User login
PCV13 vaccine benefits extend to nonimmune children
SAN DIEGO – Children who receive just one of the recommended two doses of the 13-valent pneumococcal conjugate vaccine still derive indirect protection, new data suggest.
Colonization rates were similar for immune and nonimmune children, based on surveillance data for the first 2 years after the vaccine’s introduction. The findings come from a study at the Boston Medical Center of 4,338 children under age 60 months. Roughly one-third were considered nonimmune because they did not receive sufficient doses of the vaccine.
Nasopharyngeal colonization with PCV13-unique serotypes fell in the overall population after the introduction of the vaccine. Importantly, colonization declined by at least 50% in the nonimmune group at about 1.5 years after the vaccine was introduced, when roughly 75% of eligible children had received it.
Although it took time for the nonimmune group to catch up with their immune peers, the vaccine reduced serotype-specific colonization in children regardless of their immune status, commented Dr. Stephen I. Pelton, a professor of pediatrics and epidemiology at Boston University. "No change in overall prevalence of pneumococcal colonization is observed," he said at IDWeek.
Introduction of the similar PCV7 vaccine was associated with a greater reduction of cases of pneumococcal disease in nonimmunized children than in their immunized counterparts (MBio. 2011;2:e00309-10). The researchers in that study attributed the shared benefits to the vaccine’s impact in reducing nasopharyngeal carriage and transmission of vaccine serotypes.
The records of children aged 59 months or younger who received care at the Boston Medical Center’s primary care center were reviewed to determine vaccination status. Children under age 12 months were considered immune if they received two doses of PCV13 vaccine, and older children were presumed immune if they received one dose.
Pneumococcal colonization was based on the serotypes of Streptococcus pneumoniae isolates obtained from nasopharyngeal swab samples. Colonization analyses used 25-week rolling intervals, for example, weeks 1-25, weeks 2-26, and so on.
Analyses showed good uptake of the vaccine in all age groups. The percentage of children considered immune rose steadily over the 2-year period and peaked at 80%, Dr. Pelton reported.
On average, 32% of children were considered nonimmune during the study period, but the proportion decreased over time.
The overall prevalence of colonization was essentially stable during the study period, but the prevalence of colonization specifically with PCV13-unique serotypes fell. A distinct seasonal pattern was also evident.
"By week 28, we could already see a difference between the unimmunized group and the immunized group," Dr. Pelton explained. "We saw a rapid increase in PCV13 carriage during the fall, projecting on into the winter season [in the former], whereas we have a blunting in the children who are immunized in terms of the acquisition of PCV13 serotypes."
An indirect effect of the vaccine – a reduction by at least 50% in colonization with PCV13-unique serotypes that persisted among nonimmune children during a comparable season – was achieved at week 81. At that point the prevalence stood at about 3 cases per 100 nonimmune children. This outcome occurred when vaccine uptake reached 75%.
Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
The researcher also looked at the impact of a single dose of PCV13 vaccine among children 24-59 months of age during the first half-year after the vaccine’s introduction. Colonization with PCV13-unique serotypes rose during the fall and winter months among children who did not receive any vaccine doses, but the rise was blunted among those who received one dose of vaccine.
The blunting of colonization "strongly suggests to us that a single dose is adequate to prevent colonization with PCV13 serotypes, and we can say that because [the blunting] occurs very early, before we would expect, we were able to observe a significant indirect effect in the population," Dr. Pelton said.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
Nasopharyngeal colonization with PCV13-unique serotypes, Dr. Stephen I. Pelton, pneumococcal colonization, IDWeek, PCV7 vaccine,
SAN DIEGO – Children who receive just one of the recommended two doses of the 13-valent pneumococcal conjugate vaccine still derive indirect protection, new data suggest.
Colonization rates were similar for immune and nonimmune children, based on surveillance data for the first 2 years after the vaccine’s introduction. The findings come from a study at the Boston Medical Center of 4,338 children under age 60 months. Roughly one-third were considered nonimmune because they did not receive sufficient doses of the vaccine.
Nasopharyngeal colonization with PCV13-unique serotypes fell in the overall population after the introduction of the vaccine. Importantly, colonization declined by at least 50% in the nonimmune group at about 1.5 years after the vaccine was introduced, when roughly 75% of eligible children had received it.
Although it took time for the nonimmune group to catch up with their immune peers, the vaccine reduced serotype-specific colonization in children regardless of their immune status, commented Dr. Stephen I. Pelton, a professor of pediatrics and epidemiology at Boston University. "No change in overall prevalence of pneumococcal colonization is observed," he said at IDWeek.
Introduction of the similar PCV7 vaccine was associated with a greater reduction of cases of pneumococcal disease in nonimmunized children than in their immunized counterparts (MBio. 2011;2:e00309-10). The researchers in that study attributed the shared benefits to the vaccine’s impact in reducing nasopharyngeal carriage and transmission of vaccine serotypes.
The records of children aged 59 months or younger who received care at the Boston Medical Center’s primary care center were reviewed to determine vaccination status. Children under age 12 months were considered immune if they received two doses of PCV13 vaccine, and older children were presumed immune if they received one dose.
Pneumococcal colonization was based on the serotypes of Streptococcus pneumoniae isolates obtained from nasopharyngeal swab samples. Colonization analyses used 25-week rolling intervals, for example, weeks 1-25, weeks 2-26, and so on.
Analyses showed good uptake of the vaccine in all age groups. The percentage of children considered immune rose steadily over the 2-year period and peaked at 80%, Dr. Pelton reported.
On average, 32% of children were considered nonimmune during the study period, but the proportion decreased over time.
The overall prevalence of colonization was essentially stable during the study period, but the prevalence of colonization specifically with PCV13-unique serotypes fell. A distinct seasonal pattern was also evident.
"By week 28, we could already see a difference between the unimmunized group and the immunized group," Dr. Pelton explained. "We saw a rapid increase in PCV13 carriage during the fall, projecting on into the winter season [in the former], whereas we have a blunting in the children who are immunized in terms of the acquisition of PCV13 serotypes."
An indirect effect of the vaccine – a reduction by at least 50% in colonization with PCV13-unique serotypes that persisted among nonimmune children during a comparable season – was achieved at week 81. At that point the prevalence stood at about 3 cases per 100 nonimmune children. This outcome occurred when vaccine uptake reached 75%.
Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
The researcher also looked at the impact of a single dose of PCV13 vaccine among children 24-59 months of age during the first half-year after the vaccine’s introduction. Colonization with PCV13-unique serotypes rose during the fall and winter months among children who did not receive any vaccine doses, but the rise was blunted among those who received one dose of vaccine.
The blunting of colonization "strongly suggests to us that a single dose is adequate to prevent colonization with PCV13 serotypes, and we can say that because [the blunting] occurs very early, before we would expect, we were able to observe a significant indirect effect in the population," Dr. Pelton said.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
SAN DIEGO – Children who receive just one of the recommended two doses of the 13-valent pneumococcal conjugate vaccine still derive indirect protection, new data suggest.
Colonization rates were similar for immune and nonimmune children, based on surveillance data for the first 2 years after the vaccine’s introduction. The findings come from a study at the Boston Medical Center of 4,338 children under age 60 months. Roughly one-third were considered nonimmune because they did not receive sufficient doses of the vaccine.
Nasopharyngeal colonization with PCV13-unique serotypes fell in the overall population after the introduction of the vaccine. Importantly, colonization declined by at least 50% in the nonimmune group at about 1.5 years after the vaccine was introduced, when roughly 75% of eligible children had received it.
Although it took time for the nonimmune group to catch up with their immune peers, the vaccine reduced serotype-specific colonization in children regardless of their immune status, commented Dr. Stephen I. Pelton, a professor of pediatrics and epidemiology at Boston University. "No change in overall prevalence of pneumococcal colonization is observed," he said at IDWeek.
Introduction of the similar PCV7 vaccine was associated with a greater reduction of cases of pneumococcal disease in nonimmunized children than in their immunized counterparts (MBio. 2011;2:e00309-10). The researchers in that study attributed the shared benefits to the vaccine’s impact in reducing nasopharyngeal carriage and transmission of vaccine serotypes.
The records of children aged 59 months or younger who received care at the Boston Medical Center’s primary care center were reviewed to determine vaccination status. Children under age 12 months were considered immune if they received two doses of PCV13 vaccine, and older children were presumed immune if they received one dose.
Pneumococcal colonization was based on the serotypes of Streptococcus pneumoniae isolates obtained from nasopharyngeal swab samples. Colonization analyses used 25-week rolling intervals, for example, weeks 1-25, weeks 2-26, and so on.
Analyses showed good uptake of the vaccine in all age groups. The percentage of children considered immune rose steadily over the 2-year period and peaked at 80%, Dr. Pelton reported.
On average, 32% of children were considered nonimmune during the study period, but the proportion decreased over time.
The overall prevalence of colonization was essentially stable during the study period, but the prevalence of colonization specifically with PCV13-unique serotypes fell. A distinct seasonal pattern was also evident.
"By week 28, we could already see a difference between the unimmunized group and the immunized group," Dr. Pelton explained. "We saw a rapid increase in PCV13 carriage during the fall, projecting on into the winter season [in the former], whereas we have a blunting in the children who are immunized in terms of the acquisition of PCV13 serotypes."
An indirect effect of the vaccine – a reduction by at least 50% in colonization with PCV13-unique serotypes that persisted among nonimmune children during a comparable season – was achieved at week 81. At that point the prevalence stood at about 3 cases per 100 nonimmune children. This outcome occurred when vaccine uptake reached 75%.
Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
The researcher also looked at the impact of a single dose of PCV13 vaccine among children 24-59 months of age during the first half-year after the vaccine’s introduction. Colonization with PCV13-unique serotypes rose during the fall and winter months among children who did not receive any vaccine doses, but the rise was blunted among those who received one dose of vaccine.
The blunting of colonization "strongly suggests to us that a single dose is adequate to prevent colonization with PCV13 serotypes, and we can say that because [the blunting] occurs very early, before we would expect, we were able to observe a significant indirect effect in the population," Dr. Pelton said.
IDWeek is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
Nasopharyngeal colonization with PCV13-unique serotypes, Dr. Stephen I. Pelton, pneumococcal colonization, IDWeek, PCV7 vaccine,
Nasopharyngeal colonization with PCV13-unique serotypes, Dr. Stephen I. Pelton, pneumococcal colonization, IDWeek, PCV7 vaccine,
AT IDWEEK
Major Finding: Colonization among nonimmune children fell to the levels seen among immune children at week 52 after the PCV13 vaccine was introduced. By this time, vaccine uptake hit 65%.
Data Source: A single-center surveillance study of 4,338 children under 60 months of age.
Disclosures: Dr. Pelton disclosed that he has received honoraria and research funding from Merck, GlaxoSmithKline, and Pfizer. The study was funded by the Thrasher Research Foundation and Pfizer.
SLN surgery may suffice for node-positive breast cancer
SAN ANTONIO – Women undergoing neoadjuvant chemotherapy for node-positive breast cancer may be able to have sentinel lymph node surgery instead of an axillary lymph node dissection, but proper surgical technique is critical for staging accuracy, a phase II trial from the American College of Surgeons Oncology Group suggests.
Among the 637 women studied in ACOSOG Z1071, all received chemotherapy and then underwent both sentinel lymph node (SLN) surgery and axillary lymph node dissection (ALND). SLN surgery correctly identified nodal status in 91.2% of cases, lead investigator Dr. Judy C. Boughey reported at the San Antonio Breast Cancer Symposium.
The false-negative rate of SLN averaged 12.6% in women with clinical N1 disease who had at least two sentinel nodes examined – slightly higher than the 10% set as a predefined endpoint. But it was lower when both blue dye and radiolabeled colloid were used to identify sentinel nodes (10.8%) and when three or more sentinel nodes were examined (9.1%).
"SLN surgery is a useful tool for detecting residual nodal disease in those women who present with node-positive breast cancer receiving neoadjuvant chemotherapy," Dr. Boughey commented in a press briefing. "Using SLN surgery in this patient population will enable us to reduce the extent of axillary surgery and therefore decrease morbidities for women treated for breast cancer."
Women were eligible for the trial if they had node-positive (T0-4, N1-2) breast cancer. After neoadjuvant chemotherapy, they underwent definitive breast surgery (lumpectomy or mastectomy), along with SLN surgery followed by a completion ALND. Patients who had N3 disease, inflammatory breast cancer, or prior ipsilateral axillary surgery were excluded.
Nodes were defined as being positive if they contained a tumor deposit measuring greater than 0.2 mm on sections stained with hematoxylin and eosin.
Sentinel nodes were identified in 92.7% of patients overall. A total of 40% of patients had no detectable nodal disease after neoadjuvant chemotherapy and thus would be unlikely to benefit from undergoing ALND, according to Dr. Boughey, a surgeon at the Mayo Clinic in Rochester, Minn.
Among the 60% of patients with detectable nodal disease (subsequently used to assess the false-negative rate of SLN), 85% had only positive sentinel nodes.
When only a single SLN was examined, the false-negative rate of SLN surgery was 31.5%, so Dr. Boughey recommends resecting a minimum of two nodes.
The false-negative rate was also lower when sentinel nodes were pathologically examined and determined to contain histologic changes (10.8%) and when a clip placed in the node at initial diagnosis was subsequently found at definitive surgery (7.4%).
"It’s important to collaborate with our radiology colleagues regarding potential for clip placement in lymph nodes, as well as our pathologists for review of sentinel lymph nodes for the presence of treatment effect," she said.
In the session where the results were presented, Dr. J. Michael Dixon of the Western General Hospital in Edinburgh asked whether a minimum of three nodes should be taken, because "if you take three or more nodes, your false-negative rate is within the range that is acceptable. Yet your conclusions said two. So I wonder if you get a second chance, you can tell us that we can do this technique if we use dual isotope and if we take three or more sentinel nodes and follow your other rules."
"We framed the conclusion based on the way the protocol had been written up front with two or more, but I think your comments are spot on," replied Dr. Boughey.
Dr. Steven Vogl of the Montefiore Medical Center in the Bronx, New York, asked about pathologic complete response (pCR).
"We did not require any specific degree of response for the patients to be in the study, since all of the patients were getting a completion ALND," Dr. Boughey replied. "We are currently evaluating specifically that question about whether the breast response and/or the nodal response on ultrasound can help define the appropriate patient population for us to tailor this therapy to."
Dr. Vogl noted that for patients who are HER2-positive, ER-negative, and triple-negative, pCR is very important. "So if we can figure out very carefully who didn’t achieve a pCR, especially in the node, we can probably do something for those patients some day with the right agents," he said.
"Do you use clips in all patients? Do you use dual agents always? And do you always remove two sentinel nodes? What is the impact of this on what we should do next?" asked session moderator Dr. Anthony Lucci Jr., of the University of Texas M.D. Anderson Cancer Center in Houston.
Dr. Boughey replied, "The take-home message is ... I’ll be looking at clip placement, making sure that we use dual-agent tracer for these cases, and ensuring that we do a thorough evaluation of the axilla and resecting any node that is radioactive, blue, or palpable.
"One of the concerns always when you are doing the sentinel node and you know you are doing a planned dissection [thereafter] is that the completeness of the evaluation of the axilla may not be quite as thorough as if you are closing as soon as you finish that sentinel node biopsy. So I think that is where the onus rests on the surgeon, so that we thoroughly evaluate the axilla and ensure this technique is as thorough as possible," she added.
In an additional analysis of patients with clinical N2 disease, the false-negative rate of SLN was 0%.
"Further work is under way regarding the secondary endpoint of this study, which will look at correlating the axillary ultrasound after chemotherapy with the false-negative rate. ... Maybe this can help improve patient selection for the procedure and further lower the false-negative rate," Dr. Boughey said. "We are also continuing to work to evaluate lymphedema rates and quality of life in these patients."
Dr. Boughey disclosed no relevant conflicts of interest.
SAN ANTONIO – Women undergoing neoadjuvant chemotherapy for node-positive breast cancer may be able to have sentinel lymph node surgery instead of an axillary lymph node dissection, but proper surgical technique is critical for staging accuracy, a phase II trial from the American College of Surgeons Oncology Group suggests.
Among the 637 women studied in ACOSOG Z1071, all received chemotherapy and then underwent both sentinel lymph node (SLN) surgery and axillary lymph node dissection (ALND). SLN surgery correctly identified nodal status in 91.2% of cases, lead investigator Dr. Judy C. Boughey reported at the San Antonio Breast Cancer Symposium.
The false-negative rate of SLN averaged 12.6% in women with clinical N1 disease who had at least two sentinel nodes examined – slightly higher than the 10% set as a predefined endpoint. But it was lower when both blue dye and radiolabeled colloid were used to identify sentinel nodes (10.8%) and when three or more sentinel nodes were examined (9.1%).
"SLN surgery is a useful tool for detecting residual nodal disease in those women who present with node-positive breast cancer receiving neoadjuvant chemotherapy," Dr. Boughey commented in a press briefing. "Using SLN surgery in this patient population will enable us to reduce the extent of axillary surgery and therefore decrease morbidities for women treated for breast cancer."
Women were eligible for the trial if they had node-positive (T0-4, N1-2) breast cancer. After neoadjuvant chemotherapy, they underwent definitive breast surgery (lumpectomy or mastectomy), along with SLN surgery followed by a completion ALND. Patients who had N3 disease, inflammatory breast cancer, or prior ipsilateral axillary surgery were excluded.
Nodes were defined as being positive if they contained a tumor deposit measuring greater than 0.2 mm on sections stained with hematoxylin and eosin.
Sentinel nodes were identified in 92.7% of patients overall. A total of 40% of patients had no detectable nodal disease after neoadjuvant chemotherapy and thus would be unlikely to benefit from undergoing ALND, according to Dr. Boughey, a surgeon at the Mayo Clinic in Rochester, Minn.
Among the 60% of patients with detectable nodal disease (subsequently used to assess the false-negative rate of SLN), 85% had only positive sentinel nodes.
When only a single SLN was examined, the false-negative rate of SLN surgery was 31.5%, so Dr. Boughey recommends resecting a minimum of two nodes.
The false-negative rate was also lower when sentinel nodes were pathologically examined and determined to contain histologic changes (10.8%) and when a clip placed in the node at initial diagnosis was subsequently found at definitive surgery (7.4%).
"It’s important to collaborate with our radiology colleagues regarding potential for clip placement in lymph nodes, as well as our pathologists for review of sentinel lymph nodes for the presence of treatment effect," she said.
In the session where the results were presented, Dr. J. Michael Dixon of the Western General Hospital in Edinburgh asked whether a minimum of three nodes should be taken, because "if you take three or more nodes, your false-negative rate is within the range that is acceptable. Yet your conclusions said two. So I wonder if you get a second chance, you can tell us that we can do this technique if we use dual isotope and if we take three or more sentinel nodes and follow your other rules."
"We framed the conclusion based on the way the protocol had been written up front with two or more, but I think your comments are spot on," replied Dr. Boughey.
Dr. Steven Vogl of the Montefiore Medical Center in the Bronx, New York, asked about pathologic complete response (pCR).
"We did not require any specific degree of response for the patients to be in the study, since all of the patients were getting a completion ALND," Dr. Boughey replied. "We are currently evaluating specifically that question about whether the breast response and/or the nodal response on ultrasound can help define the appropriate patient population for us to tailor this therapy to."
Dr. Vogl noted that for patients who are HER2-positive, ER-negative, and triple-negative, pCR is very important. "So if we can figure out very carefully who didn’t achieve a pCR, especially in the node, we can probably do something for those patients some day with the right agents," he said.
"Do you use clips in all patients? Do you use dual agents always? And do you always remove two sentinel nodes? What is the impact of this on what we should do next?" asked session moderator Dr. Anthony Lucci Jr., of the University of Texas M.D. Anderson Cancer Center in Houston.
Dr. Boughey replied, "The take-home message is ... I’ll be looking at clip placement, making sure that we use dual-agent tracer for these cases, and ensuring that we do a thorough evaluation of the axilla and resecting any node that is radioactive, blue, or palpable.
"One of the concerns always when you are doing the sentinel node and you know you are doing a planned dissection [thereafter] is that the completeness of the evaluation of the axilla may not be quite as thorough as if you are closing as soon as you finish that sentinel node biopsy. So I think that is where the onus rests on the surgeon, so that we thoroughly evaluate the axilla and ensure this technique is as thorough as possible," she added.
In an additional analysis of patients with clinical N2 disease, the false-negative rate of SLN was 0%.
"Further work is under way regarding the secondary endpoint of this study, which will look at correlating the axillary ultrasound after chemotherapy with the false-negative rate. ... Maybe this can help improve patient selection for the procedure and further lower the false-negative rate," Dr. Boughey said. "We are also continuing to work to evaluate lymphedema rates and quality of life in these patients."
Dr. Boughey disclosed no relevant conflicts of interest.
SAN ANTONIO – Women undergoing neoadjuvant chemotherapy for node-positive breast cancer may be able to have sentinel lymph node surgery instead of an axillary lymph node dissection, but proper surgical technique is critical for staging accuracy, a phase II trial from the American College of Surgeons Oncology Group suggests.
Among the 637 women studied in ACOSOG Z1071, all received chemotherapy and then underwent both sentinel lymph node (SLN) surgery and axillary lymph node dissection (ALND). SLN surgery correctly identified nodal status in 91.2% of cases, lead investigator Dr. Judy C. Boughey reported at the San Antonio Breast Cancer Symposium.
The false-negative rate of SLN averaged 12.6% in women with clinical N1 disease who had at least two sentinel nodes examined – slightly higher than the 10% set as a predefined endpoint. But it was lower when both blue dye and radiolabeled colloid were used to identify sentinel nodes (10.8%) and when three or more sentinel nodes were examined (9.1%).
"SLN surgery is a useful tool for detecting residual nodal disease in those women who present with node-positive breast cancer receiving neoadjuvant chemotherapy," Dr. Boughey commented in a press briefing. "Using SLN surgery in this patient population will enable us to reduce the extent of axillary surgery and therefore decrease morbidities for women treated for breast cancer."
Women were eligible for the trial if they had node-positive (T0-4, N1-2) breast cancer. After neoadjuvant chemotherapy, they underwent definitive breast surgery (lumpectomy or mastectomy), along with SLN surgery followed by a completion ALND. Patients who had N3 disease, inflammatory breast cancer, or prior ipsilateral axillary surgery were excluded.
Nodes were defined as being positive if they contained a tumor deposit measuring greater than 0.2 mm on sections stained with hematoxylin and eosin.
Sentinel nodes were identified in 92.7% of patients overall. A total of 40% of patients had no detectable nodal disease after neoadjuvant chemotherapy and thus would be unlikely to benefit from undergoing ALND, according to Dr. Boughey, a surgeon at the Mayo Clinic in Rochester, Minn.
Among the 60% of patients with detectable nodal disease (subsequently used to assess the false-negative rate of SLN), 85% had only positive sentinel nodes.
When only a single SLN was examined, the false-negative rate of SLN surgery was 31.5%, so Dr. Boughey recommends resecting a minimum of two nodes.
The false-negative rate was also lower when sentinel nodes were pathologically examined and determined to contain histologic changes (10.8%) and when a clip placed in the node at initial diagnosis was subsequently found at definitive surgery (7.4%).
"It’s important to collaborate with our radiology colleagues regarding potential for clip placement in lymph nodes, as well as our pathologists for review of sentinel lymph nodes for the presence of treatment effect," she said.
In the session where the results were presented, Dr. J. Michael Dixon of the Western General Hospital in Edinburgh asked whether a minimum of three nodes should be taken, because "if you take three or more nodes, your false-negative rate is within the range that is acceptable. Yet your conclusions said two. So I wonder if you get a second chance, you can tell us that we can do this technique if we use dual isotope and if we take three or more sentinel nodes and follow your other rules."
"We framed the conclusion based on the way the protocol had been written up front with two or more, but I think your comments are spot on," replied Dr. Boughey.
Dr. Steven Vogl of the Montefiore Medical Center in the Bronx, New York, asked about pathologic complete response (pCR).
"We did not require any specific degree of response for the patients to be in the study, since all of the patients were getting a completion ALND," Dr. Boughey replied. "We are currently evaluating specifically that question about whether the breast response and/or the nodal response on ultrasound can help define the appropriate patient population for us to tailor this therapy to."
Dr. Vogl noted that for patients who are HER2-positive, ER-negative, and triple-negative, pCR is very important. "So if we can figure out very carefully who didn’t achieve a pCR, especially in the node, we can probably do something for those patients some day with the right agents," he said.
"Do you use clips in all patients? Do you use dual agents always? And do you always remove two sentinel nodes? What is the impact of this on what we should do next?" asked session moderator Dr. Anthony Lucci Jr., of the University of Texas M.D. Anderson Cancer Center in Houston.
Dr. Boughey replied, "The take-home message is ... I’ll be looking at clip placement, making sure that we use dual-agent tracer for these cases, and ensuring that we do a thorough evaluation of the axilla and resecting any node that is radioactive, blue, or palpable.
"One of the concerns always when you are doing the sentinel node and you know you are doing a planned dissection [thereafter] is that the completeness of the evaluation of the axilla may not be quite as thorough as if you are closing as soon as you finish that sentinel node biopsy. So I think that is where the onus rests on the surgeon, so that we thoroughly evaluate the axilla and ensure this technique is as thorough as possible," she added.
In an additional analysis of patients with clinical N2 disease, the false-negative rate of SLN was 0%.
"Further work is under way regarding the secondary endpoint of this study, which will look at correlating the axillary ultrasound after chemotherapy with the false-negative rate. ... Maybe this can help improve patient selection for the procedure and further lower the false-negative rate," Dr. Boughey said. "We are also continuing to work to evaluate lymphedema rates and quality of life in these patients."
Dr. Boughey disclosed no relevant conflicts of interest.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: SLN surgery correctly identified axillary nodal status in 91.2% of patients. The false-negative rate was 12.6% among women with clinical N1 disease who had at least two sentinel nodes examined.
Data Source: A multicenter phase II trial involving 637 patients with node-positive breast cancer who received neoadjuvant chemotherapy (the ACOSOG Z1071 trial).
Disclosures: Dr. Boughey disclosed no relevant conflicts of interest.
Eribulin fails to best capecitabine in advanced breast cancer
SAN ANTONIO – Patients with locally advanced or metastatic breast cancer did not fare better if given eribulin instead of capecitabine in a randomized phase III trial. But results suggest eribulin may have an edge in certain subgroups, including women with triple-negative breast cancer.
Investigators led by Dr. Peter A. Kaufman studied more than 1,000 such patients who had had up to three prior treatments, assigning them evenly to eribulin (Halaven) or capecitabine (Xeloda). Trial results, reported at the San Antonio Breast Cancer Symposium, showed only a nonsignificant trend in overall survival in favor of eribulin, amounting to a gain of about 1.5 months, and essentially no difference in progression-free survival.
However, preplanned subgroup analyses hinted at a potential overall survival benefit among patients having the notoriously hard-to-treat triple-negative disease, as well as those with estrogen receptor–negative or HER2-negative disease.
"Eribulin and capecitabine have similar overall activity in this trial that included patients in the first-, second-, and third-line settings," Dr. Kaufman commented in a press briefing. "Our findings, I want to emphasize, are not definitive but interesting in terms of certain subsets and suggest that the triple-negative subgroup perhaps may be doing better with eribulin."
The reason for the unusual combination of a trend toward overall survival benefit in the absence of a progression-free survival benefit is unknown, he said. "This is not typical of what one sees in metastatic breast cancer, but the data are what the data are."
Given the trial’s findings, what are clinicians to do?
"I think eribulin is a reasonable option to consider therapeutically now for women. It compares reasonably to capecitabine," maintained Dr. Kaufman of the Geisel School of Medicine at Dartmouth and the Norris Cotton Cancer Center in Lebanon, N.H. "Our primary objective was to demonstrate superiority, which we did not meet. But the results are reasonably encouraging."
HER2 Therapy Questioned
In the session where the results were presented, attendee Dr. Daniel F. Hayes of the University of Michigan, Ann Arbor said, "I’m assuming that everyone who was HER2-positive received trastuzumab or some other anti-HER2 therapy, which might affect your subgroup analysis for HER2."
"A large proportion of the study population was enrolled in eastern Europe or South America, parts of the world where trastuzumab and other HER2-targeted therapies were not routinely used at the time of enrollment for this study," Dr. Kaufman replied. "We are sorting out those results and will present them at a further date."
Dr. Hayes also wondered about crossover on the trial and its possible impact on overall survival.
Although there was no planned crossover, about half of patients in the eribulin arm did switch to capecitabine, and that may have affected the results, Dr. Kaufman acknowledged. "We are pulling the data together on post-progression treatments to try to analyze that further."
FDA Requested Bone Scans
Attendee Dr. Steven Vogl of Montefiore Medical Center in the Bronx, New York, took issue with the objective response rates with both eribulin and capecitabine, which were 16% and 20%, respectively, by local investigator assessment but just 11% and 12% on independent review.
"The objective response rate on radiologic review is sort of distressing," he said. "Did someone decide that if the bone scan didn’t get better, that wasn’t a response, even though the node shrank 70%?"
"Correct. So this is one of the reasons why the response rate on the independent analysis is lower," Dr. Kaufman replied. "If the bone scan did not confirm it, those patients were not scored as having responding disease; additionally, if the confirmatory bone scan was not performed, those patients were not scored as having an objective response.
"We are still pulling all this together, but I think that is a large piece of why the independent analysis response rates were lower than the local investigator assessment. But I will highlight also that ... only about 20% of the patients were in the first-line setting, so it’s not a pure first-line trial."
"The 20% response rate in a third-line trial is credible, but the 10% response rate – some of us don’t think that’s worth pursuing," Dr. Vogl added. "I think bone scans shouldn’t be done on studies like this because they take forever to get better."
"Bone scan was actually included at the request of the FDA," Dr. Kaufman noted.
Results Not Statistically Different
Patients were eligible for the trial, formally known as Study 301, if they had locally advanced or metastatic breast cancer and had a received up to three prior regimens (up to two for their advanced disease), which must have included a taxane and an anthracycline.
In all, 1,102 patients were randomized to eribulin – the only chemotherapeutic agent shown to have a survival benefit in heavily pretreated metastatic breast cancer – or capecitabine. The former is approved by the FDA for treatment of metastatic breast cancer after at least two treatment regimens with an anthracycline and a taxane; the latter is approved for treatment of pretreated metastatic breast cancer, as well as stage III colon cancer.
Demographically, the patients had a median age of 53 years. The majority had received one or two prior chemotherapy regimens for advanced disease. More than 85% had visceral involvement.
In terms of drug exposure, the two groups had almost identical median durations of treatment and relative dose intensities.
Main results showed that median overall survival achieved with eribulin was numerically but not statistically better than that with capecitabine (15.9 vs. 14.5 months; hazard ratio, 0.88; P = .056), Dr. Kaufman reported.
In preplanned subgroup analyses, there were trends favoring eribulin over capecitabine in patients who had triple-negative disease (hazard ratio, 0.70), as well as those with HER2-negative disease (0.84) and estrogen receptor–negative disease (0.78).
The 1-, 2-, and 3-year rates of overall survival were statistically indistinguishable between groups.
There was also no significant difference in progression-free survival, with the value standing at about 4 months in each group (P = .31), and no significant difference in response rates.
The eribulin and capecitabine groups were similarly likely to experience treatment-related adverse events (85% and 77%) and serious adverse events (18% and 21%).
"The side effect profiles are consistent with previous data on the side effects of these medications," Dr. Kaufman said.
Eribulin was associated with higher rates of grade 3/4 neutropenia and leukopenia, whereas capecitabine was associated with higher rates of grade 3/4 hand-foot syndrome and diarrhea.
The investigators have not yet performed comparative cost analyses in the trial.
Dr. Kaufman disclosed that he receives research support from and is a consultant to Eisai. The trial was sponsored by Eisai.
"This study demonstrates that we have a long way to go in the treatment of metastatic breast cancer," commented Dr. C. Kent Osborne, moderator of a press briefing at which the findings were discussed. "In this study, we saw that neither drug was terribly effective in the situation of patients who have been treated before, and it has a relatively small impact on their outcome."
Dr. C. Kent Osborne |
"Metastatic breast cancer is still a very terrible disease," he added, noting that presence of even a single small detectable metastasis abruptly changes the prognosis to incurable for reasons yet unknown. "Our goal, I think, is to prevent people from having metastatic breast cancer by giving them better and better adjuvant treatment and diagnosing them earlier and earlier."
Dr. Osborne is director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine in Houston.
"This study demonstrates that we have a long way to go in the treatment of metastatic breast cancer," commented Dr. C. Kent Osborne, moderator of a press briefing at which the findings were discussed. "In this study, we saw that neither drug was terribly effective in the situation of patients who have been treated before, and it has a relatively small impact on their outcome."
Dr. C. Kent Osborne |
"Metastatic breast cancer is still a very terrible disease," he added, noting that presence of even a single small detectable metastasis abruptly changes the prognosis to incurable for reasons yet unknown. "Our goal, I think, is to prevent people from having metastatic breast cancer by giving them better and better adjuvant treatment and diagnosing them earlier and earlier."
Dr. Osborne is director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine in Houston.
"This study demonstrates that we have a long way to go in the treatment of metastatic breast cancer," commented Dr. C. Kent Osborne, moderator of a press briefing at which the findings were discussed. "In this study, we saw that neither drug was terribly effective in the situation of patients who have been treated before, and it has a relatively small impact on their outcome."
Dr. C. Kent Osborne |
"Metastatic breast cancer is still a very terrible disease," he added, noting that presence of even a single small detectable metastasis abruptly changes the prognosis to incurable for reasons yet unknown. "Our goal, I think, is to prevent people from having metastatic breast cancer by giving them better and better adjuvant treatment and diagnosing them earlier and earlier."
Dr. Osborne is director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine in Houston.
SAN ANTONIO – Patients with locally advanced or metastatic breast cancer did not fare better if given eribulin instead of capecitabine in a randomized phase III trial. But results suggest eribulin may have an edge in certain subgroups, including women with triple-negative breast cancer.
Investigators led by Dr. Peter A. Kaufman studied more than 1,000 such patients who had had up to three prior treatments, assigning them evenly to eribulin (Halaven) or capecitabine (Xeloda). Trial results, reported at the San Antonio Breast Cancer Symposium, showed only a nonsignificant trend in overall survival in favor of eribulin, amounting to a gain of about 1.5 months, and essentially no difference in progression-free survival.
However, preplanned subgroup analyses hinted at a potential overall survival benefit among patients having the notoriously hard-to-treat triple-negative disease, as well as those with estrogen receptor–negative or HER2-negative disease.
"Eribulin and capecitabine have similar overall activity in this trial that included patients in the first-, second-, and third-line settings," Dr. Kaufman commented in a press briefing. "Our findings, I want to emphasize, are not definitive but interesting in terms of certain subsets and suggest that the triple-negative subgroup perhaps may be doing better with eribulin."
The reason for the unusual combination of a trend toward overall survival benefit in the absence of a progression-free survival benefit is unknown, he said. "This is not typical of what one sees in metastatic breast cancer, but the data are what the data are."
Given the trial’s findings, what are clinicians to do?
"I think eribulin is a reasonable option to consider therapeutically now for women. It compares reasonably to capecitabine," maintained Dr. Kaufman of the Geisel School of Medicine at Dartmouth and the Norris Cotton Cancer Center in Lebanon, N.H. "Our primary objective was to demonstrate superiority, which we did not meet. But the results are reasonably encouraging."
HER2 Therapy Questioned
In the session where the results were presented, attendee Dr. Daniel F. Hayes of the University of Michigan, Ann Arbor said, "I’m assuming that everyone who was HER2-positive received trastuzumab or some other anti-HER2 therapy, which might affect your subgroup analysis for HER2."
"A large proportion of the study population was enrolled in eastern Europe or South America, parts of the world where trastuzumab and other HER2-targeted therapies were not routinely used at the time of enrollment for this study," Dr. Kaufman replied. "We are sorting out those results and will present them at a further date."
Dr. Hayes also wondered about crossover on the trial and its possible impact on overall survival.
Although there was no planned crossover, about half of patients in the eribulin arm did switch to capecitabine, and that may have affected the results, Dr. Kaufman acknowledged. "We are pulling the data together on post-progression treatments to try to analyze that further."
FDA Requested Bone Scans
Attendee Dr. Steven Vogl of Montefiore Medical Center in the Bronx, New York, took issue with the objective response rates with both eribulin and capecitabine, which were 16% and 20%, respectively, by local investigator assessment but just 11% and 12% on independent review.
"The objective response rate on radiologic review is sort of distressing," he said. "Did someone decide that if the bone scan didn’t get better, that wasn’t a response, even though the node shrank 70%?"
"Correct. So this is one of the reasons why the response rate on the independent analysis is lower," Dr. Kaufman replied. "If the bone scan did not confirm it, those patients were not scored as having responding disease; additionally, if the confirmatory bone scan was not performed, those patients were not scored as having an objective response.
"We are still pulling all this together, but I think that is a large piece of why the independent analysis response rates were lower than the local investigator assessment. But I will highlight also that ... only about 20% of the patients were in the first-line setting, so it’s not a pure first-line trial."
"The 20% response rate in a third-line trial is credible, but the 10% response rate – some of us don’t think that’s worth pursuing," Dr. Vogl added. "I think bone scans shouldn’t be done on studies like this because they take forever to get better."
"Bone scan was actually included at the request of the FDA," Dr. Kaufman noted.
Results Not Statistically Different
Patients were eligible for the trial, formally known as Study 301, if they had locally advanced or metastatic breast cancer and had a received up to three prior regimens (up to two for their advanced disease), which must have included a taxane and an anthracycline.
In all, 1,102 patients were randomized to eribulin – the only chemotherapeutic agent shown to have a survival benefit in heavily pretreated metastatic breast cancer – or capecitabine. The former is approved by the FDA for treatment of metastatic breast cancer after at least two treatment regimens with an anthracycline and a taxane; the latter is approved for treatment of pretreated metastatic breast cancer, as well as stage III colon cancer.
Demographically, the patients had a median age of 53 years. The majority had received one or two prior chemotherapy regimens for advanced disease. More than 85% had visceral involvement.
In terms of drug exposure, the two groups had almost identical median durations of treatment and relative dose intensities.
Main results showed that median overall survival achieved with eribulin was numerically but not statistically better than that with capecitabine (15.9 vs. 14.5 months; hazard ratio, 0.88; P = .056), Dr. Kaufman reported.
In preplanned subgroup analyses, there were trends favoring eribulin over capecitabine in patients who had triple-negative disease (hazard ratio, 0.70), as well as those with HER2-negative disease (0.84) and estrogen receptor–negative disease (0.78).
The 1-, 2-, and 3-year rates of overall survival were statistically indistinguishable between groups.
There was also no significant difference in progression-free survival, with the value standing at about 4 months in each group (P = .31), and no significant difference in response rates.
The eribulin and capecitabine groups were similarly likely to experience treatment-related adverse events (85% and 77%) and serious adverse events (18% and 21%).
"The side effect profiles are consistent with previous data on the side effects of these medications," Dr. Kaufman said.
Eribulin was associated with higher rates of grade 3/4 neutropenia and leukopenia, whereas capecitabine was associated with higher rates of grade 3/4 hand-foot syndrome and diarrhea.
The investigators have not yet performed comparative cost analyses in the trial.
Dr. Kaufman disclosed that he receives research support from and is a consultant to Eisai. The trial was sponsored by Eisai.
SAN ANTONIO – Patients with locally advanced or metastatic breast cancer did not fare better if given eribulin instead of capecitabine in a randomized phase III trial. But results suggest eribulin may have an edge in certain subgroups, including women with triple-negative breast cancer.
Investigators led by Dr. Peter A. Kaufman studied more than 1,000 such patients who had had up to three prior treatments, assigning them evenly to eribulin (Halaven) or capecitabine (Xeloda). Trial results, reported at the San Antonio Breast Cancer Symposium, showed only a nonsignificant trend in overall survival in favor of eribulin, amounting to a gain of about 1.5 months, and essentially no difference in progression-free survival.
However, preplanned subgroup analyses hinted at a potential overall survival benefit among patients having the notoriously hard-to-treat triple-negative disease, as well as those with estrogen receptor–negative or HER2-negative disease.
"Eribulin and capecitabine have similar overall activity in this trial that included patients in the first-, second-, and third-line settings," Dr. Kaufman commented in a press briefing. "Our findings, I want to emphasize, are not definitive but interesting in terms of certain subsets and suggest that the triple-negative subgroup perhaps may be doing better with eribulin."
The reason for the unusual combination of a trend toward overall survival benefit in the absence of a progression-free survival benefit is unknown, he said. "This is not typical of what one sees in metastatic breast cancer, but the data are what the data are."
Given the trial’s findings, what are clinicians to do?
"I think eribulin is a reasonable option to consider therapeutically now for women. It compares reasonably to capecitabine," maintained Dr. Kaufman of the Geisel School of Medicine at Dartmouth and the Norris Cotton Cancer Center in Lebanon, N.H. "Our primary objective was to demonstrate superiority, which we did not meet. But the results are reasonably encouraging."
HER2 Therapy Questioned
In the session where the results were presented, attendee Dr. Daniel F. Hayes of the University of Michigan, Ann Arbor said, "I’m assuming that everyone who was HER2-positive received trastuzumab or some other anti-HER2 therapy, which might affect your subgroup analysis for HER2."
"A large proportion of the study population was enrolled in eastern Europe or South America, parts of the world where trastuzumab and other HER2-targeted therapies were not routinely used at the time of enrollment for this study," Dr. Kaufman replied. "We are sorting out those results and will present them at a further date."
Dr. Hayes also wondered about crossover on the trial and its possible impact on overall survival.
Although there was no planned crossover, about half of patients in the eribulin arm did switch to capecitabine, and that may have affected the results, Dr. Kaufman acknowledged. "We are pulling the data together on post-progression treatments to try to analyze that further."
FDA Requested Bone Scans
Attendee Dr. Steven Vogl of Montefiore Medical Center in the Bronx, New York, took issue with the objective response rates with both eribulin and capecitabine, which were 16% and 20%, respectively, by local investigator assessment but just 11% and 12% on independent review.
"The objective response rate on radiologic review is sort of distressing," he said. "Did someone decide that if the bone scan didn’t get better, that wasn’t a response, even though the node shrank 70%?"
"Correct. So this is one of the reasons why the response rate on the independent analysis is lower," Dr. Kaufman replied. "If the bone scan did not confirm it, those patients were not scored as having responding disease; additionally, if the confirmatory bone scan was not performed, those patients were not scored as having an objective response.
"We are still pulling all this together, but I think that is a large piece of why the independent analysis response rates were lower than the local investigator assessment. But I will highlight also that ... only about 20% of the patients were in the first-line setting, so it’s not a pure first-line trial."
"The 20% response rate in a third-line trial is credible, but the 10% response rate – some of us don’t think that’s worth pursuing," Dr. Vogl added. "I think bone scans shouldn’t be done on studies like this because they take forever to get better."
"Bone scan was actually included at the request of the FDA," Dr. Kaufman noted.
Results Not Statistically Different
Patients were eligible for the trial, formally known as Study 301, if they had locally advanced or metastatic breast cancer and had a received up to three prior regimens (up to two for their advanced disease), which must have included a taxane and an anthracycline.
In all, 1,102 patients were randomized to eribulin – the only chemotherapeutic agent shown to have a survival benefit in heavily pretreated metastatic breast cancer – or capecitabine. The former is approved by the FDA for treatment of metastatic breast cancer after at least two treatment regimens with an anthracycline and a taxane; the latter is approved for treatment of pretreated metastatic breast cancer, as well as stage III colon cancer.
Demographically, the patients had a median age of 53 years. The majority had received one or two prior chemotherapy regimens for advanced disease. More than 85% had visceral involvement.
In terms of drug exposure, the two groups had almost identical median durations of treatment and relative dose intensities.
Main results showed that median overall survival achieved with eribulin was numerically but not statistically better than that with capecitabine (15.9 vs. 14.5 months; hazard ratio, 0.88; P = .056), Dr. Kaufman reported.
In preplanned subgroup analyses, there were trends favoring eribulin over capecitabine in patients who had triple-negative disease (hazard ratio, 0.70), as well as those with HER2-negative disease (0.84) and estrogen receptor–negative disease (0.78).
The 1-, 2-, and 3-year rates of overall survival were statistically indistinguishable between groups.
There was also no significant difference in progression-free survival, with the value standing at about 4 months in each group (P = .31), and no significant difference in response rates.
The eribulin and capecitabine groups were similarly likely to experience treatment-related adverse events (85% and 77%) and serious adverse events (18% and 21%).
"The side effect profiles are consistent with previous data on the side effects of these medications," Dr. Kaufman said.
Eribulin was associated with higher rates of grade 3/4 neutropenia and leukopenia, whereas capecitabine was associated with higher rates of grade 3/4 hand-foot syndrome and diarrhea.
The investigators have not yet performed comparative cost analyses in the trial.
Dr. Kaufman disclosed that he receives research support from and is a consultant to Eisai. The trial was sponsored by Eisai.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Eribulin was not significantly better than capecitabine in median overall survival (15.9 vs. 14.5 months, P = .056) or progression-free survival (4.1 vs. 4.2 months, P = .31).
Data Source: Study 301, a randomized phase III open-label trial among 1,102 patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes.
Disclosures: Dr. Kaufman disclosed that he receives research support from and is a consultant to Eisai. The trial was sponsored by Eisai.
Genetic Vulnerabilities Identified in Residual TNBC After Neoadjuvant Chemo
SAN ANTONIO – In the not-too-distant future, triple-negative breast cancer that persists after neoadjuvant chemotherapy could potentially be treated with targeted therapies, new data suggest.
At the time of resection performed after neoadjuvant chemotherapy, about 90% of residual tumors have alterations in pathways that could potentially be targeted by agents that are already on the market or in the pipeline, researchers reported at the San Antonio Breast Cancer Symposium. Thus, targeted adjuvant treatment might ultimately reduce the risk of recurrence.
"We believe (these cells) likely mirror the molecular profiles present in the clinically silent micrometastases that are ultimately destined to recur in such patients. If we had knowledge of these molecular alterations, it could provide us some therapeutic impetus to treat patients after surgical resection," according to lead investigator Justin M. Balko, Pharm.D., Ph.D., a research faculty member at the Vanderbilt-Ingram Cancer Center in Nashville, Tenn.
Additional study results suggested that some residual triple-negative breast cancers might be treated effectively with two emerging classes of agents, inhibitors of janus kinase 2 (JAK2) and MEK. Both proteins are involved in cell growth and proliferation.
"These data provide a targetable catalogue of the alterations present in the residual disease of triple-negative breast cancer after neoadjuvant chemotherapy, and we believe that they support genomically driven adjuvant trials in this patient population," Dr. Balko maintained.
"This study is a discovery exercise that to me suggests nothing has changed in clinical care, but it is a strong direction for discovery and research," commented Dr. Carlos Arteaga, moderator of a related press briefing and associate director for clinical research and director of the breast cancer program at Vanderbilt-Ingram Cancer Center.
"If you have patients who have residual disease in the breast after chemotherapy, the standard of care is just to observe those patients. But many of them are going to recur and die from their metastases," noted Dr. Arteaga, who was also a researcher on the study. "The reason we don’t treat them is because we don’t know how to treat them. So this study suggests that there are actionable molecular lesions that we may one day be able to act upon early and potentially change the natural history of that micrometastatic disease that is waiting there to recur in a few months or years."
The investigators obtained residual tumor samples from 114 patients who had completed neoadjuvant chemotherapy for triple-negative breast cancer. They measured immunohistochemical protein expression in 112 tumors and nanostring gene expression in 89 tumors, and performed next-generation sequencing in 81 tumors.
The patients were 48 years old, on average, and most had stage III disease. About half of them had received a taxane as part of their neoadjuvant chemotherapy and were postmenopausal.
The genes most commonly showing amplifications, deletions, or mutations of known or implied functional significance were p53 (altered in about 90% of tumors), MCL1 (an antiapoptotic gene, altered in 55%), and MYC (altered in 30%). But alterations were found, albeit less commonly, in more than 15 other genes as well.
"This heterogeneity highlights a need for personalized medicine in this subgroup" of patients, Dr. Balko commented.
When the altered genes were grouped into pathways, 90% of patients had tumors with at least one affected pathway that could potentially be targeted by agents that are already available or in clinical trials.
The most commonly affected pathways were the PI3 kinase and mammalian target of rapamycin (PI3K/mTOR) pathway (potentially targeted by PI3K/mTOR inhibitors) and the cell cycle pathways (potentially targeted by cell cycle or mitotic spindle inhibitors).
Additionally, alterations were found in the DNA repair pathway (potentially targeted by DNA repair–targeting agents), the Ras/MAPK pathway (potentially targeted by RAF/MEK inhibitors), and growth factor receptor pathways (potentially targeted with receptor tyrosine kinase inhibitors).
Patients whose tumors had high MEK activation and MYC amplification had poorer recurrence-free survival than others (P = .03). In vitro treatment of MYC-overexpressing cells with two MEK inhibitors in clinical trials – selumetinib and trametinib – indeed reduced colony formation.
"This may provide some justification for exploring MEK inhibition within MYC-amplified tumors," Dr. Balko proposed.
In a novel finding, 11% of tumors had amplification of JAK2, which is thought to contribute to cancer cells’ stem cell–like behavior. Compared to others, patients with these tumors also had poorer recurrence-free survival (P = .005) and overall survival (P = .002). They also had higher levels of gene expression of interleukin 6, one of the main cytokines activating this pathway.
"We would like to propose that these JAK2 amplifications may represent a biomarker for clinical trials exploring JAK2 inhibitors that are currently in clinical trials for inflammatory diseases," Dr. Balko said.
As for future research, "efforts to determine whether lesions present in the residual disease mirror those in the recurrence and whether they are selected during neoadjuvant treatment are under way," he concluded.
The research was funded by the Susan G. Komen for the Cure Foundation, the National Institutes of Health, and the Lee Jeans/Entertainment Industry Foundation Translational Breast Cancer Research Program. Dr. Balko disclosed no relevant conflicts of interest. Dr. Arteaga disclosed he is a consultant/adviser for Roche, Monogram Biosciences, AstraZeneca, GlaxoSmithKline, and Genentech, and that he receives research grants from Amgen, AstraZeneca, and Exelixis.
SAN ANTONIO – In the not-too-distant future, triple-negative breast cancer that persists after neoadjuvant chemotherapy could potentially be treated with targeted therapies, new data suggest.
At the time of resection performed after neoadjuvant chemotherapy, about 90% of residual tumors have alterations in pathways that could potentially be targeted by agents that are already on the market or in the pipeline, researchers reported at the San Antonio Breast Cancer Symposium. Thus, targeted adjuvant treatment might ultimately reduce the risk of recurrence.
"We believe (these cells) likely mirror the molecular profiles present in the clinically silent micrometastases that are ultimately destined to recur in such patients. If we had knowledge of these molecular alterations, it could provide us some therapeutic impetus to treat patients after surgical resection," according to lead investigator Justin M. Balko, Pharm.D., Ph.D., a research faculty member at the Vanderbilt-Ingram Cancer Center in Nashville, Tenn.
Additional study results suggested that some residual triple-negative breast cancers might be treated effectively with two emerging classes of agents, inhibitors of janus kinase 2 (JAK2) and MEK. Both proteins are involved in cell growth and proliferation.
"These data provide a targetable catalogue of the alterations present in the residual disease of triple-negative breast cancer after neoadjuvant chemotherapy, and we believe that they support genomically driven adjuvant trials in this patient population," Dr. Balko maintained.
"This study is a discovery exercise that to me suggests nothing has changed in clinical care, but it is a strong direction for discovery and research," commented Dr. Carlos Arteaga, moderator of a related press briefing and associate director for clinical research and director of the breast cancer program at Vanderbilt-Ingram Cancer Center.
"If you have patients who have residual disease in the breast after chemotherapy, the standard of care is just to observe those patients. But many of them are going to recur and die from their metastases," noted Dr. Arteaga, who was also a researcher on the study. "The reason we don’t treat them is because we don’t know how to treat them. So this study suggests that there are actionable molecular lesions that we may one day be able to act upon early and potentially change the natural history of that micrometastatic disease that is waiting there to recur in a few months or years."
The investigators obtained residual tumor samples from 114 patients who had completed neoadjuvant chemotherapy for triple-negative breast cancer. They measured immunohistochemical protein expression in 112 tumors and nanostring gene expression in 89 tumors, and performed next-generation sequencing in 81 tumors.
The patients were 48 years old, on average, and most had stage III disease. About half of them had received a taxane as part of their neoadjuvant chemotherapy and were postmenopausal.
The genes most commonly showing amplifications, deletions, or mutations of known or implied functional significance were p53 (altered in about 90% of tumors), MCL1 (an antiapoptotic gene, altered in 55%), and MYC (altered in 30%). But alterations were found, albeit less commonly, in more than 15 other genes as well.
"This heterogeneity highlights a need for personalized medicine in this subgroup" of patients, Dr. Balko commented.
When the altered genes were grouped into pathways, 90% of patients had tumors with at least one affected pathway that could potentially be targeted by agents that are already available or in clinical trials.
The most commonly affected pathways were the PI3 kinase and mammalian target of rapamycin (PI3K/mTOR) pathway (potentially targeted by PI3K/mTOR inhibitors) and the cell cycle pathways (potentially targeted by cell cycle or mitotic spindle inhibitors).
Additionally, alterations were found in the DNA repair pathway (potentially targeted by DNA repair–targeting agents), the Ras/MAPK pathway (potentially targeted by RAF/MEK inhibitors), and growth factor receptor pathways (potentially targeted with receptor tyrosine kinase inhibitors).
Patients whose tumors had high MEK activation and MYC amplification had poorer recurrence-free survival than others (P = .03). In vitro treatment of MYC-overexpressing cells with two MEK inhibitors in clinical trials – selumetinib and trametinib – indeed reduced colony formation.
"This may provide some justification for exploring MEK inhibition within MYC-amplified tumors," Dr. Balko proposed.
In a novel finding, 11% of tumors had amplification of JAK2, which is thought to contribute to cancer cells’ stem cell–like behavior. Compared to others, patients with these tumors also had poorer recurrence-free survival (P = .005) and overall survival (P = .002). They also had higher levels of gene expression of interleukin 6, one of the main cytokines activating this pathway.
"We would like to propose that these JAK2 amplifications may represent a biomarker for clinical trials exploring JAK2 inhibitors that are currently in clinical trials for inflammatory diseases," Dr. Balko said.
As for future research, "efforts to determine whether lesions present in the residual disease mirror those in the recurrence and whether they are selected during neoadjuvant treatment are under way," he concluded.
The research was funded by the Susan G. Komen for the Cure Foundation, the National Institutes of Health, and the Lee Jeans/Entertainment Industry Foundation Translational Breast Cancer Research Program. Dr. Balko disclosed no relevant conflicts of interest. Dr. Arteaga disclosed he is a consultant/adviser for Roche, Monogram Biosciences, AstraZeneca, GlaxoSmithKline, and Genentech, and that he receives research grants from Amgen, AstraZeneca, and Exelixis.
SAN ANTONIO – In the not-too-distant future, triple-negative breast cancer that persists after neoadjuvant chemotherapy could potentially be treated with targeted therapies, new data suggest.
At the time of resection performed after neoadjuvant chemotherapy, about 90% of residual tumors have alterations in pathways that could potentially be targeted by agents that are already on the market or in the pipeline, researchers reported at the San Antonio Breast Cancer Symposium. Thus, targeted adjuvant treatment might ultimately reduce the risk of recurrence.
"We believe (these cells) likely mirror the molecular profiles present in the clinically silent micrometastases that are ultimately destined to recur in such patients. If we had knowledge of these molecular alterations, it could provide us some therapeutic impetus to treat patients after surgical resection," according to lead investigator Justin M. Balko, Pharm.D., Ph.D., a research faculty member at the Vanderbilt-Ingram Cancer Center in Nashville, Tenn.
Additional study results suggested that some residual triple-negative breast cancers might be treated effectively with two emerging classes of agents, inhibitors of janus kinase 2 (JAK2) and MEK. Both proteins are involved in cell growth and proliferation.
"These data provide a targetable catalogue of the alterations present in the residual disease of triple-negative breast cancer after neoadjuvant chemotherapy, and we believe that they support genomically driven adjuvant trials in this patient population," Dr. Balko maintained.
"This study is a discovery exercise that to me suggests nothing has changed in clinical care, but it is a strong direction for discovery and research," commented Dr. Carlos Arteaga, moderator of a related press briefing and associate director for clinical research and director of the breast cancer program at Vanderbilt-Ingram Cancer Center.
"If you have patients who have residual disease in the breast after chemotherapy, the standard of care is just to observe those patients. But many of them are going to recur and die from their metastases," noted Dr. Arteaga, who was also a researcher on the study. "The reason we don’t treat them is because we don’t know how to treat them. So this study suggests that there are actionable molecular lesions that we may one day be able to act upon early and potentially change the natural history of that micrometastatic disease that is waiting there to recur in a few months or years."
The investigators obtained residual tumor samples from 114 patients who had completed neoadjuvant chemotherapy for triple-negative breast cancer. They measured immunohistochemical protein expression in 112 tumors and nanostring gene expression in 89 tumors, and performed next-generation sequencing in 81 tumors.
The patients were 48 years old, on average, and most had stage III disease. About half of them had received a taxane as part of their neoadjuvant chemotherapy and were postmenopausal.
The genes most commonly showing amplifications, deletions, or mutations of known or implied functional significance were p53 (altered in about 90% of tumors), MCL1 (an antiapoptotic gene, altered in 55%), and MYC (altered in 30%). But alterations were found, albeit less commonly, in more than 15 other genes as well.
"This heterogeneity highlights a need for personalized medicine in this subgroup" of patients, Dr. Balko commented.
When the altered genes were grouped into pathways, 90% of patients had tumors with at least one affected pathway that could potentially be targeted by agents that are already available or in clinical trials.
The most commonly affected pathways were the PI3 kinase and mammalian target of rapamycin (PI3K/mTOR) pathway (potentially targeted by PI3K/mTOR inhibitors) and the cell cycle pathways (potentially targeted by cell cycle or mitotic spindle inhibitors).
Additionally, alterations were found in the DNA repair pathway (potentially targeted by DNA repair–targeting agents), the Ras/MAPK pathway (potentially targeted by RAF/MEK inhibitors), and growth factor receptor pathways (potentially targeted with receptor tyrosine kinase inhibitors).
Patients whose tumors had high MEK activation and MYC amplification had poorer recurrence-free survival than others (P = .03). In vitro treatment of MYC-overexpressing cells with two MEK inhibitors in clinical trials – selumetinib and trametinib – indeed reduced colony formation.
"This may provide some justification for exploring MEK inhibition within MYC-amplified tumors," Dr. Balko proposed.
In a novel finding, 11% of tumors had amplification of JAK2, which is thought to contribute to cancer cells’ stem cell–like behavior. Compared to others, patients with these tumors also had poorer recurrence-free survival (P = .005) and overall survival (P = .002). They also had higher levels of gene expression of interleukin 6, one of the main cytokines activating this pathway.
"We would like to propose that these JAK2 amplifications may represent a biomarker for clinical trials exploring JAK2 inhibitors that are currently in clinical trials for inflammatory diseases," Dr. Balko said.
As for future research, "efforts to determine whether lesions present in the residual disease mirror those in the recurrence and whether they are selected during neoadjuvant treatment are under way," he concluded.
The research was funded by the Susan G. Komen for the Cure Foundation, the National Institutes of Health, and the Lee Jeans/Entertainment Industry Foundation Translational Breast Cancer Research Program. Dr. Balko disclosed no relevant conflicts of interest. Dr. Arteaga disclosed he is a consultant/adviser for Roche, Monogram Biosciences, AstraZeneca, GlaxoSmithKline, and Genentech, and that he receives research grants from Amgen, AstraZeneca, and Exelixis.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: About 90% of patients had tumors harboring genetic alterations in clinical pathways that could be potentially targeted, such as the PI3K/mTOR pathway and the DNA repair pathway.
Data Source: Results were taken from molecular profiling study of residual tumors from 114 patients who had undergone neoadjuvant chemotherapy for triple-negative breast cancer
Disclosures: The research was funded by the Susan G. Komen for the Cure Foundation, the National Institutes of Health, and the Lee Jeans/Entertainment Industry Foundation Translational Breast Cancer Research Program. Dr. Balko disclosed no relevant conflicts of interest. Dr. Arteaga disclosed he is a consultant/adviser for Roche, Monogram Biosciences, AstraZeneca, GlaxoSmithKline, and Genentech, and that he receives research grants from Amgen, AstraZeneca, and Exelixis.
Experimental Drug Puts ER-Positive Breast Cancer on Hold
SAN ANTONIO – An oral investigational agent that blocks cell cycle progression may expand treatment options for the most common type of breast cancer, the results of a randomized phase II trial suggest.
Combining the agent, called PD 0332991, with letrozole (Femara) as first-line therapy for advanced estrogen receptor (ER)-positive, HER2-negative disease more than tripled median progression-free survival, lead investigator Dr. Richard S. Finn reported at the San Antonio Breast Cancer Symposium.
The response rate also was better with the combination, but there were no complete responses.
PD 0332991 inhibits cyclin-dependent kinase 4/6, and thereby interferes with the action of the retinoblastoma (Rb) protein, arresting cells between the G1 and S phases of the cell cycle. Benefit appeared to be consistent regardless of the time to relapse, whether disease was recurrent or de novo, and irrespective of whether the tumor had cyclin D1 gene amplification or p16 gene loss (perturbations affecting the cell cycle).
There was an increase in grade 3/4 adverse events mainly driven by an increase in those of hematologic nature.
"PD 0332991 when added to letrozole provides a dramatic and unprecedented improvement in progression-free survival in this population," commented Dr. Finn of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles.
"These results confirm our observations made preclinically that PD 0332991 has activity in ER-positive breast cancer, as well as highlight the synergy that we observed in the laboratory. It is very important to remember that this molecule is well tolerated, that the toxicity profile was very manageable," he added.
On the basis of these positive findings, the investigators are planning a phase III trial that should open in 2013.
Many Questions for Investigator
The presentation drew comments and questions from breast cancer specialists in the audience.
Attendee Dr. Larry Norton of Memorial Sloan-Kettering Cancer Center, New York, noted, "When one sees a disconnect between progression-free survival and response rate, one must think that maybe there is an effect on metastasis or new sites of disease. There’s two ways, obviously, that you can progress: in prior sites of disease vs. new sites of disease.
"Have you divided that out in this study, and are you having an antimetastatic effect?" he asked. "This pathway clearly relates to many mechanisms of metastasis."
"Certainly, that’s a potential mechanism," Dr. Finn replied. "We have not teased that out. I would say that if we are inducing cell cycle arrest and having a cytostatic effect, that could be having an effect in the metastatic sites or the measurable sites, regardless."
Another attendee noted that in such patients, letrozole monotherapy usually achieves a median progression-free survival on the order of 9.5 months, whereas the value in the trial was 7.5 months. "Why did the control arm do so badly?" he asked.
Dr. Finn noted that the values did not differ greatly, that the patient population was heterogeneous (including some who had previously received hormonal therapy), and that cross-trial comparisons are problematic. "Even with all that taken into consideration, the magnitude of benefit that we are seeing, even if the control arm did 9 or 11 or 12 months, this magnitude of benefit is so significant that this is clearly a signal that this molecule has a role in this disease," he maintained.
Dr. Frankie Ann Holmes of Texas Oncology in Houston, who also attended the session, wondered, "So if in all of the parameters you have studied, you couldn’t find a biomarker, do you have a hypothesis? Are you going to find a biomarker, or is it just such a big difference, we don’t really need to drill down?"
"We do have a biomarker: That biomarker is ER," Dr. Finn replied. "The most important genomic event that probably is related to sensitivity to this compound is having an intact Rb pathway. And it would appear that ER positivity is a marker for an intact Rb pathway and that explains part of the dramatic benefit that we are seeing."
Most Women Had Stage IV Disease
Women were eligible for the trial, formally known as TRIO-18, if they were postmenopausal and had previously untreated, locally recurrent or metastatic ER-positive, HER2-negative breast cancer.
The trial had two parts. In the first part, 66 otherwise unselected women were randomized evenly to the combination of PD 0332991 and letrozole or to letrozole alone. In the second, exploratory part, 99 patients also known to have cyclin D1 amplification and/or p16 loss were similarly randomized.
The women had a median age of 63 years, and most had stage IV disease. Roughly two-thirds had experienced their relapse within 12 months of adjuvant treatment or had de novo disease.
A first interim analysis, conducted in the unselected patient population and previously reported at the IMPAKT Breast Cancer Conference, showed that progression-free survival, the trial’s primary endpoint, was significantly better with the combination than with letrozole alone (hazard ratio, 0.35; P = .006).
A second interim analysis, conducted in the entire trial population and the one being reported in San Antonio, showed that median progression-free survival was 26.1 months with the combination versus 7.5 months with letrozole alone (hazard ratio, 0.37; P less than .001), according to Dr. Finn.
The combination was also associated with a better overall response rate both in the entire trial population (34% vs. 26%) and in the subset having measurable disease (45% vs. 31%).
Dose modifications were more common with addition of PD 0332991. The rate of treatment discontinuation because of adverse events was 10% with the combination vs. 1% with letrozole monotherapy.
Compared with monotherapy, the combination was associated with higher rates of grade 3/4 neutropenia (51% vs. 1%), leukopenia (14% vs. 0%), and anemia (5% vs. 0%).
However, "I need to stress that this was not clinically significant neutropenia," Dr. Finn commented. "There was no evidence of neutropenic fever, there was no use of growth factors in this study either."
Alopecia was more common with the combination as well, but all cases were of grade 1 severity.
Dr. Finn disclosed that he receives research from funding from Novartis Pharmaceuticals. The trial was sponsored by Pfizer.
SAN ANTONIO – An oral investigational agent that blocks cell cycle progression may expand treatment options for the most common type of breast cancer, the results of a randomized phase II trial suggest.
Combining the agent, called PD 0332991, with letrozole (Femara) as first-line therapy for advanced estrogen receptor (ER)-positive, HER2-negative disease more than tripled median progression-free survival, lead investigator Dr. Richard S. Finn reported at the San Antonio Breast Cancer Symposium.
The response rate also was better with the combination, but there were no complete responses.
PD 0332991 inhibits cyclin-dependent kinase 4/6, and thereby interferes with the action of the retinoblastoma (Rb) protein, arresting cells between the G1 and S phases of the cell cycle. Benefit appeared to be consistent regardless of the time to relapse, whether disease was recurrent or de novo, and irrespective of whether the tumor had cyclin D1 gene amplification or p16 gene loss (perturbations affecting the cell cycle).
There was an increase in grade 3/4 adverse events mainly driven by an increase in those of hematologic nature.
"PD 0332991 when added to letrozole provides a dramatic and unprecedented improvement in progression-free survival in this population," commented Dr. Finn of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles.
"These results confirm our observations made preclinically that PD 0332991 has activity in ER-positive breast cancer, as well as highlight the synergy that we observed in the laboratory. It is very important to remember that this molecule is well tolerated, that the toxicity profile was very manageable," he added.
On the basis of these positive findings, the investigators are planning a phase III trial that should open in 2013.
Many Questions for Investigator
The presentation drew comments and questions from breast cancer specialists in the audience.
Attendee Dr. Larry Norton of Memorial Sloan-Kettering Cancer Center, New York, noted, "When one sees a disconnect between progression-free survival and response rate, one must think that maybe there is an effect on metastasis or new sites of disease. There’s two ways, obviously, that you can progress: in prior sites of disease vs. new sites of disease.
"Have you divided that out in this study, and are you having an antimetastatic effect?" he asked. "This pathway clearly relates to many mechanisms of metastasis."
"Certainly, that’s a potential mechanism," Dr. Finn replied. "We have not teased that out. I would say that if we are inducing cell cycle arrest and having a cytostatic effect, that could be having an effect in the metastatic sites or the measurable sites, regardless."
