User login
Popliteal Artery Pseudoaneurysm: An Unusual Complication of Tibial Traction
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
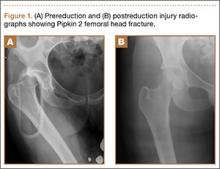
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
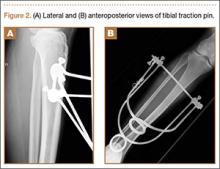
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
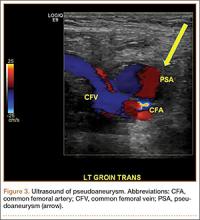
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
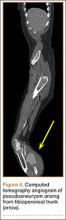
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
Traction-pin placement is a basic orthopedic skill learned in the early years of residency training. Skeletal traction historically was used as definitive treatment for long-bone fractures, and it is still in use in countries without access to modern medical care.1,2 In current orthopedic practice, proximal tibial and distal femoral traction pins are most commonly used to temporize femoral shaft and acetabular fractures, respectively, before definitive surgical intervention. Although traction-pin placement is common, there are complications that can cause morbidity ranging from skin irritation to death.
In this article, we report on a popliteal artery pseudoaneurysm, a unique complication related to pin placement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 22-year-old woman with no past medical history was driving a car at 35 miles per hour when she hit a telephone pole. She was wearing a seatbelt. She was taken by ambulance for a trauma evaluation. She sustained a right posterior hip dislocation with an associated Pipkin 2 femoral head fracture (Figures 1A, 1B), a right elbow skin avulsion, and a scalp abrasion.
The patient underwent closed hip reduction under sedation, and a proximal tibial traction pin was placed (Figures 2A, 2B). At our institution, proximal tibial traction pins are placed 2 cm distal and 2 cm posterior to the tibial tubercle without the use of fluoroscopy. The skin leg is prepared and draped in sterile fashion, and a local anesthetic is used to anesthetize the skin and periosteum on the medial and lateral aspects of the leg. A 1.5-cm incision is made laterally, and soft tissue is spread laterally down to the periosteum. Then the traction pin (always the largest nonthreaded pin available) is used to sound the anterior and posterior aspects of the tibia—the goal being to end at the anterior two-thirds/posterior one-third mark. After the pin is advanced through both cortices, the place where the pin will exit the skin is noted, and another incision is made. For this patient, no complications were noted at time of pin placement.
On postinjury day 1, she was taken to the operating room for open reduction and internal fixation of the femoral head fracture through a Smith-Petersen approach. At the same time, a traction pin was removed. Routine postoperative protocols were followed. The patient was started on enoxaparin, was seen by physical therapy on postoperative day 1, and was kept non-weight-bearing on the right lower extremity. Pain was well controlled, and the patient was discharged on postoperative day 3 (postinjury day 4).
Three weeks after surgery, she returned for a scheduled follow-up with complaints of paresthesia and decreased sensation in the right lateral cutaneous femoral nerve distribution and burning pain in the toes. A stiff right ankle was noted. A night splint, amitriptyline, and additional pain medications were ordered.
The patient returned for her scheduled 6-week follow-up reporting she had not taken the enoxaparin because her pharmacy “did not have any.” Her primary care physician had given her “another blood thinner,” which she had taken orally for 1 to 2 weeks. She had complaints of right calf pain, but her hip was pain-free. There was a large amount of calf swelling and tenderness along with mild equinus contracture of the ankle. She was sent to the emergency department (ED) for Doppler ultrasound evaluation for deep venous thrombosis (DVT).
Duplex Doppler imaging of the deep venous system of the lower extremities was performed with visualization from the common femoral veins to the popliteal veins bilaterally. There was normal compressibility, respiratory phasicity, and flow with no intraluminal thrombus. A radiograph of tibia and fibula showed soft-tissue swelling and lucencies from the traction pin but no other abnormalities.
