User login
Lessons From Statin Failure in COPD
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
Monoclonal antibody attacks new asthma target
SAN DIEGO – An investigational human monoclonal antibody suggested a new approach to asthma treatment by showing evidence of anti-inflammatory activity and partial attenuation of early and late asthmatic responses in a proof-of-concept study of 31 patients with mild asthma.
Patients were randomized to intravenous treatment with AMG 157 or placebo for up to 3 months before undergoing allergen-induced challenge and testing. AMG 157 binds human thymic stromal lymphopoietin (TSLP), an epithelial-cell–derived cytokine that might be important in initiating allergic inflammation, and prevents receptor interaction.
By day 84, the maximum percentage decrease in the forced expiratory volume at 1 second (FEV1) during late asthmatic response (3-7 hours after allergy challenge) was 46% smaller in the treatment group, compared with placebo, a significant difference, Gail M. Gauvreau, Ph.D., and her associates reported at an international conference of the American Thoracic Society.
Among secondary outcomes, the area under the curve (AUC) of the time-adjusted percent decrease in FEV1 during the early asthmatic response was significantly smaller in the treatment group than the control group on day 84. Patients who got AMG 157 showed significantly lower levels of blood and sputum eosinophils before and after allergy challenge and in the fraction of exhaled nitric oxide.
Adverse events were reported in 15 patients on AMG 157 and 12 patients on placebo. No serious adverse events were seen, reported Dr. Gauvreau of the Firestone Institute of Respiratory Health at McMaster University, Hamilton, Ontario.
The study was published online by the New England Journal of Medicine (2014 May 20 [doi:10.1056/NEJMoa1402895]). "This proof-of-concept study suggests that TSLP is a pivotal cytokine not only in allergen-induced airway responses but also in persistent airway inflammation in patients with allergic asthma," the investigators wrote.
The company that is developing AMG 157 has started a phase II clinical trial, Dr. Gauvreau said in an interview at the meeting.
The population of mild asthmatics in the proof-of-concept study are not the kind of patients who might use this treatment, she said, but this potential treatment pathway offers hope for a new therapy for patients with steroid-resistant asthma. "They use an awful lot of resources and costs in terms of hospital emergency room care. They are the folks who have an unmet need to treat their asthma," she said.
Amgen, which is developing AMG 157, funded the study and analyzed the data. Dr. Gauvreau reported financial associations with Amgen and three other companies. Fourteen of her coinvestigators reported financial associations with Amgen and multiple other companies.
The magnitude of the effect of AMG 157 was sizable, similar to what could be achieved in allergen-induced asthma studies by blocking established asthma mediators such as the cysteinyl leukotrienes, Dr. Sven-Erik Dahlén wrote in an editorial accompanying Dr. Gauvreau’s article (N. Engl. J. Med.; 2014 May 20 [doi:10.1056/NEJMe1404737]).
The evidence of an effect from this highly selective anti-thymic stromal lymphopoietin [TSLP] antibody on both the early and late asthmatic responses might be because it directly inhibits interleukin-4 or interleukin-13 (two downstream cytokines induced by TSLP), this study and previous data suggest. There may be two ways to inhibit allergen-induced asthmatic responses, he suggested – direct antagonism of mast-cell mediators to block acute response, or long-term inhibition of regulatory networks. TSLP may be a "master switch" in the signaling between airway epithelium and other inflammatory cascades. Or, it may be part of action by several parallel pathways involving interleukins.
AMG 157 has "earned our respect" in this study, but it’s unclear whether this strategy will succeed in treating patients with asthma, Dr. Dahlén wrote. "We will first have to unravel the biologic mechanisms involved in this unexpected effect of AMG 157 and show why TSLP signaling" contributes to asthmatic responses.
Dr. Dahlén is a professor at the Center for Allergy Research, Institute of Environmental Medicine, Karolinska Institutet, Stockholm. He reported a financial association with AstraZeneca unrelated to this study.
The magnitude of the effect of AMG 157 was sizable, similar to what could be achieved in allergen-induced asthma studies by blocking established asthma mediators such as the cysteinyl leukotrienes, Dr. Sven-Erik Dahlén wrote in an editorial accompanying Dr. Gauvreau’s article (N. Engl. J. Med.; 2014 May 20 [doi:10.1056/NEJMe1404737]).
The evidence of an effect from this highly selective anti-thymic stromal lymphopoietin [TSLP] antibody on both the early and late asthmatic responses might be because it directly inhibits interleukin-4 or interleukin-13 (two downstream cytokines induced by TSLP), this study and previous data suggest. There may be two ways to inhibit allergen-induced asthmatic responses, he suggested – direct antagonism of mast-cell mediators to block acute response, or long-term inhibition of regulatory networks. TSLP may be a "master switch" in the signaling between airway epithelium and other inflammatory cascades. Or, it may be part of action by several parallel pathways involving interleukins.
AMG 157 has "earned our respect" in this study, but it’s unclear whether this strategy will succeed in treating patients with asthma, Dr. Dahlén wrote. "We will first have to unravel the biologic mechanisms involved in this unexpected effect of AMG 157 and show why TSLP signaling" contributes to asthmatic responses.
Dr. Dahlén is a professor at the Center for Allergy Research, Institute of Environmental Medicine, Karolinska Institutet, Stockholm. He reported a financial association with AstraZeneca unrelated to this study.
The magnitude of the effect of AMG 157 was sizable, similar to what could be achieved in allergen-induced asthma studies by blocking established asthma mediators such as the cysteinyl leukotrienes, Dr. Sven-Erik Dahlén wrote in an editorial accompanying Dr. Gauvreau’s article (N. Engl. J. Med.; 2014 May 20 [doi:10.1056/NEJMe1404737]).
The evidence of an effect from this highly selective anti-thymic stromal lymphopoietin [TSLP] antibody on both the early and late asthmatic responses might be because it directly inhibits interleukin-4 or interleukin-13 (two downstream cytokines induced by TSLP), this study and previous data suggest. There may be two ways to inhibit allergen-induced asthmatic responses, he suggested – direct antagonism of mast-cell mediators to block acute response, or long-term inhibition of regulatory networks. TSLP may be a "master switch" in the signaling between airway epithelium and other inflammatory cascades. Or, it may be part of action by several parallel pathways involving interleukins.
AMG 157 has "earned our respect" in this study, but it’s unclear whether this strategy will succeed in treating patients with asthma, Dr. Dahlén wrote. "We will first have to unravel the biologic mechanisms involved in this unexpected effect of AMG 157 and show why TSLP signaling" contributes to asthmatic responses.
Dr. Dahlén is a professor at the Center for Allergy Research, Institute of Environmental Medicine, Karolinska Institutet, Stockholm. He reported a financial association with AstraZeneca unrelated to this study.
SAN DIEGO – An investigational human monoclonal antibody suggested a new approach to asthma treatment by showing evidence of anti-inflammatory activity and partial attenuation of early and late asthmatic responses in a proof-of-concept study of 31 patients with mild asthma.
Patients were randomized to intravenous treatment with AMG 157 or placebo for up to 3 months before undergoing allergen-induced challenge and testing. AMG 157 binds human thymic stromal lymphopoietin (TSLP), an epithelial-cell–derived cytokine that might be important in initiating allergic inflammation, and prevents receptor interaction.
By day 84, the maximum percentage decrease in the forced expiratory volume at 1 second (FEV1) during late asthmatic response (3-7 hours after allergy challenge) was 46% smaller in the treatment group, compared with placebo, a significant difference, Gail M. Gauvreau, Ph.D., and her associates reported at an international conference of the American Thoracic Society.
Among secondary outcomes, the area under the curve (AUC) of the time-adjusted percent decrease in FEV1 during the early asthmatic response was significantly smaller in the treatment group than the control group on day 84. Patients who got AMG 157 showed significantly lower levels of blood and sputum eosinophils before and after allergy challenge and in the fraction of exhaled nitric oxide.
Adverse events were reported in 15 patients on AMG 157 and 12 patients on placebo. No serious adverse events were seen, reported Dr. Gauvreau of the Firestone Institute of Respiratory Health at McMaster University, Hamilton, Ontario.
The study was published online by the New England Journal of Medicine (2014 May 20 [doi:10.1056/NEJMoa1402895]). "This proof-of-concept study suggests that TSLP is a pivotal cytokine not only in allergen-induced airway responses but also in persistent airway inflammation in patients with allergic asthma," the investigators wrote.
The company that is developing AMG 157 has started a phase II clinical trial, Dr. Gauvreau said in an interview at the meeting.
The population of mild asthmatics in the proof-of-concept study are not the kind of patients who might use this treatment, she said, but this potential treatment pathway offers hope for a new therapy for patients with steroid-resistant asthma. "They use an awful lot of resources and costs in terms of hospital emergency room care. They are the folks who have an unmet need to treat their asthma," she said.
Amgen, which is developing AMG 157, funded the study and analyzed the data. Dr. Gauvreau reported financial associations with Amgen and three other companies. Fourteen of her coinvestigators reported financial associations with Amgen and multiple other companies.
SAN DIEGO – An investigational human monoclonal antibody suggested a new approach to asthma treatment by showing evidence of anti-inflammatory activity and partial attenuation of early and late asthmatic responses in a proof-of-concept study of 31 patients with mild asthma.
Patients were randomized to intravenous treatment with AMG 157 or placebo for up to 3 months before undergoing allergen-induced challenge and testing. AMG 157 binds human thymic stromal lymphopoietin (TSLP), an epithelial-cell–derived cytokine that might be important in initiating allergic inflammation, and prevents receptor interaction.
By day 84, the maximum percentage decrease in the forced expiratory volume at 1 second (FEV1) during late asthmatic response (3-7 hours after allergy challenge) was 46% smaller in the treatment group, compared with placebo, a significant difference, Gail M. Gauvreau, Ph.D., and her associates reported at an international conference of the American Thoracic Society.
Among secondary outcomes, the area under the curve (AUC) of the time-adjusted percent decrease in FEV1 during the early asthmatic response was significantly smaller in the treatment group than the control group on day 84. Patients who got AMG 157 showed significantly lower levels of blood and sputum eosinophils before and after allergy challenge and in the fraction of exhaled nitric oxide.
