User login
Mobile Enlarging Scalp Nodule
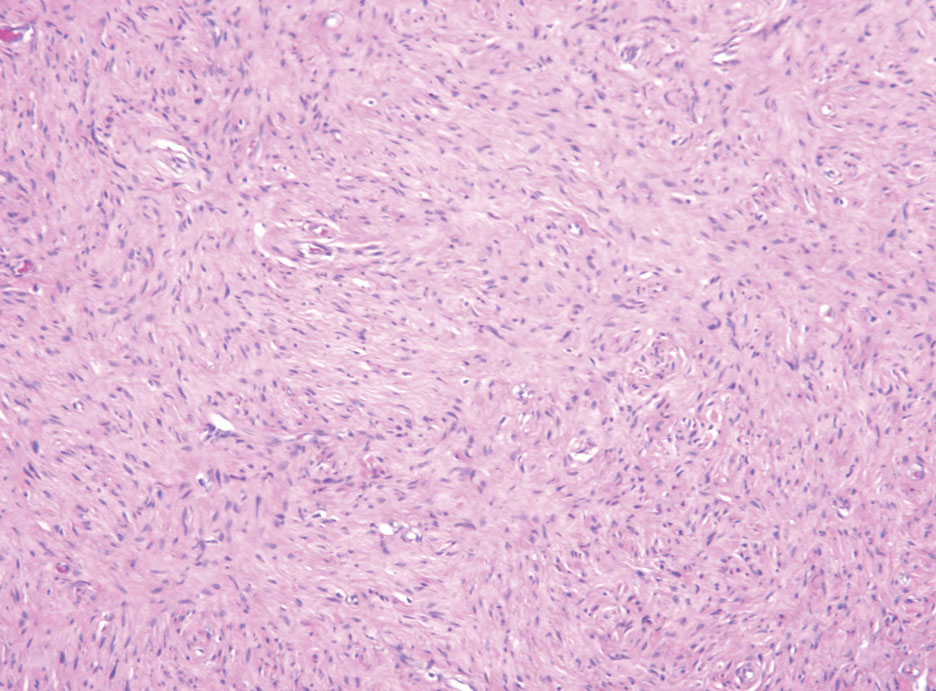
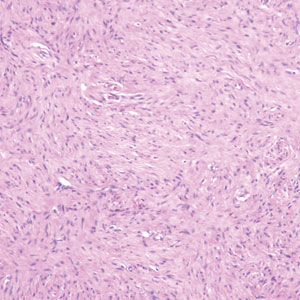
The Diagnosis: Hybrid Schwannoma-Perineurioma
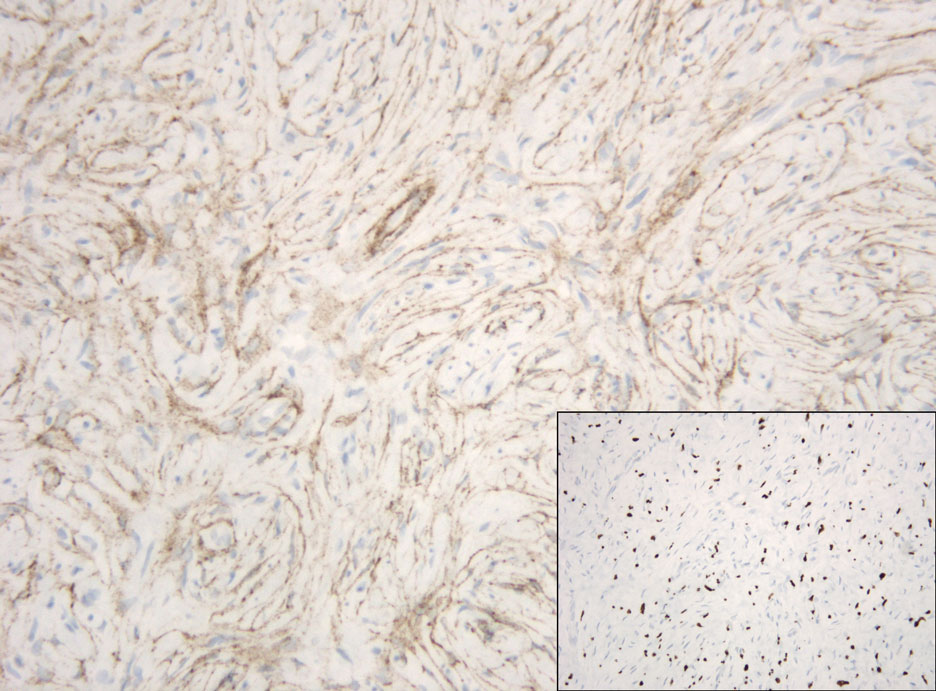
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6
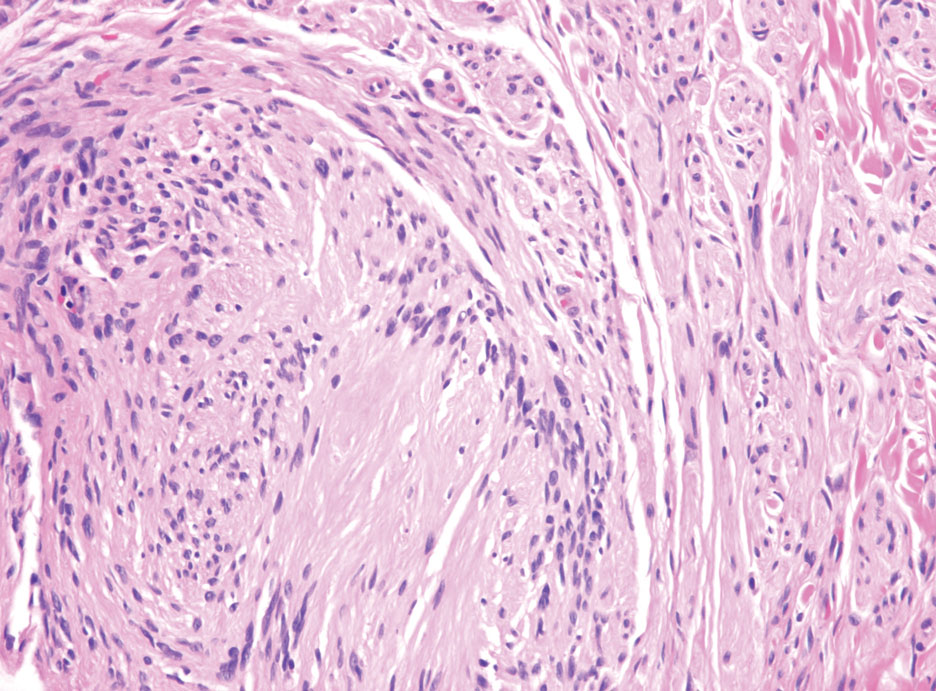
Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9
