User login
Mortality after breast cancer diagnosis found higher for men
Sex predicts mortality after a breast cancer diagnosis, with male patients about one-fifth more likely than female counterparts to have died by the 5-year mark, finds a cohort study of more than 1.8 million patients. Clinical characteristics and undertreatment explained much, but not all, of this excess mortality.
“Studies have indicated that male patients with breast cancer had worse overall survival than their female counterparts, including those with early-stage disease, although results have been inconsistent,” the investigators note. However, “few studies have systematically investigated the factors associated with mortality in male patients with breast cancer or assessed whether breast cancer prognosis for men is congruent with that for women, accounting for the differences in clinical characteristics and treatment.”
Senior investigator Xiao-Ou Shu, MD, PhD, of the Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tenn., and coinvestigators conducted a nationwide, registry-based cohort study using the National Cancer Database to identify patients receiving a breast cancer diagnosis during 2004-2014. Analyses were based on 16,025 male patients (mean age, 63.3 years) having a median follow-up of 54.0 months and 1,800,708 female patients (mean age, 59.9 years) having a median follow-up of 60.5 months.
Results reported in JAMA Oncology showed that men had higher mortality across all stages (P less than .001 for each). Male patients also had poorer relative overall survival (45.8% vs. 60.4%, P less than .001), 3-year survival (86.4% vs. 91.7%, P less than .001), and 5-year survival (77.6% vs. 86.4%, P less than .001).
Age, clinical factors (tumor size; nodal status; stage, ER, PR, and HER2 statuses; histologic type; grade; lymphovascular invasion; OncotypeDX Breast Recurrence Score; and Charlson/Deyo score), and treatment factors (surgical procedure, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy) collectively explained 63.3% of the excess mortality rate for male patients. They explained fully 66.0% of the excess mortality in the first 3 years after diagnosis, including 30.5% and 13.6% of that among patients with stage I and stage II disease, respectively.
However, even after adjustment for these factors plus race/ethnicity and access to care, men still had significantly higher risks of overall mortality (adjusted hazard ratio, 1.19), 3-year mortality (adjusted hazard ratio, 1.15), and 5-year mortality (adjusted hazard ratio, 1.19).
The database used did not contain information on causes of death or on cancer recurrence or progression events, precluding analyses of disease-free survival.
“Future research should focus on why and how clinical characteristics, as well as biological features, may have different implications for the survival of male and female patients with breast cancer,” Dr. Shu and coinvestigators recommended. “Additional factors, particularly compliance to treatment, biological attributes, and lifestyle factors (e.g., smoking, drinking, and obesity), should be assessed to help in developing treatments tailored for men, which would mitigate this sex-based disparity.”
Dr. Shu disclosed no relevant conflicts of interest. One author was funded by the program of the China Scholarship Council.
SOURCE: Wang F et al. JAMA Oncol. 2019 Sep 19. doi: 10.1001/jamaoncol.2019.2803.
Sex predicts mortality after a breast cancer diagnosis, with male patients about one-fifth more likely than female counterparts to have died by the 5-year mark, finds a cohort study of more than 1.8 million patients. Clinical characteristics and undertreatment explained much, but not all, of this excess mortality.
“Studies have indicated that male patients with breast cancer had worse overall survival than their female counterparts, including those with early-stage disease, although results have been inconsistent,” the investigators note. However, “few studies have systematically investigated the factors associated with mortality in male patients with breast cancer or assessed whether breast cancer prognosis for men is congruent with that for women, accounting for the differences in clinical characteristics and treatment.”
Senior investigator Xiao-Ou Shu, MD, PhD, of the Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tenn., and coinvestigators conducted a nationwide, registry-based cohort study using the National Cancer Database to identify patients receiving a breast cancer diagnosis during 2004-2014. Analyses were based on 16,025 male patients (mean age, 63.3 years) having a median follow-up of 54.0 months and 1,800,708 female patients (mean age, 59.9 years) having a median follow-up of 60.5 months.
Results reported in JAMA Oncology showed that men had higher mortality across all stages (P less than .001 for each). Male patients also had poorer relative overall survival (45.8% vs. 60.4%, P less than .001), 3-year survival (86.4% vs. 91.7%, P less than .001), and 5-year survival (77.6% vs. 86.4%, P less than .001).
Age, clinical factors (tumor size; nodal status; stage, ER, PR, and HER2 statuses; histologic type; grade; lymphovascular invasion; OncotypeDX Breast Recurrence Score; and Charlson/Deyo score), and treatment factors (surgical procedure, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy) collectively explained 63.3% of the excess mortality rate for male patients. They explained fully 66.0% of the excess mortality in the first 3 years after diagnosis, including 30.5% and 13.6% of that among patients with stage I and stage II disease, respectively.
However, even after adjustment for these factors plus race/ethnicity and access to care, men still had significantly higher risks of overall mortality (adjusted hazard ratio, 1.19), 3-year mortality (adjusted hazard ratio, 1.15), and 5-year mortality (adjusted hazard ratio, 1.19).
The database used did not contain information on causes of death or on cancer recurrence or progression events, precluding analyses of disease-free survival.
“Future research should focus on why and how clinical characteristics, as well as biological features, may have different implications for the survival of male and female patients with breast cancer,” Dr. Shu and coinvestigators recommended. “Additional factors, particularly compliance to treatment, biological attributes, and lifestyle factors (e.g., smoking, drinking, and obesity), should be assessed to help in developing treatments tailored for men, which would mitigate this sex-based disparity.”
Dr. Shu disclosed no relevant conflicts of interest. One author was funded by the program of the China Scholarship Council.
SOURCE: Wang F et al. JAMA Oncol. 2019 Sep 19. doi: 10.1001/jamaoncol.2019.2803.
Sex predicts mortality after a breast cancer diagnosis, with male patients about one-fifth more likely than female counterparts to have died by the 5-year mark, finds a cohort study of more than 1.8 million patients. Clinical characteristics and undertreatment explained much, but not all, of this excess mortality.
“Studies have indicated that male patients with breast cancer had worse overall survival than their female counterparts, including those with early-stage disease, although results have been inconsistent,” the investigators note. However, “few studies have systematically investigated the factors associated with mortality in male patients with breast cancer or assessed whether breast cancer prognosis for men is congruent with that for women, accounting for the differences in clinical characteristics and treatment.”
Senior investigator Xiao-Ou Shu, MD, PhD, of the Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tenn., and coinvestigators conducted a nationwide, registry-based cohort study using the National Cancer Database to identify patients receiving a breast cancer diagnosis during 2004-2014. Analyses were based on 16,025 male patients (mean age, 63.3 years) having a median follow-up of 54.0 months and 1,800,708 female patients (mean age, 59.9 years) having a median follow-up of 60.5 months.
Results reported in JAMA Oncology showed that men had higher mortality across all stages (P less than .001 for each). Male patients also had poorer relative overall survival (45.8% vs. 60.4%, P less than .001), 3-year survival (86.4% vs. 91.7%, P less than .001), and 5-year survival (77.6% vs. 86.4%, P less than .001).
Age, clinical factors (tumor size; nodal status; stage, ER, PR, and HER2 statuses; histologic type; grade; lymphovascular invasion; OncotypeDX Breast Recurrence Score; and Charlson/Deyo score), and treatment factors (surgical procedure, chemotherapy, endocrine therapy, radiation therapy, and immunotherapy) collectively explained 63.3% of the excess mortality rate for male patients. They explained fully 66.0% of the excess mortality in the first 3 years after diagnosis, including 30.5% and 13.6% of that among patients with stage I and stage II disease, respectively.
However, even after adjustment for these factors plus race/ethnicity and access to care, men still had significantly higher risks of overall mortality (adjusted hazard ratio, 1.19), 3-year mortality (adjusted hazard ratio, 1.15), and 5-year mortality (adjusted hazard ratio, 1.19).
The database used did not contain information on causes of death or on cancer recurrence or progression events, precluding analyses of disease-free survival.
“Future research should focus on why and how clinical characteristics, as well as biological features, may have different implications for the survival of male and female patients with breast cancer,” Dr. Shu and coinvestigators recommended. “Additional factors, particularly compliance to treatment, biological attributes, and lifestyle factors (e.g., smoking, drinking, and obesity), should be assessed to help in developing treatments tailored for men, which would mitigate this sex-based disparity.”
