User login
Physician burnout vs depression: Recognize the signs
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
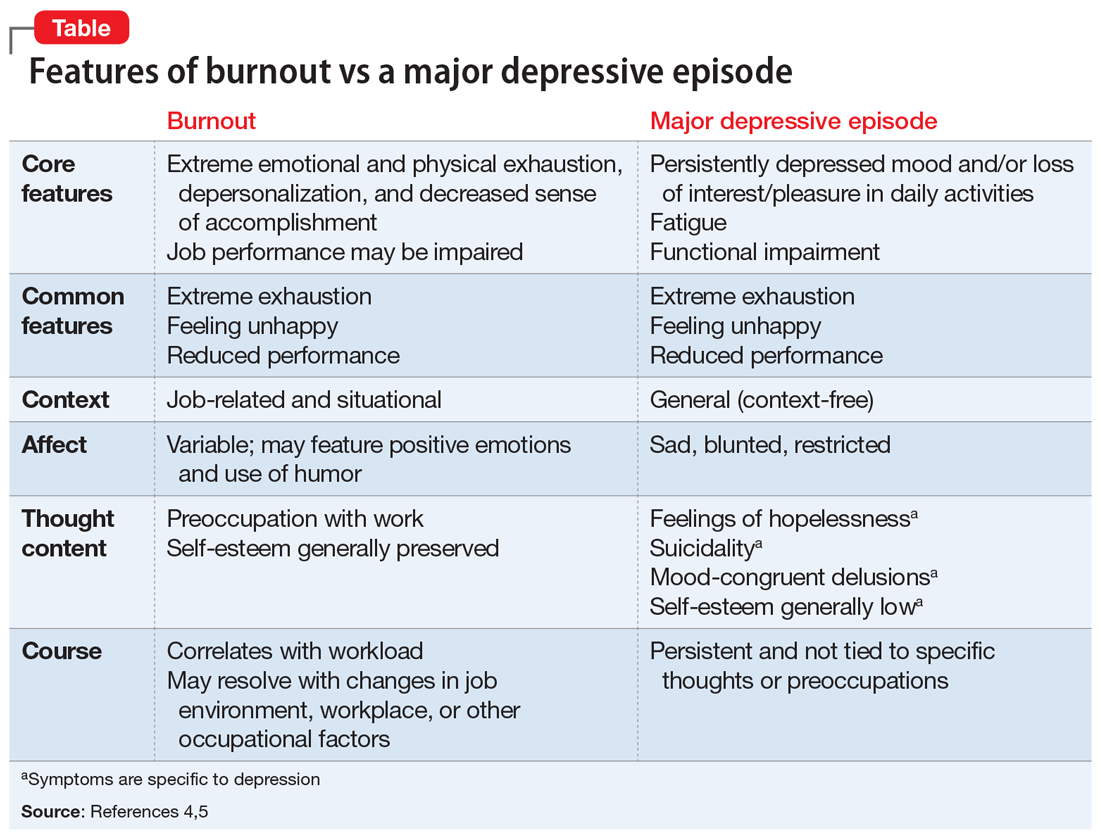
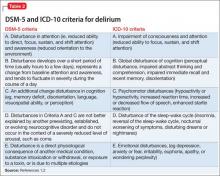
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
Although all health care professionals are at risk for burnout, physicians have especially high rates of self-reported burnout—which is commonly understood as a work-related syndrome of emotional exhaustion, depersonalization, and a decreased sense of accomplishment that develops over time.1 In a 2019 report investigating burnout in approximately 15,000 physicians, 39% of psychiatrists and nearly 50% of physicians from multiple other specialities described themselves as “burned out.”2 In addition, 15% reported symptoms of clinical depression (4%) or subclinical depression (11%). In comparison, in 2017, 7.1% of US adults experienced at least 1 major depressive episode.3 Because physician burnout and depression can be associated with adverse outcomes in patient care and personal health, rapid identification and differentiation of the 2 conditions is paramount.
Differentiating burnout and depression
Burnout and depression are distinct but overlapping entities. Although burnout can be difficult to recognize and is not currently a DSM diagnosis, physicians can learn to identify the signs with reference to the more familiar features of depression (Table4,5). Many features of burnout are work-related, while the negative feelings and thoughts of depression pertain to all areas of life. Furthermore, a major depressive episode often includes hopelessness, suicidality, or mood-congruent delusions; burnout does not. Shared symptoms of burnout and depression include extreme exhaustion, feeling unhappy, and reduced performance.
Surprisingly, there is no universally accepted definition of burnout.4,5 Some researchers have proposed that physicians who are categorized as “burned out” may actually have underlying anxiety or depressive disorders that have been misdiagnosed and not appropriately treated.4,5 Others claim that burnout is best formulated as a depressive condition in need of formal diagnostic criteria.4,5 Because the definition of burnout is in question,4,5 strategies to prevent and detect burnout in individual clinicians remain elusive.
Key areas that contribute to vulnerability to burnout include one’s sense of community, fairness, and control in the workplace; personal and organization values; and work-life balance. We propose the mnemonic WORK to help clinicians quickly assess their vulnerability to burnout in these areas.
Workload. Outside of working hours, are you satisfied with the amount of time you devote to self-care, recreation, and other activities that are important to you? Do you honor your “down time”?
Oversight. Are you satisfied with the flexibility and autonomy in your professional life? Are you able to cope with the systemic demands of your practice while upholding your priorities within these restrictions?
Reward. Are the mechanisms for feedback, opportunities for advancement, and financial compensation in your workplace fair? Do you find positive meaning in the work that you do?
Continue to: Kinship
Kinship. Does your place of work support cooperation and collaboration, rather than competition and isolation? Do you approach and receive support from your colleagues when you need assistance?
Persistent dissatisfaction in any of these aspects should prompt clinicians to further develop strategies that promote workplace engagement, job satisfaction, and resilience. We hope this mnemonic helps clinicians to take responsibility for their own well-being and ultimately reap the rewards of a fulfilling professional life.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
1. Brindley P. Psychological burnout and the intensive care practitioner: a practical and candid review for those who care. J Inten Care Soc. 2017;18(4):270-275.
2. Kane L. Medscape national physician b urnout & depression report 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#1. Published January 16, 2019. Accessed September 17, 2019.
3. National Institute of Mental Health. Prevalence of major depressive episode among adults. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Updated February 2019. Accessed September 17, 2019.
4. Messias E, Flynn V. The tired, retired, and recovered physician: professional burnout versus major depressive disorder. Am J Psychiatry. 2018;175(8):716-719.
5. Melnick ER, Powsner SM, Shanafelt TD. In reply—defining physician burnout, and differentiating between burnout and depression. Mayo Clinic Proc. 2017;92(9):1456-1458.
Counseling geriatric patients about opportunity and risk when ‘digital dating’
Baby Boomers represent a rapidly growing segment of digital device users.1 As these people age, their continued, even increasing, use of the Internet can be expected.1 At the same time, many older adults (age ≥65) are engaged in intimate relationships and regard sexuality as an important part of life.2
At this intersection, the Internet is likely to play a role in geriatric sexuality and “digital intimacy”—in that older adults can adopt patterns of using online dating sites similar to what their younger counterparts engage in. There is a need among clinicians to avoid stereotypical perceptions of “ageism” and the myth of “geriatric asexuality” as a result of older patients’ continued sexual interest and their adoption of social media technologies to facilitate the development of new intimate relationships. Acknowledgement of these realities by clinicians may assist in understanding and communication regarding these important areas of patients’ lives.
Why online dating?
Contemporary social and demographic changes (eg, higher divorce rates, increased longevity, aging of Baby Boomers) have influenced patterns of dating behaviors.3 Consistent with evolutionary theory, studies on courtship behaviors show that women remain the “choosers” of partners in relationships at all ages3; in contemporary society, however, there is an increasing ratio of women to men in later life, and the degree to which this demographic change might influence older men and women who are pursuing sexual relationships is unclear.3 Older adults might be aware of these demographic realities, and may use the Internet to increase their chances of finding a relationship.
For older homosexual men and women, demographic trends also are important because fewer available partners of similar sexual orientation might be available in their immediate communities, similarly incentivizing the use of online dating sites.
Hand in hand: Risk and vulnerability
Clinicians can discuss with geriatric patients who present with questions or concerns about sexuality and risks of online dating. Although risks associated with digital dating can involve anyone, those who are recently divorced, widowed, disabled, or elderly can be targeted by predators or fraudulent schemes, and thus become victims. Recognizing those risks and the vulnerability in the geriatric patient is crucial.
Chronic illness. Age-related physiological changes do not necessarily make one vulnerable; however, chronic diseases of aging, including major neurocognitive disorders, can impair daily function and increase disability and vulnerability. The majority of online dating sites do not discriminate among users, including those with disabilities such as incapacitating neuropsychiatric disorders. The clinician may need to assess cognitive status of patients specific to their capacity to fully understand the risks of use of social media. Inability to accomplish basic mastery of computer skills or inability to maintain appropriate boundaries and safeguards in relationships initiated and maintained using the Internet may assist in this determination. Patients with other problematic Internet use (eg, excessive devotion to online shopping or online gambling) may be prone to misusing social media and dating sites as well. Patients with clear impairment of memory or poor social judgment based on a neurocognitive disorder also might not maintain proper boundaries with social media use.
Feeling alone. Older persons might feel socially isolated, and therefore may be more willing to participate in online dating to increase their chances of establishing an intimate relationship or companionship. Research has shown that increased social ties, participation in groups, contact with friends and family, and perceived social support are associated with longer survival; on the other hand, social disengagement, low participation in leisure activities, and limited social networks are associated with higher risk of major neurocognitive disorders and increased disability.4
Little is known about social vulnerability in institutional settings, but institutional living could decrease social vulnerability in important ways (eg, access to social support, networks and activities, not living alone).4 Although the literature on older adults and “digital” or “virtual” dating is limited, there are essentially no such data from within institutional settings. It is important to separately address the issue of cognitively impaired patients’ capacity to consent to sexual activity both within institutional settings and elsewhere, as it raises numerous ethical dilemmas for clinicians.
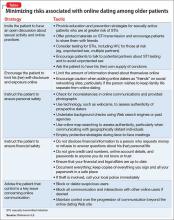
Being sexually active. Early research into online dating focused particularly on the risks of sexually transmitted infections (STIs),5 which could be acquired through failure to use condoms with a new partner.6 Older women particularly are less likely to use condoms with new sexual partners.6 Screening at-risk adults should occur regardless of age. Effective interventions are needed to increase condom use in this age group. Research in the general population has started to investigate how the use of technology can minimize the risks associated with online dating.5 The Table5,6 lists strategies that can be used to minimize some of the risks of online dating among geriatric patients, including STIs and victimization.
Clinicians working with sexually active geriatric patients need to perform sexual risk assessments, complete capacity assessments, and provide preventive measures.
Legal issues
Criminal and civil liability issues have arisen with online dating involving cases of murder, rape, fraud, identity theft, loans, theft, domestic violence, stalking, and burglary. Online dating also raises concerns around the right to fair use of the Internet in different contexts. Flirting in cyberspace can occur with e-mail, text, Twitter, Skype, and Instant Messenger. Practices likely will vary depending on whether older adults are institutionalized or living in the community, as well as their mental status (eg, having a major neurocognitive disorder).
Some questions with legal implications worth considering include:
- To what extent is there a duty to accommodate healthy sexual relationships in institutionalized settings?
- At what point does monitoring and supervision become overly intrusive?
- Are older adults fully aware of the potential ramifications of sharing sensitive information in cyberspace?
- What is the threshold for capacity to consent among older adults to understand the sexual nature of the act and consent to the act?
Nursing homes and health care providers may become concerned about potential liability if their organization provides digital devices or electronic platforms that are not closely monitored. Clinicians have a duty to protect patients under their care from risks associated with predators who target vulnerable and lonely people, whether financially, emotionally, or physically. Some patients in nursing home settings may benefit from discussing with their family members or attorney the possibility of completing a “sexual power of attorney”7 that could be completed in conjunction with an advance health care directive that addresses or authorizes an agent to make decisions about their sexual activities if cognitively impaired in the future.
One might also consider to what extent local regulatory oversight will protect your patient. Not all jurisdictions regulate online dating services similarly; many existing regulations focus on unfair contracts and pay less heed to safety concerns.
As a result, some dissatisfied clients have been known to sue an online dating service for breach of contract or misrepresentation. One of the most significant issues, however, is making sure there are appropriate background checks. Online dating services may need to change their policies to screen and verify for criminal background checks.8 Older adults interested in online dating should be made aware of these emerging issues.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/11-008-x/2009002/article/10910-eng.htm#tphp. Updated April 23, 2014. Accessed April 26, 2015.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
3. Alterovitz SS, Mendelsohn GA. Partner p across the life span: online dating by older adults. Psychol Aging. 2009;24(2):513-517.
4. Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS ONE. 2008;3(5):e2232. doi: 10.1371/journal.pone.0002232.
