User login
Restrict Mohs surgery or risk drop in reimbursement
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Dermatologists and dermatologic surgeons risk a decreased reimbursement value for Mohs surgery if they don’t review the published criteria on its appropriate use, according to Dr. Sumaira Aasi.
Although it can be difficult, it is worth the effort to convince some patients that electrodessication, a curettage procedure, or some other management strategy is appropriate for their skin lesions, she said at the annual meeting of the Pacific Dermatologic Association.
"The reason is, at the end of the day, we might be killing the goose that laid the golden egg ourselves" if too many Mohs surgeries are done for inappropriate reasons, said Dr. Aasi of Stanford (Calif.) University.
SDr. Aasi urged the audience at the meeting to study criteria published in 2012 for the appropriate use of Mohs surgery, and to pay particular attention to scenarios deemed inappropriate for the procedure (J. Am. Acad. Dermatol. 2012;67:531-50).
There are an estimated 4 million nonmelanoma skin cancers in the United States each year, and the use of Mohs surgery increased by 400% from 1995 to 2009, Dr. Aasi said (Dermatol. Clin. 2012;30:167-75).
Some experts argue that the increase is a response to the increase in skin cancer cases, and others say the increasing number of Mohs surgeons is driving the rising rate of the procedure. "Regardless, it’s a problem," Dr. Aasi said.
Federal reimbursement programs don’t care if there is an epidemic of skin cancer, she said. If a surgery is being "overused," it is considered overvalued, and reimbursement rates are subject to change, she explained.
The top two CPT codes flagged by the federal government as potentially misvalued in the July 19, 2013, Federal Register were codes 17311 for Mohs surgery on the face, head, and neck and 17313 for Mohs surgery on the trunk and extremities, Dr. Aasi noted.
The 2012 appropriate-use criteria were created to help maintain the value of Mohs surgery in Medicare reimbursements, Dr. Aasi said. The American Academy of Dermatology, the American Society of Dermatologic Surgery, the American College of Mohs Surgery, and the American Society of Mohs Surgery convened a consensus panel of eight Mohs surgeons and nine non-Mohs surgeons. The panel considered 270 clinical scenarios and rated 74% appropriate for Mohs surgery, 17% inappropriate, and 9% uncertain, meaning there were conflicting data in the literature or not enough information to make a determination of the appropriateness of Mohs surgery in that scenario.
"I still think that Mohs surgeons in this panel were a little highly represented," Dr. Aasi said. Overall, the indications the panel deemed appropriate for Mohs surgery were "pretty generous," she added.
The consensus panel was asked to implicitly consider cost in deciding on the appropriateness of Mohs surgery for each scenario. "It’s an implicit consideration but a very important consideration," said Dr. Aasi.
Invasive melanoma was not included in the scenarios because if its complexity, but some scenarios did include melanoma in situ or lentigo maligna melanoma. The scenarios included different body areas, aggressive or nonaggressive skin cancers, tumor size, and the patient’s immune status. Agreement by at least 12 panel members was considered a consensus on the use of Mohs surgery.
Examples of scenarios that were considered inappropriate for Mohs surgery included nonaggressive basal cell or squamous cell carcinomas smaller than 2 cm on the trunk or extremities. Other lesions deemed inappropriate for Mohs included primary actinic keratosis (AK) with focal squamous cell cancer in situ, bowenoid AK, or squamous cell carcinoma in situ AK-type, labels that Dr. Aasi found "worrisome" because their definitions are unclear.
"As a Mohs surgeon, I found it a little surprising that they had to consider scenarios where actinic keratoses were being considered a possibility for Mohs surgery," she said.
Physicians can use the appropriate-use criteria to talk with other health care providers, third-party payers, and patients, said Dr. Aasi. Talking with patients can be most difficult, because if they’ve heard of Mohs surgery, they often want "the technique where I [the patient] know right away that I’m going to be clear," she said.
When Mohs surgery is inappropriate, "I try to convince them that, no, you don’t need it. It’s like an atomic bomb being used to kill a flea," she said.
The appropriate-use criteria do not compare different treatment modalities with Mohs surgery, Dr. Aasi emphasized, but they aim to state simply whether or not Mohs surgery would be appropriate in selected scenarios.
"I urge everyone to read" the criteria, she said.
Dr. Aasi reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Add Education, Vitamin D to Eczema Management
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
Add education, vitamin D to eczema management
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Treatment for severe eczema should begin with education rather than first-line agents, according to Dr. Timothy G. Berger.
Data reported within the past year from a Japanese study convinced Dr. Berger to modify his therapeutic ladder for severe eczema, he said.
He now starts with greater patient and parental education before prescribing first-line treatments and checks vitamin D levels in patients with severe eczema and frequent infection. He chooses among systemic immunomodulators based on a recent practice update. And as a last resort, for the most difficult cases, he uses omalizumab or photophoresis, he said.
Dr. Berger described his treatment ladder approach at the annual meeting of the Pacific Dermatologic Association:
• First rung: education. He used to start up the therapeutic ladder by prescribing topical steroids, moisturization, and antihistamines. Data from the Japanese study persuaded him to insert more intensive education before sending patients and parents on their way.
The study randomized the mothers of 59 children with moderate to severe atopic dermatitis to attend a 2-day parental education program about managing the disease or to receive a booklet about the disease and conventional care. Six months later, patients in the education group had "dramatically better scores" on the Scoring Atopic Dermatitis (SCORAD) tool and on measures of itching, sleeplessness, and family impact, said Dr. Berger, professor of clinical dermatology at the University of California, San Francisco.
SCORAD scores improved in the education group from 40 at baseline to 15 after treatment, compared with a change from 42 to 27 in the control group (Pediatr. Dermatol. 2013;30:438-43). Parents in the education group also reported less anxiety about steroid use. The patients ranged in age from 6 months to 6 years.
"I think that education is really critical," Dr. Berger said.
• Second rung: first-line treatments. With this foundation of education, Dr. Berger starts patients on topical steroids, moisturizers, and antihistamines.
• Third rung: checking vitamin D levels. A comparison of 95 patients with atopic dermatitis and 58 control patients in another study found that low levels of vitamin D were no more likely in one group than in the other, but frequent skin infections were more likely in the subgroup of patients with atopic dermatitis and low vitamin D levels compared with patients who had the disease and normal vitamin D levels. Vitamin D supplementation in the patients with atopic dermatitis and low vitamin D levels significantly improved SCORAD scores (J. Am. Acad. Dermatol. 2013;69:238-44).
In that study, more than 80% of patients with atopic dermatitis and levels of 25-hydroxyvitamin D below 30 ng/mL developed skin infections compared with less than 20% of patients with atopic dermatitis whose vitamin D levels were above 30 ng/mL, Dr. Berger said. That 30-ng/mL cutoff is the dividing line between normal and abnormal or "insufficient" vitamin D levels, "which I’m not so sure how to interpret," he said. In general, levels below 20 ng/mL are considered vitamin D deficiency.
Now when Dr. Berger sees a patient with atopic dermatitis and frequent infections, he checks vitamin D and looks for levels that are deficient or even close to 10 ng/mL. These patients improve on vitamin D supplementation, he said. Vitamin D is required to deliver antimicrobial peptides to the skin surface, which is the presumed mechanism by which this helps.
He recently used this strategy in a patient with refractory lupus, frequent skin infections, and low vitamin D, he said. Both her lupus and skin infections improved with vitamin D supplementation.
• Fourth, fifth, and sixth rungs. Next he prescribes soak and smear, the conventional therapy of skin hydration and smearing on of topical corticosteroids. Beyond that, phototherapy and then possibly day treatment with ultraviolet light and application of coal tar remain options on his therapeutic ladder. "Day treatment with tar and light works very well for a patient that doesn’t have bullous pemphigoid," he said.
• Seventh rung: systemic immunomodulators. Guidance for choosing among these agents comes from a recent practice update from the American Academy of Allergy, Asthma, and Immunology; the American College of Allergy, Asthma, and Immunology; and the Joint Council of Allergy, Asthma, and Immunology (J. Allergy Clin. Immunol. 2013;131:295-9).
Cyclosporine works fastest, especially if started at higher doses, the recommendations say, but "my experience is that in older patients, it’s hard to use," Dr. Berger added. Oral tacrolimus also works, according to the Dr. Berger, as well as the guidelines.
Beyond that, methotrexate and azathioprine appear to be equally effective, with approximately 80% of patients responding in 3-6 weeks. Ordering an assay to detect deficiency of the thiopurine methyltransferase enzyme helps avoid toxicity from the drug in some patients. Because one study showed that increasing the dose of methotrexate does not help if the patient fails to respond to 15 mg/wk, Dr. Berger said he tries to get to 15 mg/wk for 4-6 weeks and holds that dose if there’s a response.
Another study showed that eczema cleared or almost cleared in around 60% of patients treated with mycophenolate mofetil. "I certainly have used it, but it’s unpredictable and it takes longer to start," he said. "You wait a couple of months to see if it’s going to work and then 20%-30% of the time it’s not going to be good enough."
• Final rung: newer agents. The same practice update recommends a list of newer agents "if you can afford it," Dr. Berger said. Approximately 60% of patients respond, slowly but steadily, to treatment with subcutaneous omalizumab 150-450 mg every 2 weeks. Some patients in studies managed to get off unsustainable levels of systemic steroids with the help of omalizumab. "There really isn’t a rebound" flare when omalizumab is stopped, as happens with cyclosporine, Dr. Berger said. "You kind of get better, then creep along. So if the quality of life is moderately bad, you get to less bad."
Tumor necrosis factor inhibitors work as induction therapy but not for maintenance, so they’re "probably not useful," he said. Anecdotal reports suggest that high-dose intravenous immunoglobulin may help.
For the most refractory patients, extracorporeal photophoresis can improve SCORAD scores by 50%. "The itch and SCORAD drop pretty quickly, but you have to maintain it" with omalizumab or another strategy, Dr. Berger said.
"If everything absolutely fails, that’s a backup," he said. "It does suggest that there will be new ways to manage patients that we don’t have available now."
Dr. Berger reported having no relevant financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE PDA ANNUAL MEETING
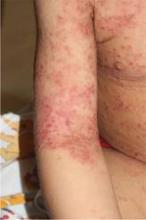
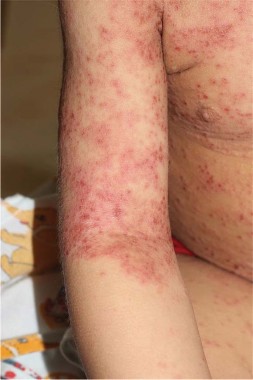
Isotretinoin duration changes game for solid facial edema
SAN FRANCISCO – None of Dr. Timothy G. Berger’s patients with solid facial edema had responded to treatment with oral isotretinoin, so he was puzzled when New York dermatologists reported success in five out of five cases of patients with Morbihan disease, considered by most clinicians as a late-stage complication of rosacea. What was the secret to their success?
