User login
Autoimmune Hemolytic Anemia: Evaluation and Diagnosis
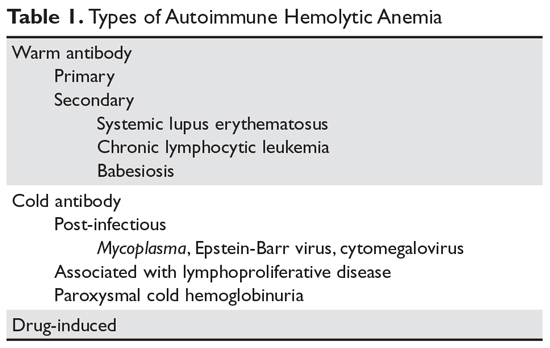
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons.1,2 AIHA is mediated by antibodies, and in the majority of cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C (Table 1). This article series reviews the most common types of AIHA, with an overview of evaluation and diagnosis presented in this article and management of warm, cold, and drug-induced AIHA reviewed in a separate article.
Pathogenesis
In most cases, the ultimate etiology of AIHA is unknown. In warm AIHA, the target epitopes in most cases are Rh proteins.2 What leads the immune system to target these proteins is unidentified, but one theory is that an initial immune response to a foreign antigen starts to cross-react with the Rh proteins and the immune system fails to suppress this autoreactive response, leading to hemolysis. In IgG-mediated (warm) hemolysis, the red cells become coated with IgG molecules, which mark the cells for uptake and destruction by splenic macrophages.3 In "cold" AIHA, IgM molecules fix complement to the surface of red blood cells. Rarely, this can lead to activation of the full complement cascade, resulting in red cell lysis, but more often it is stopped at the C3 stage, leading to C3-coated red cells which are then taken up by hepatic macrophages.4
Suspecting the Diagnosis
In many patients, it is the symptoms and signs of anemia that lead to suspicion of hemolysis. Older patients often present earlier in the course of the disease due to lack of tolerance of anemia, especially if there is a sudden drop in the red blood cell count. Dark, cola-colored urine resulting from the presence of free hemoglobin may be noted by some patients. Patients with rapid-onset hemolysis may note lumbar back pain, and those with cold agglutinins often note symptoms related to agglutination of red cells in the peripheral circulation, such as the development of acrocyanosis in cold weather.5 In rare cases, patients will have abdominal pain when eating cold food due to ischemia related to agglutination of red cells in the viscera. Some patients with cold agglutinins can have an exacerbation of their hemolysis with cold exposure.
Unlike patients with immune thrombocytopenia, those with AIHA may have mild splenomegaly on exam. The presence of enlarged lymph nodes or massive splenomegaly should raise concern about concomitant lymphoma or chronic lymphocytic leukemia.
Making the Diagnosis
The 2 key steps in diagnosis are (1) demonstrating hemolysis and (2) demonstrating the autoimmune component.
Laboratory Evaluation for Hemolysis
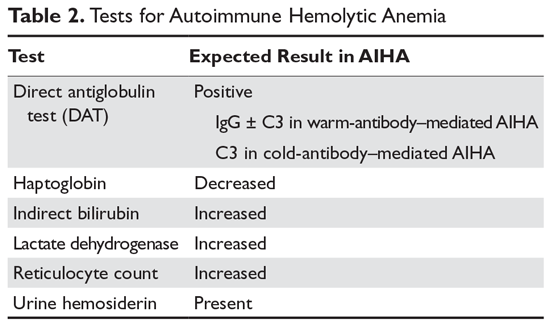
Hemolysis is proven by finding evidence of both red cell breakdown and the compensatory increase in red cell production this stimulates (Table 2). The following sections discuss the laboratory tests that are performed to investigate hemolysis.
Lactate Dehydrogenase
When red cells undergo hemolysis, they release their contents, which are mostly comprised of hemoglobin but also include lactate dehydrogenase (LDH), an enzyme found in high concentration in red cells. Most patients with hemolysis will have an elevated LDH level, making this a sensitive test. However, because many other processes, including liver disease and pneumonia, also raise the serum LDH level, this finding is not specific for hemolysis.
Serum Bilirubin
Hemoglobin is salvaged by haptoglobin, and the heme moiety is broken down first to bilirubin and then to urobilinogen, which is excreted in the urine.2 Bilirubin produced from the breakdown of heme is not conjugated, but rather is delivered to the liver, where it is conjugated and excreted into the bile. In hemolysis, the concentration of unconjugated bilirubin (indirect bilirubin) is increased, while in liver disease the level of conjugated bilirubin (direct bilirubin) is increased. However, if the patient has concomitant liver disease with an increased direct bilirubin level, the serum bilirubin test is not reliable.
Serum Haptoglobin
Haptoglobin binds free serum hemoglobin and is taken up by the liver. Haptoglobin usually falls to very low levels in hemolysis. A confounder is that haptoglobin is an acute phase reactant and can rise with systemic disease or inflammation. However, patients with advanced liver disease will have low haptoglobin levels due to lack of synthesis, and up to 2% of the population may congenitally lack haptoglobin.1
Serum Hemoglobin
If the hemolysis is very rapid, the amount of free hemoglobin released will overwhelm the binding capacity of haptoglobin and lead to free hemoglobin in the plasma. This can be crudely quantified by examining the plasma color. Even minute amounts of free hemoglobin will turn the plasma pink. In fulminant hemolysis, the plasma will be cola-colored.
Reticulocyte Count
In most patients with hemolysis, the destruction of red cells is accompanied by an increase in the reticulocyte count. Reticulocytes are red cells that still contain RNA and are a marker of red cells that are approximately 24 hours old or less. Traditionally, reticulocytes were measured manually by staining the blood smear with vital blue and counting the percentage of cells that absorb the stain; this percentage needs to be adjusted for the hematocrit. Usually a percentage above 1.5% is considered indicative of an elevated reticulocyte count. Recently, automated complete blood count machines have taken advantage of the fact that reticulocytes will absorb certain stains; these machines can directly measure the reticulocyte count via flow cytometry, which results in an “absolute” reticulocyte count. The reticulocyte count obtained using this method does not have to be corrected for hematocrit, and levels of approximately 90,000/μL are considered raised. However, the reticulocyte count can also be raised in blood loss or in patients who have other causes of anemia (eg, iron deficiency) under treatment. In addition, as many as 25% of patients with AIHA will never have raised counts for various reasons, such as nutritional deficiency, autoimmune destruction of red cell precursors, or lack of erythropoietin.
Blood Smear
The blood smear provides vital information. The hallmark laboratory parameter of AIHA is spherocytes seen on the blood smear. In AIHA, antibodies and/or complement attach to the red cells, and when the antibodies or complement are taken up by macrophages in the spleen some of the red blood cell mem-brane is removed as well, decreasing the surface area of the cell. As the surface area of the red cell decreases with each pass through the spleen, the cell's shape changes from a biconcave disk to a sphere before the cell is destroyed, reflecting the fact that a sphere has the smallest surface area for a given volume. The vast majority of patients with AIHA will have spherocytes on the blood smear. However, spherocytes are not specific to AIHA, as they can be seen in hereditary spherocytosis, Wilson’s disease, clostridial sepsis, and severe burns.
Patients with cold agglutinins will often have red cell agglutination on the blood smear. In addition, patients with AIHA will often have a raised mean corpuscular volume (MCV) for 2 reasons. In patients with brisk reticulocytosis, the MCV will be raised due to the large size of the reticulocyte. In patients with cold agglutinin disease, the MCV may be falsely raised due to clumping of the red blood cells.
Urinary Hemosiderin
When hemoglobin is excreted by the kidney, the iron is deposited in the tubules. When the tubule cells are sloughed off, they appear in the urine. The urine can be stained for iron, and a positive result is another sign of hemolysis. Hemosiderinuria is a later sign of hemolysis, as it takes 1 week for iron-laden tubule cells to be excreted in sufficient quantities to be detected in the urine.
Urinary Hemoglobin
One other sign of hemolysis is the presence of hemoglobin in the urine. A quick way to demonstrate hemoglobinuria is to check the urine with a dipstick followed by a microscopic exam. In hemolysis, the dipstick will detect “blood,” while the microscopic exam will be negative for red cells.
Laboratory Evaluation for Autoimmune Component
The autoimmune component is shown by demonstrating the presence of either IgG molecules or complement on the surface of red blood cells.4,6 This can be done by performing the direct antiglobulin test (DAT) or Coombs test. IgG bound to red cells will not agglutinate them, but if IgM that is directed against IgG or C3 is added, the red cells will agglutinate, proving that there is IgG and/or C3 on the red cell membrane. The finding of a positive DAT in the setting of a hemolytic anemia is diagnostic of AIHA. Beware of individuals with concomitant weak positive DAT and other causes of hemolysis. The strength of the DAT result and the degree of hemolysis must match in order to conclude that the hemolysis is immune-mediated.
There are several pitfalls to the DAT. One is that a positive DAT is found in 1:1000 patients in the normal population and in up to several percent of ill patients, especially those with elevated gamma globulin, such as patients with liver disease or HIV infection.6 Administration of intravenous immunoglobulin (IVIG) can also create a positive DAT. Conversely, patients can have AIHA with a negative DAT.7-9 For some patients, the number of IgG molecules bound to the red cell is below the detection limit of the DAT reagents. Other patients can have IgA or “warm” IgM as the cause of the AIHA.10 Specialty laboratories can test for these possibilities. The diagnosis of DAT-negative AIHA should be made with caution, and other causes of hemolysis, such as hereditary spherocytosis or paroxysmal hematuria, should be excluded.