5. Couch D, Liamputtong P, Pitts M. Online daters and the use of technology for surveillance and risk management. International Journal of Emerging Technologies and Society. 2011;9(2):116-134.
6. Bateson DJ, Weisberg E, McCaffery KJ, et al. When online becomes offline: attitudes to safer sex practices in older and younger women using an Australian internet dating service. Sex Health. 2012;9(2):152-159.
7. Hill E. We’ll always have Shady Pines: surrogate decision-making tools for preserving sexual autonomy in elderly nursing home residents. William Mary J Women Law. 2014;20(2):468-490.
8. Doe v Match.com, 789 F Supp 2d 1197, 1199 (CD Cal 2011).
Baby Boomers represent a rapidly growing segment of digital device users.1 As these people age, their continued, even increasing, use of the Internet can be expected.1 At the same time, many older adults (age ≥65) are engaged in intimate relationships and regard sexuality as an important part of life.2
At this intersection, the Internet is likely to play a role in geriatric sexuality and “digital intimacy”—in that older adults can adopt patterns of using online dating sites similar to what their younger counterparts engage in. There is a need among clinicians to avoid stereotypical perceptions of “ageism” and the myth of “geriatric asexuality” as a result of older patients’ continued sexual interest and their adoption of social media technologies to facilitate the development of new intimate relationships. Acknowledgement of these realities by clinicians may assist in understanding and communication regarding these important areas of patients’ lives.
Why online dating?
Contemporary social and demographic changes (eg, higher divorce rates, increased longevity, aging of Baby Boomers) have influenced patterns of dating behaviors.3 Consistent with evolutionary theory, studies on courtship behaviors show that women remain the “choosers” of partners in relationships at all ages3; in contemporary society, however, there is an increasing ratio of women to men in later life, and the degree to which this demographic change might influence older men and women who are pursuing sexual relationships is unclear.3 Older adults might be aware of these demographic realities, and may use the Internet to increase their chances of finding a relationship.
For older homosexual men and women, demographic trends also are important because fewer available partners of similar sexual orientation might be available in their immediate communities, similarly incentivizing the use of online dating sites.
Hand in hand: Risk and vulnerability
Clinicians can discuss with geriatric patients who present with questions or concerns about sexuality and risks of online dating. Although risks associated with digital dating can involve anyone, those who are recently divorced, widowed, disabled, or elderly can be targeted by predators or fraudulent schemes, and thus become victims. Recognizing those risks and the vulnerability in the geriatric patient is crucial.
Chronic illness. Age-related physiological changes do not necessarily make one vulnerable; however, chronic diseases of aging, including major neurocognitive disorders, can impair daily function and increase disability and vulnerability. The majority of online dating sites do not discriminate among users, including those with disabilities such as incapacitating neuropsychiatric disorders. The clinician may need to assess cognitive status of patients specific to their capacity to fully understand the risks of use of social media. Inability to accomplish basic mastery of computer skills or inability to maintain appropriate boundaries and safeguards in relationships initiated and maintained using the Internet may assist in this determination. Patients with other problematic Internet use (eg, excessive devotion to online shopping or online gambling) may be prone to misusing social media and dating sites as well. Patients with clear impairment of memory or poor social judgment based on a neurocognitive disorder also might not maintain proper boundaries with social media use.
Feeling alone. Older persons might feel socially isolated, and therefore may be more willing to participate in online dating to increase their chances of establishing an intimate relationship or companionship. Research has shown that increased social ties, participation in groups, contact with friends and family, and perceived social support are associated with longer survival; on the other hand, social disengagement, low participation in leisure activities, and limited social networks are associated with higher risk of major neurocognitive disorders and increased disability.4
Little is known about social vulnerability in institutional settings, but institutional living could decrease social vulnerability in important ways (eg, access to social support, networks and activities, not living alone).4 Although the literature on older adults and “digital” or “virtual” dating is limited, there are essentially no such data from within institutional settings. It is important to separately address the issue of cognitively impaired patients’ capacity to consent to sexual activity both within institutional settings and elsewhere, as it raises numerous ethical dilemmas for clinicians.
Being sexually active. Early research into online dating focused particularly on the risks of sexually transmitted infections (STIs),5 which could be acquired through failure to use condoms with a new partner.6 Older women particularly are less likely to use condoms with new sexual partners.6 Screening at-risk adults should occur regardless of age. Effective interventions are needed to increase condom use in this age group. Research in the general population has started to investigate how the use of technology can minimize the risks associated with online dating.5 The Table5,6 lists strategies that can be used to minimize some of the risks of online dating among geriatric patients, including STIs and victimization.
Clinicians working with sexually active geriatric patients need to perform sexual risk assessments, complete capacity assessments, and provide preventive measures.
Legal issues
Criminal and civil liability issues have arisen with online dating involving cases of murder, rape, fraud, identity theft, loans, theft, domestic violence, stalking, and burglary. Online dating also raises concerns around the right to fair use of the Internet in different contexts. Flirting in cyberspace can occur with e-mail, text, Twitter, Skype, and Instant Messenger. Practices likely will vary depending on whether older adults are institutionalized or living in the community, as well as their mental status (eg, having a major neurocognitive disorder).
Some questions with legal implications worth considering include:
- To what extent is there a duty to accommodate healthy sexual relationships in institutionalized settings?
- At what point does monitoring and supervision become overly intrusive?
- Are older adults fully aware of the potential ramifications of sharing sensitive information in cyberspace?
- What is the threshold for capacity to consent among older adults to understand the sexual nature of the act and consent to the act?
Nursing homes and health care providers may become concerned about potential liability if their organization provides digital devices or electronic platforms that are not closely monitored. Clinicians have a duty to protect patients under their care from risks associated with predators who target vulnerable and lonely people, whether financially, emotionally, or physically. Some patients in nursing home settings may benefit from discussing with their family members or attorney the possibility of completing a “sexual power of attorney”7 that could be completed in conjunction with an advance health care directive that addresses or authorizes an agent to make decisions about their sexual activities if cognitively impaired in the future.
One might also consider to what extent local regulatory oversight will protect your patient. Not all jurisdictions regulate online dating services similarly; many existing regulations focus on unfair contracts and pay less heed to safety concerns.
As a result, some dissatisfied clients have been known to sue an online dating service for breach of contract or misrepresentation. One of the most significant issues, however, is making sure there are appropriate background checks. Online dating services may need to change their policies to screen and verify for criminal background checks.8 Older adults interested in online dating should be made aware of these emerging issues.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Baby Boomers represent a rapidly growing segment of digital device users.1 As these people age, their continued, even increasing, use of the Internet can be expected.1 At the same time, many older adults (age ≥65) are engaged in intimate relationships and regard sexuality as an important part of life.2
At this intersection, the Internet is likely to play a role in geriatric sexuality and “digital intimacy”—in that older adults can adopt patterns of using online dating sites similar to what their younger counterparts engage in. There is a need among clinicians to avoid stereotypical perceptions of “ageism” and the myth of “geriatric asexuality” as a result of older patients’ continued sexual interest and their adoption of social media technologies to facilitate the development of new intimate relationships. Acknowledgement of these realities by clinicians may assist in understanding and communication regarding these important areas of patients’ lives.
Why online dating?
Contemporary social and demographic changes (eg, higher divorce rates, increased longevity, aging of Baby Boomers) have influenced patterns of dating behaviors.3 Consistent with evolutionary theory, studies on courtship behaviors show that women remain the “choosers” of partners in relationships at all ages3; in contemporary society, however, there is an increasing ratio of women to men in later life, and the degree to which this demographic change might influence older men and women who are pursuing sexual relationships is unclear.3 Older adults might be aware of these demographic realities, and may use the Internet to increase their chances of finding a relationship.
For older homosexual men and women, demographic trends also are important because fewer available partners of similar sexual orientation might be available in their immediate communities, similarly incentivizing the use of online dating sites.
Hand in hand: Risk and vulnerability
Clinicians can discuss with geriatric patients who present with questions or concerns about sexuality and risks of online dating. Although risks associated with digital dating can involve anyone, those who are recently divorced, widowed, disabled, or elderly can be targeted by predators or fraudulent schemes, and thus become victims. Recognizing those risks and the vulnerability in the geriatric patient is crucial.
Chronic illness. Age-related physiological changes do not necessarily make one vulnerable; however, chronic diseases of aging, including major neurocognitive disorders, can impair daily function and increase disability and vulnerability. The majority of online dating sites do not discriminate among users, including those with disabilities such as incapacitating neuropsychiatric disorders. The clinician may need to assess cognitive status of patients specific to their capacity to fully understand the risks of use of social media. Inability to accomplish basic mastery of computer skills or inability to maintain appropriate boundaries and safeguards in relationships initiated and maintained using the Internet may assist in this determination. Patients with other problematic Internet use (eg, excessive devotion to online shopping or online gambling) may be prone to misusing social media and dating sites as well. Patients with clear impairment of memory or poor social judgment based on a neurocognitive disorder also might not maintain proper boundaries with social media use.
Feeling alone. Older persons might feel socially isolated, and therefore may be more willing to participate in online dating to increase their chances of establishing an intimate relationship or companionship. Research has shown that increased social ties, participation in groups, contact with friends and family, and perceived social support are associated with longer survival; on the other hand, social disengagement, low participation in leisure activities, and limited social networks are associated with higher risk of major neurocognitive disorders and increased disability.4
Little is known about social vulnerability in institutional settings, but institutional living could decrease social vulnerability in important ways (eg, access to social support, networks and activities, not living alone).4 Although the literature on older adults and “digital” or “virtual” dating is limited, there are essentially no such data from within institutional settings. It is important to separately address the issue of cognitively impaired patients’ capacity to consent to sexual activity both within institutional settings and elsewhere, as it raises numerous ethical dilemmas for clinicians.
Being sexually active. Early research into online dating focused particularly on the risks of sexually transmitted infections (STIs),5 which could be acquired through failure to use condoms with a new partner.6 Older women particularly are less likely to use condoms with new sexual partners.6 Screening at-risk adults should occur regardless of age. Effective interventions are needed to increase condom use in this age group. Research in the general population has started to investigate how the use of technology can minimize the risks associated with online dating.5 The Table5,6 lists strategies that can be used to minimize some of the risks of online dating among geriatric patients, including STIs and victimization.
Clinicians working with sexually active geriatric patients need to perform sexual risk assessments, complete capacity assessments, and provide preventive measures.
Legal issues
Criminal and civil liability issues have arisen with online dating involving cases of murder, rape, fraud, identity theft, loans, theft, domestic violence, stalking, and burglary. Online dating also raises concerns around the right to fair use of the Internet in different contexts. Flirting in cyberspace can occur with e-mail, text, Twitter, Skype, and Instant Messenger. Practices likely will vary depending on whether older adults are institutionalized or living in the community, as well as their mental status (eg, having a major neurocognitive disorder).
Some questions with legal implications worth considering include:
- To what extent is there a duty to accommodate healthy sexual relationships in institutionalized settings?
- At what point does monitoring and supervision become overly intrusive?
- Are older adults fully aware of the potential ramifications of sharing sensitive information in cyberspace?
- What is the threshold for capacity to consent among older adults to understand the sexual nature of the act and consent to the act?
Nursing homes and health care providers may become concerned about potential liability if their organization provides digital devices or electronic platforms that are not closely monitored. Clinicians have a duty to protect patients under their care from risks associated with predators who target vulnerable and lonely people, whether financially, emotionally, or physically. Some patients in nursing home settings may benefit from discussing with their family members or attorney the possibility of completing a “sexual power of attorney”7 that could be completed in conjunction with an advance health care directive that addresses or authorizes an agent to make decisions about their sexual activities if cognitively impaired in the future.
One might also consider to what extent local regulatory oversight will protect your patient. Not all jurisdictions regulate online dating services similarly; many existing regulations focus on unfair contracts and pay less heed to safety concerns.
As a result, some dissatisfied clients have been known to sue an online dating service for breach of contract or misrepresentation. One of the most significant issues, however, is making sure there are appropriate background checks. Online dating services may need to change their policies to screen and verify for criminal background checks.8 Older adults interested in online dating should be made aware of these emerging issues.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/11-008-x/2009002/article/10910-eng.htm#tphp. Updated April 23, 2014. Accessed April 26, 2015.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
3. Alterovitz SS, Mendelsohn GA. Partner p across the life span: online dating by older adults. Psychol Aging. 2009;24(2):513-517.
4. Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS ONE. 2008;3(5):e2232. doi: 10.1371/journal.pone.0002232.
5. Couch D, Liamputtong P, Pitts M. Online daters and the use of technology for surveillance and risk management. International Journal of Emerging Technologies and Society. 2011;9(2):116-134.
6. Bateson DJ, Weisberg E, McCaffery KJ, et al. When online becomes offline: attitudes to safer sex practices in older and younger women using an Australian internet dating service. Sex Health. 2012;9(2):152-159.