Adverse events were reported in 15 patients on AMG 157 and 12 patients on placebo. No serious adverse events were seen, reported Dr. Gauvreau of the Firestone Institute of Respiratory Health at McMaster University, Hamilton, Ontario.
The study was published online by the New England Journal of Medicine (2014 May 20 [doi:10.1056/NEJMoa1402895]). "This proof-of-concept study suggests that TSLP is a pivotal cytokine not only in allergen-induced airway responses but also in persistent airway inflammation in patients with allergic asthma," the investigators wrote.
The company that is developing AMG 157 has started a phase II clinical trial, Dr. Gauvreau said in an interview at the meeting.
The population of mild asthmatics in the proof-of-concept study are not the kind of patients who might use this treatment, she said, but this potential treatment pathway offers hope for a new therapy for patients with steroid-resistant asthma. "They use an awful lot of resources and costs in terms of hospital emergency room care. They are the folks who have an unmet need to treat their asthma," she said.
Amgen, which is developing AMG 157, funded the study and analyzed the data. Dr. Gauvreau reported financial associations with Amgen and three other companies. Fourteen of her coinvestigators reported financial associations with Amgen and multiple other companies.
AT ATS 2014
Key clinical point: A proof-of-concept study suggests that there may be a new asthma treatment pathway, to be tested next in a phase II clinical trial.
Major finding: The maximum percentage decrease in the FEV1 3-7 hours after allergy challenge was 46% smaller in the treatment group, compared with placebo on day 84 (P = .02)
Data source: A double-blind, placebo-controlled study of 31 patients with mild asthma treated with 3 months of intravenous AMG 157 or placebo assessed after allergen challenge.
Disclosures: Amgen, which is developing AMG 157, funded the study and analyzed the data. Dr. Gauvreau reported financial associations with Amgen and three other companies. Fourteen of her coinvestigators reported financial associations with Amgen and multiple other companies.
VIDEO: Statins flop in COPD, but hope remains
SAN DIEGO – Two randomized, controlled trials presented at the American Thoracic Society international conference reported that statins did not improve pulmonary function or reduce exacerbations in patients with chronic obstructive pulmonary disease. But not everyone has given up on statins.
When a drug that showed promise in large observational studies doesn’t pan out in randomized, controlled trials, physicians start looking for subgroups of patients that still might benefit, Dr. Nicholas Gross told us. Hear his perspective on the STATCOPE trial (in which simvastatin did not prevent exacerbations in moderate to severe COPD) and the RODEO trial (in which rosuvastatin improved endothelial function only in a subset of patients with evidence of systemic inflammation.)
Dr. Gross is an emeritus professor of medicine and molecular biochemistry at Stritch-Loyola University, Chicago. He reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – Two randomized, controlled trials presented at the American Thoracic Society international conference reported that statins did not improve pulmonary function or reduce exacerbations in patients with chronic obstructive pulmonary disease. But not everyone has given up on statins.
When a drug that showed promise in large observational studies doesn’t pan out in randomized, controlled trials, physicians start looking for subgroups of patients that still might benefit, Dr. Nicholas Gross told us. Hear his perspective on the STATCOPE trial (in which simvastatin did not prevent exacerbations in moderate to severe COPD) and the RODEO trial (in which rosuvastatin improved endothelial function only in a subset of patients with evidence of systemic inflammation.)
Dr. Gross is an emeritus professor of medicine and molecular biochemistry at Stritch-Loyola University, Chicago. He reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – Two randomized, controlled trials presented at the American Thoracic Society international conference reported that statins did not improve pulmonary function or reduce exacerbations in patients with chronic obstructive pulmonary disease. But not everyone has given up on statins.
When a drug that showed promise in large observational studies doesn’t pan out in randomized, controlled trials, physicians start looking for subgroups of patients that still might benefit, Dr. Nicholas Gross told us. Hear his perspective on the STATCOPE trial (in which simvastatin did not prevent exacerbations in moderate to severe COPD) and the RODEO trial (in which rosuvastatin improved endothelial function only in a subset of patients with evidence of systemic inflammation.)
Dr. Gross is an emeritus professor of medicine and molecular biochemistry at Stritch-Loyola University, Chicago. He reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT ATS 2014
VIDEO: Experimental inhaler targets small airways in asthma
SAN DIEGO – An experimental inhalation system could help patients with asthma who are dependent on chronic oral corticosteroid therapy to reduce their doses of these steroids and potentially their side effects.
A phase II/III study suggests the system helped preserve lung function as oral corticosteroids were reduced, by delivering small budesonide to the peripheral lungs to target small airway inflammation in a randomized, controlled study of 199 patients. The study was presented by an employee of the system’s developer.
What we don’t know yet is whether the new system is better than existing technology such as metered dose inhalers for delivering small-molecule medications for asthma, or can be used in conjunction with them, Dr. Sally E. Wenzel told us in an interview.
Dr. Wenzel, professor of medicine at the University of Pittsburgh, does target the small airways when treating some patients. She provided perspective on this strategy and what may lie ahead.
Dr. Wenzel disclosed financial associations with multiple companies involved in asthma medications.
On Twitter @sherryboschert
SAN DIEGO – An experimental inhalation system could help patients with asthma who are dependent on chronic oral corticosteroid therapy to reduce their doses of these steroids and potentially their side effects.
A phase II/III study suggests the system helped preserve lung function as oral corticosteroids were reduced, by delivering small budesonide to the peripheral lungs to target small airway inflammation in a randomized, controlled study of 199 patients. The study was presented by an employee of the system’s developer.
What we don’t know yet is whether the new system is better than existing technology such as metered dose inhalers for delivering small-molecule medications for asthma, or can be used in conjunction with them, Dr. Sally E. Wenzel told us in an interview.
Dr. Wenzel, professor of medicine at the University of Pittsburgh, does target the small airways when treating some patients. She provided perspective on this strategy and what may lie ahead.
Dr. Wenzel disclosed financial associations with multiple companies involved in asthma medications.
On Twitter @sherryboschert
SAN DIEGO – An experimental inhalation system could help patients with asthma who are dependent on chronic oral corticosteroid therapy to reduce their doses of these steroids and potentially their side effects.
A phase II/III study suggests the system helped preserve lung function as oral corticosteroids were reduced, by delivering small budesonide to the peripheral lungs to target small airway inflammation in a randomized, controlled study of 199 patients. The study was presented by an employee of the system’s developer.
What we don’t know yet is whether the new system is better than existing technology such as metered dose inhalers for delivering small-molecule medications for asthma, or can be used in conjunction with them, Dr. Sally E. Wenzel told us in an interview.
Dr. Wenzel, professor of medicine at the University of Pittsburgh, does target the small airways when treating some patients. She provided perspective on this strategy and what may lie ahead.
Dr. Wenzel disclosed financial associations with multiple companies involved in asthma medications.
On Twitter @sherryboschert
AT THE ATS INTERNATIONAL CONFERENCE
VIDEO: Lessons from statin failure in COPD
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT THE ATS INTERNATIONAL CONFERENCE
Reference apps bring the evidence to you
There are at least two ways to consider the meaning of evidence-based apps. One is a category of apps that have clinical trial evidence showing that they are useful, a topic we cover regularly in this column.
Another category, though, is apps that take existing evidence-based guidelines or already-vetted medical information and deliver that to you in a more efficient, convenient package.
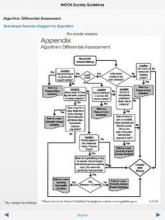
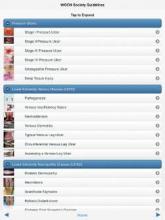
One of the latest examples of the latter is the new Evidence-Based Wound Care Guidelines and Fecal Ostomy Best Practice app, sold by the Wound, Ostomy and Continence Nurses Society (WOCN) for iPhones, iPads, or iPod Touch devices. Is it worth the $60 price?
It would cost you at least $160 to buy print copies from the WOCN of the five guidelines included in the app, on management of patients with pressure ulcers, lower-extremity arterial disease (LEAD), lower-extremity neuropathic disease (LEND), lower-extremity venous disease (LEVD), and fecal ostomy.
Or, you could spend a fair amount of time finding and printing the equivalent guidelines in the archives of medical journals or online. The Agency for Healthcare Research and Quality (AHRQ) provides detailed summaries of most of these guidelines on its website, including the major recommendations in each guideline and ratings of the evidence behind those recommendations. You can find the AHRQ pages on management of pressure ulcers, LEND, LEVD, and fecal ostomy, for example. They’re free, but it’s a time-consuming process that leaves you with bulky printouts.
Alternatively, the WOCN offers a free, 12-page, quick-reference guide for management of lower-extremity venous, arterial, or neuropathic wounds that you can download and print for free or, alternatively, consult without printing.
All in all, that’s a lot of dead trees to carry around if you want to consult these guidelines while treating patients, unless you have a bulky desktop computer handy. Compare that with the convenience of pulling out your smartphone or tablet to consult the literature on best practices at the bedside, and you can see why knowledge-delivering apps have caught physicians’ imaginations.
How can you know that an app is delivering credible information? The easiest way is to choose an app from a credible organization, such as the Cancer.Net app for patients with cancer, from the American Society of Clinical Oncology. Apps that have been vetted by the likes of the Cleveland Clinic, Mayo Clinic, or Veterans Affairs Healthcare System, for example, typically have been assessed with some quality-control measures. Every app has its flaws, however, so it’s usually worth searching for reviews online to gauge the strengths and weaknesses of various apps, especially when there is more than one available on a particular topic.
For-profit companies have developed some of the most popular evidence-driven apps for physicians. UpToDate claims that its physicians, authors, and editors synthesize the available medical evidence so the app can provide recommendations in primary care, ob.gyn., pediatrics, general surgery, emergency medicine, internal medicine, and all of the internal medicine subspecialties, among others. An individual subscription costs $499/year.
Epocrates offers a free app that provides medication information and lets you look up drug interactions and more, with additional services for a fee. Other apps do the same – Lexicomp is one subscription-based app that allows a 30-day free trial.
How to choose? Again, reviews may be helpful. The physician-run site iMedicalApps compared several medical drug reference apps in a 2010 review, but in app development time, that already seems light-years ago.
If you don’t recognize a company as a credible player in delivering mobile medical information, you can take several steps to assess the app’s trustworthiness. When you look at the app’s description online or in your mobile device’s app store, often the description is short and doesn’t list the reference information that the app relies on, though that’s often included in the app itself.