Transfusion Therapy
Performing transfusions can be very difficult in patients with AIHA.2 The presence of the autoantibody can interfere with typing of the blood and almost always interferes with the crossmatch, since this final step consists of mixing the patient’s serum or plasma with donor red cells. In most patients with AIHA, the autoantibodies will react with any donor cells, rendering a negative crossmatch impossible. Without the crossmatch, the concern is that underlying alloantibodies can be missed. Studies indicate that 15% to 32% of patients will have underlying alloantibodies, which can lead to transfusion reactions.2 However, there are 2 considerations that may mitigate these concerns.11,12 First, patients who have never been transfused or pregnant will rarely have alloantibodies. Second, a patient who has been transfused in the remote past may have an anamnestic antibody response but not an immediate hemolytic reaction.
The transfusion service can take several steps to identify alloantibodies. Occasionally, if the autoantibody is weakly reacting when the patient’s serum is tested against a panel of reagent red cells, the alloantibodies can be identified by their stronger reactions as compared with the weakly reactive autoantibody. The most common technique for identifying alloantibodies is the autoadsorption technique.4,13 This involves incu-bating the patient’s red cells with the patient’s serum to adsorb the autoantibody. After a period of incubation, the cells are pelleted and the serum is collected as the supernatant. The adsorbed serum may be incubated with another sample of the patient’s cells for a second adsorption if the initial agglutination reactions of the patient’s serum with the reagent cells were strong. After 1 to 3 adsorptions, the adsorbed serum is tested with a red cell panel in order to check for “leftover” alloantibodies.
When a patient is first suspected of having AIHA, a generous sample of blood should be given to the transfusion service to allow for adequate testing. Many centers will test the blood not only for blood groups ABO and D but also perform full Rh typing plus check for Kidd, Duffy and Kell status.14 Increasingly, this is performed by direct genetic sequencing for the appropriate genotypes. This can allow transfusion of phenotypically matched red blood cells to lessen the risk of alloantibody formation.
One difficult issue is timing of transfusion. Clinicians are often hesitant to transfuse patients with AIHA due to fear of reactions, but in patients with severe anemia, especially elderly patients or those with heart disease, transfusion can be lifesaving. Since in some cases it may take hours to screen for alloantibodies, it is often preferable to transfuse patients with severe anemia and observe carefully for reaction.
1. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
3. Seve P, Philippe P, Dufour JF, et al. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189-204.
4. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
5. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
6. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
7. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607-618.
8. Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156-164.
9. Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655-656.
10. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
11. Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503-1507.
12. Blackall DP. How do I approach patients with warm-reactive autoantibodies? Transfusion. 2011;51:14-17.
13. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668.
14. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons.1,2 AIHA is mediated by antibodies, and in the majority of cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C (Table 1). This article series reviews the most common types of AIHA, with an overview of evaluation and diagnosis presented in this article and management of warm, cold, and drug-induced AIHA reviewed in a separate article.
Pathogenesis
In most cases, the ultimate etiology of AIHA is unknown. In warm AIHA, the target epitopes in most cases are Rh proteins.2 What leads the immune system to target these proteins is unidentified, but one theory is that an initial immune response to a foreign antigen starts to cross-react with the Rh proteins and the immune system fails to suppress this autoreactive response, leading to hemolysis. In IgG-mediated (warm) hemolysis, the red cells become coated with IgG molecules, which mark the cells for uptake and destruction by splenic macrophages.3 In "cold" AIHA, IgM molecules fix complement to the surface of red blood cells. Rarely, this can lead to activation of the full complement cascade, resulting in red cell lysis, but more often it is stopped at the C3 stage, leading to C3-coated red cells which are then taken up by hepatic macrophages.4
Suspecting the Diagnosis
In many patients, it is the symptoms and signs of anemia that lead to suspicion of hemolysis. Older patients often present earlier in the course of the disease due to lack of tolerance of anemia, especially if there is a sudden drop in the red blood cell count. Dark, cola-colored urine resulting from the presence of free hemoglobin may be noted by some patients. Patients with rapid-onset hemolysis may note lumbar back pain, and those with cold agglutinins often note symptoms related to agglutination of red cells in the peripheral circulation, such as the development of acrocyanosis in cold weather.5 In rare cases, patients will have abdominal pain when eating cold food due to ischemia related to agglutination of red cells in the viscera. Some patients with cold agglutinins can have an exacerbation of their hemolysis with cold exposure.
Unlike patients with immune thrombocytopenia, those with AIHA may have mild splenomegaly on exam. The presence of enlarged lymph nodes or massive splenomegaly should raise concern about concomitant lymphoma or chronic lymphocytic leukemia.
Making the Diagnosis
The 2 key steps in diagnosis are (1) demonstrating hemolysis and (2) demonstrating the autoimmune component.
Laboratory Evaluation for Hemolysis
Hemolysis is proven by finding evidence of both red cell breakdown and the compensatory increase in red cell production this stimulates (Table 2). The following sections discuss the laboratory tests that are performed to investigate hemolysis.
Lactate Dehydrogenase
When red cells undergo hemolysis, they release their contents, which are mostly comprised of hemoglobin but also include lactate dehydrogenase (LDH), an enzyme found in high concentration in red cells. Most patients with hemolysis will have an elevated LDH level, making this a sensitive test. However, because many other processes, including liver disease and pneumonia, also raise the serum LDH level, this finding is not specific for hemolysis.
Serum Bilirubin
Hemoglobin is salvaged by haptoglobin, and the heme moiety is broken down first to bilirubin and then to urobilinogen, which is excreted in the urine.2 Bilirubin produced from the breakdown of heme is not conjugated, but rather is delivered to the liver, where it is conjugated and excreted into the bile. In hemolysis, the concentration of unconjugated bilirubin (indirect bilirubin) is increased, while in liver disease the level of conjugated bilirubin (direct bilirubin) is increased. However, if the patient has concomitant liver disease with an increased direct bilirubin level, the serum bilirubin test is not reliable.
Serum Haptoglobin
Haptoglobin binds free serum hemoglobin and is taken up by the liver. Haptoglobin usually falls to very low levels in hemolysis. A confounder is that haptoglobin is an acute phase reactant and can rise with systemic disease or inflammation. However, patients with advanced liver disease will have low haptoglobin levels due to lack of synthesis, and up to 2% of the population may congenitally lack haptoglobin.1
Serum Hemoglobin
If the hemolysis is very rapid, the amount of free hemoglobin released will overwhelm the binding capacity of haptoglobin and lead to free hemoglobin in the plasma. This can be crudely quantified by examining the plasma color. Even minute amounts of free hemoglobin will turn the plasma pink. In fulminant hemolysis, the plasma will be cola-colored.
Reticulocyte Count
In most patients with hemolysis, the destruction of red cells is accompanied by an increase in the reticulocyte count. Reticulocytes are red cells that still contain RNA and are a marker of red cells that are approximately 24 hours old or less. Traditionally, reticulocytes were measured manually by staining the blood smear with vital blue and counting the percentage of cells that absorb the stain; this percentage needs to be adjusted for the hematocrit. Usually a percentage above 1.5% is considered indicative of an elevated reticulocyte count. Recently, automated complete blood count machines have taken advantage of the fact that reticulocytes will absorb certain stains; these machines can directly measure the reticulocyte count via flow cytometry, which results in an “absolute” reticulocyte count. The reticulocyte count obtained using this method does not have to be corrected for hematocrit, and levels of approximately 90,000/μL are considered raised. However, the reticulocyte count can also be raised in blood loss or in patients who have other causes of anemia (eg, iron deficiency) under treatment. In addition, as many as 25% of patients with AIHA will never have raised counts for various reasons, such as nutritional deficiency, autoimmune destruction of red cell precursors, or lack of erythropoietin.
Blood Smear
The blood smear provides vital information. The hallmark laboratory parameter of AIHA is spherocytes seen on the blood smear. In AIHA, antibodies and/or complement attach to the red cells, and when the antibodies or complement are taken up by macrophages in the spleen some of the red blood cell mem-brane is removed as well, decreasing the surface area of the cell. As the surface area of the red cell decreases with each pass through the spleen, the cell's shape changes from a biconcave disk to a sphere before the cell is destroyed, reflecting the fact that a sphere has the smallest surface area for a given volume. The vast majority of patients with AIHA will have spherocytes on the blood smear. However, spherocytes are not specific to AIHA, as they can be seen in hereditary spherocytosis, Wilson’s disease, clostridial sepsis, and severe burns.
Patients with cold agglutinins will often have red cell agglutination on the blood smear. In addition, patients with AIHA will often have a raised mean corpuscular volume (MCV) for 2 reasons. In patients with brisk reticulocytosis, the MCV will be raised due to the large size of the reticulocyte. In patients with cold agglutinin disease, the MCV may be falsely raised due to clumping of the red blood cells.
Urinary Hemosiderin
When hemoglobin is excreted by the kidney, the iron is deposited in the tubules. When the tubule cells are sloughed off, they appear in the urine. The urine can be stained for iron, and a positive result is another sign of hemolysis. Hemosiderinuria is a later sign of hemolysis, as it takes 1 week for iron-laden tubule cells to be excreted in sufficient quantities to be detected in the urine.
