User login
Eyelid Dermatitis: Common Patterns and Contact Allergens
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
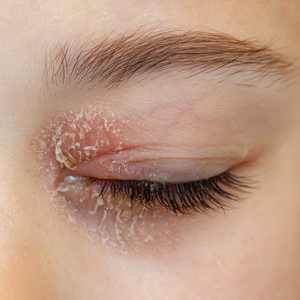
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
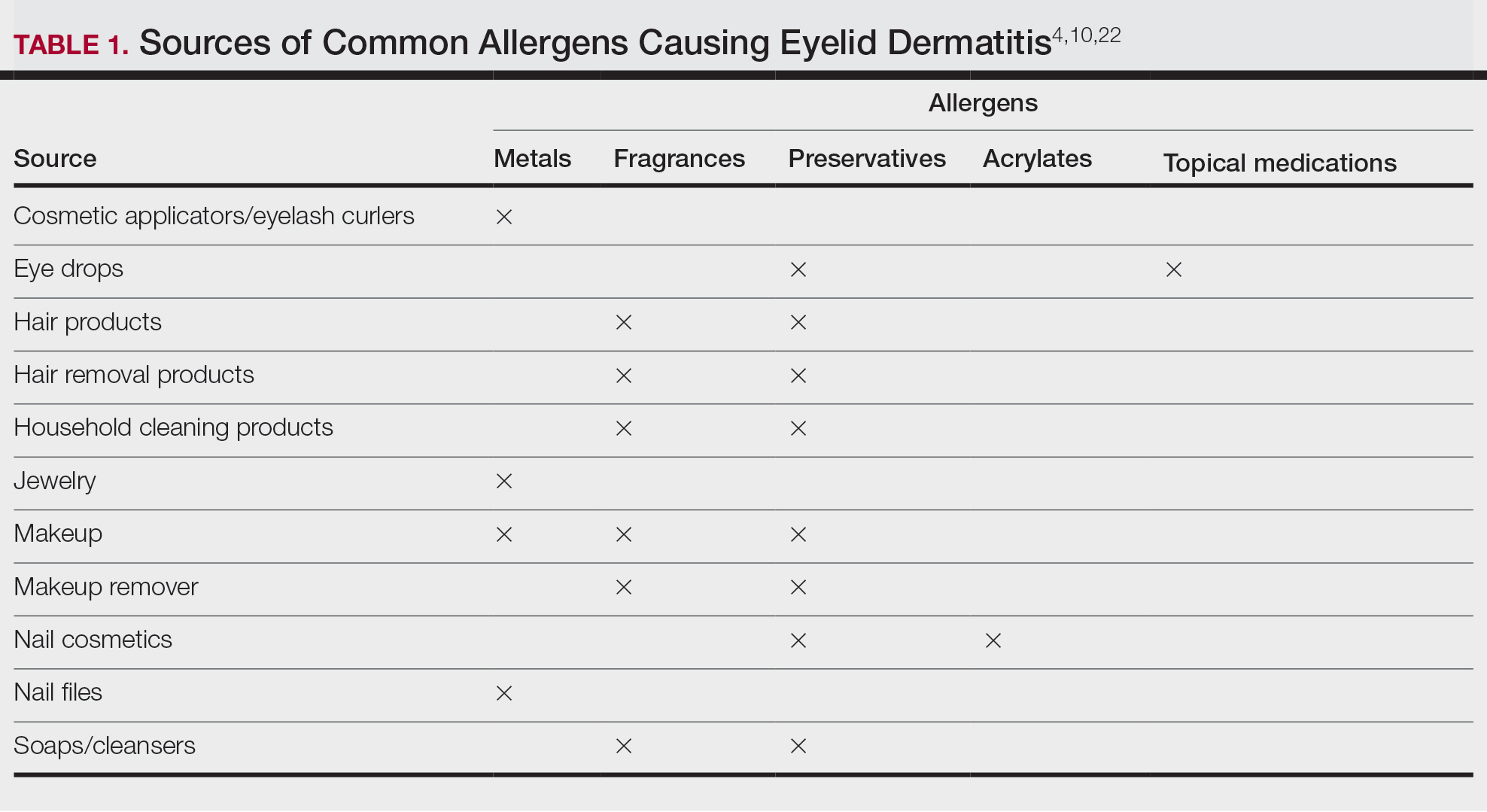
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.
Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
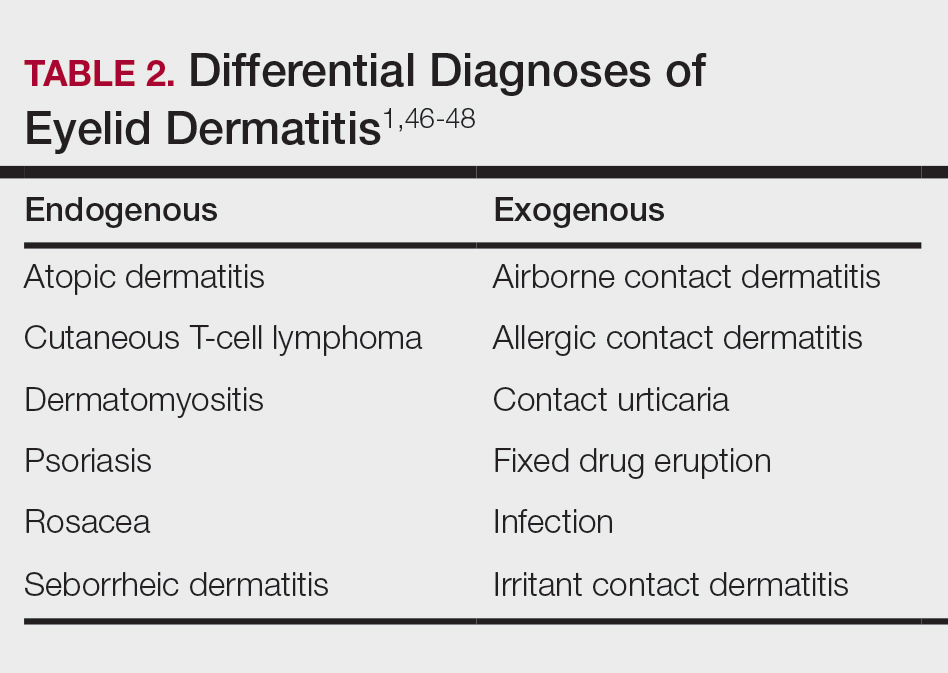
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46
Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Practice Points
- Eyelid dermatitis is a common dermatologic concern representing a broad range of inflammatory dermatoses, most often caused by allergic contact dermatitis (ACD).
- The most common contact allergens associated with eyelid dermatitis are metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications, which may be found in a variety of sources, including cosmetics, ophthalmic medications, nail lacquers, and jewelry.
- Eyelid ACD is diagnosed via patch testing, and management involves strict allergen avoidance.
A Whiff of Trouble: Navigating Allergic Contact Dermatitis to Fragrance
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Practice Points
- Fragrance allergy is common due to daily exposure from many sources, ranging from personal care products and cosmetics to cleaning products, foods/spices, and workplace materials.
- More than 100 different fragrances can cause contact allergy, but patch testing in routine practice usually is limited to a few key screening allergens with important limitations.
- Fragrance avoidance is challenging, and comprehensive patient education is critical, including the provision of a list of safe products that are truly fragrance free.
Sunscreen Safety: 2024 Updates
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Tangled Truths: Unraveling the Link Between Frontal Fibrosing Alopecia and Allergic Contact Dermatitis
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Practice Points
- Frontal fibrosing alopecia (FFA), a variant of lichen planopilaris (LPP), is an increasingly prevalent type of scarring alopecia that may have a closer relationship to contact allergy than was previously understood. However, there is no evidence of a causal association to date.
- When evaluating for FFA/LPP, clinicians should assess for use of cosmetic products or sunscreens that may have a potential impact on the disease course.
Tackling Acrylate Allergy: The Sticky Truth
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.
