User login
Program for Early Detection of Sepsis
Sepsis, the body's systemic response to infection leading to organ failure, can occur in patients throughout the hospital. However, patients initially diagnosed with sepsis on the wards experience the highest mortality for several reasons, including delayed recognition and treatment, particularly when localized infections progress to shock and organ failure. Consequently, hospitals have responded by having nurses screen patients for signs and symptoms of sepsis to identify cases earlier and improve outcomes. The intent of this article, which is based on our experience with a multihospital implementation effort, was to describe potential reasons for ward patients' poor prognosis. We provide a toolkit for how hospitals can implement a severe sepsis quality improvement (QI) program in general medicalsurgical wards.
In a previous study, we reported on our international effort, the Surviving Sepsis Campaign's (SSC) Phase III performance improvement (PI) program, targeting selected guideline recommendations (6‐ and 24‐hour bundles) in the emergency department (ED), the Intensive Care Unit (ICU), and wards in 165 volunteer hospitals in the United States, Europe, and South America.[1] The program was associated with increased bundle compliance and decreased mortality over time.[1, 2] The SSC's Phase III program, which focused on improvement efforts primarily in the ED and ICU, also exposed a need to address the high mortality in ward patients.[3] Patients admitted to the ICU directly from the ED with severe sepsis had a mortality rate of 26%, whereas those transferred to the ICU from the ward had significantly higher mortality (40.3%).[3]
Although the reasons for the higher mortality rate among ward patients have not been studied, several factors may play a role. First, the diagnosis of severe sepsis may be delayed in ward patients because physicians and nurses may not recognize the progression to sepsis and/or because hospitalized patients may not present with obvious systemic manifestations of sepsis as they do in the ED (Table 1).[4] Second, ward patients may have differences in the timing of their presentation and concurrent conditions confounding the diagnosis.[5] Third, treatment may be delayed once the diagnosis is made on the ward. The ICU and ED are designed to provide rapid high‐acuity care, whereas the wards have fewer systems and resources for rapid delivery of care needed for severe sepsis. Finally, some patients on the ward may develop sepsis from nosocomial infection, which can portend a worse prognosis.[6]
| Emergency Department Presentation | Ward Presentation | |
|---|---|---|
| Patient‐familyreported symptoms | I just feel sick, family reports disorientation, not eating | Currently hospitalized, family often not present, diagnosis may not be clear, baseline mental status unknown, lack of appetite may be linked to dislike of hospital food. |
| Systemic manifestations | Triage observed 2 or more signs of infection or patient reports temperature while at home plus additional finding on assessment. | Signs of infection may appear 1 at a time, hours apart, and may appear to be mild changes to staff or missed entirely due to staff discontinuity. |
| Organ dysfunction | Present on admission; triage nurse assesses for organ dysfunction. | Develops over hours or days; may be subtle or acute. |
| Laboratory study process | Ordered and evaluated within 1 hour. | Not routinely completed daily, may be ordered after physician evaluation or during rounds. Results within 34 hours. |
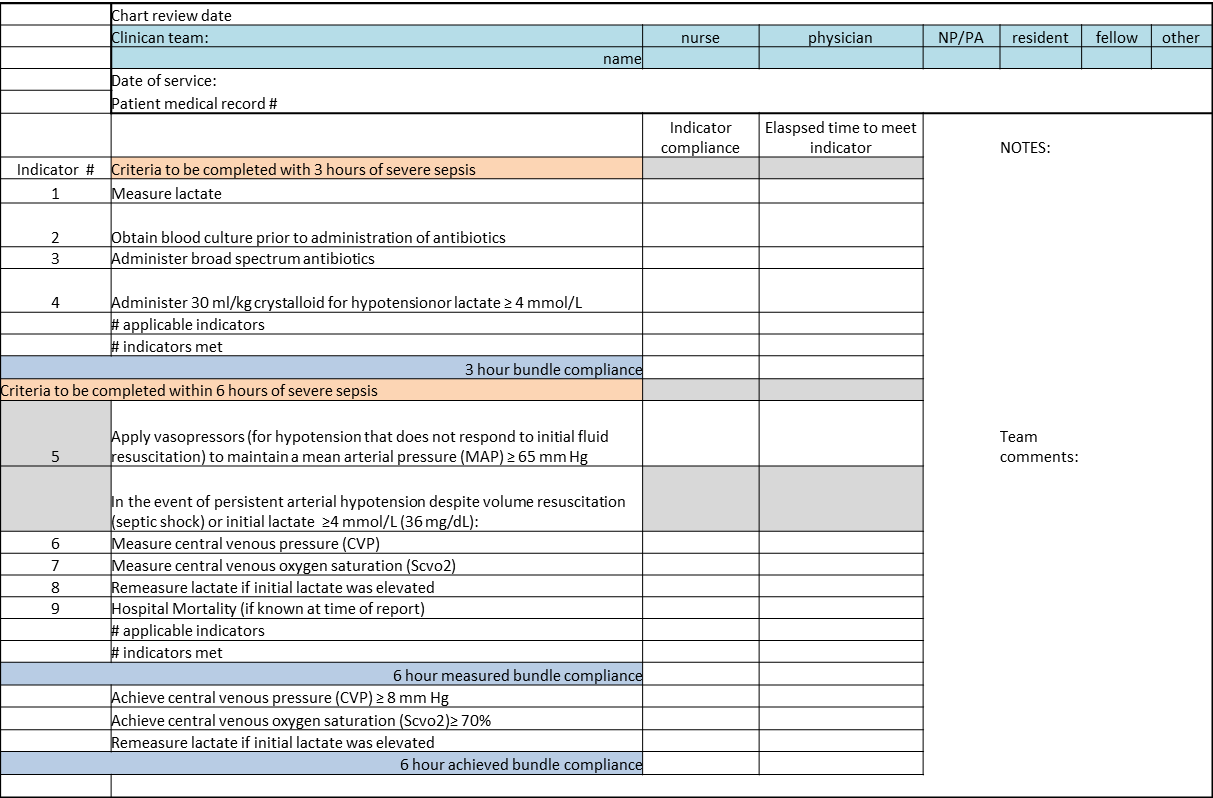
The SSC Phase III results led to the launch of a QI program, known as the SSC Phase IV Sepsis on the Wards Collaborative, funded by the Gordon and Betty Moore Foundation. This program, a partnership between the Society of Critical Care Medicine and the Society of Hospital Medicine (SHM), targeted ward patients and focused on early recognition through protocol‐driven regular nurse screening. The program applied the SSC 2012 guidelines with a primary focus on the 3‐hour bundle (Table 2).[7] The framework used for this program was the Institute for Healthcare Improvement's Plan‐Do‐Study‐Act (PDSA) model of improvement.[8, 9] The collaborative design included learning sessions designed to motivate and support improvement.[10] The program began with 60 academic and community hospitals in 4 US regions. Participating sites were required to have prior hospital experience in sepsis performance improvement as well as a formal commitment of support from their EDs and ICUs.
| To be completed within 3 hours of time of presentation |
| 1. Measure lactate level |
| 2. Obtain blood cultures prior to administration of antibiotics |
| 3. Administer broad‐spectrum antibiotics |
| 4. Administer 30 mL/kg crystalloid for hypotension or lactate 4 mmol/L (36 mg/dL) |
We provided sites with a basic screening tool and guidance for routine severe sepsis screening, monitoring, and feedback (Figure 1). Because of the anticipated challenges of implementing routine nurse screening on every shift in all inpatient wards, participants identified 1 ward to pilot the every‐shift screening program. Each pilot ward refined the nurse screening process and developed site‐specific tools based on electronic health record (EHR) capability, informatics support, and available resources. After this initial phase, the program could be implemented in a hospital's remaining wards. The slogan adopted for the program was Screen every patient, every shift, every day.

Although knowledge gained from the SSC Phase III program led to improvements in treating severe sepsis, ward patients continued to have poor outcomes. To address the potential contributions of delayed case identification, we developed an early recognition and treatment program. We outline the steps we took to develop this multisite PI program.
PREPARATORY WORK
During the planning phase, several procedural steps were taken before initiating the ward sepsis program (Table 3). These required 3 levels of involvement: senior administration, midlevel management, and patient‐level support.
| |
| 1. | Obtain administrative support (ie, funding for data collection, project lead, informatics) |
| 2. | Align with ED and ICU |
| 3. | Identify 1 ward to pilot the program |
| 4. | Establish unit‐based champions on each shift (nurse, physician) |
| 5. | Review ward workflow |
| 6. | Develop nurse screening tool |
| 7. | Provide education |
Administrative Support
In the course of our implementation effort, we found that sites that had high‐level administrative support were more likely to implement and sustain the intervention. For this reason, we consider such support to be critical. Examples of such support include chief medical officers, chief nursing officers, and chief quality officers. As an example, securing commitment from hospital leadership may be necessary to improve/change the EHR and provide funding for project management to achieve sustainable improvement in outcomes. Aligning leadership with frontline physicians, nurses, and support staff toward a common goal provides the platform for a successful program.[11]
ED and ICU Leadership Support
Maintaining lines of communication among the ED, ICU, and ward staff is critical to improving outcomes. Establishing a cohesive system (ED, ICU, and wards) aimed at early recognition and treatment of sepsis throughout the hospital stay can lead to improvement in continuity of care and outcomes. For example, when an ED severe sepsis patient is transferred to the ward and subsequently requires admission to the ICU due to declining clinical status, providing timely feedback to the ED can help improve care for subsequent patients. Collaboration between the ED and the ward can also contribute to improved transitions of care for patients with severe sepsis.
Hospitalist/Internal Medicine Leadership
Our experience with implementing sepsis bundles in the ED and ICU highlights the need for effective interdisciplinary collaboration with designated physician and nurse leaders/champions. We found that engaging local clinical leaders in the early recognition and management of a severe sepsis QI program is imperative for the program's success. Hospitalists are often the physician leaders for the inpatient wards, so it is essential to secure their early engagement, support, and leadership. Moreover, though collaboration with ED and ICU physicians may be useful, as described above, a hospitalist champion is likely to be more effective at educating other hospitalists about the program, overcoming physician resistance, and facilitating change.
Depending on a hospital's size and workflows, designated ward‐ or shift‐based hospitalists and nurses as champions can serve as key resources to support implementation. These individuals help establish mutual respect and a common mental model of how sepsis can evolve in ward patients. Even more important, by providing assistance with both the screening tool as well as with recognition itself, these individuals not only speed implementation, but also protect against rough patches (ie, those instances where workflow changes run into resistance).
EDUCATION
Diagnosing sepsis is not always easy, making education on sepsis recognition, evaluation, and treatment necessary prior to implementation. Retention of knowledge over time through review and refresher courses are methods we used in the program. Providing background material explaining why education is necessary and providing physicians and nurses with materials to help them recall the information over time were developed at several sites. Resources included sepsis posters, identification‐size badge cards with the sepsis bundle elements, and bulletin boards on the wards with information to reinforce sepsis recognition, evaluation, and treatment. Education for the ward‐centric program included an overview of the SSC guidelines, supportive literature, sepsis definitions, description of the infection's systemic manifestations, criteria for identification of new‐ onset organ dysfunction, and the details on current severe sepsis 3‐ and 6‐hour bundle requirements. We made clinicians aware of resources available on the SSC website.[12] Data emphasizing the incidence of sepsis, as well as outcomes and motives for the QI wards program, were incorporated during the collaborative meetings. Data can serve as strong motivators for action (eg, highlighting current incidence rates). Many hospitals combined presentation of these aggregate data with local review of selected cases of severe sepsis that occurred in their own wards.
Understanding that the training for and experiences of ED, ICU, and ward nurses varies, nurse education contained critical assessment skills in determining when to suspect a new or worsening infection. Training nurses to complete a comprehensive daily infection assessment may help them overcome uncertainty in judgement. Assessment skills include examination of invasive lines, surgical sites, wounds, and presence of a productive cough. Equally important, patients being treated for an infection would benefit from a daily assessment for improvement or worsening of the infection. Information uncovered may identify early signs of organ failure in addition to infections that may need further evaluation and treatment. Education provides knowledge, but achieving program success relies heavily on staff accepting that they can make a difference in sepsis patient identification, management, and outcomes.
SCREENING METHODS, COMMUNICATION, AND PROTOCOLS
The SSC tool for severe sepsis facilitates screening for (1) confirmed or suspected infection, (2) presence of 2 or more systemic manifestations of infection, and (3) acute organ dysfunction. This tool was the basis for the do (screening) portion of the PDSA model.
Continuous Screening
Technology can facilitate early recognition of severe sepsis with EHR‐based surveillance screening tools. Surveillance may include continuous review of vital signs and laboratory values with an automated alerting system. A valuable feature of the screening tool alert is the incorporation of the nurse's assessment. Decision support can improve the process by providing advice with systems requiring a reason to over‐ride the advice.[13] For example, an alert may include input from the nurse to determine if the abnormal data are thought to be related to an infectious process or due to another cause. If a suspected or confirmed infection is identified, further surveillance screening can include review of blood pressure readings and laboratory data to determine if organ dysfunction is present. If organ dysfunction criteria are identified, the alert can prompt the nurse to notify the physician to discuss whether the organ dysfunction is new and related to the infection and if implementation of the severe sepsis bundles is indicated (Figure 2). Additional continuous screening models may include variations of the example provided to include alerts to other clinicians or a response team.