You’ll find reviews and ratings in the app description, but take these with a grain of salt. These can be anonymous, reflect anecdotal experiences, and may be planted by the developer of the app to make it look good.
Click on the tab for the developer in the app description, and if you don’t recognize the name, make sure there’s a link to a website or at least an e-mail address so you can assess whether the developer has health care expertise.
It’s also worth clicking on the Updates or Version History tab to see if it’s been updated, because good-quality apps usually get frequent updates, typically at least every 6 months, several app consultants said in interviews.
Are you already using evidence-delivering apps? Which are your favorites? Let us know, and we may highlight them in a future column.
On Twitter @sherryboschert
There are at least two ways to consider the meaning of evidence-based apps. One is a category of apps that have clinical trial evidence showing that they are useful, a topic we cover regularly in this column.
Another category, though, is apps that take existing evidence-based guidelines or already-vetted medical information and deliver that to you in a more efficient, convenient package.
One of the latest examples of the latter is the new Evidence-Based Wound Care Guidelines and Fecal Ostomy Best Practice app, sold by the Wound, Ostomy and Continence Nurses Society (WOCN) for iPhones, iPads, or iPod Touch devices. Is it worth the $60 price?
It would cost you at least $160 to buy print copies from the WOCN of the five guidelines included in the app, on management of patients with pressure ulcers, lower-extremity arterial disease (LEAD), lower-extremity neuropathic disease (LEND), lower-extremity venous disease (LEVD), and fecal ostomy.
Or, you could spend a fair amount of time finding and printing the equivalent guidelines in the archives of medical journals or online. The Agency for Healthcare Research and Quality (AHRQ) provides detailed summaries of most of these guidelines on its website, including the major recommendations in each guideline and ratings of the evidence behind those recommendations. You can find the AHRQ pages on management of pressure ulcers, LEND, LEVD, and fecal ostomy, for example. They’re free, but it’s a time-consuming process that leaves you with bulky printouts.
Alternatively, the WOCN offers a free, 12-page, quick-reference guide for management of lower-extremity venous, arterial, or neuropathic wounds that you can download and print for free or, alternatively, consult without printing.
All in all, that’s a lot of dead trees to carry around if you want to consult these guidelines while treating patients, unless you have a bulky desktop computer handy. Compare that with the convenience of pulling out your smartphone or tablet to consult the literature on best practices at the bedside, and you can see why knowledge-delivering apps have caught physicians’ imaginations.
How can you know that an app is delivering credible information? The easiest way is to choose an app from a credible organization, such as the Cancer.Net app for patients with cancer, from the American Society of Clinical Oncology. Apps that have been vetted by the likes of the Cleveland Clinic, Mayo Clinic, or Veterans Affairs Healthcare System, for example, typically have been assessed with some quality-control measures. Every app has its flaws, however, so it’s usually worth searching for reviews online to gauge the strengths and weaknesses of various apps, especially when there is more than one available on a particular topic.
For-profit companies have developed some of the most popular evidence-driven apps for physicians. UpToDate claims that its physicians, authors, and editors synthesize the available medical evidence so the app can provide recommendations in primary care, ob.gyn., pediatrics, general surgery, emergency medicine, internal medicine, and all of the internal medicine subspecialties, among others. An individual subscription costs $499/year.
Epocrates offers a free app that provides medication information and lets you look up drug interactions and more, with additional services for a fee. Other apps do the same – Lexicomp is one subscription-based app that allows a 30-day free trial.
How to choose? Again, reviews may be helpful. The physician-run site iMedicalApps compared several medical drug reference apps in a 2010 review, but in app development time, that already seems light-years ago.
If you don’t recognize a company as a credible player in delivering mobile medical information, you can take several steps to assess the app’s trustworthiness. When you look at the app’s description online or in your mobile device’s app store, often the description is short and doesn’t list the reference information that the app relies on, though that’s often included in the app itself.
You’ll find reviews and ratings in the app description, but take these with a grain of salt. These can be anonymous, reflect anecdotal experiences, and may be planted by the developer of the app to make it look good.
Click on the tab for the developer in the app description, and if you don’t recognize the name, make sure there’s a link to a website or at least an e-mail address so you can assess whether the developer has health care expertise.
It’s also worth clicking on the Updates or Version History tab to see if it’s been updated, because good-quality apps usually get frequent updates, typically at least every 6 months, several app consultants said in interviews.
Are you already using evidence-delivering apps? Which are your favorites? Let us know, and we may highlight them in a future column.
On Twitter @sherryboschert
There are at least two ways to consider the meaning of evidence-based apps. One is a category of apps that have clinical trial evidence showing that they are useful, a topic we cover regularly in this column.
Another category, though, is apps that take existing evidence-based guidelines or already-vetted medical information and deliver that to you in a more efficient, convenient package.
One of the latest examples of the latter is the new Evidence-Based Wound Care Guidelines and Fecal Ostomy Best Practice app, sold by the Wound, Ostomy and Continence Nurses Society (WOCN) for iPhones, iPads, or iPod Touch devices. Is it worth the $60 price?
It would cost you at least $160 to buy print copies from the WOCN of the five guidelines included in the app, on management of patients with pressure ulcers, lower-extremity arterial disease (LEAD), lower-extremity neuropathic disease (LEND), lower-extremity venous disease (LEVD), and fecal ostomy.
Or, you could spend a fair amount of time finding and printing the equivalent guidelines in the archives of medical journals or online. The Agency for Healthcare Research and Quality (AHRQ) provides detailed summaries of most of these guidelines on its website, including the major recommendations in each guideline and ratings of the evidence behind those recommendations. You can find the AHRQ pages on management of pressure ulcers, LEND, LEVD, and fecal ostomy, for example. They’re free, but it’s a time-consuming process that leaves you with bulky printouts.
Alternatively, the WOCN offers a free, 12-page, quick-reference guide for management of lower-extremity venous, arterial, or neuropathic wounds that you can download and print for free or, alternatively, consult without printing.
All in all, that’s a lot of dead trees to carry around if you want to consult these guidelines while treating patients, unless you have a bulky desktop computer handy. Compare that with the convenience of pulling out your smartphone or tablet to consult the literature on best practices at the bedside, and you can see why knowledge-delivering apps have caught physicians’ imaginations.
How can you know that an app is delivering credible information? The easiest way is to choose an app from a credible organization, such as the Cancer.Net app for patients with cancer, from the American Society of Clinical Oncology. Apps that have been vetted by the likes of the Cleveland Clinic, Mayo Clinic, or Veterans Affairs Healthcare System, for example, typically have been assessed with some quality-control measures. Every app has its flaws, however, so it’s usually worth searching for reviews online to gauge the strengths and weaknesses of various apps, especially when there is more than one available on a particular topic.
For-profit companies have developed some of the most popular evidence-driven apps for physicians. UpToDate claims that its physicians, authors, and editors synthesize the available medical evidence so the app can provide recommendations in primary care, ob.gyn., pediatrics, general surgery, emergency medicine, internal medicine, and all of the internal medicine subspecialties, among others. An individual subscription costs $499/year.
Epocrates offers a free app that provides medication information and lets you look up drug interactions and more, with additional services for a fee. Other apps do the same – Lexicomp is one subscription-based app that allows a 30-day free trial.
How to choose? Again, reviews may be helpful. The physician-run site iMedicalApps compared several medical drug reference apps in a 2010 review, but in app development time, that already seems light-years ago.
If you don’t recognize a company as a credible player in delivering mobile medical information, you can take several steps to assess the app’s trustworthiness. When you look at the app’s description online or in your mobile device’s app store, often the description is short and doesn’t list the reference information that the app relies on, though that’s often included in the app itself.
You’ll find reviews and ratings in the app description, but take these with a grain of salt. These can be anonymous, reflect anecdotal experiences, and may be planted by the developer of the app to make it look good.
Click on the tab for the developer in the app description, and if you don’t recognize the name, make sure there’s a link to a website or at least an e-mail address so you can assess whether the developer has health care expertise.
It’s also worth clicking on the Updates or Version History tab to see if it’s been updated, because good-quality apps usually get frequent updates, typically at least every 6 months, several app consultants said in interviews.
Are you already using evidence-delivering apps? Which are your favorites? Let us know, and we may highlight them in a future column.
On Twitter @sherryboschert
Corticosteroid doses reduced with inhalation system protocol
SAN DIEGO – Adults on chronic oral corticosteroids for asthma were able to decrease their doses while maintaining lung function by adding 0.5-1 mg budesonide delivered by an experimental inhaler in a randomized study of 199 patients.
The four-arm phase II/III study compared 18 weeks of twice-daily treatment using the AKITA inhalation system or a conventional nebulizer while tapering oral corticosteroids. The compressor-driven inhalation system is designed to deliver the budesonide suspension to the small airways of the lungs. It is not approved in the United States to treat adults with asthma.
The system delivered budesonide 1 mg, budesonide 0.5 mg, or placebo, compared with an open-label treatment group that used a nebulizer to deliver 1 mg budesonide. Oral corticosteroids were tapered to week 14. Patients were followed to week 20 with lung function parameters measures every 2 weeks.
The mean expiratory volume at 1 second (FEV1) significantly improved in the AKITA 1 mg budesonide group, compared with baseline, Dr. Sebastian Canisius and his associates reported at an international conference of the American Thoracic Society.
The mean FEV1 improved by 239 mL in the 1-mg AKITA group. Mean FEV1 improvements were lower in the other groups: 126 mL in the AKITA 0.5 mg budesonide group, 93 mL in the placebo group, and 137 mL in the nebulizer group.
Another surrogate marker of small airway function improved significantly in the AKITA 1 mg group compared with the placebo group – forced expiratory flow 25%-75% of vital capacity, or FEF (25-75), said Dr. Canisius, director of clinical development and drug safety for the Vectura Group, Kassel Area, Germany, the company that developed the inhalation system.
The FEF (25-75) improved by a mean of 0.2 l/s, compared with baseline in the AKITA 1 mg group and did not change in the placebo group. Improvements in the other two groups were smaller and not significant compared with placebo.
Patients in the AKITA 1 mg group were significantly less likely to develop an asthma exacerbation, compared with the nebulizer group (8% vs. 22%, respectively) and went significantly longer before an exacerbation (96 days vs. 50 days, respectively).