The answer: The successful patients took high doses for a prolonged treatment period – at least 6 months – before there was any sign of improvement.
"I was bailing out too soon. I didn’t treat long enough," said Dr. Berger of the University of California, San Francisco. "I’m now changing the way that I approach these patients," he said during a presentation at the annual meeting of the Pacific Dermatologic Association.
The average dose for the five patients in the report was 60 mg/day (ranging from 40 to 80 mg/day), with a mean treatment period of 16 months (and a range of 10 to 24 months). Patients received a mean cumulative dose of isotretinoin of 285 mg/kg (ranging from 170 to 491 mg/kg). The mean disease-free follow-up period was 9 months (ranging from 1 to 24 months), the researchers wrote (Arch. Derm. 2012;148:1395-8). However, the researchers noted that they saw no substantial clinical improvement in the patients until at least 6 months.
Both the daily and cumulative doses used in the study are high, Dr. Berger noted. "If you have patients with solid facial edema and you give them oral retinoids, you have to tell them ahead of time, ‘We have to get you up to this dose and we have to treat you for at least 6 months,’ " he said.
Solid facial edema "is a really difficult disease to treat," Dr. Berger said. Conventional therapy for solid facial edema includes systemic anti-inflammatory drugs, but the clinical response often is unsatisfactory, he noted. The findings were limited by the small number of patients, but the results represent an encouraging option for a challenging condition.
"This paper taught me a lot," Dr. Berger added.
Dr. Berger reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – None of Dr. Timothy G. Berger’s patients with solid facial edema had responded to treatment with oral isotretinoin, so he was puzzled when New York dermatologists reported success in five out of five cases of patients with Morbihan disease, considered by most clinicians as a late-stage complication of rosacea. What was the secret to their success?
The answer: The successful patients took high doses for a prolonged treatment period – at least 6 months – before there was any sign of improvement.
"I was bailing out too soon. I didn’t treat long enough," said Dr. Berger of the University of California, San Francisco. "I’m now changing the way that I approach these patients," he said during a presentation at the annual meeting of the Pacific Dermatologic Association.
The average dose for the five patients in the report was 60 mg/day (ranging from 40 to 80 mg/day), with a mean treatment period of 16 months (and a range of 10 to 24 months). Patients received a mean cumulative dose of isotretinoin of 285 mg/kg (ranging from 170 to 491 mg/kg). The mean disease-free follow-up period was 9 months (ranging from 1 to 24 months), the researchers wrote (Arch. Derm. 2012;148:1395-8). However, the researchers noted that they saw no substantial clinical improvement in the patients until at least 6 months.
Both the daily and cumulative doses used in the study are high, Dr. Berger noted. "If you have patients with solid facial edema and you give them oral retinoids, you have to tell them ahead of time, ‘We have to get you up to this dose and we have to treat you for at least 6 months,’ " he said.
Solid facial edema "is a really difficult disease to treat," Dr. Berger said. Conventional therapy for solid facial edema includes systemic anti-inflammatory drugs, but the clinical response often is unsatisfactory, he noted. The findings were limited by the small number of patients, but the results represent an encouraging option for a challenging condition.
"This paper taught me a lot," Dr. Berger added.
Dr. Berger reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – None of Dr. Timothy G. Berger’s patients with solid facial edema had responded to treatment with oral isotretinoin, so he was puzzled when New York dermatologists reported success in five out of five cases of patients with Morbihan disease, considered by most clinicians as a late-stage complication of rosacea. What was the secret to their success?
The answer: The successful patients took high doses for a prolonged treatment period – at least 6 months – before there was any sign of improvement.
"I was bailing out too soon. I didn’t treat long enough," said Dr. Berger of the University of California, San Francisco. "I’m now changing the way that I approach these patients," he said during a presentation at the annual meeting of the Pacific Dermatologic Association.
The average dose for the five patients in the report was 60 mg/day (ranging from 40 to 80 mg/day), with a mean treatment period of 16 months (and a range of 10 to 24 months). Patients received a mean cumulative dose of isotretinoin of 285 mg/kg (ranging from 170 to 491 mg/kg). The mean disease-free follow-up period was 9 months (ranging from 1 to 24 months), the researchers wrote (Arch. Derm. 2012;148:1395-8). However, the researchers noted that they saw no substantial clinical improvement in the patients until at least 6 months.
Both the daily and cumulative doses used in the study are high, Dr. Berger noted. "If you have patients with solid facial edema and you give them oral retinoids, you have to tell them ahead of time, ‘We have to get you up to this dose and we have to treat you for at least 6 months,’ " he said.
Solid facial edema "is a really difficult disease to treat," Dr. Berger said. Conventional therapy for solid facial edema includes systemic anti-inflammatory drugs, but the clinical response often is unsatisfactory, he noted. The findings were limited by the small number of patients, but the results represent an encouraging option for a challenging condition.
"This paper taught me a lot," Dr. Berger added.
Dr. Berger reported having no financial disclosures.
On Twitter @sherryboschert
EXPERT ANALYSIS AT THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
Tumor distance from nipple boosts nomogram results
SAN FRANCISCO – The distance of a breast cancer from a woman’s nipple added to the ability of nomograms to predict sentinel lymph node positivity, based on a study of data from 401 breast cancers.
The data came from 395 patients who had clinical stage T1 (85% of the cohort) or T2 tumors and underwent prebiopsy ultrasounds at the Mayo Clinic, Rochester, Minn. The investigators performed a second review of the images to measure the tumors’ distance from the nipple.
Nomograms published online by the Memorial Sloan-Kettering Cancer Center and by the University of Texas M.D. Anderson Cancer Center do a reasonably good job of discriminating which newly diagnosed breast cancer patients who have not undergone neoadjuvant therapy are likely to have positive and negative sentinel nodes. Using those two nomograms alone on the patients in the study produced an area under the curve (AUC) of 0.71 for the Memorial Sloan-Kettering nomogram and 0.74 for the M.D. Anderson nomogram. Adding a tumor-to-nipple distance of 2 cm or less improved the AUCs to 0.73 and 0.76, respectively, Dr. Miraj Shah-Khan and her coinvestigators reported.
Sentinel lymph nodes were positive in 20% of the 401 tumors; 17 of 33 tumors within 2 cm of the nipple had positive sentinel lymph nodes (52%), compared with 17% of 368 tumors farther than 2 cm from the nipple, Dr. Shah-Khan, of the Medical College of Wisconsin, Milwaukee, and her associates reported in a poster presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In each subgroup of probability predicted by the nomograms, tumor-to-nipple distance helped to refine the probability of a positive node.
When the Memorial Sloan-Kettering nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 35%, compared with 11% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 62% if a tumor was within 2 cm of the nipple or 29% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 32% if it was farther away.
When the M.D. Anderson nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 42%, compared with 14% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 83% if a tumor was within 2 cm of the nipple or 40% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 67% if it was farther away, she reported.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Shah-Khan reported having no financial disclosures.
On Twitter @sherryboschert
The Mayo Clinic group has previously proposed that the tumor’s distance from the nipple is a predictive factor of whether there is tumor in the sentinel node. There are data showing that the distance from the nipple affects the positivity rate.
 |
|
This study factors in the M.D. Anderson and the Memorial Sloan-Kettering nomograms to create a more robust model for predicting sentinel node positivity. The distance from the nipple and the distance from the skin, when used in addition to the nomogram, increase the predictive ability.
If you are a user of nomograms or if you are a selective user of nomograms for some patients with invasive breast cancer who are still on the fence about whether or not they should undergo a sentinel node procedure, this additional measure gives you a bit more ability to predict the positivity rate with more certainty. I’m a very selective user of nomograms because the individual patient in front of me is not a statistic. For me, nomograms become useful only for those patients who are fence-sitters. It is not a routine part of my practice.
Dr. David W. Ollila is a professor of surgical oncology at the University of North Carolina, Chapel Hill. He reported having no financial disclosures.
The Mayo Clinic group has previously proposed that the tumor’s distance from the nipple is a predictive factor of whether there is tumor in the sentinel node. There are data showing that the distance from the nipple affects the positivity rate.
 |
|
This study factors in the M.D. Anderson and the Memorial Sloan-Kettering nomograms to create a more robust model for predicting sentinel node positivity. The distance from the nipple and the distance from the skin, when used in addition to the nomogram, increase the predictive ability.
If you are a user of nomograms or if you are a selective user of nomograms for some patients with invasive breast cancer who are still on the fence about whether or not they should undergo a sentinel node procedure, this additional measure gives you a bit more ability to predict the positivity rate with more certainty. I’m a very selective user of nomograms because the individual patient in front of me is not a statistic. For me, nomograms become useful only for those patients who are fence-sitters. It is not a routine part of my practice.
Dr. David W. Ollila is a professor of surgical oncology at the University of North Carolina, Chapel Hill. He reported having no financial disclosures.
The Mayo Clinic group has previously proposed that the tumor’s distance from the nipple is a predictive factor of whether there is tumor in the sentinel node. There are data showing that the distance from the nipple affects the positivity rate.
 |
|
This study factors in the M.D. Anderson and the Memorial Sloan-Kettering nomograms to create a more robust model for predicting sentinel node positivity. The distance from the nipple and the distance from the skin, when used in addition to the nomogram, increase the predictive ability.
If you are a user of nomograms or if you are a selective user of nomograms for some patients with invasive breast cancer who are still on the fence about whether or not they should undergo a sentinel node procedure, this additional measure gives you a bit more ability to predict the positivity rate with more certainty. I’m a very selective user of nomograms because the individual patient in front of me is not a statistic. For me, nomograms become useful only for those patients who are fence-sitters. It is not a routine part of my practice.
Dr. David W. Ollila is a professor of surgical oncology at the University of North Carolina, Chapel Hill. He reported having no financial disclosures.
SAN FRANCISCO – The distance of a breast cancer from a woman’s nipple added to the ability of nomograms to predict sentinel lymph node positivity, based on a study of data from 401 breast cancers.
The data came from 395 patients who had clinical stage T1 (85% of the cohort) or T2 tumors and underwent prebiopsy ultrasounds at the Mayo Clinic, Rochester, Minn. The investigators performed a second review of the images to measure the tumors’ distance from the nipple.
Nomograms published online by the Memorial Sloan-Kettering Cancer Center and by the University of Texas M.D. Anderson Cancer Center do a reasonably good job of discriminating which newly diagnosed breast cancer patients who have not undergone neoadjuvant therapy are likely to have positive and negative sentinel nodes. Using those two nomograms alone on the patients in the study produced an area under the curve (AUC) of 0.71 for the Memorial Sloan-Kettering nomogram and 0.74 for the M.D. Anderson nomogram. Adding a tumor-to-nipple distance of 2 cm or less improved the AUCs to 0.73 and 0.76, respectively, Dr. Miraj Shah-Khan and her coinvestigators reported.