An automated screening tool within the EHR can be useful because the system continuously scans to identify signs and symptoms of sepsis, thus providing screening consistency, and offers data on the back end to be used as a mechanism for feedback to monitor effectiveness. Challenges with EHR severe sepsis alert development are resource allocation, testing, education, and ongoing evaluation and feedback. Other challenges include the potential for alert fatigue (false positive) and inappropriate response (false negative) to the infection prompt, thereby halting the next step in automated screening for organ dysfunction. Time to complete an automated screening tool varies based on strategic design and user understanding.
Screening Checklist
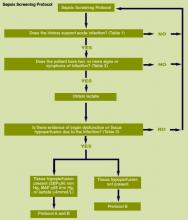
Whereas EHR tools may be effective in early recognition of sepsis, not all sites will have the capability to use these tools because of lack of informatics support, cost of development, and absence of an EHR in some hospitals.[14] An alternative to continuous screening is a sepsis checklist such as the severe sepsis screening tool (Figure 1). The checklist is designed to prompt nurses to screen every patient during every shift for new signs of sepsis and organ dysfunction.
The checklist ensures that 3 key issues are considered: presence of a suspected or confirmed infection, systemic manifestations of inflammation, and physiological manifestations of organ dysfunction. The paper tool is simple to use and can be completed in 10 to 20 minutes. It requires the nurse to review the progress notes, vital signs, and laboratory test results. Although the time investment seems onerous, the gain in consistency of screening and treatment compensates for the extra effort. Review of the checklist also provides a locus for feedback and new improvement cycles.
Scripted Communication
Once a patient with severe sepsis is identified, communicating this finding to the rest of the clinical team is essential. Because communication skills are not always emphasized in QI projects, we decided to emphasize a structured approach. We provided clinicians with scripts based on the SBAR (situation, background, assessment, and recommendation) technique aimed to improve communication (Figure 3).[15, 16] Using the SBAR technique also supports our efforts to build nurses' confidence and willingness to employ protocols that give them greater autonomy.

Nurse‐Directed Protocols
Skillful identification and management of severe sepsis patients constitute the foundation for implementation of nurse‐directed protocols in this patient population. Such protocols promote autonomy and staff ownership. Severe sepsis protocols may include increasing the frequency of vital signs, placement of laboratory orders and, in sites with an established culture of increased nurse autonomy, initiation of intravenous access and a fluid bolus when specific criteria are met. Because nursing scope of practice varies from state to state and among hospitals, nurse‐directed severe sepsis protocols generally require review of current site practice guidelines, physician agreement, and approval by the medical executive committee prior to implementation. Despite these differences, maximizing nurse leadership involvement and nurse autonomy can help propel the program forward. Protocols may be implemented based on knowledge level and resources on a particular ward. A workflow evaluation may be included in this process to define staff performing each step, what is being reported, and where and when data are recorded.
DATA COLLECTION AND FEEDBACK
Nurse screening drives the ward program and ensuring its consistency is the key to early patient identification. We made ongoing repeated evaluation of the appropriate use of the screening tool, time to physician notification, and time to follow‐up intervention, a critical part of the study phase of the PDSA cycle. Once the nursing staff is consistently accurate and compliant (>90%) with screening, random (eg, once per week) screening tool review may be more suitable, thus requiring fewer resources (see Supporting Information, Appendix 1, in the online version of this article).
Data Collection
A key to improvement is to study the process, which requires data collection to assess compliance. In our experience, timely clinician feedback, along with data, led to effective process change. Real‐time data collection and discussion with the clinical team may lead to early recognition or intervention.
In our collaborative experience, we observed varied resources and timing for data collection across hospitals. For example, several participating sites had sepsis coordinators to collect data, whereas others relied on the quality department or nursing staff to collect data. Data may be collected concurrently (within 24 hours of severe sepsis presentation) or retrospectively. Retrospective data collection may allow for staff flexibility in data collection, but limits feedback to the clinicians. For example, with retrospective review, early recognition and treatment failure may go unrecognized until the data are analyzed and reported, which can be months after the patient has been discharged or expired.
Feedback to Caregivers
A consistent feedback process, which can occur at the individual or group level, may lead to prompt improvement in severe sepsis management. An example of individual feedback would be providing the nurse with the elapsed time from antibiotic order to time of administration. Early in the implementation phase, frequent (daily or weekly) feedback is helpful to build team cohesiveness. An example of feedback to build the team may include a unit‐based report on the last 5 severe sepsis patients managed by the group. Providing overall bundle compliance and outcome reports on a weekly and monthly basis will allow the clinical team to track progress. Examples of report cards and a dashboard are provided in the supplemental material, which highlight compliance with the bundle elements as well as time to achieve the bundle elements. (see Supporting Information, Appendix 2 and Appendix 3, in the online version of this article). Resources to evaluate and provide consistent data may require up to 10 to 15 hours per week for 1 unit. Automated reports may decrease the resources needed in collating and reporting data.
OUTCOME MEASURES
Although certainly important, mortality is not the only outcome measure worthy of measurement. Other relevant outcomes include transfers to a higher level of care and need for major supportive therapies (eg, dialysis, mechanical ventilation, vasopressor infusion). Whereas it is valuable to review transfers to a higher level of care, we emphasized that these are not necessarily adverse outcomes; in fact, in many cases such transfers are highly desirable. It is also important to track the overall impact of sepsis on hospital length of stay.
SUMMARY/CONCLUSIONS
Grounded in the Institute for Healthcare Improvement's PDSA QI model, we developed a program aimed at improving outcomes for severe sepsis ward patients. Our program's cornerstone is nurse‐led checklist‐based screening. Our faculty led learning sessions that concentrated on using a collaborative approach whose key components were education in early sepsis identification, use of a sepsis screening tool, and the SBAR method for effective communication. Pitfalls identified during the program included lack of knowledge for both nurses and physicians in early severe sepsis identification, resistance to routine screening, and lack of data collection and leadership support. The most successful participating sites were those with senior leadership backing, staff engagement, informatics support, and data collection resources. Ultimately, replicating a program such as ours will depend on team cohesiveness, and nurse empowerment through the use of nurse‐driven protocols. Programs like this may lead to progression toward standardizing practice (eg, antibiotic administration, fluid resuscitation), matching patient needs to resources, and building stronger partnerships between hospitalists and nurses.
Disclosures
This work was supported by a grant provided to the Society of Critical Care Medicine by the Gordon and Betty Moore Foundation (Early Identification and Management of Sepsis on the Wards). The work was supported by a grant from the Adventist Hospital System. As part of our agreement with the Gordon and Betty Moore Foundation, we made a commitment to disseminate our findings in articles such as this one. However, the Moore Foundation played no role in how we actually structured our articles, nor did they review or preapprove any of the manuscripts submitted as part of the dissemination component; the same was the case with the other sponsors. The authors report no conflicts of interest.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- , , , et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5‐year study. Intensive Care Med. 2014;40(11):1623–1633.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8(5):243–247.
- , , , et al. Recognizing and managing sepsis: what needs to be done? BMC Med. 2015;13:98.
- , , , et al. Risk factors for hospital‐acquired pneumonia outside the intensive care unit: a case‐control study. Am J Infect Control. 2014;42(1):38–42.
- , , , et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med. 2013;41(2):580–637.
- , , , , . Understanding the components of quality improvement collaboratives: a systematic literature review. Milbank Q. 2013;91(2):354–394.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- . Learning and improving in quality improvement collaboratives: which collaborative features do participants value most? Health Serv Res. 2009;44(2 pt 1):359–378.
- , , , et al. Senior executive adopt‐a‐work unit: a model for safety improvement. Jt Comm J Qual Saf. 2004;30(2):59–68.
- Surviving Sepsis Campaign. Available at: http://survivingsepsis.org/Resources/Pages/default.aspx. Accessed September 24, 2015.
- , , , et al. Features of effective computerised clinical decision support systems: meta‐regression of 162 randomised trials. BMJ. 2013;346:f657.
- , . characteristics of hospitals associated with complete and partial implementation of electronic health records. Perspect Health Inf Manag. 2016;13:1c.
- Institute for Healthcare Improvement. SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/resources/pages/tools/sbartechniqueforcommunicationasituationalbriefingmodel.aspx. Accessed September 12, 2015.
- , , , et al. Implementing SBAR across a large multihospital health system. Jt Comm J Qual Patient Saf. 2012;38(6):261–268.
Sepsis, the body's systemic response to infection leading to organ failure, can occur in patients throughout the hospital. However, patients initially diagnosed with sepsis on the wards experience the highest mortality for several reasons, including delayed recognition and treatment, particularly when localized infections progress to shock and organ failure. Consequently, hospitals have responded by having nurses screen patients for signs and symptoms of sepsis to identify cases earlier and improve outcomes. The intent of this article, which is based on our experience with a multihospital implementation effort, was to describe potential reasons for ward patients' poor prognosis. We provide a toolkit for how hospitals can implement a severe sepsis quality improvement (QI) program in general medicalsurgical wards.
In a previous study, we reported on our international effort, the Surviving Sepsis Campaign's (SSC) Phase III performance improvement (PI) program, targeting selected guideline recommendations (6‐ and 24‐hour bundles) in the emergency department (ED), the Intensive Care Unit (ICU), and wards in 165 volunteer hospitals in the United States, Europe, and South America.[1] The program was associated with increased bundle compliance and decreased mortality over time.[1, 2] The SSC's Phase III program, which focused on improvement efforts primarily in the ED and ICU, also exposed a need to address the high mortality in ward patients.[3] Patients admitted to the ICU directly from the ED with severe sepsis had a mortality rate of 26%, whereas those transferred to the ICU from the ward had significantly higher mortality (40.3%).[3]
Although the reasons for the higher mortality rate among ward patients have not been studied, several factors may play a role. First, the diagnosis of severe sepsis may be delayed in ward patients because physicians and nurses may not recognize the progression to sepsis and/or because hospitalized patients may not present with obvious systemic manifestations of sepsis as they do in the ED (Table 1).[4] Second, ward patients may have differences in the timing of their presentation and concurrent conditions confounding the diagnosis.[5] Third, treatment may be delayed once the diagnosis is made on the ward. The ICU and ED are designed to provide rapid high‐acuity care, whereas the wards have fewer systems and resources for rapid delivery of care needed for severe sepsis. Finally, some patients on the ward may develop sepsis from nosocomial infection, which can portend a worse prognosis.[6]
| Emergency Department Presentation | Ward Presentation | |
|---|---|---|
| Patient‐familyreported symptoms | I just feel sick, family reports disorientation, not eating | Currently hospitalized, family often not present, diagnosis may not be clear, baseline mental status unknown, lack of appetite may be linked to dislike of hospital food. |
| Systemic manifestations | Triage observed 2 or more signs of infection or patient reports temperature while at home plus additional finding on assessment. | Signs of infection may appear 1 at a time, hours apart, and may appear to be mild changes to staff or missed entirely due to staff discontinuity. |
| Organ dysfunction | Present on admission; triage nurse assesses for organ dysfunction. | Develops over hours or days; may be subtle or acute. |
| Laboratory study process | Ordered and evaluated within 1 hour. | Not routinely completed daily, may be ordered after physician evaluation or during rounds. Results within 34 hours. |
The SSC Phase III results led to the launch of a QI program, known as the SSC Phase IV Sepsis on the Wards Collaborative, funded by the Gordon and Betty Moore Foundation. This program, a partnership between the Society of Critical Care Medicine and the Society of Hospital Medicine (SHM), targeted ward patients and focused on early recognition through protocol‐driven regular nurse screening. The program applied the SSC 2012 guidelines with a primary focus on the 3‐hour bundle (Table 2).[7] The framework used for this program was the Institute for Healthcare Improvement's Plan‐Do‐Study‐Act (PDSA) model of improvement.[8, 9] The collaborative design included learning sessions designed to motivate and support improvement.[10] The program began with 60 academic and community hospitals in 4 US regions. Participating sites were required to have prior hospital experience in sepsis performance improvement as well as a formal commitment of support from their EDs and ICUs.
| To be completed within 3 hours of time of presentation |
| 1. Measure lactate level |
| 2. Obtain blood cultures prior to administration of antibiotics |
| 3. Administer broad‐spectrum antibiotics |
| 4. Administer 30 mL/kg crystalloid for hypotension or lactate 4 mmol/L (36 mg/dL) |
We provided sites with a basic screening tool and guidance for routine severe sepsis screening, monitoring, and feedback (Figure 1). Because of the anticipated challenges of implementing routine nurse screening on every shift in all inpatient wards, participants identified 1 ward to pilot the every‐shift screening program. Each pilot ward refined the nurse screening process and developed site‐specific tools based on electronic health record (EHR) capability, informatics support, and available resources. After this initial phase, the program could be implemented in a hospital's remaining wards. The slogan adopted for the program was Screen every patient, every shift, every day.

Although knowledge gained from the SSC Phase III program led to improvements in treating severe sepsis, ward patients continued to have poor outcomes. To address the potential contributions of delayed case identification, we developed an early recognition and treatment program. We outline the steps we took to develop this multisite PI program.