Inhaled budesonide therapy targeted to the small airways, on top of Global Initiative for Asthma step 5 treatment, can help patients decrease their doses of oral corticosteroids, Dr. Canisius concluded. How this works isn’t exactly clear, he added.
Dr. Canisius works for and owns stock in the company that developed the inhalation device.
On Twitter @sherryboschert
SAN DIEGO – Adults on chronic oral corticosteroids for asthma were able to decrease their doses while maintaining lung function by adding 0.5-1 mg budesonide delivered by an experimental inhaler in a randomized study of 199 patients.
The four-arm phase II/III study compared 18 weeks of twice-daily treatment using the AKITA inhalation system or a conventional nebulizer while tapering oral corticosteroids. The compressor-driven inhalation system is designed to deliver the budesonide suspension to the small airways of the lungs. It is not approved in the United States to treat adults with asthma.
The system delivered budesonide 1 mg, budesonide 0.5 mg, or placebo, compared with an open-label treatment group that used a nebulizer to deliver 1 mg budesonide. Oral corticosteroids were tapered to week 14. Patients were followed to week 20 with lung function parameters measures every 2 weeks.
The mean expiratory volume at 1 second (FEV1) significantly improved in the AKITA 1 mg budesonide group, compared with baseline, Dr. Sebastian Canisius and his associates reported at an international conference of the American Thoracic Society.
The mean FEV1 improved by 239 mL in the 1-mg AKITA group. Mean FEV1 improvements were lower in the other groups: 126 mL in the AKITA 0.5 mg budesonide group, 93 mL in the placebo group, and 137 mL in the nebulizer group.
Another surrogate marker of small airway function improved significantly in the AKITA 1 mg group compared with the placebo group – forced expiratory flow 25%-75% of vital capacity, or FEF (25-75), said Dr. Canisius, director of clinical development and drug safety for the Vectura Group, Kassel Area, Germany, the company that developed the inhalation system.
The FEF (25-75) improved by a mean of 0.2 l/s, compared with baseline in the AKITA 1 mg group and did not change in the placebo group. Improvements in the other two groups were smaller and not significant compared with placebo.
Patients in the AKITA 1 mg group were significantly less likely to develop an asthma exacerbation, compared with the nebulizer group (8% vs. 22%, respectively) and went significantly longer before an exacerbation (96 days vs. 50 days, respectively).
Inhaled budesonide therapy targeted to the small airways, on top of Global Initiative for Asthma step 5 treatment, can help patients decrease their doses of oral corticosteroids, Dr. Canisius concluded. How this works isn’t exactly clear, he added.
Dr. Canisius works for and owns stock in the company that developed the inhalation device.
On Twitter @sherryboschert
SAN DIEGO – Adults on chronic oral corticosteroids for asthma were able to decrease their doses while maintaining lung function by adding 0.5-1 mg budesonide delivered by an experimental inhaler in a randomized study of 199 patients.
The four-arm phase II/III study compared 18 weeks of twice-daily treatment using the AKITA inhalation system or a conventional nebulizer while tapering oral corticosteroids. The compressor-driven inhalation system is designed to deliver the budesonide suspension to the small airways of the lungs. It is not approved in the United States to treat adults with asthma.
The system delivered budesonide 1 mg, budesonide 0.5 mg, or placebo, compared with an open-label treatment group that used a nebulizer to deliver 1 mg budesonide. Oral corticosteroids were tapered to week 14. Patients were followed to week 20 with lung function parameters measures every 2 weeks.
The mean expiratory volume at 1 second (FEV1) significantly improved in the AKITA 1 mg budesonide group, compared with baseline, Dr. Sebastian Canisius and his associates reported at an international conference of the American Thoracic Society.
The mean FEV1 improved by 239 mL in the 1-mg AKITA group. Mean FEV1 improvements were lower in the other groups: 126 mL in the AKITA 0.5 mg budesonide group, 93 mL in the placebo group, and 137 mL in the nebulizer group.
Another surrogate marker of small airway function improved significantly in the AKITA 1 mg group compared with the placebo group – forced expiratory flow 25%-75% of vital capacity, or FEF (25-75), said Dr. Canisius, director of clinical development and drug safety for the Vectura Group, Kassel Area, Germany, the company that developed the inhalation system.
The FEF (25-75) improved by a mean of 0.2 l/s, compared with baseline in the AKITA 1 mg group and did not change in the placebo group. Improvements in the other two groups were smaller and not significant compared with placebo.
Patients in the AKITA 1 mg group were significantly less likely to develop an asthma exacerbation, compared with the nebulizer group (8% vs. 22%, respectively) and went significantly longer before an exacerbation (96 days vs. 50 days, respectively).
Inhaled budesonide therapy targeted to the small airways, on top of Global Initiative for Asthma step 5 treatment, can help patients decrease their doses of oral corticosteroids, Dr. Canisius concluded. How this works isn’t exactly clear, he added.
Dr. Canisius works for and owns stock in the company that developed the inhalation device.
On Twitter @sherryboschert
AT ATS 2014
Key clinical point: A novel inhaler that targets small airways can improve budesonide delivery.
Major finding: The mean FEV1 improved significantly in the AKITA 1-mg budesonide group (by 239 mL) compared with baseline.
Data source: A randomized, four-arm placebo-controlled trial comparing budesonide delivery methods in 199 patients.
Disclosures: The study presenter is an employee of and investor in the company that developed the inhalation device.
Statin helped endothelial function in COPD subgroup
SAN DIEGO – Twelve weeks of rosuvastatin therapy was associated with reduced systemic inflammation, but no improvement in pulmonary function in a randomized, placebo-controlled trial in 99 patients with stable chronic obstructive pulmonary disease.
However, a prespecified analysis of patients with elevated results on high-sensitivity C-reactive protein tests (hsCRP) found that those with levels higher than the median of 1.7 mg/dL at baseline showed improved endothelial function with the statin, compared with placebo, Dr. Anke Neukamm reported at an international conference of the American Thoracic Society.
The findings provided a small ray of positivity for the anti-inflammatory effects of statins in chronic obstructive pulmonary disease (COPD) on a day when two larger randomized, controlled studies reported that rosuvastatin did not reduce mortality in patients with sepsis-associated acute respiratory distress syndrome and simvastatin did not reduce exacerbations in patients with moderate to severe COPD.
Dr. Neukamm’s RODEO study (Effect of Rosuvastatin Treatment in Patients With Stable Chronic Obstructive Pulmonary Disease) randomized patients with stable chronic COPD to once-daily rosuvastatin 10 mg or placebo for 12 weeks. The groups did not differ significantly in the primary endpoint peripheral vasodilator function expressed as a reactive hyperemia ratio. In the subgroup analysis of patients with elevated hsCRP levels, however, the reactive hyperemia index improved from approximately 2.5 at baseline to 2.8 after 12 weeks of rosuvastatin and worsened from 2.6 to 2.5 in the placebo group, leaving a statistically significant difference between groups at the end of treatment, said Dr. Neukamm of Akershus University Hospital, Lørenskog, Norway.
This suggested "a clear improvement of endothelial function in the statin group," she said.
Some physicians in the audience disputed the results, noting that rosuvastatin’s effects would not have reached statistical significance if endothelial function had not worsened in the placebo group.
Dr. Neukamm remained cautiously optimistic. Targeting statin therapy for patients with COPD to those with increased systemic inflammation as indicated by elevated hsCRP levels might improve vascular function, she said in an interview. Patients in the study were "a very select group with a lower cardiovascular risk" than patients in other COPD studies. Without larger prospective trials, "we don’t know if this small improvement in endothelial function would translate" into better clinical outcomes, she said.
Among secondary endpoints, circulating hsCRP levels decreased from 1.4 mg/L at baseline to 1.2 mg/L in the rosuvastatin group, compared with a change from 1.8 mg/L to 2.2 mg/L in the placebo group. The difference between groups was significant. Levels of the inflammatory marker interleukin-6 remained stable at 4.1 pg/mL in the statin group and increased from 3.4 pg/mL to 4.4 pg/mL in the placebo group, also a significant difference between groups.
The trial excluded patients with a history of coronary artery disease, other diagnosed lung disease (except asthma), uncontrolled arterial hypertension, or diabetes, or patients who had used statins in the prior 4 weeks or who had a clear indication for statin use.
Thirty-three adverse events included COPD acute exacerbations, constipation, diarrhea, nausea, myalgia, and vertigo. Seven serious adverse events included hospitalizations for COPD exacerbations, atrial flutter in one patient, and pituitary apoplexy in one patient.
Patients had a mean age of 65 years, 48% were female, and 25% had a history of hypertension. They had a smoking history of a mean 37 pack-years. Baseline patient characteristics were similar between groups except for a higher percentage of current smokers in the placebo group (49%), compared with the rosuvastatin group (26%).
Stable COPD and acute exacerbations have been associated with systemic inflammation as reflected in increased levels of CRP and other inflammatory markers. Cardiovascular disease is a major comorbidity in patients with COPD, and inflammation is a hallmark of the atherosclerotic process, Dr. Neukamm said. Endothelial dysfunction, which is an early predictor of atherosclerosis, has been shown to be increased in patients with COPD.
A previous observational study of 2,708 patients with COPD suggested that long-term statin therapy was associated with a significant 78% decrease in mortality in those with hsCRP levels greater than 3 mg/L and a nonsignificant 21% decreased mortality in those with lower hsCRP levels (Pulm. Pharmacol. Ther. 2013;26:212-7).
The study was funded by the Norwegian Extra Foundation for Health and Rehabilitation and by AstraZeneca, which makes a brand name formulation of rosuvastatin. Dr. Neukamm has been a speaker for AstraZeneca.
On Twitter @sherryboschert
SAN DIEGO – Twelve weeks of rosuvastatin therapy was associated with reduced systemic inflammation, but no improvement in pulmonary function in a randomized, placebo-controlled trial in 99 patients with stable chronic obstructive pulmonary disease.
However, a prespecified analysis of patients with elevated results on high-sensitivity C-reactive protein tests (hsCRP) found that those with levels higher than the median of 1.7 mg/dL at baseline showed improved endothelial function with the statin, compared with placebo, Dr. Anke Neukamm reported at an international conference of the American Thoracic Society.
