User login
Smartphones Poised to Revolutionize Heart Failure Monitoring
SEATTLE – Smartphones may soon be harnessed for monitoring patients with heart failure, offering advantages such as remote assessment and early prediction of decompensation.
A recent survey suggested that 88% of physicians would like to be able to monitor measures of their patients’ health status at home, including many relevant to heart failure. "Those metrics will become more and more available" with smartphone technology, noted Dr. David E. Albert, founder and chief scientific officer of AliveCor Inc., a manufacturer of mobile monitors, including the investigational AliveCor Smartphone System.
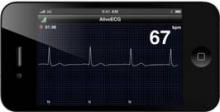
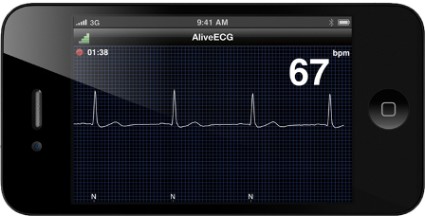
The device is an ECG monitor that is in clinical trials and under review by the Food and Drug Administration. A patient uses a smartphone and an app to record a clinical-quality ECG that is securely stored and processed in cloud computing-based server, and can be accessed by a physician anywhere in the world.
The device can evaluate at least three cardiac indices used in the monitoring of heart failure, according to Dr. Albert: cardiac rhythm, heart rate, and heart rate variability.
When it comes to cardiac rhythm, smartphones can be used to detect arrhythmias such as atrial fibrillation (J. Am. Coll. Cardiol. 2012;59:E726). He recounted the story of a man in Mumbai, India, experiencing asymptomatic ischemia-induced rhythm changes that were recorded with a smartphone. Physicians in Oklahoma City and Los Angeles identified the arrhythmia and notified the patient, who then went to his physician.
The ECG obtained with the smartphone has the same quality as a 12-lead ECG obtained with state-of-the-art equipment in the clinic, he said. Also, data suggest that a daily ECG is second only to implanted devices for detecting atrial fibrillation (Pacing Clin. Electrophysiol. 2007;30:458-62). "So it’s better than a 24-hour Holter, even now, and our very intermittent 7-day Holters."
Heart rate, the second index, may be a key therapeutic target in heart failure. Here, too, the smartphone-assessed heart rate is just as accurate as clinically measured heart rate, with sensitivity exceeding 99% for QRS detection (and thus R to R intervals), putting it on par with the 12-lead ECG, according to Dr. Albert.
Heart rate variability, the third index, potentially could be used as an index to guide the need for intervention before progression to decompensated heart failure.
Decompensation develops through a series of changes beginning with increasing preload and autonomic adaptation, and culminating in weight gain, symptoms, and hospitalization (Curr. Heart Fail. Rep. 2009;6:287-92). "Obviously, we want to operate on the left side of this graph, where filling and autonomic adaptation are the places we can intervene early," he noted.
Short-term heart rate variability obtained during 8 minutes of paced breathing has been shown to predict sudden cardiac death in patients having chronic heart failure (Circulation 2003;107:565-70), and smartphones can readily be used for such measurement.
The timing of events during the cardiac cycle may also be informative, according to Dr. Albert. These events can be assessed with seismocardiography, whereby vibrations in the chest are measured with an accelerometer placed on the sternum (Chest 1991;100:991-3) and can be combined with ECG data to derive the Tei index, a global measure of cardiac performance (J. Cardiol. 1995;26:135-6). Although the necessary data can be collected with a somewhat elaborate laboratory setup (J. Med. Biol. Engineer 2012;32:103-10), they can also be obtained easily with a smartphone placed on the chest.
"We can measure isovolumic contraction time, isovolumic relaxation time, and ejection time, and develop in 30 seconds not only rate, rhythm, variability, but now a modified Tei index, an index of performance, and as many papers have said, an index of preload status," he noted.
"With today’s smartphones, which will only get more powerful, we can evaluate cardiac rhythm, or our patients can. They can evaluate their cardiac rate, their heart rate variability, and probably potentially – unproven yet very interesting – their ventricular performance and their preload status, enabling that [information] to be injected into the network, enabling certainly intervention and maybe self-care," Dr. Albert concluded.
A session attendee said that this new technology "sounds very good. But we know even when [clinical devices] are used just to study time intervals, there were a lot of artifacts, and accuracy was not very easily determined. Certainly, with this kind of platform, there must be problems with accuracy and artifact in recordings."
It is still early in development of this technology, Dr. Albert acknowledged. "But understand that we have processing power that’s quite unbelievable. ... What I can tell you is that we can measure these variables; what I can’t tell you is how valuable they are going to be."
Another attendee expressed concern over the vast amount of data that would be generated and then require analysis. "How far are we going to go before we get to the point where we need a different layer besides the physician, the nurses, the PAs? If you are going to swamp us with this much data, there is no way a busy doctor seeing 20 heart patients a day, going to the cath lab, can possibly figure out so much data, what’s important, what’s not," he said.
Recently, the venture capitalist Vinod Khosla put forth a paper calling for fewer doctors and more algorithms, Dr. Albert replied. "I don’t think we will disenfranchise physicians, but I do believe the power of big data will become more and more important for all of us in the management of all our patients," he predicted.
"Apps will be in our pockets as professionals; they will be in our patients’ pockets. And we must figure out how to utilize them to help us deal with major health care issues of the day."
Dr. Albert disclosed that he is member of the board of directors of, a full-time salaried employee of, and an equity shareholder in AliveCor.
SEATTLE – Smartphones may soon be harnessed for monitoring patients with heart failure, offering advantages such as remote assessment and early prediction of decompensation.
A recent survey suggested that 88% of physicians would like to be able to monitor measures of their patients’ health status at home, including many relevant to heart failure. "Those metrics will become more and more available" with smartphone technology, noted Dr. David E. Albert, founder and chief scientific officer of AliveCor Inc., a manufacturer of mobile monitors, including the investigational AliveCor Smartphone System.
The device is an ECG monitor that is in clinical trials and under review by the Food and Drug Administration. A patient uses a smartphone and an app to record a clinical-quality ECG that is securely stored and processed in cloud computing-based server, and can be accessed by a physician anywhere in the world.
The device can evaluate at least three cardiac indices used in the monitoring of heart failure, according to Dr. Albert: cardiac rhythm, heart rate, and heart rate variability.
When it comes to cardiac rhythm, smartphones can be used to detect arrhythmias such as atrial fibrillation (J. Am. Coll. Cardiol. 2012;59:E726). He recounted the story of a man in Mumbai, India, experiencing asymptomatic ischemia-induced rhythm changes that were recorded with a smartphone. Physicians in Oklahoma City and Los Angeles identified the arrhythmia and notified the patient, who then went to his physician.
The ECG obtained with the smartphone has the same quality as a 12-lead ECG obtained with state-of-the-art equipment in the clinic, he said. Also, data suggest that a daily ECG is second only to implanted devices for detecting atrial fibrillation (Pacing Clin. Electrophysiol. 2007;30:458-62). "So it’s better than a 24-hour Holter, even now, and our very intermittent 7-day Holters."
Heart rate, the second index, may be a key therapeutic target in heart failure. Here, too, the smartphone-assessed heart rate is just as accurate as clinically measured heart rate, with sensitivity exceeding 99% for QRS detection (and thus R to R intervals), putting it on par with the 12-lead ECG, according to Dr. Albert.
Heart rate variability, the third index, potentially could be used as an index to guide the need for intervention before progression to decompensated heart failure.
Decompensation develops through a series of changes beginning with increasing preload and autonomic adaptation, and culminating in weight gain, symptoms, and hospitalization (Curr. Heart Fail. Rep. 2009;6:287-92). "Obviously, we want to operate on the left side of this graph, where filling and autonomic adaptation are the places we can intervene early," he noted.
Short-term heart rate variability obtained during 8 minutes of paced breathing has been shown to predict sudden cardiac death in patients having chronic heart failure (Circulation 2003;107:565-70), and smartphones can readily be used for such measurement.
The timing of events during the cardiac cycle may also be informative, according to Dr. Albert. These events can be assessed with seismocardiography, whereby vibrations in the chest are measured with an accelerometer placed on the sternum (Chest 1991;100:991-3) and can be combined with ECG data to derive the Tei index, a global measure of cardiac performance (J. Cardiol. 1995;26:135-6). Although the necessary data can be collected with a somewhat elaborate laboratory setup (J. Med. Biol. Engineer 2012;32:103-10), they can also be obtained easily with a smartphone placed on the chest.
"We can measure isovolumic contraction time, isovolumic relaxation time, and ejection time, and develop in 30 seconds not only rate, rhythm, variability, but now a modified Tei index, an index of performance, and as many papers have said, an index of preload status," he noted.
"With today’s smartphones, which will only get more powerful, we can evaluate cardiac rhythm, or our patients can. They can evaluate their cardiac rate, their heart rate variability, and probably potentially – unproven yet very interesting – their ventricular performance and their preload status, enabling that [information] to be injected into the network, enabling certainly intervention and maybe self-care," Dr. Albert concluded.
A session attendee said that this new technology "sounds very good. But we know even when [clinical devices] are used just to study time intervals, there were a lot of artifacts, and accuracy was not very easily determined. Certainly, with this kind of platform, there must be problems with accuracy and artifact in recordings."
It is still early in development of this technology, Dr. Albert acknowledged. "But understand that we have processing power that’s quite unbelievable. ... What I can tell you is that we can measure these variables; what I can’t tell you is how valuable they are going to be."
Another attendee expressed concern over the vast amount of data that would be generated and then require analysis. "How far are we going to go before we get to the point where we need a different layer besides the physician, the nurses, the PAs? If you are going to swamp us with this much data, there is no way a busy doctor seeing 20 heart patients a day, going to the cath lab, can possibly figure out so much data, what’s important, what’s not," he said.
Recently, the venture capitalist Vinod Khosla put forth a paper calling for fewer doctors and more algorithms, Dr. Albert replied. "I don’t think we will disenfranchise physicians, but I do believe the power of big data will become more and more important for all of us in the management of all our patients," he predicted.
"Apps will be in our pockets as professionals; they will be in our patients’ pockets. And we must figure out how to utilize them to help us deal with major health care issues of the day."
Dr. Albert disclosed that he is member of the board of directors of, a full-time salaried employee of, and an equity shareholder in AliveCor.
SEATTLE – Smartphones may soon be harnessed for monitoring patients with heart failure, offering advantages such as remote assessment and early prediction of decompensation.
A recent survey suggested that 88% of physicians would like to be able to monitor measures of their patients’ health status at home, including many relevant to heart failure. "Those metrics will become more and more available" with smartphone technology, noted Dr. David E. Albert, founder and chief scientific officer of AliveCor Inc., a manufacturer of mobile monitors, including the investigational AliveCor Smartphone System.
The device is an ECG monitor that is in clinical trials and under review by the Food and Drug Administration. A patient uses a smartphone and an app to record a clinical-quality ECG that is securely stored and processed in cloud computing-based server, and can be accessed by a physician anywhere in the world.
The device can evaluate at least three cardiac indices used in the monitoring of heart failure, according to Dr. Albert: cardiac rhythm, heart rate, and heart rate variability.
When it comes to cardiac rhythm, smartphones can be used to detect arrhythmias such as atrial fibrillation (J. Am. Coll. Cardiol. 2012;59:E726). He recounted the story of a man in Mumbai, India, experiencing asymptomatic ischemia-induced rhythm changes that were recorded with a smartphone. Physicians in Oklahoma City and Los Angeles identified the arrhythmia and notified the patient, who then went to his physician.
The ECG obtained with the smartphone has the same quality as a 12-lead ECG obtained with state-of-the-art equipment in the clinic, he said. Also, data suggest that a daily ECG is second only to implanted devices for detecting atrial fibrillation (Pacing Clin. Electrophysiol. 2007;30:458-62). "So it’s better than a 24-hour Holter, even now, and our very intermittent 7-day Holters."
Heart rate, the second index, may be a key therapeutic target in heart failure. Here, too, the smartphone-assessed heart rate is just as accurate as clinically measured heart rate, with sensitivity exceeding 99% for QRS detection (and thus R to R intervals), putting it on par with the 12-lead ECG, according to Dr. Albert.
Heart rate variability, the third index, potentially could be used as an index to guide the need for intervention before progression to decompensated heart failure.
Decompensation develops through a series of changes beginning with increasing preload and autonomic adaptation, and culminating in weight gain, symptoms, and hospitalization (Curr. Heart Fail. Rep. 2009;6:287-92). "Obviously, we want to operate on the left side of this graph, where filling and autonomic adaptation are the places we can intervene early," he noted.
Short-term heart rate variability obtained during 8 minutes of paced breathing has been shown to predict sudden cardiac death in patients having chronic heart failure (Circulation 2003;107:565-70), and smartphones can readily be used for such measurement.
The timing of events during the cardiac cycle may also be informative, according to Dr. Albert. These events can be assessed with seismocardiography, whereby vibrations in the chest are measured with an accelerometer placed on the sternum (Chest 1991;100:991-3) and can be combined with ECG data to derive the Tei index, a global measure of cardiac performance (J. Cardiol. 1995;26:135-6). Although the necessary data can be collected with a somewhat elaborate laboratory setup (J. Med. Biol. Engineer 2012;32:103-10), they can also be obtained easily with a smartphone placed on the chest.
"We can measure isovolumic contraction time, isovolumic relaxation time, and ejection time, and develop in 30 seconds not only rate, rhythm, variability, but now a modified Tei index, an index of performance, and as many papers have said, an index of preload status," he noted.
"With today’s smartphones, which will only get more powerful, we can evaluate cardiac rhythm, or our patients can. They can evaluate their cardiac rate, their heart rate variability, and probably potentially – unproven yet very interesting – their ventricular performance and their preload status, enabling that [information] to be injected into the network, enabling certainly intervention and maybe self-care," Dr. Albert concluded.
A session attendee said that this new technology "sounds very good. But we know even when [clinical devices] are used just to study time intervals, there were a lot of artifacts, and accuracy was not very easily determined. Certainly, with this kind of platform, there must be problems with accuracy and artifact in recordings."
It is still early in development of this technology, Dr. Albert acknowledged. "But understand that we have processing power that’s quite unbelievable. ... What I can tell you is that we can measure these variables; what I can’t tell you is how valuable they are going to be."