Urinary Hemoglobin
One other sign of hemolysis is the presence of hemoglobin in the urine. A quick way to demonstrate hemoglobinuria is to check the urine with a dipstick followed by a microscopic exam. In hemolysis, the dipstick will detect “blood,” while the microscopic exam will be negative for red cells.
Laboratory Evaluation for Autoimmune Component
The autoimmune component is shown by demonstrating the presence of either IgG molecules or complement on the surface of red blood cells.4,6 This can be done by performing the direct antiglobulin test (DAT) or Coombs test. IgG bound to red cells will not agglutinate them, but if IgM that is directed against IgG or C3 is added, the red cells will agglutinate, proving that there is IgG and/or C3 on the red cell membrane. The finding of a positive DAT in the setting of a hemolytic anemia is diagnostic of AIHA. Beware of individuals with concomitant weak positive DAT and other causes of hemolysis. The strength of the DAT result and the degree of hemolysis must match in order to conclude that the hemolysis is immune-mediated.
There are several pitfalls to the DAT. One is that a positive DAT is found in 1:1000 patients in the normal population and in up to several percent of ill patients, especially those with elevated gamma globulin, such as patients with liver disease or HIV infection.6 Administration of intravenous immunoglobulin (IVIG) can also create a positive DAT. Conversely, patients can have AIHA with a negative DAT.7-9 For some patients, the number of IgG molecules bound to the red cell is below the detection limit of the DAT reagents. Other patients can have IgA or “warm” IgM as the cause of the AIHA.10 Specialty laboratories can test for these possibilities. The diagnosis of DAT-negative AIHA should be made with caution, and other causes of hemolysis, such as hereditary spherocytosis or paroxysmal hematuria, should be excluded.
Transfusion Therapy
Performing transfusions can be very difficult in patients with AIHA.2 The presence of the autoantibody can interfere with typing of the blood and almost always interferes with the crossmatch, since this final step consists of mixing the patient’s serum or plasma with donor red cells. In most patients with AIHA, the autoantibodies will react with any donor cells, rendering a negative crossmatch impossible. Without the crossmatch, the concern is that underlying alloantibodies can be missed. Studies indicate that 15% to 32% of patients will have underlying alloantibodies, which can lead to transfusion reactions.2 However, there are 2 considerations that may mitigate these concerns.11,12 First, patients who have never been transfused or pregnant will rarely have alloantibodies. Second, a patient who has been transfused in the remote past may have an anamnestic antibody response but not an immediate hemolytic reaction.
The transfusion service can take several steps to identify alloantibodies. Occasionally, if the autoantibody is weakly reacting when the patient’s serum is tested against a panel of reagent red cells, the alloantibodies can be identified by their stronger reactions as compared with the weakly reactive autoantibody. The most common technique for identifying alloantibodies is the autoadsorption technique.4,13 This involves incu-bating the patient’s red cells with the patient’s serum to adsorb the autoantibody. After a period of incubation, the cells are pelleted and the serum is collected as the supernatant. The adsorbed serum may be incubated with another sample of the patient’s cells for a second adsorption if the initial agglutination reactions of the patient’s serum with the reagent cells were strong. After 1 to 3 adsorptions, the adsorbed serum is tested with a red cell panel in order to check for “leftover” alloantibodies.
When a patient is first suspected of having AIHA, a generous sample of blood should be given to the transfusion service to allow for adequate testing. Many centers will test the blood not only for blood groups ABO and D but also perform full Rh typing plus check for Kidd, Duffy and Kell status.14 Increasingly, this is performed by direct genetic sequencing for the appropriate genotypes. This can allow transfusion of phenotypically matched red blood cells to lessen the risk of alloantibody formation.
One difficult issue is timing of transfusion. Clinicians are often hesitant to transfuse patients with AIHA due to fear of reactions, but in patients with severe anemia, especially elderly patients or those with heart disease, transfusion can be lifesaving. Since in some cases it may take hours to screen for alloantibodies, it is often preferable to transfuse patients with severe anemia and observe carefully for reaction.
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons.1,2 AIHA is mediated by antibodies, and in the majority of cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C (Table 1). This article series reviews the most common types of AIHA, with an overview of evaluation and diagnosis presented in this article and management of warm, cold, and drug-induced AIHA reviewed in a separate article.
Pathogenesis
In most cases, the ultimate etiology of AIHA is unknown. In warm AIHA, the target epitopes in most cases are Rh proteins.2 What leads the immune system to target these proteins is unidentified, but one theory is that an initial immune response to a foreign antigen starts to cross-react with the Rh proteins and the immune system fails to suppress this autoreactive response, leading to hemolysis. In IgG-mediated (warm) hemolysis, the red cells become coated with IgG molecules, which mark the cells for uptake and destruction by splenic macrophages.3 In "cold" AIHA, IgM molecules fix complement to the surface of red blood cells. Rarely, this can lead to activation of the full complement cascade, resulting in red cell lysis, but more often it is stopped at the C3 stage, leading to C3-coated red cells which are then taken up by hepatic macrophages.4
Suspecting the Diagnosis
In many patients, it is the symptoms and signs of anemia that lead to suspicion of hemolysis. Older patients often present earlier in the course of the disease due to lack of tolerance of anemia, especially if there is a sudden drop in the red blood cell count. Dark, cola-colored urine resulting from the presence of free hemoglobin may be noted by some patients. Patients with rapid-onset hemolysis may note lumbar back pain, and those with cold agglutinins often note symptoms related to agglutination of red cells in the peripheral circulation, such as the development of acrocyanosis in cold weather.5 In rare cases, patients will have abdominal pain when eating cold food due to ischemia related to agglutination of red cells in the viscera. Some patients with cold agglutinins can have an exacerbation of their hemolysis with cold exposure.
Unlike patients with immune thrombocytopenia, those with AIHA may have mild splenomegaly on exam. The presence of enlarged lymph nodes or massive splenomegaly should raise concern about concomitant lymphoma or chronic lymphocytic leukemia.
Making the Diagnosis
The 2 key steps in diagnosis are (1) demonstrating hemolysis and (2) demonstrating the autoimmune component.
Laboratory Evaluation for Hemolysis
Hemolysis is proven by finding evidence of both red cell breakdown and the compensatory increase in red cell production this stimulates (Table 2). The following sections discuss the laboratory tests that are performed to investigate hemolysis.
Lactate Dehydrogenase
When red cells undergo hemolysis, they release their contents, which are mostly comprised of hemoglobin but also include lactate dehydrogenase (LDH), an enzyme found in high concentration in red cells. Most patients with hemolysis will have an elevated LDH level, making this a sensitive test. However, because many other processes, including liver disease and pneumonia, also raise the serum LDH level, this finding is not specific for hemolysis.
Serum Bilirubin
Hemoglobin is salvaged by haptoglobin, and the heme moiety is broken down first to bilirubin and then to urobilinogen, which is excreted in the urine.2 Bilirubin produced from the breakdown of heme is not conjugated, but rather is delivered to the liver, where it is conjugated and excreted into the bile. In hemolysis, the concentration of unconjugated bilirubin (indirect bilirubin) is increased, while in liver disease the level of conjugated bilirubin (direct bilirubin) is increased. However, if the patient has concomitant liver disease with an increased direct bilirubin level, the serum bilirubin test is not reliable.
Serum Haptoglobin
Haptoglobin binds free serum hemoglobin and is taken up by the liver. Haptoglobin usually falls to very low levels in hemolysis. A confounder is that haptoglobin is an acute phase reactant and can rise with systemic disease or inflammation. However, patients with advanced liver disease will have low haptoglobin levels due to lack of synthesis, and up to 2% of the population may congenitally lack haptoglobin.1
Serum Hemoglobin
If the hemolysis is very rapid, the amount of free hemoglobin released will overwhelm the binding capacity of haptoglobin and lead to free hemoglobin in the plasma. This can be crudely quantified by examining the plasma color. Even minute amounts of free hemoglobin will turn the plasma pink. In fulminant hemolysis, the plasma will be cola-colored.
Reticulocyte Count
In most patients with hemolysis, the destruction of red cells is accompanied by an increase in the reticulocyte count. Reticulocytes are red cells that still contain RNA and are a marker of red cells that are approximately 24 hours old or less. Traditionally, reticulocytes were measured manually by staining the blood smear with vital blue and counting the percentage of cells that absorb the stain; this percentage needs to be adjusted for the hematocrit. Usually a percentage above 1.5% is considered indicative of an elevated reticulocyte count. Recently, automated complete blood count machines have taken advantage of the fact that reticulocytes will absorb certain stains; these machines can directly measure the reticulocyte count via flow cytometry, which results in an “absolute” reticulocyte count. The reticulocyte count obtained using this method does not have to be corrected for hematocrit, and levels of approximately 90,000/μL are considered raised. However, the reticulocyte count can also be raised in blood loss or in patients who have other causes of anemia (eg, iron deficiency) under treatment. In addition, as many as 25% of patients with AIHA will never have raised counts for various reasons, such as nutritional deficiency, autoimmune destruction of red cell precursors, or lack of erythropoietin.
Blood Smear
The blood smear provides vital information. The hallmark laboratory parameter of AIHA is spherocytes seen on the blood smear. In AIHA, antibodies and/or complement attach to the red cells, and when the antibodies or complement are taken up by macrophages in the spleen some of the red blood cell mem-brane is removed as well, decreasing the surface area of the cell. As the surface area of the red cell decreases with each pass through the spleen, the cell's shape changes from a biconcave disk to a sphere before the cell is destroyed, reflecting the fact that a sphere has the smallest surface area for a given volume. The vast majority of patients with AIHA will have spherocytes on the blood smear. However, spherocytes are not specific to AIHA, as they can be seen in hereditary spherocytosis, Wilson’s disease, clostridial sepsis, and severe burns.
