User login
The medical profession and the 2022 ̶ 2023 Term of the Supreme Court

The 2022-2023 Term of the Supreme Court illustrates how important the Court has become to health-related matters, including decisions regarding the selection and training of new professionals, the daily practice of medicine, and the future availability of new drugs. The importance of several cases is reinforced by the fact that major medical organizations filed amicus curiae (“friend of the court”) briefs in those cases.
Amicus briefs are filed by individuals or organizations with something significant to say about a case to the court—most often to present a point of view, make an argument, or provide information that the parties to the case may not have communicated. Amicus briefs are burdensome in terms of the time, energy, and cost of preparing and filing. Thus, they are not undertaken lightly. Medical organizations submitted amicus briefs in the first 3 cases we consider.
Admissions, race, and diversity
The case: Students for Fair Admissions v President and Fellows of Harvard College
The American College of Obstetricians and Gynecologists (ACOG) joined an amici curiae brief in Students for Fair Admissions v President and Fellows of Harvard College (and the University of North Carolina [UNC]).1 This case challenged the use of racial preferences in college admissions. The Association of American Medical Colleges (AAMC) was the lead organization; nearly 40 other health-related organizations joined the brief.
The legal claim. Those filing the suits asserted that racial preferences by public colleges violate the 14th Amendment’s Equal Protection Clause (“no state shall deny to any person … the equal protection of the law”). That is, if a state university gives racial preferences in selective admissions, it denies some other applicant the equal protection of the law. As for private schools (in this case, Harvard), Title VI of the Civil Rights Act of 1964 has the same standards as the Equal Protection Clause. Thus, the Court consolidated the cases and used the same legal standard in considering public and private colleges (with “colleges” including professional and graduate programs as well as undergraduate institutions).
Background. For nearly 50 years, the Supreme Court has allowed limited racial preferences in college admissions. Those preferences could only operate as a plus, however, and not a negative for applicants and be narrowly tailored. The measure was instituted temporarily; in a 2003 case, the Court said, “We expect that 25 years from now, the use of racial preferences will no longer be necessary.”2
Decision. In a 6-3 decision, the Court held (in the UNC case) that racial preferences generally violate the Constitution, and by a 6-2 decision (in the Harvard case) these preferences violate the Civil Rights Act of 1964. (Justice Jackson was recused in the Harvard case because of a conflict.) The opinion covered 237 pages in the US Reports, so any summary is incomplete.
The majority concluded, “The Harvard and UNC admissions programs cannot be reconciled with the guarantees of the Equal Protection Clause. Both programs lack sufficiently focused and measurable objectives warranting the use of race, unavoidably employ race in a negative manner, involve racial stereotyping, and lack meaningful end points. We have never permitted admissions programs to work in that way, and we will not do so today.”3
There were 3 concurring opinions and 2 dissents in the case. The concurrences reviewed the history of the Equal Protection Clause and the Civil Rights Act, the damage racial preferences can do, and the explicit limits the Court said there must be on racial preferences in higher education. The dissents had a different view of the legal history of the 14th Amendment. They said the majority was turning a blind eye to segregation in society and the race-based gap in America.
As a practical matter, this case means that colleges, including professional schools, cannot use racial preferences. The Court said that universities may consider essays and the like in which applicants describe how their own experiences as an individual (including race) have affected their own lives. However, the Court cautioned that “universities may not simply establish through application essays or other means the regime we hold unlawful today.”3
Continue to: The amici brief...
The amici brief
ACOG joined 40 other health-related organizations in filing an amici brief (multiple “friends”) in Students for Fair Admission. The AAMC led the brief, with the others signing as amici.4 The brief made 3 essential points: diversity in medical education “markedly improves health outcomes,” and a loss of diversity “threaten[s] patients’ health; medical schools engage in an intense “holistic” review of applicants for admission; and medical schools must consider applicants’ “full background” (including race) to achieve their educational and professional goals.4
A powerful part of the brief described the medical school admissions process, particularly the very “holistic” review that is not entirely dependent on admissions scores. The brief effectively weaves the consideration of race into this process, mentioning race (on page 22) only after discussing many other admissions factors.
Child custody decisions related to the Indian Child Welfare Act
The case: Haaland v Brackeen
The American Medical Association (AMA) and the American Academy of Pediatrics filed a brief in Haaland v Brackeen5 involving the constitutionality of the 1978 Indian Child Welfare Act (ICWA). The statute followed a terrible history of Indian children being removed from their families inappropriately, as detailed in a concurring opinion by Justice Gorsuch.5 The two purposes of the act were to promote raising Native American children in their culture and stem the downward trend in tribal membership.
The legal claim. The Court consolidated several cases. Essentially, a 10-month-old child (A.L.M.) was placed in foster care with the Brackeens in Texas. After more than 1 year, the Brackeens sought adoption; the biological father, mother, and grandparents all supported it. The Navajo and Cherokee Nations objected and informed the Texas court that they had found alternative placement with (nonrelative) tribal members in New Mexico. The “court-appointed guardian and a psychological expert … described the strong emotional bond between A.L.M. and his foster parents.” The court denied the adoption petition based on ICWA’s preference for tribe custody, and the Brackeens filed a lawsuit.
Decision. The constitutional claim in the case was that Congress lacked the authority to impose these substantial rules on states in making child custody decisions. The Supreme Court, in a 7-2 decision, upheld the constitutionality of the ICWA.
The amici brief
The joint amici brief of the American Academy of Pediatrics (AAP) and the AMA argued that tribes are “extended families” of Native American children.6 It noted the destructive history of removing Native American children from their families and suggested that kinship care improves children’s health. To its credit, the brief also honestly noted the serious mental health and suicide rates in some tribes, which suggest issues that might arise in child custody and adoption cases.
The Court did not, in this case, take up another constitutional issue that the parties raised—whether the strong preference for Native American over non ̶ Native American custody violates the Equal Protection Clause of the 14th Amendment. The Court said the parties to this case did not have standing to raise the issue. Justice Kavanaugh, concurring, said it was a “serious” issue and invited it to be raised in another case.5
False Claims Act cases
The case: Costs for SuperValu prescriptions
The legal claim. One FCA case this Term involved billings SuperValu made for outpatient prescriptions in Medicare-Medicaid programs. As its “usual and customary” costs, it essentially reported a list price that did not include the substantial discounts it commonly gave.
Background. Subjective knowledge (what the defendant actually knows) may seem impossible to prove—the defendant could just say, “I did not know I was doing wrong.” Over time the law has developed several ways of demonstrating “knowing.” Justice Thomas, writing for a unanimous Court, held that whistleblowers or the government might prove “knowing” in 3 ways:
1. defendants “actually knew that their reported prices were not their ‘usual and customary’ prices when they reported them”
2. were aware of a substantial risk that their higher, retail prices were not their “usual and customary” prices and intentionally avoided learning whether their reports were accurate
3. were aware of such a substantial and unjustifiable risk but submitted the claims anyway.9
Of course, records of the company, information from the whistleblower, and circumstantial evidence may be used to prove any of these; it does not require the company’s admission.
The Court said that if the government or whistleblowers make a showing of any of these 3 things, it is enough.
Decision. The case was returned to the lower court to apply these rules.
The amici brief
The American Hospital Association and America’s Health Insurance Plans filed an amici brief.10 It reminded the Court that many reimbursement regulations are unclear. Therefore, it is inappropriate to impose FCA liability for guessing incorrectly what the regulations mean. Having to check on every possible ambiguity was unworkable. The Court declined, however, the suggestion that defendants should be able to use any one of many “objectively” reasonable interpretations of regulations.
Continue to: The case: Polansky v Executive Health Resources...
The case: Polansky v Executive Health Resources
Health care providers who dislike the FCA may find solace this Term in this second FCA case.11
The legal claim. Polansky, a physician employed by a medical billing company, became an “intervenor” in a suit claiming the company assisted hospitals in false billing (inpatient claims for outpatient services). The government sought to dismiss the case, but Polansky refused.
Decision. The case eventually reached the Supreme Court, which held that the government may enter an FCA case at any time and move to dismiss the case even over the objection of a whistleblower. The government does not seek to enter a case in order to file dismissal motions often. When it does so, whistleblowers are protected by the fact that the dismissal motion requires a hearing before the federal court.
An important part of this case has escaped much attention. Justices Thomas, Kavanaugh, and Barrett invited litigation to determine if allowing private whistleblowers to represent the government’s interest is consistent with Article II of the Constitution.11 The invitation will likely be accepted. We expect to see cases challenging the place of “intervenors” pursuing claims when the government has declined to take up the case. The private intervenor is a crucial provision of the current FCA, and if such a challenge were successful, it could substantially reduce FCA cases.
Criminal false claims
Dubin overbilled Medicaid for psychological testing by saying the testing was done by a licensed psychologist rather than an assistant. The government claimed the “identity theft” was using the patient’s (actual) Medicaid number in submitting the bill.12 The Court unanimously held the overbilling was not aggravated identity theft as defined in federal law. Dubin could be convicted of fraudulent billing but not aggravated identity theft, thereby avoiding the mandatory prison term.
Patents of “genus” targets
The case: Amgen v Sanofi
Background. “Genus” patents allow a single pharmaceutical company to patent every antibody that binds to a specific amino acid on a naturally occurring protein. In this case, the patent office had granted a “genus” patent on “all antibodies” that bind to the naturally occurring protein PCSK9 and block it from hindering the body’s mechanism for removing low-density lipoprotein (LDL) cholesterol from the bloodstream,13 helping to reduce LDL cholesterol levels. These patents could involve millions of antibodies—and Amgen was claiming a patent on all of them. Amgen and Sanofi marketed their products, each with their own unique amino acid sequence.13 Amgen sued Sanofi for violating its patent rights.
Decision. The Court unanimously held that Amgen did not have a valid patent on all antibodies targeting PCSK9, only those that it had explicitly described in its patent application—a ruling based on a 150-year-old technical requirement for receiving a patent. An applicant for a patent must include “a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art…to make and use the same.”13 Amgen’s patent provided the description for only a few of the antibodies, but from the description in its application others could not “make and use” all of the antibodies targeting PCSK9.
While the decision was vital for future pharmaceuticals, the patent principle on which it was based has an interesting history. The Court noted that it affected the telegraph (Morse lost part of his patent), electric lights (Edison won his case against other inventors), and the glue for wood veneering (Perkins Glue Company lost).13
Continue to: Other notable decisions...
Other notable decisions
Student loans
The Court struck down the Biden Administration’s student loan forgiveness program, which would have cost approximately $430 billion.14 The central issue was whether the administration had the authority for such massive loan forgiveness; that is, whether Congress had authorized the broad loan forgiveness. The administration claimed authority from the post ̶ 9/11 HEROES Act, which allows the Secretary of Education to “waive or modify” loan provisions during national emergencies.
Free speech and the wedding web designer
303 Creative v Elenis involved a creative website designer who did not want to be required to create a website for a gay wedding.15 The designer had strong beliefs against same-sex marriages, but Colorado sought to force her to do so under the state “public accommodations” law. In a 6-3 decision, the Court held that the designer had a “free speech” right. That is, the state could not compel her to undertake speech expressing things she did not believe. This was because the website design was an expressive, creative activity and therefore was “speech” under the First Amendment.
Wetlands and the Clean Water Act
The essential issue in Sackett v Environmental Protection Agency (EPA) was the definitions of waters of the United States and related wetlands. The broad definition the EPA used meant it had jurisdiction to regulate an extraordinary amount of territory. It had, for example, prevented the Sacketts from building a modest house claiming it was part of the “waters of the United States because they were near a ditch that fed into a creek, which fed into Priest Lake, a navigable, intrastate lake.” The Court held that the EPA exceeded its statutory authority to define “wetlands.”16
The Court held that under the Clean Water Act, for the EPA to establish jurisdiction over adjacent wetlands, it must demonstrate that16:
1. “the adjacent body of water constitutes waters of the United States (ie, a relatively permanent body of water connected to traditional interstate navigable waters)…”
2. “…the wetland has a continuous surface connection with that water, making it difficult to determine where the water ends, and the wetland begins.”
Under this definition, the Sacketts could build their house. This was a statutory interpretation case. Therefore, Congress can expand or otherwise change the EPA’s authority under the Clean Water Act and other legislation.
Conclusions: A new justice, “shadow docket,” and ethics rules
SCOTUS’ newest member. When the Marshall called the Court into session on October 3, 2022, it had a new member, Justice Ketanji Brown Jackson. She was sworn in on June 30, 2022, when her predecessor (Justice Breyer) officially retired. She had been a law clerk for Justice Breyer in 1999, as well as a district court judge and court of appeals judge. Those who count such things described her as the “chattiest justice.”17 She spoke more than any other justice—by one count, a total of 75,632 words (an average of 1,300 words in each of the 58 arguments).
A more balanced Court? Most commentators view the Court as more balanced or less conservative than the previous Term. For example, Justice Sotomayor was in the majority 40% last Term but 65% this Term. Justice Thomas was in the majority 75% last Term but 55% this Term. Put another way, this Term in the divided cases, the liberal justices were in the majority 64% of the time, compared with the conservative justices 73%.18 Of course, these differences may reflect a different set of cases rather than a change in the direction of the Court. There were 11 (or 12, depending on how 1 case is counted) 6-3 cases, but only 5 were considered ideological. That suggests that, in many cases, the coalitions were somewhat fluid.
“Shadow docket” controversy continues.19 Shadow docket refers to orders the Court makes that do not follow oral arguments and often do not have written opinions. The orders are all publicly available. This Term a close examination of the approximately 30 shadow docket opinions shows that the overwhelming majority were dissents or explanations about denials of certiorari. The Court ordered only a few stays or injunctions via the shadow docket. One shadow docket stay (that prevented a lower court order from going into effect) is particularly noteworthy. A federal judge had ordered the suspension of the distribution of mifepristone while courts considered claims that the US Food and Drug Administration (FDA) had improperly approved the drug. In a shadow docket order, the Court issued the stay to allow mifepristone to be sold while the case challenging its approval was heard.20 The only opinion was a dissent from Justice Alito. But it also demonstrates the importance of the shadow docket. Without this intervention, in at least part of the country, the distribution of mifepristone would have been interrupted pending the outcome of the FDA cases.
In August, the Court delayed a settlement in the Purdue Pharma liability bankruptcy case.21 It also stayed an injunction of a lower court, thereby permitting federal “ghost guns” regulations to go into effect at least temporarily.22
More ethics rules to come? Another area in which the Court faced criticism was formal ethics rules. The justices make financial disclosures, but these are somewhat ambiguous. There is likely to be increasing pressure for a more complete disclosure of non-financial relationships and more formal ethics rules. ●
The Court had, by September 1, 2023, accepted 22 cases for hearing next Term.1 The cases include a challenge to the extraordinary funding provision for the Consumer Financial Protection Bureau, another racial challenge to congressional districts (South Carolina), the status of Americans with Disability Act “testers” who look for violations without ever intending to use the facilities, the level of deference courts should give to interpreting federal statutes (so-called “Chevron” deference), the opioid (OxyContin ) bankruptcy, and limitations on gun ownership. This represents less than half of the cases the Court will likely hear next Term, so the Court will add many more cases to the docket. It promises to be an appealing Term.
Reference
1. October Term 2023. SCOTUSblog website. Accessed August 29, 2023. https://www.scotusblog.com/case-files/terms/ot2023/
When the Court adjourned on June 30, 2023, it had considered 60 cases, plus hundreds of petitions asking it to hear cases. Most commentators count 55 cases decided after briefing and oral argument and where there was a signed opinion. The information below uses 55 cases unless otherwise noted. During the 2022-2023 Term, the Court:
- upheld liability for the involuntary administration of psychotropic drugs in nursing home1
- permitted disabled students, in some instances, both to make Individuals with Disabilities Education Act (IDEA) claims for services and to file Americans with Disabilities Act (ADA) lawsuits against their schools2
- upheld a statute that makes it illegal to “encourage or induce an alien to come to, enter, or reside in the United States, knowing or in reckless disregard of the fact that such coming to, entry, or residence is or will be in violation of law.” The defendant had used a scam promising noncitizens “adult adoptions” (of which there is no such thing) making it legal for them to come to and stay in the United States.3
- narrowed the “fair use” of copyrighted works. It held that Andy Warhol’s use of a copyrighted photograph in his famous Prince prints was not “transformative” in a legal sense largely because the photo and prints “share the same use”—magazine illustrations.4
- in another intellectual property case, held that Jack Daniel’s might sue a dog toy maker for a rubber dog toy that looked like a Jack Daniel’s bottle5
- further expanded the Federal Arbitration Act by holding that a federal district court must immediately stay court proceedings if one party is appealing a decision not to require arbitration6
- held that two social media companies were not responsible for terrorists using their platforms to recruit others to their cause. It did not, however, decide whether §230 of the Communication Decency Act protects companies from liability.7
- made it easier for employees to receive accommodation for their religious practices and beliefs. Employers must make religious accommodations unless the employer can show that “the burden of granting an accommodation would result in substantial increased [financial and other] costs in relation to the conduct of its particular business.”8
- declined to hear an appeal from Johnson & Johnson (through a subsidiary, Ethicon) about pelvic mesh. In this case, the California Attorney General filed a lawsuit against Ethicon for false advertising by failing to detail the risks of pelvic mesh. The lower courts estimated 240,000 written violations of the law by Ethicon between 2008 and 2017. The trial and appeal to California courts resulted in a judgment of $302 million against Johnson & Johnson. The company asked the Court to review that judgment, but the Court denied certiorari. That likely means the $302 million is final.
References
1. Health and Hospital Corporation of Marion Cty. v Talevski, Docket no. 21-806; June 8, 2023.
2. Luna Perez v Sturgis Public Schools, Docket no. 21-887; March 21, 2023.
3. United States v Hansen, Docket no. 22-179; June 23, 2023.
4. Andy Warhol Foundation for Visual Arts, Inc. v Goldsmith, Docket no. 21-869; May 18, 2023.
5. Jack Daniel’s Properties, Inc. v VIP Products LLC, Docket no. 22-148; June 8, 2023.
6. Coinbase, Inc. v Bielski, Docket no. 22-105; June 23, 2023.
7. Gonzalez v Google LLC, Docket no. 21-1333; May 18, 2023.
8. Groff v DeJoy, Docket no. 22-273; June 29, 2023.
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306, 326 (2003).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___, 39 (2023).
- Brief for Amici Curiae Association of American Medical Colleges et al. in Support of Respondents, Students for Fair Admissions v University of North Carolina (July 28, 2022). Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-707/232120/20220728171307159_20 -1199%20and%2021-707%20Amicus%20Brief%20for%20 Association%20of%20American%20Medical%20Colleges%20 et%20al.pdf
- Haaland v Brackeen, Docket no. 21-376; June 15, 2023.
- Brief of American Academy of Pediatrics and American Medical Association as Amici Curiae in Support of Respondents, in Haaland v Brackeen. August 19, 2022. Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-376/234042/20220819140750948_21-376 .amics.brief.FINAL.pdf
- Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021. US Department of Justice website. February 1, 2022. Accessed August 18, 2023. https://www.justice.gov/opa/pr/justice -department-s-false-claims-act-settlements-and-judgments -exceed-56-billion-fiscal-year
- False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022. US Department of Justice website. February 7, 2023. Accessed August 18, 2023. https://www .justice.gov/opa/pr/false-claims-act-settlements-and -judgments-exceed-2-billion-fiscal-year-2022
- United States ex rel. Schutte v Supervalu Inc., Docket no. 21-1326; June 1, 2023.
- Brief of American Hospital Association and America’s Health Insurance Plans as Amici Curiae in Support of Respondents, in Schutte v Supervalu. March 2023. Accessed August 18, 2023. https://www.supremecourt.gov/DocketPDF/21/21-1326 /262428/20230331113854936_3-31-23%20AHA_AHIP _Amicus_Brief.pdf
- United States ex rel. Polansky v Executive Health Resources, Inc., Docket no. 21-1052; June 16, 2023.
- Dubin v United States, Docket no. 22-10; June 8, 2023.
- Amgen v Sanofi, 598 US ___ (2023).
- Biden v Nebraska, 600 US ___ (2023).
- 303 Creative LLC v Elenis, 600 US ___ (2023).
- Sackett v Environmental Protection Agency, Docket no. 21454; May 25, 2023.
- Krochtengel J. Jackson debuts as chattiest Justice. Law360. July 3, 2023. https://www.law360.com/articles/1692839 /jackson-debuts-as-chattiest-justice
- Feldman A. Another One Bites the Dust: End of 2022/2023 Supreme Court Term Statistics. EmpiricalScotus website. 30, 2023. Accessed August 18, 2023. https://empiricalscotus .com/2023/06/30/another-one-bites-2022/
- Vladeck S. The Shadow Docket: How the Supreme Court Uses Stealth Rulings to Amass Power and Undermine the Republic. New York, New York; Basic Books; 2023.
- Danco Laboratories, LLC v Alliance for Hippocratic Medicine. Docket no. 22A902; April 21, 2023.
- Harrington v Purdue Pharma, 23-124 (23A87).
- Garland v Vanderstok, 23-10718 (August 8, 2023).

The 2022-2023 Term of the Supreme Court illustrates how important the Court has become to health-related matters, including decisions regarding the selection and training of new professionals, the daily practice of medicine, and the future availability of new drugs. The importance of several cases is reinforced by the fact that major medical organizations filed amicus curiae (“friend of the court”) briefs in those cases.
Amicus briefs are filed by individuals or organizations with something significant to say about a case to the court—most often to present a point of view, make an argument, or provide information that the parties to the case may not have communicated. Amicus briefs are burdensome in terms of the time, energy, and cost of preparing and filing. Thus, they are not undertaken lightly. Medical organizations submitted amicus briefs in the first 3 cases we consider.
Admissions, race, and diversity
The case: Students for Fair Admissions v President and Fellows of Harvard College
The American College of Obstetricians and Gynecologists (ACOG) joined an amici curiae brief in Students for Fair Admissions v President and Fellows of Harvard College (and the University of North Carolina [UNC]).1 This case challenged the use of racial preferences in college admissions. The Association of American Medical Colleges (AAMC) was the lead organization; nearly 40 other health-related organizations joined the brief.
The legal claim. Those filing the suits asserted that racial preferences by public colleges violate the 14th Amendment’s Equal Protection Clause (“no state shall deny to any person … the equal protection of the law”). That is, if a state university gives racial preferences in selective admissions, it denies some other applicant the equal protection of the law. As for private schools (in this case, Harvard), Title VI of the Civil Rights Act of 1964 has the same standards as the Equal Protection Clause. Thus, the Court consolidated the cases and used the same legal standard in considering public and private colleges (with “colleges” including professional and graduate programs as well as undergraduate institutions).
Background. For nearly 50 years, the Supreme Court has allowed limited racial preferences in college admissions. Those preferences could only operate as a plus, however, and not a negative for applicants and be narrowly tailored. The measure was instituted temporarily; in a 2003 case, the Court said, “We expect that 25 years from now, the use of racial preferences will no longer be necessary.”2
Decision. In a 6-3 decision, the Court held (in the UNC case) that racial preferences generally violate the Constitution, and by a 6-2 decision (in the Harvard case) these preferences violate the Civil Rights Act of 1964. (Justice Jackson was recused in the Harvard case because of a conflict.) The opinion covered 237 pages in the US Reports, so any summary is incomplete.
The majority concluded, “The Harvard and UNC admissions programs cannot be reconciled with the guarantees of the Equal Protection Clause. Both programs lack sufficiently focused and measurable objectives warranting the use of race, unavoidably employ race in a negative manner, involve racial stereotyping, and lack meaningful end points. We have never permitted admissions programs to work in that way, and we will not do so today.”3
There were 3 concurring opinions and 2 dissents in the case. The concurrences reviewed the history of the Equal Protection Clause and the Civil Rights Act, the damage racial preferences can do, and the explicit limits the Court said there must be on racial preferences in higher education. The dissents had a different view of the legal history of the 14th Amendment. They said the majority was turning a blind eye to segregation in society and the race-based gap in America.
As a practical matter, this case means that colleges, including professional schools, cannot use racial preferences. The Court said that universities may consider essays and the like in which applicants describe how their own experiences as an individual (including race) have affected their own lives. However, the Court cautioned that “universities may not simply establish through application essays or other means the regime we hold unlawful today.”3
Continue to: The amici brief...
The amici brief
ACOG joined 40 other health-related organizations in filing an amici brief (multiple “friends”) in Students for Fair Admission. The AAMC led the brief, with the others signing as amici.4 The brief made 3 essential points: diversity in medical education “markedly improves health outcomes,” and a loss of diversity “threaten[s] patients’ health; medical schools engage in an intense “holistic” review of applicants for admission; and medical schools must consider applicants’ “full background” (including race) to achieve their educational and professional goals.4
A powerful part of the brief described the medical school admissions process, particularly the very “holistic” review that is not entirely dependent on admissions scores. The brief effectively weaves the consideration of race into this process, mentioning race (on page 22) only after discussing many other admissions factors.
Child custody decisions related to the Indian Child Welfare Act
The case: Haaland v Brackeen
The American Medical Association (AMA) and the American Academy of Pediatrics filed a brief in Haaland v Brackeen5 involving the constitutionality of the 1978 Indian Child Welfare Act (ICWA). The statute followed a terrible history of Indian children being removed from their families inappropriately, as detailed in a concurring opinion by Justice Gorsuch.5 The two purposes of the act were to promote raising Native American children in their culture and stem the downward trend in tribal membership.
The legal claim. The Court consolidated several cases. Essentially, a 10-month-old child (A.L.M.) was placed in foster care with the Brackeens in Texas. After more than 1 year, the Brackeens sought adoption; the biological father, mother, and grandparents all supported it. The Navajo and Cherokee Nations objected and informed the Texas court that they had found alternative placement with (nonrelative) tribal members in New Mexico. The “court-appointed guardian and a psychological expert … described the strong emotional bond between A.L.M. and his foster parents.” The court denied the adoption petition based on ICWA’s preference for tribe custody, and the Brackeens filed a lawsuit.
Decision. The constitutional claim in the case was that Congress lacked the authority to impose these substantial rules on states in making child custody decisions. The Supreme Court, in a 7-2 decision, upheld the constitutionality of the ICWA.
The amici brief
The joint amici brief of the American Academy of Pediatrics (AAP) and the AMA argued that tribes are “extended families” of Native American children.6 It noted the destructive history of removing Native American children from their families and suggested that kinship care improves children’s health. To its credit, the brief also honestly noted the serious mental health and suicide rates in some tribes, which suggest issues that might arise in child custody and adoption cases.
The Court did not, in this case, take up another constitutional issue that the parties raised—whether the strong preference for Native American over non ̶ Native American custody violates the Equal Protection Clause of the 14th Amendment. The Court said the parties to this case did not have standing to raise the issue. Justice Kavanaugh, concurring, said it was a “serious” issue and invited it to be raised in another case.5
False Claims Act cases
The case: Costs for SuperValu prescriptions
The legal claim. One FCA case this Term involved billings SuperValu made for outpatient prescriptions in Medicare-Medicaid programs. As its “usual and customary” costs, it essentially reported a list price that did not include the substantial discounts it commonly gave.
Background. Subjective knowledge (what the defendant actually knows) may seem impossible to prove—the defendant could just say, “I did not know I was doing wrong.” Over time the law has developed several ways of demonstrating “knowing.” Justice Thomas, writing for a unanimous Court, held that whistleblowers or the government might prove “knowing” in 3 ways:
1. defendants “actually knew that their reported prices were not their ‘usual and customary’ prices when they reported them”
2. were aware of a substantial risk that their higher, retail prices were not their “usual and customary” prices and intentionally avoided learning whether their reports were accurate
3. were aware of such a substantial and unjustifiable risk but submitted the claims anyway.9
Of course, records of the company, information from the whistleblower, and circumstantial evidence may be used to prove any of these; it does not require the company’s admission.
The Court said that if the government or whistleblowers make a showing of any of these 3 things, it is enough.
Decision. The case was returned to the lower court to apply these rules.
The amici brief
The American Hospital Association and America’s Health Insurance Plans filed an amici brief.10 It reminded the Court that many reimbursement regulations are unclear. Therefore, it is inappropriate to impose FCA liability for guessing incorrectly what the regulations mean. Having to check on every possible ambiguity was unworkable. The Court declined, however, the suggestion that defendants should be able to use any one of many “objectively” reasonable interpretations of regulations.
Continue to: The case: Polansky v Executive Health Resources...
The case: Polansky v Executive Health Resources
Health care providers who dislike the FCA may find solace this Term in this second FCA case.11
The legal claim. Polansky, a physician employed by a medical billing company, became an “intervenor” in a suit claiming the company assisted hospitals in false billing (inpatient claims for outpatient services). The government sought to dismiss the case, but Polansky refused.
Decision. The case eventually reached the Supreme Court, which held that the government may enter an FCA case at any time and move to dismiss the case even over the objection of a whistleblower. The government does not seek to enter a case in order to file dismissal motions often. When it does so, whistleblowers are protected by the fact that the dismissal motion requires a hearing before the federal court.
An important part of this case has escaped much attention. Justices Thomas, Kavanaugh, and Barrett invited litigation to determine if allowing private whistleblowers to represent the government’s interest is consistent with Article II of the Constitution.11 The invitation will likely be accepted. We expect to see cases challenging the place of “intervenors” pursuing claims when the government has declined to take up the case. The private intervenor is a crucial provision of the current FCA, and if such a challenge were successful, it could substantially reduce FCA cases.
Criminal false claims
Dubin overbilled Medicaid for psychological testing by saying the testing was done by a licensed psychologist rather than an assistant. The government claimed the “identity theft” was using the patient’s (actual) Medicaid number in submitting the bill.12 The Court unanimously held the overbilling was not aggravated identity theft as defined in federal law. Dubin could be convicted of fraudulent billing but not aggravated identity theft, thereby avoiding the mandatory prison term.
Patents of “genus” targets
The case: Amgen v Sanofi
Background. “Genus” patents allow a single pharmaceutical company to patent every antibody that binds to a specific amino acid on a naturally occurring protein. In this case, the patent office had granted a “genus” patent on “all antibodies” that bind to the naturally occurring protein PCSK9 and block it from hindering the body’s mechanism for removing low-density lipoprotein (LDL) cholesterol from the bloodstream,13 helping to reduce LDL cholesterol levels. These patents could involve millions of antibodies—and Amgen was claiming a patent on all of them. Amgen and Sanofi marketed their products, each with their own unique amino acid sequence.13 Amgen sued Sanofi for violating its patent rights.
Decision. The Court unanimously held that Amgen did not have a valid patent on all antibodies targeting PCSK9, only those that it had explicitly described in its patent application—a ruling based on a 150-year-old technical requirement for receiving a patent. An applicant for a patent must include “a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art…to make and use the same.”13 Amgen’s patent provided the description for only a few of the antibodies, but from the description in its application others could not “make and use” all of the antibodies targeting PCSK9.
While the decision was vital for future pharmaceuticals, the patent principle on which it was based has an interesting history. The Court noted that it affected the telegraph (Morse lost part of his patent), electric lights (Edison won his case against other inventors), and the glue for wood veneering (Perkins Glue Company lost).13
Continue to: Other notable decisions...
Other notable decisions
Student loans
The Court struck down the Biden Administration’s student loan forgiveness program, which would have cost approximately $430 billion.14 The central issue was whether the administration had the authority for such massive loan forgiveness; that is, whether Congress had authorized the broad loan forgiveness. The administration claimed authority from the post ̶ 9/11 HEROES Act, which allows the Secretary of Education to “waive or modify” loan provisions during national emergencies.
Free speech and the wedding web designer
303 Creative v Elenis involved a creative website designer who did not want to be required to create a website for a gay wedding.15 The designer had strong beliefs against same-sex marriages, but Colorado sought to force her to do so under the state “public accommodations” law. In a 6-3 decision, the Court held that the designer had a “free speech” right. That is, the state could not compel her to undertake speech expressing things she did not believe. This was because the website design was an expressive, creative activity and therefore was “speech” under the First Amendment.
Wetlands and the Clean Water Act
The essential issue in Sackett v Environmental Protection Agency (EPA) was the definitions of waters of the United States and related wetlands. The broad definition the EPA used meant it had jurisdiction to regulate an extraordinary amount of territory. It had, for example, prevented the Sacketts from building a modest house claiming it was part of the “waters of the United States because they were near a ditch that fed into a creek, which fed into Priest Lake, a navigable, intrastate lake.” The Court held that the EPA exceeded its statutory authority to define “wetlands.”16
The Court held that under the Clean Water Act, for the EPA to establish jurisdiction over adjacent wetlands, it must demonstrate that16:
1. “the adjacent body of water constitutes waters of the United States (ie, a relatively permanent body of water connected to traditional interstate navigable waters)…”
2. “…the wetland has a continuous surface connection with that water, making it difficult to determine where the water ends, and the wetland begins.”
Under this definition, the Sacketts could build their house. This was a statutory interpretation case. Therefore, Congress can expand or otherwise change the EPA’s authority under the Clean Water Act and other legislation.
Conclusions: A new justice, “shadow docket,” and ethics rules
SCOTUS’ newest member. When the Marshall called the Court into session on October 3, 2022, it had a new member, Justice Ketanji Brown Jackson. She was sworn in on June 30, 2022, when her predecessor (Justice Breyer) officially retired. She had been a law clerk for Justice Breyer in 1999, as well as a district court judge and court of appeals judge. Those who count such things described her as the “chattiest justice.”17 She spoke more than any other justice—by one count, a total of 75,632 words (an average of 1,300 words in each of the 58 arguments).
A more balanced Court? Most commentators view the Court as more balanced or less conservative than the previous Term. For example, Justice Sotomayor was in the majority 40% last Term but 65% this Term. Justice Thomas was in the majority 75% last Term but 55% this Term. Put another way, this Term in the divided cases, the liberal justices were in the majority 64% of the time, compared with the conservative justices 73%.18 Of course, these differences may reflect a different set of cases rather than a change in the direction of the Court. There were 11 (or 12, depending on how 1 case is counted) 6-3 cases, but only 5 were considered ideological. That suggests that, in many cases, the coalitions were somewhat fluid.
“Shadow docket” controversy continues.19 Shadow docket refers to orders the Court makes that do not follow oral arguments and often do not have written opinions. The orders are all publicly available. This Term a close examination of the approximately 30 shadow docket opinions shows that the overwhelming majority were dissents or explanations about denials of certiorari. The Court ordered only a few stays or injunctions via the shadow docket. One shadow docket stay (that prevented a lower court order from going into effect) is particularly noteworthy. A federal judge had ordered the suspension of the distribution of mifepristone while courts considered claims that the US Food and Drug Administration (FDA) had improperly approved the drug. In a shadow docket order, the Court issued the stay to allow mifepristone to be sold while the case challenging its approval was heard.20 The only opinion was a dissent from Justice Alito. But it also demonstrates the importance of the shadow docket. Without this intervention, in at least part of the country, the distribution of mifepristone would have been interrupted pending the outcome of the FDA cases.
In August, the Court delayed a settlement in the Purdue Pharma liability bankruptcy case.21 It also stayed an injunction of a lower court, thereby permitting federal “ghost guns” regulations to go into effect at least temporarily.22
More ethics rules to come? Another area in which the Court faced criticism was formal ethics rules. The justices make financial disclosures, but these are somewhat ambiguous. There is likely to be increasing pressure for a more complete disclosure of non-financial relationships and more formal ethics rules. ●
The Court had, by September 1, 2023, accepted 22 cases for hearing next Term.1 The cases include a challenge to the extraordinary funding provision for the Consumer Financial Protection Bureau, another racial challenge to congressional districts (South Carolina), the status of Americans with Disability Act “testers” who look for violations without ever intending to use the facilities, the level of deference courts should give to interpreting federal statutes (so-called “Chevron” deference), the opioid (OxyContin ) bankruptcy, and limitations on gun ownership. This represents less than half of the cases the Court will likely hear next Term, so the Court will add many more cases to the docket. It promises to be an appealing Term.
Reference
1. October Term 2023. SCOTUSblog website. Accessed August 29, 2023. https://www.scotusblog.com/case-files/terms/ot2023/
When the Court adjourned on June 30, 2023, it had considered 60 cases, plus hundreds of petitions asking it to hear cases. Most commentators count 55 cases decided after briefing and oral argument and where there was a signed opinion. The information below uses 55 cases unless otherwise noted. During the 2022-2023 Term, the Court:
- upheld liability for the involuntary administration of psychotropic drugs in nursing home1
- permitted disabled students, in some instances, both to make Individuals with Disabilities Education Act (IDEA) claims for services and to file Americans with Disabilities Act (ADA) lawsuits against their schools2
- upheld a statute that makes it illegal to “encourage or induce an alien to come to, enter, or reside in the United States, knowing or in reckless disregard of the fact that such coming to, entry, or residence is or will be in violation of law.” The defendant had used a scam promising noncitizens “adult adoptions” (of which there is no such thing) making it legal for them to come to and stay in the United States.3
- narrowed the “fair use” of copyrighted works. It held that Andy Warhol’s use of a copyrighted photograph in his famous Prince prints was not “transformative” in a legal sense largely because the photo and prints “share the same use”—magazine illustrations.4
- in another intellectual property case, held that Jack Daniel’s might sue a dog toy maker for a rubber dog toy that looked like a Jack Daniel’s bottle5
- further expanded the Federal Arbitration Act by holding that a federal district court must immediately stay court proceedings if one party is appealing a decision not to require arbitration6
- held that two social media companies were not responsible for terrorists using their platforms to recruit others to their cause. It did not, however, decide whether §230 of the Communication Decency Act protects companies from liability.7
- made it easier for employees to receive accommodation for their religious practices and beliefs. Employers must make religious accommodations unless the employer can show that “the burden of granting an accommodation would result in substantial increased [financial and other] costs in relation to the conduct of its particular business.”8
- declined to hear an appeal from Johnson & Johnson (through a subsidiary, Ethicon) about pelvic mesh. In this case, the California Attorney General filed a lawsuit against Ethicon for false advertising by failing to detail the risks of pelvic mesh. The lower courts estimated 240,000 written violations of the law by Ethicon between 2008 and 2017. The trial and appeal to California courts resulted in a judgment of $302 million against Johnson & Johnson. The company asked the Court to review that judgment, but the Court denied certiorari. That likely means the $302 million is final.
References
1. Health and Hospital Corporation of Marion Cty. v Talevski, Docket no. 21-806; June 8, 2023.
2. Luna Perez v Sturgis Public Schools, Docket no. 21-887; March 21, 2023.
3. United States v Hansen, Docket no. 22-179; June 23, 2023.
4. Andy Warhol Foundation for Visual Arts, Inc. v Goldsmith, Docket no. 21-869; May 18, 2023.
5. Jack Daniel’s Properties, Inc. v VIP Products LLC, Docket no. 22-148; June 8, 2023.
6. Coinbase, Inc. v Bielski, Docket no. 22-105; June 23, 2023.
7. Gonzalez v Google LLC, Docket no. 21-1333; May 18, 2023.
8. Groff v DeJoy, Docket no. 22-273; June 29, 2023.

The 2022-2023 Term of the Supreme Court illustrates how important the Court has become to health-related matters, including decisions regarding the selection and training of new professionals, the daily practice of medicine, and the future availability of new drugs. The importance of several cases is reinforced by the fact that major medical organizations filed amicus curiae (“friend of the court”) briefs in those cases.
Amicus briefs are filed by individuals or organizations with something significant to say about a case to the court—most often to present a point of view, make an argument, or provide information that the parties to the case may not have communicated. Amicus briefs are burdensome in terms of the time, energy, and cost of preparing and filing. Thus, they are not undertaken lightly. Medical organizations submitted amicus briefs in the first 3 cases we consider.
Admissions, race, and diversity
The case: Students for Fair Admissions v President and Fellows of Harvard College
The American College of Obstetricians and Gynecologists (ACOG) joined an amici curiae brief in Students for Fair Admissions v President and Fellows of Harvard College (and the University of North Carolina [UNC]).1 This case challenged the use of racial preferences in college admissions. The Association of American Medical Colleges (AAMC) was the lead organization; nearly 40 other health-related organizations joined the brief.
The legal claim. Those filing the suits asserted that racial preferences by public colleges violate the 14th Amendment’s Equal Protection Clause (“no state shall deny to any person … the equal protection of the law”). That is, if a state university gives racial preferences in selective admissions, it denies some other applicant the equal protection of the law. As for private schools (in this case, Harvard), Title VI of the Civil Rights Act of 1964 has the same standards as the Equal Protection Clause. Thus, the Court consolidated the cases and used the same legal standard in considering public and private colleges (with “colleges” including professional and graduate programs as well as undergraduate institutions).
Background. For nearly 50 years, the Supreme Court has allowed limited racial preferences in college admissions. Those preferences could only operate as a plus, however, and not a negative for applicants and be narrowly tailored. The measure was instituted temporarily; in a 2003 case, the Court said, “We expect that 25 years from now, the use of racial preferences will no longer be necessary.”2
Decision. In a 6-3 decision, the Court held (in the UNC case) that racial preferences generally violate the Constitution, and by a 6-2 decision (in the Harvard case) these preferences violate the Civil Rights Act of 1964. (Justice Jackson was recused in the Harvard case because of a conflict.) The opinion covered 237 pages in the US Reports, so any summary is incomplete.
The majority concluded, “The Harvard and UNC admissions programs cannot be reconciled with the guarantees of the Equal Protection Clause. Both programs lack sufficiently focused and measurable objectives warranting the use of race, unavoidably employ race in a negative manner, involve racial stereotyping, and lack meaningful end points. We have never permitted admissions programs to work in that way, and we will not do so today.”3
There were 3 concurring opinions and 2 dissents in the case. The concurrences reviewed the history of the Equal Protection Clause and the Civil Rights Act, the damage racial preferences can do, and the explicit limits the Court said there must be on racial preferences in higher education. The dissents had a different view of the legal history of the 14th Amendment. They said the majority was turning a blind eye to segregation in society and the race-based gap in America.
As a practical matter, this case means that colleges, including professional schools, cannot use racial preferences. The Court said that universities may consider essays and the like in which applicants describe how their own experiences as an individual (including race) have affected their own lives. However, the Court cautioned that “universities may not simply establish through application essays or other means the regime we hold unlawful today.”3
Continue to: The amici brief...
The amici brief
ACOG joined 40 other health-related organizations in filing an amici brief (multiple “friends”) in Students for Fair Admission. The AAMC led the brief, with the others signing as amici.4 The brief made 3 essential points: diversity in medical education “markedly improves health outcomes,” and a loss of diversity “threaten[s] patients’ health; medical schools engage in an intense “holistic” review of applicants for admission; and medical schools must consider applicants’ “full background” (including race) to achieve their educational and professional goals.4
A powerful part of the brief described the medical school admissions process, particularly the very “holistic” review that is not entirely dependent on admissions scores. The brief effectively weaves the consideration of race into this process, mentioning race (on page 22) only after discussing many other admissions factors.
Child custody decisions related to the Indian Child Welfare Act
The case: Haaland v Brackeen
The American Medical Association (AMA) and the American Academy of Pediatrics filed a brief in Haaland v Brackeen5 involving the constitutionality of the 1978 Indian Child Welfare Act (ICWA). The statute followed a terrible history of Indian children being removed from their families inappropriately, as detailed in a concurring opinion by Justice Gorsuch.5 The two purposes of the act were to promote raising Native American children in their culture and stem the downward trend in tribal membership.
The legal claim. The Court consolidated several cases. Essentially, a 10-month-old child (A.L.M.) was placed in foster care with the Brackeens in Texas. After more than 1 year, the Brackeens sought adoption; the biological father, mother, and grandparents all supported it. The Navajo and Cherokee Nations objected and informed the Texas court that they had found alternative placement with (nonrelative) tribal members in New Mexico. The “court-appointed guardian and a psychological expert … described the strong emotional bond between A.L.M. and his foster parents.” The court denied the adoption petition based on ICWA’s preference for tribe custody, and the Brackeens filed a lawsuit.
Decision. The constitutional claim in the case was that Congress lacked the authority to impose these substantial rules on states in making child custody decisions. The Supreme Court, in a 7-2 decision, upheld the constitutionality of the ICWA.
The amici brief
The joint amici brief of the American Academy of Pediatrics (AAP) and the AMA argued that tribes are “extended families” of Native American children.6 It noted the destructive history of removing Native American children from their families and suggested that kinship care improves children’s health. To its credit, the brief also honestly noted the serious mental health and suicide rates in some tribes, which suggest issues that might arise in child custody and adoption cases.
The Court did not, in this case, take up another constitutional issue that the parties raised—whether the strong preference for Native American over non ̶ Native American custody violates the Equal Protection Clause of the 14th Amendment. The Court said the parties to this case did not have standing to raise the issue. Justice Kavanaugh, concurring, said it was a “serious” issue and invited it to be raised in another case.5
False Claims Act cases
The case: Costs for SuperValu prescriptions
The legal claim. One FCA case this Term involved billings SuperValu made for outpatient prescriptions in Medicare-Medicaid programs. As its “usual and customary” costs, it essentially reported a list price that did not include the substantial discounts it commonly gave.
Background. Subjective knowledge (what the defendant actually knows) may seem impossible to prove—the defendant could just say, “I did not know I was doing wrong.” Over time the law has developed several ways of demonstrating “knowing.” Justice Thomas, writing for a unanimous Court, held that whistleblowers or the government might prove “knowing” in 3 ways:
1. defendants “actually knew that their reported prices were not their ‘usual and customary’ prices when they reported them”
2. were aware of a substantial risk that their higher, retail prices were not their “usual and customary” prices and intentionally avoided learning whether their reports were accurate
3. were aware of such a substantial and unjustifiable risk but submitted the claims anyway.9
Of course, records of the company, information from the whistleblower, and circumstantial evidence may be used to prove any of these; it does not require the company’s admission.
The Court said that if the government or whistleblowers make a showing of any of these 3 things, it is enough.
Decision. The case was returned to the lower court to apply these rules.
The amici brief
The American Hospital Association and America’s Health Insurance Plans filed an amici brief.10 It reminded the Court that many reimbursement regulations are unclear. Therefore, it is inappropriate to impose FCA liability for guessing incorrectly what the regulations mean. Having to check on every possible ambiguity was unworkable. The Court declined, however, the suggestion that defendants should be able to use any one of many “objectively” reasonable interpretations of regulations.
Continue to: The case: Polansky v Executive Health Resources...
The case: Polansky v Executive Health Resources
Health care providers who dislike the FCA may find solace this Term in this second FCA case.11
The legal claim. Polansky, a physician employed by a medical billing company, became an “intervenor” in a suit claiming the company assisted hospitals in false billing (inpatient claims for outpatient services). The government sought to dismiss the case, but Polansky refused.
Decision. The case eventually reached the Supreme Court, which held that the government may enter an FCA case at any time and move to dismiss the case even over the objection of a whistleblower. The government does not seek to enter a case in order to file dismissal motions often. When it does so, whistleblowers are protected by the fact that the dismissal motion requires a hearing before the federal court.
An important part of this case has escaped much attention. Justices Thomas, Kavanaugh, and Barrett invited litigation to determine if allowing private whistleblowers to represent the government’s interest is consistent with Article II of the Constitution.11 The invitation will likely be accepted. We expect to see cases challenging the place of “intervenors” pursuing claims when the government has declined to take up the case. The private intervenor is a crucial provision of the current FCA, and if such a challenge were successful, it could substantially reduce FCA cases.
Criminal false claims
Dubin overbilled Medicaid for psychological testing by saying the testing was done by a licensed psychologist rather than an assistant. The government claimed the “identity theft” was using the patient’s (actual) Medicaid number in submitting the bill.12 The Court unanimously held the overbilling was not aggravated identity theft as defined in federal law. Dubin could be convicted of fraudulent billing but not aggravated identity theft, thereby avoiding the mandatory prison term.
Patents of “genus” targets
The case: Amgen v Sanofi
Background. “Genus” patents allow a single pharmaceutical company to patent every antibody that binds to a specific amino acid on a naturally occurring protein. In this case, the patent office had granted a “genus” patent on “all antibodies” that bind to the naturally occurring protein PCSK9 and block it from hindering the body’s mechanism for removing low-density lipoprotein (LDL) cholesterol from the bloodstream,13 helping to reduce LDL cholesterol levels. These patents could involve millions of antibodies—and Amgen was claiming a patent on all of them. Amgen and Sanofi marketed their products, each with their own unique amino acid sequence.13 Amgen sued Sanofi for violating its patent rights.
Decision. The Court unanimously held that Amgen did not have a valid patent on all antibodies targeting PCSK9, only those that it had explicitly described in its patent application—a ruling based on a 150-year-old technical requirement for receiving a patent. An applicant for a patent must include “a written description of the invention, and of the manner and process of making and using it, in such full, clear, concise, and exact terms as to enable any person skilled in the art…to make and use the same.”13 Amgen’s patent provided the description for only a few of the antibodies, but from the description in its application others could not “make and use” all of the antibodies targeting PCSK9.
While the decision was vital for future pharmaceuticals, the patent principle on which it was based has an interesting history. The Court noted that it affected the telegraph (Morse lost part of his patent), electric lights (Edison won his case against other inventors), and the glue for wood veneering (Perkins Glue Company lost).13
Continue to: Other notable decisions...
Other notable decisions
Student loans
The Court struck down the Biden Administration’s student loan forgiveness program, which would have cost approximately $430 billion.14 The central issue was whether the administration had the authority for such massive loan forgiveness; that is, whether Congress had authorized the broad loan forgiveness. The administration claimed authority from the post ̶ 9/11 HEROES Act, which allows the Secretary of Education to “waive or modify” loan provisions during national emergencies.
Free speech and the wedding web designer
303 Creative v Elenis involved a creative website designer who did not want to be required to create a website for a gay wedding.15 The designer had strong beliefs against same-sex marriages, but Colorado sought to force her to do so under the state “public accommodations” law. In a 6-3 decision, the Court held that the designer had a “free speech” right. That is, the state could not compel her to undertake speech expressing things she did not believe. This was because the website design was an expressive, creative activity and therefore was “speech” under the First Amendment.
Wetlands and the Clean Water Act
The essential issue in Sackett v Environmental Protection Agency (EPA) was the definitions of waters of the United States and related wetlands. The broad definition the EPA used meant it had jurisdiction to regulate an extraordinary amount of territory. It had, for example, prevented the Sacketts from building a modest house claiming it was part of the “waters of the United States because they were near a ditch that fed into a creek, which fed into Priest Lake, a navigable, intrastate lake.” The Court held that the EPA exceeded its statutory authority to define “wetlands.”16
The Court held that under the Clean Water Act, for the EPA to establish jurisdiction over adjacent wetlands, it must demonstrate that16:
1. “the adjacent body of water constitutes waters of the United States (ie, a relatively permanent body of water connected to traditional interstate navigable waters)…”
2. “…the wetland has a continuous surface connection with that water, making it difficult to determine where the water ends, and the wetland begins.”
Under this definition, the Sacketts could build their house. This was a statutory interpretation case. Therefore, Congress can expand or otherwise change the EPA’s authority under the Clean Water Act and other legislation.
Conclusions: A new justice, “shadow docket,” and ethics rules
SCOTUS’ newest member. When the Marshall called the Court into session on October 3, 2022, it had a new member, Justice Ketanji Brown Jackson. She was sworn in on June 30, 2022, when her predecessor (Justice Breyer) officially retired. She had been a law clerk for Justice Breyer in 1999, as well as a district court judge and court of appeals judge. Those who count such things described her as the “chattiest justice.”17 She spoke more than any other justice—by one count, a total of 75,632 words (an average of 1,300 words in each of the 58 arguments).
A more balanced Court? Most commentators view the Court as more balanced or less conservative than the previous Term. For example, Justice Sotomayor was in the majority 40% last Term but 65% this Term. Justice Thomas was in the majority 75% last Term but 55% this Term. Put another way, this Term in the divided cases, the liberal justices were in the majority 64% of the time, compared with the conservative justices 73%.18 Of course, these differences may reflect a different set of cases rather than a change in the direction of the Court. There were 11 (or 12, depending on how 1 case is counted) 6-3 cases, but only 5 were considered ideological. That suggests that, in many cases, the coalitions were somewhat fluid.
“Shadow docket” controversy continues.19 Shadow docket refers to orders the Court makes that do not follow oral arguments and often do not have written opinions. The orders are all publicly available. This Term a close examination of the approximately 30 shadow docket opinions shows that the overwhelming majority were dissents or explanations about denials of certiorari. The Court ordered only a few stays or injunctions via the shadow docket. One shadow docket stay (that prevented a lower court order from going into effect) is particularly noteworthy. A federal judge had ordered the suspension of the distribution of mifepristone while courts considered claims that the US Food and Drug Administration (FDA) had improperly approved the drug. In a shadow docket order, the Court issued the stay to allow mifepristone to be sold while the case challenging its approval was heard.20 The only opinion was a dissent from Justice Alito. But it also demonstrates the importance of the shadow docket. Without this intervention, in at least part of the country, the distribution of mifepristone would have been interrupted pending the outcome of the FDA cases.
In August, the Court delayed a settlement in the Purdue Pharma liability bankruptcy case.21 It also stayed an injunction of a lower court, thereby permitting federal “ghost guns” regulations to go into effect at least temporarily.22
More ethics rules to come? Another area in which the Court faced criticism was formal ethics rules. The justices make financial disclosures, but these are somewhat ambiguous. There is likely to be increasing pressure for a more complete disclosure of non-financial relationships and more formal ethics rules. ●
The Court had, by September 1, 2023, accepted 22 cases for hearing next Term.1 The cases include a challenge to the extraordinary funding provision for the Consumer Financial Protection Bureau, another racial challenge to congressional districts (South Carolina), the status of Americans with Disability Act “testers” who look for violations without ever intending to use the facilities, the level of deference courts should give to interpreting federal statutes (so-called “Chevron” deference), the opioid (OxyContin ) bankruptcy, and limitations on gun ownership. This represents less than half of the cases the Court will likely hear next Term, so the Court will add many more cases to the docket. It promises to be an appealing Term.
Reference
1. October Term 2023. SCOTUSblog website. Accessed August 29, 2023. https://www.scotusblog.com/case-files/terms/ot2023/
When the Court adjourned on June 30, 2023, it had considered 60 cases, plus hundreds of petitions asking it to hear cases. Most commentators count 55 cases decided after briefing and oral argument and where there was a signed opinion. The information below uses 55 cases unless otherwise noted. During the 2022-2023 Term, the Court:
- upheld liability for the involuntary administration of psychotropic drugs in nursing home1
- permitted disabled students, in some instances, both to make Individuals with Disabilities Education Act (IDEA) claims for services and to file Americans with Disabilities Act (ADA) lawsuits against their schools2
- upheld a statute that makes it illegal to “encourage or induce an alien to come to, enter, or reside in the United States, knowing or in reckless disregard of the fact that such coming to, entry, or residence is or will be in violation of law.” The defendant had used a scam promising noncitizens “adult adoptions” (of which there is no such thing) making it legal for them to come to and stay in the United States.3
- narrowed the “fair use” of copyrighted works. It held that Andy Warhol’s use of a copyrighted photograph in his famous Prince prints was not “transformative” in a legal sense largely because the photo and prints “share the same use”—magazine illustrations.4
- in another intellectual property case, held that Jack Daniel’s might sue a dog toy maker for a rubber dog toy that looked like a Jack Daniel’s bottle5
- further expanded the Federal Arbitration Act by holding that a federal district court must immediately stay court proceedings if one party is appealing a decision not to require arbitration6
- held that two social media companies were not responsible for terrorists using their platforms to recruit others to their cause. It did not, however, decide whether §230 of the Communication Decency Act protects companies from liability.7
- made it easier for employees to receive accommodation for their religious practices and beliefs. Employers must make religious accommodations unless the employer can show that “the burden of granting an accommodation would result in substantial increased [financial and other] costs in relation to the conduct of its particular business.”8
- declined to hear an appeal from Johnson & Johnson (through a subsidiary, Ethicon) about pelvic mesh. In this case, the California Attorney General filed a lawsuit against Ethicon for false advertising by failing to detail the risks of pelvic mesh. The lower courts estimated 240,000 written violations of the law by Ethicon between 2008 and 2017. The trial and appeal to California courts resulted in a judgment of $302 million against Johnson & Johnson. The company asked the Court to review that judgment, but the Court denied certiorari. That likely means the $302 million is final.
References
1. Health and Hospital Corporation of Marion Cty. v Talevski, Docket no. 21-806; June 8, 2023.
2. Luna Perez v Sturgis Public Schools, Docket no. 21-887; March 21, 2023.
3. United States v Hansen, Docket no. 22-179; June 23, 2023.
4. Andy Warhol Foundation for Visual Arts, Inc. v Goldsmith, Docket no. 21-869; May 18, 2023.
5. Jack Daniel’s Properties, Inc. v VIP Products LLC, Docket no. 22-148; June 8, 2023.
6. Coinbase, Inc. v Bielski, Docket no. 22-105; June 23, 2023.
7. Gonzalez v Google LLC, Docket no. 21-1333; May 18, 2023.
8. Groff v DeJoy, Docket no. 22-273; June 29, 2023.
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306, 326 (2003).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___, 39 (2023).
- Brief for Amici Curiae Association of American Medical Colleges et al. in Support of Respondents, Students for Fair Admissions v University of North Carolina (July 28, 2022). Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-707/232120/20220728171307159_20 -1199%20and%2021-707%20Amicus%20Brief%20for%20 Association%20of%20American%20Medical%20Colleges%20 et%20al.pdf
- Haaland v Brackeen, Docket no. 21-376; June 15, 2023.
- Brief of American Academy of Pediatrics and American Medical Association as Amici Curiae in Support of Respondents, in Haaland v Brackeen. August 19, 2022. Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-376/234042/20220819140750948_21-376 .amics.brief.FINAL.pdf
- Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021. US Department of Justice website. February 1, 2022. Accessed August 18, 2023. https://www.justice.gov/opa/pr/justice -department-s-false-claims-act-settlements-and-judgments -exceed-56-billion-fiscal-year
- False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022. US Department of Justice website. February 7, 2023. Accessed August 18, 2023. https://www .justice.gov/opa/pr/false-claims-act-settlements-and -judgments-exceed-2-billion-fiscal-year-2022
- United States ex rel. Schutte v Supervalu Inc., Docket no. 21-1326; June 1, 2023.
- Brief of American Hospital Association and America’s Health Insurance Plans as Amici Curiae in Support of Respondents, in Schutte v Supervalu. March 2023. Accessed August 18, 2023. https://www.supremecourt.gov/DocketPDF/21/21-1326 /262428/20230331113854936_3-31-23%20AHA_AHIP _Amicus_Brief.pdf
- United States ex rel. Polansky v Executive Health Resources, Inc., Docket no. 21-1052; June 16, 2023.
- Dubin v United States, Docket no. 22-10; June 8, 2023.
- Amgen v Sanofi, 598 US ___ (2023).
- Biden v Nebraska, 600 US ___ (2023).
- 303 Creative LLC v Elenis, 600 US ___ (2023).
- Sackett v Environmental Protection Agency, Docket no. 21454; May 25, 2023.
- Krochtengel J. Jackson debuts as chattiest Justice. Law360. July 3, 2023. https://www.law360.com/articles/1692839 /jackson-debuts-as-chattiest-justice
- Feldman A. Another One Bites the Dust: End of 2022/2023 Supreme Court Term Statistics. EmpiricalScotus website. 30, 2023. Accessed August 18, 2023. https://empiricalscotus .com/2023/06/30/another-one-bites-2022/
- Vladeck S. The Shadow Docket: How the Supreme Court Uses Stealth Rulings to Amass Power and Undermine the Republic. New York, New York; Basic Books; 2023.
- Danco Laboratories, LLC v Alliance for Hippocratic Medicine. Docket no. 22A902; April 21, 2023.
- Harrington v Purdue Pharma, 23-124 (23A87).
- Garland v Vanderstok, 23-10718 (August 8, 2023).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306, 326 (2003).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___, 39 (2023).
- Brief for Amici Curiae Association of American Medical Colleges et al. in Support of Respondents, Students for Fair Admissions v University of North Carolina (July 28, 2022). Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-707/232120/20220728171307159_20 -1199%20and%2021-707%20Amicus%20Brief%20for%20 Association%20of%20American%20Medical%20Colleges%20 et%20al.pdf
- Haaland v Brackeen, Docket no. 21-376; June 15, 2023.
- Brief of American Academy of Pediatrics and American Medical Association as Amici Curiae in Support of Respondents, in Haaland v Brackeen. August 19, 2022. Accessed August 18, 2023. https://www.supremecourt.gov /DocketPDF/21/21-376/234042/20220819140750948_21-376 .amics.brief.FINAL.pdf
- Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021. US Department of Justice website. February 1, 2022. Accessed August 18, 2023. https://www.justice.gov/opa/pr/justice -department-s-false-claims-act-settlements-and-judgments -exceed-56-billion-fiscal-year
- False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022. US Department of Justice website. February 7, 2023. Accessed August 18, 2023. https://www .justice.gov/opa/pr/false-claims-act-settlements-and -judgments-exceed-2-billion-fiscal-year-2022
- United States ex rel. Schutte v Supervalu Inc., Docket no. 21-1326; June 1, 2023.
- Brief of American Hospital Association and America’s Health Insurance Plans as Amici Curiae in Support of Respondents, in Schutte v Supervalu. March 2023. Accessed August 18, 2023. https://www.supremecourt.gov/DocketPDF/21/21-1326 /262428/20230331113854936_3-31-23%20AHA_AHIP _Amicus_Brief.pdf
- United States ex rel. Polansky v Executive Health Resources, Inc., Docket no. 21-1052; June 16, 2023.
- Dubin v United States, Docket no. 22-10; June 8, 2023.
- Amgen v Sanofi, 598 US ___ (2023).
- Biden v Nebraska, 600 US ___ (2023).
- 303 Creative LLC v Elenis, 600 US ___ (2023).
- Sackett v Environmental Protection Agency, Docket no. 21454; May 25, 2023.
- Krochtengel J. Jackson debuts as chattiest Justice. Law360. July 3, 2023. https://www.law360.com/articles/1692839 /jackson-debuts-as-chattiest-justice
- Feldman A. Another One Bites the Dust: End of 2022/2023 Supreme Court Term Statistics. EmpiricalScotus website. 30, 2023. Accessed August 18, 2023. https://empiricalscotus .com/2023/06/30/another-one-bites-2022/
- Vladeck S. The Shadow Docket: How the Supreme Court Uses Stealth Rulings to Amass Power and Undermine the Republic. New York, New York; Basic Books; 2023.
- Danco Laboratories, LLC v Alliance for Hippocratic Medicine. Docket no. 22A902; April 21, 2023.
- Harrington v Purdue Pharma, 23-124 (23A87).
- Garland v Vanderstok, 23-10718 (August 8, 2023).
Telemedicine: Medicolegal aspects in ObGyn