Eight weeks after surgery, the patient returned to the ED because of continuing calf pain. Given that the ultrasound findings had been negative, magnetic resonance imaging (MRI) was ordered, and orthopedic follow-up scheduled. About 10 weeks after surgery, she returned to the ED, still with calf pain, and reported having been unable to go for her MRI because of financial reasons. Physical examination revealed increased calf swelling and a new ecchymosis tracking along the tibia. Repeat ultrasound, performed from the common femoral veins through the popliteal veins, showed a right posterior calf pseudoaneurysm (8.5×4.3×5.3 cm) arising from the popliteal artery about 5 cm below the popliteal fossa. In addition, a large hematoma was seen originating in the upper posterior calf and extending inferiorly (Figure 3).
Computed tomography angiogram confirmed the ultrasound diagnosis of a large (5.6×4.8-cm) right calf pseudoaneurysm arising possibly from a small muscular branch of the proximal tibioperoneal trunk, with a large surrounding hematoma in the posterior compartment of the proximal right calf (Figure 4). Vascular surgery was consulted, and coil embolization of the pseudoaneurysm was performed later that night in the interventional radiology suite.
After the coiling of the pseudoaneurysm, the calf swelling and pain slowly improved. At 12-week orthopedic follow-up, the patient was no longer using any pain medications, and she noted improvement in the right foot’s neuropathic symptoms. Serial casting was prescribed for the equinus contracture of the ankle. She was allowed to start weight-bearing on the right lower extremity. Radiographs at 12 weeks showed collapse of the superomedial aspect of the femoral head with surrounding sclerosis consistent with posttraumatic avascular necrosis.
At final orthopedic follow-up, about 16 weeks after surgery, the patient reported 0/10 pain. Sensation was noted as being intact throughout the right lower extremity but decreased in the tibial nerve distribution. Ankle range of motion was still limited, with 5° of dorsiflexion and 25° of plantar flexion. The hip was pain-free with flexion of 0° to 100°, 10° of internal rotation, and 20° of external rotation. Additional appointments were scheduled, but the patient did not follow up. Two years after initial injury, she returned to the ED for evaluation of rhinorrhea, and no orthopedic complaints were noted.
Discussion
Skeletal traction begins with the insertion of a wire or pin through a bone. It is extremely important to use proper technique in order to minimize the risks associated with pin insertion.3 Potential pitfalls involve the energy transferred into the bone during insertion, the incisions used to place the pin, and injury to surrounding neurovascular structures. For proximal tibial pins, standard technique dictates placing the pin in a lateral-to-medial direction 2 cm posterior to the tibial tubercle and avoiding the dense anterior cortical bone. At our institution, traction pins are placed with a power drill after the patient is given a local anesthetic or is placed under conscious sedation. Which type of anesthesia to use is based on the patient’s overall condition and on the ED attending physician’s willingness to administer conscious sedation.
The 2 most common types of tibial traction involve use of either a large Steinmann pin attached to a metal bow or a Kirschner wire (K-wire) placed under tension before traction. Which to use is the surgeon’s choice. Surgeons at our institution historically have used Steinmann pins. No studies have directly compared fine-wire and Steinmann-pin traction, but with this complication our institution is evaluating a change to tensioned wires. Compared with large Steinmann pins, fine-wire pins create less of a defect in the bone but also bend or break more easily if tension is not applied or if it fails. A fine wire with its smaller surface area may also cut more easily into osteopenic bone than a large-diameter pin would.
Proximal tibial traction typically is indicated for femoral shaft and acetabular fractures. Although the subcutaneous nature of the tibia makes for easier pin placement, the anatomy of the tibia can predispose this bone to complications. Its triangular shape can lead to intracortical rather than the preferred bicortical pin placement. Increased heat caused by intracortical placement can lead to osteonecrosis and even to damage of surrounding soft tissues. Green and Ripley4 found that chronic osteomyelitis typically resulted from intracortical placement of traction pins.