Sentinel lymph nodes were positive in 20% of the 401 tumors; 17 of 33 tumors within 2 cm of the nipple had positive sentinel lymph nodes (52%), compared with 17% of 368 tumors farther than 2 cm from the nipple, Dr. Shah-Khan, of the Medical College of Wisconsin, Milwaukee, and her associates reported in a poster presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In each subgroup of probability predicted by the nomograms, tumor-to-nipple distance helped to refine the probability of a positive node.
When the Memorial Sloan-Kettering nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 35%, compared with 11% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 62% if a tumor was within 2 cm of the nipple or 29% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 32% if it was farther away.
When the M.D. Anderson nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 42%, compared with 14% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 83% if a tumor was within 2 cm of the nipple or 40% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 67% if it was farther away, she reported.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Shah-Khan reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The distance of a breast cancer from a woman’s nipple added to the ability of nomograms to predict sentinel lymph node positivity, based on a study of data from 401 breast cancers.
The data came from 395 patients who had clinical stage T1 (85% of the cohort) or T2 tumors and underwent prebiopsy ultrasounds at the Mayo Clinic, Rochester, Minn. The investigators performed a second review of the images to measure the tumors’ distance from the nipple.
Nomograms published online by the Memorial Sloan-Kettering Cancer Center and by the University of Texas M.D. Anderson Cancer Center do a reasonably good job of discriminating which newly diagnosed breast cancer patients who have not undergone neoadjuvant therapy are likely to have positive and negative sentinel nodes. Using those two nomograms alone on the patients in the study produced an area under the curve (AUC) of 0.71 for the Memorial Sloan-Kettering nomogram and 0.74 for the M.D. Anderson nomogram. Adding a tumor-to-nipple distance of 2 cm or less improved the AUCs to 0.73 and 0.76, respectively, Dr. Miraj Shah-Khan and her coinvestigators reported.
Sentinel lymph nodes were positive in 20% of the 401 tumors; 17 of 33 tumors within 2 cm of the nipple had positive sentinel lymph nodes (52%), compared with 17% of 368 tumors farther than 2 cm from the nipple, Dr. Shah-Khan, of the Medical College of Wisconsin, Milwaukee, and her associates reported in a poster presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In each subgroup of probability predicted by the nomograms, tumor-to-nipple distance helped to refine the probability of a positive node.
When the Memorial Sloan-Kettering nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 35%, compared with 11% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 62% if a tumor was within 2 cm of the nipple or 29% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 32% if it was farther away.
When the M.D. Anderson nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 42%, compared with 14% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 83% if a tumor was within 2 cm of the nipple or 40% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 67% if it was farther away, she reported.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Shah-Khan reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Positive sentinel nodes were seen in 17 of 33 (52%) tumors within 2 cm of the nipple, compared with 17% of 368 tumors more than 2 cm from the nipple.
Data source: A second review of prebiopsy ultrasound images of 401 clinical T1 or T2 breast cancer tumors in 395 patients and comparison with nomograms for predicting sentinel node positivity.
Disclosures: Dr. Shah-Khan reported having no financial disclosures.
Oral therapy for metastatic breast cancer grows in Medicare patients
SAN FRANCISCO – Injectable fulvestrant and oral anastrozole were the top picks for first-, second- and third-line treatment of metastatic breast cancer in women enrolled in Medicare Part D, based on a retrospective study of 681 women.
Total treatment costs averaged $102,000/patient, including costs for physicians, inpatient and outpatient care, hospice, skilled nursing, and durable medical equipment, reported Dr. Hope S. Rugo and her associates.
Their analysis updates a similar previous study that did not include data on oral medications. Thus, this study provides a more complete picture of treatment patterns and costs for patients with metastatic breast cancer. Oral medications now comprise three of the five most common first-, second- or third-line therapies in women who developed metastatic breast cancer, according to the researchers.
The investigators analyzed SEER (Surveillance Epidemiology and End Results) cancer registry data and Medicare data for 7,905 women who were diagnosed with breast cancer in 2001-2007 with concurrent or subsequent metastases and were enrolled in Medicare from 12 months prior to diagnosis through 2009 or death. Of these, 82% received first-line treatment. Data on oral therapies were available only for the 681 patients enrolled in Medicare Part D, the federal program that subsidizes the costs of prescription drugs for Medicare beneficiaries.
In the overall sample, 48% of the women went on to second-line therapy and 26% had third-line therapy. In the Part D subgroup, 63% went on to second-line therapy and 45% had third-line therapy, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The mean total cost per patient in the cohort as a whole was $127,000. Fulvestrant was the top choice for first-line therapy, used in 19%. Injectable vinorelbine was the most common second-line chemotherapy, used in 18% of those who got second-line treatment and in 16% of those who got third-line therapy, she reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The findings were similar to those from a previous report presented by another group of investigators at the 2011 Breast Cancer Symposium. Those researchers used SEER data on women diagnosed in 2001-2005 and enrolled in Medicare until 2008 or death. Fulvestrant was most common as first-line therapy and vinorelbine was most common for second- and third-line treatment. The mean per-patient cost in that study was $110,000.
In the current analysis of the 681 patients enrolled in Medicare Part D, fulvestrant still was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Of the 427 patients in the Part D subgroup who got second-line therapy, 19% received fulvestrant, 9% got anastrozole, 8% got letrozole, 7% received vinorelbine, and 4% took tamoxifen.
For third-line treatment of 309 patients in the Part D subgroup, anastrozole and fulvestrant tied for top choice (11% each), tamoxifen or letrozole was used in 7% each, and vinorelbine was used in 6%.
The total costs per drug were highest for vinorelbine: a mean of $155,000 in second-line treatment and $134,000 in third-line treatment. In comparison, mean costs for the other top second-line therapies were $111,000 for fulvestrant, $106,000 for anastrozole, $108,000 for letrozole, and $107,000 for tamoxifen. Mean costs for other third-line treatments were $110,000 for anastrozole, $100,000 for fulvestrant, $101,000 for tamoxifen, and $71,000 for letrozole.
The study excluded patients with a history of other cancers before a diagnosis of breast cancer, patients who were not eligible for Medicare Part A or Part B benefits, patients enrolled in health maintenance organizations, and those who were first diagnosed with metastatic breast cancer at the time of death or autopsy.
The data did not identify disease progression, so the investigators used a published algorithm to identify the date of metastases. They also created an algorithm to identify first-, second- and third-line treatments in the data, based on factors such as the length of time before administering a new agent. These methods may have misclassified some treatments. Other limitations of the study include using Medicare data from 2001-2007, which may not reflect recent advances in treatment. SEER data cover just 28% of the U.S. population, and 90% of the study population was 65 years in age or older, so the cohort may not be representative of all patients with metastatic breast cancer.
The symposium was co-sponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Injectable fulvestrant and oral anastrozole were the top picks for first-, second- and third-line treatment of metastatic breast cancer in women enrolled in Medicare Part D, based on a retrospective study of 681 women.
Total treatment costs averaged $102,000/patient, including costs for physicians, inpatient and outpatient care, hospice, skilled nursing, and durable medical equipment, reported Dr. Hope S. Rugo and her associates.
Their analysis updates a similar previous study that did not include data on oral medications. Thus, this study provides a more complete picture of treatment patterns and costs for patients with metastatic breast cancer. Oral medications now comprise three of the five most common first-, second- or third-line therapies in women who developed metastatic breast cancer, according to the researchers.
The investigators analyzed SEER (Surveillance Epidemiology and End Results) cancer registry data and Medicare data for 7,905 women who were diagnosed with breast cancer in 2001-2007 with concurrent or subsequent metastases and were enrolled in Medicare from 12 months prior to diagnosis through 2009 or death. Of these, 82% received first-line treatment. Data on oral therapies were available only for the 681 patients enrolled in Medicare Part D, the federal program that subsidizes the costs of prescription drugs for Medicare beneficiaries.
In the overall sample, 48% of the women went on to second-line therapy and 26% had third-line therapy. In the Part D subgroup, 63% went on to second-line therapy and 45% had third-line therapy, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The mean total cost per patient in the cohort as a whole was $127,000. Fulvestrant was the top choice for first-line therapy, used in 19%. Injectable vinorelbine was the most common second-line chemotherapy, used in 18% of those who got second-line treatment and in 16% of those who got third-line therapy, she reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The findings were similar to those from a previous report presented by another group of investigators at the 2011 Breast Cancer Symposium. Those researchers used SEER data on women diagnosed in 2001-2005 and enrolled in Medicare until 2008 or death. Fulvestrant was most common as first-line therapy and vinorelbine was most common for second- and third-line treatment. The mean per-patient cost in that study was $110,000.
In the current analysis of the 681 patients enrolled in Medicare Part D, fulvestrant still was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Of the 427 patients in the Part D subgroup who got second-line therapy, 19% received fulvestrant, 9% got anastrozole, 8% got letrozole, 7% received vinorelbine, and 4% took tamoxifen.
For third-line treatment of 309 patients in the Part D subgroup, anastrozole and fulvestrant tied for top choice (11% each), tamoxifen or letrozole was used in 7% each, and vinorelbine was used in 6%.
The total costs per drug were highest for vinorelbine: a mean of $155,000 in second-line treatment and $134,000 in third-line treatment. In comparison, mean costs for the other top second-line therapies were $111,000 for fulvestrant, $106,000 for anastrozole, $108,000 for letrozole, and $107,000 for tamoxifen. Mean costs for other third-line treatments were $110,000 for anastrozole, $100,000 for fulvestrant, $101,000 for tamoxifen, and $71,000 for letrozole.
The study excluded patients with a history of other cancers before a diagnosis of breast cancer, patients who were not eligible for Medicare Part A or Part B benefits, patients enrolled in health maintenance organizations, and those who were first diagnosed with metastatic breast cancer at the time of death or autopsy.
The data did not identify disease progression, so the investigators used a published algorithm to identify the date of metastases. They also created an algorithm to identify first-, second- and third-line treatments in the data, based on factors such as the length of time before administering a new agent. These methods may have misclassified some treatments. Other limitations of the study include using Medicare data from 2001-2007, which may not reflect recent advances in treatment. SEER data cover just 28% of the U.S. population, and 90% of the study population was 65 years in age or older, so the cohort may not be representative of all patients with metastatic breast cancer.
The symposium was co-sponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Injectable fulvestrant and oral anastrozole were the top picks for first-, second- and third-line treatment of metastatic breast cancer in women enrolled in Medicare Part D, based on a retrospective study of 681 women.