Telemedicine (or telehealth) originated in the early 1900s, when radios were used to communicate medical advice to clinics aboard ships.1 According to the American Telemedicine Association, telemedicine is namely “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status.”2 These communications use 2-way video, email, smartphones, wireless tools, and other forms of telecommunications technology.
During the COVID-19 pandemic, many ObGyns—encouraged and advised by professional organizations—began providing telemedicine services.3 The first reported case of COVID-19 was in late 2019; the use of telemedicine was 38 times higher in February 2021 than in February 2020,4 illustrating how many physicians quickly moved to telemedicine practices.
CASE Dr. TM’s telemedicine dream
Before COVID-19, Dr. TM (an ObGyn practi-tioner) practiced in-person medicine in his home state. With the onset of the pandemic, Dr. TM struggled to switch to primarily seeing patients online (generally using Zoom or Facebook Live), with 1 day per week in the office for essential in-person visits.
After several months, however, Dr. TM’s routine became very efficient. He could see many more patients in a shorter time than with the former, in-person system. Therefore, as staff left his practice, Dr. TM did not replace them and also laid off others. Ultimately, the practice had 1 full-time records/insurance secretary who worked from home and 1 part-time nurse who helped with the in-person day and answered some patient inquiries by email. In part as an effort to add new patients, Dr. TM built an engaging website through which his current patients could receive medical information and new patients could sign up.
In late 2022, Dr. TM offered a $100 credit to any current patient who referred a friend or family member who then became a patient. This promotion was surprisingly effective and resulted in an influx of new patients. For example, Patient Z (a long-time patient) received 3 credits for referring her 3 sisters who lived out of state and became telepatients: Patient D, who lived 200 hundred miles away; Patient E, who lived 50 miles away in the adjoining state; and Patient F, who lived 150 miles away. Patient D contacted Dr. TM because she thought she was pregnant and wanted prenatal care, Patient E thought she might have a sexually transmitted infection (STI) and wanted treatment, and Patient F wanted general care and was inquiring about a medical abortion. Dr. TM agreed to treat Patient D but required 1 in-person visit. After 1 brief telemedicine session each with Patients E and F, Dr. TM wrote prescriptions for them.
By 2023, Dr. TM was enthusiastic about telemedicine as a professional practice. However, problems would ensue.
Dos and don’ts of telemedicine2
- Do take the initiative and inform patients of the availability of telemedicine/telehealth services
- Do use the services of medical malpractice insurance companies with regard to telemedicine
- Do integrate telemedicine into practice protocols and account for their limitations
- Don’t assume there are blanket exemptions or waivers in the states where your patients are located
Medical considerations
Telemedicine is endorsed by the American College of Obstetricians and Gynecologists (ACOG) as a vehicle for delivering prenatal and postpartum care.5 This represents an effort to reduce maternal and neonatal morbidity and mortality,5 as well as expandaccess to care and address the deficit in primary care providers and services, especially in rural and underserved populations.5,6 For obstetrics, prenatal care is designed to optimize pregnancy, childbirth, and postpartum care, with a focus on nutrition and genetic consultation and patient education on pregnancy, childbearing, breastfeeding, and newborn care.7
Benefits of telemedicine include its convenience for patients and providers, its efficiency and lower costs for providers (and hopefully patients, as well), and the potential improved access to care for patients.8 It is estimated that if a woman inititates obstetric care at 6 weeks, over the course of the 40-week gestation period, 15 prenatal visits will occur.9 Ultimately, the number of visits is determined based on the specifics of the pregnancy. With telemedicine, clinicians can provide those consultations, and information related to: ultrasonography, fetal echocardiography, and postpartum care services remotely.10 Using telemedicine may reduce missed visits, and remote monitoring may improve the quality of care.11
Barriers to telemedicine care include technical limitations, time constraints, and patient concerns of telehealth (visits). Technical limitations include the lack of a high speed internet connection and/or a smart device and the initial technical set-up–related problems,12 which affect providers as well as patients. Time constraints primarly refer to the ObGyn practice’s lack of time to establish telehealth services.13 Other challenges include integrating translation services, billing-related problems,10 and reimbursement and licensing barriers.14
Before the COVID-19 pandemic, obstetrics led the way in telemedicine with the development of the OB Nest model. Designed to replace in-person obstetrics care visits with telehealth,15 it includes home management tools such as blood pressure cuffs, cardiotocography, scales for weight checks, and Doppler ultrasounds.10 Patients can be instructed to measure fundal height and receive medications by mail. Anesthesia consultation can occur via this venue by having the patient complete a questionnaire prior to arriving at the labor and delivery unit.16
Legal considerations
With the COVID-19 pandemic, temporary changes were made to encourage the rapid adoption of telemedicine, including changes to licensing laws, certain prescription requirements, Health Insurance Portability and Accountability Act (HIPAA) privacy-security regulations, and reimbursement rules that required in-person visits. Thus, many ObGyns started using telemedicine during this rarified period, in which the rules appeared to be few and far between, with limited enforcement of the law and professional obligations.17 However, now that many of the legal rules that were suspended or ignored have been (or are being) reimposed and enforced, it is important for providers to become familiar with the legal issues involved in practicing telemedicine.
First, where is the patient? When discussing the legal issues of telemedicine, it is important to remember that many legal rules for medical care (ie, liability, informed consent, and licensing) vary from state to state. If the patient resides in a different state (“foreign” state) from the physician’s practice location (the physician’s “home” state), the care is considered delivered in the state where the patient is located. Thus, the patient’s location generally establishes the law covering the telemedicine transaction. In the following discussion, the rules refer to the law and professional obligations, with commentary on some key legal issues that are relevant to ObGyn telemedicine.
Continue to: Reinforcing the rules...
Reinforcing the rules
Licensing
During the height of the COVID-19 pandemic, the federal government and almost all states temporarily modified the licensing requirement to allow telemedicine based on an existing medical license in any state—disregarding the “where is the patient” rule. As those rules begin to lapse or change with the official end of the pandemic declared by President Biden as May 2023,17 the rules under which a physician began telemedicine interstate practice in 2020 also may be changing.
Simply put, “The same standards for licensure apply to health care providers regardless of whether care is delivered in-person or virtually through telehealth services.”18 When a physician is engaged in telemedicine treatment of a patient in the physician’s home state, there is generally no licensing issue. Telemedicine generally does not require a separate specific license.19 However, when the patient is in another state (a “foreign” state), there can be a substantial licensing issue.20 Ordinarily, to provide that treatment, the physician must, in some manner, be approved to practice in the patient’s state. That may occur, for example, in the following ways: (1) the physician may hold an additional regular license in the patient’s state, which allows practice there, or (2) the physician may have received permission for “temporary practice” in another state.
Many states (often adjoining states) have formal agreements with other states that allow telemedicine practice by providers in each other’s states. There also are “compacts”, or agreements that enable providers in any of the participating states to practice in the other associated states without a separate license.18 Although several websites provide information about compact licensing and the like, clinicians should not rely on simple lists or maps. Individual states may have special provisions about applying their laws to out-of-state “compact” physicians. In addition, under the Interstate Medical Licensure Compact, “physicians have to pay licensing fees and satisfy the requirements of each medical board in the states where they wish to practice.”21
Consequences. Practicing telemedicine with a patient in a state where the physician does not have a license is generally a crime. Furthermore, it may be the basis for license discipline in the physician’s home state and result in a report to the National Practi-tioner Databank.22 In addition, reimbursement often depends on the practitioner being licensed, and the absence of a license may be a basis for denying payment for services.23 Finally, malpractice insurance generally is limited to licensed practice. Thus, the insurer may decline to defend the unlicensed clinician against a malpractice claim or pay any damages.
Prescribing privileges
Prescribing privileges usually are connected to licensing, so as the rules for licensing change postpandemic, so do the rules for prescribing. In most cases, the physician must have a license in the state where care is given to prescribe medication—which in telemedicine, as noted, typically means the state where the patient is located. Exceptions vary by state, but in general, if a physician does not have a license to provide care, the physician is unlikely to be authorized to prescribe medication.24 Failure to abide by the applicable state rules may result in civil and even criminal liability for illegal prescribing activity.
In addition, the US Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA, which enforces laws concerning controlled substances) also regulate the prescription and sale of pharmaceuticals.25 There are state and federal limits on the ability of clinicians to order controlled substances without an in-person visit. The Ryan Haight Online Pharmacy Consumer Protection Act, for example, sets limits on controlled substance prescriptions without an in-person examination.26 Federal law was modified due to COVID-19 to permit prescribing of many controlled substances by telemedicine if there is synchronous audio and visual examination of the patient. Physicians who write such prescriptions also are required to have a DEA registration in the patient’s state. This is an essential consideration for physicians considering interstate telemedicine practice.27
HIPAA and privacy
Governments waived some of the legal requirements related to health information during the pandemic, but those waivers either have expired or will do so soon. Federal and state laws regarding privacy and security—notably including HIPAA—apply to telemedicine and are of particular concern given the considerable amount of communication of protected health information with telemedicine.
HIPAA security rules essentially require making sure health information cannot be hacked or intercepted. Audio-only telemedicine by landline (not cell) is acceptable under the security rules, but almost all other remote communication requires secure communications.28
Clinicians also need to adhere to the more usual HIPAA privacy rules when practicingtelehealth. State laws protecting patient privacy vary and may be more stringent than HIPAA, so clinicians also must know the requirements in any state where they practice—whether in office or telemedicine.29
Making sure telemedicine practices are consistent with these security and privacy rules often requires particular technical expertise that is outside the realm of most practicing clinicians. However, without modification, the pre-telemedicine technology of many medical offices likely is insufficient for the full range of telemedicine services.30
Reimbursement and fraud
Before COVID-19, Medicare and Medicaid reimbursement for telemedicine was limited. Government decisions to substantially broaden those reimbursement rules (at least temporarily) provided a substantial boost to telemedicine early in the pandemic.23 Federal regulations and statutes also expanded telemedicine reimbursement for various services. Some will end shortly after the health emergency, and others will be permanent. Parts of that will not be sorted out for several years, so it will likely be a changing landscape for reimbursement.
Continue to: Rules that are evolving...
Rules that are evolving
Informed consent
The ethical and legal obligations to obtain informed consent are present in telemedicineas well as in-person care, with the same basic requirements regarding risks, benefits, alternative care, etc.32 However, with telemedicine, information related to remote care should be included and is outlined in TABLE 1.
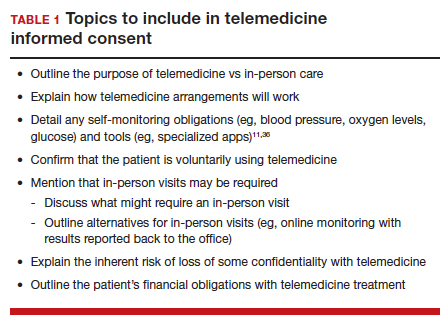
Certain states may have somewhat unique informed consent requirements—especially for reproductive care, including abortion.34 Therefore, it is important for clinicians to ensure their consent process and forms comply with any legal jurisdiction in which a patient is located.
Medical malpractice
The basics of medical malpractice (or negligence) are the same in telemedicine as in in-person care: duty, breach of duty, and injury caused by the breach. That is, there may be liability when a medical professional breaches the duty of care, causing the patient’s injury. The physician’s duty is defined by the quality of care that the profession (specialty) accepts as reasonably good. This is defined by the opinions of physicians within the specialty and formal statements from professional organizations, including ACOG.3
Maintaining the standard of care and quality. The use of telemedicine is not an excuse to lower the quality of health care. There are some circumstances for which it is medically better to have an in-person visit. In these instances, the provider should recommend the appropriate care, even if telemedicine would be more convenient for the provider and staff.35
If the patient insists and telemedicine might result in less than optimal care, the reasons for using a remote visit should be clearly documented contemporaneously with the decision. Furthermore, when the limitations of being unable to physically examine the patient result in less information than is needed for the patient’s care, the provider must find alternatives to make up for the information gap.11,36 It also may be necessary to inform patients about how to maximize telemedicine care.37 At the beginning of telemedicine care the provider should include information about the nature and limits of telehealth, and the patient’s responsibilities. (See TABLE 1) Throughout treatment of the patient, that information should be updated by the provider. That, of course, is particularly important for patients who have not previously used telemedice services.
Malpractice rules vary by state. Many states have special rules regarding malpractice cases. These differences in malpractice standards and regulations “can be problematic for physicians who use telemedicine services to provide care outside the state in which they practice.”38 Caps on noneconomic damages are an example. Those state rules would apply to telemedicine in the patient’s state.
Malpractice insurance
Malpractice insurance now commonly includes telemedicine legally practiced within the physician’s home state. Practitioners who treat patients in foreign states should carefully examine their malpractice insurance policies to confirm that the coverage extends to practice in those states.39 Malpractice carriers may require notification by a covered physician who routinely provides services to patients in another state.3
Keep in mind, malpractice insurance generally does not cover the practice of medicine that is illegal. Practicing telemedicine in a foreign state, where the physician or other provider does not have a license and where that state does not otherwise permit the practice, is illegal. Most likely, the physician’s malpractice insurance will not cover claims that arise from this illegal practice in a foreign state or provide defense for malpractice claims, including frivolous lawsuits. Thus, the physician will pay out of pocket for the costs of a defense attorney.
Telemedicine treatment of minors
Children and adolescents present special legal issues for ObGyn care, which may become more complicated with telemedicine. Historically, parents are responsible for minors (those aged <18 years): they consent to medical treatment, are responsible for paying for it, and have the right to receive information about treatment.
Over the years, though, many states have made exceptions to these principles, especially with regard to contraception and treatment of sexually transmitted diseases.40 For abortion, in particular, there is considerable variation among the states in parental consent and notification.41 The Supreme Court’s decision in Dobbs v Jackson Women’s Health42 may (depending on the state) be followed with more stringent limitations on adolescent consent to abortions, including medical abortions.43
Use of telehealth does not change any obligations regarding adolescent consent or parental notification. Because those differ considerably among states, it is important for all practitioners to know their states’ requirements and keep reasonably complete records demonstrating their compliance with state law.
Abortion
The most heated current controversy about telemedicine involves abortion—specifically medical abortion, which is the combination of mifepristone and misoprostol.44,45 The FDA approved the combination in 2000. Almost immediately, many states required in-person visits with a certified clinician to receive a prescription for mifepristone and misoprostol, and eventually, the FDA adopted similar requirements.46 However, during the pandemic from 2021 to 2022, the FDA permitted telemedicine prescriptions. Several states still require in-person physician visits, although the constitutionality of those requirements has not been established.47
With the Supreme Court’s decision in Dobbs v Jackson Women’s Health in 2022,42 disagreements have ensued about the degree to which states may regulate the prescription of FDA-approved medical abortion drugs. Thorny constitutional issues exist in the plans of both abortion opponents and proponents in the battle over medical abortion in antiabortion states. It may be that federal drug law preempts state laws limiting access to FDA-approved drugs. On the other hand, it may be that states can make it a crime within the state to possess or provide abortion-inducing drugs. Courts will probably take years to resolve the many tangled legal questions.48
Thus, while the pandemic telemedicine rules may have advanced access to abortion,34 there may be some pending downsides.49 States that prohibit abortion will likely include prohibitions on medical abortions. In addition, they may prohibit anyone in the state (including pharmacies) from selling, possessing, or obtaining any drug used for causing or inducing an abortion.50 If, for constitutional reasons, they cannot press criminal charges or undertake licensing discipline for prescribing abortion, some states will likely withdraw from telehealth licensing compacts to avoid out-of-state prescriptions. This area of telemedicine has considerable uncertainty.
Continue to: CASE Conclusion...
CASE Conclusion
Patient concerns come to the fore
By 2023, Dr. TM started receiving bad news. Patient D called complaining that after following the advice on the website, she suffered a severe reaction and had to be rushed to an emergency department. Patient E (who had only 1 in-office visit early in her pregnancy) notified the office that she developed very high blood pressure that resulted in severe placental abruption, requiring emergency care and resulting in the loss of the fetus. Patient F complained that someone hacked the TikTok direct message communication with Dr. TM and tried to “blackmail” or harass her.
Discussion. Patients D, E, and F represent potential problems of telemedicine practice. Patient D was injured because she relied on her doctor’s website (to which Dr. TM directed patients). It contained an error that caused an injury. A doctor-patient relationship existed, and bad medical advice likely caused the injury. Physicians providing advice online must ensure the advice is correct and kept current.
Patient E demonstrates the importance of monitoring patients remotely (blood pressure transmitted to the office) or with periodic in-office visits. It is not clear whether she was a no-show for office visits (and whether the office followed up on any missed appointments) or if such visits were never scheduled. Liability for failure to monitor adequately is a possibility.
Patient F’s seemingly minor complaint could be a potential problem. Dr. TM used an insecure mode of communication. Although some HIPAA security regulations were modified or suspended during the pandemic, using such an unsecure platform is problematic, especially if temporary HIPAA rules expired. The outcome of the complaint is in doubt.
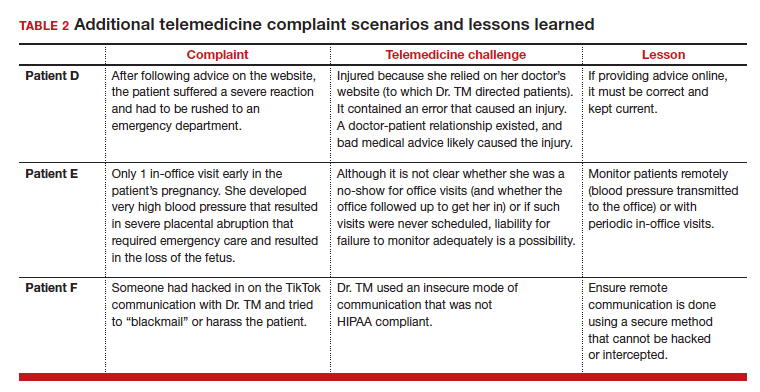
(See TABLE 2 for additional comments on patients D, E, and F.)
Out-of-state practice
Dr. TM treated 3 out-of-state residents (D, E, and F) via telemedicine. Recently Dr. TM received a complaint from the State Medical Licensure Board for practicing medicine without a license (Patient D), followed by similar charges from Patient E’s and Patient F’s state licensing boards. He has received a licensing inquiry from his home state board about those claims of illegal practice in other states and incompetent treatment.
Patient D’s pregnancy did not go well. The 1 in-person visit did not occur and she has filed a malpractice suit against Dr. TM. Patient E is threatening a malpractice case because the STI was not appropriately diagnosed and had advanced before another physician treated it.
In addition, a private citizen in Patient F’s state has filed suit against Dr. TM for abetting an illegal abortion (for Patient F).
Discussion. Patients D, E, and F illustrate the risk of even incidental out-of-state practice. The medical board inquiries arose from anonymous tips to all 4 states reporting Dr. TM was “practicing medicine without a license.” Patient E’s home state did have a licensing compact with the adjoining state (ie, Dr. TM’s home state). However, it required physicians to register and file an annual report, which Dr. TM had not done. The other 2 states did not have compacts with Dr. TM’s home state. Thus, he was illegally practicing medicine and would be subject to penalties. His home state also might impose license discipline based on his illegal practice in other states.
Continue to: What’s the verdict?...
What’s the verdict?
Dr. TM’s malpractice carrier is refusing to defend the claims of medical malpractice threatened by Patients D, E, and F. The company first notes that the terms of the malpractice policy specifically exclude the illegal practice of medicine. Furthermore, when a physician legally practices in another state, the policy requires a written notice to the insurance carrier of such practice. Dr. TM will likely have to engage and pay for a malpractice attorney for these cases. Because the claims are filed in 3 different states, more than a home-state attorney will likely be involved in the defense of these cases. Dr. TM will need to pay the attorneys and any damages from a settlement or trial.
Malpractice claims. Patient D claims that the doctor essentially abandoned her by never reaching out to her or arranging an in-person visit. Dr. TM claims the patient was responsible for scheduling the in-person visit. Patient E claims it was malpractice not to determine the specific nature of the STI and to do follow-up testing to determine that it was cured. All patients claim there was no genuine informed consent to the telemedicine. An attorney has warned Dr. TM that it is “not going to look good to the jury” that he was practicing without a license in the state and suggests he settle the cases quickly by paying damages.
Abortion-related claims. Patient F presents a different set of problems. Dr. TM’s home state is “proabortion.” Patient F’s home state is strongly “antiabortion,” making it a felony to participate in, assist, or facilitate an abortion (including medical abortion). Criminal charges have been filed against Dr. TM for the illegal practice of medicine, for aiding and facilitating an abortion, and for failure to notify a parent that a minor is seeking an abortion. For now, Dr. TM’s state is refusing to extradite on the abortion charge. Still, the patient’s state insists that it do so on the illegal practice of medicine charges and new charges of insurance fraud and failure to report suspected sexual abuse of a child. (Under the patient’s state law, anyone having sex with Patient F would have engaged in sexual abuse or “statutory rape,” so the state insists that the fact she was pregnant proves someone had sex with her.)
Patient F’s state also has a statute that allows private citizens to file civil claims against anyone procuring or assisting with an abortion (a successful private citizen can receive a minimum of $10,000 from the defendant). Several citizens from the patient’s state have already filed claims against Dr. TM in his state courts. Only one of them, probably the first to file, could succeed. Courts in the state have issued subpoenas and ordered Dr. TM to appear and reply to the civil suits. If he does not respond, there will be a default judgment.
Dr. TM’s attorney tells him that these lawsuits will not settle and will take a long time to defend and resolve. That will be expensive.
Billing and fraud. Dr. TM’s office recently received a series of notices from private health insurers stating they are investigating previously made payments as being fraudulent (unlicensed). They will not pay any new claims pending the investigation. On behalf of Medicare-Medicaid and other federal programs, the US Attorney’s office has notified Dr. TM that it has opened an investigation into fraudulent federal payments. F’s home state also is filing a (criminal) insurance fraud case, although the basis for it is unclear. (Dr. TM’s attorney believes it might be to increase pressure on the physician’s state to extradite Dr. TM for Patient F’s case.)
In addition, a disgruntled former employee of Dr. TM has filed a federal FCA case against him for filing inflated claims with various federally funded programs. The employee also made whistleblower calls to insurance companies and some state-funded medical programs. A forensic accounting investigation by Dr. TM’s accountant confirmed a pattern of very sloppy records and recurring billing for televisits that did not occur. Dr. TM believes that this was the act of one of the temporary assistants he hired in a pinch, who did not understand the system and just guessed when filing some insurance claims.
During the investigation, the federal and state attorneys are looking into a possible violation of state and federal Anti-Kickback Statutes. This is based on the original offer of a $100 credit for referrals to Dr. TM’s telemedicine practice.
The attorneys are concerned that other legal problems may present themselves. They are thoroughly reviewing Dr. TM’s practice and making several critical but somewhat modest changes to his practice. They also have insisted that Dr. TM have appropriate staff to handle the details of the practice and billing.
Conclusions
Telemedicine presents notable legal challenges to medical practice. As the pandemic status ends, ObGyn physicians practicing telemedicine need to be aware of the rules and how they are changing. For those physicians who want to continue or start a telemedicine practice, securing legal and technical support to ensure your operations are inline with the legal requirements can minimize any risk of legal troubles in the future. ●
A physician in State A, where abortion is legal, has a telemedicine patient in State B, where it is illegal to assist, provide, or procure an abortion. If the physician prescribes a medical abortion, he would violate the law of State B by using telemedicine to help the patient (located in State B) obtain an abortion. This could result in criminal charges against the prescribing physician.
- Board on Health Care Services; Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press: 2012. https://www.ncbi.nlm.nih.gov/books/NBK207145/. Accessed March 30, 2023.
- Bruhn HK. Telemedicine: dos and don’ts to mitigate liability risk. J APPOS. 2020;24:195-196. doi:10.1016/j.jaapos. 2020.07.002
- Implementing telehealth in practice: ACOG Committee Opinion Summary, number 798. Obstet Gynecol. 2020; 2135:493-494. doi:10.1097/AOG.0000000000003672
- Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. July 9, 2021. Accessed March 2, 2023. https://www.mckinsey.com/industries/healthcare/our-insights /telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- Stanley AY, Wallace JB. Telehealth to improve perinatal care access. MCN Am J Matern Child Nurs. 2022;47:281-287. doi: 10.1097/NMC.0000000000000841
- Warshaw R. Health disparities affect millions in rural US communities. Association of American Medical Colleges. Published October 31, 2017. Accessed March 31, 2023. https://www.aamc.org/news-insights/health-disparities -affect-millions-rural-us-communities
- Almuslin H, AlDossary S. Models of incorporating telehealth into obstetric care during the COVID-19 pandemic, its benefits and barriers: a scoping review. Telemed J E Health. 2022;28:24-38. doi:10.1089/tmj.2020.0553
- Gold AE, Gilbert A, McMichael BJ. Socially distant health care. Tul L Rev. 2021;96:423-468. https://scholarship .law.ua.edu/cgi/viewcontent.cgi?article=1713&context =fac_articles. Accessed March 4, 2023.
- Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
- Odibo IN, Wendel PJ, Magann EF. Telemedicine in obstetrics. Clin Obstet Gynecol. 2013;56:422-433. doi:10.1097/ GRF.0b013e318290fef0
- Shmerling A, Hoss M, Malam N, et al. Prenatal care via telehealth. Prim Care. 2022;49:609-619. doi:10.1016/j. pop.2022.05.002
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939
- Dosaj A, Thiyagarajan D, Ter Haar C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2020;27:116-120. doi:10.1089/ tmj.2020.0219
- Lurie N, Carr B. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;187:745-746. doi: 10.1001/jamainternmed.2018.1314
- Tobah YSB, LeBlanc A, Branda E, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638-e1-638.e8. doi:10.1016/j.ajog.2019.06.034
- Vivanti AJ, Deruelle P, Piccone O, et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstet Hum Reprod. 2020;49:101804. doi:10.1016/j. jogoh.2020.101804
- Ellimoottil C. Takeaways from 2 key studies on interstate telehealth use among Medicare fee-for-service beneficiaries. JAMA Health Forum. 2022;3:e223020-E223020. doi:10.1001/ jamahealthforum.2022.3020
- Harris J, Hartnett T, Hoagland GW, et al. What eliminating barriers to interstate telehealth taught us during the pandemic. Bipartisan Policy Center. Published November 2021. Accessed March 9, 2023. https://bipartisanpolicy .org/download/?file=/wp-content/uploads/2021/11/BPC -Health-Licensure-Brief_WEB.pdf.
- Center for Connected Health Policy. Cross-state licensing. Accessed February 21, 2023. https://www.cchpca.org/topic /cross-state-licensing-professional-requirements.
- US Department of Health & Human Services. Telehealth. Getting started with licensure. Published February 3, 2023. Accessed February 27, 2023. https://telehealth.hhs.gov /licensure/getting-started-licensure/
- US Department of Health & Human Services. Telehealth. Licensure. Accessed February 27, 2023. https://telehealth .hhs.gov/licensure
- US Department of Health & Human Services. National Practitioner Data Bank (NPDB) code lists. Published December 2022. Accessed March 9, 2023. https://www.npdb .hrsa.gov/software/CodeLists.pdf
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, telehealth. 2020. Accessed March 5, 2023. https://www.acog.org /clinical-information/physician-faqs/covid-19-faqs-for -ob-gyns-telehealth
- Gorman RK. Prescribing medication through the practice of telemedicine: a comparative analysis of federal and state online prescribing policies, and policy considerations for the future. S Cal Interdisc Law J. 2020;30:739-769. https://gould .usc.edu/why/students/orgs/ilj/assets/docs/30-3-Gorman. pdf. Accessed March 10, 2023.
- Farringer DR. A telehealth explosion: using lessons from the pandemic to shape the future of telehealth regulation. Tex A&M Law Rev. 2021;9:1-47. https://scholarship.law.tamu. edu/cgi/viewcontent.cgi?article=1232&context=lawreview. Accessed February 28, 2023.
- Sterba KR, Johnson EE, Douglas E, et al. Implementation of a women’s reproductive behavioral health telemedicine program: a qualitative study of barriers and facilitators in obstetric and pediatric clinics. BMC Pregnancy Childbirth. 2023;23:167, 1-10. doi:10.1186/s12884-023-05463-2.
- US Department of Justice. COVID-19 FAQ (telemedicine). https://www.deadiversion.usdoj.gov/faq/coronavirus_faq .htm#TELE_FAQ2. Accessed March 13, 2023.
- US Department of Health & Human Services. Guidance on how the HIPAA rules permit covered health care providers and health plans to use remote communication technologies for audio-only telehealth. Published June 13, 2022. Accessed February 22, 2023. https://www.hhs.gov/hipaa/for-professionals/privacy /guidance/hipaa-audio-telehealth/index.html.
- Gray JME. HIPAA, telehealth, and the treatment of mental illness in a post-COVID world. Okla City Uni Law Rev. 2021;46:1-26. https://law.okcu.edu/wp-content /uploads/2022/04/J-Michael-E-Gray-HIPAA-Telehealth -and-Treament.pdf. Accessed March 9, 2023.
- Kurzweil C. Telemental health care and data privacy: current HIPAA privacy pitfalls and a proposed solution. Ann Health L Adv Dir. 2022;31:165.
- US Department of Health & Human Services and US Department of Justice. Health care fraud and abuse control program FY 2020: annual report. July 2021. Accessed March 9, 2023. https://oig.hhs.gov/publications/docs/hcfac /FY2020-hcfac.pdf
- Copeland KB. Telemedicine scams. Iowa Law Rev. 2022: 108:69-126. https://ilr.law.uiowa.edu/sites/ilr.law.uiowa.edu /files/2023-01/A2_Copeland.pdf. Accessed March 10, 2023.
- Solimini R, Busardò FP, Gibelli F, et al. Ethical and legal challenges of telemedicine in the era of the COVID-19 pandemic. Medicina (Kaunas). 2021;57:13141324. doi:10.3390/medicina57121314
- Reed A. COVID: a silver linings playbook. mobilizing pandemic era success stories to advance reproductive justice. Berkeley J Gender Law Justice. 2022;37:221-266. https://lawcat.berkeley.edu/record/1237158/files/16%20 Reed_final.pdf. Accessed March 11, 2023.
- Women’s Preventive Services Initiative and The American College of Obstetricians and Gynecologists. FAQ for telehealth services. Accessed March 2, 2023. https://www .womenspreventivehealth.org/wp-content/uploads/WPSI -Telehealth-FAQ.pdf
- Warren L, Chen KT. Telehealth apps in ObGyn practice. OBG Manag. 2022;34:46-47. doi:10.12788/obgm.0178
- American College of Obstetricians and Gynecologists. 10 telehealth tips for an Ob-Gyn visit. 2020. Accessed March 2, 2023. https://www.acog.org/womens-health /infographics/10-telehealth-tips-for-an-ob-gyn-visit
- Wolf TD. Telemedicine and malpractice: creating uniformity at the national level. Wm Mary Law Rev. 2019;61:15051536. https://scholarship.law.wm.edu/cgi/viewcontent.cgi ?article=3862&context=wmlr. Accessed March 11, 2023.
- Cahan E. Lawsuits, reimbursement, and liability insurance— facing the realities of a post-Roe era. JAMA. 2022;328:515517. doi:10.1001/jama.2022.9193
- Heinrich L, Hernandez AK, Laurie AR. Telehealth considerations for the adolescent patient. Prim Care. 2022;49:597-607. doi:10.1016/j.pop.2022.04.006
- Guttmacher Institute. An overview of consent to reproductive health services by young people. Published March 1, 2023. Accessed April 1, 2023. https://www.guttmacher.org /state-policy/explore/overview-minors-consent-law.
- Dobbs v. Jackson Women’s Health. No. 19–1392. June 24, 2022. Accessed April 1, 2023. https://www.supremecourt .gov/opinions/21pdf/19-1392_6j37.pdf
- Lindgren Y. Dobbs v. Jackson Women’s Health and the post-Roe landscape. J Am Acad Matrimonial Law. 2022;35:235283. https://www.aaml.org/wp-content/uploads/MAT110-1 .pdf. Accessed March 11, 2023.
- Mohiuddin H. The use of telemedicine during a pandemic to provide access to medication abortion. Hous J Health Law Policy. 2021;21:483-525. https://houstonhealthlaw. scholasticahq.com/article/34611.pdf. Accessed March 10, 2023.
- Rebouché R. The public health turn in reproductive rights. Wash & Lee Law Rev. 2021;78:1355-1432. https:// scholarlycommons.law.wlu.edu/cgi/viewcontent .cgi?article=4743&context=wlulr. Accessed March 10, 2023.
- Fliegel R. Access to medication abortion: now more important than ever. Am J Law Med. 2022;48:286-304. doi:10.1017/amj.2022.24
- Guttmacher Institute. Medication abortion. March 1, 2023. Accessed April 1, 2023 https://www.guttmacher.org /state-policy/explore/medication-abortion#:~:text=In%20 January%202023%2C%20the%20FDA,order%20to%20 dispense%20the%20pills
- Cohen DS, Donley G, Rebouché R. The new abortion battleground. Columbia Law Rev. 2023;123:1-100. https:// columbialawreview.org/content/the-new-abortion -battleground/. Accessed March 1, 2023.
- Hunt SA. Call me, beep me, if you want to reach me: utilizing telemedicine to expand abortion access. Vanderbilt Law Rev. 2023;76:323-359. Accessed March 10, 2023. https:// vanderbiltlawreview.org/lawreview/wp-content/uploads /sites/278/2023/01/Call-Me-Beep-Me-If-You-Want-toReach-Me-Utilizing-Telemedicine-to-Expand-AbortionAccess.pdf
- Gleckel JA, Wulkan SL. Abortion and telemedicine: looking beyond COVID-19 and the shadow docket. UC Davis Law Rev Online. 2020;54:105-121. https://lawreview.law.ucdavis. edu/online/54/files/54-online-Gleckel_Wulkan.pdf. Accessed April 1, 2023.

Telemedicine (or telehealth) originated in the early 1900s, when radios were used to communicate medical advice to clinics aboard ships.1 According to the American Telemedicine Association, telemedicine is namely “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status.”2 These communications use 2-way video, email, smartphones, wireless tools, and other forms of telecommunications technology.
During the COVID-19 pandemic, many ObGyns—encouraged and advised by professional organizations—began providing telemedicine services.3 The first reported case of COVID-19 was in late 2019; the use of telemedicine was 38 times higher in February 2021 than in February 2020,4 illustrating how many physicians quickly moved to telemedicine practices.
CASE Dr. TM’s telemedicine dream
Before COVID-19, Dr. TM (an ObGyn practi-tioner) practiced in-person medicine in his home state. With the onset of the pandemic, Dr. TM struggled to switch to primarily seeing patients online (generally using Zoom or Facebook Live), with 1 day per week in the office for essential in-person visits.
After several months, however, Dr. TM’s routine became very efficient. He could see many more patients in a shorter time than with the former, in-person system. Therefore, as staff left his practice, Dr. TM did not replace them and also laid off others. Ultimately, the practice had 1 full-time records/insurance secretary who worked from home and 1 part-time nurse who helped with the in-person day and answered some patient inquiries by email. In part as an effort to add new patients, Dr. TM built an engaging website through which his current patients could receive medical information and new patients could sign up.
In late 2022, Dr. TM offered a $100 credit to any current patient who referred a friend or family member who then became a patient. This promotion was surprisingly effective and resulted in an influx of new patients. For example, Patient Z (a long-time patient) received 3 credits for referring her 3 sisters who lived out of state and became telepatients: Patient D, who lived 200 hundred miles away; Patient E, who lived 50 miles away in the adjoining state; and Patient F, who lived 150 miles away. Patient D contacted Dr. TM because she thought she was pregnant and wanted prenatal care, Patient E thought she might have a sexually transmitted infection (STI) and wanted treatment, and Patient F wanted general care and was inquiring about a medical abortion. Dr. TM agreed to treat Patient D but required 1 in-person visit. After 1 brief telemedicine session each with Patients E and F, Dr. TM wrote prescriptions for them.
By 2023, Dr. TM was enthusiastic about telemedicine as a professional practice. However, problems would ensue.
Dos and don’ts of telemedicine2
- Do take the initiative and inform patients of the availability of telemedicine/telehealth services
- Do use the services of medical malpractice insurance companies with regard to telemedicine
- Do integrate telemedicine into practice protocols and account for their limitations
- Don’t assume there are blanket exemptions or waivers in the states where your patients are located
Medical considerations
Telemedicine is endorsed by the American College of Obstetricians and Gynecologists (ACOG) as a vehicle for delivering prenatal and postpartum care.5 This represents an effort to reduce maternal and neonatal morbidity and mortality,5 as well as expandaccess to care and address the deficit in primary care providers and services, especially in rural and underserved populations.5,6 For obstetrics, prenatal care is designed to optimize pregnancy, childbirth, and postpartum care, with a focus on nutrition and genetic consultation and patient education on pregnancy, childbearing, breastfeeding, and newborn care.7
Benefits of telemedicine include its convenience for patients and providers, its efficiency and lower costs for providers (and hopefully patients, as well), and the potential improved access to care for patients.8 It is estimated that if a woman inititates obstetric care at 6 weeks, over the course of the 40-week gestation period, 15 prenatal visits will occur.9 Ultimately, the number of visits is determined based on the specifics of the pregnancy. With telemedicine, clinicians can provide those consultations, and information related to: ultrasonography, fetal echocardiography, and postpartum care services remotely.10 Using telemedicine may reduce missed visits, and remote monitoring may improve the quality of care.11
Barriers to telemedicine care include technical limitations, time constraints, and patient concerns of telehealth (visits). Technical limitations include the lack of a high speed internet connection and/or a smart device and the initial technical set-up–related problems,12 which affect providers as well as patients. Time constraints primarly refer to the ObGyn practice’s lack of time to establish telehealth services.13 Other challenges include integrating translation services, billing-related problems,10 and reimbursement and licensing barriers.14
Before the COVID-19 pandemic, obstetrics led the way in telemedicine with the development of the OB Nest model. Designed to replace in-person obstetrics care visits with telehealth,15 it includes home management tools such as blood pressure cuffs, cardiotocography, scales for weight checks, and Doppler ultrasounds.10 Patients can be instructed to measure fundal height and receive medications by mail. Anesthesia consultation can occur via this venue by having the patient complete a questionnaire prior to arriving at the labor and delivery unit.16
Legal considerations
With the COVID-19 pandemic, temporary changes were made to encourage the rapid adoption of telemedicine, including changes to licensing laws, certain prescription requirements, Health Insurance Portability and Accountability Act (HIPAA) privacy-security regulations, and reimbursement rules that required in-person visits. Thus, many ObGyns started using telemedicine during this rarified period, in which the rules appeared to be few and far between, with limited enforcement of the law and professional obligations.17 However, now that many of the legal rules that were suspended or ignored have been (or are being) reimposed and enforced, it is important for providers to become familiar with the legal issues involved in practicing telemedicine.
First, where is the patient? When discussing the legal issues of telemedicine, it is important to remember that many legal rules for medical care (ie, liability, informed consent, and licensing) vary from state to state. If the patient resides in a different state (“foreign” state) from the physician’s practice location (the physician’s “home” state), the care is considered delivered in the state where the patient is located. Thus, the patient’s location generally establishes the law covering the telemedicine transaction. In the following discussion, the rules refer to the law and professional obligations, with commentary on some key legal issues that are relevant to ObGyn telemedicine.
Continue to: Reinforcing the rules...
Reinforcing the rules
Licensing
During the height of the COVID-19 pandemic, the federal government and almost all states temporarily modified the licensing requirement to allow telemedicine based on an existing medical license in any state—disregarding the “where is the patient” rule. As those rules begin to lapse or change with the official end of the pandemic declared by President Biden as May 2023,17 the rules under which a physician began telemedicine interstate practice in 2020 also may be changing.
Simply put, “The same standards for licensure apply to health care providers regardless of whether care is delivered in-person or virtually through telehealth services.”18 When a physician is engaged in telemedicine treatment of a patient in the physician’s home state, there is generally no licensing issue. Telemedicine generally does not require a separate specific license.19 However, when the patient is in another state (a “foreign” state), there can be a substantial licensing issue.20 Ordinarily, to provide that treatment, the physician must, in some manner, be approved to practice in the patient’s state. That may occur, for example, in the following ways: (1) the physician may hold an additional regular license in the patient’s state, which allows practice there, or (2) the physician may have received permission for “temporary practice” in another state.
Many states (often adjoining states) have formal agreements with other states that allow telemedicine practice by providers in each other’s states. There also are “compacts”, or agreements that enable providers in any of the participating states to practice in the other associated states without a separate license.18 Although several websites provide information about compact licensing and the like, clinicians should not rely on simple lists or maps. Individual states may have special provisions about applying their laws to out-of-state “compact” physicians. In addition, under the Interstate Medical Licensure Compact, “physicians have to pay licensing fees and satisfy the requirements of each medical board in the states where they wish to practice.”21
Consequences. Practicing telemedicine with a patient in a state where the physician does not have a license is generally a crime. Furthermore, it may be the basis for license discipline in the physician’s home state and result in a report to the National Practi-tioner Databank.22 In addition, reimbursement often depends on the practitioner being licensed, and the absence of a license may be a basis for denying payment for services.23 Finally, malpractice insurance generally is limited to licensed practice. Thus, the insurer may decline to defend the unlicensed clinician against a malpractice claim or pay any damages.
Prescribing privileges
Prescribing privileges usually are connected to licensing, so as the rules for licensing change postpandemic, so do the rules for prescribing. In most cases, the physician must have a license in the state where care is given to prescribe medication—which in telemedicine, as noted, typically means the state where the patient is located. Exceptions vary by state, but in general, if a physician does not have a license to provide care, the physician is unlikely to be authorized to prescribe medication.24 Failure to abide by the applicable state rules may result in civil and even criminal liability for illegal prescribing activity.
In addition, the US Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA, which enforces laws concerning controlled substances) also regulate the prescription and sale of pharmaceuticals.25 There are state and federal limits on the ability of clinicians to order controlled substances without an in-person visit. The Ryan Haight Online Pharmacy Consumer Protection Act, for example, sets limits on controlled substance prescriptions without an in-person examination.26 Federal law was modified due to COVID-19 to permit prescribing of many controlled substances by telemedicine if there is synchronous audio and visual examination of the patient. Physicians who write such prescriptions also are required to have a DEA registration in the patient’s state. This is an essential consideration for physicians considering interstate telemedicine practice.27
HIPAA and privacy
Governments waived some of the legal requirements related to health information during the pandemic, but those waivers either have expired or will do so soon. Federal and state laws regarding privacy and security—notably including HIPAA—apply to telemedicine and are of particular concern given the considerable amount of communication of protected health information with telemedicine.
HIPAA security rules essentially require making sure health information cannot be hacked or intercepted. Audio-only telemedicine by landline (not cell) is acceptable under the security rules, but almost all other remote communication requires secure communications.28
Clinicians also need to adhere to the more usual HIPAA privacy rules when practicingtelehealth. State laws protecting patient privacy vary and may be more stringent than HIPAA, so clinicians also must know the requirements in any state where they practice—whether in office or telemedicine.29
Making sure telemedicine practices are consistent with these security and privacy rules often requires particular technical expertise that is outside the realm of most practicing clinicians. However, without modification, the pre-telemedicine technology of many medical offices likely is insufficient for the full range of telemedicine services.30
Reimbursement and fraud
Before COVID-19, Medicare and Medicaid reimbursement for telemedicine was limited. Government decisions to substantially broaden those reimbursement rules (at least temporarily) provided a substantial boost to telemedicine early in the pandemic.23 Federal regulations and statutes also expanded telemedicine reimbursement for various services. Some will end shortly after the health emergency, and others will be permanent. Parts of that will not be sorted out for several years, so it will likely be a changing landscape for reimbursement.
Continue to: Rules that are evolving...
Rules that are evolving
Informed consent
The ethical and legal obligations to obtain informed consent are present in telemedicineas well as in-person care, with the same basic requirements regarding risks, benefits, alternative care, etc.32 However, with telemedicine, information related to remote care should be included and is outlined in TABLE 1.