Injury to surrounding soft tissues, either from heat necrosis or from infection introduced through pin sites, can also have consequences. Pin-site infections increase with duration of treatment, though care seldom requires more than pin removal and antibiotics.5,6 More-invasive infections range from cellulitis surrounding the pin site to subcutaneous abscesses. There is 1 report of a Clostridium perfringens infection leading to death only 5 days after pin placement.7
Neurovascular structures are at risk with any orthopedic procedure. With proximal tibial pins in pediatric patients, the peroneal nerve, the anterior tibial artery, and the proximal physis are most at risk. The deep peroneal nerve and the anterior tibial artery run together deep to the anterior compartment, which places them at highest risk with pin insertion. The peroneal and tibial arteries run deep to the deep posterior compartment along with the tibial nerve behind the posterior cortex of the tibia, which makes injury less likely.8
Historically, long-bone fractures were often treated with traction. Kirby and Fitts9 reported on 342 transfixion pins and wires used in the treatment of 233 long-bone fractures between 1943 and 1945. Of the 305 pins/wires observed over the entire treatment period (average, 6 weeks), only 12 (3.93%) developed a complication. There were 4 loose K-wires, 1 broken wire, and 1 bow failure; Steinmann pins were involved in 1 infection and 2 transient peroneal nerve palsies; and 3 Roger Anderson pins loosened. Pin-tract drainage was not included as a complication if it did not also involve localized or general signs of inflammation. The 2 peroneal nerve palsies were associated with medial-to-lateral pin insertion creating a more posterior pin path.
Pins inserted for external fixators of the tibia have injured the anterior tibial vessels and branches of the peroneal and saphenous nerves. A proximal tibial traction pin, in essence a transfixion pin, can cause similar injuries, particularly with imperfect placement (Figure 5).3,10
A pseudoaneurysm is a pulsating, encapsulated hematoma that remains in communication with the lumen of a ruptured or injured vessel. The arterial wall itself is torn or ruptured, and the external wall of the aneurysmal sac consists of outer arterial layers, perivascular tissue, blood clot, or a layer of reactive fibrosis. This contrasts with a true aneurysm, in which all 3 arterial layers (intima, media, adventitia) remain confluent but are dilated beyond their normal diameter. Of all pseudoaneurysms, those caused iatrogenically are the most common and are typically produced by femoral artery catheterization, accounting for 70% to 80% of the incidence.11
Our patient’s injury was most likely caused by an initial error in pin placement before the pin was driven across the tibia. The typical teaching for traction-pin placement involves finding the correct starting point and then using the pin to feel the anterior and posterior surfaces of the bone (described earlier). If the pin slid posteriorly, it may have contacted the artery and caused a small tear that eventually led to the formation of the pseudoaneurysm.
The pseudoaneurysm was not the only complication in the present case. There was also the delay in diagnosis. A standard technique is used to evaluate the lower extremity venous system for DVT. The ultrasonographer starts with the probe as proximal as possible (above the inguinal ligament), ideally proximal to the saphenofemoral junction, and moves distally in 1-cm increments, checking the veins for compressibility, color, and Doppler signal. Unless advised otherwise, the ultrasonographer typically does not examine distal to the knee.12,13 As this patient’s pseudoaneurysm was distal to the knee, it was not found on initial ultrasound, and her inability to obtain her MRI compounded the delay. The second ultrasound identified the pseudoaneurysm. The ultrasonographer examined more distally, given the contrast between the clinical diagnosis of vascular pathology and the negative Doppler study. Computed tomography angiogram confirmed the diagnosis and guided the vascular surgeons in identifying the lesion as a pseudoaneurysm, allowing it to be coiled rather than bypassed.
Duplex ultrasound is the preferred diagnostic modality for imaging pseudoaneurysms. Although our patient’s scan was performed in timely fashion, it did not image the area of pathology. Instead, this patient with multiple orthopedic injuries was scanned for DVT, the most likely cause of her lower extremity swelling. Had a pseudoaneurysm been suspected, the ultrasonographer would have been instructed to image the entire extremity and not just the area where DVT might be found.
Fortunately, despite the treatment delay, the patient recovered well from both the traumatic injuries sustained in the car crash and the likely iatrogenic pseudoaneurysm. Although traction pins are easily and frequently used, they can have complications, which are often preventable. Starting with pin placement itself, there were several opportunities for improving this patient’s care or, at a minimum, reducing the time spent in diagnosis. If the pin had been noticed sliding posteriorly during insertion, extra attention during follow-up visits could have helped identify the injury sooner. Another difficulty in diagnosis was that of obtaining the appropriate outpatient radiology studies which necessitated repeat ED visits. An additional juncture was between the patient’s multiple ED visits for similar complaints. Obtaining advanced imaging sooner could have helped in diagnosing the pseudoaneurysm earlier.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
1. Gosselin RA, Heitto M, Zirkle L. Cost-effectiveness of replacing skeletal traction by interlocked intramedullary nailing for femoral shaft fractures in a provincial trauma hospital in Cambodia. Int Orthop. 2009;33(5):1445-1448.