PREPARATORY WORK
During the planning phase, several procedural steps were taken before initiating the ward sepsis program (Table 3). These required 3 levels of involvement: senior administration, midlevel management, and patient‐level support.
| |
| 1. | Obtain administrative support (ie, funding for data collection, project lead, informatics) |
| 2. | Align with ED and ICU |
| 3. | Identify 1 ward to pilot the program |
| 4. | Establish unit‐based champions on each shift (nurse, physician) |
| 5. | Review ward workflow |
| 6. | Develop nurse screening tool |
| 7. | Provide education |
Administrative Support
In the course of our implementation effort, we found that sites that had high‐level administrative support were more likely to implement and sustain the intervention. For this reason, we consider such support to be critical. Examples of such support include chief medical officers, chief nursing officers, and chief quality officers. As an example, securing commitment from hospital leadership may be necessary to improve/change the EHR and provide funding for project management to achieve sustainable improvement in outcomes. Aligning leadership with frontline physicians, nurses, and support staff toward a common goal provides the platform for a successful program.[11]
ED and ICU Leadership Support
Maintaining lines of communication among the ED, ICU, and ward staff is critical to improving outcomes. Establishing a cohesive system (ED, ICU, and wards) aimed at early recognition and treatment of sepsis throughout the hospital stay can lead to improvement in continuity of care and outcomes. For example, when an ED severe sepsis patient is transferred to the ward and subsequently requires admission to the ICU due to declining clinical status, providing timely feedback to the ED can help improve care for subsequent patients. Collaboration between the ED and the ward can also contribute to improved transitions of care for patients with severe sepsis.
Hospitalist/Internal Medicine Leadership
Our experience with implementing sepsis bundles in the ED and ICU highlights the need for effective interdisciplinary collaboration with designated physician and nurse leaders/champions. We found that engaging local clinical leaders in the early recognition and management of a severe sepsis QI program is imperative for the program's success. Hospitalists are often the physician leaders for the inpatient wards, so it is essential to secure their early engagement, support, and leadership. Moreover, though collaboration with ED and ICU physicians may be useful, as described above, a hospitalist champion is likely to be more effective at educating other hospitalists about the program, overcoming physician resistance, and facilitating change.
Depending on a hospital's size and workflows, designated ward‐ or shift‐based hospitalists and nurses as champions can serve as key resources to support implementation. These individuals help establish mutual respect and a common mental model of how sepsis can evolve in ward patients. Even more important, by providing assistance with both the screening tool as well as with recognition itself, these individuals not only speed implementation, but also protect against rough patches (ie, those instances where workflow changes run into resistance).
EDUCATION
Diagnosing sepsis is not always easy, making education on sepsis recognition, evaluation, and treatment necessary prior to implementation. Retention of knowledge over time through review and refresher courses are methods we used in the program. Providing background material explaining why education is necessary and providing physicians and nurses with materials to help them recall the information over time were developed at several sites. Resources included sepsis posters, identification‐size badge cards with the sepsis bundle elements, and bulletin boards on the wards with information to reinforce sepsis recognition, evaluation, and treatment. Education for the ward‐centric program included an overview of the SSC guidelines, supportive literature, sepsis definitions, description of the infection's systemic manifestations, criteria for identification of new‐ onset organ dysfunction, and the details on current severe sepsis 3‐ and 6‐hour bundle requirements. We made clinicians aware of resources available on the SSC website.[12] Data emphasizing the incidence of sepsis, as well as outcomes and motives for the QI wards program, were incorporated during the collaborative meetings. Data can serve as strong motivators for action (eg, highlighting current incidence rates). Many hospitals combined presentation of these aggregate data with local review of selected cases of severe sepsis that occurred in their own wards.
Understanding that the training for and experiences of ED, ICU, and ward nurses varies, nurse education contained critical assessment skills in determining when to suspect a new or worsening infection. Training nurses to complete a comprehensive daily infection assessment may help them overcome uncertainty in judgement. Assessment skills include examination of invasive lines, surgical sites, wounds, and presence of a productive cough. Equally important, patients being treated for an infection would benefit from a daily assessment for improvement or worsening of the infection. Information uncovered may identify early signs of organ failure in addition to infections that may need further evaluation and treatment. Education provides knowledge, but achieving program success relies heavily on staff accepting that they can make a difference in sepsis patient identification, management, and outcomes.
SCREENING METHODS, COMMUNICATION, AND PROTOCOLS
The SSC tool for severe sepsis facilitates screening for (1) confirmed or suspected infection, (2) presence of 2 or more systemic manifestations of infection, and (3) acute organ dysfunction. This tool was the basis for the do (screening) portion of the PDSA model.
Continuous Screening
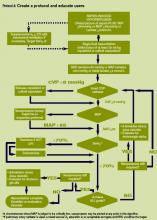
Technology can facilitate early recognition of severe sepsis with EHR‐based surveillance screening tools. Surveillance may include continuous review of vital signs and laboratory values with an automated alerting system. A valuable feature of the screening tool alert is the incorporation of the nurse's assessment. Decision support can improve the process by providing advice with systems requiring a reason to over‐ride the advice.[13] For example, an alert may include input from the nurse to determine if the abnormal data are thought to be related to an infectious process or due to another cause. If a suspected or confirmed infection is identified, further surveillance screening can include review of blood pressure readings and laboratory data to determine if organ dysfunction is present. If organ dysfunction criteria are identified, the alert can prompt the nurse to notify the physician to discuss whether the organ dysfunction is new and related to the infection and if implementation of the severe sepsis bundles is indicated (Figure 2). Additional continuous screening models may include variations of the example provided to include alerts to other clinicians or a response team.

An automated screening tool within the EHR can be useful because the system continuously scans to identify signs and symptoms of sepsis, thus providing screening consistency, and offers data on the back end to be used as a mechanism for feedback to monitor effectiveness. Challenges with EHR severe sepsis alert development are resource allocation, testing, education, and ongoing evaluation and feedback. Other challenges include the potential for alert fatigue (false positive) and inappropriate response (false negative) to the infection prompt, thereby halting the next step in automated screening for organ dysfunction. Time to complete an automated screening tool varies based on strategic design and user understanding.
Screening Checklist
Whereas EHR tools may be effective in early recognition of sepsis, not all sites will have the capability to use these tools because of lack of informatics support, cost of development, and absence of an EHR in some hospitals.[14] An alternative to continuous screening is a sepsis checklist such as the severe sepsis screening tool (Figure 1). The checklist is designed to prompt nurses to screen every patient during every shift for new signs of sepsis and organ dysfunction.
The checklist ensures that 3 key issues are considered: presence of a suspected or confirmed infection, systemic manifestations of inflammation, and physiological manifestations of organ dysfunction. The paper tool is simple to use and can be completed in 10 to 20 minutes. It requires the nurse to review the progress notes, vital signs, and laboratory test results. Although the time investment seems onerous, the gain in consistency of screening and treatment compensates for the extra effort. Review of the checklist also provides a locus for feedback and new improvement cycles.
Scripted Communication
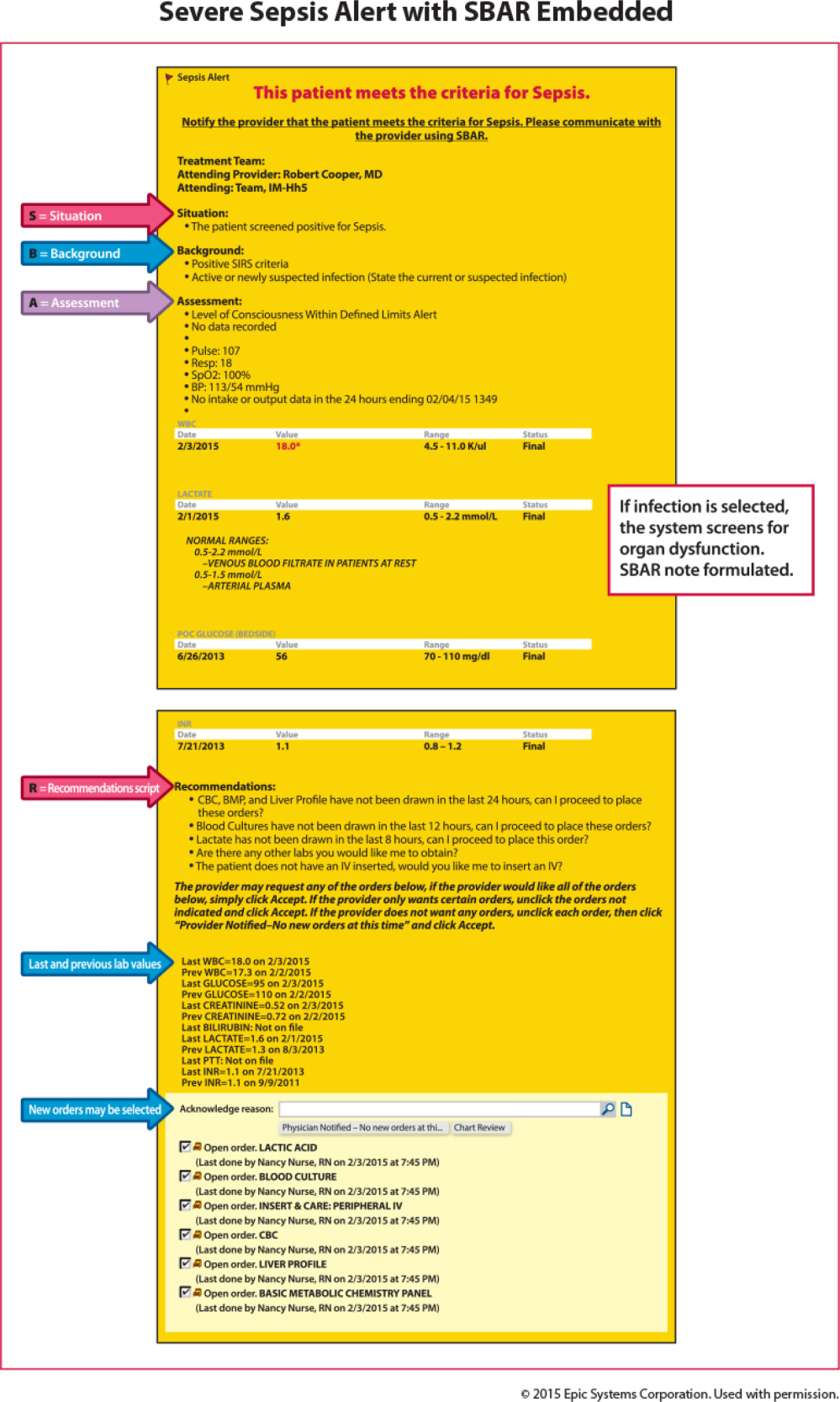
Once a patient with severe sepsis is identified, communicating this finding to the rest of the clinical team is essential. Because communication skills are not always emphasized in QI projects, we decided to emphasize a structured approach. We provided clinicians with scripts based on the SBAR (situation, background, assessment, and recommendation) technique aimed to improve communication (Figure 3).[15, 16] Using the SBAR technique also supports our efforts to build nurses' confidence and willingness to employ protocols that give them greater autonomy.

Nurse‐Directed Protocols
Skillful identification and management of severe sepsis patients constitute the foundation for implementation of nurse‐directed protocols in this patient population. Such protocols promote autonomy and staff ownership. Severe sepsis protocols may include increasing the frequency of vital signs, placement of laboratory orders and, in sites with an established culture of increased nurse autonomy, initiation of intravenous access and a fluid bolus when specific criteria are met. Because nursing scope of practice varies from state to state and among hospitals, nurse‐directed severe sepsis protocols generally require review of current site practice guidelines, physician agreement, and approval by the medical executive committee prior to implementation. Despite these differences, maximizing nurse leadership involvement and nurse autonomy can help propel the program forward. Protocols may be implemented based on knowledge level and resources on a particular ward. A workflow evaluation may be included in this process to define staff performing each step, what is being reported, and where and when data are recorded.
DATA COLLECTION AND FEEDBACK
Nurse screening drives the ward program and ensuring its consistency is the key to early patient identification. We made ongoing repeated evaluation of the appropriate use of the screening tool, time to physician notification, and time to follow‐up intervention, a critical part of the study phase of the PDSA cycle. Once the nursing staff is consistently accurate and compliant (>90%) with screening, random (eg, once per week) screening tool review may be more suitable, thus requiring fewer resources (see Supporting Information, Appendix 1, in the online version of this article).
Data Collection
A key to improvement is to study the process, which requires data collection to assess compliance. In our experience, timely clinician feedback, along with data, led to effective process change. Real‐time data collection and discussion with the clinical team may lead to early recognition or intervention.
In our collaborative experience, we observed varied resources and timing for data collection across hospitals. For example, several participating sites had sepsis coordinators to collect data, whereas others relied on the quality department or nursing staff to collect data. Data may be collected concurrently (within 24 hours of severe sepsis presentation) or retrospectively. Retrospective data collection may allow for staff flexibility in data collection, but limits feedback to the clinicians. For example, with retrospective review, early recognition and treatment failure may go unrecognized until the data are analyzed and reported, which can be months after the patient has been discharged or expired.
Feedback to Caregivers
A consistent feedback process, which can occur at the individual or group level, may lead to prompt improvement in severe sepsis management. An example of individual feedback would be providing the nurse with the elapsed time from antibiotic order to time of administration. Early in the implementation phase, frequent (daily or weekly) feedback is helpful to build team cohesiveness. An example of feedback to build the team may include a unit‐based report on the last 5 severe sepsis patients managed by the group. Providing overall bundle compliance and outcome reports on a weekly and monthly basis will allow the clinical team to track progress. Examples of report cards and a dashboard are provided in the supplemental material, which highlight compliance with the bundle elements as well as time to achieve the bundle elements. (see Supporting Information, Appendix 2 and Appendix 3, in the online version of this article). Resources to evaluate and provide consistent data may require up to 10 to 15 hours per week for 1 unit. Automated reports may decrease the resources needed in collating and reporting data.