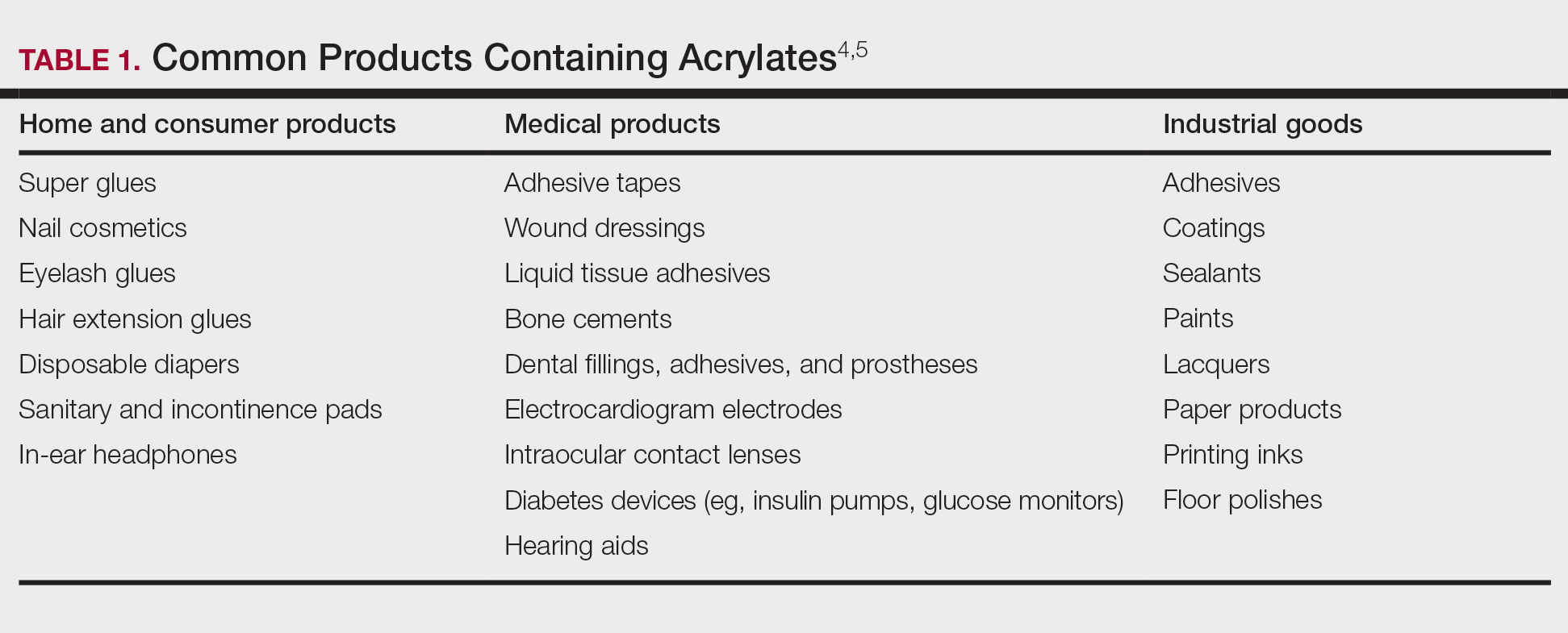
Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.
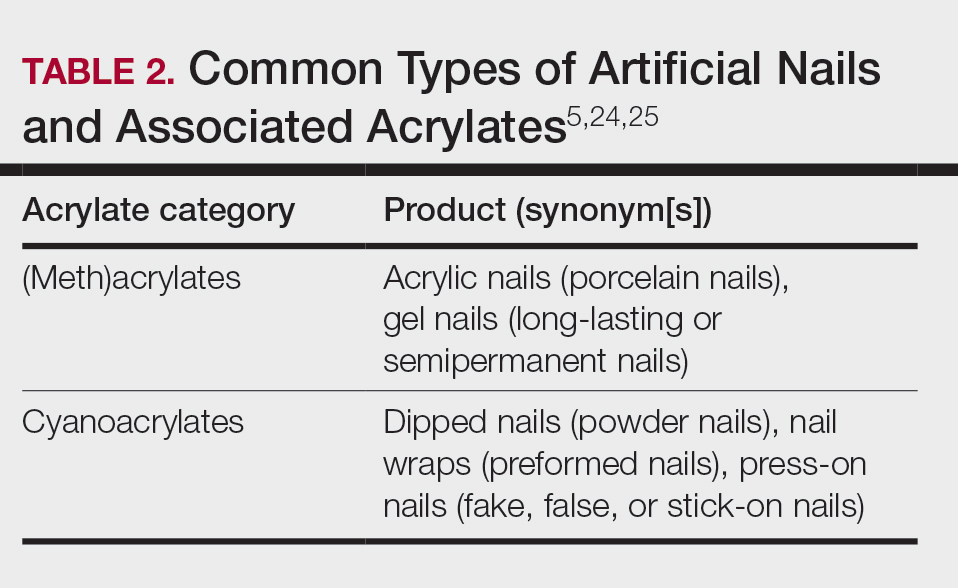
Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.
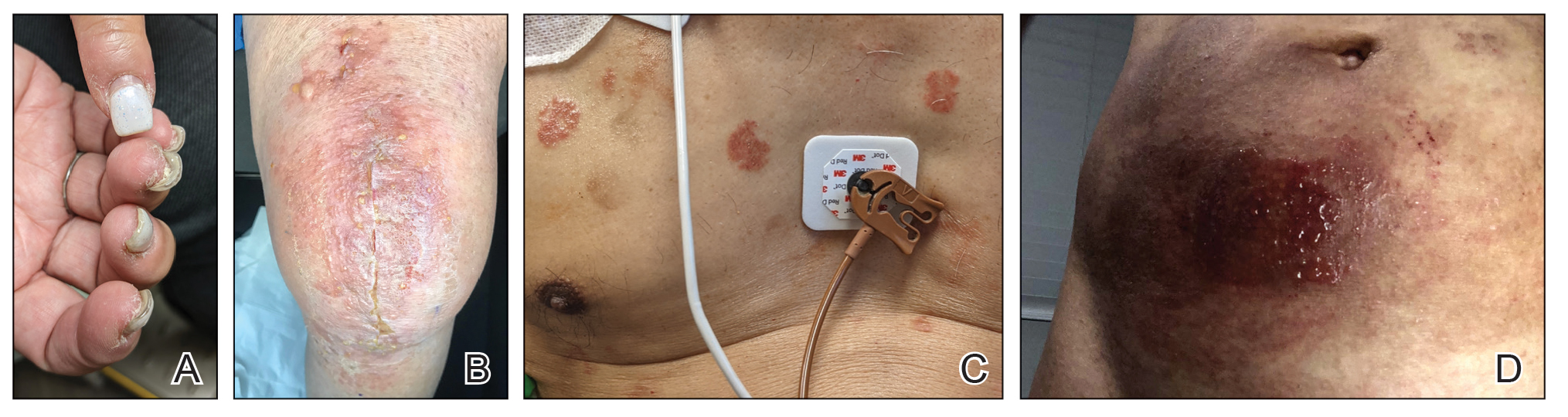
Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.

Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.

Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.

Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
Acrylates are a ubiquitous family of synthetic thermoplastic resins that are employed in a wide array of products. Since the discovery of acrylic acid in 1843 and its industrialization in the early 20th century, acrylates have been used by many different sectors of industry.1 Today, acrylates can be found in diverse sources such as adhesives, coatings, electronics, nail cosmetics, dental materials, and medical devices. Although these versatile compounds have revolutionized numerous sectors, their potential to trigger allergic contact dermatitis (ACD) has garnered considerable attention in recent years. In 2012, acrylates as a group were named Allergen of the Year by the American Contact Dermatitis Society,2 and one member—isobornyl acrylate—also was given the infamous award in 2020.3 In this article, we highlight the chemistry of acrylates, the growing prevalence of acrylate contact allergy, common sources of exposure, patch testing considerations, and management/prevention strategies.
Chemistry and Uses of Acrylates
Acrylates are widely used due to their pliable and resilient properties.4 They begin as liquid monomers of (meth)acrylic acid or cyanoacrylic acid that are molded to the desired application before being cured or hardened by one of several means: spontaneously, using chemical catalysts, or with heat, UV light, or a light-emitting diode. Once cured, the final polymers (ie, [meth]acrylates, cyanoacrylates) serve a myriad of different purposes. Table 1 includes some of the more clinically relevant sources of acrylate exposure. Although this list is not comprehensive, it offers a glimpse into the vast array of uses for acrylates.