7. Hill E. We’ll always have Shady Pines: surrogate decision-making tools for preserving sexual autonomy in elderly nursing home residents. William Mary J Women Law. 2014;20(2):468-490.
8. Doe v Match.com, 789 F Supp 2d 1197, 1199 (CD Cal 2011).
1. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/11-008-x/2009002/article/10910-eng.htm#tphp. Updated April 23, 2014. Accessed April 26, 2015.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
3. Alterovitz SS, Mendelsohn GA. Partner p across the life span: online dating by older adults. Psychol Aging. 2009;24(2):513-517.
4. Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS ONE. 2008;3(5):e2232. doi: 10.1371/journal.pone.0002232.
5. Couch D, Liamputtong P, Pitts M. Online daters and the use of technology for surveillance and risk management. International Journal of Emerging Technologies and Society. 2011;9(2):116-134.
6. Bateson DJ, Weisberg E, McCaffery KJ, et al. When online becomes offline: attitudes to safer sex practices in older and younger women using an Australian internet dating service. Sex Health. 2012;9(2):152-159.
7. Hill E. We’ll always have Shady Pines: surrogate decision-making tools for preserving sexual autonomy in elderly nursing home residents. William Mary J Women Law. 2014;20(2):468-490.
8. Doe v Match.com, 789 F Supp 2d 1197, 1199 (CD Cal 2011).
When it’s time for ‘the talk’: Sexuality and your geriatric patient
Recent studies suggest that most older adults maintain sexual interest well into late life; many, however, experience sexual dysfunction. This article provides psychiatric practitioners with current information regarding sexuality and aging, as well as psychiatric and systemic medical comorbidities and sexual side effects of medications. Practice guidelines for assessing and managing sexual dysfunction have been developed for use in many medical specialties, and such guidance would be welcome in psychiatric practice.
This article addresses the myth of “geriatric asexuality” and its potential impact on clinical practice, the effects of age-related physiological changes on sexual activity, the importance of sexuality in the lives of older adults, and sensitive questions clinicians can pose about geriatric sexuality. We also will discuss:
• the importance of including a sexual assessment in the comprehensive psychiatric evaluation
• recognizing sexual dysfunction
• providing appropriate management within a multi-disciplinary, collaborative approach.
Sexuality after 65
Regardless of age, sexual activity can provide a sense of comfort and elicit a positive emotional and physical response.1 Hillman2 defined human sexuality as any combination of sexual behavior, emotional intimacy, and sense of sexual identity.
Sexuality in the aging population generally is an understudied area, obscured by the myth of “geriatric asexuality” and subject to numerous psychosocial variables.1 Previous research, focused on a biological perspective of sexuality, has largely overlooked psychological and social influences.3 It has been assumed that, with age, physical and hormonal changes or chronic illness ordinarily reduce or eliminate sexual desire and sexual behavior.3 However, the majority of older adults (defined as age ≥65) report a moderate-to-high level of sexual interest well into late life.1,3
Sexual function remains a subject often neglected in psychiatry. Sexual dysfunctions, as described in the DSM-5,4 do not include age-related changes in sexual function. In addition to physiological changes, sexual difficulties can result from relationship strain, systemic medical or psychiatric disorders, and sexual side effects of medications.
CASE REPORT
Mr. C, age 71 and married, is being treated for a major depressive episode that followed a course of shingles and persistent postherpetic neuralgia. Medications are: escitalopram, 20 mg/d; pregabalin, 150 mg/d; and ramipril, 5 mg/d. Mr. C is physically active and involved in social activities; he has no substance use history. He attends clinic visits with his wife.
Mr. C reports that despite significant improvement of his depressive and pain symptoms, he now experiences sexual difficulties, which he seems hesitant to discuss in detail. According to his wife, Mr. C appears to lack sexual desire and has difficulty initiating and maintaining an erection. She asks Mr. C’s psychiatrist whether she should stop her estrogen treatment, intended to enhance her sexual function, given that the couple is no longer engaging in sexual intercourse.
Mr. C admits to missing physical intimacy; however, he states, “If I have to make a choice between having sex with my wife and getting this depression out of my head, I’m going to pick getting rid of the depression.” Mrs. C says she is becoming dissatisfied with their marriage and the limited time she and her husband now spend together. Mr. C’s psychiatrist suggests that Mr. C and his wife undergo couples counseling.
Physiological changes with aging
In both women and men, the reproductive system undergoes age-related physiological changes.
Women. In women, the phase of decline in ovarian function and resulting decline in sex steroid production (estradiol and progesterone) is referred to as the climacteric, with menopause being determined retrospectively by the cessation of a menstrual period for 1 year.5
Menopausal symptoms typically occur between age 40 and 58; the average age of menopause is 51.6,7 Both estradiol and progesterone levels decline with menopause, and anovulation and ovarian failure ensue. A more gradual decline of female testosterone levels also occurs with aging, starting in the fourth decade of life.8
Clinical manifestations of menopause include vasomotor symptoms (ie, “hot flushes”), sleep disturbances, anxiety and depressive symptoms, decreased bone mineral density, and increased risk of cardiovascular disease.6,7 Loss of estrogen as well as continued loss of testosterone can result in dyspareunia because of atrophy and decreased vulvar and vaginal lubrication, with sexual excitement achieved less quickly, and a decreased intensity of orgasm.7
Men. Research has shown that testosterone levels are highest in men in the second and third decades, with a subsequent gradual decline.9 Older men with a low testosterone level are described as experiencing “late-onset hypogonadism,” also known by the popularized term “andropause.”10 This is attributed to decreased activity at the testicular and hypothalamic levels.10
Nonetheless, only a small fraction of older men with confirmed androgen deficiency are clinically symptomatic.11,12 Low testosterone is associated with decreased libido; it can hinder morning erections, contribute to erectile dysfunction, and result in erections that require physical stimulation.13
Notably, erectile dysfunction involves several other etiologic factors: psychiatric (eg, relationship difficulties, depression), neurogenic (eg, spinal cord injury), endocrine (eg, hyperprolactinemia), arteriogenic (eg, hypertension, type 2 diabetes mellitus), and drug-induced (eg, antidepressants, antihypertensives).14 A low testosterone level also has been associated with potential cognitive changes, decreased bone mineral density, metabolic syndrome (eg, increased risk of type 2 diabetes mellitus), and cardiovascular mortality.10
Effects on sexual activity. How much age-related physiological changes impact sexual practices in the geriatric population is uncertain. A study following 3,302 women through menopause over 6 years found some decline in sexual activity; however, this decline was not associated with increased sexual pain, decreased desire, or lack of arousal.15 Men continue to find ways to remain sexually active despite physiological changes (eg, erectile difficulties), but the etiology of sexual dysfunction in later life remains multi-modal, involving physical, psychological, and relational factors.16,17
Sexual practices in older adults
Researchers for the National Social Life, Health, and Aging Project (NSHAP) have examined sexual activities, behaviors, and problems in >3,000 older community-dwelling men and women across the United States, using information collected from in-home interviews.18 This study found that sexual activity, defined as any mutually voluntary sexual contact with another person, declines with age; however, even in the oldest age group (age 75 to 85), 39% of men and 17% of women reported being sexually active in the last 12 months. Among these persons, 54% reported sexual activity at least 2 times per month; 23% reported having sex at least once a week; and 32% reported engaging in oral sex. Partner availability predicted sexual activity.
Respondents with self-reported poor physical health were more likely to experience sexual problems (eg, difficulty with erection or lubrication, dyspareunia, and lack of pleasure). The most commonly reported reason for sexual inactivity in those with a spouse or other intimate partner was the male partner’s poor physical health.18
A longitudinal study, part of the Women’s Healthy Ageing Project, examined changes in sexual function at late menopause compared with early menopause. Although the researchers also found an age-related decrease in sexual activity, 50% of their late-menopause respondents (mean age, 70; range, 64 to 77) still reported sexual activity in the previous month, with 35% of this subgroup reporting sexual activity at least once a week; 83% reported sexual thoughts or fantasies.19 Availability of a partner, absence of a history of depression, moderate (compared with no) alcohol consumption, and better cognitive function were significantly associated with a higher level of sexual activity.19
In the Successful Aging Evaluation study, conducted in San Diego County, California, community-dwelling older partnered adults age 50 to 99 (mean age, 75) were surveyed about their sexual health after a cognitive screen by telephone20; rating scales for depression, anxiety, and physical function also were included. Results included 41% of men and 35% of women reporting sexual activity at least once a week, and only 21% of men and 24% of women reporting no sexual activity in the previous year. Depressive symptoms were most highly correlated with lack of sexual activity.20
Overall, these studies reveal that positive physical and mental health, access to a healthy partner, and a positive attitude toward sex are correlated with sexual activity in later life, whereas barriers to sexual activity include lack of a healthy sexual partner, depression, and chronic systemic medical illnesses, such as coronary artery disease or type 2 diabetes mellitus.13,17,21-24 Sexual activity and satisfaction have been positively associated with perceived general well-being and self-esteem.25,26 Conversely, sexual difficulties secondary to disease can be a source of distress for couples.27
Possibly overlooked? It is important to note that sexuality itself is a subjective area. Psychological intimacy is a universal phenomenon, and its physical expression may evolve as couples accommodate to age-related bodily changes. Means of achieving physical closeness, other than intercourse (eg, intimate touching, hand holding, kissing, or even acts of caretaking), may not be adequately captured in studies that look specifically at sexual activity.
Taking a sexual history in a geriatric patient
Because sexuality can be an uncomfortable topic for geriatric patients to discuss, sexual problems in this population often go unrecognized. It has been suggested that psychiatrists are more likely to inquire about sexual activity in middle-aged patients than geriatric patients with the same psychiatric presentation—perhaps illustrating a bias against taking a sexual history from a geriatric patient.28 However, because many older patients can experience depression or anxiety disorders in relation to normal sexual changes or sexual dysfunction within the context of their intimate relationships, it is essential to bring these issues to light.
Although a sexual history may not be the focus of a first clinical encounter, consider making such an assessment at a relatively early stage of patient care. The importance of such dialogue is 2-fold:
• It demonstrates to the patient that talking about sexuality in a respectful and empathic manner is appropriate and can encourage patients to communicate more effectively about sexuality with clinicians and with sexual partners.
• It helps elicit medical information needed to make an accurate diagnosis and provide adequate management.
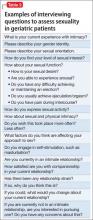
How to begin. As a starting point to taking a sexual history, an open-ended invitation for the geriatric patient to share information may be best, such as “What would you like to tell me about your sexual life?” See further suggestions (Table 1) and examples of more detailed questions to ask once a dialogue has been initiated (Table 2). Additional factors that may contribute to sexual dysfunction are presented in Table 3.1,27,29,30
CASE CONTINUED
In Mr. C’s case, an assessment of his sexual history, including risk factors for sexual dysfunction, is completed. Results from laboratory investigations, including a total testosterone level, are within normal limits.
Mr. C asks about using medications with fewer sexual side effects (he has been taking 3 medications that can contribute to sexual dysfunction). A gradual cross-taper of escitalopram, 20 mg/d, to mirtazapine, 45 mg/d, is implemented, along with tapering pregabalin to 50 mg/d.
Mr. C’s psychiatric and pain symptom improvement is maintained. He notices a boost in his sexual desire but has minimal improvement in erectile dysfunction. He is encouraged to speak with his primary care physician about an antihypertensive agent with less impact on sexual function, as well as therapeutic agents for erectile dysfunction; these, he declines.
At a subsequent visit, Mr. C reports feeling less apprehension about sexual performance. He is now willing to consider further medication options with his primary care physician, and agrees to a recommendation for couples psychotherapy.
As illustrated in Mr. C’s case, the recommended sexual assessment and management strategies to consider at a minimum in psychiatric practice are listed in Table 4.
STI risk in geriatric patients
The risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), often is overlooked in sexually active older adults. Although STIs are more common among younger adults, there is recent evidence of increased incidence in the geriatric population31 (with the highest risk of incident HIV and some STIs in older men who have sex with men32). These increased rates can be explained, at least in part, by:
• older men being less likely to use a condom during sexual activity
• promotion of viral entry in older women through a drier, thinner vaginal wall
• increased longevity of HIV-positive persons.31
Routine STI screening is not warranted in all older adults, but education and prevention strategies in sexually active seniors who are at greater risk of STIs are recommended. Particularly, clinicians should seek opportunities to discuss risk factors and safe sex practices (eg, using condoms, limiting number of sexual partners, practicing good hygiene, engaging in preventive care), and provide behavioral counseling where appropriate.31,33
Additional considerations in geriatric sexuality
Because psychiatric and systemic medical conditions can hinder sexual function, it is essential to identify and manage these conditions. Several neuropsychiatric disorders, including mood and neurocognitive disorders, can not only cause sexual dysfunction, but also can raise ethical dilemmas for clinicians, such as reduced decisional capacity in cognitively impaired patients to consent to sexual activity.1,34
In some patients, psychological, environmental, and pharmacological treatment options may help. A phosphodiesterase type 5 inhibitor for erectile dysfunction can be prescribed by the primary care physician, a psychiatrist, or another specialist, depending on the physician’s expertise and comfort level.