OUTCOME MEASURES
Although certainly important, mortality is not the only outcome measure worthy of measurement. Other relevant outcomes include transfers to a higher level of care and need for major supportive therapies (eg, dialysis, mechanical ventilation, vasopressor infusion). Whereas it is valuable to review transfers to a higher level of care, we emphasized that these are not necessarily adverse outcomes; in fact, in many cases such transfers are highly desirable. It is also important to track the overall impact of sepsis on hospital length of stay.
SUMMARY/CONCLUSIONS
Grounded in the Institute for Healthcare Improvement's PDSA QI model, we developed a program aimed at improving outcomes for severe sepsis ward patients. Our program's cornerstone is nurse‐led checklist‐based screening. Our faculty led learning sessions that concentrated on using a collaborative approach whose key components were education in early sepsis identification, use of a sepsis screening tool, and the SBAR method for effective communication. Pitfalls identified during the program included lack of knowledge for both nurses and physicians in early severe sepsis identification, resistance to routine screening, and lack of data collection and leadership support. The most successful participating sites were those with senior leadership backing, staff engagement, informatics support, and data collection resources. Ultimately, replicating a program such as ours will depend on team cohesiveness, and nurse empowerment through the use of nurse‐driven protocols. Programs like this may lead to progression toward standardizing practice (eg, antibiotic administration, fluid resuscitation), matching patient needs to resources, and building stronger partnerships between hospitalists and nurses.
Disclosures
This work was supported by a grant provided to the Society of Critical Care Medicine by the Gordon and Betty Moore Foundation (Early Identification and Management of Sepsis on the Wards). The work was supported by a grant from the Adventist Hospital System. As part of our agreement with the Gordon and Betty Moore Foundation, we made a commitment to disseminate our findings in articles such as this one. However, the Moore Foundation played no role in how we actually structured our articles, nor did they review or preapprove any of the manuscripts submitted as part of the dissemination component; the same was the case with the other sponsors. The authors report no conflicts of interest.
Sepsis, the body's systemic response to infection leading to organ failure, can occur in patients throughout the hospital. However, patients initially diagnosed with sepsis on the wards experience the highest mortality for several reasons, including delayed recognition and treatment, particularly when localized infections progress to shock and organ failure. Consequently, hospitals have responded by having nurses screen patients for signs and symptoms of sepsis to identify cases earlier and improve outcomes. The intent of this article, which is based on our experience with a multihospital implementation effort, was to describe potential reasons for ward patients' poor prognosis. We provide a toolkit for how hospitals can implement a severe sepsis quality improvement (QI) program in general medicalsurgical wards.
In a previous study, we reported on our international effort, the Surviving Sepsis Campaign's (SSC) Phase III performance improvement (PI) program, targeting selected guideline recommendations (6‐ and 24‐hour bundles) in the emergency department (ED), the Intensive Care Unit (ICU), and wards in 165 volunteer hospitals in the United States, Europe, and South America.[1] The program was associated with increased bundle compliance and decreased mortality over time.[1, 2] The SSC's Phase III program, which focused on improvement efforts primarily in the ED and ICU, also exposed a need to address the high mortality in ward patients.[3] Patients admitted to the ICU directly from the ED with severe sepsis had a mortality rate of 26%, whereas those transferred to the ICU from the ward had significantly higher mortality (40.3%).[3]
Although the reasons for the higher mortality rate among ward patients have not been studied, several factors may play a role. First, the diagnosis of severe sepsis may be delayed in ward patients because physicians and nurses may not recognize the progression to sepsis and/or because hospitalized patients may not present with obvious systemic manifestations of sepsis as they do in the ED (Table 1).[4] Second, ward patients may have differences in the timing of their presentation and concurrent conditions confounding the diagnosis.[5] Third, treatment may be delayed once the diagnosis is made on the ward. The ICU and ED are designed to provide rapid high‐acuity care, whereas the wards have fewer systems and resources for rapid delivery of care needed for severe sepsis. Finally, some patients on the ward may develop sepsis from nosocomial infection, which can portend a worse prognosis.[6]
| Emergency Department Presentation | Ward Presentation | |
|---|---|---|
| Patient‐familyreported symptoms | I just feel sick, family reports disorientation, not eating | Currently hospitalized, family often not present, diagnosis may not be clear, baseline mental status unknown, lack of appetite may be linked to dislike of hospital food. |
| Systemic manifestations | Triage observed 2 or more signs of infection or patient reports temperature while at home plus additional finding on assessment. | Signs of infection may appear 1 at a time, hours apart, and may appear to be mild changes to staff or missed entirely due to staff discontinuity. |
| Organ dysfunction | Present on admission; triage nurse assesses for organ dysfunction. | Develops over hours or days; may be subtle or acute. |
| Laboratory study process | Ordered and evaluated within 1 hour. | Not routinely completed daily, may be ordered after physician evaluation or during rounds. Results within 34 hours. |
The SSC Phase III results led to the launch of a QI program, known as the SSC Phase IV Sepsis on the Wards Collaborative, funded by the Gordon and Betty Moore Foundation. This program, a partnership between the Society of Critical Care Medicine and the Society of Hospital Medicine (SHM), targeted ward patients and focused on early recognition through protocol‐driven regular nurse screening. The program applied the SSC 2012 guidelines with a primary focus on the 3‐hour bundle (Table 2).[7] The framework used for this program was the Institute for Healthcare Improvement's Plan‐Do‐Study‐Act (PDSA) model of improvement.[8, 9] The collaborative design included learning sessions designed to motivate and support improvement.[10] The program began with 60 academic and community hospitals in 4 US regions. Participating sites were required to have prior hospital experience in sepsis performance improvement as well as a formal commitment of support from their EDs and ICUs.
| To be completed within 3 hours of time of presentation |
| 1. Measure lactate level |
| 2. Obtain blood cultures prior to administration of antibiotics |
| 3. Administer broad‐spectrum antibiotics |
| 4. Administer 30 mL/kg crystalloid for hypotension or lactate 4 mmol/L (36 mg/dL) |
We provided sites with a basic screening tool and guidance for routine severe sepsis screening, monitoring, and feedback (Figure 1). Because of the anticipated challenges of implementing routine nurse screening on every shift in all inpatient wards, participants identified 1 ward to pilot the every‐shift screening program. Each pilot ward refined the nurse screening process and developed site‐specific tools based on electronic health record (EHR) capability, informatics support, and available resources. After this initial phase, the program could be implemented in a hospital's remaining wards. The slogan adopted for the program was Screen every patient, every shift, every day.

Although knowledge gained from the SSC Phase III program led to improvements in treating severe sepsis, ward patients continued to have poor outcomes. To address the potential contributions of delayed case identification, we developed an early recognition and treatment program. We outline the steps we took to develop this multisite PI program.
PREPARATORY WORK
During the planning phase, several procedural steps were taken before initiating the ward sepsis program (Table 3). These required 3 levels of involvement: senior administration, midlevel management, and patient‐level support.
| |
| 1. | Obtain administrative support (ie, funding for data collection, project lead, informatics) |
| 2. | Align with ED and ICU |
| 3. | Identify 1 ward to pilot the program |
| 4. | Establish unit‐based champions on each shift (nurse, physician) |
| 5. | Review ward workflow |
| 6. | Develop nurse screening tool |
| 7. | Provide education |
Administrative Support
In the course of our implementation effort, we found that sites that had high‐level administrative support were more likely to implement and sustain the intervention. For this reason, we consider such support to be critical. Examples of such support include chief medical officers, chief nursing officers, and chief quality officers. As an example, securing commitment from hospital leadership may be necessary to improve/change the EHR and provide funding for project management to achieve sustainable improvement in outcomes. Aligning leadership with frontline physicians, nurses, and support staff toward a common goal provides the platform for a successful program.[11]
ED and ICU Leadership Support
Maintaining lines of communication among the ED, ICU, and ward staff is critical to improving outcomes. Establishing a cohesive system (ED, ICU, and wards) aimed at early recognition and treatment of sepsis throughout the hospital stay can lead to improvement in continuity of care and outcomes. For example, when an ED severe sepsis patient is transferred to the ward and subsequently requires admission to the ICU due to declining clinical status, providing timely feedback to the ED can help improve care for subsequent patients. Collaboration between the ED and the ward can also contribute to improved transitions of care for patients with severe sepsis.
Hospitalist/Internal Medicine Leadership
Our experience with implementing sepsis bundles in the ED and ICU highlights the need for effective interdisciplinary collaboration with designated physician and nurse leaders/champions. We found that engaging local clinical leaders in the early recognition and management of a severe sepsis QI program is imperative for the program's success. Hospitalists are often the physician leaders for the inpatient wards, so it is essential to secure their early engagement, support, and leadership. Moreover, though collaboration with ED and ICU physicians may be useful, as described above, a hospitalist champion is likely to be more effective at educating other hospitalists about the program, overcoming physician resistance, and facilitating change.
Depending on a hospital's size and workflows, designated ward‐ or shift‐based hospitalists and nurses as champions can serve as key resources to support implementation. These individuals help establish mutual respect and a common mental model of how sepsis can evolve in ward patients. Even more important, by providing assistance with both the screening tool as well as with recognition itself, these individuals not only speed implementation, but also protect against rough patches (ie, those instances where workflow changes run into resistance).
EDUCATION
Diagnosing sepsis is not always easy, making education on sepsis recognition, evaluation, and treatment necessary prior to implementation. Retention of knowledge over time through review and refresher courses are methods we used in the program. Providing background material explaining why education is necessary and providing physicians and nurses with materials to help them recall the information over time were developed at several sites. Resources included sepsis posters, identification‐size badge cards with the sepsis bundle elements, and bulletin boards on the wards with information to reinforce sepsis recognition, evaluation, and treatment. Education for the ward‐centric program included an overview of the SSC guidelines, supportive literature, sepsis definitions, description of the infection's systemic manifestations, criteria for identification of new‐ onset organ dysfunction, and the details on current severe sepsis 3‐ and 6‐hour bundle requirements. We made clinicians aware of resources available on the SSC website.[12] Data emphasizing the incidence of sepsis, as well as outcomes and motives for the QI wards program, were incorporated during the collaborative meetings. Data can serve as strong motivators for action (eg, highlighting current incidence rates). Many hospitals combined presentation of these aggregate data with local review of selected cases of severe sepsis that occurred in their own wards.
Understanding that the training for and experiences of ED, ICU, and ward nurses varies, nurse education contained critical assessment skills in determining when to suspect a new or worsening infection. Training nurses to complete a comprehensive daily infection assessment may help them overcome uncertainty in judgement. Assessment skills include examination of invasive lines, surgical sites, wounds, and presence of a productive cough. Equally important, patients being treated for an infection would benefit from a daily assessment for improvement or worsening of the infection. Information uncovered may identify early signs of organ failure in addition to infections that may need further evaluation and treatment. Education provides knowledge, but achieving program success relies heavily on staff accepting that they can make a difference in sepsis patient identification, management, and outcomes.
SCREENING METHODS, COMMUNICATION, AND PROTOCOLS
The SSC tool for severe sepsis facilitates screening for (1) confirmed or suspected infection, (2) presence of 2 or more systemic manifestations of infection, and (3) acute organ dysfunction. This tool was the basis for the do (screening) portion of the PDSA model.
Continuous Screening
Technology can facilitate early recognition of severe sepsis with EHR‐based surveillance screening tools. Surveillance may include continuous review of vital signs and laboratory values with an automated alerting system. A valuable feature of the screening tool alert is the incorporation of the nurse's assessment. Decision support can improve the process by providing advice with systems requiring a reason to over‐ride the advice.[13] For example, an alert may include input from the nurse to determine if the abnormal data are thought to be related to an infectious process or due to another cause. If a suspected or confirmed infection is identified, further surveillance screening can include review of blood pressure readings and laboratory data to determine if organ dysfunction is present. If organ dysfunction criteria are identified, the alert can prompt the nurse to notify the physician to discuss whether the organ dysfunction is new and related to the infection and if implementation of the severe sepsis bundles is indicated (Figure 2). Additional continuous screening models may include variations of the example provided to include alerts to other clinicians or a response team.

An automated screening tool within the EHR can be useful because the system continuously scans to identify signs and symptoms of sepsis, thus providing screening consistency, and offers data on the back end to be used as a mechanism for feedback to monitor effectiveness. Challenges with EHR severe sepsis alert development are resource allocation, testing, education, and ongoing evaluation and feedback. Other challenges include the potential for alert fatigue (false positive) and inappropriate response (false negative) to the infection prompt, thereby halting the next step in automated screening for organ dysfunction. Time to complete an automated screening tool varies based on strategic design and user understanding.
Screening Checklist
Whereas EHR tools may be effective in early recognition of sepsis, not all sites will have the capability to use these tools because of lack of informatics support, cost of development, and absence of an EHR in some hospitals.[14] An alternative to continuous screening is a sepsis checklist such as the severe sepsis screening tool (Figure 1). The checklist is designed to prompt nurses to screen every patient during every shift for new signs of sepsis and organ dysfunction.