The findings provided a small ray of positivity for the anti-inflammatory effects of statins in chronic obstructive pulmonary disease (COPD) on a day when two larger randomized, controlled studies reported that rosuvastatin did not reduce mortality in patients with sepsis-associated acute respiratory distress syndrome and simvastatin did not reduce exacerbations in patients with moderate to severe COPD.
Dr. Neukamm’s RODEO study (Effect of Rosuvastatin Treatment in Patients With Stable Chronic Obstructive Pulmonary Disease) randomized patients with stable chronic COPD to once-daily rosuvastatin 10 mg or placebo for 12 weeks. The groups did not differ significantly in the primary endpoint peripheral vasodilator function expressed as a reactive hyperemia ratio. In the subgroup analysis of patients with elevated hsCRP levels, however, the reactive hyperemia index improved from approximately 2.5 at baseline to 2.8 after 12 weeks of rosuvastatin and worsened from 2.6 to 2.5 in the placebo group, leaving a statistically significant difference between groups at the end of treatment, said Dr. Neukamm of Akershus University Hospital, Lørenskog, Norway.
This suggested "a clear improvement of endothelial function in the statin group," she said.
Some physicians in the audience disputed the results, noting that rosuvastatin’s effects would not have reached statistical significance if endothelial function had not worsened in the placebo group.
Dr. Neukamm remained cautiously optimistic. Targeting statin therapy for patients with COPD to those with increased systemic inflammation as indicated by elevated hsCRP levels might improve vascular function, she said in an interview. Patients in the study were "a very select group with a lower cardiovascular risk" than patients in other COPD studies. Without larger prospective trials, "we don’t know if this small improvement in endothelial function would translate" into better clinical outcomes, she said.
Among secondary endpoints, circulating hsCRP levels decreased from 1.4 mg/L at baseline to 1.2 mg/L in the rosuvastatin group, compared with a change from 1.8 mg/L to 2.2 mg/L in the placebo group. The difference between groups was significant. Levels of the inflammatory marker interleukin-6 remained stable at 4.1 pg/mL in the statin group and increased from 3.4 pg/mL to 4.4 pg/mL in the placebo group, also a significant difference between groups.
The trial excluded patients with a history of coronary artery disease, other diagnosed lung disease (except asthma), uncontrolled arterial hypertension, or diabetes, or patients who had used statins in the prior 4 weeks or who had a clear indication for statin use.
Thirty-three adverse events included COPD acute exacerbations, constipation, diarrhea, nausea, myalgia, and vertigo. Seven serious adverse events included hospitalizations for COPD exacerbations, atrial flutter in one patient, and pituitary apoplexy in one patient.
Patients had a mean age of 65 years, 48% were female, and 25% had a history of hypertension. They had a smoking history of a mean 37 pack-years. Baseline patient characteristics were similar between groups except for a higher percentage of current smokers in the placebo group (49%), compared with the rosuvastatin group (26%).
Stable COPD and acute exacerbations have been associated with systemic inflammation as reflected in increased levels of CRP and other inflammatory markers. Cardiovascular disease is a major comorbidity in patients with COPD, and inflammation is a hallmark of the atherosclerotic process, Dr. Neukamm said. Endothelial dysfunction, which is an early predictor of atherosclerosis, has been shown to be increased in patients with COPD.
A previous observational study of 2,708 patients with COPD suggested that long-term statin therapy was associated with a significant 78% decrease in mortality in those with hsCRP levels greater than 3 mg/L and a nonsignificant 21% decreased mortality in those with lower hsCRP levels (Pulm. Pharmacol. Ther. 2013;26:212-7).
The study was funded by the Norwegian Extra Foundation for Health and Rehabilitation and by AstraZeneca, which makes a brand name formulation of rosuvastatin. Dr. Neukamm has been a speaker for AstraZeneca.
On Twitter @sherryboschert
SAN DIEGO – Twelve weeks of rosuvastatin therapy was associated with reduced systemic inflammation, but no improvement in pulmonary function in a randomized, placebo-controlled trial in 99 patients with stable chronic obstructive pulmonary disease.
However, a prespecified analysis of patients with elevated results on high-sensitivity C-reactive protein tests (hsCRP) found that those with levels higher than the median of 1.7 mg/dL at baseline showed improved endothelial function with the statin, compared with placebo, Dr. Anke Neukamm reported at an international conference of the American Thoracic Society.
The findings provided a small ray of positivity for the anti-inflammatory effects of statins in chronic obstructive pulmonary disease (COPD) on a day when two larger randomized, controlled studies reported that rosuvastatin did not reduce mortality in patients with sepsis-associated acute respiratory distress syndrome and simvastatin did not reduce exacerbations in patients with moderate to severe COPD.
Dr. Neukamm’s RODEO study (Effect of Rosuvastatin Treatment in Patients With Stable Chronic Obstructive Pulmonary Disease) randomized patients with stable chronic COPD to once-daily rosuvastatin 10 mg or placebo for 12 weeks. The groups did not differ significantly in the primary endpoint peripheral vasodilator function expressed as a reactive hyperemia ratio. In the subgroup analysis of patients with elevated hsCRP levels, however, the reactive hyperemia index improved from approximately 2.5 at baseline to 2.8 after 12 weeks of rosuvastatin and worsened from 2.6 to 2.5 in the placebo group, leaving a statistically significant difference between groups at the end of treatment, said Dr. Neukamm of Akershus University Hospital, Lørenskog, Norway.
This suggested "a clear improvement of endothelial function in the statin group," she said.
Some physicians in the audience disputed the results, noting that rosuvastatin’s effects would not have reached statistical significance if endothelial function had not worsened in the placebo group.
Dr. Neukamm remained cautiously optimistic. Targeting statin therapy for patients with COPD to those with increased systemic inflammation as indicated by elevated hsCRP levels might improve vascular function, she said in an interview. Patients in the study were "a very select group with a lower cardiovascular risk" than patients in other COPD studies. Without larger prospective trials, "we don’t know if this small improvement in endothelial function would translate" into better clinical outcomes, she said.
Among secondary endpoints, circulating hsCRP levels decreased from 1.4 mg/L at baseline to 1.2 mg/L in the rosuvastatin group, compared with a change from 1.8 mg/L to 2.2 mg/L in the placebo group. The difference between groups was significant. Levels of the inflammatory marker interleukin-6 remained stable at 4.1 pg/mL in the statin group and increased from 3.4 pg/mL to 4.4 pg/mL in the placebo group, also a significant difference between groups.
The trial excluded patients with a history of coronary artery disease, other diagnosed lung disease (except asthma), uncontrolled arterial hypertension, or diabetes, or patients who had used statins in the prior 4 weeks or who had a clear indication for statin use.
Thirty-three adverse events included COPD acute exacerbations, constipation, diarrhea, nausea, myalgia, and vertigo. Seven serious adverse events included hospitalizations for COPD exacerbations, atrial flutter in one patient, and pituitary apoplexy in one patient.
Patients had a mean age of 65 years, 48% were female, and 25% had a history of hypertension. They had a smoking history of a mean 37 pack-years. Baseline patient characteristics were similar between groups except for a higher percentage of current smokers in the placebo group (49%), compared with the rosuvastatin group (26%).
Stable COPD and acute exacerbations have been associated with systemic inflammation as reflected in increased levels of CRP and other inflammatory markers. Cardiovascular disease is a major comorbidity in patients with COPD, and inflammation is a hallmark of the atherosclerotic process, Dr. Neukamm said. Endothelial dysfunction, which is an early predictor of atherosclerosis, has been shown to be increased in patients with COPD.
A previous observational study of 2,708 patients with COPD suggested that long-term statin therapy was associated with a significant 78% decrease in mortality in those with hsCRP levels greater than 3 mg/L and a nonsignificant 21% decreased mortality in those with lower hsCRP levels (Pulm. Pharmacol. Ther. 2013;26:212-7).
The study was funded by the Norwegian Extra Foundation for Health and Rehabilitation and by AstraZeneca, which makes a brand name formulation of rosuvastatin. Dr. Neukamm has been a speaker for AstraZeneca.
On Twitter @sherryboschert
AT ATS 2014
Key clinical point: Focusing statin therapy for COPD patients on those with increased systemic inflammation as indicated by elevated hsCRP might improve vascular function, but larger studies are needed.
Major finding: Rosuvastatin failed to improve pulmonary function overall, but significantly improved peripheral vasodilator function in patients with elevated hsCRP levels at baseline, indicated by a final reactive hyperemia index of 2.8 vs. 2.5 with placebo.
Data source: A randomized, controlled trial of 10 mg of rosuvastatin or placebo for 12 weeks in 99 patients with stable COPD.
Disclosures: The study was funded by the Norwegian Extra Foundation for Health and Rehabilitation and by AstraZeneca, which makes a brand name formulation of rosuvastatin. Dr. Neukamm has been a speaker for AstraZeneca.
Statins don’t help, may harm in COPD, sepsis-associated ARDS
SAN DIEGO – Two separate prospective, multicenter trials of statins stopped early when interim results showed they did not help – and potentially harmed – patients with moderate to severe chronic obstructive pulmonary disease or sepsis-associated acute respiratory distress syndrome.
The findings contradict previous observational data suggesting that the potential anti-inflammatory effects of statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (HMG-CoA) might benefit patients with these two diseases.
Statins did not significantly reduce rates of exacerbation of chronic obstructive pulmonary disease (COPD) or the time to first exacerbation in a study of 885 patients with moderate-to-severe COPD who were at high risk for exacerbations and who did not require statins for other indications. Patients in the STATCOPE trial (Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD) received a daily oral dose of either 40 mg simvastatin or placebo for 12-36 months.
The simvastatin group had a mean of 1.36 exacerbations/person-year, compared with 1.39/person-year in the placebo group. The median number of days to the first exacerbation was 223 on simvastatin and 231 on placebo, differences that were not significant, Dr. Gerard J. Criner and his associates reported at an international conference of the American Thoracic Society.
The results were published online by the New England Journal of Medicine (2014 May 18 [doi:10.1056/NEJMoa1403086]).
Among the 885 patients for whom follow-up information was available, 1,982 acute COPD exacerbations occurred, 965 in 430 patients on simvastatin and 1,017 exacerbations in 447 patients on placebo, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia.