2. Gosselin R, Lavaly D. Perkins traction for adult femoral shaft fractures: a report on 53 patients in Sierra Leone. Int Orthop. 2007;31(5):697-702.
3. Althausen PL, Hak DJ. Lower extremity traction pins: indications, technique, and complications. Am J Orthop. 2002;31(1):43-47.
4. Green SA, Ripley MJ. Chronic osteomyelitis in pin tracks. J Bone Joint Surg Am. 1984;66(7):1092-1098.
5. Nigam V, Jaiswal A, Dhaon BK. Local antibiotics: panacea for long term skeletal traction. Injury. 2005;36(1):199-202.
6. Lethaby A, Temple J, Santy J. Pin site care for preventing infections associated with external bone fixators and pins. Cochrane Database Syst Rev. 2008;(4):CD004551.
7. Taylor BC, Bramwell TJ, Formaini N. Gas gangrene as a result of femoral traction pin placement. Case Rep Orthop. 2011;(2011):459812.
8. Moskovich R. Proximal tibial transfixion for skeletal traction. An anatomic study of neurovascular structures. Clin Orthop. 1987;(214):264-268.
9. Kirby CK, Fitts WT. The incidence of complications in the use of transfixion pins and wires for skeletal traction. Ann Surg. 1946;123(1):27-31.
10. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br. 1986;68(2):246-254.
11. Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185(3):741-749.
12. Scoutt LM, Zawin ML, Taylor KJ. Doppler US. Part II. Clinical applications. Radiology. 1990;174(2):309-319.
13. Mitchell DG, Needleman L, Bezzi M, et al. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology. 1987;165(3):687-690.
Office-Based Rapid Prototyping in Orthopedic Surgery: A Novel Planning Technique and Review of the Literature
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
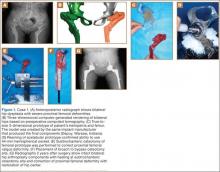
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
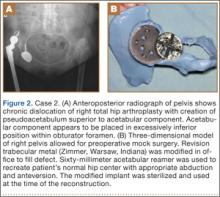
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
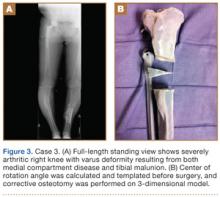
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
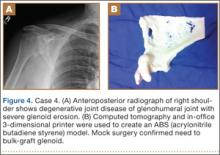
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
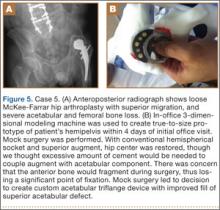
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
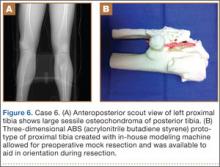
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
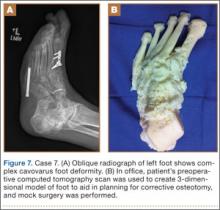
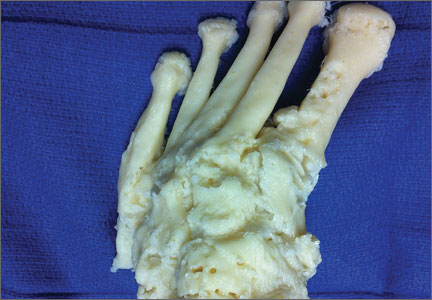
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
Three-dimensional (3-D) printing is a rapidly evolving technology with both medical and nonmedical applications.1,2 Rapid prototyping involves creating a physical model of human tissue from a 3-D computer-generated rendering.3 The method relies on export of Digital Imaging and Communications in Medicine (DICOM)–based computed tomography (CT) or magnetic resonance imaging (MRI) data into standard triangular language (STL) format. Reducing CT or MRI slice thickness increases resolution of the final model.2 Five types of rapid prototyping exist: STL, selective laser sintering, fused deposition modeling, multijet modeling, and 3-D printing.