Certain states may have somewhat unique informed consent requirements—especially for reproductive care, including abortion.34 Therefore, it is important for clinicians to ensure their consent process and forms comply with any legal jurisdiction in which a patient is located.
Medical malpractice
The basics of medical malpractice (or negligence) are the same in telemedicine as in in-person care: duty, breach of duty, and injury caused by the breach. That is, there may be liability when a medical professional breaches the duty of care, causing the patient’s injury. The physician’s duty is defined by the quality of care that the profession (specialty) accepts as reasonably good. This is defined by the opinions of physicians within the specialty and formal statements from professional organizations, including ACOG.3
Maintaining the standard of care and quality. The use of telemedicine is not an excuse to lower the quality of health care. There are some circumstances for which it is medically better to have an in-person visit. In these instances, the provider should recommend the appropriate care, even if telemedicine would be more convenient for the provider and staff.35
If the patient insists and telemedicine might result in less than optimal care, the reasons for using a remote visit should be clearly documented contemporaneously with the decision. Furthermore, when the limitations of being unable to physically examine the patient result in less information than is needed for the patient’s care, the provider must find alternatives to make up for the information gap.11,36 It also may be necessary to inform patients about how to maximize telemedicine care.37 At the beginning of telemedicine care the provider should include information about the nature and limits of telehealth, and the patient’s responsibilities. (See TABLE 1) Throughout treatment of the patient, that information should be updated by the provider. That, of course, is particularly important for patients who have not previously used telemedice services.
Malpractice rules vary by state. Many states have special rules regarding malpractice cases. These differences in malpractice standards and regulations “can be problematic for physicians who use telemedicine services to provide care outside the state in which they practice.”38 Caps on noneconomic damages are an example. Those state rules would apply to telemedicine in the patient’s state.
Malpractice insurance
Malpractice insurance now commonly includes telemedicine legally practiced within the physician’s home state. Practitioners who treat patients in foreign states should carefully examine their malpractice insurance policies to confirm that the coverage extends to practice in those states.39 Malpractice carriers may require notification by a covered physician who routinely provides services to patients in another state.3
Keep in mind, malpractice insurance generally does not cover the practice of medicine that is illegal. Practicing telemedicine in a foreign state, where the physician or other provider does not have a license and where that state does not otherwise permit the practice, is illegal. Most likely, the physician’s malpractice insurance will not cover claims that arise from this illegal practice in a foreign state or provide defense for malpractice claims, including frivolous lawsuits. Thus, the physician will pay out of pocket for the costs of a defense attorney.
Telemedicine treatment of minors
Children and adolescents present special legal issues for ObGyn care, which may become more complicated with telemedicine. Historically, parents are responsible for minors (those aged <18 years): they consent to medical treatment, are responsible for paying for it, and have the right to receive information about treatment.
Over the years, though, many states have made exceptions to these principles, especially with regard to contraception and treatment of sexually transmitted diseases.40 For abortion, in particular, there is considerable variation among the states in parental consent and notification.41 The Supreme Court’s decision in Dobbs v Jackson Women’s Health42 may (depending on the state) be followed with more stringent limitations on adolescent consent to abortions, including medical abortions.43
Use of telehealth does not change any obligations regarding adolescent consent or parental notification. Because those differ considerably among states, it is important for all practitioners to know their states’ requirements and keep reasonably complete records demonstrating their compliance with state law.
Abortion
The most heated current controversy about telemedicine involves abortion—specifically medical abortion, which is the combination of mifepristone and misoprostol.44,45 The FDA approved the combination in 2000. Almost immediately, many states required in-person visits with a certified clinician to receive a prescription for mifepristone and misoprostol, and eventually, the FDA adopted similar requirements.46 However, during the pandemic from 2021 to 2022, the FDA permitted telemedicine prescriptions. Several states still require in-person physician visits, although the constitutionality of those requirements has not been established.47
With the Supreme Court’s decision in Dobbs v Jackson Women’s Health in 2022,42 disagreements have ensued about the degree to which states may regulate the prescription of FDA-approved medical abortion drugs. Thorny constitutional issues exist in the plans of both abortion opponents and proponents in the battle over medical abortion in antiabortion states. It may be that federal drug law preempts state laws limiting access to FDA-approved drugs. On the other hand, it may be that states can make it a crime within the state to possess or provide abortion-inducing drugs. Courts will probably take years to resolve the many tangled legal questions.48
Thus, while the pandemic telemedicine rules may have advanced access to abortion,34 there may be some pending downsides.49 States that prohibit abortion will likely include prohibitions on medical abortions. In addition, they may prohibit anyone in the state (including pharmacies) from selling, possessing, or obtaining any drug used for causing or inducing an abortion.50 If, for constitutional reasons, they cannot press criminal charges or undertake licensing discipline for prescribing abortion, some states will likely withdraw from telehealth licensing compacts to avoid out-of-state prescriptions. This area of telemedicine has considerable uncertainty.
Continue to: CASE Conclusion...
CASE Conclusion
Patient concerns come to the fore
By 2023, Dr. TM started receiving bad news. Patient D called complaining that after following the advice on the website, she suffered a severe reaction and had to be rushed to an emergency department. Patient E (who had only 1 in-office visit early in her pregnancy) notified the office that she developed very high blood pressure that resulted in severe placental abruption, requiring emergency care and resulting in the loss of the fetus. Patient F complained that someone hacked the TikTok direct message communication with Dr. TM and tried to “blackmail” or harass her.
Discussion. Patients D, E, and F represent potential problems of telemedicine practice. Patient D was injured because she relied on her doctor’s website (to which Dr. TM directed patients). It contained an error that caused an injury. A doctor-patient relationship existed, and bad medical advice likely caused the injury. Physicians providing advice online must ensure the advice is correct and kept current.
Patient E demonstrates the importance of monitoring patients remotely (blood pressure transmitted to the office) or with periodic in-office visits. It is not clear whether she was a no-show for office visits (and whether the office followed up on any missed appointments) or if such visits were never scheduled. Liability for failure to monitor adequately is a possibility.
Patient F’s seemingly minor complaint could be a potential problem. Dr. TM used an insecure mode of communication. Although some HIPAA security regulations were modified or suspended during the pandemic, using such an unsecure platform is problematic, especially if temporary HIPAA rules expired. The outcome of the complaint is in doubt.

(See TABLE 2 for additional comments on patients D, E, and F.)
Out-of-state practice
Dr. TM treated 3 out-of-state residents (D, E, and F) via telemedicine. Recently Dr. TM received a complaint from the State Medical Licensure Board for practicing medicine without a license (Patient D), followed by similar charges from Patient E’s and Patient F’s state licensing boards. He has received a licensing inquiry from his home state board about those claims of illegal practice in other states and incompetent treatment.
Patient D’s pregnancy did not go well. The 1 in-person visit did not occur and she has filed a malpractice suit against Dr. TM. Patient E is threatening a malpractice case because the STI was not appropriately diagnosed and had advanced before another physician treated it.
In addition, a private citizen in Patient F’s state has filed suit against Dr. TM for abetting an illegal abortion (for Patient F).
Discussion. Patients D, E, and F illustrate the risk of even incidental out-of-state practice. The medical board inquiries arose from anonymous tips to all 4 states reporting Dr. TM was “practicing medicine without a license.” Patient E’s home state did have a licensing compact with the adjoining state (ie, Dr. TM’s home state). However, it required physicians to register and file an annual report, which Dr. TM had not done. The other 2 states did not have compacts with Dr. TM’s home state. Thus, he was illegally practicing medicine and would be subject to penalties. His home state also might impose license discipline based on his illegal practice in other states.
Continue to: What’s the verdict?...
What’s the verdict?
Dr. TM’s malpractice carrier is refusing to defend the claims of medical malpractice threatened by Patients D, E, and F. The company first notes that the terms of the malpractice policy specifically exclude the illegal practice of medicine. Furthermore, when a physician legally practices in another state, the policy requires a written notice to the insurance carrier of such practice. Dr. TM will likely have to engage and pay for a malpractice attorney for these cases. Because the claims are filed in 3 different states, more than a home-state attorney will likely be involved in the defense of these cases. Dr. TM will need to pay the attorneys and any damages from a settlement or trial.
Malpractice claims. Patient D claims that the doctor essentially abandoned her by never reaching out to her or arranging an in-person visit. Dr. TM claims the patient was responsible for scheduling the in-person visit. Patient E claims it was malpractice not to determine the specific nature of the STI and to do follow-up testing to determine that it was cured. All patients claim there was no genuine informed consent to the telemedicine. An attorney has warned Dr. TM that it is “not going to look good to the jury” that he was practicing without a license in the state and suggests he settle the cases quickly by paying damages.
Abortion-related claims. Patient F presents a different set of problems. Dr. TM’s home state is “proabortion.” Patient F’s home state is strongly “antiabortion,” making it a felony to participate in, assist, or facilitate an abortion (including medical abortion). Criminal charges have been filed against Dr. TM for the illegal practice of medicine, for aiding and facilitating an abortion, and for failure to notify a parent that a minor is seeking an abortion. For now, Dr. TM’s state is refusing to extradite on the abortion charge. Still, the patient’s state insists that it do so on the illegal practice of medicine charges and new charges of insurance fraud and failure to report suspected sexual abuse of a child. (Under the patient’s state law, anyone having sex with Patient F would have engaged in sexual abuse or “statutory rape,” so the state insists that the fact she was pregnant proves someone had sex with her.)
Patient F’s state also has a statute that allows private citizens to file civil claims against anyone procuring or assisting with an abortion (a successful private citizen can receive a minimum of $10,000 from the defendant). Several citizens from the patient’s state have already filed claims against Dr. TM in his state courts. Only one of them, probably the first to file, could succeed. Courts in the state have issued subpoenas and ordered Dr. TM to appear and reply to the civil suits. If he does not respond, there will be a default judgment.
Dr. TM’s attorney tells him that these lawsuits will not settle and will take a long time to defend and resolve. That will be expensive.
Billing and fraud. Dr. TM’s office recently received a series of notices from private health insurers stating they are investigating previously made payments as being fraudulent (unlicensed). They will not pay any new claims pending the investigation. On behalf of Medicare-Medicaid and other federal programs, the US Attorney’s office has notified Dr. TM that it has opened an investigation into fraudulent federal payments. F’s home state also is filing a (criminal) insurance fraud case, although the basis for it is unclear. (Dr. TM’s attorney believes it might be to increase pressure on the physician’s state to extradite Dr. TM for Patient F’s case.)
In addition, a disgruntled former employee of Dr. TM has filed a federal FCA case against him for filing inflated claims with various federally funded programs. The employee also made whistleblower calls to insurance companies and some state-funded medical programs. A forensic accounting investigation by Dr. TM’s accountant confirmed a pattern of very sloppy records and recurring billing for televisits that did not occur. Dr. TM believes that this was the act of one of the temporary assistants he hired in a pinch, who did not understand the system and just guessed when filing some insurance claims.
During the investigation, the federal and state attorneys are looking into a possible violation of state and federal Anti-Kickback Statutes. This is based on the original offer of a $100 credit for referrals to Dr. TM’s telemedicine practice.
The attorneys are concerned that other legal problems may present themselves. They are thoroughly reviewing Dr. TM’s practice and making several critical but somewhat modest changes to his practice. They also have insisted that Dr. TM have appropriate staff to handle the details of the practice and billing.
Conclusions
Telemedicine presents notable legal challenges to medical practice. As the pandemic status ends, ObGyn physicians practicing telemedicine need to be aware of the rules and how they are changing. For those physicians who want to continue or start a telemedicine practice, securing legal and technical support to ensure your operations are inline with the legal requirements can minimize any risk of legal troubles in the future. ●
A physician in State A, where abortion is legal, has a telemedicine patient in State B, where it is illegal to assist, provide, or procure an abortion. If the physician prescribes a medical abortion, he would violate the law of State B by using telemedicine to help the patient (located in State B) obtain an abortion. This could result in criminal charges against the prescribing physician.

Telemedicine (or telehealth) originated in the early 1900s, when radios were used to communicate medical advice to clinics aboard ships.1 According to the American Telemedicine Association, telemedicine is namely “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status.”2 These communications use 2-way video, email, smartphones, wireless tools, and other forms of telecommunications technology.
During the COVID-19 pandemic, many ObGyns—encouraged and advised by professional organizations—began providing telemedicine services.3 The first reported case of COVID-19 was in late 2019; the use of telemedicine was 38 times higher in February 2021 than in February 2020,4 illustrating how many physicians quickly moved to telemedicine practices.
CASE Dr. TM’s telemedicine dream
Before COVID-19, Dr. TM (an ObGyn practi-tioner) practiced in-person medicine in his home state. With the onset of the pandemic, Dr. TM struggled to switch to primarily seeing patients online (generally using Zoom or Facebook Live), with 1 day per week in the office for essential in-person visits.
After several months, however, Dr. TM’s routine became very efficient. He could see many more patients in a shorter time than with the former, in-person system. Therefore, as staff left his practice, Dr. TM did not replace them and also laid off others. Ultimately, the practice had 1 full-time records/insurance secretary who worked from home and 1 part-time nurse who helped with the in-person day and answered some patient inquiries by email. In part as an effort to add new patients, Dr. TM built an engaging website through which his current patients could receive medical information and new patients could sign up.
In late 2022, Dr. TM offered a $100 credit to any current patient who referred a friend or family member who then became a patient. This promotion was surprisingly effective and resulted in an influx of new patients. For example, Patient Z (a long-time patient) received 3 credits for referring her 3 sisters who lived out of state and became telepatients: Patient D, who lived 200 hundred miles away; Patient E, who lived 50 miles away in the adjoining state; and Patient F, who lived 150 miles away. Patient D contacted Dr. TM because she thought she was pregnant and wanted prenatal care, Patient E thought she might have a sexually transmitted infection (STI) and wanted treatment, and Patient F wanted general care and was inquiring about a medical abortion. Dr. TM agreed to treat Patient D but required 1 in-person visit. After 1 brief telemedicine session each with Patients E and F, Dr. TM wrote prescriptions for them.
By 2023, Dr. TM was enthusiastic about telemedicine as a professional practice. However, problems would ensue.
Dos and don’ts of telemedicine2
- Do take the initiative and inform patients of the availability of telemedicine/telehealth services
- Do use the services of medical malpractice insurance companies with regard to telemedicine
- Do integrate telemedicine into practice protocols and account for their limitations
- Don’t assume there are blanket exemptions or waivers in the states where your patients are located
Medical considerations
Telemedicine is endorsed by the American College of Obstetricians and Gynecologists (ACOG) as a vehicle for delivering prenatal and postpartum care.5 This represents an effort to reduce maternal and neonatal morbidity and mortality,5 as well as expandaccess to care and address the deficit in primary care providers and services, especially in rural and underserved populations.5,6 For obstetrics, prenatal care is designed to optimize pregnancy, childbirth, and postpartum care, with a focus on nutrition and genetic consultation and patient education on pregnancy, childbearing, breastfeeding, and newborn care.7
Benefits of telemedicine include its convenience for patients and providers, its efficiency and lower costs for providers (and hopefully patients, as well), and the potential improved access to care for patients.8 It is estimated that if a woman inititates obstetric care at 6 weeks, over the course of the 40-week gestation period, 15 prenatal visits will occur.9 Ultimately, the number of visits is determined based on the specifics of the pregnancy. With telemedicine, clinicians can provide those consultations, and information related to: ultrasonography, fetal echocardiography, and postpartum care services remotely.10 Using telemedicine may reduce missed visits, and remote monitoring may improve the quality of care.11
Barriers to telemedicine care include technical limitations, time constraints, and patient concerns of telehealth (visits). Technical limitations include the lack of a high speed internet connection and/or a smart device and the initial technical set-up–related problems,12 which affect providers as well as patients. Time constraints primarly refer to the ObGyn practice’s lack of time to establish telehealth services.13 Other challenges include integrating translation services, billing-related problems,10 and reimbursement and licensing barriers.14
Before the COVID-19 pandemic, obstetrics led the way in telemedicine with the development of the OB Nest model. Designed to replace in-person obstetrics care visits with telehealth,15 it includes home management tools such as blood pressure cuffs, cardiotocography, scales for weight checks, and Doppler ultrasounds.10 Patients can be instructed to measure fundal height and receive medications by mail. Anesthesia consultation can occur via this venue by having the patient complete a questionnaire prior to arriving at the labor and delivery unit.16
Legal considerations
With the COVID-19 pandemic, temporary changes were made to encourage the rapid adoption of telemedicine, including changes to licensing laws, certain prescription requirements, Health Insurance Portability and Accountability Act (HIPAA) privacy-security regulations, and reimbursement rules that required in-person visits. Thus, many ObGyns started using telemedicine during this rarified period, in which the rules appeared to be few and far between, with limited enforcement of the law and professional obligations.17 However, now that many of the legal rules that were suspended or ignored have been (or are being) reimposed and enforced, it is important for providers to become familiar with the legal issues involved in practicing telemedicine.
First, where is the patient? When discussing the legal issues of telemedicine, it is important to remember that many legal rules for medical care (ie, liability, informed consent, and licensing) vary from state to state. If the patient resides in a different state (“foreign” state) from the physician’s practice location (the physician’s “home” state), the care is considered delivered in the state where the patient is located. Thus, the patient’s location generally establishes the law covering the telemedicine transaction. In the following discussion, the rules refer to the law and professional obligations, with commentary on some key legal issues that are relevant to ObGyn telemedicine.
Continue to: Reinforcing the rules...
Reinforcing the rules
Licensing
During the height of the COVID-19 pandemic, the federal government and almost all states temporarily modified the licensing requirement to allow telemedicine based on an existing medical license in any state—disregarding the “where is the patient” rule. As those rules begin to lapse or change with the official end of the pandemic declared by President Biden as May 2023,17 the rules under which a physician began telemedicine interstate practice in 2020 also may be changing.
Simply put, “The same standards for licensure apply to health care providers regardless of whether care is delivered in-person or virtually through telehealth services.”18 When a physician is engaged in telemedicine treatment of a patient in the physician’s home state, there is generally no licensing issue. Telemedicine generally does not require a separate specific license.19 However, when the patient is in another state (a “foreign” state), there can be a substantial licensing issue.20 Ordinarily, to provide that treatment, the physician must, in some manner, be approved to practice in the patient’s state. That may occur, for example, in the following ways: (1) the physician may hold an additional regular license in the patient’s state, which allows practice there, or (2) the physician may have received permission for “temporary practice” in another state.
Many states (often adjoining states) have formal agreements with other states that allow telemedicine practice by providers in each other’s states. There also are “compacts”, or agreements that enable providers in any of the participating states to practice in the other associated states without a separate license.18 Although several websites provide information about compact licensing and the like, clinicians should not rely on simple lists or maps. Individual states may have special provisions about applying their laws to out-of-state “compact” physicians. In addition, under the Interstate Medical Licensure Compact, “physicians have to pay licensing fees and satisfy the requirements of each medical board in the states where they wish to practice.”21
Consequences. Practicing telemedicine with a patient in a state where the physician does not have a license is generally a crime. Furthermore, it may be the basis for license discipline in the physician’s home state and result in a report to the National Practi-tioner Databank.22 In addition, reimbursement often depends on the practitioner being licensed, and the absence of a license may be a basis for denying payment for services.23 Finally, malpractice insurance generally is limited to licensed practice. Thus, the insurer may decline to defend the unlicensed clinician against a malpractice claim or pay any damages.
Prescribing privileges
Prescribing privileges usually are connected to licensing, so as the rules for licensing change postpandemic, so do the rules for prescribing. In most cases, the physician must have a license in the state where care is given to prescribe medication—which in telemedicine, as noted, typically means the state where the patient is located. Exceptions vary by state, but in general, if a physician does not have a license to provide care, the physician is unlikely to be authorized to prescribe medication.24 Failure to abide by the applicable state rules may result in civil and even criminal liability for illegal prescribing activity.
In addition, the US Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA, which enforces laws concerning controlled substances) also regulate the prescription and sale of pharmaceuticals.25 There are state and federal limits on the ability of clinicians to order controlled substances without an in-person visit. The Ryan Haight Online Pharmacy Consumer Protection Act, for example, sets limits on controlled substance prescriptions without an in-person examination.26 Federal law was modified due to COVID-19 to permit prescribing of many controlled substances by telemedicine if there is synchronous audio and visual examination of the patient. Physicians who write such prescriptions also are required to have a DEA registration in the patient’s state. This is an essential consideration for physicians considering interstate telemedicine practice.27
HIPAA and privacy
Governments waived some of the legal requirements related to health information during the pandemic, but those waivers either have expired or will do so soon. Federal and state laws regarding privacy and security—notably including HIPAA—apply to telemedicine and are of particular concern given the considerable amount of communication of protected health information with telemedicine.
HIPAA security rules essentially require making sure health information cannot be hacked or intercepted. Audio-only telemedicine by landline (not cell) is acceptable under the security rules, but almost all other remote communication requires secure communications.28
Clinicians also need to adhere to the more usual HIPAA privacy rules when practicingtelehealth. State laws protecting patient privacy vary and may be more stringent than HIPAA, so clinicians also must know the requirements in any state where they practice—whether in office or telemedicine.29
Making sure telemedicine practices are consistent with these security and privacy rules often requires particular technical expertise that is outside the realm of most practicing clinicians. However, without modification, the pre-telemedicine technology of many medical offices likely is insufficient for the full range of telemedicine services.30
Reimbursement and fraud
Before COVID-19, Medicare and Medicaid reimbursement for telemedicine was limited. Government decisions to substantially broaden those reimbursement rules (at least temporarily) provided a substantial boost to telemedicine early in the pandemic.23 Federal regulations and statutes also expanded telemedicine reimbursement for various services. Some will end shortly after the health emergency, and others will be permanent. Parts of that will not be sorted out for several years, so it will likely be a changing landscape for reimbursement.
Continue to: Rules that are evolving...
Rules that are evolving
Informed consent
The ethical and legal obligations to obtain informed consent are present in telemedicineas well as in-person care, with the same basic requirements regarding risks, benefits, alternative care, etc.32 However, with telemedicine, information related to remote care should be included and is outlined in TABLE 1.

Certain states may have somewhat unique informed consent requirements—especially for reproductive care, including abortion.34 Therefore, it is important for clinicians to ensure their consent process and forms comply with any legal jurisdiction in which a patient is located.
Medical malpractice
The basics of medical malpractice (or negligence) are the same in telemedicine as in in-person care: duty, breach of duty, and injury caused by the breach. That is, there may be liability when a medical professional breaches the duty of care, causing the patient’s injury. The physician’s duty is defined by the quality of care that the profession (specialty) accepts as reasonably good. This is defined by the opinions of physicians within the specialty and formal statements from professional organizations, including ACOG.3
Maintaining the standard of care and quality. The use of telemedicine is not an excuse to lower the quality of health care. There are some circumstances for which it is medically better to have an in-person visit. In these instances, the provider should recommend the appropriate care, even if telemedicine would be more convenient for the provider and staff.35
If the patient insists and telemedicine might result in less than optimal care, the reasons for using a remote visit should be clearly documented contemporaneously with the decision. Furthermore, when the limitations of being unable to physically examine the patient result in less information than is needed for the patient’s care, the provider must find alternatives to make up for the information gap.11,36 It also may be necessary to inform patients about how to maximize telemedicine care.37 At the beginning of telemedicine care the provider should include information about the nature and limits of telehealth, and the patient’s responsibilities. (See TABLE 1) Throughout treatment of the patient, that information should be updated by the provider. That, of course, is particularly important for patients who have not previously used telemedice services.
Malpractice rules vary by state. Many states have special rules regarding malpractice cases. These differences in malpractice standards and regulations “can be problematic for physicians who use telemedicine services to provide care outside the state in which they practice.”38 Caps on noneconomic damages are an example. Those state rules would apply to telemedicine in the patient’s state.
Malpractice insurance
Malpractice insurance now commonly includes telemedicine legally practiced within the physician’s home state. Practitioners who treat patients in foreign states should carefully examine their malpractice insurance policies to confirm that the coverage extends to practice in those states.39 Malpractice carriers may require notification by a covered physician who routinely provides services to patients in another state.3
Keep in mind, malpractice insurance generally does not cover the practice of medicine that is illegal. Practicing telemedicine in a foreign state, where the physician or other provider does not have a license and where that state does not otherwise permit the practice, is illegal. Most likely, the physician’s malpractice insurance will not cover claims that arise from this illegal practice in a foreign state or provide defense for malpractice claims, including frivolous lawsuits. Thus, the physician will pay out of pocket for the costs of a defense attorney.
Telemedicine treatment of minors
Children and adolescents present special legal issues for ObGyn care, which may become more complicated with telemedicine. Historically, parents are responsible for minors (those aged <18 years): they consent to medical treatment, are responsible for paying for it, and have the right to receive information about treatment.
Over the years, though, many states have made exceptions to these principles, especially with regard to contraception and treatment of sexually transmitted diseases.40 For abortion, in particular, there is considerable variation among the states in parental consent and notification.41 The Supreme Court’s decision in Dobbs v Jackson Women’s Health42 may (depending on the state) be followed with more stringent limitations on adolescent consent to abortions, including medical abortions.43
Use of telehealth does not change any obligations regarding adolescent consent or parental notification. Because those differ considerably among states, it is important for all practitioners to know their states’ requirements and keep reasonably complete records demonstrating their compliance with state law.
Abortion
The most heated current controversy about telemedicine involves abortion—specifically medical abortion, which is the combination of mifepristone and misoprostol.44,45 The FDA approved the combination in 2000. Almost immediately, many states required in-person visits with a certified clinician to receive a prescription for mifepristone and misoprostol, and eventually, the FDA adopted similar requirements.46 However, during the pandemic from 2021 to 2022, the FDA permitted telemedicine prescriptions. Several states still require in-person physician visits, although the constitutionality of those requirements has not been established.47
With the Supreme Court’s decision in Dobbs v Jackson Women’s Health in 2022,42 disagreements have ensued about the degree to which states may regulate the prescription of FDA-approved medical abortion drugs. Thorny constitutional issues exist in the plans of both abortion opponents and proponents in the battle over medical abortion in antiabortion states. It may be that federal drug law preempts state laws limiting access to FDA-approved drugs. On the other hand, it may be that states can make it a crime within the state to possess or provide abortion-inducing drugs. Courts will probably take years to resolve the many tangled legal questions.48
Thus, while the pandemic telemedicine rules may have advanced access to abortion,34 there may be some pending downsides.49 States that prohibit abortion will likely include prohibitions on medical abortions. In addition, they may prohibit anyone in the state (including pharmacies) from selling, possessing, or obtaining any drug used for causing or inducing an abortion.50 If, for constitutional reasons, they cannot press criminal charges or undertake licensing discipline for prescribing abortion, some states will likely withdraw from telehealth licensing compacts to avoid out-of-state prescriptions. This area of telemedicine has considerable uncertainty.
Continue to: CASE Conclusion...
CASE Conclusion
Patient concerns come to the fore
By 2023, Dr. TM started receiving bad news. Patient D called complaining that after following the advice on the website, she suffered a severe reaction and had to be rushed to an emergency department. Patient E (who had only 1 in-office visit early in her pregnancy) notified the office that she developed very high blood pressure that resulted in severe placental abruption, requiring emergency care and resulting in the loss of the fetus. Patient F complained that someone hacked the TikTok direct message communication with Dr. TM and tried to “blackmail” or harass her.
Discussion. Patients D, E, and F represent potential problems of telemedicine practice. Patient D was injured because she relied on her doctor’s website (to which Dr. TM directed patients). It contained an error that caused an injury. A doctor-patient relationship existed, and bad medical advice likely caused the injury. Physicians providing advice online must ensure the advice is correct and kept current.
Patient E demonstrates the importance of monitoring patients remotely (blood pressure transmitted to the office) or with periodic in-office visits. It is not clear whether she was a no-show for office visits (and whether the office followed up on any missed appointments) or if such visits were never scheduled. Liability for failure to monitor adequately is a possibility.
Patient F’s seemingly minor complaint could be a potential problem. Dr. TM used an insecure mode of communication. Although some HIPAA security regulations were modified or suspended during the pandemic, using such an unsecure platform is problematic, especially if temporary HIPAA rules expired. The outcome of the complaint is in doubt.

(See TABLE 2 for additional comments on patients D, E, and F.)
Out-of-state practice
Dr. TM treated 3 out-of-state residents (D, E, and F) via telemedicine. Recently Dr. TM received a complaint from the State Medical Licensure Board for practicing medicine without a license (Patient D), followed by similar charges from Patient E’s and Patient F’s state licensing boards. He has received a licensing inquiry from his home state board about those claims of illegal practice in other states and incompetent treatment.
Patient D’s pregnancy did not go well. The 1 in-person visit did not occur and she has filed a malpractice suit against Dr. TM. Patient E is threatening a malpractice case because the STI was not appropriately diagnosed and had advanced before another physician treated it.
In addition, a private citizen in Patient F’s state has filed suit against Dr. TM for abetting an illegal abortion (for Patient F).
Discussion. Patients D, E, and F illustrate the risk of even incidental out-of-state practice. The medical board inquiries arose from anonymous tips to all 4 states reporting Dr. TM was “practicing medicine without a license.” Patient E’s home state did have a licensing compact with the adjoining state (ie, Dr. TM’s home state). However, it required physicians to register and file an annual report, which Dr. TM had not done. The other 2 states did not have compacts with Dr. TM’s home state. Thus, he was illegally practicing medicine and would be subject to penalties. His home state also might impose license discipline based on his illegal practice in other states.
Continue to: What’s the verdict?...
What’s the verdict?
Dr. TM’s malpractice carrier is refusing to defend the claims of medical malpractice threatened by Patients D, E, and F. The company first notes that the terms of the malpractice policy specifically exclude the illegal practice of medicine. Furthermore, when a physician legally practices in another state, the policy requires a written notice to the insurance carrier of such practice. Dr. TM will likely have to engage and pay for a malpractice attorney for these cases. Because the claims are filed in 3 different states, more than a home-state attorney will likely be involved in the defense of these cases. Dr. TM will need to pay the attorneys and any damages from a settlement or trial.
Malpractice claims. Patient D claims that the doctor essentially abandoned her by never reaching out to her or arranging an in-person visit. Dr. TM claims the patient was responsible for scheduling the in-person visit. Patient E claims it was malpractice not to determine the specific nature of the STI and to do follow-up testing to determine that it was cured. All patients claim there was no genuine informed consent to the telemedicine. An attorney has warned Dr. TM that it is “not going to look good to the jury” that he was practicing without a license in the state and suggests he settle the cases quickly by paying damages.
Abortion-related claims. Patient F presents a different set of problems. Dr. TM’s home state is “proabortion.” Patient F’s home state is strongly “antiabortion,” making it a felony to participate in, assist, or facilitate an abortion (including medical abortion). Criminal charges have been filed against Dr. TM for the illegal practice of medicine, for aiding and facilitating an abortion, and for failure to notify a parent that a minor is seeking an abortion. For now, Dr. TM’s state is refusing to extradite on the abortion charge. Still, the patient’s state insists that it do so on the illegal practice of medicine charges and new charges of insurance fraud and failure to report suspected sexual abuse of a child. (Under the patient’s state law, anyone having sex with Patient F would have engaged in sexual abuse or “statutory rape,” so the state insists that the fact she was pregnant proves someone had sex with her.)
Patient F’s state also has a statute that allows private citizens to file civil claims against anyone procuring or assisting with an abortion (a successful private citizen can receive a minimum of $10,000 from the defendant). Several citizens from the patient’s state have already filed claims against Dr. TM in his state courts. Only one of them, probably the first to file, could succeed. Courts in the state have issued subpoenas and ordered Dr. TM to appear and reply to the civil suits. If he does not respond, there will be a default judgment.
Dr. TM’s attorney tells him that these lawsuits will not settle and will take a long time to defend and resolve. That will be expensive.
Billing and fraud. Dr. TM’s office recently received a series of notices from private health insurers stating they are investigating previously made payments as being fraudulent (unlicensed). They will not pay any new claims pending the investigation. On behalf of Medicare-Medicaid and other federal programs, the US Attorney’s office has notified Dr. TM that it has opened an investigation into fraudulent federal payments. F’s home state also is filing a (criminal) insurance fraud case, although the basis for it is unclear. (Dr. TM’s attorney believes it might be to increase pressure on the physician’s state to extradite Dr. TM for Patient F’s case.)
In addition, a disgruntled former employee of Dr. TM has filed a federal FCA case against him for filing inflated claims with various federally funded programs. The employee also made whistleblower calls to insurance companies and some state-funded medical programs. A forensic accounting investigation by Dr. TM’s accountant confirmed a pattern of very sloppy records and recurring billing for televisits that did not occur. Dr. TM believes that this was the act of one of the temporary assistants he hired in a pinch, who did not understand the system and just guessed when filing some insurance claims.
During the investigation, the federal and state attorneys are looking into a possible violation of state and federal Anti-Kickback Statutes. This is based on the original offer of a $100 credit for referrals to Dr. TM’s telemedicine practice.
The attorneys are concerned that other legal problems may present themselves. They are thoroughly reviewing Dr. TM’s practice and making several critical but somewhat modest changes to his practice. They also have insisted that Dr. TM have appropriate staff to handle the details of the practice and billing.
Conclusions
Telemedicine presents notable legal challenges to medical practice. As the pandemic status ends, ObGyn physicians practicing telemedicine need to be aware of the rules and how they are changing. For those physicians who want to continue or start a telemedicine practice, securing legal and technical support to ensure your operations are inline with the legal requirements can minimize any risk of legal troubles in the future. ●
A physician in State A, where abortion is legal, has a telemedicine patient in State B, where it is illegal to assist, provide, or procure an abortion. If the physician prescribes a medical abortion, he would violate the law of State B by using telemedicine to help the patient (located in State B) obtain an abortion. This could result in criminal charges against the prescribing physician.
- Board on Health Care Services; Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press: 2012. https://www.ncbi.nlm.nih.gov/books/NBK207145/. Accessed March 30, 2023.
- Bruhn HK. Telemedicine: dos and don’ts to mitigate liability risk. J APPOS. 2020;24:195-196. doi:10.1016/j.jaapos. 2020.07.002
- Implementing telehealth in practice: ACOG Committee Opinion Summary, number 798. Obstet Gynecol. 2020; 2135:493-494. doi:10.1097/AOG.0000000000003672
- Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. July 9, 2021. Accessed March 2, 2023. https://www.mckinsey.com/industries/healthcare/our-insights /telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- Stanley AY, Wallace JB. Telehealth to improve perinatal care access. MCN Am J Matern Child Nurs. 2022;47:281-287. doi: 10.1097/NMC.0000000000000841
- Warshaw R. Health disparities affect millions in rural US communities. Association of American Medical Colleges. Published October 31, 2017. Accessed March 31, 2023. https://www.aamc.org/news-insights/health-disparities -affect-millions-rural-us-communities
- Almuslin H, AlDossary S. Models of incorporating telehealth into obstetric care during the COVID-19 pandemic, its benefits and barriers: a scoping review. Telemed J E Health. 2022;28:24-38. doi:10.1089/tmj.2020.0553
- Gold AE, Gilbert A, McMichael BJ. Socially distant health care. Tul L Rev. 2021;96:423-468. https://scholarship .law.ua.edu/cgi/viewcontent.cgi?article=1713&context =fac_articles. Accessed March 4, 2023.
- Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
- Odibo IN, Wendel PJ, Magann EF. Telemedicine in obstetrics. Clin Obstet Gynecol. 2013;56:422-433. doi:10.1097/ GRF.0b013e318290fef0
- Shmerling A, Hoss M, Malam N, et al. Prenatal care via telehealth. Prim Care. 2022;49:609-619. doi:10.1016/j. pop.2022.05.002
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939
- Dosaj A, Thiyagarajan D, Ter Haar C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2020;27:116-120. doi:10.1089/ tmj.2020.0219
- Lurie N, Carr B. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;187:745-746. doi: 10.1001/jamainternmed.2018.1314
- Tobah YSB, LeBlanc A, Branda E, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638-e1-638.e8. doi:10.1016/j.ajog.2019.06.034
- Vivanti AJ, Deruelle P, Piccone O, et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstet Hum Reprod. 2020;49:101804. doi:10.1016/j. jogoh.2020.101804
- Ellimoottil C. Takeaways from 2 key studies on interstate telehealth use among Medicare fee-for-service beneficiaries. JAMA Health Forum. 2022;3:e223020-E223020. doi:10.1001/ jamahealthforum.2022.3020
- Harris J, Hartnett T, Hoagland GW, et al. What eliminating barriers to interstate telehealth taught us during the pandemic. Bipartisan Policy Center. Published November 2021. Accessed March 9, 2023. https://bipartisanpolicy .org/download/?file=/wp-content/uploads/2021/11/BPC -Health-Licensure-Brief_WEB.pdf.
- Center for Connected Health Policy. Cross-state licensing. Accessed February 21, 2023. https://www.cchpca.org/topic /cross-state-licensing-professional-requirements.
- US Department of Health & Human Services. Telehealth. Getting started with licensure. Published February 3, 2023. Accessed February 27, 2023. https://telehealth.hhs.gov /licensure/getting-started-licensure/
- US Department of Health & Human Services. Telehealth. Licensure. Accessed February 27, 2023. https://telehealth .hhs.gov/licensure
- US Department of Health & Human Services. National Practitioner Data Bank (NPDB) code lists. Published December 2022. Accessed March 9, 2023. https://www.npdb .hrsa.gov/software/CodeLists.pdf
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, telehealth. 2020. Accessed March 5, 2023. https://www.acog.org /clinical-information/physician-faqs/covid-19-faqs-for -ob-gyns-telehealth
- Gorman RK. Prescribing medication through the practice of telemedicine: a comparative analysis of federal and state online prescribing policies, and policy considerations for the future. S Cal Interdisc Law J. 2020;30:739-769. https://gould .usc.edu/why/students/orgs/ilj/assets/docs/30-3-Gorman. pdf. Accessed March 10, 2023.
- Farringer DR. A telehealth explosion: using lessons from the pandemic to shape the future of telehealth regulation. Tex A&M Law Rev. 2021;9:1-47. https://scholarship.law.tamu. edu/cgi/viewcontent.cgi?article=1232&context=lawreview. Accessed February 28, 2023.
- Sterba KR, Johnson EE, Douglas E, et al. Implementation of a women’s reproductive behavioral health telemedicine program: a qualitative study of barriers and facilitators in obstetric and pediatric clinics. BMC Pregnancy Childbirth. 2023;23:167, 1-10. doi:10.1186/s12884-023-05463-2.
- US Department of Justice. COVID-19 FAQ (telemedicine). https://www.deadiversion.usdoj.gov/faq/coronavirus_faq .htm#TELE_FAQ2. Accessed March 13, 2023.
- US Department of Health & Human Services. Guidance on how the HIPAA rules permit covered health care providers and health plans to use remote communication technologies for audio-only telehealth. Published June 13, 2022. Accessed February 22, 2023. https://www.hhs.gov/hipaa/for-professionals/privacy /guidance/hipaa-audio-telehealth/index.html.
- Gray JME. HIPAA, telehealth, and the treatment of mental illness in a post-COVID world. Okla City Uni Law Rev. 2021;46:1-26. https://law.okcu.edu/wp-content /uploads/2022/04/J-Michael-E-Gray-HIPAA-Telehealth -and-Treament.pdf. Accessed March 9, 2023.
- Kurzweil C. Telemental health care and data privacy: current HIPAA privacy pitfalls and a proposed solution. Ann Health L Adv Dir. 2022;31:165.
- US Department of Health & Human Services and US Department of Justice. Health care fraud and abuse control program FY 2020: annual report. July 2021. Accessed March 9, 2023. https://oig.hhs.gov/publications/docs/hcfac /FY2020-hcfac.pdf
- Copeland KB. Telemedicine scams. Iowa Law Rev. 2022: 108:69-126. https://ilr.law.uiowa.edu/sites/ilr.law.uiowa.edu /files/2023-01/A2_Copeland.pdf. Accessed March 10, 2023.
- Solimini R, Busardò FP, Gibelli F, et al. Ethical and legal challenges of telemedicine in the era of the COVID-19 pandemic. Medicina (Kaunas). 2021;57:13141324. doi:10.3390/medicina57121314
- Reed A. COVID: a silver linings playbook. mobilizing pandemic era success stories to advance reproductive justice. Berkeley J Gender Law Justice. 2022;37:221-266. https://lawcat.berkeley.edu/record/1237158/files/16%20 Reed_final.pdf. Accessed March 11, 2023.
- Women’s Preventive Services Initiative and The American College of Obstetricians and Gynecologists. FAQ for telehealth services. Accessed March 2, 2023. https://www .womenspreventivehealth.org/wp-content/uploads/WPSI -Telehealth-FAQ.pdf
- Warren L, Chen KT. Telehealth apps in ObGyn practice. OBG Manag. 2022;34:46-47. doi:10.12788/obgm.0178
- American College of Obstetricians and Gynecologists. 10 telehealth tips for an Ob-Gyn visit. 2020. Accessed March 2, 2023. https://www.acog.org/womens-health /infographics/10-telehealth-tips-for-an-ob-gyn-visit
- Wolf TD. Telemedicine and malpractice: creating uniformity at the national level. Wm Mary Law Rev. 2019;61:15051536. https://scholarship.law.wm.edu/cgi/viewcontent.cgi ?article=3862&context=wmlr. Accessed March 11, 2023.
- Cahan E. Lawsuits, reimbursement, and liability insurance— facing the realities of a post-Roe era. JAMA. 2022;328:515517. doi:10.1001/jama.2022.9193
- Heinrich L, Hernandez AK, Laurie AR. Telehealth considerations for the adolescent patient. Prim Care. 2022;49:597-607. doi:10.1016/j.pop.2022.04.006
- Guttmacher Institute. An overview of consent to reproductive health services by young people. Published March 1, 2023. Accessed April 1, 2023. https://www.guttmacher.org /state-policy/explore/overview-minors-consent-law.
- Dobbs v. Jackson Women’s Health. No. 19–1392. June 24, 2022. Accessed April 1, 2023. https://www.supremecourt .gov/opinions/21pdf/19-1392_6j37.pdf
- Lindgren Y. Dobbs v. Jackson Women’s Health and the post-Roe landscape. J Am Acad Matrimonial Law. 2022;35:235283. https://www.aaml.org/wp-content/uploads/MAT110-1 .pdf. Accessed March 11, 2023.
- Mohiuddin H. The use of telemedicine during a pandemic to provide access to medication abortion. Hous J Health Law Policy. 2021;21:483-525. https://houstonhealthlaw. scholasticahq.com/article/34611.pdf. Accessed March 10, 2023.
- Rebouché R. The public health turn in reproductive rights. Wash & Lee Law Rev. 2021;78:1355-1432. https:// scholarlycommons.law.wlu.edu/cgi/viewcontent .cgi?article=4743&context=wlulr. Accessed March 10, 2023.
- Fliegel R. Access to medication abortion: now more important than ever. Am J Law Med. 2022;48:286-304. doi:10.1017/amj.2022.24
- Guttmacher Institute. Medication abortion. March 1, 2023. Accessed April 1, 2023 https://www.guttmacher.org /state-policy/explore/medication-abortion#:~:text=In%20 January%202023%2C%20the%20FDA,order%20to%20 dispense%20the%20pills
- Cohen DS, Donley G, Rebouché R. The new abortion battleground. Columbia Law Rev. 2023;123:1-100. https:// columbialawreview.org/content/the-new-abortion -battleground/. Accessed March 1, 2023.
- Hunt SA. Call me, beep me, if you want to reach me: utilizing telemedicine to expand abortion access. Vanderbilt Law Rev. 2023;76:323-359. Accessed March 10, 2023. https:// vanderbiltlawreview.org/lawreview/wp-content/uploads /sites/278/2023/01/Call-Me-Beep-Me-If-You-Want-toReach-Me-Utilizing-Telemedicine-to-Expand-AbortionAccess.pdf
- Gleckel JA, Wulkan SL. Abortion and telemedicine: looking beyond COVID-19 and the shadow docket. UC Davis Law Rev Online. 2020;54:105-121. https://lawreview.law.ucdavis. edu/online/54/files/54-online-Gleckel_Wulkan.pdf. Accessed April 1, 2023.
- Board on Health Care Services; Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press: 2012. https://www.ncbi.nlm.nih.gov/books/NBK207145/. Accessed March 30, 2023.
- Bruhn HK. Telemedicine: dos and don’ts to mitigate liability risk. J APPOS. 2020;24:195-196. doi:10.1016/j.jaapos. 2020.07.002
- Implementing telehealth in practice: ACOG Committee Opinion Summary, number 798. Obstet Gynecol. 2020; 2135:493-494. doi:10.1097/AOG.0000000000003672
- Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. July 9, 2021. Accessed March 2, 2023. https://www.mckinsey.com/industries/healthcare/our-insights /telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- Stanley AY, Wallace JB. Telehealth to improve perinatal care access. MCN Am J Matern Child Nurs. 2022;47:281-287. doi: 10.1097/NMC.0000000000000841
- Warshaw R. Health disparities affect millions in rural US communities. Association of American Medical Colleges. Published October 31, 2017. Accessed March 31, 2023. https://www.aamc.org/news-insights/health-disparities -affect-millions-rural-us-communities
- Almuslin H, AlDossary S. Models of incorporating telehealth into obstetric care during the COVID-19 pandemic, its benefits and barriers: a scoping review. Telemed J E Health. 2022;28:24-38. doi:10.1089/tmj.2020.0553
- Gold AE, Gilbert A, McMichael BJ. Socially distant health care. Tul L Rev. 2021;96:423-468. https://scholarship .law.ua.edu/cgi/viewcontent.cgi?article=1713&context =fac_articles. Accessed March 4, 2023.
- Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
- Odibo IN, Wendel PJ, Magann EF. Telemedicine in obstetrics. Clin Obstet Gynecol. 2013;56:422-433. doi:10.1097/ GRF.0b013e318290fef0
- Shmerling A, Hoss M, Malam N, et al. Prenatal care via telehealth. Prim Care. 2022;49:609-619. doi:10.1016/j. pop.2022.05.002
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939
- Dosaj A, Thiyagarajan D, Ter Haar C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2020;27:116-120. doi:10.1089/ tmj.2020.0219
- Lurie N, Carr B. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;187:745-746. doi: 10.1001/jamainternmed.2018.1314
- Tobah YSB, LeBlanc A, Branda E, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638-e1-638.e8. doi:10.1016/j.ajog.2019.06.034
- Vivanti AJ, Deruelle P, Piccone O, et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstet Hum Reprod. 2020;49:101804. doi:10.1016/j. jogoh.2020.101804
- Ellimoottil C. Takeaways from 2 key studies on interstate telehealth use among Medicare fee-for-service beneficiaries. JAMA Health Forum. 2022;3:e223020-E223020. doi:10.1001/ jamahealthforum.2022.3020
- Harris J, Hartnett T, Hoagland GW, et al. What eliminating barriers to interstate telehealth taught us during the pandemic. Bipartisan Policy Center. Published November 2021. Accessed March 9, 2023. https://bipartisanpolicy .org/download/?file=/wp-content/uploads/2021/11/BPC -Health-Licensure-Brief_WEB.pdf.
- Center for Connected Health Policy. Cross-state licensing. Accessed February 21, 2023. https://www.cchpca.org/topic /cross-state-licensing-professional-requirements.
- US Department of Health & Human Services. Telehealth. Getting started with licensure. Published February 3, 2023. Accessed February 27, 2023. https://telehealth.hhs.gov /licensure/getting-started-licensure/
- US Department of Health & Human Services. Telehealth. Licensure. Accessed February 27, 2023. https://telehealth .hhs.gov/licensure
- US Department of Health & Human Services. National Practitioner Data Bank (NPDB) code lists. Published December 2022. Accessed March 9, 2023. https://www.npdb .hrsa.gov/software/CodeLists.pdf
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, telehealth. 2020. Accessed March 5, 2023. https://www.acog.org /clinical-information/physician-faqs/covid-19-faqs-for -ob-gyns-telehealth
- Gorman RK. Prescribing medication through the practice of telemedicine: a comparative analysis of federal and state online prescribing policies, and policy considerations for the future. S Cal Interdisc Law J. 2020;30:739-769. https://gould .usc.edu/why/students/orgs/ilj/assets/docs/30-3-Gorman. pdf. Accessed March 10, 2023.
- Farringer DR. A telehealth explosion: using lessons from the pandemic to shape the future of telehealth regulation. Tex A&M Law Rev. 2021;9:1-47. https://scholarship.law.tamu. edu/cgi/viewcontent.cgi?article=1232&context=lawreview. Accessed February 28, 2023.
- Sterba KR, Johnson EE, Douglas E, et al. Implementation of a women’s reproductive behavioral health telemedicine program: a qualitative study of barriers and facilitators in obstetric and pediatric clinics. BMC Pregnancy Childbirth. 2023;23:167, 1-10. doi:10.1186/s12884-023-05463-2.
- US Department of Justice. COVID-19 FAQ (telemedicine). https://www.deadiversion.usdoj.gov/faq/coronavirus_faq .htm#TELE_FAQ2. Accessed March 13, 2023.
- US Department of Health & Human Services. Guidance on how the HIPAA rules permit covered health care providers and health plans to use remote communication technologies for audio-only telehealth. Published June 13, 2022. Accessed February 22, 2023. https://www.hhs.gov/hipaa/for-professionals/privacy /guidance/hipaa-audio-telehealth/index.html.
- Gray JME. HIPAA, telehealth, and the treatment of mental illness in a post-COVID world. Okla City Uni Law Rev. 2021;46:1-26. https://law.okcu.edu/wp-content /uploads/2022/04/J-Michael-E-Gray-HIPAA-Telehealth -and-Treament.pdf. Accessed March 9, 2023.
- Kurzweil C. Telemental health care and data privacy: current HIPAA privacy pitfalls and a proposed solution. Ann Health L Adv Dir. 2022;31:165.
- US Department of Health & Human Services and US Department of Justice. Health care fraud and abuse control program FY 2020: annual report. July 2021. Accessed March 9, 2023. https://oig.hhs.gov/publications/docs/hcfac /FY2020-hcfac.pdf
- Copeland KB. Telemedicine scams. Iowa Law Rev. 2022: 108:69-126. https://ilr.law.uiowa.edu/sites/ilr.law.uiowa.edu /files/2023-01/A2_Copeland.pdf. Accessed March 10, 2023.
- Solimini R, Busardò FP, Gibelli F, et al. Ethical and legal challenges of telemedicine in the era of the COVID-19 pandemic. Medicina (Kaunas). 2021;57:13141324. doi:10.3390/medicina57121314
- Reed A. COVID: a silver linings playbook. mobilizing pandemic era success stories to advance reproductive justice. Berkeley J Gender Law Justice. 2022;37:221-266. https://lawcat.berkeley.edu/record/1237158/files/16%20 Reed_final.pdf. Accessed March 11, 2023.
- Women’s Preventive Services Initiative and The American College of Obstetricians and Gynecologists. FAQ for telehealth services. Accessed March 2, 2023. https://www .womenspreventivehealth.org/wp-content/uploads/WPSI -Telehealth-FAQ.pdf
- Warren L, Chen KT. Telehealth apps in ObGyn practice. OBG Manag. 2022;34:46-47. doi:10.12788/obgm.0178
- American College of Obstetricians and Gynecologists. 10 telehealth tips for an Ob-Gyn visit. 2020. Accessed March 2, 2023. https://www.acog.org/womens-health /infographics/10-telehealth-tips-for-an-ob-gyn-visit
- Wolf TD. Telemedicine and malpractice: creating uniformity at the national level. Wm Mary Law Rev. 2019;61:15051536. https://scholarship.law.wm.edu/cgi/viewcontent.cgi ?article=3862&context=wmlr. Accessed March 11, 2023.
- Cahan E. Lawsuits, reimbursement, and liability insurance— facing the realities of a post-Roe era. JAMA. 2022;328:515517. doi:10.1001/jama.2022.9193
- Heinrich L, Hernandez AK, Laurie AR. Telehealth considerations for the adolescent patient. Prim Care. 2022;49:597-607. doi:10.1016/j.pop.2022.04.006
- Guttmacher Institute. An overview of consent to reproductive health services by young people. Published March 1, 2023. Accessed April 1, 2023. https://www.guttmacher.org /state-policy/explore/overview-minors-consent-law.
- Dobbs v. Jackson Women’s Health. No. 19–1392. June 24, 2022. Accessed April 1, 2023. https://www.supremecourt .gov/opinions/21pdf/19-1392_6j37.pdf
- Lindgren Y. Dobbs v. Jackson Women’s Health and the post-Roe landscape. J Am Acad Matrimonial Law. 2022;35:235283. https://www.aaml.org/wp-content/uploads/MAT110-1 .pdf. Accessed March 11, 2023.
- Mohiuddin H. The use of telemedicine during a pandemic to provide access to medication abortion. Hous J Health Law Policy. 2021;21:483-525. https://houstonhealthlaw. scholasticahq.com/article/34611.pdf. Accessed March 10, 2023.
- Rebouché R. The public health turn in reproductive rights. Wash & Lee Law Rev. 2021;78:1355-1432. https:// scholarlycommons.law.wlu.edu/cgi/viewcontent .cgi?article=4743&context=wlulr. Accessed March 10, 2023.
- Fliegel R. Access to medication abortion: now more important than ever. Am J Law Med. 2022;48:286-304. doi:10.1017/amj.2022.24
- Guttmacher Institute. Medication abortion. March 1, 2023. Accessed April 1, 2023 https://www.guttmacher.org /state-policy/explore/medication-abortion#:~:text=In%20 January%202023%2C%20the%20FDA,order%20to%20 dispense%20the%20pills
- Cohen DS, Donley G, Rebouché R. The new abortion battleground. Columbia Law Rev. 2023;123:1-100. https:// columbialawreview.org/content/the-new-abortion -battleground/. Accessed March 1, 2023.
- Hunt SA. Call me, beep me, if you want to reach me: utilizing telemedicine to expand abortion access. Vanderbilt Law Rev. 2023;76:323-359. Accessed March 10, 2023. https:// vanderbiltlawreview.org/lawreview/wp-content/uploads /sites/278/2023/01/Call-Me-Beep-Me-If-You-Want-toReach-Me-Utilizing-Telemedicine-to-Expand-AbortionAccess.pdf
- Gleckel JA, Wulkan SL. Abortion and telemedicine: looking beyond COVID-19 and the shadow docket. UC Davis Law Rev Online. 2020;54:105-121. https://lawreview.law.ucdavis. edu/online/54/files/54-online-Gleckel_Wulkan.pdf. Accessed April 1, 2023.
Liability in robotic gyn surgery
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
Criminal liability: What are the risks for medical professionals?
Medical professionals are well aware that civil liability (malpractice) may incur when a patient is harmed because of carelessness (negligence). Recent criminal charges against physicians and a nurse, however, have called medical professionals’ attention to the fact that they also may face criminal charges for inappropriate practice.
We cite 2 cases in which criminal liability resulted from bad medical practice. In both instances, there was considerable concern among medical professionals that criminal charges for making a mistake would make it difficult to practice without fear of criminal charges. One concern is that criminal charges could drive good people out of the profession or make them too cautious.1
We look more closely at those 2 cases in which criminal liability was imposed. These cases are outliers. Relatively few criminal cases against medical professionals are based on the quality of care. (There are, however, more criminal charges related to fraudulent billing and other insurance fraud, kickbacks, Medicare and Medicaid abuse, and the like.2) At the same time, the criminal law does not stop at the front door of a clinic or hospital.3 When medical professionals engage in seriously inappropriate health care conduct that directly harms someone, criminal liability may result.4
Anatomy of a crime
Crimes generally require a specific mental state (mens rea) and an act (actus reus). The law specifies the mental state required for conviction. It can range from premeditation—once commonly called “malice aforethought”—to negligence. The mens rea requirement is an essential element of the crime—as we will see in the discussion of the prescription drug cases. A few offenses do not require even negligence, but overwhelmingly, crimes require something more than simple negligence.5
The act requirement is generally obvious, such as firing a gun, driving while intoxicated, or recklessly giving inappropriate medication to a patient. It may include “attempts,” crimes where an act was not completed. For example, attempted murder or conspiracy to commit do not require a completed offense, only intent plus overt acts toward carrying out the crime. Similarly, the wrongful act usually has to produce some harm, but again there are exceptions (attempts). To obtain a conviction, the prosecution must prove all of the elements of the crime, including the required mens rea, beyond a reasonable doubt.6
With this general background, we turn to the first case, in which the charge was a form of homicide. Please note that the following case description was derived from news descriptions of the case, because juries do not publish opinions concerning their conclusions and court documents are unavailable. The public reports therefore may contain factual gaps and errors.
CASE 1 Patient dies after nurse administers wrong drug
RaDonda Vaught, a 38-year-old experienced registered nurse employed at Vanderbilt University Medical Center (VUMC) in the intensive care unit (ICU), was providing care for a 76-year-old patient who was admitted to VUMC’s ICU in December 2017 in association with a brain injury. The brain injury involved a fall with resultant subdural hematoma. In preparation for a positron emission tomography (PET) scan to assess the patient’s injury, the physician team prescribed the sedative Versed (midazolam) because of the patient’s claustrophobia. During the course of treatment, Ms. Vaught inadvertently administered the wrong drug, a fatal dose of the muscle relaxant vecuronium, to the patient, which resulted in the patient being unable to breathe. Apparently, Ms. Vaught had been unable to find the midazolam and disengaged a safeguard, proceeding into override mode, and thus vecuronium was dispensed. By the time the error was noticed, the patient was already in cardiac arrest with resultant brain damage (partial brain death). The patient died soon thereafter.
How this medication error occurred
The medication error occurred when Ms. Vaught overrode a computer in the medical system when she could not find the “Versed” entry and typed in “VE,” which was the abbreviation for vecuronium. The prosecutors in the case stated that she failed to distinguish that vecuronium is dispensed as a powder and Versed as a liquid formula. The vecuronium has a red cap, which warns that it is a paralyzing agent. Ms. Vaught ignored these red flags, according to the prosecutors. Furthermore, the lawsuit filing documented her discussion that she was “distracted with something” at the time and admitted to overriding the medication warning.
Continue to: The charges in this case...
The charges in this case
The charges revolved around “criminally negligent homicide and gross neglect of an impaired adult,” the most notable charge being criminally negligent homicide. Potential consequences were up to an 8 years’ prison sentence.7
Furthermore, the Tennessee Board of Nursing revoked Ms. Vaught’s license in July 2021.8 The Board also reportedly fined her $3,000.9
The criminal proceedings were filed in Davidson County Criminal Court, with Judge Jennifer Smith presiding. Ms. Vaught repeatedly manifested remorse for the event. The patient’s family, including her son Michael and her daughters-in-law, provided tearful testimonies at the hearing. Ms. Vaught repeatedly cried during the testimonies. The nurse did not provide an apology, according to one daughter-in-law. The news media reported that the family did not want jail time for Ms. Vaught.7 Nurses across the country were “jolted,” as expressed by the news media.10
Why the controversy?
The entire issue of medical errors continues to be discussed among both the medical and the legal professions. To have a nursing personnel held to the level of criminal liability is unusual.
It was clear that Ms. Vaught took responsibility for her actions, and neither the prosecutors nor defendant attorneys sensed any evidence of malice on her part. On the other hand, there was enough evidence and concern for District Attorney Glenn Funk to proceed with prosecution-related action. Ms. Vaught was facing years in prison if convicted.
WHAT’S THE VERDICT?
In March 2022, the jury convicted Ms. Vaught of criminally negligent homicide—but not of reckless homicide, a more serious offense.
Judge Smith granted a judicial diversion, that is, the conviction would be expunged from the record if Ms. Vaught completed a 3-year probation. Judge Smith noted the “credible remorse expressed by Nurse Vaught” and went on to state, “this is a terrible, terrible, mistake and there have been consequences to the defendant.” In the courtroom, Ms. Vaught apologized to the patient’s family and conveyed that she will “forever be haunted by her role in the (patient’s) passing.”
Overall, this served as an opportunity for health care workers to address oftentimes poor working conditions, which have been exacerbated by the COVID-19 pandemic.
The Davidson County District Attorney’s office conveyed that this was one case of a careless nurse and not a reflection of the nursing profession. The prosecutors were in accord with a probation verdict. The family felt that their mother, the patient, would not want to see the nurse serve a jail sentence: “Mom was a very forgiving person.”
The patient’s cause of death was listed as “intracerebral hemorrhage and cardiac arrest.” One year later, a new death certificate was issued and noted vecuronium intoxication as the cause of death.
Continue to: The health care institution’s involvement...
The health care institution’s involvement
Approximately 1 year after an apparent anonymous tip was made to health care officials, an unscheduled state and federal investigation, with the threat of possible sanctions, occurred at the VUMC. This was predicated on the criminal indictment related to Ms. Vaught. In the end, her nursing license was revoked, as noted earlier. The family earlier reached an out-of-court settlement with the hospital and there were a number of problems identified at the university medical center.11
Legal principles in the case
Most criminal cases are state cases. Crimes are defined in state statutes, and the trial takes place in state courts. Thus, crimes are defined a little differently from state to state. Ms. Vaught, for example, was tried in Tennessee under the laws of that state.
Homicide involves the killing of a human being. It may not be a crime. For example, there is “justifiable homicide,” such as self-defense. At the other extreme is first-degree murder, an intentional and planned killing. In this case, Ms. Vaught was charged with criminally negligent homicide, which is usually the least serious of criminal homicides but is still a felony. (Some states have misdemeanor manslaughter, which was not an issue in this case.) In some states, criminally negligent homicide is sometimes referred to as involuntary manslaughter. The mens rea for involuntary manslaughter is generally recklessness or “criminal negligence.” This crime goes by various names depending on the state, but involuntary manslaughter and criminally negligent homicide are common names.
Ordinary negligence versus criminal negligence. Criminal negligence is usually considered a more serious mistake than ordinary negligence. This is where there is a difference between civil malpractice negligence and criminal negligence. Criminal negligence is somewhat more careless than ordinary negligence. To use a driving example, if Dr. A was driving home from the hospital, missed seeing a red light, and killed Joe Pedestrian, it could be ordinary negligence. If, however, Dr. B was texting or drinking while driving, causing Dr. B to be distracted and miss seeing the red light, killing a pedestrian, it could be criminal negligence and result in the conviction for the homicide. Of course, in either case there could be civil liability for causing the death.
Applying these legal principles to the reported facts in Ms. Vaught’s case, it appears there was more than simple negligence. That is, the nurse was more than careless. Using “VE” for the wrong drug might have been negligent. In addition, however, she disengaged a safeguard meant to prevent wrongful use of the drug, failed to notice that the drug was a powder instead of a liquid, and ignored the red cap warning that the drug was a paralyzing agent. It becomes apparent why the jury could have found aggravated or criminal negligence.
It is worth emphasizing that in this case, the criminal charges were unusual. For years, studies have suggested that many deaths result from medical errors. The Institute of Medicine famously said that the number of deaths from medical errors was equivalent to that of a 747 airplane crashing every day.12,13 These events result in a relatively small number of malpractice actions but an infinitesimally small number of homicide charges. Among other things, prosecutors are reluctant to pursue such cases regarding acts carried out as part of clinical duties unless there is strong evidence, and grand juries may be reluctant to indict medical professionals.14
Nonetheless, medical professionals ultimately can be criminally responsible for deaths resulting from intentional, or criminally negligent, careless practice. Such liability should not dissuade nurses or others from medical practice any more than the much more common homicide charges that can occur from driving an automobile carelessly that results in someone’s death. A fundamental purpose of the criminal law is to disincentivize unnecessarily harmful (deadly) conduct, whether it is distracted driving or distracted nursing.
Continue to: The drug-prescribing crimes...
The drug-prescribing crimes
The US Supreme Court considered a much different kind of criminal medical practice in 2 (consolidated) cases in its 2021–2022 Term. Physicians in 2 states were each tried and convicted of federal charges of illegally dispensing or distributing (prescribing) controlled substances.15 A federal statute makes it a felony for a physician, or others, “except as authorized” to “knowingly or intentionally distribute, or dispense a controlled substance.”16 Federal regulations clarify the statute. The regulation provides that a prescription is authorized only if a doctor issues it “for a legitimate medical purpose . . . acting in the usual course of professional practice.”17
CASE 2 Physicians charged with overprescribing controlled substances
In these 2 drug-prescribing cases, the physicians had grossly overprescribed the opioids. One reportedly wrote prescriptions in 2 states in exchange for payments in cash or, infrequently, firearms, approximating the cost of the prescriptions to street drugs. The other had a clinic that, over about 4 years, issued 300,000 prescriptions for controlled substances and was a significant source for some kinds of fentanyl.18
WHAT’S THE VERDICT?
In each trial, the juries found the defendant guilty of improper distribution of controlled substances. Although the charges were not homicides, the sentencing judges were much more severe than the court had been in the nursing case discussed above. One physician received a prison term of 20 years, the other, a 25-year term. These undoubtedly reflect both the outrageous conduct and the likely great harm the defendants did.
The Supreme Court heard the cases
The Supreme Court reversed these physicians’ convictions. The Court held that the lower courts had not correctly described for the juries the mens rea required for a conviction under these charges. The Supreme Court held that to be convicted of these offenses, the government had to prove “beyond a reasonable doubt that the defendant [physician] knew that he or she was acting in an unauthorized manner.”19 Both can be retried and probably will be unless they reach a plea agreement with the federal government. Nonetheless, the Court established a very high standard. Carelessness is not enough, but rather “knowingly” acting in an unauthorized way is required. Although these physicians were prosecuted under federal law, other physicians have been prosecuted under state laws limiting the distribution of controlled substances.20
Some physicians have expressed concern that the Supreme Court, in these cases, made the practice of medicine more dangerous for physicians (the threat of criminal sanctions) and patients (making it more difficult to obtain pain control, for example).21,22 That view may be overly pessimistic for 2 reasons. First, the Court actually made it more difficult to convict physicians of writing excessive prescriptions. It did so by setting a higher mens rea standard than lower courts were using, that is, the physician had to “knowingly” act in an unauthorized way. Because “knowingly” can be implied by the circumstances, taking guns or cash would be evidence that the physician knowingly misprescribed.
More fundamentally, the actions of these physicians appear to be well outside even a generous legitimate level of controlled substance prescription. These convictions should not be misunderstood as a way of federal courts to crack down on pain medications. However, the original convictions are a warning to the small handful who grossly overprescribe controlled substances.
Lessons about criminal law and the practice of medicine
Medical professionals’ strong reaction to criminal charges is understandable. Criminal charges can result in jail time (the physicians involved in the controlled substance case were sentenced to 20 years or more) and hefty fines; bring social and professional disapprobation; may lead to license discipline; and are terribly disruptive even for those found not guilty. To make matters worse, malpractice insurance ordinarily does not cover criminal charges, so any fines and the cost of defense are likely out of pocket for those charged—and that can be very expensive. Therefore, the strong reaction to the cases we have described is understandable.
At the same time, the probability of criminal charges against medical personnel for their medical treatment is very low compared with, for example, fraudulent billing, their driving habits, or tax avoidance. Criminal charges are much more likely to arise from insurance fraud, Medicare or Medicaid dishonesty, kickbacks, false statements, and similar corruption crimes rather than inadequate practice. In the cases we examined here, there is an enhanced or aggravated negligence in one case and grossly inappropriate prescribing in the others (which the Supreme Court held must be “knowingly” wrong).
Finally, there is an irony. Medical professionals worried about practice-related criminal charges should be thankful for the malpractice system. Civil malpractice is, as a practical matter, an alternative for patients who believe they were mistreated or harmed by physicians or other providers. They have the option of finding a private attorney to file a civil complaint. In the absence of that system, they would be much more likely to take their grievance and complaint to the prosecutor to seek answers and retribution. Criminal law and civil liability are each a way of allowing someone harmed by another to seek redress. Both are intended to deter harmful conduct and provide some individual and social retribution for such behavior. The civil system, of course, also provides the potential for compensation to those injured. An injured patient without the possibility of a civil suit sometimes would turn to the criminal system for satisfaction. This way, the malpractice system is a better alternative to criminal charges. ●
- Kelman B. As a nurse faces prison for a deadly error, her colleagues worry: could I be next? NPR. March 22, 2022. Accessed November 7, 2022. https://www.npr.org/sections/health-shots/2022/03/22/1087903348/as-a-nurse-faces-prison-for-a-deadly-error-her-colleagues-worry-could-i-be-next
- US Department of Justice. National health care fraud enforcement action results in charges involving over $1.4 billion in alleged losses. September 17, 2021. Accessed November 7, 2022. https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-charges-involving-over-14-billion
- Steinman G. Stuff of nightmares: criminal prosecution for malpractice. OBG Manag. 2008;20(8):35-45.
- Maher V, Cwiek M. Criminal liability for nursing and medical harm. Hosp Top. 2022 July 13;1-8.
- Singer RG. The resurgence of mens rea: III—the rise and fall of strict criminal liability. Boston Coll Law Rev. 1989;30:337-408. Accessed November 7, 2022. https://lawdigitalcommons.bc.edu/cgi/viewcontent.cgi?article=2431&context=bclr
- Sarch AF. Knowledge, recklessness and the connection requirement between actus reus and mens rea. Penn State Law Rev. 2015;120:1-51. Accessed November 7, 2022. https://ideas.dickinsonlaw.psu.edu/cgi/viewcontent.cgi?article=4120&context=dlra
- Timms M, Gluck F, Wegner R, et al. RaDonda Vaught sentenced to three years probation on a diverted sentence, could see record wiped. Tennessean. May 13, 2022. Accessed November 7, 2022. http://www.tennessean.com/story/news/crime/2022/05/13/radonda-vaught-sentened-vanderbilt-nurse/9717529002/
- Tennessee Board of Nursing. Disciplinary hearing: RaDonda Vaught, RN #205702, minutes. July 22-23, 2021. Accessed November 7, 2022. https://www.tn.gov/content/dam/tn/health/healthprofboards/nursing/meeting-minutes/Nursing%20Meeting%20Minutes%20July%2022-23,%202021.pdf
- Institute for Safe Medication Practices. TN Board of Nursing’s unjust decision to revoke nurse’s license: travesty on top of tragedy! August 12, 2021. Accessed November 7, 2022. https://www.ismp.org/resources/tn-board-nursings-unjust-decision-revoke-nurses-license-travesty-top-tragedy
- Medina E. Ex-nurse convicted in fatal medication error gets probation. New York Times. May 15, 2022. Accessed November 7, 2022. https://www.nytimes.com/2022/05/15/us/tennessee-nurse-sentencing.html
- Kelman B. In nurse’s trial, investigator says hospital bears ‘heavy’ responsibility for patient death. KHN. March 24, 2022. Accessed November 15, 2022. https://khn.org/news/article/radonda-vaught-fatal-drug-error-vanderbilt-hospital-responsibility/
- Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, ed. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
- Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Affairs. 2018;37:1736-1743.
- Eisenberg RL, Berlin L. When does malpractice become manslaughter? Am J Roentgenol. 2002;179:331-335.
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1410_1an2.pdf
- 84 Stat. 1260, 21 U. S. C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Liptak A. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022.
- Xiulu Ruan v United States, at 2 (slip opinion).
- Pedemonte S. State v. Christensen: criminalizing medical malpractice. Montana Law Rev. 2022;83(1):183-193. Accessed November 7, 2022. https://scholarworks.umt.edu/cgi/view content.cgi?article=2497&context=mlr
- Szalavitz M. A recent Supreme Court ruling will help people in pain. Scientific American. September 19, 2022. Accessed November 15, 2022. https://www.scientificamerican.com/ article/a-recent-supreme-court-ruling-will-help-people-in -pain/
- Lopez I. Opioid pill peddling case threatens future of pain treatment. Bloomberg Law. March 29, 2022. Accessed November 15, 2022. https://news.bloomberglaw.com/health -law-and-business/opioid-pill-peddling-case-threatens -future-of-pain-treatment
Medical professionals are well aware that civil liability (malpractice) may incur when a patient is harmed because of carelessness (negligence). Recent criminal charges against physicians and a nurse, however, have called medical professionals’ attention to the fact that they also may face criminal charges for inappropriate practice.
We cite 2 cases in which criminal liability resulted from bad medical practice. In both instances, there was considerable concern among medical professionals that criminal charges for making a mistake would make it difficult to practice without fear of criminal charges. One concern is that criminal charges could drive good people out of the profession or make them too cautious.1
We look more closely at those 2 cases in which criminal liability was imposed. These cases are outliers. Relatively few criminal cases against medical professionals are based on the quality of care. (There are, however, more criminal charges related to fraudulent billing and other insurance fraud, kickbacks, Medicare and Medicaid abuse, and the like.2) At the same time, the criminal law does not stop at the front door of a clinic or hospital.3 When medical professionals engage in seriously inappropriate health care conduct that directly harms someone, criminal liability may result.4
Anatomy of a crime
Crimes generally require a specific mental state (mens rea) and an act (actus reus). The law specifies the mental state required for conviction. It can range from premeditation—once commonly called “malice aforethought”—to negligence. The mens rea requirement is an essential element of the crime—as we will see in the discussion of the prescription drug cases. A few offenses do not require even negligence, but overwhelmingly, crimes require something more than simple negligence.5
The act requirement is generally obvious, such as firing a gun, driving while intoxicated, or recklessly giving inappropriate medication to a patient. It may include “attempts,” crimes where an act was not completed. For example, attempted murder or conspiracy to commit do not require a completed offense, only intent plus overt acts toward carrying out the crime. Similarly, the wrongful act usually has to produce some harm, but again there are exceptions (attempts). To obtain a conviction, the prosecution must prove all of the elements of the crime, including the required mens rea, beyond a reasonable doubt.6
With this general background, we turn to the first case, in which the charge was a form of homicide. Please note that the following case description was derived from news descriptions of the case, because juries do not publish opinions concerning their conclusions and court documents are unavailable. The public reports therefore may contain factual gaps and errors.
CASE 1 Patient dies after nurse administers wrong drug
RaDonda Vaught, a 38-year-old experienced registered nurse employed at Vanderbilt University Medical Center (VUMC) in the intensive care unit (ICU), was providing care for a 76-year-old patient who was admitted to VUMC’s ICU in December 2017 in association with a brain injury. The brain injury involved a fall with resultant subdural hematoma. In preparation for a positron emission tomography (PET) scan to assess the patient’s injury, the physician team prescribed the sedative Versed (midazolam) because of the patient’s claustrophobia. During the course of treatment, Ms. Vaught inadvertently administered the wrong drug, a fatal dose of the muscle relaxant vecuronium, to the patient, which resulted in the patient being unable to breathe. Apparently, Ms. Vaught had been unable to find the midazolam and disengaged a safeguard, proceeding into override mode, and thus vecuronium was dispensed. By the time the error was noticed, the patient was already in cardiac arrest with resultant brain damage (partial brain death). The patient died soon thereafter.
How this medication error occurred
The medication error occurred when Ms. Vaught overrode a computer in the medical system when she could not find the “Versed” entry and typed in “VE,” which was the abbreviation for vecuronium. The prosecutors in the case stated that she failed to distinguish that vecuronium is dispensed as a powder and Versed as a liquid formula. The vecuronium has a red cap, which warns that it is a paralyzing agent. Ms. Vaught ignored these red flags, according to the prosecutors. Furthermore, the lawsuit filing documented her discussion that she was “distracted with something” at the time and admitted to overriding the medication warning.
Continue to: The charges in this case...
The charges in this case
The charges revolved around “criminally negligent homicide and gross neglect of an impaired adult,” the most notable charge being criminally negligent homicide. Potential consequences were up to an 8 years’ prison sentence.7
Furthermore, the Tennessee Board of Nursing revoked Ms. Vaught’s license in July 2021.8 The Board also reportedly fined her $3,000.9
The criminal proceedings were filed in Davidson County Criminal Court, with Judge Jennifer Smith presiding. Ms. Vaught repeatedly manifested remorse for the event. The patient’s family, including her son Michael and her daughters-in-law, provided tearful testimonies at the hearing. Ms. Vaught repeatedly cried during the testimonies. The nurse did not provide an apology, according to one daughter-in-law. The news media reported that the family did not want jail time for Ms. Vaught.7 Nurses across the country were “jolted,” as expressed by the news media.10
Why the controversy?
The entire issue of medical errors continues to be discussed among both the medical and the legal professions. To have a nursing personnel held to the level of criminal liability is unusual.
It was clear that Ms. Vaught took responsibility for her actions, and neither the prosecutors nor defendant attorneys sensed any evidence of malice on her part. On the other hand, there was enough evidence and concern for District Attorney Glenn Funk to proceed with prosecution-related action. Ms. Vaught was facing years in prison if convicted.
WHAT’S THE VERDICT?
In March 2022, the jury convicted Ms. Vaught of criminally negligent homicide—but not of reckless homicide, a more serious offense.
Judge Smith granted a judicial diversion, that is, the conviction would be expunged from the record if Ms. Vaught completed a 3-year probation. Judge Smith noted the “credible remorse expressed by Nurse Vaught” and went on to state, “this is a terrible, terrible, mistake and there have been consequences to the defendant.” In the courtroom, Ms. Vaught apologized to the patient’s family and conveyed that she will “forever be haunted by her role in the (patient’s) passing.”
Overall, this served as an opportunity for health care workers to address oftentimes poor working conditions, which have been exacerbated by the COVID-19 pandemic.
The Davidson County District Attorney’s office conveyed that this was one case of a careless nurse and not a reflection of the nursing profession. The prosecutors were in accord with a probation verdict. The family felt that their mother, the patient, would not want to see the nurse serve a jail sentence: “Mom was a very forgiving person.”
The patient’s cause of death was listed as “intracerebral hemorrhage and cardiac arrest.” One year later, a new death certificate was issued and noted vecuronium intoxication as the cause of death.
Continue to: The health care institution’s involvement...
The health care institution’s involvement
Approximately 1 year after an apparent anonymous tip was made to health care officials, an unscheduled state and federal investigation, with the threat of possible sanctions, occurred at the VUMC. This was predicated on the criminal indictment related to Ms. Vaught. In the end, her nursing license was revoked, as noted earlier. The family earlier reached an out-of-court settlement with the hospital and there were a number of problems identified at the university medical center.11
Legal principles in the case
Most criminal cases are state cases. Crimes are defined in state statutes, and the trial takes place in state courts. Thus, crimes are defined a little differently from state to state. Ms. Vaught, for example, was tried in Tennessee under the laws of that state.
Homicide involves the killing of a human being. It may not be a crime. For example, there is “justifiable homicide,” such as self-defense. At the other extreme is first-degree murder, an intentional and planned killing. In this case, Ms. Vaught was charged with criminally negligent homicide, which is usually the least serious of criminal homicides but is still a felony. (Some states have misdemeanor manslaughter, which was not an issue in this case.) In some states, criminally negligent homicide is sometimes referred to as involuntary manslaughter. The mens rea for involuntary manslaughter is generally recklessness or “criminal negligence.” This crime goes by various names depending on the state, but involuntary manslaughter and criminally negligent homicide are common names.
Ordinary negligence versus criminal negligence. Criminal negligence is usually considered a more serious mistake than ordinary negligence. This is where there is a difference between civil malpractice negligence and criminal negligence. Criminal negligence is somewhat more careless than ordinary negligence. To use a driving example, if Dr. A was driving home from the hospital, missed seeing a red light, and killed Joe Pedestrian, it could be ordinary negligence. If, however, Dr. B was texting or drinking while driving, causing Dr. B to be distracted and miss seeing the red light, killing a pedestrian, it could be criminal negligence and result in the conviction for the homicide. Of course, in either case there could be civil liability for causing the death.
Applying these legal principles to the reported facts in Ms. Vaught’s case, it appears there was more than simple negligence. That is, the nurse was more than careless. Using “VE” for the wrong drug might have been negligent. In addition, however, she disengaged a safeguard meant to prevent wrongful use of the drug, failed to notice that the drug was a powder instead of a liquid, and ignored the red cap warning that the drug was a paralyzing agent. It becomes apparent why the jury could have found aggravated or criminal negligence.
It is worth emphasizing that in this case, the criminal charges were unusual. For years, studies have suggested that many deaths result from medical errors. The Institute of Medicine famously said that the number of deaths from medical errors was equivalent to that of a 747 airplane crashing every day.12,13 These events result in a relatively small number of malpractice actions but an infinitesimally small number of homicide charges. Among other things, prosecutors are reluctant to pursue such cases regarding acts carried out as part of clinical duties unless there is strong evidence, and grand juries may be reluctant to indict medical professionals.14
Nonetheless, medical professionals ultimately can be criminally responsible for deaths resulting from intentional, or criminally negligent, careless practice. Such liability should not dissuade nurses or others from medical practice any more than the much more common homicide charges that can occur from driving an automobile carelessly that results in someone’s death. A fundamental purpose of the criminal law is to disincentivize unnecessarily harmful (deadly) conduct, whether it is distracted driving or distracted nursing.
Continue to: The drug-prescribing crimes...
The drug-prescribing crimes
The US Supreme Court considered a much different kind of criminal medical practice in 2 (consolidated) cases in its 2021–2022 Term. Physicians in 2 states were each tried and convicted of federal charges of illegally dispensing or distributing (prescribing) controlled substances.15 A federal statute makes it a felony for a physician, or others, “except as authorized” to “knowingly or intentionally distribute, or dispense a controlled substance.”16 Federal regulations clarify the statute. The regulation provides that a prescription is authorized only if a doctor issues it “for a legitimate medical purpose . . . acting in the usual course of professional practice.”17
CASE 2 Physicians charged with overprescribing controlled substances
In these 2 drug-prescribing cases, the physicians had grossly overprescribed the opioids. One reportedly wrote prescriptions in 2 states in exchange for payments in cash or, infrequently, firearms, approximating the cost of the prescriptions to street drugs. The other had a clinic that, over about 4 years, issued 300,000 prescriptions for controlled substances and was a significant source for some kinds of fentanyl.18
WHAT’S THE VERDICT?
In each trial, the juries found the defendant guilty of improper distribution of controlled substances. Although the charges were not homicides, the sentencing judges were much more severe than the court had been in the nursing case discussed above. One physician received a prison term of 20 years, the other, a 25-year term. These undoubtedly reflect both the outrageous conduct and the likely great harm the defendants did.
The Supreme Court heard the cases
The Supreme Court reversed these physicians’ convictions. The Court held that the lower courts had not correctly described for the juries the mens rea required for a conviction under these charges. The Supreme Court held that to be convicted of these offenses, the government had to prove “beyond a reasonable doubt that the defendant [physician] knew that he or she was acting in an unauthorized manner.”19 Both can be retried and probably will be unless they reach a plea agreement with the federal government. Nonetheless, the Court established a very high standard. Carelessness is not enough, but rather “knowingly” acting in an unauthorized way is required. Although these physicians were prosecuted under federal law, other physicians have been prosecuted under state laws limiting the distribution of controlled substances.20
Some physicians have expressed concern that the Supreme Court, in these cases, made the practice of medicine more dangerous for physicians (the threat of criminal sanctions) and patients (making it more difficult to obtain pain control, for example).21,22 That view may be overly pessimistic for 2 reasons. First, the Court actually made it more difficult to convict physicians of writing excessive prescriptions. It did so by setting a higher mens rea standard than lower courts were using, that is, the physician had to “knowingly” act in an unauthorized way. Because “knowingly” can be implied by the circumstances, taking guns or cash would be evidence that the physician knowingly misprescribed.
More fundamentally, the actions of these physicians appear to be well outside even a generous legitimate level of controlled substance prescription. These convictions should not be misunderstood as a way of federal courts to crack down on pain medications. However, the original convictions are a warning to the small handful who grossly overprescribe controlled substances.
Lessons about criminal law and the practice of medicine
Medical professionals’ strong reaction to criminal charges is understandable. Criminal charges can result in jail time (the physicians involved in the controlled substance case were sentenced to 20 years or more) and hefty fines; bring social and professional disapprobation; may lead to license discipline; and are terribly disruptive even for those found not guilty. To make matters worse, malpractice insurance ordinarily does not cover criminal charges, so any fines and the cost of defense are likely out of pocket for those charged—and that can be very expensive. Therefore, the strong reaction to the cases we have described is understandable.
At the same time, the probability of criminal charges against medical personnel for their medical treatment is very low compared with, for example, fraudulent billing, their driving habits, or tax avoidance. Criminal charges are much more likely to arise from insurance fraud, Medicare or Medicaid dishonesty, kickbacks, false statements, and similar corruption crimes rather than inadequate practice. In the cases we examined here, there is an enhanced or aggravated negligence in one case and grossly inappropriate prescribing in the others (which the Supreme Court held must be “knowingly” wrong).
Finally, there is an irony. Medical professionals worried about practice-related criminal charges should be thankful for the malpractice system. Civil malpractice is, as a practical matter, an alternative for patients who believe they were mistreated or harmed by physicians or other providers. They have the option of finding a private attorney to file a civil complaint. In the absence of that system, they would be much more likely to take their grievance and complaint to the prosecutor to seek answers and retribution. Criminal law and civil liability are each a way of allowing someone harmed by another to seek redress. Both are intended to deter harmful conduct and provide some individual and social retribution for such behavior. The civil system, of course, also provides the potential for compensation to those injured. An injured patient without the possibility of a civil suit sometimes would turn to the criminal system for satisfaction. This way, the malpractice system is a better alternative to criminal charges. ●
Medical professionals are well aware that civil liability (malpractice) may incur when a patient is harmed because of carelessness (negligence). Recent criminal charges against physicians and a nurse, however, have called medical professionals’ attention to the fact that they also may face criminal charges for inappropriate practice.
We cite 2 cases in which criminal liability resulted from bad medical practice. In both instances, there was considerable concern among medical professionals that criminal charges for making a mistake would make it difficult to practice without fear of criminal charges. One concern is that criminal charges could drive good people out of the profession or make them too cautious.1
We look more closely at those 2 cases in which criminal liability was imposed. These cases are outliers. Relatively few criminal cases against medical professionals are based on the quality of care. (There are, however, more criminal charges related to fraudulent billing and other insurance fraud, kickbacks, Medicare and Medicaid abuse, and the like.2) At the same time, the criminal law does not stop at the front door of a clinic or hospital.3 When medical professionals engage in seriously inappropriate health care conduct that directly harms someone, criminal liability may result.4
Anatomy of a crime
Crimes generally require a specific mental state (mens rea) and an act (actus reus). The law specifies the mental state required for conviction. It can range from premeditation—once commonly called “malice aforethought”—to negligence. The mens rea requirement is an essential element of the crime—as we will see in the discussion of the prescription drug cases. A few offenses do not require even negligence, but overwhelmingly, crimes require something more than simple negligence.5
The act requirement is generally obvious, such as firing a gun, driving while intoxicated, or recklessly giving inappropriate medication to a patient. It may include “attempts,” crimes where an act was not completed. For example, attempted murder or conspiracy to commit do not require a completed offense, only intent plus overt acts toward carrying out the crime. Similarly, the wrongful act usually has to produce some harm, but again there are exceptions (attempts). To obtain a conviction, the prosecution must prove all of the elements of the crime, including the required mens rea, beyond a reasonable doubt.6
With this general background, we turn to the first case, in which the charge was a form of homicide. Please note that the following case description was derived from news descriptions of the case, because juries do not publish opinions concerning their conclusions and court documents are unavailable. The public reports therefore may contain factual gaps and errors.
CASE 1 Patient dies after nurse administers wrong drug
RaDonda Vaught, a 38-year-old experienced registered nurse employed at Vanderbilt University Medical Center (VUMC) in the intensive care unit (ICU), was providing care for a 76-year-old patient who was admitted to VUMC’s ICU in December 2017 in association with a brain injury. The brain injury involved a fall with resultant subdural hematoma. In preparation for a positron emission tomography (PET) scan to assess the patient’s injury, the physician team prescribed the sedative Versed (midazolam) because of the patient’s claustrophobia. During the course of treatment, Ms. Vaught inadvertently administered the wrong drug, a fatal dose of the muscle relaxant vecuronium, to the patient, which resulted in the patient being unable to breathe. Apparently, Ms. Vaught had been unable to find the midazolam and disengaged a safeguard, proceeding into override mode, and thus vecuronium was dispensed. By the time the error was noticed, the patient was already in cardiac arrest with resultant brain damage (partial brain death). The patient died soon thereafter.
How this medication error occurred
The medication error occurred when Ms. Vaught overrode a computer in the medical system when she could not find the “Versed” entry and typed in “VE,” which was the abbreviation for vecuronium. The prosecutors in the case stated that she failed to distinguish that vecuronium is dispensed as a powder and Versed as a liquid formula. The vecuronium has a red cap, which warns that it is a paralyzing agent. Ms. Vaught ignored these red flags, according to the prosecutors. Furthermore, the lawsuit filing documented her discussion that she was “distracted with something” at the time and admitted to overriding the medication warning.
Continue to: The charges in this case...
The charges in this case
The charges revolved around “criminally negligent homicide and gross neglect of an impaired adult,” the most notable charge being criminally negligent homicide. Potential consequences were up to an 8 years’ prison sentence.7
Furthermore, the Tennessee Board of Nursing revoked Ms. Vaught’s license in July 2021.8 The Board also reportedly fined her $3,000.9
The criminal proceedings were filed in Davidson County Criminal Court, with Judge Jennifer Smith presiding. Ms. Vaught repeatedly manifested remorse for the event. The patient’s family, including her son Michael and her daughters-in-law, provided tearful testimonies at the hearing. Ms. Vaught repeatedly cried during the testimonies. The nurse did not provide an apology, according to one daughter-in-law. The news media reported that the family did not want jail time for Ms. Vaught.7 Nurses across the country were “jolted,” as expressed by the news media.10
Why the controversy?
The entire issue of medical errors continues to be discussed among both the medical and the legal professions. To have a nursing personnel held to the level of criminal liability is unusual.
It was clear that Ms. Vaught took responsibility for her actions, and neither the prosecutors nor defendant attorneys sensed any evidence of malice on her part. On the other hand, there was enough evidence and concern for District Attorney Glenn Funk to proceed with prosecution-related action. Ms. Vaught was facing years in prison if convicted.
WHAT’S THE VERDICT?
In March 2022, the jury convicted Ms. Vaught of criminally negligent homicide—but not of reckless homicide, a more serious offense.
Judge Smith granted a judicial diversion, that is, the conviction would be expunged from the record if Ms. Vaught completed a 3-year probation. Judge Smith noted the “credible remorse expressed by Nurse Vaught” and went on to state, “this is a terrible, terrible, mistake and there have been consequences to the defendant.” In the courtroom, Ms. Vaught apologized to the patient’s family and conveyed that she will “forever be haunted by her role in the (patient’s) passing.”
Overall, this served as an opportunity for health care workers to address oftentimes poor working conditions, which have been exacerbated by the COVID-19 pandemic.
The Davidson County District Attorney’s office conveyed that this was one case of a careless nurse and not a reflection of the nursing profession. The prosecutors were in accord with a probation verdict. The family felt that their mother, the patient, would not want to see the nurse serve a jail sentence: “Mom was a very forgiving person.”
The patient’s cause of death was listed as “intracerebral hemorrhage and cardiac arrest.” One year later, a new death certificate was issued and noted vecuronium intoxication as the cause of death.
Continue to: The health care institution’s involvement...
The health care institution’s involvement
Approximately 1 year after an apparent anonymous tip was made to health care officials, an unscheduled state and federal investigation, with the threat of possible sanctions, occurred at the VUMC. This was predicated on the criminal indictment related to Ms. Vaught. In the end, her nursing license was revoked, as noted earlier. The family earlier reached an out-of-court settlement with the hospital and there were a number of problems identified at the university medical center.11
Legal principles in the case
Most criminal cases are state cases. Crimes are defined in state statutes, and the trial takes place in state courts. Thus, crimes are defined a little differently from state to state. Ms. Vaught, for example, was tried in Tennessee under the laws of that state.
Homicide involves the killing of a human being. It may not be a crime. For example, there is “justifiable homicide,” such as self-defense. At the other extreme is first-degree murder, an intentional and planned killing. In this case, Ms. Vaught was charged with criminally negligent homicide, which is usually the least serious of criminal homicides but is still a felony. (Some states have misdemeanor manslaughter, which was not an issue in this case.) In some states, criminally negligent homicide is sometimes referred to as involuntary manslaughter. The mens rea for involuntary manslaughter is generally recklessness or “criminal negligence.” This crime goes by various names depending on the state, but involuntary manslaughter and criminally negligent homicide are common names.
Ordinary negligence versus criminal negligence. Criminal negligence is usually considered a more serious mistake than ordinary negligence. This is where there is a difference between civil malpractice negligence and criminal negligence. Criminal negligence is somewhat more careless than ordinary negligence. To use a driving example, if Dr. A was driving home from the hospital, missed seeing a red light, and killed Joe Pedestrian, it could be ordinary negligence. If, however, Dr. B was texting or drinking while driving, causing Dr. B to be distracted and miss seeing the red light, killing a pedestrian, it could be criminal negligence and result in the conviction for the homicide. Of course, in either case there could be civil liability for causing the death.
Applying these legal principles to the reported facts in Ms. Vaught’s case, it appears there was more than simple negligence. That is, the nurse was more than careless. Using “VE” for the wrong drug might have been negligent. In addition, however, she disengaged a safeguard meant to prevent wrongful use of the drug, failed to notice that the drug was a powder instead of a liquid, and ignored the red cap warning that the drug was a paralyzing agent. It becomes apparent why the jury could have found aggravated or criminal negligence.
It is worth emphasizing that in this case, the criminal charges were unusual. For years, studies have suggested that many deaths result from medical errors. The Institute of Medicine famously said that the number of deaths from medical errors was equivalent to that of a 747 airplane crashing every day.12,13 These events result in a relatively small number of malpractice actions but an infinitesimally small number of homicide charges. Among other things, prosecutors are reluctant to pursue such cases regarding acts carried out as part of clinical duties unless there is strong evidence, and grand juries may be reluctant to indict medical professionals.14
Nonetheless, medical professionals ultimately can be criminally responsible for deaths resulting from intentional, or criminally negligent, careless practice. Such liability should not dissuade nurses or others from medical practice any more than the much more common homicide charges that can occur from driving an automobile carelessly that results in someone’s death. A fundamental purpose of the criminal law is to disincentivize unnecessarily harmful (deadly) conduct, whether it is distracted driving or distracted nursing.
Continue to: The drug-prescribing crimes...
The drug-prescribing crimes
The US Supreme Court considered a much different kind of criminal medical practice in 2 (consolidated) cases in its 2021–2022 Term. Physicians in 2 states were each tried and convicted of federal charges of illegally dispensing or distributing (prescribing) controlled substances.15 A federal statute makes it a felony for a physician, or others, “except as authorized” to “knowingly or intentionally distribute, or dispense a controlled substance.”16 Federal regulations clarify the statute. The regulation provides that a prescription is authorized only if a doctor issues it “for a legitimate medical purpose . . . acting in the usual course of professional practice.”17
CASE 2 Physicians charged with overprescribing controlled substances
In these 2 drug-prescribing cases, the physicians had grossly overprescribed the opioids. One reportedly wrote prescriptions in 2 states in exchange for payments in cash or, infrequently, firearms, approximating the cost of the prescriptions to street drugs. The other had a clinic that, over about 4 years, issued 300,000 prescriptions for controlled substances and was a significant source for some kinds of fentanyl.18
WHAT’S THE VERDICT?
In each trial, the juries found the defendant guilty of improper distribution of controlled substances. Although the charges were not homicides, the sentencing judges were much more severe than the court had been in the nursing case discussed above. One physician received a prison term of 20 years, the other, a 25-year term. These undoubtedly reflect both the outrageous conduct and the likely great harm the defendants did.
The Supreme Court heard the cases
The Supreme Court reversed these physicians’ convictions. The Court held that the lower courts had not correctly described for the juries the mens rea required for a conviction under these charges. The Supreme Court held that to be convicted of these offenses, the government had to prove “beyond a reasonable doubt that the defendant [physician] knew that he or she was acting in an unauthorized manner.”19 Both can be retried and probably will be unless they reach a plea agreement with the federal government. Nonetheless, the Court established a very high standard. Carelessness is not enough, but rather “knowingly” acting in an unauthorized way is required. Although these physicians were prosecuted under federal law, other physicians have been prosecuted under state laws limiting the distribution of controlled substances.20
Some physicians have expressed concern that the Supreme Court, in these cases, made the practice of medicine more dangerous for physicians (the threat of criminal sanctions) and patients (making it more difficult to obtain pain control, for example).21,22 That view may be overly pessimistic for 2 reasons. First, the Court actually made it more difficult to convict physicians of writing excessive prescriptions. It did so by setting a higher mens rea standard than lower courts were using, that is, the physician had to “knowingly” act in an unauthorized way. Because “knowingly” can be implied by the circumstances, taking guns or cash would be evidence that the physician knowingly misprescribed.
More fundamentally, the actions of these physicians appear to be well outside even a generous legitimate level of controlled substance prescription. These convictions should not be misunderstood as a way of federal courts to crack down on pain medications. However, the original convictions are a warning to the small handful who grossly overprescribe controlled substances.
Lessons about criminal law and the practice of medicine
Medical professionals’ strong reaction to criminal charges is understandable. Criminal charges can result in jail time (the physicians involved in the controlled substance case were sentenced to 20 years or more) and hefty fines; bring social and professional disapprobation; may lead to license discipline; and are terribly disruptive even for those found not guilty. To make matters worse, malpractice insurance ordinarily does not cover criminal charges, so any fines and the cost of defense are likely out of pocket for those charged—and that can be very expensive. Therefore, the strong reaction to the cases we have described is understandable.
At the same time, the probability of criminal charges against medical personnel for their medical treatment is very low compared with, for example, fraudulent billing, their driving habits, or tax avoidance. Criminal charges are much more likely to arise from insurance fraud, Medicare or Medicaid dishonesty, kickbacks, false statements, and similar corruption crimes rather than inadequate practice. In the cases we examined here, there is an enhanced or aggravated negligence in one case and grossly inappropriate prescribing in the others (which the Supreme Court held must be “knowingly” wrong).
Finally, there is an irony. Medical professionals worried about practice-related criminal charges should be thankful for the malpractice system. Civil malpractice is, as a practical matter, an alternative for patients who believe they were mistreated or harmed by physicians or other providers. They have the option of finding a private attorney to file a civil complaint. In the absence of that system, they would be much more likely to take their grievance and complaint to the prosecutor to seek answers and retribution. Criminal law and civil liability are each a way of allowing someone harmed by another to seek redress. Both are intended to deter harmful conduct and provide some individual and social retribution for such behavior. The civil system, of course, also provides the potential for compensation to those injured. An injured patient without the possibility of a civil suit sometimes would turn to the criminal system for satisfaction. This way, the malpractice system is a better alternative to criminal charges. ●
- Kelman B. As a nurse faces prison for a deadly error, her colleagues worry: could I be next? NPR. March 22, 2022. Accessed November 7, 2022. https://www.npr.org/sections/health-shots/2022/03/22/1087903348/as-a-nurse-faces-prison-for-a-deadly-error-her-colleagues-worry-could-i-be-next
- US Department of Justice. National health care fraud enforcement action results in charges involving over $1.4 billion in alleged losses. September 17, 2021. Accessed November 7, 2022. https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-charges-involving-over-14-billion
- Steinman G. Stuff of nightmares: criminal prosecution for malpractice. OBG Manag. 2008;20(8):35-45.
- Maher V, Cwiek M. Criminal liability for nursing and medical harm. Hosp Top. 2022 July 13;1-8.
- Singer RG. The resurgence of mens rea: III—the rise and fall of strict criminal liability. Boston Coll Law Rev. 1989;30:337-408. Accessed November 7, 2022. https://lawdigitalcommons.bc.edu/cgi/viewcontent.cgi?article=2431&context=bclr
- Sarch AF. Knowledge, recklessness and the connection requirement between actus reus and mens rea. Penn State Law Rev. 2015;120:1-51. Accessed November 7, 2022. https://ideas.dickinsonlaw.psu.edu/cgi/viewcontent.cgi?article=4120&context=dlra
- Timms M, Gluck F, Wegner R, et al. RaDonda Vaught sentenced to three years probation on a diverted sentence, could see record wiped. Tennessean. May 13, 2022. Accessed November 7, 2022. http://www.tennessean.com/story/news/crime/2022/05/13/radonda-vaught-sentened-vanderbilt-nurse/9717529002/
- Tennessee Board of Nursing. Disciplinary hearing: RaDonda Vaught, RN #205702, minutes. July 22-23, 2021. Accessed November 7, 2022. https://www.tn.gov/content/dam/tn/health/healthprofboards/nursing/meeting-minutes/Nursing%20Meeting%20Minutes%20July%2022-23,%202021.pdf
- Institute for Safe Medication Practices. TN Board of Nursing’s unjust decision to revoke nurse’s license: travesty on top of tragedy! August 12, 2021. Accessed November 7, 2022. https://www.ismp.org/resources/tn-board-nursings-unjust-decision-revoke-nurses-license-travesty-top-tragedy
- Medina E. Ex-nurse convicted in fatal medication error gets probation. New York Times. May 15, 2022. Accessed November 7, 2022. https://www.nytimes.com/2022/05/15/us/tennessee-nurse-sentencing.html
- Kelman B. In nurse’s trial, investigator says hospital bears ‘heavy’ responsibility for patient death. KHN. March 24, 2022. Accessed November 15, 2022. https://khn.org/news/article/radonda-vaught-fatal-drug-error-vanderbilt-hospital-responsibility/
- Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, ed. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
- Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Affairs. 2018;37:1736-1743.
- Eisenberg RL, Berlin L. When does malpractice become manslaughter? Am J Roentgenol. 2002;179:331-335.
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1410_1an2.pdf
- 84 Stat. 1260, 21 U. S. C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Liptak A. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022.
- Xiulu Ruan v United States, at 2 (slip opinion).
- Pedemonte S. State v. Christensen: criminalizing medical malpractice. Montana Law Rev. 2022;83(1):183-193. Accessed November 7, 2022. https://scholarworks.umt.edu/cgi/view content.cgi?article=2497&context=mlr
- Szalavitz M. A recent Supreme Court ruling will help people in pain. Scientific American. September 19, 2022. Accessed November 15, 2022. https://www.scientificamerican.com/ article/a-recent-supreme-court-ruling-will-help-people-in -pain/
- Lopez I. Opioid pill peddling case threatens future of pain treatment. Bloomberg Law. March 29, 2022. Accessed November 15, 2022. https://news.bloomberglaw.com/health -law-and-business/opioid-pill-peddling-case-threatens -future-of-pain-treatment
- Kelman B. As a nurse faces prison for a deadly error, her colleagues worry: could I be next? NPR. March 22, 2022. Accessed November 7, 2022. https://www.npr.org/sections/health-shots/2022/03/22/1087903348/as-a-nurse-faces-prison-for-a-deadly-error-her-colleagues-worry-could-i-be-next
- US Department of Justice. National health care fraud enforcement action results in charges involving over $1.4 billion in alleged losses. September 17, 2021. Accessed November 7, 2022. https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-charges-involving-over-14-billion
- Steinman G. Stuff of nightmares: criminal prosecution for malpractice. OBG Manag. 2008;20(8):35-45.
- Maher V, Cwiek M. Criminal liability for nursing and medical harm. Hosp Top. 2022 July 13;1-8.
- Singer RG. The resurgence of mens rea: III—the rise and fall of strict criminal liability. Boston Coll Law Rev. 1989;30:337-408. Accessed November 7, 2022. https://lawdigitalcommons.bc.edu/cgi/viewcontent.cgi?article=2431&context=bclr
- Sarch AF. Knowledge, recklessness and the connection requirement between actus reus and mens rea. Penn State Law Rev. 2015;120:1-51. Accessed November 7, 2022. https://ideas.dickinsonlaw.psu.edu/cgi/viewcontent.cgi?article=4120&context=dlra
- Timms M, Gluck F, Wegner R, et al. RaDonda Vaught sentenced to three years probation on a diverted sentence, could see record wiped. Tennessean. May 13, 2022. Accessed November 7, 2022. http://www.tennessean.com/story/news/crime/2022/05/13/radonda-vaught-sentened-vanderbilt-nurse/9717529002/
- Tennessee Board of Nursing. Disciplinary hearing: RaDonda Vaught, RN #205702, minutes. July 22-23, 2021. Accessed November 7, 2022. https://www.tn.gov/content/dam/tn/health/healthprofboards/nursing/meeting-minutes/Nursing%20Meeting%20Minutes%20July%2022-23,%202021.pdf
- Institute for Safe Medication Practices. TN Board of Nursing’s unjust decision to revoke nurse’s license: travesty on top of tragedy! August 12, 2021. Accessed November 7, 2022. https://www.ismp.org/resources/tn-board-nursings-unjust-decision-revoke-nurses-license-travesty-top-tragedy
- Medina E. Ex-nurse convicted in fatal medication error gets probation. New York Times. May 15, 2022. Accessed November 7, 2022. https://www.nytimes.com/2022/05/15/us/tennessee-nurse-sentencing.html
- Kelman B. In nurse’s trial, investigator says hospital bears ‘heavy’ responsibility for patient death. KHN. March 24, 2022. Accessed November 15, 2022. https://khn.org/news/article/radonda-vaught-fatal-drug-error-vanderbilt-hospital-responsibility/
- Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, ed. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
- Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Affairs. 2018;37:1736-1743.
- Eisenberg RL, Berlin L. When does malpractice become manslaughter? Am J Roentgenol. 2002;179:331-335.
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1410_1an2.pdf
- 84 Stat. 1260, 21 U. S. C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Liptak A. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022.
- Xiulu Ruan v United States, at 2 (slip opinion).
- Pedemonte S. State v. Christensen: criminalizing medical malpractice. Montana Law Rev. 2022;83(1):183-193. Accessed November 7, 2022. https://scholarworks.umt.edu/cgi/view content.cgi?article=2497&context=mlr
- Szalavitz M. A recent Supreme Court ruling will help people in pain. Scientific American. September 19, 2022. Accessed November 15, 2022. https://www.scientificamerican.com/ article/a-recent-supreme-court-ruling-will-help-people-in -pain/
- Lopez I. Opioid pill peddling case threatens future of pain treatment. Bloomberg Law. March 29, 2022. Accessed November 15, 2022. https://news.bloomberglaw.com/health -law-and-business/opioid-pill-peddling-case-threatens -future-of-pain-treatment
The SCOTUS 2021–2022 Term: Decisions and analysis
The 2021–2022 US Supreme Court Term was a blockbuster medical Term. The bookends of the Term were COVID-19 vaccinations and abortion rights. Between the bookends were Medicare reimbursement, criminal liability for prescribing controlled substances, gun control, and carbon dioxide emissions. In this article, we focus on the significant medical issues, briefly note other important decisions, and consider the implications of this Term.
Abortion decisions
Dobbs v Jackson Women’s Health Organization1 was the most controversial decision and, for ObGyns, perhaps the most important decision in decades. The basic holding of the case can be stated simply: Roe v Wade2 and Planned Parenthood of Southeastern Pennsylvania v Casey3 (which essentially created a constitutional right to abortion) are overruled. The law related to abortion is for the states and Congress to determine, not federal courts. (For a review of earlier reproductive freedom cases in the Court, see our previous article, “The Supreme Court and reproductive rights.”4)
Dobbs arose from a Mississippi statute that made it illegal to perform abortions after 15 weeks of gestation, well before viability. Six members of the Court held that the Mississippi law was constitutional and 3 would have struck down the state law. There were 5 opinions, covering a total of 213 pages in the U.S. Reports. The Court fell into 4 camps, ranging from the most to the least protective of abortion rights, as follows:
- Three justices (Breyer, Kagan, and Sotomayor) voted to strike down the Mississippi statute and uphold Roe and Casey and wrote a joint dissent. They believe the Constitution makes abortion an issue “off limits to majority rule.” They also warned that other areas of “substantive due process” (discussed below), including contraception and same-sex marriage, might be under threat.
- The Chief Justice voted to uphold the statute but wanted an incremental approach; that is, not to overturn Roe and Casey entirely in this case because the Dobbs case required the Court only to determine the more limited question of whether the 15-week limit on abortion was constitutional. He found that the viability standard did not make sense, but he suggested that the Court “leave for another day” whether to overturn Roe.
- Five justices joined the opinion to uphold the statute and overturn Roe. Justice Alito wrote the decision joined by Justices Thomas, Kavanaugh, Gorsuch, and Barrett. They found that a right to abortion was not “deeply rooted in our Nation’s history,” as evidenced by the fact that when the 14th Amendment was adopted, abortion was a criminal offense in most states and not a protected right in any state. In 2 lengthy appendices, the Court reviewed the criminalization of abortion in the states in 1868 and in the territories that later became states. Even when Roe was decided in 1973, abortion was not “deeply rooted” because it was not generally legal in the states. Justice Kavanaugh joined this opinion and wrote separately to emphasize that the majority opinion does not outlaw abortion, but rather leaves the issue to “the people and their representatives.” He also emphasized that the case did not overturn all of the substantive due process cases.
- Justice Thomas would have gone further and abandoned “substantive due process” completely.
The constitutional issue
The majority said that the issue before the Court was not whether the law should permit or prohibit abortions—that is a question for the political branches. Rather, the question was only whether the Constitution precludes the political branches from allowing abortions. There is no mention of abortion in the Constitution and no specific reference to a right to privacy that includes medical decisions. A central constitutional question has been to identify where exactly in the Constitution the right to privacy resides. The Court has generally used “substantive due process” to locate privacy rights. The 14th Amendment provides, in part, that no state may “deprive any person of life, liberty, or property, without due process of law.” “Process” generally refers to procedural protections, but the Court sometimes has used it to encompass substantive rights (for example, privacy)—hence, “substantive due process.”
Over the decades, the legitimacy of substantive due process has remained controversial. Justice Thomas called it an “oxymoron” to turn “process” into substantive rights. And its use has a somewhat checkered history. For nearly 50 years (1890–1937), it was used to preclude states from protecting employees (for example, hour and wage laws violated “the right to contract”) and was discredited. More recently the Court has used substantive due process to protect contraception access, abortion, and same-sex marriages.
A critical question is knowing what rights substantive due process protects. The Court sometimes has said that it protects rights “deeply rooted in the Nation’s history and traditions” and “implicit in the concept of ordered liberty,”5 although in other cases suggested a more ambiguous definition.6 The next constitutional question is how to state or define the right to be protected. For example, is it the right to intimately personal decisions, bodily integrity, reproductive choice, abortion, or late-term abortion? Some of those may be deeply rooted in history and traditions (intimate decisions), and others not so much (late-term abortion). Finally, a question is whether a substantive right is defined at the time the 14th Amendment was adopted (1868) or now—is it a “living Constitution” that, without much guidance, means whatever 5 justices believe at the moment, or is it a Constitution grounded in the distant past?
The future of substantive due process is uncertain following Dobbs. Although the majority said it was not disclaiming substantive due process, the dissent said it doubted that claim because other rights are “part of the same constitutional fabric” (substantive due process). The Court might, in future cases, find some other constitutional provision in which to ground rights. The source of those rights might be the 9th Amendment (in addition to the Constitution’s enumerated rights, there are “others retained by the people”) or another provision of the 14th (“No State shall make or enforce any law which shall abridge the privileges or immunities of citizens of the United States…”). Each of these possibilities has its problems, many of which are similar to substantive due process, but they avoid the “oxymoron” issue.
Among the other important cases this Term, the Court made these determinations:
- Held that the 2nd Amendment, as applied to the states through the 14th Amendment, includes a general right to carry a gun for self-defense outside the home.1 It struck down a New York law that required people to show a special need to have and carry a gun.
- Determined that the US Environmental Protection Agency exceeded the authority Congress had granted it with a “Clean Power Plan” that was intended to reduce carbon dioxide emissions.2 It is up to Congress, not the agency, to expand agency authority.
- Gave trial courts discretion in determining whether (and under what conditions) children in international custody disputes must be returned to their home countries where there is a serious risk of harm to them.3
- Held that there is an implied right of action to sue medical providers for disability discrimination, but under the Rehabilitation Act and the Affordable Care Act the damages do not include emotional harm.4
- Decided several “free exercise of religion” cases, and in each found the state had violated religious rights, holding that: A state improperly prevented religious schools from being eligible for a state tuition grant system,5 a coach was wrongfully fired for kneeling in prayer following football games,6 Boston denied free speech in allowing other organizations to fly their flags but denying a Christian flag to be displayed,7 and a state must permit prisoners to have a spiritual advisor to be present and pray and touch them during their execution.8
- Held that the administration’s rescission of the “stay in Mexico” immigration policy was permitted by existing statutes.9
References
1. New York State Rifle & Pistol Association, Inc. v Bruen, 20-843, decided June 23, 2022. https://www.supremecourt.gov/opinions/21pdf/20-843_7j80.pdf
2. West Virginia v Environmental Protection Agency, 20-1530, decided June 30, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1530_new_l537.pdf
3. Golan v Saada, 20-1034, decided June 15, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1034_b8dg.pdf
4. Cummings v Premier Rehab Keller, 20-219, decided April 28, 2022. https://www.supremecourt.gov/opinions/21pdf/20-219_1b82.pdf
5. Carson v Makin, 20-1088, decided June 21, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1088_dbfi.pdf
6. Kennedy v Bremerton School District., 21-418, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/21-418_new_onkq.pdf
7. Shurtleff v Boston, 20-1800, decided May 2, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1800_7lho.pdf
8. Ramirez v Collier, 21-5592, decided March 24, 2022. https://www.supremecourt.gov/opinions/21pdf/21-5592_feah.pdf
9. Biden v Texas, 21-9 54, decided June 30, 2022. https://www.supremecourt.gov/opinions/21pdf/21-954_7l48.pdf
Continue to: ObGyn briefs in the case...
ObGyn briefs in the case
The medical profession filed several amicus curiae briefs in the Dobbs case. (These are “friends of the court” briefs filed by nonparties to the litigation. The purpose is to give a court a perspective on the case not presented by the parties.) The American College of Obstetricians and Gynecologists (ACOG) took the lead in filing an amicus brief.7 Nearly 2 dozen other medical organizations joined the brief, including the American Academy of Pediatrics, American College of Osteopathic Obstetricians and Gynecologists, American Gynecological and Obstetrical Society, American Society for Reproductive Medicine, Council of University Chairs of Obstetrics and Gynecology, North American Society for Pediatric and Adolescent Gynecology, Society for Academic Specialists in General Obstetrics and Gynecology, Society of Gynecologic Oncology, and Society of OB/GYN Hospitalists.
The brief argued that abortion is a safe procedure, an abortion ban would harm the health of pregnant patients, and it would undermine the physician-patient relationship and interfere with patient autonomy. It also discussed the issue of fetal pain,8 telling the Court that “Every major medical organization that has examined the issue of fetal pain and peer-reviewed studies on the matter have consistently concluded that pre-viability abortion does not result in fetal pain perception.”9 The brief was cited in the dissent for the fact that “About 18 percent of pregnancies in this country end in abortion, and about one-quarter of American women will have an abortion before the age of 45.”10
The Court received a different view from an amicus brief filed by the American Association of Pro-Life Obstetricians and Gynecologists.11 It told the Court that abortion, especially later-term, poses health risks: the abortion process itself may injure the woman, abortion puts women at risk for future preterm births, later-term abortion raises a woman’s risk of developing breast cancer, and abortions (especially those later in the pregnancy) are linked to a greater risk of psychological harm.12 The brief also noted that 93% of obstetrician-gynecologists do not perform abortions, and “abortion has been deemed contrary to sound medicine for thousands of years” (citing the Hippocratic oath).13 The brief was not cited by the Court.
Many other medical and pro-life medical groups presented amicus briefs. A list of and links to all the briefs is available on the SCOTUSblog website at https://www.scotusblog.com/case-files/cases/dobbs-v-jackson-womens-health-organization/.
Ramifications
The Court decision does not make abortion illegal but allows states (and possibly Congress) to decide whether, when, and how abortions may be performed. Some states may ban most abortions (making it illegal to have or to perform abortions). Thirteen states had “trigger laws” to go into effect limiting abortion if the Court permitted such limitations. Most of those states were almost immediately entangled in lawsuits challenging the state laws. Some states, for example, have privacy provisions in their state constitution that state courts could interpret as allowing abortion, thereby voiding the state statutes prohibiting abortion.
At least a few states have abortion laws still on the books that were passed decades ago (perhaps before Roe) and were never repealed. Those laws may once again be valid, although state courts might hold that those statutes were repealed by Roe and must be passed again to be valid. Some experts anticipate that 28 states will eventually have significant limitations on abortion.
The Guttmacher Institute maintains a frequently updated table on the abortion laws in each state.14 According to one estimate, 29 states are hostile to abortion rights (or lean that way), with about 40 million women aged 13 to 44 (58% of the United States) living in states with some hostility to abortion.15 Congress may pass some national abortion laws, but that seems unlikely and there may be some limits on its ability to control private medical practice within states.
An additional legal issue will arise from medication-induced abortions, generally through the use of mifepristone and misoprostol. They now account for the majority of abortions. These medications might be used for abortion, up to about 9 weeks of pregnancy, in states prohibiting abortion. The drugs once were available only with an in-person visit, but now the US Food and Drug Administration (FDA) permits mail-order delivery. The potential exists, therefore, to circumvent states’ prohibition on abortion through mail-order postal shipments. The FDA controls the licensing of pharmaceuticals in interstate commerce, but not the practice of medicine within a state. Therefore the ability of individuals (within a state) to possess or use drugs is unclear.
The abortion wars of the last 50 years gave rise to state laws related to abortion, including consent by minors, information to parents, special informed consent, and facilities requirements. If these laws were once struck down because they were inconsistent with Roe, but were never formally repealed, they may now become legal requirements.
In the foreseeable future, abortion laws generally will not be determined by federal courts but by state law, generally legislatures. In legislative hearings, town hall meetings, and conversations with lawmakers, ObGyns should engage the topic of abortion with scientific expertise, reason, openness, and humility. It will be impossible for the profession to speak with a single voice, as the briefs filed this Term demonstrate. Where there are honest differences in science, the reasons for the different interpretations should be explainable to lay decision makers. The profession, who are not being pseudo-lobbyists, can contribute a great deal to the rational consideration of this emotional topic.
On January 27, 2022, Justice Stephen Breyer informed President Biden of his intention to retire from the Court at the end of the Term. At age 84, he was the oldest member of the Court, but he continued to be among the most active of the justices and seemed to relish the work of the Court. He had been under pressure from liberal groups to retire earlier so a successor could be confirmed by a Democratic Senate. In many ways he was the Renaissance man of the Court: he spoke fluent French, wrote books, and famously sprinkled his questions with complex and funny hypotheticals.
Justice Breyer was a law professor before becoming a judge and enjoyed presentations to many groups, from children to law professors. He loved the Court and defended it—most recently against partisan attacks from both the right and the left. In the decisions of the Court, he was one of the more liberal justices. He had, for example, indicated that the death penalty is unconstitutional.
In his January retirement letter, he said that he would step down at the end of the Term if his replacement had been appointed and confirmed. She had. The new justice had clerked for Justice Breyer in 1999–2000.
Ketanji Brown Jackson was nominated by President Biden on February 28, confirmed by the Senate on April 7 by a 53–47 margin, and sworn in on June 30, 2022. Justice Jackson had previously been a federal district court judge and on the Court of Appeals for the D.C. Circuit. She attended Harvard-Radcliffe College and received her law degree from Harvard Law School. She worked as a criminal defense attorney and was active in the US Sentencing Commission.
Continue to: What is a practitioner to do?...
What is a practitioner to do?
For many practitioners, the Dobbs decision will have little effect because their state laws are consistent with Roe, and the legislature is not going to change the law. They may, of course, see an influx of patients from other states (that restrict abortion) seeking treatment. At the other extreme, in some states, most abortions will become prohibited. State courts may ease the restrictions. In many states, there will be an ongoing battle over when abortion is legal and when it is not, resulting in shifting laws and regulations. Keeping up with the shifts that affect practice will be a challenge.
All states are likely to permit abortions “to save the life of the mother,” and many will have a version of “to preserve the health of the mother.” Other exceptions may be for pregnancy resulting from rape or incest or in the case of serious fetal abnormality. ObGyns, of course, will be called on to certify that one of these exceptions exists. Determining that pregnancy resulted from rape or incest, of course, can be challenging. Before Roe, there was a cottage industry opining that pregnancy seriously affected the health of the mother, which often involved physical manifestations of mental health. ObGyns in some states may be asked once again to make such determinations.
Laws not directly related to abortions will, in some states, be changed as a way of discouraging abortion. For example, child abuse reporting laws may be modified to require reporting of any known or suspected abortion or attempted abortion, and medical licensing standards may make it a violation to participate in or facilitate abortion in any way.
Particularly in states where the rules keep shifting, practitioners must keep up with the current law. Professional organizations can help with that, but there is no substitute for practitioners having an ongoing professional relationship with an attorney who has expertise in health law.
Other abortion decisions this Term
In other abortion decisions this Term, the Court refused to suspend a Texas law that prohibited abortions after a fetal heartbeat could be detected.16 The law has remarkable enforcement mechanisms that preclude state officers from enforcing it; instead, it creates what amounts to a private attorney general (PAG) provision that allows private citizens to file suit against anyone performing or assisting in performing abortions. This PAG made pre-enforcement challenges to the law difficult.17
In a Kentucky case, the Court allowed the Kentucky attorney general to intervene in a case that challenged a Kentucky law that prohibits physicians from using dilation and evacuation procedures to end second-trimester pregnancies.18
Criminal convictions for physicians’ overprescription of controlled substances
Perhaps the least sympathetic of the physicians involved with the Court this Term were the 2 in Ruan v U.S.19 Their trials indicate that Dr. Ruan’s clinic issued more than 300,000 controlled substance prescriptions over 4 years and was one of the most frequent prescribers of fentanyl. Dr. Kahn prescribed controlled substances without an examination, falsified notes, and sold controlled substances for cash and guns.20
Both physicians were convicted of “knowingly or intentionally” dispensing a controlled substance without authorization.21 They were authorized to prescribe drugs, but only “for a legitimate medical purpose.”22 Appeals to their respective Circuit courts confirmed their convictions. The Supreme Court, however, held that to convict them, the government must prove that they knowingly or intentionally acted in an unauthorized manner. That proof can be by circumstantial evidence, but it must be beyond a reasonable doubt.
Health care reimbursement
Hospitals won one and lost one Medicare-Medicaid reimbursement case that involved payments for low-income patients.
In the loss, the Court held that the US Department of Health and Human Services (HHS) properly calculated the disproportionate share adjustments (DSH), or Medicare fraction,23 that provides a supplemental payment for hospitals with a large proportion of low-income patients. The lower DSH payments calculated by HHS were upheld, thereby reducing the number of hospitals receiving DSH payments and decreasing the amounts others will receive.
The win involved payments for prescription drugs that hospitals provide to outpatients in safety-net hospitals.24 HHS determined that it was overpaying hospitals for drugs and cut the reimbursement rate. The Court held that before HHS can change the drug rate, it must conduct a survey of hospitals regarding actual costs. It had not done that, so the rate reduction was not permitted by the law.
An accidental disincentive to (some) malpractice suits
Medicaid requires states to obtain part of a tort recovery that recipients obtain if Medicaid is covering medical expenses related to their injuries. In implementing that law, a state may provide a disincentive for injured beneficiaries to file malpractice cases. At issue was a Florida law that provided the Medicaid state would take 37.5% of the beneficiary’s total tort recovery (being one-half of the recovery after deducting 25% for attorney’s fees and costs). In a 7-2 decision, the Court upheld the Florida law.25
The disincentive to filing a lawsuit is that the state is taking 37.5%, plus contingency fee attorneys will typically take 33.3% (and there will be some fees). This is especially true when there is a state cap on noneconomic damages. In the case the Court decided, the plaintiff received a settlement of $850,000. If we assume a typical contingency fee, less the state’s Medicaid claim of $300,000, the plaintiff possibly received $266,667. That is not trivial, but it is only 31% of the settlement.
The Medicaid expectation of reimbursement and the Florida approach, however, impose heavy burdens on severely injured beneficiaries. The plaintiff had catastrophic injuries and was in a vegetative state. There are some things Medicaid does not pay for, as well as nonmedical expenses. The amount left for such expenses is likely well below what the family will need.
Continue to: COVID-19 vaccinations...
COVID-19 vaccinations
Had it not been for the abortion decisions, 2021–2022 might have been “the COVID Term.” Two of the most anticipated decisions involved mandatory vaccinations (or masking/testing instead). The question in each of these cases was whether Congress had authorized 2 federal agencies to issue the emergency regulations requiring vaccination. Emergency regulations are held to higher standards because they bypass the usual protections of the Administrative Procedure Act.
One case involved a regulation issued by the Occupational Safety and Health Administration (OSHA) that employers (with more than 100 employees) must require their employees to be vaccinated. In a 6-3 decision, the Court held that OSHA did not have the authority to enforce this as an emergency regulation. The other case was a regulation issued by HHS that health care institutions receiving Medicare and Medicaid funding must require all staff to be vaccinated.26 In a 5-4 decision, the Court upheld this emergency regulation because of the very broad authority Congress had given HHS to ensure the safety of patients and the quality of Medicare- and Medicaid-funded programs.27
In another case, in the shadow docket (orders and opinions in cases without full arguments), the Court struck down the Centers for Disease Control and Prevention’s eviction moratorium.28 The Court said the government claimed “a breathtaking amount of authority” that Congress did not intend. In other shadow docket cases, the Court refused to hold unconstitutional state laws that require COVID-19 vaccination but did not have religious exemptions.29
Analysis of this Term
It was an extraordinary Term. The Court decided 66 cases (excluding most cases in the shadow docket), a low number historically. Not only were there many seminal cases but also the Court appears to be shifting toward a new direction. That direction may be oriented more toward the original understanding of the words of the Constitution and statutes and less toward policy; Congress rather than administrative agencies; racial nondiscrimination rather than preferences; and the free exercise rather than the establishment of religion. Whether there is such a shift or not, of course, only time will tell.
Chief Justice Roberts and Justice Kavanaugh were in the majority most often (95% of the cases), followed by Justices Barrett (90%), Alito (85%), Thomas (80%), and Gorsuch (75%). Justices Kagan (69%) and Breyer (68%) were not far behind. Justice Sotomayor was in the majority 58%. The Court was unanimous 29% of the time, well below the decade average (43%), and 6-3 accounted for 30% of the decisions.
A major, potentially scarring, event this Term was the leak of an early draft of the majority opinion in Dobbs. Although leaks have occurred before, the early leak of an opinion was unprecedented. It will almost inevitably change the openness and candor within the Court and the justices’ clerks. Although not unprecedented, the attempt on the life of Justice Kavanaugh and the organized efforts to harass some justices in their homes are likely to have lasting impact. Almost certainly it means that justices and their families will have constant security and their movements and connection with the general public will become less frequent, which is sad for the justices and our democracy.
Looking toward the next Term
When the Court next convenes, Justice Ketanji Brown Jackson will take her seat on the left end of the Court (the traditional seat for a new justice, not a commentary on judicial philosophy). The Court has already taken many cases, including issues about university affirmative action programs, web designers and same-sex couples, redistricting and voting rights, DNA testing in criminal cases, and overtime pay for someone making over $200,000 per year. It begins Monday, October 3, and promises to be another interesting Term. ●
- Dobbs v Jackson Women’s Health Organization, 19-1392, decided June 24, 2022. https://www.supremecourt.gov /opinions/21pdf/19-1392_6j37.pdf
- Roe v Wade, 410 U.S. 113, 163 (1973).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Smith SR, Sanfilippo JR. The Supreme Court and reproductive rights. OBG Manag. 2022;34(1):36-41, 46. https://cdn.mdedge. com/files/s3fs-public/issues/articles/obgm0340136_smith.pdf
- Washington v Glucksberg, 521 U.S. 702 (1997).
- Obergefell v Hodges, 576 U.S. 644, 654-70 (2015).
- Brief of amici curiae of American College of Obstetricians and Gynecologists, American Medical Association, et al, in Dobbs v Jackson Women’s Health Organization, in Support of Respondents (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/193074/20210920174518042 _19-1392%20bsacACOGetal.pdf
- Id. at 8, 13-15.
- Id. at 14.
- Justices Breyer, Kagan, and Sotomayor, dissenting, at 48, note 22.
- Brief for American Association of Pro-Life Obstetricians And Gynecologists as Amicus Curiae, in Dobbs v Jackson Women’s Health Organization, in Support of Petitioners (July 2021). https://www.supremecourt.gov /DocketPDF/19/19-1392/185350/20210729163532595_No. %2019-1392%20-%20American%20Association%20of%20 Pro-Life%20Obstetricians%20and%20Gynecologists%20-%20 Amicus%20Brief%20in%20Support%20of%20Petitioner%20-%20 7-29-21.pdf
- Id. at 3-4, 7-29.
- Id. at 30.
- Guttmacher Institute. An overview of abortion laws. July 11, 2022. https://www.guttmacher.org/state-policy/explore/overview -abortion-laws
- Guttmacher Institute. State abortion policy landscape: from hostile to supportive. Dec. 2020. https://www .guttmacher.org/article/2019/08/state-abortion-policy -landscape-hostile-supportive
- Whole Woman’s Health v Jackson, 21-463, decided Dec. 10, 2021. https://www.supremecourt.gov/opinions/21pdf/21-463_ new_8o6b.pdf
- United States v Texas, 21-588, decided Dec. 10, 2021. (Per curiam, Sotomayor dissenting). https://www.supremecourt.gov /opinions/21pdf/21-588_c07d.pdf
- Cameron v EMW Women’s Surgical Center, 20-601, decided Mar. 3, 2022. https://www.supremecourt.gov/opinions/21pdf/20-601 _new_g20h.pdf
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https:// www.supremecourt.gov/opinions/21pdf/20-1410_1an2.pdf
- Adam Liptak. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022. https://www .nytimes.com/2022/06/27/us/politics/supreme-court-controlled -substance-act.html
- 84 Stat. 1260, 21 U.S.C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Becerra v Empire Health Foundation, For Valley Hospital Medical Center, 20-1312, decided June 24, 2022. https://www.supremecourt .gov/opinions/21pdf/20-1312_j42l.pdf
- American Hospital Association v Becerra, 20-1114, decided June 15, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1114_09m1.pdf
- Gallardo v Marstiller, 20-1263, decided June 6, 2022. https:// www.supremecourt.gov/opinions/21pdf/20-1263_new_hfci.pdf
- National Federation of Independent Business v Department of Labor, Occupational Safety and Health Administration, 21A244, decided Jan. 13, 2022. https://www.supremecourt.gov /opinions/21pdf/21a244_hgci.pdf
- Biden v Missouri, 21A240, decided Jan. 13, 2022. https://www .supremecourt.gov/opinions/21pdf/21a240_d18e.pdf
- Alabama Association of Realtors v Department of Health and Human Services, 21A23, decided Aug. 26, 2021. https://www .supremecourt.gov/opinions/20pdf/21a23_ap6c.pdf
- Does v Mills, 21A90, decided Oct. 29, 2021. https://www .supremecourt.gov/opinions/21pdf/21a90_6j37.pdf
The 2021–2022 US Supreme Court Term was a blockbuster medical Term. The bookends of the Term were COVID-19 vaccinations and abortion rights. Between the bookends were Medicare reimbursement, criminal liability for prescribing controlled substances, gun control, and carbon dioxide emissions. In this article, we focus on the significant medical issues, briefly note other important decisions, and consider the implications of this Term.
Abortion decisions
Dobbs v Jackson Women’s Health Organization1 was the most controversial decision and, for ObGyns, perhaps the most important decision in decades. The basic holding of the case can be stated simply: Roe v Wade2 and Planned Parenthood of Southeastern Pennsylvania v Casey3 (which essentially created a constitutional right to abortion) are overruled. The law related to abortion is for the states and Congress to determine, not federal courts. (For a review of earlier reproductive freedom cases in the Court, see our previous article, “The Supreme Court and reproductive rights.”4)
Dobbs arose from a Mississippi statute that made it illegal to perform abortions after 15 weeks of gestation, well before viability. Six members of the Court held that the Mississippi law was constitutional and 3 would have struck down the state law. There were 5 opinions, covering a total of 213 pages in the U.S. Reports. The Court fell into 4 camps, ranging from the most to the least protective of abortion rights, as follows:
- Three justices (Breyer, Kagan, and Sotomayor) voted to strike down the Mississippi statute and uphold Roe and Casey and wrote a joint dissent. They believe the Constitution makes abortion an issue “off limits to majority rule.” They also warned that other areas of “substantive due process” (discussed below), including contraception and same-sex marriage, might be under threat.
- The Chief Justice voted to uphold the statute but wanted an incremental approach; that is, not to overturn Roe and Casey entirely in this case because the Dobbs case required the Court only to determine the more limited question of whether the 15-week limit on abortion was constitutional. He found that the viability standard did not make sense, but he suggested that the Court “leave for another day” whether to overturn Roe.
- Five justices joined the opinion to uphold the statute and overturn Roe. Justice Alito wrote the decision joined by Justices Thomas, Kavanaugh, Gorsuch, and Barrett. They found that a right to abortion was not “deeply rooted in our Nation’s history,” as evidenced by the fact that when the 14th Amendment was adopted, abortion was a criminal offense in most states and not a protected right in any state. In 2 lengthy appendices, the Court reviewed the criminalization of abortion in the states in 1868 and in the territories that later became states. Even when Roe was decided in 1973, abortion was not “deeply rooted” because it was not generally legal in the states. Justice Kavanaugh joined this opinion and wrote separately to emphasize that the majority opinion does not outlaw abortion, but rather leaves the issue to “the people and their representatives.” He also emphasized that the case did not overturn all of the substantive due process cases.
- Justice Thomas would have gone further and abandoned “substantive due process” completely.
The constitutional issue
The majority said that the issue before the Court was not whether the law should permit or prohibit abortions—that is a question for the political branches. Rather, the question was only whether the Constitution precludes the political branches from allowing abortions. There is no mention of abortion in the Constitution and no specific reference to a right to privacy that includes medical decisions. A central constitutional question has been to identify where exactly in the Constitution the right to privacy resides. The Court has generally used “substantive due process” to locate privacy rights. The 14th Amendment provides, in part, that no state may “deprive any person of life, liberty, or property, without due process of law.” “Process” generally refers to procedural protections, but the Court sometimes has used it to encompass substantive rights (for example, privacy)—hence, “substantive due process.”
Over the decades, the legitimacy of substantive due process has remained controversial. Justice Thomas called it an “oxymoron” to turn “process” into substantive rights. And its use has a somewhat checkered history. For nearly 50 years (1890–1937), it was used to preclude states from protecting employees (for example, hour and wage laws violated “the right to contract”) and was discredited. More recently the Court has used substantive due process to protect contraception access, abortion, and same-sex marriages.
A critical question is knowing what rights substantive due process protects. The Court sometimes has said that it protects rights “deeply rooted in the Nation’s history and traditions” and “implicit in the concept of ordered liberty,”5 although in other cases suggested a more ambiguous definition.6 The next constitutional question is how to state or define the right to be protected. For example, is it the right to intimately personal decisions, bodily integrity, reproductive choice, abortion, or late-term abortion? Some of those may be deeply rooted in history and traditions (intimate decisions), and others not so much (late-term abortion). Finally, a question is whether a substantive right is defined at the time the 14th Amendment was adopted (1868) or now—is it a “living Constitution” that, without much guidance, means whatever 5 justices believe at the moment, or is it a Constitution grounded in the distant past?
The future of substantive due process is uncertain following Dobbs. Although the majority said it was not disclaiming substantive due process, the dissent said it doubted that claim because other rights are “part of the same constitutional fabric” (substantive due process). The Court might, in future cases, find some other constitutional provision in which to ground rights. The source of those rights might be the 9th Amendment (in addition to the Constitution’s enumerated rights, there are “others retained by the people”) or another provision of the 14th (“No State shall make or enforce any law which shall abridge the privileges or immunities of citizens of the United States…”). Each of these possibilities has its problems, many of which are similar to substantive due process, but they avoid the “oxymoron” issue.
Among the other important cases this Term, the Court made these determinations:
- Held that the 2nd Amendment, as applied to the states through the 14th Amendment, includes a general right to carry a gun for self-defense outside the home.1 It struck down a New York law that required people to show a special need to have and carry a gun.
- Determined that the US Environmental Protection Agency exceeded the authority Congress had granted it with a “Clean Power Plan” that was intended to reduce carbon dioxide emissions.2 It is up to Congress, not the agency, to expand agency authority.
- Gave trial courts discretion in determining whether (and under what conditions) children in international custody disputes must be returned to their home countries where there is a serious risk of harm to them.3
- Held that there is an implied right of action to sue medical providers for disability discrimination, but under the Rehabilitation Act and the Affordable Care Act the damages do not include emotional harm.4
- Decided several “free exercise of religion” cases, and in each found the state had violated religious rights, holding that: A state improperly prevented religious schools from being eligible for a state tuition grant system,5 a coach was wrongfully fired for kneeling in prayer following football games,6 Boston denied free speech in allowing other organizations to fly their flags but denying a Christian flag to be displayed,7 and a state must permit prisoners to have a spiritual advisor to be present and pray and touch them during their execution.8
- Held that the administration’s rescission of the “stay in Mexico” immigration policy was permitted by existing statutes.9
References
1. New York State Rifle & Pistol Association, Inc. v Bruen, 20-843, decided June 23, 2022. https://www.supremecourt.gov/opinions/21pdf/20-843_7j80.pdf
2. West Virginia v Environmental Protection Agency, 20-1530, decided June 30, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1530_new_l537.pdf
3. Golan v Saada, 20-1034, decided June 15, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1034_b8dg.pdf
4. Cummings v Premier Rehab Keller, 20-219, decided April 28, 2022. https://www.supremecourt.gov/opinions/21pdf/20-219_1b82.pdf
5. Carson v Makin, 20-1088, decided June 21, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1088_dbfi.pdf
6. Kennedy v Bremerton School District., 21-418, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/21-418_new_onkq.pdf
7. Shurtleff v Boston, 20-1800, decided May 2, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1800_7lho.pdf
8. Ramirez v Collier, 21-5592, decided March 24, 2022. https://www.supremecourt.gov/opinions/21pdf/21-5592_feah.pdf
9. Biden v Texas, 21-9 54, decided June 30, 2022. https://www.supremecourt.gov/opinions/21pdf/21-954_7l48.pdf
Continue to: ObGyn briefs in the case...
ObGyn briefs in the case
The medical profession filed several amicus curiae briefs in the Dobbs case. (These are “friends of the court” briefs filed by nonparties to the litigation. The purpose is to give a court a perspective on the case not presented by the parties.) The American College of Obstetricians and Gynecologists (ACOG) took the lead in filing an amicus brief.7 Nearly 2 dozen other medical organizations joined the brief, including the American Academy of Pediatrics, American College of Osteopathic Obstetricians and Gynecologists, American Gynecological and Obstetrical Society, American Society for Reproductive Medicine, Council of University Chairs of Obstetrics and Gynecology, North American Society for Pediatric and Adolescent Gynecology, Society for Academic Specialists in General Obstetrics and Gynecology, Society of Gynecologic Oncology, and Society of OB/GYN Hospitalists.
The brief argued that abortion is a safe procedure, an abortion ban would harm the health of pregnant patients, and it would undermine the physician-patient relationship and interfere with patient autonomy. It also discussed the issue of fetal pain,8 telling the Court that “Every major medical organization that has examined the issue of fetal pain and peer-reviewed studies on the matter have consistently concluded that pre-viability abortion does not result in fetal pain perception.”9 The brief was cited in the dissent for the fact that “About 18 percent of pregnancies in this country end in abortion, and about one-quarter of American women will have an abortion before the age of 45.”10
The Court received a different view from an amicus brief filed by the American Association of Pro-Life Obstetricians and Gynecologists.11 It told the Court that abortion, especially later-term, poses health risks: the abortion process itself may injure the woman, abortion puts women at risk for future preterm births, later-term abortion raises a woman’s risk of developing breast cancer, and abortions (especially those later in the pregnancy) are linked to a greater risk of psychological harm.12 The brief also noted that 93% of obstetrician-gynecologists do not perform abortions, and “abortion has been deemed contrary to sound medicine for thousands of years” (citing the Hippocratic oath).13 The brief was not cited by the Court.
Many other medical and pro-life medical groups presented amicus briefs. A list of and links to all the briefs is available on the SCOTUSblog website at https://www.scotusblog.com/case-files/cases/dobbs-v-jackson-womens-health-organization/.
Ramifications
The Court decision does not make abortion illegal but allows states (and possibly Congress) to decide whether, when, and how abortions may be performed. Some states may ban most abortions (making it illegal to have or to perform abortions). Thirteen states had “trigger laws” to go into effect limiting abortion if the Court permitted such limitations. Most of those states were almost immediately entangled in lawsuits challenging the state laws. Some states, for example, have privacy provisions in their state constitution that state courts could interpret as allowing abortion, thereby voiding the state statutes prohibiting abortion.
At least a few states have abortion laws still on the books that were passed decades ago (perhaps before Roe) and were never repealed. Those laws may once again be valid, although state courts might hold that those statutes were repealed by Roe and must be passed again to be valid. Some experts anticipate that 28 states will eventually have significant limitations on abortion.
The Guttmacher Institute maintains a frequently updated table on the abortion laws in each state.14 According to one estimate, 29 states are hostile to abortion rights (or lean that way), with about 40 million women aged 13 to 44 (58% of the United States) living in states with some hostility to abortion.15 Congress may pass some national abortion laws, but that seems unlikely and there may be some limits on its ability to control private medical practice within states.
An additional legal issue will arise from medication-induced abortions, generally through the use of mifepristone and misoprostol. They now account for the majority of abortions. These medications might be used for abortion, up to about 9 weeks of pregnancy, in states prohibiting abortion. The drugs once were available only with an in-person visit, but now the US Food and Drug Administration (FDA) permits mail-order delivery. The potential exists, therefore, to circumvent states’ prohibition on abortion through mail-order postal shipments. The FDA controls the licensing of pharmaceuticals in interstate commerce, but not the practice of medicine within a state. Therefore the ability of individuals (within a state) to possess or use drugs is unclear.
The abortion wars of the last 50 years gave rise to state laws related to abortion, including consent by minors, information to parents, special informed consent, and facilities requirements. If these laws were once struck down because they were inconsistent with Roe, but were never formally repealed, they may now become legal requirements.
In the foreseeable future, abortion laws generally will not be determined by federal courts but by state law, generally legislatures. In legislative hearings, town hall meetings, and conversations with lawmakers, ObGyns should engage the topic of abortion with scientific expertise, reason, openness, and humility. It will be impossible for the profession to speak with a single voice, as the briefs filed this Term demonstrate. Where there are honest differences in science, the reasons for the different interpretations should be explainable to lay decision makers. The profession, who are not being pseudo-lobbyists, can contribute a great deal to the rational consideration of this emotional topic.
On January 27, 2022, Justice Stephen Breyer informed President Biden of his intention to retire from the Court at the end of the Term. At age 84, he was the oldest member of the Court, but he continued to be among the most active of the justices and seemed to relish the work of the Court. He had been under pressure from liberal groups to retire earlier so a successor could be confirmed by a Democratic Senate. In many ways he was the Renaissance man of the Court: he spoke fluent French, wrote books, and famously sprinkled his questions with complex and funny hypotheticals.
Justice Breyer was a law professor before becoming a judge and enjoyed presentations to many groups, from children to law professors. He loved the Court and defended it—most recently against partisan attacks from both the right and the left. In the decisions of the Court, he was one of the more liberal justices. He had, for example, indicated that the death penalty is unconstitutional.
In his January retirement letter, he said that he would step down at the end of the Term if his replacement had been appointed and confirmed. She had. The new justice had clerked for Justice Breyer in 1999–2000.
Ketanji Brown Jackson was nominated by President Biden on February 28, confirmed by the Senate on April 7 by a 53–47 margin, and sworn in on June 30, 2022. Justice Jackson had previously been a federal district court judge and on the Court of Appeals for the D.C. Circuit. She attended Harvard-Radcliffe College and received her law degree from Harvard Law School. She worked as a criminal defense attorney and was active in the US Sentencing Commission.
Continue to: What is a practitioner to do?...
What is a practitioner to do?
For many practitioners, the Dobbs decision will have little effect because their state laws are consistent with Roe, and the legislature is not going to change the law. They may, of course, see an influx of patients from other states (that restrict abortion) seeking treatment. At the other extreme, in some states, most abortions will become prohibited. State courts may ease the restrictions. In many states, there will be an ongoing battle over when abortion is legal and when it is not, resulting in shifting laws and regulations. Keeping up with the shifts that affect practice will be a challenge.
All states are likely to permit abortions “to save the life of the mother,” and many will have a version of “to preserve the health of the mother.” Other exceptions may be for pregnancy resulting from rape or incest or in the case of serious fetal abnormality. ObGyns, of course, will be called on to certify that one of these exceptions exists. Determining that pregnancy resulted from rape or incest, of course, can be challenging. Before Roe, there was a cottage industry opining that pregnancy seriously affected the health of the mother, which often involved physical manifestations of mental health. ObGyns in some states may be asked once again to make such determinations.
Laws not directly related to abortions will, in some states, be changed as a way of discouraging abortion. For example, child abuse reporting laws may be modified to require reporting of any known or suspected abortion or attempted abortion, and medical licensing standards may make it a violation to participate in or facilitate abortion in any way.
Particularly in states where the rules keep shifting, practitioners must keep up with the current law. Professional organizations can help with that, but there is no substitute for practitioners having an ongoing professional relationship with an attorney who has expertise in health law.
Other abortion decisions this Term
In other abortion decisions this Term, the Court refused to suspend a Texas law that prohibited abortions after a fetal heartbeat could be detected.16 The law has remarkable enforcement mechanisms that preclude state officers from enforcing it; instead, it creates what amounts to a private attorney general (PAG) provision that allows private citizens to file suit against anyone performing or assisting in performing abortions. This PAG made pre-enforcement challenges to the law difficult.17
In a Kentucky case, the Court allowed the Kentucky attorney general to intervene in a case that challenged a Kentucky law that prohibits physicians from using dilation and evacuation procedures to end second-trimester pregnancies.18
Criminal convictions for physicians’ overprescription of controlled substances
Perhaps the least sympathetic of the physicians involved with the Court this Term were the 2 in Ruan v U.S.19 Their trials indicate that Dr. Ruan’s clinic issued more than 300,000 controlled substance prescriptions over 4 years and was one of the most frequent prescribers of fentanyl. Dr. Kahn prescribed controlled substances without an examination, falsified notes, and sold controlled substances for cash and guns.20
Both physicians were convicted of “knowingly or intentionally” dispensing a controlled substance without authorization.21 They were authorized to prescribe drugs, but only “for a legitimate medical purpose.”22 Appeals to their respective Circuit courts confirmed their convictions. The Supreme Court, however, held that to convict them, the government must prove that they knowingly or intentionally acted in an unauthorized manner. That proof can be by circumstantial evidence, but it must be beyond a reasonable doubt.
Health care reimbursement
Hospitals won one and lost one Medicare-Medicaid reimbursement case that involved payments for low-income patients.
In the loss, the Court held that the US Department of Health and Human Services (HHS) properly calculated the disproportionate share adjustments (DSH), or Medicare fraction,23 that provides a supplemental payment for hospitals with a large proportion of low-income patients. The lower DSH payments calculated by HHS were upheld, thereby reducing the number of hospitals receiving DSH payments and decreasing the amounts others will receive.
The win involved payments for prescription drugs that hospitals provide to outpatients in safety-net hospitals.24 HHS determined that it was overpaying hospitals for drugs and cut the reimbursement rate. The Court held that before HHS can change the drug rate, it must conduct a survey of hospitals regarding actual costs. It had not done that, so the rate reduction was not permitted by the law.
An accidental disincentive to (some) malpractice suits
Medicaid requires states to obtain part of a tort recovery that recipients obtain if Medicaid is covering medical expenses related to their injuries. In implementing that law, a state may provide a disincentive for injured beneficiaries to file malpractice cases. At issue was a Florida law that provided the Medicaid state would take 37.5% of the beneficiary’s total tort recovery (being one-half of the recovery after deducting 25% for attorney’s fees and costs). In a 7-2 decision, the Court upheld the Florida law.25
The disincentive to filing a lawsuit is that the state is taking 37.5%, plus contingency fee attorneys will typically take 33.3% (and there will be some fees). This is especially true when there is a state cap on noneconomic damages. In the case the Court decided, the plaintiff received a settlement of $850,000. If we assume a typical contingency fee, less the state’s Medicaid claim of $300,000, the plaintiff possibly received $266,667. That is not trivial, but it is only 31% of the settlement.
The Medicaid expectation of reimbursement and the Florida approach, however, impose heavy burdens on severely injured beneficiaries. The plaintiff had catastrophic injuries and was in a vegetative state. There are some things Medicaid does not pay for, as well as nonmedical expenses. The amount left for such expenses is likely well below what the family will need.
Continue to: COVID-19 vaccinations...
COVID-19 vaccinations
Had it not been for the abortion decisions, 2021–2022 might have been “the COVID Term.” Two of the most anticipated decisions involved mandatory vaccinations (or masking/testing instead). The question in each of these cases was whether Congress had authorized 2 federal agencies to issue the emergency regulations requiring vaccination. Emergency regulations are held to higher standards because they bypass the usual protections of the Administrative Procedure Act.
One case involved a regulation issued by the Occupational Safety and Health Administration (OSHA) that employers (with more than 100 employees) must require their employees to be vaccinated. In a 6-3 decision, the Court held that OSHA did not have the authority to enforce this as an emergency regulation. The other case was a regulation issued by HHS that health care institutions receiving Medicare and Medicaid funding must require all staff to be vaccinated.26 In a 5-4 decision, the Court upheld this emergency regulation because of the very broad authority Congress had given HHS to ensure the safety of patients and the quality of Medicare- and Medicaid-funded programs.27
In another case, in the shadow docket (orders and opinions in cases without full arguments), the Court struck down the Centers for Disease Control and Prevention’s eviction moratorium.28 The Court said the government claimed “a breathtaking amount of authority” that Congress did not intend. In other shadow docket cases, the Court refused to hold unconstitutional state laws that require COVID-19 vaccination but did not have religious exemptions.29
Analysis of this Term
It was an extraordinary Term. The Court decided 66 cases (excluding most cases in the shadow docket), a low number historically. Not only were there many seminal cases but also the Court appears to be shifting toward a new direction. That direction may be oriented more toward the original understanding of the words of the Constitution and statutes and less toward policy; Congress rather than administrative agencies; racial nondiscrimination rather than preferences; and the free exercise rather than the establishment of religion. Whether there is such a shift or not, of course, only time will tell.
Chief Justice Roberts and Justice Kavanaugh were in the majority most often (95% of the cases), followed by Justices Barrett (90%), Alito (85%), Thomas (80%), and Gorsuch (75%). Justices Kagan (69%) and Breyer (68%) were not far behind. Justice Sotomayor was in the majority 58%. The Court was unanimous 29% of the time, well below the decade average (43%), and 6-3 accounted for 30% of the decisions.
A major, potentially scarring, event this Term was the leak of an early draft of the majority opinion in Dobbs. Although leaks have occurred before, the early leak of an opinion was unprecedented. It will almost inevitably change the openness and candor within the Court and the justices’ clerks. Although not unprecedented, the attempt on the life of Justice Kavanaugh and the organized efforts to harass some justices in their homes are likely to have lasting impact. Almost certainly it means that justices and their families will have constant security and their movements and connection with the general public will become less frequent, which is sad for the justices and our democracy.
Looking toward the next Term
When the Court next convenes, Justice Ketanji Brown Jackson will take her seat on the left end of the Court (the traditional seat for a new justice, not a commentary on judicial philosophy). The Court has already taken many cases, including issues about university affirmative action programs, web designers and same-sex couples, redistricting and voting rights, DNA testing in criminal cases, and overtime pay for someone making over $200,000 per year. It begins Monday, October 3, and promises to be another interesting Term. ●
The 2021–2022 US Supreme Court Term was a blockbuster medical Term. The bookends of the Term were COVID-19 vaccinations and abortion rights. Between the bookends were Medicare reimbursement, criminal liability for prescribing controlled substances, gun control, and carbon dioxide emissions. In this article, we focus on the significant medical issues, briefly note other important decisions, and consider the implications of this Term.
Abortion decisions
Dobbs v Jackson Women’s Health Organization1 was the most controversial decision and, for ObGyns, perhaps the most important decision in decades. The basic holding of the case can be stated simply: Roe v Wade2 and Planned Parenthood of Southeastern Pennsylvania v Casey3 (which essentially created a constitutional right to abortion) are overruled. The law related to abortion is for the states and Congress to determine, not federal courts. (For a review of earlier reproductive freedom cases in the Court, see our previous article, “The Supreme Court and reproductive rights.”4)
Dobbs arose from a Mississippi statute that made it illegal to perform abortions after 15 weeks of gestation, well before viability. Six members of the Court held that the Mississippi law was constitutional and 3 would have struck down the state law. There were 5 opinions, covering a total of 213 pages in the U.S. Reports. The Court fell into 4 camps, ranging from the most to the least protective of abortion rights, as follows:
- Three justices (Breyer, Kagan, and Sotomayor) voted to strike down the Mississippi statute and uphold Roe and Casey and wrote a joint dissent. They believe the Constitution makes abortion an issue “off limits to majority rule.” They also warned that other areas of “substantive due process” (discussed below), including contraception and same-sex marriage, might be under threat.
- The Chief Justice voted to uphold the statute but wanted an incremental approach; that is, not to overturn Roe and Casey entirely in this case because the Dobbs case required the Court only to determine the more limited question of whether the 15-week limit on abortion was constitutional. He found that the viability standard did not make sense, but he suggested that the Court “leave for another day” whether to overturn Roe.
- Five justices joined the opinion to uphold the statute and overturn Roe. Justice Alito wrote the decision joined by Justices Thomas, Kavanaugh, Gorsuch, and Barrett. They found that a right to abortion was not “deeply rooted in our Nation’s history,” as evidenced by the fact that when the 14th Amendment was adopted, abortion was a criminal offense in most states and not a protected right in any state. In 2 lengthy appendices, the Court reviewed the criminalization of abortion in the states in 1868 and in the territories that later became states. Even when Roe was decided in 1973, abortion was not “deeply rooted” because it was not generally legal in the states. Justice Kavanaugh joined this opinion and wrote separately to emphasize that the majority opinion does not outlaw abortion, but rather leaves the issue to “the people and their representatives.” He also emphasized that the case did not overturn all of the substantive due process cases.
- Justice Thomas would have gone further and abandoned “substantive due process” completely.
The constitutional issue
The majority said that the issue before the Court was not whether the law should permit or prohibit abortions—that is a question for the political branches. Rather, the question was only whether the Constitution precludes the political branches from allowing abortions. There is no mention of abortion in the Constitution and no specific reference to a right to privacy that includes medical decisions. A central constitutional question has been to identify where exactly in the Constitution the right to privacy resides. The Court has generally used “substantive due process” to locate privacy rights. The 14th Amendment provides, in part, that no state may “deprive any person of life, liberty, or property, without due process of law.” “Process” generally refers to procedural protections, but the Court sometimes has used it to encompass substantive rights (for example, privacy)—hence, “substantive due process.”
Over the decades, the legitimacy of substantive due process has remained controversial. Justice Thomas called it an “oxymoron” to turn “process” into substantive rights. And its use has a somewhat checkered history. For nearly 50 years (1890–1937), it was used to preclude states from protecting employees (for example, hour and wage laws violated “the right to contract”) and was discredited. More recently the Court has used substantive due process to protect contraception access, abortion, and same-sex marriages.
A critical question is knowing what rights substantive due process protects. The Court sometimes has said that it protects rights “deeply rooted in the Nation’s history and traditions” and “implicit in the concept of ordered liberty,”5 although in other cases suggested a more ambiguous definition.6 The next constitutional question is how to state or define the right to be protected. For example, is it the right to intimately personal decisions, bodily integrity, reproductive choice, abortion, or late-term abortion? Some of those may be deeply rooted in history and traditions (intimate decisions), and others not so much (late-term abortion). Finally, a question is whether a substantive right is defined at the time the 14th Amendment was adopted (1868) or now—is it a “living Constitution” that, without much guidance, means whatever 5 justices believe at the moment, or is it a Constitution grounded in the distant past?
The future of substantive due process is uncertain following Dobbs. Although the majority said it was not disclaiming substantive due process, the dissent said it doubted that claim because other rights are “part of the same constitutional fabric” (substantive due process). The Court might, in future cases, find some other constitutional provision in which to ground rights. The source of those rights might be the 9th Amendment (in addition to the Constitution’s enumerated rights, there are “others retained by the people”) or another provision of the 14th (“No State shall make or enforce any law which shall abridge the privileges or immunities of citizens of the United States…”). Each of these possibilities has its problems, many of which are similar to substantive due process, but they avoid the “oxymoron” issue.
Among the other important cases this Term, the Court made these determinations:
- Held that the 2nd Amendment, as applied to the states through the 14th Amendment, includes a general right to carry a gun for self-defense outside the home.1 It struck down a New York law that required people to show a special need to have and carry a gun.
- Determined that the US Environmental Protection Agency exceeded the authority Congress had granted it with a “Clean Power Plan” that was intended to reduce carbon dioxide emissions.2 It is up to Congress, not the agency, to expand agency authority.
- Gave trial courts discretion in determining whether (and under what conditions) children in international custody disputes must be returned to their home countries where there is a serious risk of harm to them.3
- Held that there is an implied right of action to sue medical providers for disability discrimination, but under the Rehabilitation Act and the Affordable Care Act the damages do not include emotional harm.4
- Decided several “free exercise of religion” cases, and in each found the state had violated religious rights, holding that: A state improperly prevented religious schools from being eligible for a state tuition grant system,5 a coach was wrongfully fired for kneeling in prayer following football games,6 Boston denied free speech in allowing other organizations to fly their flags but denying a Christian flag to be displayed,7 and a state must permit prisoners to have a spiritual advisor to be present and pray and touch them during their execution.8
- Held that the administration’s rescission of the “stay in Mexico” immigration policy was permitted by existing statutes.9
References
1. New York State Rifle & Pistol Association, Inc. v Bruen, 20-843, decided June 23, 2022. https://www.supremecourt.gov/opinions/21pdf/20-843_7j80.pdf
2. West Virginia v Environmental Protection Agency, 20-1530, decided June 30, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1530_new_l537.pdf
3. Golan v Saada, 20-1034, decided June 15, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1034_b8dg.pdf
4. Cummings v Premier Rehab Keller, 20-219, decided April 28, 2022. https://www.supremecourt.gov/opinions/21pdf/20-219_1b82.pdf
5. Carson v Makin, 20-1088, decided June 21, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1088_dbfi.pdf
6. Kennedy v Bremerton School District., 21-418, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/21-418_new_onkq.pdf
7. Shurtleff v Boston, 20-1800, decided May 2, 2022. https://www.supremecourt.gov/opinions/21pdf/20-1800_7lho.pdf
8. Ramirez v Collier, 21-5592, decided March 24, 2022. https://www.supremecourt.gov/opinions/21pdf/21-5592_feah.pdf
9. Biden v Texas, 21-9 54, decided June 30, 2022. https://www.supremecourt.gov/opinions/21pdf/21-954_7l48.pdf
Continue to: ObGyn briefs in the case...
ObGyn briefs in the case
The medical profession filed several amicus curiae briefs in the Dobbs case. (These are “friends of the court” briefs filed by nonparties to the litigation. The purpose is to give a court a perspective on the case not presented by the parties.) The American College of Obstetricians and Gynecologists (ACOG) took the lead in filing an amicus brief.7 Nearly 2 dozen other medical organizations joined the brief, including the American Academy of Pediatrics, American College of Osteopathic Obstetricians and Gynecologists, American Gynecological and Obstetrical Society, American Society for Reproductive Medicine, Council of University Chairs of Obstetrics and Gynecology, North American Society for Pediatric and Adolescent Gynecology, Society for Academic Specialists in General Obstetrics and Gynecology, Society of Gynecologic Oncology, and Society of OB/GYN Hospitalists.
The brief argued that abortion is a safe procedure, an abortion ban would harm the health of pregnant patients, and it would undermine the physician-patient relationship and interfere with patient autonomy. It also discussed the issue of fetal pain,8 telling the Court that “Every major medical organization that has examined the issue of fetal pain and peer-reviewed studies on the matter have consistently concluded that pre-viability abortion does not result in fetal pain perception.”9 The brief was cited in the dissent for the fact that “About 18 percent of pregnancies in this country end in abortion, and about one-quarter of American women will have an abortion before the age of 45.”10
The Court received a different view from an amicus brief filed by the American Association of Pro-Life Obstetricians and Gynecologists.11 It told the Court that abortion, especially later-term, poses health risks: the abortion process itself may injure the woman, abortion puts women at risk for future preterm births, later-term abortion raises a woman’s risk of developing breast cancer, and abortions (especially those later in the pregnancy) are linked to a greater risk of psychological harm.12 The brief also noted that 93% of obstetrician-gynecologists do not perform abortions, and “abortion has been deemed contrary to sound medicine for thousands of years” (citing the Hippocratic oath).13 The brief was not cited by the Court.
Many other medical and pro-life medical groups presented amicus briefs. A list of and links to all the briefs is available on the SCOTUSblog website at https://www.scotusblog.com/case-files/cases/dobbs-v-jackson-womens-health-organization/.
Ramifications
The Court decision does not make abortion illegal but allows states (and possibly Congress) to decide whether, when, and how abortions may be performed. Some states may ban most abortions (making it illegal to have or to perform abortions). Thirteen states had “trigger laws” to go into effect limiting abortion if the Court permitted such limitations. Most of those states were almost immediately entangled in lawsuits challenging the state laws. Some states, for example, have privacy provisions in their state constitution that state courts could interpret as allowing abortion, thereby voiding the state statutes prohibiting abortion.
At least a few states have abortion laws still on the books that were passed decades ago (perhaps before Roe) and were never repealed. Those laws may once again be valid, although state courts might hold that those statutes were repealed by Roe and must be passed again to be valid. Some experts anticipate that 28 states will eventually have significant limitations on abortion.
The Guttmacher Institute maintains a frequently updated table on the abortion laws in each state.14 According to one estimate, 29 states are hostile to abortion rights (or lean that way), with about 40 million women aged 13 to 44 (58% of the United States) living in states with some hostility to abortion.15 Congress may pass some national abortion laws, but that seems unlikely and there may be some limits on its ability to control private medical practice within states.
An additional legal issue will arise from medication-induced abortions, generally through the use of mifepristone and misoprostol. They now account for the majority of abortions. These medications might be used for abortion, up to about 9 weeks of pregnancy, in states prohibiting abortion. The drugs once were available only with an in-person visit, but now the US Food and Drug Administration (FDA) permits mail-order delivery. The potential exists, therefore, to circumvent states’ prohibition on abortion through mail-order postal shipments. The FDA controls the licensing of pharmaceuticals in interstate commerce, but not the practice of medicine within a state. Therefore the ability of individuals (within a state) to possess or use drugs is unclear.
The abortion wars of the last 50 years gave rise to state laws related to abortion, including consent by minors, information to parents, special informed consent, and facilities requirements. If these laws were once struck down because they were inconsistent with Roe, but were never formally repealed, they may now become legal requirements.
In the foreseeable future, abortion laws generally will not be determined by federal courts but by state law, generally legislatures. In legislative hearings, town hall meetings, and conversations with lawmakers, ObGyns should engage the topic of abortion with scientific expertise, reason, openness, and humility. It will be impossible for the profession to speak with a single voice, as the briefs filed this Term demonstrate. Where there are honest differences in science, the reasons for the different interpretations should be explainable to lay decision makers. The profession, who are not being pseudo-lobbyists, can contribute a great deal to the rational consideration of this emotional topic.
On January 27, 2022, Justice Stephen Breyer informed President Biden of his intention to retire from the Court at the end of the Term. At age 84, he was the oldest member of the Court, but he continued to be among the most active of the justices and seemed to relish the work of the Court. He had been under pressure from liberal groups to retire earlier so a successor could be confirmed by a Democratic Senate. In many ways he was the Renaissance man of the Court: he spoke fluent French, wrote books, and famously sprinkled his questions with complex and funny hypotheticals.
Justice Breyer was a law professor before becoming a judge and enjoyed presentations to many groups, from children to law professors. He loved the Court and defended it—most recently against partisan attacks from both the right and the left. In the decisions of the Court, he was one of the more liberal justices. He had, for example, indicated that the death penalty is unconstitutional.
In his January retirement letter, he said that he would step down at the end of the Term if his replacement had been appointed and confirmed. She had. The new justice had clerked for Justice Breyer in 1999–2000.
Ketanji Brown Jackson was nominated by President Biden on February 28, confirmed by the Senate on April 7 by a 53–47 margin, and sworn in on June 30, 2022. Justice Jackson had previously been a federal district court judge and on the Court of Appeals for the D.C. Circuit. She attended Harvard-Radcliffe College and received her law degree from Harvard Law School. She worked as a criminal defense attorney and was active in the US Sentencing Commission.
Continue to: What is a practitioner to do?...
What is a practitioner to do?
For many practitioners, the Dobbs decision will have little effect because their state laws are consistent with Roe, and the legislature is not going to change the law. They may, of course, see an influx of patients from other states (that restrict abortion) seeking treatment. At the other extreme, in some states, most abortions will become prohibited. State courts may ease the restrictions. In many states, there will be an ongoing battle over when abortion is legal and when it is not, resulting in shifting laws and regulations. Keeping up with the shifts that affect practice will be a challenge.
All states are likely to permit abortions “to save the life of the mother,” and many will have a version of “to preserve the health of the mother.” Other exceptions may be for pregnancy resulting from rape or incest or in the case of serious fetal abnormality. ObGyns, of course, will be called on to certify that one of these exceptions exists. Determining that pregnancy resulted from rape or incest, of course, can be challenging. Before Roe, there was a cottage industry opining that pregnancy seriously affected the health of the mother, which often involved physical manifestations of mental health. ObGyns in some states may be asked once again to make such determinations.
Laws not directly related to abortions will, in some states, be changed as a way of discouraging abortion. For example, child abuse reporting laws may be modified to require reporting of any known or suspected abortion or attempted abortion, and medical licensing standards may make it a violation to participate in or facilitate abortion in any way.
Particularly in states where the rules keep shifting, practitioners must keep up with the current law. Professional organizations can help with that, but there is no substitute for practitioners having an ongoing professional relationship with an attorney who has expertise in health law.
Other abortion decisions this Term
In other abortion decisions this Term, the Court refused to suspend a Texas law that prohibited abortions after a fetal heartbeat could be detected.16 The law has remarkable enforcement mechanisms that preclude state officers from enforcing it; instead, it creates what amounts to a private attorney general (PAG) provision that allows private citizens to file suit against anyone performing or assisting in performing abortions. This PAG made pre-enforcement challenges to the law difficult.17
In a Kentucky case, the Court allowed the Kentucky attorney general to intervene in a case that challenged a Kentucky law that prohibits physicians from using dilation and evacuation procedures to end second-trimester pregnancies.18
Criminal convictions for physicians’ overprescription of controlled substances
Perhaps the least sympathetic of the physicians involved with the Court this Term were the 2 in Ruan v U.S.19 Their trials indicate that Dr. Ruan’s clinic issued more than 300,000 controlled substance prescriptions over 4 years and was one of the most frequent prescribers of fentanyl. Dr. Kahn prescribed controlled substances without an examination, falsified notes, and sold controlled substances for cash and guns.20
Both physicians were convicted of “knowingly or intentionally” dispensing a controlled substance without authorization.21 They were authorized to prescribe drugs, but only “for a legitimate medical purpose.”22 Appeals to their respective Circuit courts confirmed their convictions. The Supreme Court, however, held that to convict them, the government must prove that they knowingly or intentionally acted in an unauthorized manner. That proof can be by circumstantial evidence, but it must be beyond a reasonable doubt.
Health care reimbursement
Hospitals won one and lost one Medicare-Medicaid reimbursement case that involved payments for low-income patients.
In the loss, the Court held that the US Department of Health and Human Services (HHS) properly calculated the disproportionate share adjustments (DSH), or Medicare fraction,23 that provides a supplemental payment for hospitals with a large proportion of low-income patients. The lower DSH payments calculated by HHS were upheld, thereby reducing the number of hospitals receiving DSH payments and decreasing the amounts others will receive.
The win involved payments for prescription drugs that hospitals provide to outpatients in safety-net hospitals.24 HHS determined that it was overpaying hospitals for drugs and cut the reimbursement rate. The Court held that before HHS can change the drug rate, it must conduct a survey of hospitals regarding actual costs. It had not done that, so the rate reduction was not permitted by the law.
An accidental disincentive to (some) malpractice suits
Medicaid requires states to obtain part of a tort recovery that recipients obtain if Medicaid is covering medical expenses related to their injuries. In implementing that law, a state may provide a disincentive for injured beneficiaries to file malpractice cases. At issue was a Florida law that provided the Medicaid state would take 37.5% of the beneficiary’s total tort recovery (being one-half of the recovery after deducting 25% for attorney’s fees and costs). In a 7-2 decision, the Court upheld the Florida law.25
The disincentive to filing a lawsuit is that the state is taking 37.5%, plus contingency fee attorneys will typically take 33.3% (and there will be some fees). This is especially true when there is a state cap on noneconomic damages. In the case the Court decided, the plaintiff received a settlement of $850,000. If we assume a typical contingency fee, less the state’s Medicaid claim of $300,000, the plaintiff possibly received $266,667. That is not trivial, but it is only 31% of the settlement.
The Medicaid expectation of reimbursement and the Florida approach, however, impose heavy burdens on severely injured beneficiaries. The plaintiff had catastrophic injuries and was in a vegetative state. There are some things Medicaid does not pay for, as well as nonmedical expenses. The amount left for such expenses is likely well below what the family will need.
Continue to: COVID-19 vaccinations...
COVID-19 vaccinations
Had it not been for the abortion decisions, 2021–2022 might have been “the COVID Term.” Two of the most anticipated decisions involved mandatory vaccinations (or masking/testing instead). The question in each of these cases was whether Congress had authorized 2 federal agencies to issue the emergency regulations requiring vaccination. Emergency regulations are held to higher standards because they bypass the usual protections of the Administrative Procedure Act.
One case involved a regulation issued by the Occupational Safety and Health Administration (OSHA) that employers (with more than 100 employees) must require their employees to be vaccinated. In a 6-3 decision, the Court held that OSHA did not have the authority to enforce this as an emergency regulation. The other case was a regulation issued by HHS that health care institutions receiving Medicare and Medicaid funding must require all staff to be vaccinated.26 In a 5-4 decision, the Court upheld this emergency regulation because of the very broad authority Congress had given HHS to ensure the safety of patients and the quality of Medicare- and Medicaid-funded programs.27
In another case, in the shadow docket (orders and opinions in cases without full arguments), the Court struck down the Centers for Disease Control and Prevention’s eviction moratorium.28 The Court said the government claimed “a breathtaking amount of authority” that Congress did not intend. In other shadow docket cases, the Court refused to hold unconstitutional state laws that require COVID-19 vaccination but did not have religious exemptions.29
Analysis of this Term
It was an extraordinary Term. The Court decided 66 cases (excluding most cases in the shadow docket), a low number historically. Not only were there many seminal cases but also the Court appears to be shifting toward a new direction. That direction may be oriented more toward the original understanding of the words of the Constitution and statutes and less toward policy; Congress rather than administrative agencies; racial nondiscrimination rather than preferences; and the free exercise rather than the establishment of religion. Whether there is such a shift or not, of course, only time will tell.
Chief Justice Roberts and Justice Kavanaugh were in the majority most often (95% of the cases), followed by Justices Barrett (90%), Alito (85%), Thomas (80%), and Gorsuch (75%). Justices Kagan (69%) and Breyer (68%) were not far behind. Justice Sotomayor was in the majority 58%. The Court was unanimous 29% of the time, well below the decade average (43%), and 6-3 accounted for 30% of the decisions.
A major, potentially scarring, event this Term was the leak of an early draft of the majority opinion in Dobbs. Although leaks have occurred before, the early leak of an opinion was unprecedented. It will almost inevitably change the openness and candor within the Court and the justices’ clerks. Although not unprecedented, the attempt on the life of Justice Kavanaugh and the organized efforts to harass some justices in their homes are likely to have lasting impact. Almost certainly it means that justices and their families will have constant security and their movements and connection with the general public will become less frequent, which is sad for the justices and our democracy.
Looking toward the next Term
When the Court next convenes, Justice Ketanji Brown Jackson will take her seat on the left end of the Court (the traditional seat for a new justice, not a commentary on judicial philosophy). The Court has already taken many cases, including issues about university affirmative action programs, web designers and same-sex couples, redistricting and voting rights, DNA testing in criminal cases, and overtime pay for someone making over $200,000 per year. It begins Monday, October 3, and promises to be another interesting Term. ●
- Dobbs v Jackson Women’s Health Organization, 19-1392, decided June 24, 2022. https://www.supremecourt.gov /opinions/21pdf/19-1392_6j37.pdf
- Roe v Wade, 410 U.S. 113, 163 (1973).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Smith SR, Sanfilippo JR. The Supreme Court and reproductive rights. OBG Manag. 2022;34(1):36-41, 46. https://cdn.mdedge. com/files/s3fs-public/issues/articles/obgm0340136_smith.pdf
- Washington v Glucksberg, 521 U.S. 702 (1997).
- Obergefell v Hodges, 576 U.S. 644, 654-70 (2015).
- Brief of amici curiae of American College of Obstetricians and Gynecologists, American Medical Association, et al, in Dobbs v Jackson Women’s Health Organization, in Support of Respondents (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/193074/20210920174518042 _19-1392%20bsacACOGetal.pdf
- Id. at 8, 13-15.
- Id. at 14.
- Justices Breyer, Kagan, and Sotomayor, dissenting, at 48, note 22.
- Brief for American Association of Pro-Life Obstetricians And Gynecologists as Amicus Curiae, in Dobbs v Jackson Women’s Health Organization, in Support of Petitioners (July 2021). https://www.supremecourt.gov /DocketPDF/19/19-1392/185350/20210729163532595_No. %2019-1392%20-%20American%20Association%20of%20 Pro-Life%20Obstetricians%20and%20Gynecologists%20-%20 Amicus%20Brief%20in%20Support%20of%20Petitioner%20-%20 7-29-21.pdf
- Id. at 3-4, 7-29.
- Id. at 30.
- Guttmacher Institute. An overview of abortion laws. July 11, 2022. https://www.guttmacher.org/state-policy/explore/overview -abortion-laws
- Guttmacher Institute. State abortion policy landscape: from hostile to supportive. Dec. 2020. https://www .guttmacher.org/article/2019/08/state-abortion-policy -landscape-hostile-supportive
- Whole Woman’s Health v Jackson, 21-463, decided Dec. 10, 2021. https://www.supremecourt.gov/opinions/21pdf/21-463_ new_8o6b.pdf
- United States v Texas, 21-588, decided Dec. 10, 2021. (Per curiam, Sotomayor dissenting). https://www.supremecourt.gov /opinions/21pdf/21-588_c07d.pdf
- Cameron v EMW Women’s Surgical Center, 20-601, decided Mar. 3, 2022. https://www.supremecourt.gov/opinions/21pdf/20-601 _new_g20h.pdf
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https:// www.supremecourt.gov/opinions/21pdf/20-1410_1an2.pdf
- Adam Liptak. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022. https://www .nytimes.com/2022/06/27/us/politics/supreme-court-controlled -substance-act.html
- 84 Stat. 1260, 21 U.S.C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Becerra v Empire Health Foundation, For Valley Hospital Medical Center, 20-1312, decided June 24, 2022. https://www.supremecourt .gov/opinions/21pdf/20-1312_j42l.pdf
- American Hospital Association v Becerra, 20-1114, decided June 15, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1114_09m1.pdf
- Gallardo v Marstiller, 20-1263, decided June 6, 2022. https:// www.supremecourt.gov/opinions/21pdf/20-1263_new_hfci.pdf
- National Federation of Independent Business v Department of Labor, Occupational Safety and Health Administration, 21A244, decided Jan. 13, 2022. https://www.supremecourt.gov /opinions/21pdf/21a244_hgci.pdf
- Biden v Missouri, 21A240, decided Jan. 13, 2022. https://www .supremecourt.gov/opinions/21pdf/21a240_d18e.pdf
- Alabama Association of Realtors v Department of Health and Human Services, 21A23, decided Aug. 26, 2021. https://www .supremecourt.gov/opinions/20pdf/21a23_ap6c.pdf
- Does v Mills, 21A90, decided Oct. 29, 2021. https://www .supremecourt.gov/opinions/21pdf/21a90_6j37.pdf
- Dobbs v Jackson Women’s Health Organization, 19-1392, decided June 24, 2022. https://www.supremecourt.gov /opinions/21pdf/19-1392_6j37.pdf
- Roe v Wade, 410 U.S. 113, 163 (1973).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Smith SR, Sanfilippo JR. The Supreme Court and reproductive rights. OBG Manag. 2022;34(1):36-41, 46. https://cdn.mdedge. com/files/s3fs-public/issues/articles/obgm0340136_smith.pdf
- Washington v Glucksberg, 521 U.S. 702 (1997).
- Obergefell v Hodges, 576 U.S. 644, 654-70 (2015).
- Brief of amici curiae of American College of Obstetricians and Gynecologists, American Medical Association, et al, in Dobbs v Jackson Women’s Health Organization, in Support of Respondents (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/193074/20210920174518042 _19-1392%20bsacACOGetal.pdf
- Id. at 8, 13-15.
- Id. at 14.
- Justices Breyer, Kagan, and Sotomayor, dissenting, at 48, note 22.
- Brief for American Association of Pro-Life Obstetricians And Gynecologists as Amicus Curiae, in Dobbs v Jackson Women’s Health Organization, in Support of Petitioners (July 2021). https://www.supremecourt.gov /DocketPDF/19/19-1392/185350/20210729163532595_No. %2019-1392%20-%20American%20Association%20of%20 Pro-Life%20Obstetricians%20and%20Gynecologists%20-%20 Amicus%20Brief%20in%20Support%20of%20Petitioner%20-%20 7-29-21.pdf
- Id. at 3-4, 7-29.
- Id. at 30.
- Guttmacher Institute. An overview of abortion laws. July 11, 2022. https://www.guttmacher.org/state-policy/explore/overview -abortion-laws
- Guttmacher Institute. State abortion policy landscape: from hostile to supportive. Dec. 2020. https://www .guttmacher.org/article/2019/08/state-abortion-policy -landscape-hostile-supportive
- Whole Woman’s Health v Jackson, 21-463, decided Dec. 10, 2021. https://www.supremecourt.gov/opinions/21pdf/21-463_ new_8o6b.pdf
- United States v Texas, 21-588, decided Dec. 10, 2021. (Per curiam, Sotomayor dissenting). https://www.supremecourt.gov /opinions/21pdf/21-588_c07d.pdf
- Cameron v EMW Women’s Surgical Center, 20-601, decided Mar. 3, 2022. https://www.supremecourt.gov/opinions/21pdf/20-601 _new_g20h.pdf
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https:// www.supremecourt.gov/opinions/21pdf/20-1410_1an2.pdf
- Adam Liptak. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022. https://www .nytimes.com/2022/06/27/us/politics/supreme-court-controlled -substance-act.html
- 84 Stat. 1260, 21 U.S.C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Becerra v Empire Health Foundation, For Valley Hospital Medical Center, 20-1312, decided June 24, 2022. https://www.supremecourt .gov/opinions/21pdf/20-1312_j42l.pdf
- American Hospital Association v Becerra, 20-1114, decided June 15, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1114_09m1.pdf
- Gallardo v Marstiller, 20-1263, decided June 6, 2022. https:// www.supremecourt.gov/opinions/21pdf/20-1263_new_hfci.pdf
- National Federation of Independent Business v Department of Labor, Occupational Safety and Health Administration, 21A244, decided Jan. 13, 2022. https://www.supremecourt.gov /opinions/21pdf/21a244_hgci.pdf
- Biden v Missouri, 21A240, decided Jan. 13, 2022. https://www .supremecourt.gov/opinions/21pdf/21a240_d18e.pdf
- Alabama Association of Realtors v Department of Health and Human Services, 21A23, decided Aug. 26, 2021. https://www .supremecourt.gov/opinions/20pdf/21a23_ap6c.pdf
- Does v Mills, 21A90, decided Oct. 29, 2021. https://www .supremecourt.gov/opinions/21pdf/21a90_6j37.pdf
Suing patients: Medical, ethical, and legal considerations
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
Embryo mix-up debacles: Is there liability?
CASE Embryo mix-up with 2 couples
A lawsuit was recently filed in California by a couple after the woman carried and gave birth to “the wrong child.” This was the second full-term pregnancy for the couple. The couple had undergone an unsuccessful in vitro fertilization (IVF) cycle in October 2018. The next IVF cycle in 2019 led to the birth of a daughter on September 24, 2019, who is the subject of this case.1
At the time of birth, the couple suspected something was wrong because the baby had “jet-black hair and a complexion that was darker” than their complexions. The couple eventually obtained a DNA test, which confirmed in November 2019 that this was not their biological child.1
A few weeks later, they learned that another woman who went to the same IVF clinic gave birth to a female baby 1 week after their daughter was born. Similarly, that baby did not resemble the parents, and DNA testing confirmed the baby belonged to the first couple. The couples ultimately exchanged the babies.1
The legal claim filed against the IVF center and its owner (an obstetrician) was for breach of contract, medical malpractice, and infliction of emotional distress, including experiencing “disassociation” on the part of the couple(s). Each couple felt they did not get to experience the birth of their biological child, and, of course there was considerable distress in the process of learning that the child was not theirs and exchanging the birth child for the biological child. In addition, the couple who filed the suit had another child (now age 7 years), who begged them to keep the baby to whom they gave birth. The couple also reported experiencing panic attacks as a result of the events.1
Medical considerations
As of 2018, more than 8 million IVF babies had been born, with the first in 1978 in the United Kingdom.2 Advances in science and technology have improved the process. Storage tanks now have alarms and several safeguards to monitor the level of liquid nitrogen and immediately notify key personnel if levels are low (FIGURES 1 and 2). Preimplantation genetic testing is also readily available to assess the embryo prior to transfer into the uterus and identify various genetic problems.


Guidelines for embryo straw labelling are provided by the College of American Pathologists and the Centers for Disease Control and Prevention. The American Society for Reproductive Medicine (ASRM) also provides guidelines. When an error occurs, disclosure is recommended and ethical and legal counsel should be involved. Failing to disclose can lead to professional penalties.4
Unfortunately, despite these advances and guidelines, embryo mix-ups like the one in the above case do occur and receive public notice (See “Cross country embryo mix-up cases”).5,6 A report from the University of Nevada assessed liability for embryo mix-ups in US fertility practices from 2000 to 2020.7 They evaluated 184,015 IVF cycles with 176 claims. Payments were made to plaintiffs in 21 cases, resulting in $15 million of awarded damages (average award was $199,188).7 The most common problem was in the embryology laboratory with an overall incidence of 0.03% of the total number of IVF cycles.7 To avoid damages, the authors emphasized the importance of following labeling guidelines when storing embryos, considering a 2-step read-back method prior to embryo transfer, and offering genetic testing when a discrepancy is noted in the record (TABLE).7

Other medical liability considerations
Embryo mix-ups are not the only source of problems and potential liability in IVF. At the 2021 Association of Sexual and Reproductive Medicine Annual Meeting, Applebaum et al presented results from a comprehensive review of malpractice litigation involving IVF in the United States.8 Using the legal database NEXIS Uni they identified 50 cases between 1986 and 2020 (32% of which were filed in New York state). Common thematic elements among patient allegations were embryology errors (eg, lost or destroyed embryos or incorrect sperm or egg donor), errors in preimplantation genetics, surgical or medical errors/complications, or misdiagnosis (eg, sexually transmitted disease screening or malignancy).8 Overall, the most common plaintiff complaint was negligence (26 cases) due to informed consent–related issues (9 cases), wrongful life or birth (9 cases), or negligent or intentional infliction of emotional distress (5 cases).8
In 48% of cases, the verdict was in favor of the defendant; it was for the plaintiff in 36% of cases and ongoing proceedings or partial judgement accounted for the remaining cases.8 Damages ranged from $4,171.45 to $50 million. The authors emphasized specific defense strategies, including the importance of careful labeling and handling of embryos, prompt disclosure when an error does occur, and awareness of the specific state statute(s) of limitations for medical malpractice claims.8
Continue to: Legal considerations...
Legal considerations
The case at the beginning of this article is a “mix-up” case, in which an IVF center implanted the wrong embryo, resulting in the birth parents not being the biological parents.1 As in that case, there may be (but are not always)6,9 2 mix-ups, so that 2 couples have each other’s biological children. These cases may go unnoticed by the birth parent if the physical appearance is not unexpected and the parents never do genetic testing, or if the IVF center does not discover the error and inform the parents. Infrequently the cases make the news or the courts.10,11
News accounts are not trials, and we do not suggest that all the facts discussed in news reports on the case described here are complete—or even accurate in the details reported. They are generally 1-sided, so there are other perspectives. To consider the legal issues, however, we will assume for discussion only that the facts are as they have been reported in the news coverage—with the understanding that the discovery and trial processes would undoubtedly bring to light many other important facts or corrections.
Negligence
Although there are several potential bases for liability (ie, contract or warranty claims, a form of product liability/defect) in mix-up and other artificial reproductive technology (ART), negligence or malpractice seem most likely.12 “Negligence” here is intended to be simple negligence but may also include gross negligence or recklessness.
Although the incidence of errors in ART is unknown, there is limited evidence that suggests it is not a rare event. One study suggested >20% of fertility clinics knew of errors in processing or handling donor samples and embryos for implantation.13 Another study in the United Kingdom found that 1 in 1,000 IVF embryos were implanted in the wrong woman.14
Was there negligence? The first question in a malpractice or negligence-type action is, was there a professional relationship between the plaintiff who is claiming harm and the professional or organization defendant? The next question is whether the defendant was reasonably careful given the circumstances—that is, did the physician meet the “standard of care”? This is sometimes described as whether the professional’s actions would be acceptable (ie, reasonably prudent within the profession or specialty). If there was negligence, then the next question is, did that negligence cause an injury to the plaintiff?15
Determining the standard of care. The nature of the expected standard of care is dependent, in part, on the potential consequences of an error. For example, the care required when there is a significant risk of death from an error would be considerably more cautious than for an error that might result in small property damage. In this case study, a mix-up error is likely to be less severe than death, but is very substantial in terms of emotional harm and disruption. Thus, considerable care and attention would be expected to avoid these errors. They should be a “never” event. Institutions and physicians should give considerable attention to their processes and procedures to avoid the possibility of a mix-up error.16
Where did the negligence occur? There is an old tort doctrine “Res ipsa loquitor” (RIL) that means, “The thing speaks for itself.” Although there are several technical rules around the application of RIL as a presumption of negligence, it comes down to the proposition that some injuries do not occur without negligence. A traditional medical example is the sponge left in a patient during surgery—ordinarily that does not happen without some negligence. For RIL to be applied, usually the mechanism by which the injury occurred had to be under the control of the defendant (or the agents of the defendant).
The “mix-up” of embryos is an example of the kind of error that would not likely occur without negligence.17 But the embryo may not be in the exclusive control of any 1 institution. For example, the mistake could be made by the IVF center (or its employees), a separate facility that has processed or cryogenically stored the genetic materials, and independent physicians (not employees or agents of the center). Therefore, it is necessary to pinpoint where the negligence occurred and who is legally responsible. In some cases, a health care provider must take steps to ensure that its contractors have sufficient safeguards to avoid unnecessary harms. For example, an IVF center that uses an external cryogenic storage facility may have some obligation to know that the genetic material returned to the center is the same material that the center provided the storage facility in the first place and is properly identified.18
Assessing damages
From the facts as we have them, it appears that there must have been negligence that caused the mix-up of the embryos in the original case. It also appears reasonably clear that the negligence resulted in harm to both sets of parents and their families. This would suggest that the families should recover substantial damages. But that, somewhat surprisingly, may not be the case.19 Several legal principles may limit the availability or size of damages in mix-up cases. Also, it is worth remembering that there are differences in how states treat the different types of damages in these cases. Although the case was filed in California, we’ll take a more national view of the damages issue.
Not all harm is treated as equal. The first problem facing plaintiffs in mix-up cases may be the fact that they have suffered only emotional harm, without any physical injury. Traditionally, the courts have been reluctant to allow recovery in negligence for purely emotional injuries. Also “intentional” infliction of emotional distress does permit financial recovery, but generally “negligent” infliction of emotional harm traditionally has not. In part, this was because of the fear of unwarranted (and difficult-to-assess) claims of emotional harm that are not related to a physical harm. Some states developed a “zone of danger” exception (eg, where someone was almost hit by a car) or allowed some emotional injury recovery if there were “physical manifestations” of the emotional harm. In short, depending on the state’s rules, negligence that causes purely emotional harm may not be compensable.20
State-based malpractice “caps.” Another limitation on emotional injuries is the “caps” on malpractice damages enacted by several states (including California, where this mix-up case occurred). Therefore, if a mix-up case is determined to be a malpractice case under state law, emotional suffering damages (which are non-economic damages) may be limited to the cap—$250,000 in California, for example—even if the state allows damages for emotional injuries without physical injuries.
The rare exception. Very careless labeling or handling of the identity of the embryo could at the extreme be considered gross negligence or recklessness. There are relatively inexpensive and easy procedures that could easily avoid what is likely to be significant harm to families (including emotional upset).21 Institutions that callously fail to use those procedures might be seen by some courts as reckless, or in outrageous cases, even intentional. An example would be the University of California Irvine Center for Reproductive Health case, in which physicians intentionally (without consent) used patients’ ova, fertilized them, and then implanted them in other patients, with at least 15 births, many lawsuits, and multimillion dollar settlements.22 In “intentional” cases, limitations on emotional injuries would usually not be major barriers to recovery of damages. However, those are legal stretches, and recovery is the exception rather than the rule.23
Continue to: Additional legal concerns with IVF...
Additional legal concerns with IVF
Reproduction negligence cases include a large range of errors and injuries—not just embryo mix-ups. Courts have struggled with when it is appropriate to allow damages, even when there have been clear injuries. For the most part courts have been reluctant to find liability in many areas of new IVF technology.12 One problem in determining how to assess damages is determining how incidental benefits should be used to offset some or all of the damages. For example, how should the joy of having a child offset the costs of raising the child?
There are more than a dozen kinds of current and likely future claims arising from problems with ART. It is tempting to conclude, “Oh, what a tangled legal web we weave when first we practice to artificially conceive.” There are various groupings of such claims, with several examples of cases presented in this article. It is not possible to consider those in detail in this article. As a general proposition, however, “our legal system treats wrongfully disrupted plans concerning reproduction like one of those life adversities that people are expected to abide without remedy.”24
This is not to say, however, that there is no compensation for IVF-related injuries. Applebaum and colleagues found more than 100 cases in the 35 years covered by the study (1984-2020).8 However, only 50 of those cases fit the criteria for inclusion in their data. The successful cases for the plaintiffs involved medical or surgical error, while it appeared that various forms of wrongful life or birth were much less successful. It would be a mistake to conclude from these data that there are not, and will not be, meaningful risks of liability in the areas of IVF and ART more generally.
First, claims that fit with existing legal doctrine are producing liability. About half of the claims (25 over the 34 years) examined by Applebaum et al resulted in liability. Admittedly, that number was small because ART use was increasing. Where the claims fit well-recognized legal forms of damages and forms of action (primarily negligence), the liability could be substantial. A remarkable example of this is the case of Wuth v Lab. Corp (see “Liability for genetic testing errors”),25 which was the largest verdict ($50 million) in the Applebaum and colleagues’ study.8 The large verdict was due to the failure of the testing company and a medical center to properly perform and assess a genetic test, which resulted in the birth of a child with an unbalanced chromosome translocation.8,25 The child’s serious disabilities would require a great deal of expensive care. Although the jury held the testing laboratory and medical center liable, they did not find liability against the physician.25 Ultimately, this case would be considered a failure of genetic testing rather than an IVF case.
More than 2 couples
In a second case from California, a couples’ son was born to another couple in New York—along with another boy from a third couple. The woman in New York thought she had carried biological twins but genetic testing confirmed the twins were not related to the couple or to each other (the second couple filed a separate medical malpractice and negligence lawsuit in New York). All 3 couples had sought care at the same IVF clinic. The babies were eventually returned to their biological parents.1
Different races
In a New York case, a Korean couple had twin White boys after consenting to a single embryo transfer. Meanwhile a couple in Los Angeles who went to the same in vitro fertilization clinic gave birth to a child that did not match their appearance. Both couples had undergone embryo transfers on the same day. The court arranged for the Korean couple to surrender their twins to their biological parents when they were 6 months of age in exchange for their biological child.2
References
1. Couple claims clinic implanted their embryo in wrong woman. Associated Press. July 10, 2019. https://apnews.com/article/de32d537c6e34808b28834c23f00e272. Accessed January 6, 2022.
2. In the matter of accusation against Steven L. Katz. Case no. 03-20001-122617.OAH no. N2004080093. Sacramento, CA. Medical Board of California Department of Consumer Affairs 2005.
Future challenges
The future is likely to bring substantially expanded IVF/ART liability for several reasons. ART is becoming more common. Although courts have struggled with how to apply existing liability rules to the new technologies and related novel legal claims, the absence of established legal principles into which IVF injuries fit will not last forever. The legal system eventually finds ways of adjusting old rules or adopting new ones to cover injuries from new technology.
Although IVF injuries that most people feel deserve compensation currently are not cognizable in law, that will undoubtedly change. Either the courts will find new ways of assessing ART claims, or state legislatures and Congress will step in with legislation. To date, Congress has been relatively “hands off” on the ART processes, with the Fertility Clinic Success Rate and Certification Act of 1992 being a notable exception.24 This law requires ART programs to report success rates and directs the Centers for Disease Control and Prevention (CDC) to publish reported success rates and laboratory incidents. It also establishes a model state laboratory certification program.24 The CDC has an outline of the work under the statute,26 as well as state-specific data regarding ART27 and lists of publications in key areas.28 In addition there are various state laws related to recordkeeping, donor qualifications, licensing, and family law issues.29 Ultimately, physicians, scientists, and legal professionals can perform a valuable role in helping to fashion IVF liability principles that are workable and reasonable, that will not interfere with the progress of medicine, and that will ensure that those injured through carelessness or bad medicine receive compensation. ●
Although not technically an in vitro fertilization (IVF) case, Wuth v Lab. Corp. involved an infant born through IVF with a translocation defect chromosome 2 (ie, deleted material) and extra chromatin on 9. The father’s family history included birth defects, including a female cousin with profound developmental disabilities, seizures, and antisocial behavior. He had undergone genetic testing that revealed an asymptomatic balanced, 2;9 translocation. As part of the IVF process, the couple had a genetic consultation and were told there was a 50% chance that the fetus would have an unbalanced 2;9 translocation given the father’s family history and that chorionic villus sampling or amniocentesis could detect this in the fetus.1
Amniocentesis had been performed, with the specimen sent to Lab. Corp. The result was “normal male karyotype.” However, when the baby was born, it was immediately apparent that he had severe physical defects and subsequently cognitive defects. Genetic testing of the child revealed an unbalanced 2;9 translocation. The couple filed a suit for wrongful birth and wrongful life, which went to a jury. The child was awarded $25 million and the parents/family were awarded another $25 million in general damages. The verdict reflected errors in genetic (laboratory) testing.
Reference
1. Wuth v Lab. Corp. of Am., 189 Wash. App. 660, 359 P.3d 841 (2015).
- Mark J. California couple sues fertility clinic following IVF embryo mix-up. Washington Post. November 9, 2021. https://www.washingtonpost.com/nation/2021/11/09/in-vitro-fertilization-ivf-mix-up-daphna-cardinale. Accessed January 5, 2022.
- More than 8 million babies born from IVF since the world’s first in 1978. Science Daily. July 3, 2018. https://www.sciencedaily.com/releases/2018/07/180703084127.htm. Accessed January 11, 2022.
- ESCO Medical. In vitro fertilization (IVF) as fertility treatment. https://www.esco-medical.com/resource/in-vitro-fertilization-ivf-as-fertility-treatment.
- Vigdor N. “We had their baby, and they had our baby”: couple sues over embryo “mix-up.” NY Times. November 9, 2021. https://www.nytimes.com/2021/11/09/us/fertility-clinic-embryo-mixup.html. Accessed January 11, 2022.
- Couple claims clinic implanted their embryo in wrong woman. Associated Press. July 10, 2019. https://apnews.com/article/de32d537c6e34808b28834c23f00e2728. Accessed January 6, 2022.
- In the matter of accusation against Steven L. Katz. Case no. 03-20001-122617.OAH no. N2004080093. Sacramento, CA. Medical Board of California Department of Consumer Affairs 2005
- Rasouli MA, Moutos CP, Phelps JY. Liability for embryo mix-ups in fertility practices in the USA. J Assist Reprod Genet. 2021;38:1101-1107. doi:10.1007/s10815-021-02108-1
- Applebaum J, Berger D, O’Neill K. Can a reproductive endocrinologist be sued for 50 million dollars? A comprehensive review of malpractice litigation involving in vitro fertilization in the U.S. Fertil Steril. 2021;116(3s):e19. doi:10.1016/j.fertnstert.2021.07.059
- Andrews v Keltz, 838 N.Y.S.2d 363, 365 (Sup. Ct. 2007).
- Chichi DV. In vitro fertilization, fertility frustrations, and the lack of regulation. Hofstra L Rev. 2021;49:535-568. https://www.hofstralawreview.org/wp-content/uploads/2021/04/bb.2.chichi.pdf. Accessed January 11, 2022.
- Lewin T. Sperm banks accused of losing samples and lying about donors. NY Times. July 21, 2016. https://www.nytimes.com/2016/07/22/us/sperm-banks-accused-of-losing-samples-and-lying-about-donors.html. Accessed January 11, 2022.
- Bender L. To err is human ART mix-ups: labor-based, relational proposal. J Gender Race Justice. 2006;9:443-508. https://surface.syr.edu/cgi/viewcontent.cgi?article=1050&context=lawpub. Accessed January 11, 2022.
- Baruch S, Kaufman D, Hudson KL. Genetic testing of embryos: practices and perspectives of U.S. in vitro fertilization clinics. Fertil Steril. 2007;89:1053-1058. doi:10.1016/j.fertnstert.2007.05.048
- Liebler R. Are you my parent? Are you my child? The role of genetics and race in defining relationships after reproductive technological mistakes. DePaul J Health Care Law. 2002;5:15-56. https://via.library.depaul.edu/cgi/viewcontent.cgi?article=1202&context=jhcl. Accessed January 11, 2022.
- Crockin SL, Altman AB, Edmonds MA. The history and future trends of art medicine and law. Fam Court Rev. 2021;59:22-45. doi:10.1111/fcre.12550
- Fernandes JS. Perfecting pregnancy via preimplantation genetic screening: the quest for an elusive standard of care. UC Irvine L Rev. 2014;4:1295-1326. https://www.law.uci.edu/lawreview/vol4/no4/Fernandes.pdf. Accessed January 11, 2022.
- VanGessel MM. Wrongful surrogacy: the need for right of action in cases of clear negligence. U Toledo L Rev. 2015;46:681-706.
- Reich J, Swink D. Outsourcing human reproduction: embryos and surrogacy services in the cyberprocreation era. J Health Care L Policy. 2011;14:241-298. https://core.ac.uk/download/pdf/217156567.pdf. Accessed January 11, 2022.
- Strasser M. Prenatal tort slippage. Health Matrix. 2021;31:221-262. https://scholarlycommons.law.case.edu/healthmatrix/vol31/iss1/9. Accessed January 11, 2022.
- Heide IH. Negligence in the creation of healthy babies: negligent infliction of emotional distress in cases of alternative reproductive technology malpractice without physical injury. J Med L. 2005;9:55-94.
- Novo S, Nogués C, Penon O, et al. Barcode tagging of human oocytes and embryos to prevent mix-ups in assisted reproduction technologies. Hum Reprod. 2014;29:18-28. doi: 10.1093/humrep/det409
- Yoshino K. UCI Settles Dozens of Fertility Suits. LA Times. September 11, 2009. https://www.latimes.com/archives/la-xpm-2009-sep-11-me-uci-fertility11-story.html. Accessed January 11, 2022.
- Fox D. Reproductive negligence. Columbia L Rev. 2017;117:149-242. https://columbialawreview.org/wp-content/uploads/2017/01/149.pdf. Accessed January 11, 2022.
- 42 U.S.C.S. §263a-1-263a-7; Public Law 102-493. https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3146.pdf. Accessed January 11, 2022.
- Wuth v Lab. Corp. of Am., 189 Wash. App. 660, 359 P.3d 841 (2015).
- Centers for Disease Control and Prevention. The Fertility Clinic Success Rate and Certification Act. December 14, 2020. https://www.cdc.gov/art/nass/policy.html#act. Accessed January 11, 2022.
- Centers for Disease Control and Prevention. State-specific assisted reproductive technology surveillance. December 17, 2020. https://www.cdc.gov/art/state-specific-surveillance/index.html. Accessed January 11, 2022.
- Centers for Disease Control and Prevention. Key findings. March 12, 2021. https://www.cdc.gov/art/key-findings/index.html. Accessed January 11, 2022.
- Cohen EN. 5 Treatise on Health Care Law §22.04, (ed. Hooper, Lundy & Bookman, & Robert W. Lundy, Jr. RW.) (Matthew Bender-LexisNexis)
CASE Embryo mix-up with 2 couples
A lawsuit was recently filed in California by a couple after the woman carried and gave birth to “the wrong child.” This was the second full-term pregnancy for the couple. The couple had undergone an unsuccessful in vitro fertilization (IVF) cycle in October 2018. The next IVF cycle in 2019 led to the birth of a daughter on September 24, 2019, who is the subject of this case.1
At the time of birth, the couple suspected something was wrong because the baby had “jet-black hair and a complexion that was darker” than their complexions. The couple eventually obtained a DNA test, which confirmed in November 2019 that this was not their biological child.1
A few weeks later, they learned that another woman who went to the same IVF clinic gave birth to a female baby 1 week after their daughter was born. Similarly, that baby did not resemble the parents, and DNA testing confirmed the baby belonged to the first couple. The couples ultimately exchanged the babies.1
The legal claim filed against the IVF center and its owner (an obstetrician) was for breach of contract, medical malpractice, and infliction of emotional distress, including experiencing “disassociation” on the part of the couple(s). Each couple felt they did not get to experience the birth of their biological child, and, of course there was considerable distress in the process of learning that the child was not theirs and exchanging the birth child for the biological child. In addition, the couple who filed the suit had another child (now age 7 years), who begged them to keep the baby to whom they gave birth. The couple also reported experiencing panic attacks as a result of the events.1
Medical considerations
As of 2018, more than 8 million IVF babies had been born, with the first in 1978 in the United Kingdom.2 Advances in science and technology have improved the process. Storage tanks now have alarms and several safeguards to monitor the level of liquid nitrogen and immediately notify key personnel if levels are low (FIGURES 1 and 2). Preimplantation genetic testing is also readily available to assess the embryo prior to transfer into the uterus and identify various genetic problems.


Guidelines for embryo straw labelling are provided by the College of American Pathologists and the Centers for Disease Control and Prevention. The American Society for Reproductive Medicine (ASRM) also provides guidelines. When an error occurs, disclosure is recommended and ethical and legal counsel should be involved. Failing to disclose can lead to professional penalties.4
Unfortunately, despite these advances and guidelines, embryo mix-ups like the one in the above case do occur and receive public notice (See “Cross country embryo mix-up cases”).5,6 A report from the University of Nevada assessed liability for embryo mix-ups in US fertility practices from 2000 to 2020.7 They evaluated 184,015 IVF cycles with 176 claims. Payments were made to plaintiffs in 21 cases, resulting in $15 million of awarded damages (average award was $199,188).7 The most common problem was in the embryology laboratory with an overall incidence of 0.03% of the total number of IVF cycles.7 To avoid damages, the authors emphasized the importance of following labeling guidelines when storing embryos, considering a 2-step read-back method prior to embryo transfer, and offering genetic testing when a discrepancy is noted in the record (TABLE).7

Other medical liability considerations
Embryo mix-ups are not the only source of problems and potential liability in IVF. At the 2021 Association of Sexual and Reproductive Medicine Annual Meeting, Applebaum et al presented results from a comprehensive review of malpractice litigation involving IVF in the United States.8 Using the legal database NEXIS Uni they identified 50 cases between 1986 and 2020 (32% of which were filed in New York state). Common thematic elements among patient allegations were embryology errors (eg, lost or destroyed embryos or incorrect sperm or egg donor), errors in preimplantation genetics, surgical or medical errors/complications, or misdiagnosis (eg, sexually transmitted disease screening or malignancy).8 Overall, the most common plaintiff complaint was negligence (26 cases) due to informed consent–related issues (9 cases), wrongful life or birth (9 cases), or negligent or intentional infliction of emotional distress (5 cases).8
In 48% of cases, the verdict was in favor of the defendant; it was for the plaintiff in 36% of cases and ongoing proceedings or partial judgement accounted for the remaining cases.8 Damages ranged from $4,171.45 to $50 million. The authors emphasized specific defense strategies, including the importance of careful labeling and handling of embryos, prompt disclosure when an error does occur, and awareness of the specific state statute(s) of limitations for medical malpractice claims.8
Continue to: Legal considerations...
Legal considerations
The case at the beginning of this article is a “mix-up” case, in which an IVF center implanted the wrong embryo, resulting in the birth parents not being the biological parents.1 As in that case, there may be (but are not always)6,9 2 mix-ups, so that 2 couples have each other’s biological children. These cases may go unnoticed by the birth parent if the physical appearance is not unexpected and the parents never do genetic testing, or if the IVF center does not discover the error and inform the parents. Infrequently the cases make the news or the courts.10,11
News accounts are not trials, and we do not suggest that all the facts discussed in news reports on the case described here are complete—or even accurate in the details reported. They are generally 1-sided, so there are other perspectives. To consider the legal issues, however, we will assume for discussion only that the facts are as they have been reported in the news coverage—with the understanding that the discovery and trial processes would undoubtedly bring to light many other important facts or corrections.
Negligence
Although there are several potential bases for liability (ie, contract or warranty claims, a form of product liability/defect) in mix-up and other artificial reproductive technology (ART), negligence or malpractice seem most likely.12 “Negligence” here is intended to be simple negligence but may also include gross negligence or recklessness.
Although the incidence of errors in ART is unknown, there is limited evidence that suggests it is not a rare event. One study suggested >20% of fertility clinics knew of errors in processing or handling donor samples and embryos for implantation.13 Another study in the United Kingdom found that 1 in 1,000 IVF embryos were implanted in the wrong woman.14
Was there negligence? The first question in a malpractice or negligence-type action is, was there a professional relationship between the plaintiff who is claiming harm and the professional or organization defendant? The next question is whether the defendant was reasonably careful given the circumstances—that is, did the physician meet the “standard of care”? This is sometimes described as whether the professional’s actions would be acceptable (ie, reasonably prudent within the profession or specialty). If there was negligence, then the next question is, did that negligence cause an injury to the plaintiff?15
Determining the standard of care. The nature of the expected standard of care is dependent, in part, on the potential consequences of an error. For example, the care required when there is a significant risk of death from an error would be considerably more cautious than for an error that might result in small property damage. In this case study, a mix-up error is likely to be less severe than death, but is very substantial in terms of emotional harm and disruption. Thus, considerable care and attention would be expected to avoid these errors. They should be a “never” event. Institutions and physicians should give considerable attention to their processes and procedures to avoid the possibility of a mix-up error.16
Where did the negligence occur? There is an old tort doctrine “Res ipsa loquitor” (RIL) that means, “The thing speaks for itself.” Although there are several technical rules around the application of RIL as a presumption of negligence, it comes down to the proposition that some injuries do not occur without negligence. A traditional medical example is the sponge left in a patient during surgery—ordinarily that does not happen without some negligence. For RIL to be applied, usually the mechanism by which the injury occurred had to be under the control of the defendant (or the agents of the defendant).
The “mix-up” of embryos is an example of the kind of error that would not likely occur without negligence.17 But the embryo may not be in the exclusive control of any 1 institution. For example, the mistake could be made by the IVF center (or its employees), a separate facility that has processed or cryogenically stored the genetic materials, and independent physicians (not employees or agents of the center). Therefore, it is necessary to pinpoint where the negligence occurred and who is legally responsible. In some cases, a health care provider must take steps to ensure that its contractors have sufficient safeguards to avoid unnecessary harms. For example, an IVF center that uses an external cryogenic storage facility may have some obligation to know that the genetic material returned to the center is the same material that the center provided the storage facility in the first place and is properly identified.18
Assessing damages
From the facts as we have them, it appears that there must have been negligence that caused the mix-up of the embryos in the original case. It also appears reasonably clear that the negligence resulted in harm to both sets of parents and their families. This would suggest that the families should recover substantial damages. But that, somewhat surprisingly, may not be the case.19 Several legal principles may limit the availability or size of damages in mix-up cases. Also, it is worth remembering that there are differences in how states treat the different types of damages in these cases. Although the case was filed in California, we’ll take a more national view of the damages issue.
Not all harm is treated as equal. The first problem facing plaintiffs in mix-up cases may be the fact that they have suffered only emotional harm, without any physical injury. Traditionally, the courts have been reluctant to allow recovery in negligence for purely emotional injuries. Also “intentional” infliction of emotional distress does permit financial recovery, but generally “negligent” infliction of emotional harm traditionally has not. In part, this was because of the fear of unwarranted (and difficult-to-assess) claims of emotional harm that are not related to a physical harm. Some states developed a “zone of danger” exception (eg, where someone was almost hit by a car) or allowed some emotional injury recovery if there were “physical manifestations” of the emotional harm. In short, depending on the state’s rules, negligence that causes purely emotional harm may not be compensable.20
State-based malpractice “caps.” Another limitation on emotional injuries is the “caps” on malpractice damages enacted by several states (including California, where this mix-up case occurred). Therefore, if a mix-up case is determined to be a malpractice case under state law, emotional suffering damages (which are non-economic damages) may be limited to the cap—$250,000 in California, for example—even if the state allows damages for emotional injuries without physical injuries.
The rare exception. Very careless labeling or handling of the identity of the embryo could at the extreme be considered gross negligence or recklessness. There are relatively inexpensive and easy procedures that could easily avoid what is likely to be significant harm to families (including emotional upset).21 Institutions that callously fail to use those procedures might be seen by some courts as reckless, or in outrageous cases, even intentional. An example would be the University of California Irvine Center for Reproductive Health case, in which physicians intentionally (without consent) used patients’ ova, fertilized them, and then implanted them in other patients, with at least 15 births, many lawsuits, and multimillion dollar settlements.22 In “intentional” cases, limitations on emotional injuries would usually not be major barriers to recovery of damages. However, those are legal stretches, and recovery is the exception rather than the rule.23
Continue to: Additional legal concerns with IVF...
Additional legal concerns with IVF
Reproduction negligence cases include a large range of errors and injuries—not just embryo mix-ups. Courts have struggled with when it is appropriate to allow damages, even when there have been clear injuries. For the most part courts have been reluctant to find liability in many areas of new IVF technology.12 One problem in determining how to assess damages is determining how incidental benefits should be used to offset some or all of the damages. For example, how should the joy of having a child offset the costs of raising the child?
There are more than a dozen kinds of current and likely future claims arising from problems with ART. It is tempting to conclude, “Oh, what a tangled legal web we weave when first we practice to artificially conceive.” There are various groupings of such claims, with several examples of cases presented in this article. It is not possible to consider those in detail in this article. As a general proposition, however, “our legal system treats wrongfully disrupted plans concerning reproduction like one of those life adversities that people are expected to abide without remedy.”24
This is not to say, however, that there is no compensation for IVF-related injuries. Applebaum and colleagues found more than 100 cases in the 35 years covered by the study (1984-2020).8 However, only 50 of those cases fit the criteria for inclusion in their data. The successful cases for the plaintiffs involved medical or surgical error, while it appeared that various forms of wrongful life or birth were much less successful. It would be a mistake to conclude from these data that there are not, and will not be, meaningful risks of liability in the areas of IVF and ART more generally.
First, claims that fit with existing legal doctrine are producing liability. About half of the claims (25 over the 34 years) examined by Applebaum et al resulted in liability. Admittedly, that number was small because ART use was increasing. Where the claims fit well-recognized legal forms of damages and forms of action (primarily negligence), the liability could be substantial. A remarkable example of this is the case of Wuth v Lab. Corp (see “Liability for genetic testing errors”),25 which was the largest verdict ($50 million) in the Applebaum and colleagues’ study.8 The large verdict was due to the failure of the testing company and a medical center to properly perform and assess a genetic test, which resulted in the birth of a child with an unbalanced chromosome translocation.8,25 The child’s serious disabilities would require a great deal of expensive care. Although the jury held the testing laboratory and medical center liable, they did not find liability against the physician.25 Ultimately, this case would be considered a failure of genetic testing rather than an IVF case.
More than 2 couples
In a second case from California, a couples’ son was born to another couple in New York—along with another boy from a third couple. The woman in New York thought she had carried biological twins but genetic testing confirmed the twins were not related to the couple or to each other (the second couple filed a separate medical malpractice and negligence lawsuit in New York). All 3 couples had sought care at the same IVF clinic. The babies were eventually returned to their biological parents.1
Different races
In a New York case, a Korean couple had twin White boys after consenting to a single embryo transfer. Meanwhile a couple in Los Angeles who went to the same in vitro fertilization clinic gave birth to a child that did not match their appearance. Both couples had undergone embryo transfers on the same day. The court arranged for the Korean couple to surrender their twins to their biological parents when they were 6 months of age in exchange for their biological child.2
References
1. Couple claims clinic implanted their embryo in wrong woman. Associated Press. July 10, 2019. https://apnews.com/article/de32d537c6e34808b28834c23f00e272. Accessed January 6, 2022.
2. In the matter of accusation against Steven L. Katz. Case no. 03-20001-122617.OAH no. N2004080093. Sacramento, CA. Medical Board of California Department of Consumer Affairs 2005.
Future challenges
The future is likely to bring substantially expanded IVF/ART liability for several reasons. ART is becoming more common. Although courts have struggled with how to apply existing liability rules to the new technologies and related novel legal claims, the absence of established legal principles into which IVF injuries fit will not last forever. The legal system eventually finds ways of adjusting old rules or adopting new ones to cover injuries from new technology.
Although IVF injuries that most people feel deserve compensation currently are not cognizable in law, that will undoubtedly change. Either the courts will find new ways of assessing ART claims, or state legislatures and Congress will step in with legislation. To date, Congress has been relatively “hands off” on the ART processes, with the Fertility Clinic Success Rate and Certification Act of 1992 being a notable exception.24 This law requires ART programs to report success rates and directs the Centers for Disease Control and Prevention (CDC) to publish reported success rates and laboratory incidents. It also establishes a model state laboratory certification program.24 The CDC has an outline of the work under the statute,26 as well as state-specific data regarding ART27 and lists of publications in key areas.28 In addition there are various state laws related to recordkeeping, donor qualifications, licensing, and family law issues.29 Ultimately, physicians, scientists, and legal professionals can perform a valuable role in helping to fashion IVF liability principles that are workable and reasonable, that will not interfere with the progress of medicine, and that will ensure that those injured through carelessness or bad medicine receive compensation. ●
Although not technically an in vitro fertilization (IVF) case, Wuth v Lab. Corp. involved an infant born through IVF with a translocation defect chromosome 2 (ie, deleted material) and extra chromatin on 9. The father’s family history included birth defects, including a female cousin with profound developmental disabilities, seizures, and antisocial behavior. He had undergone genetic testing that revealed an asymptomatic balanced, 2;9 translocation. As part of the IVF process, the couple had a genetic consultation and were told there was a 50% chance that the fetus would have an unbalanced 2;9 translocation given the father’s family history and that chorionic villus sampling or amniocentesis could detect this in the fetus.1
Amniocentesis had been performed, with the specimen sent to Lab. Corp. The result was “normal male karyotype.” However, when the baby was born, it was immediately apparent that he had severe physical defects and subsequently cognitive defects. Genetic testing of the child revealed an unbalanced 2;9 translocation. The couple filed a suit for wrongful birth and wrongful life, which went to a jury. The child was awarded $25 million and the parents/family were awarded another $25 million in general damages. The verdict reflected errors in genetic (laboratory) testing.
Reference
1. Wuth v Lab. Corp. of Am., 189 Wash. App. 660, 359 P.3d 841 (2015).
CASE Embryo mix-up with 2 couples
A lawsuit was recently filed in California by a couple after the woman carried and gave birth to “the wrong child.” This was the second full-term pregnancy for the couple. The couple had undergone an unsuccessful in vitro fertilization (IVF) cycle in October 2018. The next IVF cycle in 2019 led to the birth of a daughter on September 24, 2019, who is the subject of this case.1
At the time of birth, the couple suspected something was wrong because the baby had “jet-black hair and a complexion that was darker” than their complexions. The couple eventually obtained a DNA test, which confirmed in November 2019 that this was not their biological child.1
A few weeks later, they learned that another woman who went to the same IVF clinic gave birth to a female baby 1 week after their daughter was born. Similarly, that baby did not resemble the parents, and DNA testing confirmed the baby belonged to the first couple. The couples ultimately exchanged the babies.1
The legal claim filed against the IVF center and its owner (an obstetrician) was for breach of contract, medical malpractice, and infliction of emotional distress, including experiencing “disassociation” on the part of the couple(s). Each couple felt they did not get to experience the birth of their biological child, and, of course there was considerable distress in the process of learning that the child was not theirs and exchanging the birth child for the biological child. In addition, the couple who filed the suit had another child (now age 7 years), who begged them to keep the baby to whom they gave birth. The couple also reported experiencing panic attacks as a result of the events.1
Medical considerations
As of 2018, more than 8 million IVF babies had been born, with the first in 1978 in the United Kingdom.2 Advances in science and technology have improved the process. Storage tanks now have alarms and several safeguards to monitor the level of liquid nitrogen and immediately notify key personnel if levels are low (FIGURES 1 and 2). Preimplantation genetic testing is also readily available to assess the embryo prior to transfer into the uterus and identify various genetic problems.


Guidelines for embryo straw labelling are provided by the College of American Pathologists and the Centers for Disease Control and Prevention. The American Society for Reproductive Medicine (ASRM) also provides guidelines. When an error occurs, disclosure is recommended and ethical and legal counsel should be involved. Failing to disclose can lead to professional penalties.4
Unfortunately, despite these advances and guidelines, embryo mix-ups like the one in the above case do occur and receive public notice (See “Cross country embryo mix-up cases”).5,6 A report from the University of Nevada assessed liability for embryo mix-ups in US fertility practices from 2000 to 2020.7 They evaluated 184,015 IVF cycles with 176 claims. Payments were made to plaintiffs in 21 cases, resulting in $15 million of awarded damages (average award was $199,188).7 The most common problem was in the embryology laboratory with an overall incidence of 0.03% of the total number of IVF cycles.7 To avoid damages, the authors emphasized the importance of following labeling guidelines when storing embryos, considering a 2-step read-back method prior to embryo transfer, and offering genetic testing when a discrepancy is noted in the record (TABLE).7

Other medical liability considerations
Embryo mix-ups are not the only source of problems and potential liability in IVF. At the 2021 Association of Sexual and Reproductive Medicine Annual Meeting, Applebaum et al presented results from a comprehensive review of malpractice litigation involving IVF in the United States.8 Using the legal database NEXIS Uni they identified 50 cases between 1986 and 2020 (32% of which were filed in New York state). Common thematic elements among patient allegations were embryology errors (eg, lost or destroyed embryos or incorrect sperm or egg donor), errors in preimplantation genetics, surgical or medical errors/complications, or misdiagnosis (eg, sexually transmitted disease screening or malignancy).8 Overall, the most common plaintiff complaint was negligence (26 cases) due to informed consent–related issues (9 cases), wrongful life or birth (9 cases), or negligent or intentional infliction of emotional distress (5 cases).8
In 48% of cases, the verdict was in favor of the defendant; it was for the plaintiff in 36% of cases and ongoing proceedings or partial judgement accounted for the remaining cases.8 Damages ranged from $4,171.45 to $50 million. The authors emphasized specific defense strategies, including the importance of careful labeling and handling of embryos, prompt disclosure when an error does occur, and awareness of the specific state statute(s) of limitations for medical malpractice claims.8
Continue to: Legal considerations...
Legal considerations
The case at the beginning of this article is a “mix-up” case, in which an IVF center implanted the wrong embryo, resulting in the birth parents not being the biological parents.1 As in that case, there may be (but are not always)6,9 2 mix-ups, so that 2 couples have each other’s biological children. These cases may go unnoticed by the birth parent if the physical appearance is not unexpected and the parents never do genetic testing, or if the IVF center does not discover the error and inform the parents. Infrequently the cases make the news or the courts.10,11
News accounts are not trials, and we do not suggest that all the facts discussed in news reports on the case described here are complete—or even accurate in the details reported. They are generally 1-sided, so there are other perspectives. To consider the legal issues, however, we will assume for discussion only that the facts are as they have been reported in the news coverage—with the understanding that the discovery and trial processes would undoubtedly bring to light many other important facts or corrections.
Negligence
Although there are several potential bases for liability (ie, contract or warranty claims, a form of product liability/defect) in mix-up and other artificial reproductive technology (ART), negligence or malpractice seem most likely.12 “Negligence” here is intended to be simple negligence but may also include gross negligence or recklessness.
Although the incidence of errors in ART is unknown, there is limited evidence that suggests it is not a rare event. One study suggested >20% of fertility clinics knew of errors in processing or handling donor samples and embryos for implantation.13 Another study in the United Kingdom found that 1 in 1,000 IVF embryos were implanted in the wrong woman.14
Was there negligence? The first question in a malpractice or negligence-type action is, was there a professional relationship between the plaintiff who is claiming harm and the professional or organization defendant? The next question is whether the defendant was reasonably careful given the circumstances—that is, did the physician meet the “standard of care”? This is sometimes described as whether the professional’s actions would be acceptable (ie, reasonably prudent within the profession or specialty). If there was negligence, then the next question is, did that negligence cause an injury to the plaintiff?15
Determining the standard of care. The nature of the expected standard of care is dependent, in part, on the potential consequences of an error. For example, the care required when there is a significant risk of death from an error would be considerably more cautious than for an error that might result in small property damage. In this case study, a mix-up error is likely to be less severe than death, but is very substantial in terms of emotional harm and disruption. Thus, considerable care and attention would be expected to avoid these errors. They should be a “never” event. Institutions and physicians should give considerable attention to their processes and procedures to avoid the possibility of a mix-up error.16
Where did the negligence occur? There is an old tort doctrine “Res ipsa loquitor” (RIL) that means, “The thing speaks for itself.” Although there are several technical rules around the application of RIL as a presumption of negligence, it comes down to the proposition that some injuries do not occur without negligence. A traditional medical example is the sponge left in a patient during surgery—ordinarily that does not happen without some negligence. For RIL to be applied, usually the mechanism by which the injury occurred had to be under the control of the defendant (or the agents of the defendant).
The “mix-up” of embryos is an example of the kind of error that would not likely occur without negligence.17 But the embryo may not be in the exclusive control of any 1 institution. For example, the mistake could be made by the IVF center (or its employees), a separate facility that has processed or cryogenically stored the genetic materials, and independent physicians (not employees or agents of the center). Therefore, it is necessary to pinpoint where the negligence occurred and who is legally responsible. In some cases, a health care provider must take steps to ensure that its contractors have sufficient safeguards to avoid unnecessary harms. For example, an IVF center that uses an external cryogenic storage facility may have some obligation to know that the genetic material returned to the center is the same material that the center provided the storage facility in the first place and is properly identified.18
Assessing damages
From the facts as we have them, it appears that there must have been negligence that caused the mix-up of the embryos in the original case. It also appears reasonably clear that the negligence resulted in harm to both sets of parents and their families. This would suggest that the families should recover substantial damages. But that, somewhat surprisingly, may not be the case.19 Several legal principles may limit the availability or size of damages in mix-up cases. Also, it is worth remembering that there are differences in how states treat the different types of damages in these cases. Although the case was filed in California, we’ll take a more national view of the damages issue.
Not all harm is treated as equal. The first problem facing plaintiffs in mix-up cases may be the fact that they have suffered only emotional harm, without any physical injury. Traditionally, the courts have been reluctant to allow recovery in negligence for purely emotional injuries. Also “intentional” infliction of emotional distress does permit financial recovery, but generally “negligent” infliction of emotional harm traditionally has not. In part, this was because of the fear of unwarranted (and difficult-to-assess) claims of emotional harm that are not related to a physical harm. Some states developed a “zone of danger” exception (eg, where someone was almost hit by a car) or allowed some emotional injury recovery if there were “physical manifestations” of the emotional harm. In short, depending on the state’s rules, negligence that causes purely emotional harm may not be compensable.20
State-based malpractice “caps.” Another limitation on emotional injuries is the “caps” on malpractice damages enacted by several states (including California, where this mix-up case occurred). Therefore, if a mix-up case is determined to be a malpractice case under state law, emotional suffering damages (which are non-economic damages) may be limited to the cap—$250,000 in California, for example—even if the state allows damages for emotional injuries without physical injuries.
The rare exception. Very careless labeling or handling of the identity of the embryo could at the extreme be considered gross negligence or recklessness. There are relatively inexpensive and easy procedures that could easily avoid what is likely to be significant harm to families (including emotional upset).21 Institutions that callously fail to use those procedures might be seen by some courts as reckless, or in outrageous cases, even intentional. An example would be the University of California Irvine Center for Reproductive Health case, in which physicians intentionally (without consent) used patients’ ova, fertilized them, and then implanted them in other patients, with at least 15 births, many lawsuits, and multimillion dollar settlements.22 In “intentional” cases, limitations on emotional injuries would usually not be major barriers to recovery of damages. However, those are legal stretches, and recovery is the exception rather than the rule.23
Continue to: Additional legal concerns with IVF...
Additional legal concerns with IVF
Reproduction negligence cases include a large range of errors and injuries—not just embryo mix-ups. Courts have struggled with when it is appropriate to allow damages, even when there have been clear injuries. For the most part courts have been reluctant to find liability in many areas of new IVF technology.12 One problem in determining how to assess damages is determining how incidental benefits should be used to offset some or all of the damages. For example, how should the joy of having a child offset the costs of raising the child?
There are more than a dozen kinds of current and likely future claims arising from problems with ART. It is tempting to conclude, “Oh, what a tangled legal web we weave when first we practice to artificially conceive.” There are various groupings of such claims, with several examples of cases presented in this article. It is not possible to consider those in detail in this article. As a general proposition, however, “our legal system treats wrongfully disrupted plans concerning reproduction like one of those life adversities that people are expected to abide without remedy.”24
This is not to say, however, that there is no compensation for IVF-related injuries. Applebaum and colleagues found more than 100 cases in the 35 years covered by the study (1984-2020).8 However, only 50 of those cases fit the criteria for inclusion in their data. The successful cases for the plaintiffs involved medical or surgical error, while it appeared that various forms of wrongful life or birth were much less successful. It would be a mistake to conclude from these data that there are not, and will not be, meaningful risks of liability in the areas of IVF and ART more generally.
First, claims that fit with existing legal doctrine are producing liability. About half of the claims (25 over the 34 years) examined by Applebaum et al resulted in liability. Admittedly, that number was small because ART use was increasing. Where the claims fit well-recognized legal forms of damages and forms of action (primarily negligence), the liability could be substantial. A remarkable example of this is the case of Wuth v Lab. Corp (see “Liability for genetic testing errors”),25 which was the largest verdict ($50 million) in the Applebaum and colleagues’ study.8 The large verdict was due to the failure of the testing company and a medical center to properly perform and assess a genetic test, which resulted in the birth of a child with an unbalanced chromosome translocation.8,25 The child’s serious disabilities would require a great deal of expensive care. Although the jury held the testing laboratory and medical center liable, they did not find liability against the physician.25 Ultimately, this case would be considered a failure of genetic testing rather than an IVF case.
More than 2 couples
In a second case from California, a couples’ son was born to another couple in New York—along with another boy from a third couple. The woman in New York thought she had carried biological twins but genetic testing confirmed the twins were not related to the couple or to each other (the second couple filed a separate medical malpractice and negligence lawsuit in New York). All 3 couples had sought care at the same IVF clinic. The babies were eventually returned to their biological parents.1
Different races
In a New York case, a Korean couple had twin White boys after consenting to a single embryo transfer. Meanwhile a couple in Los Angeles who went to the same in vitro fertilization clinic gave birth to a child that did not match their appearance. Both couples had undergone embryo transfers on the same day. The court arranged for the Korean couple to surrender their twins to their biological parents when they were 6 months of age in exchange for their biological child.2
References
1. Couple claims clinic implanted their embryo in wrong woman. Associated Press. July 10, 2019. https://apnews.com/article/de32d537c6e34808b28834c23f00e272. Accessed January 6, 2022.
2. In the matter of accusation against Steven L. Katz. Case no. 03-20001-122617.OAH no. N2004080093. Sacramento, CA. Medical Board of California Department of Consumer Affairs 2005.
Future challenges
The future is likely to bring substantially expanded IVF/ART liability for several reasons. ART is becoming more common. Although courts have struggled with how to apply existing liability rules to the new technologies and related novel legal claims, the absence of established legal principles into which IVF injuries fit will not last forever. The legal system eventually finds ways of adjusting old rules or adopting new ones to cover injuries from new technology.
Although IVF injuries that most people feel deserve compensation currently are not cognizable in law, that will undoubtedly change. Either the courts will find new ways of assessing ART claims, or state legislatures and Congress will step in with legislation. To date, Congress has been relatively “hands off” on the ART processes, with the Fertility Clinic Success Rate and Certification Act of 1992 being a notable exception.24 This law requires ART programs to report success rates and directs the Centers for Disease Control and Prevention (CDC) to publish reported success rates and laboratory incidents. It also establishes a model state laboratory certification program.24 The CDC has an outline of the work under the statute,26 as well as state-specific data regarding ART27 and lists of publications in key areas.28 In addition there are various state laws related to recordkeeping, donor qualifications, licensing, and family law issues.29 Ultimately, physicians, scientists, and legal professionals can perform a valuable role in helping to fashion IVF liability principles that are workable and reasonable, that will not interfere with the progress of medicine, and that will ensure that those injured through carelessness or bad medicine receive compensation. ●
Although not technically an in vitro fertilization (IVF) case, Wuth v Lab. Corp. involved an infant born through IVF with a translocation defect chromosome 2 (ie, deleted material) and extra chromatin on 9. The father’s family history included birth defects, including a female cousin with profound developmental disabilities, seizures, and antisocial behavior. He had undergone genetic testing that revealed an asymptomatic balanced, 2;9 translocation. As part of the IVF process, the couple had a genetic consultation and were told there was a 50% chance that the fetus would have an unbalanced 2;9 translocation given the father’s family history and that chorionic villus sampling or amniocentesis could detect this in the fetus.1
Amniocentesis had been performed, with the specimen sent to Lab. Corp. The result was “normal male karyotype.” However, when the baby was born, it was immediately apparent that he had severe physical defects and subsequently cognitive defects. Genetic testing of the child revealed an unbalanced 2;9 translocation. The couple filed a suit for wrongful birth and wrongful life, which went to a jury. The child was awarded $25 million and the parents/family were awarded another $25 million in general damages. The verdict reflected errors in genetic (laboratory) testing.
Reference
1. Wuth v Lab. Corp. of Am., 189 Wash. App. 660, 359 P.3d 841 (2015).
- Mark J. California couple sues fertility clinic following IVF embryo mix-up. Washington Post. November 9, 2021. https://www.washingtonpost.com/nation/2021/11/09/in-vitro-fertilization-ivf-mix-up-daphna-cardinale. Accessed January 5, 2022.
- More than 8 million babies born from IVF since the world’s first in 1978. Science Daily. July 3, 2018. https://www.sciencedaily.com/releases/2018/07/180703084127.htm. Accessed January 11, 2022.
- ESCO Medical. In vitro fertilization (IVF) as fertility treatment. https://www.esco-medical.com/resource/in-vitro-fertilization-ivf-as-fertility-treatment.
- Vigdor N. “We had their baby, and they had our baby”: couple sues over embryo “mix-up.” NY Times. November 9, 2021. https://www.nytimes.com/2021/11/09/us/fertility-clinic-embryo-mixup.html. Accessed January 11, 2022.
- Couple claims clinic implanted their embryo in wrong woman. Associated Press. July 10, 2019. https://apnews.com/article/de32d537c6e34808b28834c23f00e2728. Accessed January 6, 2022.
- In the matter of accusation against Steven L. Katz. Case no. 03-20001-122617.OAH no. N2004080093. Sacramento, CA. Medical Board of California Department of Consumer Affairs 2005
- Rasouli MA, Moutos CP, Phelps JY. Liability for embryo mix-ups in fertility practices in the USA. J Assist Reprod Genet. 2021;38:1101-1107. doi:10.1007/s10815-021-02108-1
- Applebaum J, Berger D, O’Neill K. Can a reproductive endocrinologist be sued for 50 million dollars? A comprehensive review of malpractice litigation involving in vitro fertilization in the U.S. Fertil Steril. 2021;116(3s):e19. doi:10.1016/j.fertnstert.2021.07.059
- Andrews v Keltz, 838 N.Y.S.2d 363, 365 (Sup. Ct. 2007).
- Chichi DV. In vitro fertilization, fertility frustrations, and the lack of regulation. Hofstra L Rev. 2021;49:535-568. https://www.hofstralawreview.org/wp-content/uploads/2021/04/bb.2.chichi.pdf. Accessed January 11, 2022.
- Lewin T. Sperm banks accused of losing samples and lying about donors. NY Times. July 21, 2016. https://www.nytimes.com/2016/07/22/us/sperm-banks-accused-of-losing-samples-and-lying-about-donors.html. Accessed January 11, 2022.
- Bender L. To err is human ART mix-ups: labor-based, relational proposal. J Gender Race Justice. 2006;9:443-508. https://surface.syr.edu/cgi/viewcontent.cgi?article=1050&context=lawpub. Accessed January 11, 2022.
- Baruch S, Kaufman D, Hudson KL. Genetic testing of embryos: practices and perspectives of U.S. in vitro fertilization clinics. Fertil Steril. 2007;89:1053-1058. doi:10.1016/j.fertnstert.2007.05.048
- Liebler R. Are you my parent? Are you my child? The role of genetics and race in defining relationships after reproductive technological mistakes. DePaul J Health Care Law. 2002;5:15-56. https://via.library.depaul.edu/cgi/viewcontent.cgi?article=1202&context=jhcl. Accessed January 11, 2022.
- Crockin SL, Altman AB, Edmonds MA. The history and future trends of art medicine and law. Fam Court Rev. 2021;59:22-45. doi:10.1111/fcre.12550
- Fernandes JS. Perfecting pregnancy via preimplantation genetic screening: the quest for an elusive standard of care. UC Irvine L Rev. 2014;4:1295-1326. https://www.law.uci.edu/lawreview/vol4/no4/Fernandes.pdf. Accessed January 11, 2022.
- VanGessel MM. Wrongful surrogacy: the need for right of action in cases of clear negligence. U Toledo L Rev. 2015;46:681-706.
- Reich J, Swink D. Outsourcing human reproduction: embryos and surrogacy services in the cyberprocreation era. J Health Care L Policy. 2011;14:241-298. https://core.ac.uk/download/pdf/217156567.pdf. Accessed January 11, 2022.
- Strasser M. Prenatal tort slippage. Health Matrix. 2021;31:221-262. https://scholarlycommons.law.case.edu/healthmatrix/vol31/iss1/9. Accessed January 11, 2022.
- Heide IH. Negligence in the creation of healthy babies: negligent infliction of emotional distress in cases of alternative reproductive technology malpractice without physical injury. J Med L. 2005;9:55-94.
- Novo S, Nogués C, Penon O, et al. Barcode tagging of human oocytes and embryos to prevent mix-ups in assisted reproduction technologies. Hum Reprod. 2014;29:18-28. doi: 10.1093/humrep/det409
- Yoshino K. UCI Settles Dozens of Fertility Suits. LA Times. September 11, 2009. https://www.latimes.com/archives/la-xpm-2009-sep-11-me-uci-fertility11-story.html. Accessed January 11, 2022.
- Fox D. Reproductive negligence. Columbia L Rev. 2017;117:149-242. https://columbialawreview.org/wp-content/uploads/2017/01/149.pdf. Accessed January 11, 2022.
- 42 U.S.C.S. §263a-1-263a-7; Public Law 102-493. https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3146.pdf. Accessed January 11, 2022.
- Wuth v Lab. Corp. of Am., 189 Wash. App. 660, 359 P.3d 841 (2015).
- Centers for Disease Control and Prevention. The Fertility Clinic Success Rate and Certification Act. December 14, 2020. https://www.cdc.gov/art/nass/policy.html#act. Accessed January 11, 2022.
- Centers for Disease Control and Prevention. State-specific assisted reproductive technology surveillance. December 17, 2020. https://www.cdc.gov/art/state-specific-surveillance/index.html. Accessed January 11, 2022.
- Centers for Disease Control and Prevention. Key findings. March 12, 2021. https://www.cdc.gov/art/key-findings/index.html. Accessed January 11, 2022.
- Cohen EN. 5 Treatise on Health Care Law §22.04, (ed. Hooper, Lundy & Bookman, & Robert W. Lundy, Jr. RW.) (Matthew Bender-LexisNexis)
- Mark J. California couple sues fertility clinic following IVF embryo mix-up. Washington Post. November 9, 2021. https://www.washingtonpost.com/nation/2021/11/09/in-vitro-fertilization-ivf-mix-up-daphna-cardinale. Accessed January 5, 2022.
- More than 8 million babies born from IVF since the world’s first in 1978. Science Daily. July 3, 2018. https://www.sciencedaily.com/releases/2018/07/180703084127.htm. Accessed January 11, 2022.
- ESCO Medical. In vitro fertilization (IVF) as fertility treatment. https://www.esco-medical.com/resource/in-vitro-fertilization-ivf-as-fertility-treatment.
- Vigdor N. “We had their baby, and they had our baby”: couple sues over embryo “mix-up.” NY Times. November 9, 2021. https://www.nytimes.com/2021/11/09/us/fertility-clinic-embryo-mixup.html. Accessed January 11, 2022.
- Couple claims clinic implanted their embryo in wrong woman. Associated Press. July 10, 2019. https://apnews.com/article/de32d537c6e34808b28834c23f00e2728. Accessed January 6, 2022.
- In the matter of accusation against Steven L. Katz. Case no. 03-20001-122617.OAH no. N2004080093. Sacramento, CA. Medical Board of California Department of Consumer Affairs 2005
- Rasouli MA, Moutos CP, Phelps JY. Liability for embryo mix-ups in fertility practices in the USA. J Assist Reprod Genet. 2021;38:1101-1107. doi:10.1007/s10815-021-02108-1
- Applebaum J, Berger D, O’Neill K. Can a reproductive endocrinologist be sued for 50 million dollars? A comprehensive review of malpractice litigation involving in vitro fertilization in the U.S. Fertil Steril. 2021;116(3s):e19. doi:10.1016/j.fertnstert.2021.07.059
- Andrews v Keltz, 838 N.Y.S.2d 363, 365 (Sup. Ct. 2007).
- Chichi DV. In vitro fertilization, fertility frustrations, and the lack of regulation. Hofstra L Rev. 2021;49:535-568. https://www.hofstralawreview.org/wp-content/uploads/2021/04/bb.2.chichi.pdf. Accessed January 11, 2022.
- Lewin T. Sperm banks accused of losing samples and lying about donors. NY Times. July 21, 2016. https://www.nytimes.com/2016/07/22/us/sperm-banks-accused-of-losing-samples-and-lying-about-donors.html. Accessed January 11, 2022.
- Bender L. To err is human ART mix-ups: labor-based, relational proposal. J Gender Race Justice. 2006;9:443-508. https://surface.syr.edu/cgi/viewcontent.cgi?article=1050&context=lawpub. Accessed January 11, 2022.
- Baruch S, Kaufman D, Hudson KL. Genetic testing of embryos: practices and perspectives of U.S. in vitro fertilization clinics. Fertil Steril. 2007;89:1053-1058. doi:10.1016/j.fertnstert.2007.05.048
- Liebler R. Are you my parent? Are you my child? The role of genetics and race in defining relationships after reproductive technological mistakes. DePaul J Health Care Law. 2002;5:15-56. https://via.library.depaul.edu/cgi/viewcontent.cgi?article=1202&context=jhcl. Accessed January 11, 2022.
- Crockin SL, Altman AB, Edmonds MA. The history and future trends of art medicine and law. Fam Court Rev. 2021;59:22-45. doi:10.1111/fcre.12550
- Fernandes JS. Perfecting pregnancy via preimplantation genetic screening: the quest for an elusive standard of care. UC Irvine L Rev. 2014;4:1295-1326. https://www.law.uci.edu/lawreview/vol4/no4/Fernandes.pdf. Accessed January 11, 2022.
- VanGessel MM. Wrongful surrogacy: the need for right of action in cases of clear negligence. U Toledo L Rev. 2015;46:681-706.
- Reich J, Swink D. Outsourcing human reproduction: embryos and surrogacy services in the cyberprocreation era. J Health Care L Policy. 2011;14:241-298. https://core.ac.uk/download/pdf/217156567.pdf. Accessed January 11, 2022.
- Strasser M. Prenatal tort slippage. Health Matrix. 2021;31:221-262. https://scholarlycommons.law.case.edu/healthmatrix/vol31/iss1/9. Accessed January 11, 2022.
- Heide IH. Negligence in the creation of healthy babies: negligent infliction of emotional distress in cases of alternative reproductive technology malpractice without physical injury. J Med L. 2005;9:55-94.
- Novo S, Nogués C, Penon O, et al. Barcode tagging of human oocytes and embryos to prevent mix-ups in assisted reproduction technologies. Hum Reprod. 2014;29:18-28. doi: 10.1093/humrep/det409
- Yoshino K. UCI Settles Dozens of Fertility Suits. LA Times. September 11, 2009. https://www.latimes.com/archives/la-xpm-2009-sep-11-me-uci-fertility11-story.html. Accessed January 11, 2022.
- Fox D. Reproductive negligence. Columbia L Rev. 2017;117:149-242. https://columbialawreview.org/wp-content/uploads/2017/01/149.pdf. Accessed January 11, 2022.
- 42 U.S.C.S. §263a-1-263a-7; Public Law 102-493. https://www.govinfo.gov/content/pkg/STATUTE-106/pdf/STATUTE-106-Pg3146.pdf. Accessed January 11, 2022.
- Wuth v Lab. Corp. of Am., 189 Wash. App. 660, 359 P.3d 841 (2015).
- Centers for Disease Control and Prevention. The Fertility Clinic Success Rate and Certification Act. December 14, 2020. https://www.cdc.gov/art/nass/policy.html#act. Accessed January 11, 2022.
- Centers for Disease Control and Prevention. State-specific assisted reproductive technology surveillance. December 17, 2020. https://www.cdc.gov/art/state-specific-surveillance/index.html. Accessed January 11, 2022.
- Centers for Disease Control and Prevention. Key findings. March 12, 2021. https://www.cdc.gov/art/key-findings/index.html. Accessed January 11, 2022.
- Cohen EN. 5 Treatise on Health Care Law §22.04, (ed. Hooper, Lundy & Bookman, & Robert W. Lundy, Jr. RW.) (Matthew Bender-LexisNexis)
The Supreme Court and reproductive rights
There is now great interest in the Supreme Court’s handling of cases that involve a woman’s ability to have an abortion. Recent decisions, and those planned in the next few months will be the source of intense scrutiny. But the Court’s involvement in reproductive rights did not begin with abortion. In fact, the Supreme Court has a long history of controversial decisions dealing with reproductive rights.
Involuntary sterilization
A notable, even infamous, case was Buck v Bell (1927)—later discredited—in which the Court reviewed a state law that provided for the involuntary sterilization of the “feeble minded.”1 The 8-1 decision was that the state could choose to have such a law to protect the so-called genetic health of the state. The law was based on a theory of eugenics. The opinion by the highly respected Justice Oliver Wendell Holmes included the unfortunate conclusion, “Three generations of imbeciles are enough.”2 As mentioned, the law has since been thoroughly discredited. In 1942, the Court did come to a different result, holding in Skinner v Oklahoma that it was unconstitutional for a state to involuntarily sterilize “habitual criminals.”3
Contraception
Forty years after Buck, in Griswold v Connecticut, the Court reviewed a state law that prohibited the distribution of any drug or device used for contraception (even for married couples).4 In a 7-2 decision, the Supreme Court struck down the state law as violating a marital right of privacy. Beyond its specific holding, Griswold was important in several ways. First, a physician was raising the rights of patients (not specifically his own rights). This is notable because, ordinarily in court, litigants may argue their own rights, not the rights of others. This has been important in later reproductive rights cases because often it has been physicians raising and arguing the rights of patients.
A second interesting part of Griswold was the source of this constitutional right of privacy. The Constitution contains no express privacy provision. In Griswold, the Court found that the 1st, 3rd, 4th, and 9th Amendments create the right to privacy in marital relations. Writing for the majority, Justice Douglas found that “emanations” from these amendments have “penumbras” that create a right of marital privacy.
Although Griswold was based on marital privacy, a few years later, in 1972, the Court essentially converted that right to one of reproductive privacy (“the decision whether to bear or beget a child.”) In Eisenstadt v Baird, the Court held that it was a violation of equal protection (the 14th Amendment) for a state to allow contraception to the married but deny it to an unmarried person.5
Continue to: Abortion...
Abortion
In 1971, the Court had heard arguments in 2 cases that raised issues regarding whether state laws prohibiting abortion were constitutional. The first oral argument in Roe v Wade is widely considered one of the worst oral arguments in modern history, and for several reasons the Court set the case for rehearing the following Term (October 1972). In January 1973, the Court decided Roe v Wade.6 The 7-2 decision was written by Justice Blackmun, who had at one point been the attorney for the Mayo Clinic and might be considered one of the first “health lawyers.” The Court held that the Constitution (perhaps in the 14th or 9th Amendment) includes a right of privacy that includes the right of a woman to choose to have, or not to have, an abortion. In implementing the right, the Court held that a state may impose only modest medical safeguards for the mother (eg, requiring that abortions be performed by a licensed physician). In the second trimester, to the point of viability, a state could impose only limitations on abortion that were reasonably directed to ensure the health of the mother. After a fetus was viable (could live outside the mother’s body), the state was free to regulate or prohibit abortions and protect the fetus. At the time, viability was approximately the beginning of the third trimester.6
The clear majority of the Court in Roe (7-2) may have suggested that there was not strong opposition to the decision. That, of course, was not the case. Legal and political conflict surrounding the case has been, and remains, intense. Since 1973, the Court has been called upon to decide many abortion cases, and each case seems to beget more controversy and still more cases.
Some of the legal objections to Roe and other abortion decisions are that the constitutional basis for the decision remains unclear—a specific right of privacy is not contained in the text of the Constitution. Several locations of a possible right of privacy have been mentioned by various justices, but “substantive due process” became the common constitutional basis for the right. Critics note that “substantive” due process (as opposed to procedural due process) is not mentioned in the Constitution, and it is short on clear guiding principles. Beyond those jurisprudential issues, of course, there were strong religious and philosophical objections to abortion. What followed Roe has been a long series of efforts to limit or discourage access to abortion, and the Supreme Court has had to decide a great many abortion cases (and a few contraception cases) over the last 50 years. Most years (except from 2008‒2013) the Court has heard, on average, at least one abortion case.
By way of examples, here are some of the issues related to abortion that the Court has decided:
- Payment and facilities. States and the federal government are not required to pay for abortions for women who cannot afford them or to provide facilities for abortion.7-10
- Informed consent. Some states’ special informed consent requirements for abortion were upheld, but complex consents that required the father’s participation were not.
- Ability to advertise. Prohibitions on advertisement of abortion services were struck down.
- Location. Requirements for hospital-only abortions (or similar regulations) were struck down.
- Anti-abortion protests. Several cases addressed guidelines involving demonstrations near abortion clinics.
Of particular importance was the case of Planned Parenthood of Southeastern Pennsylvania v Casey—“Casey.”11 In 1992 that case reaffirmed the “essential” holding of Roe v Wade. A plurality in that case de-emphasized the trimester framework and applied an “undue burden” test on limitations on abortion. In the more recent cases argued before the Court, Casey is frequently referred to as specifically reaffirming, and therefore solidifying, Roe.
Consent for minors
There have been several cases since 1973 that involved contraceptives or abortions and “minors” (generally, adolescents aged <18 years, although there are some state-defined exceptions). These cases typically involve 2 issues: the right of minors to consent to treatment and the obligation of the physician to provide information to parents about treatment to their minor daughter. In 1977 the Court struck down a New York law that prohibited the distribution of contraceptives to minors.12 However, abortion issues involving minors have been more complicated. While the Court has struck down “2-parent” consent statutes,13 it has generally upheld 1-parent consent statutes, but only if those statutes contain a “judicial bypass” provision and an emergency medical provision.11,14,15 (This bypass allows a minor to “bypass” parental consent to abortion in some circumstances, and instead seek judicial authorization for an abortion.) Generally, the Court has upheld parental notification for abortions, with exceptions where it would be harmful to the minor who is seeking the abortion.16-19
Continue to: Who can perform an abortion...
Who can perform an abortion
Over the years there have also been several cases raising questions about the professionals who can perform abortions, their hospital privileges, and what facilities can perform abortions. Two of those cases in recent years have, for example, seen the Court strike down state statutes that required the physicians who perform abortions to have admitting privileges at least in 1 nearby hospital.20,21 The basis for these decisions is that the admitting qualification is an “undue burden” because it serves almost no health purpose, while significantly limiting the number of professionals who can perform abortions.
Cases this Term
The current Term of the Court (officially the “October 2021 Term”) may be one of the most significant for reproductive rights in recent history. The Court accepted 6 abortion-related cases to hear. It dismissed 3 of those cases, which had become “moot” because the Biden administration changed the rules that had been legally challenged.22-24 It has heard arguments in the 3 (technically 4) remaining cases, in which decisions will be announced over the next several months.
The first of these cases (involving the Texas Heartbeat Act) raises very important, but vexing, procedural issues about a Texas abortion law. The second (Whole Woman’s Health v Jackson) is a direct challenge to Roe v Wade. The third case (Cameron v EMW Women’s Surgical Center) involves the narrow question of whether a state attorney general can intervene in a case to uphold a state abortion law when another state official refuses to defend the law.25 It is worthwhile taking a look at the first 2 of these cases.
Texas Heartbeat Act
In the first case (technically, it is 2 cases, as we will see), the Texas legislature adopted a law that prohibits abortions after there is a discernable heartbeat (around 6 weeks of pregnancy). The law precludes state officials from enforcing the law. Instead, it allows almost any private citizen to seek monetary damages ($10,000 plus fees) from anyone who performs an abortion or “aids and abets” an abortion. (This is in some ways similar to “private attorney general” actions found in the False Claims Act, and in some civil rights and labor laws.) This statute is clearly inconsistent with Roe in that it prohibits abortions before the end of the second trimester. If it were a usual law—a Texas law being enforced by state officials—federal courts would issue injunctions to state officials against enforcing the law. The difficulty with the Texas law (and its very purpose) is that there are procedural limitations in federal law that make it very difficult to find a path for federal courts to review the Texas statute quickly. For example, would federal courts enjoin every private citizen of the state? There is a longstanding Constitutional doctrine that precludes federal courts from enjoining state courts.26 Therefore, it is difficult to challenge the law before someone performing or aiding an abortion has been ordered to pay the private citizen who is enforcing it. In the interim, which could be months or even years, health care providers face uncertainty about continuing to provide abortion services. Some providers would stop providing abortion services, reducing the availability of those services.
Two cases challenge this Texas procedure. In the first, Whole Woman’s Health v Jackson, 27 abortion providers seek to find some way through the procedural thicket to allow an immediate challenge to the statute. It is important because this technique of exclusive private enforcement could be used in any number of ways by the state to chill important constitutional rights (beyond abortion—to speech, to bear arms). In the second case involving the Texas law, U.S. v Texas, the federal government seeks to intervene in the case, which is another unusual procedure.28 The Court found these questions so important and difficult that it allowed 3 hours of argument (and 4 sets of lawyers). It seems likely that the Court will find a mechanism for allowing some early federal court review of individual enforcement of state laws, while minimizing harm to the state-national federalism that is at the heart of the Constitution.
For the recent procedural decisions in the Texas cases, see the “Current Court Decisions” box below.
On December 10, 2021, the Court handed down two decisions in reproductive freedom (abortion) cases, both involving the Texas abortion law (which prohibits most abortions after a fetal heartbeat can be detected and allows only private individuals to enforce the law). The more significant of the two cases, Whole Woman’s Health v Jackson,1 was the request of abortion providers (and others) to allow them to challenge the constitutionality of the Texas law by suing various state officials or a private individual, before the enforcement of the new Texas law.
The decision of the Court was somewhat complex because of the split among justices. Overall:
- The Court held 8-1 that before the law is enforced, providers have the ability to sue executives of medical licensing boards. This was based on the possibility that there could be licensure discipline for professionals who violate the new abortion law. Only Justice Thomas dissented from this part of the decision, which was written by Justice Gorsuch.
- The Court unanimously held that state-court judges could not be sued to stop enforcement of the law, and dismissed them from the suit.
- In a 5-4 split the Court held that state court clerks (and the state attorney general) could not be brought into federal court as a way of challenging the law. This was based on the 11th Amendment, sovereign immunity, and an important precedent from 1908.2 Chief Justice Roberts wrote from the justices who were essentially in dissent (Justices Bryer, Kagan, and Sotomayor). Justice Sotomayor also wrote a dissent (joined by Justices Breyer and Kagan) urging that there should be some way for providers to test the constitutionality of the law before enforcement. Allowing an action against state court clerks would be a good way to do that. She also expressly noted the problem of the Texas law approach being used by other states to attack any number of constitutional rights.
- The Court unanimously dismissed (for lack of standing) the one private citizen who had been sued. He had signed a sworn statement that he did not intend to seek the damages against abortion providers under the Texas law.
- The Court declined again to stay the Texas law while it is being challenged. That is, it left standing the 5th Circuit order allowing the law to go into effect.
- In a second, related case, the Court dismissed, without deciding, the Biden administration’s request to become a party in the Texas abortion case.3
References
- Whole Woman’s Health v Jackson, No. 21–463 (Dec. 10, 2021). https://www.supremecourt.gov /opinions/21pdf/21-463_new_8o6b.pdf.
- Ex parte Young, 209 U.S. 123 (1908).
- U.S. v Texas, 21-588 (Dec. 10, 2021). https://www.supremecourt.gov/opinions/21pdf/21-588 _c07d.pdf
Continue to: Re-evaluating the viability standard...
Re-evaluating the viability standard
The substantive abortion issue in Dobbs v Jackson Women’s Health Organization is the constitutionality of the Mississippi Gestational Age Act, which allows abortions after 15 weeks of pregnancy only for medical emergencies or severe fetal abnormality.29 This case is likely the most watched and controversial case of the Term. Many medical organizations have filed amicus curiae briefs, including the American College of Obstetricians and Gynecologists joined by the American Medical Association,30 the International Federation of Gynecology and Obstetrics,31 and the American Association of Pro-Life Obstetricians and Gynecologists.32 The reason for all this attention is that the Court has accepted to resolve “whether all pre-viability prohibitions on elective abortions are unconstitutional.” Thus, it represents a direct challenge to the trimester/viability structure of Roe.
It appears that there are 3 justices ready to outright overrule Roe, 3 that would uphold it as is, and 3 who are not in favor of Roe, but feel bound by precedent or are not in favor of a traumatic move. For that reason, there may be a narrow decision in this case. For example, the Court might find a procedural way to avoid directly deciding the abortion issue in this case, or it might uphold the right to abortion but change the viability standard. It is also true that predicting what the Court will do in controversial cases is a fool’s errand.
The complexity of reproductive rights and the ObGyn practice
These cases and policies affect the day-to-day practice of obstetrics. It is the most legally complex area of medical practice for several reasons. The law varies considerably from state to state. The clinician who practices both in California and across the border in Arizona will face substantially different laws, especially regarding the treatment of adolescents. And the reproductive rights laws in many states are a complicated mix of state statutes and state court decisions, with an overlay of federal statutes and court decisions, and a series of both state and federal regulations. This article demonstrates an additional complexity for practitioners—the continuous change in the law surrounding reproductive rights—and practice involving adolescent patients is especially difficult.
There are some good state-by-state reviews of laws related to abortion and contraception. We find the Guttmacher Institute particularly helpful. (See “State-by-state reviews of laws related to abortion and contraception”.) Although these are good resources, they are not the basis for legal practice with the current law in a state. The complexity and ever-changing nature of reproductive rights is one of the reasons we believe that it is important that anyone in active ObGyn practice maintain an ongoing professional relationship with a lawyer with expertise in this area of practice. This relationship should establish and update policies and procedures consistent with local law, consent and other forms, reporting of possible child abuse, and the like. An annual legal checkup may be as important for physicians as a physical checkup is for their patients.

Future outcomes
At the end of the Term, we will review the outcome of the cases noted above—and the possibility of follow-on cases. Whatever the Court does this Term, it will not be the end of the legal and political struggles over abortion and other reproductive issues. These questions deeply divide our society, and the cases and controversies reflect that continuing division. ●
- Buck v Bell, 274 U.S. 200 (1927).
- Id. at 207.
- Skinner v State of Oklahoma, ex rel. Williamson, 316 U.S. 535 (1942).
- Griswold v Connecticut, 381 U.S. 479 (1965)
- Eisenstadt v Baird, 405 U.S. 438 (1972).
- Roe v Wade, 410 U.S. 113 (1973).
- Harris v McRae, 448 U.S. 297 (1980).
- Williams v Zbaraz, 448 U.S 358 (1980).
- Webster v Reproductive Health Services, 492 U.S. 490 (1989).
- Rust v Sullivan, 500 U.S. 173 (1991).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Carey v Population Services, 431 U.S. 678 (1977).
- Bellotti v Baird, 443 U.S. 622 (1979).
- Planned Parenthood of Kansas City v Ashcroft, 462 U.S. 476 (1983).
- Planned Parenthood of Northern New England v Ayotte, 546 U.S. 320 (2006).
- H.L. v Matheson, 450 U.S. 398 (1981).
- Hodgson v Minnesota, 497 U.S. 417 (1990).
- Ohio v Akron Center for Reproductive Health, 497 U.S. 502 (1990).
- Lambert v Wicklund, 520 U.S. 292 (1997).
- June Medical Services v Russo, 591 U.S. ___ (2020), https:// www.supremecourt.gov/opinions/19pdf/18-1323_c07d.pdf.
- Whole Woman’s Health v Hellerstedt, 579 U.S. 582 (2016).
- AMA v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/american-medical -association-v-cochran.
- Becerra v Baltimore, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/cochran-v-mayor-and-city-council-of-baltimore.
- Oregon v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/oregon-v-cochran.
- Cameron v EMW Women’s Surgical Center, 20-601. https:// www.scotusblog.com/case-files/cases/cameron-v-emw -womens-surgical-center-p-s-c.
- Ex parte Young, 209 U.S. 123 (1908).
- Whole Woman’s Health v Jackson, 21-463. https://www .scotusblog.com/case-files/cases/whole-womans-health-v -jackson.
- U.S. v Texas, 21-588. https://www.scotusblog.com/case-files /cases/united-states-v-texas-3.
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.scotusblog.com/case-files/cases/dobbs-v -jackson-womens-health-organization.
- Brief of Amici Curiae American College of Obstetricians and Gynecologists et al., Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.acog.org/ -/media/project/acog/acogorg/files/advocacy/amicus -briefs/2021/20210920-dobbs-v-jwho-amicus-brief.pdf?la=e n&hash=717DFDD07A03B93A04490E66835BB8C5.
- Brief Amicus Curiae of International Federation of Gynecology and Obstetrics, Dobbs v Jackson Women’s Health Organization (Sep. 20, 2021). https://www.supremecourt. gov/DocketPDF/19/19-1392/193019/20210920155508744 _41426%20pdf%20Chen.pdf.
- Brief Amicus Curiae of American Association of Pro-Life Obstetricians and Gynecologists. Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/185350/20210729163532595 _No.%2019-1392%20-%20American%20Association %20of%20Pro-Life%20Obstetricians%20and%20 Gynecologists%20-%20Amicus%20Brief%20in%20Support %20of%20Petitioner%20-%207-29-21.pdf.

There is now great interest in the Supreme Court’s handling of cases that involve a woman’s ability to have an abortion. Recent decisions, and those planned in the next few months will be the source of intense scrutiny. But the Court’s involvement in reproductive rights did not begin with abortion. In fact, the Supreme Court has a long history of controversial decisions dealing with reproductive rights.
Involuntary sterilization
A notable, even infamous, case was Buck v Bell (1927)—later discredited—in which the Court reviewed a state law that provided for the involuntary sterilization of the “feeble minded.”1 The 8-1 decision was that the state could choose to have such a law to protect the so-called genetic health of the state. The law was based on a theory of eugenics. The opinion by the highly respected Justice Oliver Wendell Holmes included the unfortunate conclusion, “Three generations of imbeciles are enough.”2 As mentioned, the law has since been thoroughly discredited. In 1942, the Court did come to a different result, holding in Skinner v Oklahoma that it was unconstitutional for a state to involuntarily sterilize “habitual criminals.”3
Contraception
Forty years after Buck, in Griswold v Connecticut, the Court reviewed a state law that prohibited the distribution of any drug or device used for contraception (even for married couples).4 In a 7-2 decision, the Supreme Court struck down the state law as violating a marital right of privacy. Beyond its specific holding, Griswold was important in several ways. First, a physician was raising the rights of patients (not specifically his own rights). This is notable because, ordinarily in court, litigants may argue their own rights, not the rights of others. This has been important in later reproductive rights cases because often it has been physicians raising and arguing the rights of patients.
A second interesting part of Griswold was the source of this constitutional right of privacy. The Constitution contains no express privacy provision. In Griswold, the Court found that the 1st, 3rd, 4th, and 9th Amendments create the right to privacy in marital relations. Writing for the majority, Justice Douglas found that “emanations” from these amendments have “penumbras” that create a right of marital privacy.
Although Griswold was based on marital privacy, a few years later, in 1972, the Court essentially converted that right to one of reproductive privacy (“the decision whether to bear or beget a child.”) In Eisenstadt v Baird, the Court held that it was a violation of equal protection (the 14th Amendment) for a state to allow contraception to the married but deny it to an unmarried person.5
Continue to: Abortion...
Abortion
In 1971, the Court had heard arguments in 2 cases that raised issues regarding whether state laws prohibiting abortion were constitutional. The first oral argument in Roe v Wade is widely considered one of the worst oral arguments in modern history, and for several reasons the Court set the case for rehearing the following Term (October 1972). In January 1973, the Court decided Roe v Wade.6 The 7-2 decision was written by Justice Blackmun, who had at one point been the attorney for the Mayo Clinic and might be considered one of the first “health lawyers.” The Court held that the Constitution (perhaps in the 14th or 9th Amendment) includes a right of privacy that includes the right of a woman to choose to have, or not to have, an abortion. In implementing the right, the Court held that a state may impose only modest medical safeguards for the mother (eg, requiring that abortions be performed by a licensed physician). In the second trimester, to the point of viability, a state could impose only limitations on abortion that were reasonably directed to ensure the health of the mother. After a fetus was viable (could live outside the mother’s body), the state was free to regulate or prohibit abortions and protect the fetus. At the time, viability was approximately the beginning of the third trimester.6
The clear majority of the Court in Roe (7-2) may have suggested that there was not strong opposition to the decision. That, of course, was not the case. Legal and political conflict surrounding the case has been, and remains, intense. Since 1973, the Court has been called upon to decide many abortion cases, and each case seems to beget more controversy and still more cases.
Some of the legal objections to Roe and other abortion decisions are that the constitutional basis for the decision remains unclear—a specific right of privacy is not contained in the text of the Constitution. Several locations of a possible right of privacy have been mentioned by various justices, but “substantive due process” became the common constitutional basis for the right. Critics note that “substantive” due process (as opposed to procedural due process) is not mentioned in the Constitution, and it is short on clear guiding principles. Beyond those jurisprudential issues, of course, there were strong religious and philosophical objections to abortion. What followed Roe has been a long series of efforts to limit or discourage access to abortion, and the Supreme Court has had to decide a great many abortion cases (and a few contraception cases) over the last 50 years. Most years (except from 2008‒2013) the Court has heard, on average, at least one abortion case.
By way of examples, here are some of the issues related to abortion that the Court has decided:
- Payment and facilities. States and the federal government are not required to pay for abortions for women who cannot afford them or to provide facilities for abortion.7-10
- Informed consent. Some states’ special informed consent requirements for abortion were upheld, but complex consents that required the father’s participation were not.
- Ability to advertise. Prohibitions on advertisement of abortion services were struck down.
- Location. Requirements for hospital-only abortions (or similar regulations) were struck down.
- Anti-abortion protests. Several cases addressed guidelines involving demonstrations near abortion clinics.
Of particular importance was the case of Planned Parenthood of Southeastern Pennsylvania v Casey—“Casey.”11 In 1992 that case reaffirmed the “essential” holding of Roe v Wade. A plurality in that case de-emphasized the trimester framework and applied an “undue burden” test on limitations on abortion. In the more recent cases argued before the Court, Casey is frequently referred to as specifically reaffirming, and therefore solidifying, Roe.
Consent for minors
There have been several cases since 1973 that involved contraceptives or abortions and “minors” (generally, adolescents aged <18 years, although there are some state-defined exceptions). These cases typically involve 2 issues: the right of minors to consent to treatment and the obligation of the physician to provide information to parents about treatment to their minor daughter. In 1977 the Court struck down a New York law that prohibited the distribution of contraceptives to minors.12 However, abortion issues involving minors have been more complicated. While the Court has struck down “2-parent” consent statutes,13 it has generally upheld 1-parent consent statutes, but only if those statutes contain a “judicial bypass” provision and an emergency medical provision.11,14,15 (This bypass allows a minor to “bypass” parental consent to abortion in some circumstances, and instead seek judicial authorization for an abortion.) Generally, the Court has upheld parental notification for abortions, with exceptions where it would be harmful to the minor who is seeking the abortion.16-19
Continue to: Who can perform an abortion...
Who can perform an abortion
Over the years there have also been several cases raising questions about the professionals who can perform abortions, their hospital privileges, and what facilities can perform abortions. Two of those cases in recent years have, for example, seen the Court strike down state statutes that required the physicians who perform abortions to have admitting privileges at least in 1 nearby hospital.20,21 The basis for these decisions is that the admitting qualification is an “undue burden” because it serves almost no health purpose, while significantly limiting the number of professionals who can perform abortions.
Cases this Term
The current Term of the Court (officially the “October 2021 Term”) may be one of the most significant for reproductive rights in recent history. The Court accepted 6 abortion-related cases to hear. It dismissed 3 of those cases, which had become “moot” because the Biden administration changed the rules that had been legally challenged.22-24 It has heard arguments in the 3 (technically 4) remaining cases, in which decisions will be announced over the next several months.
The first of these cases (involving the Texas Heartbeat Act) raises very important, but vexing, procedural issues about a Texas abortion law. The second (Whole Woman’s Health v Jackson) is a direct challenge to Roe v Wade. The third case (Cameron v EMW Women’s Surgical Center) involves the narrow question of whether a state attorney general can intervene in a case to uphold a state abortion law when another state official refuses to defend the law.25 It is worthwhile taking a look at the first 2 of these cases.
Texas Heartbeat Act
In the first case (technically, it is 2 cases, as we will see), the Texas legislature adopted a law that prohibits abortions after there is a discernable heartbeat (around 6 weeks of pregnancy). The law precludes state officials from enforcing the law. Instead, it allows almost any private citizen to seek monetary damages ($10,000 plus fees) from anyone who performs an abortion or “aids and abets” an abortion. (This is in some ways similar to “private attorney general” actions found in the False Claims Act, and in some civil rights and labor laws.) This statute is clearly inconsistent with Roe in that it prohibits abortions before the end of the second trimester. If it were a usual law—a Texas law being enforced by state officials—federal courts would issue injunctions to state officials against enforcing the law. The difficulty with the Texas law (and its very purpose) is that there are procedural limitations in federal law that make it very difficult to find a path for federal courts to review the Texas statute quickly. For example, would federal courts enjoin every private citizen of the state? There is a longstanding Constitutional doctrine that precludes federal courts from enjoining state courts.26 Therefore, it is difficult to challenge the law before someone performing or aiding an abortion has been ordered to pay the private citizen who is enforcing it. In the interim, which could be months or even years, health care providers face uncertainty about continuing to provide abortion services. Some providers would stop providing abortion services, reducing the availability of those services.
Two cases challenge this Texas procedure. In the first, Whole Woman’s Health v Jackson, 27 abortion providers seek to find some way through the procedural thicket to allow an immediate challenge to the statute. It is important because this technique of exclusive private enforcement could be used in any number of ways by the state to chill important constitutional rights (beyond abortion—to speech, to bear arms). In the second case involving the Texas law, U.S. v Texas, the federal government seeks to intervene in the case, which is another unusual procedure.28 The Court found these questions so important and difficult that it allowed 3 hours of argument (and 4 sets of lawyers). It seems likely that the Court will find a mechanism for allowing some early federal court review of individual enforcement of state laws, while minimizing harm to the state-national federalism that is at the heart of the Constitution.
For the recent procedural decisions in the Texas cases, see the “Current Court Decisions” box below.
On December 10, 2021, the Court handed down two decisions in reproductive freedom (abortion) cases, both involving the Texas abortion law (which prohibits most abortions after a fetal heartbeat can be detected and allows only private individuals to enforce the law). The more significant of the two cases, Whole Woman’s Health v Jackson,1 was the request of abortion providers (and others) to allow them to challenge the constitutionality of the Texas law by suing various state officials or a private individual, before the enforcement of the new Texas law.
The decision of the Court was somewhat complex because of the split among justices. Overall:
- The Court held 8-1 that before the law is enforced, providers have the ability to sue executives of medical licensing boards. This was based on the possibility that there could be licensure discipline for professionals who violate the new abortion law. Only Justice Thomas dissented from this part of the decision, which was written by Justice Gorsuch.
- The Court unanimously held that state-court judges could not be sued to stop enforcement of the law, and dismissed them from the suit.
- In a 5-4 split the Court held that state court clerks (and the state attorney general) could not be brought into federal court as a way of challenging the law. This was based on the 11th Amendment, sovereign immunity, and an important precedent from 1908.2 Chief Justice Roberts wrote from the justices who were essentially in dissent (Justices Bryer, Kagan, and Sotomayor). Justice Sotomayor also wrote a dissent (joined by Justices Breyer and Kagan) urging that there should be some way for providers to test the constitutionality of the law before enforcement. Allowing an action against state court clerks would be a good way to do that. She also expressly noted the problem of the Texas law approach being used by other states to attack any number of constitutional rights.
- The Court unanimously dismissed (for lack of standing) the one private citizen who had been sued. He had signed a sworn statement that he did not intend to seek the damages against abortion providers under the Texas law.
- The Court declined again to stay the Texas law while it is being challenged. That is, it left standing the 5th Circuit order allowing the law to go into effect.
- In a second, related case, the Court dismissed, without deciding, the Biden administration’s request to become a party in the Texas abortion case.3
References
- Whole Woman’s Health v Jackson, No. 21–463 (Dec. 10, 2021). https://www.supremecourt.gov /opinions/21pdf/21-463_new_8o6b.pdf.
- Ex parte Young, 209 U.S. 123 (1908).
- U.S. v Texas, 21-588 (Dec. 10, 2021). https://www.supremecourt.gov/opinions/21pdf/21-588 _c07d.pdf
Continue to: Re-evaluating the viability standard...
Re-evaluating the viability standard
The substantive abortion issue in Dobbs v Jackson Women’s Health Organization is the constitutionality of the Mississippi Gestational Age Act, which allows abortions after 15 weeks of pregnancy only for medical emergencies or severe fetal abnormality.29 This case is likely the most watched and controversial case of the Term. Many medical organizations have filed amicus curiae briefs, including the American College of Obstetricians and Gynecologists joined by the American Medical Association,30 the International Federation of Gynecology and Obstetrics,31 and the American Association of Pro-Life Obstetricians and Gynecologists.32 The reason for all this attention is that the Court has accepted to resolve “whether all pre-viability prohibitions on elective abortions are unconstitutional.” Thus, it represents a direct challenge to the trimester/viability structure of Roe.
It appears that there are 3 justices ready to outright overrule Roe, 3 that would uphold it as is, and 3 who are not in favor of Roe, but feel bound by precedent or are not in favor of a traumatic move. For that reason, there may be a narrow decision in this case. For example, the Court might find a procedural way to avoid directly deciding the abortion issue in this case, or it might uphold the right to abortion but change the viability standard. It is also true that predicting what the Court will do in controversial cases is a fool’s errand.
The complexity of reproductive rights and the ObGyn practice
These cases and policies affect the day-to-day practice of obstetrics. It is the most legally complex area of medical practice for several reasons. The law varies considerably from state to state. The clinician who practices both in California and across the border in Arizona will face substantially different laws, especially regarding the treatment of adolescents. And the reproductive rights laws in many states are a complicated mix of state statutes and state court decisions, with an overlay of federal statutes and court decisions, and a series of both state and federal regulations. This article demonstrates an additional complexity for practitioners—the continuous change in the law surrounding reproductive rights—and practice involving adolescent patients is especially difficult.
There are some good state-by-state reviews of laws related to abortion and contraception. We find the Guttmacher Institute particularly helpful. (See “State-by-state reviews of laws related to abortion and contraception”.) Although these are good resources, they are not the basis for legal practice with the current law in a state. The complexity and ever-changing nature of reproductive rights is one of the reasons we believe that it is important that anyone in active ObGyn practice maintain an ongoing professional relationship with a lawyer with expertise in this area of practice. This relationship should establish and update policies and procedures consistent with local law, consent and other forms, reporting of possible child abuse, and the like. An annual legal checkup may be as important for physicians as a physical checkup is for their patients.

Future outcomes
At the end of the Term, we will review the outcome of the cases noted above—and the possibility of follow-on cases. Whatever the Court does this Term, it will not be the end of the legal and political struggles over abortion and other reproductive issues. These questions deeply divide our society, and the cases and controversies reflect that continuing division. ●

There is now great interest in the Supreme Court’s handling of cases that involve a woman’s ability to have an abortion. Recent decisions, and those planned in the next few months will be the source of intense scrutiny. But the Court’s involvement in reproductive rights did not begin with abortion. In fact, the Supreme Court has a long history of controversial decisions dealing with reproductive rights.
Involuntary sterilization
A notable, even infamous, case was Buck v Bell (1927)—later discredited—in which the Court reviewed a state law that provided for the involuntary sterilization of the “feeble minded.”1 The 8-1 decision was that the state could choose to have such a law to protect the so-called genetic health of the state. The law was based on a theory of eugenics. The opinion by the highly respected Justice Oliver Wendell Holmes included the unfortunate conclusion, “Three generations of imbeciles are enough.”2 As mentioned, the law has since been thoroughly discredited. In 1942, the Court did come to a different result, holding in Skinner v Oklahoma that it was unconstitutional for a state to involuntarily sterilize “habitual criminals.”3
Contraception
Forty years after Buck, in Griswold v Connecticut, the Court reviewed a state law that prohibited the distribution of any drug or device used for contraception (even for married couples).4 In a 7-2 decision, the Supreme Court struck down the state law as violating a marital right of privacy. Beyond its specific holding, Griswold was important in several ways. First, a physician was raising the rights of patients (not specifically his own rights). This is notable because, ordinarily in court, litigants may argue their own rights, not the rights of others. This has been important in later reproductive rights cases because often it has been physicians raising and arguing the rights of patients.
A second interesting part of Griswold was the source of this constitutional right of privacy. The Constitution contains no express privacy provision. In Griswold, the Court found that the 1st, 3rd, 4th, and 9th Amendments create the right to privacy in marital relations. Writing for the majority, Justice Douglas found that “emanations” from these amendments have “penumbras” that create a right of marital privacy.
Although Griswold was based on marital privacy, a few years later, in 1972, the Court essentially converted that right to one of reproductive privacy (“the decision whether to bear or beget a child.”) In Eisenstadt v Baird, the Court held that it was a violation of equal protection (the 14th Amendment) for a state to allow contraception to the married but deny it to an unmarried person.5
Continue to: Abortion...
Abortion
In 1971, the Court had heard arguments in 2 cases that raised issues regarding whether state laws prohibiting abortion were constitutional. The first oral argument in Roe v Wade is widely considered one of the worst oral arguments in modern history, and for several reasons the Court set the case for rehearing the following Term (October 1972). In January 1973, the Court decided Roe v Wade.6 The 7-2 decision was written by Justice Blackmun, who had at one point been the attorney for the Mayo Clinic and might be considered one of the first “health lawyers.” The Court held that the Constitution (perhaps in the 14th or 9th Amendment) includes a right of privacy that includes the right of a woman to choose to have, or not to have, an abortion. In implementing the right, the Court held that a state may impose only modest medical safeguards for the mother (eg, requiring that abortions be performed by a licensed physician). In the second trimester, to the point of viability, a state could impose only limitations on abortion that were reasonably directed to ensure the health of the mother. After a fetus was viable (could live outside the mother’s body), the state was free to regulate or prohibit abortions and protect the fetus. At the time, viability was approximately the beginning of the third trimester.6
The clear majority of the Court in Roe (7-2) may have suggested that there was not strong opposition to the decision. That, of course, was not the case. Legal and political conflict surrounding the case has been, and remains, intense. Since 1973, the Court has been called upon to decide many abortion cases, and each case seems to beget more controversy and still more cases.
Some of the legal objections to Roe and other abortion decisions are that the constitutional basis for the decision remains unclear—a specific right of privacy is not contained in the text of the Constitution. Several locations of a possible right of privacy have been mentioned by various justices, but “substantive due process” became the common constitutional basis for the right. Critics note that “substantive” due process (as opposed to procedural due process) is not mentioned in the Constitution, and it is short on clear guiding principles. Beyond those jurisprudential issues, of course, there were strong religious and philosophical objections to abortion. What followed Roe has been a long series of efforts to limit or discourage access to abortion, and the Supreme Court has had to decide a great many abortion cases (and a few contraception cases) over the last 50 years. Most years (except from 2008‒2013) the Court has heard, on average, at least one abortion case.
By way of examples, here are some of the issues related to abortion that the Court has decided:
- Payment and facilities. States and the federal government are not required to pay for abortions for women who cannot afford them or to provide facilities for abortion.7-10
- Informed consent. Some states’ special informed consent requirements for abortion were upheld, but complex consents that required the father’s participation were not.
- Ability to advertise. Prohibitions on advertisement of abortion services were struck down.
- Location. Requirements for hospital-only abortions (or similar regulations) were struck down.
- Anti-abortion protests. Several cases addressed guidelines involving demonstrations near abortion clinics.
Of particular importance was the case of Planned Parenthood of Southeastern Pennsylvania v Casey—“Casey.”11 In 1992 that case reaffirmed the “essential” holding of Roe v Wade. A plurality in that case de-emphasized the trimester framework and applied an “undue burden” test on limitations on abortion. In the more recent cases argued before the Court, Casey is frequently referred to as specifically reaffirming, and therefore solidifying, Roe.
Consent for minors
There have been several cases since 1973 that involved contraceptives or abortions and “minors” (generally, adolescents aged <18 years, although there are some state-defined exceptions). These cases typically involve 2 issues: the right of minors to consent to treatment and the obligation of the physician to provide information to parents about treatment to their minor daughter. In 1977 the Court struck down a New York law that prohibited the distribution of contraceptives to minors.12 However, abortion issues involving minors have been more complicated. While the Court has struck down “2-parent” consent statutes,13 it has generally upheld 1-parent consent statutes, but only if those statutes contain a “judicial bypass” provision and an emergency medical provision.11,14,15 (This bypass allows a minor to “bypass” parental consent to abortion in some circumstances, and instead seek judicial authorization for an abortion.) Generally, the Court has upheld parental notification for abortions, with exceptions where it would be harmful to the minor who is seeking the abortion.16-19
Continue to: Who can perform an abortion...
Who can perform an abortion
Over the years there have also been several cases raising questions about the professionals who can perform abortions, their hospital privileges, and what facilities can perform abortions. Two of those cases in recent years have, for example, seen the Court strike down state statutes that required the physicians who perform abortions to have admitting privileges at least in 1 nearby hospital.20,21 The basis for these decisions is that the admitting qualification is an “undue burden” because it serves almost no health purpose, while significantly limiting the number of professionals who can perform abortions.
Cases this Term
The current Term of the Court (officially the “October 2021 Term”) may be one of the most significant for reproductive rights in recent history. The Court accepted 6 abortion-related cases to hear. It dismissed 3 of those cases, which had become “moot” because the Biden administration changed the rules that had been legally challenged.22-24 It has heard arguments in the 3 (technically 4) remaining cases, in which decisions will be announced over the next several months.
The first of these cases (involving the Texas Heartbeat Act) raises very important, but vexing, procedural issues about a Texas abortion law. The second (Whole Woman’s Health v Jackson) is a direct challenge to Roe v Wade. The third case (Cameron v EMW Women’s Surgical Center) involves the narrow question of whether a state attorney general can intervene in a case to uphold a state abortion law when another state official refuses to defend the law.25 It is worthwhile taking a look at the first 2 of these cases.
Texas Heartbeat Act
In the first case (technically, it is 2 cases, as we will see), the Texas legislature adopted a law that prohibits abortions after there is a discernable heartbeat (around 6 weeks of pregnancy). The law precludes state officials from enforcing the law. Instead, it allows almost any private citizen to seek monetary damages ($10,000 plus fees) from anyone who performs an abortion or “aids and abets” an abortion. (This is in some ways similar to “private attorney general” actions found in the False Claims Act, and in some civil rights and labor laws.) This statute is clearly inconsistent with Roe in that it prohibits abortions before the end of the second trimester. If it were a usual law—a Texas law being enforced by state officials—federal courts would issue injunctions to state officials against enforcing the law. The difficulty with the Texas law (and its very purpose) is that there are procedural limitations in federal law that make it very difficult to find a path for federal courts to review the Texas statute quickly. For example, would federal courts enjoin every private citizen of the state? There is a longstanding Constitutional doctrine that precludes federal courts from enjoining state courts.26 Therefore, it is difficult to challenge the law before someone performing or aiding an abortion has been ordered to pay the private citizen who is enforcing it. In the interim, which could be months or even years, health care providers face uncertainty about continuing to provide abortion services. Some providers would stop providing abortion services, reducing the availability of those services.
Two cases challenge this Texas procedure. In the first, Whole Woman’s Health v Jackson, 27 abortion providers seek to find some way through the procedural thicket to allow an immediate challenge to the statute. It is important because this technique of exclusive private enforcement could be used in any number of ways by the state to chill important constitutional rights (beyond abortion—to speech, to bear arms). In the second case involving the Texas law, U.S. v Texas, the federal government seeks to intervene in the case, which is another unusual procedure.28 The Court found these questions so important and difficult that it allowed 3 hours of argument (and 4 sets of lawyers). It seems likely that the Court will find a mechanism for allowing some early federal court review of individual enforcement of state laws, while minimizing harm to the state-national federalism that is at the heart of the Constitution.
For the recent procedural decisions in the Texas cases, see the “Current Court Decisions” box below.
On December 10, 2021, the Court handed down two decisions in reproductive freedom (abortion) cases, both involving the Texas abortion law (which prohibits most abortions after a fetal heartbeat can be detected and allows only private individuals to enforce the law). The more significant of the two cases, Whole Woman’s Health v Jackson,1 was the request of abortion providers (and others) to allow them to challenge the constitutionality of the Texas law by suing various state officials or a private individual, before the enforcement of the new Texas law.
The decision of the Court was somewhat complex because of the split among justices. Overall:
- The Court held 8-1 that before the law is enforced, providers have the ability to sue executives of medical licensing boards. This was based on the possibility that there could be licensure discipline for professionals who violate the new abortion law. Only Justice Thomas dissented from this part of the decision, which was written by Justice Gorsuch.
- The Court unanimously held that state-court judges could not be sued to stop enforcement of the law, and dismissed them from the suit.
- In a 5-4 split the Court held that state court clerks (and the state attorney general) could not be brought into federal court as a way of challenging the law. This was based on the 11th Amendment, sovereign immunity, and an important precedent from 1908.2 Chief Justice Roberts wrote from the justices who were essentially in dissent (Justices Bryer, Kagan, and Sotomayor). Justice Sotomayor also wrote a dissent (joined by Justices Breyer and Kagan) urging that there should be some way for providers to test the constitutionality of the law before enforcement. Allowing an action against state court clerks would be a good way to do that. She also expressly noted the problem of the Texas law approach being used by other states to attack any number of constitutional rights.
- The Court unanimously dismissed (for lack of standing) the one private citizen who had been sued. He had signed a sworn statement that he did not intend to seek the damages against abortion providers under the Texas law.
- The Court declined again to stay the Texas law while it is being challenged. That is, it left standing the 5th Circuit order allowing the law to go into effect.
- In a second, related case, the Court dismissed, without deciding, the Biden administration’s request to become a party in the Texas abortion case.3
References
- Whole Woman’s Health v Jackson, No. 21–463 (Dec. 10, 2021). https://www.supremecourt.gov /opinions/21pdf/21-463_new_8o6b.pdf.
- Ex parte Young, 209 U.S. 123 (1908).
- U.S. v Texas, 21-588 (Dec. 10, 2021). https://www.supremecourt.gov/opinions/21pdf/21-588 _c07d.pdf
Continue to: Re-evaluating the viability standard...
Re-evaluating the viability standard
The substantive abortion issue in Dobbs v Jackson Women’s Health Organization is the constitutionality of the Mississippi Gestational Age Act, which allows abortions after 15 weeks of pregnancy only for medical emergencies or severe fetal abnormality.29 This case is likely the most watched and controversial case of the Term. Many medical organizations have filed amicus curiae briefs, including the American College of Obstetricians and Gynecologists joined by the American Medical Association,30 the International Federation of Gynecology and Obstetrics,31 and the American Association of Pro-Life Obstetricians and Gynecologists.32 The reason for all this attention is that the Court has accepted to resolve “whether all pre-viability prohibitions on elective abortions are unconstitutional.” Thus, it represents a direct challenge to the trimester/viability structure of Roe.
It appears that there are 3 justices ready to outright overrule Roe, 3 that would uphold it as is, and 3 who are not in favor of Roe, but feel bound by precedent or are not in favor of a traumatic move. For that reason, there may be a narrow decision in this case. For example, the Court might find a procedural way to avoid directly deciding the abortion issue in this case, or it might uphold the right to abortion but change the viability standard. It is also true that predicting what the Court will do in controversial cases is a fool’s errand.
The complexity of reproductive rights and the ObGyn practice
These cases and policies affect the day-to-day practice of obstetrics. It is the most legally complex area of medical practice for several reasons. The law varies considerably from state to state. The clinician who practices both in California and across the border in Arizona will face substantially different laws, especially regarding the treatment of adolescents. And the reproductive rights laws in many states are a complicated mix of state statutes and state court decisions, with an overlay of federal statutes and court decisions, and a series of both state and federal regulations. This article demonstrates an additional complexity for practitioners—the continuous change in the law surrounding reproductive rights—and practice involving adolescent patients is especially difficult.
There are some good state-by-state reviews of laws related to abortion and contraception. We find the Guttmacher Institute particularly helpful. (See “State-by-state reviews of laws related to abortion and contraception”.) Although these are good resources, they are not the basis for legal practice with the current law in a state. The complexity and ever-changing nature of reproductive rights is one of the reasons we believe that it is important that anyone in active ObGyn practice maintain an ongoing professional relationship with a lawyer with expertise in this area of practice. This relationship should establish and update policies and procedures consistent with local law, consent and other forms, reporting of possible child abuse, and the like. An annual legal checkup may be as important for physicians as a physical checkup is for their patients.

Future outcomes
At the end of the Term, we will review the outcome of the cases noted above—and the possibility of follow-on cases. Whatever the Court does this Term, it will not be the end of the legal and political struggles over abortion and other reproductive issues. These questions deeply divide our society, and the cases and controversies reflect that continuing division. ●
- Buck v Bell, 274 U.S. 200 (1927).
- Id. at 207.
- Skinner v State of Oklahoma, ex rel. Williamson, 316 U.S. 535 (1942).
- Griswold v Connecticut, 381 U.S. 479 (1965)
- Eisenstadt v Baird, 405 U.S. 438 (1972).
- Roe v Wade, 410 U.S. 113 (1973).
- Harris v McRae, 448 U.S. 297 (1980).
- Williams v Zbaraz, 448 U.S 358 (1980).
- Webster v Reproductive Health Services, 492 U.S. 490 (1989).
- Rust v Sullivan, 500 U.S. 173 (1991).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Carey v Population Services, 431 U.S. 678 (1977).
- Bellotti v Baird, 443 U.S. 622 (1979).
- Planned Parenthood of Kansas City v Ashcroft, 462 U.S. 476 (1983).
- Planned Parenthood of Northern New England v Ayotte, 546 U.S. 320 (2006).
- H.L. v Matheson, 450 U.S. 398 (1981).
- Hodgson v Minnesota, 497 U.S. 417 (1990).
- Ohio v Akron Center for Reproductive Health, 497 U.S. 502 (1990).
- Lambert v Wicklund, 520 U.S. 292 (1997).
- June Medical Services v Russo, 591 U.S. ___ (2020), https:// www.supremecourt.gov/opinions/19pdf/18-1323_c07d.pdf.
- Whole Woman’s Health v Hellerstedt, 579 U.S. 582 (2016).
- AMA v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/american-medical -association-v-cochran.
- Becerra v Baltimore, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/cochran-v-mayor-and-city-council-of-baltimore.
- Oregon v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/oregon-v-cochran.
- Cameron v EMW Women’s Surgical Center, 20-601. https:// www.scotusblog.com/case-files/cases/cameron-v-emw -womens-surgical-center-p-s-c.
- Ex parte Young, 209 U.S. 123 (1908).
- Whole Woman’s Health v Jackson, 21-463. https://www .scotusblog.com/case-files/cases/whole-womans-health-v -jackson.
- U.S. v Texas, 21-588. https://www.scotusblog.com/case-files /cases/united-states-v-texas-3.
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.scotusblog.com/case-files/cases/dobbs-v -jackson-womens-health-organization.
- Brief of Amici Curiae American College of Obstetricians and Gynecologists et al., Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.acog.org/ -/media/project/acog/acogorg/files/advocacy/amicus -briefs/2021/20210920-dobbs-v-jwho-amicus-brief.pdf?la=e n&hash=717DFDD07A03B93A04490E66835BB8C5.
- Brief Amicus Curiae of International Federation of Gynecology and Obstetrics, Dobbs v Jackson Women’s Health Organization (Sep. 20, 2021). https://www.supremecourt. gov/DocketPDF/19/19-1392/193019/20210920155508744 _41426%20pdf%20Chen.pdf.
- Brief Amicus Curiae of American Association of Pro-Life Obstetricians and Gynecologists. Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/185350/20210729163532595 _No.%2019-1392%20-%20American%20Association %20of%20Pro-Life%20Obstetricians%20and%20 Gynecologists%20-%20Amicus%20Brief%20in%20Support %20of%20Petitioner%20-%207-29-21.pdf.
- Buck v Bell, 274 U.S. 200 (1927).
- Id. at 207.
- Skinner v State of Oklahoma, ex rel. Williamson, 316 U.S. 535 (1942).
- Griswold v Connecticut, 381 U.S. 479 (1965)
- Eisenstadt v Baird, 405 U.S. 438 (1972).
- Roe v Wade, 410 U.S. 113 (1973).
- Harris v McRae, 448 U.S. 297 (1980).
- Williams v Zbaraz, 448 U.S 358 (1980).
- Webster v Reproductive Health Services, 492 U.S. 490 (1989).
- Rust v Sullivan, 500 U.S. 173 (1991).
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U.S. 833 (1992).
- Carey v Population Services, 431 U.S. 678 (1977).
- Bellotti v Baird, 443 U.S. 622 (1979).
- Planned Parenthood of Kansas City v Ashcroft, 462 U.S. 476 (1983).
- Planned Parenthood of Northern New England v Ayotte, 546 U.S. 320 (2006).
- H.L. v Matheson, 450 U.S. 398 (1981).
- Hodgson v Minnesota, 497 U.S. 417 (1990).
- Ohio v Akron Center for Reproductive Health, 497 U.S. 502 (1990).
- Lambert v Wicklund, 520 U.S. 292 (1997).
- June Medical Services v Russo, 591 U.S. ___ (2020), https:// www.supremecourt.gov/opinions/19pdf/18-1323_c07d.pdf.
- Whole Woman’s Health v Hellerstedt, 579 U.S. 582 (2016).
- AMA v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/american-medical -association-v-cochran.
- Becerra v Baltimore, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/cochran-v-mayor-and-city-council-of-baltimore.
- Oregon v Becerra, dismissed May 17, 2021, https://www .scotusblog.com/case-files/cases/oregon-v-cochran.
- Cameron v EMW Women’s Surgical Center, 20-601. https:// www.scotusblog.com/case-files/cases/cameron-v-emw -womens-surgical-center-p-s-c.
- Ex parte Young, 209 U.S. 123 (1908).
- Whole Woman’s Health v Jackson, 21-463. https://www .scotusblog.com/case-files/cases/whole-womans-health-v -jackson.
- U.S. v Texas, 21-588. https://www.scotusblog.com/case-files /cases/united-states-v-texas-3.
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.scotusblog.com/case-files/cases/dobbs-v -jackson-womens-health-organization.
- Brief of Amici Curiae American College of Obstetricians and Gynecologists et al., Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.acog.org/ -/media/project/acog/acogorg/files/advocacy/amicus -briefs/2021/20210920-dobbs-v-jwho-amicus-brief.pdf?la=e n&hash=717DFDD07A03B93A04490E66835BB8C5.
- Brief Amicus Curiae of International Federation of Gynecology and Obstetrics, Dobbs v Jackson Women’s Health Organization (Sep. 20, 2021). https://www.supremecourt. gov/DocketPDF/19/19-1392/193019/20210920155508744 _41426%20pdf%20Chen.pdf.
- Brief Amicus Curiae of American Association of Pro-Life Obstetricians and Gynecologists. Dobbs v Jackson Women’s Health Organization (Sep. 2021). https://www.supremecourt .gov/DocketPDF/19/19-1392/185350/20210729163532595 _No.%2019-1392%20-%20American%20Association %20of%20Pro-Life%20Obstetricians%20and%20 Gynecologists%20-%20Amicus%20Brief%20in%20Support %20of%20Petitioner%20-%207-29-21.pdf.
The Supreme Court 2020‒2021: What will affect ObGyns?
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
Genetic testing and the future of cerebral palsy malpractice cases
CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.
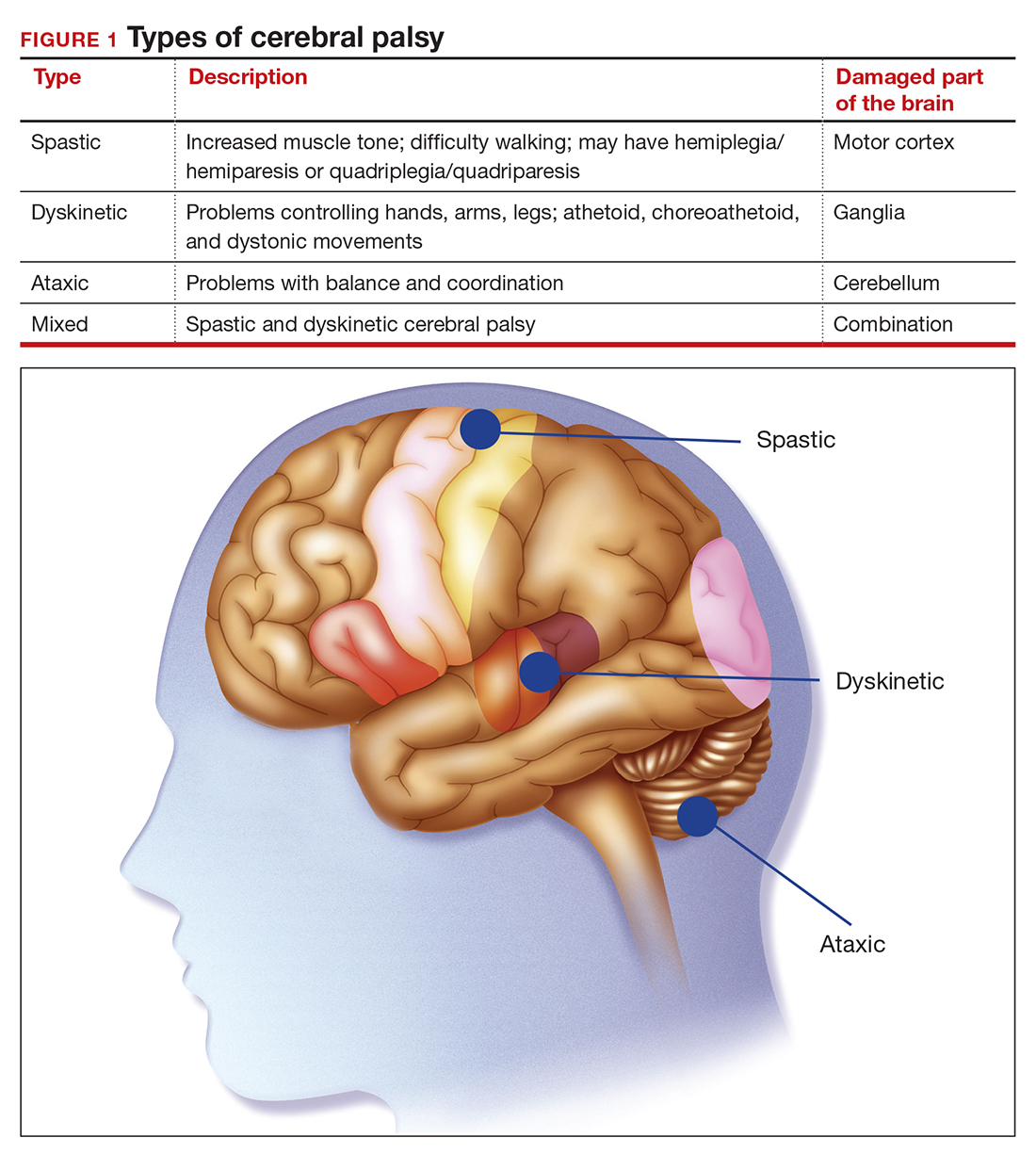
A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7
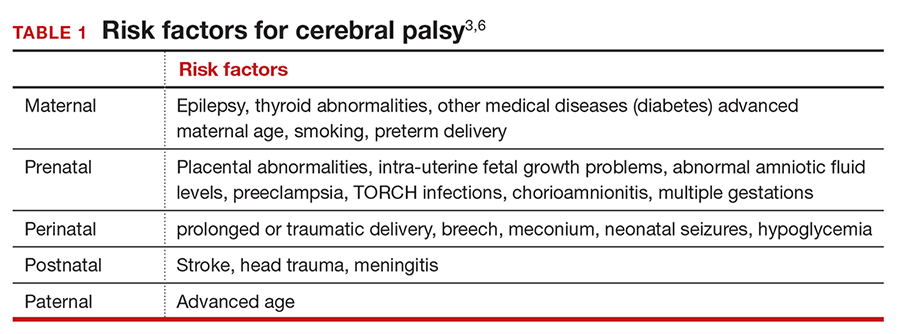
DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).
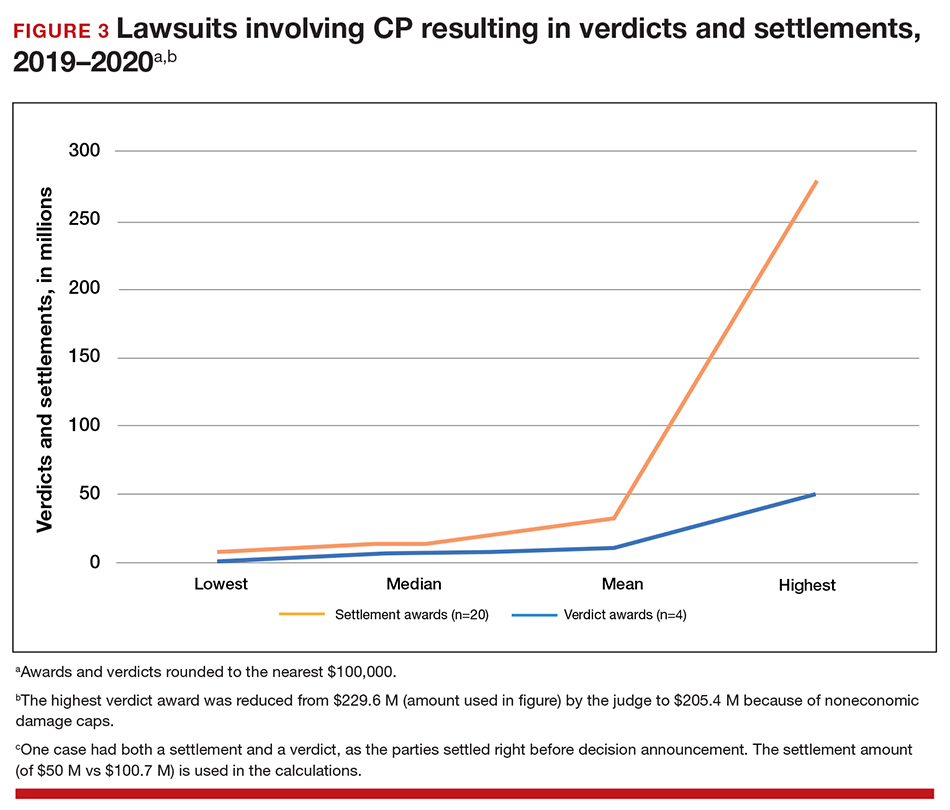
These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.