Dr. Shu disclosed no relevant conflicts of interest. One author was funded by the program of the China Scholarship Council.
SOURCE: Wang F et al. JAMA Oncol. 2019 Sep 19. doi: 10.1001/jamaoncol.2019.2803.
FROM JAMA ONCOLOGY
ASCT may cure follicular lymphoma for some rituximab-naive patients
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY
Meta-analysis supports hormone therapy-targeted therapy combo for advanced breast cancer
Combined hormone therapy and targeted therapy should be the first choice for most postmenopausal women with recently diagnosed hormone receptor–positive, HER2-negative metastatic breast cancer, new data suggest.
Although guidelines support use of hormone therapies with or without targeted therapies in this population, up-front chemotherapy is still commonly used even in the absence of a visceral crisis, noted lead investigator Mario Giuliano, MD, PhD, of the department of clinical medicine and surgery at the University of Naples (Italy) Federico II, and colleagues.
The investigators undertook a systematic review and network meta-analysis of 140 randomized, controlled trials among 50,029 postmenopausal patients with hormone receptor–positive, HER2-negative metastatic breast cancer treated in the first- and/or second-line setting.
Study results, reported in Lancet Oncology, showed that relative to standard hormone therapy alone, the combination of hormone therapy with a targeted therapy – a CDK4/6 inhibitor, an mammalian target of rapamycin inhibitor, or an indicated phosphoinositide 3-kinase inhibitor – had significantly better efficacy, reducing the risk of progression-free survival events by more than half. In addition, no chemotherapy regimen, with or without targeted therapy, significantly outperformed the combination of hormone therapy with a CDK4/6 inhibitor.
Meanwhile, the hormone therapy–targeted therapy combinations had manageable toxicity, with the severity of adverse effects intermediate between that of hormone therapy alone and that of chemotherapy with or without targeted therapies.
“This study is, to our knowledge, the first to compare the efficacy and activity of all currently available chemotherapy and hormone therapy regimens, in combination with or without targeted therapies,” Dr. Giuliano and coinvestigators wrote. “[O]ur results corroborate the treatment algorithms recommended by the official oncology guidelines, supporting the use of new combinations of hormone therapies plus targeted therapies in the first-line or second-line setting in patients with hormone receptor-positive, HER2-negative metastatic breast cancer without visceral crisis.”
For the study, the investigators identified relevant phase 2 and phase 3 randomized, controlled trials published between 2000 and 2017, with the addition of several recently reported trials such as BOLERO-6 (JAMA Oncol. 2018;4:1367-74). Of the 140 trials ultimately included, 114 were used in the analysis of progression-free survival and time to progression, and 135 were used in the analysis of overall response.
Study results showed that when anastrozole (Arimidex) alone was the comparator, progression-free survival was significantly better with palbociclib (Ibrance) plus letrozole (Femara) (hazard ratio for events, 0.42); ribociclib (Kisqali) plus letrozole (HR, 0.43); abemaciclib (Verzenio) plus anastrozole or letrozole (HR, 0.42); palbociclib plus fulvestrant (Faslodex) (HR, 0.37); ribociclib plus fulvestrant (HR, 0.48); abemaciclib plus fulvestrant (HR, 0.44); everolimus (Afinitor) plus exemestane (Aromasin) (HR, 0.42); and, in patients with a PIK3CA mutation, the PI3K inhibitor alpelisib (Piqray) plus fulvestrant (HR, 0.39).
Several chemotherapy-based regimens, including anthracycline- and taxane-containing regimens, were also superior to anastrozole alone (hazard ratios, 0.41-0.47).
When palbociclib plus letrozole was the comparator, no chemotherapy or hormone therapy regimen yielded significantly better progression-free survival.
For the outcome of overall response, paclitaxel (Taxol) plus bevacizumab (Avastin) was the only clinically relevant regimen that significantly improved the likelihood of response relative to palbociclib plus letrozole (odds ratio, 8.95).
Dr. Giuliano reported that he receives honoraria from Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer,and Roche. The study did not receive any funding.
SOURCE: Giuliano M et al. Lancet Oncol. 2019 Sep 4. doi: 10.1016/S1470-2045(19)30420-6.
“This meta-analysis solidifies an increasingly accepted role for CDK4/6 inhibitors in the upfront treatment of postmenopausal women with hormone receptor–positive, HER2-negative metastatic breast cancer,” Azadeh Nasrazadani, PhD, MD, and Adam M. Brufsky, MD, PhD, wrote in an editorial published in Lancet Oncology. “These agents are starting to displace more toxic chemotherapy agents as frontline treatments for estrogen receptor–positive metastatic breast cancer in general practice because of, to some extent, their similar efficacy with substantially more favorable side-effect profiles.”
Nonetheless, chemotherapy still has a role in this population, typically in the later-line setting and in patients experiencing a visceral crisis, they noted. However, the better overall response rate seen with a chemotherapy regimen (paclitaxel plus bevacizumab), compared with a combination targeted and hormonal therapy regimen (palbociclib plus letrozole), may hinge on the specific CDK4/6 inhibitor. “Future studies are needed to identify the appropriate setting and sequence for chemotherapy in these patients,” they maintained.
Outcomes were similar for comparable regimens containing the three currently approved CDK4/6 inhibitors, but individual resistance mechanisms have yet to be elucidated, according to Dr. Nasrazadani and Dr. Brufsky. In addition, it is unclear how these agents may affect duration of response to subsequent therapies, as evidence of a broad overall survival benefit is lacking.
“With the ever-increasing range of targeted therapies gaining approval for metastatic breast cancer, the clinical and research community continues to move further away from a monotherapy approach,” they concluded. “Trials are currently in progress evaluating the role of inhibitors of phosphoinositide 3-kinase, mammalian target of rapamycin, and MEK, among others. How these agents will compare to previously frontline chemotherapy, and whether we will gain more success with the combinations of these targeted therapies in addition to CDK4/6 inhibitors remains to be seen.”
Dr. Nasrazadani is a fellow in the division of hematology/oncology at the University of Pittsburgh Medical Center–Magee-Women’s Hospital. Dr. Brufsky is associate chief of the division of hematology/oncology and codirector of the Comprehensive Breast Cancer Center at Magee-Women’s Hospital. Dr. Brufsky reported receiving personal fees from numerous pharmaceutical companies; Dr. Nasrazadani reported no conflicts of interest.
“This meta-analysis solidifies an increasingly accepted role for CDK4/6 inhibitors in the upfront treatment of postmenopausal women with hormone receptor–positive, HER2-negative metastatic breast cancer,” Azadeh Nasrazadani, PhD, MD, and Adam M. Brufsky, MD, PhD, wrote in an editorial published in Lancet Oncology. “These agents are starting to displace more toxic chemotherapy agents as frontline treatments for estrogen receptor–positive metastatic breast cancer in general practice because of, to some extent, their similar efficacy with substantially more favorable side-effect profiles.”
Nonetheless, chemotherapy still has a role in this population, typically in the later-line setting and in patients experiencing a visceral crisis, they noted. However, the better overall response rate seen with a chemotherapy regimen (paclitaxel plus bevacizumab), compared with a combination targeted and hormonal therapy regimen (palbociclib plus letrozole), may hinge on the specific CDK4/6 inhibitor. “Future studies are needed to identify the appropriate setting and sequence for chemotherapy in these patients,” they maintained.
Outcomes were similar for comparable regimens containing the three currently approved CDK4/6 inhibitors, but individual resistance mechanisms have yet to be elucidated, according to Dr. Nasrazadani and Dr. Brufsky. In addition, it is unclear how these agents may affect duration of response to subsequent therapies, as evidence of a broad overall survival benefit is lacking.
“With the ever-increasing range of targeted therapies gaining approval for metastatic breast cancer, the clinical and research community continues to move further away from a monotherapy approach,” they concluded. “Trials are currently in progress evaluating the role of inhibitors of phosphoinositide 3-kinase, mammalian target of rapamycin, and MEK, among others. How these agents will compare to previously frontline chemotherapy, and whether we will gain more success with the combinations of these targeted therapies in addition to CDK4/6 inhibitors remains to be seen.”