Sequencing of sexual dysfunction. Notably, there is a common paradox in mood disorders. Decreased sexual interest or performance may represent an aspect of anhedonia associated with depression, whereas sexual dysfunction could also result from medication use (particularly that of serotonergic antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine inhibitors), even as other depressive symptoms improve. Therefore, it is critical to analyze sequencing of sexual dysfunction—as part of the presenting mood symptoms or dysfunction induced by antidepressant treatment.
Geriatric sexuality in the digital age. Because older adults represent a rapidly growing segment of digital device users,35 Internet use is likely to play a role in the future of sexuality and “digital intimacy,” in that older adults can engage in online sexual activities. The Internet also can be a tool to access medical education.
Related Resources
• Burghardt KJ, Gardner KN. Sildenafil for SSRI-induced sexual dysfunction. Current Psychiatry. 2013;12(4):29-32,A.
• Maciel M, Laganà L. Older women’s sexual desire problems: biopsychosocial factors impacting them and barriers to their clinical assessment [published online January 5, 2014]. Biomed Res Int. 2014;2014:107217. doi: 10.1155/2014/107217.
Drug Brand Names
Bupropion • Wellbutrin, Zyban Mirtazapine • Remeron
Carbamazepine • Tegretol Oxcarbazepine • Trileptal
Clonidine • Catapres Phenobarbital • Luminal
Donepezil • Aricept Phenytoin • Dilantin
Escitalopram • Lexapro Pregabalin • Lyrica
Gabapentin • Neurontin Ramipril • Altace
Lamotrigine • Lamictal Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Trazodone • Desyrel
Memantine • Namenda Valproic acid • Depakote
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jagus CE, Benbow SM. Sexuality in older men with mental health problems. Sex Relation Ther. 2002;17(3):271-279.
2. Hillman JL. Clinical perspectives on elderly sexuality. New York, NY: Springer; 2000.
3. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42(2):138-149.
4. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Laufer LR, Gambone JC. Climacteric: menopause and peri-and postmenopause. In: Hacker NF, Gambone JC, Hobel CJ. Hacker and Moore’s essentials of obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2010:379-385.
6. Wilson MM. Menopause. Clin Geriatr Med. 2003;19(3): 483-506.
7. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830-838.
8. Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140-1152.
9. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
10. Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42(2):255-270.
11. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135.
12. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
13. Lochlainn MN, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565-572.
14. McMahon CG. Erectile dysfunction. Intern Med J. 2014;44(1):18-26.
15. Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442-452.
16. Perelman M, Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a cross-national survey. J Sex Med. 2005;2(3):397-406.
17. Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581-601.
18. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
19. Lonnèe-Hoffmann RA, Dennerstein L, Lehert P, et al. Sexual function in the late postmenopause: a decade of follow-up in a population-based cohort of Australian women. J Sex Med. 2014;11(8):2029-2038.
20. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. Am J Geriatr Psychiatry. 2015;23(3):227-233.
21. Garrett D. Psychosocial barriers to sexual intimacy for older people. Br J Nurs. 2014;23(6):327-331.
22. DeLamater J, Karraker A. Sexual functioning in older adults. Curr Psychiatry Rep. 2009;11(1):6-11.
23. DeLamater J. Sexual expression in later life: a review and synthesis. J Sex Res. 2012;49(2-3):125-141.
24. Inelmen EM, Sergi G, Girardi A, et al. The importance of sexual health in the elderly: breaking down barriers and taboos. Aging Clin Exp Res. 2012;24(suppl 3):31-34.
25. Choi KB, Jang SH, Lee MY, et al. Sexual life and self-esteem in married elderly. Arch Gerontol Geriatr. 2011;53(1):e17-e20.
26. Davison SL, Bell RJ, LaChina M, et al. The relationship between self-reported sexual satisfaction and general well-being in women. J Sex Med. 2009;6(10):2690-2697.
27. Morley JE, Tariq SH. Sexuality and disease. Clin Geriatr Med. 2003;19(3):563-573.
28. Bouman WP, Arcelus J. Are psychiatrists guilty of “ageism” when it comes to taking a sexual history? Int J Geriatr Psychiatry. 2001;16(1):27-31.
29. La Torre A, Giupponi G, Duffy DM, et al. Sexual dysfunction related to psychotropic drugs: a critical review. Part III: mood stabilizers and anxiolytic drugs. Pharmacopsychiatry. 2014;47(1):1-6.
30. Tucker I. Management of inappropriate sexual behaviors in dementia: a literature review. Int Psychogeriatr. 2010; 22(5):683-692.
31. Imparato T, Sanders D. STD prevalence demands clinical awareness. Aging Well. 2012;5(1):14.
32. Poynten IM, Grulich AE, Templeton DJ. Sexually transmitted infections in older populations. Curr Opin Infect Dis. 2013;26(1):80-85.
33. Talashek ML, Tichy AM, Epping H. Sexually transmitted diseases in the elderly—issues and recommendations. J Gerontol Nurs. 1990;16(4):33-40.
34. Benbow SM, Jagus CE. Sexuality in older women with mental health problems. Sex Relation Ther. 2002;17(3):261-270.
35. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/ 11-008-x/2009002/article/10910-eng.htm#tphp. Accessed March 26, 2015.
Recent studies suggest that most older adults maintain sexual interest well into late life; many, however, experience sexual dysfunction. This article provides psychiatric practitioners with current information regarding sexuality and aging, as well as psychiatric and systemic medical comorbidities and sexual side effects of medications. Practice guidelines for assessing and managing sexual dysfunction have been developed for use in many medical specialties, and such guidance would be welcome in psychiatric practice.
This article addresses the myth of “geriatric asexuality” and its potential impact on clinical practice, the effects of age-related physiological changes on sexual activity, the importance of sexuality in the lives of older adults, and sensitive questions clinicians can pose about geriatric sexuality. We also will discuss:
• the importance of including a sexual assessment in the comprehensive psychiatric evaluation
• recognizing sexual dysfunction
• providing appropriate management within a multi-disciplinary, collaborative approach.
Sexuality after 65
Regardless of age, sexual activity can provide a sense of comfort and elicit a positive emotional and physical response.1 Hillman2 defined human sexuality as any combination of sexual behavior, emotional intimacy, and sense of sexual identity.
Sexuality in the aging population generally is an understudied area, obscured by the myth of “geriatric asexuality” and subject to numerous psychosocial variables.1 Previous research, focused on a biological perspective of sexuality, has largely overlooked psychological and social influences.3 It has been assumed that, with age, physical and hormonal changes or chronic illness ordinarily reduce or eliminate sexual desire and sexual behavior.3 However, the majority of older adults (defined as age ≥65) report a moderate-to-high level of sexual interest well into late life.1,3
Sexual function remains a subject often neglected in psychiatry. Sexual dysfunctions, as described in the DSM-5,4 do not include age-related changes in sexual function. In addition to physiological changes, sexual difficulties can result from relationship strain, systemic medical or psychiatric disorders, and sexual side effects of medications.
CASE REPORT
Mr. C, age 71 and married, is being treated for a major depressive episode that followed a course of shingles and persistent postherpetic neuralgia. Medications are: escitalopram, 20 mg/d; pregabalin, 150 mg/d; and ramipril, 5 mg/d. Mr. C is physically active and involved in social activities; he has no substance use history. He attends clinic visits with his wife.
Mr. C reports that despite significant improvement of his depressive and pain symptoms, he now experiences sexual difficulties, which he seems hesitant to discuss in detail. According to his wife, Mr. C appears to lack sexual desire and has difficulty initiating and maintaining an erection. She asks Mr. C’s psychiatrist whether she should stop her estrogen treatment, intended to enhance her sexual function, given that the couple is no longer engaging in sexual intercourse.
Mr. C admits to missing physical intimacy; however, he states, “If I have to make a choice between having sex with my wife and getting this depression out of my head, I’m going to pick getting rid of the depression.” Mrs. C says she is becoming dissatisfied with their marriage and the limited time she and her husband now spend together. Mr. C’s psychiatrist suggests that Mr. C and his wife undergo couples counseling.
Physiological changes with aging
In both women and men, the reproductive system undergoes age-related physiological changes.
Women. In women, the phase of decline in ovarian function and resulting decline in sex steroid production (estradiol and progesterone) is referred to as the climacteric, with menopause being determined retrospectively by the cessation of a menstrual period for 1 year.5
Menopausal symptoms typically occur between age 40 and 58; the average age of menopause is 51.6,7 Both estradiol and progesterone levels decline with menopause, and anovulation and ovarian failure ensue. A more gradual decline of female testosterone levels also occurs with aging, starting in the fourth decade of life.8
Clinical manifestations of menopause include vasomotor symptoms (ie, “hot flushes”), sleep disturbances, anxiety and depressive symptoms, decreased bone mineral density, and increased risk of cardiovascular disease.6,7 Loss of estrogen as well as continued loss of testosterone can result in dyspareunia because of atrophy and decreased vulvar and vaginal lubrication, with sexual excitement achieved less quickly, and a decreased intensity of orgasm.7
Men. Research has shown that testosterone levels are highest in men in the second and third decades, with a subsequent gradual decline.9 Older men with a low testosterone level are described as experiencing “late-onset hypogonadism,” also known by the popularized term “andropause.”10 This is attributed to decreased activity at the testicular and hypothalamic levels.10
Nonetheless, only a small fraction of older men with confirmed androgen deficiency are clinically symptomatic.11,12 Low testosterone is associated with decreased libido; it can hinder morning erections, contribute to erectile dysfunction, and result in erections that require physical stimulation.13
Notably, erectile dysfunction involves several other etiologic factors: psychiatric (eg, relationship difficulties, depression), neurogenic (eg, spinal cord injury), endocrine (eg, hyperprolactinemia), arteriogenic (eg, hypertension, type 2 diabetes mellitus), and drug-induced (eg, antidepressants, antihypertensives).14 A low testosterone level also has been associated with potential cognitive changes, decreased bone mineral density, metabolic syndrome (eg, increased risk of type 2 diabetes mellitus), and cardiovascular mortality.10
Effects on sexual activity. How much age-related physiological changes impact sexual practices in the geriatric population is uncertain. A study following 3,302 women through menopause over 6 years found some decline in sexual activity; however, this decline was not associated with increased sexual pain, decreased desire, or lack of arousal.15 Men continue to find ways to remain sexually active despite physiological changes (eg, erectile difficulties), but the etiology of sexual dysfunction in later life remains multi-modal, involving physical, psychological, and relational factors.16,17
Sexual practices in older adults
Researchers for the National Social Life, Health, and Aging Project (NSHAP) have examined sexual activities, behaviors, and problems in >3,000 older community-dwelling men and women across the United States, using information collected from in-home interviews.18 This study found that sexual activity, defined as any mutually voluntary sexual contact with another person, declines with age; however, even in the oldest age group (age 75 to 85), 39% of men and 17% of women reported being sexually active in the last 12 months. Among these persons, 54% reported sexual activity at least 2 times per month; 23% reported having sex at least once a week; and 32% reported engaging in oral sex. Partner availability predicted sexual activity.
Respondents with self-reported poor physical health were more likely to experience sexual problems (eg, difficulty with erection or lubrication, dyspareunia, and lack of pleasure). The most commonly reported reason for sexual inactivity in those with a spouse or other intimate partner was the male partner’s poor physical health.18
A longitudinal study, part of the Women’s Healthy Ageing Project, examined changes in sexual function at late menopause compared with early menopause. Although the researchers also found an age-related decrease in sexual activity, 50% of their late-menopause respondents (mean age, 70; range, 64 to 77) still reported sexual activity in the previous month, with 35% of this subgroup reporting sexual activity at least once a week; 83% reported sexual thoughts or fantasies.19 Availability of a partner, absence of a history of depression, moderate (compared with no) alcohol consumption, and better cognitive function were significantly associated with a higher level of sexual activity.19
In the Successful Aging Evaluation study, conducted in San Diego County, California, community-dwelling older partnered adults age 50 to 99 (mean age, 75) were surveyed about their sexual health after a cognitive screen by telephone20; rating scales for depression, anxiety, and physical function also were included. Results included 41% of men and 35% of women reporting sexual activity at least once a week, and only 21% of men and 24% of women reporting no sexual activity in the previous year. Depressive symptoms were most highly correlated with lack of sexual activity.20
Overall, these studies reveal that positive physical and mental health, access to a healthy partner, and a positive attitude toward sex are correlated with sexual activity in later life, whereas barriers to sexual activity include lack of a healthy sexual partner, depression, and chronic systemic medical illnesses, such as coronary artery disease or type 2 diabetes mellitus.13,17,21-24 Sexual activity and satisfaction have been positively associated with perceived general well-being and self-esteem.25,26 Conversely, sexual difficulties secondary to disease can be a source of distress for couples.27
Possibly overlooked? It is important to note that sexuality itself is a subjective area. Psychological intimacy is a universal phenomenon, and its physical expression may evolve as couples accommodate to age-related bodily changes. Means of achieving physical closeness, other than intercourse (eg, intimate touching, hand holding, kissing, or even acts of caretaking), may not be adequately captured in studies that look specifically at sexual activity.
