User login
First-line or BiV backup? Conduction system pacing for CRT in heart failure
Pacing as a device therapy for heart failure (HF) is headed for what is probably its next big advance.
After decades of biventricular (BiV) pacemaker success in resynchronizing the ventricles and improving clinical outcomes, relatively new conduction-system pacing (CSP) techniques that avoid the pitfalls of right-ventricular (RV) pacing using BiV lead systems have been supplanting traditional cardiac resynchronization therapy (CRT) in selected patients at some major centers. In fact, they are solidly ensconced in a new guideline document addressing indications for CSP and BiV pacing in HF.
But , an alternative when BiV pacing isn’t appropriate or can’t be engaged.
That’s mainly because the limited, mostly observational evidence supporting CSP in the document can’t measure up to the clinical experience and plethora of large, randomized trials behind BiV-CRT.
But that shortfall is headed for change. Several new comparative studies, including a small, randomized trial, have added significantly to evidence suggesting that CSP is at least as effective as traditional CRT for procedural, functional safety, and clinical outcomes.
The new studies “are inherently prone to bias, but their results are really good,” observed Juan C. Diaz, MD. They show improvements in left ventricular ejection fraction (LVEF) and symptoms with CSP that are “outstanding compared to what we have been doing for the last 20 years,” he said in an interview.
Dr. Diaz, Clínica Las Vegas, Medellin, Colombia, is an investigator with the observational SYNCHRONY, which is among the new CSP studies formally presented at the annual scientific sessions of the Heart Rhythm Society. He is also lead author on its same-day publication in JACC: Clinical Electrophysiology.
Dr. Diaz said that CSP, which sustains pacing via the native conduction system, makes more “physiologic sense” than BiV pacing and represents “a step forward” for HF device therapy.
SYNCHRONY compared LBB-area with BiV pacing as the initial strategy for achieving cardiac resynchronization in patients with ischemic or nonischemic cardiomyopathy.
CSP is “a long way” from replacing conventional CRT, he said. But the new studies at the HRS sessions should help extend His-bundle and LBB-area pacing to more patients, he added, given the significant long-term “drawbacks” of BiV pacing. These include inevitable RV pacing, multiple leads, and the risks associated with chronic transvenous leads.
Zachary Goldberger, MD, University of Wisconsin–Madison, went a bit further in support of CSP as invited discussant for the SYNCHRONY presentation.
Given that it improved LVEF, heart failure class, HF hospitalizations (HFH), and mortality in that study and others, Dr. Goldberger said, CSP could potentially “become the dominant mode of resynchronization going forward.”
Other experts at the meeting saw CSP’s potential more as one of several pacing techniques that could be brought to bear for patients with CRT indications.
“Conduction system pacing is going to be a huge complement to biventricular pacing,” to which about 30% of patients have a “less than optimal response,” said Pugazhendhi Vijayaraman, MD, chief of clinical electrophysiology, Geisinger Heart Institute, Danville, Pa.
“I don’t think it needs to replace biventricular pacing, because biventricular pacing is a well-established, incredibly powerful therapy,” he told this news organization. But CSP is likely to provide “a good alternative option” in patients with poor responses to BiV-CRT.
It may, however, render some current BiV-pacing alternatives “obsolete,” Dr. Vijayaraman observed. “At our center, at least for the last 5 years, no patient has needed epicardial surgical left ventricular lead placement” because CSP was a better backup option.
Dr. Vijayaraman presented two of the meeting’s CSP vs. BiV pacing comparisons. In one, the 100-patient randomized HOT-CRT trial, contractile function improved significantly on CSP, which could be either His-bundle or LBB-area pacing.
He also presented an observational study of LBB-area pacing at 15 centers in Asia, Europe, and North America and led the authors of its simultaneous publication in the Journal of the American College of Cardiology.
“I think left-bundle conduction system pacing is the future, for sure,” Jagmeet P. Singh, MD, DPhil, told this news organization. Still, it doesn’t always work and when it does, it “doesn’t work equally in all patients,” he said.
“Conduction system pacing certainly makes a lot of sense,” especially in patients with left-bundle-branch block (LBBB), and “maybe not as a primary approach but certainly as a secondary approach,” said Dr. Singh, Massachusetts General Hospital, Boston, who is not a coauthor on any of the three studies.
He acknowledged that CSP may work well as a first-line option in patients with LBBB at some experienced centers. For those without LBBB or who have an intraventricular conduction delay, who represent 45%-50% of current CRT cases, Dr. Singh observed, “there’s still more evidence” that BiV-CRT is a more appropriate initial approach.
Standard CRT may fail, however, even in some patients who otherwise meet guideline-based indications. “We don’t really understand all the mechanisms for nonresponse in conventional biventricular pacing,” observed Niraj Varma, MD, PhD, Cleveland Clinic, also not involved with any of the three studies.
In some groups, including “patients with larger ventricles,” for example, BiV-CRT doesn’t always narrow the electrocardiographic QRS complex or preexcite delayed left ventricular (LV) activation, hallmarks of successful CRT, he said in an interview.
“I think we need to understand why this occurs in both situations,” but in such cases, CSP alone or as an adjunct to direct LV pacing may be successful. “Sometimes we need both an LV lead and the conduction-system pacing lead.”
Narrower, more efficient use of CSP as a BiV-CRT alternative may also boost its chances for success, Dr. Varma added. “I think we need to refine patient selection.”
HOT-CRT: Randomized CSP vs. BiV pacing trial
Conducted at three centers in a single health system, the His-optimized cardiac resynchronization therapy study (HOT-CRT) randomly assigned 100 patients with primary or secondary CRT indications to either to CSP – by either His-bundle or LBB-area pacing – or to standard BiV-CRT as the first-line resynchronization method.
Treatment crossovers, allowed for either pacing modality in the event of implantation failure, occurred in two patients and nine patients initially assigned to CSP and BiV pacing, respectively (4% vs. 18%), Dr. Vijayaraman reported.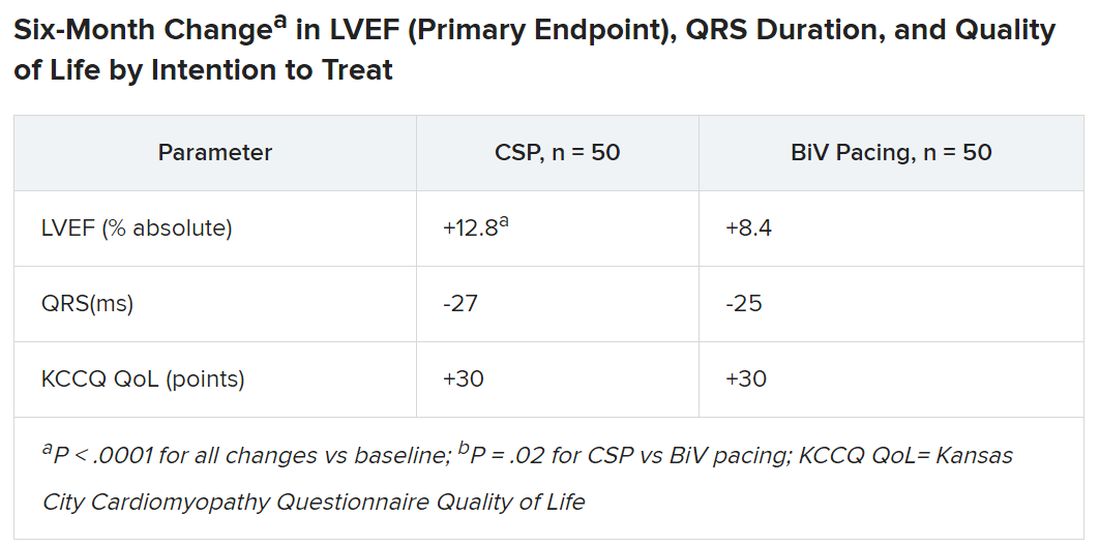
Historically in trials, BiV pacing has elevated LVEF by about 7%, he said. The mean 12-point increase observed with CSP “is huge, in that sense.” HOT-CRT enrolled a predominantly male and White population at centers highly experienced in both CSP and BiV pacing, limiting its broad relevance to practice, as pointed out by both Dr. Vijayaraman and his presentation’s invited discussant, Yong-Mei Cha, MD, Mayo Clinic, Rochester, Minn. Dr. Cha, who is director of cardiac device services at her center, also highlighted the greater rate of crossover from BiV pacing to CSP, 18% vs. 4% in the other direction. “This is a very encouraging result,” because the implant-failure rate for LBB-area pacing may drop once more operators become “familiar and skilled with conduction-system pacing.” Overall, the study supports CSP as “a very good alternative for heart failure patients when BiV pacing fails.”
International comparison of CSP and BiV pacing
In Dr. Vijayaraman’s other study, the observational comparison of LBB-area pacing and BiV-CRT, the CSP technique emerged as a “reasonable alternative to biventricular pacing, not only for improvement in LV function but also to reduce adverse clinical outcomes.”
Indeed, in the international study of 1,778 mostly male patients with primary or secondary CRT indications who received LBB-area or BiV pacing (797 and 981 patients, respectively), those on CSP saw a significant drop in risk for the primary endpoint, death or HFH.
Mean LVEF improved from 27% to 41% in the LBB-area pacing group and 27% to 37% with BiV pacing (P < .001 for both changes) over a follow-up averaging 33 months. The difference in improvement between CSP and BiV pacing was significant at P < .001.
In adjusted analysis, the risk for death or HFH was greater for BiV-pacing patients, a difference driven by HFH events.
- Death or HF: hazard ratio, 1.49 (95% confidence interval, 1.21-1.84; P < .001).
- Death: HR, 1.14 (95% CI, 0.88-1.48; P = .313).
- HFH: HR, 1.49 (95% CI, 1.16-1.92; P = .002)
The analysis has all the “inherent biases” of an observational study. The risk for patient-selection bias, however, was somewhat mitigated by consistent practice patterns at participating centers, Dr. Vijayaraman told this news organization.
For example, he said, operators at six of the institutions were most likely to use CSP as the first-line approach, and the same number of centers usually went with BiV pacing.
SYNCHRONY: First-line LBB-area pacing vs. BiV-CRT
Outcomes using the two approaches were similar in the prospective, international, observational study of 371 patients with ischemic or nonischemic cardiomyopathy and standard CRT indications. Allocation of 128 patients to LBB-area pacing and 243 to BiV-CRT was based on patient and operator preferences, reported Jorge Romero Jr, MD, Brigham and Women’s Hospital, Boston, at the HRS sessions.
Risk for the death-HFH primary endpoint dropped 38% for those initially treated with LBB-area pacing, compared with BiV pacing, primarily because of a lower HFH risk:
- Death or HFH: HR, 0.62 (95% CI, 0.41-0.93; P = .02).
- Death: HR, 0.57 (95% CI, 0.25-1.32; P = .19).
- HFH: HR, 0.61 (95% CI, 0.34-0.93; P = .02)
Patients in the CSP group were also more likely to improve by at least one NYHA (New York Heart Association) class (80.4% vs. 67.9%; P < .001), consistent with their greater absolute change in LVEF (8.0 vs. 3.9 points; P < .01).
The findings “suggest that LBBAP [left-bundle branch area pacing] is an excellent alternative to BiV pacing,” with a comparable safety profile, write Jayanthi N. Koneru, MBBS, and Kenneth A. Ellenbogen, MD, in an editorial accompanying the published SYNCHRONY report.
“The differences in improvement of LVEF are encouraging for both groups,” but were superior for LBB-area pacing, continue Dr. Koneru and Dr. Ellenbogen, both with Virginia Commonwealth University Medical Center, Richmond. “Whether these results would have regressed to the mean over a longer period of follow-up or diverge further with LBB-area pacing continuing to be superior is unknown.”
Years for an answer?
A large randomized comparison of CSP and BiV-CRT, called Left vs. Left, is currently in early stages, Sana M. Al-Khatib, MD, MHS, Duke University Medical Center, Durham, N.C., said in a media presentation on two of the presented studies. It has a planned enrollment of more than 2,100 patients on optimal meds with an LVEF of 50% or lower and either a QRS duration of at least 130 ms or an anticipated burden of RV pacing exceeding 40%.
The trial, she said, “will take years to give an answer, but it is actually designed to address the question of whether a composite endpoint of time to death or heart failure hospitalization can be improved with conduction system pacing vs. biventricular pacing.”
Dr. Al-Khatib is a coauthor on the new guideline covering both CSP and BiV-CRT in HF, as are Dr. Cha, Dr. Varma, Dr. Singh, Dr. Vijayaraman, and Dr. Goldberger; Dr. Ellenbogen is one of the reviewers.
Dr. Diaz discloses receiving honoraria or fees for speaking or teaching from Bayer Healthcare, Pfizer, AstraZeneca, Boston Scientific, and Medtronic. Dr. Vijayaraman discloses receiving honoraria or fees for speaking, teaching, or consulting for Abbott, Medtronic, Biotronik, and Boston Scientific; and receiving research grants from Medtronic. Dr. Varma discloses receiving honoraria or fees for speaking or consulting as an independent contractor for Medtronic, Boston Scientific, Biotronik, Impulse Dynamics USA, Cardiologs, Abbott, Pacemate, Implicity, and EP Solutions. Dr. Singh discloses receiving fees for consulting from EBR Systems, Merit Medical Systems, New Century Health, Biotronik, Abbott, Medtronic, MicroPort Scientific, Cardiologs, Sanofi, CVRx, Impulse Dynamics USA, Octagos, Implicity, Orchestra Biomed, Rhythm Management Group, and Biosense Webster; and receiving honoraria or fees for speaking and teaching from Medscape. Dr. Cha had no relevant financial relationships. Dr. Romero discloses receiving research grants from Biosense Webster; and speaking or receiving honoraria or fees for consulting, speaking, or teaching, or serving on a board for Sanofi, Boston Scientific, and AtriCure. Dr. Koneru discloses consulting for Medtronic and receiving honoraria from Abbott. Dr. Ellenbogen discloses consulting or lecturing for or receiving honoraria from Medtronic, Boston Scientific, and Abbott. Dr. Goldberger discloses receiving royalty income from and serving as an independent contractor for Elsevier. Dr. Al-Khatib discloses receiving research grants from Medtronic and Boston Scientific.
A version of this article first appeared on Medscape.com.
Pacing as a device therapy for heart failure (HF) is headed for what is probably its next big advance.
After decades of biventricular (BiV) pacemaker success in resynchronizing the ventricles and improving clinical outcomes, relatively new conduction-system pacing (CSP) techniques that avoid the pitfalls of right-ventricular (RV) pacing using BiV lead systems have been supplanting traditional cardiac resynchronization therapy (CRT) in selected patients at some major centers. In fact, they are solidly ensconced in a new guideline document addressing indications for CSP and BiV pacing in HF.
But , an alternative when BiV pacing isn’t appropriate or can’t be engaged.