The checklist ensures that 3 key issues are considered: presence of a suspected or confirmed infection, systemic manifestations of inflammation, and physiological manifestations of organ dysfunction. The paper tool is simple to use and can be completed in 10 to 20 minutes. It requires the nurse to review the progress notes, vital signs, and laboratory test results. Although the time investment seems onerous, the gain in consistency of screening and treatment compensates for the extra effort. Review of the checklist also provides a locus for feedback and new improvement cycles.
Scripted Communication
Once a patient with severe sepsis is identified, communicating this finding to the rest of the clinical team is essential. Because communication skills are not always emphasized in QI projects, we decided to emphasize a structured approach. We provided clinicians with scripts based on the SBAR (situation, background, assessment, and recommendation) technique aimed to improve communication (Figure 3).[15, 16] Using the SBAR technique also supports our efforts to build nurses' confidence and willingness to employ protocols that give them greater autonomy.

Nurse‐Directed Protocols
Skillful identification and management of severe sepsis patients constitute the foundation for implementation of nurse‐directed protocols in this patient population. Such protocols promote autonomy and staff ownership. Severe sepsis protocols may include increasing the frequency of vital signs, placement of laboratory orders and, in sites with an established culture of increased nurse autonomy, initiation of intravenous access and a fluid bolus when specific criteria are met. Because nursing scope of practice varies from state to state and among hospitals, nurse‐directed severe sepsis protocols generally require review of current site practice guidelines, physician agreement, and approval by the medical executive committee prior to implementation. Despite these differences, maximizing nurse leadership involvement and nurse autonomy can help propel the program forward. Protocols may be implemented based on knowledge level and resources on a particular ward. A workflow evaluation may be included in this process to define staff performing each step, what is being reported, and where and when data are recorded.
DATA COLLECTION AND FEEDBACK
Nurse screening drives the ward program and ensuring its consistency is the key to early patient identification. We made ongoing repeated evaluation of the appropriate use of the screening tool, time to physician notification, and time to follow‐up intervention, a critical part of the study phase of the PDSA cycle. Once the nursing staff is consistently accurate and compliant (>90%) with screening, random (eg, once per week) screening tool review may be more suitable, thus requiring fewer resources (see Supporting Information, Appendix 1, in the online version of this article).
Data Collection
A key to improvement is to study the process, which requires data collection to assess compliance. In our experience, timely clinician feedback, along with data, led to effective process change. Real‐time data collection and discussion with the clinical team may lead to early recognition or intervention.
In our collaborative experience, we observed varied resources and timing for data collection across hospitals. For example, several participating sites had sepsis coordinators to collect data, whereas others relied on the quality department or nursing staff to collect data. Data may be collected concurrently (within 24 hours of severe sepsis presentation) or retrospectively. Retrospective data collection may allow for staff flexibility in data collection, but limits feedback to the clinicians. For example, with retrospective review, early recognition and treatment failure may go unrecognized until the data are analyzed and reported, which can be months after the patient has been discharged or expired.
Feedback to Caregivers
A consistent feedback process, which can occur at the individual or group level, may lead to prompt improvement in severe sepsis management. An example of individual feedback would be providing the nurse with the elapsed time from antibiotic order to time of administration. Early in the implementation phase, frequent (daily or weekly) feedback is helpful to build team cohesiveness. An example of feedback to build the team may include a unit‐based report on the last 5 severe sepsis patients managed by the group. Providing overall bundle compliance and outcome reports on a weekly and monthly basis will allow the clinical team to track progress. Examples of report cards and a dashboard are provided in the supplemental material, which highlight compliance with the bundle elements as well as time to achieve the bundle elements. (see Supporting Information, Appendix 2 and Appendix 3, in the online version of this article). Resources to evaluate and provide consistent data may require up to 10 to 15 hours per week for 1 unit. Automated reports may decrease the resources needed in collating and reporting data.
OUTCOME MEASURES
Although certainly important, mortality is not the only outcome measure worthy of measurement. Other relevant outcomes include transfers to a higher level of care and need for major supportive therapies (eg, dialysis, mechanical ventilation, vasopressor infusion). Whereas it is valuable to review transfers to a higher level of care, we emphasized that these are not necessarily adverse outcomes; in fact, in many cases such transfers are highly desirable. It is also important to track the overall impact of sepsis on hospital length of stay.
SUMMARY/CONCLUSIONS
Grounded in the Institute for Healthcare Improvement's PDSA QI model, we developed a program aimed at improving outcomes for severe sepsis ward patients. Our program's cornerstone is nurse‐led checklist‐based screening. Our faculty led learning sessions that concentrated on using a collaborative approach whose key components were education in early sepsis identification, use of a sepsis screening tool, and the SBAR method for effective communication. Pitfalls identified during the program included lack of knowledge for both nurses and physicians in early severe sepsis identification, resistance to routine screening, and lack of data collection and leadership support. The most successful participating sites were those with senior leadership backing, staff engagement, informatics support, and data collection resources. Ultimately, replicating a program such as ours will depend on team cohesiveness, and nurse empowerment through the use of nurse‐driven protocols. Programs like this may lead to progression toward standardizing practice (eg, antibiotic administration, fluid resuscitation), matching patient needs to resources, and building stronger partnerships between hospitalists and nurses.
Disclosures
This work was supported by a grant provided to the Society of Critical Care Medicine by the Gordon and Betty Moore Foundation (Early Identification and Management of Sepsis on the Wards). The work was supported by a grant from the Adventist Hospital System. As part of our agreement with the Gordon and Betty Moore Foundation, we made a commitment to disseminate our findings in articles such as this one. However, the Moore Foundation played no role in how we actually structured our articles, nor did they review or preapprove any of the manuscripts submitted as part of the dissemination component; the same was the case with the other sponsors. The authors report no conflicts of interest.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- , , , et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5‐year study. Intensive Care Med. 2014;40(11):1623–1633.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8(5):243–247.
- , , , et al. Recognizing and managing sepsis: what needs to be done? BMC Med. 2015;13:98.
- , , , et al. Risk factors for hospital‐acquired pneumonia outside the intensive care unit: a case‐control study. Am J Infect Control. 2014;42(1):38–42.
- , , , et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med. 2013;41(2):580–637.
- , , , , . Understanding the components of quality improvement collaboratives: a systematic literature review. Milbank Q. 2013;91(2):354–394.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- . Learning and improving in quality improvement collaboratives: which collaborative features do participants value most? Health Serv Res. 2009;44(2 pt 1):359–378.
- , , , et al. Senior executive adopt‐a‐work unit: a model for safety improvement. Jt Comm J Qual Saf. 2004;30(2):59–68.
- Surviving Sepsis Campaign. Available at: http://survivingsepsis.org/Resources/Pages/default.aspx. Accessed September 24, 2015.
- , , , et al. Features of effective computerised clinical decision support systems: meta‐regression of 162 randomised trials. BMJ. 2013;346:f657.
- , . characteristics of hospitals associated with complete and partial implementation of electronic health records. Perspect Health Inf Manag. 2016;13:1c.
- Institute for Healthcare Improvement. SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/resources/pages/tools/sbartechniqueforcommunicationasituationalbriefingmodel.aspx. Accessed September 12, 2015.
- , , , et al. Implementing SBAR across a large multihospital health system. Jt Comm J Qual Patient Saf. 2012;38(6):261–268.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- , , , et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5‐year study. Intensive Care Med. 2014;40(11):1623–1633.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8(5):243–247.
- , , , et al. Recognizing and managing sepsis: what needs to be done? BMC Med. 2015;13:98.
- , , , et al. Risk factors for hospital‐acquired pneumonia outside the intensive care unit: a case‐control study. Am J Infect Control. 2014;42(1):38–42.
- , , , et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med. 2013;41(2):580–637.
- , , , , . Understanding the components of quality improvement collaboratives: a systematic literature review. Milbank Q. 2013;91(2):354–394.
- , , , , , . The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- . Learning and improving in quality improvement collaboratives: which collaborative features do participants value most? Health Serv Res. 2009;44(2 pt 1):359–378.
- , , , et al. Senior executive adopt‐a‐work unit: a model for safety improvement. Jt Comm J Qual Saf. 2004;30(2):59–68.
- Surviving Sepsis Campaign. Available at: http://survivingsepsis.org/Resources/Pages/default.aspx. Accessed September 24, 2015.
- , , , et al. Features of effective computerised clinical decision support systems: meta‐regression of 162 randomised trials. BMJ. 2013;346:f657.
- , . characteristics of hospitals associated with complete and partial implementation of electronic health records. Perspect Health Inf Manag. 2016;13:1c.
- Institute for Healthcare Improvement. SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/resources/pages/tools/sbartechniqueforcommunicationasituationalbriefingmodel.aspx. Accessed September 12, 2015.
- , , , et al. Implementing SBAR across a large multihospital health system. Jt Comm J Qual Patient Saf. 2012;38(6):261–268.
© 2016 Society of Hospital Medicine

The Surviving Sepsis Campaign
The incidence of severe sepsis (sepsis with organ dysfunction) is increasing.1 The initial diagnosis and management of severe sepsis may occur in the ED, the ICU, or the hospital ward.
Several recently published studies have demonstrated decreased mortality and morbidity as a result of interventions and therapeutics applied to patients with sepsis.2-5 These new data, resulting from rigorously performed, randomized controlled trials, combined with previous data for beneficial interventions not specific to sepsis management (such as DVT and stress ulcer prophylaxis) and consensus opinion where no evidence exists lend significant weight to the belief that critical care clinicians can now significantly reduce mortality in patients with severe sepsis and septic shock.6-9
Protocolized care now exists for heart attack and stroke, which is based on recent advances as demonstrated by the medical literature. Until now there has been no attempt to reproduce such an approach in severe sepsis. The Surviving Sepsis Campaign hopes to change that.
The Surviving Sepsis Campaign is administered by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), and the International Sepsis Forum (ISF) and is open to all industry for funding through unrestricted educational grants. Contributors to date include Baxter, Edwards, and Eli Lilly.
The first phase was the introduction of the campaign at several major international critical care medicine conferences, the ESICM meeting in Barcelona in 2002, and the SCCM meeting in 2003. The stated goal of the campaign is to decrease the mortality from severe sepsis by 25% in five years.
Phase 2 of the campaign was aimed at producing guidelines for the management of sepsis. In 2003, critical care and infectious disease experts representing 11 international organizations developed evidence-based management guidelines for severe sepsis and septic shock for practical use for the bedside clinician, under the auspices of the Surviving Sepsis Campaign.
Pediatric considerations were provided to contrast adult and pediatric management. The resulting recommendations represent an attempt to facilitate a rapid change in the standard of care for management of sepsis, based on the quality of available published data and expert opinion where no literature guidance is available. The guidelines manuscript was published in both Critical Care Medicine and Intensive Care Medicine.10,11 The publication of this manuscript represents an historic step for critical care worldwide. These guidelines represent an international consensus on the best available standard for management of sepsis.
Key Recommendations
Key recommendations (listed by category and not by hierarchy) include:
- Early goal-directed resuscitation of the septic patient during the first six hours after recognition;
- Appropriate diagnostic studies to ascertain causative organisms before starting antibiotics;
- Early administration of broad-spectrum antibiotic therapy;
- Reassessment of antibiotic therapy with microbiology and clinical data to narrow coverage, when appropriate;
- A usual seven to 10 days of antibiotic therapy guided by clinical response;
- Source control with attention to the method that balances risks and benefits;
- Equivalence of crystalloid and colloid resuscitation;
- Aggressive fluid challenge to restore mean circulating filling pressure;
- Vasopressor preference for norepinephrine and dopamine;
- Cautious use of vasopressin pending further studies;
- Avoidance of low-dose dopamine administration for renal protection;
- Consideration of dobutamine inotropic therapy in some clinical situations;
- Avoidance of supranormal oxygen delivery as a goal of therapy;
- Stress-dose steroid therapy for septic shock;
- Use of recombinant activated protein C in patients with severe sepsis and high risk for death;
- Resolution of tissue hypoperfusion and targeting a hemoglobin of 7-9 g/dL in the absence of coronary artery disease or acute hemorrhage;
- Appropriate use of fresh frozen plasma and platelets;
- A low tidal volume and limitation of inspiratory plateau pressure strategy for acute lung injury and acute respiratory distress syndrome;
- Application of a minimal amount of positive end expiratory pressure in acute lung injury/acute respiratory distress syndrome;
- A semi-recumbent bed position unless contraindicated;
- Protocols for weaning and sedation/analgesia, using either intermittent bolus sedation or continuous infusion sedation with daily interruptions/lightening;
- Avoidance of neuromuscular blockers, if at all possible;
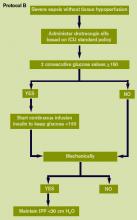
- Maintenance of blood glucose <150 mg/dL after initial stabilization;
- Equivalence of continuous veno-veno hemofiltration (CVVH) and intermittent hemodialysis;
- Lack of utility of bicarbonate use for pH 7.15 or greater;
- Use of DVT/stress ulcer prophylaxis; and
- Consideration of limitation of support where appropriate.
Pediatric considerations include a more likely need for intubation due to low functional residual capacity; more difficult intravenous access; fluid resuscitation based on weight with 40-60 mL/kg or higher needed; decreased cardiac output and increased systemic vascular resistance as the most common hemodynamic profile; greater use of physical examination therapeutic endpoints; unsettled issue of high-dose steroids for therapy of septic shock; and greater risk of hypoglycemia with aggressive glucose control.