Taking a sexual history in a geriatric patient
Because sexuality can be an uncomfortable topic for geriatric patients to discuss, sexual problems in this population often go unrecognized. It has been suggested that psychiatrists are more likely to inquire about sexual activity in middle-aged patients than geriatric patients with the same psychiatric presentation—perhaps illustrating a bias against taking a sexual history from a geriatric patient.28 However, because many older patients can experience depression or anxiety disorders in relation to normal sexual changes or sexual dysfunction within the context of their intimate relationships, it is essential to bring these issues to light.
Although a sexual history may not be the focus of a first clinical encounter, consider making such an assessment at a relatively early stage of patient care. The importance of such dialogue is 2-fold:
• It demonstrates to the patient that talking about sexuality in a respectful and empathic manner is appropriate and can encourage patients to communicate more effectively about sexuality with clinicians and with sexual partners.
• It helps elicit medical information needed to make an accurate diagnosis and provide adequate management.
How to begin. As a starting point to taking a sexual history, an open-ended invitation for the geriatric patient to share information may be best, such as “What would you like to tell me about your sexual life?” See further suggestions (Table 1) and examples of more detailed questions to ask once a dialogue has been initiated (Table 2). Additional factors that may contribute to sexual dysfunction are presented in Table 3.1,27,29,30
CASE CONTINUED
In Mr. C’s case, an assessment of his sexual history, including risk factors for sexual dysfunction, is completed. Results from laboratory investigations, including a total testosterone level, are within normal limits.
Mr. C asks about using medications with fewer sexual side effects (he has been taking 3 medications that can contribute to sexual dysfunction). A gradual cross-taper of escitalopram, 20 mg/d, to mirtazapine, 45 mg/d, is implemented, along with tapering pregabalin to 50 mg/d.
Mr. C’s psychiatric and pain symptom improvement is maintained. He notices a boost in his sexual desire but has minimal improvement in erectile dysfunction. He is encouraged to speak with his primary care physician about an antihypertensive agent with less impact on sexual function, as well as therapeutic agents for erectile dysfunction; these, he declines.
At a subsequent visit, Mr. C reports feeling less apprehension about sexual performance. He is now willing to consider further medication options with his primary care physician, and agrees to a recommendation for couples psychotherapy.
As illustrated in Mr. C’s case, the recommended sexual assessment and management strategies to consider at a minimum in psychiatric practice are listed in Table 4.
STI risk in geriatric patients
The risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), often is overlooked in sexually active older adults. Although STIs are more common among younger adults, there is recent evidence of increased incidence in the geriatric population31 (with the highest risk of incident HIV and some STIs in older men who have sex with men32). These increased rates can be explained, at least in part, by:
• older men being less likely to use a condom during sexual activity
• promotion of viral entry in older women through a drier, thinner vaginal wall
• increased longevity of HIV-positive persons.31
Routine STI screening is not warranted in all older adults, but education and prevention strategies in sexually active seniors who are at greater risk of STIs are recommended. Particularly, clinicians should seek opportunities to discuss risk factors and safe sex practices (eg, using condoms, limiting number of sexual partners, practicing good hygiene, engaging in preventive care), and provide behavioral counseling where appropriate.31,33
Additional considerations in geriatric sexuality
Because psychiatric and systemic medical conditions can hinder sexual function, it is essential to identify and manage these conditions. Several neuropsychiatric disorders, including mood and neurocognitive disorders, can not only cause sexual dysfunction, but also can raise ethical dilemmas for clinicians, such as reduced decisional capacity in cognitively impaired patients to consent to sexual activity.1,34
In some patients, psychological, environmental, and pharmacological treatment options may help. A phosphodiesterase type 5 inhibitor for erectile dysfunction can be prescribed by the primary care physician, a psychiatrist, or another specialist, depending on the physician’s expertise and comfort level.
Sequencing of sexual dysfunction. Notably, there is a common paradox in mood disorders. Decreased sexual interest or performance may represent an aspect of anhedonia associated with depression, whereas sexual dysfunction could also result from medication use (particularly that of serotonergic antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine inhibitors), even as other depressive symptoms improve. Therefore, it is critical to analyze sequencing of sexual dysfunction—as part of the presenting mood symptoms or dysfunction induced by antidepressant treatment.
Geriatric sexuality in the digital age. Because older adults represent a rapidly growing segment of digital device users,35 Internet use is likely to play a role in the future of sexuality and “digital intimacy,” in that older adults can engage in online sexual activities. The Internet also can be a tool to access medical education.
Related Resources
• Burghardt KJ, Gardner KN. Sildenafil for SSRI-induced sexual dysfunction. Current Psychiatry. 2013;12(4):29-32,A.
• Maciel M, Laganà L. Older women’s sexual desire problems: biopsychosocial factors impacting them and barriers to their clinical assessment [published online January 5, 2014]. Biomed Res Int. 2014;2014:107217. doi: 10.1155/2014/107217.
Drug Brand Names
Bupropion • Wellbutrin, Zyban Mirtazapine • Remeron
Carbamazepine • Tegretol Oxcarbazepine • Trileptal
Clonidine • Catapres Phenobarbital • Luminal
Donepezil • Aricept Phenytoin • Dilantin
Escitalopram • Lexapro Pregabalin • Lyrica
Gabapentin • Neurontin Ramipril • Altace
Lamotrigine • Lamictal Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Trazodone • Desyrel
Memantine • Namenda Valproic acid • Depakote
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Recent studies suggest that most older adults maintain sexual interest well into late life; many, however, experience sexual dysfunction. This article provides psychiatric practitioners with current information regarding sexuality and aging, as well as psychiatric and systemic medical comorbidities and sexual side effects of medications. Practice guidelines for assessing and managing sexual dysfunction have been developed for use in many medical specialties, and such guidance would be welcome in psychiatric practice.
This article addresses the myth of “geriatric asexuality” and its potential impact on clinical practice, the effects of age-related physiological changes on sexual activity, the importance of sexuality in the lives of older adults, and sensitive questions clinicians can pose about geriatric sexuality. We also will discuss:
• the importance of including a sexual assessment in the comprehensive psychiatric evaluation
• recognizing sexual dysfunction
• providing appropriate management within a multi-disciplinary, collaborative approach.
Sexuality after 65
Regardless of age, sexual activity can provide a sense of comfort and elicit a positive emotional and physical response.1 Hillman2 defined human sexuality as any combination of sexual behavior, emotional intimacy, and sense of sexual identity.
Sexuality in the aging population generally is an understudied area, obscured by the myth of “geriatric asexuality” and subject to numerous psychosocial variables.1 Previous research, focused on a biological perspective of sexuality, has largely overlooked psychological and social influences.3 It has been assumed that, with age, physical and hormonal changes or chronic illness ordinarily reduce or eliminate sexual desire and sexual behavior.3 However, the majority of older adults (defined as age ≥65) report a moderate-to-high level of sexual interest well into late life.1,3
Sexual function remains a subject often neglected in psychiatry. Sexual dysfunctions, as described in the DSM-5,4 do not include age-related changes in sexual function. In addition to physiological changes, sexual difficulties can result from relationship strain, systemic medical or psychiatric disorders, and sexual side effects of medications.
CASE REPORT
Mr. C, age 71 and married, is being treated for a major depressive episode that followed a course of shingles and persistent postherpetic neuralgia. Medications are: escitalopram, 20 mg/d; pregabalin, 150 mg/d; and ramipril, 5 mg/d. Mr. C is physically active and involved in social activities; he has no substance use history. He attends clinic visits with his wife.
Mr. C reports that despite significant improvement of his depressive and pain symptoms, he now experiences sexual difficulties, which he seems hesitant to discuss in detail. According to his wife, Mr. C appears to lack sexual desire and has difficulty initiating and maintaining an erection. She asks Mr. C’s psychiatrist whether she should stop her estrogen treatment, intended to enhance her sexual function, given that the couple is no longer engaging in sexual intercourse.
Mr. C admits to missing physical intimacy; however, he states, “If I have to make a choice between having sex with my wife and getting this depression out of my head, I’m going to pick getting rid of the depression.” Mrs. C says she is becoming dissatisfied with their marriage and the limited time she and her husband now spend together. Mr. C’s psychiatrist suggests that Mr. C and his wife undergo couples counseling.
Physiological changes with aging
In both women and men, the reproductive system undergoes age-related physiological changes.
Women. In women, the phase of decline in ovarian function and resulting decline in sex steroid production (estradiol and progesterone) is referred to as the climacteric, with menopause being determined retrospectively by the cessation of a menstrual period for 1 year.5
Menopausal symptoms typically occur between age 40 and 58; the average age of menopause is 51.6,7 Both estradiol and progesterone levels decline with menopause, and anovulation and ovarian failure ensue. A more gradual decline of female testosterone levels also occurs with aging, starting in the fourth decade of life.8
Clinical manifestations of menopause include vasomotor symptoms (ie, “hot flushes”), sleep disturbances, anxiety and depressive symptoms, decreased bone mineral density, and increased risk of cardiovascular disease.6,7 Loss of estrogen as well as continued loss of testosterone can result in dyspareunia because of atrophy and decreased vulvar and vaginal lubrication, with sexual excitement achieved less quickly, and a decreased intensity of orgasm.7
Men. Research has shown that testosterone levels are highest in men in the second and third decades, with a subsequent gradual decline.9 Older men with a low testosterone level are described as experiencing “late-onset hypogonadism,” also known by the popularized term “andropause.”10 This is attributed to decreased activity at the testicular and hypothalamic levels.10
Nonetheless, only a small fraction of older men with confirmed androgen deficiency are clinically symptomatic.11,12 Low testosterone is associated with decreased libido; it can hinder morning erections, contribute to erectile dysfunction, and result in erections that require physical stimulation.13
Notably, erectile dysfunction involves several other etiologic factors: psychiatric (eg, relationship difficulties, depression), neurogenic (eg, spinal cord injury), endocrine (eg, hyperprolactinemia), arteriogenic (eg, hypertension, type 2 diabetes mellitus), and drug-induced (eg, antidepressants, antihypertensives).14 A low testosterone level also has been associated with potential cognitive changes, decreased bone mineral density, metabolic syndrome (eg, increased risk of type 2 diabetes mellitus), and cardiovascular mortality.10
Effects on sexual activity. How much age-related physiological changes impact sexual practices in the geriatric population is uncertain. A study following 3,302 women through menopause over 6 years found some decline in sexual activity; however, this decline was not associated with increased sexual pain, decreased desire, or lack of arousal.15 Men continue to find ways to remain sexually active despite physiological changes (eg, erectile difficulties), but the etiology of sexual dysfunction in later life remains multi-modal, involving physical, psychological, and relational factors.16,17
Sexual practices in older adults
Researchers for the National Social Life, Health, and Aging Project (NSHAP) have examined sexual activities, behaviors, and problems in >3,000 older community-dwelling men and women across the United States, using information collected from in-home interviews.18 This study found that sexual activity, defined as any mutually voluntary sexual contact with another person, declines with age; however, even in the oldest age group (age 75 to 85), 39% of men and 17% of women reported being sexually active in the last 12 months. Among these persons, 54% reported sexual activity at least 2 times per month; 23% reported having sex at least once a week; and 32% reported engaging in oral sex. Partner availability predicted sexual activity.
Respondents with self-reported poor physical health were more likely to experience sexual problems (eg, difficulty with erection or lubrication, dyspareunia, and lack of pleasure). The most commonly reported reason for sexual inactivity in those with a spouse or other intimate partner was the male partner’s poor physical health.18
A longitudinal study, part of the Women’s Healthy Ageing Project, examined changes in sexual function at late menopause compared with early menopause. Although the researchers also found an age-related decrease in sexual activity, 50% of their late-menopause respondents (mean age, 70; range, 64 to 77) still reported sexual activity in the previous month, with 35% of this subgroup reporting sexual activity at least once a week; 83% reported sexual thoughts or fantasies.19 Availability of a partner, absence of a history of depression, moderate (compared with no) alcohol consumption, and better cognitive function were significantly associated with a higher level of sexual activity.19
In the Successful Aging Evaluation study, conducted in San Diego County, California, community-dwelling older partnered adults age 50 to 99 (mean age, 75) were surveyed about their sexual health after a cognitive screen by telephone20; rating scales for depression, anxiety, and physical function also were included. Results included 41% of men and 35% of women reporting sexual activity at least once a week, and only 21% of men and 24% of women reporting no sexual activity in the previous year. Depressive symptoms were most highly correlated with lack of sexual activity.20
Overall, these studies reveal that positive physical and mental health, access to a healthy partner, and a positive attitude toward sex are correlated with sexual activity in later life, whereas barriers to sexual activity include lack of a healthy sexual partner, depression, and chronic systemic medical illnesses, such as coronary artery disease or type 2 diabetes mellitus.13,17,21-24 Sexual activity and satisfaction have been positively associated with perceived general well-being and self-esteem.25,26 Conversely, sexual difficulties secondary to disease can be a source of distress for couples.27
Possibly overlooked? It is important to note that sexuality itself is a subjective area. Psychological intimacy is a universal phenomenon, and its physical expression may evolve as couples accommodate to age-related bodily changes. Means of achieving physical closeness, other than intercourse (eg, intimate touching, hand holding, kissing, or even acts of caretaking), may not be adequately captured in studies that look specifically at sexual activity.