Dr. Nasrazadani is a fellow in the division of hematology/oncology at the University of Pittsburgh Medical Center–Magee-Women’s Hospital. Dr. Brufsky is associate chief of the division of hematology/oncology and codirector of the Comprehensive Breast Cancer Center at Magee-Women’s Hospital. Dr. Brufsky reported receiving personal fees from numerous pharmaceutical companies; Dr. Nasrazadani reported no conflicts of interest.
“This meta-analysis solidifies an increasingly accepted role for CDK4/6 inhibitors in the upfront treatment of postmenopausal women with hormone receptor–positive, HER2-negative metastatic breast cancer,” Azadeh Nasrazadani, PhD, MD, and Adam M. Brufsky, MD, PhD, wrote in an editorial published in Lancet Oncology. “These agents are starting to displace more toxic chemotherapy agents as frontline treatments for estrogen receptor–positive metastatic breast cancer in general practice because of, to some extent, their similar efficacy with substantially more favorable side-effect profiles.”
Nonetheless, chemotherapy still has a role in this population, typically in the later-line setting and in patients experiencing a visceral crisis, they noted. However, the better overall response rate seen with a chemotherapy regimen (paclitaxel plus bevacizumab), compared with a combination targeted and hormonal therapy regimen (palbociclib plus letrozole), may hinge on the specific CDK4/6 inhibitor. “Future studies are needed to identify the appropriate setting and sequence for chemotherapy in these patients,” they maintained.
Outcomes were similar for comparable regimens containing the three currently approved CDK4/6 inhibitors, but individual resistance mechanisms have yet to be elucidated, according to Dr. Nasrazadani and Dr. Brufsky. In addition, it is unclear how these agents may affect duration of response to subsequent therapies, as evidence of a broad overall survival benefit is lacking.
“With the ever-increasing range of targeted therapies gaining approval for metastatic breast cancer, the clinical and research community continues to move further away from a monotherapy approach,” they concluded. “Trials are currently in progress evaluating the role of inhibitors of phosphoinositide 3-kinase, mammalian target of rapamycin, and MEK, among others. How these agents will compare to previously frontline chemotherapy, and whether we will gain more success with the combinations of these targeted therapies in addition to CDK4/6 inhibitors remains to be seen.”
Dr. Nasrazadani is a fellow in the division of hematology/oncology at the University of Pittsburgh Medical Center–Magee-Women’s Hospital. Dr. Brufsky is associate chief of the division of hematology/oncology and codirector of the Comprehensive Breast Cancer Center at Magee-Women’s Hospital. Dr. Brufsky reported receiving personal fees from numerous pharmaceutical companies; Dr. Nasrazadani reported no conflicts of interest.
Combined hormone therapy and targeted therapy should be the first choice for most postmenopausal women with recently diagnosed hormone receptor–positive, HER2-negative metastatic breast cancer, new data suggest.
Although guidelines support use of hormone therapies with or without targeted therapies in this population, up-front chemotherapy is still commonly used even in the absence of a visceral crisis, noted lead investigator Mario Giuliano, MD, PhD, of the department of clinical medicine and surgery at the University of Naples (Italy) Federico II, and colleagues.
The investigators undertook a systematic review and network meta-analysis of 140 randomized, controlled trials among 50,029 postmenopausal patients with hormone receptor–positive, HER2-negative metastatic breast cancer treated in the first- and/or second-line setting.
Study results, reported in Lancet Oncology, showed that relative to standard hormone therapy alone, the combination of hormone therapy with a targeted therapy – a CDK4/6 inhibitor, an mammalian target of rapamycin inhibitor, or an indicated phosphoinositide 3-kinase inhibitor – had significantly better efficacy, reducing the risk of progression-free survival events by more than half. In addition, no chemotherapy regimen, with or without targeted therapy, significantly outperformed the combination of hormone therapy with a CDK4/6 inhibitor.
Meanwhile, the hormone therapy–targeted therapy combinations had manageable toxicity, with the severity of adverse effects intermediate between that of hormone therapy alone and that of chemotherapy with or without targeted therapies.
“This study is, to our knowledge, the first to compare the efficacy and activity of all currently available chemotherapy and hormone therapy regimens, in combination with or without targeted therapies,” Dr. Giuliano and coinvestigators wrote. “[O]ur results corroborate the treatment algorithms recommended by the official oncology guidelines, supporting the use of new combinations of hormone therapies plus targeted therapies in the first-line or second-line setting in patients with hormone receptor-positive, HER2-negative metastatic breast cancer without visceral crisis.”
For the study, the investigators identified relevant phase 2 and phase 3 randomized, controlled trials published between 2000 and 2017, with the addition of several recently reported trials such as BOLERO-6 (JAMA Oncol. 2018;4:1367-74). Of the 140 trials ultimately included, 114 were used in the analysis of progression-free survival and time to progression, and 135 were used in the analysis of overall response.
Study results showed that when anastrozole (Arimidex) alone was the comparator, progression-free survival was significantly better with palbociclib (Ibrance) plus letrozole (Femara) (hazard ratio for events, 0.42); ribociclib (Kisqali) plus letrozole (HR, 0.43); abemaciclib (Verzenio) plus anastrozole or letrozole (HR, 0.42); palbociclib plus fulvestrant (Faslodex) (HR, 0.37); ribociclib plus fulvestrant (HR, 0.48); abemaciclib plus fulvestrant (HR, 0.44); everolimus (Afinitor) plus exemestane (Aromasin) (HR, 0.42); and, in patients with a PIK3CA mutation, the PI3K inhibitor alpelisib (Piqray) plus fulvestrant (HR, 0.39).
Several chemotherapy-based regimens, including anthracycline- and taxane-containing regimens, were also superior to anastrozole alone (hazard ratios, 0.41-0.47).
When palbociclib plus letrozole was the comparator, no chemotherapy or hormone therapy regimen yielded significantly better progression-free survival.
For the outcome of overall response, paclitaxel (Taxol) plus bevacizumab (Avastin) was the only clinically relevant regimen that significantly improved the likelihood of response relative to palbociclib plus letrozole (odds ratio, 8.95).
Dr. Giuliano reported that he receives honoraria from Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer,and Roche. The study did not receive any funding.
SOURCE: Giuliano M et al. Lancet Oncol. 2019 Sep 4. doi: 10.1016/S1470-2045(19)30420-6.
Combined hormone therapy and targeted therapy should be the first choice for most postmenopausal women with recently diagnosed hormone receptor–positive, HER2-negative metastatic breast cancer, new data suggest.
Although guidelines support use of hormone therapies with or without targeted therapies in this population, up-front chemotherapy is still commonly used even in the absence of a visceral crisis, noted lead investigator Mario Giuliano, MD, PhD, of the department of clinical medicine and surgery at the University of Naples (Italy) Federico II, and colleagues.
The investigators undertook a systematic review and network meta-analysis of 140 randomized, controlled trials among 50,029 postmenopausal patients with hormone receptor–positive, HER2-negative metastatic breast cancer treated in the first- and/or second-line setting.
Study results, reported in Lancet Oncology, showed that relative to standard hormone therapy alone, the combination of hormone therapy with a targeted therapy – a CDK4/6 inhibitor, an mammalian target of rapamycin inhibitor, or an indicated phosphoinositide 3-kinase inhibitor – had significantly better efficacy, reducing the risk of progression-free survival events by more than half. In addition, no chemotherapy regimen, with or without targeted therapy, significantly outperformed the combination of hormone therapy with a CDK4/6 inhibitor.
Meanwhile, the hormone therapy–targeted therapy combinations had manageable toxicity, with the severity of adverse effects intermediate between that of hormone therapy alone and that of chemotherapy with or without targeted therapies.
“This study is, to our knowledge, the first to compare the efficacy and activity of all currently available chemotherapy and hormone therapy regimens, in combination with or without targeted therapies,” Dr. Giuliano and coinvestigators wrote. “[O]ur results corroborate the treatment algorithms recommended by the official oncology guidelines, supporting the use of new combinations of hormone therapies plus targeted therapies in the first-line or second-line setting in patients with hormone receptor-positive, HER2-negative metastatic breast cancer without visceral crisis.”
For the study, the investigators identified relevant phase 2 and phase 3 randomized, controlled trials published between 2000 and 2017, with the addition of several recently reported trials such as BOLERO-6 (JAMA Oncol. 2018;4:1367-74). Of the 140 trials ultimately included, 114 were used in the analysis of progression-free survival and time to progression, and 135 were used in the analysis of overall response.