Overall, 34% of patients had three or more exacerbations of COPD, with similar numbers in each group – 141 in the simvastatin group and 155 in the placebo groups. The proportions of patients who received glucocorticoid therapy or antibiotics for exacerbations did not differ significantly between groups.
The simvastatin group had a significantly higher rate of nonfatal serious adverse events involving the GI tract (mainly nausea and bloating from simvastatin) in 30 patients (0.05 events/person-year), compared with events in 17 patients on placebo (0.02 events/person-year). Rates of other nonfatal adverse events were similar between groups, with pneumonia and other respiratory and cardiovascular events most common. Twenty-eight patients on simvastatin and 30 on placebo died.
Asked why he thinks the STATCOPE trial’s negative results didn’t confirm previous positive findings from observational studies including thousands of patients, Dr. Criner speculated that excluding patients with indications for statins may have removed patients with cardiovascular risks who were included in other studies. "I think a lot of the problems we’re seeing for exacerbations in COPD might be related to cardiovascular events in patients who aren’t appropriately treated with statins, who should be," he said.
Rosuvastatin sags in SAILS trial
In the separate double-blind SAILS trial (Statins for Acutely Injured Lungs [ARDS] From Sepsis), enteral rosuvastatin did not decrease mortality, compared with placebo, in 745 patients with sepsis-associated acute respiratory distress syndrome (ARDS). Dr. Jonathon D. Truwit and his associates are scheduled to report the results at the meeting on Tuesday. The findings also appeared online in the New England Journal of Medicine (2014 May 18 [doi:10.1056/NEJMoa1401520]).
Researchers found that 28.5% of patients on rosuvastatin and 25% on placebo died before hospital discharge or within 60 days if the patient was still in a health care facility, reported Dr. Truwit, professor of medicine at the Medical College of Wisconsin, Madison.
Patients in the rosuvastatin group received a loading dose of 40 mg followed by daily maintenance doses of 20 mg (or 10 mg for patients with a morning serum creatinine level of 2.8 mg/dL or more who were not receiving renal replacement therapy. Treatment continued until the third day of discharge from the ICU, hospital discharge, or death, whichever came first.
Both the rosuvastatin and placebo groups had a mean of 15 ventilator-free days.
Results also did not differ significantly between groups for the 339 patients who were in shock at the start of the study or for 109 patients who had used statins before the study and who underwent a 48-hour washout period before randomization.
Patients on rosuvastatin had a mean of 10.1 days free of renal failure and 10.8 days free of hepatic failure within the first 14 days, both significantly fewer compared with patients on placebo (11 and 11.8 days, respectively). "These differences in organ-failure-free days were small, and their significance may be spurious owing to the number of secondary endpoints analyzed. However, we cannot rule out a detrimental effect of rosuvastatin," the investigators wrote.
The results, combined with previous smaller randomized trials of other statins, suggest no benefits from starting or continuing statin therapy for sepsis-associated ARDS, Dr. Truwit said.
"The finding in observational studies that previous statin use provides a benefit may reflect better access to health care among patients who use statins than among those who do not, with a shorter time to the initiation of antibiotic therapy at the onset of symptoms of infection in statin users," according to the journal article.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes simvastatin. The SAILS trial was sponsored by the NHLBI and by AstraZeneca, which makes rosuvastatin. Dr. Truwit reported having no financial disclosures. Three of his coinvestigators reported financial ties to AstraZeneca, and four reported associations with a total of 14 other companies.
On Twitter @sherryboschert
Dr. Jeffrey M. Drazen and Annetine C. Gelijns, Ph.D., comment:
Finding out that statins did not help in these two trials may be
disappointing, but not knowing that – if the studies had not been done –
would have been worse, Dr. Drazen and Dr. Gelijns wrote in an
accompanying editorial in the New England Journal of Medicine.
"The cynic would say that the public’s money had been wasted – we had struck out swinging," they wrote (N. Engl. J. Med. 2014 May 18 [doi: 10.1056/NEJMe1405032]).
Treatment
with statins seemed reasonable and, on the face of it, valid for both
moderate to severe chronic obstructive pulmonary disease (COPD) and
sepsis-associated acute respiratory distress syndrome (ARDS), conditions
in which inflammatory events are thought to drive disease pathobiology.
The known pleomorphic anti-inflammatory properties of statins made them
candidates, and previous observational data suggested that they helped
patients with these conditions. Because of meager therapeutic options
for these diseases, the studies of statins had to be done, Dr. Drazen
and Dr. Gelijns argued.
"It would have been a big mistake" to
accept the observational findings without a test, they wrote. "Had we
accepted the observational data at face value, we might have spent the
cost of the trials many times over in useless treatments before
recognizing our errors."
Dr. Jeffrey M. Drazen is editor in
chief of the Journal. He reported having no disclosures. Dr. Gelijns is a
professor and chair of Health Evidence and Policy at Mount Sinai School
of Medicine, New York. She reported a financial association with MERS
International, which developed a medical event reporting system.
Dr. Jeffrey M. Drazen and Annetine C. Gelijns, Ph.D., comment:
Finding out that statins did not help in these two trials may be
disappointing, but not knowing that – if the studies had not been done –
would have been worse, Dr. Drazen and Dr. Gelijns wrote in an
accompanying editorial in the New England Journal of Medicine.
"The cynic would say that the public’s money had been wasted – we had struck out swinging," they wrote (N. Engl. J. Med. 2014 May 18 [doi: 10.1056/NEJMe1405032]).
Treatment
with statins seemed reasonable and, on the face of it, valid for both
moderate to severe chronic obstructive pulmonary disease (COPD) and
sepsis-associated acute respiratory distress syndrome (ARDS), conditions
in which inflammatory events are thought to drive disease pathobiology.
The known pleomorphic anti-inflammatory properties of statins made them
candidates, and previous observational data suggested that they helped
patients with these conditions. Because of meager therapeutic options
for these diseases, the studies of statins had to be done, Dr. Drazen
and Dr. Gelijns argued.
"It would have been a big mistake" to
accept the observational findings without a test, they wrote. "Had we
accepted the observational data at face value, we might have spent the
cost of the trials many times over in useless treatments before
recognizing our errors."
Dr. Jeffrey M. Drazen is editor in
chief of the Journal. He reported having no disclosures. Dr. Gelijns is a
professor and chair of Health Evidence and Policy at Mount Sinai School
of Medicine, New York. She reported a financial association with MERS
International, which developed a medical event reporting system.
Dr. Jeffrey M. Drazen and Annetine C. Gelijns, Ph.D., comment:
Finding out that statins did not help in these two trials may be
disappointing, but not knowing that – if the studies had not been done –
would have been worse, Dr. Drazen and Dr. Gelijns wrote in an
accompanying editorial in the New England Journal of Medicine.
"The cynic would say that the public’s money had been wasted – we had struck out swinging," they wrote (N. Engl. J. Med. 2014 May 18 [doi: 10.1056/NEJMe1405032]).
Treatment
with statins seemed reasonable and, on the face of it, valid for both
moderate to severe chronic obstructive pulmonary disease (COPD) and
sepsis-associated acute respiratory distress syndrome (ARDS), conditions
in which inflammatory events are thought to drive disease pathobiology.
The known pleomorphic anti-inflammatory properties of statins made them
candidates, and previous observational data suggested that they helped
patients with these conditions. Because of meager therapeutic options
for these diseases, the studies of statins had to be done, Dr. Drazen
and Dr. Gelijns argued.
"It would have been a big mistake" to
accept the observational findings without a test, they wrote. "Had we
accepted the observational data at face value, we might have spent the
cost of the trials many times over in useless treatments before
recognizing our errors."
Dr. Jeffrey M. Drazen is editor in
chief of the Journal. He reported having no disclosures. Dr. Gelijns is a
professor and chair of Health Evidence and Policy at Mount Sinai School
of Medicine, New York. She reported a financial association with MERS
International, which developed a medical event reporting system.
SAN DIEGO – Two separate prospective, multicenter trials of statins stopped early when interim results showed they did not help – and potentially harmed – patients with moderate to severe chronic obstructive pulmonary disease or sepsis-associated acute respiratory distress syndrome.
The findings contradict previous observational data suggesting that the potential anti-inflammatory effects of statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (HMG-CoA) might benefit patients with these two diseases.
Statins did not significantly reduce rates of exacerbation of chronic obstructive pulmonary disease (COPD) or the time to first exacerbation in a study of 885 patients with moderate-to-severe COPD who were at high risk for exacerbations and who did not require statins for other indications. Patients in the STATCOPE trial (Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD) received a daily oral dose of either 40 mg simvastatin or placebo for 12-36 months.
The simvastatin group had a mean of 1.36 exacerbations/person-year, compared with 1.39/person-year in the placebo group. The median number of days to the first exacerbation was 223 on simvastatin and 231 on placebo, differences that were not significant, Dr. Gerard J. Criner and his associates reported at an international conference of the American Thoracic Society.
The results were published online by the New England Journal of Medicine (2014 May 18 [doi:10.1056/NEJMoa1403086]).
Among the 885 patients for whom follow-up information was available, 1,982 acute COPD exacerbations occurred, 965 in 430 patients on simvastatin and 1,017 exacerbations in 447 patients on placebo, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia.
Overall, 34% of patients had three or more exacerbations of COPD, with similar numbers in each group – 141 in the simvastatin group and 155 in the placebo groups. The proportions of patients who received glucocorticoid therapy or antibiotics for exacerbations did not differ significantly between groups.
The simvastatin group had a significantly higher rate of nonfatal serious adverse events involving the GI tract (mainly nausea and bloating from simvastatin) in 30 patients (0.05 events/person-year), compared with events in 17 patients on placebo (0.02 events/person-year). Rates of other nonfatal adverse events were similar between groups, with pneumonia and other respiratory and cardiovascular events most common. Twenty-eight patients on simvastatin and 30 on placebo died.
Asked why he thinks the STATCOPE trial’s negative results didn’t confirm previous positive findings from observational studies including thousands of patients, Dr. Criner speculated that excluding patients with indications for statins may have removed patients with cardiovascular risks who were included in other studies. "I think a lot of the problems we’re seeing for exacerbations in COPD might be related to cardiovascular events in patients who aren’t appropriately treated with statins, who should be," he said.
