User login
Obtaining informed consent for research in an acute inpatient psychiatric setting
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
Taking the spice route: Psychoactive properties of culinary spices
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
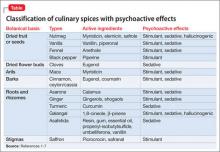
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
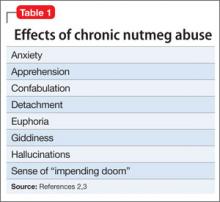
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
Out of the cupboard and into the clinic: Nutmeg-induced mood disorder
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.