Study results showed that when anastrozole (Arimidex) alone was the comparator, progression-free survival was significantly better with palbociclib (Ibrance) plus letrozole (Femara) (hazard ratio for events, 0.42); ribociclib (Kisqali) plus letrozole (HR, 0.43); abemaciclib (Verzenio) plus anastrozole or letrozole (HR, 0.42); palbociclib plus fulvestrant (Faslodex) (HR, 0.37); ribociclib plus fulvestrant (HR, 0.48); abemaciclib plus fulvestrant (HR, 0.44); everolimus (Afinitor) plus exemestane (Aromasin) (HR, 0.42); and, in patients with a PIK3CA mutation, the PI3K inhibitor alpelisib (Piqray) plus fulvestrant (HR, 0.39).
Several chemotherapy-based regimens, including anthracycline- and taxane-containing regimens, were also superior to anastrozole alone (hazard ratios, 0.41-0.47).
When palbociclib plus letrozole was the comparator, no chemotherapy or hormone therapy regimen yielded significantly better progression-free survival.
For the outcome of overall response, paclitaxel (Taxol) plus bevacizumab (Avastin) was the only clinically relevant regimen that significantly improved the likelihood of response relative to palbociclib plus letrozole (odds ratio, 8.95).
Dr. Giuliano reported that he receives honoraria from Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer,and Roche. The study did not receive any funding.
SOURCE: Giuliano M et al. Lancet Oncol. 2019 Sep 4. doi: 10.1016/S1470-2045(19)30420-6.
FROM LANCET ONCOLOGY
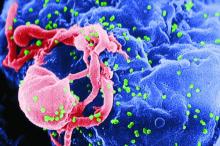
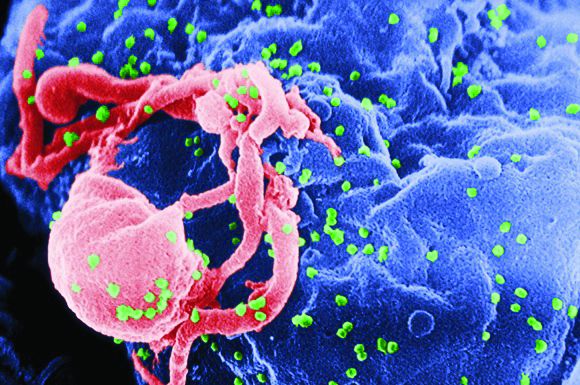
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
Staging PET/CT better defines extent of mantle cell lymphoma
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
Use of staging PET/CT better defines the extent of mantle cell lymphoma (MCL) in certain compartments, adding important information for treatment decisions, a cohort study suggests. However, patient selection and evaluation criteria are important for its utility.
“A correct and early identification of initial disease could be crucial because it could affect patient management and therapeutic choice,” Domenico Albano, MD, a nuclear medicine physician at the University of Brescia (Italy) and Spedali Civili Brescia, and colleagues wrote in Clinical Lymphoma, Myeloma, & Leukemia.
Using retrospective data from two centers and 122 patients with MCL, the investigators compared the utility of staging 18F-fluorodeoxyglucose (18F-FDG) PET/CT with that of other modalities used for detecting disease in specific compartments. They also assessed its impact on patient management.
The study results showed that, with the exception of a single patient having bone marrow involvement, all patients had positive PET/CT results, with detection of at least one hypermetabolic lesion.
For assessing nodal involvement, compared with CT alone, PET/CT detected additional lesions in 21% of patients. Splenic lesions were detected by PET/CT in 49% of patients and by CT alone in 47%.
For assessing bone marrow involvement, compared with bone marrow biopsy, PET/CT had a sensitivity of 52%, a specificity of 98%, a positive predictive value of 97%, a negative predictive value of 65%, and an overall accuracy of 74%.
For gastrointestinal involvement, compared with endoscopy, PET/CT had a sensitivity of 64% (78% after excluding diabetic patients taking metformin), a specificity of 91% (92%), a positive predictive value of 69% (72%), a negative predictive value of 90% (94%), and an overall accuracy of 85% (89%).
Ultimately, relative to CT alone, PET/CT altered stage and management in 19% of patients. Specifically, this imaging led to up-staging in 17% of patients, prompting clinicians to switch to more-aggressive chemotherapy, and down-staging in 2% of patients, prompting clinicians to skip unnecessary invasive therapies.
“We demonstrated that 18F-FDG pathologic uptake in MCL occurred in almost all patients, with excellent detection rate in nodal and splenic disease – indeed, better than CT,” the researchers wrote. “When we analyzed bone marrow and gastrointestinal involvement, the selection of patients excluding all conditions potentially affecting organ uptake and the use of specific criteria for the evaluation of these organs seemed to be crucial. Within these caveats, PET/CT showed good specificity for bone marrow and gastrointestinal evaluation.”
Study funding was not disclosed. Dr. Albano reported having no relevant conflicts of interest.
SOURCE: Albano D et al. Clin Lymphoma Myeloma Leuk. 2019 Aug;19(8):e457-64.
FROM CLINICAL LYMPHOMA, MYELOMA, & LEUKEMIA
Tamoxifen benefit in lower-risk breast cancer varies by intrinsic subtype
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
FROM JAMA ONCOLOGY
Center’s experience casts doubt on clinical utility of NGS
Next-generation sequencing (NGS) of tumor samples seldom changes patient management, and even when it does prompt off-label therapy, outcomes are usually poor, one center’s experience suggests.
“NGS has allowed more personalized medicine in oncology. It is well established that treatment of certain actionable mutations improves outcomes in many cancer types,” wrote Gregory J. Kubicek, MD, of MD Anderson Cancer Center at Cooper in Camden, N.J., and colleagues.
However, evidence of its utility to date has been mixed, and key trials – NCI-MPACT (National Cancer Institute Molecular Profiling–Based Assignment of Cancer Therapy) and NCI-MATCH (National Cancer Institute Molecular Analysis for Therapy Choice)—are still ongoing. “In the interim, the oncologist must make clinical decisions with limited empiric data but an exponentially increasing number of options,” they noted.
The investigators studied outcomes of the first 305 consecutive patients at their institution for whom tissue samples were sent to FoundationOne for NGS testing between March 2014 and April 2017. On average, the patients had received two lines of therapy, and the test was ordered 1.1 years from diagnosis of metastatic disease.
Study findings reported in the Journal of Oncology Practice showed that 116 of the tests were unusable because they did not yield a report (most often as a result of insufficient tissue) or yielded a report that could not be acted on owing to follow-up issues (patient loss of contact, transfer to hospice, or death).
Of the 189 potentially usable tests, 40.2% and 66.7% showed an aberration targetable by on-label therapies and off-label therapies, respectively. And fully 89.9% had actionable aberrations via all potential avenues, including clinical trials.
However, only 11.1% of the 189 potentially usable tests (and merely 8.3% of 253 completed tests and 6.9% of all 305 ordered tests) yielded a change in management, including use of on-label or off-label therapies, enrollment in clinical trials, or discontinuation of medications with a predicted poor response.
Of the six patients who were started on an off-label therapy, the median duration of treatment was 46 days, with half of these patients each stopping therapy because of death or because of progression.
“A vast majority of NGS assay results were not actively incorporated into clinical decision making, despite many assays indicating potential on- or off-label therapies,” Dr. Kubicek and coinvestigators wrote. “Given the escalating cost of medical care and scrutiny thereof, it is important to analyze whether tests are changing management and order tests appropriately.”
Several factors may explain the observed low use of NGS test results, they noted. For example, many patients were heavily pretreated, so some NGS-detected mutations would have already been known. Also, clinicians at the center had little experience with NGS testing.
“A variety of factors make precisely defining the utility of these assays in clinical decision making difficult, but we can certainly conclude that we have observed substantial costs with few discernible benefits,” the investigators stated. “It is possible that there will be greater use in the future as familiarity with these assays increases. Similarly, although we found poor outcomes with NGS-directed off-label therapies, we will eagerly await the results of NCI-MPACT and NCI-MATCH.”
Dr. Kubicek disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Davis W et al. J Oncol Pract. 2019 Aug 2. doi: 10.1200/JOP.19.00269.
Next-generation sequencing (NGS) of tumor samples seldom changes patient management, and even when it does prompt off-label therapy, outcomes are usually poor, one center’s experience suggests.