Total treatment costs averaged $102,000/patient, including costs for physicians, inpatient and outpatient care, hospice, skilled nursing, and durable medical equipment, reported Dr. Hope S. Rugo and her associates.
Their analysis updates a similar previous study that did not include data on oral medications. Thus, this study provides a more complete picture of treatment patterns and costs for patients with metastatic breast cancer. Oral medications now comprise three of the five most common first-, second- or third-line therapies in women who developed metastatic breast cancer, according to the researchers.
The investigators analyzed SEER (Surveillance Epidemiology and End Results) cancer registry data and Medicare data for 7,905 women who were diagnosed with breast cancer in 2001-2007 with concurrent or subsequent metastases and were enrolled in Medicare from 12 months prior to diagnosis through 2009 or death. Of these, 82% received first-line treatment. Data on oral therapies were available only for the 681 patients enrolled in Medicare Part D, the federal program that subsidizes the costs of prescription drugs for Medicare beneficiaries.
In the overall sample, 48% of the women went on to second-line therapy and 26% had third-line therapy. In the Part D subgroup, 63% went on to second-line therapy and 45% had third-line therapy, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The mean total cost per patient in the cohort as a whole was $127,000. Fulvestrant was the top choice for first-line therapy, used in 19%. Injectable vinorelbine was the most common second-line chemotherapy, used in 18% of those who got second-line treatment and in 16% of those who got third-line therapy, she reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The findings were similar to those from a previous report presented by another group of investigators at the 2011 Breast Cancer Symposium. Those researchers used SEER data on women diagnosed in 2001-2005 and enrolled in Medicare until 2008 or death. Fulvestrant was most common as first-line therapy and vinorelbine was most common for second- and third-line treatment. The mean per-patient cost in that study was $110,000.
In the current analysis of the 681 patients enrolled in Medicare Part D, fulvestrant still was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Of the 427 patients in the Part D subgroup who got second-line therapy, 19% received fulvestrant, 9% got anastrozole, 8% got letrozole, 7% received vinorelbine, and 4% took tamoxifen.
For third-line treatment of 309 patients in the Part D subgroup, anastrozole and fulvestrant tied for top choice (11% each), tamoxifen or letrozole was used in 7% each, and vinorelbine was used in 6%.
The total costs per drug were highest for vinorelbine: a mean of $155,000 in second-line treatment and $134,000 in third-line treatment. In comparison, mean costs for the other top second-line therapies were $111,000 for fulvestrant, $106,000 for anastrozole, $108,000 for letrozole, and $107,000 for tamoxifen. Mean costs for other third-line treatments were $110,000 for anastrozole, $100,000 for fulvestrant, $101,000 for tamoxifen, and $71,000 for letrozole.
The study excluded patients with a history of other cancers before a diagnosis of breast cancer, patients who were not eligible for Medicare Part A or Part B benefits, patients enrolled in health maintenance organizations, and those who were first diagnosed with metastatic breast cancer at the time of death or autopsy.
The data did not identify disease progression, so the investigators used a published algorithm to identify the date of metastases. They also created an algorithm to identify first-, second- and third-line treatments in the data, based on factors such as the length of time before administering a new agent. These methods may have misclassified some treatments. Other limitations of the study include using Medicare data from 2001-2007, which may not reflect recent advances in treatment. SEER data cover just 28% of the U.S. population, and 90% of the study population was 65 years in age or older, so the cohort may not be representative of all patients with metastatic breast cancer.
The symposium was co-sponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In patients enrolled in Medicare Part D, fulvestrant was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Data source: A retrospective study of 681 women who were diagnosed in 2001-2007 with breast cancer, had concurrent or subsequent metastases, and were enrolled in Medicare Part D until 2009 or death.
Disclosures: Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
‘Eczema coxsackium’ cutaneous eruptions characterized
SAN FRANCISCO – A study of cases at seven academic pediatric dermatology centers has more precisely described the cutaneous eruptions caused by a severe form of hand, foot, and mouth disease in U.S. children.
Dermatologists don’t see many cases of hand, foot, and mouth disease because these usually are routine viral infections managed by pediatricians. In the spring of 2012, however, Dr. Erin Mathes and her associates started seeing children with the fevers and GI symptoms typical of hand, foot, and mouth disease, but with a very severe rash with severe blisters, vesicles, and erosions instead of the milder papulovesicles of the hands, feet, buttocks, and mouth seen with the classic disease.
"We were left scratching our heads wondering exactly what was going on" until the Centers for Disease Control and Prevention reported an outbreak of severe hand, foot, and mouth disease associated with coxsackievirus A6 (CVA6) that also had been reported in several other countries (MMWR 2012;61:213-4).
"These reports said that the rash was severe, but they didn’t characterize it very carefully," so she and her colleagues across the country set out to describe the cutaneous manifestations in U.S. patients more specifically, said Dr. Mathes of the University of California, San Francisco.
The 80 cases they reviewed were treated between July 2011 and June 2012 at seven pediatric academic centers. Seventeen were caused by CVA6 infection that was confirmed by polymerase chain reaction (PCR) testing and 63 cases met predefined clinical criteria for inclusion as likely CVA6 infection, she reported at the annual meeting of the Pacific Dermatologic Association. Patients ranged in age from 4 months to 16 years.
Nearly all of the patients had vesiculobullous and erosive eruptions (99% of patients). "Some of the smaller vesicles were more widespread than in classic hand, foot, and mouth disease. We saw a striking perioral distribution," she said. "And then some of our younger patients had quite large blisters," such as a 4-cm blister on the foot of a 4-month-old baby (Pediatrics 2013;132:e149-57 [doi: 10.1542/peds.2012-3175]).
The most interesting finding was an eczema herpeticum–like rash in 55% of patients. From a distance, all of the patients looked like they had eczema herpeticum – erosions and vesicles in areas that previously were affected by atopic dermatitis but closer inspection showed that they weren’t as monomorphous or as "punched out" as erosions from eczema herpeticum. "So, there are some morphologic clues that this is not eczema herpeticum," she said. Most patients tested negative for herpes simplex virus (HSV).
CVA6 now can be added to the short list of HSV-1, varicella, and coxsackievirus A16 as viruses that have been reported to cause a Kaposi’s varicelliform eruption, in which preexisting dermatitis becomes superinfected with virus. Dr. Mathes proposed that the CVA6 eruption be called "eczema coxsackium." "We like this [term] because it brings to mind eczema herpeticum and yet indicates that the virus is coxsackie rather than a herpes virus," she said.
Nineteen percent of patients had locus minoris infection without previous atopic dermatitis and developed blisters and erosions in areas of sunburn, laceration, or irritant dermatitis.
Gianotti-Crosti–like eruptions with papulovesicles on only the cheeks, arms, and legs were seen in 36% of patients.
One of the rarer morphologies that "was confusing to both the pediatricians and the dermatologists" was a petechial and purpuric eruption in 17% of patients, more commonly in older children and in acral areas, she said. Biopsies from several patients showed no evidence of vasculitis, and several patients had confirmed CVA6 infection. "It was more consistent with a viral eruption," she said.
Among nail changes in 25% of patients, Beau’s lines and onychomadesis were common. These probably would have been seen more if patients were followed longitudinally, but the study captured data from just one visit, she noted.
Only half of patients had oropharyngeal ulcerations (50%), a lower percentage than with classic hand, foot, and mouth disease. Dr. Mathes’ impression was that these ulcers tended to occur more commonly in anterior locations, compared with classic disease.
Ten patients were hospitalized for an average of 12 days, mainly for diagnostic work-ups and empiric antibiotic treatment. All recovered with supportive care. There were no cases of severe systemic illness, in contrast with published case series from Asia in which several patients developed aseptic meningitis and other neurologic complications, she said.
Dr. Mathes and her colleagues were wondering if they’d see eczema coxsackium in 2013 until she saw her first case of the year recently. "We think we’ll probably see it in lower numbers given that there is some herd immunity now, and some children will have immunity to it," she said.
The diagnosis is a clinical one. Look for features of hand, foot, and mouth disease with severe rash, and do bacterial and viral cultures to rule out other infections. "We did have some patients who had a superinfection with Staph in addition to their viral infection," she said. Coxsackievirus will not grow in culture; if you want to confirm the diagnosis, use PCR. If you see a severe case in a patient hospitalized with neurologic or cardiac complications, send specimens for diagnostic confirmation to your local health department or the CDC, she advised.
The international reports of severe hand, foot, and mouth disease from CVA6 infection began in 2012 in Finland, and then came from Singapore and Japan. The international reports noted a severe rash, onychomadesis and desquamation, rare neurologic complications, and a high prevalence of infection in school-age children or adults, probably because of low herd immunity. The international investigators were able to obtain the virus for analysis from nail clippings from the patients who had onychomadesis, Dr. Mathes noted.
She reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A study of cases at seven academic pediatric dermatology centers has more precisely described the cutaneous eruptions caused by a severe form of hand, foot, and mouth disease in U.S. children.
Dermatologists don’t see many cases of hand, foot, and mouth disease because these usually are routine viral infections managed by pediatricians. In the spring of 2012, however, Dr. Erin Mathes and her associates started seeing children with the fevers and GI symptoms typical of hand, foot, and mouth disease, but with a very severe rash with severe blisters, vesicles, and erosions instead of the milder papulovesicles of the hands, feet, buttocks, and mouth seen with the classic disease.
"We were left scratching our heads wondering exactly what was going on" until the Centers for Disease Control and Prevention reported an outbreak of severe hand, foot, and mouth disease associated with coxsackievirus A6 (CVA6) that also had been reported in several other countries (MMWR 2012;61:213-4).
"These reports said that the rash was severe, but they didn’t characterize it very carefully," so she and her colleagues across the country set out to describe the cutaneous manifestations in U.S. patients more specifically, said Dr. Mathes of the University of California, San Francisco.
The 80 cases they reviewed were treated between July 2011 and June 2012 at seven pediatric academic centers. Seventeen were caused by CVA6 infection that was confirmed by polymerase chain reaction (PCR) testing and 63 cases met predefined clinical criteria for inclusion as likely CVA6 infection, she reported at the annual meeting of the Pacific Dermatologic Association. Patients ranged in age from 4 months to 16 years.
Nearly all of the patients had vesiculobullous and erosive eruptions (99% of patients). "Some of the smaller vesicles were more widespread than in classic hand, foot, and mouth disease. We saw a striking perioral distribution," she said. "And then some of our younger patients had quite large blisters," such as a 4-cm blister on the foot of a 4-month-old baby (Pediatrics 2013;132:e149-57 [doi: 10.1542/peds.2012-3175]).
The most interesting finding was an eczema herpeticum–like rash in 55% of patients. From a distance, all of the patients looked like they had eczema herpeticum – erosions and vesicles in areas that previously were affected by atopic dermatitis but closer inspection showed that they weren’t as monomorphous or as "punched out" as erosions from eczema herpeticum. "So, there are some morphologic clues that this is not eczema herpeticum," she said. Most patients tested negative for herpes simplex virus (HSV).