Most implant manufacturers can produce a 3-D model based on surgeon-provided DICOM images. The ability to produce anatomical models in an office-based setting is a more recent development. Three-dimensional modeling may allow for more accurate and extensive preoperative planning than radiographic examination alone does, and may even allow surgeons to perform procedures as part of preoperative preparation. This can allow for early recognition of unanticipated intraoperative problems or of the need for special techniques and implants that would not have been otherwise available, all of which may ultimately reduce operative time.
The breadth of applications for office-based 3-D prototyping is not well described in the orthopedic surgery literature. In this article, we describe 7 cases of complex orthopedic disorders that were surgically treated after preoperative planning in which use of a 3-D printer allowed for “mock” surgery before the actual procedures. In 3 of the cases, the models were made by the implant manufacturers. Working with these models prompted us to buy a 3-D printer (Fortus 250; Stratasys, Eden Prairie, Minnesota) for in-office use. In the other 4 cases, we used this printer to create our own models. As indicated in the manufacturer’s literature, the printer uses fused deposition modeling, which builds a model layer by layer by heating thermoplastic material to a semi-liquid state and extruding it according to computer-controlled pathways.
We present preoperative images, preoperative 3-D modeling, and intraoperative and postoperative images along with brief case descriptions (Table). The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 28-year-old woman with a history of spondyloepiphyseal dysplasia presented to our clinic with bilateral hip pain. About 8 years earlier, she had undergone bilateral proximal and distal femoral osteotomies. Her function had initially improved, but over the 2 to 3 years before presentation she began having more pain and stiffness with activity. At time of initial evaluation, she was able to walk only 1 to 2 blocks and had difficulty getting in and out of a car and up out of a seated position.
On physical examination, the patient was 3 feet 10 inches tall and weighed 77 pounds. She ambulated with decreased stance phase on both lower extremities and had developed a significant amount of increased forward pelvic inclination and increased lumbar lordosis. Both hips and thighs had multiple healed scars from prior surgeries and pin tracts. Range of motion (ROM) on both sides was restricted to 85° of flexion, 10° of internal rotation, 15° of external rotation, and 15° of abduction.
Plain radiographs showed advanced degenerative joint disease (DJD) of both hips with dysplastic acetabuli and evidence of healed osteotomies (Figure 1). Femoral deformities, noted bilaterally, consisted of marked valgus proximally and varus distally. Preoperative CT was used to create a 3-D model of the pelvis and femur. The model was created by the same implant manufacturer that produced the final components (Depuy, Warsaw, Indiana). Corrective femoral osteotomy was performed on the model to allow for design and use of a custom implant, while the modeled pelvis confirmed the ability to reproduce the normal hip center with a 44-mm conventional hemispherical socket.
After surgery, the patient was able to ambulate without a limp and return to work. Her hip ROM was pain-free passively and actively with flexion to 100°, internal rotation to 35°, external rotation to 20°, and abduction to 30°.
Case 2
A 48-year-old woman with a history of Crowe IV hip dysplasia presented to our clinic with a chronically dislocated right total hip arthroplasty (THA) (Figure 2). Her initial THA was revised 1 year later because of acetabular component failure. Two years later, she was diagnosed with a deep periprosthetic infection, which was ultimately treated with 2-stage reimplantation. She subsequently dislocated and underwent re-revision of the S-ROM body and stem (DePuy Synthes, Warsaw, Indiana). At a visit after that revision, she was noted to be chronically dislocated, and was sent to our clinic for further management.
Preoperative radiographs showed a right uncemented THA with the femoral head dislocated toward the false acetabulum, retained hardware, and an old ununited trochanteric fragment. Both the femoral and acetabular components appeared well-fixed, though the acetabular component was positioned inferior, toward the obturator foramen.
Preoperative CT with metal artifact subtraction was used to create a 3-D model of the residual bony pelvis. The model was made by an implant manufacturer (Zimmer, Warsaw, Indiana). The shape of the superior defect was amenable to reconstruction using a modified revision trabecular metal socket. The pelvic model was reamed to accept a conventional hemispherical socket. The defect was reamed to accept a modified revision trabecular metal socket. The real implant was fashioned before surgery and was sterilized to avoid the need for intraoperative modification. Use of the preoperative model significantly reduced the time that would have been needed to modify the implant during actual surgery.