Another attendee expressed concern over the vast amount of data that would be generated and then require analysis. "How far are we going to go before we get to the point where we need a different layer besides the physician, the nurses, the PAs? If you are going to swamp us with this much data, there is no way a busy doctor seeing 20 heart patients a day, going to the cath lab, can possibly figure out so much data, what’s important, what’s not," he said.
Recently, the venture capitalist Vinod Khosla put forth a paper calling for fewer doctors and more algorithms, Dr. Albert replied. "I don’t think we will disenfranchise physicians, but I do believe the power of big data will become more and more important for all of us in the management of all our patients," he predicted.
"Apps will be in our pockets as professionals; they will be in our patients’ pockets. And we must figure out how to utilize them to help us deal with major health care issues of the day."
Dr. Albert disclosed that he is member of the board of directors of, a full-time salaried employee of, and an equity shareholder in AliveCor.
AT THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Underuse of Aldosterone Antagonists Contributes to Heart Failure Deaths
SEATTLE – Aldosterone antagonists can be used safely and effectively to treat heart failure in real-world practice, and their underuse likely accounts for a sizable share of deaths in this patient population, according to Dr. Gregg C. Fonarow, cochief of the division of cardiology at the University of California, Los Angeles.
These agents are among six heart failure therapies having a class I (highest) recommendation in national guidelines for managing heart failure, he said at the annual meeting of the Heart Failure Society of America. Yet, nearly 68,000 patients with heart failure and reduced left ventricular ejection fraction (LVEF) die each year in the United States because they do not receive these therapies (Am. Heart J. 2011;161:1024-30).
"If we bridged that gap, and assuming the efficacy and effectiveness match, you can see the potential number of lives saved each year. ... And, in fact, the greatest number of lives saved [roughly 21,400] could result from the improved use of aldosterone antagonists, if there were optimal implementation," he said.
Indeed, analyses show that only about one-third of eligible heart failure outpatients (Circ. Heart Fail. 2008;1:98-106) and inpatients (JAMA 2009;302:1658-65) in real-world U.S. practice receive drugs in this class.
Some resistance to the use of aldosterone antagonists – such as spironolactone (brand name Aldactone) and eplerenone (Inspra) – has stemmed from uncertainty as to whether the risk-benefit profile in clinical trials translates to real-world practice, according to Dr. Fonarow.
A recent analysis suggests that among patients with heart failure and reduced LVEF, aldosterone antagonist therapy decreases the risk of death by about 30% and the risk of hospitalization by 35% (Am. Heart J. 2011;161:1024-30). The number needed to treat to prevent a single death in a 36-month period is just eight, and the benefit is incremental to that of other standard therapies.
"There was just a moderate increase in the risk of hyperkalemia and increased creatinine, but overall, safe and well tolerated," he said. "They were shown to be highly cost effective even with fully branded medications at full cost, and the benefits greatly outweighed the potential risks."
However, an analysis of Canadian administrative data showed that after publication of the Randomized Aldactone Evaluation Study (RALES), which found significant benefit of spironolactone in heart failure, there was an increase in prescriptions for the drug but also a concomitant increase in hyperkalemia-related hospitalizations and deaths (N. Engl. J. Med. 2004;351:543-51).
The analysis did not assess benefits and had some limitations, according to Dr. Fonarow. "But after that publication and a few others, a number of notable heart failure experts stated pretty strong viewpoints that aldosterone antagonists are really not safe in real-world clinical practice, the risks outweigh any potential benefits, and they should be reserved only for the most severe heart failure patients and only after all other therapies have been tried and failed."
Yet, other studies have suggested, for example, that every 10% increase in aldosterone antagonist use among hospitalized patients is associated with a 6% reduction in all-cause mortality over the following year (Am. Heart J. 2010;159:406-13). Furthermore, in the context of careful monitoring, with increasing use of spironolactone, hyperkalemia is not problematic (BMJ 2010;340:c1768).
"Certainly, some of the data have had a number of individuals saying, ‘We need to get away from evidence guidelines and performance measures, and individualize therapy,’ and ‘I should pick and choose therapy as I think,’ and ‘Findings from clinical trials are not applicable to my patients, they are much more complex, and I know my patients are going to do well because I give them highly personalized care rather than cookbook medicine,’" Dr. Fonarow commented. "That is certainly not a very scientific or satisfying type of approach."
Experience from IMPROVE HF suggests that a performance improvement program can safely and effectively increase the use of aldosterone antagonists for heart failure in a real-world setting (Circulation 2010;122:585-96). The program led to a near doubling of use of these agents over 2 years. "At the same time, did we see any increase in inappropriate or ineligible patients being treated? No, the rates for patients with absolute contraindications or relative contraindications was very small, below 1%, and did not increase," he said.
"Treatment gaps between guidelines and practice exist for heart failure and, as a result, large numbers of patients are having hospitalizations and fatal events that could have been prevented. This is particularly true for aldosterone antagonists," Dr. Fonarow concluded. "Bridging the gap between evidence and clinical practice systems is needed, and by applying these evidence-based therapies with the appropriate monitoring and the appropriate selection of patients, we can do this in a way that truly will improve outcomes."
Dr. Fonarow disclosed that he is a consultant to and/or receives honoraria or research funding from Novartis and Medtronic.
SEATTLE – Aldosterone antagonists can be used safely and effectively to treat heart failure in real-world practice, and their underuse likely accounts for a sizable share of deaths in this patient population, according to Dr. Gregg C. Fonarow, cochief of the division of cardiology at the University of California, Los Angeles.
These agents are among six heart failure therapies having a class I (highest) recommendation in national guidelines for managing heart failure, he said at the annual meeting of the Heart Failure Society of America. Yet, nearly 68,000 patients with heart failure and reduced left ventricular ejection fraction (LVEF) die each year in the United States because they do not receive these therapies (Am. Heart J. 2011;161:1024-30).
"If we bridged that gap, and assuming the efficacy and effectiveness match, you can see the potential number of lives saved each year. ... And, in fact, the greatest number of lives saved [roughly 21,400] could result from the improved use of aldosterone antagonists, if there were optimal implementation," he said.
Indeed, analyses show that only about one-third of eligible heart failure outpatients (Circ. Heart Fail. 2008;1:98-106) and inpatients (JAMA 2009;302:1658-65) in real-world U.S. practice receive drugs in this class.
Some resistance to the use of aldosterone antagonists – such as spironolactone (brand name Aldactone) and eplerenone (Inspra) – has stemmed from uncertainty as to whether the risk-benefit profile in clinical trials translates to real-world practice, according to Dr. Fonarow.
A recent analysis suggests that among patients with heart failure and reduced LVEF, aldosterone antagonist therapy decreases the risk of death by about 30% and the risk of hospitalization by 35% (Am. Heart J. 2011;161:1024-30). The number needed to treat to prevent a single death in a 36-month period is just eight, and the benefit is incremental to that of other standard therapies.
"There was just a moderate increase in the risk of hyperkalemia and increased creatinine, but overall, safe and well tolerated," he said. "They were shown to be highly cost effective even with fully branded medications at full cost, and the benefits greatly outweighed the potential risks."
However, an analysis of Canadian administrative data showed that after publication of the Randomized Aldactone Evaluation Study (RALES), which found significant benefit of spironolactone in heart failure, there was an increase in prescriptions for the drug but also a concomitant increase in hyperkalemia-related hospitalizations and deaths (N. Engl. J. Med. 2004;351:543-51).
The analysis did not assess benefits and had some limitations, according to Dr. Fonarow. "But after that publication and a few others, a number of notable heart failure experts stated pretty strong viewpoints that aldosterone antagonists are really not safe in real-world clinical practice, the risks outweigh any potential benefits, and they should be reserved only for the most severe heart failure patients and only after all other therapies have been tried and failed."
Yet, other studies have suggested, for example, that every 10% increase in aldosterone antagonist use among hospitalized patients is associated with a 6% reduction in all-cause mortality over the following year (Am. Heart J. 2010;159:406-13). Furthermore, in the context of careful monitoring, with increasing use of spironolactone, hyperkalemia is not problematic (BMJ 2010;340:c1768).
"Certainly, some of the data have had a number of individuals saying, ‘We need to get away from evidence guidelines and performance measures, and individualize therapy,’ and ‘I should pick and choose therapy as I think,’ and ‘Findings from clinical trials are not applicable to my patients, they are much more complex, and I know my patients are going to do well because I give them highly personalized care rather than cookbook medicine,’" Dr. Fonarow commented. "That is certainly not a very scientific or satisfying type of approach."
Experience from IMPROVE HF suggests that a performance improvement program can safely and effectively increase the use of aldosterone antagonists for heart failure in a real-world setting (Circulation 2010;122:585-96). The program led to a near doubling of use of these agents over 2 years. "At the same time, did we see any increase in inappropriate or ineligible patients being treated? No, the rates for patients with absolute contraindications or relative contraindications was very small, below 1%, and did not increase," he said.
"Treatment gaps between guidelines and practice exist for heart failure and, as a result, large numbers of patients are having hospitalizations and fatal events that could have been prevented. This is particularly true for aldosterone antagonists," Dr. Fonarow concluded. "Bridging the gap between evidence and clinical practice systems is needed, and by applying these evidence-based therapies with the appropriate monitoring and the appropriate selection of patients, we can do this in a way that truly will improve outcomes."
Dr. Fonarow disclosed that he is a consultant to and/or receives honoraria or research funding from Novartis and Medtronic.
SEATTLE – Aldosterone antagonists can be used safely and effectively to treat heart failure in real-world practice, and their underuse likely accounts for a sizable share of deaths in this patient population, according to Dr. Gregg C. Fonarow, cochief of the division of cardiology at the University of California, Los Angeles.
These agents are among six heart failure therapies having a class I (highest) recommendation in national guidelines for managing heart failure, he said at the annual meeting of the Heart Failure Society of America. Yet, nearly 68,000 patients with heart failure and reduced left ventricular ejection fraction (LVEF) die each year in the United States because they do not receive these therapies (Am. Heart J. 2011;161:1024-30).
"If we bridged that gap, and assuming the efficacy and effectiveness match, you can see the potential number of lives saved each year. ... And, in fact, the greatest number of lives saved [roughly 21,400] could result from the improved use of aldosterone antagonists, if there were optimal implementation," he said.
Indeed, analyses show that only about one-third of eligible heart failure outpatients (Circ. Heart Fail. 2008;1:98-106) and inpatients (JAMA 2009;302:1658-65) in real-world U.S. practice receive drugs in this class.
Some resistance to the use of aldosterone antagonists – such as spironolactone (brand name Aldactone) and eplerenone (Inspra) – has stemmed from uncertainty as to whether the risk-benefit profile in clinical trials translates to real-world practice, according to Dr. Fonarow.
A recent analysis suggests that among patients with heart failure and reduced LVEF, aldosterone antagonist therapy decreases the risk of death by about 30% and the risk of hospitalization by 35% (Am. Heart J. 2011;161:1024-30). The number needed to treat to prevent a single death in a 36-month period is just eight, and the benefit is incremental to that of other standard therapies.
"There was just a moderate increase in the risk of hyperkalemia and increased creatinine, but overall, safe and well tolerated," he said. "They were shown to be highly cost effective even with fully branded medications at full cost, and the benefits greatly outweighed the potential risks."
However, an analysis of Canadian administrative data showed that after publication of the Randomized Aldactone Evaluation Study (RALES), which found significant benefit of spironolactone in heart failure, there was an increase in prescriptions for the drug but also a concomitant increase in hyperkalemia-related hospitalizations and deaths (N. Engl. J. Med. 2004;351:543-51).
The analysis did not assess benefits and had some limitations, according to Dr. Fonarow. "But after that publication and a few others, a number of notable heart failure experts stated pretty strong viewpoints that aldosterone antagonists are really not safe in real-world clinical practice, the risks outweigh any potential benefits, and they should be reserved only for the most severe heart failure patients and only after all other therapies have been tried and failed."
Yet, other studies have suggested, for example, that every 10% increase in aldosterone antagonist use among hospitalized patients is associated with a 6% reduction in all-cause mortality over the following year (Am. Heart J. 2010;159:406-13). Furthermore, in the context of careful monitoring, with increasing use of spironolactone, hyperkalemia is not problematic (BMJ 2010;340:c1768).
"Certainly, some of the data have had a number of individuals saying, ‘We need to get away from evidence guidelines and performance measures, and individualize therapy,’ and ‘I should pick and choose therapy as I think,’ and ‘Findings from clinical trials are not applicable to my patients, they are much more complex, and I know my patients are going to do well because I give them highly personalized care rather than cookbook medicine,’" Dr. Fonarow commented. "That is certainly not a very scientific or satisfying type of approach."
Experience from IMPROVE HF suggests that a performance improvement program can safely and effectively increase the use of aldosterone antagonists for heart failure in a real-world setting (Circulation 2010;122:585-96). The program led to a near doubling of use of these agents over 2 years. "At the same time, did we see any increase in inappropriate or ineligible patients being treated? No, the rates for patients with absolute contraindications or relative contraindications was very small, below 1%, and did not increase," he said.
"Treatment gaps between guidelines and practice exist for heart failure and, as a result, large numbers of patients are having hospitalizations and fatal events that could have been prevented. This is particularly true for aldosterone antagonists," Dr. Fonarow concluded. "Bridging the gap between evidence and clinical practice systems is needed, and by applying these evidence-based therapies with the appropriate monitoring and the appropriate selection of patients, we can do this in a way that truly will improve outcomes."
Dr. Fonarow disclosed that he is a consultant to and/or receives honoraria or research funding from Novartis and Medtronic.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Intervention Halves Off-Guideline Antibiotic Use in Children
SAN DIEGO – An outpatient antimicrobial stewardship intervention dramatically reduced inappropriate use of antibiotics for acute respiratory tract infections in children, judging from the findings of a trial reported at IDWeek.
In the cluster-randomized trial, which involved 185,212 pediatric patients making more than 1.4 million outpatient visits, the rate of inappropriate prescribing for these infections – use of a broad-spectrum antibiotic when guidelines recommended a narrow-spectrum one – fell by nearly half in the intervention group over a year, compared with about one-fifth in the control group.
Led by Dr. Jeffrey Gerber, the investigators enrolled 18 practices in a large pediatric primary care network that share an electronic health record, randomizing them evenly to intervention and control groups.