CVA6 now can be added to the short list of HSV-1, varicella, and coxsackievirus A16 as viruses that have been reported to cause a Kaposi’s varicelliform eruption, in which preexisting dermatitis becomes superinfected with virus. Dr. Mathes proposed that the CVA6 eruption be called "eczema coxsackium." "We like this [term] because it brings to mind eczema herpeticum and yet indicates that the virus is coxsackie rather than a herpes virus," she said.
Nineteen percent of patients had locus minoris infection without previous atopic dermatitis and developed blisters and erosions in areas of sunburn, laceration, or irritant dermatitis.
Gianotti-Crosti–like eruptions with papulovesicles on only the cheeks, arms, and legs were seen in 36% of patients.
One of the rarer morphologies that "was confusing to both the pediatricians and the dermatologists" was a petechial and purpuric eruption in 17% of patients, more commonly in older children and in acral areas, she said. Biopsies from several patients showed no evidence of vasculitis, and several patients had confirmed CVA6 infection. "It was more consistent with a viral eruption," she said.
Among nail changes in 25% of patients, Beau’s lines and onychomadesis were common. These probably would have been seen more if patients were followed longitudinally, but the study captured data from just one visit, she noted.
Only half of patients had oropharyngeal ulcerations (50%), a lower percentage than with classic hand, foot, and mouth disease. Dr. Mathes’ impression was that these ulcers tended to occur more commonly in anterior locations, compared with classic disease.
Ten patients were hospitalized for an average of 12 days, mainly for diagnostic work-ups and empiric antibiotic treatment. All recovered with supportive care. There were no cases of severe systemic illness, in contrast with published case series from Asia in which several patients developed aseptic meningitis and other neurologic complications, she said.
Dr. Mathes and her colleagues were wondering if they’d see eczema coxsackium in 2013 until she saw her first case of the year recently. "We think we’ll probably see it in lower numbers given that there is some herd immunity now, and some children will have immunity to it," she said.
The diagnosis is a clinical one. Look for features of hand, foot, and mouth disease with severe rash, and do bacterial and viral cultures to rule out other infections. "We did have some patients who had a superinfection with Staph in addition to their viral infection," she said. Coxsackievirus will not grow in culture; if you want to confirm the diagnosis, use PCR. If you see a severe case in a patient hospitalized with neurologic or cardiac complications, send specimens for diagnostic confirmation to your local health department or the CDC, she advised.
The international reports of severe hand, foot, and mouth disease from CVA6 infection began in 2012 in Finland, and then came from Singapore and Japan. The international reports noted a severe rash, onychomadesis and desquamation, rare neurologic complications, and a high prevalence of infection in school-age children or adults, probably because of low herd immunity. The international investigators were able to obtain the virus for analysis from nail clippings from the patients who had onychomadesis, Dr. Mathes noted.
She reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A study of cases at seven academic pediatric dermatology centers has more precisely described the cutaneous eruptions caused by a severe form of hand, foot, and mouth disease in U.S. children.
Dermatologists don’t see many cases of hand, foot, and mouth disease because these usually are routine viral infections managed by pediatricians. In the spring of 2012, however, Dr. Erin Mathes and her associates started seeing children with the fevers and GI symptoms typical of hand, foot, and mouth disease, but with a very severe rash with severe blisters, vesicles, and erosions instead of the milder papulovesicles of the hands, feet, buttocks, and mouth seen with the classic disease.
"We were left scratching our heads wondering exactly what was going on" until the Centers for Disease Control and Prevention reported an outbreak of severe hand, foot, and mouth disease associated with coxsackievirus A6 (CVA6) that also had been reported in several other countries (MMWR 2012;61:213-4).
"These reports said that the rash was severe, but they didn’t characterize it very carefully," so she and her colleagues across the country set out to describe the cutaneous manifestations in U.S. patients more specifically, said Dr. Mathes of the University of California, San Francisco.
The 80 cases they reviewed were treated between July 2011 and June 2012 at seven pediatric academic centers. Seventeen were caused by CVA6 infection that was confirmed by polymerase chain reaction (PCR) testing and 63 cases met predefined clinical criteria for inclusion as likely CVA6 infection, she reported at the annual meeting of the Pacific Dermatologic Association. Patients ranged in age from 4 months to 16 years.
Nearly all of the patients had vesiculobullous and erosive eruptions (99% of patients). "Some of the smaller vesicles were more widespread than in classic hand, foot, and mouth disease. We saw a striking perioral distribution," she said. "And then some of our younger patients had quite large blisters," such as a 4-cm blister on the foot of a 4-month-old baby (Pediatrics 2013;132:e149-57 [doi: 10.1542/peds.2012-3175]).
The most interesting finding was an eczema herpeticum–like rash in 55% of patients. From a distance, all of the patients looked like they had eczema herpeticum – erosions and vesicles in areas that previously were affected by atopic dermatitis but closer inspection showed that they weren’t as monomorphous or as "punched out" as erosions from eczema herpeticum. "So, there are some morphologic clues that this is not eczema herpeticum," she said. Most patients tested negative for herpes simplex virus (HSV).
CVA6 now can be added to the short list of HSV-1, varicella, and coxsackievirus A16 as viruses that have been reported to cause a Kaposi’s varicelliform eruption, in which preexisting dermatitis becomes superinfected with virus. Dr. Mathes proposed that the CVA6 eruption be called "eczema coxsackium." "We like this [term] because it brings to mind eczema herpeticum and yet indicates that the virus is coxsackie rather than a herpes virus," she said.
Nineteen percent of patients had locus minoris infection without previous atopic dermatitis and developed blisters and erosions in areas of sunburn, laceration, or irritant dermatitis.
Gianotti-Crosti–like eruptions with papulovesicles on only the cheeks, arms, and legs were seen in 36% of patients.
One of the rarer morphologies that "was confusing to both the pediatricians and the dermatologists" was a petechial and purpuric eruption in 17% of patients, more commonly in older children and in acral areas, she said. Biopsies from several patients showed no evidence of vasculitis, and several patients had confirmed CVA6 infection. "It was more consistent with a viral eruption," she said.
Among nail changes in 25% of patients, Beau’s lines and onychomadesis were common. These probably would have been seen more if patients were followed longitudinally, but the study captured data from just one visit, she noted.
Only half of patients had oropharyngeal ulcerations (50%), a lower percentage than with classic hand, foot, and mouth disease. Dr. Mathes’ impression was that these ulcers tended to occur more commonly in anterior locations, compared with classic disease.
Ten patients were hospitalized for an average of 12 days, mainly for diagnostic work-ups and empiric antibiotic treatment. All recovered with supportive care. There were no cases of severe systemic illness, in contrast with published case series from Asia in which several patients developed aseptic meningitis and other neurologic complications, she said.
Dr. Mathes and her colleagues were wondering if they’d see eczema coxsackium in 2013 until she saw her first case of the year recently. "We think we’ll probably see it in lower numbers given that there is some herd immunity now, and some children will have immunity to it," she said.
The diagnosis is a clinical one. Look for features of hand, foot, and mouth disease with severe rash, and do bacterial and viral cultures to rule out other infections. "We did have some patients who had a superinfection with Staph in addition to their viral infection," she said. Coxsackievirus will not grow in culture; if you want to confirm the diagnosis, use PCR. If you see a severe case in a patient hospitalized with neurologic or cardiac complications, send specimens for diagnostic confirmation to your local health department or the CDC, she advised.
The international reports of severe hand, foot, and mouth disease from CVA6 infection began in 2012 in Finland, and then came from Singapore and Japan. The international reports noted a severe rash, onychomadesis and desquamation, rare neurologic complications, and a high prevalence of infection in school-age children or adults, probably because of low herd immunity. The international investigators were able to obtain the virus for analysis from nail clippings from the patients who had onychomadesis, Dr. Mathes noted.
She reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
Major finding: The most common cutaneous findings were vesiculobullous and erosive eruptions in 99% of patients, eczema herpeticum-like rash in 55%, Gianotti-Crosti–like eruptions in 36%, nail changes in 25%, and oropharyngeal ulcerations in 50%.
Data source: Retrospective, multicenter review of 80 pediatric patients with atypical cases of hand, foot, and mouth disease caused by a confirmed CVA6 infection or that met predefined criteria for infection.
Disclosures: Dr. Mathes reported having no financial disclosures.
Everolimus effective in women with early failure of adjuvant therapy
SAN FRANCISCO – In patients who had hormone receptor–positive, HER2-negative advanced breast cancer, everolimus plus exemestane was just as effective among women who had a recurrence during or within a year of adjuvant therapy.
The results were seen in a secondary, subgroup analysis of 137 patients in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus 2) study. The subset that had recurrences during or within a year of adjuvant therapy comprised 19% of the patients in the study, and their outcomes were similar to the overall results of the trial, which support the use of everolimus plus exemestane as first-line therapy in postmenopausal patients with hormone receptor–positive advanced breast cancer that recurs after adjuvant therapy, Dr. Hope S. Rugo and her associates reported in a poster session at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In the women who had a recurrence during or within 12 months of adjuvant therapy, everolimus plus exemestane improved median progression-free survival to 11.5 months, compared with 4.1 months with placebo plus exemestane.
The multicenter, double-blind BOLERO-2 study randomized 724 postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. Patients received open-label exemestane plus blinded therapy with either placebo or everolimus, which inhibits the mammalian target of rapamycin (mTOR) signaling pathway. At 18 months of follow-up, patients in the exemestane-plus-everolimus group had a significantly longer median progression-free survival of 11 months as compared to 4 months for the control group (N. Engl. J. Med. 2012;366:520-529).
Similar efficacy was found in various analyses of subgroups in the study, including patients with visceral metastases, patients with bone-only metastases, and patients whose disease recurred after adjuvant therapy.
In the subgroup analysis of patients who entered the study after a recurrence during or within a year of adjuvant therapy, 100 patients received everolimus plus exemestane and 37 received placebo and exemestane. Almost all of the 137 patients had received nonsteroidal aromatase inhibitor therapy as their last therapy before entering the BOLERO-2 trial.
The median progression-free survival rates of 11.5 months with the everolimus combination and 4.1 months with the placebo combination were based on investigators’ local radiologic assessments. Central radiologic assessment by an independent radiology committee confirmed these results, finding that median progression-free survival reached 15.2 months in the everolimus group, compared with 4.2 months in the placebo group. Hazard ratios were 0.39 under the local assessment and 0.32 under the central assessment, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
Rates of the most common grade 3 or 4 adverse events seen in the everolimus-plus-exemestane group in the subset analysis – hyperglycemia in 8%, stomatitis in 4%, diarrhea in 4%, and fatigue in 3% – were similar to rates seen in the overall BOLERO-2 population and were within the known safety profile of everolimus. Some patients in the everolimus group (but not the placebo group) developed pneumonitis or interstitial lung disease, but these were mostly low grade and manageable by conventional strategies.