That’s mainly because the limited, mostly observational evidence supporting CSP in the document can’t measure up to the clinical experience and plethora of large, randomized trials behind BiV-CRT.
But that shortfall is headed for change. Several new comparative studies, including a small, randomized trial, have added significantly to evidence suggesting that CSP is at least as effective as traditional CRT for procedural, functional safety, and clinical outcomes.
The new studies “are inherently prone to bias, but their results are really good,” observed Juan C. Diaz, MD. They show improvements in left ventricular ejection fraction (LVEF) and symptoms with CSP that are “outstanding compared to what we have been doing for the last 20 years,” he said in an interview.
Dr. Diaz, Clínica Las Vegas, Medellin, Colombia, is an investigator with the observational SYNCHRONY, which is among the new CSP studies formally presented at the annual scientific sessions of the Heart Rhythm Society. He is also lead author on its same-day publication in JACC: Clinical Electrophysiology.
Dr. Diaz said that CSP, which sustains pacing via the native conduction system, makes more “physiologic sense” than BiV pacing and represents “a step forward” for HF device therapy.
SYNCHRONY compared LBB-area with BiV pacing as the initial strategy for achieving cardiac resynchronization in patients with ischemic or nonischemic cardiomyopathy.
CSP is “a long way” from replacing conventional CRT, he said. But the new studies at the HRS sessions should help extend His-bundle and LBB-area pacing to more patients, he added, given the significant long-term “drawbacks” of BiV pacing. These include inevitable RV pacing, multiple leads, and the risks associated with chronic transvenous leads.
Zachary Goldberger, MD, University of Wisconsin–Madison, went a bit further in support of CSP as invited discussant for the SYNCHRONY presentation.
Given that it improved LVEF, heart failure class, HF hospitalizations (HFH), and mortality in that study and others, Dr. Goldberger said, CSP could potentially “become the dominant mode of resynchronization going forward.”
Other experts at the meeting saw CSP’s potential more as one of several pacing techniques that could be brought to bear for patients with CRT indications.
“Conduction system pacing is going to be a huge complement to biventricular pacing,” to which about 30% of patients have a “less than optimal response,” said Pugazhendhi Vijayaraman, MD, chief of clinical electrophysiology, Geisinger Heart Institute, Danville, Pa.
“I don’t think it needs to replace biventricular pacing, because biventricular pacing is a well-established, incredibly powerful therapy,” he told this news organization. But CSP is likely to provide “a good alternative option” in patients with poor responses to BiV-CRT.
It may, however, render some current BiV-pacing alternatives “obsolete,” Dr. Vijayaraman observed. “At our center, at least for the last 5 years, no patient has needed epicardial surgical left ventricular lead placement” because CSP was a better backup option.
Dr. Vijayaraman presented two of the meeting’s CSP vs. BiV pacing comparisons. In one, the 100-patient randomized HOT-CRT trial, contractile function improved significantly on CSP, which could be either His-bundle or LBB-area pacing.
He also presented an observational study of LBB-area pacing at 15 centers in Asia, Europe, and North America and led the authors of its simultaneous publication in the Journal of the American College of Cardiology.
“I think left-bundle conduction system pacing is the future, for sure,” Jagmeet P. Singh, MD, DPhil, told this news organization. Still, it doesn’t always work and when it does, it “doesn’t work equally in all patients,” he said.
“Conduction system pacing certainly makes a lot of sense,” especially in patients with left-bundle-branch block (LBBB), and “maybe not as a primary approach but certainly as a secondary approach,” said Dr. Singh, Massachusetts General Hospital, Boston, who is not a coauthor on any of the three studies.
He acknowledged that CSP may work well as a first-line option in patients with LBBB at some experienced centers. For those without LBBB or who have an intraventricular conduction delay, who represent 45%-50% of current CRT cases, Dr. Singh observed, “there’s still more evidence” that BiV-CRT is a more appropriate initial approach.
Standard CRT may fail, however, even in some patients who otherwise meet guideline-based indications. “We don’t really understand all the mechanisms for nonresponse in conventional biventricular pacing,” observed Niraj Varma, MD, PhD, Cleveland Clinic, also not involved with any of the three studies.
In some groups, including “patients with larger ventricles,” for example, BiV-CRT doesn’t always narrow the electrocardiographic QRS complex or preexcite delayed left ventricular (LV) activation, hallmarks of successful CRT, he said in an interview.
“I think we need to understand why this occurs in both situations,” but in such cases, CSP alone or as an adjunct to direct LV pacing may be successful. “Sometimes we need both an LV lead and the conduction-system pacing lead.”
Narrower, more efficient use of CSP as a BiV-CRT alternative may also boost its chances for success, Dr. Varma added. “I think we need to refine patient selection.”
HOT-CRT: Randomized CSP vs. BiV pacing trial
Conducted at three centers in a single health system, the His-optimized cardiac resynchronization therapy study (HOT-CRT) randomly assigned 100 patients with primary or secondary CRT indications to either to CSP – by either His-bundle or LBB-area pacing – or to standard BiV-CRT as the first-line resynchronization method.
Treatment crossovers, allowed for either pacing modality in the event of implantation failure, occurred in two patients and nine patients initially assigned to CSP and BiV pacing, respectively (4% vs. 18%), Dr. Vijayaraman reported.
Historically in trials, BiV pacing has elevated LVEF by about 7%, he said. The mean 12-point increase observed with CSP “is huge, in that sense.” HOT-CRT enrolled a predominantly male and White population at centers highly experienced in both CSP and BiV pacing, limiting its broad relevance to practice, as pointed out by both Dr. Vijayaraman and his presentation’s invited discussant, Yong-Mei Cha, MD, Mayo Clinic, Rochester, Minn. Dr. Cha, who is director of cardiac device services at her center, also highlighted the greater rate of crossover from BiV pacing to CSP, 18% vs. 4% in the other direction. “This is a very encouraging result,” because the implant-failure rate for LBB-area pacing may drop once more operators become “familiar and skilled with conduction-system pacing.” Overall, the study supports CSP as “a very good alternative for heart failure patients when BiV pacing fails.”
International comparison of CSP and BiV pacing
In Dr. Vijayaraman’s other study, the observational comparison of LBB-area pacing and BiV-CRT, the CSP technique emerged as a “reasonable alternative to biventricular pacing, not only for improvement in LV function but also to reduce adverse clinical outcomes.”
Indeed, in the international study of 1,778 mostly male patients with primary or secondary CRT indications who received LBB-area or BiV pacing (797 and 981 patients, respectively), those on CSP saw a significant drop in risk for the primary endpoint, death or HFH.
Mean LVEF improved from 27% to 41% in the LBB-area pacing group and 27% to 37% with BiV pacing (P < .001 for both changes) over a follow-up averaging 33 months. The difference in improvement between CSP and BiV pacing was significant at P < .001.
In adjusted analysis, the risk for death or HFH was greater for BiV-pacing patients, a difference driven by HFH events.
- Death or HF: hazard ratio, 1.49 (95% confidence interval, 1.21-1.84; P < .001).
- Death: HR, 1.14 (95% CI, 0.88-1.48; P = .313).
- HFH: HR, 1.49 (95% CI, 1.16-1.92; P = .002)
The analysis has all the “inherent biases” of an observational study. The risk for patient-selection bias, however, was somewhat mitigated by consistent practice patterns at participating centers, Dr. Vijayaraman told this news organization.
For example, he said, operators at six of the institutions were most likely to use CSP as the first-line approach, and the same number of centers usually went with BiV pacing.
SYNCHRONY: First-line LBB-area pacing vs. BiV-CRT
Outcomes using the two approaches were similar in the prospective, international, observational study of 371 patients with ischemic or nonischemic cardiomyopathy and standard CRT indications. Allocation of 128 patients to LBB-area pacing and 243 to BiV-CRT was based on patient and operator preferences, reported Jorge Romero Jr, MD, Brigham and Women’s Hospital, Boston, at the HRS sessions.
Risk for the death-HFH primary endpoint dropped 38% for those initially treated with LBB-area pacing, compared with BiV pacing, primarily because of a lower HFH risk:
- Death or HFH: HR, 0.62 (95% CI, 0.41-0.93; P = .02).
- Death: HR, 0.57 (95% CI, 0.25-1.32; P = .19).
- HFH: HR, 0.61 (95% CI, 0.34-0.93; P = .02)
Patients in the CSP group were also more likely to improve by at least one NYHA (New York Heart Association) class (80.4% vs. 67.9%; P < .001), consistent with their greater absolute change in LVEF (8.0 vs. 3.9 points; P < .01).
The findings “suggest that LBBAP [left-bundle branch area pacing] is an excellent alternative to BiV pacing,” with a comparable safety profile, write Jayanthi N. Koneru, MBBS, and Kenneth A. Ellenbogen, MD, in an editorial accompanying the published SYNCHRONY report.
“The differences in improvement of LVEF are encouraging for both groups,” but were superior for LBB-area pacing, continue Dr. Koneru and Dr. Ellenbogen, both with Virginia Commonwealth University Medical Center, Richmond. “Whether these results would have regressed to the mean over a longer period of follow-up or diverge further with LBB-area pacing continuing to be superior is unknown.”
Years for an answer?
A large randomized comparison of CSP and BiV-CRT, called Left vs. Left, is currently in early stages, Sana M. Al-Khatib, MD, MHS, Duke University Medical Center, Durham, N.C., said in a media presentation on two of the presented studies. It has a planned enrollment of more than 2,100 patients on optimal meds with an LVEF of 50% or lower and either a QRS duration of at least 130 ms or an anticipated burden of RV pacing exceeding 40%.
The trial, she said, “will take years to give an answer, but it is actually designed to address the question of whether a composite endpoint of time to death or heart failure hospitalization can be improved with conduction system pacing vs. biventricular pacing.”
Dr. Al-Khatib is a coauthor on the new guideline covering both CSP and BiV-CRT in HF, as are Dr. Cha, Dr. Varma, Dr. Singh, Dr. Vijayaraman, and Dr. Goldberger; Dr. Ellenbogen is one of the reviewers.
Dr. Diaz discloses receiving honoraria or fees for speaking or teaching from Bayer Healthcare, Pfizer, AstraZeneca, Boston Scientific, and Medtronic. Dr. Vijayaraman discloses receiving honoraria or fees for speaking, teaching, or consulting for Abbott, Medtronic, Biotronik, and Boston Scientific; and receiving research grants from Medtronic. Dr. Varma discloses receiving honoraria or fees for speaking or consulting as an independent contractor for Medtronic, Boston Scientific, Biotronik, Impulse Dynamics USA, Cardiologs, Abbott, Pacemate, Implicity, and EP Solutions. Dr. Singh discloses receiving fees for consulting from EBR Systems, Merit Medical Systems, New Century Health, Biotronik, Abbott, Medtronic, MicroPort Scientific, Cardiologs, Sanofi, CVRx, Impulse Dynamics USA, Octagos, Implicity, Orchestra Biomed, Rhythm Management Group, and Biosense Webster; and receiving honoraria or fees for speaking and teaching from Medscape. Dr. Cha had no relevant financial relationships. Dr. Romero discloses receiving research grants from Biosense Webster; and speaking or receiving honoraria or fees for consulting, speaking, or teaching, or serving on a board for Sanofi, Boston Scientific, and AtriCure. Dr. Koneru discloses consulting for Medtronic and receiving honoraria from Abbott. Dr. Ellenbogen discloses consulting or lecturing for or receiving honoraria from Medtronic, Boston Scientific, and Abbott. Dr. Goldberger discloses receiving royalty income from and serving as an independent contractor for Elsevier. Dr. Al-Khatib discloses receiving research grants from Medtronic and Boston Scientific.
A version of this article first appeared on Medscape.com.
Pacing as a device therapy for heart failure (HF) is headed for what is probably its next big advance.
After decades of biventricular (BiV) pacemaker success in resynchronizing the ventricles and improving clinical outcomes, relatively new conduction-system pacing (CSP) techniques that avoid the pitfalls of right-ventricular (RV) pacing using BiV lead systems have been supplanting traditional cardiac resynchronization therapy (CRT) in selected patients at some major centers. In fact, they are solidly ensconced in a new guideline document addressing indications for CSP and BiV pacing in HF.
But , an alternative when BiV pacing isn’t appropriate or can’t be engaged.
That’s mainly because the limited, mostly observational evidence supporting CSP in the document can’t measure up to the clinical experience and plethora of large, randomized trials behind BiV-CRT.
But that shortfall is headed for change. Several new comparative studies, including a small, randomized trial, have added significantly to evidence suggesting that CSP is at least as effective as traditional CRT for procedural, functional safety, and clinical outcomes.
The new studies “are inherently prone to bias, but their results are really good,” observed Juan C. Diaz, MD. They show improvements in left ventricular ejection fraction (LVEF) and symptoms with CSP that are “outstanding compared to what we have been doing for the last 20 years,” he said in an interview.
Dr. Diaz, Clínica Las Vegas, Medellin, Colombia, is an investigator with the observational SYNCHRONY, which is among the new CSP studies formally presented at the annual scientific sessions of the Heart Rhythm Society. He is also lead author on its same-day publication in JACC: Clinical Electrophysiology.
Dr. Diaz said that CSP, which sustains pacing via the native conduction system, makes more “physiologic sense” than BiV pacing and represents “a step forward” for HF device therapy.
SYNCHRONY compared LBB-area with BiV pacing as the initial strategy for achieving cardiac resynchronization in patients with ischemic or nonischemic cardiomyopathy.
CSP is “a long way” from replacing conventional CRT, he said. But the new studies at the HRS sessions should help extend His-bundle and LBB-area pacing to more patients, he added, given the significant long-term “drawbacks” of BiV pacing. These include inevitable RV pacing, multiple leads, and the risks associated with chronic transvenous leads.
Zachary Goldberger, MD, University of Wisconsin–Madison, went a bit further in support of CSP as invited discussant for the SYNCHRONY presentation.
Given that it improved LVEF, heart failure class, HF hospitalizations (HFH), and mortality in that study and others, Dr. Goldberger said, CSP could potentially “become the dominant mode of resynchronization going forward.”
Other experts at the meeting saw CSP’s potential more as one of several pacing techniques that could be brought to bear for patients with CRT indications.
“Conduction system pacing is going to be a huge complement to biventricular pacing,” to which about 30% of patients have a “less than optimal response,” said Pugazhendhi Vijayaraman, MD, chief of clinical electrophysiology, Geisinger Heart Institute, Danville, Pa.
“I don’t think it needs to replace biventricular pacing, because biventricular pacing is a well-established, incredibly powerful therapy,” he told this news organization. But CSP is likely to provide “a good alternative option” in patients with poor responses to BiV-CRT.
It may, however, render some current BiV-pacing alternatives “obsolete,” Dr. Vijayaraman observed. “At our center, at least for the last 5 years, no patient has needed epicardial surgical left ventricular lead placement” because CSP was a better backup option.
Dr. Vijayaraman presented two of the meeting’s CSP vs. BiV pacing comparisons. In one, the 100-patient randomized HOT-CRT trial, contractile function improved significantly on CSP, which could be either His-bundle or LBB-area pacing.