“NGS has allowed more personalized medicine in oncology. It is well established that treatment of certain actionable mutations improves outcomes in many cancer types,” wrote Gregory J. Kubicek, MD, of MD Anderson Cancer Center at Cooper in Camden, N.J., and colleagues.
However, evidence of its utility to date has been mixed, and key trials – NCI-MPACT (National Cancer Institute Molecular Profiling–Based Assignment of Cancer Therapy) and NCI-MATCH (National Cancer Institute Molecular Analysis for Therapy Choice)—are still ongoing. “In the interim, the oncologist must make clinical decisions with limited empiric data but an exponentially increasing number of options,” they noted.
The investigators studied outcomes of the first 305 consecutive patients at their institution for whom tissue samples were sent to FoundationOne for NGS testing between March 2014 and April 2017. On average, the patients had received two lines of therapy, and the test was ordered 1.1 years from diagnosis of metastatic disease.
Study findings reported in the Journal of Oncology Practice showed that 116 of the tests were unusable because they did not yield a report (most often as a result of insufficient tissue) or yielded a report that could not be acted on owing to follow-up issues (patient loss of contact, transfer to hospice, or death).
Of the 189 potentially usable tests, 40.2% and 66.7% showed an aberration targetable by on-label therapies and off-label therapies, respectively. And fully 89.9% had actionable aberrations via all potential avenues, including clinical trials.
However, only 11.1% of the 189 potentially usable tests (and merely 8.3% of 253 completed tests and 6.9% of all 305 ordered tests) yielded a change in management, including use of on-label or off-label therapies, enrollment in clinical trials, or discontinuation of medications with a predicted poor response.
Of the six patients who were started on an off-label therapy, the median duration of treatment was 46 days, with half of these patients each stopping therapy because of death or because of progression.
“A vast majority of NGS assay results were not actively incorporated into clinical decision making, despite many assays indicating potential on- or off-label therapies,” Dr. Kubicek and coinvestigators wrote. “Given the escalating cost of medical care and scrutiny thereof, it is important to analyze whether tests are changing management and order tests appropriately.”
Several factors may explain the observed low use of NGS test results, they noted. For example, many patients were heavily pretreated, so some NGS-detected mutations would have already been known. Also, clinicians at the center had little experience with NGS testing.
“A variety of factors make precisely defining the utility of these assays in clinical decision making difficult, but we can certainly conclude that we have observed substantial costs with few discernible benefits,” the investigators stated. “It is possible that there will be greater use in the future as familiarity with these assays increases. Similarly, although we found poor outcomes with NGS-directed off-label therapies, we will eagerly await the results of NCI-MPACT and NCI-MATCH.”
Dr. Kubicek disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Davis W et al. J Oncol Pract. 2019 Aug 2. doi: 10.1200/JOP.19.00269.
Next-generation sequencing (NGS) of tumor samples seldom changes patient management, and even when it does prompt off-label therapy, outcomes are usually poor, one center’s experience suggests.
“NGS has allowed more personalized medicine in oncology. It is well established that treatment of certain actionable mutations improves outcomes in many cancer types,” wrote Gregory J. Kubicek, MD, of MD Anderson Cancer Center at Cooper in Camden, N.J., and colleagues.
However, evidence of its utility to date has been mixed, and key trials – NCI-MPACT (National Cancer Institute Molecular Profiling–Based Assignment of Cancer Therapy) and NCI-MATCH (National Cancer Institute Molecular Analysis for Therapy Choice)—are still ongoing. “In the interim, the oncologist must make clinical decisions with limited empiric data but an exponentially increasing number of options,” they noted.
The investigators studied outcomes of the first 305 consecutive patients at their institution for whom tissue samples were sent to FoundationOne for NGS testing between March 2014 and April 2017. On average, the patients had received two lines of therapy, and the test was ordered 1.1 years from diagnosis of metastatic disease.
Study findings reported in the Journal of Oncology Practice showed that 116 of the tests were unusable because they did not yield a report (most often as a result of insufficient tissue) or yielded a report that could not be acted on owing to follow-up issues (patient loss of contact, transfer to hospice, or death).
Of the 189 potentially usable tests, 40.2% and 66.7% showed an aberration targetable by on-label therapies and off-label therapies, respectively. And fully 89.9% had actionable aberrations via all potential avenues, including clinical trials.
However, only 11.1% of the 189 potentially usable tests (and merely 8.3% of 253 completed tests and 6.9% of all 305 ordered tests) yielded a change in management, including use of on-label or off-label therapies, enrollment in clinical trials, or discontinuation of medications with a predicted poor response.
Of the six patients who were started on an off-label therapy, the median duration of treatment was 46 days, with half of these patients each stopping therapy because of death or because of progression.
“A vast majority of NGS assay results were not actively incorporated into clinical decision making, despite many assays indicating potential on- or off-label therapies,” Dr. Kubicek and coinvestigators wrote. “Given the escalating cost of medical care and scrutiny thereof, it is important to analyze whether tests are changing management and order tests appropriately.”
Several factors may explain the observed low use of NGS test results, they noted. For example, many patients were heavily pretreated, so some NGS-detected mutations would have already been known. Also, clinicians at the center had little experience with NGS testing.
“A variety of factors make precisely defining the utility of these assays in clinical decision making difficult, but we can certainly conclude that we have observed substantial costs with few discernible benefits,” the investigators stated. “It is possible that there will be greater use in the future as familiarity with these assays increases. Similarly, although we found poor outcomes with NGS-directed off-label therapies, we will eagerly await the results of NCI-MPACT and NCI-MATCH.”
Dr. Kubicek disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Davis W et al. J Oncol Pract. 2019 Aug 2. doi: 10.1200/JOP.19.00269.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Bevacizumab-erlotinib combo falls short in EGFR-mutant advanced NSCLC
according to a multicenter, phase 2, randomized, controlled trial.
“The development of erlotinib as a first-line therapy was an important advance, but there was interest in improving the efficacy of erlotinib,” noted the investigators, led by Thomas E. Stinchcombe, MD, of the Duke Cancer Institute in Durham, N.C. Preclinical data suggested that adding an antiangiogenic agent could overcome resistance, and subgroup analyses of a phase 3 trial hinted at greater efficacy of this combination in patients with EGFR-mutant disease (Lancet. 2011;377:1846-54).
The new trial was conducted among 88 patients with previously untreated stage 4 EGFR-mutant NSCLC eligible to receive bevacizumab. Two-thirds had an EGFR exon 19 deletion.
The patients were assigned evenly to receive erlotinib (Tarceva), an EGFR tyrosine kinase inhibitor, either alone or in combination with bevacizumab (Avastin), an antibody directed against vascular endothelial growth factor.
Results reported in JAMA Oncology showed that, during a median follow-up of 33 months, median progression-free survival (PFS) – the trial’s primary endpoint – was 17.9 months with the combination and 13.5 months with erlotinib alone, a nonsignificant difference (hazard ratio, 0.81; P = .39).
There was also no significant difference between groups in objective response rate (81% vs. 83%; P = .81) and overall survival (median, 32.4 vs. 50.6 months; HR, 1.41; P = .33).
Relative to the erlotinib monotherapy group, the combination therapy group had higher rates of grade 3 or worse acneiform rash (26% vs. 16%), hypertension (40% vs. 20%), and proteinuria (12% vs. 0%), but a lower rate of grade 3 or worse diarrhea (9% vs. 13%).
“Our study, unlike previous randomized clinical trials, did not reveal a significant improvement in PFS with the combination of erlotinib and bevacizumab. ... One consideration is that our trial used investigator assessment of response and disease progression, whereas previous randomized trials used blinded independent radiologic review,” Dr. Stinchcombe and coinvestigators summarized.
“Future studies will investigate novel osimertinib [Tagrisso] combinations and molecular markers to identify patients most likely to experience disease progression with single-agent EGFR TKIs,” they concluded.
Dr. Stinchcombe reported no relevant conflicts of interest. The trial was supported by Genentech/Roche.
SOURCE: Stinchcombe TE et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1847.
according to a multicenter, phase 2, randomized, controlled trial.