Baseline characteristics were well balanced in the randomized groups in the subset analysis.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – In patients who had hormone receptor–positive, HER2-negative advanced breast cancer, everolimus plus exemestane was just as effective among women who had a recurrence during or within a year of adjuvant therapy.
The results were seen in a secondary, subgroup analysis of 137 patients in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus 2) study. The subset that had recurrences during or within a year of adjuvant therapy comprised 19% of the patients in the study, and their outcomes were similar to the overall results of the trial, which support the use of everolimus plus exemestane as first-line therapy in postmenopausal patients with hormone receptor–positive advanced breast cancer that recurs after adjuvant therapy, Dr. Hope S. Rugo and her associates reported in a poster session at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In the women who had a recurrence during or within 12 months of adjuvant therapy, everolimus plus exemestane improved median progression-free survival to 11.5 months, compared with 4.1 months with placebo plus exemestane.
The multicenter, double-blind BOLERO-2 study randomized 724 postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. Patients received open-label exemestane plus blinded therapy with either placebo or everolimus, which inhibits the mammalian target of rapamycin (mTOR) signaling pathway. At 18 months of follow-up, patients in the exemestane-plus-everolimus group had a significantly longer median progression-free survival of 11 months as compared to 4 months for the control group (N. Engl. J. Med. 2012;366:520-529).
Similar efficacy was found in various analyses of subgroups in the study, including patients with visceral metastases, patients with bone-only metastases, and patients whose disease recurred after adjuvant therapy.
In the subgroup analysis of patients who entered the study after a recurrence during or within a year of adjuvant therapy, 100 patients received everolimus plus exemestane and 37 received placebo and exemestane. Almost all of the 137 patients had received nonsteroidal aromatase inhibitor therapy as their last therapy before entering the BOLERO-2 trial.
The median progression-free survival rates of 11.5 months with the everolimus combination and 4.1 months with the placebo combination were based on investigators’ local radiologic assessments. Central radiologic assessment by an independent radiology committee confirmed these results, finding that median progression-free survival reached 15.2 months in the everolimus group, compared with 4.2 months in the placebo group. Hazard ratios were 0.39 under the local assessment and 0.32 under the central assessment, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
Rates of the most common grade 3 or 4 adverse events seen in the everolimus-plus-exemestane group in the subset analysis – hyperglycemia in 8%, stomatitis in 4%, diarrhea in 4%, and fatigue in 3% – were similar to rates seen in the overall BOLERO-2 population and were within the known safety profile of everolimus. Some patients in the everolimus group (but not the placebo group) developed pneumonitis or interstitial lung disease, but these were mostly low grade and manageable by conventional strategies.
Baseline characteristics were well balanced in the randomized groups in the subset analysis.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – In patients who had hormone receptor–positive, HER2-negative advanced breast cancer, everolimus plus exemestane was just as effective among women who had a recurrence during or within a year of adjuvant therapy.
The results were seen in a secondary, subgroup analysis of 137 patients in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus 2) study. The subset that had recurrences during or within a year of adjuvant therapy comprised 19% of the patients in the study, and their outcomes were similar to the overall results of the trial, which support the use of everolimus plus exemestane as first-line therapy in postmenopausal patients with hormone receptor–positive advanced breast cancer that recurs after adjuvant therapy, Dr. Hope S. Rugo and her associates reported in a poster session at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In the women who had a recurrence during or within 12 months of adjuvant therapy, everolimus plus exemestane improved median progression-free survival to 11.5 months, compared with 4.1 months with placebo plus exemestane.
The multicenter, double-blind BOLERO-2 study randomized 724 postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. Patients received open-label exemestane plus blinded therapy with either placebo or everolimus, which inhibits the mammalian target of rapamycin (mTOR) signaling pathway. At 18 months of follow-up, patients in the exemestane-plus-everolimus group had a significantly longer median progression-free survival of 11 months as compared to 4 months for the control group (N. Engl. J. Med. 2012;366:520-529).
Similar efficacy was found in various analyses of subgroups in the study, including patients with visceral metastases, patients with bone-only metastases, and patients whose disease recurred after adjuvant therapy.
In the subgroup analysis of patients who entered the study after a recurrence during or within a year of adjuvant therapy, 100 patients received everolimus plus exemestane and 37 received placebo and exemestane. Almost all of the 137 patients had received nonsteroidal aromatase inhibitor therapy as their last therapy before entering the BOLERO-2 trial.
The median progression-free survival rates of 11.5 months with the everolimus combination and 4.1 months with the placebo combination were based on investigators’ local radiologic assessments. Central radiologic assessment by an independent radiology committee confirmed these results, finding that median progression-free survival reached 15.2 months in the everolimus group, compared with 4.2 months in the placebo group. Hazard ratios were 0.39 under the local assessment and 0.32 under the central assessment, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
Rates of the most common grade 3 or 4 adverse events seen in the everolimus-plus-exemestane group in the subset analysis – hyperglycemia in 8%, stomatitis in 4%, diarrhea in 4%, and fatigue in 3% – were similar to rates seen in the overall BOLERO-2 population and were within the known safety profile of everolimus. Some patients in the everolimus group (but not the placebo group) developed pneumonitis or interstitial lung disease, but these were mostly low grade and manageable by conventional strategies.
Baseline characteristics were well balanced in the randomized groups in the subset analysis.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Median progression-free survival was 11.5 months in the everolimus-plus-exemestane group and 4.1 months in the placebo-plus exemestane group.
Data source: A secondary analysis of a subset of 137 patients who had a recurrence of hormone receptor–positive, HER2-negative breast cancer during or within 12 months of adjuvant therapy before randomization in the BOLERO-2 trial.
Disclosures: Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
Genetic alterations may predict everolimus efficacy
SAN FRANCISCO – Among postmenopausal women with advanced hormone receptor–positive breast cancer, everolimus appeared to be more effective in the patients with one or no alterations in four key genes.
In a secondary analysis of data from the Breast Cancer Trials of Oral Everolimus–2 (BOLERO-2) study, one or no alterations in the PIK3CA; CCND1; FGFR1 and FGFR2 genes were seen in 76% of 227 tumor samples from women who took everolimus. These patients had significantly better progression-free survival than did patients with two or more alterations in these genes, Dr. Hope S. Rugo reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The number of patients with multiple gene alterations was admittedly small, but "potentially we have identified a population of patients who are less likely to benefit from everolimus," Dr. Rugo said.
She and her associates performed next-generation sequencing on DNA extracted tumor samples taken at diagnosis in BOLERO-2. Point mutations, short insertions or deletions, copy number alterations, or gene rearrangements were assessed in 219 tumors. Each tumor averaged 4 somatic mutations, with a range of 0-15 somatic mutations. Of the 182 genes sequenced, 104 had at least one known somatic mutation.
In the primary data analysis, all patients on everolimus had a 55% improvement in progression-free survival. In the secondary analysis of patients with minimal or no genetic alterations, 73% had an improvement in progression-free survival, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The genetic analysis found alterations in PIK3CA in 48% of samples, altered CCND1 in 31% of samples, altered P53 in 23%, and altered FGFR1 in 18%, so the researchers used these common alterations to seek predictors of treatment response. The four most commonly altered genes and the overall "tumor genetic landscape" in the current analysis were similar to those seen in previous large analyses such as The Cancer Genome Atlas Network (Nature 2012;490:61-70).
There was a hint that patients with alterations of FGFR1 or FGFR2 might be less likely to benefit from everolimus therapy. These numbers were small, however, and so the finding is only a hypothesis-generating observation, she said.
The sample group comprised approximately one-third of the cohort in BOLERO-2, the trial data that was the basis for the approval of everolimus. Baseline characteristics and outcomes were similar between the subgroup in the secondary analysis and the overall cohort.
The BOLERO-2 study comprised 724 postmenopausal women with advanced hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. The women were randomized to treatment with exemestane plus everolimus or placebo. At 18 months of follow-up, median progression-free survival was 11 months in the patients in the exemestane-plus-everolimus group, significantly longer than the 4-month median progression-free survival seen in the exemestane-plus-placebo group (N. Engl. J. Med. 2012;366:520-29).
As in other recent trials, most of the tumor samples for the genetic analysis were archived from the time of initial diagnosis rather than after metastasis. "Although the impact on the results shown here is unknown, we do know that additional mutations in these pathways are acquired with metastatic progression," Dr. Rugo said. "I think it’s important for all of us to try to obtain metastatic tumor tissue for these analyses moving forward."
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Among postmenopausal women with advanced hormone receptor–positive breast cancer, everolimus appeared to be more effective in the patients with one or no alterations in four key genes.
In a secondary analysis of data from the Breast Cancer Trials of Oral Everolimus–2 (BOLERO-2) study, one or no alterations in the PIK3CA; CCND1; FGFR1 and FGFR2 genes were seen in 76% of 227 tumor samples from women who took everolimus. These patients had significantly better progression-free survival than did patients with two or more alterations in these genes, Dr. Hope S. Rugo reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The number of patients with multiple gene alterations was admittedly small, but "potentially we have identified a population of patients who are less likely to benefit from everolimus," Dr. Rugo said.
She and her associates performed next-generation sequencing on DNA extracted tumor samples taken at diagnosis in BOLERO-2. Point mutations, short insertions or deletions, copy number alterations, or gene rearrangements were assessed in 219 tumors. Each tumor averaged 4 somatic mutations, with a range of 0-15 somatic mutations. Of the 182 genes sequenced, 104 had at least one known somatic mutation.
In the primary data analysis, all patients on everolimus had a 55% improvement in progression-free survival. In the secondary analysis of patients with minimal or no genetic alterations, 73% had an improvement in progression-free survival, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The genetic analysis found alterations in PIK3CA in 48% of samples, altered CCND1 in 31% of samples, altered P53 in 23%, and altered FGFR1 in 18%, so the researchers used these common alterations to seek predictors of treatment response. The four most commonly altered genes and the overall "tumor genetic landscape" in the current analysis were similar to those seen in previous large analyses such as The Cancer Genome Atlas Network (Nature 2012;490:61-70).
There was a hint that patients with alterations of FGFR1 or FGFR2 might be less likely to benefit from everolimus therapy. These numbers were small, however, and so the finding is only a hypothesis-generating observation, she said.
The sample group comprised approximately one-third of the cohort in BOLERO-2, the trial data that was the basis for the approval of everolimus. Baseline characteristics and outcomes were similar between the subgroup in the secondary analysis and the overall cohort.