He also presented an observational study of LBB-area pacing at 15 centers in Asia, Europe, and North America and led the authors of its simultaneous publication in the Journal of the American College of Cardiology.
“I think left-bundle conduction system pacing is the future, for sure,” Jagmeet P. Singh, MD, DPhil, told this news organization. Still, it doesn’t always work and when it does, it “doesn’t work equally in all patients,” he said.
“Conduction system pacing certainly makes a lot of sense,” especially in patients with left-bundle-branch block (LBBB), and “maybe not as a primary approach but certainly as a secondary approach,” said Dr. Singh, Massachusetts General Hospital, Boston, who is not a coauthor on any of the three studies.
He acknowledged that CSP may work well as a first-line option in patients with LBBB at some experienced centers. For those without LBBB or who have an intraventricular conduction delay, who represent 45%-50% of current CRT cases, Dr. Singh observed, “there’s still more evidence” that BiV-CRT is a more appropriate initial approach.
Standard CRT may fail, however, even in some patients who otherwise meet guideline-based indications. “We don’t really understand all the mechanisms for nonresponse in conventional biventricular pacing,” observed Niraj Varma, MD, PhD, Cleveland Clinic, also not involved with any of the three studies.
In some groups, including “patients with larger ventricles,” for example, BiV-CRT doesn’t always narrow the electrocardiographic QRS complex or preexcite delayed left ventricular (LV) activation, hallmarks of successful CRT, he said in an interview.
“I think we need to understand why this occurs in both situations,” but in such cases, CSP alone or as an adjunct to direct LV pacing may be successful. “Sometimes we need both an LV lead and the conduction-system pacing lead.”
Narrower, more efficient use of CSP as a BiV-CRT alternative may also boost its chances for success, Dr. Varma added. “I think we need to refine patient selection.”
HOT-CRT: Randomized CSP vs. BiV pacing trial
Conducted at three centers in a single health system, the His-optimized cardiac resynchronization therapy study (HOT-CRT) randomly assigned 100 patients with primary or secondary CRT indications to either to CSP – by either His-bundle or LBB-area pacing – or to standard BiV-CRT as the first-line resynchronization method.
Treatment crossovers, allowed for either pacing modality in the event of implantation failure, occurred in two patients and nine patients initially assigned to CSP and BiV pacing, respectively (4% vs. 18%), Dr. Vijayaraman reported.
Historically in trials, BiV pacing has elevated LVEF by about 7%, he said. The mean 12-point increase observed with CSP “is huge, in that sense.” HOT-CRT enrolled a predominantly male and White population at centers highly experienced in both CSP and BiV pacing, limiting its broad relevance to practice, as pointed out by both Dr. Vijayaraman and his presentation’s invited discussant, Yong-Mei Cha, MD, Mayo Clinic, Rochester, Minn. Dr. Cha, who is director of cardiac device services at her center, also highlighted the greater rate of crossover from BiV pacing to CSP, 18% vs. 4% in the other direction. “This is a very encouraging result,” because the implant-failure rate for LBB-area pacing may drop once more operators become “familiar and skilled with conduction-system pacing.” Overall, the study supports CSP as “a very good alternative for heart failure patients when BiV pacing fails.”
International comparison of CSP and BiV pacing
In Dr. Vijayaraman’s other study, the observational comparison of LBB-area pacing and BiV-CRT, the CSP technique emerged as a “reasonable alternative to biventricular pacing, not only for improvement in LV function but also to reduce adverse clinical outcomes.”
Indeed, in the international study of 1,778 mostly male patients with primary or secondary CRT indications who received LBB-area or BiV pacing (797 and 981 patients, respectively), those on CSP saw a significant drop in risk for the primary endpoint, death or HFH.
Mean LVEF improved from 27% to 41% in the LBB-area pacing group and 27% to 37% with BiV pacing (P < .001 for both changes) over a follow-up averaging 33 months. The difference in improvement between CSP and BiV pacing was significant at P < .001.
In adjusted analysis, the risk for death or HFH was greater for BiV-pacing patients, a difference driven by HFH events.
- Death or HF: hazard ratio, 1.49 (95% confidence interval, 1.21-1.84; P < .001).
- Death: HR, 1.14 (95% CI, 0.88-1.48; P = .313).
- HFH: HR, 1.49 (95% CI, 1.16-1.92; P = .002)
The analysis has all the “inherent biases” of an observational study. The risk for patient-selection bias, however, was somewhat mitigated by consistent practice patterns at participating centers, Dr. Vijayaraman told this news organization.
For example, he said, operators at six of the institutions were most likely to use CSP as the first-line approach, and the same number of centers usually went with BiV pacing.
SYNCHRONY: First-line LBB-area pacing vs. BiV-CRT
Outcomes using the two approaches were similar in the prospective, international, observational study of 371 patients with ischemic or nonischemic cardiomyopathy and standard CRT indications. Allocation of 128 patients to LBB-area pacing and 243 to BiV-CRT was based on patient and operator preferences, reported Jorge Romero Jr, MD, Brigham and Women’s Hospital, Boston, at the HRS sessions.
Risk for the death-HFH primary endpoint dropped 38% for those initially treated with LBB-area pacing, compared with BiV pacing, primarily because of a lower HFH risk:
- Death or HFH: HR, 0.62 (95% CI, 0.41-0.93; P = .02).
- Death: HR, 0.57 (95% CI, 0.25-1.32; P = .19).
- HFH: HR, 0.61 (95% CI, 0.34-0.93; P = .02)
Patients in the CSP group were also more likely to improve by at least one NYHA (New York Heart Association) class (80.4% vs. 67.9%; P < .001), consistent with their greater absolute change in LVEF (8.0 vs. 3.9 points; P < .01).
The findings “suggest that LBBAP [left-bundle branch area pacing] is an excellent alternative to BiV pacing,” with a comparable safety profile, write Jayanthi N. Koneru, MBBS, and Kenneth A. Ellenbogen, MD, in an editorial accompanying the published SYNCHRONY report.
“The differences in improvement of LVEF are encouraging for both groups,” but were superior for LBB-area pacing, continue Dr. Koneru and Dr. Ellenbogen, both with Virginia Commonwealth University Medical Center, Richmond. “Whether these results would have regressed to the mean over a longer period of follow-up or diverge further with LBB-area pacing continuing to be superior is unknown.”
Years for an answer?
A large randomized comparison of CSP and BiV-CRT, called Left vs. Left, is currently in early stages, Sana M. Al-Khatib, MD, MHS, Duke University Medical Center, Durham, N.C., said in a media presentation on two of the presented studies. It has a planned enrollment of more than 2,100 patients on optimal meds with an LVEF of 50% or lower and either a QRS duration of at least 130 ms or an anticipated burden of RV pacing exceeding 40%.
The trial, she said, “will take years to give an answer, but it is actually designed to address the question of whether a composite endpoint of time to death or heart failure hospitalization can be improved with conduction system pacing vs. biventricular pacing.”
Dr. Al-Khatib is a coauthor on the new guideline covering both CSP and BiV-CRT in HF, as are Dr. Cha, Dr. Varma, Dr. Singh, Dr. Vijayaraman, and Dr. Goldberger; Dr. Ellenbogen is one of the reviewers.
Dr. Diaz discloses receiving honoraria or fees for speaking or teaching from Bayer Healthcare, Pfizer, AstraZeneca, Boston Scientific, and Medtronic. Dr. Vijayaraman discloses receiving honoraria or fees for speaking, teaching, or consulting for Abbott, Medtronic, Biotronik, and Boston Scientific; and receiving research grants from Medtronic. Dr. Varma discloses receiving honoraria or fees for speaking or consulting as an independent contractor for Medtronic, Boston Scientific, Biotronik, Impulse Dynamics USA, Cardiologs, Abbott, Pacemate, Implicity, and EP Solutions. Dr. Singh discloses receiving fees for consulting from EBR Systems, Merit Medical Systems, New Century Health, Biotronik, Abbott, Medtronic, MicroPort Scientific, Cardiologs, Sanofi, CVRx, Impulse Dynamics USA, Octagos, Implicity, Orchestra Biomed, Rhythm Management Group, and Biosense Webster; and receiving honoraria or fees for speaking and teaching from Medscape. Dr. Cha had no relevant financial relationships. Dr. Romero discloses receiving research grants from Biosense Webster; and speaking or receiving honoraria or fees for consulting, speaking, or teaching, or serving on a board for Sanofi, Boston Scientific, and AtriCure. Dr. Koneru discloses consulting for Medtronic and receiving honoraria from Abbott. Dr. Ellenbogen discloses consulting or lecturing for or receiving honoraria from Medtronic, Boston Scientific, and Abbott. Dr. Goldberger discloses receiving royalty income from and serving as an independent contractor for Elsevier. Dr. Al-Khatib discloses receiving research grants from Medtronic and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
ECG implant tightens AFib management, improves outcomes in MONITOR-AF
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.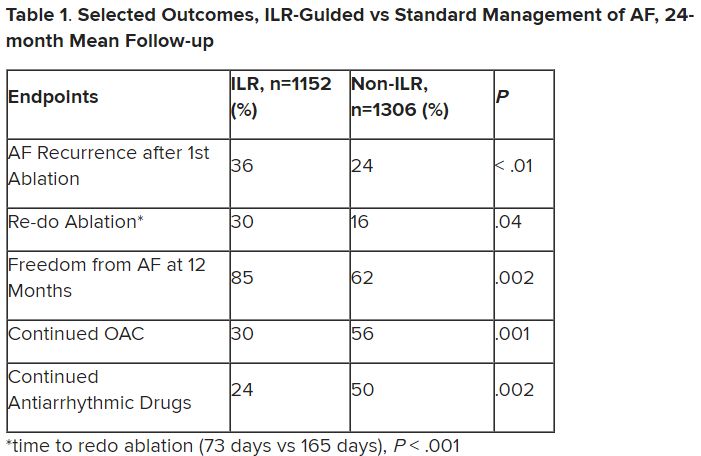
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.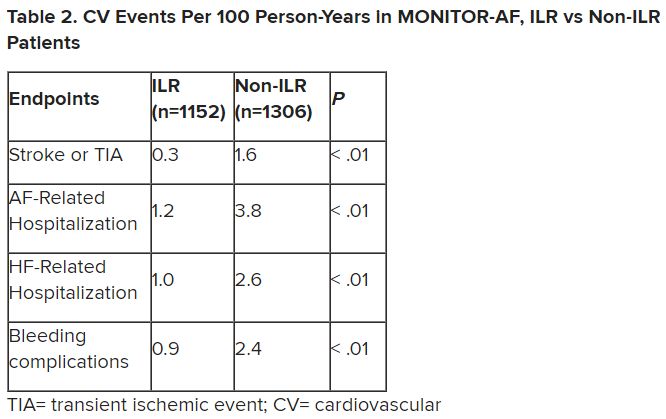
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
Leadless dual-chamber pacemaker clears early safety, performance hurdles
Cardiology, well into the age of leadless pacemakers, could be headed for an age of leadless pacemaker systems in which various pacing functions are achieved by multiple implants that “talk” to each other.
Even now, a leadless two-part pacemaker system has shown it can safely achieve atrioventricular (AV) synchrony in patients with standard indications for a dual-chamber device, at least over the short term, suggests a prospective observational study. Currently available leadless pacemakers can stimulate only the right ventricle.
Experienced operators achieved a 98% implantation success rate in 300 patients who received an investigational dual-chamber leadless system, the AVEIR DR i2i (Abbott).
Its two separately implanted miniature pulse generators achieve AV synchrony via “beat-to-beat wireless bidirectional communication,” Daniel J. Cantillon, MD, said when presenting the study at the annual scientific sessions of the Heart Rhythm Societyin New Orleans.
The system seemed to work well regardless of the patient’s body orientation. “Sitting, supine, left lateral, right lateral, standing, normal walk, fast walk – we demonstrated robust AV synchrony in all of those positions and with movement,” said Dr. Cantillon, of the Cleveland Clinic.
Should the device be approved, it could “expand the use case for leadless cardiac pacing” to include atrial-only, ventricular-only, fully functional dual-chamber pacing scenarios.”
Dr. Cantillon is senior author on the study’s online publication in the New England Journal of Medicine, timed to coincide with his HRS presentation, with first author Reinoud E. Knops, MD, PhD, Amsterdam University Medical Center.
“The electrical performance of both the atrial and ventricular leadless pacemakers appears to be similar to that of transvenous dual-chamber pacemakers,” the published report states.
More data needed
The study is important and has “significant implications for our pacing field,” Jonathan P. Piccini, MD, MHS, said in an interview. It suggests that “dual-chamber pacing can be achieved with leadless technology” and “with a very high degree” of AV synchrony.
“Obviously, more data as the technology moves into clinical practice will be critical,” said Dr. Piccini, who directs cardiac electrophysiology at Duke University Medical Center, Durham, N.C. “We will also need to understand which patients are best served by leadless technology and which will be better served with traditional transvenous devices.”
The AVEIR DR i2i system consists of two leadless pulse generators for percutaneous implantation in the right atrium and right ventricle, respectively. They link like components of a wireless network to coordinate their separate sensing and rate-adaptive, AV-synchronous pacing functions.
The right ventricular implant “is physically identical to a commercially available single-chamber leadless pacemaker” from Abbott, the published report states.
Leadless pacemaker systems inherently avoid the two main sources of transvenous devices’ major complication – infection – by not requiring such leads or surgery for creating a pulse-generator subcutaneous pocket.
The first such systems consisted of one implant that could provide single-chamber ventricular pacing but not atrial pacing or AV synchronous pacing. The transcatheter single-chamber leadless Micra (Medtronic) for example, was approved in the United States in April 2016 for ventricular-only pacing.
A successor, the Micra AV, approved in 2020, was designed to simulate AV-synchronous pacing by stimulating the ventricle in sequence with mechanically sensed atrial contractions, as described by Dr. Cantillon and associates. But it could not directly pace the atrium, “rendering it inappropriate for patients with sinus-node dysfunction.”
The AVEIR DR i2i system doesn’t have those limitations. It was, however, associated with 35 device- or procedure-related complications in the study, of which the most common was procedural arrhythmia, “namely atrial fibrillation,” Dr. Cantillon said.
Atrial fibrillation can develop during implantation of pacemakers with transvenous leads but is generally terminated without being considered an important event. Yet the study classified it as a serious complication, inflating the complication rate, because “the patients had to be restored to sinus rhythm so we could assess the AV synchrony and also the atrial electrical performance,” he said.
Some of the devices dislodged from their implantation site within a month of the procedure, but “all of those patients were successfully managed percutaneously,” said Dr. Cantillon.
“The 1.7% dislodgement rate is something that we will need to keep an eye on, as embolization of devices is always a significant concern,” Dr. Piccini said. Still, the observed total complication rate “was certainly in line” with rates associated with conventional pacemaker implantation.
