User login
Tangled Truths: Unraveling the Link Between Frontal Fibrosing Alopecia and Allergic Contact Dermatitis
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
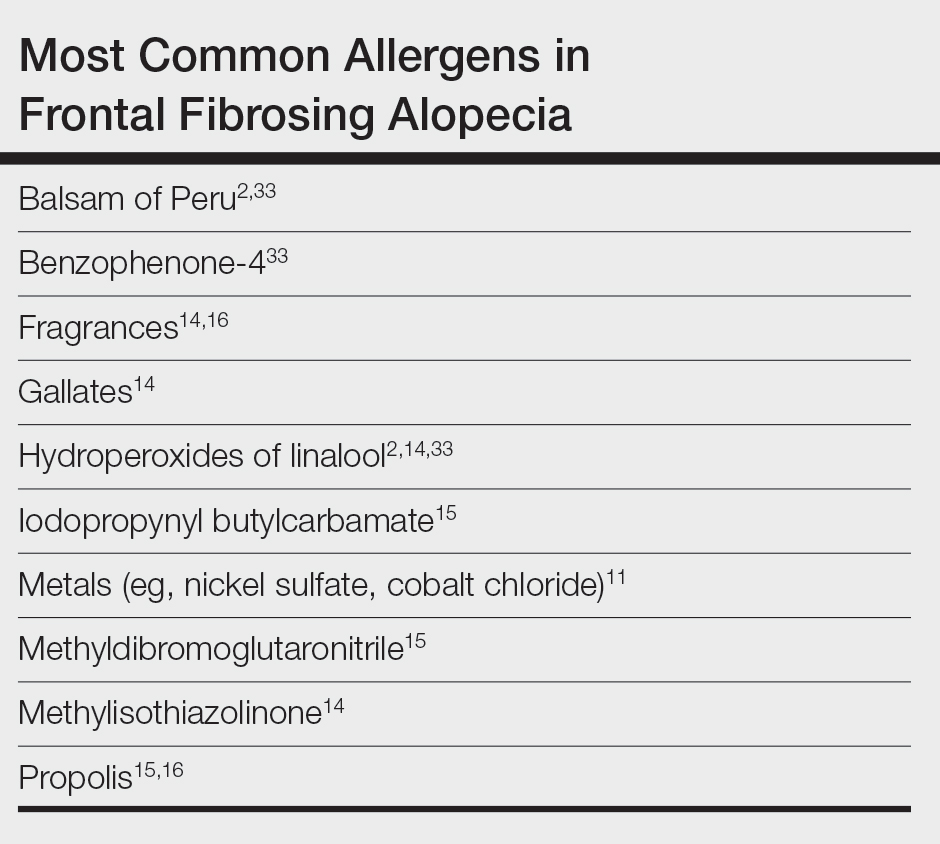
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Practice Points
- Frontal fibrosing alopecia (FFA), a variant of lichen planopilaris (LPP), is an increasingly prevalent type of scarring alopecia that may have a closer relationship to contact allergy than was previously understood. However, there is no evidence of a causal association to date.
- When evaluating for FFA/LPP, clinicians should assess for use of cosmetic products or sunscreens that may have a potential impact on the disease course.
Tackling Acrylate Allergy: The Sticky Truth
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
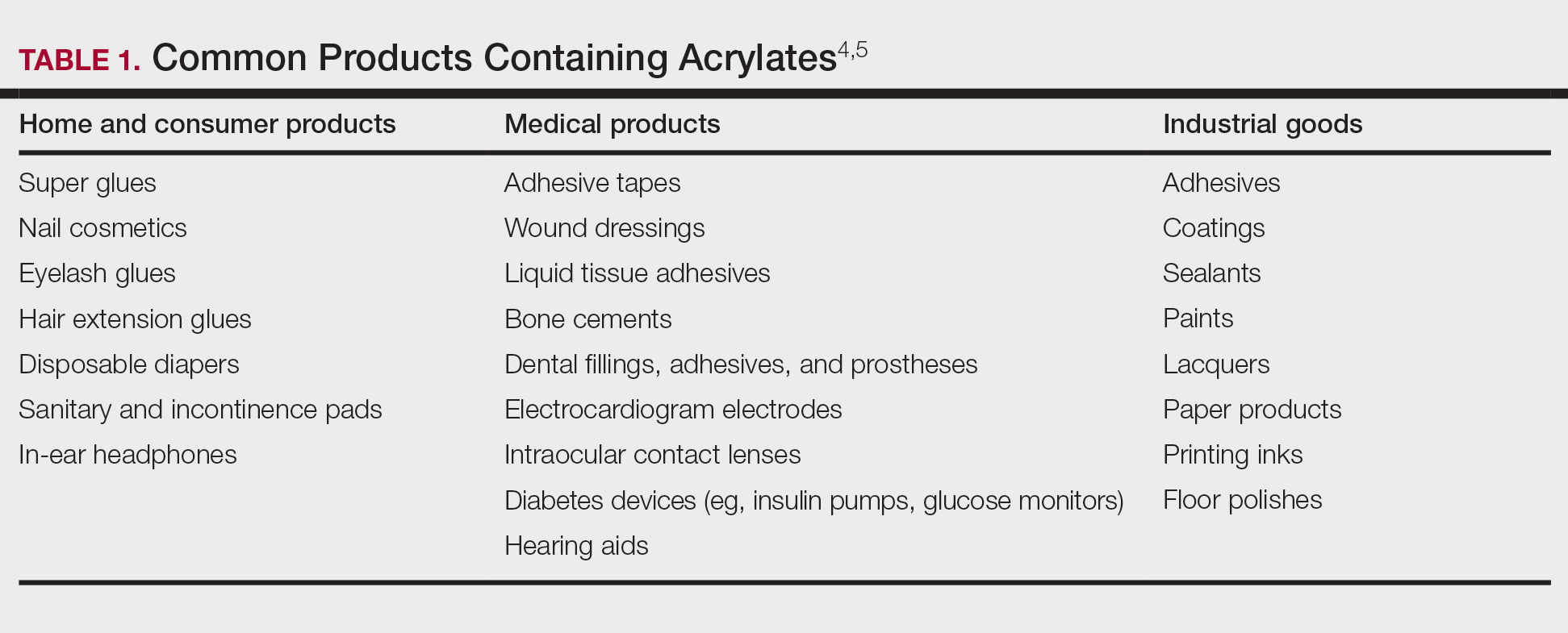
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.
Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
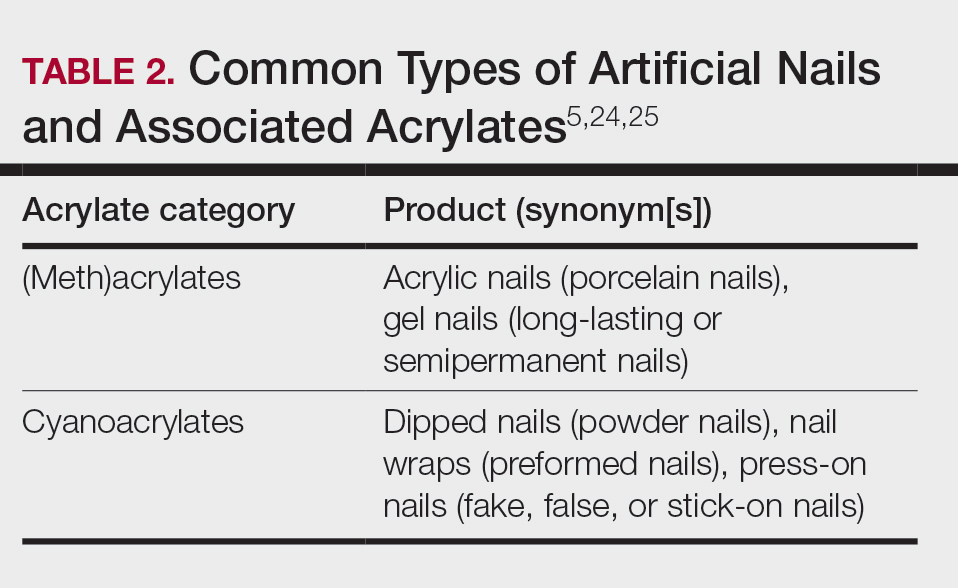
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.
Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
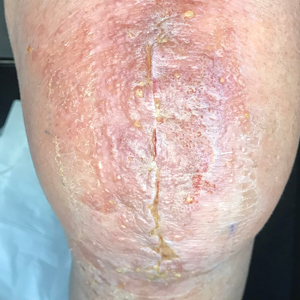
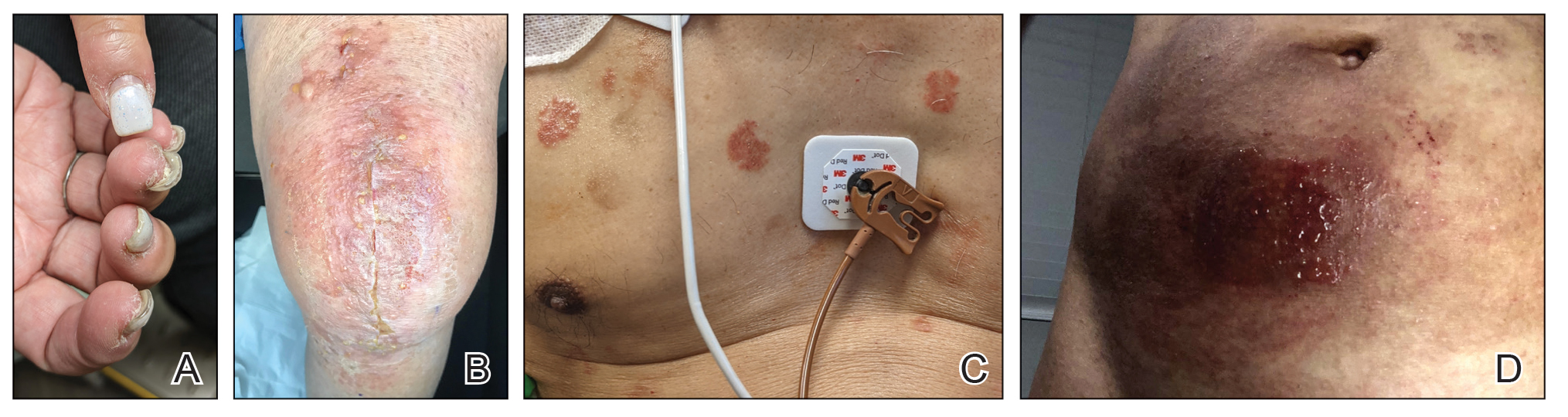
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.
Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.

Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.

Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.

Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.

Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.

Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.

Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
Practice Points
- Acrylates are thermoplastic resins used in a variety of products ranging from cosmetics to adhesives and industrial materials. Acrylic monomers are strong contact allergens, whereas fully polymerized forms are inert, provided they are completely cured.
- The use of home gel nail kits may increase the risk for sensitization to acrylates, which are the most common modern nail cosmetic allergens.
- When patch testing for suspected acrylate allergy, 2-hydroxyethyl methacrylate (HEMA) is the most important screening allergen. Expanded testing to additional acrylates should be considered depending on the clinical scenario.