The BOLERO-2 study comprised 724 postmenopausal women with advanced hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. The women were randomized to treatment with exemestane plus everolimus or placebo. At 18 months of follow-up, median progression-free survival was 11 months in the patients in the exemestane-plus-everolimus group, significantly longer than the 4-month median progression-free survival seen in the exemestane-plus-placebo group (N. Engl. J. Med. 2012;366:520-29).
As in other recent trials, most of the tumor samples for the genetic analysis were archived from the time of initial diagnosis rather than after metastasis. "Although the impact on the results shown here is unknown, we do know that additional mutations in these pathways are acquired with metastatic progression," Dr. Rugo said. "I think it’s important for all of us to try to obtain metastatic tumor tissue for these analyses moving forward."
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Among postmenopausal women with advanced hormone receptor–positive breast cancer, everolimus appeared to be more effective in the patients with one or no alterations in four key genes.
In a secondary analysis of data from the Breast Cancer Trials of Oral Everolimus–2 (BOLERO-2) study, one or no alterations in the PIK3CA; CCND1; FGFR1 and FGFR2 genes were seen in 76% of 227 tumor samples from women who took everolimus. These patients had significantly better progression-free survival than did patients with two or more alterations in these genes, Dr. Hope S. Rugo reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The number of patients with multiple gene alterations was admittedly small, but "potentially we have identified a population of patients who are less likely to benefit from everolimus," Dr. Rugo said.
She and her associates performed next-generation sequencing on DNA extracted tumor samples taken at diagnosis in BOLERO-2. Point mutations, short insertions or deletions, copy number alterations, or gene rearrangements were assessed in 219 tumors. Each tumor averaged 4 somatic mutations, with a range of 0-15 somatic mutations. Of the 182 genes sequenced, 104 had at least one known somatic mutation.
In the primary data analysis, all patients on everolimus had a 55% improvement in progression-free survival. In the secondary analysis of patients with minimal or no genetic alterations, 73% had an improvement in progression-free survival, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The genetic analysis found alterations in PIK3CA in 48% of samples, altered CCND1 in 31% of samples, altered P53 in 23%, and altered FGFR1 in 18%, so the researchers used these common alterations to seek predictors of treatment response. The four most commonly altered genes and the overall "tumor genetic landscape" in the current analysis were similar to those seen in previous large analyses such as The Cancer Genome Atlas Network (Nature 2012;490:61-70).
There was a hint that patients with alterations of FGFR1 or FGFR2 might be less likely to benefit from everolimus therapy. These numbers were small, however, and so the finding is only a hypothesis-generating observation, she said.
The sample group comprised approximately one-third of the cohort in BOLERO-2, the trial data that was the basis for the approval of everolimus. Baseline characteristics and outcomes were similar between the subgroup in the secondary analysis and the overall cohort.
The BOLERO-2 study comprised 724 postmenopausal women with advanced hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. The women were randomized to treatment with exemestane plus everolimus or placebo. At 18 months of follow-up, median progression-free survival was 11 months in the patients in the exemestane-plus-everolimus group, significantly longer than the 4-month median progression-free survival seen in the exemestane-plus-placebo group (N. Engl. J. Med. 2012;366:520-29).
As in other recent trials, most of the tumor samples for the genetic analysis were archived from the time of initial diagnosis rather than after metastasis. "Although the impact on the results shown here is unknown, we do know that additional mutations in these pathways are acquired with metastatic progression," Dr. Rugo said. "I think it’s important for all of us to try to obtain metastatic tumor tissue for these analyses moving forward."
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In a secondary analysis, patients with one or no alterations in four genes had a 73% improvement in progression-free survival with everolimus therapy, compared with the 55% improvement in the general study population.
Data source: A retrospective, secondary analysis of genetic alterations in 227 tumor samples from women with advanced hormone receptor–positive breast cancer in the BOLERO-2 trial.
Disclosures: Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
Breast cancer receptor change may predict outcomes
SAN FRANCISCO – A change in the status of any breast cancer tumor biomarker after neoadjuvant therapy was independently associated with a 37% decreased likelihood of recurrence in 5 years, a multivariate analysis showed.
The subtyping of breast cancer by receptor status changed in 41% of 398 samples between the initial tumor and residual disease after neoadjuvant chemotherapy.
In patients with any change in the status of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), after neoadjuvant chemotherapy, 63% were relapse free 5 years later as compared with 48% of patients with no receptor change, a significant difference.
Overall survival at 5 years, however, did not differ significantly based on receptor status change, Dr. Napa Parinyanitikul and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. At 5 years, 73% of patients with a receptor status change and 63% with no change were alive.
The study analyzed data on patients treated at her institution in 1992-2012 who had tumor biomarker results from pretreatment biopsies and from samples of residual disease after neoadjuvant chemotherapy. Most patients in the study were white, with clinical stage II or III disease.
Of the tumors, 49% were hormone receptor–positive and HER2 negative; 18% were HER2 positive, and 33% were negative for all three receptors (triple-negative breast cancer). Neoadjuvant chemotherapy consisted of a taxane-based regimen in 10%, an anthracycline-based regimen in 19%, an anthracycline/taxane-based regimen in 71%, and trastuzumab-based chemotherapy in 49%. The 88% of patients with hormone receptor-positive disease received adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor.
After a median of 40 months of follow-up, 32% of women had died and 42% had a recurrence of breast cancer, reported Dr. Parinyanitikul, a fellow at the University of Texas MD Anderson Cancer Center, Houston.
The likelihood of receptor status changes differed significantly by the subtype of breast cancer. Of hormone receptor–positive cancers, 51% had a change in receptor status, compared with 27% of HER2-positive cancers and 22% of triple-negative breast cancers.
Other factors did not significantly affect the likelihood of receptor status change, including patient age or race, histology, tumor stage or grade, the presence of lymphovascular invasion, or the class of neoadjuvant chemotherapeutic agents.
Of ER-positive tumors, 11% changed to ER negative after neoadjuvant chemotherapy; of ER-negative tumors, 21% changed to ER positive. Of the PR-positive tumors, 35% changed to PR-negative; of PR-negative tumors, 12% changed to PR positive. Of HER2-positive tumors, 40% changed to HER2 negative; of HER2-negative tumors, 3% changed to HER2 positive. Among 35 patients treated with trastuzumab, the HER2 status changed in 16 (46%).
Patients who had tumors with an ER-negative status that did not change had the worst 5-year rates for overall survival (47%) and relapse-free survival (40%), compared with patients whose tumors had an ER-positive status did not change (81% overall survival and 63% relapse-free survival), patients whose ER status changed from positive to negative (67% and 66% survival rates, respectively), and patients whose ER status changed from negative to positive (75% and 59%, respectively).
Similarly, patients who had PR-negative tumors whose status did not change had significantly lower rates of overall survival (51%) and relapse-free survival (41%), compared with patients whose PR-positive tumor status did not change (87% and 67%, respectively), those who changed from PR positive to PR negative (78% and 73%, respectively), and patients whose PR-negative status changed to PR positive (69% and 55%, respectively).
Changes or lack of change in HER2 subgroups were not significantly associated with varying outcomes.
The investigators also looked at the absolute percent changes in ER or PR status using cutoffs in 10% increments from 10% to 50%. The 5-year overall survival rate was significantly greater in ER-positive patients who had at least a 20% change in ER status, compared with those with less than a 20% change (87% vs. 73%, respectively). The 5-year relapse-free survival rate also was significantly greater if the ER status changed by at least 20% (71%), compared with smaller ER changes (59%).
Of cancers that were hormone receptor–positive and HER2 negative before neoadjuvant chemotherapy, 20% changed to HER2-positive or triple-negative breast cancer after treatment, 12% of tumors that had been HER2 positive changed to triple negative tumors, and 2% of triple negative breast cancers changed to HER2-positive after treatment. These changes produced only one significant effect on outcomes: 5-year overall survival rates were significantly lower in patients who changed from HER2-positive to triple-negative breast cancer (23%), compared with those whose HER2-positive cancer did not change to triple negative (71%).
Larger prospective studies are needed to confirm the findings and to determine any impact of biomarker changes on long-term survival, she said.
Previous studies have reported discordance in hormone receptor status in up to 51% of breast cancers between the primary tumor and residual disease and changes in HER2 status in up to 43% of cases. Conclusions about associations between changes in receptor status and clinical outcomes have been inconsistent.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Parinyanitikul reported having no financial disclosures.
sherryboschert@frontlinemedcom.com
On Twitter @sherryboschert
Technical artifacts when testing receptor status in breast cancer complicate assessments of their potential ramifications on outcomes.
 |
|
ER and PR status can change if you repeat the testing on the same specimen without any change in the actual marker status. In one "classic" study, the HER2 or ER status changed in 23% of samples after they were air-mailed from one center to another. You can’t conclude that it was the airplane flight that changed receptor status.
Similarly, in the current study, you can’t conclude that it was the chemotherapy that changed the receptor status, because part of this discordance is from the technical imprecision of the test. If a test is 90% accurate, for example, 10% of results on first testing will be "noise" and approximately 20% of results on repeat testing will be discordant.
The study also did not report the proportion of cases in which initial receptor status tests were conducted in one laboratory and the second test was conducted at a different center, as is often the case in the real world. That also introduces a very large, obvious cause for receptor discordance independent of therapy.
Dr. Lajos Pusztai is a professor of medicine and director of the Breast Medical Oncology Section at Yale University, New Haven, Conn. He made his remarks during a question-and-answer session at the meeting. Dr. Pusztai reported financial associations with BiPar Sciences/Sanofi, Bristol-Myers Squibb, Pfizer, AstraZeneca, Roche/Genentech, and Foundation Medicine.
Technical artifacts when testing receptor status in breast cancer complicate assessments of their potential ramifications on outcomes.
 |
|
ER and PR status can change if you repeat the testing on the same specimen without any change in the actual marker status. In one "classic" study, the HER2 or ER status changed in 23% of samples after they were air-mailed from one center to another. You can’t conclude that it was the airplane flight that changed receptor status.
Similarly, in the current study, you can’t conclude that it was the chemotherapy that changed the receptor status, because part of this discordance is from the technical imprecision of the test. If a test is 90% accurate, for example, 10% of results on first testing will be "noise" and approximately 20% of results on repeat testing will be discordant.
The study also did not report the proportion of cases in which initial receptor status tests were conducted in one laboratory and the second test was conducted at a different center, as is often the case in the real world. That also introduces a very large, obvious cause for receptor discordance independent of therapy.
Dr. Lajos Pusztai is a professor of medicine and director of the Breast Medical Oncology Section at Yale University, New Haven, Conn. He made his remarks during a question-and-answer session at the meeting. Dr. Pusztai reported financial associations with BiPar Sciences/Sanofi, Bristol-Myers Squibb, Pfizer, AstraZeneca, Roche/Genentech, and Foundation Medicine.