Reliable AV synchrony
Fred M. Kusumoto, MD, Mayo Clinic, Jacksonville, Fla., lauded what seems to be the system’s “incredibly reliable AV synchrony in different conditions, albeit in a very controlled environment.”
Of interest will be whether its performance, including maintenance of AV synchrony, holds up in “a more long-term evaluation in the outpatient setting,” said Dr. Kusumoto, speaking as the invited discussant for Dr. Cantillon’s presentation.
Also missing or in short supply from the study, he observed, are insights about long-term efficacy and complications, battery longevity, effectiveness of its rate-responsive capability, and any effect on clinical outcomes.
Local body network
Of the study’s 300 patients (mean age 69 years; 38% female) at 55 sites in Canada, Europe, and the United States, 63.3% had sinus-node dysfunction and 33.3% had AV block as their primary dual-chamber pacing indication; 298 were successfully implanted with both devices.
About 45% had a history of supraventricular arrhythmia, 4.3% had prior ventricular arrhythmia, and 20% had a history of arrhythmia ablation.
By 3 months, the group reported, the primary safety endpoint (freedom from device- or procedure-related serious adverse events) occurred in 90.3%, compared with the performance goal of 78% (P < .001).
The first of two primary performance endpoints (adequate atrial capture threshold and sensing amplitude by predefined criteria) was met in 90.2%, surpassing the 82.5% performance goal (P < .001).
The second primary performance goal (at least 70% AV synchrony with the patient sitting) was seen in 97.3% against the performance goal of 83% (P < .001).
What shouldn’t be “glossed over” from the study, Dr. Kusumoto offered, is that it’s possible to achieve a wireless connection “between two devices that are actually intracardiac.” That raises the prospect of a “local body network” that could be “expanded even more dramatically with other types of devices. I mean, think of the paradigm shift.”
The AVEIR DR i2i trial was funded by Abbott. Dr. Cantillon discloses receiving honoraria or fees for speaking or consulting from Abbott Laboratories, Boston Scientific, Biosense Webster, and Shockwave Medical, as well as holding royalty rights with AirStrip. Dr. Piccini has disclosed relationships with Abbott, Medtronic, Biotronik, Boston Scientific, and other drug and medical device companies. Dr. Kusumoto reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiology, well into the age of leadless pacemakers, could be headed for an age of leadless pacemaker systems in which various pacing functions are achieved by multiple implants that “talk” to each other.
Even now, a leadless two-part pacemaker system has shown it can safely achieve atrioventricular (AV) synchrony in patients with standard indications for a dual-chamber device, at least over the short term, suggests a prospective observational study. Currently available leadless pacemakers can stimulate only the right ventricle.
Experienced operators achieved a 98% implantation success rate in 300 patients who received an investigational dual-chamber leadless system, the AVEIR DR i2i (Abbott).
Its two separately implanted miniature pulse generators achieve AV synchrony via “beat-to-beat wireless bidirectional communication,” Daniel J. Cantillon, MD, said when presenting the study at the annual scientific sessions of the Heart Rhythm Societyin New Orleans.
The system seemed to work well regardless of the patient’s body orientation. “Sitting, supine, left lateral, right lateral, standing, normal walk, fast walk – we demonstrated robust AV synchrony in all of those positions and with movement,” said Dr. Cantillon, of the Cleveland Clinic.
Should the device be approved, it could “expand the use case for leadless cardiac pacing” to include atrial-only, ventricular-only, fully functional dual-chamber pacing scenarios.”
Dr. Cantillon is senior author on the study’s online publication in the New England Journal of Medicine, timed to coincide with his HRS presentation, with first author Reinoud E. Knops, MD, PhD, Amsterdam University Medical Center.
“The electrical performance of both the atrial and ventricular leadless pacemakers appears to be similar to that of transvenous dual-chamber pacemakers,” the published report states.
More data needed
The study is important and has “significant implications for our pacing field,” Jonathan P. Piccini, MD, MHS, said in an interview. It suggests that “dual-chamber pacing can be achieved with leadless technology” and “with a very high degree” of AV synchrony.
“Obviously, more data as the technology moves into clinical practice will be critical,” said Dr. Piccini, who directs cardiac electrophysiology at Duke University Medical Center, Durham, N.C. “We will also need to understand which patients are best served by leadless technology and which will be better served with traditional transvenous devices.”
The AVEIR DR i2i system consists of two leadless pulse generators for percutaneous implantation in the right atrium and right ventricle, respectively. They link like components of a wireless network to coordinate their separate sensing and rate-adaptive, AV-synchronous pacing functions.
The right ventricular implant “is physically identical to a commercially available single-chamber leadless pacemaker” from Abbott, the published report states.
Leadless pacemaker systems inherently avoid the two main sources of transvenous devices’ major complication – infection – by not requiring such leads or surgery for creating a pulse-generator subcutaneous pocket.
The first such systems consisted of one implant that could provide single-chamber ventricular pacing but not atrial pacing or AV synchronous pacing. The transcatheter single-chamber leadless Micra (Medtronic) for example, was approved in the United States in April 2016 for ventricular-only pacing.
A successor, the Micra AV, approved in 2020, was designed to simulate AV-synchronous pacing by stimulating the ventricle in sequence with mechanically sensed atrial contractions, as described by Dr. Cantillon and associates. But it could not directly pace the atrium, “rendering it inappropriate for patients with sinus-node dysfunction.”
The AVEIR DR i2i system doesn’t have those limitations. It was, however, associated with 35 device- or procedure-related complications in the study, of which the most common was procedural arrhythmia, “namely atrial fibrillation,” Dr. Cantillon said.
Atrial fibrillation can develop during implantation of pacemakers with transvenous leads but is generally terminated without being considered an important event. Yet the study classified it as a serious complication, inflating the complication rate, because “the patients had to be restored to sinus rhythm so we could assess the AV synchrony and also the atrial electrical performance,” he said.
Some of the devices dislodged from their implantation site within a month of the procedure, but “all of those patients were successfully managed percutaneously,” said Dr. Cantillon.
“The 1.7% dislodgement rate is something that we will need to keep an eye on, as embolization of devices is always a significant concern,” Dr. Piccini said. Still, the observed total complication rate “was certainly in line” with rates associated with conventional pacemaker implantation.
Reliable AV synchrony
Fred M. Kusumoto, MD, Mayo Clinic, Jacksonville, Fla., lauded what seems to be the system’s “incredibly reliable AV synchrony in different conditions, albeit in a very controlled environment.”
Of interest will be whether its performance, including maintenance of AV synchrony, holds up in “a more long-term evaluation in the outpatient setting,” said Dr. Kusumoto, speaking as the invited discussant for Dr. Cantillon’s presentation.
Also missing or in short supply from the study, he observed, are insights about long-term efficacy and complications, battery longevity, effectiveness of its rate-responsive capability, and any effect on clinical outcomes.
Local body network
Of the study’s 300 patients (mean age 69 years; 38% female) at 55 sites in Canada, Europe, and the United States, 63.3% had sinus-node dysfunction and 33.3% had AV block as their primary dual-chamber pacing indication; 298 were successfully implanted with both devices.
About 45% had a history of supraventricular arrhythmia, 4.3% had prior ventricular arrhythmia, and 20% had a history of arrhythmia ablation.
By 3 months, the group reported, the primary safety endpoint (freedom from device- or procedure-related serious adverse events) occurred in 90.3%, compared with the performance goal of 78% (P < .001).
The first of two primary performance endpoints (adequate atrial capture threshold and sensing amplitude by predefined criteria) was met in 90.2%, surpassing the 82.5% performance goal (P < .001).
The second primary performance goal (at least 70% AV synchrony with the patient sitting) was seen in 97.3% against the performance goal of 83% (P < .001).
What shouldn’t be “glossed over” from the study, Dr. Kusumoto offered, is that it’s possible to achieve a wireless connection “between two devices that are actually intracardiac.” That raises the prospect of a “local body network” that could be “expanded even more dramatically with other types of devices. I mean, think of the paradigm shift.”
The AVEIR DR i2i trial was funded by Abbott. Dr. Cantillon discloses receiving honoraria or fees for speaking or consulting from Abbott Laboratories, Boston Scientific, Biosense Webster, and Shockwave Medical, as well as holding royalty rights with AirStrip. Dr. Piccini has disclosed relationships with Abbott, Medtronic, Biotronik, Boston Scientific, and other drug and medical device companies. Dr. Kusumoto reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiology, well into the age of leadless pacemakers, could be headed for an age of leadless pacemaker systems in which various pacing functions are achieved by multiple implants that “talk” to each other.
Even now, a leadless two-part pacemaker system has shown it can safely achieve atrioventricular (AV) synchrony in patients with standard indications for a dual-chamber device, at least over the short term, suggests a prospective observational study. Currently available leadless pacemakers can stimulate only the right ventricle.
Experienced operators achieved a 98% implantation success rate in 300 patients who received an investigational dual-chamber leadless system, the AVEIR DR i2i (Abbott).
Its two separately implanted miniature pulse generators achieve AV synchrony via “beat-to-beat wireless bidirectional communication,” Daniel J. Cantillon, MD, said when presenting the study at the annual scientific sessions of the Heart Rhythm Societyin New Orleans.
The system seemed to work well regardless of the patient’s body orientation. “Sitting, supine, left lateral, right lateral, standing, normal walk, fast walk – we demonstrated robust AV synchrony in all of those positions and with movement,” said Dr. Cantillon, of the Cleveland Clinic.
Should the device be approved, it could “expand the use case for leadless cardiac pacing” to include atrial-only, ventricular-only, fully functional dual-chamber pacing scenarios.”
Dr. Cantillon is senior author on the study’s online publication in the New England Journal of Medicine, timed to coincide with his HRS presentation, with first author Reinoud E. Knops, MD, PhD, Amsterdam University Medical Center.
“The electrical performance of both the atrial and ventricular leadless pacemakers appears to be similar to that of transvenous dual-chamber pacemakers,” the published report states.
More data needed
The study is important and has “significant implications for our pacing field,” Jonathan P. Piccini, MD, MHS, said in an interview. It suggests that “dual-chamber pacing can be achieved with leadless technology” and “with a very high degree” of AV synchrony.
“Obviously, more data as the technology moves into clinical practice will be critical,” said Dr. Piccini, who directs cardiac electrophysiology at Duke University Medical Center, Durham, N.C. “We will also need to understand which patients are best served by leadless technology and which will be better served with traditional transvenous devices.”
The AVEIR DR i2i system consists of two leadless pulse generators for percutaneous implantation in the right atrium and right ventricle, respectively. They link like components of a wireless network to coordinate their separate sensing and rate-adaptive, AV-synchronous pacing functions.
The right ventricular implant “is physically identical to a commercially available single-chamber leadless pacemaker” from Abbott, the published report states.
Leadless pacemaker systems inherently avoid the two main sources of transvenous devices’ major complication – infection – by not requiring such leads or surgery for creating a pulse-generator subcutaneous pocket.
The first such systems consisted of one implant that could provide single-chamber ventricular pacing but not atrial pacing or AV synchronous pacing. The transcatheter single-chamber leadless Micra (Medtronic) for example, was approved in the United States in April 2016 for ventricular-only pacing.
A successor, the Micra AV, approved in 2020, was designed to simulate AV-synchronous pacing by stimulating the ventricle in sequence with mechanically sensed atrial contractions, as described by Dr. Cantillon and associates. But it could not directly pace the atrium, “rendering it inappropriate for patients with sinus-node dysfunction.”
The AVEIR DR i2i system doesn’t have those limitations. It was, however, associated with 35 device- or procedure-related complications in the study, of which the most common was procedural arrhythmia, “namely atrial fibrillation,” Dr. Cantillon said.
Atrial fibrillation can develop during implantation of pacemakers with transvenous leads but is generally terminated without being considered an important event. Yet the study classified it as a serious complication, inflating the complication rate, because “the patients had to be restored to sinus rhythm so we could assess the AV synchrony and also the atrial electrical performance,” he said.
Some of the devices dislodged from their implantation site within a month of the procedure, but “all of those patients were successfully managed percutaneously,” said Dr. Cantillon.
“The 1.7% dislodgement rate is something that we will need to keep an eye on, as embolization of devices is always a significant concern,” Dr. Piccini said. Still, the observed total complication rate “was certainly in line” with rates associated with conventional pacemaker implantation.
Reliable AV synchrony
Fred M. Kusumoto, MD, Mayo Clinic, Jacksonville, Fla., lauded what seems to be the system’s “incredibly reliable AV synchrony in different conditions, albeit in a very controlled environment.”
Of interest will be whether its performance, including maintenance of AV synchrony, holds up in “a more long-term evaluation in the outpatient setting,” said Dr. Kusumoto, speaking as the invited discussant for Dr. Cantillon’s presentation.
Also missing or in short supply from the study, he observed, are insights about long-term efficacy and complications, battery longevity, effectiveness of its rate-responsive capability, and any effect on clinical outcomes.
Local body network
Of the study’s 300 patients (mean age 69 years; 38% female) at 55 sites in Canada, Europe, and the United States, 63.3% had sinus-node dysfunction and 33.3% had AV block as their primary dual-chamber pacing indication; 298 were successfully implanted with both devices.
About 45% had a history of supraventricular arrhythmia, 4.3% had prior ventricular arrhythmia, and 20% had a history of arrhythmia ablation.
By 3 months, the group reported, the primary safety endpoint (freedom from device- or procedure-related serious adverse events) occurred in 90.3%, compared with the performance goal of 78% (P < .001).
The first of two primary performance endpoints (adequate atrial capture threshold and sensing amplitude by predefined criteria) was met in 90.2%, surpassing the 82.5% performance goal (P < .001).
The second primary performance goal (at least 70% AV synchrony with the patient sitting) was seen in 97.3% against the performance goal of 83% (P < .001).
What shouldn’t be “glossed over” from the study, Dr. Kusumoto offered, is that it’s possible to achieve a wireless connection “between two devices that are actually intracardiac.” That raises the prospect of a “local body network” that could be “expanded even more dramatically with other types of devices. I mean, think of the paradigm shift.”
The AVEIR DR i2i trial was funded by Abbott. Dr. Cantillon discloses receiving honoraria or fees for speaking or consulting from Abbott Laboratories, Boston Scientific, Biosense Webster, and Shockwave Medical, as well as holding royalty rights with AirStrip. Dr. Piccini has disclosed relationships with Abbott, Medtronic, Biotronik, Boston Scientific, and other drug and medical device companies. Dr. Kusumoto reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HRS 2023
Cut in AFib burden gains traction as gauge of ablation success: PULSED-AF
suggests a new analysis.
It’s the first study tying those outcomes to residual AFib burden after ablation achieved using the emerging pulsed-field ablation (PFA) technology, say researchers. These associations are already established for cath ablation using traditional radiofrequency energy or cryoablation.
The new findings come from a secondary analysis of the recently published PULSED-AF study, which highlighted the ablation efficacy of Medtronic’s investigational PulseSelect PFA system in patients with either paroxysmal AFib (PAF) or persistent AFib.