“The development of erlotinib as a first-line therapy was an important advance, but there was interest in improving the efficacy of erlotinib,” noted the investigators, led by Thomas E. Stinchcombe, MD, of the Duke Cancer Institute in Durham, N.C. Preclinical data suggested that adding an antiangiogenic agent could overcome resistance, and subgroup analyses of a phase 3 trial hinted at greater efficacy of this combination in patients with EGFR-mutant disease (Lancet. 2011;377:1846-54).
The new trial was conducted among 88 patients with previously untreated stage 4 EGFR-mutant NSCLC eligible to receive bevacizumab. Two-thirds had an EGFR exon 19 deletion.
The patients were assigned evenly to receive erlotinib (Tarceva), an EGFR tyrosine kinase inhibitor, either alone or in combination with bevacizumab (Avastin), an antibody directed against vascular endothelial growth factor.
Results reported in JAMA Oncology showed that, during a median follow-up of 33 months, median progression-free survival (PFS) – the trial’s primary endpoint – was 17.9 months with the combination and 13.5 months with erlotinib alone, a nonsignificant difference (hazard ratio, 0.81; P = .39).
There was also no significant difference between groups in objective response rate (81% vs. 83%; P = .81) and overall survival (median, 32.4 vs. 50.6 months; HR, 1.41; P = .33).
Relative to the erlotinib monotherapy group, the combination therapy group had higher rates of grade 3 or worse acneiform rash (26% vs. 16%), hypertension (40% vs. 20%), and proteinuria (12% vs. 0%), but a lower rate of grade 3 or worse diarrhea (9% vs. 13%).
“Our study, unlike previous randomized clinical trials, did not reveal a significant improvement in PFS with the combination of erlotinib and bevacizumab. ... One consideration is that our trial used investigator assessment of response and disease progression, whereas previous randomized trials used blinded independent radiologic review,” Dr. Stinchcombe and coinvestigators summarized.
“Future studies will investigate novel osimertinib [Tagrisso] combinations and molecular markers to identify patients most likely to experience disease progression with single-agent EGFR TKIs,” they concluded.
Dr. Stinchcombe reported no relevant conflicts of interest. The trial was supported by Genentech/Roche.
SOURCE: Stinchcombe TE et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1847.
according to a multicenter, phase 2, randomized, controlled trial.
“The development of erlotinib as a first-line therapy was an important advance, but there was interest in improving the efficacy of erlotinib,” noted the investigators, led by Thomas E. Stinchcombe, MD, of the Duke Cancer Institute in Durham, N.C. Preclinical data suggested that adding an antiangiogenic agent could overcome resistance, and subgroup analyses of a phase 3 trial hinted at greater efficacy of this combination in patients with EGFR-mutant disease (Lancet. 2011;377:1846-54).
The new trial was conducted among 88 patients with previously untreated stage 4 EGFR-mutant NSCLC eligible to receive bevacizumab. Two-thirds had an EGFR exon 19 deletion.
The patients were assigned evenly to receive erlotinib (Tarceva), an EGFR tyrosine kinase inhibitor, either alone or in combination with bevacizumab (Avastin), an antibody directed against vascular endothelial growth factor.
Results reported in JAMA Oncology showed that, during a median follow-up of 33 months, median progression-free survival (PFS) – the trial’s primary endpoint – was 17.9 months with the combination and 13.5 months with erlotinib alone, a nonsignificant difference (hazard ratio, 0.81; P = .39).
There was also no significant difference between groups in objective response rate (81% vs. 83%; P = .81) and overall survival (median, 32.4 vs. 50.6 months; HR, 1.41; P = .33).
Relative to the erlotinib monotherapy group, the combination therapy group had higher rates of grade 3 or worse acneiform rash (26% vs. 16%), hypertension (40% vs. 20%), and proteinuria (12% vs. 0%), but a lower rate of grade 3 or worse diarrhea (9% vs. 13%).
“Our study, unlike previous randomized clinical trials, did not reveal a significant improvement in PFS with the combination of erlotinib and bevacizumab. ... One consideration is that our trial used investigator assessment of response and disease progression, whereas previous randomized trials used blinded independent radiologic review,” Dr. Stinchcombe and coinvestigators summarized.
“Future studies will investigate novel osimertinib [Tagrisso] combinations and molecular markers to identify patients most likely to experience disease progression with single-agent EGFR TKIs,” they concluded.
Dr. Stinchcombe reported no relevant conflicts of interest. The trial was supported by Genentech/Roche.
SOURCE: Stinchcombe TE et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1847.
FROM JAMA ONCOLOGY
CVD risk after breast cancer: Adipose distribution trumps BMI
When it comes to cardiovascular disease (CVD) risk after a breast cancer diagnosis, it’s not so much a matter of the amount of body fat carried but rather where it is located, results of a retrospective cohort study of nearly 3,000 survivors suggest.
“It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual-level adiposity and does not describe adipose tissue distribution,” note lead investigator Elizabeth M. Cespedes Feliciano, ScD, of Kaiser Permanente Northern California, Oakland, Calif., and coinvestigators.
The investigators studied 2,943 survivors of nonmetastatic breast cancer having a mean age of 56 years who were initially CVD free, using clinically acquired CT scans obtained near diagnosis to measure adiposity in three compartments: visceral, subcutaneous, and intramuscular.
The cohort experienced 328 CVD events (nonfatal stroke, myocardial infarction, heart failure, or CVD death) during a median follow-up of 6 years, the investigators reported in Journal of Clinical Oncology. The 10-year cumulative incidence was 15%.
In analyses that were adjusted for potential confounders and took into account competing risks, survivors’ CVD risk increased significantly with each standard deviation (SD) increase in visceral adiposity (hazard ratio, 1.15; 95% confidence interval, 1.03-1.29) and each SD increase in intramuscular adiposity (HR, 1.21; 95% CI, 1.06-1.37). The association for subcutaneous adiposity was not significant.
Findings were similar across all BMI categories. Of particular note, among survivors having a normal BMI, risk of CVD events increased by 70% with each SD greater visceral adiposity (HR, 1.70; 95% CI, 1.10-2.62).
Risk also rose with BMI exceeding the normal range, but the association became significant only in survivors with a BMI placing them in obesity class II (35 kg/m2 or greater) (HR, 1.70; 95% CI, 1.20-2.42).
“Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI,” Dr. Cespedes Feliciano and coinvestigators note.
“Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration,” they note. “Measures of adipose tissue distribution from CT or anthropometry (e.g., waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients’ body composition.”
Dr. Cespedes Feliciano disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Cespedes Feliciano EM et al. J Clin Oncol. 2019 Aug 1. doi: 10.1200/JCO.19.00286.
When it comes to cardiovascular disease (CVD) risk after a breast cancer diagnosis, it’s not so much a matter of the amount of body fat carried but rather where it is located, results of a retrospective cohort study of nearly 3,000 survivors suggest.
“It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual-level adiposity and does not describe adipose tissue distribution,” note lead investigator Elizabeth M. Cespedes Feliciano, ScD, of Kaiser Permanente Northern California, Oakland, Calif., and coinvestigators.
The investigators studied 2,943 survivors of nonmetastatic breast cancer having a mean age of 56 years who were initially CVD free, using clinically acquired CT scans obtained near diagnosis to measure adiposity in three compartments: visceral, subcutaneous, and intramuscular.
The cohort experienced 328 CVD events (nonfatal stroke, myocardial infarction, heart failure, or CVD death) during a median follow-up of 6 years, the investigators reported in Journal of Clinical Oncology. The 10-year cumulative incidence was 15%.
In analyses that were adjusted for potential confounders and took into account competing risks, survivors’ CVD risk increased significantly with each standard deviation (SD) increase in visceral adiposity (hazard ratio, 1.15; 95% confidence interval, 1.03-1.29) and each SD increase in intramuscular adiposity (HR, 1.21; 95% CI, 1.06-1.37). The association for subcutaneous adiposity was not significant.
Findings were similar across all BMI categories. Of particular note, among survivors having a normal BMI, risk of CVD events increased by 70% with each SD greater visceral adiposity (HR, 1.70; 95% CI, 1.10-2.62).
Risk also rose with BMI exceeding the normal range, but the association became significant only in survivors with a BMI placing them in obesity class II (35 kg/m2 or greater) (HR, 1.70; 95% CI, 1.20-2.42).
“Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI,” Dr. Cespedes Feliciano and coinvestigators note.
“Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration,” they note. “Measures of adipose tissue distribution from CT or anthropometry (e.g., waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients’ body composition.”