Technical artifacts when testing receptor status in breast cancer complicate assessments of their potential ramifications on outcomes.
 |
|
ER and PR status can change if you repeat the testing on the same specimen without any change in the actual marker status. In one "classic" study, the HER2 or ER status changed in 23% of samples after they were air-mailed from one center to another. You can’t conclude that it was the airplane flight that changed receptor status.
Similarly, in the current study, you can’t conclude that it was the chemotherapy that changed the receptor status, because part of this discordance is from the technical imprecision of the test. If a test is 90% accurate, for example, 10% of results on first testing will be "noise" and approximately 20% of results on repeat testing will be discordant.
The study also did not report the proportion of cases in which initial receptor status tests were conducted in one laboratory and the second test was conducted at a different center, as is often the case in the real world. That also introduces a very large, obvious cause for receptor discordance independent of therapy.
Dr. Lajos Pusztai is a professor of medicine and director of the Breast Medical Oncology Section at Yale University, New Haven, Conn. He made his remarks during a question-and-answer session at the meeting. Dr. Pusztai reported financial associations with BiPar Sciences/Sanofi, Bristol-Myers Squibb, Pfizer, AstraZeneca, Roche/Genentech, and Foundation Medicine.
SAN FRANCISCO – A change in the status of any breast cancer tumor biomarker after neoadjuvant therapy was independently associated with a 37% decreased likelihood of recurrence in 5 years, a multivariate analysis showed.
The subtyping of breast cancer by receptor status changed in 41% of 398 samples between the initial tumor and residual disease after neoadjuvant chemotherapy.
In patients with any change in the status of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), after neoadjuvant chemotherapy, 63% were relapse free 5 years later as compared with 48% of patients with no receptor change, a significant difference.
Overall survival at 5 years, however, did not differ significantly based on receptor status change, Dr. Napa Parinyanitikul and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. At 5 years, 73% of patients with a receptor status change and 63% with no change were alive.
The study analyzed data on patients treated at her institution in 1992-2012 who had tumor biomarker results from pretreatment biopsies and from samples of residual disease after neoadjuvant chemotherapy. Most patients in the study were white, with clinical stage II or III disease.
Of the tumors, 49% were hormone receptor–positive and HER2 negative; 18% were HER2 positive, and 33% were negative for all three receptors (triple-negative breast cancer). Neoadjuvant chemotherapy consisted of a taxane-based regimen in 10%, an anthracycline-based regimen in 19%, an anthracycline/taxane-based regimen in 71%, and trastuzumab-based chemotherapy in 49%. The 88% of patients with hormone receptor-positive disease received adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor.
After a median of 40 months of follow-up, 32% of women had died and 42% had a recurrence of breast cancer, reported Dr. Parinyanitikul, a fellow at the University of Texas MD Anderson Cancer Center, Houston.
The likelihood of receptor status changes differed significantly by the subtype of breast cancer. Of hormone receptor–positive cancers, 51% had a change in receptor status, compared with 27% of HER2-positive cancers and 22% of triple-negative breast cancers.
Other factors did not significantly affect the likelihood of receptor status change, including patient age or race, histology, tumor stage or grade, the presence of lymphovascular invasion, or the class of neoadjuvant chemotherapeutic agents.
Of ER-positive tumors, 11% changed to ER negative after neoadjuvant chemotherapy; of ER-negative tumors, 21% changed to ER positive. Of the PR-positive tumors, 35% changed to PR-negative; of PR-negative tumors, 12% changed to PR positive. Of HER2-positive tumors, 40% changed to HER2 negative; of HER2-negative tumors, 3% changed to HER2 positive. Among 35 patients treated with trastuzumab, the HER2 status changed in 16 (46%).
Patients who had tumors with an ER-negative status that did not change had the worst 5-year rates for overall survival (47%) and relapse-free survival (40%), compared with patients whose tumors had an ER-positive status did not change (81% overall survival and 63% relapse-free survival), patients whose ER status changed from positive to negative (67% and 66% survival rates, respectively), and patients whose ER status changed from negative to positive (75% and 59%, respectively).
Similarly, patients who had PR-negative tumors whose status did not change had significantly lower rates of overall survival (51%) and relapse-free survival (41%), compared with patients whose PR-positive tumor status did not change (87% and 67%, respectively), those who changed from PR positive to PR negative (78% and 73%, respectively), and patients whose PR-negative status changed to PR positive (69% and 55%, respectively).
Changes or lack of change in HER2 subgroups were not significantly associated with varying outcomes.
The investigators also looked at the absolute percent changes in ER or PR status using cutoffs in 10% increments from 10% to 50%. The 5-year overall survival rate was significantly greater in ER-positive patients who had at least a 20% change in ER status, compared with those with less than a 20% change (87% vs. 73%, respectively). The 5-year relapse-free survival rate also was significantly greater if the ER status changed by at least 20% (71%), compared with smaller ER changes (59%).
Of cancers that were hormone receptor–positive and HER2 negative before neoadjuvant chemotherapy, 20% changed to HER2-positive or triple-negative breast cancer after treatment, 12% of tumors that had been HER2 positive changed to triple negative tumors, and 2% of triple negative breast cancers changed to HER2-positive after treatment. These changes produced only one significant effect on outcomes: 5-year overall survival rates were significantly lower in patients who changed from HER2-positive to triple-negative breast cancer (23%), compared with those whose HER2-positive cancer did not change to triple negative (71%).
Larger prospective studies are needed to confirm the findings and to determine any impact of biomarker changes on long-term survival, she said.
Previous studies have reported discordance in hormone receptor status in up to 51% of breast cancers between the primary tumor and residual disease and changes in HER2 status in up to 43% of cases. Conclusions about associations between changes in receptor status and clinical outcomes have been inconsistent.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Parinyanitikul reported having no financial disclosures.
sherryboschert@frontlinemedcom.com
On Twitter @sherryboschert
SAN FRANCISCO – A change in the status of any breast cancer tumor biomarker after neoadjuvant therapy was independently associated with a 37% decreased likelihood of recurrence in 5 years, a multivariate analysis showed.
The subtyping of breast cancer by receptor status changed in 41% of 398 samples between the initial tumor and residual disease after neoadjuvant chemotherapy.
In patients with any change in the status of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), after neoadjuvant chemotherapy, 63% were relapse free 5 years later as compared with 48% of patients with no receptor change, a significant difference.
Overall survival at 5 years, however, did not differ significantly based on receptor status change, Dr. Napa Parinyanitikul and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. At 5 years, 73% of patients with a receptor status change and 63% with no change were alive.
The study analyzed data on patients treated at her institution in 1992-2012 who had tumor biomarker results from pretreatment biopsies and from samples of residual disease after neoadjuvant chemotherapy. Most patients in the study were white, with clinical stage II or III disease.
Of the tumors, 49% were hormone receptor–positive and HER2 negative; 18% were HER2 positive, and 33% were negative for all three receptors (triple-negative breast cancer). Neoadjuvant chemotherapy consisted of a taxane-based regimen in 10%, an anthracycline-based regimen in 19%, an anthracycline/taxane-based regimen in 71%, and trastuzumab-based chemotherapy in 49%. The 88% of patients with hormone receptor-positive disease received adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor.
After a median of 40 months of follow-up, 32% of women had died and 42% had a recurrence of breast cancer, reported Dr. Parinyanitikul, a fellow at the University of Texas MD Anderson Cancer Center, Houston.
The likelihood of receptor status changes differed significantly by the subtype of breast cancer. Of hormone receptor–positive cancers, 51% had a change in receptor status, compared with 27% of HER2-positive cancers and 22% of triple-negative breast cancers.
Other factors did not significantly affect the likelihood of receptor status change, including patient age or race, histology, tumor stage or grade, the presence of lymphovascular invasion, or the class of neoadjuvant chemotherapeutic agents.
Of ER-positive tumors, 11% changed to ER negative after neoadjuvant chemotherapy; of ER-negative tumors, 21% changed to ER positive. Of the PR-positive tumors, 35% changed to PR-negative; of PR-negative tumors, 12% changed to PR positive. Of HER2-positive tumors, 40% changed to HER2 negative; of HER2-negative tumors, 3% changed to HER2 positive. Among 35 patients treated with trastuzumab, the HER2 status changed in 16 (46%).
Patients who had tumors with an ER-negative status that did not change had the worst 5-year rates for overall survival (47%) and relapse-free survival (40%), compared with patients whose tumors had an ER-positive status did not change (81% overall survival and 63% relapse-free survival), patients whose ER status changed from positive to negative (67% and 66% survival rates, respectively), and patients whose ER status changed from negative to positive (75% and 59%, respectively).
Similarly, patients who had PR-negative tumors whose status did not change had significantly lower rates of overall survival (51%) and relapse-free survival (41%), compared with patients whose PR-positive tumor status did not change (87% and 67%, respectively), those who changed from PR positive to PR negative (78% and 73%, respectively), and patients whose PR-negative status changed to PR positive (69% and 55%, respectively).
Changes or lack of change in HER2 subgroups were not significantly associated with varying outcomes.
The investigators also looked at the absolute percent changes in ER or PR status using cutoffs in 10% increments from 10% to 50%. The 5-year overall survival rate was significantly greater in ER-positive patients who had at least a 20% change in ER status, compared with those with less than a 20% change (87% vs. 73%, respectively). The 5-year relapse-free survival rate also was significantly greater if the ER status changed by at least 20% (71%), compared with smaller ER changes (59%).
Of cancers that were hormone receptor–positive and HER2 negative before neoadjuvant chemotherapy, 20% changed to HER2-positive or triple-negative breast cancer after treatment, 12% of tumors that had been HER2 positive changed to triple negative tumors, and 2% of triple negative breast cancers changed to HER2-positive after treatment. These changes produced only one significant effect on outcomes: 5-year overall survival rates were significantly lower in patients who changed from HER2-positive to triple-negative breast cancer (23%), compared with those whose HER2-positive cancer did not change to triple negative (71%).
Larger prospective studies are needed to confirm the findings and to determine any impact of biomarker changes on long-term survival, she said.
Previous studies have reported discordance in hormone receptor status in up to 51% of breast cancers between the primary tumor and residual disease and changes in HER2 status in up to 43% of cases. Conclusions about associations between changes in receptor status and clinical outcomes have been inconsistent.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Parinyanitikul reported having no financial disclosures.
sherryboschert@frontlinemedcom.com
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Receptor status changed in 41% of breast cancers after neoadjuvant chemotherapy. Relapse within 5 years was significantly less likely in patients with a receptor change (63%) than in those with no receptor change (48%).
Data source: A retrospective study of 398 women with data on ER, PR, and HER2 status in the primary tumor and in residual disease after neoadjuvant chemotherapy.
Disclosures: Dr. Parinyanitikul reported having no financial disclosures.