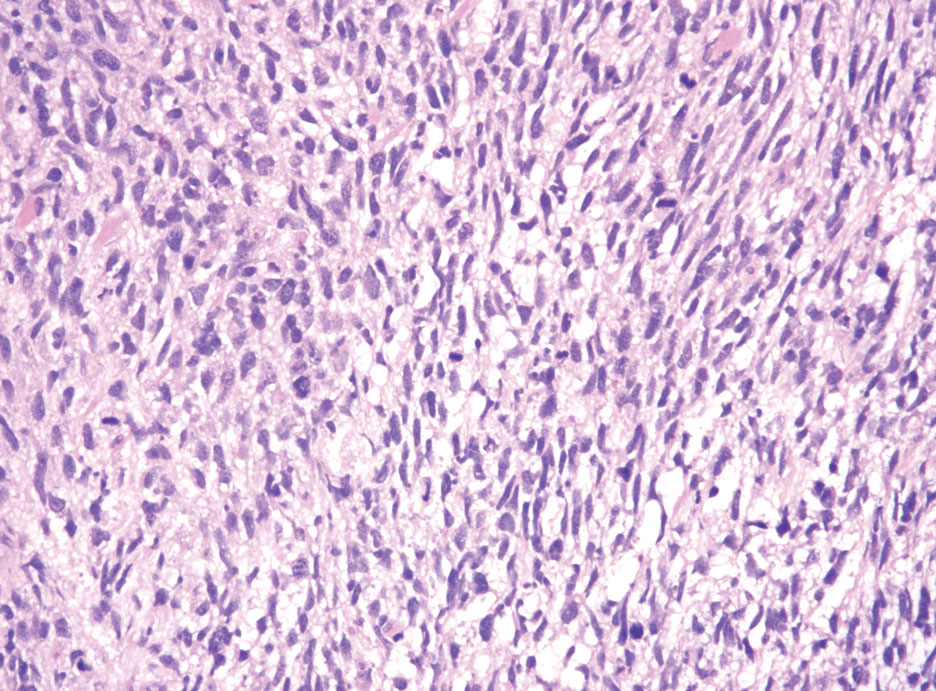
Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7
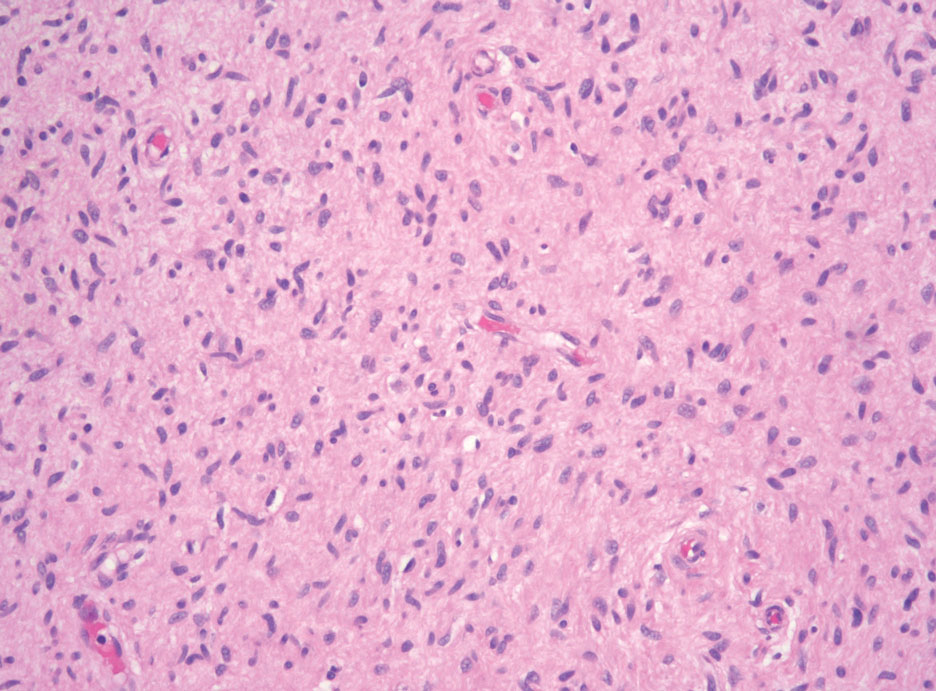
Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15
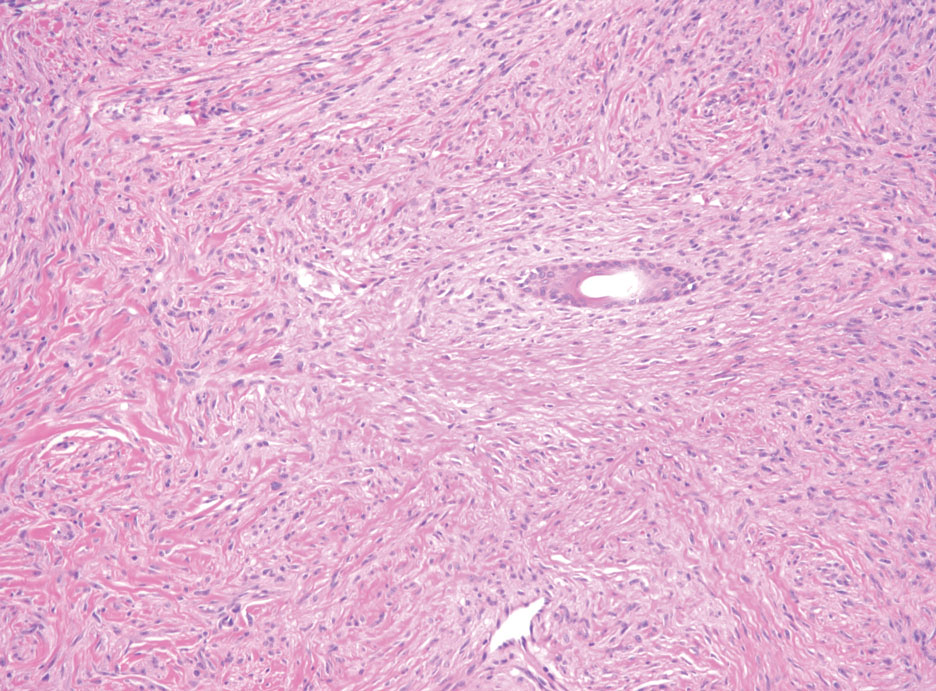
Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
A 50-year-old man presented with a 2.5-cm, subcutaneous, freely mobile nodule on the occipital scalp that first appeared 35 years prior but recently had started enlarging. Histologically the lesion was well circumscribed. Immunohistochemical staining was positive for SRY-box transcription factor 10 in some of the spindle cells, and staining for epithelial membrane antigen was positive in a separate population of intermixed spindle cells.