User login
The perimenopausal period and the benefits of progestin IUDs
Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.
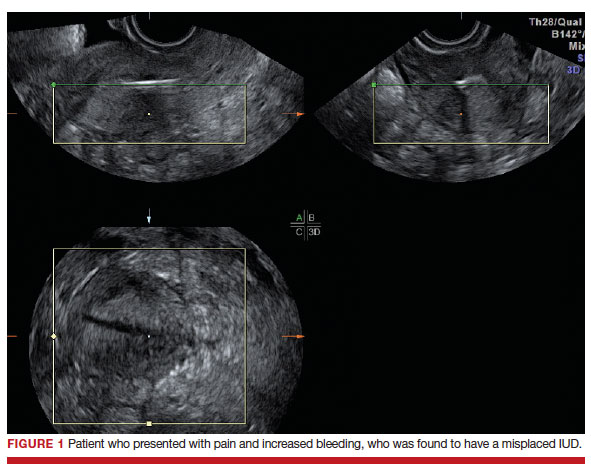
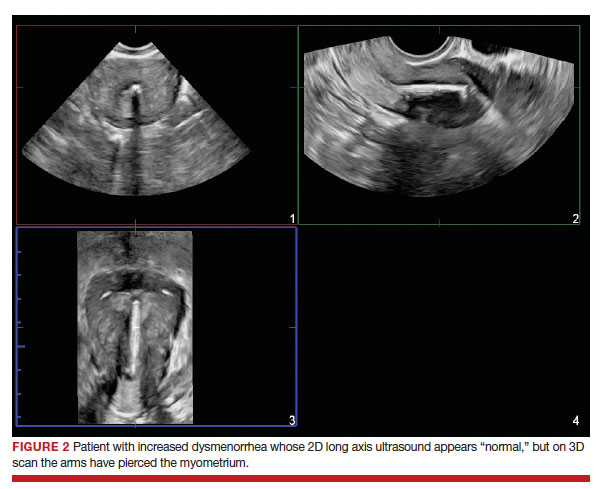
It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●

Intrauterine devices (IUDs) are now used by more than 15% of US contraceptors. The majority of these IUDs release the progestin levonorgestrel, and with now longer extended use of the IUDs approved by the US Food and Drug Administration (FDA),1-3 they become even more attractive for use for contraception,control of menorrhagia or heavy menstrual bleeding (HMB) during reproductive years and perimenopause, and potentially, although not FDA approved for this purpose, postmenopause for endometrial protection in estrogen users. In this roundtable discussion, we will look at some of the benefits of the IUD for contraception effectiveness and control of bleeding, as well as the potential risks if used for postmenopausal women.
Progestin IUDs and contraception
JoAnn V. Pinkerton, MD, NCMP: Dr. Kaunitz, what are the contraceptive benefits of progestin IUDs during perimenopause?
Andrew M. Kaunitz, MD, NCMP: We know fertility declines as women approach menopause. However, when pregnancy occurs in older reproductive-age women, the pregnancies are often unintended, as reflected by high rates of induced abortion in this population. In addition, the prevalence of maternal comorbidities (during pregnancy and delivery) is higher in older reproductive-age women, with the maternal mortality rate more than 5 times higher compared with that of younger women.4 Two recently published clinical trials assessed the extended use of full-size IUDs containing 52 mg of levonor-gestrel (LNG), with the brand names Mirena and Liletta.1,2 The data from these trials confirmed that both IUDs remain highly effective for up to 8 years of use, and currently, both devices are approved for up to 8 years of use. One caveat is that, in the unusual occurrence of a pregnancy being diagnosed in a woman using an IUD, we as clinicians, must be alert to the high prevalence of ectopic pregnancies in this setting.
Progestin IUDs and HMB
Dr. Pinkerton: Dr. Goldstein, can you comment on how well progestin IUDs work for HMB?
Steven R. Goldstein, MD, NCMP, CCD: Many women who need contraception will use these devices for suppressing HMB, and they can be quite effective, if the diagnosis truly is HMB, at reducing bleeding.5 But that efficacy in bleeding reduction may not be quite as long as the efficacy in pregnancy prevention.6 In my experience, among women using IUDs specifically for their HMB, good bleeding control may require changing the IUD at 3 to 5 years.
Barbara S. Levy, MD: When inserting a LNG-IUD for menorrhagia in the perimenopausal time frame, sometimes I will do a progestin withdrawal first, which will thin the endometrium and induce withdrawal bleeding because, in my experience, if you place an IUD in someone with perimenopausal bleeding, you may end up with a lot of breakthrough bleeding.
Perimenopause and hot flashes
Dr. Pinkerton: Dr. Kaunitz, we have learned that hot flashes often occur and become bothersome to women during perimenopause. Many women have IUDs placed during perimenopause for bleeding. Can you comment about IUD use during perimenopause and postmenopause?
Dr. Kaunitz: In older reproductive-age women who already have a progestin-releasing IUD placed, as they get closer to menopause when vasomotor symptoms (VMS) might occur, if these symptoms are bothersome, the presence or placement of a progestin-releasing IUD can facilitate treatment of perimenopausal VMS with estrogen therapy.
Progestin IUDs cause profound endometrial suppression, reduce bleeding and often, over time, cause users to become amenorrheic.7
The Mirena package insert states, “Amenorrhea develops in about 20% of users by one year.”2 By year 3 and continuing through year 8, the prevalence of amenorrhea with the 52-mg LNG-IUD is 35% to 40%.8 From a study by Nanette Santoro, MD, and colleagues, we know that, in perimenopausal women with a progestin-releasing IUD in place, who are experiencing bothersome VMS, adding transdermal estrogen is very effective in treating and suppressing those hot flashes. In her small clinical trial, among participants with perimenopausal bothersome VMS with an IUD in place, half were randomized to use of transdermal estradiol and then compared with women who did not get the estradiol patch. There was excellent relief of perimenopausal hot flashes with the combination of the progestin IUD for endometrial suppression and transdermal estrogen to relieve hot flashes.9
Dr. Pinkerton: Which women would not be good candidates for the use of this combination?
Dr. Kaunitz: We know that, as women age, the prevalence of conditions that are contraindications to combination contraceptives (estrogen-progestin pills, patches, or rings) starts to increase. Specifically, we see more: hypertension, diabetes, and high body mass index (BMI), or obesity. We also know that migraine headaches in women older than age 35 years is another condition in which ACOG and the Centers for Disease Control and Prevention (CDC) would not recommend use of combination contraceptives.10,11 These older perimenopausal women may be excellent candidates for a progestin-only releasing IUD combined with use of transdermal menopausal doses of estradiol if needed for VMS.
Dr. Goldstein: I do want to add that, in those patients who don’t have these comorbidities, combination estrogen-progestin contraceptives do a very nice job of ovarian suppression and will prevent the erratic production of estradiol, which, in my experience, often results in not only irregular bleeding but also possible exacerbation of perimenopausal mood symptoms.
Dr. Kaunitz: I agree, Steve. The ideal older reproductive-age candidate for combination pills, patch, or ring would be a slender, healthy, nonsmoking woman with normal blood pressure. Such women would be a fairly small subgroup of my practice, but they can safely continue combination contraceptives right through menopause. Consistent with CDC and ACOG guidance, rather than checking gonadotropins to “determine when menopause has occurred,” (which is, in fact, not an evidence-based approach to diagnosing menopause in this setting), such women can continue the combination contraceptive right up until age 55—the likelihood that women are still going to be ovulating or at risk for pregnancy becomes vanishingly small at that age.11,12 Women in their mid-50s can either seamlessly transition to use of systemic estrogen-progestin menopausal therapy or go off hormones completely.
Continue to: The IUD and HMB...
The IUD and HMB
Dr. Pinkerton: Dr. Goldstein, there’s been some good literature on the best management options for women with HMB. What is the most current evidence?
Dr. Goldstein: I think that the retiring of the terms menorrhagia and metrorrhagia may have been premature because HMB implies cyclical bleeding, and this population of women with HMB will typically do quite well. Women who have what we used to call metrorrhagia or irregular bleeding, by definition, need endometrial evaluation to be sure they don’t have some sort of organic pathology. It would be a mistake for clinicians to use an LNG-IUD in patients with abnormal uterine bleeding (AUB) that has not been appropriately evaluated.
If we understand that we are discussing HMB, a Cochrane Review from 202213 suggests that an LNG intrauterine system is the best first-line treatment for reducing menstrual blood loss in perimenopausal women with HMB. Antifibrinolytics appeared second best, while long-cycle progestogens came in third place. Evidence on perception of improvement in satisfaction was ranked as low certainty. That same review found that hysterectomy was the best treatment for reducing bleeding, obviously, followed by resectoscopic endometrial ablation or a nonresectoscopic global endometrial ablation.
The evidence rating was low certainty regarding the likelihood that placing an LNG-IUD in women with HMB will result in amenorrhea, and I think that’s a very important point. The expectation of patients should be reduced or a significantly reduced amount of their HMB, not necessarily amenorrhea. Certainly, minimally invasive hysterectomy will result in total amenorrhea and may have a larger increase in satisfaction, but it has its own set of other kinds of possible complications.
Dr. Kaunitz: In an industry-funded, international multicenter trial,14 women with documented HMB (hemoglobin was eluted from soiled sanitary products), with menstrual blood loss of 80 mL or more per cycle, were randomized to placement of an LNG 52-mg IUD (Mirena) or cyclical medroxyprogesterone acetate (MPA)—oral progestin use.
Although menstrual blood loss declined in both groups, it declined dramatically more in women with an IUD placed, and specifically with the IUD, menstrual blood loss declined by 129 mL on average, whereas the decline in menstrual blood loss with cyclical MPA was 18 mL. This data, along with earlier European data,15 which showed similar findings in women with HMB led to the approval of the Mirena progestin IUD for a second indication to treat HMB in 2009.
I also want to point out that, in the May 2023 issue of Obstetrics & Gynecology, Creinin and colleagues published a similar trial in women with HMB showing, once again, that progestin IUDs (52-mg LNG-IUD, Liletta) are extremely effective in reducing HMB.16 There is crystal clear evidence from randomized trials that both 52-mg LNG-IUDs, Mirena and Liletta, are very effective in reducing HMB and, in fact, are contributing to many women who in the past would have proceeded with surgery, such as ablation or hysterectomy, to control their HMB.
Oral contraception
Dr. Pinkerton: What about using low-dose continuous oral contraceptives noncyclically for women with HMB?
Dr. Goldstein: I do that all the time. It is interesting that Dr. Kaunitz mentions his patient population. It’s why we understand that one size does not fit all. You need to see patients one at a time, and if they are good candidates for a combined estrogen-progestin contraception, whether it’s pills, patches, or rings, giving that continuously does a very nice job in reducing HMB and straightening out some of the other symptoms that these perimenopausal women will have.
IUD risks
Dr. Pinkerton: We all know about use of low-dose oral contraceptives for management of AUB, and we use them, although we worry a little bit about breast cancer risk. Dr. Levy, please comment on the risks with IUDs of expulsions and perforations. What are the downsides of IUDs?
Dr. Levy: Beyond the cost, although it is a minimally invasive procedure, IUD insertion can be an invasive procedure for a patient to undergo; expulsions can occur.17 We know that a substantial percentage of perimenopausal women will have fibroids. Although many fibroids are not located in the uterine cavity, the expulsion rate with HMB for an LNG-IUD can be higher,13,16,18,19 perhaps because of local prostaglandin release with an increase in uterine contractility. There is a low incidence of perforations, but they do happen, particularly among women with scars in the uterus or who have a severely anteflexed or retroflexed uterus, and women with cervical stenosis, for example, if they have had a LEEP procedure, etc. Even though progestin IUDs are outstanding tools in our toolbox, they are invasive to some extent, and they do have the possibility of complications.
Dr. Kaunitz: As Dr. Levy points out, although placement of an IUD may be considered an invasive procedure, it is also an office-based procedure, so women can drive home or drive back to work afterwards without the disruption in their life and the potential complications associated with surgery and anesthesia.
Continue to: Concerns with malpositioning...
Concerns with malpositioning
Dr. Pinkerton: After placement of an IUD, during a follow-up visit, sometimes you can’t visualize the string. The ultrasonography report may reveal, “IUD appears to be in the right place within the endometrium.” Dr. Goldstein, can you comment on how we should use ultrasound when we can’t visualize or find the IUD string, or if the patient complains of abdominal pain, lower abdominal discomfort, or irregular bleeding or spotting and we become concerned about IUD malposition?
Dr. Goldstein: Ultrasound is not really required after an uncomplicated placement of an IUD or during routine management of women who have no problems who are using an IUD. In patients who present with pain or some abnormal bleeding, however, sometimes it is the IUD being malpositioned. A very interesting study by the late great Beryl Benacerraf20 showed that there was a statistically significant higher incidence of the IUD being poorly positioned when patients have pain or bleeding (FIGURE 1). It was not always apparent on 2D ultrasonography. Using a standard transvaginal ultrasound of the long access plane, the IUD may appear to be very centrally located. However, if you do a 3D coronal section, not infrequently in these patients with any pain or bleeding, one of the arms has pierced the myometrium (FIGURE 2). This can actually be a source of pain and bleeding.