Patients with cold agglutinins will often have red cell agglutination on the blood smear. In addition, patients with AIHA will often have a raised mean corpuscular volume (MCV) for 2 reasons. In patients with brisk reticulocytosis, the MCV will be raised due to the large size of the reticulocyte. In patients with cold agglutinin disease, the MCV may be falsely raised due to clumping of the red blood cells.
Urinary Hemosiderin
When hemoglobin is excreted by the kidney, the iron is deposited in the tubules. When the tubule cells are sloughed off, they appear in the urine. The urine can be stained for iron, and a positive result is another sign of hemolysis. Hemosiderinuria is a later sign of hemolysis, as it takes 1 week for iron-laden tubule cells to be excreted in sufficient quantities to be detected in the urine.
Urinary Hemoglobin
One other sign of hemolysis is the presence of hemoglobin in the urine. A quick way to demonstrate hemoglobinuria is to check the urine with a dipstick followed by a microscopic exam. In hemolysis, the dipstick will detect “blood,” while the microscopic exam will be negative for red cells.
Laboratory Evaluation for Autoimmune Component
The autoimmune component is shown by demonstrating the presence of either IgG molecules or complement on the surface of red blood cells.4,6 This can be done by performing the direct antiglobulin test (DAT) or Coombs test. IgG bound to red cells will not agglutinate them, but if IgM that is directed against IgG or C3 is added, the red cells will agglutinate, proving that there is IgG and/or C3 on the red cell membrane. The finding of a positive DAT in the setting of a hemolytic anemia is diagnostic of AIHA. Beware of individuals with concomitant weak positive DAT and other causes of hemolysis. The strength of the DAT result and the degree of hemolysis must match in order to conclude that the hemolysis is immune-mediated.
There are several pitfalls to the DAT. One is that a positive DAT is found in 1:1000 patients in the normal population and in up to several percent of ill patients, especially those with elevated gamma globulin, such as patients with liver disease or HIV infection.6 Administration of intravenous immunoglobulin (IVIG) can also create a positive DAT. Conversely, patients can have AIHA with a negative DAT.7-9 For some patients, the number of IgG molecules bound to the red cell is below the detection limit of the DAT reagents. Other patients can have IgA or “warm” IgM as the cause of the AIHA.10 Specialty laboratories can test for these possibilities. The diagnosis of DAT-negative AIHA should be made with caution, and other causes of hemolysis, such as hereditary spherocytosis or paroxysmal hematuria, should be excluded.
Transfusion Therapy
Performing transfusions can be very difficult in patients with AIHA.2 The presence of the autoantibody can interfere with typing of the blood and almost always interferes with the crossmatch, since this final step consists of mixing the patient’s serum or plasma with donor red cells. In most patients with AIHA, the autoantibodies will react with any donor cells, rendering a negative crossmatch impossible. Without the crossmatch, the concern is that underlying alloantibodies can be missed. Studies indicate that 15% to 32% of patients will have underlying alloantibodies, which can lead to transfusion reactions.2 However, there are 2 considerations that may mitigate these concerns.11,12 First, patients who have never been transfused or pregnant will rarely have alloantibodies. Second, a patient who has been transfused in the remote past may have an anamnestic antibody response but not an immediate hemolytic reaction.
The transfusion service can take several steps to identify alloantibodies. Occasionally, if the autoantibody is weakly reacting when the patient’s serum is tested against a panel of reagent red cells, the alloantibodies can be identified by their stronger reactions as compared with the weakly reactive autoantibody. The most common technique for identifying alloantibodies is the autoadsorption technique.4,13 This involves incu-bating the patient’s red cells with the patient’s serum to adsorb the autoantibody. After a period of incubation, the cells are pelleted and the serum is collected as the supernatant. The adsorbed serum may be incubated with another sample of the patient’s cells for a second adsorption if the initial agglutination reactions of the patient’s serum with the reagent cells were strong. After 1 to 3 adsorptions, the adsorbed serum is tested with a red cell panel in order to check for “leftover” alloantibodies.
When a patient is first suspected of having AIHA, a generous sample of blood should be given to the transfusion service to allow for adequate testing. Many centers will test the blood not only for blood groups ABO and D but also perform full Rh typing plus check for Kidd, Duffy and Kell status.14 Increasingly, this is performed by direct genetic sequencing for the appropriate genotypes. This can allow transfusion of phenotypically matched red blood cells to lessen the risk of alloantibody formation.
One difficult issue is timing of transfusion. Clinicians are often hesitant to transfuse patients with AIHA due to fear of reactions, but in patients with severe anemia, especially elderly patients or those with heart disease, transfusion can be lifesaving. Since in some cases it may take hours to screen for alloantibodies, it is often preferable to transfuse patients with severe anemia and observe carefully for reaction.
1. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
3. Seve P, Philippe P, Dufour JF, et al. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189-204.
4. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
5. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
6. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
7. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607-618.
8. Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156-164.
9. Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655-656.
10. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
11. Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503-1507.
12. Blackall DP. How do I approach patients with warm-reactive autoantibodies? Transfusion. 2011;51:14-17.
13. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668.
14. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
1. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
3. Seve P, Philippe P, Dufour JF, et al. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189-204.
4. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
5. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
6. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
7. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607-618.
8. Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156-164.
9. Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655-656.
10. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
11. Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503-1507.
12. Blackall DP. How do I approach patients with warm-reactive autoantibodies? Transfusion. 2011;51:14-17.
13. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668.
14. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
Transfusion Medicine
INTRODUCTION
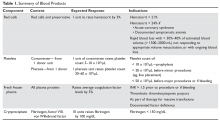
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
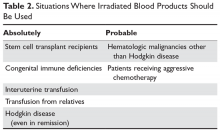
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
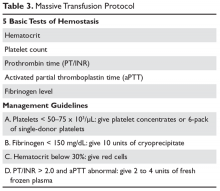
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
INTRODUCTION
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transmission of infections and transfusion-related reactions. Controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products (Table 1) and indications for transfusion along with the associated risks, and also discusses specific clinical situations, such as massive transfusion.
BLOOD PRODUCTS
WHOLE BLOOD
Whole blood is the product of 1 unit of donated blood plus anticoagulant/preservative, and by definition contains 1 unit of plasma and red cells. Whole blood can be stored for 5 weeks. Although it was the standard product in the past, whole blood is rarely used since 1 unit of donated blood can now be fractionated into 1 unit of red blood cells (RBC), 1 unit of platelets, and 1 unit of fresh frozen plasma (FFP). Thus, the use of whole blood for just a single transfusion represents a waste of resources. There are 2 exceptions. One is autologous blood donations, which are whole blood units. Second, whole blood is increasingly being used in massive transfusions for trauma patients, with the rationale being that all essential blood components are being transfused at once.1
PACKED RED CELLS
The remaining red cell mass after most of the plasma is removed is called the “packed” red cell unit (hematocrit = 70%–80%), and so red cells are often called “packed” red cells, or PRBC. A preservative is added to improve the flow of blood and to provide “nutrients” for the red cells, and this reduces the hematocrit to approximately 60%. The volume of a red cell unit is approximately 340 mL. In the average adult, 1 unit of RBC raises the hematocrit by 3%. The indications for transfusion of red cells are to increase red cell mass, and thus oxygen delivery, in patients who are compromised by their anemia.
Several randomized trials have helped define the indications for red cell transfusions and justify lower hematocrit thresholds for initiating transfusion. The TRICC (Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group) trial showed that in critical care patients (30-day mortality, 18.7%–23.3%), a conservative transfusion strategy of waiting until the hematocrit was below 21% had the same outcomes as transfusing at a threshold of 24%.2 The TRACS (Transfusion Requirements After Cardiac Surgery) trial showed that a hematocrit target of 24% had the same benefit as a target of 30% in patients who had undergone cardiac bypass surgery.3 For patients with acute myocardial infarction, the outcomes were worse with aggressive transfusion at a hematocrit of 30% compared to 24%.4 In patients with upper gastrointestinal bleeding, a hemoglobin transfusion trigger of 7 g/dL was associated with a lower mortality than a trigger of 9 g/dL (5% versus 9%).5 Finally, the FOCUS (Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair) trial showed that in older patients (average age 80 years) who had undergone hip fracture surgery, transfusions based on symptoms and not a fixed trigger of 30% had the same outcomes but considerable savings in blood products.6 Based on these trials, decisions regarding when to transfuse patients should be based on symptoms and not “numbers.” Young patients, especially those with reversible anemias, can tolerate low blood counts and should not be transfused based on an arbitrary number.
PLATELETS
Several types of platelet products exist. One unit of platelet concentrate is derived from 1 unit of donor blood. Plateletpheresis from volunteer donors is also used to harvest platelets, with the resulting product referred to as plateletpheresis platelets. One unit of single-donor (pheresis) platelets is equivalent to 6 platelet concentrates. Finally, HLA-matched platelets are single-donor pheresis units that are obtained from an HLA-matched donor. This product should be ordered only if there is evidence of HLA antibodies (see Platelet Alloimmunization section).