Operationalizing the Guidelines
Unfortunately, clinicians change slowly. Historically, transfer of research from the bench to the bedside is a long, tortuous process—one that is not driven by anything clear and that seems to be based more on fad and coincidence than on a keen, evidence-based evaluation of the literature. Phase 3 of the campaign hopes to change that.
Phase 3 of the campaign (www.survivingsepsis.org) aims to operationalize the guidelines to create a global standard of care for sepsis management.12 The guidelines will be transformed into user-friendly tools that allow clinicians to easily incorporate these new recommendations into bedside care. The first step in this next phase has been a joint effort with the Institute of Healthcare Improvement (IHI) to deploy a “change bundle” based on a core set of the previous recommendations into the IHI’s collaborative system. Chart review or concurrent data gathering will identify and track changes in practice and clinical outcomes. Engendering evidence-based change through motivational strategies while monitoring and sharing the results with healthcare practitioners is the key to improving outcomes in severe sepsis.
The severe sepsis bundles form the core of the Surviving Sepsis Campaign. A “bundle” is a group of interventions related to a disease process. When executed together, the interventions produce better outcomes than when implemented individually. The individual bundle elements are built on evidence-based practices. The science behind the elements of a bundle is so well established that their implementation should be considered a generally accepted practice. Develop a bundle process in the following way:
- Identify a set of four to six evidence-based interventions that apply to a cohort of patients with a common disease or a common location. An example might be patients with sepsis admitted to the ICU;
- Develop the will in the providers to deliver the interventions every time they are indicated;
- Redesign the delivery system to ensure the interventions in the bundle are delivered; and
- Measure related outcomes to ascertain the effects of the changes in the delivery system.
The sepsis bundles were developed in just such a manner, based on the experience of the ventilator bundle. The goal now is to motivate providers to deliver the sepsis interventions every time they are indicated and measure them in an all-or-nothing way. We believe that if the bundle elements are reliably performed we can achieve the desired outcome of reducing sepsis-related deaths by 25%.
These elements distill the Surviving Sepsis Campaign practice guidelines into a manageable format for use at most institutions. The bundles represent the specific changes the campaign has identified as essential to the care of severely septic patients. Following the severe sepsis bundles will eliminate the piecemeal or inappropriate application of standards for sepsis care that characterize most clinical environments today.
Hospitals should implement two different severe sepsis bundles. Each bundle articulates objectives to be accomplished within specific time frames.
Sepsis Resuscitation Bundle
The severe sepsis resuscitation bundle describes seven tasks that should begin immediately but must be accomplished within the first six hours of presentation for patients with severe sepsis or septic shock. Some items may not be completed if the clinical conditions described in the bundle do not prevail in a particular case, but clinicians must assess for them. The goal is to perform all indicated tasks 100% of the time within the first six hours of identification of severe sepsis. The tasks are:
- Measure serum lactate;
- Obtain blood cultures prior to antibiotic administration;
- Administer broad-spectrum antibiotics within three hours from time of presentation for ED admissions and one hour for non-ED ICU admissions;
- In the event of hypotension and/or lactate >4 mmol/L (36 mg/dL):
- Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent); and
- Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65 mm Hg;
- In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L (36 mg/dL):
- Achieve central venous pressure (CVP) of ≥8-12 mm Hg; and
- Achieve central venous oxygen saturation (ScvO2) of Surviving Sepsis Campaign70%. (Achieving a mixed venous oxygen saturation (SvO2) of 65% is an acceptable alternative.)
Sepsis Management Bundle
The severe sepsis management bundle lists four management goals. Efforts to accomplish these tasks should also begin immediately, but these items may be completed within 24 hours of presentation for patients with severe sepsis or septic shock.
- Administer low-dose steroids for septic shock in accordance with a standardized ICU policy;
- Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy;
- Maintain glucose control ≥ lower limit of normal, but <150 mg/dL (8.3 mmol/L); and
- Maintain inspiratory plateau pressures <30 cm H2O for mechanically ventilated patients.
Team Effort
To achieve the goal of reducing mortality by 25% by 2008, everyone involved with the care of severe sepsis patients must be included, work processes must be carefully scripted and standardized, and commitment to this effort must be elevated. This must be a team effort that crosses disciplines and departments; it requires leadership, support from the entire organization, and buy-in from all stakeholders involved with the care of these patients.
Three levels of participation exist in creating successful change:
1) Active working teams are responsible for daily planning, documentation, communication, education, monitoring, and evaluation of activities. The working team must have representation from all departments involved in the change processes ICU, ED, pulmonary department, pharmacy, etc. The team should also be multidisciplinary, comprising physicians, nurses, pharmacists, respiratory therapists, and other staff with roles in the specific change process, such as clerks and technicians. Team members should be knowledgeable about the specific aims, the current local work processes, the associated literature, and any environmental issues that will be affected by these changes.
2) A leadership group or person within the team helps remove barriers, provides resources, monitors global progress, and gives suggestions from an institutional perspective. The working team needs someone with authority in the organization to overcome barriers and to allocate the time and resources the team needs to achieve its aim. Leadership needs to understand how the proposed changes will affect various parts of the system and the more remote consequences such changes might trigger.
3) Providers and stakeholders must be kept informed. Procedures are needed to keep them informed, to receive their feedback, and to ensure them that their responses are respected. This gives stakeholders a sense of ownership and facilitates implementation of the new processes.
Protocols
Teams should use the bundles to create customized protocols and pathways that will function well within their institutions. However, all of the elements in the bundles must be incorporated into the protocols. The protocols should mirror the bundles but allow flexibility to accommodate the specific needs of a local hospital. The severe sepsis bundles (and thereby the hospital’s protocol) form the basis for the measurements the team will conduct. If all of the elements of the bundles are not incorporated into your customized protocol, your performance on the measures will suffer.
A strong protocol will accomplish all of the items listed in the severe sepsis bundles. If the protocol designer pays careful attention to the details in the bundles, the protocol will score well on the severe sepsis quality indicators. Hospitals will want to publicize their efforts with regard to improving sepsis care and make the protocol an integral part of their rollout strategy. It is imperative to launch an educational initiative regarding the effort.
Examples of sepsis screening and management protocols are available on the Surviving Sepsis Campaign IHI Web site and are rendered on this page as “Protocol A: Create a protocol and educate users” and as “Prot0col B.” The easiest way to get to that page of the IHI Web site is through the home page link from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. These highly visual and easy-to-follow pathways exemplify ways to encourage adherence to a protocol. Notice that the “Sepsis Screening Protocol” (p. 25) complies with the terms of the severe sepsis bundles. Posting these types of algorithms prominently in the ED, hospital wards, and ICU, and making them readily available in laminated and PDA format, can have a significant impact on performance improvement programs.
These flow diagrams may be incorporated into lectures and training programs to support your efforts to change care at the bedside. You can adapt the algorithms to fit the needs of your individual institution, but keep in mind the need to comply with the overall structure of the severe sepsis bundles.
Data Collection
Data collection can seem like an onerous duty in any quality improvement project. Nevertheless, it is essential for improvement. Without attention to measurement, how will you know that your efforts are leading to improvement? At most hospitals, the magnitude of the data collection effort will not be huge as it will be relative to the number of severely septic patients cared for in the ICU.
Generally, hospitals report three to four severely septic patients are treated in one week’s time. This means that zero, one, or two severely septic patients’ charts will need to be abstracted each day in an average-size hospital. If abstraction takes between 20–30 minutes per chart, the daily time for this effort may range from 30–90 minutes daily. This relatively small burden is likely to represent an initial challenge to anyone unfamiliar with the organization of the chart and the measurement forms, or tools, used by the Surviving Sepsis Campaign for data collection. In time, however, data collection will become easier as the chart and the tools provided by the Surviving Sepsis Campaign will become more familiar. Bundle implementation and data collection have begun in hospitals throughout Europe, Latin America, the United Kingdom, and the United States.
The measurement tools were created to achieve a uniform system of data gathering, collation, and calculation across hospitals. Without the measurement tools, teams armed with only the concepts in the severe sepsis bundles would need to decide how to gather data from charts and put it in a format consistent with the calculations listed in the severe sepsis quality indicators. If any hospital were to undertake such a task on its own, it would quickly find that its results were not comparable across institutions because scores of other hospitals would have derived their results by entirely different means.
The Surviving Sepsis Campaign aims to make using the measurement tools as easy as possible for those involved in collecting data. Several basic tools organize data from the patient’s chart. Initially, a paper set of measurement tools was developed to help hospitals orchestrate data collection. Although a database now performs much of the work formerly done on paper, some use of paper tools may be helpful.
For example, the Surviving Sepsis Campaign’s screening tool for severe sepsis is integrated into the database. However, a paper version readily accessible to nurses and clinicians in the ED triage area, the medical and surgical nursing stations, and even the ICU itself will still be practical.
Likewise, some data collectors might find that first capturing on paper the data abstracted from the chart and subsequently entering it into the database is preferable. Most users are likely to find, however, that bringing the database to the ICU on a laptop and directly entering data is the easiest solution.
The most up-to-date paper versions of the tools and the Surviving Sepsis Campaign database can be found on the Institute of Healthcare Improvement Web site. The easiest way to get to that page of the Institute of Healthcare Improvement Web site is by home page linkage from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. An implementation manual is also available that will facilitate initiation of the Surviving Sepsis Campaign performance improvement program as well as installation and use of the associated electronic database.
Conclusion
The Surviving Sepsis Campaign represents an important step for international critical care societies. Recognizing the long history of delay in incorporating research into bedside care, these critical care societies have committed to working together to facilitate bench-to-bedside transfer of recent research. Thus, the campaign represents an ongoing commitment to excellence in patient care. The Surviving Sepsis Campaign has established a target of a 25% reduction in mortality worldwide from sepsis over the next five years. If the Surviving Sepsis Campaign is able to bring the guidelines into routine use, it is possible to achieve this goal. For the campaign to be successful, it will require more than good publicity. It will require a further commitment from bedside clinicians to appraise new research critically and adopt interventions proven to be effective rapidly.
Hospitalists interested in more information about instituting the Surviving Sepsis Campaign and performance improvement package in their hospital should e-mail the Surviving Sepsis Campaign user group program manager at dskbranch@mac.com. TH
References
- Angus DC, Linde-Zwirble WT, Lidicer J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10)699-709.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med. 1999;340:409-4178.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793-800.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-55.
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(suppl):S595-S597.
The incidence of severe sepsis (sepsis with organ dysfunction) is increasing.1 The initial diagnosis and management of severe sepsis may occur in the ED, the ICU, or the hospital ward.
Several recently published studies have demonstrated decreased mortality and morbidity as a result of interventions and therapeutics applied to patients with sepsis.2-5 These new data, resulting from rigorously performed, randomized controlled trials, combined with previous data for beneficial interventions not specific to sepsis management (such as DVT and stress ulcer prophylaxis) and consensus opinion where no evidence exists lend significant weight to the belief that critical care clinicians can now significantly reduce mortality in patients with severe sepsis and septic shock.6-9
Protocolized care now exists for heart attack and stroke, which is based on recent advances as demonstrated by the medical literature. Until now there has been no attempt to reproduce such an approach in severe sepsis. The Surviving Sepsis Campaign hopes to change that.
The Surviving Sepsis Campaign is administered by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), and the International Sepsis Forum (ISF) and is open to all industry for funding through unrestricted educational grants. Contributors to date include Baxter, Edwards, and Eli Lilly.
The first phase was the introduction of the campaign at several major international critical care medicine conferences, the ESICM meeting in Barcelona in 2002, and the SCCM meeting in 2003. The stated goal of the campaign is to decrease the mortality from severe sepsis by 25% in five years.
Phase 2 of the campaign was aimed at producing guidelines for the management of sepsis. In 2003, critical care and infectious disease experts representing 11 international organizations developed evidence-based management guidelines for severe sepsis and septic shock for practical use for the bedside clinician, under the auspices of the Surviving Sepsis Campaign.
Pediatric considerations were provided to contrast adult and pediatric management. The resulting recommendations represent an attempt to facilitate a rapid change in the standard of care for management of sepsis, based on the quality of available published data and expert opinion where no literature guidance is available. The guidelines manuscript was published in both Critical Care Medicine and Intensive Care Medicine.10,11 The publication of this manuscript represents an historic step for critical care worldwide. These guidelines represent an international consensus on the best available standard for management of sepsis.
Key Recommendations
Key recommendations (listed by category and not by hierarchy) include:
- Early goal-directed resuscitation of the septic patient during the first six hours after recognition;
- Appropriate diagnostic studies to ascertain causative organisms before starting antibiotics;
- Early administration of broad-spectrum antibiotic therapy;
- Reassessment of antibiotic therapy with microbiology and clinical data to narrow coverage, when appropriate;
- A usual seven to 10 days of antibiotic therapy guided by clinical response;
- Source control with attention to the method that balances risks and benefits;
- Equivalence of crystalloid and colloid resuscitation;
- Aggressive fluid challenge to restore mean circulating filling pressure;
- Vasopressor preference for norepinephrine and dopamine;
- Cautious use of vasopressin pending further studies;
- Avoidance of low-dose dopamine administration for renal protection;
- Consideration of dobutamine inotropic therapy in some clinical situations;
- Avoidance of supranormal oxygen delivery as a goal of therapy;
- Stress-dose steroid therapy for septic shock;
- Use of recombinant activated protein C in patients with severe sepsis and high risk for death;
- Resolution of tissue hypoperfusion and targeting a hemoglobin of 7-9 g/dL in the absence of coronary artery disease or acute hemorrhage;
- Appropriate use of fresh frozen plasma and platelets;
- A low tidal volume and limitation of inspiratory plateau pressure strategy for acute lung injury and acute respiratory distress syndrome;
- Application of a minimal amount of positive end expiratory pressure in acute lung injury/acute respiratory distress syndrome;
- A semi-recumbent bed position unless contraindicated;
- Protocols for weaning and sedation/analgesia, using either intermittent bolus sedation or continuous infusion sedation with daily interruptions/lightening;
- Avoidance of neuromuscular blockers, if at all possible;
- Maintenance of blood glucose <150 mg/dL after initial stabilization;
- Equivalence of continuous veno-veno hemofiltration (CVVH) and intermittent hemodialysis;
- Lack of utility of bicarbonate use for pH 7.15 or greater;
- Use of DVT/stress ulcer prophylaxis; and
- Consideration of limitation of support where appropriate.