The trial had entered 300 adult candidates for catheter ablation of recurrent, symptomatic PAF or persistent AFib at 41 centers in Australia, Canada, Europe, Japan, and the United States.
After ablation, 69% of PAF patients and 62% of those who had persistent AFib showed no sign of atrial arrhythmia (AA) over 12 months, based on the trial’s method for estimating AA burden.
Residual AA burden less than 10% was seen in 87% and 82% of those initially with PAF and persistent AFib, respectively. Burdens in that lowest range, compared with greater AA burden, predicted a “clinically meaningful” improvement in QoL scores in PAF patients.
Those who entered the study with persistent AFib showed such improvement – defined as a more than 19-point gain on the Atrial Fibrillation Effect on Quality-of-Life Questionnaire – regardless of postablation AA burden.
Moreover, patients initially with either type of AFib and residual burdens in the lowest range went on to have fewer cardioversions and repeat ablations (P < .01), Atul Verma, MD, McGill University Health Centre, Montreal, reported at the annual scientific sessions of the Heart Rhythm Society.
Dr. Verma, the trial’s principal investigator, is also lead author on the same-day publication of the secondary analysis in Heart Rhythm.
Binary endpoint lacks relevance
The PULSED-AF primary analysis defined ablation efficacy partly as freedom from AA recurrence lasting at least 30 seconds, with or without symptoms, a traditional AFib-ablation trial endpoint that is nonetheless considered clinically unhelpful.
The secondary analysis recasts that binary endpoint as degree of reduction in AFib burden, a continuous variable. That potentially allows AFib ablation efficacy to be assessed in a more nuanced way likely to be more meaningful to patients and the health care system, observed Dr. Verma and colleagues.
The “30-second endpoint” is limited in clinical usefulness and “doesn’t mean much to the patient,” he said at a press conference on the analysis before formally presenting it at the HRS sessions.
Recent AFib ablation trials have explored AA burden as possibly a superior way to assess the procedure’s success “but also to see if it’s better correlated with quality of life and health care outcomes,” Dr. Verma said. “So that’s exactly what we’ve tried to do here using the PULSED-AF data.”
In the secondary analysis, he said, patients’ rate of freedom from the 30-second endpoint was about 70%, but “more than 85% of them had an AFib burden of less than 10%.”
“This efficacy endpoint of 30 seconds of atrial arrhythmia has been challenged and has been seen clinically as insignificant,” agreed Rajeev Pathak, MBBS, PhD, of Australian National University and director of cardiac electrophysiology at Canberra (Australia) Hospital.
In AFib radiofrequency ablation and cryoablation studies “there is clear disconnect between these 30-second episodes of atrial arrhythmias we see and the clinical relevance of health care utilization and quality of life,” said Dr. Pathak, invited discussant for Dr. Verma’s presentation at the sessions.
Now an AFib ablation trial using PFA catheters has yielded similar results, finding AA burden to be “a more objective and relevant measure of success,” he said. “A 30-second endpoint is arbitrary, lacks significance, and is highly dependent on the monitoring strategy.”
The more you look, the more you see
The new secondary analysis included a demonstration that success rates based on the 30-second endpoint indeed vary depending on how subsequent arrhythmias are monitored.
As described by Dr. Verma, PULSED-AF data were assessed for the 30-second endpoint captured using three separate intermittent monitoring strategies that it and other recent ablation trials have used:
- Strategy A: Transtelephonic monitoring weekly and in the event of symptoms, plus 24-hour Holter monitoring at 6 and 12 months and 12-lead ECG at 3, 6, and 12 months
- Strategy B: Transtelephonic monitoring weekly and at symptoms for 3-6 months followed by monthly and at symptoms from 6 to 12 months, plus 24-hour Holter monitoring at 6 and 12 months, plus 12-lead ECG at 3, 6, and 12 months
- Strategy C: The median of two 24-hour Holter monitoring sessions per patient over 12 months
As Dr. Verma reported, rates of freedom from the 30-second endpoint climbed with successive monitoring strategies. The rates for PAF and persistent AFib patients, respectively, were: Strategy A – 70% and 62%, Strategy B – 71% and 68%, Strategy C – 91% and 86%.
“If you’re using the ‘freedom-from-30-seconds’ endpoint, the results that you are going to get are highly dependent on the monitoring strategy,” Dr. Verma said. “The more you look, the more you see.”
Valid estimation of burden
For the main PULSED-AF secondary analysis, the investigators defined AA burden according to findings on either Holter monitoring or the 12-lead ECG. “So as not to bias these results,” Dr. Verma said, “for every patient, we picked the method that gave us the highest atrial arrhythmia burden.”
Ideally, Dr. Verma said in an interview, arrhythmia burden would be determined using devices such as implantable loop recorders. “The problem is, this is expensive and not practical” in both clinical practice and many trials, so PULSED-AF investigators went with the intermittent monitoring strategy to estimate burdens.
Their method appears valid, he said, given that the study identified a statistically relevant 10% AA burden cut off for predicting quality of life improvement or less health care resource use.
“If their residual atrial arrhythmia burden was greater than 10%, they did not have a statistically significant improvement in quality of life,” Dr. Verma observed. And “very few” of them had cardioversions or repeat ablation.
“I couldn’t agree more” that residual AA burden is preferable to the 30-second endpoint for gauging AFib ablation success, Kenneth Ellenbogen, MD, Virginia Commonwealth University Medical Center, Richmond, said in an interview. Dr. Ellenbogen is also director of clinical cardiac electrophysiology and pacing at VCU Health Pauley Heart Center and not associated with PULSED-AF.
That AA burden was linked to health care resource use in the study “is absolutely brilliant,” he said, “because that’s what the bean counters really want at the end of the day. And as doctors we care about patients feeling better – improving quality of life.”
PULSED-AF was funded by Medtronic. Dr. Verma disclosed financial relationships with Bayer, Biosense Webster, Medtronic, Thermedical, Kardium, and Galaxy Medical, as well as and research grants from Adagio Medical. Dr. Ellenbogen disclosed financial relationships with Boston Scientific, Medtronic, Kestra, Hylomorph, Biotronik, MediLynx, Impulse Dynamics USA, Abbott, Biosense Webster, Milestone Pharmaceuticals, Sanofi, Medpace, and Elsevier. Dr. Pathak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggests a new analysis.
It’s the first study tying those outcomes to residual AFib burden after ablation achieved using the emerging pulsed-field ablation (PFA) technology, say researchers. These associations are already established for cath ablation using traditional radiofrequency energy or cryoablation.
The new findings come from a secondary analysis of the recently published PULSED-AF study, which highlighted the ablation efficacy of Medtronic’s investigational PulseSelect PFA system in patients with either paroxysmal AFib (PAF) or persistent AFib.
The trial had entered 300 adult candidates for catheter ablation of recurrent, symptomatic PAF or persistent AFib at 41 centers in Australia, Canada, Europe, Japan, and the United States.
After ablation, 69% of PAF patients and 62% of those who had persistent AFib showed no sign of atrial arrhythmia (AA) over 12 months, based on the trial’s method for estimating AA burden.
Residual AA burden less than 10% was seen in 87% and 82% of those initially with PAF and persistent AFib, respectively. Burdens in that lowest range, compared with greater AA burden, predicted a “clinically meaningful” improvement in QoL scores in PAF patients.
Those who entered the study with persistent AFib showed such improvement – defined as a more than 19-point gain on the Atrial Fibrillation Effect on Quality-of-Life Questionnaire – regardless of postablation AA burden.
Moreover, patients initially with either type of AFib and residual burdens in the lowest range went on to have fewer cardioversions and repeat ablations (P < .01), Atul Verma, MD, McGill University Health Centre, Montreal, reported at the annual scientific sessions of the Heart Rhythm Society.
Dr. Verma, the trial’s principal investigator, is also lead author on the same-day publication of the secondary analysis in Heart Rhythm.
Binary endpoint lacks relevance
The PULSED-AF primary analysis defined ablation efficacy partly as freedom from AA recurrence lasting at least 30 seconds, with or without symptoms, a traditional AFib-ablation trial endpoint that is nonetheless considered clinically unhelpful.
The secondary analysis recasts that binary endpoint as degree of reduction in AFib burden, a continuous variable. That potentially allows AFib ablation efficacy to be assessed in a more nuanced way likely to be more meaningful to patients and the health care system, observed Dr. Verma and colleagues.
The “30-second endpoint” is limited in clinical usefulness and “doesn’t mean much to the patient,” he said at a press conference on the analysis before formally presenting it at the HRS sessions.
Recent AFib ablation trials have explored AA burden as possibly a superior way to assess the procedure’s success “but also to see if it’s better correlated with quality of life and health care outcomes,” Dr. Verma said. “So that’s exactly what we’ve tried to do here using the PULSED-AF data.”
In the secondary analysis, he said, patients’ rate of freedom from the 30-second endpoint was about 70%, but “more than 85% of them had an AFib burden of less than 10%.”
“This efficacy endpoint of 30 seconds of atrial arrhythmia has been challenged and has been seen clinically as insignificant,” agreed Rajeev Pathak, MBBS, PhD, of Australian National University and director of cardiac electrophysiology at Canberra (Australia) Hospital.
In AFib radiofrequency ablation and cryoablation studies “there is clear disconnect between these 30-second episodes of atrial arrhythmias we see and the clinical relevance of health care utilization and quality of life,” said Dr. Pathak, invited discussant for Dr. Verma’s presentation at the sessions.
Now an AFib ablation trial using PFA catheters has yielded similar results, finding AA burden to be “a more objective and relevant measure of success,” he said. “A 30-second endpoint is arbitrary, lacks significance, and is highly dependent on the monitoring strategy.”
The more you look, the more you see
The new secondary analysis included a demonstration that success rates based on the 30-second endpoint indeed vary depending on how subsequent arrhythmias are monitored.
As described by Dr. Verma, PULSED-AF data were assessed for the 30-second endpoint captured using three separate intermittent monitoring strategies that it and other recent ablation trials have used:
- Strategy A: Transtelephonic monitoring weekly and in the event of symptoms, plus 24-hour Holter monitoring at 6 and 12 months and 12-lead ECG at 3, 6, and 12 months
- Strategy B: Transtelephonic monitoring weekly and at symptoms for 3-6 months followed by monthly and at symptoms from 6 to 12 months, plus 24-hour Holter monitoring at 6 and 12 months, plus 12-lead ECG at 3, 6, and 12 months
- Strategy C: The median of two 24-hour Holter monitoring sessions per patient over 12 months
As Dr. Verma reported, rates of freedom from the 30-second endpoint climbed with successive monitoring strategies. The rates for PAF and persistent AFib patients, respectively, were: Strategy A – 70% and 62%, Strategy B – 71% and 68%, Strategy C – 91% and 86%.
“If you’re using the ‘freedom-from-30-seconds’ endpoint, the results that you are going to get are highly dependent on the monitoring strategy,” Dr. Verma said. “The more you look, the more you see.”
Valid estimation of burden
For the main PULSED-AF secondary analysis, the investigators defined AA burden according to findings on either Holter monitoring or the 12-lead ECG. “So as not to bias these results,” Dr. Verma said, “for every patient, we picked the method that gave us the highest atrial arrhythmia burden.”
Ideally, Dr. Verma said in an interview, arrhythmia burden would be determined using devices such as implantable loop recorders. “The problem is, this is expensive and not practical” in both clinical practice and many trials, so PULSED-AF investigators went with the intermittent monitoring strategy to estimate burdens.
Their method appears valid, he said, given that the study identified a statistically relevant 10% AA burden cut off for predicting quality of life improvement or less health care resource use.
“If their residual atrial arrhythmia burden was greater than 10%, they did not have a statistically significant improvement in quality of life,” Dr. Verma observed. And “very few” of them had cardioversions or repeat ablation.
“I couldn’t agree more” that residual AA burden is preferable to the 30-second endpoint for gauging AFib ablation success, Kenneth Ellenbogen, MD, Virginia Commonwealth University Medical Center, Richmond, said in an interview. Dr. Ellenbogen is also director of clinical cardiac electrophysiology and pacing at VCU Health Pauley Heart Center and not associated with PULSED-AF.
That AA burden was linked to health care resource use in the study “is absolutely brilliant,” he said, “because that’s what the bean counters really want at the end of the day. And as doctors we care about patients feeling better – improving quality of life.”
PULSED-AF was funded by Medtronic. Dr. Verma disclosed financial relationships with Bayer, Biosense Webster, Medtronic, Thermedical, Kardium, and Galaxy Medical, as well as and research grants from Adagio Medical. Dr. Ellenbogen disclosed financial relationships with Boston Scientific, Medtronic, Kestra, Hylomorph, Biotronik, MediLynx, Impulse Dynamics USA, Abbott, Biosense Webster, Milestone Pharmaceuticals, Sanofi, Medpace, and Elsevier. Dr. Pathak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggests a new analysis.
It’s the first study tying those outcomes to residual AFib burden after ablation achieved using the emerging pulsed-field ablation (PFA) technology, say researchers. These associations are already established for cath ablation using traditional radiofrequency energy or cryoablation.
The new findings come from a secondary analysis of the recently published PULSED-AF study, which highlighted the ablation efficacy of Medtronic’s investigational PulseSelect PFA system in patients with either paroxysmal AFib (PAF) or persistent AFib.
The trial had entered 300 adult candidates for catheter ablation of recurrent, symptomatic PAF or persistent AFib at 41 centers in Australia, Canada, Europe, Japan, and the United States.
After ablation, 69% of PAF patients and 62% of those who had persistent AFib showed no sign of atrial arrhythmia (AA) over 12 months, based on the trial’s method for estimating AA burden.
Residual AA burden less than 10% was seen in 87% and 82% of those initially with PAF and persistent AFib, respectively. Burdens in that lowest range, compared with greater AA burden, predicted a “clinically meaningful” improvement in QoL scores in PAF patients.
Those who entered the study with persistent AFib showed such improvement – defined as a more than 19-point gain on the Atrial Fibrillation Effect on Quality-of-Life Questionnaire – regardless of postablation AA burden.
Moreover, patients initially with either type of AFib and residual burdens in the lowest range went on to have fewer cardioversions and repeat ablations (P < .01), Atul Verma, MD, McGill University Health Centre, Montreal, reported at the annual scientific sessions of the Heart Rhythm Society.
Dr. Verma, the trial’s principal investigator, is also lead author on the same-day publication of the secondary analysis in Heart Rhythm.
Binary endpoint lacks relevance
The PULSED-AF primary analysis defined ablation efficacy partly as freedom from AA recurrence lasting at least 30 seconds, with or without symptoms, a traditional AFib-ablation trial endpoint that is nonetheless considered clinically unhelpful.
The secondary analysis recasts that binary endpoint as degree of reduction in AFib burden, a continuous variable. That potentially allows AFib ablation efficacy to be assessed in a more nuanced way likely to be more meaningful to patients and the health care system, observed Dr. Verma and colleagues.
The “30-second endpoint” is limited in clinical usefulness and “doesn’t mean much to the patient,” he said at a press conference on the analysis before formally presenting it at the HRS sessions.
Recent AFib ablation trials have explored AA burden as possibly a superior way to assess the procedure’s success “but also to see if it’s better correlated with quality of life and health care outcomes,” Dr. Verma said. “So that’s exactly what we’ve tried to do here using the PULSED-AF data.”
In the secondary analysis, he said, patients’ rate of freedom from the 30-second endpoint was about 70%, but “more than 85% of them had an AFib burden of less than 10%.”
“This efficacy endpoint of 30 seconds of atrial arrhythmia has been challenged and has been seen clinically as insignificant,” agreed Rajeev Pathak, MBBS, PhD, of Australian National University and director of cardiac electrophysiology at Canberra (Australia) Hospital.
In AFib radiofrequency ablation and cryoablation studies “there is clear disconnect between these 30-second episodes of atrial arrhythmias we see and the clinical relevance of health care utilization and quality of life,” said Dr. Pathak, invited discussant for Dr. Verma’s presentation at the sessions.
Now an AFib ablation trial using PFA catheters has yielded similar results, finding AA burden to be “a more objective and relevant measure of success,” he said. “A 30-second endpoint is arbitrary, lacks significance, and is highly dependent on the monitoring strategy.”
The more you look, the more you see
The new secondary analysis included a demonstration that success rates based on the 30-second endpoint indeed vary depending on how subsequent arrhythmias are monitored.
As described by Dr. Verma, PULSED-AF data were assessed for the 30-second endpoint captured using three separate intermittent monitoring strategies that it and other recent ablation trials have used:
- Strategy A: Transtelephonic monitoring weekly and in the event of symptoms, plus 24-hour Holter monitoring at 6 and 12 months and 12-lead ECG at 3, 6, and 12 months
- Strategy B: Transtelephonic monitoring weekly and at symptoms for 3-6 months followed by monthly and at symptoms from 6 to 12 months, plus 24-hour Holter monitoring at 6 and 12 months, plus 12-lead ECG at 3, 6, and 12 months
- Strategy C: The median of two 24-hour Holter monitoring sessions per patient over 12 months
As Dr. Verma reported, rates of freedom from the 30-second endpoint climbed with successive monitoring strategies. The rates for PAF and persistent AFib patients, respectively, were: Strategy A – 70% and 62%, Strategy B – 71% and 68%, Strategy C – 91% and 86%.
“If you’re using the ‘freedom-from-30-seconds’ endpoint, the results that you are going to get are highly dependent on the monitoring strategy,” Dr. Verma said. “The more you look, the more you see.”
Valid estimation of burden
For the main PULSED-AF secondary analysis, the investigators defined AA burden according to findings on either Holter monitoring or the 12-lead ECG. “So as not to bias these results,” Dr. Verma said, “for every patient, we picked the method that gave us the highest atrial arrhythmia burden.”
Ideally, Dr. Verma said in an interview, arrhythmia burden would be determined using devices such as implantable loop recorders. “The problem is, this is expensive and not practical” in both clinical practice and many trials, so PULSED-AF investigators went with the intermittent monitoring strategy to estimate burdens.
Their method appears valid, he said, given that the study identified a statistically relevant 10% AA burden cut off for predicting quality of life improvement or less health care resource use.
“If their residual atrial arrhythmia burden was greater than 10%, they did not have a statistically significant improvement in quality of life,” Dr. Verma observed. And “very few” of them had cardioversions or repeat ablation.
“I couldn’t agree more” that residual AA burden is preferable to the 30-second endpoint for gauging AFib ablation success, Kenneth Ellenbogen, MD, Virginia Commonwealth University Medical Center, Richmond, said in an interview. Dr. Ellenbogen is also director of clinical cardiac electrophysiology and pacing at VCU Health Pauley Heart Center and not associated with PULSED-AF.
That AA burden was linked to health care resource use in the study “is absolutely brilliant,” he said, “because that’s what the bean counters really want at the end of the day. And as doctors we care about patients feeling better – improving quality of life.”
PULSED-AF was funded by Medtronic. Dr. Verma disclosed financial relationships with Bayer, Biosense Webster, Medtronic, Thermedical, Kardium, and Galaxy Medical, as well as and research grants from Adagio Medical. Dr. Ellenbogen disclosed financial relationships with Boston Scientific, Medtronic, Kestra, Hylomorph, Biotronik, MediLynx, Impulse Dynamics USA, Abbott, Biosense Webster, Milestone Pharmaceuticals, Sanofi, Medpace, and Elsevier. Dr. Pathak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HRS 2023
Losing weight may bolster AFib ablation’s chances for success: LEAF interim results
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
Gestational HTN, preeclampsia worsen long-term risk for ischemic, nonischemic heart failure
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
NSAID use in diabetes may worsen risk for first HF hospitalization
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Statins don’t worsen muscle injury from moderately intense exercise
People who are physically active and on statins may have one less potential concern about the drugs. Despite their reputation for causing muscle injury, a study suggests statins won’t worsen the toll that sustained, moderately intensive exercise already takes on patients’ muscles.
Statin therapy in this prospective, controlled study wasn’t seen to aggravate normal muscle fatigue or pain from sustained exercise or adversely affect enzymes or other biomarkers associated with muscle injury.
The findings come from 100 individuals, of whom about two-thirds were on statins, participating in a public, 4-day, long-distance walking event held annually in the Netherlands. Results were published in the Journal of the American College of Cardiology with Neeltje A.E. Allard, MD, Radboud University Medical Center, Nijmegen, the Netherlands, as lead author.
For all of statins’ common use in adults with cardiovascular (CV) risk factors, the drugs are often blamed for causing excessive muscle pain or injury as a side effect. Yet there is a predominance of evidence to the contrary based on meta-analyses and clinical trials, suggesting that the drugs are taking the rap for many entirely unrelated muscle symptoms.
The new findings, from people ranging widely in fitness levels, suggest that “exercise of moderate intensity is feasible and safe” in statin users, that the drugs won’t exacerbate normal muscle symptoms from exercise, Dr. Allard told this news organization.
And that exercise doesn’t have to be on an unusual scale. Regular exercise in statin users can simply be consistent with broader guidelines, say 30 minutes of walking per day, she noted.
The study has such broad applicability, Dr. Allard said, because participants represented the spectrum of the thousands who signed up for the walking event, who varied in age, level of physical fitness, and number of CV risk factors. They included CV patients, the physically fit, “recreational walkers who didn’t really exercise regularly,” and “habitual nonexercisers.”
It enrolled three groups of participants in the Four Days Marches in Nijmegen, which in a typical year attracts tens of thousands of participants who walk up to 30 km, 40 km, or 50 km per day for 4 consecutive days.
They included 35 statin users who walked the event despite muscle symptoms, 34 on statins but without such symptoms, and 31 non–statin-using controls. Their mean ages ranged from 65 to 68 years.
Statin users were overwhelmingly on simvastatin or atorvastatin. The average statin therapy durations were 60 months and 96 months for those with and without symptoms, respectively.
Assessments were performed several days before the event, at baseline, and after the end of walking on days 1, 2, and 3.
Scores for muscle pain on the Brief Pain Inventory were higher at baseline for the symptomatic-on-statins group (P < .001) compared with the other two groups, and went up (P < .001) similarly across the three groups during each of the 3 days, the report notes. Fatigue scores on the Brief Fatigue Inventory followed the same pattern.
All biomarkers of muscle injury or stress were at comparable levels at baseline in the three groups and went up similarly (P < .001) with no significant differences at the end of day 3. Biomarkers included lactate dehydrogenase, creatine kinase, myoglobin, cardiac troponin I, and N-terminal pro-brain natriuretic peptide.
Statin-related reductions in levels of coenzyme Q 10 (CoQ10) have been thought to exacerbate muscle injury, the authors note. But levels of CoQ10 weren’t significantly different across the three groups at any point in the study, and they did not show any significant associations with measures of muscle injury, symptoms, or fatigue.
Patients with statin-associated muscle symptoms (SAMS) often limit physical activity because of muscle pain or weakness, but also “concerns that exercise will exacerbate muscle injury,” an accompanying editorial notes. “Therefore, exercise, a foundation of improving and maintaining cardiometabolic health, is often avoided or limited.”
But the current study, writes Robert S. Rosenson, MD, of Mount Sinai Heart, New York, indeed suggests that “many patients who develop SAMS may engage in a moderately intensive walking program without concern for worsened muscle biomarkers or performance.”
The exercise didn’t seem to improve muscle function in symptomatic statin users, compared with the other groups over the study’s very short follow-up, Dr. Rosenson observes. But “it remains uncertain from this study whether sustained exercise in SAMS patients will effectuate improved metabolic biomarkers or exercise capacity in the long term.”
Dr. Allard is supported by a grant from the Radboud Institute for Health Sciences; the other authors have disclosed no relevant financial relationships. Dr. Rosenson disclosed receiving research funding to his institution from Amgen, Arrowhead, Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, Lilly, Lipigon, Novartis, CRISPR Therapeutics, Precision BioSciences, Verve, Ultragenyx Pharmaceutical, and Regeneron; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer (UpToDate); and that he holds stock in MediMergent.
A version of this article first appeared on Medscape.com.
People who are physically active and on statins may have one less potential concern about the drugs. Despite their reputation for causing muscle injury, a study suggests statins won’t worsen the toll that sustained, moderately intensive exercise already takes on patients’ muscles.
Statin therapy in this prospective, controlled study wasn’t seen to aggravate normal muscle fatigue or pain from sustained exercise or adversely affect enzymes or other biomarkers associated with muscle injury.
The findings come from 100 individuals, of whom about two-thirds were on statins, participating in a public, 4-day, long-distance walking event held annually in the Netherlands. Results were published in the Journal of the American College of Cardiology with Neeltje A.E. Allard, MD, Radboud University Medical Center, Nijmegen, the Netherlands, as lead author.
For all of statins’ common use in adults with cardiovascular (CV) risk factors, the drugs are often blamed for causing excessive muscle pain or injury as a side effect. Yet there is a predominance of evidence to the contrary based on meta-analyses and clinical trials, suggesting that the drugs are taking the rap for many entirely unrelated muscle symptoms.
The new findings, from people ranging widely in fitness levels, suggest that “exercise of moderate intensity is feasible and safe” in statin users, that the drugs won’t exacerbate normal muscle symptoms from exercise, Dr. Allard told this news organization.
And that exercise doesn’t have to be on an unusual scale. Regular exercise in statin users can simply be consistent with broader guidelines, say 30 minutes of walking per day, she noted.
The study has such broad applicability, Dr. Allard said, because participants represented the spectrum of the thousands who signed up for the walking event, who varied in age, level of physical fitness, and number of CV risk factors. They included CV patients, the physically fit, “recreational walkers who didn’t really exercise regularly,” and “habitual nonexercisers.”
It enrolled three groups of participants in the Four Days Marches in Nijmegen, which in a typical year attracts tens of thousands of participants who walk up to 30 km, 40 km, or 50 km per day for 4 consecutive days.
They included 35 statin users who walked the event despite muscle symptoms, 34 on statins but without such symptoms, and 31 non–statin-using controls. Their mean ages ranged from 65 to 68 years.
Statin users were overwhelmingly on simvastatin or atorvastatin. The average statin therapy durations were 60 months and 96 months for those with and without symptoms, respectively.
Assessments were performed several days before the event, at baseline, and after the end of walking on days 1, 2, and 3.
Scores for muscle pain on the Brief Pain Inventory were higher at baseline for the symptomatic-on-statins group (P < .001) compared with the other two groups, and went up (P < .001) similarly across the three groups during each of the 3 days, the report notes. Fatigue scores on the Brief Fatigue Inventory followed the same pattern.
All biomarkers of muscle injury or stress were at comparable levels at baseline in the three groups and went up similarly (P < .001) with no significant differences at the end of day 3. Biomarkers included lactate dehydrogenase, creatine kinase, myoglobin, cardiac troponin I, and N-terminal pro-brain natriuretic peptide.
Statin-related reductions in levels of coenzyme Q 10 (CoQ10) have been thought to exacerbate muscle injury, the authors note. But levels of CoQ10 weren’t significantly different across the three groups at any point in the study, and they did not show any significant associations with measures of muscle injury, symptoms, or fatigue.
Patients with statin-associated muscle symptoms (SAMS) often limit physical activity because of muscle pain or weakness, but also “concerns that exercise will exacerbate muscle injury,” an accompanying editorial notes. “Therefore, exercise, a foundation of improving and maintaining cardiometabolic health, is often avoided or limited.”
But the current study, writes Robert S. Rosenson, MD, of Mount Sinai Heart, New York, indeed suggests that “many patients who develop SAMS may engage in a moderately intensive walking program without concern for worsened muscle biomarkers or performance.”
The exercise didn’t seem to improve muscle function in symptomatic statin users, compared with the other groups over the study’s very short follow-up, Dr. Rosenson observes. But “it remains uncertain from this study whether sustained exercise in SAMS patients will effectuate improved metabolic biomarkers or exercise capacity in the long term.”
Dr. Allard is supported by a grant from the Radboud Institute for Health Sciences; the other authors have disclosed no relevant financial relationships. Dr. Rosenson disclosed receiving research funding to his institution from Amgen, Arrowhead, Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, Lilly, Lipigon, Novartis, CRISPR Therapeutics, Precision BioSciences, Verve, Ultragenyx Pharmaceutical, and Regeneron; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer (UpToDate); and that he holds stock in MediMergent.
A version of this article first appeared on Medscape.com.
People who are physically active and on statins may have one less potential concern about the drugs. Despite their reputation for causing muscle injury, a study suggests statins won’t worsen the toll that sustained, moderately intensive exercise already takes on patients’ muscles.
Statin therapy in this prospective, controlled study wasn’t seen to aggravate normal muscle fatigue or pain from sustained exercise or adversely affect enzymes or other biomarkers associated with muscle injury.
The findings come from 100 individuals, of whom about two-thirds were on statins, participating in a public, 4-day, long-distance walking event held annually in the Netherlands. Results were published in the Journal of the American College of Cardiology with Neeltje A.E. Allard, MD, Radboud University Medical Center, Nijmegen, the Netherlands, as lead author.
For all of statins’ common use in adults with cardiovascular (CV) risk factors, the drugs are often blamed for causing excessive muscle pain or injury as a side effect. Yet there is a predominance of evidence to the contrary based on meta-analyses and clinical trials, suggesting that the drugs are taking the rap for many entirely unrelated muscle symptoms.
The new findings, from people ranging widely in fitness levels, suggest that “exercise of moderate intensity is feasible and safe” in statin users, that the drugs won’t exacerbate normal muscle symptoms from exercise, Dr. Allard told this news organization.
And that exercise doesn’t have to be on an unusual scale. Regular exercise in statin users can simply be consistent with broader guidelines, say 30 minutes of walking per day, she noted.