Taking a sexual history in a geriatric patient
Because sexuality can be an uncomfortable topic for geriatric patients to discuss, sexual problems in this population often go unrecognized. It has been suggested that psychiatrists are more likely to inquire about sexual activity in middle-aged patients than geriatric patients with the same psychiatric presentation—perhaps illustrating a bias against taking a sexual history from a geriatric patient.28 However, because many older patients can experience depression or anxiety disorders in relation to normal sexual changes or sexual dysfunction within the context of their intimate relationships, it is essential to bring these issues to light.
Although a sexual history may not be the focus of a first clinical encounter, consider making such an assessment at a relatively early stage of patient care. The importance of such dialogue is 2-fold:
• It demonstrates to the patient that talking about sexuality in a respectful and empathic manner is appropriate and can encourage patients to communicate more effectively about sexuality with clinicians and with sexual partners.
• It helps elicit medical information needed to make an accurate diagnosis and provide adequate management.
How to begin. As a starting point to taking a sexual history, an open-ended invitation for the geriatric patient to share information may be best, such as “What would you like to tell me about your sexual life?” See further suggestions (Table 1) and examples of more detailed questions to ask once a dialogue has been initiated (Table 2). Additional factors that may contribute to sexual dysfunction are presented in Table 3.1,27,29,30
CASE CONTINUED
In Mr. C’s case, an assessment of his sexual history, including risk factors for sexual dysfunction, is completed. Results from laboratory investigations, including a total testosterone level, are within normal limits.
Mr. C asks about using medications with fewer sexual side effects (he has been taking 3 medications that can contribute to sexual dysfunction). A gradual cross-taper of escitalopram, 20 mg/d, to mirtazapine, 45 mg/d, is implemented, along with tapering pregabalin to 50 mg/d.
Mr. C’s psychiatric and pain symptom improvement is maintained. He notices a boost in his sexual desire but has minimal improvement in erectile dysfunction. He is encouraged to speak with his primary care physician about an antihypertensive agent with less impact on sexual function, as well as therapeutic agents for erectile dysfunction; these, he declines.
At a subsequent visit, Mr. C reports feeling less apprehension about sexual performance. He is now willing to consider further medication options with his primary care physician, and agrees to a recommendation for couples psychotherapy.
As illustrated in Mr. C’s case, the recommended sexual assessment and management strategies to consider at a minimum in psychiatric practice are listed in Table 4.
STI risk in geriatric patients
The risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), often is overlooked in sexually active older adults. Although STIs are more common among younger adults, there is recent evidence of increased incidence in the geriatric population31 (with the highest risk of incident HIV and some STIs in older men who have sex with men32). These increased rates can be explained, at least in part, by:
• older men being less likely to use a condom during sexual activity
• promotion of viral entry in older women through a drier, thinner vaginal wall
• increased longevity of HIV-positive persons.31
Routine STI screening is not warranted in all older adults, but education and prevention strategies in sexually active seniors who are at greater risk of STIs are recommended. Particularly, clinicians should seek opportunities to discuss risk factors and safe sex practices (eg, using condoms, limiting number of sexual partners, practicing good hygiene, engaging in preventive care), and provide behavioral counseling where appropriate.31,33
Additional considerations in geriatric sexuality
Because psychiatric and systemic medical conditions can hinder sexual function, it is essential to identify and manage these conditions. Several neuropsychiatric disorders, including mood and neurocognitive disorders, can not only cause sexual dysfunction, but also can raise ethical dilemmas for clinicians, such as reduced decisional capacity in cognitively impaired patients to consent to sexual activity.1,34
In some patients, psychological, environmental, and pharmacological treatment options may help. A phosphodiesterase type 5 inhibitor for erectile dysfunction can be prescribed by the primary care physician, a psychiatrist, or another specialist, depending on the physician’s expertise and comfort level.
Sequencing of sexual dysfunction. Notably, there is a common paradox in mood disorders. Decreased sexual interest or performance may represent an aspect of anhedonia associated with depression, whereas sexual dysfunction could also result from medication use (particularly that of serotonergic antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine inhibitors), even as other depressive symptoms improve. Therefore, it is critical to analyze sequencing of sexual dysfunction—as part of the presenting mood symptoms or dysfunction induced by antidepressant treatment.
Geriatric sexuality in the digital age. Because older adults represent a rapidly growing segment of digital device users,35 Internet use is likely to play a role in the future of sexuality and “digital intimacy,” in that older adults can engage in online sexual activities. The Internet also can be a tool to access medical education.
Related Resources
• Burghardt KJ, Gardner KN. Sildenafil for SSRI-induced sexual dysfunction. Current Psychiatry. 2013;12(4):29-32,A.
• Maciel M, Laganà L. Older women’s sexual desire problems: biopsychosocial factors impacting them and barriers to their clinical assessment [published online January 5, 2014]. Biomed Res Int. 2014;2014:107217. doi: 10.1155/2014/107217.
Drug Brand Names
Bupropion • Wellbutrin, Zyban Mirtazapine • Remeron
Carbamazepine • Tegretol Oxcarbazepine • Trileptal
Clonidine • Catapres Phenobarbital • Luminal
Donepezil • Aricept Phenytoin • Dilantin
Escitalopram • Lexapro Pregabalin • Lyrica
Gabapentin • Neurontin Ramipril • Altace
Lamotrigine • Lamictal Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Trazodone • Desyrel
Memantine • Namenda Valproic acid • Depakote
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jagus CE, Benbow SM. Sexuality in older men with mental health problems. Sex Relation Ther. 2002;17(3):271-279.
2. Hillman JL. Clinical perspectives on elderly sexuality. New York, NY: Springer; 2000.
3. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42(2):138-149.
4. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Laufer LR, Gambone JC. Climacteric: menopause and peri-and postmenopause. In: Hacker NF, Gambone JC, Hobel CJ. Hacker and Moore’s essentials of obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2010:379-385.
6. Wilson MM. Menopause. Clin Geriatr Med. 2003;19(3): 483-506.
7. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830-838.
8. Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140-1152.
9. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
10. Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42(2):255-270.
11. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135.
12. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
13. Lochlainn MN, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565-572.
14. McMahon CG. Erectile dysfunction. Intern Med J. 2014;44(1):18-26.
15. Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442-452.
16. Perelman M, Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a cross-national survey. J Sex Med. 2005;2(3):397-406.
17. Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581-601.
18. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
19. Lonnèe-Hoffmann RA, Dennerstein L, Lehert P, et al. Sexual function in the late postmenopause: a decade of follow-up in a population-based cohort of Australian women. J Sex Med. 2014;11(8):2029-2038.
20. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. Am J Geriatr Psychiatry. 2015;23(3):227-233.
21. Garrett D. Psychosocial barriers to sexual intimacy for older people. Br J Nurs. 2014;23(6):327-331.
22. DeLamater J, Karraker A. Sexual functioning in older adults. Curr Psychiatry Rep. 2009;11(1):6-11.
23. DeLamater J. Sexual expression in later life: a review and synthesis. J Sex Res. 2012;49(2-3):125-141.
24. Inelmen EM, Sergi G, Girardi A, et al. The importance of sexual health in the elderly: breaking down barriers and taboos. Aging Clin Exp Res. 2012;24(suppl 3):31-34.
25. Choi KB, Jang SH, Lee MY, et al. Sexual life and self-esteem in married elderly. Arch Gerontol Geriatr. 2011;53(1):e17-e20.
26. Davison SL, Bell RJ, LaChina M, et al. The relationship between self-reported sexual satisfaction and general well-being in women. J Sex Med. 2009;6(10):2690-2697.
27. Morley JE, Tariq SH. Sexuality and disease. Clin Geriatr Med. 2003;19(3):563-573.
28. Bouman WP, Arcelus J. Are psychiatrists guilty of “ageism” when it comes to taking a sexual history? Int J Geriatr Psychiatry. 2001;16(1):27-31.
29. La Torre A, Giupponi G, Duffy DM, et al. Sexual dysfunction related to psychotropic drugs: a critical review. Part III: mood stabilizers and anxiolytic drugs. Pharmacopsychiatry. 2014;47(1):1-6.
30. Tucker I. Management of inappropriate sexual behaviors in dementia: a literature review. Int Psychogeriatr. 2010; 22(5):683-692.
31. Imparato T, Sanders D. STD prevalence demands clinical awareness. Aging Well. 2012;5(1):14.
32. Poynten IM, Grulich AE, Templeton DJ. Sexually transmitted infections in older populations. Curr Opin Infect Dis. 2013;26(1):80-85.
33. Talashek ML, Tichy AM, Epping H. Sexually transmitted diseases in the elderly—issues and recommendations. J Gerontol Nurs. 1990;16(4):33-40.
34. Benbow SM, Jagus CE. Sexuality in older women with mental health problems. Sex Relation Ther. 2002;17(3):261-270.
35. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/ 11-008-x/2009002/article/10910-eng.htm#tphp. Accessed March 26, 2015.
1. Jagus CE, Benbow SM. Sexuality in older men with mental health problems. Sex Relation Ther. 2002;17(3):271-279.
2. Hillman JL. Clinical perspectives on elderly sexuality. New York, NY: Springer; 2000.
3. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42(2):138-149.
4. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Laufer LR, Gambone JC. Climacteric: menopause and peri-and postmenopause. In: Hacker NF, Gambone JC, Hobel CJ. Hacker and Moore’s essentials of obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2010:379-385.
6. Wilson MM. Menopause. Clin Geriatr Med. 2003;19(3): 483-506.
7. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830-838.
8. Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140-1152.
9. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
10. Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42(2):255-270.
11. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135.
12. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
13. Lochlainn MN, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565-572.
14. McMahon CG. Erectile dysfunction. Intern Med J. 2014;44(1):18-26.
15. Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442-452.
16. Perelman M, Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a cross-national survey. J Sex Med. 2005;2(3):397-406.
17. Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581-601.
18. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
19. Lonnèe-Hoffmann RA, Dennerstein L, Lehert P, et al. Sexual function in the late postmenopause: a decade of follow-up in a population-based cohort of Australian women. J Sex Med. 2014;11(8):2029-2038.
20. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. Am J Geriatr Psychiatry. 2015;23(3):227-233.
21. Garrett D. Psychosocial barriers to sexual intimacy for older people. Br J Nurs. 2014;23(6):327-331.
22. DeLamater J, Karraker A. Sexual functioning in older adults. Curr Psychiatry Rep. 2009;11(1):6-11.
23. DeLamater J. Sexual expression in later life: a review and synthesis. J Sex Res. 2012;49(2-3):125-141.
24. Inelmen EM, Sergi G, Girardi A, et al. The importance of sexual health in the elderly: breaking down barriers and taboos. Aging Clin Exp Res. 2012;24(suppl 3):31-34.
25. Choi KB, Jang SH, Lee MY, et al. Sexual life and self-esteem in married elderly. Arch Gerontol Geriatr. 2011;53(1):e17-e20.
26. Davison SL, Bell RJ, LaChina M, et al. The relationship between self-reported sexual satisfaction and general well-being in women. J Sex Med. 2009;6(10):2690-2697.
27. Morley JE, Tariq SH. Sexuality and disease. Clin Geriatr Med. 2003;19(3):563-573.
28. Bouman WP, Arcelus J. Are psychiatrists guilty of “ageism” when it comes to taking a sexual history? Int J Geriatr Psychiatry. 2001;16(1):27-31.
29. La Torre A, Giupponi G, Duffy DM, et al. Sexual dysfunction related to psychotropic drugs: a critical review. Part III: mood stabilizers and anxiolytic drugs. Pharmacopsychiatry. 2014;47(1):1-6.
30. Tucker I. Management of inappropriate sexual behaviors in dementia: a literature review. Int Psychogeriatr. 2010; 22(5):683-692.
31. Imparato T, Sanders D. STD prevalence demands clinical awareness. Aging Well. 2012;5(1):14.
32. Poynten IM, Grulich AE, Templeton DJ. Sexually transmitted infections in older populations. Curr Opin Infect Dis. 2013;26(1):80-85.
33. Talashek ML, Tichy AM, Epping H. Sexually transmitted diseases in the elderly—issues and recommendations. J Gerontol Nurs. 1990;16(4):33-40.