The dose of platelets for the average patient is 6 units of platelet concentrate or 1 pheresis unit. In theory, 1 unit of platelet concentrate can raise the count by 5 to 7 × 103/µL, but often this response is blunted by concurrent illness or bleeding. In patients who appear to have a poor response, the platelet count can be checked 15 minutes after platelet infusion. No rise or a minimal rise (< 2 × 103/µL) in the platelet count is suggestive of platelet refractoriness, while a good 15-minute response but poor 24-hour count is more suggestive of consumption—fever, sepsis, drug, or splenomegaly—and not refractoriness.
The indication for platelet transfusion depends on the clinical situation. For patients with immune thrombocytopenia, platelets should not be transfused unless there is life-threatening bleeding. For stable patients with marrow aplasia from chemotherapy, a cut-off of a morning platelet count of less than 10 × 103/µL has been shown to be as safe as higher levels for prophylactic transfusions.7 For patients with active bleeding, the platelet count should be kept above 50 × 103/µL. Patients with acquired or inherited platelet dysfunction may benefit from transfusion no matter the platelet count.
Platelet Alloimmunization
Patients exposed to transfused white cells with different HLA antigens can develop antibodies to these antigens.8 Anti-HLA antibodies are common in patients who previously have received transfused blood that is not leukodepleted and in patients who have been pregnant. Since platelets carry class I HLA antigens, they will be rapidly destroyed by anti-HLA antibodies when transfused into these patients. In patients transfused for aplastic anemia or myelodysplasia, as many as 90% will become HLA-immunized. The incidence is lower in patients receiving chemotherapy but still can be as high as 60% to 90%.9,10 Patients who have developed anti-HLA antibodies can respond to transfused platelets matched for HLA antigens. Unfortunately, some patients will either be a rare HLA type or be so heavily immunized that they will not respond to any platelet transfusion.
The significance of alloimmunization centers on 2 concepts: recognition and avoidance. Patients with HLA antibodies will fail to have an increment of their platelet counts with transfusions. Accordingly, patients who do not experience an increase in their count 15 minutes after the transfusion may have HLA antibodies. One can test for the presence of anti-HLA antibodies, although some patients instead have specific antiplatelet antibodies that will not respond to HLA-matched platelets. In patients who have been pregnant or previously transfused and are scheduled to undergo transplant or aggressive chemotherapy, it is wise to test for anti-HLA antibodies in order to plan their transfusion needs. The evidence suggests that transfused white cells are responsible for initiating the anti-HLA response. Trials have shown that giving leukodepleted blood products may reduce the incidence of alloimmunization, so patients who are not HLA-alloimmunized should receive only leukodepleted products.11A difficult problem is bleeding in patients who are refractory to platelet transfusion.12 Patients who test positive for the presence of anti-HLA antibodies can receive transfusions of HLA-matched platelets.13 Unfortunately, matched platelet transfusions are not effective in 20% to 70% of these patients. Also, since some loci are difficult to match, effective products may be unavailable. Finally, as many as 25% of patients have antiplatelet antibodies in which HLA-matched products will be ineffective. Platelet cross-matching can be performed to find compatible units for these patients, but this may not always be successful. In the patient who is totally refractory to platelet transfusion, consider drugs as an etiology of antiplatelet antibodies (especially vancomycin).14 Use of antifibrinolytic agents such as epsilon-aminocaproic acid or tranexamic acid may decrease the incidence of minor bleeding, but these are ineffective for major bleeding. “Platelet drips”—infusing either a platelet concentrate per hour or 1 plateletpheresis unit every 6 hours—may be given as a continuous infusion, but there is no evidence that this is helpful.15
FRESH FROZEN PLASMA
FFP is made from 1 unit of donated whole blood, with an average volume of 225 mL per unit. One unit of FFP can increase coagulation factor levels by 5% and fibrinogen by 10 mg/dL in the average stable patient. FFP can take 20 to 30 minutes to thaw before use, so in situations where FFP is needed quickly, the blood bank must be informed to “keep ahead” some units. Units of FFP that have been thawed but not used can be stored refrigerated for 5 days to prevent wasting blood products.
The indications for FFP are limited to several situations. These include a documented coagulation defect that can be corrected by a reasonable amount of FFP, such as factor V deficiency and factor XI deficiency, disseminated intravascular coagulation (DIC), reversal of warfarin, and massive transfusions. FFP is also used for the therapy of thrombotic thrombocytopenic purpura.
There is little justification for FFP transfusion in many of the clinical settings in which it is commonly used. For example, FFP is given for minor elevations of the INR in patients with liver disease, despite literature showing not only that the INR rise is not reflective of coagulation defects, but also that patients with liver disease may even be thrombophilic.16,17 Reviews of FFP use found limited evidence-based indications for its use.18,19 Also, several studies have shown that transfusion of FFP is not effective at reversing minor elevations of the INR (1.3–1.8).20 In a meta-analysis, FFP was associated with increased risk for lung injury and a trend toward increased mortality.18
CRYOPRECIPITATE
Cryoprecipitate is produced from 1 unit of FFP that is thawed at 4°C. The precipitate is resuspended with 10 mL of saline or FFP and refrozen for storage. One unit contains at least 150 mg of fibrinogen and 80 units of factor VIII, along with von Willebrand factor. Thawing time for cryoprecipitate is approximately 20 minutes.
Cryoprecipitate is used to raise the fibrinogen level in patients with DIC or massive transfusion with hemodilution. It is third-line therapy in the treatment of type 1 von Willebrand disease and is second-line therapy in the treatment of patients with other types of von Willebrand disease. Currently, von Willebrand factor concentrates are the preferred replacement product for von Willebrand disease. Cryoprecipitate can be used as a source of factor VIII for hemophiliacs, but the preferred product for these patients is the super pure factor VIII concentrates or recombinant products. Cryoprecipitate can also be used to shorten the bleeding time of uremic patients, but its effectiveness for this is controversial.
GRANULOCYTES
Granulocytes are harvested by leukopheresis of normal donors, with a target yield of 1010 granulocytes from each donor. To reach this target, the donors are often “stimulated” with neutrophil growth factors. The harvesting procedure can take 3 hours and is associated with some risks to the donor (eg, citrate toxicity). The current indications for granulocytes are very limited since the advent of neutrophil growth factors and improved antimicrobials.21 They can be useful in the neutropenic patient with a documented bacterial infection in whom the white blood cell count is not expected to recover in the near future. Given the difficulty of keeping the count up, these transfusions have been mainly used in treating small children.
SPECIAL BLOOD PRODUCTS
IRRADIATED BLOOD PRODUCTS
Irradiation of blood is performed for only one reason: to prevent transfusion-related graft-versus-host disease (TGVHD) (Table 2).22 The irradiation can be performed at the blood center or in the transfusion service of larger hospitals. The units are not radioactive and can be transfused safely to other patients. There is increased leakage of potassium in irradiated units of blood, so the units need to be transfused within 14 days; in patients potentially sensitive to potassium (eg, neonates), the units must be transfused within 24 hours. Patients undergoing stem cell transplant, those receiving either interuterine transfusions or products from relatives, any patient with Hodgkin disease or receiving purine analogs or alemtuzumab, and patients with severe congenital immune deficiencies should receive irradiated blood. Most would also advocate that patients with hematologic malignances receiving chemotherapy receive irradiated products, but this is more controversial.
LEUKPDEPLETED BLOOD
Contamination of blood products by white blood cells is increasingly being recognized as a possible cause of adverse effects in transfused patients, including febrile transfusion reactions, inducing HLA alloimmunization, immunosuppression, disease transmission, and TGVHD. Reducing white cells can reduce the incidence of all of these complications except TGVHD. Currently, white cells are removed by infusion through filters that trap the cells. This can be done either at the bedside, in the blood bank, or at the donor center. The majority of red cells provided by blood centers in many areas of the country are already leukoreduced, eliminating the need for labor-intensive filtration at the transfusion center or bedside. Platelets collected by plateletpheresis methods can also be made leukocyte-poor. The current indications for leukodepleted productions are:
- Prevention of febrile transfusion reactions in patients with previous documented reactions
- Prevention of HLA alloimmunization (ineffective if patient has received 1 or more blood products not leukodepleted or is already HLA immunized)
- Prevention of cytomegalovirus (CMV) infection
CMV-NEGATIVE BLOOD
CMV can be transmitted through any cellular blood product—red cells and platelets. For patients who are CMV-negative and receiving transplants, especially stem cell transplants, a new CMV infection can be devastating.21 For years only blood from CMV-negative donors was used to transfuse CMV-negative patients. This policy is effective in preventing CMV infection, but because 50% of the population is positive for CMV antibodies, it may potentially lead to shortages of products that could be transfused to the patient. Currently, leukoreduced blood products are used since leukofiltration of the blood is just as effective as transfusion of CMV-negative blood in preventing infections and allows greater use of all blood products.23
COMPLICATIONS OF TRANSFUSIONS
HEMOLYTIC TRANSFUSION REACTION
There are 2 forms of hemolytic reactions—immediate and delayed.24 The immediate reaction is associated with fevers, hypotension, back pain, and oliguria. In severe cases, DIC and renal failure may occur. The immediate reaction is due to transfusion of blood that reacts with the recipient’s preformed high-titer blood antigen antibodies, most often to ABO. This is fatal 2% of the time and occurs almost always as a result of errors in correct identification of the patient. Reactions are due to recipient antibodies attacking donated RBCs, resulting in release of hemoglobin and red cell membrane–antigen complexes. These complexes are believed to lead to the hypotension, fevers, chills, and renal damage associated with the hemolytic reaction. Treatment consists of immediately stopping the transfusion, notifying the blood bank, vigorous intravenous hydration to keep the urine output over 100 mL/hr, and supportive therapy.