Dr. Cespedes Feliciano disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Cespedes Feliciano EM et al. J Clin Oncol. 2019 Aug 1. doi: 10.1200/JCO.19.00286.
When it comes to cardiovascular disease (CVD) risk after a breast cancer diagnosis, it’s not so much a matter of the amount of body fat carried but rather where it is located, results of a retrospective cohort study of nearly 3,000 survivors suggest.
“It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual-level adiposity and does not describe adipose tissue distribution,” note lead investigator Elizabeth M. Cespedes Feliciano, ScD, of Kaiser Permanente Northern California, Oakland, Calif., and coinvestigators.
The investigators studied 2,943 survivors of nonmetastatic breast cancer having a mean age of 56 years who were initially CVD free, using clinically acquired CT scans obtained near diagnosis to measure adiposity in three compartments: visceral, subcutaneous, and intramuscular.
The cohort experienced 328 CVD events (nonfatal stroke, myocardial infarction, heart failure, or CVD death) during a median follow-up of 6 years, the investigators reported in Journal of Clinical Oncology. The 10-year cumulative incidence was 15%.
In analyses that were adjusted for potential confounders and took into account competing risks, survivors’ CVD risk increased significantly with each standard deviation (SD) increase in visceral adiposity (hazard ratio, 1.15; 95% confidence interval, 1.03-1.29) and each SD increase in intramuscular adiposity (HR, 1.21; 95% CI, 1.06-1.37). The association for subcutaneous adiposity was not significant.
Findings were similar across all BMI categories. Of particular note, among survivors having a normal BMI, risk of CVD events increased by 70% with each SD greater visceral adiposity (HR, 1.70; 95% CI, 1.10-2.62).
Risk also rose with BMI exceeding the normal range, but the association became significant only in survivors with a BMI placing them in obesity class II (35 kg/m2 or greater) (HR, 1.70; 95% CI, 1.20-2.42).
“Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI,” Dr. Cespedes Feliciano and coinvestigators note.
“Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration,” they note. “Measures of adipose tissue distribution from CT or anthropometry (e.g., waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients’ body composition.”
Dr. Cespedes Feliciano disclosed no relevant conflicts of interest. The study did not receive any specific funding.
SOURCE: Cespedes Feliciano EM et al. J Clin Oncol. 2019 Aug 1. doi: 10.1200/JCO.19.00286.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Benefit of chemo + RT for high-risk endometrial cancer increases with time
Benefits of adding chemotherapy to pelvic radiotherapy in localized high-risk endometrial cancer increase with follow-up, finds an updated analysis of the randomized PORTEC-3 trial.
The relative risk-benefit profile of chemoradiotherapy is uncertain, with some evidence suggesting it varies according to disease histology and stage, noted lead investigator Stephanie de Boer, MD, department of radiation oncology, Leiden (the Netherlands) University Medical Center, and coinvestigators.
PORTEC-3, a multicenter phase 3 trial, enrolled women who had undergone surgery for high-risk endometrial cancer: FIGO 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion, lymphovascular space invasion, or both; stage II or III disease; or stage I to III disease with serous or clear cell histology. In all, 660 women were evenly assigned to external-beam radiotherapy alone (48.6 Gy in 1.8-Gy fractions, on 5 days per week) or radiotherapy and chemotherapy (two cycles of cisplatin given during radiotherapy, followed by four cycles of carboplatin and paclitaxel).
A previous analysis, at a median follow-up of 60.2 months (5.0 years), showed a significant 5-year failure-free survival benefit of chemoradiotherapy over radiotherapy (hazard ratio [HR], 0.71; P = .022) but only a trend toward an overall survival benefit (Lancet Oncol. 2018;19[3]:295-309).
The updated analysis, now at a median follow-up of 72.6 months (6.1 years), was reported in Lancet Oncology and showed that chemoradiotherapy was still significantly superior to radiotherapy alone in terms of 5-year failure-free survival (76.5% vs. 69.1%; adjusted HR, 0.70; P = .016) but was now also significantly superior in terms of 5-year overall survival (81.4% vs. 76.1%; adjusted HR, 0.70; P = .034)
The 5-year probability of distant metastasis was lower with chemoradiotherapy than with radiotherapy alone (21.4% vs. 29.1%; HR, 0.74; P = .047). The two groups did not differ significantly with respect to isolated vaginal recurrence as first site (0.3% in each group) or isolated pelvic recurrence as first site (0.9% in each group).
Only a single patient, in the chemoradiotherapy group, experienced a grade 4 adverse event (ileus or obstruction). The chemoradiotherapy and radiotherapy-only groups were similar on the rate of grade 3 adverse events (8% vs. 5%; P = .24), the most common of which was hypertension. However, the former had a higher rate of grade 2 or worse adverse events (38% vs. 23%; P = .002), such as persistent sensory neuropathy (6% vs. 0%). None of the patients died from treatment-related causes.
“Combined adjuvant chemotherapy and radiotherapy should be discussed and recommended as a new standard of care, especially for women with stage III endometrial cancer or serous cancers, or both,” Dr. de Boer and coinvestigators maintained. “Shared decision making between doctors and their patients remains essential to weigh the costs and benefits for individual patients.
“Molecular analysis has the potential to improve risk stratification and should be used to identify subgroups that can derive the greatest benefit from chemotherapy and to select patients for targeted therapies; molecular studies on tissue samples donated by PORTEC-3 trial participants are ongoing,” they noted.
Dr. de Boer disclosed no competing interests in relation to the study. The study was supported in part by the Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Cancer Australia, the Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
SOURCE: de Boer SM et al. Lancet Oncol. 2019 July 22. doi: 10.1016/S1470-2045(19)30395-X.
Results of PORTEC-3 are potentially practice changing but generate several questions relevant to optimizing adjuvant therapy for high-risk endometrial cancer, Marcus Randall, MD, contends in a commentary (Lancet Oncol. 2019 Jul 22. doi: 10.1016/S1470-2045[19]30416-4).
One question is applicability of the findings across trial subgroups, which is still uncertain. “However, taking into account the statistical limitations of subgroup analyses, the therapeutic benefit of combined chemotherapy and radiotherapy (vs. radiotherapy alone) appeared to remain confined to patients with stage III disease and those with serous carcinomas of all stages,” he noted.
Another question is whether chemotherapy alone is sufficient. Here, results from trials conducted by the Gynecologic Oncology Group (now NRG Oncology) suggest that omitting pelvic radiotherapy increases the risk of locoregional failure, according to Dr. Randall.
A final question is whether there is a preferred approach for combining chemotherapy with radiotherapy. “Increasing evidence supports the use of upfront systemic therapy, when combined with radiotherapy, as a strategy to maximise both systemic and local control. ... Many clinicians often use this regimen as a preferred adjuvant approach in locally advanced endometrial cancer,” he noted.
“Based on outstanding work done by the PORTEC Study Group and others, we have made good progress in improving outcomes for women with high-risk and locally advanced endometrial cancers. However, we are not there yet,” Dr. Randall concludes.
Marcus Randall, MD, is with the department of radiation medicine, University of Kentucky, Lexington. He has no disclosures related to the commentary.
Results of PORTEC-3 are potentially practice changing but generate several questions relevant to optimizing adjuvant therapy for high-risk endometrial cancer, Marcus Randall, MD, contends in a commentary (Lancet Oncol. 2019 Jul 22. doi: 10.1016/S1470-2045[19]30416-4).
One question is applicability of the findings across trial subgroups, which is still uncertain. “However, taking into account the statistical limitations of subgroup analyses, the therapeutic benefit of combined chemotherapy and radiotherapy (vs. radiotherapy alone) appeared to remain confined to patients with stage III disease and those with serous carcinomas of all stages,” he noted.
Another question is whether chemotherapy alone is sufficient. Here, results from trials conducted by the Gynecologic Oncology Group (now NRG Oncology) suggest that omitting pelvic radiotherapy increases the risk of locoregional failure, according to Dr. Randall.
A final question is whether there is a preferred approach for combining chemotherapy with radiotherapy. “Increasing evidence supports the use of upfront systemic therapy, when combined with radiotherapy, as a strategy to maximise both systemic and local control. ... Many clinicians often use this regimen as a preferred adjuvant approach in locally advanced endometrial cancer,” he noted.