The patient’s right THA was revised. At time of surgery, the modified revision trabecular metal acetabular component was noted to seat appropriately in the superior defect. The true acetabulum was reestablished, and a hemispherical socket was placed with multiple screws. The 2 components were then unitized using cement in the same manner as would be done with an off-the-shelf augment.
Case 3
A 57-year-old man presented with a 10-year history of right knee pain. About 30 years before presentation at our clinic, he was treated for an open right tibia fracture sustained in a motorcycle accident. He had been treated nonsurgically, with injections, but they failed to provide sustained relief.
Preoperative radiographs showed severe advanced DJD in conjunction with an extra-articular posttraumatic varus tibial shaft deformity (Figure 3). An implant manufacturer (Zimmer) used a CT scan to create a model of the deformity. The resultant center of rotation angle was calculated using preoperative images and conventional techniques for deformity correction, and a lateral closing-wedge osteotomy was performed on the CT-based model. The initial attempt at deformity correction was slightly excessive, and the amount of resected bone slightly thicker than the calculated wedge, resulting in a valgus deformity. This error was noted, and the decision was made to recut a new model with a slight amount of residual varus that could be corrected during the final knee arthroplasty procedure.
Corrective osteotomy was performed with a lateral plate. Six months later, the patient had no residual pain, and CT confirmed union at the osteotomy site and a slight amount of residual varus. The patient then underwent routine total knee arthroplasty (TKA) using an abbreviated keel to avoid the need for removal of the previously placed hardware. The varus deformity was completely corrected.
Case 4
A 73-year-old man had a history of shoulder pain dating back to his childhood. Despite treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and injections, his debilitating pain persisted. Physical examination revealed limited ROM and an intact rotator cuff.
Plain radiographs showed severe DJD of the glenohumeral joint (Figure 4). Severe erosions of the glenoid were noted, prompting further workup with CT, which showed significant bone loss, particularly along the posterior margin of the glenoid. We used our 3-D printer to create a model of the scapula from CT images. The model was then reamed in the usual fashion to accept a 3-pegged glenoid component. On placement of a trial implant, a large deficiency was seen posteriorly. We thought the size and location of the defect made it amenable to grafting using the patient’s humeral head.
The patient elected to undergo right total shoulder arthroplasty. During the procedure, the glenoid defect was found to be identical to what was encountered with the model before surgery. A portion of the patient’s humeral head was then fashioned to fit the defect, and was secured with three 2.7-mm screws, after provisional fixation using 2.0-mm Kirschner wires. The screws were countersunk, and the graft was contoured by hand to match the previous reaming. A 3-pegged 52-mm glenoid component was then cemented into position with excellent stability.
Case 5
A 64-year-old man presented to our clinic with left hip pain 40 years after THA. The original procedure was performed for resolved proximal femoral osteomyelitis. Plain radiographs showed a loose cemented McKee-Farrar hip arthroplasty (Figure 5). Because of the elevated position of the acetabular component relative to the native hip center, CT was used to determine the amount of femoral bone loss.
We used our 3-D printer to create a model and tried to recreate the native hip center with conventional off-the-shelf implants. A 50-mm hemispherical socket trial was placed in the appropriate location, along with a trabecular metal augment trial to provide extended coverage over the superolateral portion of the socket. Noted between the socket and the augment was a large gap; a substantial amount of cement would have been needed to unitize the construct. We thought a custom acetabular component would avoid the need for cement. In addition, given the patient’s small stature, the conventional acetabular component would allow a head only 32 mm in diameter. With a custom implant, the head could be enlarged to 36 mm, providing improved ROM and stability.
The patient underwent revision left hip arthroplasty using a custom acetabular component. A 3-D model available at time of surgery was used to aid implant placement.
Case 6
A 23-year-old man with multiple hereditary exostoses presented to our clinic with a painful mass in the left calf. Plain radiographs showed extensive osteochondromatosis involving the left proximal tibiofibular joint (Figure 6). The exostosis extended posteromedially, displacing the arterial trifurcation. MRI showed a small cartilage cap without evidence of malignant transformation.
CT angiogram allowed the vasculature to be modeled along with the deformity. A 3-D model was fabricated. The model included the entire proximal tibiofibular joint, as well as the anterior tibial, peroneal, and posterior tibial arteries. Cautious intralesional resection was recommended because of the proximity to all 3 vessels.
The patient underwent tumor resection through a longitudinal posterior approach. The interval between the medial and lateral heads of the gastrocnemius muscles was developed to expose the underlying soleus muscle. The soleus was split longitudinally from its hiatus to the inferior portion of the exostosis. This allowed for identification of the trifurcation and the tibial nerve, which were protected. Osteotomes were used to resect the mass at its base, the edges were carefully trimmed, and bone wax was placed over the defect. Anterior and lateral to this mass was another large mass (under the soleus muscle), which was also transected using an osteotome. The gastrocnemius and soleus muscles were then reflected off the fibula in order to remove 2 other exostoses, beneath the neck and head of the fibula.
Case 7
A 71-year-old man with a history of idiopathic lymphedema and peripheral neuropathy presented to our clinic with a left cavovarus foot deformity and a history of recurrent neuropathic foot ulcers (Figure 7). Physical examination revealed a callus over the lateral aspect of the base of the fifth metatarsal. Preoperative radiograph showed evidence of prior triple arthrodesis with a cavovarus foot deformity. CT scan was used to create a 3-D model of the foot. The model was then used to identify an appropriate location for lateral midtarsal and calcaneal closing-wedge osteotomies.
The patient underwent midfoot and hindfoot surgical correction. At surgery, the lateral closing-wedge osteotomies were performed according to the preoperative model. Radiographs 1 year after surgery showed correction of the forefoot varus.
Discussion
Three-dimensional printing for medical applications of anatomical modeling is not a new concept.1,3,4 Its use has been reported for a variety of applications in orthopedic surgery, including the printing of porous and metallic surfaces5 and bone-tissue engineering.6-9 Rapid prototyping for medical application was first reported in 1990 when a CT-based model was used to create a cranial bone.10 Reports of using the technique are becoming more widespread, particularly in the dental and maxillofacial literature, which includes reports on a variety of applications, including patient-specific drill guides, splints, and implants.11-14 The ability to perform mock surgery in advance of an actual procedure provides an invaluable opportunity to anticipate potential intraoperative problems, reduce operative time, and improve the accuracy of reconstruction.
Office-based rapid prototyping that uses an in-house 3-D printer is a novel application of this technology. It allows for creation of a patient-specific model for preoperative planning purposes. We are unaware of any other reports demonstrating the breadth and utility of office-based rapid prototyping in orthopedic surgery. For general reference, a printer similar to ours requires an initial investment of $52,000 to $56,000. This cost generally covers the printer, printer base cabinet, installation, training, and printer software (different from the 3-D modeling software), plus a 1-year warranty. A service agreement costs about $4000 annually. Printer and model supply expenses depend on the material used for the model (eg, ABS [acrylonitrile butadiene styrene]) and on the size and complexity of the 3-D models created. Average time to generate an appropriately formatted 3-D printing file is about 1 hour, though times can vary largely, according to amount of metal artifact subtraction necessary and the experience of the software user. For the rare, extremely complex deformities that require a significant amount of metal artifact subtraction, file preparation times can exceed 3 or 4 hours. We think these preparation times will decrease as communication between radiology file export format and modeling software ultimately allows for metal artifact subtraction images to function within the modeling software environment. Once an appropriately formatted file has been created, typical printing times vary according to the size of the to-be-modeled bone. For a hemipelvis, printing time is 30 to 40 hours; printing that is started on a Friday afternoon will be complete by Monday morning.
There are few reports of rapid prototyping in orthopedic surgery. In 2003, Minns and colleagues15 used a 3-D model in the planning of a tibial resection for TKA. They found the model to be accurate at time of surgery, resulting in appropriate tibial coverage by a conventional meniscal-bearing implant. Munjal and colleagues16 reported on 10 complex failed hip arthroplasty cases in which patients had revision surgery after preoperative planning using 3-D modeling techniques. The authors found that, in 8 of the 10 cases, conventional classification systems of bone loss were inaccurate in comparison with the prototype. Four cases required reconstruction with a custom triflange when conventional implants were not deemed reasonable based on the pelvic model. Tam and colleagues17 reported using a 3-D prototype as an aid in surgical planning for resection of a scapular osteochondroma in a 6-year-old patient. They found the rapid prototype to be useful at time of resection—similar to what we found with 1 patient (case 6). Adding contrast media to our patient’s scan allowed for 3-D visualization of the lesion and the encased vasculature. Fu and colleagues18 reported using a patient-specific drill template to insert anterior transpedicular screws. They constructed 24 prototypes of a formalin-preserved cervical vertebra to create a patient-specific biocompatible drill template for use in correcting multilevel cervical instability. They found the technique to be highly reproducible and accurate. Zein and colleagues19 used a rapid prototype of 3 consecutive human livers to preoperatively identify the vascular and biliary tract anatomy. They reported a high degree of accuracy—mean dimensional errors of less than 4 mm for the entire model and 1.3 mm for the vascular diameter.
The models created by implant manufacturers in this series were used to perform “mock” surgery before the actual procedures. Working with these models prompted us to buy our own 3-D printer. The learning curve can be steep, but commercially available 3-D printers allow for prompt in-office production of high-quality realistic prototypes at relatively low per-case cost (Figure 8). Three-dimensional modeling allows surgeons to assess the accuracy of their original surgical plans and, if necessary, correct them before surgery. Although computer-aided design models are useful, the ability to “perform surgery preoperatively” adds another element to surgeons’ understanding of the potential issues that may arise. Also, an in-office printer can help improve surgeons’ understanding and control over the process by which images are translated from radiographic file to 3-D model. Disadvantages of an in-office system include start-up and maintenance costs, office space requirements, and a significant learning curve for software and hardware applications. In addition, creation of 3-D models requires close interaction with radiologists who can provide appropriately formatted DICOM images, as metal artifact subtraction can be challenging. We think that, as image formatting and software capabilities are continually refined, this technology will become an invaluable part of multiple subspecialties across orthopedic surgery, with potentially infinite clinical, educational, and research applications.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.
1. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997;79(3):169-174.
2. Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24(4):149-153.
3. Esses SJ, Berman P, Bloom AI, Sosna J. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol. 2011;196(6):W683-W688.
4. Torres K, Staśkiewicz G, Śnieżyński M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 2011;70(1):1-4.
5. Melican MC, Zimmerman MC, Dhillon MS, Ponnambalam AR, Curodeau A, Parsons JR. Three-dimensional printing and porous metallic surfaces: a new orthopedic application. J Biomed Mater Res. 2001;55(2):194-202.
6. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011;7(3):907-920.
7. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009;33(1):58-62.
8. Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121-1124.
9. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74(2):782-788.
10. Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3(3):200-203.
11. Flügge TV, Nelson K, Schmelzeisen R, Metzger MC. Three-dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. J Oral Maxillofac Surg. 2013;71(8):1340-1346.
12. Goiato MC, Santos MR, Pesqueira AA, Moreno A, dos Santos DM, Haddad MF. Prototyping for surgical and prosthetic treatment. J Craniofac Surg. 2011;22(3):914-917.
13. Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1-e7.
14. Robiony M, Salvo I, Costa F, et al. Virtual reality surgical planning for maxillofacial distraction osteogenesis: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65(6):1198-1208.
15. Minns RJ, Bibb R, Banks R, Sutton RA. The use of a reconstructed three-dimensional solid model from CT to aid the surgical management of a total knee arthroplasty: a case study. Med Eng Phys. 2003;25(6):523-526.
16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15(5):644-653.
17. Tam MD, Laycock SD, Bell D, Chojnowski A. 3-D printout of a DICOM file to aid surgical planning in a 6 year old patient with a large scapular osteochondroma complicating congenital diaphyseal aclasia. J Radiol Case Rep. 2012;6(1):31-37.
18. Fu M, Lin L, Kong X, et al. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One. 2013;8(1):e53580.
19. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304-1310.