The intervention had two parts: an on-site clinician education session, including a refresher in current guidelines for treating sinusitis, group A streptococcal pharyngitis, and pneumonia, and then private quarterly audit and feedback reports to physicians of their antibiotic prescribing for these conditions.
The reports "showed how they were prescribing relative to national guidelines at baseline and over time throughout the intervention for 12 months, compared with those in the practice group and across the network," explained Dr. Gerber of Children’s Hospital of Philadelphia. "So the idea is to not only show providers how they prescribe relative to national recommendations, but also to have what we call achievable benchmarks available, to show how other folks in busy practices just like theirs are prescribing."
The rate of off-guideline antibiotic prescribing for acute respiratory tract infections was 28% overall, with a range of 15%-60% across practices, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
One year after the intervention, the rate had fallen by 48% in the intervention group and by 18% in the control group (P = .001).
In stratified analyses, the greatest reduction was seen for pneumonia: The rate of off-guideline prescribing fell by 75% in the intervention group, compared with 6% in the control group.
For acute respiratory tract infections overall, azithromycin was the greatest contributor to off-guideline antibiotic prescribing. For pneumonia specifically, amoxicillin plus clavulanic acid (Augmentin) was the greatest contributor.
"We are really encouraged by these results. We think that it is a relatively simple intervention that we hope will be scalable to other pediatric practices that have electronic health records," Dr. Gerber commented in a related press briefing. The Agency for Healthcare Research and Quality, which funded the study, has provided an extra year of funding so that the intervention can be packaged and disseminated for use by other practices.
He predicted that the findings would be largely generalizable, given the good mix of practices and patients in the trial. "We don’t think yet it’s broadly applicable to every practice, but at the same time, to practices that use electronic health records," he said, noting that the simple algorithms used in the study draw on data routinely captured in these records.
The findings could have a major impact nationally, given that broad-spectrum antibiotics account for roughly half of the 40 million antibiotic prescriptions written per year for children in the United States for acute respiratory tract infections in the outpatient setting, according to Dr. Gerber. Not only are they more expensive than narrow-spectrum ones, but they also more rapidly promote resistance.
The study’s findings are "very powerful," commented Dr. Liise-Anne Pirofski, moderator of the press briefing and IDWeek Chair. "This is a fairly unique study and probably pretty groundbreaking."
She wondered whether factors such as better taste or greater ease of once-daily dosing of several broad-spectrum antibiotics contribute to off-guideline prescribing and need to be taken into account. "In children, multidosing is difficult because most schools don’t administer antibiotics to children in school," she pointed out. Also, parents sometimes request specific antibiotics.
"These are real issues," and likely driving forces behind at least some off-guideline prescribing, Dr. Gerber agreed. "However, the societies that recommend antibiotics really have to take into account the spectrum of activity to be careful because of the development of antibiotic resistance."
In addition, emerging research suggests that, in at least some cases, more frequent dosing of narrow-spectrum agents is not necessary. For example, the American Academy of Pediatrics now endorses once-daily amoxicillin for treating strep throat. "That used to be dosed more frequently, but studies have shown that once-daily dosing is actually as effective. So that’s helped combat this issue a bit," he said.
Dr. Pirofski noted that it’s nice to see evidence of the benefits of electronic health records, as putting them in place is typically a major undertaking. "It will be very interesting if good studies are done to determine how much that watchdog effect is driving people’s behavior. If it is, then I think the electronic component is absolutely essential because it’s the only way that you can really generate that data and data that people will believe."
That said, face-to-face interaction should not be underestimated in such interventions, she maintained. "The personal interaction, I believe, is really what drove the early success of some of these antibiotic stewardships, because medicine can be very lonely. If somebody comes in and chats you up a little bit, you feel like you are more in tune with what’s going on. ... My own feeling is that personal interaction always drives change better than these other things," said Dr. Pirofski, who is chief of the division of infectious diseases at Albert Einstein College of Medicine, New York.
Ascertaining the cost effectiveness of the intervention would be complicated because of copayment and reimbursement issues, according to Dr. Gerber, but "most of these broad-spectrum agents are between five and ten times more expensive than the narrow-spectrum agents." He predicted that, as insurers move toward a bundled-payment model, costs will get greater attention. "Maybe insurance companies won’t reimburse for broad-spectrum agents if we can show that recommendations should be followed, and there are no differences in outcomes," he said.
The investigators plan to assess the impact of the intervention on health outcomes and will monitor the durability of its efficacy, Dr. Gerber said. "We are going to follow up for at least another year, now that there are no more feedback reports coming in, to see if it continues or if people revert back to their initial prescribing patterns," he explained. In addition, they are interviewing participating clinicians to obtain their viewpoints on prescribing and auditing, along with suggestions for improving the intervention.
Neither Dr. Gerber nor Dr. Pirofski disclosed any relevant conflicts of interest.
SAN DIEGO – An outpatient antimicrobial stewardship intervention dramatically reduced inappropriate use of antibiotics for acute respiratory tract infections in children, judging from the findings of a trial reported at IDWeek.
In the cluster-randomized trial, which involved 185,212 pediatric patients making more than 1.4 million outpatient visits, the rate of inappropriate prescribing for these infections – use of a broad-spectrum antibiotic when guidelines recommended a narrow-spectrum one – fell by nearly half in the intervention group over a year, compared with about one-fifth in the control group.
Led by Dr. Jeffrey Gerber, the investigators enrolled 18 practices in a large pediatric primary care network that share an electronic health record, randomizing them evenly to intervention and control groups.
The intervention had two parts: an on-site clinician education session, including a refresher in current guidelines for treating sinusitis, group A streptococcal pharyngitis, and pneumonia, and then private quarterly audit and feedback reports to physicians of their antibiotic prescribing for these conditions.
The reports "showed how they were prescribing relative to national guidelines at baseline and over time throughout the intervention for 12 months, compared with those in the practice group and across the network," explained Dr. Gerber of Children’s Hospital of Philadelphia. "So the idea is to not only show providers how they prescribe relative to national recommendations, but also to have what we call achievable benchmarks available, to show how other folks in busy practices just like theirs are prescribing."
The rate of off-guideline antibiotic prescribing for acute respiratory tract infections was 28% overall, with a range of 15%-60% across practices, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
One year after the intervention, the rate had fallen by 48% in the intervention group and by 18% in the control group (P = .001).
In stratified analyses, the greatest reduction was seen for pneumonia: The rate of off-guideline prescribing fell by 75% in the intervention group, compared with 6% in the control group.
For acute respiratory tract infections overall, azithromycin was the greatest contributor to off-guideline antibiotic prescribing. For pneumonia specifically, amoxicillin plus clavulanic acid (Augmentin) was the greatest contributor.
"We are really encouraged by these results. We think that it is a relatively simple intervention that we hope will be scalable to other pediatric practices that have electronic health records," Dr. Gerber commented in a related press briefing. The Agency for Healthcare Research and Quality, which funded the study, has provided an extra year of funding so that the intervention can be packaged and disseminated for use by other practices.
He predicted that the findings would be largely generalizable, given the good mix of practices and patients in the trial. "We don’t think yet it’s broadly applicable to every practice, but at the same time, to practices that use electronic health records," he said, noting that the simple algorithms used in the study draw on data routinely captured in these records.
The findings could have a major impact nationally, given that broad-spectrum antibiotics account for roughly half of the 40 million antibiotic prescriptions written per year for children in the United States for acute respiratory tract infections in the outpatient setting, according to Dr. Gerber. Not only are they more expensive than narrow-spectrum ones, but they also more rapidly promote resistance.
The study’s findings are "very powerful," commented Dr. Liise-Anne Pirofski, moderator of the press briefing and IDWeek Chair. "This is a fairly unique study and probably pretty groundbreaking."
She wondered whether factors such as better taste or greater ease of once-daily dosing of several broad-spectrum antibiotics contribute to off-guideline prescribing and need to be taken into account. "In children, multidosing is difficult because most schools don’t administer antibiotics to children in school," she pointed out. Also, parents sometimes request specific antibiotics.
"These are real issues," and likely driving forces behind at least some off-guideline prescribing, Dr. Gerber agreed. "However, the societies that recommend antibiotics really have to take into account the spectrum of activity to be careful because of the development of antibiotic resistance."
In addition, emerging research suggests that, in at least some cases, more frequent dosing of narrow-spectrum agents is not necessary. For example, the American Academy of Pediatrics now endorses once-daily amoxicillin for treating strep throat. "That used to be dosed more frequently, but studies have shown that once-daily dosing is actually as effective. So that’s helped combat this issue a bit," he said.
Dr. Pirofski noted that it’s nice to see evidence of the benefits of electronic health records, as putting them in place is typically a major undertaking. "It will be very interesting if good studies are done to determine how much that watchdog effect is driving people’s behavior. If it is, then I think the electronic component is absolutely essential because it’s the only way that you can really generate that data and data that people will believe."
That said, face-to-face interaction should not be underestimated in such interventions, she maintained. "The personal interaction, I believe, is really what drove the early success of some of these antibiotic stewardships, because medicine can be very lonely. If somebody comes in and chats you up a little bit, you feel like you are more in tune with what’s going on. ... My own feeling is that personal interaction always drives change better than these other things," said Dr. Pirofski, who is chief of the division of infectious diseases at Albert Einstein College of Medicine, New York.
Ascertaining the cost effectiveness of the intervention would be complicated because of copayment and reimbursement issues, according to Dr. Gerber, but "most of these broad-spectrum agents are between five and ten times more expensive than the narrow-spectrum agents." He predicted that, as insurers move toward a bundled-payment model, costs will get greater attention. "Maybe insurance companies won’t reimburse for broad-spectrum agents if we can show that recommendations should be followed, and there are no differences in outcomes," he said.
The investigators plan to assess the impact of the intervention on health outcomes and will monitor the durability of its efficacy, Dr. Gerber said. "We are going to follow up for at least another year, now that there are no more feedback reports coming in, to see if it continues or if people revert back to their initial prescribing patterns," he explained. In addition, they are interviewing participating clinicians to obtain their viewpoints on prescribing and auditing, along with suggestions for improving the intervention.
Neither Dr. Gerber nor Dr. Pirofski disclosed any relevant conflicts of interest.
SAN DIEGO – An outpatient antimicrobial stewardship intervention dramatically reduced inappropriate use of antibiotics for acute respiratory tract infections in children, judging from the findings of a trial reported at IDWeek.
In the cluster-randomized trial, which involved 185,212 pediatric patients making more than 1.4 million outpatient visits, the rate of inappropriate prescribing for these infections – use of a broad-spectrum antibiotic when guidelines recommended a narrow-spectrum one – fell by nearly half in the intervention group over a year, compared with about one-fifth in the control group.
Led by Dr. Jeffrey Gerber, the investigators enrolled 18 practices in a large pediatric primary care network that share an electronic health record, randomizing them evenly to intervention and control groups.
The intervention had two parts: an on-site clinician education session, including a refresher in current guidelines for treating sinusitis, group A streptococcal pharyngitis, and pneumonia, and then private quarterly audit and feedback reports to physicians of their antibiotic prescribing for these conditions.
The reports "showed how they were prescribing relative to national guidelines at baseline and over time throughout the intervention for 12 months, compared with those in the practice group and across the network," explained Dr. Gerber of Children’s Hospital of Philadelphia. "So the idea is to not only show providers how they prescribe relative to national recommendations, but also to have what we call achievable benchmarks available, to show how other folks in busy practices just like theirs are prescribing."
The rate of off-guideline antibiotic prescribing for acute respiratory tract infections was 28% overall, with a range of 15%-60% across practices, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
One year after the intervention, the rate had fallen by 48% in the intervention group and by 18% in the control group (P = .001).
In stratified analyses, the greatest reduction was seen for pneumonia: The rate of off-guideline prescribing fell by 75% in the intervention group, compared with 6% in the control group.
For acute respiratory tract infections overall, azithromycin was the greatest contributor to off-guideline antibiotic prescribing. For pneumonia specifically, amoxicillin plus clavulanic acid (Augmentin) was the greatest contributor.
"We are really encouraged by these results. We think that it is a relatively simple intervention that we hope will be scalable to other pediatric practices that have electronic health records," Dr. Gerber commented in a related press briefing. The Agency for Healthcare Research and Quality, which funded the study, has provided an extra year of funding so that the intervention can be packaged and disseminated for use by other practices.
He predicted that the findings would be largely generalizable, given the good mix of practices and patients in the trial. "We don’t think yet it’s broadly applicable to every practice, but at the same time, to practices that use electronic health records," he said, noting that the simple algorithms used in the study draw on data routinely captured in these records.
The findings could have a major impact nationally, given that broad-spectrum antibiotics account for roughly half of the 40 million antibiotic prescriptions written per year for children in the United States for acute respiratory tract infections in the outpatient setting, according to Dr. Gerber. Not only are they more expensive than narrow-spectrum ones, but they also more rapidly promote resistance.
The study’s findings are "very powerful," commented Dr. Liise-Anne Pirofski, moderator of the press briefing and IDWeek Chair. "This is a fairly unique study and probably pretty groundbreaking."
She wondered whether factors such as better taste or greater ease of once-daily dosing of several broad-spectrum antibiotics contribute to off-guideline prescribing and need to be taken into account. "In children, multidosing is difficult because most schools don’t administer antibiotics to children in school," she pointed out. Also, parents sometimes request specific antibiotics.
"These are real issues," and likely driving forces behind at least some off-guideline prescribing, Dr. Gerber agreed. "However, the societies that recommend antibiotics really have to take into account the spectrum of activity to be careful because of the development of antibiotic resistance."
In addition, emerging research suggests that, in at least some cases, more frequent dosing of narrow-spectrum agents is not necessary. For example, the American Academy of Pediatrics now endorses once-daily amoxicillin for treating strep throat. "That used to be dosed more frequently, but studies have shown that once-daily dosing is actually as effective. So that’s helped combat this issue a bit," he said.
Dr. Pirofski noted that it’s nice to see evidence of the benefits of electronic health records, as putting them in place is typically a major undertaking. "It will be very interesting if good studies are done to determine how much that watchdog effect is driving people’s behavior. If it is, then I think the electronic component is absolutely essential because it’s the only way that you can really generate that data and data that people will believe."