Another attendee noted that in such patients, letrozole monotherapy usually achieves a median progression-free survival on the order of 9.5 months, whereas the value in the trial was 7.5 months. "Why did the control arm do so badly?" he asked.
Dr. Finn noted that the values did not differ greatly, that the patient population was heterogeneous (including some who had previously received hormonal therapy), and that cross-trial comparisons are problematic. "Even with all that taken into consideration, the magnitude of benefit that we are seeing, even if the control arm did 9 or 11 or 12 months, this magnitude of benefit is so significant that this is clearly a signal that this molecule has a role in this disease," he maintained.
Dr. Frankie Ann Holmes of Texas Oncology in Houston, who also attended the session, wondered, "So if in all of the parameters you have studied, you couldn’t find a biomarker, do you have a hypothesis? Are you going to find a biomarker, or is it just such a big difference, we don’t really need to drill down?"
"We do have a biomarker: That biomarker is ER," Dr. Finn replied. "The most important genomic event that probably is related to sensitivity to this compound is having an intact Rb pathway. And it would appear that ER positivity is a marker for an intact Rb pathway and that explains part of the dramatic benefit that we are seeing."
Most Women Had Stage IV Disease
Women were eligible for the trial, formally known as TRIO-18, if they were postmenopausal and had previously untreated, locally recurrent or metastatic ER-positive, HER2-negative breast cancer.
The trial had two parts. In the first part, 66 otherwise unselected women were randomized evenly to the combination of PD 0332991 and letrozole or to letrozole alone. In the second, exploratory part, 99 patients also known to have cyclin D1 amplification and/or p16 loss were similarly randomized.
The women had a median age of 63 years, and most had stage IV disease. Roughly two-thirds had experienced their relapse within 12 months of adjuvant treatment or had de novo disease.
A first interim analysis, conducted in the unselected patient population and previously reported at the IMPAKT Breast Cancer Conference, showed that progression-free survival, the trial’s primary endpoint, was significantly better with the combination than with letrozole alone (hazard ratio, 0.35; P = .006).
A second interim analysis, conducted in the entire trial population and the one being reported in San Antonio, showed that median progression-free survival was 26.1 months with the combination versus 7.5 months with letrozole alone (hazard ratio, 0.37; P less than .001), according to Dr. Finn.
The combination was also associated with a better overall response rate both in the entire trial population (34% vs. 26%) and in the subset having measurable disease (45% vs. 31%).
Dose modifications were more common with addition of PD 0332991. The rate of treatment discontinuation because of adverse events was 10% with the combination vs. 1% with letrozole monotherapy.
Compared with monotherapy, the combination was associated with higher rates of grade 3/4 neutropenia (51% vs. 1%), leukopenia (14% vs. 0%), and anemia (5% vs. 0%).
However, "I need to stress that this was not clinically significant neutropenia," Dr. Finn commented. "There was no evidence of neutropenic fever, there was no use of growth factors in this study either."
Alopecia was more common with the combination as well, but all cases were of grade 1 severity.
Dr. Finn disclosed that he receives research from funding from Novartis Pharmaceuticals. The trial was sponsored by Pfizer.
SAN ANTONIO – An oral investigational agent that blocks cell cycle progression may expand treatment options for the most common type of breast cancer, the results of a randomized phase II trial suggest.
Combining the agent, called PD 0332991, with letrozole (Femara) as first-line therapy for advanced estrogen receptor (ER)-positive, HER2-negative disease more than tripled median progression-free survival, lead investigator Dr. Richard S. Finn reported at the San Antonio Breast Cancer Symposium.
The response rate also was better with the combination, but there were no complete responses.
PD 0332991 inhibits cyclin-dependent kinase 4/6, and thereby interferes with the action of the retinoblastoma (Rb) protein, arresting cells between the G1 and S phases of the cell cycle. Benefit appeared to be consistent regardless of the time to relapse, whether disease was recurrent or de novo, and irrespective of whether the tumor had cyclin D1 gene amplification or p16 gene loss (perturbations affecting the cell cycle).
There was an increase in grade 3/4 adverse events mainly driven by an increase in those of hematologic nature.
"PD 0332991 when added to letrozole provides a dramatic and unprecedented improvement in progression-free survival in this population," commented Dr. Finn of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles.
"These results confirm our observations made preclinically that PD 0332991 has activity in ER-positive breast cancer, as well as highlight the synergy that we observed in the laboratory. It is very important to remember that this molecule is well tolerated, that the toxicity profile was very manageable," he added.
On the basis of these positive findings, the investigators are planning a phase III trial that should open in 2013.
Many Questions for Investigator
The presentation drew comments and questions from breast cancer specialists in the audience.
Attendee Dr. Larry Norton of Memorial Sloan-Kettering Cancer Center, New York, noted, "When one sees a disconnect between progression-free survival and response rate, one must think that maybe there is an effect on metastasis or new sites of disease. There’s two ways, obviously, that you can progress: in prior sites of disease vs. new sites of disease.
"Have you divided that out in this study, and are you having an antimetastatic effect?" he asked. "This pathway clearly relates to many mechanisms of metastasis."
"Certainly, that’s a potential mechanism," Dr. Finn replied. "We have not teased that out. I would say that if we are inducing cell cycle arrest and having a cytostatic effect, that could be having an effect in the metastatic sites or the measurable sites, regardless."
Another attendee noted that in such patients, letrozole monotherapy usually achieves a median progression-free survival on the order of 9.5 months, whereas the value in the trial was 7.5 months. "Why did the control arm do so badly?" he asked.
Dr. Finn noted that the values did not differ greatly, that the patient population was heterogeneous (including some who had previously received hormonal therapy), and that cross-trial comparisons are problematic. "Even with all that taken into consideration, the magnitude of benefit that we are seeing, even if the control arm did 9 or 11 or 12 months, this magnitude of benefit is so significant that this is clearly a signal that this molecule has a role in this disease," he maintained.
Dr. Frankie Ann Holmes of Texas Oncology in Houston, who also attended the session, wondered, "So if in all of the parameters you have studied, you couldn’t find a biomarker, do you have a hypothesis? Are you going to find a biomarker, or is it just such a big difference, we don’t really need to drill down?"
"We do have a biomarker: That biomarker is ER," Dr. Finn replied. "The most important genomic event that probably is related to sensitivity to this compound is having an intact Rb pathway. And it would appear that ER positivity is a marker for an intact Rb pathway and that explains part of the dramatic benefit that we are seeing."
Most Women Had Stage IV Disease
Women were eligible for the trial, formally known as TRIO-18, if they were postmenopausal and had previously untreated, locally recurrent or metastatic ER-positive, HER2-negative breast cancer.
The trial had two parts. In the first part, 66 otherwise unselected women were randomized evenly to the combination of PD 0332991 and letrozole or to letrozole alone. In the second, exploratory part, 99 patients also known to have cyclin D1 amplification and/or p16 loss were similarly randomized.
The women had a median age of 63 years, and most had stage IV disease. Roughly two-thirds had experienced their relapse within 12 months of adjuvant treatment or had de novo disease.
A first interim analysis, conducted in the unselected patient population and previously reported at the IMPAKT Breast Cancer Conference, showed that progression-free survival, the trial’s primary endpoint, was significantly better with the combination than with letrozole alone (hazard ratio, 0.35; P = .006).
A second interim analysis, conducted in the entire trial population and the one being reported in San Antonio, showed that median progression-free survival was 26.1 months with the combination versus 7.5 months with letrozole alone (hazard ratio, 0.37; P less than .001), according to Dr. Finn.
The combination was also associated with a better overall response rate both in the entire trial population (34% vs. 26%) and in the subset having measurable disease (45% vs. 31%).
Dose modifications were more common with addition of PD 0332991. The rate of treatment discontinuation because of adverse events was 10% with the combination vs. 1% with letrozole monotherapy.
Compared with monotherapy, the combination was associated with higher rates of grade 3/4 neutropenia (51% vs. 1%), leukopenia (14% vs. 0%), and anemia (5% vs. 0%).
However, "I need to stress that this was not clinically significant neutropenia," Dr. Finn commented. "There was no evidence of neutropenic fever, there was no use of growth factors in this study either."
Alopecia was more common with the combination as well, but all cases were of grade 1 severity.
Dr. Finn disclosed that he receives research from funding from Novartis Pharmaceuticals. The trial was sponsored by Pfizer.
AT THE SAN ANTONIO BREAST CANCER SYMPOSIUM
Major Finding: Compared with letrozole alone, letrozole plus PD 0332991 was associated with significantly better progression-free survival (26.1 vs. 7.5 months; hazard ratio, 0.37).
Data Source: This was a randomized phase II trial among 165 patients with advanced ER-positive, HER2-negative breast cancer (the TRIO-18 trial).
Disclosures: Dr. Finn disclosed that he receives research funding from Novartis Pharmaceuticals. The trial was sponsored by Pfizer.
Early results promising for micropump in heart failure
SEATTLE – A tiny pump that provides partial support to patients with heart failure may improve outcomes, at least in the short term, for those in the earlier stages of the disease, clinical experience with the device has shown.
The 20 patients in Belgium and Germany who underwent implantation of the newest version of the investigational device had significantly improved hemodynamics, exercise tolerance, and end organ function at a median of 12 weeks’ follow-up, Dr. Daniel Burkhoff reported at the annual meeting of the Heart Failure Society of America.
The rate of adverse events was about 10 per patient-year in the first month after implantation and roughly 3 per patient-year thereafter.
The findings suggest that "significant and sustained improvements in hemodynamics, exercise capacity, and quality of life can be achieved in this population," commented Dr. Burkhoff of Columbia University in New York and also medical director of CircuLite.
The micropump (known as the Synergy System and manufactured by CircuLite in Saddle Brook, N.J.) received the CE mark for use in Europe in September.
Discussant Dr. Robert L. Kormos of the University of Pittsburgh Medical Center maintained that longer-term data will be needed to assess true sustainability of the results. He also questioned whether the 20 patients described were similar clinically to the entire group of 58 patients who have received some version of the device.
It’s unclear at this point if a U.S. trial designed for Food and Drug Administration approval will focus on a combined heart failure indication versus that of a separate indication as a bridge to transplant or destination therapy, he said.
"The trial suggested that a major reduction in adverse events compared to those seen with contemporary LVADs [left ventricular assist devices] can be achieved through the strategy of early implantation in the spectrum of heart failure and the minimally invasive approach that was used," Dr. Kormos commented. However, the relative contributions of patient selection and the device to this favorable outcome were unclear, he added.
"I do consider this in many respects a landmark [study]. This opens a window to a new therapy that many of us, especially as surgeons, are looking forward to, specifically because it allows us to operate on a group of patients who have fewer comorbidities than we currently see," he concluded.
The patients in the study had heart failure with Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 4 or higher disease, corresponding to New York Heart Association class III or IV, and were symptomatic despite appropriate medical therapy and cardiac resynchronization therapy. They were ambulatory, were not dependent on inotropes, and had recently had a hospitalization and/or increasing medical visits.
The device was used for any of three management strategies, Dr. Burkhoff noted: bridge to transplant, destination therapy, or bridge to decision. "We have not made a distinction between these because especially in Europe, the wait times for heart transplant are so long that these traditional boundaries are really being blurred," he explained.
The patients underwent implantation of the micropump, which is the size of a AA battery, weighs 25 g, and pumps 1.5-4.25 L/min. It is implanted subcutaneously and extrathoracically in a pacemaker pocket with an off-pump minithoracotomy procedure. The device pumps blood from the left atrium to the subclavian artery to increase circulation.
Of the 20 patients who received the most current version of the device, 82% had an implantable cardioverter-defibrillator and 65% had had cardiac resynchronization therapy.
"In terms of the postoperative care, it’s important to consider the differences between this and other VADs [ventricular assist devices]," Dr. Burkhoff maintained, noting the typically short times with the micropump to extubation (within hours), chest tube removal (1 day), and ambulation (1-2 days).
"In terms of the hemodynamic effectiveness, these data show that use of this partial support device can really interrupt and reverse the hemodynamic derangements of heart failure," he said.
Specifically, at a median follow-up of 12 weeks, patients had significant improvements from baseline in cardiac output (by about 1 L/min), pulmonary capillary wedge pressure (10 mm Hg), central venous pressure (5 mm Hg), pulmonary artery pressures (5-15 mm Hg), and pulmonary vascular resistance (1 Wood unit).
Because the device provides only partial cardiac support, the heart still beats regularly; thus, arterial systolic and diastolic pressures did not change significantly, and normal pulsatility in the arterial system was preserved.
Patients also had significant improvements in exercise tolerance as assessed with the 6-minute walk test (by 120 m) and peak VO2 (1.6 mL/kg per minute), and in end organ function, as assessed from creatinine level (0.5 mg/dL), but not in total bilirubin level (0.2 m/dL.
The rate of adverse events, predominantly bleeding, was 9.9 per patient-year in the first month after implantation and 2.5 per patient-year thereafter.
There were three deaths due to perioperative complications; two were related to bleeding, and one was related to a stroke after administration of two doses of vitamin K.
Taken together, the adverse events and deaths "remind us that this is still a surgical procedure, and patients are exposed to certain risks," he said.
Dr. Burkhoff disclosed that he is medical director of and a stockholder in CircuLite. Dr. Kormos disclosed that he receives research support from HeartWare. The study was sponsored by CircuLite.
SEATTLE – A tiny pump that provides partial support to patients with heart failure may improve outcomes, at least in the short term, for those in the earlier stages of the disease, clinical experience with the device has shown.
The 20 patients in Belgium and Germany who underwent implantation of the newest version of the investigational device had significantly improved hemodynamics, exercise tolerance, and end organ function at a median of 12 weeks’ follow-up, Dr. Daniel Burkhoff reported at the annual meeting of the Heart Failure Society of America.
The rate of adverse events was about 10 per patient-year in the first month after implantation and roughly 3 per patient-year thereafter.
The findings suggest that "significant and sustained improvements in hemodynamics, exercise capacity, and quality of life can be achieved in this population," commented Dr. Burkhoff of Columbia University in New York and also medical director of CircuLite.
The micropump (known as the Synergy System and manufactured by CircuLite in Saddle Brook, N.J.) received the CE mark for use in Europe in September.
Discussant Dr. Robert L. Kormos of the University of Pittsburgh Medical Center maintained that longer-term data will be needed to assess true sustainability of the results. He also questioned whether the 20 patients described were similar clinically to the entire group of 58 patients who have received some version of the device.
It’s unclear at this point if a U.S. trial designed for Food and Drug Administration approval will focus on a combined heart failure indication versus that of a separate indication as a bridge to transplant or destination therapy, he said.
"The trial suggested that a major reduction in adverse events compared to those seen with contemporary LVADs [left ventricular assist devices] can be achieved through the strategy of early implantation in the spectrum of heart failure and the minimally invasive approach that was used," Dr. Kormos commented. However, the relative contributions of patient selection and the device to this favorable outcome were unclear, he added.
"I do consider this in many respects a landmark [study]. This opens a window to a new therapy that many of us, especially as surgeons, are looking forward to, specifically because it allows us to operate on a group of patients who have fewer comorbidities than we currently see," he concluded.
The patients in the study had heart failure with Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 4 or higher disease, corresponding to New York Heart Association class III or IV, and were symptomatic despite appropriate medical therapy and cardiac resynchronization therapy. They were ambulatory, were not dependent on inotropes, and had recently had a hospitalization and/or increasing medical visits.
The device was used for any of three management strategies, Dr. Burkhoff noted: bridge to transplant, destination therapy, or bridge to decision. "We have not made a distinction between these because especially in Europe, the wait times for heart transplant are so long that these traditional boundaries are really being blurred," he explained.
The patients underwent implantation of the micropump, which is the size of a AA battery, weighs 25 g, and pumps 1.5-4.25 L/min. It is implanted subcutaneously and extrathoracically in a pacemaker pocket with an off-pump minithoracotomy procedure. The device pumps blood from the left atrium to the subclavian artery to increase circulation.
Of the 20 patients who received the most current version of the device, 82% had an implantable cardioverter-defibrillator and 65% had had cardiac resynchronization therapy.
"In terms of the postoperative care, it’s important to consider the differences between this and other VADs [ventricular assist devices]," Dr. Burkhoff maintained, noting the typically short times with the micropump to extubation (within hours), chest tube removal (1 day), and ambulation (1-2 days).
"In terms of the hemodynamic effectiveness, these data show that use of this partial support device can really interrupt and reverse the hemodynamic derangements of heart failure," he said.
Specifically, at a median follow-up of 12 weeks, patients had significant improvements from baseline in cardiac output (by about 1 L/min), pulmonary capillary wedge pressure (10 mm Hg), central venous pressure (5 mm Hg), pulmonary artery pressures (5-15 mm Hg), and pulmonary vascular resistance (1 Wood unit).
Because the device provides only partial cardiac support, the heart still beats regularly; thus, arterial systolic and diastolic pressures did not change significantly, and normal pulsatility in the arterial system was preserved.
Patients also had significant improvements in exercise tolerance as assessed with the 6-minute walk test (by 120 m) and peak VO2 (1.6 mL/kg per minute), and in end organ function, as assessed from creatinine level (0.5 mg/dL), but not in total bilirubin level (0.2 m/dL.
The rate of adverse events, predominantly bleeding, was 9.9 per patient-year in the first month after implantation and 2.5 per patient-year thereafter.
There were three deaths due to perioperative complications; two were related to bleeding, and one was related to a stroke after administration of two doses of vitamin K.
Taken together, the adverse events and deaths "remind us that this is still a surgical procedure, and patients are exposed to certain risks," he said.
Dr. Burkhoff disclosed that he is medical director of and a stockholder in CircuLite. Dr. Kormos disclosed that he receives research support from HeartWare. The study was sponsored by CircuLite.
SEATTLE – A tiny pump that provides partial support to patients with heart failure may improve outcomes, at least in the short term, for those in the earlier stages of the disease, clinical experience with the device has shown.
The 20 patients in Belgium and Germany who underwent implantation of the newest version of the investigational device had significantly improved hemodynamics, exercise tolerance, and end organ function at a median of 12 weeks’ follow-up, Dr. Daniel Burkhoff reported at the annual meeting of the Heart Failure Society of America.
The rate of adverse events was about 10 per patient-year in the first month after implantation and roughly 3 per patient-year thereafter.
The findings suggest that "significant and sustained improvements in hemodynamics, exercise capacity, and quality of life can be achieved in this population," commented Dr. Burkhoff of Columbia University in New York and also medical director of CircuLite.
The micropump (known as the Synergy System and manufactured by CircuLite in Saddle Brook, N.J.) received the CE mark for use in Europe in September.
Discussant Dr. Robert L. Kormos of the University of Pittsburgh Medical Center maintained that longer-term data will be needed to assess true sustainability of the results. He also questioned whether the 20 patients described were similar clinically to the entire group of 58 patients who have received some version of the device.
It’s unclear at this point if a U.S. trial designed for Food and Drug Administration approval will focus on a combined heart failure indication versus that of a separate indication as a bridge to transplant or destination therapy, he said.
"The trial suggested that a major reduction in adverse events compared to those seen with contemporary LVADs [left ventricular assist devices] can be achieved through the strategy of early implantation in the spectrum of heart failure and the minimally invasive approach that was used," Dr. Kormos commented. However, the relative contributions of patient selection and the device to this favorable outcome were unclear, he added.
"I do consider this in many respects a landmark [study]. This opens a window to a new therapy that many of us, especially as surgeons, are looking forward to, specifically because it allows us to operate on a group of patients who have fewer comorbidities than we currently see," he concluded.
The patients in the study had heart failure with Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 4 or higher disease, corresponding to New York Heart Association class III or IV, and were symptomatic despite appropriate medical therapy and cardiac resynchronization therapy. They were ambulatory, were not dependent on inotropes, and had recently had a hospitalization and/or increasing medical visits.
The device was used for any of three management strategies, Dr. Burkhoff noted: bridge to transplant, destination therapy, or bridge to decision. "We have not made a distinction between these because especially in Europe, the wait times for heart transplant are so long that these traditional boundaries are really being blurred," he explained.
The patients underwent implantation of the micropump, which is the size of a AA battery, weighs 25 g, and pumps 1.5-4.25 L/min. It is implanted subcutaneously and extrathoracically in a pacemaker pocket with an off-pump minithoracotomy procedure. The device pumps blood from the left atrium to the subclavian artery to increase circulation.
Of the 20 patients who received the most current version of the device, 82% had an implantable cardioverter-defibrillator and 65% had had cardiac resynchronization therapy.
"In terms of the postoperative care, it’s important to consider the differences between this and other VADs [ventricular assist devices]," Dr. Burkhoff maintained, noting the typically short times with the micropump to extubation (within hours), chest tube removal (1 day), and ambulation (1-2 days).
"In terms of the hemodynamic effectiveness, these data show that use of this partial support device can really interrupt and reverse the hemodynamic derangements of heart failure," he said.
Specifically, at a median follow-up of 12 weeks, patients had significant improvements from baseline in cardiac output (by about 1 L/min), pulmonary capillary wedge pressure (10 mm Hg), central venous pressure (5 mm Hg), pulmonary artery pressures (5-15 mm Hg), and pulmonary vascular resistance (1 Wood unit).
Because the device provides only partial cardiac support, the heart still beats regularly; thus, arterial systolic and diastolic pressures did not change significantly, and normal pulsatility in the arterial system was preserved.
Patients also had significant improvements in exercise tolerance as assessed with the 6-minute walk test (by 120 m) and peak VO2 (1.6 mL/kg per minute), and in end organ function, as assessed from creatinine level (0.5 mg/dL), but not in total bilirubin level (0.2 m/dL.
The rate of adverse events, predominantly bleeding, was 9.9 per patient-year in the first month after implantation and 2.5 per patient-year thereafter.
There were three deaths due to perioperative complications; two were related to bleeding, and one was related to a stroke after administration of two doses of vitamin K.
Taken together, the adverse events and deaths "remind us that this is still a surgical procedure, and patients are exposed to certain risks," he said.
Dr. Burkhoff disclosed that he is medical director of and a stockholder in CircuLite. Dr. Kormos disclosed that he receives research support from HeartWare. The study was sponsored by CircuLite.
AT THE ANNUAL MEETING OF THE HEART FAILURE SOCIETY OF AMERICA
Major Finding: Patients had significant improvements in hemodynamics, exercise tolerance, and end organ function. The adverse event rates were 9.9 and 2.5 per patient-year in the first month and thereafter, respectively.
Data Source: Data are from a series of 20 patients with heart failure who underwent implantation of the newest version of the micropump system.
Disclosures: Dr. Burkhoff disclosed that he is medical director of and a stockholder in CircuLite. Dr. Kormos disclosed that he receives research support from HeartWare. The study was sponsored by CircuLite.
Gastric Acid Drugs, Antibiotics Tied to Recurrent C. difficile Risk
SAN DIEGO – A variety of factors, some of them modifiable, increase the risk of recurrence of Clostridium difficile infection among inpatients, researchers reported at IDWeek.
The team retrospectively studied 4,200 adult inpatients who had an initial C. difficile infection, defined as a positive toxin assay at hospital admission in the setting of unformed stools. Overall, 10% went on to experience a recurrence within 6 weeks.
Results showed that patients had a more than one-third increase in recurrence risk if they were started on a new gastric acid–suppressing agent during treatment of their initial infection, and a near tripling of the risk if they were started on a high-risk antibiotic after completing treatment.
"Recognizing both modifiable and nonmodifiable recurrent C. difficile infection risk factors may help clinicians tailor the therapy for the initial episode of C. difficile infection more precisely," presenting author Dr. Erik R. Dubberke commented.
"Some characteristics, such as age, were not modifiable, but there were several modifiable exposures, such as gastric acid suppression as well as antimicrobial exposures during as well as after the treatment for the initial C. difficile infection," he said. "Reducing these exposures may help decrease the risk of developing future episodes of C. difficile infection." Dr. Dubberke is with the department of medicine at the Washington University in St. Louis.
Audience members inquired about the finer points of the investigation.
One attendee was "curious" about the elevated risk seen after receipt of intravenous vancomycin, "since this is a drug that doesn’t get into the stool at all." He wondered if perhaps other antibiotics given with vancomycin were actually at play.
Dr. Dubberke noted that multivariate analysis took this into consideration, and receipt of vancomycin after completing treatment was still a risk factor. "There are data to suggest that even intravenous vancomycin – though you don’t get adequate levels to treat C. difficile in the colon – can potentially get into the colon and affect the microbiome. So it’s possible there may be some direct causation," he said. "Conversely ... people who receive intravenous vancomycin tend to get other antibiotics. They also tend to be sicker patients, patients at increased risk for having drug-resistant organisms. So it is possible this is just a marker for recurrent C. difficile based on the patient characteristics."
Regarding whether severity of the initial infection affected recurrence risk, the research did not find an association on multivariate analysis with white count, new-onset renal dysfunction, baseline renal dysfunction, being in the ICU or being transferred to the ICU, or initiation of vasopressors, Dr. Dubberke explained. He speculated that lack of association was due in part to the competing risk of death.
"If a patient is very sick from C. difficile, they may die. Our [group] has demonstrated that you have an increased risk of death even after that initial episode is treated. So maybe that is why were not seeing [severity] as a risk for recurrent C. difficile."
Another attendee asked about the finding of elevated risk associated with gastric acid–suppressing medications. "[Have you] taken any practical steps at your hospital or in your health system to try to modify that risk factor because it’s medication that we clearly use and prescribe, and it’s questionable as to whether these prescriptions actually meet the indications ... " she said.
"We have not done anything in regard to interventions at this point based on the data," Dr. Dubberke replied.
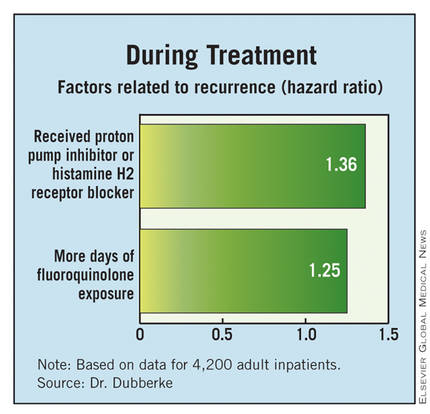
The investigators identified patients having an initial C. difficile infection on admission to Barnes-Jewish Hospital, St. Louis, between 2003 and 2009, and classified those infections according to standard definitions as health care–onset infections; community-onset, health care facility–associated infections; or community-associated infections.
They classified antibiotics received as high risk (clindamycin, cephalosporins, and aminopenicillins), intravenous vancomycin, fluoroquinolones, and low risk (all others).
Study results shows that, compared with their peers who did not have a recurrence, patients who did were older and more likely to have diabetes, had higher levels of comorbidity, were more likely to have a community-onset, health care facility–associated initial infection, and were more likely to have been hospitalized in the past 60 days, Dr. Dubberke reported at the meeting, which is a the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society..
In multivariate analyses, patients were significantly more likely to have a recurrence of C. difficile if their initial infection was a community-onset, health care facility–associated infection as compared with a health care–onset infection (hazard ratio, 1.80) or they had at least two hospitalizations in the 60 days before that infection (HR, 1.40). Additionally, risk increased with age (HR, 1.01).
With respect to factors during the treatment of the initial infection, patients were more likely to have a recurrence if they received a new gastric acid–suppressing agent (a proton pump inhibitor or histamine H2 receptor blocker) (HR, 1.36) or had more days of fluoroquinolone exposure (1.24).
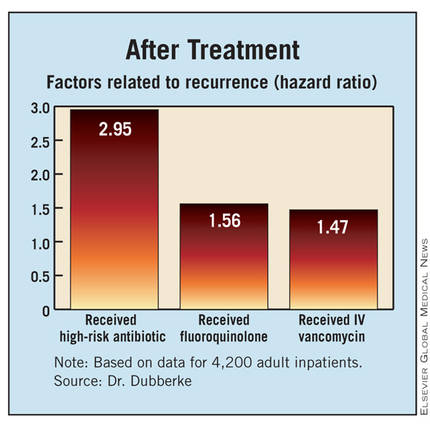
Finally, with respect to factors after completing treatment of the initial infection, patients were more likely to have a recurrence if they received a high-risk antibiotic (HR, 2.95), received a fluoroquinolone (HR, 1.56), or received intravenous vancomycin (HR, 1.45).
Discussing the study’s limitations, Dr. Dubberke noted that the investigators relied on the hospital database to identify cases of C. difficile recurrence. "We likely missed recurrences that were diagnosed and managed as outpatients," he acknowledged. "However, conversely, the recurrences that were identified were presumably more severe in that these patients ended up having another contact with our hospital, either in the emergency room" or through direct admission.
Dr. Dubberke disclosed that he has received research funding from Optimer Pharmaceuticals, ViroPharma, Merck, and Sanofi Pasteur, and that he has been a consultant to Optimer, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
SAN DIEGO – A variety of factors, some of them modifiable, increase the risk of recurrence of Clostridium difficile infection among inpatients, researchers reported at IDWeek.
The team retrospectively studied 4,200 adult inpatients who had an initial C. difficile infection, defined as a positive toxin assay at hospital admission in the setting of unformed stools. Overall, 10% went on to experience a recurrence within 6 weeks.
Results showed that patients had a more than one-third increase in recurrence risk if they were started on a new gastric acid–suppressing agent during treatment of their initial infection, and a near tripling of the risk if they were started on a high-risk antibiotic after completing treatment.
"Recognizing both modifiable and nonmodifiable recurrent C. difficile infection risk factors may help clinicians tailor the therapy for the initial episode of C. difficile infection more precisely," presenting author Dr. Erik R. Dubberke commented.
"Some characteristics, such as age, were not modifiable, but there were several modifiable exposures, such as gastric acid suppression as well as antimicrobial exposures during as well as after the treatment for the initial C. difficile infection," he said. "Reducing these exposures may help decrease the risk of developing future episodes of C. difficile infection." Dr. Dubberke is with the department of medicine at the Washington University in St. Louis.
Audience members inquired about the finer points of the investigation.
One attendee was "curious" about the elevated risk seen after receipt of intravenous vancomycin, "since this is a drug that doesn’t get into the stool at all." He wondered if perhaps other antibiotics given with vancomycin were actually at play.
Dr. Dubberke noted that multivariate analysis took this into consideration, and receipt of vancomycin after completing treatment was still a risk factor. "There are data to suggest that even intravenous vancomycin – though you don’t get adequate levels to treat C. difficile in the colon – can potentially get into the colon and affect the microbiome. So it’s possible there may be some direct causation," he said. "Conversely ... people who receive intravenous vancomycin tend to get other antibiotics. They also tend to be sicker patients, patients at increased risk for having drug-resistant organisms. So it is possible this is just a marker for recurrent C. difficile based on the patient characteristics."
Regarding whether severity of the initial infection affected recurrence risk, the research did not find an association on multivariate analysis with white count, new-onset renal dysfunction, baseline renal dysfunction, being in the ICU or being transferred to the ICU, or initiation of vasopressors, Dr. Dubberke explained. He speculated that lack of association was due in part to the competing risk of death.
"If a patient is very sick from C. difficile, they may die. Our [group] has demonstrated that you have an increased risk of death even after that initial episode is treated. So maybe that is why were not seeing [severity] as a risk for recurrent C. difficile."
Another attendee asked about the finding of elevated risk associated with gastric acid–suppressing medications. "[Have you] taken any practical steps at your hospital or in your health system to try to modify that risk factor because it’s medication that we clearly use and prescribe, and it’s questionable as to whether these prescriptions actually meet the indications ... " she said.
"We have not done anything in regard to interventions at this point based on the data," Dr. Dubberke replied.

The investigators identified patients having an initial C. difficile infection on admission to Barnes-Jewish Hospital, St. Louis, between 2003 and 2009, and classified those infections according to standard definitions as health care–onset infections; community-onset, health care facility–associated infections; or community-associated infections.
They classified antibiotics received as high risk (clindamycin, cephalosporins, and aminopenicillins), intravenous vancomycin, fluoroquinolones, and low risk (all others).
Study results shows that, compared with their peers who did not have a recurrence, patients who did were older and more likely to have diabetes, had higher levels of comorbidity, were more likely to have a community-onset, health care facility–associated initial infection, and were more likely to have been hospitalized in the past 60 days, Dr. Dubberke reported at the meeting, which is a the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society..
In multivariate analyses, patients were significantly more likely to have a recurrence of C. difficile if their initial infection was a community-onset, health care facility–associated infection as compared with a health care–onset infection (hazard ratio, 1.80) or they had at least two hospitalizations in the 60 days before that infection (HR, 1.40). Additionally, risk increased with age (HR, 1.01).
With respect to factors during the treatment of the initial infection, patients were more likely to have a recurrence if they received a new gastric acid–suppressing agent (a proton pump inhibitor or histamine H2 receptor blocker) (HR, 1.36) or had more days of fluoroquinolone exposure (1.24).

Finally, with respect to factors after completing treatment of the initial infection, patients were more likely to have a recurrence if they received a high-risk antibiotic (HR, 2.95), received a fluoroquinolone (HR, 1.56), or received intravenous vancomycin (HR, 1.45).
Discussing the study’s limitations, Dr. Dubberke noted that the investigators relied on the hospital database to identify cases of C. difficile recurrence. "We likely missed recurrences that were diagnosed and managed as outpatients," he acknowledged. "However, conversely, the recurrences that were identified were presumably more severe in that these patients ended up having another contact with our hospital, either in the emergency room" or through direct admission.
Dr. Dubberke disclosed that he has received research funding from Optimer Pharmaceuticals, ViroPharma, Merck, and Sanofi Pasteur, and that he has been a consultant to Optimer, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
SAN DIEGO – A variety of factors, some of them modifiable, increase the risk of recurrence of Clostridium difficile infection among inpatients, researchers reported at IDWeek.
The team retrospectively studied 4,200 adult inpatients who had an initial C. difficile infection, defined as a positive toxin assay at hospital admission in the setting of unformed stools. Overall, 10% went on to experience a recurrence within 6 weeks.
Results showed that patients had a more than one-third increase in recurrence risk if they were started on a new gastric acid–suppressing agent during treatment of their initial infection, and a near tripling of the risk if they were started on a high-risk antibiotic after completing treatment.
"Recognizing both modifiable and nonmodifiable recurrent C. difficile infection risk factors may help clinicians tailor the therapy for the initial episode of C. difficile infection more precisely," presenting author Dr. Erik R. Dubberke commented.
"Some characteristics, such as age, were not modifiable, but there were several modifiable exposures, such as gastric acid suppression as well as antimicrobial exposures during as well as after the treatment for the initial C. difficile infection," he said. "Reducing these exposures may help decrease the risk of developing future episodes of C. difficile infection." Dr. Dubberke is with the department of medicine at the Washington University in St. Louis.
Audience members inquired about the finer points of the investigation.
One attendee was "curious" about the elevated risk seen after receipt of intravenous vancomycin, "since this is a drug that doesn’t get into the stool at all." He wondered if perhaps other antibiotics given with vancomycin were actually at play.
Dr. Dubberke noted that multivariate analysis took this into consideration, and receipt of vancomycin after completing treatment was still a risk factor. "There are data to suggest that even intravenous vancomycin – though you don’t get adequate levels to treat C. difficile in the colon – can potentially get into the colon and affect the microbiome. So it’s possible there may be some direct causation," he said. "Conversely ... people who receive intravenous vancomycin tend to get other antibiotics. They also tend to be sicker patients, patients at increased risk for having drug-resistant organisms. So it is possible this is just a marker for recurrent C. difficile based on the patient characteristics."
Regarding whether severity of the initial infection affected recurrence risk, the research did not find an association on multivariate analysis with white count, new-onset renal dysfunction, baseline renal dysfunction, being in the ICU or being transferred to the ICU, or initiation of vasopressors, Dr. Dubberke explained. He speculated that lack of association was due in part to the competing risk of death.
"If a patient is very sick from C. difficile, they may die. Our [group] has demonstrated that you have an increased risk of death even after that initial episode is treated. So maybe that is why were not seeing [severity] as a risk for recurrent C. difficile."
Another attendee asked about the finding of elevated risk associated with gastric acid–suppressing medications. "[Have you] taken any practical steps at your hospital or in your health system to try to modify that risk factor because it’s medication that we clearly use and prescribe, and it’s questionable as to whether these prescriptions actually meet the indications ... " she said.
"We have not done anything in regard to interventions at this point based on the data," Dr. Dubberke replied.

The investigators identified patients having an initial C. difficile infection on admission to Barnes-Jewish Hospital, St. Louis, between 2003 and 2009, and classified those infections according to standard definitions as health care–onset infections; community-onset, health care facility–associated infections; or community-associated infections.
They classified antibiotics received as high risk (clindamycin, cephalosporins, and aminopenicillins), intravenous vancomycin, fluoroquinolones, and low risk (all others).
Study results shows that, compared with their peers who did not have a recurrence, patients who did were older and more likely to have diabetes, had higher levels of comorbidity, were more likely to have a community-onset, health care facility–associated initial infection, and were more likely to have been hospitalized in the past 60 days, Dr. Dubberke reported at the meeting, which is a the combined annual meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society..
In multivariate analyses, patients were significantly more likely to have a recurrence of C. difficile if their initial infection was a community-onset, health care facility–associated infection as compared with a health care–onset infection (hazard ratio, 1.80) or they had at least two hospitalizations in the 60 days before that infection (HR, 1.40). Additionally, risk increased with age (HR, 1.01).
With respect to factors during the treatment of the initial infection, patients were more likely to have a recurrence if they received a new gastric acid–suppressing agent (a proton pump inhibitor or histamine H2 receptor blocker) (HR, 1.36) or had more days of fluoroquinolone exposure (1.24).

Finally, with respect to factors after completing treatment of the initial infection, patients were more likely to have a recurrence if they received a high-risk antibiotic (HR, 2.95), received a fluoroquinolone (HR, 1.56), or received intravenous vancomycin (HR, 1.45).
Discussing the study’s limitations, Dr. Dubberke noted that the investigators relied on the hospital database to identify cases of C. difficile recurrence. "We likely missed recurrences that were diagnosed and managed as outpatients," he acknowledged. "However, conversely, the recurrences that were identified were presumably more severe in that these patients ended up having another contact with our hospital, either in the emergency room" or through direct admission.
Dr. Dubberke disclosed that he has received research funding from Optimer Pharmaceuticals, ViroPharma, Merck, and Sanofi Pasteur, and that he has been a consultant to Optimer, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
AT IDWEEK
Major Finding: Risk factors for recurrence included starting gastric acid suppression during treatment of the initial infection (hazard ratio, 1.36) and receipt of a high-risk antibiotic after completing treatment (HR, 2.95).
Data Source: Results were taken from a retrospective cohort study of 4,200 adults having C. difficile infection on hospital admission
Disclosures: Dr. Dubberke disclosed that he has received research funding from and/or been a consultant for Optimer Pharmaceuticals, ViroPharma, Merck, Pfizer, and Sanofi Pasteur. The study was supported by Optimer.
Infliximab Not Cardioprotective in Kawasaki
SAN DIEGO – Intensifying primary therapy for Kawasaki disease by adding infliximab, an antibody to tumor necrosis factor–alpha, improves certain outcomes but not others, finds a phase III randomized trial reported at IDWeek.
The 196 children studied, all of whom were given intravenous immunoglobulin (IVIG), had a 22% lower erythrocyte sedimentation rate and a 50% or 1-day shorter duration of fever if they also received infliximab. But there was no effect on the rate of treatment resistance (the trial’s primary outcome) or the development of coronary aneurysms.
The findings "really beg the question of what is the role of infliximab in Kawasaki disease," commented Dr. Adriana Tremoulet, a pediatric infectious disease specialist at the Rady Children’s Hospital in San Diego.
"For primary therapy, what I would say is that it’s safe to use. The data do suggest a biological and clinical effect, but there is no reduction in treatment resistance or coronary artery abnormalities that we have proved to date," she said. "In addition, many of you may wonder what we will do about rescue therapy for IVIG resistance. Again, I think we definitely have enough data to say that it’s a safe alternative to a second IVIG [treatment], but that the efficacy is unproved at this time."
In the trial, children aged 4 weeks to 7 years with acute Kawasaki disease were randomized to infliximab (Remicade, 5 mg/kg) or placebo, each followed by IVIG (2 g/kg).
The children had a median age of 2.8 years (10% were younger than 1 year), and 62% were boys. The median duration of illness at trial enrollment was 6 days, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Results showed that, compared with their counterparts in the placebo group, patients in the infliximab group had less inflammation, as indicated by lower levels of C-reactive protein at 24 hours (4.1 vs. 5.3 mg/dL; P = .01) and a lower absolute neutrophil count (2,271 mm3 vs. 2,706 mm3; P = .001) and erythrocyte sedimentation rate (36 vs. 46; P = .003) at 2 weeks.
On the other hand, the infliximab group had a higher absolute lymphocyte count at 2 weeks (3,630 vs. 2,996; P = .002). "This is something we do see in children as they are going from an acute to a subacute change in their illness," Dr. Tremoulet commented.
The median duration of fever after enrollment was 1 day in the infliximab group, compared with 2 days in the placebo group (P less than .0001). The rate of IVIG infusion reactions, despite premedication, was also lower in the infliximab group (0% vs. 13%).
However, the rate of treatment resistance – defined as presence of fever between 36 hours and 7 days after the end of the first IVIG infusion – did not differ significantly between the groups (11% vs. 11%). And the same was true of the rate of coronary aneurysms detected by echocardiography (4% vs. 6%).
"It was safe to use infliximab even in children less than 1 year of age. Infliximab was tolerated well, even in the infants," Dr. Tremoulet maintained. Rates of serious adverse did not differ between treatment groups.
The investigators are now evaluating levels of various inflammatory cytokines, such as alpha1-antitrypsin and tumor necrosis factor–receptors, to better understand the biological effects of infliximab in this population, she noted.
Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech, the manufacturer.
SAN DIEGO – Intensifying primary therapy for Kawasaki disease by adding infliximab, an antibody to tumor necrosis factor–alpha, improves certain outcomes but not others, finds a phase III randomized trial reported at IDWeek.
The 196 children studied, all of whom were given intravenous immunoglobulin (IVIG), had a 22% lower erythrocyte sedimentation rate and a 50% or 1-day shorter duration of fever if they also received infliximab. But there was no effect on the rate of treatment resistance (the trial’s primary outcome) or the development of coronary aneurysms.
The findings "really beg the question of what is the role of infliximab in Kawasaki disease," commented Dr. Adriana Tremoulet, a pediatric infectious disease specialist at the Rady Children’s Hospital in San Diego.
"For primary therapy, what I would say is that it’s safe to use. The data do suggest a biological and clinical effect, but there is no reduction in treatment resistance or coronary artery abnormalities that we have proved to date," she said. "In addition, many of you may wonder what we will do about rescue therapy for IVIG resistance. Again, I think we definitely have enough data to say that it’s a safe alternative to a second IVIG [treatment], but that the efficacy is unproved at this time."
In the trial, children aged 4 weeks to 7 years with acute Kawasaki disease were randomized to infliximab (Remicade, 5 mg/kg) or placebo, each followed by IVIG (2 g/kg).
The children had a median age of 2.8 years (10% were younger than 1 year), and 62% were boys. The median duration of illness at trial enrollment was 6 days, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Results showed that, compared with their counterparts in the placebo group, patients in the infliximab group had less inflammation, as indicated by lower levels of C-reactive protein at 24 hours (4.1 vs. 5.3 mg/dL; P = .01) and a lower absolute neutrophil count (2,271 mm3 vs. 2,706 mm3; P = .001) and erythrocyte sedimentation rate (36 vs. 46; P = .003) at 2 weeks.
On the other hand, the infliximab group had a higher absolute lymphocyte count at 2 weeks (3,630 vs. 2,996; P = .002). "This is something we do see in children as they are going from an acute to a subacute change in their illness," Dr. Tremoulet commented.
The median duration of fever after enrollment was 1 day in the infliximab group, compared with 2 days in the placebo group (P less than .0001). The rate of IVIG infusion reactions, despite premedication, was also lower in the infliximab group (0% vs. 13%).
However, the rate of treatment resistance – defined as presence of fever between 36 hours and 7 days after the end of the first IVIG infusion – did not differ significantly between the groups (11% vs. 11%). And the same was true of the rate of coronary aneurysms detected by echocardiography (4% vs. 6%).
"It was safe to use infliximab even in children less than 1 year of age. Infliximab was tolerated well, even in the infants," Dr. Tremoulet maintained. Rates of serious adverse did not differ between treatment groups.
The investigators are now evaluating levels of various inflammatory cytokines, such as alpha1-antitrypsin and tumor necrosis factor–receptors, to better understand the biological effects of infliximab in this population, she noted.
Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech, the manufacturer.
SAN DIEGO – Intensifying primary therapy for Kawasaki disease by adding infliximab, an antibody to tumor necrosis factor–alpha, improves certain outcomes but not others, finds a phase III randomized trial reported at IDWeek.
The 196 children studied, all of whom were given intravenous immunoglobulin (IVIG), had a 22% lower erythrocyte sedimentation rate and a 50% or 1-day shorter duration of fever if they also received infliximab. But there was no effect on the rate of treatment resistance (the trial’s primary outcome) or the development of coronary aneurysms.
The findings "really beg the question of what is the role of infliximab in Kawasaki disease," commented Dr. Adriana Tremoulet, a pediatric infectious disease specialist at the Rady Children’s Hospital in San Diego.
"For primary therapy, what I would say is that it’s safe to use. The data do suggest a biological and clinical effect, but there is no reduction in treatment resistance or coronary artery abnormalities that we have proved to date," she said. "In addition, many of you may wonder what we will do about rescue therapy for IVIG resistance. Again, I think we definitely have enough data to say that it’s a safe alternative to a second IVIG [treatment], but that the efficacy is unproved at this time."
In the trial, children aged 4 weeks to 7 years with acute Kawasaki disease were randomized to infliximab (Remicade, 5 mg/kg) or placebo, each followed by IVIG (2 g/kg).
The children had a median age of 2.8 years (10% were younger than 1 year), and 62% were boys. The median duration of illness at trial enrollment was 6 days, according to data reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Results showed that, compared with their counterparts in the placebo group, patients in the infliximab group had less inflammation, as indicated by lower levels of C-reactive protein at 24 hours (4.1 vs. 5.3 mg/dL; P = .01) and a lower absolute neutrophil count (2,271 mm3 vs. 2,706 mm3; P = .001) and erythrocyte sedimentation rate (36 vs. 46; P = .003) at 2 weeks.
On the other hand, the infliximab group had a higher absolute lymphocyte count at 2 weeks (3,630 vs. 2,996; P = .002). "This is something we do see in children as they are going from an acute to a subacute change in their illness," Dr. Tremoulet commented.
The median duration of fever after enrollment was 1 day in the infliximab group, compared with 2 days in the placebo group (P less than .0001). The rate of IVIG infusion reactions, despite premedication, was also lower in the infliximab group (0% vs. 13%).
However, the rate of treatment resistance – defined as presence of fever between 36 hours and 7 days after the end of the first IVIG infusion – did not differ significantly between the groups (11% vs. 11%). And the same was true of the rate of coronary aneurysms detected by echocardiography (4% vs. 6%).
"It was safe to use infliximab even in children less than 1 year of age. Infliximab was tolerated well, even in the infants," Dr. Tremoulet maintained. Rates of serious adverse did not differ between treatment groups.
The investigators are now evaluating levels of various inflammatory cytokines, such as alpha1-antitrypsin and tumor necrosis factor–receptors, to better understand the biological effects of infliximab in this population, she noted.
Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech, the manufacturer.
AT IDWEEK
Major Finding: Compared with placebo, infliximab reduced the erythrocyte sedimentation rate by 22% and shortened the duration of fever by 50%, but did not alter rates of treatment resistance or development of coronary artery aneurysms.
Data Source: These findings come from a phase III randomized trial among 196 children with acute Kawasaki disease also given IVIG.
Disclosures: Dr. Tremoulet disclosed no relevant conflicts of interest. Infliximab was donated by Janssen Biotech.
Pertussis Immunity Drop Linked to Whole Cell Vaccine Withdrawal
SAN DIEGO – The declining herd immunity to pertussis seen in the United States community may be related to withdrawal of the whole cell vaccine from the market about a decade ago, a study of more than 450,000 vaccinated patients from Kaiser Permanente Medical Center has shown.
Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had at least a tripling of the risk of acquiring the disease, lead author Maxwell Witt reported at IDWeek. The association was still present but weaker among patients who had received a total of six doses versus five.
"Acellular pertussis vaccine offered significantly less protection when compared with the whole cell vaccine," commented Mr. Witt of Kaiser Permanente in San Rafael, Calif. "The risk of pertussis was mitigated, but not eliminated, by a sixth dose of pertussis vaccine, the Tdap [tetanus, diphtheria, and acellular pertussis] vaccine.
"The current generation of children is the first to have been vaccinated solely with the acellular pertussis vaccine," Mr. Witt said. "Our findings would predict a significant population of underprotected children in this group." Recent outbreaks of pertussis in the United States "had peak attack rates among those who are exactly in the same age group," he noted.
"The waning immunity associated with these outbreaks is clearly a call for development of more effective and durable pertussis vaccines," Mr. Witt said. "In the shorter term, strategies to prevent these outbreaks of pertussis could include either earlier or additional booster doses, and targeted vaccination programs to address insufficient immunity in outbreak situations."
The field of pertussis vaccination has undergone transition, with the introduction of the acellular vaccine in 1991 and retirement of the whole cell vaccine in 2001. "In 2010, we saw the largest epidemic of Bordatella pertussis in California in more than 50 years, and that epidemic subsequently spread across the United States," he said at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In a previous study, the investigators noted waning pertussis immunity among preadolescents (with a peak attack rate among 8- to 12-year-olds) but also robust immunity among adolescents (with a sharply lower attack rate among 13-year-olds) (Clin. Infect. Dis. 2010;54:1730-35), suggesting a possible role for the vaccine transition.
In the new study, the investigators used electronic health records to identify vaccines administered and cases of laboratory-confirmed pertussis among Kaiser Permanente members aged 8-20 years in Northern California.
This age range was chosen to include patients who were old enough to have received whole cell vaccine and young enough to have received acellular vaccine, explained Mr. Witt. The search identified 465,059 patients, among whom there were 1,424 cases of pertussis.
A total of 253,005 patients had received all of their vaccine doses at Kaiser Permanente. Analyses restricted to this group showed that among patients who had received five total doses of vaccine, the pertussis attack rate was 786/100,000 for those who had received only acellular vaccine, compared with 92/100,000 for those who had received at least one dose of whole cell vaccine. The difference corresponded to an 8.57-fold higher risk for the former group (P less than .0001).
Similarly, among patients who had received six total doses of vaccine, the attack rate was 378 vs. 106/100,000 for those who received only acellular vaccine compared with those who had received at least one dose of whole cell vaccine. Here, the difference amounted to a smaller but still significant 3.55-fold higher risk.
The study findings were essentially the same when analyses were based on all patients, including those who had received at least some doses of vaccine outside of Kaiser Permanente, with respective 6.76- and 2.46-fold higher risks for patients given only acellular vaccine, depending on the total number of doses.
Mr. Witt disclosed no relevant financial conflicts.
SAN DIEGO – The declining herd immunity to pertussis seen in the United States community may be related to withdrawal of the whole cell vaccine from the market about a decade ago, a study of more than 450,000 vaccinated patients from Kaiser Permanente Medical Center has shown.
Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had at least a tripling of the risk of acquiring the disease, lead author Maxwell Witt reported at IDWeek. The association was still present but weaker among patients who had received a total of six doses versus five.
"Acellular pertussis vaccine offered significantly less protection when compared with the whole cell vaccine," commented Mr. Witt of Kaiser Permanente in San Rafael, Calif. "The risk of pertussis was mitigated, but not eliminated, by a sixth dose of pertussis vaccine, the Tdap [tetanus, diphtheria, and acellular pertussis] vaccine.
"The current generation of children is the first to have been vaccinated solely with the acellular pertussis vaccine," Mr. Witt said. "Our findings would predict a significant population of underprotected children in this group." Recent outbreaks of pertussis in the United States "had peak attack rates among those who are exactly in the same age group," he noted.
"The waning immunity associated with these outbreaks is clearly a call for development of more effective and durable pertussis vaccines," Mr. Witt said. "In the shorter term, strategies to prevent these outbreaks of pertussis could include either earlier or additional booster doses, and targeted vaccination programs to address insufficient immunity in outbreak situations."
The field of pertussis vaccination has undergone transition, with the introduction of the acellular vaccine in 1991 and retirement of the whole cell vaccine in 2001. "In 2010, we saw the largest epidemic of Bordatella pertussis in California in more than 50 years, and that epidemic subsequently spread across the United States," he said at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In a previous study, the investigators noted waning pertussis immunity among preadolescents (with a peak attack rate among 8- to 12-year-olds) but also robust immunity among adolescents (with a sharply lower attack rate among 13-year-olds) (Clin. Infect. Dis. 2010;54:1730-35), suggesting a possible role for the vaccine transition.
In the new study, the investigators used electronic health records to identify vaccines administered and cases of laboratory-confirmed pertussis among Kaiser Permanente members aged 8-20 years in Northern California.
This age range was chosen to include patients who were old enough to have received whole cell vaccine and young enough to have received acellular vaccine, explained Mr. Witt. The search identified 465,059 patients, among whom there were 1,424 cases of pertussis.
A total of 253,005 patients had received all of their vaccine doses at Kaiser Permanente. Analyses restricted to this group showed that among patients who had received five total doses of vaccine, the pertussis attack rate was 786/100,000 for those who had received only acellular vaccine, compared with 92/100,000 for those who had received at least one dose of whole cell vaccine. The difference corresponded to an 8.57-fold higher risk for the former group (P less than .0001).
Similarly, among patients who had received six total doses of vaccine, the attack rate was 378 vs. 106/100,000 for those who received only acellular vaccine compared with those who had received at least one dose of whole cell vaccine. Here, the difference amounted to a smaller but still significant 3.55-fold higher risk.
The study findings were essentially the same when analyses were based on all patients, including those who had received at least some doses of vaccine outside of Kaiser Permanente, with respective 6.76- and 2.46-fold higher risks for patients given only acellular vaccine, depending on the total number of doses.
Mr. Witt disclosed no relevant financial conflicts.
SAN DIEGO – The declining herd immunity to pertussis seen in the United States community may be related to withdrawal of the whole cell vaccine from the market about a decade ago, a study of more than 450,000 vaccinated patients from Kaiser Permanente Medical Center has shown.
Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had at least a tripling of the risk of acquiring the disease, lead author Maxwell Witt reported at IDWeek. The association was still present but weaker among patients who had received a total of six doses versus five.
"Acellular pertussis vaccine offered significantly less protection when compared with the whole cell vaccine," commented Mr. Witt of Kaiser Permanente in San Rafael, Calif. "The risk of pertussis was mitigated, but not eliminated, by a sixth dose of pertussis vaccine, the Tdap [tetanus, diphtheria, and acellular pertussis] vaccine.
"The current generation of children is the first to have been vaccinated solely with the acellular pertussis vaccine," Mr. Witt said. "Our findings would predict a significant population of underprotected children in this group." Recent outbreaks of pertussis in the United States "had peak attack rates among those who are exactly in the same age group," he noted.
"The waning immunity associated with these outbreaks is clearly a call for development of more effective and durable pertussis vaccines," Mr. Witt said. "In the shorter term, strategies to prevent these outbreaks of pertussis could include either earlier or additional booster doses, and targeted vaccination programs to address insufficient immunity in outbreak situations."
The field of pertussis vaccination has undergone transition, with the introduction of the acellular vaccine in 1991 and retirement of the whole cell vaccine in 2001. "In 2010, we saw the largest epidemic of Bordatella pertussis in California in more than 50 years, and that epidemic subsequently spread across the United States," he said at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In a previous study, the investigators noted waning pertussis immunity among preadolescents (with a peak attack rate among 8- to 12-year-olds) but also robust immunity among adolescents (with a sharply lower attack rate among 13-year-olds) (Clin. Infect. Dis. 2010;54:1730-35), suggesting a possible role for the vaccine transition.
In the new study, the investigators used electronic health records to identify vaccines administered and cases of laboratory-confirmed pertussis among Kaiser Permanente members aged 8-20 years in Northern California.
This age range was chosen to include patients who were old enough to have received whole cell vaccine and young enough to have received acellular vaccine, explained Mr. Witt. The search identified 465,059 patients, among whom there were 1,424 cases of pertussis.
A total of 253,005 patients had received all of their vaccine doses at Kaiser Permanente. Analyses restricted to this group showed that among patients who had received five total doses of vaccine, the pertussis attack rate was 786/100,000 for those who had received only acellular vaccine, compared with 92/100,000 for those who had received at least one dose of whole cell vaccine. The difference corresponded to an 8.57-fold higher risk for the former group (P less than .0001).
Similarly, among patients who had received six total doses of vaccine, the attack rate was 378 vs. 106/100,000 for those who received only acellular vaccine compared with those who had received at least one dose of whole cell vaccine. Here, the difference amounted to a smaller but still significant 3.55-fold higher risk.
The study findings were essentially the same when analyses were based on all patients, including those who had received at least some doses of vaccine outside of Kaiser Permanente, with respective 6.76- and 2.46-fold higher risks for patients given only acellular vaccine, depending on the total number of doses.
Mr. Witt disclosed no relevant financial conflicts.
AT IDWEEK
Major Finding: Compared with their peers who had received at least one dose of whole cell vaccine, patients who had received only acellular vaccine had a 3.55- to 8.57-fold higher risk of pertussis.
Data Source: A cross-sectional study of 465,059 vaccinated patients aged 8-20 years from a single health care system
Disclosures: Mr. Witt disclosed no relevant financial conflicts.
Nasal Povidone-Iodine Cuts Postop Infections
SAN DIEGO – Preoperative nasal application of a povidone-iodine solution may be more efficacious than nasal mupirocin for preventing deep surgical site infections caused by Staphylococcus aureus, a study has shown.
Investigators led by Dr. Michael Phillips of the New York University Langone Medical Center conducted a randomized trial, assigning 1,697 patients undergoing arthroplasty or spine fusion surgery evenly to the two treatments. All patients in the study also underwent chlorhexidine cleansing of the skin and received standard antimicrobial prophylaxis.
Trial results, reported at IDWeek, showed that the rate of S. aureus deep surgical site infections was one-sixth as high in the povidone-iodine group as in the mupirocin group. The povidone-iodine group also had a lower rate of adverse events and less often rated their treatment as unpleasant.
"We feel that individuals should consider the use of nasal povidone-iodine as a component of a multifaceted approach to prevent S. aureus infection after high-risk surgery," Dr. Phillips said.
In the trial, adult patients undergoing arthroplasty or spine fusion surgery at the NYU Hospital for Joint Diseases had nasal cultures for S. aureus before surgery and were then assigned to two groups.
In one group, patients were given 2% mupirocin nasal ointment and told to apply it twice daily for the 5 days leading up to surgery. The mupirocin was provided directly to the patients because a survey suggested that some patients were skipping the mupirocin because of its cost, as it is not routinely covered by insurance, he explained at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the other group, research personnel applied a 5% solution of povidone-iodine to both nostrils in patients up to 2 hours before incision.
Both groups also were given chlorhexidine wipes and told to use them from chin to toes the evening before and again the morning of surgery. Both groups received standard perioperative antimicrobial prophylaxis (cefazolin or clindamycin, plus vancomycin for those having a positive nasal culture for methicillin-resistant S. aureus). Both also had surgical site prepping with 2% chlorhexidine and alcohol.
The patients’ median age was 62 years; 60% were women and 80% were white. Preoperative nasal culture results showed that one in five patients was colonized with S. aureus.
The rate of noncompletion of the protocol was 11% in the mupirocin group and 8% in the povidone-iodine group, Dr. Phillips reported. The leading reason for noncompletion in the mupirocin group was failure to apply mupirocin at least seven times. In the povidone-iodine group, it was failure to use the chlorhexidine wipes.
In intent-to-treat analyses, patients in the nasal povidone-iodine group had a 0.7% rate of any deep surgical site infection at 3 months; those in the mupirocin group had a 1.6% rate. The rate of S. aureus deep surgical site infections was 0.1% and. 0.6%, respectively.
In a univariate analysis, patients had a higher risk of S. aureus deep surgical site infection if they were in the mupirocin group (relative risk, 1.01; P = .04) or were colonized with S. aureus preoperatively (relative risk, 6.79; P = .02).
When patients were stratified by colonization status, there was a trend toward a lower rate of S. aureus deep surgical site infection with povidone-iodine versus mupirocin for those who were colonized (P = .08) but not for those who were not.
Patients in the povidone-iodine group had a lower rate of study drug adverse events overall (2% vs. 9%, P less than .0001). The difference was significant for rhinorrhea, headache, congestion, and pharyngeal pain individually.
In addition, patients in the povidone-iodine group were less likely to report that application of the study medication was unpleasant or very unpleasant (4% vs. 38%).
Dr. Phillips disclosed that he received a research grant from 3M Corp., manufacturer of the povidone-iodine solution. The study was supported by 3M.
SAN DIEGO – Preoperative nasal application of a povidone-iodine solution may be more efficacious than nasal mupirocin for preventing deep surgical site infections caused by Staphylococcus aureus, a study has shown.
Investigators led by Dr. Michael Phillips of the New York University Langone Medical Center conducted a randomized trial, assigning 1,697 patients undergoing arthroplasty or spine fusion surgery evenly to the two treatments. All patients in the study also underwent chlorhexidine cleansing of the skin and received standard antimicrobial prophylaxis.
Trial results, reported at IDWeek, showed that the rate of S. aureus deep surgical site infections was one-sixth as high in the povidone-iodine group as in the mupirocin group. The povidone-iodine group also had a lower rate of adverse events and less often rated their treatment as unpleasant.
"We feel that individuals should consider the use of nasal povidone-iodine as a component of a multifaceted approach to prevent S. aureus infection after high-risk surgery," Dr. Phillips said.
In the trial, adult patients undergoing arthroplasty or spine fusion surgery at the NYU Hospital for Joint Diseases had nasal cultures for S. aureus before surgery and were then assigned to two groups.
In one group, patients were given 2% mupirocin nasal ointment and told to apply it twice daily for the 5 days leading up to surgery. The mupirocin was provided directly to the patients because a survey suggested that some patients were skipping the mupirocin because of its cost, as it is not routinely covered by insurance, he explained at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the other group, research personnel applied a 5% solution of povidone-iodine to both nostrils in patients up to 2 hours before incision.
Both groups also were given chlorhexidine wipes and told to use them from chin to toes the evening before and again the morning of surgery. Both groups received standard perioperative antimicrobial prophylaxis (cefazolin or clindamycin, plus vancomycin for those having a positive nasal culture for methicillin-resistant S. aureus). Both also had surgical site prepping with 2% chlorhexidine and alcohol.
The patients’ median age was 62 years; 60% were women and 80% were white. Preoperative nasal culture results showed that one in five patients was colonized with S. aureus.
The rate of noncompletion of the protocol was 11% in the mupirocin group and 8% in the povidone-iodine group, Dr. Phillips reported. The leading reason for noncompletion in the mupirocin group was failure to apply mupirocin at least seven times. In the povidone-iodine group, it was failure to use the chlorhexidine wipes.
In intent-to-treat analyses, patients in the nasal povidone-iodine group had a 0.7% rate of any deep surgical site infection at 3 months; those in the mupirocin group had a 1.6% rate. The rate of S. aureus deep surgical site infections was 0.1% and. 0.6%, respectively.
In a univariate analysis, patients had a higher risk of S. aureus deep surgical site infection if they were in the mupirocin group (relative risk, 1.01; P = .04) or were colonized with S. aureus preoperatively (relative risk, 6.79; P = .02).
When patients were stratified by colonization status, there was a trend toward a lower rate of S. aureus deep surgical site infection with povidone-iodine versus mupirocin for those who were colonized (P = .08) but not for those who were not.
Patients in the povidone-iodine group had a lower rate of study drug adverse events overall (2% vs. 9%, P less than .0001). The difference was significant for rhinorrhea, headache, congestion, and pharyngeal pain individually.
In addition, patients in the povidone-iodine group were less likely to report that application of the study medication was unpleasant or very unpleasant (4% vs. 38%).
Dr. Phillips disclosed that he received a research grant from 3M Corp., manufacturer of the povidone-iodine solution. The study was supported by 3M.
SAN DIEGO – Preoperative nasal application of a povidone-iodine solution may be more efficacious than nasal mupirocin for preventing deep surgical site infections caused by Staphylococcus aureus, a study has shown.
Investigators led by Dr. Michael Phillips of the New York University Langone Medical Center conducted a randomized trial, assigning 1,697 patients undergoing arthroplasty or spine fusion surgery evenly to the two treatments. All patients in the study also underwent chlorhexidine cleansing of the skin and received standard antimicrobial prophylaxis.
Trial results, reported at IDWeek, showed that the rate of S. aureus deep surgical site infections was one-sixth as high in the povidone-iodine group as in the mupirocin group. The povidone-iodine group also had a lower rate of adverse events and less often rated their treatment as unpleasant.
"We feel that individuals should consider the use of nasal povidone-iodine as a component of a multifaceted approach to prevent S. aureus infection after high-risk surgery," Dr. Phillips said.
In the trial, adult patients undergoing arthroplasty or spine fusion surgery at the NYU Hospital for Joint Diseases had nasal cultures for S. aureus before surgery and were then assigned to two groups.
In one group, patients were given 2% mupirocin nasal ointment and told to apply it twice daily for the 5 days leading up to surgery. The mupirocin was provided directly to the patients because a survey suggested that some patients were skipping the mupirocin because of its cost, as it is not routinely covered by insurance, he explained at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the other group, research personnel applied a 5% solution of povidone-iodine to both nostrils in patients up to 2 hours before incision.
Both groups also were given chlorhexidine wipes and told to use them from chin to toes the evening before and again the morning of surgery. Both groups received standard perioperative antimicrobial prophylaxis (cefazolin or clindamycin, plus vancomycin for those having a positive nasal culture for methicillin-resistant S. aureus). Both also had surgical site prepping with 2% chlorhexidine and alcohol.
The patients’ median age was 62 years; 60% were women and 80% were white. Preoperative nasal culture results showed that one in five patients was colonized with S. aureus.
The rate of noncompletion of the protocol was 11% in the mupirocin group and 8% in the povidone-iodine group, Dr. Phillips reported. The leading reason for noncompletion in the mupirocin group was failure to apply mupirocin at least seven times. In the povidone-iodine group, it was failure to use the chlorhexidine wipes.
In intent-to-treat analyses, patients in the nasal povidone-iodine group had a 0.7% rate of any deep surgical site infection at 3 months; those in the mupirocin group had a 1.6% rate. The rate of S. aureus deep surgical site infections was 0.1% and. 0.6%, respectively.
In a univariate analysis, patients had a higher risk of S. aureus deep surgical site infection if they were in the mupirocin group (relative risk, 1.01; P = .04) or were colonized with S. aureus preoperatively (relative risk, 6.79; P = .02).
When patients were stratified by colonization status, there was a trend toward a lower rate of S. aureus deep surgical site infection with povidone-iodine versus mupirocin for those who were colonized (P = .08) but not for those who were not.
Patients in the povidone-iodine group had a lower rate of study drug adverse events overall (2% vs. 9%, P less than .0001). The difference was significant for rhinorrhea, headache, congestion, and pharyngeal pain individually.
In addition, patients in the povidone-iodine group were less likely to report that application of the study medication was unpleasant or very unpleasant (4% vs. 38%).
Dr. Phillips disclosed that he received a research grant from 3M Corp., manufacturer of the povidone-iodine solution. The study was supported by 3M.
AT IDWEEK
Major Finding: S. aureus deep surgical site infections were seen in 0.6% of patients given nasal mupirocin and 0.1% of patients given nasal povidone-iodine solution.
Data Source: Results are from a randomized trial of 1,697 patients undergoing arthroplasty or spine fusion surgery.
Disclosures: Dr. Phillips disclosed that he received a research grant from 3M Corp., which made the povidone-iodine solution. The study was supported by 3M.