Acrylate Contact Allergy
Acrylic monomers are potent contact allergens, but the polymerized final products are not considered allergenic, assuming they are completely cured; however, ACD can occur with incomplete curing.6 It is of clinical importance that once an individual becomes sensitized to one type of acrylate, they may develop cross-reactions to others contained in different products. Notably, cyanoacrylates generally do not cross-react with (meth)acrylates; this has important implications for choosing safe alternative products in sensitized patients, though independent sensitization to cyanoacrylates is possible.7,8
Epidemiology and Risk Factors
The prevalence of acrylate allergy in the general population is unknown; however, there is a trend of increased patch test positivity in studies of patients referred for patch testing. A 2018 study by the European Environmental Contact Dermatitis Research Group reported positive patch tests to acrylates in 1.1% of 18,228 patients tested from 2013 to 2015.9 More recently, a multicenter European study (2019-2020) reported a 2.3% patch test positivity to 2-hydroxyethyl methacrylate (HEMA) among 7675 tested individuals,10 and even higher HEMA positivity was reported in Spain (3.7% of 1884 patients in 2019-2020).11 In addition, the North American Contact Dermatitis Group (NACDG) reported positive patch test reactions to HEMA in 3.2% of 4111 patients tested from 2019 to 2020, a statistically significant increase compared with those tested in 2009 to 2018 (odds ratio, 1.25 [95% CI, 1.03-1.51]; P=.02).12
Historically, acrylate sensitization primarily stemmed from occupational exposure. A retrospective analysis of occupational dermatitis performed by the NACDG (2001-2016) showed that HEMA was among the top 10 most common occupational allergens (3.4% positivity [83/2461]) and had the fifth highest percentage of occupationally relevant reactions (73.5% [83/113]).13 High-risk occupations include dental providers and nail technicians. Dentistry utilizes many materials containing acrylates, including uncured plastic resins used in dental prostheses, dentin bonding materials, and glass ionomers.14 A retrospective analysis of 585 dental personnel who were patch tested by the NACDG (2001-2018) found that more than 20% of occupational ACD cases were related to acrylates.15 Nail technicians are another group routinely exposed to acrylates through a variety of modern nail cosmetics. In a 7-year study from Portugal evaluating acrylate ACD, 68% (25/37) of cases were attributed to occupation, 80% (20/25) of which were in nail technicians.16 Likewise, among 28 nail technicians in Sweden who were referred for patch testing, 57% (16/28) tested positive for at least 1 acrylate.17
Modern Sources of Acrylate Exposure
Once thought to be a predominantly occupational exposure, acrylates have rapidly made their way into everyday consumer products. Clinicians should be aware of several sources of clinically relevant acrylate exposure, including nail cosmetics, consumer electronics, and medical/surgical adhesives.
A 2016 study found a shift to nail cosmetics as the most common source of acrylate sensitization.18 Nail cosmetics that contain acrylates include traditional acrylic, gel (shellac), dipped, and press-on (false) nails.19 The NACDG found that the most common allergen in patients experiencing ACD associated with nail products (2001-2016) was HEMA (56.6% [273/482]), far ahead of the traditional nail polish allergen tosylamide (36.2% [273/755]). Over the study period, the frequency of positive patch tests statistically increased for HEMA (P=.0069) and decreased for tosylamide (P<.0001).20 There is concern that the use of home gel nail kits, which can be purchased online at the click of a button, may be associated with a risk for acrylate sensitization.21,22 A recent study surveyed a Facebook support group for individuals with self-reported reactions to nail cosmetics, finding that 78% of the 199 individuals had used at-home gel nail kits, and more than 80% of them first developed skin reactions after starting to use at-home kits.23 The risks for sensitization are thought to be greater when self-applying nail acrylates compared to having them done professionally because individuals are more likely to spill allergenic monomers onto the skin at home; it also is possible that home techniques could lead to incomplete curing. Table 2 reviews the different types of acrylic nail cosmetics.

Medical adhesives and equipment are other important areas where acrylates can be encountered in abundance. A review by Spencer et al18 cautioned wound dressings as an up-and-coming source of sensitization, and this has been demonstrated in the literature as coming to fruition.26 Another study identified acrylates in 15 of 16 (94%) tested medical adhesives; among 7 medical adhesives labeled as hypoallergenic, 100% still contained acrylates and/or abietic acid.27 Multiple case reports have described ACD to adhesives of electrocardiogram electrodes containing acrylates.28-31 Physicians providing care to patients with diabetes mellitus also must be aware of acrylates in glucose monitors and insulin pumps, either found in the adhesives or leaching from the inside of the device to reach the skin.32 Isobornyl acrylate in particular has made quite the name for itself in this sector, being crowned the 2020 Allergen of the Year owing to its key role in cases of ACD to diabetes devices.3
Cyanoacrylate-based tissue adhesives (eg, 2‐octyl cyanoacrylate) are now well documented to cause postoperative ACD.33,34 Although robust prospective data are limited, studies suggest that 2% to 14% of patients develop postoperative skin reactions following 2-octyl cyanoacrylate application.35-37 It has been shown that sensitization to tissue adhesives often occurs after the first application, followed by an eruption of ACD as long as a month later, which can create confusion about the nature of the rash for patients and health care providers alike, who may for instance attribute it to infection rather than allergy.38 In the orthopedic literature, a woman with a known history of acrylic nail ACD had knee arthroplasty failure attributed to acrylic bone cement with resolution of the joint symptoms after changing to a cementless device.39
Awareness of the common use of acrylates is important to identify the cause of reactions from products that would otherwise seem nonallergenic. A case of occupational ACD to isobornyl acrylate in UV-cured phone screen protectors has been reported40; several cases of ACD to acrylates in headphones41,42 as well as one related to a wearable fitness device also have been reported.43 Given all these possible sources of exposure, ACD to acrylates should be on your radar.
When to Consider Acrylate ACD
When working up a patient with dermatitis, it is essential to ask about occupational history and hobbies to get a sense of potential contact allergen exposures. The typical presentation of occupational acrylate-associated ACD is hand eczema, specifically involving the fingertips.5,24,25,44 Acrylate ACD should be considered in patients with nail dystrophy and a history of wearing acrylic nails.45 There can even be involvement of the face and eyelids secondary to airborne contact or ectopic spread from the hands.24 Spreading vesicular eruptions associated with adhesives also should raise concern. The Figure depicts several possible presentations of ACD to acrylates. In a time of abundant access to products containing acrylates, dermatologists should consider this allergy in their differential diagnosis and consider patch testing.

Patch Testing to Acrylates
The gold standard for ACD diagnosis is patch testing. It should be noted that no acrylates are included in the thin-layer rapid use epicutaneous (T.R.U.E.) test series. Several acrylates are tested in expanded patch test series including the American Contact Dermatitis Society Core Allergen series and North American 80 Comprehensive Series. 2-Hydroxyethyl methacrylate is thought to be the most important screening allergen to test. Ramos et al16 reported a positive patch test to HEMA in 81% (30/37) of patients who had any type of acrylate allergy.
If initial testing to a limited number of acrylates is negative but clinical suspicion remains high, expanded acrylates/plastics and glue series also are available from commercial patch test suppliers. Testing to an expanded panel of acrylates is especially pertinent to consider in suspected occupational cases given the risk of workplace absenteeism and even disability that come with continued exposure to the allergen. Of note, isobornyl acrylate is not included in the baseline patch test series and must be tested separately, particularly because it usually does not cross-react with other acrylates, and therefore allergy could be missed if not tested on its own.
Acrylates are volatile substances that have been shown to degrade at room temperature and to a lesser degree when refrigerated. Ideally, they should be stored in a freezer and not used beyond their expiration date. Furthermore, it is advised that acrylate patch tests be prepared immediately prior to placement on the patient and to discard the initial extrusion from the syringe, as the concentration at the tip may be decreased.46,47
With regard to tissue adhesives, the actual product should be tested as-is because these are not commercially available patch test substances.48 Occasionally, patients who are sensitized to the tissue adhesive will not react when patch tested on intact skin. If clinical suspicion remains high, scratch patch testing may confirm contact allergy in cases of negative testing on intact skin.49
Management and Prevention
Once a diagnosis of ACD secondary to acrylates has been established, counseling patients on allergen avoidance strategies is essential. For (meth)acrylate-allergic patients who want to continue using modern nail products, cyanoacrylate-based options (eg, dipped, press-on nails) can be considered as an alternative, as they do not cross-react, though independent sensitization is still possible. However, traditional nail polish is the safest option to recommend.