The study has such broad applicability, Dr. Allard said, because participants represented the spectrum of the thousands who signed up for the walking event, who varied in age, level of physical fitness, and number of CV risk factors. They included CV patients, the physically fit, “recreational walkers who didn’t really exercise regularly,” and “habitual nonexercisers.”
It enrolled three groups of participants in the Four Days Marches in Nijmegen, which in a typical year attracts tens of thousands of participants who walk up to 30 km, 40 km, or 50 km per day for 4 consecutive days.
They included 35 statin users who walked the event despite muscle symptoms, 34 on statins but without such symptoms, and 31 non–statin-using controls. Their mean ages ranged from 65 to 68 years.
Statin users were overwhelmingly on simvastatin or atorvastatin. The average statin therapy durations were 60 months and 96 months for those with and without symptoms, respectively.
Assessments were performed several days before the event, at baseline, and after the end of walking on days 1, 2, and 3.
Scores for muscle pain on the Brief Pain Inventory were higher at baseline for the symptomatic-on-statins group (P < .001) compared with the other two groups, and went up (P < .001) similarly across the three groups during each of the 3 days, the report notes. Fatigue scores on the Brief Fatigue Inventory followed the same pattern.
All biomarkers of muscle injury or stress were at comparable levels at baseline in the three groups and went up similarly (P < .001) with no significant differences at the end of day 3. Biomarkers included lactate dehydrogenase, creatine kinase, myoglobin, cardiac troponin I, and N-terminal pro-brain natriuretic peptide.
Statin-related reductions in levels of coenzyme Q 10 (CoQ10) have been thought to exacerbate muscle injury, the authors note. But levels of CoQ10 weren’t significantly different across the three groups at any point in the study, and they did not show any significant associations with measures of muscle injury, symptoms, or fatigue.
Patients with statin-associated muscle symptoms (SAMS) often limit physical activity because of muscle pain or weakness, but also “concerns that exercise will exacerbate muscle injury,” an accompanying editorial notes. “Therefore, exercise, a foundation of improving and maintaining cardiometabolic health, is often avoided or limited.”
But the current study, writes Robert S. Rosenson, MD, of Mount Sinai Heart, New York, indeed suggests that “many patients who develop SAMS may engage in a moderately intensive walking program without concern for worsened muscle biomarkers or performance.”
The exercise didn’t seem to improve muscle function in symptomatic statin users, compared with the other groups over the study’s very short follow-up, Dr. Rosenson observes. But “it remains uncertain from this study whether sustained exercise in SAMS patients will effectuate improved metabolic biomarkers or exercise capacity in the long term.”
Dr. Allard is supported by a grant from the Radboud Institute for Health Sciences; the other authors have disclosed no relevant financial relationships. Dr. Rosenson disclosed receiving research funding to his institution from Amgen, Arrowhead, Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, Lilly, Lipigon, Novartis, CRISPR Therapeutics, Precision BioSciences, Verve, Ultragenyx Pharmaceutical, and Regeneron; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer (UpToDate); and that he holds stock in MediMergent.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
AHA, ACC push supervised exercise training for HFpEF
A statement released by the American Heart Association and the American College of Cardiology advocates use of supervised exercise training in patients with heart failure with preserved ejection fraction (HFpEF), as well as coverage for these services by third-party payers.
The authors hope to boost the stature of supervised exercise training (SET) in HFpEF among practitioners and show Medicare and insurers that it deserves reimbursement. Currently, they noted, clinicians tend to recognize exercise as therapy more in HF with reduced ejection fraction (HFrEF). And Medicare covers exercise training within broader cardiac rehabilitation programs for patients with HFrEF but not HFpEF.
Yet exercise has been broadly effective in HFpEF clinical trials, as outlined in the document. And there are good mechanistic reasons to believe that patients with the disorder can gain as much or more from SET than those with HFrEF.
“The signals for improvement from exercise training, in symptoms and objective measures of exercise capacity, are considerably larger for HFpEF than for HFrEF,” Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C., said in an interview.
So, it’s a bit of a paradox that clinicians don’t prescribe it as often in HFpEF, probably because of the lack of reimbursement but also from less “awareness” and understanding of the disease itself, he proposed.
Dr. Kitzman is senior author on the statement sponsored by the AHA and the ACC. It was published in the societies’ flagship journals Circulation and the Journal of the American College of Cardiology. The statement was also endorsed by the Heart Failure Society of America, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the American Association of Heart Failure Nurses.
Carefully chosen words
The statement makes its case in HFpEF specifically for SET rather than cardiac rehabilitation, the latter typically a comprehensive program that goes beyond exercise, Dr. Kitzman noted. And SET is closer to the exercise interventions used in the supportive HFpEF trials.
“Also, Medicare in recent years has approved something called ‘supervised exercise training’ for other disorders, such as peripheral artery disease.” So, the document specifies SET “to be fully aligned with the evidence base,” he said, as well as “align it with a type of treatment that Medicare has a precedent for approving for other disorders.”
Data and physiologic basis
Core features of the AHA/ACC statement is its review of HFpEF exercise physiology, survey of randomized trials supporting SET in the disease, and characterization of exercise as an especially suitable pleiotropic therapy.
Increasingly, “HFpEF is now accepted as a systemic disorder that affects and impacts all organs,” Dr. Kitzman observed. “With a systemic multiorgan disorder, it would make sense that a broad treatment like exercise might be just the right thing. We think that’s the reason that its benefits are really quite large in magnitude.”
The document notes that exercise seems “potentially well suited for the treatment of both the cardiac and, in particular, the extracardiac abnormalities that contribute to exercise intolerance in HFpEF.”
Its effects in the disorder are “anti-inflammatory, rheological, lipid lowering, antihypertensive, positive inotropic, positive lusitropic, negative chronotropic, vasodilation, diuretic, weight-reducing, hypoglycemic, hypnotic, and antidepressive,” the statement notes. It achieves them via multiple pathways involving the heart, lungs, vasculature and, notably, the skeletal muscles.
“It’s been widely overlooked that at least 50% of low exercise capacity and symptoms in HFpEF are due to skeletal muscle dysfunction,” said Dr. Kitzman, an authority on exercise physiology in heart failure.
“But we’ve spent about 95% of our attention trying to modify and understand the cardiac component.” Skeletal muscles, he said, “are not an innocent bystander. They’re part of the problem. And that’s why we should really spend more time focusing on them.”
Dr. Kitzman disclosed receiving consulting fees from Bayer, Medtronic, Corvia Medical, Boehringer Ingelheim, Keyto, Rivus, NovoNordisk, AstraZeneca, and Pfizer; holding stock in Gilead; and receiving grants to his institution from Bayer, Novo Nordisk, AstraZeneca, Rivus, and Pfizer.
A version of this article first appeared on Medscape.com.
A statement released by the American Heart Association and the American College of Cardiology advocates use of supervised exercise training in patients with heart failure with preserved ejection fraction (HFpEF), as well as coverage for these services by third-party payers.
The authors hope to boost the stature of supervised exercise training (SET) in HFpEF among practitioners and show Medicare and insurers that it deserves reimbursement. Currently, they noted, clinicians tend to recognize exercise as therapy more in HF with reduced ejection fraction (HFrEF). And Medicare covers exercise training within broader cardiac rehabilitation programs for patients with HFrEF but not HFpEF.
Yet exercise has been broadly effective in HFpEF clinical trials, as outlined in the document. And there are good mechanistic reasons to believe that patients with the disorder can gain as much or more from SET than those with HFrEF.
“The signals for improvement from exercise training, in symptoms and objective measures of exercise capacity, are considerably larger for HFpEF than for HFrEF,” Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C., said in an interview.
So, it’s a bit of a paradox that clinicians don’t prescribe it as often in HFpEF, probably because of the lack of reimbursement but also from less “awareness” and understanding of the disease itself, he proposed.
Dr. Kitzman is senior author on the statement sponsored by the AHA and the ACC. It was published in the societies’ flagship journals Circulation and the Journal of the American College of Cardiology. The statement was also endorsed by the Heart Failure Society of America, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the American Association of Heart Failure Nurses.
Carefully chosen words
The statement makes its case in HFpEF specifically for SET rather than cardiac rehabilitation, the latter typically a comprehensive program that goes beyond exercise, Dr. Kitzman noted. And SET is closer to the exercise interventions used in the supportive HFpEF trials.
“Also, Medicare in recent years has approved something called ‘supervised exercise training’ for other disorders, such as peripheral artery disease.” So, the document specifies SET “to be fully aligned with the evidence base,” he said, as well as “align it with a type of treatment that Medicare has a precedent for approving for other disorders.”
Data and physiologic basis
Core features of the AHA/ACC statement is its review of HFpEF exercise physiology, survey of randomized trials supporting SET in the disease, and characterization of exercise as an especially suitable pleiotropic therapy.
Increasingly, “HFpEF is now accepted as a systemic disorder that affects and impacts all organs,” Dr. Kitzman observed. “With a systemic multiorgan disorder, it would make sense that a broad treatment like exercise might be just the right thing. We think that’s the reason that its benefits are really quite large in magnitude.”
The document notes that exercise seems “potentially well suited for the treatment of both the cardiac and, in particular, the extracardiac abnormalities that contribute to exercise intolerance in HFpEF.”
Its effects in the disorder are “anti-inflammatory, rheological, lipid lowering, antihypertensive, positive inotropic, positive lusitropic, negative chronotropic, vasodilation, diuretic, weight-reducing, hypoglycemic, hypnotic, and antidepressive,” the statement notes. It achieves them via multiple pathways involving the heart, lungs, vasculature and, notably, the skeletal muscles.
“It’s been widely overlooked that at least 50% of low exercise capacity and symptoms in HFpEF are due to skeletal muscle dysfunction,” said Dr. Kitzman, an authority on exercise physiology in heart failure.
“But we’ve spent about 95% of our attention trying to modify and understand the cardiac component.” Skeletal muscles, he said, “are not an innocent bystander. They’re part of the problem. And that’s why we should really spend more time focusing on them.”
Dr. Kitzman disclosed receiving consulting fees from Bayer, Medtronic, Corvia Medical, Boehringer Ingelheim, Keyto, Rivus, NovoNordisk, AstraZeneca, and Pfizer; holding stock in Gilead; and receiving grants to his institution from Bayer, Novo Nordisk, AstraZeneca, Rivus, and Pfizer.
A version of this article first appeared on Medscape.com.
A statement released by the American Heart Association and the American College of Cardiology advocates use of supervised exercise training in patients with heart failure with preserved ejection fraction (HFpEF), as well as coverage for these services by third-party payers.
The authors hope to boost the stature of supervised exercise training (SET) in HFpEF among practitioners and show Medicare and insurers that it deserves reimbursement. Currently, they noted, clinicians tend to recognize exercise as therapy more in HF with reduced ejection fraction (HFrEF). And Medicare covers exercise training within broader cardiac rehabilitation programs for patients with HFrEF but not HFpEF.
Yet exercise has been broadly effective in HFpEF clinical trials, as outlined in the document. And there are good mechanistic reasons to believe that patients with the disorder can gain as much or more from SET than those with HFrEF.
“The signals for improvement from exercise training, in symptoms and objective measures of exercise capacity, are considerably larger for HFpEF than for HFrEF,” Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C., said in an interview.
So, it’s a bit of a paradox that clinicians don’t prescribe it as often in HFpEF, probably because of the lack of reimbursement but also from less “awareness” and understanding of the disease itself, he proposed.
Dr. Kitzman is senior author on the statement sponsored by the AHA and the ACC. It was published in the societies’ flagship journals Circulation and the Journal of the American College of Cardiology. The statement was also endorsed by the Heart Failure Society of America, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the American Association of Heart Failure Nurses.
Carefully chosen words
The statement makes its case in HFpEF specifically for SET rather than cardiac rehabilitation, the latter typically a comprehensive program that goes beyond exercise, Dr. Kitzman noted. And SET is closer to the exercise interventions used in the supportive HFpEF trials.
“Also, Medicare in recent years has approved something called ‘supervised exercise training’ for other disorders, such as peripheral artery disease.” So, the document specifies SET “to be fully aligned with the evidence base,” he said, as well as “align it with a type of treatment that Medicare has a precedent for approving for other disorders.”
Data and physiologic basis
Core features of the AHA/ACC statement is its review of HFpEF exercise physiology, survey of randomized trials supporting SET in the disease, and characterization of exercise as an especially suitable pleiotropic therapy.
Increasingly, “HFpEF is now accepted as a systemic disorder that affects and impacts all organs,” Dr. Kitzman observed. “With a systemic multiorgan disorder, it would make sense that a broad treatment like exercise might be just the right thing. We think that’s the reason that its benefits are really quite large in magnitude.”
The document notes that exercise seems “potentially well suited for the treatment of both the cardiac and, in particular, the extracardiac abnormalities that contribute to exercise intolerance in HFpEF.”
Its effects in the disorder are “anti-inflammatory, rheological, lipid lowering, antihypertensive, positive inotropic, positive lusitropic, negative chronotropic, vasodilation, diuretic, weight-reducing, hypoglycemic, hypnotic, and antidepressive,” the statement notes. It achieves them via multiple pathways involving the heart, lungs, vasculature and, notably, the skeletal muscles.
“It’s been widely overlooked that at least 50% of low exercise capacity and symptoms in HFpEF are due to skeletal muscle dysfunction,” said Dr. Kitzman, an authority on exercise physiology in heart failure.
“But we’ve spent about 95% of our attention trying to modify and understand the cardiac component.” Skeletal muscles, he said, “are not an innocent bystander. They’re part of the problem. And that’s why we should really spend more time focusing on them.”
Dr. Kitzman disclosed receiving consulting fees from Bayer, Medtronic, Corvia Medical, Boehringer Ingelheim, Keyto, Rivus, NovoNordisk, AstraZeneca, and Pfizer; holding stock in Gilead; and receiving grants to his institution from Bayer, Novo Nordisk, AstraZeneca, Rivus, and Pfizer.
A version of this article first appeared on Medscape.com.
Subclinical CAD by CT predicts MI risk, with or without stenoses
About half of middle-aged adults in the community without cardiovascular (CV) symptoms have coronary atherosclerosis by CT angiography (CTA) that puts them at substantial risk for myocardial infarction (MI), suggests a prospective cohort study.
The 10% of participants who had subclinical disease considered obstructive at CTA showed a ninefold increased risk for MI over several years. Obstructive disease seemed to elevate risk more than subclinical disease that wasn’t obstructive but still considered extensive within the coronary arteries.
The findings, based on a Copenhagen General Population Study cohort, are new for CTA but consistent with research based on coronary artery calcium (CAC) scores and other ways to assess CV risk, say researchers.
Although all participants underwent CTA, such imaging isn’t used in the general population for atherosclerosis screening. But the findings may have implications for “opportunistic screening” for subclinical coronary disease at CTA conducted for other reasons, notes the study’s report, published online in the Annals of Internal Medicine.