That said, face-to-face interaction should not be underestimated in such interventions, she maintained. "The personal interaction, I believe, is really what drove the early success of some of these antibiotic stewardships, because medicine can be very lonely. If somebody comes in and chats you up a little bit, you feel like you are more in tune with what’s going on. ... My own feeling is that personal interaction always drives change better than these other things," said Dr. Pirofski, who is chief of the division of infectious diseases at Albert Einstein College of Medicine, New York.
Ascertaining the cost effectiveness of the intervention would be complicated because of copayment and reimbursement issues, according to Dr. Gerber, but "most of these broad-spectrum agents are between five and ten times more expensive than the narrow-spectrum agents." He predicted that, as insurers move toward a bundled-payment model, costs will get greater attention. "Maybe insurance companies won’t reimburse for broad-spectrum agents if we can show that recommendations should be followed, and there are no differences in outcomes," he said.
The investigators plan to assess the impact of the intervention on health outcomes and will monitor the durability of its efficacy, Dr. Gerber said. "We are going to follow up for at least another year, now that there are no more feedback reports coming in, to see if it continues or if people revert back to their initial prescribing patterns," he explained. In addition, they are interviewing participating clinicians to obtain their viewpoints on prescribing and auditing, along with suggestions for improving the intervention.
Neither Dr. Gerber nor Dr. Pirofski disclosed any relevant conflicts of interest.
AT IDWEEK
Major Finding: There was a greater reduction in the rate of off-guideline prescribing of antibiotics for acute respiratory tract infections in the intervention group than in the control group (48% vs. 18%).
Data Source: This finding came from a cluster-randomized trial of 185,212 patients making more than 1.4 million outpatient visits.
Disclosures: Neither Dr. Gerber nor Dr. Pirofski disclosed any relevant conflicts of interest.
Ablation Therapy for AF Effective Despite Isolated Diastolic Dysfunction
SEATTLE – Isolated left ventricular diastolic dysfunction should not be viewed as a reason to withhold ablation therapy in patients with atrial fibrillation and preserved ejection fraction, new data suggest.
Investigators led by Dr. Rosita Zakeri studied a series of 707 patients who were undergoing a first catheter ablation at the Mayo Clinic for symptomatic, drug-refractory atrial fibrillation and who had preserved left ventricular ejection fraction.
Fully 34% had isolated diastolic dysfunction (a left atrial pressure of greater than 15 mm as measured directly by the transseptal approach), according to results reported at the annual meeting of the Heart Failure Society of America.
Patients with isolated diastolic dysfunction had a marginally elevated risk of recurrence of atrial fibrillation at 1 year in a univariate analysis but not after multivariate adjustment. In addition, they derived a similar improvement in quality of life from ablation.
"Although diastolic dysfunction was associated with an increased risk of atrial fibrillation recurrence, in and of itself, it does not appear to be a key arrhythmogenic factor nor strongly related to quality of life at 1 year after ablation," commented Dr. Zakeri, who is a cardiology fellow at the Mayo Clinic in Rochester, Minn.
"Therefore ... the presence of left ventricular diastolic dysfunction should not necessarily discourage the use of catheter ablation for treatment of symptomatic atrial fibrillation in this patient group," she said.
Diastolic dysfunction increases susceptibility to atrial fibrillation, but it is unclear whether it has a continued proarrhythmic effect after ablation, Dr. Zakeri noted.
"We also know that diastolic dysfunction predisposes to heart failure and is one of the hemodynamic hallmarks of heart failure with preserved ejection fraction," she continued. And some patients with atrial fibrillation having diastolic dysfunction may have early heart failure, with the arrhythmia accelerating the severity or presentation of symptoms.
Study results showed that relative to other patients, the patients with isolated diastolic dysfunction had a higher body mass index; had greater prevalences of diabetes, hypertension, and heart failure; were more likely to have nonparoxysmal atrial fibrillation; and had poorer quality of life as assessed with both the Medical Outcomes Study 36-item questionnaire (SF-36) and the Mayo AF-specific Symptom Inventory (MAFSI).
Overall, 21.1% of patients had a recurrence of atrial fibrillation at 1 year, Dr. Zakeri reported. The rate was 25.3% in patients with diastolic dysfunction and 18.9% in patients without it.
The difference corresponded to a marginally elevated risk of recurrence for the group with diastolic dysfunction in unadjusted analyses (odds ratio, 1.45; P = .05), but after adjustment for age, sex, type of atrial fibrillation, and left atrial volume index, there was no significant association.
When patients were stratified by baseline left atrial pressure – less than 12 mm Hg, 12-15 mm Hg, or greater than 15 mm Hg – all three groups had significant improvements at 1 year in scores on the quality of life scales, with no significant difference in the magnitude of improvement between them.
Dr. Zakeri disclosed no relevant conflicts of interest.
SEATTLE – Isolated left ventricular diastolic dysfunction should not be viewed as a reason to withhold ablation therapy in patients with atrial fibrillation and preserved ejection fraction, new data suggest.
Investigators led by Dr. Rosita Zakeri studied a series of 707 patients who were undergoing a first catheter ablation at the Mayo Clinic for symptomatic, drug-refractory atrial fibrillation and who had preserved left ventricular ejection fraction.
Fully 34% had isolated diastolic dysfunction (a left atrial pressure of greater than 15 mm as measured directly by the transseptal approach), according to results reported at the annual meeting of the Heart Failure Society of America.
Patients with isolated diastolic dysfunction had a marginally elevated risk of recurrence of atrial fibrillation at 1 year in a univariate analysis but not after multivariate adjustment. In addition, they derived a similar improvement in quality of life from ablation.
"Although diastolic dysfunction was associated with an increased risk of atrial fibrillation recurrence, in and of itself, it does not appear to be a key arrhythmogenic factor nor strongly related to quality of life at 1 year after ablation," commented Dr. Zakeri, who is a cardiology fellow at the Mayo Clinic in Rochester, Minn.
"Therefore ... the presence of left ventricular diastolic dysfunction should not necessarily discourage the use of catheter ablation for treatment of symptomatic atrial fibrillation in this patient group," she said.
Diastolic dysfunction increases susceptibility to atrial fibrillation, but it is unclear whether it has a continued proarrhythmic effect after ablation, Dr. Zakeri noted.
"We also know that diastolic dysfunction predisposes to heart failure and is one of the hemodynamic hallmarks of heart failure with preserved ejection fraction," she continued. And some patients with atrial fibrillation having diastolic dysfunction may have early heart failure, with the arrhythmia accelerating the severity or presentation of symptoms.
Study results showed that relative to other patients, the patients with isolated diastolic dysfunction had a higher body mass index; had greater prevalences of diabetes, hypertension, and heart failure; were more likely to have nonparoxysmal atrial fibrillation; and had poorer quality of life as assessed with both the Medical Outcomes Study 36-item questionnaire (SF-36) and the Mayo AF-specific Symptom Inventory (MAFSI).
Overall, 21.1% of patients had a recurrence of atrial fibrillation at 1 year, Dr. Zakeri reported. The rate was 25.3% in patients with diastolic dysfunction and 18.9% in patients without it.
The difference corresponded to a marginally elevated risk of recurrence for the group with diastolic dysfunction in unadjusted analyses (odds ratio, 1.45; P = .05), but after adjustment for age, sex, type of atrial fibrillation, and left atrial volume index, there was no significant association.
When patients were stratified by baseline left atrial pressure – less than 12 mm Hg, 12-15 mm Hg, or greater than 15 mm Hg – all three groups had significant improvements at 1 year in scores on the quality of life scales, with no significant difference in the magnitude of improvement between them.
Dr. Zakeri disclosed no relevant conflicts of interest.
SEATTLE – Isolated left ventricular diastolic dysfunction should not be viewed as a reason to withhold ablation therapy in patients with atrial fibrillation and preserved ejection fraction, new data suggest.
Investigators led by Dr. Rosita Zakeri studied a series of 707 patients who were undergoing a first catheter ablation at the Mayo Clinic for symptomatic, drug-refractory atrial fibrillation and who had preserved left ventricular ejection fraction.
Fully 34% had isolated diastolic dysfunction (a left atrial pressure of greater than 15 mm as measured directly by the transseptal approach), according to results reported at the annual meeting of the Heart Failure Society of America.
Patients with isolated diastolic dysfunction had a marginally elevated risk of recurrence of atrial fibrillation at 1 year in a univariate analysis but not after multivariate adjustment. In addition, they derived a similar improvement in quality of life from ablation.
"Although diastolic dysfunction was associated with an increased risk of atrial fibrillation recurrence, in and of itself, it does not appear to be a key arrhythmogenic factor nor strongly related to quality of life at 1 year after ablation," commented Dr. Zakeri, who is a cardiology fellow at the Mayo Clinic in Rochester, Minn.
"Therefore ... the presence of left ventricular diastolic dysfunction should not necessarily discourage the use of catheter ablation for treatment of symptomatic atrial fibrillation in this patient group," she said.
Diastolic dysfunction increases susceptibility to atrial fibrillation, but it is unclear whether it has a continued proarrhythmic effect after ablation, Dr. Zakeri noted.
"We also know that diastolic dysfunction predisposes to heart failure and is one of the hemodynamic hallmarks of heart failure with preserved ejection fraction," she continued. And some patients with atrial fibrillation having diastolic dysfunction may have early heart failure, with the arrhythmia accelerating the severity or presentation of symptoms.
Study results showed that relative to other patients, the patients with isolated diastolic dysfunction had a higher body mass index; had greater prevalences of diabetes, hypertension, and heart failure; were more likely to have nonparoxysmal atrial fibrillation; and had poorer quality of life as assessed with both the Medical Outcomes Study 36-item questionnaire (SF-36) and the Mayo AF-specific Symptom Inventory (MAFSI).
Overall, 21.1% of patients had a recurrence of atrial fibrillation at 1 year, Dr. Zakeri reported. The rate was 25.3% in patients with diastolic dysfunction and 18.9% in patients without it.
The difference corresponded to a marginally elevated risk of recurrence for the group with diastolic dysfunction in unadjusted analyses (odds ratio, 1.45; P = .05), but after adjustment for age, sex, type of atrial fibrillation, and left atrial volume index, there was no significant association.
When patients were stratified by baseline left atrial pressure – less than 12 mm Hg, 12-15 mm Hg, or greater than 15 mm Hg – all three groups had significant improvements at 1 year in scores on the quality of life scales, with no significant difference in the magnitude of improvement between them.
Dr. Zakeri disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Major Finding: Isolated diastolic dysfunction did not independently increase the risk of recurrence of atrial fibrillation after catheter ablation or diminish improvements in quality of life.
Data Source: This series involved 707 patients with preserved ejection fraction who were undergoing a first catheter ablation for symptomatic atrial fibrillation.
Disclosures: Dr. Zakeri disclosed no relevant conflicts of interest.
Spironolactone's Heart Failure Benefit Outweighs Hyperkalemia Risk
SEATTLE – Patients with heart failure still benefit from spironolactone therapy even when they develop elevated potassium levels, a well-known and potentially life-threatening side effect of the medication, new data suggest.
Investigators led by Orly Vardeny, Pharm.D., conducted a secondary analysis of data from the RALES (Randomized Aldactone Evaluation Study) trial, in which more than 1,500 patients with heart failure were assigned to spironolactone (Aldactone) or placebo.
The spironolactone group had nearly quadruple the risk of hyperkalemia compared with the placebo group, she reported at the annual meeting of the Heart Failure Society of America. And hyperkalemia was indeed associated with a higher mortality rate.
But relative to their placebo-treated counterparts who did not have elevated potassium levels, spironolactone-treated patients who had elevated potassium levels, up to a serum potassium level of at least 5.5 mEq/L, were still less likely to die.
"The benefits of spironolactone are maintained in patients with mild to moderate hyperkalemia, and even in patients with potassium levels approaching 6.0 mEq/L," commented Dr. Vardeny, who is an associate professor of pharmacy at the University of Wisconsin in Madison.
"Our data argue for strategies that maintain spironolactone or aldosterone antagonist therapy even in the setting of mild to moderate hyperkalemia," she maintained.
"How many of these patients were on digoxin, and did you factor that into the analysis?" asked session comoderator Dr. Carl V. Leier of the Ohio State University Medical Center in Columbus.
Dr. Vardeny replied that analyses did not adjust for digoxin use, but they did adjust for age, estimated glomerular filtration rate, baseline potassium level, and diabetes.
"It seems that hypokalemia was more dangerous than hyperkalemia," commented attendee Dr. Edward Gilbert of the University of Utah in Salt Lake City. Might some of the benefit of spironolactone actually be related to reduced hypokalemia, he wondered.
"My personal bias is that there could be an association. This is really difficult to prove, only because potassium will vary so much, so the data will inform multiple categories of potassium," Dr. Vardeny replied. "But yes, we are investigating that as well."
The 1,663 patients in RALES had moderate to severe heart failure with a left ventricular ejection fraction of less than 35%, and were already receiving an angiotensin-converting enzyme inhibitor with or without a diuretic. They were randomized evenly to 25 mg of spironolactone daily, with optional titration up to 50 mg daily or placebo.
Serum potassium levels were monitored regularly. If patients developed hyperkalemia, investigators could reduce the dose of study medication but were encouraged to first adjust doses of other medications.
Main RALES results, previously reported, showed that spironolactone treatment was associated with a 30% reduction in the risk of all-cause mortality and a 35% reduction in the risk of hospitalization for worsening of heart failure (N. Engl. J. Med. 1999;341:709-17).
The new analysis showed that both treatment groups had mean potassium levels of 4.3 mEq/L at baseline. But levels increased within the first month in the spironolactone group and remained persistently higher than those in the placebo group, by 0.25 mEq/L on average, throughout the trial.
Relative to their peers in the placebo group, patients in the spironolactone group were significantly less likely to develop hypokalemia (6.5% vs. 16.2%) and more likely to develop hyperkalemia (14.5% vs. 4.2%), Dr. Vardeny reported. Hypokalemia was defined as a potassium level of less than 3.5 mEq/L; hyperkalemia equalled a potassium level of greater than 5.5 mEq/L.
In the spironolactone group, the rate of death was fairly stable – at about 17 per 100 patient-years – for patients with potassium levels ranging from 3.5 to 6.0 mEq/L. But it was higher among patients with lower levels (about 42 per 100 patient-years) or higher levels (about 25 per 100 patient-years).
In a multivariate analysis, patients were more likely to develop hypokalemia if they were black (hazard ratio, 2.0) or had a higher New York Heart Association functional class (HR, 1.5), whereas they were less likely if they had a higher baseline potassium level (HR, 0.25) or received spironolactone (HR, 0.36).
Patients were more likely to develop hyperkalemia if they received spironolactone (HR, 3.7), had a higher baseline potassium level (HR, 2.4), or had a history of diabetes (HR, 1.9).
Compared with placebo-treated patients having potassium levels of less than 5.0 mEq/L, spironolactone-treated patients with elevated potassium levels still had a significantly lower risk of death with potassium levels up to 5.5 mEq/L, and they had a nonsignificantly lower risk with levels up to about 6.0 mEq/L.
Dr. Vardeny reported having no relevant conflicts of interest. The RALES trial was sponsored by Searle, manufacturer of Aldactone.
SEATTLE – Patients with heart failure still benefit from spironolactone therapy even when they develop elevated potassium levels, a well-known and potentially life-threatening side effect of the medication, new data suggest.
Investigators led by Orly Vardeny, Pharm.D., conducted a secondary analysis of data from the RALES (Randomized Aldactone Evaluation Study) trial, in which more than 1,500 patients with heart failure were assigned to spironolactone (Aldactone) or placebo.
The spironolactone group had nearly quadruple the risk of hyperkalemia compared with the placebo group, she reported at the annual meeting of the Heart Failure Society of America. And hyperkalemia was indeed associated with a higher mortality rate.
But relative to their placebo-treated counterparts who did not have elevated potassium levels, spironolactone-treated patients who had elevated potassium levels, up to a serum potassium level of at least 5.5 mEq/L, were still less likely to die.
"The benefits of spironolactone are maintained in patients with mild to moderate hyperkalemia, and even in patients with potassium levels approaching 6.0 mEq/L," commented Dr. Vardeny, who is an associate professor of pharmacy at the University of Wisconsin in Madison.
"Our data argue for strategies that maintain spironolactone or aldosterone antagonist therapy even in the setting of mild to moderate hyperkalemia," she maintained.
"How many of these patients were on digoxin, and did you factor that into the analysis?" asked session comoderator Dr. Carl V. Leier of the Ohio State University Medical Center in Columbus.
Dr. Vardeny replied that analyses did not adjust for digoxin use, but they did adjust for age, estimated glomerular filtration rate, baseline potassium level, and diabetes.
"It seems that hypokalemia was more dangerous than hyperkalemia," commented attendee Dr. Edward Gilbert of the University of Utah in Salt Lake City. Might some of the benefit of spironolactone actually be related to reduced hypokalemia, he wondered.
"My personal bias is that there could be an association. This is really difficult to prove, only because potassium will vary so much, so the data will inform multiple categories of potassium," Dr. Vardeny replied. "But yes, we are investigating that as well."
The 1,663 patients in RALES had moderate to severe heart failure with a left ventricular ejection fraction of less than 35%, and were already receiving an angiotensin-converting enzyme inhibitor with or without a diuretic. They were randomized evenly to 25 mg of spironolactone daily, with optional titration up to 50 mg daily or placebo.
Serum potassium levels were monitored regularly. If patients developed hyperkalemia, investigators could reduce the dose of study medication but were encouraged to first adjust doses of other medications.
Main RALES results, previously reported, showed that spironolactone treatment was associated with a 30% reduction in the risk of all-cause mortality and a 35% reduction in the risk of hospitalization for worsening of heart failure (N. Engl. J. Med. 1999;341:709-17).
The new analysis showed that both treatment groups had mean potassium levels of 4.3 mEq/L at baseline. But levels increased within the first month in the spironolactone group and remained persistently higher than those in the placebo group, by 0.25 mEq/L on average, throughout the trial.
Relative to their peers in the placebo group, patients in the spironolactone group were significantly less likely to develop hypokalemia (6.5% vs. 16.2%) and more likely to develop hyperkalemia (14.5% vs. 4.2%), Dr. Vardeny reported. Hypokalemia was defined as a potassium level of less than 3.5 mEq/L; hyperkalemia equalled a potassium level of greater than 5.5 mEq/L.
In the spironolactone group, the rate of death was fairly stable – at about 17 per 100 patient-years – for patients with potassium levels ranging from 3.5 to 6.0 mEq/L. But it was higher among patients with lower levels (about 42 per 100 patient-years) or higher levels (about 25 per 100 patient-years).
In a multivariate analysis, patients were more likely to develop hypokalemia if they were black (hazard ratio, 2.0) or had a higher New York Heart Association functional class (HR, 1.5), whereas they were less likely if they had a higher baseline potassium level (HR, 0.25) or received spironolactone (HR, 0.36).
Patients were more likely to develop hyperkalemia if they received spironolactone (HR, 3.7), had a higher baseline potassium level (HR, 2.4), or had a history of diabetes (HR, 1.9).
Compared with placebo-treated patients having potassium levels of less than 5.0 mEq/L, spironolactone-treated patients with elevated potassium levels still had a significantly lower risk of death with potassium levels up to 5.5 mEq/L, and they had a nonsignificantly lower risk with levels up to about 6.0 mEq/L.
Dr. Vardeny reported having no relevant conflicts of interest. The RALES trial was sponsored by Searle, manufacturer of Aldactone.
SEATTLE – Patients with heart failure still benefit from spironolactone therapy even when they develop elevated potassium levels, a well-known and potentially life-threatening side effect of the medication, new data suggest.
Investigators led by Orly Vardeny, Pharm.D., conducted a secondary analysis of data from the RALES (Randomized Aldactone Evaluation Study) trial, in which more than 1,500 patients with heart failure were assigned to spironolactone (Aldactone) or placebo.
The spironolactone group had nearly quadruple the risk of hyperkalemia compared with the placebo group, she reported at the annual meeting of the Heart Failure Society of America. And hyperkalemia was indeed associated with a higher mortality rate.
But relative to their placebo-treated counterparts who did not have elevated potassium levels, spironolactone-treated patients who had elevated potassium levels, up to a serum potassium level of at least 5.5 mEq/L, were still less likely to die.
"The benefits of spironolactone are maintained in patients with mild to moderate hyperkalemia, and even in patients with potassium levels approaching 6.0 mEq/L," commented Dr. Vardeny, who is an associate professor of pharmacy at the University of Wisconsin in Madison.
"Our data argue for strategies that maintain spironolactone or aldosterone antagonist therapy even in the setting of mild to moderate hyperkalemia," she maintained.
"How many of these patients were on digoxin, and did you factor that into the analysis?" asked session comoderator Dr. Carl V. Leier of the Ohio State University Medical Center in Columbus.
Dr. Vardeny replied that analyses did not adjust for digoxin use, but they did adjust for age, estimated glomerular filtration rate, baseline potassium level, and diabetes.
"It seems that hypokalemia was more dangerous than hyperkalemia," commented attendee Dr. Edward Gilbert of the University of Utah in Salt Lake City. Might some of the benefit of spironolactone actually be related to reduced hypokalemia, he wondered.
"My personal bias is that there could be an association. This is really difficult to prove, only because potassium will vary so much, so the data will inform multiple categories of potassium," Dr. Vardeny replied. "But yes, we are investigating that as well."
The 1,663 patients in RALES had moderate to severe heart failure with a left ventricular ejection fraction of less than 35%, and were already receiving an angiotensin-converting enzyme inhibitor with or without a diuretic. They were randomized evenly to 25 mg of spironolactone daily, with optional titration up to 50 mg daily or placebo.
Serum potassium levels were monitored regularly. If patients developed hyperkalemia, investigators could reduce the dose of study medication but were encouraged to first adjust doses of other medications.
Main RALES results, previously reported, showed that spironolactone treatment was associated with a 30% reduction in the risk of all-cause mortality and a 35% reduction in the risk of hospitalization for worsening of heart failure (N. Engl. J. Med. 1999;341:709-17).
The new analysis showed that both treatment groups had mean potassium levels of 4.3 mEq/L at baseline. But levels increased within the first month in the spironolactone group and remained persistently higher than those in the placebo group, by 0.25 mEq/L on average, throughout the trial.
Relative to their peers in the placebo group, patients in the spironolactone group were significantly less likely to develop hypokalemia (6.5% vs. 16.2%) and more likely to develop hyperkalemia (14.5% vs. 4.2%), Dr. Vardeny reported. Hypokalemia was defined as a potassium level of less than 3.5 mEq/L; hyperkalemia equalled a potassium level of greater than 5.5 mEq/L.
In the spironolactone group, the rate of death was fairly stable – at about 17 per 100 patient-years – for patients with potassium levels ranging from 3.5 to 6.0 mEq/L. But it was higher among patients with lower levels (about 42 per 100 patient-years) or higher levels (about 25 per 100 patient-years).
In a multivariate analysis, patients were more likely to develop hypokalemia if they were black (hazard ratio, 2.0) or had a higher New York Heart Association functional class (HR, 1.5), whereas they were less likely if they had a higher baseline potassium level (HR, 0.25) or received spironolactone (HR, 0.36).
Patients were more likely to develop hyperkalemia if they received spironolactone (HR, 3.7), had a higher baseline potassium level (HR, 2.4), or had a history of diabetes (HR, 1.9).
Compared with placebo-treated patients having potassium levels of less than 5.0 mEq/L, spironolactone-treated patients with elevated potassium levels still had a significantly lower risk of death with potassium levels up to 5.5 mEq/L, and they had a nonsignificantly lower risk with levels up to about 6.0 mEq/L.
Dr. Vardeny reported having no relevant conflicts of interest. The RALES trial was sponsored by Searle, manufacturer of Aldactone.
AT THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Major Finding: Patients receiving spironolactone were more likely to develop hyperkalemia than were those on placebo (14.5% vs. 4.2%), but still had a net reduction in the risk of death with potassium levels up to at least 5.5 mEq/L.
Data Source: This was an analysis performed on data from 1,663 patients with heart failure in a randomized trial of spironolactone vs. placebo (the RALES trial).
Disclosures: Dr. Vardeny disclosed no relevant conflicts of interest. The RALES trial was sponsored by Searle, manufacturer of Aldactone.
Agent Shunts Fluid Overload Via GI Tract
SEATTLE – An investigational agent that eliminates excess fluid through the gastrointestinal tract appears promising for treatment in patients with heart failure–related fluid overload who also have chronic kidney disease, new data suggest.
In a phase II trial among 111 such patients who were starting spironolactone, the new agent – known as cross-linked polyelectrolyte (CLP) – did not improve serum potassium levels when compared with placebo, the trial’s primary end point, investigators reported at the annual meeting of the Heart Failure Society of America. However, it did improve body weight and New York Heart Association (NYHA) functional class.
The CLP group had a higher rate of gastrointestinal adverse effects. Additionally, the mortality rate was about 7% with the medication, compared with 0% with placebo. None of the deaths appeared to be related to treatment, according to the study’s lead investigator, Dr. Barry M. Massie, chief of the cardiology division at the San Francisco Veterans Affairs Medical Center.
"Use of CLP in the gastrointestinal tract may be an effective means for removal of sodium and water in heart failure patients already treated with guideline therapy including diuretics," said Dr. Massie. "Such extrarenal removal of fluid appears to result in meaningful improvements in function and symptoms in these patients, and warrants further investigation."
"An adequately powered trial is needed both to confirm the efficacy and further evaluate safety of the compound," he added.
Dr. Stephen S. Gottlieb of the University of Maryland, Baltimore, who was invited to discuss the trial, said that it addressed two critical issues in this population: hyperkalemia (a major barrier to using and adequately titrating cardiovascular medications) and fluid status (an ongoing problem as existing diuretics have known limitations).
"Unfortunately, CLP did not prevent hyperkalemia, and we clearly need ways to safely control potassium," he said. "We know that CLP caused sustained fluid loss. What we don’t know is how that compares with other ways of removing fluid: Would patients have done as well if they were given loop diuretics? Clearly, if we are going to address this issue, we have to decide whether, as compared to loop diuretics, is it really more efficacious, is it really safer, and does it lead to better outcomes."
The trial was conducted at 24 sites in Armenia, Georgia, and Moldova. Patients were eligible if they had heart failure with New York Heart Association class III or IV plus chronic kidney disease, and had recently been hospitalized for decompensation associated with fluid overload.
They were randomized to double-blind treatment with CLP (manufactured by Sorbent Therapeutics) or placebo for 8 weeks. Patients in both groups were also started on spironolactone.
CLP is an orally administered superabsorbent polymer that is neither metabolized nor absorbed, explained Dr. Massie, who also is professor of medicine at the University of California, San Francisco. It binds cations and water, and is excreted in stool.
Trial results showed that changes in serum potassium levels from baseline did not differ significantly between treatment groups. Also, the rate of trial discontinuation due to hyperkalemia was about 10% in each group.
However, patients in the CLP group had a significant reduction in body weight relative to their counterparts in the placebo group at weeks 1 and 2, and there was a trend toward a greater reduction over the entire 8 weeks (P = .065).
The CLP group was more likely to rate their dyspnea as moderately or markedly better (37% vs. 22%). According to physician ratings, the proportion having marked or disabling dyspnea on exertion fell in both groups over time, but to a greater extent among those in the CLP group.
Patients in the CLP group were also more likely to have an improvement of at least one NYHA class from baseline (49% vs. 17%, P = .0018).
Both groups had better 6-minute walk test results at 8 weeks, but the magnitude of improvement was 24% with CLP vs. 14% with placebo, corresponding to an absolute difference of 20 meters (P = .07). CLP also was associated with better scores on the Kansas City Cardiomyopathy Questionnaire.
In terms of safety, there were no clinically important changes in various ions or in serum aldosterone levels, according to Dr. Massie.
The overall rate of adverse events was 36% in the CLP group and 31% in the placebo group, a difference largely caused by a higher rate of gastrointestinal events (mainly abdominal discomfort) in the former (24% vs. 14%).
There were four deaths, all in the CLP group. However, there did not appear to be any common pattern, and investigators did not judge any deaths to be related to the study medication, according to Dr. Massie.
Yet, the higher death rate with CLP "is certainly something to think about down the road," he acknowledged. "Obviously, as we proceed with clinical trials, we will try to decipher that and hope that it is a fluke and not an effect of the drug," he said.
Dr. Massie disclosed that he is a consultant to Sorbent Therapeutics, which sponsored the trial. Dr. Gottlieb disclosed no relevant conflicts of interest.
SEATTLE – An investigational agent that eliminates excess fluid through the gastrointestinal tract appears promising for treatment in patients with heart failure–related fluid overload who also have chronic kidney disease, new data suggest.
In a phase II trial among 111 such patients who were starting spironolactone, the new agent – known as cross-linked polyelectrolyte (CLP) – did not improve serum potassium levels when compared with placebo, the trial’s primary end point, investigators reported at the annual meeting of the Heart Failure Society of America. However, it did improve body weight and New York Heart Association (NYHA) functional class.
The CLP group had a higher rate of gastrointestinal adverse effects. Additionally, the mortality rate was about 7% with the medication, compared with 0% with placebo. None of the deaths appeared to be related to treatment, according to the study’s lead investigator, Dr. Barry M. Massie, chief of the cardiology division at the San Francisco Veterans Affairs Medical Center.
"Use of CLP in the gastrointestinal tract may be an effective means for removal of sodium and water in heart failure patients already treated with guideline therapy including diuretics," said Dr. Massie. "Such extrarenal removal of fluid appears to result in meaningful improvements in function and symptoms in these patients, and warrants further investigation."
"An adequately powered trial is needed both to confirm the efficacy and further evaluate safety of the compound," he added.
Dr. Stephen S. Gottlieb of the University of Maryland, Baltimore, who was invited to discuss the trial, said that it addressed two critical issues in this population: hyperkalemia (a major barrier to using and adequately titrating cardiovascular medications) and fluid status (an ongoing problem as existing diuretics have known limitations).
"Unfortunately, CLP did not prevent hyperkalemia, and we clearly need ways to safely control potassium," he said. "We know that CLP caused sustained fluid loss. What we don’t know is how that compares with other ways of removing fluid: Would patients have done as well if they were given loop diuretics? Clearly, if we are going to address this issue, we have to decide whether, as compared to loop diuretics, is it really more efficacious, is it really safer, and does it lead to better outcomes."
The trial was conducted at 24 sites in Armenia, Georgia, and Moldova. Patients were eligible if they had heart failure with New York Heart Association class III or IV plus chronic kidney disease, and had recently been hospitalized for decompensation associated with fluid overload.
They were randomized to double-blind treatment with CLP (manufactured by Sorbent Therapeutics) or placebo for 8 weeks. Patients in both groups were also started on spironolactone.
CLP is an orally administered superabsorbent polymer that is neither metabolized nor absorbed, explained Dr. Massie, who also is professor of medicine at the University of California, San Francisco. It binds cations and water, and is excreted in stool.
Trial results showed that changes in serum potassium levels from baseline did not differ significantly between treatment groups. Also, the rate of trial discontinuation due to hyperkalemia was about 10% in each group.
However, patients in the CLP group had a significant reduction in body weight relative to their counterparts in the placebo group at weeks 1 and 2, and there was a trend toward a greater reduction over the entire 8 weeks (P = .065).
The CLP group was more likely to rate their dyspnea as moderately or markedly better (37% vs. 22%). According to physician ratings, the proportion having marked or disabling dyspnea on exertion fell in both groups over time, but to a greater extent among those in the CLP group.
Patients in the CLP group were also more likely to have an improvement of at least one NYHA class from baseline (49% vs. 17%, P = .0018).
Both groups had better 6-minute walk test results at 8 weeks, but the magnitude of improvement was 24% with CLP vs. 14% with placebo, corresponding to an absolute difference of 20 meters (P = .07). CLP also was associated with better scores on the Kansas City Cardiomyopathy Questionnaire.
In terms of safety, there were no clinically important changes in various ions or in serum aldosterone levels, according to Dr. Massie.
The overall rate of adverse events was 36% in the CLP group and 31% in the placebo group, a difference largely caused by a higher rate of gastrointestinal events (mainly abdominal discomfort) in the former (24% vs. 14%).
There were four deaths, all in the CLP group. However, there did not appear to be any common pattern, and investigators did not judge any deaths to be related to the study medication, according to Dr. Massie.
Yet, the higher death rate with CLP "is certainly something to think about down the road," he acknowledged. "Obviously, as we proceed with clinical trials, we will try to decipher that and hope that it is a fluke and not an effect of the drug," he said.
Dr. Massie disclosed that he is a consultant to Sorbent Therapeutics, which sponsored the trial. Dr. Gottlieb disclosed no relevant conflicts of interest.
SEATTLE – An investigational agent that eliminates excess fluid through the gastrointestinal tract appears promising for treatment in patients with heart failure–related fluid overload who also have chronic kidney disease, new data suggest.
In a phase II trial among 111 such patients who were starting spironolactone, the new agent – known as cross-linked polyelectrolyte (CLP) – did not improve serum potassium levels when compared with placebo, the trial’s primary end point, investigators reported at the annual meeting of the Heart Failure Society of America. However, it did improve body weight and New York Heart Association (NYHA) functional class.
The CLP group had a higher rate of gastrointestinal adverse effects. Additionally, the mortality rate was about 7% with the medication, compared with 0% with placebo. None of the deaths appeared to be related to treatment, according to the study’s lead investigator, Dr. Barry M. Massie, chief of the cardiology division at the San Francisco Veterans Affairs Medical Center.
"Use of CLP in the gastrointestinal tract may be an effective means for removal of sodium and water in heart failure patients already treated with guideline therapy including diuretics," said Dr. Massie. "Such extrarenal removal of fluid appears to result in meaningful improvements in function and symptoms in these patients, and warrants further investigation."
"An adequately powered trial is needed both to confirm the efficacy and further evaluate safety of the compound," he added.
Dr. Stephen S. Gottlieb of the University of Maryland, Baltimore, who was invited to discuss the trial, said that it addressed two critical issues in this population: hyperkalemia (a major barrier to using and adequately titrating cardiovascular medications) and fluid status (an ongoing problem as existing diuretics have known limitations).
"Unfortunately, CLP did not prevent hyperkalemia, and we clearly need ways to safely control potassium," he said. "We know that CLP caused sustained fluid loss. What we don’t know is how that compares with other ways of removing fluid: Would patients have done as well if they were given loop diuretics? Clearly, if we are going to address this issue, we have to decide whether, as compared to loop diuretics, is it really more efficacious, is it really safer, and does it lead to better outcomes."
The trial was conducted at 24 sites in Armenia, Georgia, and Moldova. Patients were eligible if they had heart failure with New York Heart Association class III or IV plus chronic kidney disease, and had recently been hospitalized for decompensation associated with fluid overload.
They were randomized to double-blind treatment with CLP (manufactured by Sorbent Therapeutics) or placebo for 8 weeks. Patients in both groups were also started on spironolactone.
CLP is an orally administered superabsorbent polymer that is neither metabolized nor absorbed, explained Dr. Massie, who also is professor of medicine at the University of California, San Francisco. It binds cations and water, and is excreted in stool.
Trial results showed that changes in serum potassium levels from baseline did not differ significantly between treatment groups. Also, the rate of trial discontinuation due to hyperkalemia was about 10% in each group.
However, patients in the CLP group had a significant reduction in body weight relative to their counterparts in the placebo group at weeks 1 and 2, and there was a trend toward a greater reduction over the entire 8 weeks (P = .065).
The CLP group was more likely to rate their dyspnea as moderately or markedly better (37% vs. 22%). According to physician ratings, the proportion having marked or disabling dyspnea on exertion fell in both groups over time, but to a greater extent among those in the CLP group.
Patients in the CLP group were also more likely to have an improvement of at least one NYHA class from baseline (49% vs. 17%, P = .0018).
Both groups had better 6-minute walk test results at 8 weeks, but the magnitude of improvement was 24% with CLP vs. 14% with placebo, corresponding to an absolute difference of 20 meters (P = .07). CLP also was associated with better scores on the Kansas City Cardiomyopathy Questionnaire.
In terms of safety, there were no clinically important changes in various ions or in serum aldosterone levels, according to Dr. Massie.
The overall rate of adverse events was 36% in the CLP group and 31% in the placebo group, a difference largely caused by a higher rate of gastrointestinal events (mainly abdominal discomfort) in the former (24% vs. 14%).
There were four deaths, all in the CLP group. However, there did not appear to be any common pattern, and investigators did not judge any deaths to be related to the study medication, according to Dr. Massie.
Yet, the higher death rate with CLP "is certainly something to think about down the road," he acknowledged. "Obviously, as we proceed with clinical trials, we will try to decipher that and hope that it is a fluke and not an effect of the drug," he said.
Dr. Massie disclosed that he is a consultant to Sorbent Therapeutics, which sponsored the trial. Dr. Gottlieb disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Major Finding: Compared with placebo, CLP was associated with improvements in body weight and New York Heart Association functional class, but not serum potassium levels.
Data Source: A randomized phase II trial among 111 patients experiencing heart failure with fluid overload and chronic kidney disease.
Disclosures: Dr. Massie disclosed that he is a consultant to Sorbent Therapeutics. Dr. Gottlieb disclosed no relevant conflicts of interest. The trial was sponsored by Sorbent Therapeutics.
Heart Failure Hospitalization, Deaths Decline with Depression Intervention
SEATTLE – A brief cognitive biobehavioral intervention focused on preventing and managing depression reduces adverse outcomes of heart failure, according to results from a randomized trial reported at the annual meeting of the Heart Failure Society of America.
"A 6-week biobehavioral intervention in patients with heart failure really was successful in severing the link between depression and poor outcomes," according to the study’s lead investigator, Debra K. Moser, D.N.Sc., professor and chair of the College of Nursing at the University of Kentucky, Lexington. "These findings provide evidence for the effectiveness of biobehavioral approaches" in this population.
Dr. Moser also noted that more than one-fifth of patients with heart failure have depression, as well as higher depressive symptoms – not necessarily clinical depression. Evidence suggests that in the heart failure population, such symptoms triple the risk of rehospitalization and double the risk of death (J. Am. Coll. Cardiol. 2006;48:1527-37).
Dr. Moser and her colleagues studied 278 patients with heart failure, assigning them to a biobehavioral intervention group (given both cognitive-behavioral therapy and biofeedback-relaxation therapy), an attention control group, or a usual-care control group. At baseline, patients were an average of 60 years old, roughly a third were women, and nearly half had New York Heart Association class III or IV heart failure. Mean depression scores on the 27-point Patient Health Questionnaire-9 were about 5.5; one-fourth of patients were taking antidepressants. Patients with heart failure were eligible for the trial if they had been on stable medication doses for at least 1 month, had not experienced a myocardial infarction or stroke in the previous 3 months, and did not have any cognitive impairment.
At 12 months’ follow-up, the biobehavioral intervention patients were about one-third less likely to have had a cardiac hospitalization or to die (28%) compared with the attention control group (40%) and the usual-care control group (38%).
Patients in the biobehavioral intervention group also had an improvement at 1 year in scores for health-related quality of life, measured with the Minnesota Living with Heart Failure questionnaire, whereas scores worsened in the other groups (P = .005).
Similarly, patients in the biobehavioral intervention group had an improvement at 1 year in scores for depression symptoms, whereas scores worsened in the other groups (P = .001). Some 13% of patients in the biobehavioral intervention group had depression at this time point, compared with 21% of those in the attention control group and 24% of those in the usual-care control group.
The biobehavioral intervention lasted 6 weeks, and consisted of 1-hour weekly sessions conducted by a therapist; it was designed to address depression and comorbid anxiety. "The therapist was a psychiatric nurse practitioner with extensive cardiac experience, and she was well suited to this project because she is from the part of Kentucky where many of the patients come from, so ... there is a lot of concordance between her style and her patients’," Dr. Moser explained.
The attention control condition also lasted 6 weeks, and consisted of 1-hour weekly sessions with the same nurse practitioner present, but entailed only unguided relaxation and the opportunity to speak with her.
Christine Moravec, Ph.D., of the Cleveland Clinic, pointed out that at this point it’s difficult to dissect out whether the benefits seen in the biobehavioral intervention were due to the cognitive-behavioral therapy or the biofeedback.
"That’s our next trial" Dr. Moser noted. "We will be looking at the combination of the two and [each] separately."
Reproducibility is another issue, noted Dr. Javed Butler of Emory University in Atlanta. "Can anybody who does this biobehavioral intervention expect the same results?"
"That’s actually the major question that I have," Dr. Moser acknowledged. The particular nurse practitioner who ran the biobehavioral intervention "is probably the best therapist for these people that I have ever seen, so I really do worry about the reproducibility.
"But also, it sort of speaks to maybe some of the failure of the other clinical trials that we have seen in treating depression for cardiac patients. We have had some colossal failures," she continued. "I don’t think that any therapist can do it. It takes a special therapist who can really connect with patients, and a lot of patients, who can individualize therapy and is concordant with the group. ... The negative impact of depression is so powerful that it bears looking at to try to really define what it is in the therapist-patient relationship that is important and that does need to be reproduced."
Interestingly, depression levels in the biobehavioral intervention group continued to decline over a year, whereas they increased in the control groups, according to Dr. Moser. "We think that that is how cognitive-behavioral therapy should work. It doesn’t of course work for everyone, but if it works, it should give you the skills to manage depressive symptoms in the long term," she said.
"Future research should examine the combined effects of pharmacological and biobehavioral therapy, and ... we need to think about the practicality of this intensive intervention in widespread clinical practice," she added.
Dr. Moser reported having no relevant conflicts of interest.
SEATTLE – A brief cognitive biobehavioral intervention focused on preventing and managing depression reduces adverse outcomes of heart failure, according to results from a randomized trial reported at the annual meeting of the Heart Failure Society of America.
"A 6-week biobehavioral intervention in patients with heart failure really was successful in severing the link between depression and poor outcomes," according to the study’s lead investigator, Debra K. Moser, D.N.Sc., professor and chair of the College of Nursing at the University of Kentucky, Lexington. "These findings provide evidence for the effectiveness of biobehavioral approaches" in this population.
Dr. Moser also noted that more than one-fifth of patients with heart failure have depression, as well as higher depressive symptoms – not necessarily clinical depression. Evidence suggests that in the heart failure population, such symptoms triple the risk of rehospitalization and double the risk of death (J. Am. Coll. Cardiol. 2006;48:1527-37).
Dr. Moser and her colleagues studied 278 patients with heart failure, assigning them to a biobehavioral intervention group (given both cognitive-behavioral therapy and biofeedback-relaxation therapy), an attention control group, or a usual-care control group. At baseline, patients were an average of 60 years old, roughly a third were women, and nearly half had New York Heart Association class III or IV heart failure. Mean depression scores on the 27-point Patient Health Questionnaire-9 were about 5.5; one-fourth of patients were taking antidepressants. Patients with heart failure were eligible for the trial if they had been on stable medication doses for at least 1 month, had not experienced a myocardial infarction or stroke in the previous 3 months, and did not have any cognitive impairment.
At 12 months’ follow-up, the biobehavioral intervention patients were about one-third less likely to have had a cardiac hospitalization or to die (28%) compared with the attention control group (40%) and the usual-care control group (38%).
Patients in the biobehavioral intervention group also had an improvement at 1 year in scores for health-related quality of life, measured with the Minnesota Living with Heart Failure questionnaire, whereas scores worsened in the other groups (P = .005).
Similarly, patients in the biobehavioral intervention group had an improvement at 1 year in scores for depression symptoms, whereas scores worsened in the other groups (P = .001). Some 13% of patients in the biobehavioral intervention group had depression at this time point, compared with 21% of those in the attention control group and 24% of those in the usual-care control group.
The biobehavioral intervention lasted 6 weeks, and consisted of 1-hour weekly sessions conducted by a therapist; it was designed to address depression and comorbid anxiety. "The therapist was a psychiatric nurse practitioner with extensive cardiac experience, and she was well suited to this project because she is from the part of Kentucky where many of the patients come from, so ... there is a lot of concordance between her style and her patients’," Dr. Moser explained.
The attention control condition also lasted 6 weeks, and consisted of 1-hour weekly sessions with the same nurse practitioner present, but entailed only unguided relaxation and the opportunity to speak with her.
Christine Moravec, Ph.D., of the Cleveland Clinic, pointed out that at this point it’s difficult to dissect out whether the benefits seen in the biobehavioral intervention were due to the cognitive-behavioral therapy or the biofeedback.
"That’s our next trial" Dr. Moser noted. "We will be looking at the combination of the two and [each] separately."
Reproducibility is another issue, noted Dr. Javed Butler of Emory University in Atlanta. "Can anybody who does this biobehavioral intervention expect the same results?"
"That’s actually the major question that I have," Dr. Moser acknowledged. The particular nurse practitioner who ran the biobehavioral intervention "is probably the best therapist for these people that I have ever seen, so I really do worry about the reproducibility.
"But also, it sort of speaks to maybe some of the failure of the other clinical trials that we have seen in treating depression for cardiac patients. We have had some colossal failures," she continued. "I don’t think that any therapist can do it. It takes a special therapist who can really connect with patients, and a lot of patients, who can individualize therapy and is concordant with the group. ... The negative impact of depression is so powerful that it bears looking at to try to really define what it is in the therapist-patient relationship that is important and that does need to be reproduced."
Interestingly, depression levels in the biobehavioral intervention group continued to decline over a year, whereas they increased in the control groups, according to Dr. Moser. "We think that that is how cognitive-behavioral therapy should work. It doesn’t of course work for everyone, but if it works, it should give you the skills to manage depressive symptoms in the long term," she said.
"Future research should examine the combined effects of pharmacological and biobehavioral therapy, and ... we need to think about the practicality of this intensive intervention in widespread clinical practice," she added.
Dr. Moser reported having no relevant conflicts of interest.
SEATTLE – A brief cognitive biobehavioral intervention focused on preventing and managing depression reduces adverse outcomes of heart failure, according to results from a randomized trial reported at the annual meeting of the Heart Failure Society of America.
"A 6-week biobehavioral intervention in patients with heart failure really was successful in severing the link between depression and poor outcomes," according to the study’s lead investigator, Debra K. Moser, D.N.Sc., professor and chair of the College of Nursing at the University of Kentucky, Lexington. "These findings provide evidence for the effectiveness of biobehavioral approaches" in this population.
Dr. Moser also noted that more than one-fifth of patients with heart failure have depression, as well as higher depressive symptoms – not necessarily clinical depression. Evidence suggests that in the heart failure population, such symptoms triple the risk of rehospitalization and double the risk of death (J. Am. Coll. Cardiol. 2006;48:1527-37).
Dr. Moser and her colleagues studied 278 patients with heart failure, assigning them to a biobehavioral intervention group (given both cognitive-behavioral therapy and biofeedback-relaxation therapy), an attention control group, or a usual-care control group. At baseline, patients were an average of 60 years old, roughly a third were women, and nearly half had New York Heart Association class III or IV heart failure. Mean depression scores on the 27-point Patient Health Questionnaire-9 were about 5.5; one-fourth of patients were taking antidepressants. Patients with heart failure were eligible for the trial if they had been on stable medication doses for at least 1 month, had not experienced a myocardial infarction or stroke in the previous 3 months, and did not have any cognitive impairment.
At 12 months’ follow-up, the biobehavioral intervention patients were about one-third less likely to have had a cardiac hospitalization or to die (28%) compared with the attention control group (40%) and the usual-care control group (38%).
Patients in the biobehavioral intervention group also had an improvement at 1 year in scores for health-related quality of life, measured with the Minnesota Living with Heart Failure questionnaire, whereas scores worsened in the other groups (P = .005).
Similarly, patients in the biobehavioral intervention group had an improvement at 1 year in scores for depression symptoms, whereas scores worsened in the other groups (P = .001). Some 13% of patients in the biobehavioral intervention group had depression at this time point, compared with 21% of those in the attention control group and 24% of those in the usual-care control group.
The biobehavioral intervention lasted 6 weeks, and consisted of 1-hour weekly sessions conducted by a therapist; it was designed to address depression and comorbid anxiety. "The therapist was a psychiatric nurse practitioner with extensive cardiac experience, and she was well suited to this project because she is from the part of Kentucky where many of the patients come from, so ... there is a lot of concordance between her style and her patients’," Dr. Moser explained.
The attention control condition also lasted 6 weeks, and consisted of 1-hour weekly sessions with the same nurse practitioner present, but entailed only unguided relaxation and the opportunity to speak with her.
Christine Moravec, Ph.D., of the Cleveland Clinic, pointed out that at this point it’s difficult to dissect out whether the benefits seen in the biobehavioral intervention were due to the cognitive-behavioral therapy or the biofeedback.
"That’s our next trial" Dr. Moser noted. "We will be looking at the combination of the two and [each] separately."
Reproducibility is another issue, noted Dr. Javed Butler of Emory University in Atlanta. "Can anybody who does this biobehavioral intervention expect the same results?"
"That’s actually the major question that I have," Dr. Moser acknowledged. The particular nurse practitioner who ran the biobehavioral intervention "is probably the best therapist for these people that I have ever seen, so I really do worry about the reproducibility.
"But also, it sort of speaks to maybe some of the failure of the other clinical trials that we have seen in treating depression for cardiac patients. We have had some colossal failures," she continued. "I don’t think that any therapist can do it. It takes a special therapist who can really connect with patients, and a lot of patients, who can individualize therapy and is concordant with the group. ... The negative impact of depression is so powerful that it bears looking at to try to really define what it is in the therapist-patient relationship that is important and that does need to be reproduced."
Interestingly, depression levels in the biobehavioral intervention group continued to decline over a year, whereas they increased in the control groups, according to Dr. Moser. "We think that that is how cognitive-behavioral therapy should work. It doesn’t of course work for everyone, but if it works, it should give you the skills to manage depressive symptoms in the long term," she said.
"Future research should examine the combined effects of pharmacological and biobehavioral therapy, and ... we need to think about the practicality of this intensive intervention in widespread clinical practice," she added.
Dr. Moser reported having no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Major Finding: Patients in the biobehavioral intervention group had a lower rate of cardiac hospitalization or death (28%) than their counterparts in the attention control group (40%) or the usual-care control group (38%).
Data Source: This was a randomized trial of 278 patients with heart failure on stable medical therapy.
Disclosures: Dr. Moser reported having no relevant conflicts of interest.
Rising Migraine Prevalence Observed in Denmark
LOS ANGELES – The lifetime prevalence of self-reported migraine in the general population rose considerably in a recent 8-year period, suggested a study of roughly 37,000 middle-aged adults in Denmark.
From 1994 to 2002, the proportion reporting that they had ever had migraine rose by 32%, from a prevalence of 18.5% to 24.5% (P less than .001), Dr. Han Le of the University of Copenhagen reported at the annual meeting of the American Headache Society.
By type, there was a 68% increase in the prevalence of migraine with aura (from 5.6% to 9.4%; P less than .001) and a 16% increase in the prevalence of migraine without aura (from 13% to 15.1%; P less than .001), although the latter remained considerably more common (BMJ 2012 July 2 [doi:10.1136/bmjopen-2012-000962]).
"So are we having a micro-epidemic? ... We do believe that there is an increase, but [it] may not be as high as we are finding," Dr. Le commented, noting that that short time span over which the change took place would favor environmental explanations over genetic ones.
"Some part may be explained by more awareness or increased medical consultation. We do know for a fact that medical consultation has increased in Denmark. But there aren’t any campaigns to make people more aware of migraine," she elaborated. "A small part of the subjects participated in both surveys, so perhaps when they saw the migraine questions for the first time, they kind of thought more about it and went to a physician. That’s hard to tell."
In additional study findings, low education and high physical workload and activity increased the risk only of migraine without aura, whereas low body mass index increased the risk only of migraine with aura. Thus "different environmental factors seem to increase the development of migraine with aura and migraine without aura," she commented.
The investigators analyzed data from the Danish Twin Study, in which 20- to 41-year-olds in the general population were asked if they had ever experienced migraine and, if so, if they had had antecedent visual disturbances. Analyses were based on data from 22,053 adults in 1994 and from 14,810 adults in 2002.
Age-stratified analyses showed that most of the increase occurred among individuals aged older than 32 years. There was a significant increase among men and women individually, with the relative increase comparable for the sexes.
The investigators conducted an additional analysis of migraine risk factors among 13,498 adults aged 18-41 years who were free of migraine in 1994 and completed surveys in both study years.
Results showed that these adults were more likely to develop migraine if they had low back pain (odds ratio, 1.3), low education (OR, 1.3), hard physical workload (OR, 1.1), hard physical activity (OR, 1.2), and a body mass index of less than 18.5 kg/m2 (OR, 1.3).
In stratified analyses, only one of these factors (low back pain) was a risk factor for both types of migraine, according to Dr. Le. Low education as well as high physical workload and activity were risk factors only for migraine without aura, whereas low BMI was a risk factor only for migraine with aura.
Dr. Le disclosed no relevant conflicts of interest.
LOS ANGELES – The lifetime prevalence of self-reported migraine in the general population rose considerably in a recent 8-year period, suggested a study of roughly 37,000 middle-aged adults in Denmark.
From 1994 to 2002, the proportion reporting that they had ever had migraine rose by 32%, from a prevalence of 18.5% to 24.5% (P less than .001), Dr. Han Le of the University of Copenhagen reported at the annual meeting of the American Headache Society.
By type, there was a 68% increase in the prevalence of migraine with aura (from 5.6% to 9.4%; P less than .001) and a 16% increase in the prevalence of migraine without aura (from 13% to 15.1%; P less than .001), although the latter remained considerably more common (BMJ 2012 July 2 [doi:10.1136/bmjopen-2012-000962]).
"So are we having a micro-epidemic? ... We do believe that there is an increase, but [it] may not be as high as we are finding," Dr. Le commented, noting that that short time span over which the change took place would favor environmental explanations over genetic ones.
"Some part may be explained by more awareness or increased medical consultation. We do know for a fact that medical consultation has increased in Denmark. But there aren’t any campaigns to make people more aware of migraine," she elaborated. "A small part of the subjects participated in both surveys, so perhaps when they saw the migraine questions for the first time, they kind of thought more about it and went to a physician. That’s hard to tell."
In additional study findings, low education and high physical workload and activity increased the risk only of migraine without aura, whereas low body mass index increased the risk only of migraine with aura. Thus "different environmental factors seem to increase the development of migraine with aura and migraine without aura," she commented.
The investigators analyzed data from the Danish Twin Study, in which 20- to 41-year-olds in the general population were asked if they had ever experienced migraine and, if so, if they had had antecedent visual disturbances. Analyses were based on data from 22,053 adults in 1994 and from 14,810 adults in 2002.
Age-stratified analyses showed that most of the increase occurred among individuals aged older than 32 years. There was a significant increase among men and women individually, with the relative increase comparable for the sexes.
The investigators conducted an additional analysis of migraine risk factors among 13,498 adults aged 18-41 years who were free of migraine in 1994 and completed surveys in both study years.
Results showed that these adults were more likely to develop migraine if they had low back pain (odds ratio, 1.3), low education (OR, 1.3), hard physical workload (OR, 1.1), hard physical activity (OR, 1.2), and a body mass index of less than 18.5 kg/m2 (OR, 1.3).
In stratified analyses, only one of these factors (low back pain) was a risk factor for both types of migraine, according to Dr. Le. Low education as well as high physical workload and activity were risk factors only for migraine without aura, whereas low BMI was a risk factor only for migraine with aura.
Dr. Le disclosed no relevant conflicts of interest.
LOS ANGELES – The lifetime prevalence of self-reported migraine in the general population rose considerably in a recent 8-year period, suggested a study of roughly 37,000 middle-aged adults in Denmark.
From 1994 to 2002, the proportion reporting that they had ever had migraine rose by 32%, from a prevalence of 18.5% to 24.5% (P less than .001), Dr. Han Le of the University of Copenhagen reported at the annual meeting of the American Headache Society.
By type, there was a 68% increase in the prevalence of migraine with aura (from 5.6% to 9.4%; P less than .001) and a 16% increase in the prevalence of migraine without aura (from 13% to 15.1%; P less than .001), although the latter remained considerably more common (BMJ 2012 July 2 [doi:10.1136/bmjopen-2012-000962]).
"So are we having a micro-epidemic? ... We do believe that there is an increase, but [it] may not be as high as we are finding," Dr. Le commented, noting that that short time span over which the change took place would favor environmental explanations over genetic ones.
"Some part may be explained by more awareness or increased medical consultation. We do know for a fact that medical consultation has increased in Denmark. But there aren’t any campaigns to make people more aware of migraine," she elaborated. "A small part of the subjects participated in both surveys, so perhaps when they saw the migraine questions for the first time, they kind of thought more about it and went to a physician. That’s hard to tell."
In additional study findings, low education and high physical workload and activity increased the risk only of migraine without aura, whereas low body mass index increased the risk only of migraine with aura. Thus "different environmental factors seem to increase the development of migraine with aura and migraine without aura," she commented.
The investigators analyzed data from the Danish Twin Study, in which 20- to 41-year-olds in the general population were asked if they had ever experienced migraine and, if so, if they had had antecedent visual disturbances. Analyses were based on data from 22,053 adults in 1994 and from 14,810 adults in 2002.
Age-stratified analyses showed that most of the increase occurred among individuals aged older than 32 years. There was a significant increase among men and women individually, with the relative increase comparable for the sexes.
The investigators conducted an additional analysis of migraine risk factors among 13,498 adults aged 18-41 years who were free of migraine in 1994 and completed surveys in both study years.
Results showed that these adults were more likely to develop migraine if they had low back pain (odds ratio, 1.3), low education (OR, 1.3), hard physical workload (OR, 1.1), hard physical activity (OR, 1.2), and a body mass index of less than 18.5 kg/m2 (OR, 1.3).
In stratified analyses, only one of these factors (low back pain) was a risk factor for both types of migraine, according to Dr. Le. Low education as well as high physical workload and activity were risk factors only for migraine without aura, whereas low BMI was a risk factor only for migraine with aura.
Dr. Le disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN HEADACHE SOCIETY
Major Finding: From 1994 to 2002, the lifetime prevalence of self-reported migraine increased from 18.5% to 24.5% (P less than .001), with increases for both migraine with aura and migraine without aura individually.
Data Source: This was an observational study of 36,863 young and middle-aged adults from the Danish general population.
Disclosures: Dr. Le disclosed no relevant conflicts of interest.
Secondary Headaches Flag Medical Conditions in Pregnancy
LOS ANGELES – Secondary headaches in pregnant women are likely to be associated with an additional medical complaint, according to a retrospective study of patients who presented to a tertiary-care center.
A team led by Dr. Matthew S. Robbins of Montefiore Headache Center in the Bronx, N.Y., assessed characteristics of 68 consecutive women who had an inpatient neurologic consultation for acute headache in pregnancy over a 2.5-year period at Montefiore Medical Center.
Secondary headaches were diagnosed in 57% of these women. Women with secondary headaches were more likely to have hypertension and some other chief complaint along with headache. Women with primary headaches were more likely to have a history of migraine and to have photophobia, phonophobia, and lacrimation on exam.
Three cases (4%) were noteworthy in that the headache led to discovery of an unrecognized pregnancy. All of these patients had secondary headache, and life-threatening but treatable medical conditions.
"In the acute care setting, vigilance for secondary headache in the pregnant population should be heightened, particularly in those patients who may have headache in addition to other symptoms, the lack of a migraine history, and elevated blood pressure acutely," Dr. Robbins recommended. "Because of our sad corollary [of three cases], we should ... always consider a pregnancy test in all women presenting with headache during childbearing years."
He acknowledged that a study limitation was the highly selected patient population. "This is not only patients presenting to the acute care setting, but patients who are then referred by the [emergency department] doctors or obstetricians for a neurologic consultation. So we are probably not capturing the routine cases of preeclampsia that are obvious, or other diagnoses that, in our center, the obstetricians don’t feel warrant neurologic consultation," he commented at the annual meeting of the American Headache Society.
Session attendee Dr. Peter J. Goadsby of the University of California, San Francisco, noted that about a fifth of patients with primary headache were classified as having an abnormal neurologic exam. "That wouldn’t be consistent with primary headache, would it? So what’s abnormal mean?" he asked.
"Most of the abnormal exam findings in the primary headache group were sensory abnormalities," such as unilateral facial numbness, which – although documented as abnormal – might not be clinically relevant. "It was up to the discretion of the treating team whether that patient had a primary or secondary diagnosis with the exam and abnormality combined," Dr. Robbins replied.
"Clinical experience, at least ours, suggests that consultation in the acute care setting for headache in pregnant women is not such an uncommon experience," he noted, explaining the study’s rationale. However, "there are very few guidelines that address this. It is mostly review articles in the obstetrics and gynecology literature that are not really validated, and clinical series are not well reported."
On average, the 68 women the investigators studied were 29 years old, and they were predominantly Hispanic (43%) and black (41%). The mean gestational age was 28.6 weeks, with 60% of women in their third trimester.
In terms of diagnoses, made via the ICHD-II (International Classification of Headache Disorders, second edition) system, 57% of the women had secondary headache and 43% had primary headache.
Headache class was predominantly migraine (43%), preeclampsia or eclampsia (25%), intercurrent infection (7%), and pituitary adenoma/apoplexy (6%).
The patients with primary headache and the patients with secondary headache were statistically indistinguishable in terms of most demographic, pregnancy, and clinical characteristics, according to Dr. Robbins.
But patients in the secondary headache group were less likely to have a history of migraine (39% vs. 90%; P less than .0001) and, on exam, photophobia (67% vs. 93%; P = .02), phonophobia (36% vs. 79%; P = .0004), and lacrimation (0% vs. 17%; P = .01).
On the flip side, those in the secondary headache group were more likely to have headache plus some other chief complaint such as seizure, shortness of breath, or visual disturbances (54% vs. 14%; P = .0009) and hypertension (49% vs. 0%; P less than .0001).
Of the three cases in which headache led to the discovery of an unrecognized pregnancy, one had diagnoses of PRES (posterior reversible encephalopathy syndrome) and eclampsia; one had diagnoses of PRES, RCVS (reversible cerebral vasoconstriction syndrome), and eclampsia; and one had diagnoses of hyperkalemia and renal failure in systemic lupus erythematous.
Dr. Robbins disclosed no relevant conflicts of interest.
LOS ANGELES – Secondary headaches in pregnant women are likely to be associated with an additional medical complaint, according to a retrospective study of patients who presented to a tertiary-care center.
A team led by Dr. Matthew S. Robbins of Montefiore Headache Center in the Bronx, N.Y., assessed characteristics of 68 consecutive women who had an inpatient neurologic consultation for acute headache in pregnancy over a 2.5-year period at Montefiore Medical Center.
Secondary headaches were diagnosed in 57% of these women. Women with secondary headaches were more likely to have hypertension and some other chief complaint along with headache. Women with primary headaches were more likely to have a history of migraine and to have photophobia, phonophobia, and lacrimation on exam.
Three cases (4%) were noteworthy in that the headache led to discovery of an unrecognized pregnancy. All of these patients had secondary headache, and life-threatening but treatable medical conditions.
"In the acute care setting, vigilance for secondary headache in the pregnant population should be heightened, particularly in those patients who may have headache in addition to other symptoms, the lack of a migraine history, and elevated blood pressure acutely," Dr. Robbins recommended. "Because of our sad corollary [of three cases], we should ... always consider a pregnancy test in all women presenting with headache during childbearing years."
He acknowledged that a study limitation was the highly selected patient population. "This is not only patients presenting to the acute care setting, but patients who are then referred by the [emergency department] doctors or obstetricians for a neurologic consultation. So we are probably not capturing the routine cases of preeclampsia that are obvious, or other diagnoses that, in our center, the obstetricians don’t feel warrant neurologic consultation," he commented at the annual meeting of the American Headache Society.
Session attendee Dr. Peter J. Goadsby of the University of California, San Francisco, noted that about a fifth of patients with primary headache were classified as having an abnormal neurologic exam. "That wouldn’t be consistent with primary headache, would it? So what’s abnormal mean?" he asked.
"Most of the abnormal exam findings in the primary headache group were sensory abnormalities," such as unilateral facial numbness, which – although documented as abnormal – might not be clinically relevant. "It was up to the discretion of the treating team whether that patient had a primary or secondary diagnosis with the exam and abnormality combined," Dr. Robbins replied.
"Clinical experience, at least ours, suggests that consultation in the acute care setting for headache in pregnant women is not such an uncommon experience," he noted, explaining the study’s rationale. However, "there are very few guidelines that address this. It is mostly review articles in the obstetrics and gynecology literature that are not really validated, and clinical series are not well reported."
On average, the 68 women the investigators studied were 29 years old, and they were predominantly Hispanic (43%) and black (41%). The mean gestational age was 28.6 weeks, with 60% of women in their third trimester.
In terms of diagnoses, made via the ICHD-II (International Classification of Headache Disorders, second edition) system, 57% of the women had secondary headache and 43% had primary headache.
Headache class was predominantly migraine (43%), preeclampsia or eclampsia (25%), intercurrent infection (7%), and pituitary adenoma/apoplexy (6%).
The patients with primary headache and the patients with secondary headache were statistically indistinguishable in terms of most demographic, pregnancy, and clinical characteristics, according to Dr. Robbins.
But patients in the secondary headache group were less likely to have a history of migraine (39% vs. 90%; P less than .0001) and, on exam, photophobia (67% vs. 93%; P = .02), phonophobia (36% vs. 79%; P = .0004), and lacrimation (0% vs. 17%; P = .01).
On the flip side, those in the secondary headache group were more likely to have headache plus some other chief complaint such as seizure, shortness of breath, or visual disturbances (54% vs. 14%; P = .0009) and hypertension (49% vs. 0%; P less than .0001).
Of the three cases in which headache led to the discovery of an unrecognized pregnancy, one had diagnoses of PRES (posterior reversible encephalopathy syndrome) and eclampsia; one had diagnoses of PRES, RCVS (reversible cerebral vasoconstriction syndrome), and eclampsia; and one had diagnoses of hyperkalemia and renal failure in systemic lupus erythematous.
Dr. Robbins disclosed no relevant conflicts of interest.
LOS ANGELES – Secondary headaches in pregnant women are likely to be associated with an additional medical complaint, according to a retrospective study of patients who presented to a tertiary-care center.
A team led by Dr. Matthew S. Robbins of Montefiore Headache Center in the Bronx, N.Y., assessed characteristics of 68 consecutive women who had an inpatient neurologic consultation for acute headache in pregnancy over a 2.5-year period at Montefiore Medical Center.
Secondary headaches were diagnosed in 57% of these women. Women with secondary headaches were more likely to have hypertension and some other chief complaint along with headache. Women with primary headaches were more likely to have a history of migraine and to have photophobia, phonophobia, and lacrimation on exam.
Three cases (4%) were noteworthy in that the headache led to discovery of an unrecognized pregnancy. All of these patients had secondary headache, and life-threatening but treatable medical conditions.
"In the acute care setting, vigilance for secondary headache in the pregnant population should be heightened, particularly in those patients who may have headache in addition to other symptoms, the lack of a migraine history, and elevated blood pressure acutely," Dr. Robbins recommended. "Because of our sad corollary [of three cases], we should ... always consider a pregnancy test in all women presenting with headache during childbearing years."
He acknowledged that a study limitation was the highly selected patient population. "This is not only patients presenting to the acute care setting, but patients who are then referred by the [emergency department] doctors or obstetricians for a neurologic consultation. So we are probably not capturing the routine cases of preeclampsia that are obvious, or other diagnoses that, in our center, the obstetricians don’t feel warrant neurologic consultation," he commented at the annual meeting of the American Headache Society.
Session attendee Dr. Peter J. Goadsby of the University of California, San Francisco, noted that about a fifth of patients with primary headache were classified as having an abnormal neurologic exam. "That wouldn’t be consistent with primary headache, would it? So what’s abnormal mean?" he asked.
"Most of the abnormal exam findings in the primary headache group were sensory abnormalities," such as unilateral facial numbness, which – although documented as abnormal – might not be clinically relevant. "It was up to the discretion of the treating team whether that patient had a primary or secondary diagnosis with the exam and abnormality combined," Dr. Robbins replied.
"Clinical experience, at least ours, suggests that consultation in the acute care setting for headache in pregnant women is not such an uncommon experience," he noted, explaining the study’s rationale. However, "there are very few guidelines that address this. It is mostly review articles in the obstetrics and gynecology literature that are not really validated, and clinical series are not well reported."
On average, the 68 women the investigators studied were 29 years old, and they were predominantly Hispanic (43%) and black (41%). The mean gestational age was 28.6 weeks, with 60% of women in their third trimester.
In terms of diagnoses, made via the ICHD-II (International Classification of Headache Disorders, second edition) system, 57% of the women had secondary headache and 43% had primary headache.
Headache class was predominantly migraine (43%), preeclampsia or eclampsia (25%), intercurrent infection (7%), and pituitary adenoma/apoplexy (6%).
The patients with primary headache and the patients with secondary headache were statistically indistinguishable in terms of most demographic, pregnancy, and clinical characteristics, according to Dr. Robbins.
But patients in the secondary headache group were less likely to have a history of migraine (39% vs. 90%; P less than .0001) and, on exam, photophobia (67% vs. 93%; P = .02), phonophobia (36% vs. 79%; P = .0004), and lacrimation (0% vs. 17%; P = .01).
On the flip side, those in the secondary headache group were more likely to have headache plus some other chief complaint such as seizure, shortness of breath, or visual disturbances (54% vs. 14%; P = .0009) and hypertension (49% vs. 0%; P less than .0001).
Of the three cases in which headache led to the discovery of an unrecognized pregnancy, one had diagnoses of PRES (posterior reversible encephalopathy syndrome) and eclampsia; one had diagnoses of PRES, RCVS (reversible cerebral vasoconstriction syndrome), and eclampsia; and one had diagnoses of hyperkalemia and renal failure in systemic lupus erythematous.
Dr. Robbins disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN HEADACHE SOCIETY
Major Finding: Secondary headaches were seen in 57% of 68 consecutive women who had an inpatient neurologic consultation for acute headache in pregnancy.
Data Source: Researchers conducted a retrospective study of 68 women who were referred for inpatient neurologic consultation because of acute headache in pregnancy.
Disclosures: Dr. Robbins disclosed no relevant conflicts of interest.
Vemurafenib After Ipilimumab Linked to Rash
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Four patients (25%) developed grade 3 maculopapular skin rash that histologically had features of a drug hypersensitivity rash.
Data Source: A single-center retrospective case series of 16 patients with BRAFV600E-mutant metastatic melanoma treated with vemurafenib after ipilimumab
Disclosures: Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma Science, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.