34. Benbow SM, Jagus CE. Sexuality in older women with mental health problems. Sex Relation Ther. 2002;17(3):261-270.
35. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/ 11-008-x/2009002/article/10910-eng.htm#tphp. Accessed March 26, 2015.
Should lithium and ECT be used concurrently in geriatric patients?
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Delirium has been described as a potential complication of concurrent lithium and electroconvulsive therapy (ECT) for depression, in association with a range of serum lithium levels. Although debate persists about the safety of continuing previously established lithium therapy during a course of ECT for mood symptoms, withholding lithium for 24 hours before administering ECT and measuring the serum lithium level before ECT were found to decrease the risk of post-ECT neurocognitive effects.1
We have found that the conventional practice of holding lithium for 24 hours before ECT might need to be re-evaluated in geriatric patients, as the following case demonstrates. Only 24 hours of holding lithium therapy might result in a lithium level sufficient to contribute to delirium after ECT.
CASE REPORT
An older woman with recurrent unipolar psychotic depression
Mrs. A, age 81, was admitted to the hospital with a 1-week history of depressed mood, anhedonia, insomnia, anergia, anorexia, and nihilistic somatic delusions that her organs were “rotting and shutting down.” Treatment included nortriptyline, 40 mg/d; lithium, 150 mg/d; and haloperidol, 0.5 mg/d. Her serum lithium level was 0.3 mEq/L (reference range, 0.6 to 1.2 mEq/L); the serum nortriptyline level was 68 ng/mL (reference range, 50 to 150 ng/mL). CT of the head and an electrocardiogram were unremarkable.
A twice-weekly course of ECT was initiated.
The day before Treatment 1 of ECT, the serum lithium level (drawn 12 hours after the last dose) was 0.4 mEq/L. Lithium was withheld 24 hours before ECT; nortriptyline and haloperidol were continued at prescribed dosages.
Right unilateral stimulation was used at 50%/mC energy (Thymatron DG, with methohexital anesthesia, and succinylcholine for muscle relaxation). Seizure duration, measured by EEG, was 57 seconds.
Mrs. A developed postictal delirium after the first 2 ECT sessions. The serum lithium level was unchanged. Subsequently, lithium treatment was discontinued and ECT was continued; once lithium was stopped, delirium resolved. ECT sessions 3 and 4 were uneventful, with no post-treatment delirium. Seizure duration for Treatment 4 was 58 seconds. She started breathing easily after all ECT sessions.
After Treatment 4, Mrs. A experienced full remission of depressive and psychotic symptoms. Repeat CT of head, after Treatment 4, was unchanged from baseline.
What is the role of lithium?
Mrs. A did not exhibit typical signs of lithium intoxication (diarrhea, vomiting, tremor). Notably, lithium has an intrinsic anticholinergic activity2; concurrent nortriptyline, a secondary amine tricyclic antidepressant with fewer anticholinergic side effects than other tricyclics,2 could precipitate delirium in a vulnerable patient secondary to excessive cumulative anticholinergic exposure.
No prolonged time-to-respiration or time-to-awakening occurred during treatments in which concurrent lithium and ECT were used; seizure duration with and without concurrent lithium was relatively similar.
There are potential complications of concurrent use of lithium and ECT:
• prolongation of the duration of muscle paralysis and apnea induced by commonly used neuromuscular-blocking agents (eg, succinylcholine)
• post-ECT cognitive disturbance.1,3,4
There is debate about the safety of continuing lithium during, or in close proximity to, ECT. In a case series of 12 patients who underwent combined lithium therapy and ECT, the authors concluded that this combination can be safe, regardless of age, as long as appropriate clinical monitoring is provided.4 In Mrs. A’s case, once post-ECT delirium was noted, lithium was discontinued for subsequent ECT sessions.
Because further ECT was uneventful without lithium, and no other clear acute cause of delirium could be identified, we concluded that lithium likely played a role in Mrs. A’s delirium. Notably, nortriptyline had been continued, suggesting that the degree of anticholinergic blockade provided by nortriptyline was insufficient to provoke delirium post-ECT in the absence of potentiation of this effect, as it had been when lithium also was used initially.
Guidelines for dosing and serum lithium concentrations in geriatric patients are not well-established; the current traditional range of 0.6 to 1.2 mEq/L, is too high for geriatric patients and can result in episodes of lithium toxicity, including delirium.5 Although our patient’s lithium level was below the reference range for all patients, a level of 0.3 mEq/L can be considered at the low end of the reference range for geriatric patients.5 Inasmuch as the lithium-assisted post-ECT delirium could represent a clinical sign of lithium toxicity, perhaps even a subtherapeutic level in a certain patient could be paradoxically “toxic.”
Although the serum lithium level in our patient remained below the toxic level for the general population (>1.5 mEq/L), delirium in a geriatric patient could result from:
• age-related changes in the pharmacokinetics of lithium, a water-soluble drug; these changes reduce renal clearance of the drug and extend plasma elimination half-life of a single dose to 36 hours, with the result that lithium remains in the body longer and necessitating a lower dosage (ie, a dosage that yields a serum level of approximately 0.5 mEq/L)
• the CNS tissue concentration of lithium, which can be high even though the serum level is not toxic
• an age-related increase in blood-brain barrier permeability, making the barrier more porous for drugs
• changes in blood-brain barrier permeability by post-ECT biochemical induction, with subsequent increased drug availability in the CNS.5,6
What we recommend
Possible interactions between lithium and ECT that lead to ECT-associated delirium need further elucidation, but discontinuing lithium during the course of ECT in a geriatric patient warrants your consideration. Following a safe interval after the last ECT session, lithium likely can be safely re-introduced 1) if there is clinical need and 2) as long as clinical surveillance for cognitive side effects is provided— especially if ECT will need to be reconsidered in the future.
Two additional considerations:
• Actively reassess lithium dosing in all geriatric psychiatric patients, especially those with renal insufficiency and other systemic metabolic considerations.
• Actively examine the use of all other anticholinergic agents in the course of evaluating a patient’s candidacy for ECT.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
1. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging. A task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Publishing; 2001.
2. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341.
3. Hill GE, Wong KC, Hodges MR. Potentiation of succinylcholine neuromuscular blockade by lithium carbonate. Anesthesiology. 1976;44(5):439-442.
4. Dolenc TJ, Rasmussen KG. The safety of electroconvulsive therapy and lithium in combination: a case series and review of the literature. J ECT. 2005;21(3):165-170.
5. Shulman KI. Lithium for older adults with bipolar disorder: should it still be considered a first line agent? Drugs Aging. 2010;27(8):607-615.
6. Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: clinical pharmacology and therapeutic monitoring. CNS Drugs. 2009;23(4):331-349.
Delirium in the hospital: Emphasis on the management of geriatric patients
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Although delirium has many descriptive terms (Table 1), a common unifying term is “acute global cognitive dysfunction,” now recognized as delirium; a consensus supported by DSM-51 and ICD-102 (Table 2). According to DSM-5, the essential feature is a disturbance of attention or awareness that is accompanied by a change in baseline cognition that cannot be explained by another preexisting, established, or evolving neurocognitive disorder (the newly named DSM-5 entity for dementia syndromes).1 Because delirium affects the cortex diffusely, psychiatric symptoms can include cognitive, mood, anxiety, or psychotic symptoms. Because many systemic illnesses can induce delirium, the differential diagnosis spans all organ systems.
Three subtypes
Delirium can be classified, based on symptoms,3,4 into 3 subtypes: hyperactive-hyperalert, hypoactive-hypoalert, and mixed delirium. Hyperactive patients present with restlessness and agitation. Hypoactive patients are lethargic, confused, slow to respond to questions, and often appear depressed. The differential prognostic significance of these subtypes has been examined in the literature, with conflicting results. Rabinowitz5 reported that hypoactive delirium has the worst prognosis, while Marcantonio et al6 indicated that the hyperactive subtype is associated with the highest mortality rate. Mixed delirium, with periods of both hyperactivity and hypoactivity, is the most common type of delirium.7
A prodromal phase, characterized by anxiety, frequent requests for nursing and medical assistance, decreased attention, restlessness, vivid dreams, disorientation immediately after awakening, and hallucinations, can occur before an episode of full-spectrum delirium; this prodromal state often is identified retrospectively —after the patient is in an episode of delirium.8,9
Evidence-based guidelines aim to improve recognition and clinical management.10-13 Disruptive behavior is the main reason for psychiatric referral in delirium.14,15 Delayed psychiatric consultation because of non-recognition of delirium is related to variables such as older age; history of a pre-existing, comorbid neurocognitive disorder; and the clinical appearance of hypoactive delirium.14
The case of Mr. D (Box),16 illustrates how the emergence of antipsychotic-associated neuroleptic malignant syndrome (NMS) can complicate antipsychotic treatment of delirium in a geriatric medical patient, although delirium also is a common presentation in NMS.17 Delirium developed after an increase in carbidopa/levodopa, which has central dopaminergic effects that can precipitate delirium, particularly in a geriatric patient with preexisting comorbid neurocognitive disorder. Further complicating Mr. D’s delirium presentation was the development of NMS, which had a multifactorial causation, such as the use of dopamine antagonists (ie, quetiapine, metoclopramide), and an abrupt decrease of a dopaminergic agent (ie, carbidopa/levodopa), all inducing a central dopamine relative hypoactivity.
Epidemiology
Delirium is more common in older patients,15 and is seen in 30% to 40% of hospitalized geriatric patients.18 Delirium in older patients, compared with other adults, is associated with more severe cognitive impairment.19 It is common among geriatric surgical patients (15% to 62%)20 with a peak 2 to 5 days postoperatively for hip fracture,21 and often is seen in ICU patients (70% to 87%).20 However, Spronk et al22 found that delirium is significantly under-recognized in the ICU. Nearly 90% of terminally ill patients become delirious before death.23 Terminal delirium often is unrecognized and can interfere with assessment of other clinical problems.24 A preexisting history of comorbid neurocognitive disorder was evident in as many as two-thirds of delirium cases.25
Pathophysiology and risk factors
The pathophysiology of delirium has been characterized as an imbalance of CNS metabolism, including decreased blood flow in various regions of the brain that may normalize once delirium resolves.26 Studies describe the simultaneous decrease of cholinergic transmission and dopaminergic excess.27,28 Predisposing and precipitating factors for delirium that are of particular importance in geriatric patients include:
• advanced age
• CNS disease
• infection
• cognitive impairment
• male sex
• poor nutrition
• dehydration and other metabolic abnormalities
• cardiovascular events
• substance use
• medication
• sensory deprivation (eg, impaired vision or hearing)
• sleep deprivation
• low level of physical activity.27,29,30
Table 3 lists the most common delirium-provocative medications.27
Evaluation and psychometric scales
The EEG can be useful in evaluating delirium, especially in clinically ambiguous cases. EEG findings may indicate generalized slowing or dropout of the posterior dominant rhythm, and generalized slow theta and delta waves, findings that are more common in delirium than in other neurocognitive disorders and other psychiatric illnesses. The EEG must be interpreted in the context of the delirium diagnostic workup, because abnormalities seen in other neurocognitive disorders can overlap with those of delirium.31
The EEG referral should specify the clinical suspicion of delirium to help interpret the results. Delirium cases in which the patient’s previous cognitive status is unknown may benefit from EEG evaluation, such as:
• in possible status epilepticus
• when delirium improvement has reached a plateau at a lower level of cognitive function than before onset of delirium
• when the patient is unable or unwilling to complete a psychiatric interview.27
Assessment instruments are available to diagnose and monitor delirium (Table 4). Typically, delirium assessment includes examining levels of arousal, psychomotor activity, cognition (ie, orientation, attention, and memory), and perceptual disturbances.
Psychometrically, a review of Table 4 suggests that validity appeared stable with adequate specificity (64% to 99%) but more variable sensitivity (36% to 100%). These reliability parameters also will be affected by the classification system (ie, DSM vs ICD) and the cut-off score employed.32 Most measures (eg, Confusion Assessment Method [CAM], CAM-ICU) provide an adequate sample of behavioral (ie, level of alertness), motor (ie, psychomotor activity), and cognitive (ie, orientation, attention, memory, and receptive language) function, with the exception of the Global Attentiveness Rating, which is a 2-minute open conversation protocol between physician and patient.
Some measures are stand-alone instruments, such as the Memorial Delirium Assessment Scale, whereas the CAM requires administration of separate cognitive screens, including the Mini-Mental State Examination (MMSE) and Digit Span.33 Instruments to detect delirium in critically ill patients are a more recent development. Wong et al34 reported that the most widely studied tool was the CAM. Obtaining collateral information from family, caregivers, and hospital staff is essential, particularly given the fluctuating nature of delirium.
Management
Prevention. Identify patients at high risk of delirium so that preventive strategies can be employed. Multi-component, nonpharmacotherapeutic interventions are used in clinical settings but few randomized trials have been conducted. The contributing effectiveness of individual components is not well-studied, but most include staff education to increase awareness of delirium. Of 3 multi-component intervention randomized trials, 2 reported a significantly lower incidence of delirium in the intervention group.35-37 Implementation of a multi-component protocol in medical/ surgical units was associated with a significant reduction in use of restraints.38
As in Mr. D’s case, complex drug regimens, particularly for CNS illness, can increase the risk of delirium. Considering the medication profile for patients with complex systemic illness—in particular, minimizing the use anticholinergics and dopamine agonists— may be crucial in preventing delirium.
Prophylactic administration of antipsychotics may reduce the risk of developing postoperative delirium.39 Studies of the use of these agents were characterized by small sample sizes and selected groups of patient populations. Of the 4 randomized studies evaluating prophylactic antipsychotics (vs placebo), 3 found a lower incidence of delirium in the intervention groups.39-41
A study of haloperidol in post-GI surgery patients showed a reduced occurrence of delirium,40 whereas its prophylactic use in patients undergoing hip surgery42 did not reduce the incidence of delirium compared with placebo, but did decrease severity when delirium occurred.42
Risperidone39 in post-cardiac surgery and olanzapine41 perioperatively in patients undergoing total knee or hip replacement have been shown to decrease delirium severity and duration. Targeted prophylaxis with risperidone43 in post-cardiac surgery patients who showed disturbed cognition but did not meet criteria for delirium reduced the number of patients requiring medication, compared with placebo.43
Dexmedetomidine, an α-2 adrenergic receptor agonist, compared with propofol or midazolam in post-cardiac valve surgery patients, resulted in a decreased incidence of delirium but no difference in delirium duration, hospital length of stay, or use of other medications.44 However, other studies have shown that dexmedetomidine reduces ICU length of stay and duration of mechanical ventilation.45
Treatment. Management of hospitalized medically ill geriatric patients with delirium is challenging and requires a comprehensive approach. The first step in delirium management is prompt identification and management of systemic medical disturbances associated with the delirium episode. First-line, nonpharmacotherapeutic strategies for patients with delirium include:
• reorientation
• behavioral interventions (eg, use of clear instructions and frequent eye contact with patients)
• environmental interventions (eg, minimal noise, adequate lighting, and limited room and staff changes)
• avoidance of physical restraints.46
Consider employing family members or hospital staff sitters to stay with the patient and to reassure, reorient, and watch for agitation and other unsafe behaviors (eg, attempted elopement). Psychoeducation for the patient and family on the phenomenology of delirium can be helpful.
The use of drug treatment strategies should be integrated into a comprehensive approach that includes the routine use of nondrug measures.46 Using medications for treating hypoactive delirium, formerly controversial, now has wider acceptance.47,48 A few high-quality randomized trials have been performed.25,49,50
Pharmacotherapy, especially in frail patients, should be initiated at the lowest starting dosage and titrated cautiously to clinical effect and for the shortest period of time necessary. Antipsychotics are preferred agents for treating all subtypes of delirium; haloperidol is widely used.46,51,52 However, antipsychotics, including haloperidol, can be associated with adverse neurologic effects such as extrapyramidal symptoms (EPS) and NMS.
Although reported less frequently than with haloperidol, other agents have been implicated in development of EPS and NMS, including atypical antipsychotics and antiemetic dopamine antagonists, particularly in parkinsonism-prone patients.53 Strategies that can minimize such risks in geriatric inpatients with delirium include oral, rather than parenteral, use of antipsychotics—preferential use of atypical over typical antipsychotics— and lowest effective dosages.54
In controlled trials, atypical antipsychotics for delirium showed efficacy compared with haloperidol.52,55 However, there is no research that demonstrates any advantage of one atypical over another.25
In Mr. D’s case, the most important intervention for managing delirium caused by NMS is to discontinue all dopamine antagonists and treat agitation with judicious doses of a benzodiazepine, with supportive care.17 In cases of sudden discontinuation or a dosage decrease of dopamine agonists, these medications should be resumed or optimized to minimize the risk of NMS-associated rhabdomyolysis and subsequent renal failure.17 Antipsychotics carry an increased risk of stroke and mortality in older patients with established or evolving neurocognitive disorders56,57 and can cause prolongation of the QTc interval.57
Other medications that could be used for delirium include cholinesterase inhibitors58,59 (although larger trials and a systematic review did not support this use60), and 5-HT receptor antagonists,61 such as trazodone. Benzodiazepines, such as lorazepam, are first-line treatment for delirium associated with seizures or withdrawal from alcohol, sedatives, hypnotics, and anxiolytics and for delirium caused by NMS. Be cautious about using benzodiazepines in geriatric patients because of a risk of respiratory depression, falls, sedation, and amnesia.
Geriatric patients with alcoholism and those with malnutrition are prone to thiamine and vitamin B12 deficiencies, which can induce delirium. Laboratory assessment and consideration of supplementation is recommended. Despite high occurrence of delirium in hospitalized older adults with preexisting comorbid neurocognitive disorders, there is no standard care for delirium comorbid with another neurocognitive disorder.62 Clinical practice guidelines for older patients receiving palliative care have been developed63; the goal is to minimize suffering and discomfort in patients in palliative care.64
Post-delirium prophylaxis. Medications for delirium usually can be tapered and discontinued once the episode has resolved and the patient is stable; it is common to discontinue medications when the patient has been symptom-free for 1 week.65 Some patients (eg, with end-stage liver disease, disseminated cancer) are prone to recurrent or to prolonged or chronic delirium. A period of post-recovery treatment with antipsychotics—even indefinite treatment in some cases—should be considered.
Post-delirium debriefing and aftercare. The psychological complications of delirium are distressing for the patient and his (her) caregivers. Psychiatric complications associated with delirium, including acute stress disorder—which might predict posttraumatic stress disorder—have been explored; early recognition and treatment may improve long-term outcomes.66 After recovery from acute delirium, cognitive assessment (eg, MMSE67 or Montreal Cognitive Assessment68) is recommended to validate current cognitive status because patients may have persistent decrement in cognitive function compared with pre-delirium condition, even after recovery from the acute episode.
Post-delirium debriefing may help patients who have recovered from a delirium episode. Patients may fear that their brief period of hallucinations might represent the onset of a chronic-relapsing psychotic disorder. Allow patients to communicate their distress about the delirium episode and give them the opportunity to talk through the experience. Brief them on the possibility that delirium will recur and advise them to seek emergency medical care in case of recurrence. Advise patients to monitor and maintain a normal sleep-wake cycle.
Family members can watch for syndromal recurrence of delirium. They should be encouraged to discuss their reaction to having seen their relative in a delirious state.
Health care systems with integrated electronic medical records should list “delirium, resolved” on the patient’s illness profile or problem list and alert the patient’s primary care provider to the delirium history to avoid future exposure to delirium-provocative medications, and to prompt the provider to assume an active role in post-delirium care, including delirium recurrence surveillance, medication adjustment, risk factor management, and post-recovery cognitive assessment.
Bottom Line
Evaluation of delirium in geriatric patients includes clinical vigilance and screening, differentiating delirium from other neurocognitive disorders, and identifying and treating underlying causes. Perioperative use of antipsychotics may reduce the incidence of delirium, although hospital length of stay generally has not been reduced with prophylaxis. Management interventions include staff education, systematic screening, use of multicomponent interventions, and pharmacologic interventions.
Related Resources
• Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15(6):365.
• Brooks PB. Postoperative delirium in elderly patients. Am J Nurs. 2012;112(9):38-49.
Drug Brand Names
Carbidopa/levodopa • Sinemet Midazolam • Versed
Dexmedetomidine • Precedex Olanzapine • Zyprexa
Haloperidol • Haldol Propofol • Diprivan
Lithium • Eskalith, Lithobid Quetiapine • Seroquel
Lorazepam • Ativan Risperidone • Risperdal
Metoclopramide • Reglan Trazodone • Desyrel
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva, Switzerland: WHO; 1993.
3. Lipowski ZJ. Delirium in the elderly patient. N Engl J Med. 1989;320(9):578-582.
4. Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75-85.
5. Rabinowitz T. Delirium: an important (but often unrecognized) clinical syndrome. Curr Psychiatry Rep. 2002;4(3):202-208.
6. Marcantonio ER, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Am J Geriatr Soc. 2002;50(5):850-857.
7. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
8. Duppils GS, Wikblad K. Delirium: behavioural changes before and during the prodromal phase. J Clin Nurs. 2004;13(5):609-616.
9. de Jonghe JF, Kalisvaart KJ, Dijkstra M, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry. 2007;15(2):112-121.
10. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
11. Hogan D, Gage L, Bruto V, et al. National guidelines for seniors’ mental health: the assessment and treatment of delirium. Canadian Journal of Geriatrics. 2006;9(suppl 2):S42-51.
12. Leentjens AF, Diefenbacher A. A survey of delirium guidelines in Europe. J Psychosom Res. 2006;61(1):123-128.
13. Tropea J, Slee JA, Brand CA, et al. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27(3):150-156.
14. Mittal D, Majithia D, Kennedy R, et al. Differences in characteristics and outcome of delirium as based on referral patterns. Psychosomatics. 2006;47(5):367-375.
15. Grover S, Subodh BN, Avasthi A, et al. Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009;31(1): 25-29.
16. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12): 941-948.
17. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
18. Dobmejer K. Delirium in elderly medical patients. Clinical Geriatrics. 1996;4:43-68.
19. Leentjens AF, Maclullich AM, Meagher DJ. Delirium, Cinderella no more...? J Psychosom Res. 2008;65(3):205.
20. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
21. Streubel PN, Ricci WM, Gardner MJ. Fragility fractures: preoperative, perioperative, and postoperative management. Current Orthopaedic Practice. 2009;20(5):482-489.
22. Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
23. Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160(6):786-794.
24. Ganzini L. Care of patients with delirium at the end of life. Annals of Long-Term Care. 2007;15(3):35-40.
25. Bourne RS, Tahir TA, Borthwick M, et al. Drug treatment of delirium: past, present and future. J Psychosom Res. 2008;65(3):273-282.
26. Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337-339.
27. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
28. Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132-148.
29. Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97(3):278-288.
30. Laurila JV, Laakkonen ML, Tilvis RS, et al. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65(3):249-254.
31. Morandi A, McCurley J, Vasilevskis EE, et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005-2013.
32. Kazmierski J, Kowman M, Banach M, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010; 22(4):426-432.
33. Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Rating Scale. J Pain Symptom Manage. 1997;13(3):128-137.
34. Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779-786.
35. Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2011;49(5):516-522.
36. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628.
37. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007; 19(3):178-186.
38. Kratz A. Use of the acute confusion protocol: a research utilization project. J Nurs Care Qual. 2008;23(4):331-337.
39. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719.
40. Kaneko T, Cai J, Ishikura T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179-184.
41. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418.
42. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666.
43. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997.
44. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3): 206-217.
45. Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse. 2010;30(1): 29-38; quiz 39.
46. Practice guideline for the treatment of patients with delirium. American Psychiatric Association [Comment in: Treatment of patients with delirium. Am J Psychiatry. 2000.]. Am J Psychiatry. 1999;156(suppl 5):1-20.
47. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis, and treatment. Crit Care Clin. 2008;24(4):657-722, vii.
48. Platt MM, Breitbart W, Smith M, et al. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6(1):66-67.
49. Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594.
50. Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11-21.
51. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231-237.
52. Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chinese Journal of Clinical Rehabilitation. 2006;10(42): 188-190.
53. Friedman JH, Fernandez HH. Atypical antipsychotics in Parkinson-sensitive populations. J Geriatr Psychiatry Neurol. 2002;15(3):156-170.
54. Seitz DP, Gill SS. Neuroleptic malignant syndrome complicating antipsychotic treatment of delirium or agitation in medical and surgical patients: case reports and a review of the literature. Psychosomatics. 2009; 50(1):8-15.
55. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
56. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
57. Hermann N, Lanctôt KL. Atypical antipsychotics for neuropsychiatric symptoms of dementia: malignant or maligned? Drug Saf. 2006;29(10):833-843.
58. Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):885-887.
59. Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44(5):437-438.
60. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1): CD005317.
61. Davis MP. Does trazodone have a role in palliating symptoms? Support Care Cancer. 2007;15(2):221-224.
62. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002; 50(10):1723-1732.
63. Brajtman S, Wright D, Hogan D, et al. Developing guidelines for the assessment and treatment of delirium in older adults at the end of life. Can Geriatr J. 2011;14(2):40-50.
64. Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10(2):164-172.
65. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
66. Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801-2809.
67. Ringdal GI, Ringdal K, Juliebø V, et al. Using the Mini- Mental State Examination to screen for delirium in elderly patients with hip fracture. Dement Geriatr Cogn Disord. 2011;32(6):394-400.
68. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273-1278.
Obtaining informed consent for research in an acute inpatient psychiatric setting
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
Taking the spice route: Psychoactive properties of culinary spices
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
Out of the cupboard and into the clinic: Nutmeg-induced mood disorder
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.