“Identification of luminal obstructive or extensive subclinical coronary atherosclerosis” could potentially provide “clinically relevant, incremental risk assessment” in nonischemic patients who undergo cardiac CT or electrocardiogram-gated chest CT before procedures such as arrhythmia ablation or valve repair, it states.
Such patients found with subclinical coronary atherosclerosis might potentially “benefit from referral to intensified cardiovascular primary prevention therapy,” write the authors, led by Andreas Fuchs, MD, PhD, Copenhagen University Hospital-Rigshospitalet.
The group acknowledges the findings may not entirely apply to a non-Danish population.
A screening role for CTA?
Whether CTA has a role to play in adults without symptoms “is a big, open question in the field right now,” observed Ron Blankstein, MD, not associated with the current analysis, for this news organization.
Most population studies of CV risk prediction, such as MESA, have looked at CAC scores, not CTA, and have shown that “the more plaque individuals have, the higher the risk.” The current findings are similar but novel in coming from coronary CTA in a large asymptomatic community population, said Dr. Blankstein, who is director of cardiac CT at Brigham and Women’s Hospital, Boston.
“It’s possible that patients who have obstructive plaque in general tend to have a larger amount of plaque as well,” he said. So, while the study suggests that “the more plaque individuals have, the worse their overall risk,” it also shows that the risk “is enhanced even more if they have obstructive disease.”
The Danish cohort analysis “provides a unique opportunity to study the contemporary natural history of coronary artery disease in the absence of intervention,” notes an accompanying editorial.
For example, both patients and clinicians were blinded to CTA results, and CV preventive therapies weren’t common, observe Michael McDermott, MBChB, and David E. Newby, DM, PhD, of the BHF Centre for Cardiovascular Science, University of Edinburgh.
The analysis suggests that subclinical coronary disease that is obstructive predicts MI risk more strongly than extensive coronary disease, they note, and may be present in two-thirds of MI patients. “This contrasts with symptomatic populations, where nonobstructive disease accounts for most future myocardial infarctions, presumably from plaque rupture.”
It also points to “strong associations between nonobstructive extensive disease and adverse plaque characteristics,” write Dr. McDermott and Dr. Newby. “This underscores the major importance of plaque burden” for the prediction of coronary events.
Graded risk
The analysis included 9,533 persons aged 40 and older without known ischemic heart disease or symptoms with available CTA assessments.
Obstructive disease, defined as presence of a luminal stenosis of at least 50%, was seen in 10% and nonobstructive disease in 36% of the total cohort, the report states.
Disease occupying more than one-third of the coronary tree was considered extensive and less than one-third of the coronaries nonextensive, occurring in 10.5% and 35.8% of the cohort, respectively.
There were 71 MIs and 193 deaths over a median of 3.5 years. The adjusted relative risk for MI, compared with those without coronary atherosclerosis, was:
- 7.65 (95% confidence interval, 3.53-16.57) overall in patients with extensive disease.
- 8.28 (95% CI, 3.75-18.32) in those with obstructive but nonextensive disease.
- 9.19 (95% CI, 4.49-18.82) overall in those with obstructive disease.
- 12.48 (95% CI, 5.50-28.12) in those with or obstructive and extensive disease.
The adjusted RR for the composite of death or MI was also elevated in persons with extensive disease:
- 2.70 (95% CI, 1.72-4.25) in those with extensive but nonobstructive disease.
- 3.15 (95% CI, 2.05-4.83) in those with extensive and obstructive disease.
“It’s one thing to show that the more plaque, the higher the risk,” Dr. Blankstein said. But “does the information ultimately lead to better outcomes? Do patients have fewer MIs or fewer deaths?” Several ongoing randomized trials are exploring these questions.
They include DANE-HEART (Computed Tomography Coronary Angiography for Primary Prevention), projected to enroll about 6,000 participants from the Copenhagen General Population Study cohort who have at least one CV risk factor, and SCOT-HEART 2 (second Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction), enrolling a similar cohort in Scotland.
The study was supported by grants from AP Møller og Hustru Chastine Mc-Kinney Møllers Fond, the Research Council of Rigshospitalet, and Danish Heart Foundation. Dr. Fuchs reports no relevant financial relationships. Disclosures for the other authors can be found here. Dr. Blankstein recently disclosed serving as a consultant to Amgen, Caristo Diagnostics, Novartis, and Silence Therapeutics. Disclosures for Dr. McDermott and Dr. Newby, who are SCOT-HEART 2 investigators, can be found here.
A version of this article originally appeared on Medscape.com.
About half of middle-aged adults in the community without cardiovascular (CV) symptoms have coronary atherosclerosis by CT angiography (CTA) that puts them at substantial risk for myocardial infarction (MI), suggests a prospective cohort study.
The 10% of participants who had subclinical disease considered obstructive at CTA showed a ninefold increased risk for MI over several years. Obstructive disease seemed to elevate risk more than subclinical disease that wasn’t obstructive but still considered extensive within the coronary arteries.
The findings, based on a Copenhagen General Population Study cohort, are new for CTA but consistent with research based on coronary artery calcium (CAC) scores and other ways to assess CV risk, say researchers.
Although all participants underwent CTA, such imaging isn’t used in the general population for atherosclerosis screening. But the findings may have implications for “opportunistic screening” for subclinical coronary disease at CTA conducted for other reasons, notes the study’s report, published online in the Annals of Internal Medicine.
“Identification of luminal obstructive or extensive subclinical coronary atherosclerosis” could potentially provide “clinically relevant, incremental risk assessment” in nonischemic patients who undergo cardiac CT or electrocardiogram-gated chest CT before procedures such as arrhythmia ablation or valve repair, it states.
Such patients found with subclinical coronary atherosclerosis might potentially “benefit from referral to intensified cardiovascular primary prevention therapy,” write the authors, led by Andreas Fuchs, MD, PhD, Copenhagen University Hospital-Rigshospitalet.
The group acknowledges the findings may not entirely apply to a non-Danish population.
A screening role for CTA?
Whether CTA has a role to play in adults without symptoms “is a big, open question in the field right now,” observed Ron Blankstein, MD, not associated with the current analysis, for this news organization.
Most population studies of CV risk prediction, such as MESA, have looked at CAC scores, not CTA, and have shown that “the more plaque individuals have, the higher the risk.” The current findings are similar but novel in coming from coronary CTA in a large asymptomatic community population, said Dr. Blankstein, who is director of cardiac CT at Brigham and Women’s Hospital, Boston.
“It’s possible that patients who have obstructive plaque in general tend to have a larger amount of plaque as well,” he said. So, while the study suggests that “the more plaque individuals have, the worse their overall risk,” it also shows that the risk “is enhanced even more if they have obstructive disease.”
The Danish cohort analysis “provides a unique opportunity to study the contemporary natural history of coronary artery disease in the absence of intervention,” notes an accompanying editorial.
For example, both patients and clinicians were blinded to CTA results, and CV preventive therapies weren’t common, observe Michael McDermott, MBChB, and David E. Newby, DM, PhD, of the BHF Centre for Cardiovascular Science, University of Edinburgh.
The analysis suggests that subclinical coronary disease that is obstructive predicts MI risk more strongly than extensive coronary disease, they note, and may be present in two-thirds of MI patients. “This contrasts with symptomatic populations, where nonobstructive disease accounts for most future myocardial infarctions, presumably from plaque rupture.”
It also points to “strong associations between nonobstructive extensive disease and adverse plaque characteristics,” write Dr. McDermott and Dr. Newby. “This underscores the major importance of plaque burden” for the prediction of coronary events.
Graded risk
The analysis included 9,533 persons aged 40 and older without known ischemic heart disease or symptoms with available CTA assessments.
Obstructive disease, defined as presence of a luminal stenosis of at least 50%, was seen in 10% and nonobstructive disease in 36% of the total cohort, the report states.
Disease occupying more than one-third of the coronary tree was considered extensive and less than one-third of the coronaries nonextensive, occurring in 10.5% and 35.8% of the cohort, respectively.
There were 71 MIs and 193 deaths over a median of 3.5 years. The adjusted relative risk for MI, compared with those without coronary atherosclerosis, was:
- 7.65 (95% confidence interval, 3.53-16.57) overall in patients with extensive disease.
- 8.28 (95% CI, 3.75-18.32) in those with obstructive but nonextensive disease.
- 9.19 (95% CI, 4.49-18.82) overall in those with obstructive disease.
- 12.48 (95% CI, 5.50-28.12) in those with or obstructive and extensive disease.
The adjusted RR for the composite of death or MI was also elevated in persons with extensive disease:
- 2.70 (95% CI, 1.72-4.25) in those with extensive but nonobstructive disease.
- 3.15 (95% CI, 2.05-4.83) in those with extensive and obstructive disease.
“It’s one thing to show that the more plaque, the higher the risk,” Dr. Blankstein said. But “does the information ultimately lead to better outcomes? Do patients have fewer MIs or fewer deaths?” Several ongoing randomized trials are exploring these questions.
They include DANE-HEART (Computed Tomography Coronary Angiography for Primary Prevention), projected to enroll about 6,000 participants from the Copenhagen General Population Study cohort who have at least one CV risk factor, and SCOT-HEART 2 (second Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction), enrolling a similar cohort in Scotland.
The study was supported by grants from AP Møller og Hustru Chastine Mc-Kinney Møllers Fond, the Research Council of Rigshospitalet, and Danish Heart Foundation. Dr. Fuchs reports no relevant financial relationships. Disclosures for the other authors can be found here. Dr. Blankstein recently disclosed serving as a consultant to Amgen, Caristo Diagnostics, Novartis, and Silence Therapeutics. Disclosures for Dr. McDermott and Dr. Newby, who are SCOT-HEART 2 investigators, can be found here.
A version of this article originally appeared on Medscape.com.
About half of middle-aged adults in the community without cardiovascular (CV) symptoms have coronary atherosclerosis by CT angiography (CTA) that puts them at substantial risk for myocardial infarction (MI), suggests a prospective cohort study.
The 10% of participants who had subclinical disease considered obstructive at CTA showed a ninefold increased risk for MI over several years. Obstructive disease seemed to elevate risk more than subclinical disease that wasn’t obstructive but still considered extensive within the coronary arteries.
The findings, based on a Copenhagen General Population Study cohort, are new for CTA but consistent with research based on coronary artery calcium (CAC) scores and other ways to assess CV risk, say researchers.
Although all participants underwent CTA, such imaging isn’t used in the general population for atherosclerosis screening. But the findings may have implications for “opportunistic screening” for subclinical coronary disease at CTA conducted for other reasons, notes the study’s report, published online in the Annals of Internal Medicine.
“Identification of luminal obstructive or extensive subclinical coronary atherosclerosis” could potentially provide “clinically relevant, incremental risk assessment” in nonischemic patients who undergo cardiac CT or electrocardiogram-gated chest CT before procedures such as arrhythmia ablation or valve repair, it states.
Such patients found with subclinical coronary atherosclerosis might potentially “benefit from referral to intensified cardiovascular primary prevention therapy,” write the authors, led by Andreas Fuchs, MD, PhD, Copenhagen University Hospital-Rigshospitalet.
The group acknowledges the findings may not entirely apply to a non-Danish population.
A screening role for CTA?
Whether CTA has a role to play in adults without symptoms “is a big, open question in the field right now,” observed Ron Blankstein, MD, not associated with the current analysis, for this news organization.
Most population studies of CV risk prediction, such as MESA, have looked at CAC scores, not CTA, and have shown that “the more plaque individuals have, the higher the risk.” The current findings are similar but novel in coming from coronary CTA in a large asymptomatic community population, said Dr. Blankstein, who is director of cardiac CT at Brigham and Women’s Hospital, Boston.
“It’s possible that patients who have obstructive plaque in general tend to have a larger amount of plaque as well,” he said. So, while the study suggests that “the more plaque individuals have, the worse their overall risk,” it also shows that the risk “is enhanced even more if they have obstructive disease.”
The Danish cohort analysis “provides a unique opportunity to study the contemporary natural history of coronary artery disease in the absence of intervention,” notes an accompanying editorial.
For example, both patients and clinicians were blinded to CTA results, and CV preventive therapies weren’t common, observe Michael McDermott, MBChB, and David E. Newby, DM, PhD, of the BHF Centre for Cardiovascular Science, University of Edinburgh.
The analysis suggests that subclinical coronary disease that is obstructive predicts MI risk more strongly than extensive coronary disease, they note, and may be present in two-thirds of MI patients. “This contrasts with symptomatic populations, where nonobstructive disease accounts for most future myocardial infarctions, presumably from plaque rupture.”
It also points to “strong associations between nonobstructive extensive disease and adverse plaque characteristics,” write Dr. McDermott and Dr. Newby. “This underscores the major importance of plaque burden” for the prediction of coronary events.
Graded risk
The analysis included 9,533 persons aged 40 and older without known ischemic heart disease or symptoms with available CTA assessments.
Obstructive disease, defined as presence of a luminal stenosis of at least 50%, was seen in 10% and nonobstructive disease in 36% of the total cohort, the report states.
Disease occupying more than one-third of the coronary tree was considered extensive and less than one-third of the coronaries nonextensive, occurring in 10.5% and 35.8% of the cohort, respectively.
There were 71 MIs and 193 deaths over a median of 3.5 years. The adjusted relative risk for MI, compared with those without coronary atherosclerosis, was:
- 7.65 (95% confidence interval, 3.53-16.57) overall in patients with extensive disease.
- 8.28 (95% CI, 3.75-18.32) in those with obstructive but nonextensive disease.
- 9.19 (95% CI, 4.49-18.82) overall in those with obstructive disease.
- 12.48 (95% CI, 5.50-28.12) in those with or obstructive and extensive disease.
The adjusted RR for the composite of death or MI was also elevated in persons with extensive disease:
- 2.70 (95% CI, 1.72-4.25) in those with extensive but nonobstructive disease.
- 3.15 (95% CI, 2.05-4.83) in those with extensive and obstructive disease.
“It’s one thing to show that the more plaque, the higher the risk,” Dr. Blankstein said. But “does the information ultimately lead to better outcomes? Do patients have fewer MIs or fewer deaths?” Several ongoing randomized trials are exploring these questions.
They include DANE-HEART (Computed Tomography Coronary Angiography for Primary Prevention), projected to enroll about 6,000 participants from the Copenhagen General Population Study cohort who have at least one CV risk factor, and SCOT-HEART 2 (second Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction), enrolling a similar cohort in Scotland.
The study was supported by grants from AP Møller og Hustru Chastine Mc-Kinney Møllers Fond, the Research Council of Rigshospitalet, and Danish Heart Foundation. Dr. Fuchs reports no relevant financial relationships. Disclosures for the other authors can be found here. Dr. Blankstein recently disclosed serving as a consultant to Amgen, Caristo Diagnostics, Novartis, and Silence Therapeutics. Disclosures for Dr. McDermott and Dr. Newby, who are SCOT-HEART 2 investigators, can be found here.
A version of this article originally appeared on Medscape.com.