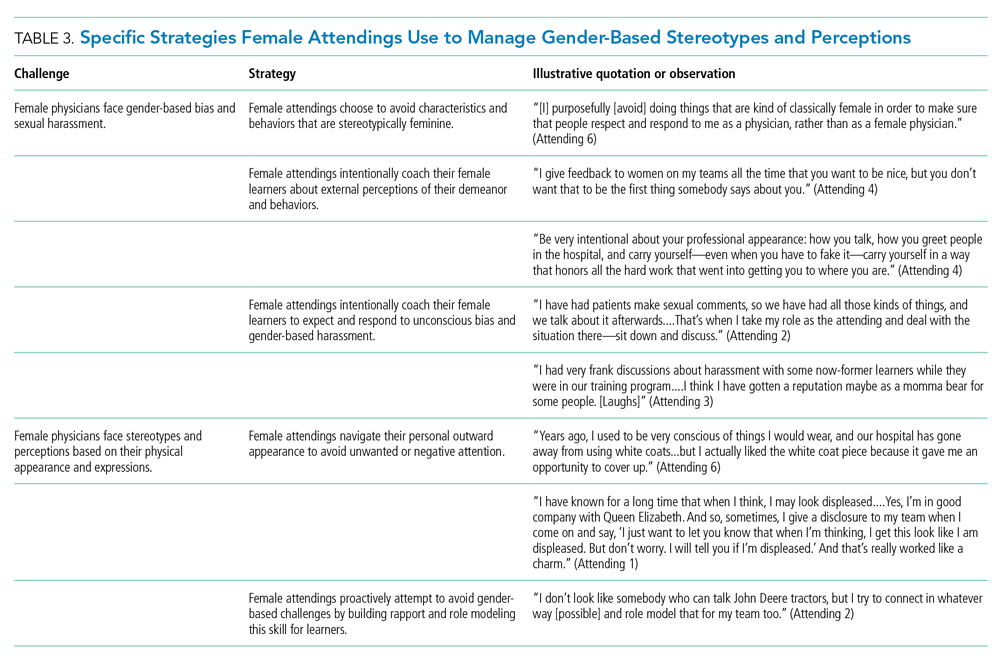
Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”
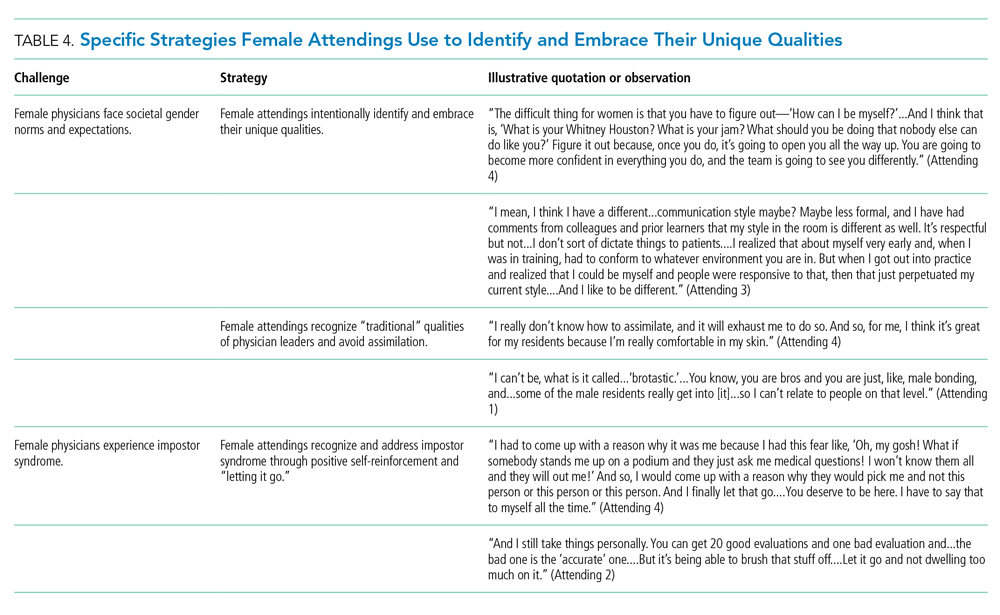
Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
© 2020 Society of Hospital Medicine
A Jaw-Dropping Diagnosis
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.
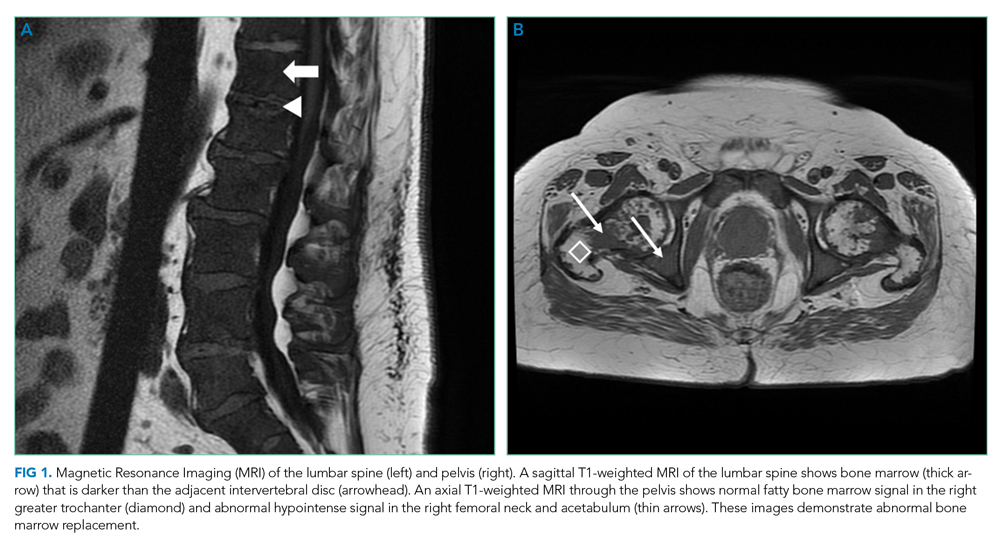
The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.
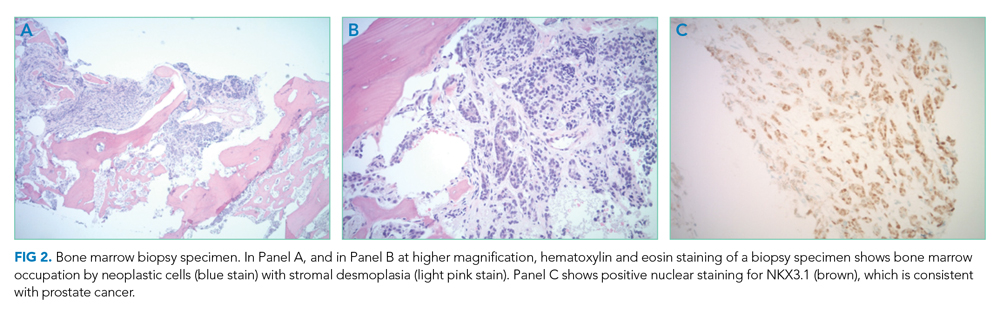
The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.

The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.

The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
A 73-year-old man presented to primary care for an annual examination. Four days prior, he noted right-sided sharp jaw pain such that he could not open his mouth nor chew solid food; it radiated from the right mandible to the ipsilateral temple. He also noted bilateral aching hip pain for several years that increased in severity in the prior 2 months. He reported an intentional weight loss of 9 kg over the past year, achieved through dietary modification. He denied fever, chills, and visual disturbance.
Acute onset of unilateral jaw pain that is worsened by chewing is a feature consistent with a temporomandibular disorder (TMD). TMD consists of musculoskeletal and neuromuscular conditions that affect the temporomandibular joints (TMJs), masticatory muscles, and associated tissues. Common symptoms of TMD include facial or ear pain, temporal headache, and TMJ dysfunction or discomfort. In addition to TMD, craniofacial pain has many possible etiologies such as dental pathology, neuralgias, sinus and otologic disorders, headache and migraine disorders, infections, rheumatologic conditions, and neoplasms.
Systemic etiologies for this patient’s symptoms are a consideration given his age and concomitant worsening of chronic hip pain. Rheumatologic conditions such as giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) are more common in adults older than 50 years of age and cause headache, jaw claudication, and pelvic girdle pain. Rarely, hematologic malignancies (eg, lymphoma), solid tumor metastases (eg, breast cancer, melanoma), and primary tumors of the head and neck (eg, nasopharyngeal carcinoma) can involve the mandible, TMJ, or parotid gland and result in symptoms of TMD.
Medical history was notable for hypertension and type 2 diabetes mellitus complicated by peripheral neuropathy. He smoked one pack of cigarettes daily for 40 years but quit 15 years prior. He drank 4 ounces of vodka each night.
On examination, temperature was 36.5°C, heart rate 92 beats per minute, blood pressure 127/60 mmHg, respiratory rate 12 breaths per minute, oxygen saturation 98% on ambient air, and weight 118 kg. Extraocular movements were intact, pupils were equal and reactive to light and accommodation, and there were no visual field deficits. Nondilated funduscopic examination revealed normal blood vessels, optic disc, and optic cup-to-disc ratio. Dentition was good with pink gingiva. Bilateral temples were nontender. There was normal range of motion and strength in the shoulders, hips, and lower extremities with no tenderness over the trochanters. Patellar and ankle reflexes were present and symmetric bilaterally. He had no rashes or ecchymoses.
The history of smoking, especially with concomitant alcohol intake, is a risk factor for head and neck cancer, and these malignancies can lead to facial pain. While the normal oral cavity exam argues against localized oral and dental causes of the patient’s symptoms, direct fiberoptic endoscopy should be considered. The neck should be examined for lymphadenopathy. Normal vital signs point away from severe infection. The lack of findings in the head and musculoskeletal regions does not exclude systemic etiologies such as rheumatologic conditions or neoplasm. Complete blood cell count and markers of inflammation including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels should be obtained. Hip and pelvic radiographs should be obtained to evaluate for hip osteoarthritis, fractures, or osseous lesions.
The appointment occurred during evening hours and the patient declined further evaluation until the following morning, at which time laboratory studies revealed normal serum levels of electrolytes, blood urea nitrogen, and creatinine. White blood cell (WBC) count was 6,800/mm3 with an immature granulocyte ratio of 1.8% (normal, 0.0-0.5%), hemoglobin 13.2 g/dL, and platelet count 163,000/mm3. ESR was 118 mm/hr (normal, 0-15 mm/hr) and CRP was 1.5 mg/dL (normal, 0-0.75 mg/dL). Radiographs of the hips and pelvis showed osteoarthritis of the bilateral hip joints and degenerative disc disease of the lower lumbar spine.
Granulocytosis may occur in response to infection, rheumatologic conditions, and hematologic malignancies such as chronic myelogenous leukemia. While infectious etiologies (eg, abscess, osteomyelitis) are the most common cause of an extremely elevated ESR level, this patient does not have other signs or symptoms of infection such as fever or leukocytosis. Therefore, other common causes for an extremely elevated ESR level should be considered, including malignancy (eg, multiple myeloma, lymphoma, metastatic solid tumor) and autoimmune conditions (eg, rheumatoid arthritis, vasculitis). While multiple myeloma is the most common malignant etiology for extremely elevated ESR, the patient lacks signs of this condition such as anemia, elevated creatinine, or osteolytic lesions on radiographic imaging. Osteoarthritis identified on the radiographs may contribute to the patient’s hip pain but would not explain the patient’s jaw pain, weight loss, granulocytosis, and elevated ESR. These findings, taken together with the patient’s age, are most suggestive of GCA with possible coexisting PMR. Temporal artery biopsy should be obtained as it is the gold standard test for diagnosing GCA.
The patient was contacted by telephone that same day with laboratory test results. During the call, he endorsed increased jaw and temple pain. He was advised to proceed to the emergency department (ED) for timely evaluation and treatment.
Because GCA was being considered, ophthalmology performed an ocular examination in the ED, which demonstrated no signs of optic nerve or retinal ischemia. Computed tomography (CT) scan of the head and neck with intravenous contrast revealed no abscess or soft tissue abnormalities. Right temporal artery biopsy was performed.
The normal ocular examination does not exclude GCA, and temporal artery biopsy is appropriate. The mainstay of treatment for GCA is high-dose systemic glucocorticoids, which should not be withheld while awaiting biopsy results since ophthalmic artery inflammation may occur and threaten vision.
While GCA remains the leading diagnosis, malignant etiologies warrant further consideration because they are a common cause of extreme ESR elevation, particularly among older patients. The patient’s cancer screening history should be reviewed. The normal CT scan of the head and neck reduces the likelihood of localized solid tumor etiologies; however, additional CT imaging of the chest, abdomen, and pelvis is warranted to evaluate for metastatic solid tumors or lymphoma.
A 10-day course of prednisone 60 mg daily was prescribed for empiric treatment of GCA. The patient was discharged home with follow-up scheduled in rheumatology and primary care clinics. Pain in the jaw and temple resolved within several days.
Two weeks later, he presented to the rheumatology clinic. He noted 1 week of lower right back pain described as dull, aching, radiating to the lateral right hip, and occurring when transitioning from sitting to standing. He had no leg numbness, weakness, or change in bowel habits. Bladder habits were also unchanged, although he reported chronic urinary frequency and occasional incontinence. He reported further weight loss, this time an unintentional loss of 9 kg. He noted frequent sweating but no fever.
He reported a normal colonoscopy within the prior 5 years. Because these records were not available for review, a fecal immunochemical test was obtained and negative for hemoglobin. He had previously declined prostate cancer screening.
The resolution of jaw and temple pain with prednisone supports the presumed diagnosis of GCA. Up to half of patients with GCA may also have PMR, which can cause aching and stiffness in the arms, hips, and lumbar region, and pain may be abrupt in onset. However, PMR-related pain would be expected to improve rather than develop or worsen in the setting of high-dose glucocorticoid use. Therefore, other causes of acute-onset back pain must be considered.
While localized musculoskeletal etiologies such as lumbar muscle strain, radiculopathy, and vertebral compression fracture are possible, co-occurrence of unintentional weight loss and diaphoresis with elevated inflammatory markers suggests a systemic etiology. A neoplastic process with bony metastasis is possible. The reportedly normal colonoscopy and the negative fecal immunochemical test make colorectal cancer less likely. Inflammatory conditions such as ankylosing spondylitis and rheumatoid arthritis are also possible. Ankylosing spondylitis usually presents at a much younger age, however, and axial skeletal involvement in rheumatoid arthritis often involves the cervical spine and is usually seen after longstanding disease. Additionally, the hallmark of inflammatory back pain is morning stiffness which the patient does not endorse. Nonetheless, additional laboratory testing should include antinuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) antibody. Vertebral osteomyelitis remains on the differential diagnosis, and repeat WBC count and inflammatory markers should be assessed. Lumbosacral radiographs should be obtained to rule out fracture.
Physical examination in the rheumatology clinic revealed a temperature of 37.0°C, heart rate 100 beats per minute, blood pressure 146/72 mmHg, respiratory rate 12 breaths per minute, and oxygen saturation 98% on ambient air. Weight was 109 kg. He was pale and diaphoretic. There was diffuse tenderness to palpation of the right-sided lumbar paraspinal muscles. Straight leg raise was negative bilaterally. Patellar reflexes and gait were normal.
Blood chemistries, renal function, and aminotransferase levels were normal. WBC count was 7,100/mm3, hemoglobin 8.0 g/dL, mean corpuscular volume 88.9 fL, platelet count 128,000/mm3, ESR 66 mm/hr, CRP 0.57 mg/dL, alkaline phosphatase 438 IU/L (normal, 30-130 IU/L), and thyroid-stimulating hormone 0.925 mU/L (normal, 0.34-5.60 mU/L). Testing for antinuclear antibodies, rheumatoid factor, and anti-CCP antibody was unremarkable. Prostate-specific antigen (PSA) level was 2.2 ng/mL (normal, 0-4 ng/mL). Urinalysis was unremarkable. Antibodies to hepatitis C and Treponema pallidum were negative. Interferon gamma release assay was negative.
Findings of new onset anemia and thrombocytopenia, in combination with elevated ESR and alkaline phosphatase level, are concerning for disseminated intravascular coagulation (DIC) and microangiopathic hemolytic anemia (MAHA), bone marrow infiltration of a metastatic neoplasm, or ineffective hematopoiesis caused by myelodysplastic syndromes or myelofibrosis.
Laboratory evaluation should include iron studies, lactate dehydrogenase (LDH), haptoglobin, fibrinogen, D-dimer, reticulocyte count, and peripheral blood smear to assess for hemolysis and erythrocyte morphology. Advanced imaging with lumbosacral magnetic resonance imaging (MRI) should be obtained to evaluate for focal etiologies of back pain such as disc herniation, abscess, marrow infiltration, and infarction.
Additional laboratory studies revealed a gamma-glutamyl transferase level of 49 IU/L (normal, 8-56 IU/L), LDH 288 IU/L (normal, 98-192 IU/L), haptoglobin 495 mg/dL (normal, 32-240 mg/dL), fibrinogen >700 mg/dL (normal, 225-550 mg/dL), D-dimer 693 ng/mL (normal, 200-250 ng/mL), serum iron 57 mcg/dL (normal, 33-150 mcg/dL), total iron binding capacity 286 mcg/dL (normal, 250-450 mcg/dL), ferritin 1,012 ng/mL (normal, 17.9-464 ng/mL), and reticulocyte count 2.9% (normal, 0.5-2.5%). Coagulation studies and serum protein electrophoresis were normal. Erythropoietin level was 109 mIU/mL (normal, 4.0-20.0 mIU/mL). Peripheral blood smear demonstrated moderate anemia with 8% nucleated erythrocytes per white blood cell (normal, 0%) and no circulating blasts.
MRI of the thoracolumbar spine and pelvis revealed diffusely abnormal bone marrow signal with multiple superimposed focal and poorly defined enhancing lesions along the lumbar spine marrow, sacrum, and bilateral iliac bones (Figure 1). Positron emission tomography/computed tomography (PET/CT) scan showed no scintigraphic evidence of metabolically active neoplastic, paraneoplastic, or inflammatory disorder.

The elevated haptoglobin, normal coagulation studies, and absence of fragmented erythrocytes on peripheral smear exclude an intravascular hemolytic process. The patient’s lower than expected reticulocyte count for the degree of anemia, elevated erythropoietin, and nucleated erythrocytes constitute a pattern that can be seen with bone marrow infiltration. There are no circulating blasts, making leukemia less likely. A solid organ tumor with bone metastases may cause enhancing lesions on MRI since this form of imaging is more sensitive than radiography for detecting skeletal malignancies. The negative PET/CT, however, does not reveal a primary tumor. Myelofibrosis is an infiltrative myeloproliferative disorder associated with nonspecific laboratory abnormalities, bone pain, weight loss, and night sweats that could cause diffuse MRI bone marrow signal alterations with normal PET/CT findings. However, myelofibrosis would not typically cause a significantly elevated ESR, and thus would be an unlikely cause for this patient’s presentation.
Given the constellation of symptoms, hematologic abnormalities, and bone marrow infiltration on imaging, hematology should be consulted to perform a bone marrow biopsy to assist with definitive diagnosis.
Bone marrow biopsy demonstrated metastatic adenocarcinoma consistent with prostatic origin (Figure 2). Bone scan demonstrated widespread osteoblastic metastases, which included the skull and temporal regions. These lesions were thought to be the cause of the patient’s original presenting symptom of jaw pain.

The patient was started on androgen deprivation therapy, initially with degarelix and subsequently leuprolide shots and abiraterone with prednisone. PSA was 0.08 ng/mL after 3 months of androgen deprivation therapy. His back and hip pain slowly improved.
DISCUSSION
Prostate cancer is the most common cancer in men with one out of every nine men diagnosed in his lifetime.1 While most men initially present with localized, curable disease,1 4% present with metastatic disease, an incidence that has been increasing since 2004.2 Despite available treatments, metastatic prostate cancer has a poor prognosis, with an average overall survival of approximately 5 years.3
Prostate cancer can be challenging to diagnose. Men with prostate cancer are commonly asymptomatic. Rarely, patients may present with hematuria, bony pain caused by metastasis, or obstructive urinary symptoms like hesitancy or incomplete bladder emptying. Our patient presented with jaw pain, which was ultimately attributed to osteoblastic lesions of the skull. Additionally, his history of urinary frequency and incontinence may have been clues to his underlying diagnosis of prostate cancer.
Prostate cancer screening remains highly nuanced and relies on shared decision-making between patients and healthcare providers. Clinical practice guidelines for early detection of prostate cancer recommend individualized PSA-based serologic screening.4,5 Specifically, the United States Preventive Services Task Force recommends screening men aged 55 to 69 years who desire screening and understand the potential harms associated with a positive test result. These harms may include psychological distress and complications from prostate biopsy (eg, pain or infection) or prostate cancer treatment (eg, erectile, urinary, and/or bowel dysfunction).4-6 The decision to screen can be guided by individuals’ risk factors including African American race, family history, and older age.
While our patient elected not to undergo routine prostate cancer screening, a PSA level was obtained during his diagnostic evaluation and highlights the limitations of PSA-based screening. A PSA level ≤4.0 ng/mL has 21% sensitivity and 91% specificity for detecting prostate cancer.7 PSA levels above 4.0 ng/mL warrant repeat testing and, if persistently elevated, referral to urology for possible prostate biopsy. PSA levels often correlate with burden of disease, and patients with PSA levels >20 ng/mL are referred for CT imaging to evaluate for metastatic disease.8 PSA’s poor sensitivity was underscored in a study by Thompson et al who evaluated the incidence of prostate cancer in men participating in the Prostate Cancer Prevention Trial with PSA levels of <4 ng/mL.9 In this study, 15% of men diagnosed with prostate cancer never had a PSA level >4 ng/mL.9 While most of the cancers in this study were low grade and may have been clinically insignificant, 15% demonstrated histologic signs of at least intermediate-risk disease. Our patient’s PSA level of 2.2 ng/mL was below the threshold that triggers additional evaluation even though he had widely metastatic prostate cancer.
Our patient’s severe jaw and temple pain, weight loss, and progressive hip pain were concerning for GCA. This vasculitis of large- and medium-sized arteries predominantly affects older adults with greatest incidence among those 70 years of age and older.10 Symptoms occur because of cranial artery inflammation and may include headache, visual disturbance, erythema or tenderness of the temporal artery, and jaw claudication. Extracranial inflammation may affect the thoracic aorta and its branches and rarely the abdominal aorta and lower limb arteries. Pelvic girdle pain more typically results from associated PMR. Patients may also note systemic symptoms such as fever, weight loss, and fatigue.
Prompt diagnostic testing is important when considering GCA. Most patients with GCA have ESR levels greater than 40 mm/hr.11 ESR is a laboratory test that measures the vertical distance erythrocytes travel in a column of blood over 1 hour; in the setting of inflammation, cells form clumps and travel more quickly than individual cells, resulting in a higher value. While moderate elevations in ESR may occur without an identifiable cause, extreme ESR levels—those above 100 mm/hr, as observed in our patient—are highly suggestive of certain serious conditions, including infection, malignancy, and autoimmune disease such as GCA.12,13 Temporal artery biopsy is the gold standard test to diagnose GCA. However, because of noncontiguous inflammation of the temporal artery, biopsies may be falsely negative. Thus, sampling of the contralateral temporal artery may be warranted if suspicion remains high.
As was the case for our patient, PET/CT is not reliable for diagnosing prostate cancer. In contrast to other malignancies (eg, lymphoma, lung cancer), prostate cancer typically does not display increased glucose metabolism. Moreover, the close proximity of the bladder and prostate can interfere with imaging interpretation because the fluorodeoxyglucose (FDG) tracer is excreted in the urine.14 The reported sensitivity of PET/CT for the diagnosis of prostate cancer ranges from 17%-65%.15,16 In a small study of men with metastatic prostate cancer, only 18% of bony metastases were FDG avid, and there was no correlation between FDG avidity and PSA level.15 Notably, although PET/CT includes CT imaging, this CT is used to map anatomic landmarks and is not separately interpreted by the radiologist. Thus, even if evidence of prostate cancer was apparent on traditional CT, it may be overlooked on PET/CT.
Several important points regarding diagnostic testing are raised by this case. First, PSA-based screening for prostate cancer may be falsely negative, even in the setting of widely metastatic disease. Second, extreme ESR elevation is a marker for serious underlying disease and warrants a thorough diagnostic evaluation. Finally, PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer because of the normal rates of glucose metabolism. Our patient initially presented with jaw pain, yet his progressive physical symptoms and laboratory abnormalities prompted an evaluation which ultimately revealed the jaw-dropping diagnosis of PSA-negative, metastatic prostate cancer.
KEY TEACHING POINTS
- ESR levels greater than 100 mm/hr are highly suggestive of certain serious conditions including infection, autoimmune disease, and malignancy.
- PSA-based screening for prostate cancer can result in false negative test results. In one study, 15% of men diagnosed with prostate cancer never had a PSA level greater than 4 ng/mL (ie, the level at which repeat laboratory testing and/or referral to urology for possible prostate biopsy is advisable).
- PET/CT has limited diagnostic utility in evaluating metastatic prostate cancer, because prostate cancer cells typically demonstrate normal glucose metabolism.
Disclosures
Drs Griauzde, Northway, Yentz, and Houchens have nothing to disclose. Dr Saint reports personal fees from ISMIE Mutual Insurance Company during the conduct of the study, as well as personal fees from Jvion and Doximity outside the submitted work.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
1. Prostate Cancer - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed October 23, 2018.
2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann Epidemiol. 2018;28(5):328-330. https://doi.org/10.1016/j.annepidem.2018.03.001.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. https://doi.org/10.1056/NEJMoa1503747.
4. US Preventive Services Task Force. Final Recommendation Statement: Prostate Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed August 8, 2018.
5. American Urological Association. http://www.auanet.org/guidelines/prostate-cancer-early-detection. Accessed August 8, 2018.
6. American Cancer Society. American Cancer Society Recommendations for Prostate Cancer Early Detection. https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html. Accessed August 8, 2018.
7. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. https://doi.org/10.3322/caac.20066.
8. Mohler JL, Lee RJ, Antonarakis ES, Higano CS, Richey S. NCCN Guidelines Index Table of Contents. Prostate Cancer. 2018:151.
9. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246. https://doi.org/10.1056/NEJMoa031918.
10. Pioro MH. Primary care vasculitis: Polymyalgia rheumatica and giant cell arteritis. Prim Care. 2018;45(2):305-323. https://doi.org/10.1016/j.pop.2018.02.007.
11. Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140-145. https://doi.org/10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
12. Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician. 1999;60(5):1443-1450.
13. Daniels LM, Tosh PK, Fiala JA, Schleck CD, Mandrekar JN, Beckman TJ. Extremely elevated erythrocyte sedimentation rates: Associations with patients’ diagnoses, demographic dharacteristics, and comorbidities. Mayo Clin Proc. 2017;92(11):1636-1643. https://doi.org/10.1016/j.mayocp.2017.07.018.
14. Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51(6):1511-1521. http://doi.org/10.1016/j.eururo.2007.01.061.
15. Yeh SDJ, Imbriaco M, Larson SM, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693-697. https://doi.org/10.1016/0969-8051(96)00044-3.
16. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926-937. https://doi.org/10.1016/j.eururo.2016.06.021.
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Make a Friend Before You Need One
“Takers believe in a zero-sum world, and they end up creating one where bosses, colleagues and clients don’t trust them. Givers build deeper and broader relationships—people are rooting for them instead of gunning for them.”
—Adam Grant
To succeed in a hospital, leaders need a generous supply of social and political capital. House officers learn this very quickly, especially when they are relying on other members of the healthcare team to obtain tests and studies for their patients and calling for specialty consultations. To be successful and efficient, building relationships and trust is key. Such capital, unfortunately, takes time to develop. Therefore, healthcare leaders and clinicians at all levels of training need to make an everyday investment of goodwill and friendliness with those they encounter. The dividends may be slow in coming, but they are substantial and sustained. Friends give you the benefit of the doubt—and help you when you are most in need.
Having friends (or friendly colleagues) at work is beneficial both professionally and personally. The benefits of social interactions have been studied for years and even more so in recent times with the dramatic increase in the use of handheld devices. Eye contact between casual acquaintances passing each other in the hallway is replaced with eyes focused downward on smartphones. The result? We are becoming more socially isolated. Our personal solution? When we see professional colleagues (or patients and families in the hallways of our hospital), we nod in acknowledgement with appropriate eye contact and say “Good morning” or “Hello” even if we don’t know them—even if their eyes are focused on their devices as they walk past you in the hallway. You get a gold star if you remember the names of the professional colleagues you see frequently in the hallways or around the hospital.
This isn’t soft science; it’s backed by hard data. When we conduct site visits of different hospitals around the country to help them improve their care quality and performance, we informally divide hospitals into two groups: The “How ya doin’?” hospitals vs the “Rec-Ignore” hospitals (in which employees recognize a colleague in the hallway but choose to not acknowledge them). Most prefer to work at a “How ya doin’?” hospital. Being friendly has been linked to increased team spirit and morale, knowledge sharing, trust, prevention of burnout, and sense of a positive working environment. It also makes you feel better about yourself—and makes other people feel similarly as well.
We’ll share an example from a search for a new department chair. The dean went on reverse site visits to meet the two finalists in their home institutions and asked them for tours of their hospitals. Candidate A walked around and it seemed like everyone knew her. She smiled and said hello to the people she came in contact with during the tour. Not so for candidate B—just the opposite. Guess which candidate the dean hired?
Put away your phone, interact with your colleagues, and learn to make small talk, and not just with your supervisors or peers. Chitchat is an important “social lubricant,” fostering a sense of community and teamwork. It helps bring down the divides that come from organizational hierarchies. It helps endear you to your staff.
Developing a reputation as a nice person who is quick with a smile and even quicker with a “How ya doin’?” pays off in the end. This reputation also makes it easier to give bad news, something that all leaders must do at some point. So make a friend before you need one—it usually will pay dividends.
“Takers believe in a zero-sum world, and they end up creating one where bosses, colleagues and clients don’t trust them. Givers build deeper and broader relationships—people are rooting for them instead of gunning for them.”
—Adam Grant
To succeed in a hospital, leaders need a generous supply of social and political capital. House officers learn this very quickly, especially when they are relying on other members of the healthcare team to obtain tests and studies for their patients and calling for specialty consultations. To be successful and efficient, building relationships and trust is key. Such capital, unfortunately, takes time to develop. Therefore, healthcare leaders and clinicians at all levels of training need to make an everyday investment of goodwill and friendliness with those they encounter. The dividends may be slow in coming, but they are substantial and sustained. Friends give you the benefit of the doubt—and help you when you are most in need.
Having friends (or friendly colleagues) at work is beneficial both professionally and personally. The benefits of social interactions have been studied for years and even more so in recent times with the dramatic increase in the use of handheld devices. Eye contact between casual acquaintances passing each other in the hallway is replaced with eyes focused downward on smartphones. The result? We are becoming more socially isolated. Our personal solution? When we see professional colleagues (or patients and families in the hallways of our hospital), we nod in acknowledgement with appropriate eye contact and say “Good morning” or “Hello” even if we don’t know them—even if their eyes are focused on their devices as they walk past you in the hallway. You get a gold star if you remember the names of the professional colleagues you see frequently in the hallways or around the hospital.
This isn’t soft science; it’s backed by hard data. When we conduct site visits of different hospitals around the country to help them improve their care quality and performance, we informally divide hospitals into two groups: The “How ya doin’?” hospitals vs the “Rec-Ignore” hospitals (in which employees recognize a colleague in the hallway but choose to not acknowledge them). Most prefer to work at a “How ya doin’?” hospital. Being friendly has been linked to increased team spirit and morale, knowledge sharing, trust, prevention of burnout, and sense of a positive working environment. It also makes you feel better about yourself—and makes other people feel similarly as well.
We’ll share an example from a search for a new department chair. The dean went on reverse site visits to meet the two finalists in their home institutions and asked them for tours of their hospitals. Candidate A walked around and it seemed like everyone knew her. She smiled and said hello to the people she came in contact with during the tour. Not so for candidate B—just the opposite. Guess which candidate the dean hired?
Put away your phone, interact with your colleagues, and learn to make small talk, and not just with your supervisors or peers. Chitchat is an important “social lubricant,” fostering a sense of community and teamwork. It helps bring down the divides that come from organizational hierarchies. It helps endear you to your staff.
Developing a reputation as a nice person who is quick with a smile and even quicker with a “How ya doin’?” pays off in the end. This reputation also makes it easier to give bad news, something that all leaders must do at some point. So make a friend before you need one—it usually will pay dividends.
“Takers believe in a zero-sum world, and they end up creating one where bosses, colleagues and clients don’t trust them. Givers build deeper and broader relationships—people are rooting for them instead of gunning for them.”
—Adam Grant
To succeed in a hospital, leaders need a generous supply of social and political capital. House officers learn this very quickly, especially when they are relying on other members of the healthcare team to obtain tests and studies for their patients and calling for specialty consultations. To be successful and efficient, building relationships and trust is key. Such capital, unfortunately, takes time to develop. Therefore, healthcare leaders and clinicians at all levels of training need to make an everyday investment of goodwill and friendliness with those they encounter. The dividends may be slow in coming, but they are substantial and sustained. Friends give you the benefit of the doubt—and help you when you are most in need.
Having friends (or friendly colleagues) at work is beneficial both professionally and personally. The benefits of social interactions have been studied for years and even more so in recent times with the dramatic increase in the use of handheld devices. Eye contact between casual acquaintances passing each other in the hallway is replaced with eyes focused downward on smartphones. The result? We are becoming more socially isolated. Our personal solution? When we see professional colleagues (or patients and families in the hallways of our hospital), we nod in acknowledgement with appropriate eye contact and say “Good morning” or “Hello” even if we don’t know them—even if their eyes are focused on their devices as they walk past you in the hallway. You get a gold star if you remember the names of the professional colleagues you see frequently in the hallways or around the hospital.
This isn’t soft science; it’s backed by hard data. When we conduct site visits of different hospitals around the country to help them improve their care quality and performance, we informally divide hospitals into two groups: The “How ya doin’?” hospitals vs the “Rec-Ignore” hospitals (in which employees recognize a colleague in the hallway but choose to not acknowledge them). Most prefer to work at a “How ya doin’?” hospital. Being friendly has been linked to increased team spirit and morale, knowledge sharing, trust, prevention of burnout, and sense of a positive working environment. It also makes you feel better about yourself—and makes other people feel similarly as well.
We’ll share an example from a search for a new department chair. The dean went on reverse site visits to meet the two finalists in their home institutions and asked them for tours of their hospitals. Candidate A walked around and it seemed like everyone knew her. She smiled and said hello to the people she came in contact with during the tour. Not so for candidate B—just the opposite. Guess which candidate the dean hired?
Put away your phone, interact with your colleagues, and learn to make small talk, and not just with your supervisors or peers. Chitchat is an important “social lubricant,” fostering a sense of community and teamwork. It helps bring down the divides that come from organizational hierarchies. It helps endear you to your staff.
Developing a reputation as a nice person who is quick with a smile and even quicker with a “How ya doin’?” pays off in the end. This reputation also makes it easier to give bad news, something that all leaders must do at some point. So make a friend before you need one—it usually will pay dividends.
Improving Hand Hygiene Adherence in Healthcare Workers Before Patient Contact: A Multimodal Intervention in Four Tertiary Care Hospitals in Japan
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
In the era of multidrug resistant organisms spreading to healthcare facilities, as well as in the community, prevention of healthcare-associated infections (HAIs) has become one of the most important issues in the world. HAIs impact morbidity and mortality of patients, increase healthcare costs,1,2 and are associated with a longer length of stay in the hospital.3,4 In Japan, HAIs are a salient problem; more than 9% of patients admitted to the intensive care unit (ICU) developed an infection during their ICU stay,5 and the numbers of multidrug resistant organism isolates causing HAIs have been increasing annually.6
Hand hygiene is the most important strategy for preventing the spread of MDROs and reducing HAIs.7 Heightened attention to hand hygiene has occurred because of the recent global outbreak of coronavirus disease 2019 (COVID-19), which first appeared in Wuhan, China.8 Because no proven antiviral or vaccine is currently available for the disease, hand hygiene, appropriate cough etiquette, and physical distancing, including school closures, are the only way to prevent spread of the illness.9,10 The virus appears to be highly contagious and spread by droplet or contact routes. The spread of COVID-19 in healthcare facilities has been significant,11 and it could be a source of further spread of the disease in the community.
Unfortunately, hand hygiene adherence remains low in most settings.12 The World Health Organization (WHO) created a strategy to improve hand hygiene adherence,13 which has been implemented in many countries.14 This strategy consists of five key components: (1) system change, (2) training/education, (3) evaluation and feedback, (4) reminders in the workplace, and (5) institutional safety climate.13 Implementing a multimodal intervention including these five elements has increased hand hygiene adherence among healthcare workers (HCWs) and appears to reduce HAIs in different locations.15-17 Improving hand hygiene practice among HCWs is considered one of the most important ways to decrease the incidence of HAIs.15,18,19
There are two types of practice for hand hygiene: either hand washing with soap and water or using alcohol-based hand rub (AHR). The former requires water, soap, a sink, and paper towels, whereas the latter requires only hand rub, which is easy to use and requires one-third the length of time as the former.20 Therefore, AHR is strongly recommended, especially in acute and intensive care settings in hospitals, which require urgent care of patients. Importantly, previous studies demonstrated that greater use of AHR resulted in significant reductions in HAIs.7,14
In Japan, the data related to hand hygiene adherence is limited. Previous studies at four hospitals in different regions of Japan demonstrated that hand hygiene rates were suboptimal21 and lower than reported adherence rates from other international studies.14 One study at three hospitals showed rates could be improved by a multimodal intervention tailored by each institution.22 A 5-year follow-up study demonstrated the sustainability of the multimodal intervention23; however, hand hygiene adherence rates remained low at approximately 32%.
We hypothesized that perhaps focusing attention on just one single region (or prefecture) could boost hand hygiene rates. Niigata prefecture is located 200 miles north of Tokyo and is the largest prefecture facing the Japan Sea. There are five major tertiary hospitals in Niigata, and they communicate frequently and discuss infection control issues as a group. To investigate hand hygiene adherence before touching patients, and to evaluate the improvement of hand hygiene adherence induced by a multimodal intervention, we performed a pre- and postintervention study among HCWs at four of these tertiary care hospitals in Niigata.
METHODS
Participating hospitals
Four tertiary care hospitals in Niigata, Japan, volunteered to participate in the study. The characteristics of the four participating hospitals are summarized in Table 1. All hospitals are public or community based. Hospital A included two units, consisting of a cardiovascular-cerebral ICU and an emergency department (ED), and Hospitals B, C, and D included various units containing surgical or medical wards, an ICU, or an ED. All four hospitals have at least one designated infection-prevention nurse and an infection-prevention department. In addition, there is an infection control network system among the hospitals, and they communicate well to update the information related to local, domestic, or global infectious diseases through regular seminars and by distributing and exchanging electronic communication.
Preintervention
The preintervention infrastructure and existing activities to improve HCW hand hygiene in each hospital are summarized in Table 1. These activities were developed by each individual hospital and had been in place for at least 6 months before the study intervention. All hospitals used AHR and did direct observation for hand washing in designated wards or units and monitoring of AHR consumption; however, Hospital B did not have a wash basin in each room and no use of portable AHR. Preintervention hand hygiene data were collected from June to August 2018.
Intervention
To improve hand hygiene adherence, we initiated a multimodal intervention from September 2018 to February 2019 based on WHO recommendations13 and the findings from prior hand hygiene studies.22 Each facility was provided the same guidance on how to improve hand hygiene adherence and was asked to tailor their intervention to their settings (Table 2 and Appendix Figure). Suggested interventions included feedback regarding hand hygiene adherence observed during the preintervention period, interventions related to AHR, direct observation of and feedback regarding hand hygiene, new posters promoting hand hygiene in the workplace, a 1-month campaign for hand hygiene, seminars for HCWs related to hand hygiene, creation of a handbook for education/training, feedback regarding hand hygiene adherence during the intervention period, and others. The infection control team at each hospital designed the plans and strategies to improve hand hygiene adherence. Postintervention data were collected from February 2019 to March 2019.
Observation of Hand Hygiene Adherence
Hand hygiene adherence before patient contact was evaluated by board-certified infection control nurses. To reduce observation bias, external nurses from other participating hospitals conducted the observations. To minimize intraobserver variation, the same training as the previous study in Japan21 was provided. Hand hygiene observations were usually performed during the day Monday to Friday from 8
Use of either AHR or soap and water before patient contact was defined as appropriate hand hygiene.24,25 Hand hygiene adherence before patient contact for each provider-patient encounter was observed and recorded using a data collection form used in the previous studies.19,26 The following information was obtained: unit name, time of initiation and completion of observations, HCW type (physician or nurse), and the type of hand hygiene (ie, AHR, hand washing with soap and water, or none). The observers kept an appropriate distance from the observed HCWs to avoid interfering with their regular clinical practice. In addition, we informed HCWs in the hospital that their clinical practices were going to be observed; however, they were not informed their hand hygiene adherence was going to be monitored.
Statistical Analysis
Overall hand hygiene adherence rates from the pre- and postintervention periods were compared based on hospitals and HCW subgroups. The Pearson’s chi-square test was used for the comparison of hand hygiene adherence rates between pre- and postintervention periods, and 95% CIs were estimated using binomial distribution. Poisson regression was used to look at changes in hand hygiene adherence with adjustment for HCW type. A two-tailed P value of <.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at all participating hospitals.
RESULTS
Overall Changes
In total, there were 2,018 and 1,630 observations of hand hygiene during the preintervention and postintervention periods, respectively. Most observations were of nurses: 1,643 of the 2,018 preintervention observations (81.4%) and 1,245 of the 1,630 postintervention observations (76.4%).
Findings from the HCW observations are summarized in Figure A. The overall postintervention hand hygiene adherence rate (548 of 1,630 observations; 33.6%; 95% CI, 31.3%-35.9%) was significantly higher than the preintervention rate (453 of 2,018 observations; 22.4%; 95% CI, 20.6%-24.3%; P < .001). This finding persisted after adjustment for the type of HCW (nurse vs physician), with proper hand hygiene adherence occurring 1.55 times more often after the intervention than before (95% CI, 1.37-1.76; P < .001). The overall improvement in hand hygiene adherence rates in the postintervention period was seen in all four hospitals (Figure B). However, the hand hygiene adherence rates of nurses in Hospitals C and D were lower than those in Hospitals A and B both before and after the intervention.
Use of AHR was the dominant appropriate hand hygiene practice vs hand washing with soap and water. Of those that practiced appropriate hand hygiene, the rate of AHR use was high and unchanged between preintervention (424 of 453; 93.6%) and postintervention periods (513 of 548; 93.6%; P = .99).
Changes by HCW Type
The rates of hand hygiene adherence in both physicians and nurses were higher in the postintervention period than in the preintervention period. However, the improvement of hand hygiene adherence among nurses—from 415 of 1,643 (25.2%) to 487 of 1,245 (39.1%) for an increase of 13.9 percentage points (95% CI,10.4-17.3)—was greater than that in physicians—from 38 of 375 (10.1%) to 61 of 385 (15.8%) for an increase of 5.7 percentage points (95% CI, 1.0-8.1; P < .001; Figure B). In general, nurse hand hygiene adherence was higher than that in physicians both in the preintervention period, with nurses at 25.2% (95% CI, 23.2%-27.4%) vs physicians at 10.1% (95% CI, 7.1%-13.2%; P < .001), and in the postintervention period, with nurses at 39.1% (95% CI, 36.4%-41.8%) vs physicians at 15.8% (95% CI, 12.2%-19.5%; P < .001).
Changes by Hospital
Overall, improvement of hand hygiene adherence was observed in all hospitals. However, the improvement rates differed in each hospital: They were 6.5 percentage points in Hospital A, 11.3 percentage points in Hospital C, 11.4 percentage points in Hospital D, and 18.4 percentage points in Hospital B. Hospital B achieved the highest postintervention adherence rates (42.6%), along with the highest improvement. The improvements of hand hygiene adherence in physicians were higher in Hospitals B (8.4 percentage points) and D (8.3 percentage points) than they were in Hospitals A (4.1 percentage points) and C (4.0 percentage points).
Interventions performed at each hospital to improve hand hygiene adherence are summarized in Table 2 and the Appendix Figure. All hospitals performed feedback of hand hygiene adherence after the preintervention period. Interventions related to AHR were frequently initiated; self-carry AHR was provided in two hospitals (Hospitals C and D), and location of AHR was moved (Hospitals B and D). In addition, new AHR products that caused less skin irritation were introduced in Hospital B. Direct observation by hospital staff (separate from our study observers) was also done as part of Hospital A and D’s improvement efforts. Other interventions included a 1-month campaign for hand hygiene including a contest for senryu (humorous 17-syllable poems; Table 2; Appendix Table), posters, seminars, and creation of a handbook related to hand hygiene. Posters emphasizing the importance of hand hygiene created by the local hospital infection control teams were put on the wall in several locations near wash basins. Seminars (1-hour lectures to emphasize the importance of hand hygiene) were provided to nurses. A 10-page hand hygiene handbook was created by one local infection control team and provided to nurses.
DISCUSSION
Our study demonstrated that the overall rate of hand hygiene adherence improved from 22.4% to 33.6% after multimodal intervention; however, the adherence rates even after intervention were suboptimal. The results were comparable with those of a previous study in Japan,22 which underscores how suboptimal HCW hand hygiene in Japan threatens patient safety. Hand hygiene among HCWs is one of the most important methods to prevent HAIs and to reduce spread of multidrug resistant organisms. High adherence has proven challenging because it requires behavior modification. We implemented WHO hand hygiene adherence strategies27 and evaluated the efficacy of a multimodal intervention in hopes of finding the specific factors that could be related to behavior modification for HCWs.
We observed several important relationships between the intervention components and their improvement in hand hygiene adherence. Among the four participating hospitals, Hospital B was the most successful with improvement of hand hygiene adherence from 24.2% to 42.6%. One unique intervention for Hospital B was the introduction of new AHR products for the people who had felt uncomfortable with current products. Frequent hand washing or the use of certain AHR products could irritate skin causing dry or rough hands, which could reduce hand hygiene practices. In Japan, there are several AHR products available. Among them, a few products contain skin moisturizing elements; these products are 10%-20% higher in cost than nonmoisturizing products. The HCWs in our study stated that the new products were more comfortable to use, and they requested to introduce them as daily use products. Thus, use of a product containing a hand moisturizer may reduce some factors negatively affecting hand hygiene practice and improve adherence rates.
Although this study was unable to determine which components are definitively associated with improving hand hygiene adherence, the findings suggest initiation of multiple intervention components simultaneously may provide more motivation for change than initiating only one or two components at a time. It is also possible that certain intervention components were more beneficial than others. Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introducing portable AHR) alone, but rather depends on altering the behavior of physicians and nurses.
This study was performed at four tertiary care hospitals in Niigata that are affiliated with Niigata University. They are located closely in the region, within 100 km, have quarterly conferences, and use a mutual monitoring system related to infection prevention. The members of infection control communicate regularly, which we thought would optimize improvements in hand hygiene adherence, compared with the circumstances of previous studies. In this setting, HCWs have similar education and share knowledge related to infection control, and the effects of interventions in each hospital were equally evaluated if similar interventions were implemented. In the current study, the interventions at each hospital were similar, and there was limited variety; therefore, specific, novel interventions that could affect hand hygiene adherence significantly were difficult to find.
There are a few possible reasons why hand hygiene adherence rates were low in the current study. First, part of this study was conducted during the summer so that the consciousness and caution for hand hygiene might be lower, compared with that in winter. In general, HCWs become more cautious for hand hygiene practice when they take care of patients diagnosed with influenza or respiratory syncytial virus infection. Second, the infrastructure for hand hygiene practice in the hospitals in Japan is inadequate and not well designed. Because of safety reasons, a single dispenser of AHR is placed at the entrance of each room in general and not at each bedside. The number of private rooms is limited, and most of the rooms in wards have multiple beds per room, with no access to AHR within the room. In fact, the interventions at all four hospitals included a change in the location and/or access of AHR. Easier access to AHR is likely a key step to improving hand hygiene adherence rates. Finally, there was not an active intervention to include hospital or unit leaders. This is important given the involvement of leaders in hand hygiene practice significantly changed the hand adherence rates in a previous study.19
Given the suboptimal hand hygiene adherence rates in Japan noted in this and previous Japanese studies,21,22 the spread of COVID-19 within the hospital setting is a concern. Transmission of COVID-19 by asymptomatic carriers has been suggested,11 which emphasizes the importance of regular standard precautions with good hand hygiene practice to prevent further transmission.
Although the hand hygiene rate was suboptimal, we were able to achieve a few sustainable, structural modifications in the clinical environment after the intervention. These include adding AHR in new locations, changing the location of existing AHR to more appropriate locations, and introducing new products. These will remain in the clinical environment and will contribute to hand hygiene adherence in the future.
This study has several limitations. First, the presence of external observers in their clinical settings might have affected the behavior of HCWs.28 Although they were not informed that their hand hygiene adherence was going to be monitored, the existence of an external observer in their clinical setting might have changed normal behavior. Second, the infrastructure and interventions for hand hygiene adherence before the intervention were different in each hospital, so there is a possibility that hospitals with less infrastructure for hand hygiene adherence had more room for improvement with the interventions. Third, we included observations at different units at each hospital, which might affect the results of the study because of the inclusion of different medical settings and HCWs. Fourth, the number of physician hand hygiene observations was limited: We conducted our observations between 8
In conclusion, a multimodal intervention to improve hand hygiene adherence successfully improved HCWs’ hand hygiene adherence in Niigata, Japan; however, the adherence rates are still relatively low compared with those reported from other countries. Further intervention is required to improve hand hygiene adherence.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
1. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
2. Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150.
3. Vrijens F, Hulstaert F, Van de Sande S, Devriese S, Morales I, Parmentier Y. Hospital-acquired, laboratory-confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158-162. https://doi.org/10.1016/j.jhin.2009.12.006.
4. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. https://doi.org/10.1016/j.ajic.2008.12.010.
5. Suka M, Yoshida K, Takezawa J. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30-35. https:// doi.org/10.1007/s12199-007-0004-y.
6. Japan Nosocomial Infection Surveillance. JANIS Open Report. 2018. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. Accessed April 2, 2020.
7. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315. https://doi.org/10.1016/j.jhin.2009.04.019.
8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017.
9. World Health Organization. Coronavirus disease (COVID-19) advice for the public. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed February 28, 2020.
10. Centers for Disease Control and Prevention. Interim Guidance for Preventing the Spread of Coronavirus Disease 2019 (COVID-19) in Homes and Residential Communities. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html. Accessed February 28, 2020.
11. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565.
12. Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651-656. https://doi.org/10.1056/NEJMhpr020557.
13. World Health Organization. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2013. https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf. Accessed February 28, 2020.
14. Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843-851. https://doi.org/10.1016/S1473-3099(13)70163-4.
15. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312. https://doi.org/10.1016/s0140-6736(00)02814-2.
16. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415-423. https://doi.org/10.1086/669860.
17. Pincock T, Bernstein P, Warthman S, Holst E. Bundling hand hygiene interventions and measurement to decrease health care-associated infections. Am J Infect Control. 2012;40(4 Suppl 1):S18-S27. https://doi.org/10.1016/j.ajic.2012.02.008.
18. Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251-269. https://doi.org/10.1016/0196-6553(95)90070-5.
19. Saint S, Conti A, Bartoloni A, et al. Improving healthcare worker hand hygiene adherence before patient contact: a before-and-after five-unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429-433. https://doi.org/10.1136/qshc.2009.032771.
20. Bolon MK. Hand hygiene: an update. Infect Dis Clin North Am. 2016;30(3):591-607. https://doi.org/10.1016/j.idc.2016.04.007.
21. Sakihama T, Honda H, Saint S, et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan. J Patient Saf. 2016;12(1):11-17. https://doi.org/10.1097/PTS.0000000000000108.
22. Sakihama T, Honda H, Saint S, et al. Improving healthcare worker hand hygiene adherence before patient contact: a multimodal intervention of hand hygiene practice in three Japanese tertiary care centers. J Hosp Med. 2016;11(3):199-205. https://doi.org/10.1002/jhm.2491.
23. Sakihama T, Kayauchi N, Kamiya T, et al. Assessing sustainability of hand hygiene adherence 5 years after a contest-based intervention in 3 Japanese hospitals. Am J Infect Control. 2020;48(1):77-81. https://doi.org/10.1016/j.ajic.2019.06.017.
24. World Health Organization. My 5 Moments for Hand Hygiene. https://www.who.int/infection-prevention/campaigns/clean-hands/5moments/en/. Accessed April 2, 2020.
25. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. 2009. https://www.who.int/gpsc/5may/tools/9789241597906/en/. Accessed February 28, 2020.
26. Saint S, Bartoloni A, Virgili G, et al. Marked variability in adherence to hand hygiene: a 5-unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306-310. https://doi.org/10.1016/j.ajic.2008.08.004.
27. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144013/pdf/Bookshelf_NBK144013.pdf. Accessed February 28, 2020.
28. Pan SC, Tien KL, Hung IC, et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746. https://doi.org/10.1371/journal.pone.0053746.
© 2020 Society of Hospital Medicine
Patient Preferences for Physician Attire: A Multicenter Study in Japan
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
The patient-physician relationship is critical for ensuring the delivery of high-quality healthcare. Successful patient-physician relationships arise from shared trust, knowledge, mutual respect, and effective verbal and nonverbal communication. The ways in which patients experience healthcare and their satisfaction with physicians affect a myriad of important health outcomes, such as adherence to treatment and outcomes for conditions such as hypertension and diabetes mellitus.1-5 One method for potentially enhancing patient satisfaction is through understanding how patients wish their physicians to dress6-8 and tailoring attire to match these expectations. In addition to our systematic review,9 a recent large-scale, multicenter study in the United States revealed that most patients perceive physician attire as important, but that preferences for specific types of attire are contextual.9,10 For example, elderly patients preferred physicians in formal attire and white coat, while scrubs with white coat or scrubs alone were preferred for emergency department (ED) physicians and surgeons, respectively. Moreover, regional variation regarding attire preference was also observed in the US, with preferences for more formal attire in the South and less formal in the Midwest.
Geographic variation, regarding patient preferences for physician dress, is perhaps even more relevant internationally. In particular, Japan is considered to have a highly contextualized culture that relies on nonverbal and implicit communication. However, medical professionals have no specific dress code and, thus, don many different kinds of attire. In part, this may be because it is not clear whether or how physician attire impacts patient satisfaction and perceived healthcare quality in Japan.11-13 Although previous studies in Japan have suggested that physician attire has a considerable influence on patient satisfaction, these studies either involved a single department in one hospital or a small number of respondents.14-17 Therefore, we performed a multicenter, cross-sectional study to understand patients’ preferences for physician attire in different clinical settings and in different geographic regions in Japan.
METHODS
Study Population
We conducted a cross-sectional, questionnaire-based study from 2015 to 2017, in four geographically diverse hospitals in Japan. Two of these hospitals, Tokyo Joto Hospital and Juntendo University Hospital, are located in eastern Japan whereas the others, Kurashiki Central Hospital and Akashi Medical Center, are in western Japan.
Questionnaires were printed and randomly distributed by research staff to outpatients in waiting rooms and inpatients in medical wards who were 20 years of age or older. We placed no restriction on ward site or time of questionnaire distribution. Research staff, including physicians, nurses, and medical clerks, were instructed to avoid guiding or influencing participants’ responses. Informed consent was obtained by the staff; only those who provided informed consent participated in the study. Respondents could request assistance with form completion from persons accompanying them if they had difficulties, such as physical, visual, or hearing impairments. All responses were collected anonymously. The study was approved by the ethics committees of all four hospitals.
Questionnaire
We used a modified version of the survey instrument from a prior study.10 The first section of the survey showed photographs of either a male or female physician with 7 unique forms of attire, including casual, casual with white coat, scrubs, scrubs with white coat, formal, formal with white coat, and business suit (Figure 1). Given the Japanese context of this study, the language was translated to Japanese and photographs of physicians of Japanese descent were used. Photographs were taken with attention paid to achieving constant facial expressions on the physicians as well as in other visual cues (eg, lighting, background, pose). The physician’s gender and attire in the first photograph seen by each respondent were randomized to prevent bias in ordering, priming, and anchoring; all other sections of the survey were identical.
Respondents were first asked to rate the standalone, randomized physician photograph using a 1-10 scale across five domains (ie, how knowledgeable, trustworthy, caring, and approachable the physician appeared and how comfortable the physician’s appearance made the respondent feel), with a score of 10 representing the highest rating. Respondents were subsequently given 7 photographs of the same physician wearing various forms of attire. Questions were asked regarding preference of attire in varied clinical settings (ie, primary care, ED, hospital, surgery, overall preference). To identify the influence of and respondent preferences for physician dress and white coats, a Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) was employed. The scale was trichotomized into “disagree” (1, 2), “neither agree nor disagree” (3), and “agree” (4, 5) for analysis. Demographic data, including age, gender, education level, nationality (Japanese or non-Japanese), and number of physicians seen in the past year were collected.
Outcomes and Sample Size Calculation
The primary outcome of attire preference was calculated as the mean composite score of the five individual rating domains (ie, knowledgeable, trustworthy, caring, approachable, and comfortable), with the highest score representing the most preferred form of attire. We also assessed variation in preferences for physician attire by respondent characteristics, such as age and gender.
Sample size estimation was based on previous survey methodology.10 The Likert scale range for identifying influence of and respondent preferences for physician dress and white coats was 1-5 (“strongly disagree” to “strongly agree”). The scale range for measuring preferences for the randomized attire photograph was 1-10. An assumption of normality was made regarding responses on the 1-10 scale. An estimated standard deviation of 2.2 was assumed, based on prior findings.10 Based on these assumptions and the inclusion of at least 816 respondents (assuming a two-sided alpha error of 0.05), we expected to have 90% capacity to detect differences for effect sizes of 0.50 on the 1-10 scale.
Statistical Analyses
Paper-based survey data were entered independently and in duplicate by the study team. Respondents were not required to answer all questions; therefore, the denominator for each question varied. Data were reported as mean and standard deviation (SD) or percentages, where appropriate. Differences in the mean composite rating scores were assessed using one-way ANOVA with the Tukey method for pairwise comparisons. Differences in proportions for categorical data were compared using the Z-test. Chi-squared tests were used for bivariate comparisons between respondent age, gender, and level of education and corresponding respondent preferences. All analyses were performed using Stata 14 MP/SE (Stata Corp., College Station, Texas, USA).
RESULTS
Characteristics of Participants
Between December 1, 2015 and October 30, 2017, a total of 2,020 surveys were completed by patients across four academic hospitals in Japan. Of those, 1,960 patients (97.0%) completed the survey in its entirety. Approximately half of the respondents were 65 years of age or older (49%), of female gender (52%), and reported receiving care in the outpatient setting (53%). Regarding use of healthcare, 91% had seen more than one physician in the year preceding the time of survey completion (Table 1).
Ratings of Physician Attire
Compared with all forms of attire depicted in the survey’s first standalone photograph, respondents rated “casual attire with white coat” the highest (Figure 2). The mean composite score for “casual attire with white coat” was 7.1 (standard deviation [SD] = 1.8), and this attire was set as the referent group. Cronbach’s alpha, for the five items included in the composite score, was 0.95. However, “formal attire with white coat” was rated almost as highly as “casual attire with white coat” with an overall mean composite score of 7.0 (SD = 1.6).
Variation in Preference for Physician Attire by Clinical Setting
Preferences for physician attire varied by clinical care setting. Most respondents preferred “casual attire with white coat” or “formal attire with white coat” in both primary care and hospital settings, with a slight preference for “casual attire with white coat.” In contrast, respondents preferred “scrubs without white coat” in the ED and surgical settings. When asked about their overall preference, respondents reported they felt their physician should wear “formal attire with white coat” (35%) or “casual attire with white coat” (30%; Table 2). When comparing the group of photographs of physicians with white coats to the group without white coats (Figure 1), respondents preferred physicians wearing white coats overall and specifically when providing care in primary care and hospital settings. However, they preferred physicians without white coats when providing care in the ED (P < .001). With respect to surgeons, there was no statistically significant difference between preference for white coats and no white coats. These results were similar for photographs of both male and female physicians.
When asked whether physician dress was important to them and if physician attire influenced their satisfaction with the care received, 61% of participants agreed that physician dress was important, and 47% agreed that physician attire influenced satisfaction (Appendix Table 1). With respect to appropriateness of physicians dressing casually over the weekend in clinical settings, 52% responded that casual wear was inappropriate, while 31% had a neutral opinion.
Participants were asked whether physicians should wear a white coat in different clinical settings. Nearly two-thirds indicated a preference for white coats in the office and hospital (65% and 64%, respectively). Responses regarding whether emergency physicians should wear white coats were nearly equally divided (Agree, 37%; Disagree, 32%; Neither Agree nor Disagree, 31%). However, “scrubs without white coat” was most preferred (56%) when patients were given photographs of various attire and asked, “Which physician would you prefer to see when visiting the ER?” Responses to the question “Physicians should always wear a white coat when seeing patients in any setting” varied equally (Agree, 32%; Disagree, 34%; Neither Agree nor Disagree, 34%).
Variation in Preference for Physician Attire by Respondent Demographics
When comparing respondents by age, those 65 years or older preferred “formal attire with white coat” more so than respondents younger than 65 years (Appendix Table 2). This finding was identified in both primary care (36% vs 31%, P < .001) and hospital settings (37% vs 30%, P < .001). Additionally, physician attire had a greater impact on older respondents’ satisfaction and experience (Appendix Table 3). For example, 67% of respondents 65 years and older agreed that physician attire was important, and 54% agreed that attire influenced satisfaction. Conversely, for respondents younger than 65 years, the proportion agreeing with these statements was lower (56% and 41%, both P < .001). When comparing older and younger respondents, those 65 years and older more often preferred physicians wearing white coats in any setting (39% vs 26%, P < .001) and specifically in their office (68% vs 61%, P = .002), the ED (40% vs 34%, P < .001), and the hospital (69% vs 60%, P < .001).
When comparing male and female respondents, male respondents more often stated that physician dress was important to them (men, 64%; women, 58%; P = .002). When comparing responses to the question “Overall, which clothes do you feel a doctor should wear?”, between the eastern and western Japanese hospitals, preferences for physician attire varied.
Variation in Expectations Between Male and Female Physicians
When comparing the ratings of male and female physicians, female physicians were rated higher in how caring (P = .005) and approachable (P < .001) they appeared. However, there were no significant differences in the ratings of the three remaining domains (ie, knowledgeable, trustworthy, and comfortable) or the composite score.
DISCUSSION
Since we employed the same methodology as previous studies conducted in the US10 and Switzerland,18 a notable strength of our approach is that comparisons among these countries can be drawn. For example, physician attire appears to hold greater importance in Japan than in the US and Switzerland. Among Japanese participants, 61% agreed that physician dress is important (US, 53%; Switzerland, 36%), and 47% agreed that physician dress influenced how satisfied they were with their care (US, 36%; Switzerland, 23%).10 This result supports the notion that nonverbal and implicit communications (such as physician dress) may carry more importance among Japanese people.11-13
Regarding preference ratings for type of dress among respondents in Japan, “casual attire with white coat” received the highest mean composite score rating, with “formal attire with white coat” rated second overall. In contrast, US respondents rated “formal attire with white coat” highest and “scrubs with white coat” second.10 Our result runs counter to our expectation in that we expected Japanese respondents to prefer formal attire, since Japan is one of the most formal cultures in the world. One potential explanation for this difference is that the casual style chosen for this study was close to the smart casual style (slightly casual). Most hospitals and clinics in Japan do not allow physicians to wear jeans or polo shirts, which were chosen as the casual attire in the previous US study.
When examining various care settings and physician types, both Japanese and US respondents were more likely to prefer physicians wearing a white coat in the office or hospital.10 However, Japanese participants preferred both “casual attire with white coat” and “formal attire with white coat” equally in primary care or hospital settings. A smaller proportion of US respondents preferred “casual attire with white coat” in primary care (11%) and hospital settings (9%), but more preferred “formal attire with white coat” for primary care (44%) and hospital physicians (39%). In the ED setting, 32% of participants in Japan and 18% in the US disagreed with the idea that physicians should wear a white coat. Among Japanese participants, “scrubs without white coat” was rated highest for emergency physicians (56%) and surgeons (47%), while US preferences were 40% and 42%, respectively.10 One potential explanation is that scrubs-based attire became popular among Japanese ED and surgical contexts as a result of cultural influence and spread from western countries.19, 20
With respect to perceptions regarding physician attire on weekends, 52% of participants considered it inappropriate for a physician to dress casually over the weekend, compared with only 30% in Switzerland and 21% in the US.11,12 Given Japan’s level of formality and the fact that most Japanese physicians continue to work over the weekend,21-23 Japanese patients tend to expect their physicians to dress in more formal attire during these times.
Previous studies in Japan have demonstrated that older patients gave low ratings to scrubs and high ratings to white coat with any attire,15,17 and this was also the case in our study. Perhaps elderly patients reflect conservative values in their preferences of physician dress. Their perceptions may be less influenced by scenes portraying physicians in popular media when compared with the perceptions of younger patients. Though a 2015 systematic review and studies in other countries revealed white coats were preferred regardless of exact dress,9,24-26 they also showed variation in preferences for physician attire. For example, patients in Saudi Arabia preferred white coat and traditional ethnic dress,25 whereas mothers of pediatric patients in Saudi Arabia preferred scrubs for their pediatricians.27 Therefore, it is recommended for internationally mobile physicians to choose their dress depending on a variety of factors including country, context, and patient age group.
Our study has limitations. First, because some physicians presented the surveys to the patients, participants may have responded differently. Second, participants may have identified photographs of the male physician model as their personal healthcare provider (one author, K.K.). To avoid this possible bias, we randomly distributed 14 different versions of physician photographs in the questionnaire. Third, although physician photographs were strictly controlled, the “formal attire and white coat” and “casual attire and white coat” photographs appeared similar, especially given that the white coats were buttoned. Also, the female physician depicted in the photographs did not have the scrub shirt tucked in, while the male physician did. These nuances may have affected participant ratings between groups. Fourth,
In conclusion, patient preferences for physician attire were examined using a multicenter survey with a large sample size and robust survey methodology, thus overcoming weaknesses of previous studies into Japanese attire. Japanese patients perceive that physician attire is important and influences satisfaction with their care, more so than patients in other countries, like the US and Switzerland. Geography, settings of care, and patient age play a role in preferences. As a result, hospitals and health systems may use these findings to inform dress code policy based on patient population and context, recognizing that the appearance of their providers affects the patient-physician relationship. Future research should focus on better understanding the various cultural and societal customs that lead to patient expectations of physician attire.
Acknowledgments
The authors thank Drs. Fumi Takemoto, Masayuki Ueno, Kazuya Sakai, Saori Kinami, and Toshio Naito for their assistance with data collection at their respective sites. Additionally, the authors thank Dr. Yoko Kanamitsu for serving as a model for photographs.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
1. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201-203. https://doi.org/ 10.1056/NEJMp1211775.
2. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48.
3. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
4. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-31. https://doi.org/10.1056/NEJMsa080411.
5. O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17(2):144-54. https://doi.org/10.1046/j.1525-1497.2002.10431.x.
6. Chung H, Lee H, Chang DS, Kim HS, Park HJ, Chae Y. Doctor’s attire influences perceived empathy in the patient-doctor relationship. Patient Educ Couns. 2012;89(3):387-391. https://doi.org/10.1016/j.pec.2012.02.017.
7. Bianchi MT. Desiderata or dogma: what the evidence reveals about physician attire. J Gen Intern Med. 2008;23(5):641-643. https://doi.org/10.1007/s11606-008-0546-8.
8. Brandt LJ. On the value of an old dress code in the new millennium. Arch Intern Med. 2003;163(11):1277-1281. https://doi.org/10.1001/archinte.163.11.1277.
9. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. https://doi.org/10.1136/bmjopen-2014-006578.
10. Petrilli CM, Saint S, Jennings JJ, et al. Understanding patient preference for physician attire: a cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open. 2018;8(5):e021239. https://doi.org/10.1136/bmjopen-2017-021239.
11. Rowbury R. The need for more proactive communications. Low trust and changing values mean Japan can no longer fall back on its homogeneity. The Japan Times. 2017, Oct 15;Sect. Opinion. https://www.japantimes.co.jp/opinion/2017/10/15/commentary/japan-commentary/need-proactive-communications/#.Xej7lC3MzUI. Accessed December 5, 2019.
12. Shoji Nishimura ANaST. Communication Style and Cultural Features in High/Low Context Communication Cultures: A Case Study of Finland, Japan and India. Nov 22nd, 2009.
13. Smith RMRSW. The influence of high/low-context culture and power distance on choice of communication media: Students’ media choice to communicate with Professors in Japan and America. Int J Intercultural Relations. 2007;31(4):479-501.
14. Yamada Y, Takahashi O, Ohde S, Deshpande GA, Fukui T. Patients’ preferences for doctors’ attire in Japan. Intern Med. 2010;49(15):1521-1526. https://doi.org/10.2169/internalmedicine.49.3572.
15. Ikusaka M, Kamegai M, Sunaga T, et al. Patients’ attitude toward consultations by a physician without a white coat in Japan. Intern Med. 1999;38(7):533-536. https://doi.org/10.2169/internalmedicine.38.533.
16. Lefor AK, Ohnuma T, Nunomiya S, Yokota S, Makino J, Sanui M. Physician attire in the intensive care unit in Japan influences visitors’ perception of care. J Crit Care. 2018;43:288-293.
17. Kurihara H, Maeno T. Importance of physicians’ attire: factors influencing the impression it makes on patients, a cross-sectional study. Asia Pac Fam Med. 2014;13(1):2. https://doi.org/10.1186/1447-056X-13-2.
18. Zollinger M, Houchens N, Chopra V, et al. Understanding patient preference for physician attire in ambulatory clinics: a cross-sectional observational study. BMJ Open. 2019;9(5):e026009. https://doi.org/10.1136/bmjopen-2018-026009.
19. Chung JE. Medical Dramas and Viewer Perception of Health: Testing Cultivation Effects. Hum Commun Res. 2014;40(3):333-349.
20. Michael Pfau LJM, Kirsten Garrow. The influence of television viewing on public perceptions of physicians. J Broadcast Electron Media. 1995;39(4):441-458.
21. Suzuki S. Exhausting physicians employed in hospitals in Japan assessed by a health questionnaire [in Japanese]. Sangyo Eiseigaku Zasshi. 2017;59(4):107-118. https://doi.org/10.1539/sangyoeisei.
22. Ogawa R, Seo E, Maeno T, Ito M, Sanuki M. The relationship between long working hours and depression among first-year residents in Japan. BMC Med Educ. 2018;18(1):50. https://doi.org/10.1186/s12909-018-1171-9.
23. Saijo Y, Chiba S, Yoshioka E, et al. Effects of work burden, job strain and support on depressive symptoms and burnout among Japanese physicians. Int J Occup Med Environ Health. 2014;27(6):980-992. https://doi.org/10.2478/s13382-014-0324-2.
24. Tiang KW, Razack AH, Ng KL. The ‘auxiliary’ white coat effect in hospitals: perceptions of patients and doctors. Singapore Med J. 2017;58(10):574-575. https://doi.org/10.11622/smedj.2017023.
25. Al Amry KM, Al Farrah M, Ur Rahman S, Abdulmajeed I. Patient perceptions and preferences of physicians’ attire in Saudi primary healthcare setting. J Community Hosp Intern Med Perspect. 2018;8(6):326-330. https://doi.org/10.1080/20009666.2018.1551026.
26. Healy WL. Letter to the editor: editor’s spotlight/take 5: physicians’ attire influences patients’ perceptions in the urban outpatient orthopaedic surgery setting. Clin Orthop Relat Res. 2016;474(11):2545-2546. https://doi.org/10.1007/s11999-016-5049-z.
27. Aldrees T, Alsuhaibani R, Alqaryan S, et al. Physicians’ attire. Parents preferences in a tertiary hospital. Saudi Med J. 2017;38(4):435-439. https://doi.org/10.15537/smj.2017.4.15853.
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Be the Change You Want to See
“…a truly strong, powerful man isn’t threatened by a strong, powerful woman. Instead, he is challenged by her, he is inspired by her, he is pleased to relate to her as an equal.”
—Michelle Obama
Mentorship is essential to success in hospital medicine and may be particularly important for women. Cross-gender mentorship is especially salient since roughly equal proportions of women and men enter the medical pipeline, but men occupy over 75% of senior leadership roles in healthcare companies.
Cross-gender mentorship poses challenges but can be done successfully.1 We’ve made cross-gender mentoring work well in our own mentoring relationship. We describe three practices for effective mentoring that are especially important for men who mentor women given how common the female mentee-male mentor dyad is in medicine. We make generalizations that don’t apply universally but illustrate the social context in which such mentorship resides.