The concern with acrylate sensitization extends beyond the immediate issue that brought the patient into your clinic. Dermatologists must counsel patients who are sensitized to acrylates on the possible sequelae of acrylate-containing dental or orthopedic procedures. Oral lichenoid lesions, denture stomatitis, burning mouth syndrome, or even acute facial swelling have been reported following dental work in patients with acrylate allergy.50-53 Dentists of patients with acrylate ACD should be informed of the diagnosis so acrylates can be avoided during dental work; if unavoidable, all possible steps should be taken to ensure complete curing of the monomers. In the surgical setting, patients sensitized to cyanoacrylate-based tissue adhesives should be offered wound closure alternatives such as sutures or staples.34
In patients with diabetes mellitus who develop ACD to their glucose monitor or insulin pump, ideally they should be switched to a device that does not contain acrylates. Problematically, these devices are constantly being reformulated, and manufacturers do not always divulge their components, which can make it challenging to determine safe alternative options.32,54 Various barrier products may help on a case-by-case basis.55Preventative measures should be implemented in workplaces that utilize acrylates, including dental practices and nail salons. Acrylic monomers have been shown to penetrate most gloves within minutes of exposure.56,57 Double gloving with nitrile gloves affords some protection for no longer than 60 minutes.6 4H gloves have been shown to provide true protection but result in a loss of dexterity.58 The fingerstall technique involves removing the fingers from a 4H glove, inserting them on the fingers, and applying a more flexible glove on top to hold them in place; this offers a hybrid between protection and finger dexterity.59
Final Interpretation
In a world characterized by technological advancements and increasing accessibility to acrylate-containing products, we hope this brief review serves as a resource and reminder to dermatologists to consider acrylates as a potential cause of ACD with diverse presentations and important future implications for affected individuals. The rising trend of acrylate allergy necessitates comprehensive assessment and shared decision-making between physicians and patients. As we navigate the ever-changing landscape of materials and technologies, clinicians must remain vigilant to avoid some potentially sticky situations for patients.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
- Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent. 2021;23:397-406.
- Militello M, Hu S, Laughter M, et al. American Contact Dermatitis Society Allergens of the Year 2000 to 2020. Dermatol Clin. 2020;38:309-320.
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Society Allergen of the Year. Cutis. 2020;105:283-285.
- Ajekwene KK. Properties and applications of acrylates. In: Serrano-Aroca A, Deb S, eds. Acrylate Polymers for Advanced Applications. IntechOpen; 2020:35-46. https://doi.org/10.5772/intechopen.89867
- Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45:277-283.
- Sasseville D. Acrylates in contact dermatitis. Dermat Contact Atopic Occup Drug. 2012;23:6-16.
- Gardeen S, Hylwa S. A review of acrylates: super glue, nail adhesives, and diabetic pump adhesives increasing sensitization risk in women and children. Int J Womens Dermatol. 2020;6:263-267.
- Chou M, Dhingra N, Strugar TL. Contact sensitization to allergens in nail cosmetics. Dermat Contact Atopic Occup Drug. 2017;28:231-240.
- Gonçalo M, Pinho A, Agner T, et al. Allergic contact dermatitis caused by nail acrylates in Europe. an EECDRG study. Contact Dermatitis. 2018;78:254-260.
- Uter W, Wilkinson SM, Aerts O, et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermatitis. 2022;87:343-355.
- Hernández-Fernández CP, Mercader-García P, Silvestre Salvador JF, et al. Candidate allergens for inclusion in the Spanish standard series based on data from the Spanish Contact Dermatitis Registry. Actas Dermosifiliogr. 2021;112:798-805.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group patch test results: 2019-2020. Dermat Contact Atopic Occup Drug. 2023;34:90-104.
- DeKoven JG, DeKoven BM, Warshaw EM, et al. Occupational contact dermatitis: retrospective analysis of North American Contact Dermatitis Group Data, 2001 to 2016. J Am Acad Dermatol. 2022;86:782-790.
- Heratizadeh A, Werfel T, Schubert S, et al. Contact sensitization in dental technicians with occupational contact dermatitis. data of the Information Network of Departments of Dermatology (IVDK) 2001-2015. Contact Dermatitis. 2018;78:266-273.
- Warshaw EM, Ruggiero JL, Atwater AR, et al. Occupational contact dermatitis in dental personnel: a retrospective analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermat Contact Atopic Occup Drug. 2022;33:80-90.
- Ramos L, Cabral R, Gonçalo M. Allergic contact dermatitis caused by acrylates and methacrylates—a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Fisch A, Hamnerius N, Isaksson M. Dermatitis and occupational (meth)acrylate contact allergy in nail technicians—a 10-year study. Contact Dermatitis. 2019;81:58-60.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review. Contact Dermatitis. 2016;75:157-164.
- DeKoven S, DeKoven J, Holness DL. (Meth)acrylate occupational contact dermatitis in nail salon workers: a case series. J Cutan Med Surg. 2017;21:340-344.
- Warshaw EM, Voller LM, Silverberg JI, et al. Contact dermatitis associated with nail care products: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. Dermat Contact Atopic Occup Drug. 2020;31:191-201.
- Le Q, Cahill J, Palmer-Le A, et al. The rising trend in allergic contact dermatitis to acrylic nail products. Australas J Dermatol. 2015;56:221-223.
- Gatica-Ortega ME, Pastor-Nieto M. The present and future burden of contact dermatitis from acrylates in manicure. Curr Treat Options Allergy. 2020;7:1-21.
- Guenther J, Norman T, Wee C, et al. A survey of skin reactions associated with acrylic nail cosmetics, with a focus on home kits: is there a need for regulation [published online October 16, 2023]? Dermatitis. doi:10.1089/derm.2023.0204
- Calado R, Gomes T, Matos A, et al. Contact dermatitis to nail cosmetics. Curr Dermatol Rep. 2021;10:173-181.
- Draelos ZD. Nail cosmetics and adornment. Dermatol Clin. 2021;39:351-359.
- Mestach L, Huygens S, Goossens A, et al. Allergic contact dermatitis caused by acrylic-based medical dressings and adhesives. Contact Dermatitis. 2018;79:81-84.
- Tam I, Wang JX, Yu JD. Identifying acrylates in medical adhesives. Dermat Contact Atopic Occup Drug. 2020;31:E40-E42.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes. Contact Dermatitis. 2015;73:44-48.
- Ozkaya E, Kavlak Bozkurt P. Allergic contact dermatitis caused by self-adhesive electrocardiography electrodes: a rare case with concomitant roles of nickel and acrylates. Contact Dermatitis. 2014;70:121-123.
- Lyons G, Nixon R. Allergic contact dermatitis to methacrylates in ECG electrode dots. Australas J Dermatol. 2013;54:39-40.
- Jelen G. Acrylate, a hidden allergen of electrocardiogram electrodes. Contact Dermatitis. 2001;45:315-316.
- Bembry R, Brys AK, Atwater AR. Medical device contact allergy: glucose monitors and insulin pumps. Curr Dermatol Rep. 2022;11:13-20.
- Liu T, Wan J, McKenna RA, et al. Allergic contact dermatitis caused by Dermabond in a paediatric patient undergoing skin surgery. Contact Dermatitis. 2019;80:61-62.
- Ricciardo BM, Nixon RL, Tam MM, et al. Allergic contact dermatitis to Dermabond Prineo after elective orthopedic surgery. Orthopedics. 2020;43:E515-E522.
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? the incidence of dermatitis after 2-octyl cyanoacrylate exposure in 102 consecutive breast cases. Plast Reconstr Surg. 2020;145:32-37.
- Alotaibi NN, Ahmad T, Rabah SM, et al. Type IV hypersensitivity reaction to Dermabond (2-octyl cyanoacrylate) in plastic surgical patients: a retrospective study. Plast Surg Oakv Ont. 2022;30:222-226.
- Durando D, Porubsky C, Winter S, et al. Allergic contact dermatitis to dermabond (2-octyl cyanoacrylate) after total knee arthroplasty. Dermat Contact Atopic Occup Drug. 2014;25:99-100.
- Asai C, Inomata N, Sato M, et al. Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure. Contact Dermatitis. 2021;84:103-108.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59:S123-S124.