“Based on outstanding work done by the PORTEC Study Group and others, we have made good progress in improving outcomes for women with high-risk and locally advanced endometrial cancers. However, we are not there yet,” Dr. Randall concludes.
Marcus Randall, MD, is with the department of radiation medicine, University of Kentucky, Lexington. He has no disclosures related to the commentary.
Results of PORTEC-3 are potentially practice changing but generate several questions relevant to optimizing adjuvant therapy for high-risk endometrial cancer, Marcus Randall, MD, contends in a commentary (Lancet Oncol. 2019 Jul 22. doi: 10.1016/S1470-2045[19]30416-4).
One question is applicability of the findings across trial subgroups, which is still uncertain. “However, taking into account the statistical limitations of subgroup analyses, the therapeutic benefit of combined chemotherapy and radiotherapy (vs. radiotherapy alone) appeared to remain confined to patients with stage III disease and those with serous carcinomas of all stages,” he noted.
Another question is whether chemotherapy alone is sufficient. Here, results from trials conducted by the Gynecologic Oncology Group (now NRG Oncology) suggest that omitting pelvic radiotherapy increases the risk of locoregional failure, according to Dr. Randall.
A final question is whether there is a preferred approach for combining chemotherapy with radiotherapy. “Increasing evidence supports the use of upfront systemic therapy, when combined with radiotherapy, as a strategy to maximise both systemic and local control. ... Many clinicians often use this regimen as a preferred adjuvant approach in locally advanced endometrial cancer,” he noted.
“Based on outstanding work done by the PORTEC Study Group and others, we have made good progress in improving outcomes for women with high-risk and locally advanced endometrial cancers. However, we are not there yet,” Dr. Randall concludes.
Marcus Randall, MD, is with the department of radiation medicine, University of Kentucky, Lexington. He has no disclosures related to the commentary.
Benefits of adding chemotherapy to pelvic radiotherapy in localized high-risk endometrial cancer increase with follow-up, finds an updated analysis of the randomized PORTEC-3 trial.
The relative risk-benefit profile of chemoradiotherapy is uncertain, with some evidence suggesting it varies according to disease histology and stage, noted lead investigator Stephanie de Boer, MD, department of radiation oncology, Leiden (the Netherlands) University Medical Center, and coinvestigators.
PORTEC-3, a multicenter phase 3 trial, enrolled women who had undergone surgery for high-risk endometrial cancer: FIGO 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion, lymphovascular space invasion, or both; stage II or III disease; or stage I to III disease with serous or clear cell histology. In all, 660 women were evenly assigned to external-beam radiotherapy alone (48.6 Gy in 1.8-Gy fractions, on 5 days per week) or radiotherapy and chemotherapy (two cycles of cisplatin given during radiotherapy, followed by four cycles of carboplatin and paclitaxel).
A previous analysis, at a median follow-up of 60.2 months (5.0 years), showed a significant 5-year failure-free survival benefit of chemoradiotherapy over radiotherapy (hazard ratio [HR], 0.71; P = .022) but only a trend toward an overall survival benefit (Lancet Oncol. 2018;19[3]:295-309).
The updated analysis, now at a median follow-up of 72.6 months (6.1 years), was reported in Lancet Oncology and showed that chemoradiotherapy was still significantly superior to radiotherapy alone in terms of 5-year failure-free survival (76.5% vs. 69.1%; adjusted HR, 0.70; P = .016) but was now also significantly superior in terms of 5-year overall survival (81.4% vs. 76.1%; adjusted HR, 0.70; P = .034)
The 5-year probability of distant metastasis was lower with chemoradiotherapy than with radiotherapy alone (21.4% vs. 29.1%; HR, 0.74; P = .047). The two groups did not differ significantly with respect to isolated vaginal recurrence as first site (0.3% in each group) or isolated pelvic recurrence as first site (0.9% in each group).
Only a single patient, in the chemoradiotherapy group, experienced a grade 4 adverse event (ileus or obstruction). The chemoradiotherapy and radiotherapy-only groups were similar on the rate of grade 3 adverse events (8% vs. 5%; P = .24), the most common of which was hypertension. However, the former had a higher rate of grade 2 or worse adverse events (38% vs. 23%; P = .002), such as persistent sensory neuropathy (6% vs. 0%). None of the patients died from treatment-related causes.
“Combined adjuvant chemotherapy and radiotherapy should be discussed and recommended as a new standard of care, especially for women with stage III endometrial cancer or serous cancers, or both,” Dr. de Boer and coinvestigators maintained. “Shared decision making between doctors and their patients remains essential to weigh the costs and benefits for individual patients.
“Molecular analysis has the potential to improve risk stratification and should be used to identify subgroups that can derive the greatest benefit from chemotherapy and to select patients for targeted therapies; molecular studies on tissue samples donated by PORTEC-3 trial participants are ongoing,” they noted.
Dr. de Boer disclosed no competing interests in relation to the study. The study was supported in part by the Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Cancer Australia, the Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
SOURCE: de Boer SM et al. Lancet Oncol. 2019 July 22. doi: 10.1016/S1470-2045(19)30395-X.
Benefits of adding chemotherapy to pelvic radiotherapy in localized high-risk endometrial cancer increase with follow-up, finds an updated analysis of the randomized PORTEC-3 trial.
The relative risk-benefit profile of chemoradiotherapy is uncertain, with some evidence suggesting it varies according to disease histology and stage, noted lead investigator Stephanie de Boer, MD, department of radiation oncology, Leiden (the Netherlands) University Medical Center, and coinvestigators.
PORTEC-3, a multicenter phase 3 trial, enrolled women who had undergone surgery for high-risk endometrial cancer: FIGO 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion, lymphovascular space invasion, or both; stage II or III disease; or stage I to III disease with serous or clear cell histology. In all, 660 women were evenly assigned to external-beam radiotherapy alone (48.6 Gy in 1.8-Gy fractions, on 5 days per week) or radiotherapy and chemotherapy (two cycles of cisplatin given during radiotherapy, followed by four cycles of carboplatin and paclitaxel).
A previous analysis, at a median follow-up of 60.2 months (5.0 years), showed a significant 5-year failure-free survival benefit of chemoradiotherapy over radiotherapy (hazard ratio [HR], 0.71; P = .022) but only a trend toward an overall survival benefit (Lancet Oncol. 2018;19[3]:295-309).
The updated analysis, now at a median follow-up of 72.6 months (6.1 years), was reported in Lancet Oncology and showed that chemoradiotherapy was still significantly superior to radiotherapy alone in terms of 5-year failure-free survival (76.5% vs. 69.1%; adjusted HR, 0.70; P = .016) but was now also significantly superior in terms of 5-year overall survival (81.4% vs. 76.1%; adjusted HR, 0.70; P = .034)
The 5-year probability of distant metastasis was lower with chemoradiotherapy than with radiotherapy alone (21.4% vs. 29.1%; HR, 0.74; P = .047). The two groups did not differ significantly with respect to isolated vaginal recurrence as first site (0.3% in each group) or isolated pelvic recurrence as first site (0.9% in each group).
Only a single patient, in the chemoradiotherapy group, experienced a grade 4 adverse event (ileus or obstruction). The chemoradiotherapy and radiotherapy-only groups were similar on the rate of grade 3 adverse events (8% vs. 5%; P = .24), the most common of which was hypertension. However, the former had a higher rate of grade 2 or worse adverse events (38% vs. 23%; P = .002), such as persistent sensory neuropathy (6% vs. 0%). None of the patients died from treatment-related causes.
“Combined adjuvant chemotherapy and radiotherapy should be discussed and recommended as a new standard of care, especially for women with stage III endometrial cancer or serous cancers, or both,” Dr. de Boer and coinvestigators maintained. “Shared decision making between doctors and their patients remains essential to weigh the costs and benefits for individual patients.
“Molecular analysis has the potential to improve risk stratification and should be used to identify subgroups that can derive the greatest benefit from chemotherapy and to select patients for targeted therapies; molecular studies on tissue samples donated by PORTEC-3 trial participants are ongoing,” they noted.
Dr. de Boer disclosed no competing interests in relation to the study. The study was supported in part by the Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Cancer Australia, the Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
SOURCE: de Boer SM et al. Lancet Oncol. 2019 July 22. doi: 10.1016/S1470-2045(19)30395-X.
FROM LANCET ONCOLOGY