Rosuvastatin sags in SAILS trial
In the separate double-blind SAILS trial (Statins for Acutely Injured Lungs [ARDS] From Sepsis), enteral rosuvastatin did not decrease mortality, compared with placebo, in 745 patients with sepsis-associated acute respiratory distress syndrome (ARDS). Dr. Jonathon D. Truwit and his associates are scheduled to report the results at the meeting on Tuesday. The findings also appeared online in the New England Journal of Medicine (2014 May 18 [doi:10.1056/NEJMoa1401520]).
Researchers found that 28.5% of patients on rosuvastatin and 25% on placebo died before hospital discharge or within 60 days if the patient was still in a health care facility, reported Dr. Truwit, professor of medicine at the Medical College of Wisconsin, Madison.
Patients in the rosuvastatin group received a loading dose of 40 mg followed by daily maintenance doses of 20 mg (or 10 mg for patients with a morning serum creatinine level of 2.8 mg/dL or more who were not receiving renal replacement therapy. Treatment continued until the third day of discharge from the ICU, hospital discharge, or death, whichever came first.
Both the rosuvastatin and placebo groups had a mean of 15 ventilator-free days.
Results also did not differ significantly between groups for the 339 patients who were in shock at the start of the study or for 109 patients who had used statins before the study and who underwent a 48-hour washout period before randomization.
Patients on rosuvastatin had a mean of 10.1 days free of renal failure and 10.8 days free of hepatic failure within the first 14 days, both significantly fewer compared with patients on placebo (11 and 11.8 days, respectively). "These differences in organ-failure-free days were small, and their significance may be spurious owing to the number of secondary endpoints analyzed. However, we cannot rule out a detrimental effect of rosuvastatin," the investigators wrote.
The results, combined with previous smaller randomized trials of other statins, suggest no benefits from starting or continuing statin therapy for sepsis-associated ARDS, Dr. Truwit said.
"The finding in observational studies that previous statin use provides a benefit may reflect better access to health care among patients who use statins than among those who do not, with a shorter time to the initiation of antibiotic therapy at the onset of symptoms of infection in statin users," according to the journal article.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes simvastatin. The SAILS trial was sponsored by the NHLBI and by AstraZeneca, which makes rosuvastatin. Dr. Truwit reported having no financial disclosures. Three of his coinvestigators reported financial ties to AstraZeneca, and four reported associations with a total of 14 other companies.
On Twitter @sherryboschert
SAN DIEGO – Two separate prospective, multicenter trials of statins stopped early when interim results showed they did not help – and potentially harmed – patients with moderate to severe chronic obstructive pulmonary disease or sepsis-associated acute respiratory distress syndrome.
The findings contradict previous observational data suggesting that the potential anti-inflammatory effects of statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (HMG-CoA) might benefit patients with these two diseases.
Statins did not significantly reduce rates of exacerbation of chronic obstructive pulmonary disease (COPD) or the time to first exacerbation in a study of 885 patients with moderate-to-severe COPD who were at high risk for exacerbations and who did not require statins for other indications. Patients in the STATCOPE trial (Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD) received a daily oral dose of either 40 mg simvastatin or placebo for 12-36 months.
The simvastatin group had a mean of 1.36 exacerbations/person-year, compared with 1.39/person-year in the placebo group. The median number of days to the first exacerbation was 223 on simvastatin and 231 on placebo, differences that were not significant, Dr. Gerard J. Criner and his associates reported at an international conference of the American Thoracic Society.
The results were published online by the New England Journal of Medicine (2014 May 18 [doi:10.1056/NEJMoa1403086]).
Among the 885 patients for whom follow-up information was available, 1,982 acute COPD exacerbations occurred, 965 in 430 patients on simvastatin and 1,017 exacerbations in 447 patients on placebo, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia.
Overall, 34% of patients had three or more exacerbations of COPD, with similar numbers in each group – 141 in the simvastatin group and 155 in the placebo groups. The proportions of patients who received glucocorticoid therapy or antibiotics for exacerbations did not differ significantly between groups.
The simvastatin group had a significantly higher rate of nonfatal serious adverse events involving the GI tract (mainly nausea and bloating from simvastatin) in 30 patients (0.05 events/person-year), compared with events in 17 patients on placebo (0.02 events/person-year). Rates of other nonfatal adverse events were similar between groups, with pneumonia and other respiratory and cardiovascular events most common. Twenty-eight patients on simvastatin and 30 on placebo died.
Asked why he thinks the STATCOPE trial’s negative results didn’t confirm previous positive findings from observational studies including thousands of patients, Dr. Criner speculated that excluding patients with indications for statins may have removed patients with cardiovascular risks who were included in other studies. "I think a lot of the problems we’re seeing for exacerbations in COPD might be related to cardiovascular events in patients who aren’t appropriately treated with statins, who should be," he said.
Rosuvastatin sags in SAILS trial
In the separate double-blind SAILS trial (Statins for Acutely Injured Lungs [ARDS] From Sepsis), enteral rosuvastatin did not decrease mortality, compared with placebo, in 745 patients with sepsis-associated acute respiratory distress syndrome (ARDS). Dr. Jonathon D. Truwit and his associates are scheduled to report the results at the meeting on Tuesday. The findings also appeared online in the New England Journal of Medicine (2014 May 18 [doi:10.1056/NEJMoa1401520]).
Researchers found that 28.5% of patients on rosuvastatin and 25% on placebo died before hospital discharge or within 60 days if the patient was still in a health care facility, reported Dr. Truwit, professor of medicine at the Medical College of Wisconsin, Madison.
Patients in the rosuvastatin group received a loading dose of 40 mg followed by daily maintenance doses of 20 mg (or 10 mg for patients with a morning serum creatinine level of 2.8 mg/dL or more who were not receiving renal replacement therapy. Treatment continued until the third day of discharge from the ICU, hospital discharge, or death, whichever came first.
Both the rosuvastatin and placebo groups had a mean of 15 ventilator-free days.
Results also did not differ significantly between groups for the 339 patients who were in shock at the start of the study or for 109 patients who had used statins before the study and who underwent a 48-hour washout period before randomization.
Patients on rosuvastatin had a mean of 10.1 days free of renal failure and 10.8 days free of hepatic failure within the first 14 days, both significantly fewer compared with patients on placebo (11 and 11.8 days, respectively). "These differences in organ-failure-free days were small, and their significance may be spurious owing to the number of secondary endpoints analyzed. However, we cannot rule out a detrimental effect of rosuvastatin," the investigators wrote.
The results, combined with previous smaller randomized trials of other statins, suggest no benefits from starting or continuing statin therapy for sepsis-associated ARDS, Dr. Truwit said.
"The finding in observational studies that previous statin use provides a benefit may reflect better access to health care among patients who use statins than among those who do not, with a shorter time to the initiation of antibiotic therapy at the onset of symptoms of infection in statin users," according to the journal article.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes simvastatin. The SAILS trial was sponsored by the NHLBI and by AstraZeneca, which makes rosuvastatin. Dr. Truwit reported having no financial disclosures. Three of his coinvestigators reported financial ties to AstraZeneca, and four reported associations with a total of 14 other companies.
On Twitter @sherryboschert
AT ATS 2014
Key clinical point: Statins did not help, and possibly harmed, patients with moderate to severe COPD or sepsis-associated acute respiratory distress syndrome who did not require statins for other indications.
Major finding: The mean rate of COPD exacerbations was 1.36 in the simvastatin group and 1.39 in the placebo group, not significantly different.
Data source: STATCOPE, a multicenter prospective randomized, placebo-controlled trial of daily oral simvastatin 40 mg or placebo in 885 patients with COPD at high risk for exacerbations.
Disclosures: The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. Several coinvestigators reported financial associations with dozens of companies, including with Merck, which makes simvastatin.
Picosecond laser makes its mark for tattoo removal
PHOENIX – Three small case series reported at the annual meeting of the American Society for Laser Medicine and Surgery reported good results using a picosecond laser for tattoo removal and explored different regimens for treatment.
One study at a single center used a 532-nm Nd-YAG laser with a pulse duration of 450-500 picoseconds (ps) in all 17 patients. Up to 10 treatments spaced every 4-8 weeks used a spot size of 2.5-5 mm, an energy density of 0.3-2.2 J/cm2, and a frequency of 5 Hz in a single pass. Some patients also were treated with a 1,064-nm Nd:YAG laser with a pulse duration of 500-600 ns or a 755-nm alexandrite laser with a pulse duration of 550-750 ns.
A single treatment cleared 75% of red pigment in tattoos on nine patients treated with the 532-nm picosecond laser. Red ink cleared completely in two patients after a second or third session, Dr. Hamad Al-Abdulrazzaq and his associates reported at the meeting.
Difficult-to-remove yellow pigment lightened by 75% in three patients after two to four treatments with the 532-ns picosecond laser.
Black ink cleared by 50%-75% after one or two treatments in four patients using the 1,064-ps laser, reported Dr. Al-Abdulrazzaq, a fellow at the Laser and Skin Surgery Center of New York.
Although there were no signs of scarring or textural skin changes after treatment, one patient developed paradoxical darkening that was removed using the 1,064-nm picosecond laser. Other adverse events were as expected with laser treatment, including erythema, edema, hypopigmentation, pain, mild blistering, and crust formation that healed within a week, Dr. Al-Abdulrazzaq said.
Nearly 90% of patients reported satisfaction with treatment, and nearly 70% said they were extremely satisfied.
A separate study compared single-pass to dual-pass treatments for 26 tattoos on 20 patients using a 755-nm, 750-ps alexandrite laser. Half of each tattoo received a single pass and the other half underwent two passes separated by a 20-minute interval at multiple treatment sessions.
After the first session, the two-pass method produced a 3.4-fold improvement in pigment, significantly better than a 2.1-fold improvement seen after a single-pass treatment, Dr. Trenton Custis and his associates reported. After three treatment sessions, pigment had improved 4.5-fold using the dual-pass method and 3.3-fold using the single-pass method, a statistically significant difference.
Lasers whose pulse durations are in the picosecond range produce a very high peak power that yields more rapid heating and photoacoustic shattering of the ink, which may allow for fewer treatments compared with conventional laser methods, said Dr. Custis, a procedural dermatology fellow at the University of California, Davis.