It’s also very interesting when you talk about perforation. I became aware of a big to-do in the medical/legal world about the possibility of the IUD migrating through the uterine cavity.21 This just does not exist, as was already pointed out. If the IUD is really going to go anywhere, if it’s properly placed, it’s going to be expelled through an open cervix. I do believe that, if you have pierced the myometrium through uterine contractility over time, some of these IUDs could work their way through the myometrium and somehow come out of the uterus either totally or partially. I think ultrasound is invaluable in patients with pain and bleeding, but I think you need to have an ultrasound lab capable of doing a 3D coronal section.
Progestin IUDs for HT replacement: Benefits/risks
Dr. Pinkerton: Many clinicians are excited that they can use essentially estrogen alone for women who have a progestin IUD in place. What about the possible off-label use of the progestin IUD to replace oral progestogen for hormone therapy (HT)? Dr. Kaunitz, are there any studies using this for postmenopausal HT (with a reminder that the IUD is not FDA approved for this purpose)?
Dr. Kaunitz: We have data from Europe indicating that, in menopausal women using systemic estrogen, the full-size LNG 52 IUD—Mirena or Liletta—provides excellent endometrial suppression.22 Where we don’t have data is with the smaller IUDs, which would be Kyleena and Skyla, which release smaller amounts of progestin each day into the endometrial cavity.
I have a number of patients, most of them women who started use of a progestin IUD as older reproductive-age women and then started systemic estrogen for treatment of perimenopausal hot flashes and then continued the use of their IUD plus systemic estrogen in treating postmenopausal hot flashes. The IUD is very useful in this setting, but as you pointed out, Dr. Pinkerton, this does represent off-label use.
Dr. Pinkerton: I know this use does not affect plasma lipids or cardiovascular risk markers, although users seem to report that the IUD has improved their quality of life. The question comes up, what are the benefits on cancer risk for using an IUD?
Dr. Levy: It’s such a great question because, as we talk about the balance of risks and benefits for anything that we are offering to our patients, it is really important to focus on some of the benefits. For both the copper and the LNG-IUD, there is a reduction in endometrial cancer,22 as well as pretty good data with the copper IUD about a reduction in cervical cancer.23 Those data are a little bit less clear for the LNG-IUD.
Interestingly, at least one meta-analysis published in 2020 shows about a 30% reduction in ovarian cancer risk with the LNG-IUD.24 We need to focus our patients on these other benefits. They tend to focus on the risks, and, of course, the media blows up the risks, but the benefits are quite substantial beyond just reducing HMB and providing contraception.
Dr. Pinkerton: As Dr. Kaunitz said, when you use this IUD, with its primarily local uterine progestin effects, it’s more like using estrogen alone without as much systemic progestin. Recently I wrote an editorial on the benefits of estrogen alone on the risk of breast cancer, primarily based on the Women’s Health Initiative (WHI) observational long-term 18-year cumulative follow-up. When estrogen alone was prescribed to women after a hysterectomy, estrogen therapy used at menopause did not increase the risk of invasive breast cancer, and was associated with decreased mortality.25 However, the nurse’s health study has suggested that longer-term use may be increased with estrogen alone.26 For women in the WHI with an intact uterus who used estrogen, oral MPA slightly increased the risk for breast cancer, and this elevated risk persisted even after discontinuation. This leads us to the question, what are the risks of breast cancer with progestin IUD use?
I recently reviewed the literature, and the answer is, it’s mixed. The FDA has put language into the package label that acknowledges a potential breast cancer risk for women who use a progestin IUD,27 and that warning states, “Women who currently have or have had breast cancer or suspect breast cancer should not use hormonal contraception because some breast cancers are hormone sensitive.” The label goes on to say, “Observational studies of the risk of breast cancer with the use of a levonorgestrel-releasing IUS don’t provide conclusive evidence of increased risk.” Thus, there is no conclusive answer as to whether there is a possible link of progestin IUDs to breast cancer.
What I tell my patients is that research is inconclusive. However, it’s unlikely for a 52-mg LNG-IUD to significantly increase a woman’s breast cancer risk, except possibly in those already at high risk from other risk factors. I tell them that breast cancer is listed in the package insert as a potential risk. I could not find any data on whether adding a low-dose estradiol patch would further increase that risk. So I counsel women about potential risk, but tell them that I don’t have any strong evidence of risk.
Continue to: Dr. Goldstein...
Dr. Goldstein: If you look in the package insert for Mirena,2 similar to Liletta, certainly the serum levels of LNG are lower than that for combination oral contraceptives. For the IUD progestins, they are not localized only to the uterus, and LNG levels range from about 150 to 200 µg/mL up to 60 months. It’s greater at 12 months, at about 180 µg/mL,at 24 months it was 192 µg/mL, and by 60 months it was 159 µg/mL. It’s important to realize that there is some systemic absorption of progestin with progestin IUDs, and it is not completely a local effect.
JoAnn, you mentioned the WHI data,25 and just to specify, it was not the estrogen-only arm, it was the conjugated equine estrogen-only arm of the WHI. I don’t think that estradiol alone increases breast cancer risk (although there are no good prospective, follow-through, 18-year study data, like the WHI), but I think readers need to understand the difference in the estrogen type.
Endometrial evaluation. My question for the panel is as follows. I agree that the use of the progestin-releasing IUD is very nice for that transition to menopause. I do believe it provides endometrial protection, but we know from other studies that, when we give continuous combined HT, about 21% to 26% of patients will experience some bleeding/staining, responding in the first 4-week cycles, and it can be as high as 9% at 1 year. If I have a patient who bleeds on continuous combined HT, I will evaluate her endometrium, usually just with a simple transvaginal ultrasound. If an IUD is in place, and the patient now begins to have some irregular bleeding, how do you evaluate her with the IUD in place?
Dr. Levy: That is a huge challenge. We know from a recent paper,28 that the endometrial thickness, while an excellent measure for Caucasian and European women, may be a poor marker for endometrial pathology in African-American women. What we thought we knew, which was, if the stripe is 4 mL or less, we can forget about it, I think in our more recent research that is not so true. So you bring up a great point, what do you do? The most reliable evaluation will be with an office hysteroscopy, where you can really look at the entire cavity and for tiny, little polyps and other things. But then we are off label because the use of hysteroscopy with an IUD in place is off label. So we are really in a conundrum.
Dr. Pinkerton: Also, if you do an endometrial biopsy, you might dislodge the IUD. If you think that you are going to take the IUD out, it may not matter if you dislodge it. I will often obtain a transvaginal ultrasound to help me figure out the next step, and maybe look at the dosing of the estrogen and progestin—but you can’t monitor an IUD with blood levels. You are in a vacuum of trying to figure out the best thing to do.
Dr. Kaunitz: One of the hats I wear here in Jacksonville is Director of GYN Ultrasound. I have a fair amount of experience doing endometrial biopsies in women with progestin IUDs in place under abdominal ultrasound guidance and keeping a close eye on the position of the IUD. In the first dozen or so such procedures I did, I was quite concerned about dislodging the IUD. It hasn’t happened yet, and it gives me some reassurance to be able to image the IUD and your endometrial suction curette inside the cavity as you are obtaining endometrial sampling. I have substantial experience now doing that, and so far, no problems. I do counsel all such women in advance that there is some chance I could dislodge their IUD.
Dr. Goldstein: In addition to dislodging the IUD, are you not concerned that, if the pathology is not global, that a blind endometrial sampling may be fraught with some error?
Dr. Kaunitz: The endometrium in women with a progestin-releasing IUD in place tends to be very well suppressed. Although one might occasionally find, for instance, a polyp in that setting, I have not run into, and I don’t expect to encounter going forward, endometrial hyperplasia or cancer in women with current use of a progestin IUD. It’s possible but unlikely.
Dr. Levy: The progestin IUD will counterbalance a type-1 endometrial cancer—an endometrial cancer related to hyperstimulation by estrogen. It will not do anything, to my knowledge, to counterbalance a type 2. I think the art of medicine is, you do the best you can with the first episode of bleeding, and if she persists in her bleeding, we have to persevere and continue to evaluate her.
Dr. Goldstein: I agree 100%.
Dr. Pinkerton: We all agree with you. That’s a really good point.
Continue to: Case examinations...
Case examinations
CASE 1 Woman with intramural fibroids
Dr. Pinkerton: Dr. Goldstein, you have a 48-year-old Black woman who has heavy but regular menstrual bleeding with multiple fibroids (the largest is about 4 to 5 cm, they look intramural, with some distortion of the cavity but not a submucous myoma, and the endometrial depth is 9 cm). Would you insert an IUD, and would you recommend an endometrial biopsy first?
Dr. Goldstein: I am not a huge fan of blind endometrial sampling, and I do think that we use the “biopsy” somewhat inappropriately since sampling is not a directed biopsy. This became obvious in the landmark paper by Guido et al in 1995 and was adopted by ACOG only in 2012.29 Cancers that occupy less than 50% of the endometrial surface area are often missed with such blind sampling. Thus I would not perform an endometrial biopsy first, but would rather rely on properly timed and performed transvaginal ultrasound to rule out any concurrent endometrial disease. I think a lot of patients who have HMB, not only because of their fibroids but also often just due to the surface area of their uterine cavity being increased—so essentially there is more blood volume when they bleed. However, you said that in this case the patient has regular menstrual bleeding, so I am assuming that she is still ovulatory. She may have some adenomyosis. She may have a large uterine cavity. I think she is an excellent candidate for an LNG-releasing IUD to reduce menstrual blood flow significantly. It will not necessarily give her amenorrhea, and it may give her some irregular bleeding. Then at some distant point, say in 5 or 6 months if she does have some irregular staining or bleeding, I would feel much better about the fact that nothing has developed as long as I knew that the endometrium was devoid of pathology when I started.
CASE 2 Woman with family history of breast cancer