Pediatric considerations include a more likely need for intubation due to low functional residual capacity; more difficult intravenous access; fluid resuscitation based on weight with 40-60 mL/kg or higher needed; decreased cardiac output and increased systemic vascular resistance as the most common hemodynamic profile; greater use of physical examination therapeutic endpoints; unsettled issue of high-dose steroids for therapy of septic shock; and greater risk of hypoglycemia with aggressive glucose control.
Operationalizing the Guidelines
Unfortunately, clinicians change slowly. Historically, transfer of research from the bench to the bedside is a long, tortuous process—one that is not driven by anything clear and that seems to be based more on fad and coincidence than on a keen, evidence-based evaluation of the literature. Phase 3 of the campaign hopes to change that.
Phase 3 of the campaign (www.survivingsepsis.org) aims to operationalize the guidelines to create a global standard of care for sepsis management.12 The guidelines will be transformed into user-friendly tools that allow clinicians to easily incorporate these new recommendations into bedside care. The first step in this next phase has been a joint effort with the Institute of Healthcare Improvement (IHI) to deploy a “change bundle” based on a core set of the previous recommendations into the IHI’s collaborative system. Chart review or concurrent data gathering will identify and track changes in practice and clinical outcomes. Engendering evidence-based change through motivational strategies while monitoring and sharing the results with healthcare practitioners is the key to improving outcomes in severe sepsis.
The severe sepsis bundles form the core of the Surviving Sepsis Campaign. A “bundle” is a group of interventions related to a disease process. When executed together, the interventions produce better outcomes than when implemented individually. The individual bundle elements are built on evidence-based practices. The science behind the elements of a bundle is so well established that their implementation should be considered a generally accepted practice. Develop a bundle process in the following way:
- Identify a set of four to six evidence-based interventions that apply to a cohort of patients with a common disease or a common location. An example might be patients with sepsis admitted to the ICU;
- Develop the will in the providers to deliver the interventions every time they are indicated;
- Redesign the delivery system to ensure the interventions in the bundle are delivered; and
- Measure related outcomes to ascertain the effects of the changes in the delivery system.
The sepsis bundles were developed in just such a manner, based on the experience of the ventilator bundle. The goal now is to motivate providers to deliver the sepsis interventions every time they are indicated and measure them in an all-or-nothing way. We believe that if the bundle elements are reliably performed we can achieve the desired outcome of reducing sepsis-related deaths by 25%.
These elements distill the Surviving Sepsis Campaign practice guidelines into a manageable format for use at most institutions. The bundles represent the specific changes the campaign has identified as essential to the care of severely septic patients. Following the severe sepsis bundles will eliminate the piecemeal or inappropriate application of standards for sepsis care that characterize most clinical environments today.
Hospitals should implement two different severe sepsis bundles. Each bundle articulates objectives to be accomplished within specific time frames.
Sepsis Resuscitation Bundle
The severe sepsis resuscitation bundle describes seven tasks that should begin immediately but must be accomplished within the first six hours of presentation for patients with severe sepsis or septic shock. Some items may not be completed if the clinical conditions described in the bundle do not prevail in a particular case, but clinicians must assess for them. The goal is to perform all indicated tasks 100% of the time within the first six hours of identification of severe sepsis. The tasks are:
- Measure serum lactate;
- Obtain blood cultures prior to antibiotic administration;
- Administer broad-spectrum antibiotics within three hours from time of presentation for ED admissions and one hour for non-ED ICU admissions;
- In the event of hypotension and/or lactate >4 mmol/L (36 mg/dL):
- Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent); and
- Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65 mm Hg;
- In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L (36 mg/dL):
- Achieve central venous pressure (CVP) of ≥8-12 mm Hg; and
- Achieve central venous oxygen saturation (ScvO2) of Surviving Sepsis Campaign70%. (Achieving a mixed venous oxygen saturation (SvO2) of 65% is an acceptable alternative.)
Sepsis Management Bundle
The severe sepsis management bundle lists four management goals. Efforts to accomplish these tasks should also begin immediately, but these items may be completed within 24 hours of presentation for patients with severe sepsis or septic shock.
- Administer low-dose steroids for septic shock in accordance with a standardized ICU policy;
- Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy;
- Maintain glucose control ≥ lower limit of normal, but <150 mg/dL (8.3 mmol/L); and
- Maintain inspiratory plateau pressures <30 cm H2O for mechanically ventilated patients.
Team Effort
To achieve the goal of reducing mortality by 25% by 2008, everyone involved with the care of severe sepsis patients must be included, work processes must be carefully scripted and standardized, and commitment to this effort must be elevated. This must be a team effort that crosses disciplines and departments; it requires leadership, support from the entire organization, and buy-in from all stakeholders involved with the care of these patients.
Three levels of participation exist in creating successful change:
1) Active working teams are responsible for daily planning, documentation, communication, education, monitoring, and evaluation of activities. The working team must have representation from all departments involved in the change processes ICU, ED, pulmonary department, pharmacy, etc. The team should also be multidisciplinary, comprising physicians, nurses, pharmacists, respiratory therapists, and other staff with roles in the specific change process, such as clerks and technicians. Team members should be knowledgeable about the specific aims, the current local work processes, the associated literature, and any environmental issues that will be affected by these changes.
2) A leadership group or person within the team helps remove barriers, provides resources, monitors global progress, and gives suggestions from an institutional perspective. The working team needs someone with authority in the organization to overcome barriers and to allocate the time and resources the team needs to achieve its aim. Leadership needs to understand how the proposed changes will affect various parts of the system and the more remote consequences such changes might trigger.
3) Providers and stakeholders must be kept informed. Procedures are needed to keep them informed, to receive their feedback, and to ensure them that their responses are respected. This gives stakeholders a sense of ownership and facilitates implementation of the new processes.
Protocols
Teams should use the bundles to create customized protocols and pathways that will function well within their institutions. However, all of the elements in the bundles must be incorporated into the protocols. The protocols should mirror the bundles but allow flexibility to accommodate the specific needs of a local hospital. The severe sepsis bundles (and thereby the hospital’s protocol) form the basis for the measurements the team will conduct. If all of the elements of the bundles are not incorporated into your customized protocol, your performance on the measures will suffer.
A strong protocol will accomplish all of the items listed in the severe sepsis bundles. If the protocol designer pays careful attention to the details in the bundles, the protocol will score well on the severe sepsis quality indicators. Hospitals will want to publicize their efforts with regard to improving sepsis care and make the protocol an integral part of their rollout strategy. It is imperative to launch an educational initiative regarding the effort.
Examples of sepsis screening and management protocols are available on the Surviving Sepsis Campaign IHI Web site and are rendered on this page as “Protocol A: Create a protocol and educate users” and as “Prot0col B.” The easiest way to get to that page of the IHI Web site is through the home page link from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. These highly visual and easy-to-follow pathways exemplify ways to encourage adherence to a protocol. Notice that the “Sepsis Screening Protocol” (p. 25) complies with the terms of the severe sepsis bundles. Posting these types of algorithms prominently in the ED, hospital wards, and ICU, and making them readily available in laminated and PDA format, can have a significant impact on performance improvement programs.
These flow diagrams may be incorporated into lectures and training programs to support your efforts to change care at the bedside. You can adapt the algorithms to fit the needs of your individual institution, but keep in mind the need to comply with the overall structure of the severe sepsis bundles.
Data Collection
Data collection can seem like an onerous duty in any quality improvement project. Nevertheless, it is essential for improvement. Without attention to measurement, how will you know that your efforts are leading to improvement? At most hospitals, the magnitude of the data collection effort will not be huge as it will be relative to the number of severely septic patients cared for in the ICU.
Generally, hospitals report three to four severely septic patients are treated in one week’s time. This means that zero, one, or two severely septic patients’ charts will need to be abstracted each day in an average-size hospital. If abstraction takes between 20–30 minutes per chart, the daily time for this effort may range from 30–90 minutes daily. This relatively small burden is likely to represent an initial challenge to anyone unfamiliar with the organization of the chart and the measurement forms, or tools, used by the Surviving Sepsis Campaign for data collection. In time, however, data collection will become easier as the chart and the tools provided by the Surviving Sepsis Campaign will become more familiar. Bundle implementation and data collection have begun in hospitals throughout Europe, Latin America, the United Kingdom, and the United States.
The measurement tools were created to achieve a uniform system of data gathering, collation, and calculation across hospitals. Without the measurement tools, teams armed with only the concepts in the severe sepsis bundles would need to decide how to gather data from charts and put it in a format consistent with the calculations listed in the severe sepsis quality indicators. If any hospital were to undertake such a task on its own, it would quickly find that its results were not comparable across institutions because scores of other hospitals would have derived their results by entirely different means.
The Surviving Sepsis Campaign aims to make using the measurement tools as easy as possible for those involved in collecting data. Several basic tools organize data from the patient’s chart. Initially, a paper set of measurement tools was developed to help hospitals orchestrate data collection. Although a database now performs much of the work formerly done on paper, some use of paper tools may be helpful.
For example, the Surviving Sepsis Campaign’s screening tool for severe sepsis is integrated into the database. However, a paper version readily accessible to nurses and clinicians in the ED triage area, the medical and surgical nursing stations, and even the ICU itself will still be practical.
Likewise, some data collectors might find that first capturing on paper the data abstracted from the chart and subsequently entering it into the database is preferable. Most users are likely to find, however, that bringing the database to the ICU on a laptop and directly entering data is the easiest solution.
The most up-to-date paper versions of the tools and the Surviving Sepsis Campaign database can be found on the Institute of Healthcare Improvement Web site. The easiest way to get to that page of the Institute of Healthcare Improvement Web site is by home page linkage from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. An implementation manual is also available that will facilitate initiation of the Surviving Sepsis Campaign performance improvement program as well as installation and use of the associated electronic database.
Conclusion
The Surviving Sepsis Campaign represents an important step for international critical care societies. Recognizing the long history of delay in incorporating research into bedside care, these critical care societies have committed to working together to facilitate bench-to-bedside transfer of recent research. Thus, the campaign represents an ongoing commitment to excellence in patient care. The Surviving Sepsis Campaign has established a target of a 25% reduction in mortality worldwide from sepsis over the next five years. If the Surviving Sepsis Campaign is able to bring the guidelines into routine use, it is possible to achieve this goal. For the campaign to be successful, it will require more than good publicity. It will require a further commitment from bedside clinicians to appraise new research critically and adopt interventions proven to be effective rapidly.
Hospitalists interested in more information about instituting the Surviving Sepsis Campaign and performance improvement package in their hospital should e-mail the Surviving Sepsis Campaign user group program manager at dskbranch@mac.com. TH
References
- Angus DC, Linde-Zwirble WT, Lidicer J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10)699-709.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med. 1999;340:409-4178.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793-800.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-55.
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(suppl):S595-S597.
The incidence of severe sepsis (sepsis with organ dysfunction) is increasing.1 The initial diagnosis and management of severe sepsis may occur in the ED, the ICU, or the hospital ward.
Several recently published studies have demonstrated decreased mortality and morbidity as a result of interventions and therapeutics applied to patients with sepsis.2-5 These new data, resulting from rigorously performed, randomized controlled trials, combined with previous data for beneficial interventions not specific to sepsis management (such as DVT and stress ulcer prophylaxis) and consensus opinion where no evidence exists lend significant weight to the belief that critical care clinicians can now significantly reduce mortality in patients with severe sepsis and septic shock.6-9
Protocolized care now exists for heart attack and stroke, which is based on recent advances as demonstrated by the medical literature. Until now there has been no attempt to reproduce such an approach in severe sepsis. The Surviving Sepsis Campaign hopes to change that.
The Surviving Sepsis Campaign is administered by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), and the International Sepsis Forum (ISF) and is open to all industry for funding through unrestricted educational grants. Contributors to date include Baxter, Edwards, and Eli Lilly.
The first phase was the introduction of the campaign at several major international critical care medicine conferences, the ESICM meeting in Barcelona in 2002, and the SCCM meeting in 2003. The stated goal of the campaign is to decrease the mortality from severe sepsis by 25% in five years.
Phase 2 of the campaign was aimed at producing guidelines for the management of sepsis. In 2003, critical care and infectious disease experts representing 11 international organizations developed evidence-based management guidelines for severe sepsis and septic shock for practical use for the bedside clinician, under the auspices of the Surviving Sepsis Campaign.
Pediatric considerations were provided to contrast adult and pediatric management. The resulting recommendations represent an attempt to facilitate a rapid change in the standard of care for management of sepsis, based on the quality of available published data and expert opinion where no literature guidance is available. The guidelines manuscript was published in both Critical Care Medicine and Intensive Care Medicine.10,11 The publication of this manuscript represents an historic step for critical care worldwide. These guidelines represent an international consensus on the best available standard for management of sepsis.