BE MINDFUL OF GENDER SCRIPTS
Gender scripts refer to social norms relating to gender identities and behaviors. Archetypal scripts include the father/daughter relationship and the knight/damsel-in-distress. Gender scripts often frame women as powerless—waiting to be rescued. By unconsciously activating a gender script, a mentor may reinforce a stereotype that women need rescuing (eg, “She’s really upset—I’ll email her Division Chief and help fix it for her”) or underestimate a mentee’s readiness for independence (eg, “She’s written four papers on this, but she’s still not ready to be senior author”). Astute mentors use reflection to combat gender scripts, asking themselves, “Am I allowing latent biases to affect my judgement?” They also consider when to intervene and when to let the mentee “rescue” herself (eg, “This is challenging, but I trust your judgement. What do you think you should do next?”).
PROMOTE RECIPROCAL LEARNING
Many women value collaborative behaviors and gravitate towards egalitarian learning environments at odds with a traditional, “top-down” mentorship model. Additionally, women may be penalized for demonstrating competitive behaviors, while identical behaviors are chalked up to confidence in men. A critical task, then, is for mentors to coach women to hone their natural leadership style, whether it be more commanding or more communal. A mentor can provide key feedback to the mentee about how her approach might be perceived and how to tweak it for optimal success. Mentors may wish to share missteps and even ask the mentee for advice. Pointing to her competence promotes “relational mentoring” and reciprocal learning, where mentor and mentee can learn positive behaviors from each other.
BE THE CHANGE YOU WANT TO SEE
Mentors will ideally wield their social capital to advance policies that promote gender equity—including fair recruiting, promotion, salary, paid leave, and breastfeeding policies. Exceptional mentors recognize that women may generally have less social capital than men in many organizations, and they proactively make women’s accomplishments more visible.2 They broadcast women’s strengths and nominate women for talks, national committees, honorific societies, and leadership positions. Effective mentors recognize that 30% of female medical faculty report experiencing sexual harassment at work,3 and thus maintain extremely high standards for professional integrity, for both themselves and others who interact with their mentees. They call out sexist remarks in the workplace as unacceptable, making it clear that such behavior won’t be tolerated. As Mohandas Gandhi said: “Be the change that you wish to see in the world.”
Cross-gender mentorship is critical to get right—nearly half our medical workforce depends on it. Men who mentor women help their organizations and gain satisfaction from playing a pivotal role in women’s advancement. When women succeed, we all do.
Disclosures
Dr. Moniz and Dr. Saint have nothing to disclose.
1. Byerley JS. Mentoring in the Era of #MeToo. JAMA. 2018;319(12):1199-1200. PubMed
2. Chopra V, Arora VM, Saint S. Will You Be My Mentor?-Four Archetypes to Help Mentees Succeed in Academic Medicine. JAMA Intern Med. 2018;178(2):175-176. PubMed
3. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual Harassment and Discrimination Experiences of Academic Medical Faculty. JAMA. 2016;315(19):2120-2121. PubMed
“…a truly strong, powerful man isn’t threatened by a strong, powerful woman. Instead, he is challenged by her, he is inspired by her, he is pleased to relate to her as an equal.”
—Michelle Obama
Mentorship is essential to success in hospital medicine and may be particularly important for women. Cross-gender mentorship is especially salient since roughly equal proportions of women and men enter the medical pipeline, but men occupy over 75% of senior leadership roles in healthcare companies.
Cross-gender mentorship poses challenges but can be done successfully.1 We’ve made cross-gender mentoring work well in our own mentoring relationship. We describe three practices for effective mentoring that are especially important for men who mentor women given how common the female mentee-male mentor dyad is in medicine. We make generalizations that don’t apply universally but illustrate the social context in which such mentorship resides.
BE MINDFUL OF GENDER SCRIPTS
Gender scripts refer to social norms relating to gender identities and behaviors. Archetypal scripts include the father/daughter relationship and the knight/damsel-in-distress. Gender scripts often frame women as powerless—waiting to be rescued. By unconsciously activating a gender script, a mentor may reinforce a stereotype that women need rescuing (eg, “She’s really upset—I’ll email her Division Chief and help fix it for her”) or underestimate a mentee’s readiness for independence (eg, “She’s written four papers on this, but she’s still not ready to be senior author”). Astute mentors use reflection to combat gender scripts, asking themselves, “Am I allowing latent biases to affect my judgement?” They also consider when to intervene and when to let the mentee “rescue” herself (eg, “This is challenging, but I trust your judgement. What do you think you should do next?”).
PROMOTE RECIPROCAL LEARNING
Many women value collaborative behaviors and gravitate towards egalitarian learning environments at odds with a traditional, “top-down” mentorship model. Additionally, women may be penalized for demonstrating competitive behaviors, while identical behaviors are chalked up to confidence in men. A critical task, then, is for mentors to coach women to hone their natural leadership style, whether it be more commanding or more communal. A mentor can provide key feedback to the mentee about how her approach might be perceived and how to tweak it for optimal success. Mentors may wish to share missteps and even ask the mentee for advice. Pointing to her competence promotes “relational mentoring” and reciprocal learning, where mentor and mentee can learn positive behaviors from each other.
BE THE CHANGE YOU WANT TO SEE
Mentors will ideally wield their social capital to advance policies that promote gender equity—including fair recruiting, promotion, salary, paid leave, and breastfeeding policies. Exceptional mentors recognize that women may generally have less social capital than men in many organizations, and they proactively make women’s accomplishments more visible.2 They broadcast women’s strengths and nominate women for talks, national committees, honorific societies, and leadership positions. Effective mentors recognize that 30% of female medical faculty report experiencing sexual harassment at work,3 and thus maintain extremely high standards for professional integrity, for both themselves and others who interact with their mentees. They call out sexist remarks in the workplace as unacceptable, making it clear that such behavior won’t be tolerated. As Mohandas Gandhi said: “Be the change that you wish to see in the world.”
Cross-gender mentorship is critical to get right—nearly half our medical workforce depends on it. Men who mentor women help their organizations and gain satisfaction from playing a pivotal role in women’s advancement. When women succeed, we all do.
Disclosures
Dr. Moniz and Dr. Saint have nothing to disclose.
“…a truly strong, powerful man isn’t threatened by a strong, powerful woman. Instead, he is challenged by her, he is inspired by her, he is pleased to relate to her as an equal.”
—Michelle Obama
Mentorship is essential to success in hospital medicine and may be particularly important for women. Cross-gender mentorship is especially salient since roughly equal proportions of women and men enter the medical pipeline, but men occupy over 75% of senior leadership roles in healthcare companies.
Cross-gender mentorship poses challenges but can be done successfully.1 We’ve made cross-gender mentoring work well in our own mentoring relationship. We describe three practices for effective mentoring that are especially important for men who mentor women given how common the female mentee-male mentor dyad is in medicine. We make generalizations that don’t apply universally but illustrate the social context in which such mentorship resides.
BE MINDFUL OF GENDER SCRIPTS
Gender scripts refer to social norms relating to gender identities and behaviors. Archetypal scripts include the father/daughter relationship and the knight/damsel-in-distress. Gender scripts often frame women as powerless—waiting to be rescued. By unconsciously activating a gender script, a mentor may reinforce a stereotype that women need rescuing (eg, “She’s really upset—I’ll email her Division Chief and help fix it for her”) or underestimate a mentee’s readiness for independence (eg, “She’s written four papers on this, but she’s still not ready to be senior author”). Astute mentors use reflection to combat gender scripts, asking themselves, “Am I allowing latent biases to affect my judgement?” They also consider when to intervene and when to let the mentee “rescue” herself (eg, “This is challenging, but I trust your judgement. What do you think you should do next?”).
PROMOTE RECIPROCAL LEARNING
Many women value collaborative behaviors and gravitate towards egalitarian learning environments at odds with a traditional, “top-down” mentorship model. Additionally, women may be penalized for demonstrating competitive behaviors, while identical behaviors are chalked up to confidence in men. A critical task, then, is for mentors to coach women to hone their natural leadership style, whether it be more commanding or more communal. A mentor can provide key feedback to the mentee about how her approach might be perceived and how to tweak it for optimal success. Mentors may wish to share missteps and even ask the mentee for advice. Pointing to her competence promotes “relational mentoring” and reciprocal learning, where mentor and mentee can learn positive behaviors from each other.
BE THE CHANGE YOU WANT TO SEE
Mentors will ideally wield their social capital to advance policies that promote gender equity—including fair recruiting, promotion, salary, paid leave, and breastfeeding policies. Exceptional mentors recognize that women may generally have less social capital than men in many organizations, and they proactively make women’s accomplishments more visible.2 They broadcast women’s strengths and nominate women for talks, national committees, honorific societies, and leadership positions. Effective mentors recognize that 30% of female medical faculty report experiencing sexual harassment at work,3 and thus maintain extremely high standards for professional integrity, for both themselves and others who interact with their mentees. They call out sexist remarks in the workplace as unacceptable, making it clear that such behavior won’t be tolerated. As Mohandas Gandhi said: “Be the change that you wish to see in the world.”
Cross-gender mentorship is critical to get right—nearly half our medical workforce depends on it. Men who mentor women help their organizations and gain satisfaction from playing a pivotal role in women’s advancement. When women succeed, we all do.
Disclosures
Dr. Moniz and Dr. Saint have nothing to disclose.
1. Byerley JS. Mentoring in the Era of #MeToo. JAMA. 2018;319(12):1199-1200. PubMed
2. Chopra V, Arora VM, Saint S. Will You Be My Mentor?-Four Archetypes to Help Mentees Succeed in Academic Medicine. JAMA Intern Med. 2018;178(2):175-176. PubMed
3. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual Harassment and Discrimination Experiences of Academic Medical Faculty. JAMA. 2016;315(19):2120-2121. PubMed
1. Byerley JS. Mentoring in the Era of #MeToo. JAMA. 2018;319(12):1199-1200. PubMed
2. Chopra V, Arora VM, Saint S. Will You Be My Mentor?-Four Archetypes to Help Mentees Succeed in Academic Medicine. JAMA Intern Med. 2018;178(2):175-176. PubMed
3. Jagsi R, Griffith KA, Jones R, Perumalswami CR, Ubel P, Stewart A. Sexual Harassment and Discrimination Experiences of Academic Medical Faculty. JAMA. 2016;315(19):2120-2121. PubMed
© 2019 Society of Hospital Medicine
Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
© 2019 Society of Hospital Medicine
Leadership & Professional Development: Know Your TLR
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
Hire Hard, Manage Easy
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
© 2019 Society of Hospital Medicine