Dr. Pinkerton: Dr. Levy, a 44-year-old woman has a family history of breast cancer in her mother at age 72, but she still needs contraceptionbecause of that unintended pregnancy risk in the 40s, and she wants something that is not going to increase her risk of breast cancer. What would you use, and how would you counsel her if you decided to use a progestin IUD?
Dr. Levy: The data are mixed,30-33 but whatever the risk, it is miniscule, and I would bring up the CDC Medical Eligibility Criteria.11 For a patient with a family history of breast cancer, for use of the progestin IUD, it is a 1—no contraindications. What I tend to tell my patients is, if you are worried about breast cancer, watch how much alcohol you are drinking and maintain regular exercise. There are so many preventive things that we can do to reduce risk of breast cancer when she needs contraception. If there is any increase in risk, it is so miniscule that I would very strongly recommend a progestin IUD for her.
Dr. Pinkerton: In addition, in recognizing the different densities of breast, dense breast density could lead to supplemental screening, which also could give her some reassurance that we are adequately screening for breast cancer.
CASE 3 Woman with IUD and VMS
Dr. Pinkerton: Dr. Kaunitz, you have a 52-year-old overweight female. She has been using a progestin IUD for 4 years, is amenorrheic, but now she is having moderate to severe vasomotor symptoms despite the IUD in place. You have talked to her about risks and benefits of HT, and she is interested in starting it. I know we talked about the studies, but I want to know what you are going to tell her. How do you counsel her about off-label use?
Dr. Kaunitz: The most important issue related to treating vasomotor symptoms in this patient is the route of systemic estrogen. Understandably, women’s biggest concern regarding the risks of systemic estrogen-progestin therapy is breast cancer. However, statistically, by far the biggest risk associated with oral estrogen-progestogen therapy, is elevated risk of venous thrombosis and pulmonary embolism. We have seen this, with a number of studies, and the WHI made it crystal clear with risks of oral conjugated equine estrogen at the dose of 0.625 mg daily. Oral estradiol 1 mg daily is also associated with a similar elevated risk of venous thrombosis. We also know that age and BMI are both independent risk factors for thrombosis. So, for a woman in her 50s who has a BMI > 30 mg/kg2, I don’t want to further elevate her risk of thrombosis by giving her oral estrogen, whether it is estradiol or conjugated equine estrogen. This is a patient in whom I would be comfortable using transdermal (patch) estradiol, perhaps starting with a standard dose of 0.05 mg weekly or twice weekly patch, keeping in mind that 0.05 mg in the setting of transdermal estrogen refer to the daily or to the 24-hour release rate. The 1.0 mg of oral estradiol and 0.625 mg of conjugated equine estrogen refers to the mg quantity of estrogen in each tablet. This is a source of great confusion for clinicians.
If, during follow-up, the 0.05 mg estradiol patch is not sufficient to substantially reduce symptoms, we could go up, for instance, to a 0.075 mg estradiol patch. We know very clearly from a variety of observational studies, including a very large UK study,34 that in contrast with oral estrogen, transdermal estradiol is safer from the perspective of thrombosis.
Insurance coverage for IUDs
Dr. Pinkerton: Dr. Levy: Can you discuss IUDs and the Affordable Care Act’s requirement to cover contraceptive services?
Dr. Levy: Unfortunately, we do not know whether this benefit will continue based on a very recent finding from a judge in Texas that ruled the preventive benefits of the ACA were illegal.35 We don’t know what will happen going forward. What I will say is that, unfortunately, many insurance companies have not preserved the meaning of “cover all things,” so what we are finding is that, for example, they only have to cover one type in a class. The FDA defined 18 classes of contraceptives, and a hormonal IUD is one class, so they can decide that they are only going to cover one of the four IUDS. And then women don’t have access to the other three, some of which might be more appropriate for them than another.
The other thing very relevant to this conversation is that, if you use an ICD-10 code for menorrhagia, for HMB, it no longer lives within that ACA preventive care requirement of coverage for contraceptives, and now she is going to owe a big deductible or a copay. If you are practicing in an institution that does not allow the use of IUDs for contraception, like a Catholic institution where I used to practice, you will want to use that ICD-10 code for HMB. But if you want it offered with no out-of-pocket cost for the patient, you need to use the preventive medicine codes and the contraception code. These little nuances for us can make a huge difference for our patients.
Dr. Pinkerton: Thank you for that reminder. I want to thank our panelists, Dr. Levy, Dr. Goldstein, and Dr. Kaunitz, for providing us with such a great mix of evidence and expert opinion and also giving a benefit of their vast experience as award-winning gynecologists. Hopefully, today you have learned the benefits of the progestin IUD not only for contraception in reproductive years and perimenopause but also for treatment of HMB, and the potential benefit due to the more prolonged effectiveness of the IUDs for endometrial protection in postmenopause. This allows less progestin risk, essentially estrogen alone for postmenopausal HT. Unsolved questions remain about whether there is a risk of breast cancer with their use, but there is a clear benefit of protecting against pregnancy and endometrial cancer. ●
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
- Liletta [package insert]. Allergan; Irvine, California. November 2022.
- Mirena [package insert]. Bayer; Whippany, New Jersey. 2000.
- Kaunitz AM. Safe extended use of levonorgestrel 52-mg IUDs. November 11, 2022. https://www.medscape.com/ viewarticle/983680. Accessed May 8, 2023.
- Kaunitz AM. Clinical practice. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270. doi: 10.1056/NEJMcp0708481.
- Tucker ME. IUD-released levonorgestrel eases heavy menstrual periods. Medscape. April 10, 2023. https://www .medscape.com/viewarticle/777406. Accessed May 2, 2023.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114:1434-1438.
- Critchley HO, Wang H, Jones RL, et al. Morphological and functional features of endometrial decidualization following long-term intrauterine levonorgestrel delivery. Hum Reprod. 1998;13:1218-1224. doi:10.1093/humrep/13.5.1218.
- Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;227:871.e1-871.e7. doi: 10.1016/j.ajog.2022.05.022.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrelcontaining intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22:1301-1307. doi: 10.1097 /GME.0000000000000557.
- ACOG Committee on Practice Bulletins-Gynecology ACOG Practice Bulletin. The use of hormonal contraception in women with coexisting medical conditions. Number 18, July 2000. Int J Gynaecol Obstet. 2001;75:93-106. doi: 10.1016 /s0020-7292(01)00520-3.
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. doi: 10.15585 /mmwr.rr6503a1.
- ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions [published correction appears in: Obstet Gynecol. 2019;133:1288.] Obstet Gynecol. 2019;133:e128-e150. doi:10.1097/AOG.0000000000003072.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180. doi: 10.1002/14651858.CD013180.pub2.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrelreleasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial [published correction appears in: Obstet Gynecol. 2010;116:999]. Obstet Gynecol. 2010;116:625-632. doi: 10.1097 /AOG.0b013e3181ec622b.
- Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879883. doi: 10.1016/s0002-9378(11)90533-x.
- Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi: 10.1097 /AOG.0000000000005137.
- 1Madden T. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014:718-726. doi:10.1097 /aog.0000000000000475.
- Kaunitz AM, Stern L, Doyle J, et al. Use of the levonorgestrelIUD in the treatment of menorrhagia: improving patient outcomes while reducing the need for surgical management. Manag Care Interface. 2007;20:47-50.
- Getahun D, Fassett MJ, Gatz J, et al. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. Am J Obstet Gynecol. 2022;227:59.e1-59.e9.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110115.
- Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med. 2010;29:1453-1456.
- Depypere H, Inki P. The levonorgestrel-releasing intrauterine system for endometrial protection during estrogen replacement therapy: a clinical review. Climacteric. 2015;18:470-482.
- Minalt N, Caldwell A, Yedlicka GM, et al. Association of intrauterine device use and endometrial, cervical, and ovarian cancer: an expert review. Am J Obstet Gynecol. 2023:S0002-9378(23)00224-7.
- Balayla J, Gil Y, Lasry A, et al. Ever-use of the intra-uterine device and the risk of ovarian cancer. J Obstet Gynaecol. 2021;41:848-853. doi: 10.1080/01443615.2020.1789960.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi:10.1001/jama.2017.11217.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032. doi: 10.1001 /archinte.166.9.1027.
- Pinkerton JV, Wilson CS, Kaunitz AM. Reassuring data regarding the use of hormone therapy at menopause and risk of breast cancer. Menopause. 2022;29:1001-1004.doi:10.1097 /GME.0000000000002057.
- Romano SS, Doll KM. The impact of fibroids and histologic subtype on the performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30:543-552. doi: 10.18865/ed.30.4.543.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206. doi: 10.1097/AOG.0b013e318262e320.
- Backman T, Rauramo I, Jaakkola Kimmo, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813-817.
- Conz L, Mota BS, Bahamondes L, et al. Levonorgestrelreleasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970-982.
- Al Kiyumi MH, Al Battashi K, Al-Riyami HA. Levonorgestrelreleasing intrauterine system and breast cancer. Is there an association? Acta Obstet Gynecol Scand. 2021;100:1749.
- Marsden J. Hormonal contraception and breast cancer, what more do we need to know? Post Reprod Health. 2017;23:116127. doi: 10.1177/2053369117715370.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810 doi:10.1136/bmj.k4810.
- Levitt L, Cox C, Dawson L. Q&A: implications of the ruling on the ACA’s preventive services requirement. KFF.org. https://www .kff.org/policy-watch/qa-implications-of-the-ruling-on -the-acas-preventive-services-requirement/#:~:text=On%20 March%2030%2C%202023%2C%20a,cost%2Dsharing%20 for%20their%20enrollees. Accessed May 2, 2023.
2023 Update on bone health
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
SERMs revisited: Can they improve menopausal care?
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).
In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32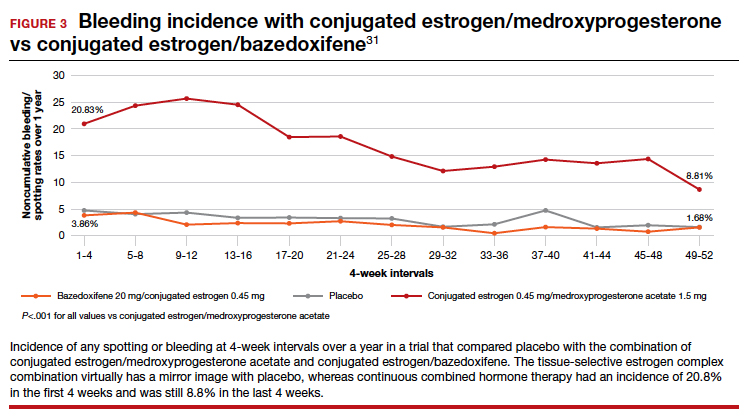
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Uterine incision closure: Is it the culprit in the cesarean scar niche and related complications?
While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25
Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.
In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.
Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
2021 Update on bone health
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
Remote and in-home prenatal care: Safe, inclusive, and here to stay