Key Recommendations
Key recommendations (listed by category and not by hierarchy) include:
- Early goal-directed resuscitation of the septic patient during the first six hours after recognition;
- Appropriate diagnostic studies to ascertain causative organisms before starting antibiotics;
- Early administration of broad-spectrum antibiotic therapy;
- Reassessment of antibiotic therapy with microbiology and clinical data to narrow coverage, when appropriate;
- A usual seven to 10 days of antibiotic therapy guided by clinical response;
- Source control with attention to the method that balances risks and benefits;
- Equivalence of crystalloid and colloid resuscitation;
- Aggressive fluid challenge to restore mean circulating filling pressure;
- Vasopressor preference for norepinephrine and dopamine;
- Cautious use of vasopressin pending further studies;
- Avoidance of low-dose dopamine administration for renal protection;
- Consideration of dobutamine inotropic therapy in some clinical situations;
- Avoidance of supranormal oxygen delivery as a goal of therapy;
- Stress-dose steroid therapy for septic shock;
- Use of recombinant activated protein C in patients with severe sepsis and high risk for death;
- Resolution of tissue hypoperfusion and targeting a hemoglobin of 7-9 g/dL in the absence of coronary artery disease or acute hemorrhage;
- Appropriate use of fresh frozen plasma and platelets;
- A low tidal volume and limitation of inspiratory plateau pressure strategy for acute lung injury and acute respiratory distress syndrome;
- Application of a minimal amount of positive end expiratory pressure in acute lung injury/acute respiratory distress syndrome;
- A semi-recumbent bed position unless contraindicated;
- Protocols for weaning and sedation/analgesia, using either intermittent bolus sedation or continuous infusion sedation with daily interruptions/lightening;
- Avoidance of neuromuscular blockers, if at all possible;
- Maintenance of blood glucose <150 mg/dL after initial stabilization;
- Equivalence of continuous veno-veno hemofiltration (CVVH) and intermittent hemodialysis;
- Lack of utility of bicarbonate use for pH 7.15 or greater;
- Use of DVT/stress ulcer prophylaxis; and
- Consideration of limitation of support where appropriate.
Pediatric considerations include a more likely need for intubation due to low functional residual capacity; more difficult intravenous access; fluid resuscitation based on weight with 40-60 mL/kg or higher needed; decreased cardiac output and increased systemic vascular resistance as the most common hemodynamic profile; greater use of physical examination therapeutic endpoints; unsettled issue of high-dose steroids for therapy of septic shock; and greater risk of hypoglycemia with aggressive glucose control.
Operationalizing the Guidelines
Unfortunately, clinicians change slowly. Historically, transfer of research from the bench to the bedside is a long, tortuous process—one that is not driven by anything clear and that seems to be based more on fad and coincidence than on a keen, evidence-based evaluation of the literature. Phase 3 of the campaign hopes to change that.
Phase 3 of the campaign (www.survivingsepsis.org) aims to operationalize the guidelines to create a global standard of care for sepsis management.12 The guidelines will be transformed into user-friendly tools that allow clinicians to easily incorporate these new recommendations into bedside care. The first step in this next phase has been a joint effort with the Institute of Healthcare Improvement (IHI) to deploy a “change bundle” based on a core set of the previous recommendations into the IHI’s collaborative system. Chart review or concurrent data gathering will identify and track changes in practice and clinical outcomes. Engendering evidence-based change through motivational strategies while monitoring and sharing the results with healthcare practitioners is the key to improving outcomes in severe sepsis.
The severe sepsis bundles form the core of the Surviving Sepsis Campaign. A “bundle” is a group of interventions related to a disease process. When executed together, the interventions produce better outcomes than when implemented individually. The individual bundle elements are built on evidence-based practices. The science behind the elements of a bundle is so well established that their implementation should be considered a generally accepted practice. Develop a bundle process in the following way:
- Identify a set of four to six evidence-based interventions that apply to a cohort of patients with a common disease or a common location. An example might be patients with sepsis admitted to the ICU;
- Develop the will in the providers to deliver the interventions every time they are indicated;
- Redesign the delivery system to ensure the interventions in the bundle are delivered; and
- Measure related outcomes to ascertain the effects of the changes in the delivery system.
The sepsis bundles were developed in just such a manner, based on the experience of the ventilator bundle. The goal now is to motivate providers to deliver the sepsis interventions every time they are indicated and measure them in an all-or-nothing way. We believe that if the bundle elements are reliably performed we can achieve the desired outcome of reducing sepsis-related deaths by 25%.
These elements distill the Surviving Sepsis Campaign practice guidelines into a manageable format for use at most institutions. The bundles represent the specific changes the campaign has identified as essential to the care of severely septic patients. Following the severe sepsis bundles will eliminate the piecemeal or inappropriate application of standards for sepsis care that characterize most clinical environments today.
Hospitals should implement two different severe sepsis bundles. Each bundle articulates objectives to be accomplished within specific time frames.
Sepsis Resuscitation Bundle
The severe sepsis resuscitation bundle describes seven tasks that should begin immediately but must be accomplished within the first six hours of presentation for patients with severe sepsis or septic shock. Some items may not be completed if the clinical conditions described in the bundle do not prevail in a particular case, but clinicians must assess for them. The goal is to perform all indicated tasks 100% of the time within the first six hours of identification of severe sepsis. The tasks are:
- Measure serum lactate;
- Obtain blood cultures prior to antibiotic administration;
- Administer broad-spectrum antibiotics within three hours from time of presentation for ED admissions and one hour for non-ED ICU admissions;
- In the event of hypotension and/or lactate >4 mmol/L (36 mg/dL):
- Deliver an initial minimum of 20 ml/kg of crystalloid (or colloid equivalent); and
- Apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥65 mm Hg;
- In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L (36 mg/dL):
- Achieve central venous pressure (CVP) of ≥8-12 mm Hg; and
- Achieve central venous oxygen saturation (ScvO2) of Surviving Sepsis Campaign70%. (Achieving a mixed venous oxygen saturation (SvO2) of 65% is an acceptable alternative.)
Sepsis Management Bundle
The severe sepsis management bundle lists four management goals. Efforts to accomplish these tasks should also begin immediately, but these items may be completed within 24 hours of presentation for patients with severe sepsis or septic shock.
- Administer low-dose steroids for septic shock in accordance with a standardized ICU policy;
- Administer drotrecogin alfa (activated) in accordance with a standardized ICU policy;
- Maintain glucose control ≥ lower limit of normal, but <150 mg/dL (8.3 mmol/L); and
- Maintain inspiratory plateau pressures <30 cm H2O for mechanically ventilated patients.
Team Effort
To achieve the goal of reducing mortality by 25% by 2008, everyone involved with the care of severe sepsis patients must be included, work processes must be carefully scripted and standardized, and commitment to this effort must be elevated. This must be a team effort that crosses disciplines and departments; it requires leadership, support from the entire organization, and buy-in from all stakeholders involved with the care of these patients.
Three levels of participation exist in creating successful change:
1) Active working teams are responsible for daily planning, documentation, communication, education, monitoring, and evaluation of activities. The working team must have representation from all departments involved in the change processes ICU, ED, pulmonary department, pharmacy, etc. The team should also be multidisciplinary, comprising physicians, nurses, pharmacists, respiratory therapists, and other staff with roles in the specific change process, such as clerks and technicians. Team members should be knowledgeable about the specific aims, the current local work processes, the associated literature, and any environmental issues that will be affected by these changes.
2) A leadership group or person within the team helps remove barriers, provides resources, monitors global progress, and gives suggestions from an institutional perspective. The working team needs someone with authority in the organization to overcome barriers and to allocate the time and resources the team needs to achieve its aim. Leadership needs to understand how the proposed changes will affect various parts of the system and the more remote consequences such changes might trigger.
3) Providers and stakeholders must be kept informed. Procedures are needed to keep them informed, to receive their feedback, and to ensure them that their responses are respected. This gives stakeholders a sense of ownership and facilitates implementation of the new processes.
Protocols
Teams should use the bundles to create customized protocols and pathways that will function well within their institutions. However, all of the elements in the bundles must be incorporated into the protocols. The protocols should mirror the bundles but allow flexibility to accommodate the specific needs of a local hospital. The severe sepsis bundles (and thereby the hospital’s protocol) form the basis for the measurements the team will conduct. If all of the elements of the bundles are not incorporated into your customized protocol, your performance on the measures will suffer.
A strong protocol will accomplish all of the items listed in the severe sepsis bundles. If the protocol designer pays careful attention to the details in the bundles, the protocol will score well on the severe sepsis quality indicators. Hospitals will want to publicize their efforts with regard to improving sepsis care and make the protocol an integral part of their rollout strategy. It is imperative to launch an educational initiative regarding the effort.
Examples of sepsis screening and management protocols are available on the Surviving Sepsis Campaign IHI Web site and are rendered on this page as “Protocol A: Create a protocol and educate users” and as “Prot0col B.” The easiest way to get to that page of the IHI Web site is through the home page link from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. These highly visual and easy-to-follow pathways exemplify ways to encourage adherence to a protocol. Notice that the “Sepsis Screening Protocol” (p. 25) complies with the terms of the severe sepsis bundles. Posting these types of algorithms prominently in the ED, hospital wards, and ICU, and making them readily available in laminated and PDA format, can have a significant impact on performance improvement programs.
These flow diagrams may be incorporated into lectures and training programs to support your efforts to change care at the bedside. You can adapt the algorithms to fit the needs of your individual institution, but keep in mind the need to comply with the overall structure of the severe sepsis bundles.
Data Collection
Data collection can seem like an onerous duty in any quality improvement project. Nevertheless, it is essential for improvement. Without attention to measurement, how will you know that your efforts are leading to improvement? At most hospitals, the magnitude of the data collection effort will not be huge as it will be relative to the number of severely septic patients cared for in the ICU.
Generally, hospitals report three to four severely septic patients are treated in one week’s time. This means that zero, one, or two severely septic patients’ charts will need to be abstracted each day in an average-size hospital. If abstraction takes between 20–30 minutes per chart, the daily time for this effort may range from 30–90 minutes daily. This relatively small burden is likely to represent an initial challenge to anyone unfamiliar with the organization of the chart and the measurement forms, or tools, used by the Surviving Sepsis Campaign for data collection. In time, however, data collection will become easier as the chart and the tools provided by the Surviving Sepsis Campaign will become more familiar. Bundle implementation and data collection have begun in hospitals throughout Europe, Latin America, the United Kingdom, and the United States.
The measurement tools were created to achieve a uniform system of data gathering, collation, and calculation across hospitals. Without the measurement tools, teams armed with only the concepts in the severe sepsis bundles would need to decide how to gather data from charts and put it in a format consistent with the calculations listed in the severe sepsis quality indicators. If any hospital were to undertake such a task on its own, it would quickly find that its results were not comparable across institutions because scores of other hospitals would have derived their results by entirely different means.
The Surviving Sepsis Campaign aims to make using the measurement tools as easy as possible for those involved in collecting data. Several basic tools organize data from the patient’s chart. Initially, a paper set of measurement tools was developed to help hospitals orchestrate data collection. Although a database now performs much of the work formerly done on paper, some use of paper tools may be helpful.
For example, the Surviving Sepsis Campaign’s screening tool for severe sepsis is integrated into the database. However, a paper version readily accessible to nurses and clinicians in the ED triage area, the medical and surgical nursing stations, and even the ICU itself will still be practical.
Likewise, some data collectors might find that first capturing on paper the data abstracted from the chart and subsequently entering it into the database is preferable. Most users are likely to find, however, that bringing the database to the ICU on a laptop and directly entering data is the easiest solution.
The most up-to-date paper versions of the tools and the Surviving Sepsis Campaign database can be found on the Institute of Healthcare Improvement Web site. The easiest way to get to that page of the Institute of Healthcare Improvement Web site is by home page linkage from the Surviving Sepsis Campaign Web site, www.survivingsepsis.org. An implementation manual is also available that will facilitate initiation of the Surviving Sepsis Campaign performance improvement program as well as installation and use of the associated electronic database.
Conclusion
The Surviving Sepsis Campaign represents an important step for international critical care societies. Recognizing the long history of delay in incorporating research into bedside care, these critical care societies have committed to working together to facilitate bench-to-bedside transfer of recent research. Thus, the campaign represents an ongoing commitment to excellence in patient care. The Surviving Sepsis Campaign has established a target of a 25% reduction in mortality worldwide from sepsis over the next five years. If the Surviving Sepsis Campaign is able to bring the guidelines into routine use, it is possible to achieve this goal. For the campaign to be successful, it will require more than good publicity. It will require a further commitment from bedside clinicians to appraise new research critically and adopt interventions proven to be effective rapidly.
Hospitalists interested in more information about instituting the Surviving Sepsis Campaign and performance improvement package in their hospital should e-mail the Surviving Sepsis Campaign user group program manager at dskbranch@mac.com. TH
References
- Angus DC, Linde-Zwirble WT, Lidicer J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10)699-709.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion in critical care. N Engl J Med. 1999;340:409-4178.
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793-800.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858-873.
- Dellinger RP, Carlet JM, Masur H, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536-55.
- Levy MM, Pronovost PJ, Dellinger RP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32(suppl):S595-S597.