- Amat-Samaranch V, Garcia-Melendo C, Tubau C, et al. Occupational allergic contact dermatitis to isobornyl acrylate present in cell phone screen protectors. Contact Dermatitis. 2021;84:352-354.
- Chan J, Rabi S, Adler BL. Allergic contact dermatitis to (meth)acrylates in Apple AirPods headphones. Dermatitis. 2021;32:E111-E112.
- Shaver RL, Buonomo M, Scherman JA, et al. Contact allergy to acrylates in Apple AirPods Pro® headphones: a case series. Int J Dermatol. 2022;61:E459-E461.
- Winston FK, Yan AC. Wearable health device dermatitis: a case of acrylate-related contact allergy. Cutis. 2017;100:97-99.
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Postepy Dermatol Alergol. 2021;38:555-560.
- Nanda S. Nail salon safety: from nail dystrophy to acrylate contact allergies. Cutis. 2022;110:E32-E33.
- Joy NM, Rice KR, Atwater AR. Stability of patch test allergens. Dermat Contact Atopic Occup Drug. 2013;24:227-236.
- Jou PC, Siegel PD, Warshaw EM. Vapor pressure and predicted stability of American Contact Dermatitis Society core allergens. Dermat Contact Atopic Occup Drug. 2016;27:193-201.
- Cook KA, White AA, Shaw DW. Patch testing ingredients of Dermabond and other cyanoacrylate-containing adhesives. Dermat Contact Atopic Occup Drug. 2019;30:314-322.
- Patel K, Nixon R. Scratch patch testing to Dermabond in a patient with suspected allergic contact dermatitis. Dermat Contact Atopic Occup Drug. 2023;34:250-251.
- Ditrichova D, Kapralova S, Tichy M, et al. Oral lichenoid lesions and allergy to dental materials. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslov. 2007;151:333-339.
- Chen AYY, Zirwas MJ. Denture stomatitis. Skinmed. 2007;6:92-94.
- Marino R, Capaccio P, Pignataro L, et al. Burning mouth syndrome: the role of contact hypersensitivity. Oral Dis. 2009;15:255-258.
- Obayashi N, Shintani T, Kamegashira A, et al. A case report of allergic reaction with acute facial swelling: a rare complication of dental acrylic resin. J Int Med Res. 2023;51:3000605231187819.
- Cameli N, Silvestri M, Mariano M, et al. Allergic contact dermatitis, an important skin reaction in diabetes device users: a systematic review. Dermat Contact Atopic Occup Drug. 20221;33:110-115.
- Ng KL, Nixon RL, Grills C, et al. Solution using Stomahesive® wafers for allergic contact dermatitis caused by isobornyl acrylate in glucose monitoring sensors. Australas J Dermatol. 2022;63:E56-E59.
- Lönnroth EC, Wellendorf H, Ruyter E. Permeability of different types of medical protective gloves to acrylic monomers. Eur J Oral Sci. 2003;111:440-446.
- Sananez A, Sanchez A, Davis L, et al. Allergic reaction from dental bonding material through nitrile gloves: clinical case study and glove permeability testing. J Esthet Restor Dent. 2020;32:371-379.
- Andersson T, Bruze M, Björkner B. In vivo testing of the protection of gloves against acrylates in dentin-bonding systems on patients with known contact allergy to acrylates. Contact Dermatitis. 1999;41:254-259.
- Roche E, Cuadra J, Alegre V. Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases. Actas Dermo-Sifiliográficas. 2009;99:788-794.
Practice Points
- Acrylates are thermoplastic resins used in a variety of products ranging from cosmetics to adhesives and industrial materials. Acrylic monomers are strong contact allergens, whereas fully polymerized forms are inert, provided they are completely cured.
- The use of home gel nail kits may increase the risk for sensitization to acrylates, which are the most common modern nail cosmetic allergens.
- When patch testing for suspected acrylate allergy, 2-hydroxyethyl methacrylate (HEMA) is the most important screening allergen. Expanded testing to additional acrylates should be considered depending on the clinical scenario.
Lanolin: The 2023 American Contact Dermatitis Society Allergen of the Year
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
Practice Points
- Lanolin is a common ingredient in personal care products (PCPs), cosmetics, topical medicaments, and industrial materials.
- Allergic contact dermatitis to lanolin appears to be most common in patients with stasis dermatitis, chronic leg ulcers, atopic dermatitis, and perianal/genital dermatitis.
- There is no single best lanolin patch test formulation. Patch testing and repeat open application testing to PCPs containing lanolin also may be of benefit.
Is Laundry Detergent a Common Cause of Allergic Contact Dermatitis?
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13
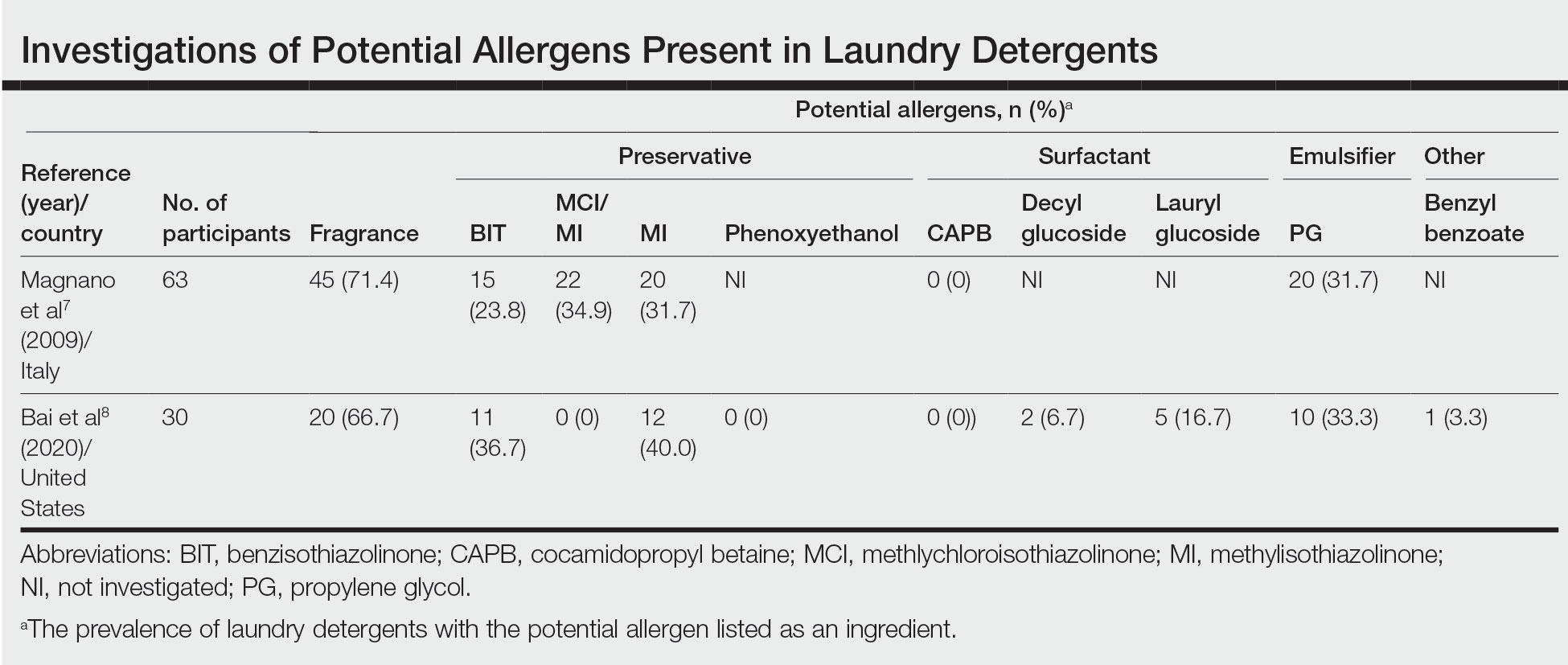
Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Practice Points
- Although laundry detergent commonly is believed to be a cause of allergic contact dermatitis (ACD), the actual prevalence is quite low (<1%).
- Common allergens present in laundry detergent such as fragrances and isothiazolinone preservatives likely are reduced to clinically irrelevant levels during routine machine washing.
- Other diagnoses to consider when laundry detergent–associated ACD is suspected include textile ACD, atopic dermatitis, and cutaneous T-cell lymphoma.
Mpox (Monkeypox) Clinical Pearls
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13
A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
A Dermatology Hospitalist Team’s Response to the Inpatient Consult Flowchart
To the Editor:
We read with interest the Cutis article by Dobkin et al1 (Cutis. 2022;109:218-220) regarding guidelines for inpatient and emergency department dermatology consultations. We agree with the authors that dermatology training is lacking in other medical specialties, which makes it challenging for teams to assess the appropriateness of a dermatology consultation in the inpatient setting. Inpatient dermatology consultation can be utilized in a hospital system to aid in rapid and accurate diagnosis, avoid inappropriate therapies, and decrease length of stay2 and readmission rates3 while providing education to the primary teams. This is precisely why in many instances the availability of inpatient dermatology consultation is so important because nondermatologists often are unable to determine whether a rash is life-threatening, benign, or something in between. From the perspective of dermatology hospitalists, there is room for improvement in the flowchart Dobkin et al1 presented to guide inpatient dermatology consultation.
To have a productive relationship with our internal medicine, surgery, pediatrics, psychiatry, and other hospital-based colleagues, we must keep an open mind when a consultation is received. We feel that the flowchart proposed by Dobkin et al1 presents too narrow a viewpoint on the utility of inpatient dermatology. It rests on assertions that other teams will be able to determine the appropriate dermatologic diagnosis without involving a dermatologist, which often is not the case.
We disagree with several recommendations in the flowchart, the first being the assertion that patients who are “hemodynamically unstable due to [a] nondermatologic problem (eg, intubated on pressors, febrile, and hypotensive)” are not appropriate for inpatient dermatology consultation.1 Although dermatologists do not commonly encounter patients with critical illness in the outpatient clinic, dermatology consultation can be extremely helpful and even lifesaving in the inpatient setting. It is unrealistic to expect the primary teams to know whether cutaneous manifestations potentially could be related to the patient’s overall clinical picture. On the contrary, we would encourage the primary team in charge of a hemodynamically unstable patient to consult dermatology at the first sign of an unexplained rash. Take for example an acutely ill patient who develops retiform purpura. There are well-established dermatology guidelines for the workup of retiform purpura,4 including prompt biopsy and assessment of broad, potentially life-threatening differential diagnoses from calciphylaxis to angioinvasive fungal infection. In this scenario, the dermatology consultant may render the correct diagnosis and recommend immediate treatment that could be lifesaving.
Secondly, we do not agree with the recommendation that a patient in hospice care is not appropriate for inpatient dermatology consultation. Patients receiving hospice or palliative care have high rates of potentially symptomatic cutaneous diseases,5 including intertrigo and dermatitis—comprising stasis, seborrheic, and contact dermatitis.6 Although aggressive intervention for asymptomatic benign or malignant skin conditions may not be in line with their goals of care, an inpatient dermatology consultation can reduce symptoms and improve quality of life. This population also is one that is unlikely to be able to attend an outpatient dermatology clinic appointment and therefore are good candidates for inpatient consultation.
Lastly, we want to highlight the difference between a stable chronic dermatologic disease and an acute flare that may occur while a patient is hospitalized, regardless of whether it is the reason for admission. For example, a patient with psoriasis affecting limited body surface area who is hospitalized for a myocardial infarction is not appropriate for a dermatology consultation. However, if that same patient develops erythroderma while they are hospitalized for cardiac monitoring, it would certainly be appropriate for dermatology to be consulted. Additionally, there are times when a chronic skin disease is the reason for hospitalization; dermatology, although technically a consulting service, would be the primary decision-maker for the patient’s care in this situation. In these scenarios, it is important for the patient to be able to establish care for long-term outpatient management of their condition; however, it is prudent to involve dermatology while the patient is acutely hospitalized to guide their treatment plan until they are able to see a dermatologist after discharge.
In conclusion, we believe that hospital dermatology is a valuable tool that can be utilized in many different scenarios. Although there are certainly situations more appropriate for outpatient dermatology referral, we would caution against overly simplified algorithms that could discourage valuable inpatient dermatology consultations. It often is worth a conversation with your dermatology consultant (when available at an institution) to determine the best course of action for each patient. Additionally, we recognize the need for more formalized guidelines on when to pursue inpatient dermatology consultation. We are members of the Society of Dermatology Hospitalists and encourage readers to reference their website, which provides additional resources on inpatient dermatology (https://societydermatologyhospitalists.com/inpatient-dermatology-literature/).
Authors’ Response
We appreciate the letter in response to our commentary on the appropriateness of inpatient dermatology consultations. It is the continued refining and re-evaluation of concepts such as these that allow our field to grow and improve knowledge and patient care.
We sought to provide a nonpatronizing yet simple consultation flowchart that would help guide triage of patients in need or not in need of dermatologic evaluation by the inpatient teams. Understandably, the impressions of our flowchart have been variable based on different readers’ medical backgrounds and experiences. It is certainly possible that our flowchart lacked certain exceptions and oversimplified certain concepts, and we welcome further refining of this flowchart to better guide inpatient dermatology consultations.
We do, however, disagree that the primary team would not know whether a patient is intubated in the intensive care unit for a dermatology reason. If the patient is in such a status, it would be pertinent for the primary team to conduct a timely workup that could include consultations until a diagnosis is made. We were not implying that every dermatology consultation in the intensive care unit is unwarranted, especially if it can lead to a primary dermatologic diagnosis. We do believe that a thorough history could elicit an allergy or other chronic skin condition that could save resources and spending within a hospital. Likewise, psoriasis comes in many different presentations, and although we do not believe a consultation for chronic psoriatic plaques is appropriate in the hospital, it is absolutely appropriate for a patient who is erythrodermic from any cause.
Our flowchart was intended to be the first step to providing education on when consultations are appropriate, and further refinement will be necessary.
Hershel Dobkin, MD; Timothy Blackwell, BS; Robin Ashinoff, MD
Drs. Dobkin and Ashinoff are from Hackensack University Medical Center, New Jersey. Mr. Blackwell is from the Rowan University School of Osteopathic Medicine, Stratford, New Jersey.
The authors report no conflict of interest.
Correspondence: Hershel Dobkin, MD, Hackensack University Medical Center, 30 Prospect Ave, Hackensack, NJ 07601 (hersheldobkinpublic@gmail.com).
- Dobkin H, Blackwell T, Ashinoff R. When are inpatient and emergency dermatologic consultations appropriate? Cutis. 2022;109:218-220. doi:10.12788/cutis.0492
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536. doi:10.1001/jamadermatol.2017.6196
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Pisano C, Paladichuk H, Keeling B. Dermatology in palliative medicine [published online October 14, 2021]. BMJ Support Palliat Care. doi:10.1136/bmjspcare-2021-003342
- Barnabé C, Daeninck P. “Beauty is only skin deep”: prevalence of dermatologic disease on a palliative care unit. J Pain Symptom Manage. 2005;29:419-422. doi:10.1016/j.jpainsymman.2004.08.009
To the Editor:
We read with interest the Cutis article by Dobkin et al1 (Cutis. 2022;109:218-220) regarding guidelines for inpatient and emergency department dermatology consultations. We agree with the authors that dermatology training is lacking in other medical specialties, which makes it challenging for teams to assess the appropriateness of a dermatology consultation in the inpatient setting. Inpatient dermatology consultation can be utilized in a hospital system to aid in rapid and accurate diagnosis, avoid inappropriate therapies, and decrease length of stay2 and readmission rates3 while providing education to the primary teams. This is precisely why in many instances the availability of inpatient dermatology consultation is so important because nondermatologists often are unable to determine whether a rash is life-threatening, benign, or something in between. From the perspective of dermatology hospitalists, there is room for improvement in the flowchart Dobkin et al1 presented to guide inpatient dermatology consultation.
To have a productive relationship with our internal medicine, surgery, pediatrics, psychiatry, and other hospital-based colleagues, we must keep an open mind when a consultation is received. We feel that the flowchart proposed by Dobkin et al1 presents too narrow a viewpoint on the utility of inpatient dermatology. It rests on assertions that other teams will be able to determine the appropriate dermatologic diagnosis without involving a dermatologist, which often is not the case.
We disagree with several recommendations in the flowchart, the first being the assertion that patients who are “hemodynamically unstable due to [a] nondermatologic problem (eg, intubated on pressors, febrile, and hypotensive)” are not appropriate for inpatient dermatology consultation.1 Although dermatologists do not commonly encounter patients with critical illness in the outpatient clinic, dermatology consultation can be extremely helpful and even lifesaving in the inpatient setting. It is unrealistic to expect the primary teams to know whether cutaneous manifestations potentially could be related to the patient’s overall clinical picture. On the contrary, we would encourage the primary team in charge of a hemodynamically unstable patient to consult dermatology at the first sign of an unexplained rash. Take for example an acutely ill patient who develops retiform purpura. There are well-established dermatology guidelines for the workup of retiform purpura,4 including prompt biopsy and assessment of broad, potentially life-threatening differential diagnoses from calciphylaxis to angioinvasive fungal infection. In this scenario, the dermatology consultant may render the correct diagnosis and recommend immediate treatment that could be lifesaving.
Secondly, we do not agree with the recommendation that a patient in hospice care is not appropriate for inpatient dermatology consultation. Patients receiving hospice or palliative care have high rates of potentially symptomatic cutaneous diseases,5 including intertrigo and dermatitis—comprising stasis, seborrheic, and contact dermatitis.6 Although aggressive intervention for asymptomatic benign or malignant skin conditions may not be in line with their goals of care, an inpatient dermatology consultation can reduce symptoms and improve quality of life. This population also is one that is unlikely to be able to attend an outpatient dermatology clinic appointment and therefore are good candidates for inpatient consultation.
Lastly, we want to highlight the difference between a stable chronic dermatologic disease and an acute flare that may occur while a patient is hospitalized, regardless of whether it is the reason for admission. For example, a patient with psoriasis affecting limited body surface area who is hospitalized for a myocardial infarction is not appropriate for a dermatology consultation. However, if that same patient develops erythroderma while they are hospitalized for cardiac monitoring, it would certainly be appropriate for dermatology to be consulted. Additionally, there are times when a chronic skin disease is the reason for hospitalization; dermatology, although technically a consulting service, would be the primary decision-maker for the patient’s care in this situation. In these scenarios, it is important for the patient to be able to establish care for long-term outpatient management of their condition; however, it is prudent to involve dermatology while the patient is acutely hospitalized to guide their treatment plan until they are able to see a dermatologist after discharge.
In conclusion, we believe that hospital dermatology is a valuable tool that can be utilized in many different scenarios. Although there are certainly situations more appropriate for outpatient dermatology referral, we would caution against overly simplified algorithms that could discourage valuable inpatient dermatology consultations. It often is worth a conversation with your dermatology consultant (when available at an institution) to determine the best course of action for each patient. Additionally, we recognize the need for more formalized guidelines on when to pursue inpatient dermatology consultation. We are members of the Society of Dermatology Hospitalists and encourage readers to reference their website, which provides additional resources on inpatient dermatology (https://societydermatologyhospitalists.com/inpatient-dermatology-literature/).
Authors’ Response
We appreciate the letter in response to our commentary on the appropriateness of inpatient dermatology consultations. It is the continued refining and re-evaluation of concepts such as these that allow our field to grow and improve knowledge and patient care.
We sought to provide a nonpatronizing yet simple consultation flowchart that would help guide triage of patients in need or not in need of dermatologic evaluation by the inpatient teams. Understandably, the impressions of our flowchart have been variable based on different readers’ medical backgrounds and experiences. It is certainly possible that our flowchart lacked certain exceptions and oversimplified certain concepts, and we welcome further refining of this flowchart to better guide inpatient dermatology consultations.
We do, however, disagree that the primary team would not know whether a patient is intubated in the intensive care unit for a dermatology reason. If the patient is in such a status, it would be pertinent for the primary team to conduct a timely workup that could include consultations until a diagnosis is made. We were not implying that every dermatology consultation in the intensive care unit is unwarranted, especially if it can lead to a primary dermatologic diagnosis. We do believe that a thorough history could elicit an allergy or other chronic skin condition that could save resources and spending within a hospital. Likewise, psoriasis comes in many different presentations, and although we do not believe a consultation for chronic psoriatic plaques is appropriate in the hospital, it is absolutely appropriate for a patient who is erythrodermic from any cause.
Our flowchart was intended to be the first step to providing education on when consultations are appropriate, and further refinement will be necessary.
Hershel Dobkin, MD; Timothy Blackwell, BS; Robin Ashinoff, MD
Drs. Dobkin and Ashinoff are from Hackensack University Medical Center, New Jersey. Mr. Blackwell is from the Rowan University School of Osteopathic Medicine, Stratford, New Jersey.
The authors report no conflict of interest.
Correspondence: Hershel Dobkin, MD, Hackensack University Medical Center, 30 Prospect Ave, Hackensack, NJ 07601 (hersheldobkinpublic@gmail.com).
To the Editor:
We read with interest the Cutis article by Dobkin et al1 (Cutis. 2022;109:218-220) regarding guidelines for inpatient and emergency department dermatology consultations. We agree with the authors that dermatology training is lacking in other medical specialties, which makes it challenging for teams to assess the appropriateness of a dermatology consultation in the inpatient setting. Inpatient dermatology consultation can be utilized in a hospital system to aid in rapid and accurate diagnosis, avoid inappropriate therapies, and decrease length of stay2 and readmission rates3 while providing education to the primary teams. This is precisely why in many instances the availability of inpatient dermatology consultation is so important because nondermatologists often are unable to determine whether a rash is life-threatening, benign, or something in between. From the perspective of dermatology hospitalists, there is room for improvement in the flowchart Dobkin et al1 presented to guide inpatient dermatology consultation.
To have a productive relationship with our internal medicine, surgery, pediatrics, psychiatry, and other hospital-based colleagues, we must keep an open mind when a consultation is received. We feel that the flowchart proposed by Dobkin et al1 presents too narrow a viewpoint on the utility of inpatient dermatology. It rests on assertions that other teams will be able to determine the appropriate dermatologic diagnosis without involving a dermatologist, which often is not the case.
We disagree with several recommendations in the flowchart, the first being the assertion that patients who are “hemodynamically unstable due to [a] nondermatologic problem (eg, intubated on pressors, febrile, and hypotensive)” are not appropriate for inpatient dermatology consultation.1 Although dermatologists do not commonly encounter patients with critical illness in the outpatient clinic, dermatology consultation can be extremely helpful and even lifesaving in the inpatient setting. It is unrealistic to expect the primary teams to know whether cutaneous manifestations potentially could be related to the patient’s overall clinical picture. On the contrary, we would encourage the primary team in charge of a hemodynamically unstable patient to consult dermatology at the first sign of an unexplained rash. Take for example an acutely ill patient who develops retiform purpura. There are well-established dermatology guidelines for the workup of retiform purpura,4 including prompt biopsy and assessment of broad, potentially life-threatening differential diagnoses from calciphylaxis to angioinvasive fungal infection. In this scenario, the dermatology consultant may render the correct diagnosis and recommend immediate treatment that could be lifesaving.
Secondly, we do not agree with the recommendation that a patient in hospice care is not appropriate for inpatient dermatology consultation. Patients receiving hospice or palliative care have high rates of potentially symptomatic cutaneous diseases,5 including intertrigo and dermatitis—comprising stasis, seborrheic, and contact dermatitis.6 Although aggressive intervention for asymptomatic benign or malignant skin conditions may not be in line with their goals of care, an inpatient dermatology consultation can reduce symptoms and improve quality of life. This population also is one that is unlikely to be able to attend an outpatient dermatology clinic appointment and therefore are good candidates for inpatient consultation.
Lastly, we want to highlight the difference between a stable chronic dermatologic disease and an acute flare that may occur while a patient is hospitalized, regardless of whether it is the reason for admission. For example, a patient with psoriasis affecting limited body surface area who is hospitalized for a myocardial infarction is not appropriate for a dermatology consultation. However, if that same patient develops erythroderma while they are hospitalized for cardiac monitoring, it would certainly be appropriate for dermatology to be consulted. Additionally, there are times when a chronic skin disease is the reason for hospitalization; dermatology, although technically a consulting service, would be the primary decision-maker for the patient’s care in this situation. In these scenarios, it is important for the patient to be able to establish care for long-term outpatient management of their condition; however, it is prudent to involve dermatology while the patient is acutely hospitalized to guide their treatment plan until they are able to see a dermatologist after discharge.
In conclusion, we believe that hospital dermatology is a valuable tool that can be utilized in many different scenarios. Although there are certainly situations more appropriate for outpatient dermatology referral, we would caution against overly simplified algorithms that could discourage valuable inpatient dermatology consultations. It often is worth a conversation with your dermatology consultant (when available at an institution) to determine the best course of action for each patient. Additionally, we recognize the need for more formalized guidelines on when to pursue inpatient dermatology consultation. We are members of the Society of Dermatology Hospitalists and encourage readers to reference their website, which provides additional resources on inpatient dermatology (https://societydermatologyhospitalists.com/inpatient-dermatology-literature/).
Authors’ Response
We appreciate the letter in response to our commentary on the appropriateness of inpatient dermatology consultations. It is the continued refining and re-evaluation of concepts such as these that allow our field to grow and improve knowledge and patient care.
We sought to provide a nonpatronizing yet simple consultation flowchart that would help guide triage of patients in need or not in need of dermatologic evaluation by the inpatient teams. Understandably, the impressions of our flowchart have been variable based on different readers’ medical backgrounds and experiences. It is certainly possible that our flowchart lacked certain exceptions and oversimplified certain concepts, and we welcome further refining of this flowchart to better guide inpatient dermatology consultations.
We do, however, disagree that the primary team would not know whether a patient is intubated in the intensive care unit for a dermatology reason. If the patient is in such a status, it would be pertinent for the primary team to conduct a timely workup that could include consultations until a diagnosis is made. We were not implying that every dermatology consultation in the intensive care unit is unwarranted, especially if it can lead to a primary dermatologic diagnosis. We do believe that a thorough history could elicit an allergy or other chronic skin condition that could save resources and spending within a hospital. Likewise, psoriasis comes in many different presentations, and although we do not believe a consultation for chronic psoriatic plaques is appropriate in the hospital, it is absolutely appropriate for a patient who is erythrodermic from any cause.
Our flowchart was intended to be the first step to providing education on when consultations are appropriate, and further refinement will be necessary.
Hershel Dobkin, MD; Timothy Blackwell, BS; Robin Ashinoff, MD
Drs. Dobkin and Ashinoff are from Hackensack University Medical Center, New Jersey. Mr. Blackwell is from the Rowan University School of Osteopathic Medicine, Stratford, New Jersey.
The authors report no conflict of interest.
Correspondence: Hershel Dobkin, MD, Hackensack University Medical Center, 30 Prospect Ave, Hackensack, NJ 07601 (hersheldobkinpublic@gmail.com).
- Dobkin H, Blackwell T, Ashinoff R. When are inpatient and emergency dermatologic consultations appropriate? Cutis. 2022;109:218-220. doi:10.12788/cutis.0492
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536. doi:10.1001/jamadermatol.2017.6196
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Pisano C, Paladichuk H, Keeling B. Dermatology in palliative medicine [published online October 14, 2021]. BMJ Support Palliat Care. doi:10.1136/bmjspcare-2021-003342
- Barnabé C, Daeninck P. “Beauty is only skin deep”: prevalence of dermatologic disease on a palliative care unit. J Pain Symptom Manage. 2005;29:419-422. doi:10.1016/j.jpainsymman.2004.08.009
- Dobkin H, Blackwell T, Ashinoff R. When are inpatient and emergency dermatologic consultations appropriate? Cutis. 2022;109:218-220. doi:10.12788/cutis.0492
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536. doi:10.1001/jamadermatol.2017.6196
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Pisano C, Paladichuk H, Keeling B. Dermatology in palliative medicine [published online October 14, 2021]. BMJ Support Palliat Care. doi:10.1136/bmjspcare-2021-003342
- Barnabé C, Daeninck P. “Beauty is only skin deep”: prevalence of dermatologic disease on a palliative care unit. J Pain Symptom Manage. 2005;29:419-422. doi:10.1016/j.jpainsymman.2004.08.009
Janus Kinase Inhibitors: A Promising Therapeutic Option for Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29
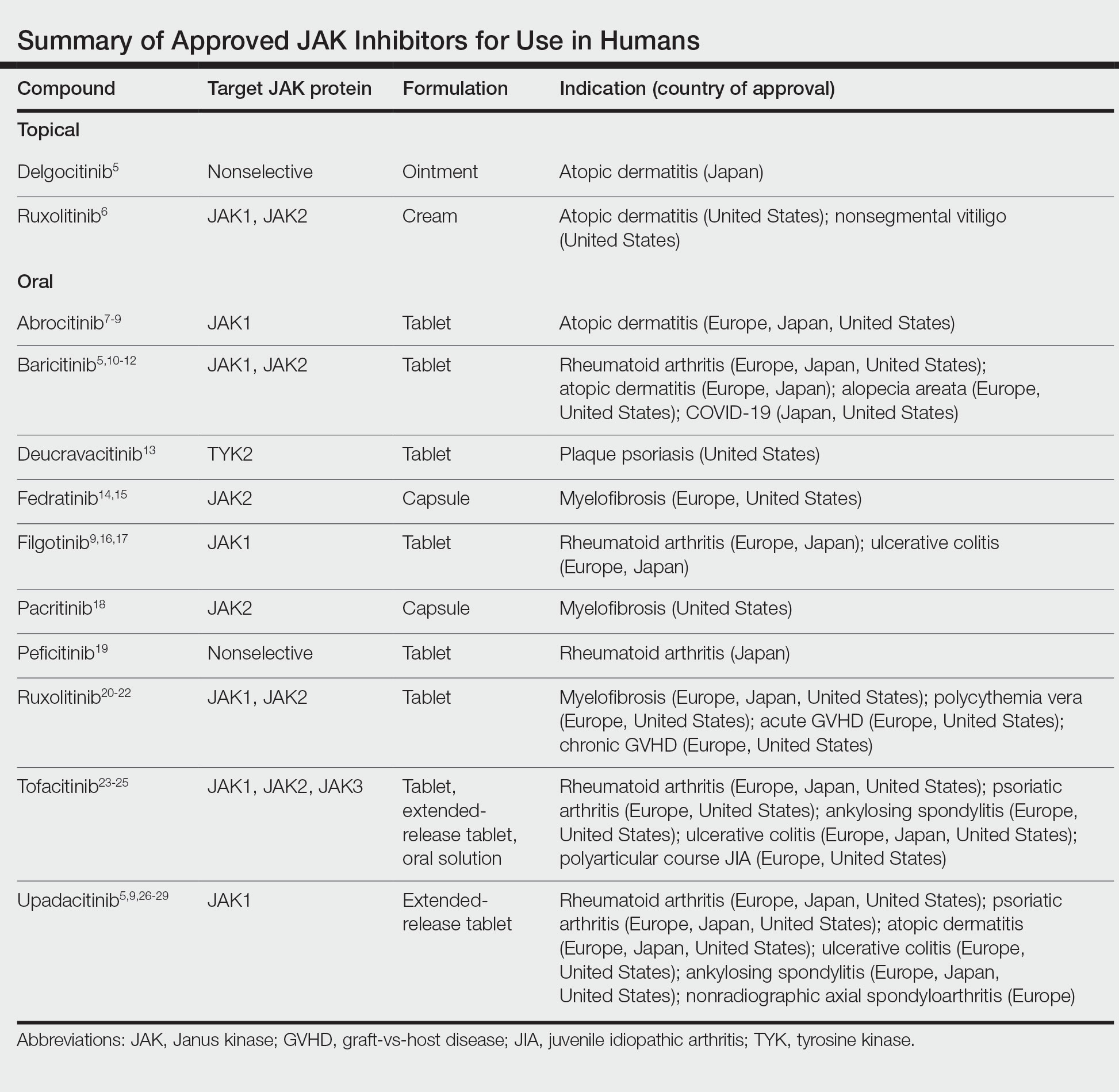
Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
PRACTICE POINTS
- Janus kinase (JAK) inhibitors are a novel class of small molecule inhibitors that modulate the JAK/signal transducer and activator of transcription signaling pathway.
- Select JAK inhibitors have been approved by the US Food and Drug Administration for the management of atopic dermatitis. Their use in allergic contact dermatitis is under active investigation.
- Regular follow-up and laboratory monitoring for patients on oral JAK inhibitors is recommended, given the potential for treatment-related adverse effects.