The delayed reaction can range in severity from an abrupt drop in the hematocrit to normal response to transfusion but the patient developing a positive Coombs’ test. The delayed response is due to an anamnestic response to blood-group antigens. When the patient is exposed to the same antigen, there is a rise in antibody titer leading to the reaction. Some alloantibodies can lead to a brisk reaction, most often anti-Kidd. The frequency with which delayed transfusion reactions occur is underestimated because mild reactions often do not get worked up or even discovered.
ALLERGIC REACTIONS
Allergic reactions are common (1%–3% of transfusions) and occur in patients having antibodies to proteins in donor blood, which can lead to hives and itching with transfusions. Most of the time these allergic reactions are mild and can be treated with antihistamines. Prophylaxis with antihistamines is not indicated for future transfusions unless the reactions are frequent. Rarely these reactions can be associated with shock and hypotension. Patients who are immunoglobulin (Ig) A–deficient can develop anaphylactic reactions to IgA-containing blood products. Patients with severe allergic reactions need to have their IgA measured and, if deficient, receive only washed units or plasma from IgA-deficient donors to prevent future severe reactions.
FEBRILE REACTIONS
The most common transfusion reaction is a febrile reaction that occurs after the transfusion starts and that sometimes can be complicated by chills. This reaction often occurs due to the presence of leukocyte debris and cytokines in the donated blood. Therapy is supportive and involves stopping the transfusion and administering acetaminophen, but since hemolytic transfusion reactions can present with fever all patients need to be thoroughly evaluated. The incidence of reactions can be decreased by using leukodepleted blood and plateletpheresis platelets. Most patients do not benefit from receiving prophylactic acetaminophen for future transfusion unless they have multiple reactions.
TRANSFUSION-RELATED ACUTE LUNG INJURY
Once thought a rare complication, transfusion-related acute lung injury (TRALI) is increasingly being recognized, with an incidence of approximately 1:5000 patients; it is now the most frequent cause of transfusion-related death.24,25 TRALI is noncardiac pulmonary edema and typically manifests clinically with hypoxemia, fever, bilateral infiltrates, and hypotension 2 to 6 hours after blood is given. Ventilatory support is often required. Recovery is usually rapid (24–48 hours) and complete. The etiology is complex. In many cases, transfused anti-HLA antibodies react with the recipient’s white cells leading to pulmonary damage. Another theory is that transfusion of preformed cytokines leads to pulmonary damage. Because plasma products from multiparous women are most often associated with anti-HLA antibodies, the restricted use of blood products from women has decreased the incidence of TRALI over the past few years.26
TRANSFUSION-ASSOCIATED CIRCULATORY OVERLOAD
Increasingly it being recognized that volume overload resulting from transfusions can lead to significant morbidity.27 Patients with heart or renal disease or patients who already have compromised fluid status are at risk for transfusion-associated circulatory overload (TACO). Another risk factor is transfusion of multiple blood products. Patients with TACO develop dyspnea within 6 hours of transfusion, but do not have fever or rash with the dyspnea. The diagnosis is made by demonstrating circulatory overload (eg, high venous pressure, B-type natriuretic peptide). Treatment is aggressive diuresis. Strategies to prevent TACO include judicious use of blood products, especially in patients at risk for TACO, and the use of prophylactic diuretics, especially with red cell or plasma transfusions.28
TRANSFUSION-RELATED GRAFT-VERSUS-HOST DISEASE
TGVHD is a rare reaction, but one that is most often fatal.29 TGVHD occurs when donor lymphocytes attack the blood recipient’s organs—skin, liver, intestines, and marrow. This is very rare in the normal blood recipient unless the donor and recipient share some HLA haplotypes.30 In immunosuppressed patients, TGVHD can occur with lesser degrees of HLA similarity, with cases reported in blood recipients who are mainly patients with Hodgkin disease or acute leukemia undergoing chemotherapy, and in patients receiving purine analogs. TGVHD had not been reported in AIDS patients despite profound immunosuppression, perhaps because the milieu of the patient does not allow lymphocyte expansion. Symptoms of TGVHD are an erythematous rash that may progress to epidermal toxic necrolysis, liver dysfunction, diarrhea, and pancytopenia. TGVHD is prevented by irradiating blood products given to at-risk patients with 2500 to 3500 rads. Directed blood donation from all blood relatives should also be irradiated. TGVHD cannot be prevented by leukopoor blood because the minute amount of lymphocytes that are not filtered still can lead to these complications.
POST-TRANSFUSION PURPURA
Patients with post-transfusion purpura (PTP) develop severe thrombocytopenia (< 10 × 103/µL) with often severe bleeding 1 to 2 weeks after receiving any type of blood product.31 Patients who develop PTP most often lack platelet antigen PLA1 or other platelet antigens. For unknown reasons, exposure to the antigens from the transfusion leads to rapid destruction of the patient’s own platelets. The diagnostic clue is thrombocytopenia in a patient, typically female, who has received a red cell or platelet blood product in the past 7 to 10 days. Treatment consists of intravenous immunoglobulin32 and plasmapheresis to remove the offending antibody. If patients with a history of PTP require further transfusions, only PLA1-negative platelets should be given.
IRON OVERLOAD
Every transfusion of red cells delivers approximately 250 mg of iron to the recipient. Since there is no natural way of ridding the body of iron, heavily transfused patients are at risk of iron overload. This is most often seen in children heavily transfused for thalassemia. Starting in the second decade of life, these individuals will develop endocrinopathies due to iron overload, liver problems, and often fatal cardiomyopathies. Studies have shown that chelation of iron with deferoxamine can be effective in preventing this fatal complication.33 Oral iron chelators such as deferasirox and deferiprone are also effective. The risk of iron overload in heavily transfused patients with myelodysplasia or other transfusion-dependent anemias is unclear, and uncertainty exists about the need for chelation.34
Young patients who face years of transfusions should be started on iron chelation to avoid iron overload. For older patients with transfusion-dependent anemia, iron chelation therapy should be considered if their life expectancy is long (years to decades) or special studies such as T2-weighted cardiac magnetic resonance imaging showing iron overloading.35
INFECTIOUS COMPLICATIONS
Concern over transmission of HIV infection via blood products in the late 1980s led to both a reduction in blood product use and a greater awareness of infectious complications of transfusion and their prevention. However, no blood product can ever be assumed to be safe for 2 reasons. One is that blood products can transmit infections during a “window period”—the time before a contaminated product can be detected by testing. The second is that blood is not screened for all potential infections (eg, babesiosis or new infections such as West Nile virus at the start of the outbreak). Risk of infection is reduced in 2 ways: deferral of potential infectious donors and blood product testing.
As part of the donation process, potential blood donors are asked a series of questions to see if they have risk factors for infections (eg, recent travel to malarious areas, recent tattoos), and if they answer positive are deferred from donating blood. Blood products are then tested for infectious agents by a combination of methods including detection of viral antigen, antibody response to infections, and more recently polymerase chain reaction (PCR).36 Current screening includes syphilis testing; testing for antibodies to HIV, HTLV (human T-lymphotropic virus), hepatitis C virus, hepatitis B core antigen (HBcAg), hepatitis B surface antigen, and PCR for HIV, hepatitis B virus, HCV, and West Nile virus. Some centers also test for Trypanosoma cruzi, the cause of Chagas disease.
In the past, the numerically most common transfusion-related disease was hepatitis, first B and then C.37,38 The first step in eliminating these infections was to stop paying donors for blood products. With the introduction of effective testing for hepatitis B and then C, the incidence of transfusion-related hepatitis has plummeted.36 For example, with the introduction of a diagnostic test for hepatitis C, the estimated risk has fallen from 5% to less than 1 per million. Currently, the risk of transmission of hepatitis B and C, HIV, and HTLV is less than 1 in a million.38
Despite this testing, blood transfusions can transmit a variety of infections, including malaria and babesiosis.39 Any new blood-borne infection introduced into the population can get into the blood supply as well. For example, at the start of the West Nile virus epidemic, there was a cluster of transfusion-transmitted cases that resulted in severe and sometimes fatal illness in immunosuppressed patients, but this issue has been addressed with the development of a PCR assay for screening blood.40 The rate of transfusion-related babesiosis has been increasing and screening for the causative parasite is being considered.
MASSIVE TRANSFUSIONS
Acutely bleeding patients can require large amounts of transfusion products. Early data showed high mortality rates with transfusion of more than 20 units of blood,41 but with modern blood banking techniques and improved laboratory testing, this rate has decreased dramatically, with survival rates of 43% to 70% in patients transfused with more than 50 units of blood.42
The basic approach to massive transfusions is to first transfuse the patient to maintain hemodynamic stability while specific blood tests are being obtained, and then to use the results of these early tests to guide the rest of the resuscitation. An important component is the ability to rapidly deliver standard packages of red cells, usually 6 to 10 units at a time, to the bleeding patient. To avoid delay while the patient’s blood is being typed, the first products delivered are blood group O Rh-positive units. Given the shortage of Rh-negative blood, this should be reserved for only empiric therapy of women of child-bearing age. Once the blood type is known, the patient can be switched over to type-specific blood.