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
Dynamic ultrasonography: An idea whose time has come
Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3
Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.
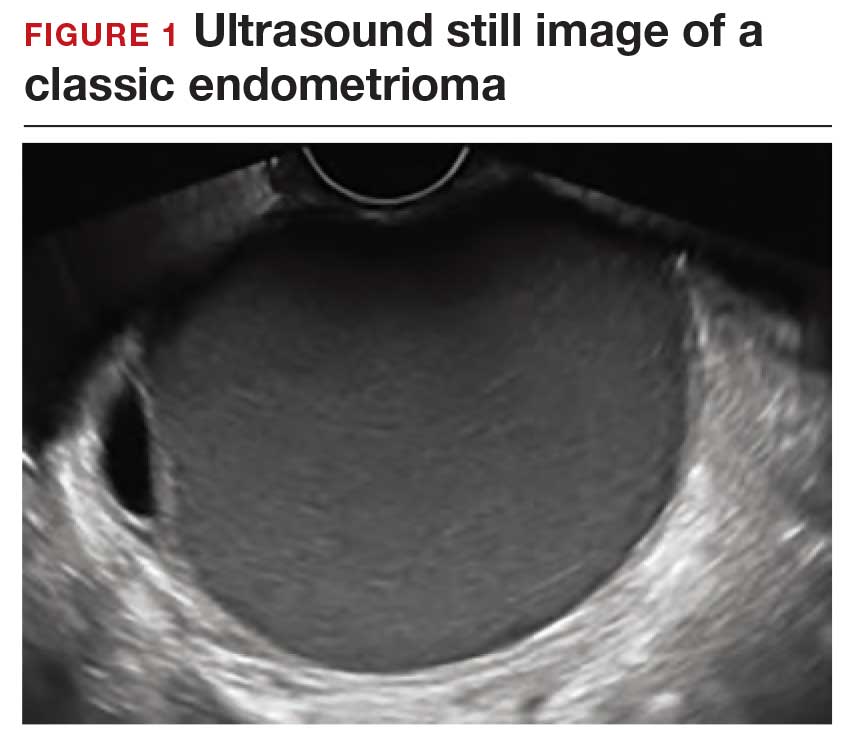
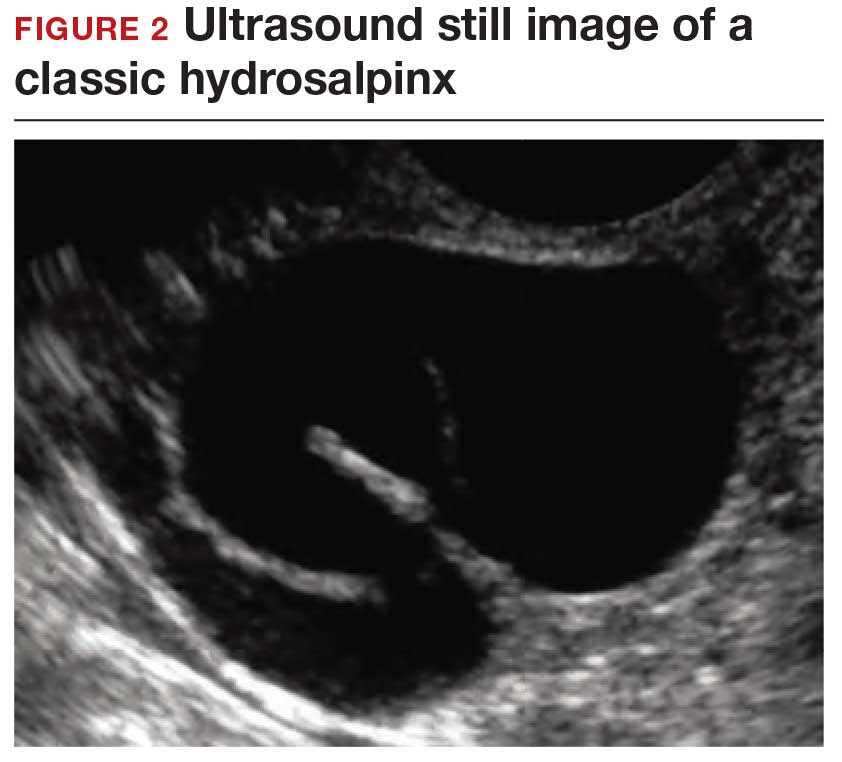
Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
Dynamic ultrasonography: An idea whose time has come (videos)
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--
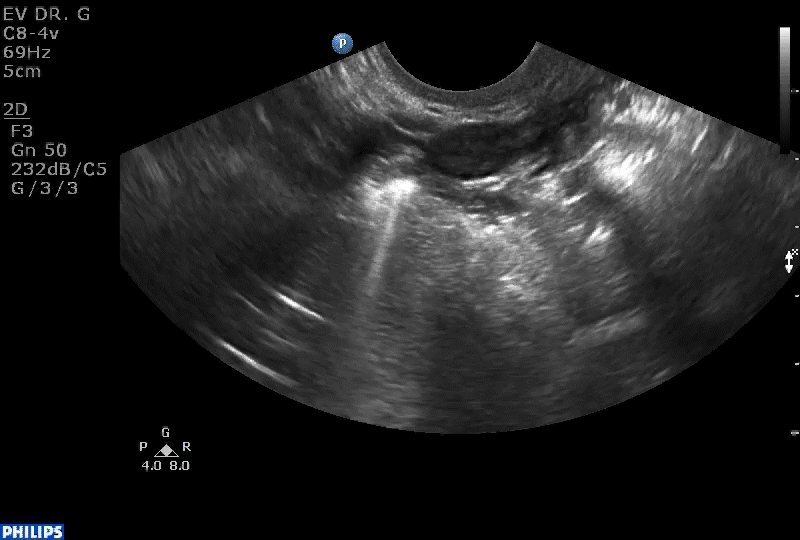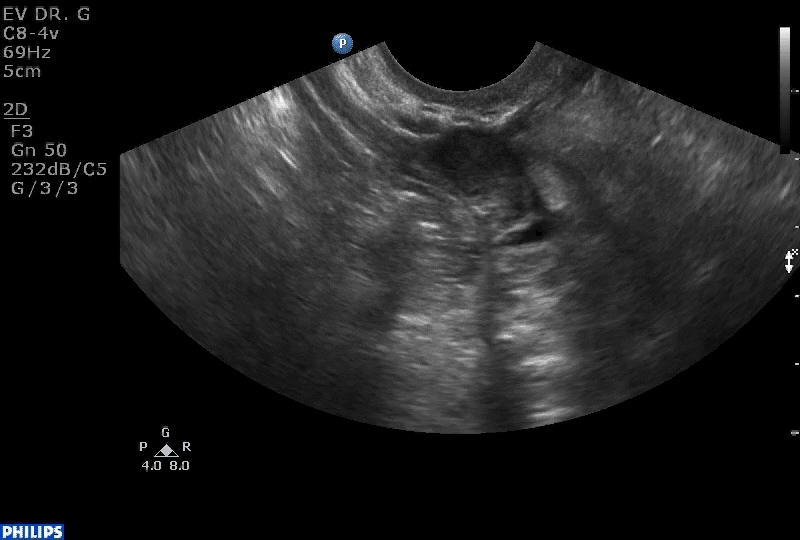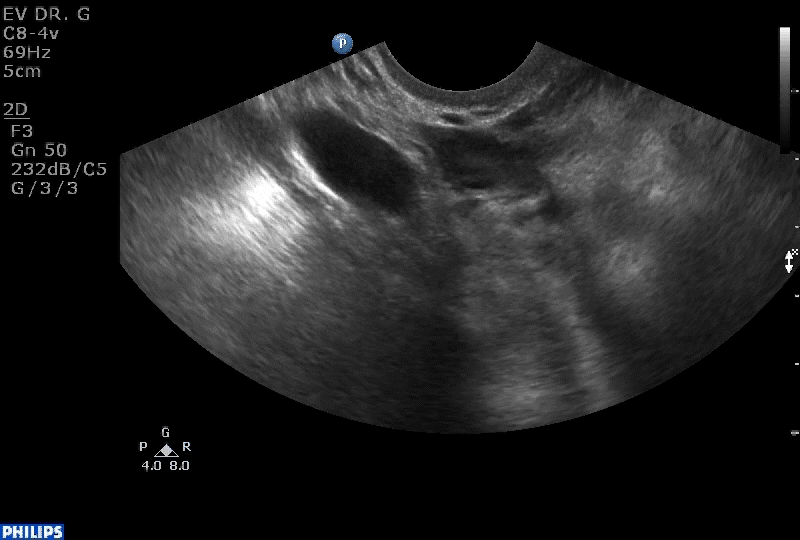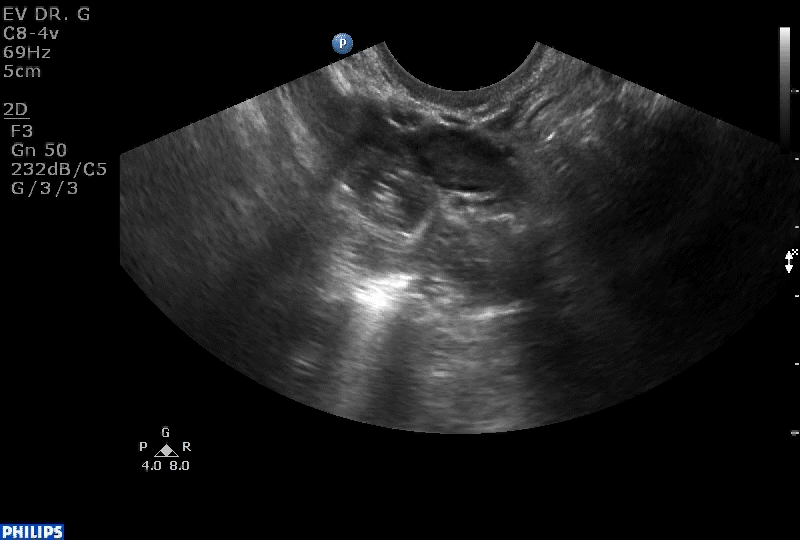
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--
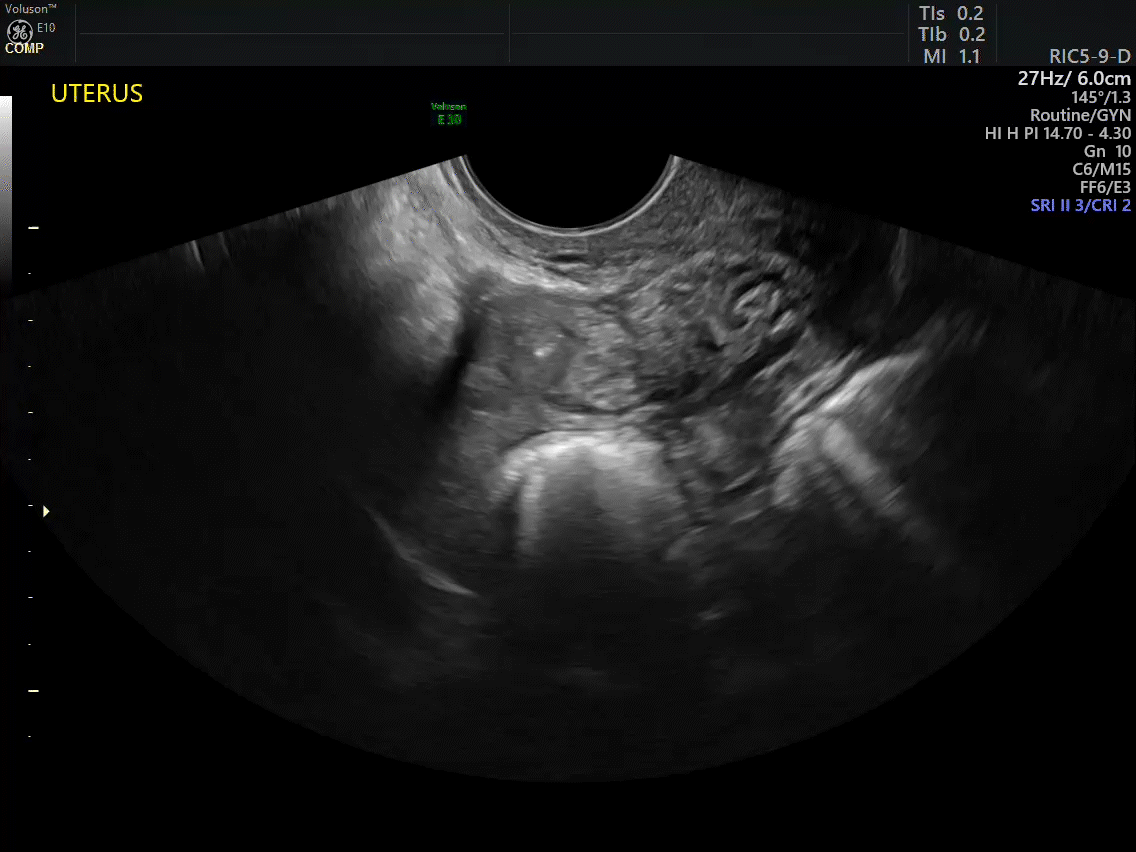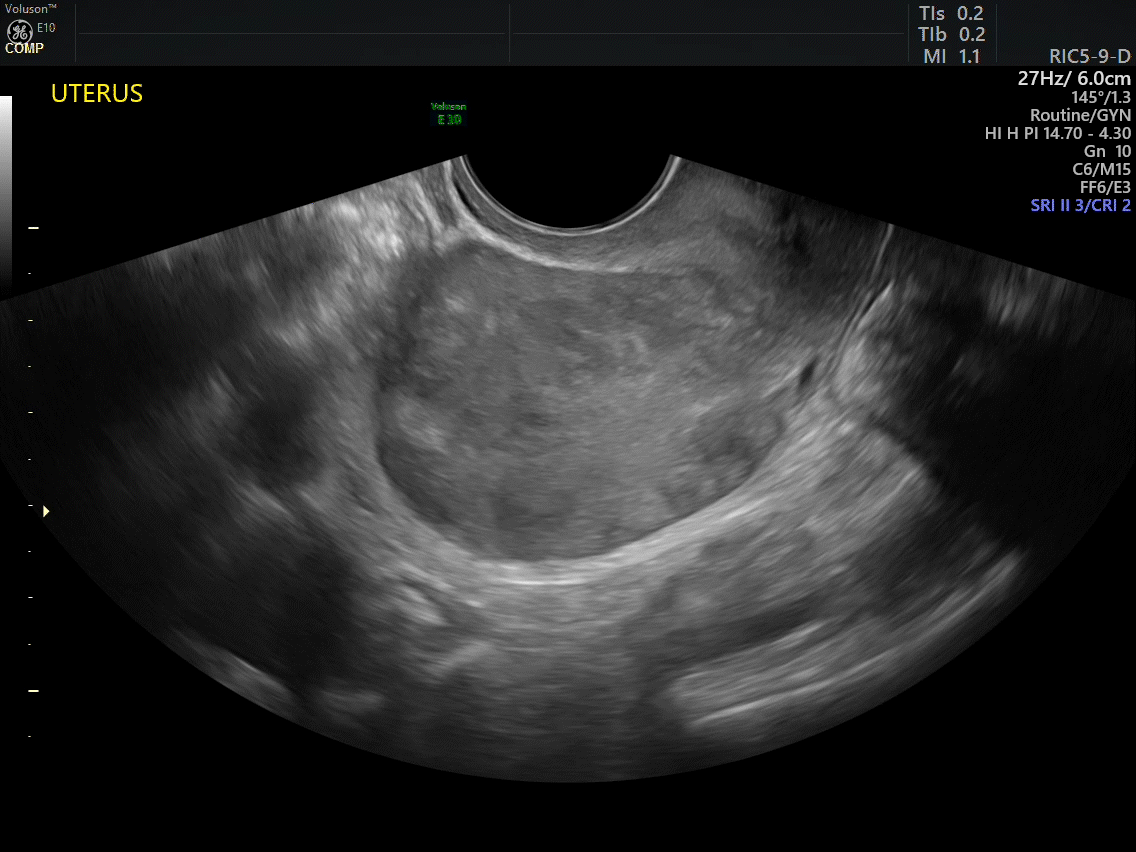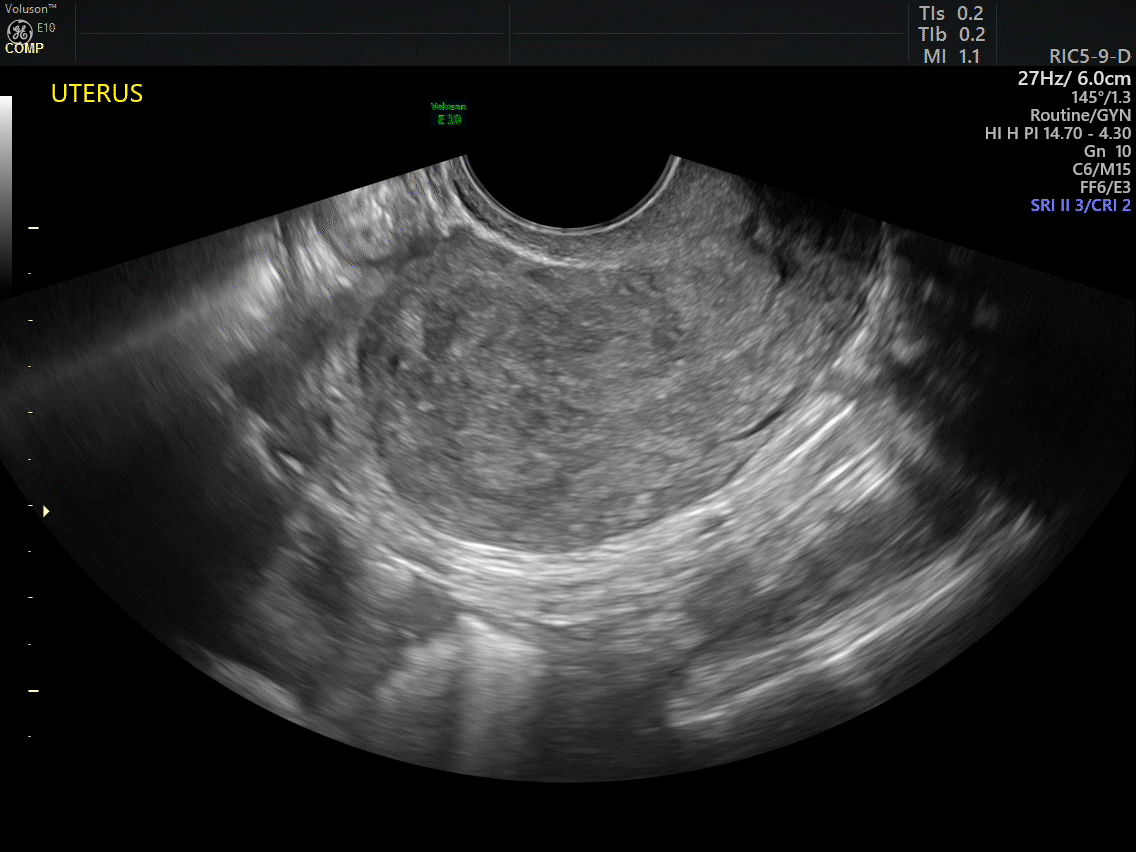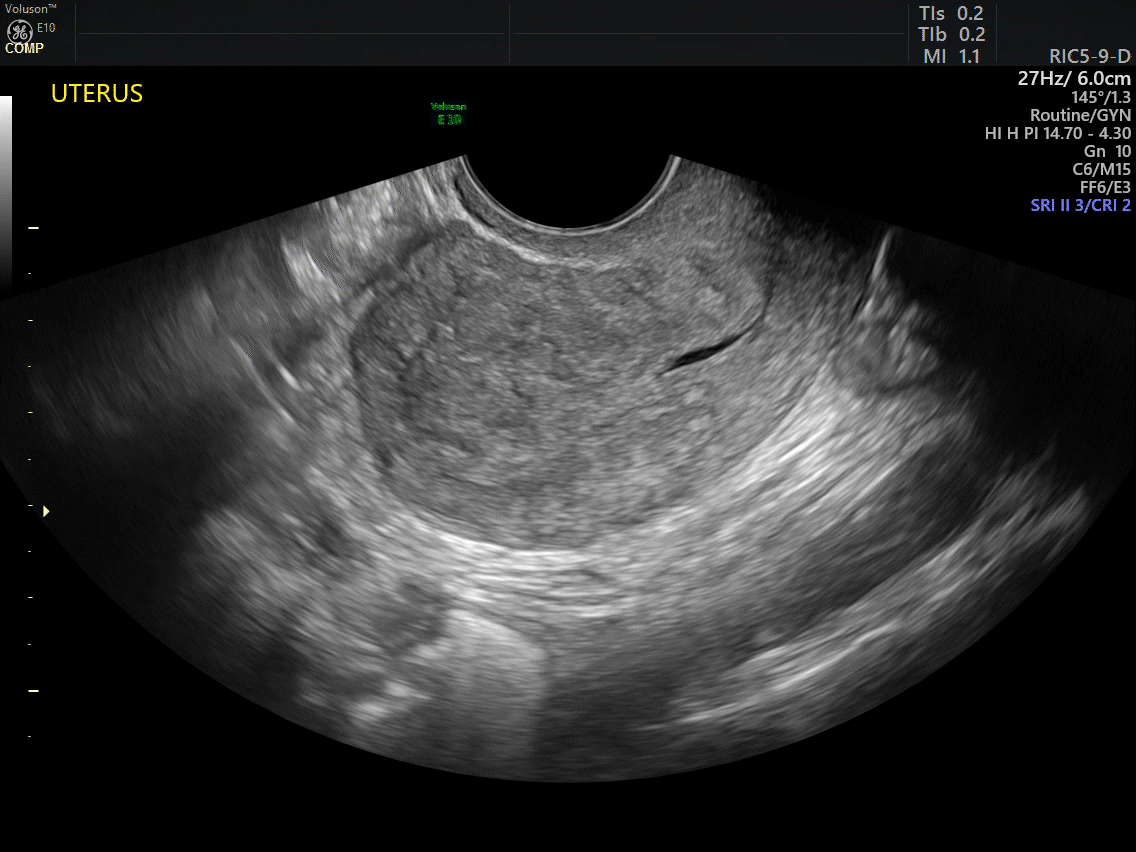
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--
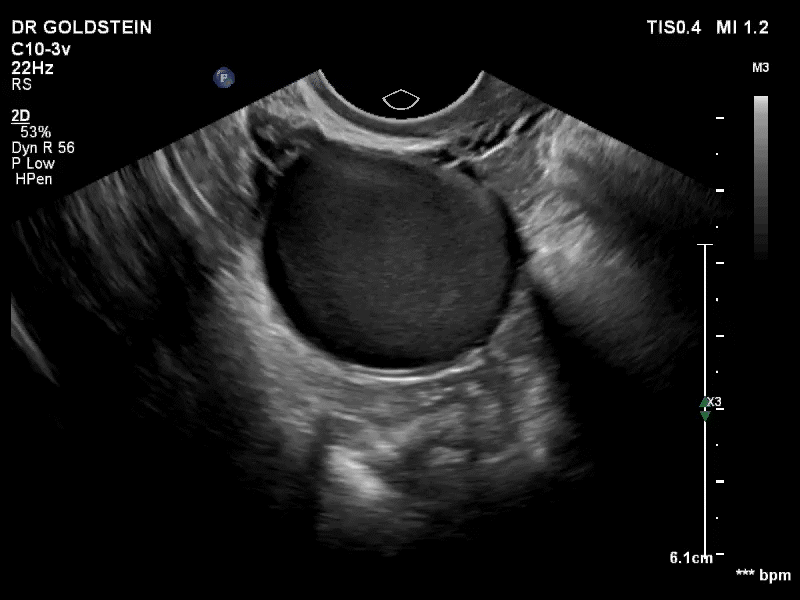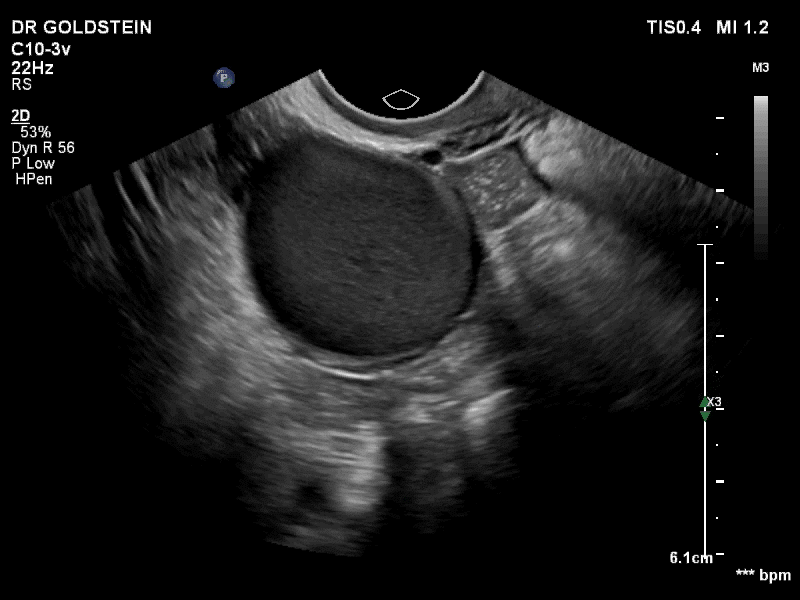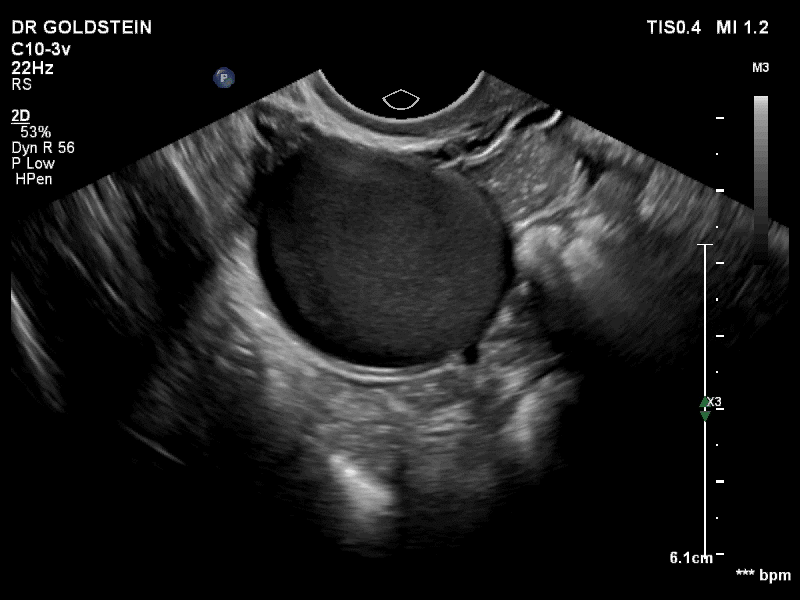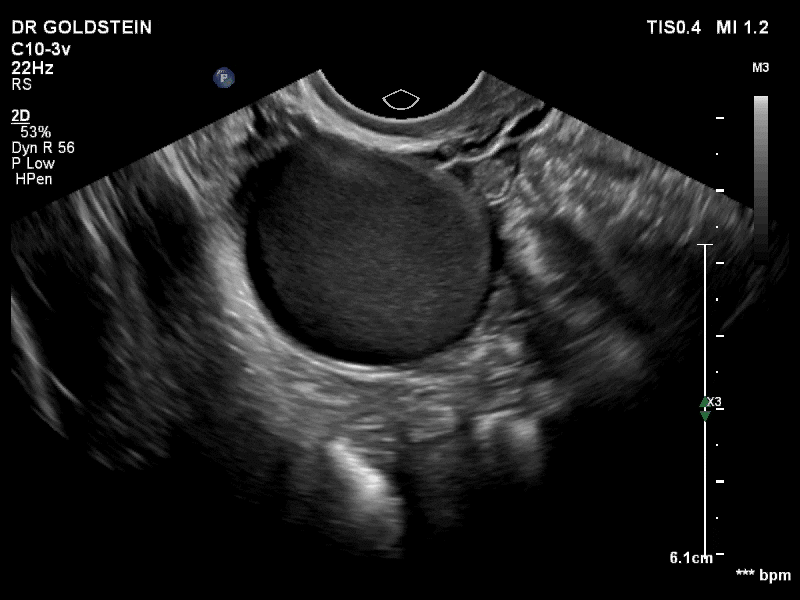
VIDEO 3A Video clip of a classic endometrioma
--
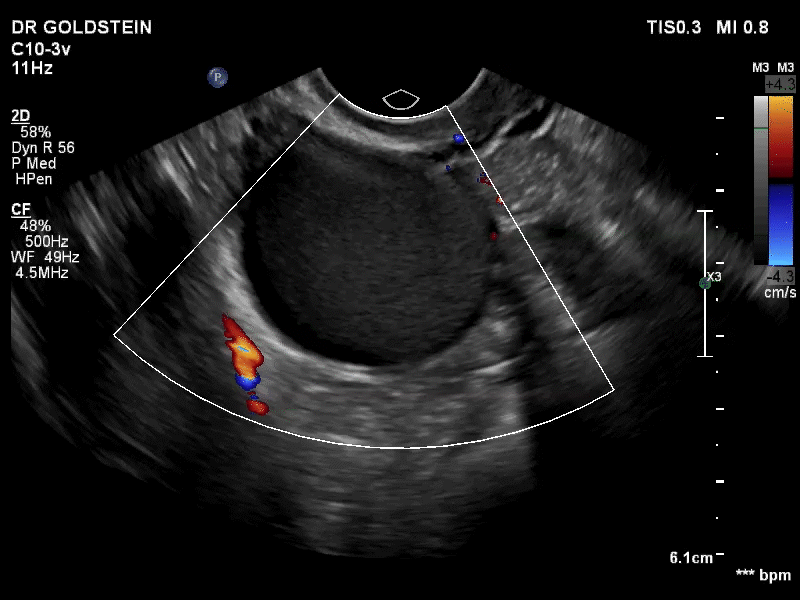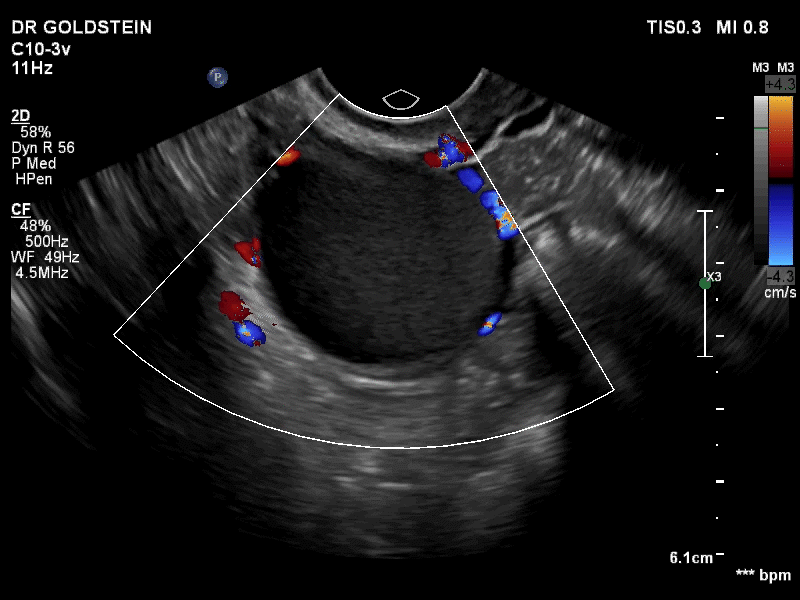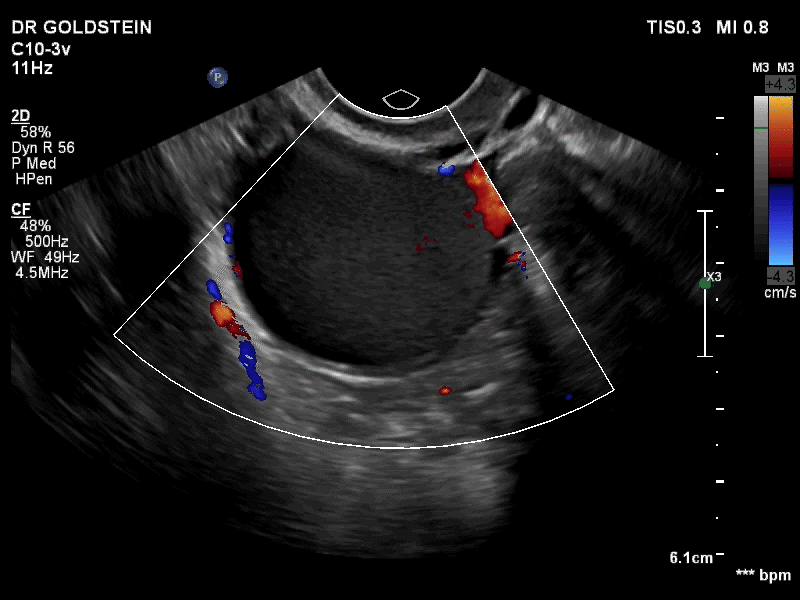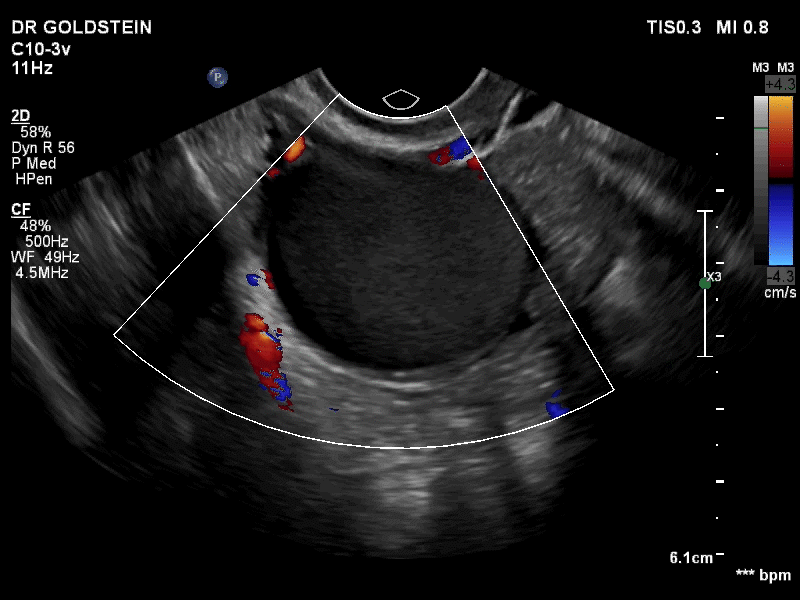
VIDEO 3B Classic endometrioma showing no Doppler flow internally
--
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--
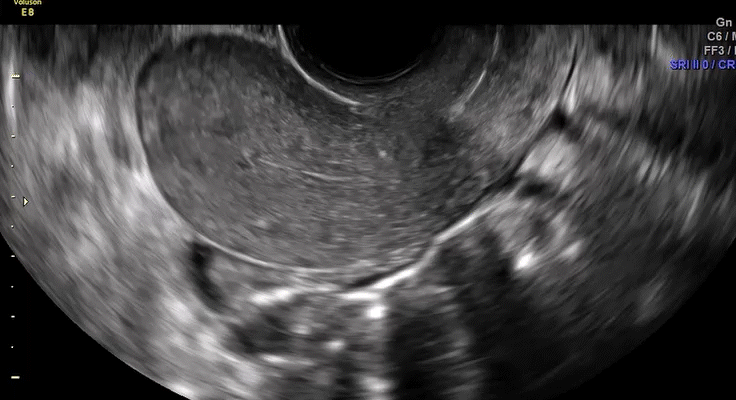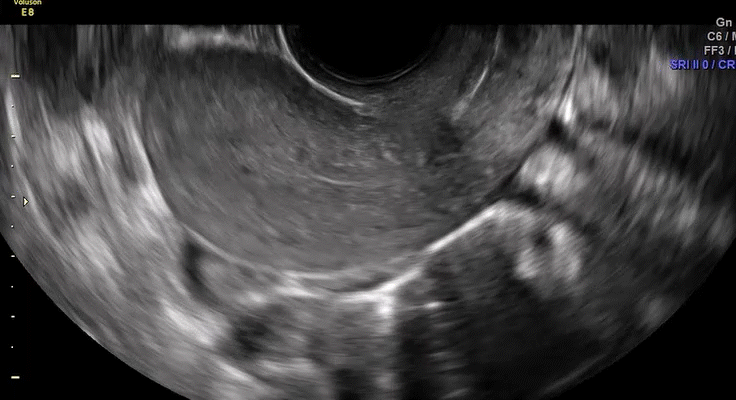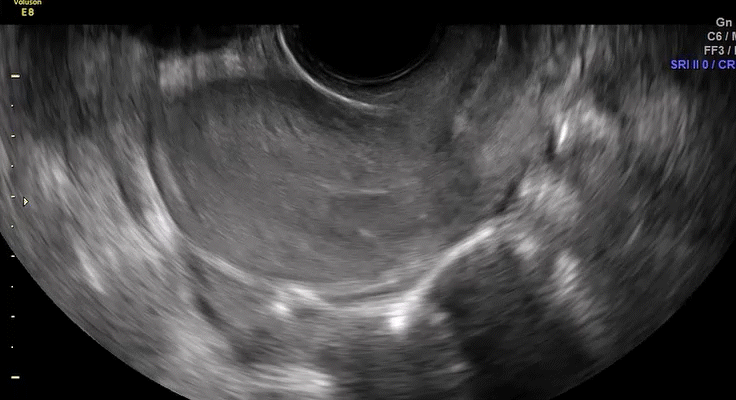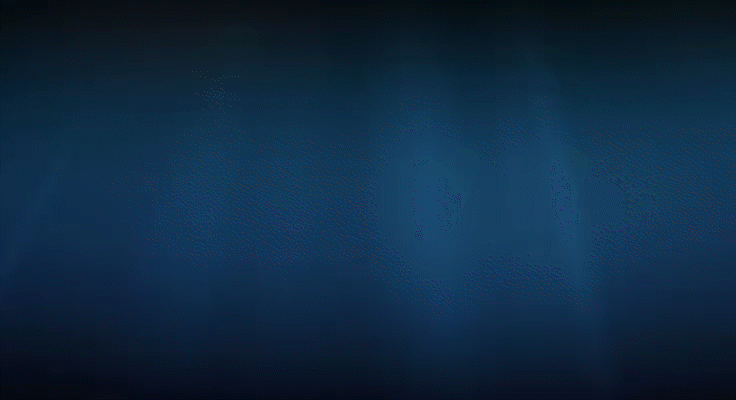
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
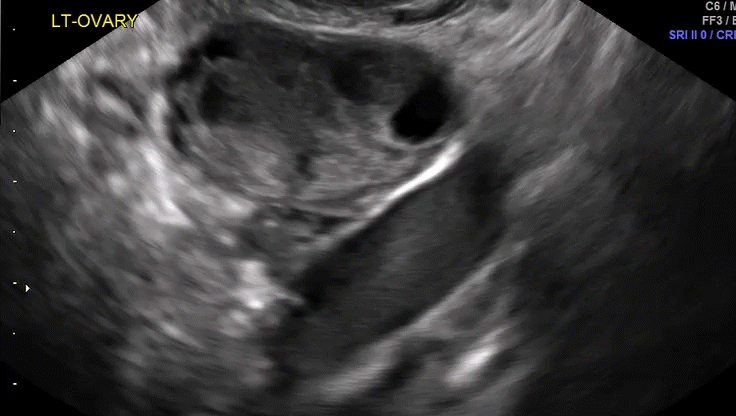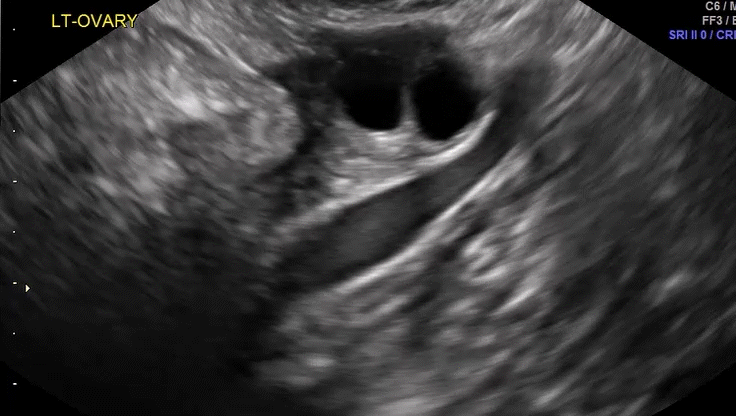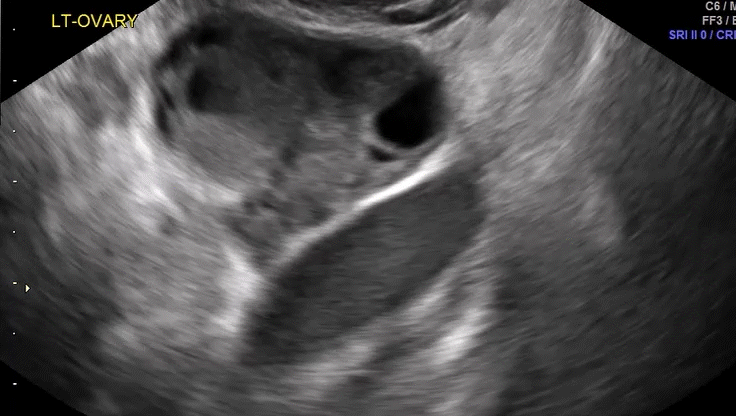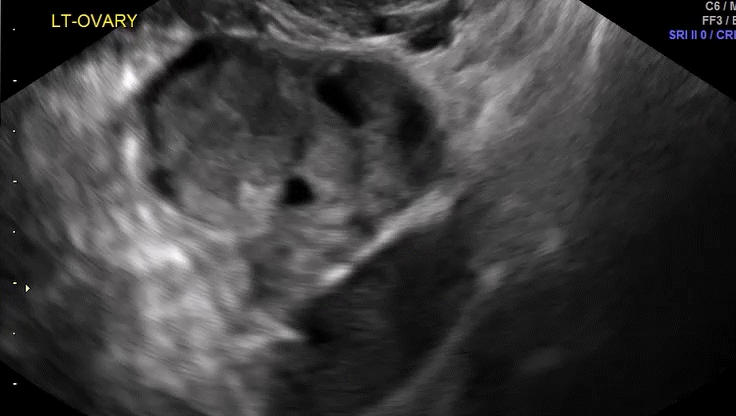
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
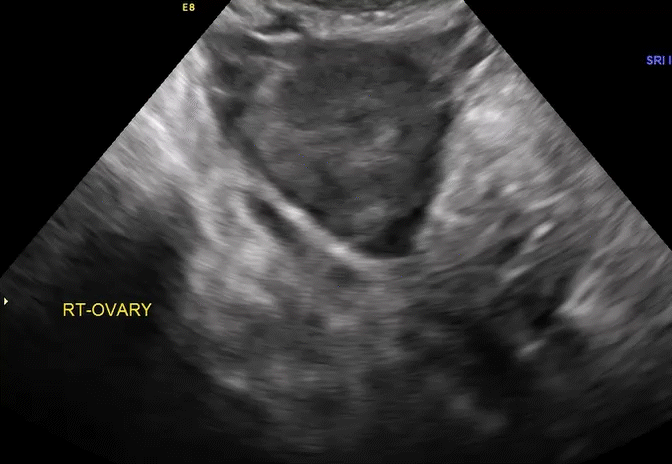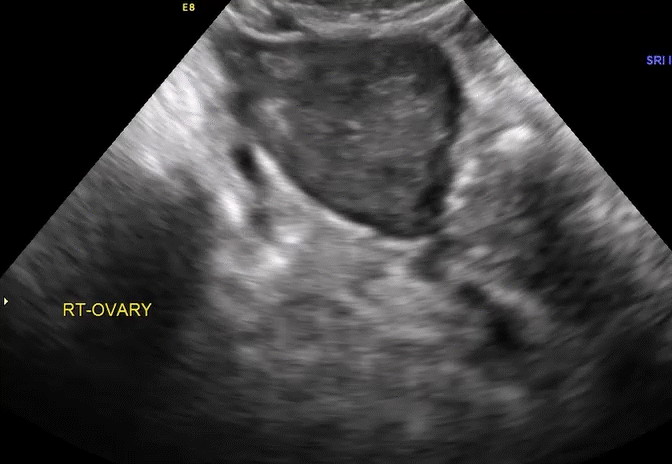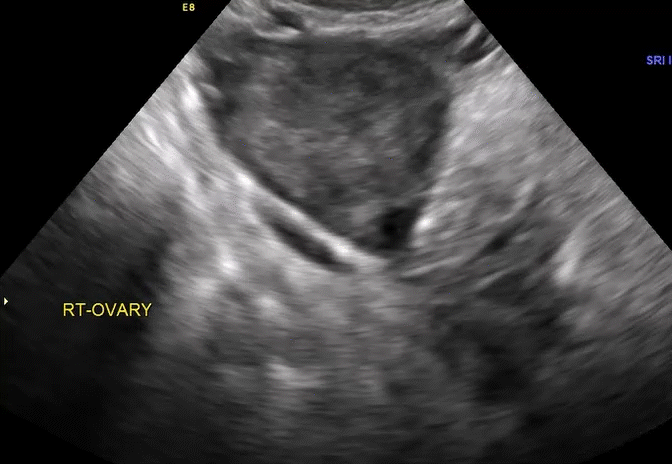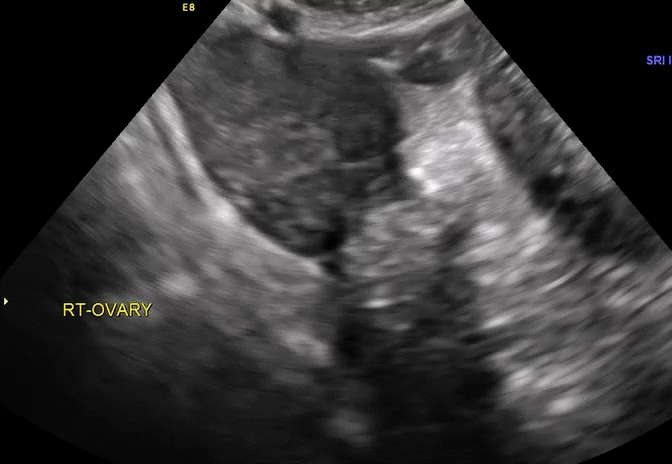
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--
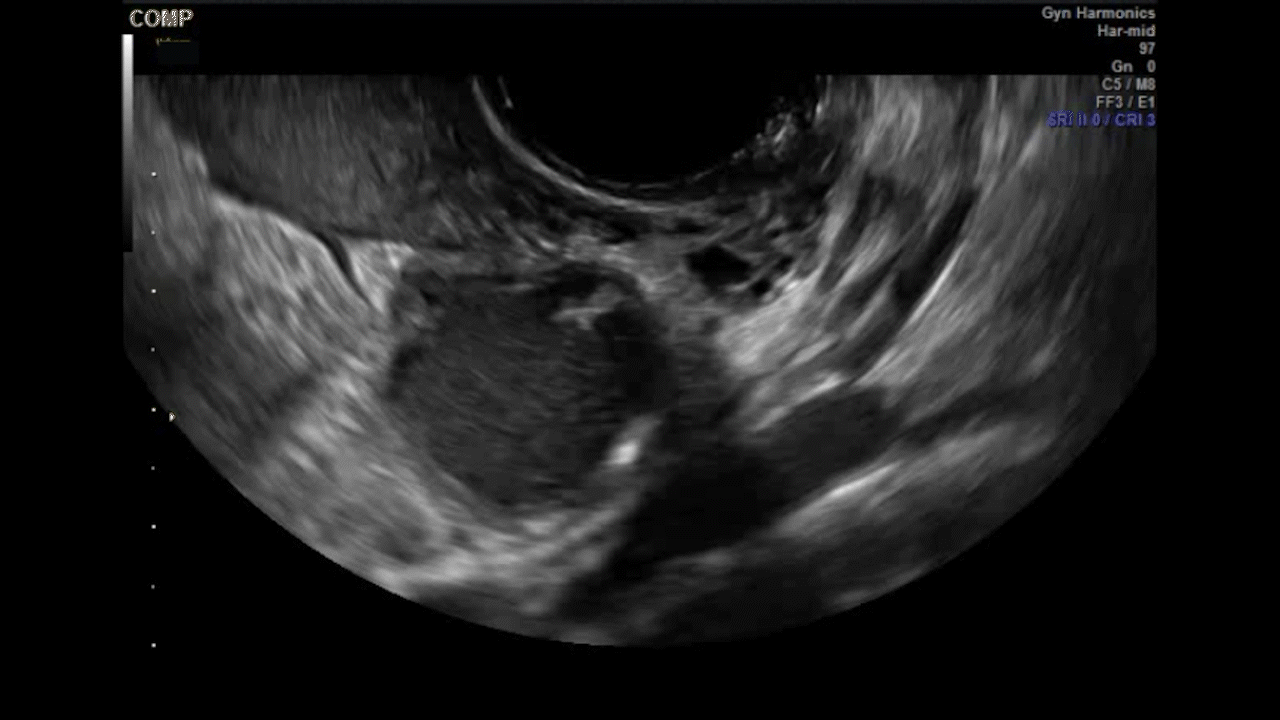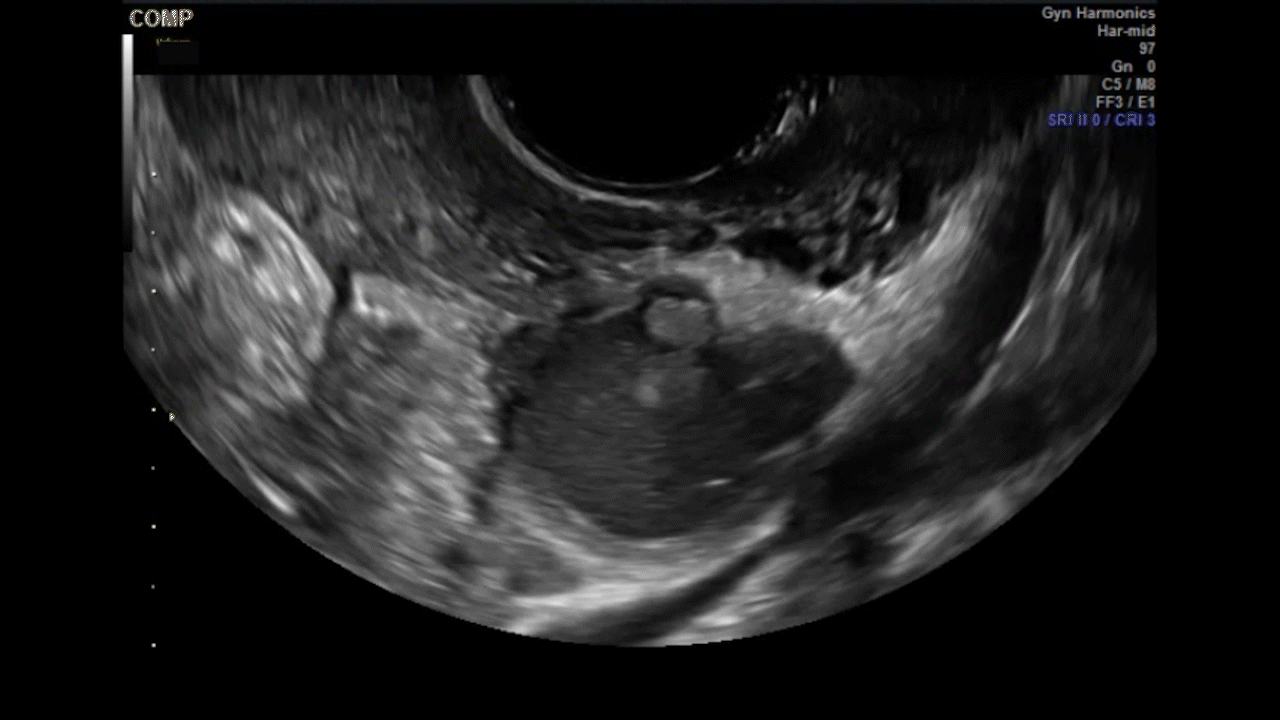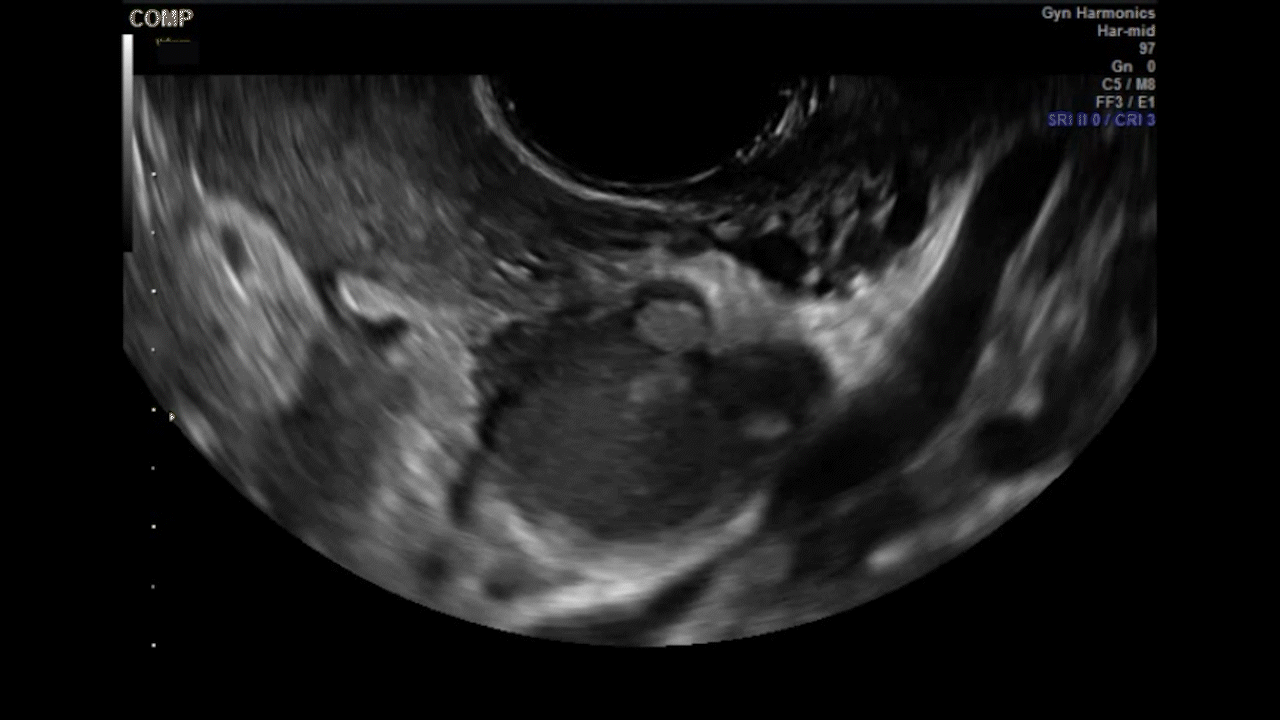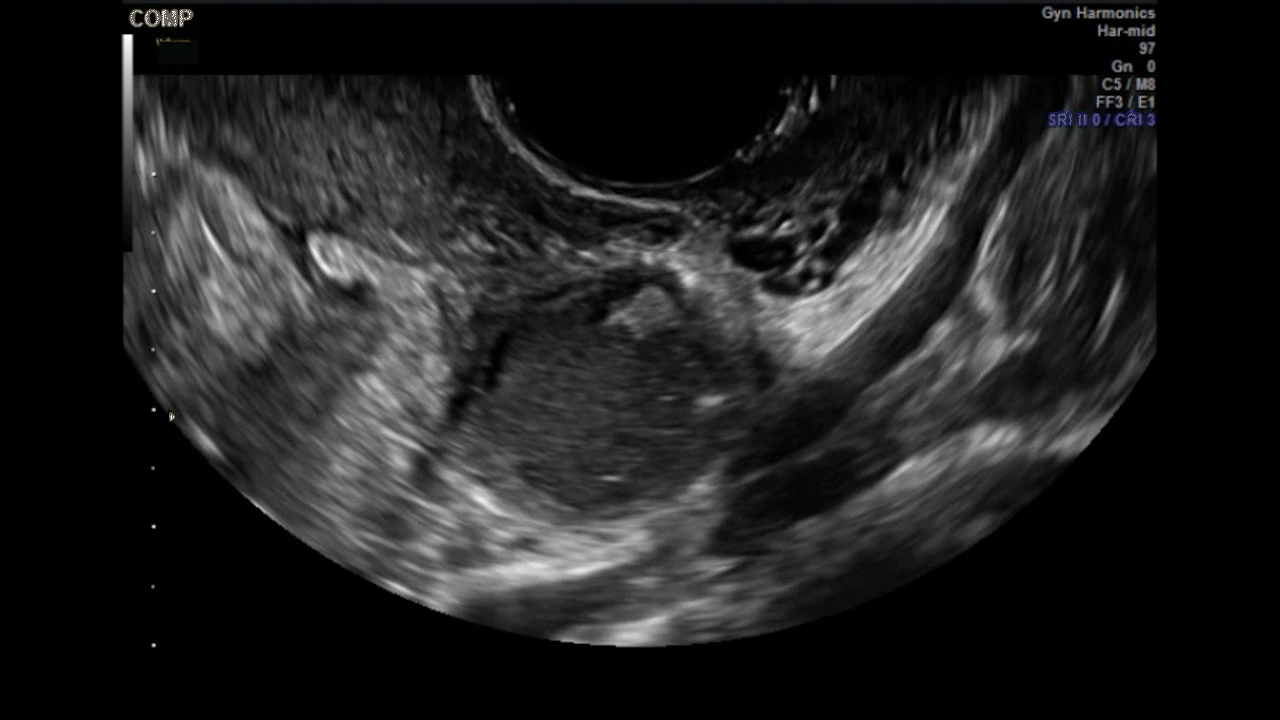
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--
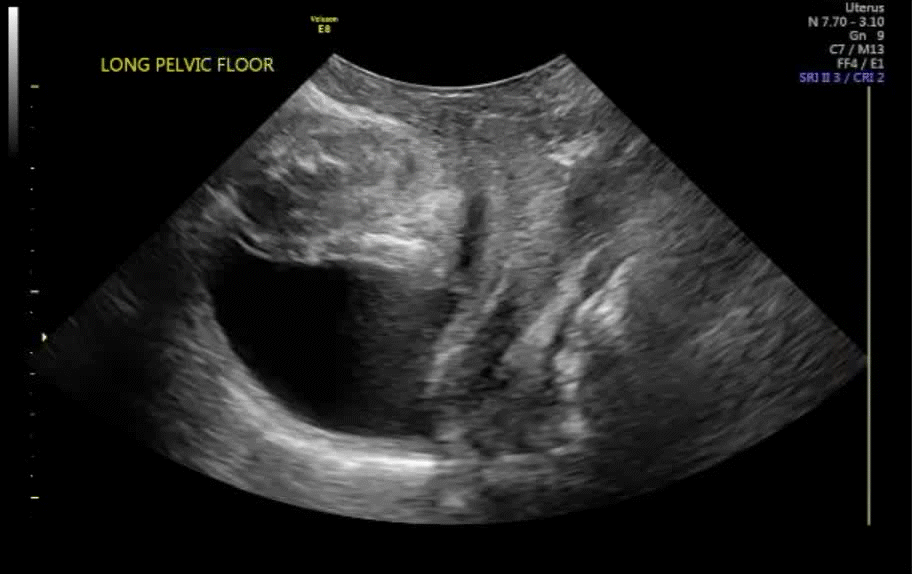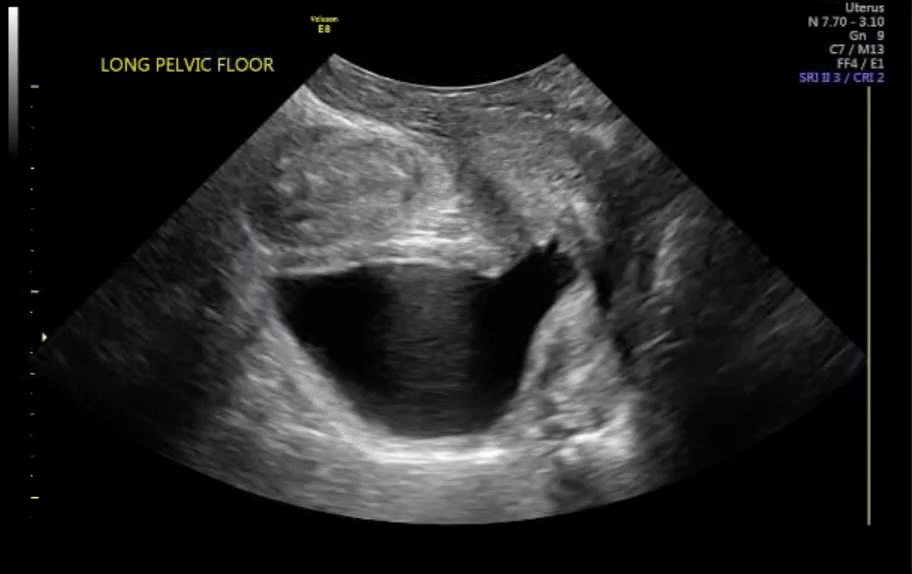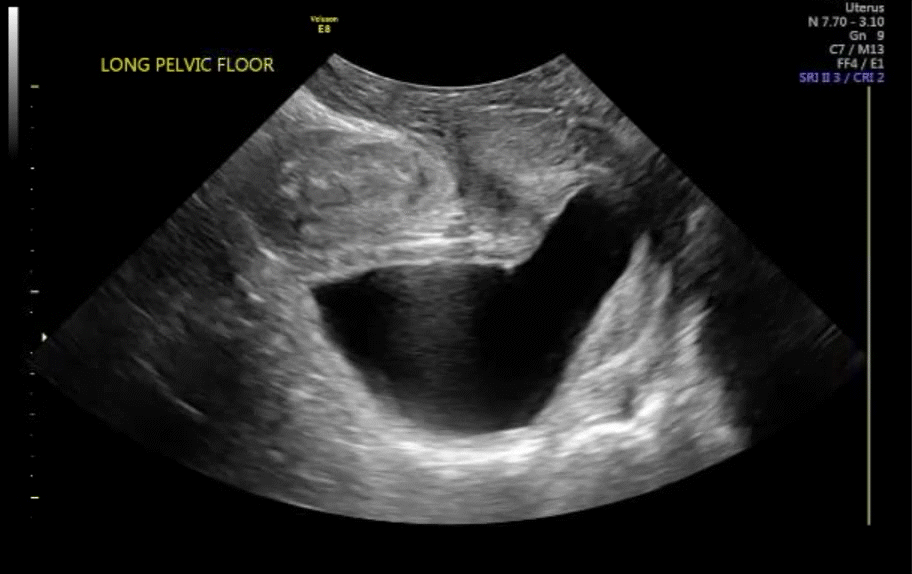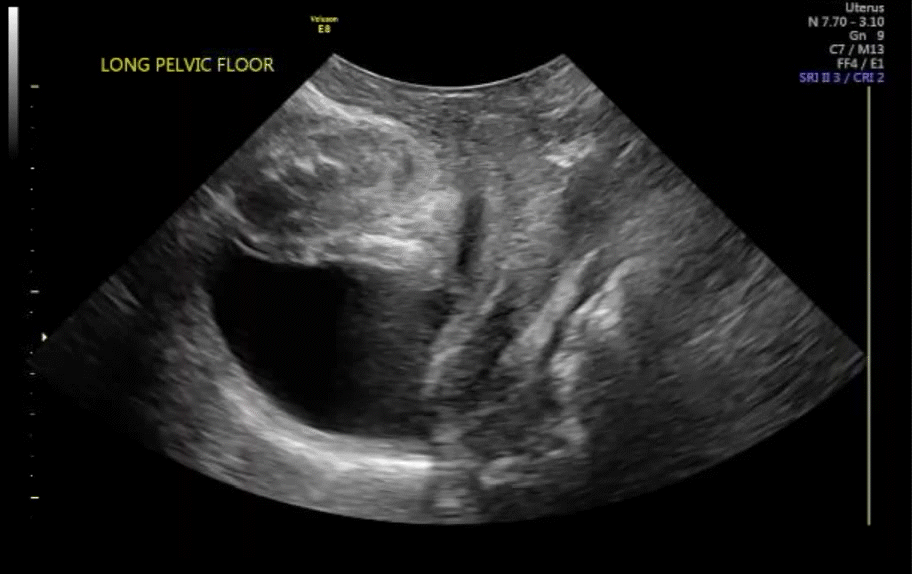
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--

VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--

VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--

VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--

VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--

VIDEO 3A Video clip of a classic endometrioma
--

VIDEO 3B Classic endometrioma showing no Doppler flow internally
--

VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--

VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--

VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
--

VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
--

VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
--

VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in Video 2A showing what appears to be a solid adnexal mass
--

VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
--

VIDEO 3A Video clip of a classic endometrioma
--

VIDEO 3B Classic endometrioma showing no Doppler flow internally
--

VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
--

VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
--

VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
--

VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
--

VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
--

VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
Can a once-daily oral formulation treat symptoms of uterine fibroids without causing hot flashes or bone loss?
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
2020 Update on bone health
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.