A separate dose-ranging study applied test spots of 1.3-4.1 J/cm2 over tattoos and adjacent normal skin on 18 patients using a 755-nm alexandrite laser. The best-tolerated fluence based on the test spots was used for half of each tattoo over the course of four treatments, and the other half of each tattoo received escalating doses not to exceed 4.1 J/cm2.
Initial laser treatment using lower fluences (1.3-2.0 J/cm2) were best tolerated, Dr. Emil A. Tanghetti and his associates reported. Higher initial doses were associated with discomfort, blistering, transient hypopigmentation, and postinflammatory hyperpigmentation, said Dr. Tanghetti of the Center for Dermatology and Laser Surgery, Sacramento, Calif.
The 1.3- to 2.0-J/cm2 fluences produced significant clearing of the tattoo, but dose escalation up to 4.1 J/cm2 appeared to better clear the tattoos with minimal side effects. "Dose escalation rather than high-dose treatment is tolerated best by patients with less downtime and blistering," he said. Fluences of 2 J/cm2 seemed to be necessary for consistent clearing of tattoos over four treatments. Blue, green, and light-black ink often disappeared after one treatment.
"Based upon this data and our experience, the picosecond alexandrite is our laser of choice for most of our tattoo patients," Dr. Tanghetti said. It’s important to consider skin type, tanning history, and other variables when choosing a fluence, he added.
An estimated 20% of U.S. residents have a tattoo. Requests for laser tattoo removal increased by 52% from 2012 to 2013, Dr. Al-Abdulrazzaq said.
Dr. Tanghetti reported financial associations with Cynosure. Dr. Al-Abdulrazzaq reported having no disclosures; one of his coinvestigators reported financial associations with Cynosure and four other companies. Dr. Custis reported financial associations with Cynosure and 12 other companies.
On Twitter @sherryboschert
PHOENIX – Three small case series reported at the annual meeting of the American Society for Laser Medicine and Surgery reported good results using a picosecond laser for tattoo removal and explored different regimens for treatment.
One study at a single center used a 532-nm Nd-YAG laser with a pulse duration of 450-500 picoseconds (ps) in all 17 patients. Up to 10 treatments spaced every 4-8 weeks used a spot size of 2.5-5 mm, an energy density of 0.3-2.2 J/cm2, and a frequency of 5 Hz in a single pass. Some patients also were treated with a 1,064-nm Nd:YAG laser with a pulse duration of 500-600 ns or a 755-nm alexandrite laser with a pulse duration of 550-750 ns.
A single treatment cleared 75% of red pigment in tattoos on nine patients treated with the 532-nm picosecond laser. Red ink cleared completely in two patients after a second or third session, Dr. Hamad Al-Abdulrazzaq and his associates reported at the meeting.
Difficult-to-remove yellow pigment lightened by 75% in three patients after two to four treatments with the 532-ns picosecond laser.
Black ink cleared by 50%-75% after one or two treatments in four patients using the 1,064-ps laser, reported Dr. Al-Abdulrazzaq, a fellow at the Laser and Skin Surgery Center of New York.
Although there were no signs of scarring or textural skin changes after treatment, one patient developed paradoxical darkening that was removed using the 1,064-nm picosecond laser. Other adverse events were as expected with laser treatment, including erythema, edema, hypopigmentation, pain, mild blistering, and crust formation that healed within a week, Dr. Al-Abdulrazzaq said.
Nearly 90% of patients reported satisfaction with treatment, and nearly 70% said they were extremely satisfied.
A separate study compared single-pass to dual-pass treatments for 26 tattoos on 20 patients using a 755-nm, 750-ps alexandrite laser. Half of each tattoo received a single pass and the other half underwent two passes separated by a 20-minute interval at multiple treatment sessions.
After the first session, the two-pass method produced a 3.4-fold improvement in pigment, significantly better than a 2.1-fold improvement seen after a single-pass treatment, Dr. Trenton Custis and his associates reported. After three treatment sessions, pigment had improved 4.5-fold using the dual-pass method and 3.3-fold using the single-pass method, a statistically significant difference.
Lasers whose pulse durations are in the picosecond range produce a very high peak power that yields more rapid heating and photoacoustic shattering of the ink, which may allow for fewer treatments compared with conventional laser methods, said Dr. Custis, a procedural dermatology fellow at the University of California, Davis.
A separate dose-ranging study applied test spots of 1.3-4.1 J/cm2 over tattoos and adjacent normal skin on 18 patients using a 755-nm alexandrite laser. The best-tolerated fluence based on the test spots was used for half of each tattoo over the course of four treatments, and the other half of each tattoo received escalating doses not to exceed 4.1 J/cm2.
Initial laser treatment using lower fluences (1.3-2.0 J/cm2) were best tolerated, Dr. Emil A. Tanghetti and his associates reported. Higher initial doses were associated with discomfort, blistering, transient hypopigmentation, and postinflammatory hyperpigmentation, said Dr. Tanghetti of the Center for Dermatology and Laser Surgery, Sacramento, Calif.
The 1.3- to 2.0-J/cm2 fluences produced significant clearing of the tattoo, but dose escalation up to 4.1 J/cm2 appeared to better clear the tattoos with minimal side effects. "Dose escalation rather than high-dose treatment is tolerated best by patients with less downtime and blistering," he said. Fluences of 2 J/cm2 seemed to be necessary for consistent clearing of tattoos over four treatments. Blue, green, and light-black ink often disappeared after one treatment.
"Based upon this data and our experience, the picosecond alexandrite is our laser of choice for most of our tattoo patients," Dr. Tanghetti said. It’s important to consider skin type, tanning history, and other variables when choosing a fluence, he added.
An estimated 20% of U.S. residents have a tattoo. Requests for laser tattoo removal increased by 52% from 2012 to 2013, Dr. Al-Abdulrazzaq said.
Dr. Tanghetti reported financial associations with Cynosure. Dr. Al-Abdulrazzaq reported having no disclosures; one of his coinvestigators reported financial associations with Cynosure and four other companies. Dr. Custis reported financial associations with Cynosure and 12 other companies.
On Twitter @sherryboschert
PHOENIX – Three small case series reported at the annual meeting of the American Society for Laser Medicine and Surgery reported good results using a picosecond laser for tattoo removal and explored different regimens for treatment.
One study at a single center used a 532-nm Nd-YAG laser with a pulse duration of 450-500 picoseconds (ps) in all 17 patients. Up to 10 treatments spaced every 4-8 weeks used a spot size of 2.5-5 mm, an energy density of 0.3-2.2 J/cm2, and a frequency of 5 Hz in a single pass. Some patients also were treated with a 1,064-nm Nd:YAG laser with a pulse duration of 500-600 ns or a 755-nm alexandrite laser with a pulse duration of 550-750 ns.
A single treatment cleared 75% of red pigment in tattoos on nine patients treated with the 532-nm picosecond laser. Red ink cleared completely in two patients after a second or third session, Dr. Hamad Al-Abdulrazzaq and his associates reported at the meeting.
Difficult-to-remove yellow pigment lightened by 75% in three patients after two to four treatments with the 532-ns picosecond laser.
Black ink cleared by 50%-75% after one or two treatments in four patients using the 1,064-ps laser, reported Dr. Al-Abdulrazzaq, a fellow at the Laser and Skin Surgery Center of New York.
Although there were no signs of scarring or textural skin changes after treatment, one patient developed paradoxical darkening that was removed using the 1,064-nm picosecond laser. Other adverse events were as expected with laser treatment, including erythema, edema, hypopigmentation, pain, mild blistering, and crust formation that healed within a week, Dr. Al-Abdulrazzaq said.
Nearly 90% of patients reported satisfaction with treatment, and nearly 70% said they were extremely satisfied.
A separate study compared single-pass to dual-pass treatments for 26 tattoos on 20 patients using a 755-nm, 750-ps alexandrite laser. Half of each tattoo received a single pass and the other half underwent two passes separated by a 20-minute interval at multiple treatment sessions.
After the first session, the two-pass method produced a 3.4-fold improvement in pigment, significantly better than a 2.1-fold improvement seen after a single-pass treatment, Dr. Trenton Custis and his associates reported. After three treatment sessions, pigment had improved 4.5-fold using the dual-pass method and 3.3-fold using the single-pass method, a statistically significant difference.
Lasers whose pulse durations are in the picosecond range produce a very high peak power that yields more rapid heating and photoacoustic shattering of the ink, which may allow for fewer treatments compared with conventional laser methods, said Dr. Custis, a procedural dermatology fellow at the University of California, Davis.
A separate dose-ranging study applied test spots of 1.3-4.1 J/cm2 over tattoos and adjacent normal skin on 18 patients using a 755-nm alexandrite laser. The best-tolerated fluence based on the test spots was used for half of each tattoo over the course of four treatments, and the other half of each tattoo received escalating doses not to exceed 4.1 J/cm2.
Initial laser treatment using lower fluences (1.3-2.0 J/cm2) were best tolerated, Dr. Emil A. Tanghetti and his associates reported. Higher initial doses were associated with discomfort, blistering, transient hypopigmentation, and postinflammatory hyperpigmentation, said Dr. Tanghetti of the Center for Dermatology and Laser Surgery, Sacramento, Calif.
The 1.3- to 2.0-J/cm2 fluences produced significant clearing of the tattoo, but dose escalation up to 4.1 J/cm2 appeared to better clear the tattoos with minimal side effects. "Dose escalation rather than high-dose treatment is tolerated best by patients with less downtime and blistering," he said. Fluences of 2 J/cm2 seemed to be necessary for consistent clearing of tattoos over four treatments. Blue, green, and light-black ink often disappeared after one treatment.
"Based upon this data and our experience, the picosecond alexandrite is our laser of choice for most of our tattoo patients," Dr. Tanghetti said. It’s important to consider skin type, tanning history, and other variables when choosing a fluence, he added.
An estimated 20% of U.S. residents have a tattoo. Requests for laser tattoo removal increased by 52% from 2012 to 2013, Dr. Al-Abdulrazzaq said.
Dr. Tanghetti reported financial associations with Cynosure. Dr. Al-Abdulrazzaq reported having no disclosures; one of his coinvestigators reported financial associations with Cynosure and four other companies. Dr. Custis reported financial associations with Cynosure and 12 other companies.
On Twitter @sherryboschert
AT LASER 2014