In the past decade, there has been a shift toward increasing the amount of plasma given to patients receiving massive transfusions. This shift has occurred for 2 reasons. One is that modeling of coagulation changes in massive bleeding suggests the need for larger amounts of plasma to correct defects than have previously been recommended.43 The other reason is based on analysis of resuscitation protocols used in military and civilian trauma centers showing that giving red cells and plasma units in a 1:1 ratio appears to be associated with improved outcomes in massive transfusion. Several studies have extended this concept to platelets, again suggesting improved survival with 1 unit of random donor platelets given 1:1 with red cells and plasma units. The PROPPR (Prospective Observational Multicenter Major Trauma Transfusion) study compared a 1:1:1 to 1:1:2 ratio in patients with severe trauma and major bleeding and found less exsanguination and faster achievement of hemostasis in the first 24 hours.44 This has led to the widespread adoption of the 1:1 ratio by most trauma centers, and by default to other massive transfusion situations despite the lack of clinical trial data.45
One barrier to increased use is that plasma is kept frozen and requires 20 minutes to thaw. Many institutions are now keeping inventories of thawed plasma available for immediate use, ranging from 2 to 4 units of group AB plasma to keeping their entire inventory as liquid plasma.46 Plasma that is thawed but not used can be relabeled as “thawed plasma” and kept for up to 5 days. Also, many centers now use group A plasma for massive transfusions as this rarely leads to transfusion reactions and is much more available.47 Research is currently under way on lyophilized plasma, which can be stored at room temperature and can be rapidly reconstituted for emergency use.
The standard approach for laboratory testing is obtaining 5 tests: hematocrit, platelet count, INR/prothrombin time, activated partial thromboplastin time (aPTT), and fibrinogen.48 Product selection is guided by these tests, and they are repeated at regular intervals during the massive transfusion. A typical protocol is shown in Table 3. It is important as part of any protocol to have a flow chart that records laboratory results and products given that any member of the team can easily view.
The transfusion threshold for a low hematocrit depends on the stability of the patient. If the hematocrit is below 30% and the patient is bleeding or hemodynamically unstable, one should transfuse packed red cells. Stable patients can tolerate lower hematocrits, and an aggressive transfusion policy may even be detrimental.2,49 If the patient is bleeding, has florid DIC, or has received platelet aggregation inhibitors, then keeping the platelet count above 50 ×
While in the past fibrinogen targets of 50 to 100 mg/dL were recommended, recent data indicate that a target of 150 mg/dL or higher may be more appropriate.51–53 Severe fibrinolysis may occur in certain clinical situations such as brain injuries, hepatic trauma, or ischemic limb reperfusion, and the use of large amounts of cryoprecipitate can be anticipated. In patients with an INR greater than 2 and an abnormal aPTT, one can give 2 to 4 units of FFP. For an aPTT greater than 1.5 times normal, 2 to 4 units of plasma should be given. Elevation of the aPTT above 1.8 times normal control is associated with microvascular bleeding in trauma patients.54 Patients with marked abnormalities (eg, anaPTT more than 2 times normal) may require aggressive therapy with at least 15 to 30 mL/kg (4–8 units for an average adult) of plasma.55
Recently there has been increasing interest in the use of thromboelastography (TEG) in massive transfusion.56 This is a point-of-care assay performed on fresh whole blood that can assess multiple facets of hemostasis, including coagulation, platelet function, and fibrinolysis.57,58 TEG is performed by placing a 0.35-mL sample of whole blood into an oscillating container with a sensor pin that measures the force of thrombus formation. TEG measures 5 parameters:
- r time: time from starting TEG until clot formation
- K time: time needed for tracing to go from 2 mm to 20 mm
- alpha angle: slope of tracing between r and K time
- MA: greatest amplitude of TEG tracing
- Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Several centers have incorporated TEG into resuscitation protocols that include standardized strategies for responding to abnormalities. Data suggest that use of TEG may decrease the use of blood products, especially in cardiac surgery, but this has not been prospectively studied in massive transfusions.56,59
COMPLICATIONS OF MASSIVE TRANSFUSIONS
Electrolyte abnormalities are unusual even in patients who receive massive transfusions.60 Platelet concentrates and plasma contain citrate that can chelate calcium. However, the citrate is rapidly metabolized, and it is rare to see clinically significant hypocalcemia. Although empiric calcium replacement is often recommended, one study suggests that this is associated with a worse outcome and should not be done.61 If hypocalcemia is a clinical concern, then levels should be drawn to guide therapy. Stored blood is acidic, with a pH of 6.5 to 6.9. However, acidosis attributed solely to transfused blood is rare and most often is a reflection of the patient’s stability. Empirical bicarbonate replacement has been associated with severe alkalosis and is not recom mended.62,63 Although potassium leaks out of stored red cells, even older units of blood contain only 8 mEq/L of potassium, so hyperkalemia is usually not a concern.
PATIENTS WITH AUTOIMMUNE HEMOLYTIC ANEMIA
Patients with autoimmune hemolytic anemia can be difficult to transfuse,64 because the autoantibody can interfere with several aspects of the transfusion services evaluation. In some patients the autoantibody can be so strong that the patient’s blood type cannot be determined. In most patients, the final step of the cross-match—mixing the donor blood with recipient plasma—will show noncompatibility due to the autoantibodies reacting with any red cells.
The first step when transfusing a patient with autoimmune hemolytic anemia is to draw several tubes of blood for the transfusion service before any potential transfusions. This allows the transfusion service to remove the autoantibodies so they can screen for underlying alloantibodies. Second, if the patient requires immediate transfusion, then type-specific or O-negative blood should be given. If the patient has not been recently (months) transfused, the incidence of a severe transfusion reaction is low. The first unit should be infused slowly with close observation of the patient. For patients who have been multiply transfused, the use of an “in-vivo” cross-match may be helpful. This is where the patient is slowly transfused 10 to 15 mL of blood over 15 minutes. The the plasma and urine are then assessed for signs of hemolysis and, if negative, the remaining product is given.
REFUSAL OF BLOOD PRODUCTS
The initial step in managing patients who refuse blood products is to find out why they are refusing them. Many patients have an exaggerated fear of HIV and other infectious agents, so discussing the very low risk for infection transmission can often resolve the situation. The most common reason for refusal of blood products is religious belief. Jehovah’s Witness patients will refuse blood products due to their interpretation of the Bible.65 All members will refuse red cells, plasma, and platelets, while decisions about “derived” blood products—products made by manipulation of the original donated units—are a matter of conscience. These include cryoprecipitate, intravenous gammaglobulin, and albumin.
In an elective situation, the first step is to discuss with the patient those products that are a matter of conscience and clearly document this. The patient’s blood count and iron stores should be assessed to identify any correctible causes of anemia or low iron stores before surgery. The use of erythropoietin to correct blood counts before surgery is controversial, as this may increase thrombosis risk and is contraindicated in patients with curable tumors.
For patients with acute blood loss, use of intravenous iron combined with high-dose erythropoietin is the most common approach to raise the blood count.65 A recommended erythropoietin dose is 300 units/kg 3 times a week, dropping to 100 units/kg 3 times weekly until the goal hematocrit is reached. Another often overlooked step is to consolidate and minimize laboratory testing. The most important step is to be respectful of the patient and their beliefs. Many larger cities have liaisons that can help with interactions between Jehovah’s Witness patients and the health care system.
NON-TRANSFUSION THERAPIES FOR ACUTE BLEEDING
DESMOPRESSIN
Desmopressin (DDAVP) is a synthetic analog of antidiuretic hormone that raises the levels of both factor VIII and von Willebrand protein severalfold.66 Desmopressin is effective in supporting hemostasis in patients with a wide variety of congenital and acquired bleeding disorders. However, desmopressin does not reduce blood loss before routine surgery in a healthy patient and should not be used for this purpose.67
TRANEXAMIC ACID
Tranexamic acid is an antifibrinolytic agent that blocks the binding of plasmin to fibrin.68 This agent was first shown to be useful in disorders that involve excessive fibrinolysis69–73 or as adjunctive therapy for oral or dental procedures in patients with a bleeding diathesis. In patients with severe thrombocytopenia, the use of antifibrinolytic agents may reduce bleeding. Increasing data shows that tranexamic acid can prevent blood loss in a variety of surgeries including heart bypass, liver transplantation, and orthopedic surgery.74 Patients across these settings have decreased blood loss and need for transfusion with no increased risk of thrombosis. The CRASH-2 study showed that the use of tranexamic acid significantly reduced mortality in trauma patients.75 The WOMEN trial demonstrated that 1 g of tranexamic acid given to women with blood loss of more than 500 mL after vaginal delivery or 1000 mL after cesarean section has a risk reduction of death of 0.81 with no increased risk of thrombosis.76 Given this abundant data, it is clear tranexamic acid needs to be part of any massive transfusion protocol.77
RECOMBINANT FACTOR VIIa
Recombinant factor VIIa (rVIIa) was originally developed as a “bypass” agent to support hemostasis in hemophiliacs.78 However, the use of rVIIa for a wide array of bleeding disorders, including patients with factor VII and XI deficiency and Glanzmann thrombasthenia, has been reported.79 Increasingly, rVIIa is being used as a “universal hemostatic agent” for patients with uncontrolled bleeding from any mechanism.80 Multiple case reports have described the use of rVIIa for bleeding in cardiac surgery patients, obstetrical bleeding, reversal of anticoagulation, and trauma.81 Unfortunately, little formal trial data exists to put these anecdotes into perspective, and formal review of clinical trial results has shown no benefit.82,83 However, when used in older patients, especially those with vascular risk factors, the risk of arterial thrombosis appears to increase.84 In the trials for intracranial hemorrhage, the thrombosis rate was 5% to 9%, and rates up to 10% for arterial events were seen in older patients in a review of all trials.85–87 Given the lack of data but the evidence of risk, rVIIa use should be restricted to patients with documented bleeding disorders that have been shown to benefit by its use.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
1. Yazer MH, Cap AP, Spinella PC, et al. How do I implement a whole blood program for massively bleeding patients? Transfusion 2018;58:622–8.
2. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17.
3. Hajjar LA, Vincent JL, Galas FR, et al. Tranfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67.
4. Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108–11.
5. Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21.
6. Carson JL, Terrin ML, Noveck H, et al; the FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011 Dec 4. [Epub ahead of print]
7. Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519–38.
8. Schiffer CA. Prevention of alloimmunization against platelets. Blood 1991;77:1–4.
9. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348–60.
10. Novotny VMJ, Van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: A prospective study. Blood 1995;85:1736–41.
11. McFarland J, Menitove J, Kagen L et al. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861–9.
12. Juskewitch JE, Norgan AP, De Goey SR, et al. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017;57:2828–35.
13. Schiffer CA. Diagnosis and management of refractoriness to platelet transfusion. Blood Rev 2001;15:175–80.
14. Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990;75:518–23.
15. Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374–8.
16. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56.
17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost 2017;1:150–61.
18. Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010;50:1370–83.
19. Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol 2018;181:54–67.
20. Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85.
21. Price TH. Granulocyte transfusion: current status. Semin Hematol 2007;44:15–23.
22. Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol 2011;152:35–51.
23. Thiele T, Kruger W, Zimmermann K, et al. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: a single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011;51:2620–6.
24. Delaney M, Wendel S, Bercovitz RS, et al; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388:2825–36.
25. Toy P, Gajic O, Bacchetti P, et al. Transfusion related acute lung injury: incidence and risk factors. Blood 2011 Nov 23. [Epub ahead of print]
26. Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion 2011;51:1278–83.
27. Friedman T, Javidroozi M, Lobel G, Shander A. Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth 2017;35:159–73.
28. Lin CR, Armali C, Callum J, et al. Transfusion-associated circulatory overload prevention: a retrospective observational study of diuretic use. Vox Sang 2018;113:386–92.
29. Sun X, Yu H, Xu Z, et al. Transfusion-associated graft-versus-host-disease: case report and review of literature. Transfus Apher Sci 2010;43:331–4.
30. Petz LD, Calhoun L, Yam P, et al. Transfusion-associated graft-versus-host disease in immunocompetent patients: report of a fatal case associated with transfusion of blood from a second-degree relative, and a survey of predisposing factors. Transfusion 1993;33:742–50.
31. Mueller-Eckhardt C. Post-transfusion purpura. Br J Hematol 1986;64:419–24.
32. Mueller-Eckhardt C, Kiefel V. High-dose IgG for posttransfusion purpura-revisited. Blut 1988;57:163–7.
33. Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051–2.
34. Zeidan AM, Griffiths EA. To chelate or not to chelate in MDS: That is the question! Blood Rev 2018. pii: S0268-960X(17)30128-5.
35. Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007;82:1013–16.
36. Squires JE. Risks of transfusion. South Med J 2011;104:762–9.
37. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician 2011;83:719–24.
38. Jacquot C, Delaney M. Efforts toward elimination of infectious agents in blood products. J Intensive Care Med 2018 Jan 1:885066618756589 [Epub ahead of print].
39. Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155:509–19.
40. Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002;42:1019–26.
41. Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma 1971;11:275–85.
42. Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma 2011;71(2 Suppl 3):S389–S393.
43. Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454–63.
44. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82.
45. Pasquier P, Gayat E, Rackelboom T, et al. An observational study of the fresh frozen plasma: red blood cell ratio in postpartum hemorrhage. Anesth Analg 2013;116:155–61.
46. Yuan S, Ziman A, Anthony MA, et al. How do we provide blood products to trauma patients? Transfusion 2009;49:1045–9.
47. Stevens WT, Morse BC, Bernard A, et al. Incompatible type A plasma transfusion in patients requiring massive transfusion protocol: Outcomes of an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg 2017;83:25–9.
48. DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin 2004;20:13–24.
49. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg 1986;73:783–5.
50. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 1979;190:91–9.
51. Gerlach R, Tolle F, Raabe A, et al. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke 2002;33:1618–23.
52. Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth 2008;101:769–73.
53. Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost 2007;5:266–73.
54. Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol 1987;67:365–8.
55. Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73.
56. Curry NS, Davenport R, Pavord S, et al.The use of viscoelastic haemostatic assays in the management of major bleeding: A British Society for Haematology Guideline. Br J Haematol 2018. doi: 10.1111/bjh.15524. [Epub ahead of print].
57. Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg 2010;251:604–14.
58. Whitten CW, Greilich PE. Thromboelastography: past, present, and future. Anesthesiology 2000;92:1223–5.
59. Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24.
60. Goskowicz R. The complications of massive tranfusion. Anesthesiology Clin North Am 1999;17:959–78.
61. Howland WS, Schwiezer O, Boyan CP. Massive blood replacement without calcuim administration. Surg Gynecol Obstet 1964;159:171–7.
62. Miller RD, Tong MJ, Robbins TO. Effects of massive transfusion of blood on acid-base balance. JAMA 1971;216:1762–5.
63. Collins JA. Problems associated with the massive transfusion of stored blood. Surgery 1974;75:274–95.
64. Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol 2004;124:712–6.
65. Scharman CD, Burger D, Shatzel JJ, et al. Treatment of individuals who cannot receive blood products for religious or other reasons. Am J Hematol 2017;92:1370–81
66. Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158–67.
67. Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017;15:263–72.
68. Ng W, Jerath A, Wa˛sowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50.
69. Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83–96.
70. Chang JC, Kane KK. Pathologic hyperfibrinolysis associated with amyloidosis: clinical response to epsilon amino caproic acid. Am J Clin Pathol 1984;81:382–7.
71. Anonymous. Tranexamic acid. Med Letter Drugs Therapeutics 1987;29:89–90.
72. Schwartz BS, Williams EC, Conlan MG, Mosher DF. Epsilon-aminocaproic acid in the treatment of patients with acute promyelocytic leukemia and acquired alpha-2-plasmin inhibitor defiency. Ann Intern Med 1986;105:873–7.
73. Takahashi H, Tatewaki W, Wada K, et al. Fibrinolysis and fibrinogenolysis in liver disease. Am J Hematol 1990;34:241-–5.
74. Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012;344:e3054.
75. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23–32.
76. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16.
77. Godbey EA, Schwartz J. ‘Massive transfusion protocols and the use of tranexamic acid’. Curr Opin Hematol 2018 Aug 16. doi: 10.1097/MOH.0000000000000457. [Epub ahead of print]
78. Hay CR, Negrier C, Ludlam CA. The treatment of bleeding in acquired haemophilia with recombinant factor VIIa: a multicentre study. Thromb Haemost 1997;78:1463–7.
79. DeLoughery TG. Management of bleeding emergencies: when to use recombinant activated factor VII. Expert Opin Pharmacother 2006;7:25–34.
80. Aledort L. Recombinant factor VIIa Is a pan-hemostatic agent? Thromb Haemost 2000;83:637–8.
81. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med 2011;154:516–22.
82. Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ 2011;183:E9–19.
83. Yank V, Tuohy CV, Logan AC et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011;154:529–40.
84. Pavese P, Bonadona A, Beaubien J, et al. FVIIa corrects the coagulopathy of fulminant hepatic failure but may be associated with thrombosis: a report of four cases. Can J Anaesth 2005;52:26–29.
85. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85.
86. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37.
87. Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010;363:1791–1800.
Autoimmune Hemolytic Anemia
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons. AIHA is mediated by antibodies, and in the majority of cases immunglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C. This manual reviews the most common types of AIHA, with emphasis on diagnosis and treatment.
To read the full article in PDF:
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons. AIHA is mediated by antibodies, and in the majority of cases immunglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C. This manual reviews the most common types of AIHA, with emphasis on diagnosis and treatment.
To read the full article in PDF:
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons. AIHA is mediated by antibodies, and in the majority of cases immunglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C. This manual reviews the most common types of AIHA, with emphasis on diagnosis and treatment.
To read the full article in PDF:
Transfusion Medicine
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transfusion-transmitted infections and transfusion-related reactions, and controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products and indications for transfusion along with the associated risks and also discusses specific clinical situations, such as massive transfusion.
To read the full article in PDF:
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transfusion-transmitted infections and transfusion-related reactions, and controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products and indications for transfusion along with the associated risks and also discusses specific clinical situations, such as massive transfusion.
To read the full article in PDF:
Transfusion therapy is an essential part of hematology practice, allowing for curative therapy of diseases such as leukemia, aplastic anemia, and aggressive lymphomas. Nonetheless, transfusions are associated with significant risks, including transfusion-transmitted infections and transfusion-related reactions, and controversy remains about key issues in transfusion therapy, such as triggers for red cell transfusions. This article reviews the available blood products and indications for transfusion along with the associated risks and also discusses specific clinical situations, such as massive transfusion.
To read the full article in PDF: