User login
Melanoma in US Hispanics: Recommended Strategies to Reduce Disparities in Outcomes
Cutaneous melanoma is a considerable public health concern. In the United States, an estimated 87,110 cases were diagnosed in 2017, and more than 9000 deaths are expected as result of this disease in 2018.1 Early diagnosis of melanoma is associated with favorable survival rates (5-year overall survival rates for melanoma in situ and stage IA melanoma, 99% and 97%, respectively).2 In contrast, the prognosis for advanced-stage melanoma is poor, with a 5-year survival rate of 16% for patients with stage IV disease. Therefore, early detection is critical to reducing mortality in melanoma patients.3
The term Hispanic refers to a panethnic category primarily encompassing Mexican-Americans, Cubans, and Puerto Ricans, as well as individuals from the Caribbean and Central and South America. These populations are diverse in birth origin, primary language, acculturation, distinct ethnic traditions, education level, and occupation. Hispanics in the United States are heterogeneous in many dimensions related to health risks, health care use, and health outcomes.4 Genetic predisposition, lifestyle risks, and access to and use of health care services can shape melanoma diagnosis, treatment, and progression across Hispanic populations differently than in other populations.
In this review, the epidemiology and clinical presentation of melanoma in US Hispanics is summarized, and recommendations for a research agenda to advance understanding of this disease in the most rapidly growing segment of the US population is provided.
In the period from 2008 to 2012, the age-adjusted incidence of melanoma in US Hispanics (4.6 per 100,00 men and 4.2 per 100,00 women) was lower than in NHWs.5 Garnett et al5 reported a decline in melanoma incidence in US Hispanics between 2003 and 2012—an observation that stands in contrast to state-level studies in California and Florida, in which small but substantial increases in melanoma incidence among Hispanics were reported.6,7 The rising incidence of melanomas thicker than 1.5 mm at presentation among Hispanic men living in California is particularly worrisome.6 Discrepancies in incidence trends might reflect changes in incidence over time or differences in state-level registry reporting of melanoma.5
Despite a lower overall incidence of melanoma in US Hispanics, those who do develop the disease are 2.4 times more likely (age-adjusted odds ratio) to present with stage III disease (confidence interval, 1.89-3.05)8 and are 3.64 times more likely to develop distant metastases (confidence interval, 2.65-5.0) than NHWs.3,7,9-13 Disparities also exist in the diagnosis of childhood melanoma: Hispanic children and adolescents who have a diagnosis of melanoma are 3 times more likely to present with advanced disease than NHW counterparts.14 Survival analyses by age and stage show considerably lower survival among Hispanic patients compared to NHW patients with stage I and II disease. In part, worse survival outcomes among Hispanics are the result of the pattern of more advanced disease at presentation.8,14,15
Late presentation for evaluation of melanomas in Hispanics has been attributed to a number of variables, including a lack of skin cancer awareness and knowledge,9,16 a lower rate of self- and physician-performed skin examinations,10 differences in tumor biology,9 and socioeconomic forces.7,17
In a previous study investigating the relationship between neighborhood characteristics and tumor stage at melanoma diagnosis in Hispanic men in California, Texas, and Florida, several key findings emerged.17 First, residency in a census tract with a high density of immigrants (California, Texas) and a high composition of Hispanics (California, Florida) was an important predictor of a late-stage melanoma diagnosis in fully adjusted models. Additionally, the strength of association between measures of socioeconomic status (ie, poverty and education) and tumor stage at melanoma diagnosis was attenuated in multivariate models when enclaves and availability of primary care resources were taken into account. Hispanic melanoma cases in areas with a low density of primary care physicians had an increased likelihood of late-stage diagnosis in California and Texas. The probability of late-stage diagnosis was concentrated in specific regions along the United States–Mexico border, in south central California, and along the southeastern coast of Florida. Lastly, in Texas, Hispanic men aged 18 to 34 years and 35 to 49 years were at an increased risk of late-stage melanoma diagnosis compared to men 65 years and older.17
Demographic and Clinical Characteristics of Melanoma in Hispanic Patients
Among Hispanics, white Hispanics comprise the majority of melanoma cases.5 Median age at diagnosis is younger in Hispanics compared to whites.5,6 Hispanic men typically are older (median age, 61 years) than Hispanic women (median age, 52 years) at diagnosis.5 Similar to what is seen in NHWs, young Hispanic women experience a higher melanoma incidence than young Hispanic men.5 Among older Hispanics, melanoma is more common in men.5,8
Melanomas located on the lower extremities and hips are more prevalent in Hispanics than in NHWs.5,8,18 Among Hispanics, there are age- and sex-based variations in the anatomic location of primary tumors: in Hispanic men, truncal tumors predominate, and in Hispanic women, tumors of the lower extremities are most common across all age groups.5 The incidence of melanomas located in the head and neck region increases with age for both Hispanic men and women.
For melanomas in which the histologic type is known, superficial spreading melanoma is the most common subtype among Hispanics.5,17,19 Acral lentiginous melanomas and nodular melanomas are more common among Hispanics than among NHWs.5,17,19
The observation that Hispanics with melanoma are more prone to lower-extremity tumors and nodular and acral lentiginous melanoma subtypes than NHWs suggests that UV exposure history may be of less importance in this population. Although numerous studies have explored melanoma risk factors in NHWs, there is a striking paucity of such studies in Hispanics. For example, there are conflicting data regarding the role of UV exposure in melanoma risk among Hispanics. Hu et al20 found that UV index and latitude correlated with melanoma risk in this population, whereas Eide et al21 found no association between UV exposure and melanoma incidence in Hispanics. A prospective study involving a multiethnic cohort (of whom 40 of the 107 participants were Hispanic) found no clear association between a history of sunburn and melanoma risk in Hispanics.18
Strategies for Reducing Disparities in Outcomes
Our knowledge of melanoma epidemiology in Hispanics derives mainly from secondary analyses of state-level and national cancer registry data sets.5-8,13-15,17,19,20 These administrative data sources often are limited by missing data (eg, tumor thickness, histologic subtype) or lack important patient-level information (eg, self-identified race and ethnicity, health insurance status). Additionally, the manner in which data are collected and integrated into research varies; for example, socioeconomic measures often are reported as either area-based or composite measures.
The host phenotypic characteristics of melanoma in NHWs are well understood, but the biological and environmental determinants of melanoma risk in Hispanics and other minorities are unknown. For example, fair complexion, red hair, blue eyes, increased freckling density, and the presence of numerous dysplastic and common melanocytic nevi indicate a propensity toward cutaneous melanoma.23,24 However, the relevance of such risk factors in Hispanics is unknown and has not been widely investigated in this patient population. Park et al18 found that a person’s sunburn susceptibility phenotype (defined as hair and eye color, ability to tan, and skin reaction to sunlight) was associated with an increased risk of melanoma among nonwhite, multiracial individuals. However, this study was limited by a small number of minority cases, which included only 40 Hispanic participants with melanoma.18 There is a need for rigorous observational studies to clearly define the phenotypic characteristics, sun-exposure behavior patterns, and genetic contributors to melanoma genesis in Hispanics.
The biologic determinants of postdiagnosis survival in Hispanics with melanoma are not well understood. It is unknown if genetic predisposition modifies melanoma risk in Hispanics. For example, the frequency of BRAF gene mutation or other driver mutations in US Hispanics has been understudied. It is important to know if mutation frequency patterns differ in Hispanics patients compared to NHWs because this knowledge could have considerable implications for treatment. Several recommendations should be considered to address these knowledge gaps. First, there is a need for development or enhancement of melanoma biorepositories, which should include tumor and nontumor specimens from a diverse sample of melanoma patients.
Conclusion
- American Cancer Society. Key statistics for melanoma skin cancer. www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html. Accessed January 13, 2018.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? Cancer. 2012;118:5395-5402.
- Bergad LW, Klein HS. Hispanics in the United States: A Demographic, Social, and Economic History, 1980-2005. New York, NY: Cambridge University Press; 2010.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152-161.
- Hu S, Parmet, Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites,Hispanics, and blacks in Florida. JAMA Dermatology. 2010;145:1369-1374.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Pollitt RA, Swetter SM, Johnson TM, et al. Examining the pathways linking lower socioeconomic status and advanced melanoma. Cancer. 2012;118:4004-4013.
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163-RA172.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S58-S68.
- Pollitt RA, Clarke CA, Shema SJ, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
- Clairwood M, Ricketts J, Grant-Kels J, et al. Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics. Int J Dermatol. 2014;53:425-433.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Harvey VM, Enos CW, Chen JT, et al. The role of neighborhood characteristics in late stage melanoma diagnosis among Hispanic men in California, Texas, and Florida, 1996-2012 [published online June 18, 2017]. J Cancer Epidemiol. 2017;2017:8418904.
- Park SL, Le Marchand L, Wilkens LR, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res. 2012;5:423-434.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Hu S, Ma F, Collado-Mesa F, et al. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819-824.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) program, 1992 to 2001. Arch Dermatol. 2005;141:477-481.
- Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77:4548-4555.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040-2059.
- Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420-428.
- Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309-319.
- Rapkin BD, Weiss E, Lounsbury D, et al. Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. Am J Community Psychol. 2017;60:145-159.
Cutaneous melanoma is a considerable public health concern. In the United States, an estimated 87,110 cases were diagnosed in 2017, and more than 9000 deaths are expected as result of this disease in 2018.1 Early diagnosis of melanoma is associated with favorable survival rates (5-year overall survival rates for melanoma in situ and stage IA melanoma, 99% and 97%, respectively).2 In contrast, the prognosis for advanced-stage melanoma is poor, with a 5-year survival rate of 16% for patients with stage IV disease. Therefore, early detection is critical to reducing mortality in melanoma patients.3
The term Hispanic refers to a panethnic category primarily encompassing Mexican-Americans, Cubans, and Puerto Ricans, as well as individuals from the Caribbean and Central and South America. These populations are diverse in birth origin, primary language, acculturation, distinct ethnic traditions, education level, and occupation. Hispanics in the United States are heterogeneous in many dimensions related to health risks, health care use, and health outcomes.4 Genetic predisposition, lifestyle risks, and access to and use of health care services can shape melanoma diagnosis, treatment, and progression across Hispanic populations differently than in other populations.
In this review, the epidemiology and clinical presentation of melanoma in US Hispanics is summarized, and recommendations for a research agenda to advance understanding of this disease in the most rapidly growing segment of the US population is provided.
In the period from 2008 to 2012, the age-adjusted incidence of melanoma in US Hispanics (4.6 per 100,00 men and 4.2 per 100,00 women) was lower than in NHWs.5 Garnett et al5 reported a decline in melanoma incidence in US Hispanics between 2003 and 2012—an observation that stands in contrast to state-level studies in California and Florida, in which small but substantial increases in melanoma incidence among Hispanics were reported.6,7 The rising incidence of melanomas thicker than 1.5 mm at presentation among Hispanic men living in California is particularly worrisome.6 Discrepancies in incidence trends might reflect changes in incidence over time or differences in state-level registry reporting of melanoma.5
Despite a lower overall incidence of melanoma in US Hispanics, those who do develop the disease are 2.4 times more likely (age-adjusted odds ratio) to present with stage III disease (confidence interval, 1.89-3.05)8 and are 3.64 times more likely to develop distant metastases (confidence interval, 2.65-5.0) than NHWs.3,7,9-13 Disparities also exist in the diagnosis of childhood melanoma: Hispanic children and adolescents who have a diagnosis of melanoma are 3 times more likely to present with advanced disease than NHW counterparts.14 Survival analyses by age and stage show considerably lower survival among Hispanic patients compared to NHW patients with stage I and II disease. In part, worse survival outcomes among Hispanics are the result of the pattern of more advanced disease at presentation.8,14,15
Late presentation for evaluation of melanomas in Hispanics has been attributed to a number of variables, including a lack of skin cancer awareness and knowledge,9,16 a lower rate of self- and physician-performed skin examinations,10 differences in tumor biology,9 and socioeconomic forces.7,17
In a previous study investigating the relationship between neighborhood characteristics and tumor stage at melanoma diagnosis in Hispanic men in California, Texas, and Florida, several key findings emerged.17 First, residency in a census tract with a high density of immigrants (California, Texas) and a high composition of Hispanics (California, Florida) was an important predictor of a late-stage melanoma diagnosis in fully adjusted models. Additionally, the strength of association between measures of socioeconomic status (ie, poverty and education) and tumor stage at melanoma diagnosis was attenuated in multivariate models when enclaves and availability of primary care resources were taken into account. Hispanic melanoma cases in areas with a low density of primary care physicians had an increased likelihood of late-stage diagnosis in California and Texas. The probability of late-stage diagnosis was concentrated in specific regions along the United States–Mexico border, in south central California, and along the southeastern coast of Florida. Lastly, in Texas, Hispanic men aged 18 to 34 years and 35 to 49 years were at an increased risk of late-stage melanoma diagnosis compared to men 65 years and older.17
Demographic and Clinical Characteristics of Melanoma in Hispanic Patients
Among Hispanics, white Hispanics comprise the majority of melanoma cases.5 Median age at diagnosis is younger in Hispanics compared to whites.5,6 Hispanic men typically are older (median age, 61 years) than Hispanic women (median age, 52 years) at diagnosis.5 Similar to what is seen in NHWs, young Hispanic women experience a higher melanoma incidence than young Hispanic men.5 Among older Hispanics, melanoma is more common in men.5,8
Melanomas located on the lower extremities and hips are more prevalent in Hispanics than in NHWs.5,8,18 Among Hispanics, there are age- and sex-based variations in the anatomic location of primary tumors: in Hispanic men, truncal tumors predominate, and in Hispanic women, tumors of the lower extremities are most common across all age groups.5 The incidence of melanomas located in the head and neck region increases with age for both Hispanic men and women.
For melanomas in which the histologic type is known, superficial spreading melanoma is the most common subtype among Hispanics.5,17,19 Acral lentiginous melanomas and nodular melanomas are more common among Hispanics than among NHWs.5,17,19
The observation that Hispanics with melanoma are more prone to lower-extremity tumors and nodular and acral lentiginous melanoma subtypes than NHWs suggests that UV exposure history may be of less importance in this population. Although numerous studies have explored melanoma risk factors in NHWs, there is a striking paucity of such studies in Hispanics. For example, there are conflicting data regarding the role of UV exposure in melanoma risk among Hispanics. Hu et al20 found that UV index and latitude correlated with melanoma risk in this population, whereas Eide et al21 found no association between UV exposure and melanoma incidence in Hispanics. A prospective study involving a multiethnic cohort (of whom 40 of the 107 participants were Hispanic) found no clear association between a history of sunburn and melanoma risk in Hispanics.18
Strategies for Reducing Disparities in Outcomes
Our knowledge of melanoma epidemiology in Hispanics derives mainly from secondary analyses of state-level and national cancer registry data sets.5-8,13-15,17,19,20 These administrative data sources often are limited by missing data (eg, tumor thickness, histologic subtype) or lack important patient-level information (eg, self-identified race and ethnicity, health insurance status). Additionally, the manner in which data are collected and integrated into research varies; for example, socioeconomic measures often are reported as either area-based or composite measures.
The host phenotypic characteristics of melanoma in NHWs are well understood, but the biological and environmental determinants of melanoma risk in Hispanics and other minorities are unknown. For example, fair complexion, red hair, blue eyes, increased freckling density, and the presence of numerous dysplastic and common melanocytic nevi indicate a propensity toward cutaneous melanoma.23,24 However, the relevance of such risk factors in Hispanics is unknown and has not been widely investigated in this patient population. Park et al18 found that a person’s sunburn susceptibility phenotype (defined as hair and eye color, ability to tan, and skin reaction to sunlight) was associated with an increased risk of melanoma among nonwhite, multiracial individuals. However, this study was limited by a small number of minority cases, which included only 40 Hispanic participants with melanoma.18 There is a need for rigorous observational studies to clearly define the phenotypic characteristics, sun-exposure behavior patterns, and genetic contributors to melanoma genesis in Hispanics.
The biologic determinants of postdiagnosis survival in Hispanics with melanoma are not well understood. It is unknown if genetic predisposition modifies melanoma risk in Hispanics. For example, the frequency of BRAF gene mutation or other driver mutations in US Hispanics has been understudied. It is important to know if mutation frequency patterns differ in Hispanics patients compared to NHWs because this knowledge could have considerable implications for treatment. Several recommendations should be considered to address these knowledge gaps. First, there is a need for development or enhancement of melanoma biorepositories, which should include tumor and nontumor specimens from a diverse sample of melanoma patients.
Conclusion
Cutaneous melanoma is a considerable public health concern. In the United States, an estimated 87,110 cases were diagnosed in 2017, and more than 9000 deaths are expected as result of this disease in 2018.1 Early diagnosis of melanoma is associated with favorable survival rates (5-year overall survival rates for melanoma in situ and stage IA melanoma, 99% and 97%, respectively).2 In contrast, the prognosis for advanced-stage melanoma is poor, with a 5-year survival rate of 16% for patients with stage IV disease. Therefore, early detection is critical to reducing mortality in melanoma patients.3
The term Hispanic refers to a panethnic category primarily encompassing Mexican-Americans, Cubans, and Puerto Ricans, as well as individuals from the Caribbean and Central and South America. These populations are diverse in birth origin, primary language, acculturation, distinct ethnic traditions, education level, and occupation. Hispanics in the United States are heterogeneous in many dimensions related to health risks, health care use, and health outcomes.4 Genetic predisposition, lifestyle risks, and access to and use of health care services can shape melanoma diagnosis, treatment, and progression across Hispanic populations differently than in other populations.
In this review, the epidemiology and clinical presentation of melanoma in US Hispanics is summarized, and recommendations for a research agenda to advance understanding of this disease in the most rapidly growing segment of the US population is provided.
In the period from 2008 to 2012, the age-adjusted incidence of melanoma in US Hispanics (4.6 per 100,00 men and 4.2 per 100,00 women) was lower than in NHWs.5 Garnett et al5 reported a decline in melanoma incidence in US Hispanics between 2003 and 2012—an observation that stands in contrast to state-level studies in California and Florida, in which small but substantial increases in melanoma incidence among Hispanics were reported.6,7 The rising incidence of melanomas thicker than 1.5 mm at presentation among Hispanic men living in California is particularly worrisome.6 Discrepancies in incidence trends might reflect changes in incidence over time or differences in state-level registry reporting of melanoma.5
Despite a lower overall incidence of melanoma in US Hispanics, those who do develop the disease are 2.4 times more likely (age-adjusted odds ratio) to present with stage III disease (confidence interval, 1.89-3.05)8 and are 3.64 times more likely to develop distant metastases (confidence interval, 2.65-5.0) than NHWs.3,7,9-13 Disparities also exist in the diagnosis of childhood melanoma: Hispanic children and adolescents who have a diagnosis of melanoma are 3 times more likely to present with advanced disease than NHW counterparts.14 Survival analyses by age and stage show considerably lower survival among Hispanic patients compared to NHW patients with stage I and II disease. In part, worse survival outcomes among Hispanics are the result of the pattern of more advanced disease at presentation.8,14,15
Late presentation for evaluation of melanomas in Hispanics has been attributed to a number of variables, including a lack of skin cancer awareness and knowledge,9,16 a lower rate of self- and physician-performed skin examinations,10 differences in tumor biology,9 and socioeconomic forces.7,17
In a previous study investigating the relationship between neighborhood characteristics and tumor stage at melanoma diagnosis in Hispanic men in California, Texas, and Florida, several key findings emerged.17 First, residency in a census tract with a high density of immigrants (California, Texas) and a high composition of Hispanics (California, Florida) was an important predictor of a late-stage melanoma diagnosis in fully adjusted models. Additionally, the strength of association between measures of socioeconomic status (ie, poverty and education) and tumor stage at melanoma diagnosis was attenuated in multivariate models when enclaves and availability of primary care resources were taken into account. Hispanic melanoma cases in areas with a low density of primary care physicians had an increased likelihood of late-stage diagnosis in California and Texas. The probability of late-stage diagnosis was concentrated in specific regions along the United States–Mexico border, in south central California, and along the southeastern coast of Florida. Lastly, in Texas, Hispanic men aged 18 to 34 years and 35 to 49 years were at an increased risk of late-stage melanoma diagnosis compared to men 65 years and older.17
Demographic and Clinical Characteristics of Melanoma in Hispanic Patients
Among Hispanics, white Hispanics comprise the majority of melanoma cases.5 Median age at diagnosis is younger in Hispanics compared to whites.5,6 Hispanic men typically are older (median age, 61 years) than Hispanic women (median age, 52 years) at diagnosis.5 Similar to what is seen in NHWs, young Hispanic women experience a higher melanoma incidence than young Hispanic men.5 Among older Hispanics, melanoma is more common in men.5,8
Melanomas located on the lower extremities and hips are more prevalent in Hispanics than in NHWs.5,8,18 Among Hispanics, there are age- and sex-based variations in the anatomic location of primary tumors: in Hispanic men, truncal tumors predominate, and in Hispanic women, tumors of the lower extremities are most common across all age groups.5 The incidence of melanomas located in the head and neck region increases with age for both Hispanic men and women.
For melanomas in which the histologic type is known, superficial spreading melanoma is the most common subtype among Hispanics.5,17,19 Acral lentiginous melanomas and nodular melanomas are more common among Hispanics than among NHWs.5,17,19
The observation that Hispanics with melanoma are more prone to lower-extremity tumors and nodular and acral lentiginous melanoma subtypes than NHWs suggests that UV exposure history may be of less importance in this population. Although numerous studies have explored melanoma risk factors in NHWs, there is a striking paucity of such studies in Hispanics. For example, there are conflicting data regarding the role of UV exposure in melanoma risk among Hispanics. Hu et al20 found that UV index and latitude correlated with melanoma risk in this population, whereas Eide et al21 found no association between UV exposure and melanoma incidence in Hispanics. A prospective study involving a multiethnic cohort (of whom 40 of the 107 participants were Hispanic) found no clear association between a history of sunburn and melanoma risk in Hispanics.18
Strategies for Reducing Disparities in Outcomes
Our knowledge of melanoma epidemiology in Hispanics derives mainly from secondary analyses of state-level and national cancer registry data sets.5-8,13-15,17,19,20 These administrative data sources often are limited by missing data (eg, tumor thickness, histologic subtype) or lack important patient-level information (eg, self-identified race and ethnicity, health insurance status). Additionally, the manner in which data are collected and integrated into research varies; for example, socioeconomic measures often are reported as either area-based or composite measures.
The host phenotypic characteristics of melanoma in NHWs are well understood, but the biological and environmental determinants of melanoma risk in Hispanics and other minorities are unknown. For example, fair complexion, red hair, blue eyes, increased freckling density, and the presence of numerous dysplastic and common melanocytic nevi indicate a propensity toward cutaneous melanoma.23,24 However, the relevance of such risk factors in Hispanics is unknown and has not been widely investigated in this patient population. Park et al18 found that a person’s sunburn susceptibility phenotype (defined as hair and eye color, ability to tan, and skin reaction to sunlight) was associated with an increased risk of melanoma among nonwhite, multiracial individuals. However, this study was limited by a small number of minority cases, which included only 40 Hispanic participants with melanoma.18 There is a need for rigorous observational studies to clearly define the phenotypic characteristics, sun-exposure behavior patterns, and genetic contributors to melanoma genesis in Hispanics.
The biologic determinants of postdiagnosis survival in Hispanics with melanoma are not well understood. It is unknown if genetic predisposition modifies melanoma risk in Hispanics. For example, the frequency of BRAF gene mutation or other driver mutations in US Hispanics has been understudied. It is important to know if mutation frequency patterns differ in Hispanics patients compared to NHWs because this knowledge could have considerable implications for treatment. Several recommendations should be considered to address these knowledge gaps. First, there is a need for development or enhancement of melanoma biorepositories, which should include tumor and nontumor specimens from a diverse sample of melanoma patients.
Conclusion
- American Cancer Society. Key statistics for melanoma skin cancer. www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html. Accessed January 13, 2018.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? Cancer. 2012;118:5395-5402.
- Bergad LW, Klein HS. Hispanics in the United States: A Demographic, Social, and Economic History, 1980-2005. New York, NY: Cambridge University Press; 2010.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152-161.
- Hu S, Parmet, Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites,Hispanics, and blacks in Florida. JAMA Dermatology. 2010;145:1369-1374.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Pollitt RA, Swetter SM, Johnson TM, et al. Examining the pathways linking lower socioeconomic status and advanced melanoma. Cancer. 2012;118:4004-4013.
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163-RA172.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S58-S68.
- Pollitt RA, Clarke CA, Shema SJ, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
- Clairwood M, Ricketts J, Grant-Kels J, et al. Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics. Int J Dermatol. 2014;53:425-433.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Harvey VM, Enos CW, Chen JT, et al. The role of neighborhood characteristics in late stage melanoma diagnosis among Hispanic men in California, Texas, and Florida, 1996-2012 [published online June 18, 2017]. J Cancer Epidemiol. 2017;2017:8418904.
- Park SL, Le Marchand L, Wilkens LR, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res. 2012;5:423-434.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Hu S, Ma F, Collado-Mesa F, et al. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819-824.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) program, 1992 to 2001. Arch Dermatol. 2005;141:477-481.
- Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77:4548-4555.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040-2059.
- Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420-428.
- Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309-319.
- Rapkin BD, Weiss E, Lounsbury D, et al. Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. Am J Community Psychol. 2017;60:145-159.
- American Cancer Society. Key statistics for melanoma skin cancer. www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html. Accessed January 13, 2018.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? Cancer. 2012;118:5395-5402.
- Bergad LW, Klein HS. Hispanics in the United States: A Demographic, Social, and Economic History, 1980-2005. New York, NY: Cambridge University Press; 2010.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152-161.
- Hu S, Parmet, Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites,Hispanics, and blacks in Florida. JAMA Dermatology. 2010;145:1369-1374.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Pollitt RA, Swetter SM, Johnson TM, et al. Examining the pathways linking lower socioeconomic status and advanced melanoma. Cancer. 2012;118:4004-4013.
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163-RA172.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S58-S68.
- Pollitt RA, Clarke CA, Shema SJ, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
- Clairwood M, Ricketts J, Grant-Kels J, et al. Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics. Int J Dermatol. 2014;53:425-433.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Harvey VM, Enos CW, Chen JT, et al. The role of neighborhood characteristics in late stage melanoma diagnosis among Hispanic men in California, Texas, and Florida, 1996-2012 [published online June 18, 2017]. J Cancer Epidemiol. 2017;2017:8418904.
- Park SL, Le Marchand L, Wilkens LR, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res. 2012;5:423-434.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Hu S, Ma F, Collado-Mesa F, et al. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819-824.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) program, 1992 to 2001. Arch Dermatol. 2005;141:477-481.
- Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77:4548-4555.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040-2059.
- Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420-428.
- Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309-319.
- Rapkin BD, Weiss E, Lounsbury D, et al. Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. Am J Community Psychol. 2017;60:145-159.
Practice Points
- Although the age-adjusted incidence of melanoma among US Hispanics is lower than among non-Hispanic whites, Hispanics with melanoma are more likely to present with stage III disease and have distant metastases.
- Late presentation of melanoma in Hispanics is not completely understood but may be attributed to socioeconomic factors, lack of skin cancer awareness and knowledge, lower rate of self- and physician-performed skin examinations, and differences in tumor biology, among other variables.
- Research is needed to address gaps in knowledge about the risk of melanoma and comparatively poor outcomes among Hispanics so interventional efforts for prevention, early detection, and treatment can be implemented.
Laugier-Hunziker Syndrome
To the Editor:
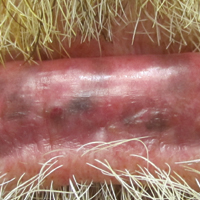
A 55-year-old man presented with hyperpigmented brown macules on the lips, hands, and fingertips of 6 years’ duration. The spots were persistent, asymptomatic, and had not changed in size. The patient denied a history of alopecia or dystrophic nails. He also denied a family history of similar skin findings. He had no personal history of cancer and a colonoscopy performed 5 years prior revealed no notable abnormalities. His medications included amlodipine and hydrocodone-acetaminophen. His mother died of “abdominal bleeding” at 74 years of age and his father died of a brain tumor at 64 years of age. Physical examination demonstrated numerous well-defined, dark brown macules of variable size distributed on the lower and upper mucosal lips (Figure 1A), buccal mucosa, hard palate, and gingiva, as well as the dorsal aspect of the fingers (Figure 1B) and volar aspect of the fingertips (Figure 1C).

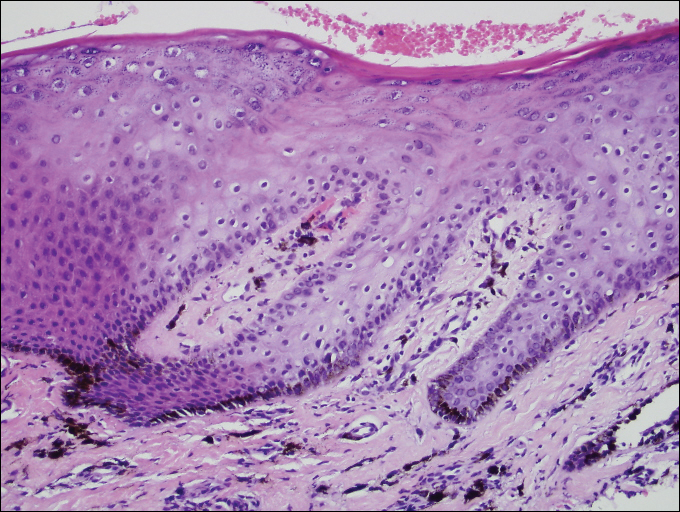
A shave biopsy of a dark brown macule from the lower lip (Figure 2) was performed. Histopathologic examination revealed pigmentation of the basal layer of the epidermis with pigment-laden cells in the dermis immediately deep to the surface epithelium. Immunoperoxidase stains showed a normal number and distribution of melanocytes.
A diagnosis of Laugier-Hunziker syndrome (LHS) was made given the age of onset; distribution of pigmentation; and lack of pathologic colonoscopic findings, personal history of cancer, or gastrointestinal tract symptoms.
Benign hyperpigmentation of the lips and fingers has been reported.1 The average age of onset of LHS is 52 years, and it typically is diagnosed in white adults.1,2 In LHS, pigmentation is most commonly distributed on the lips, especially the lower lips and oral mucosa.2 Pigmentation of the nails in the form of longitudinal melanonychia is present in approximately half of cases.2,3 There also may be pigmentation of the neck; thorax; abdomen; and acral surfaces, especially the fingertips.1-3 Rarely, pigmented macules can occur on the genitalia or sclera.1,2 Unlike Peutz-Jeghers syndrome, the diagnosis of LHS does not result from a germline mutation and carries no risk of gastrointestinal polyposis or internal malignancy.3,4 The histopathology of a pigmented macule of LHS shows a normal number and morphology of melanocytes. Epidermal basement membrane pigmentation is common, with pigment-laden macrophages evident in the papillary dermis.3
RELATED ARTICLE: Asymptomatic Lower Lip Hyperpigmentation From Laugier-Hunziker Syndrome
The differential diagnosis of multiple lentigines is broad and includes Peutz-Jeghers syndrome; LEOPARD (lentigines, electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of genitalia, retardation of growth, deafness) syndrome; Carney complexes, including LAMB (lentigines, atrial myxoma, mucocutaneous myxoma, blue nevi) and NAME (nevi, atrial myxoma, myxoid neurofibroma, ephelide) syndromes5; primary adrenocortical insufficiency (Addison disease); and idiopathic melanoplakia.2 Peutz-Jeghers syndrome, an autosomal-dominant syndrome with mucocutaneous lentigines, has a similar clinical appearance to LHS; therefore, it is necessary to exclude this diagnosis due to its association with intestinal hamartomatous polyps and internal malignancies (Table).3,6,7
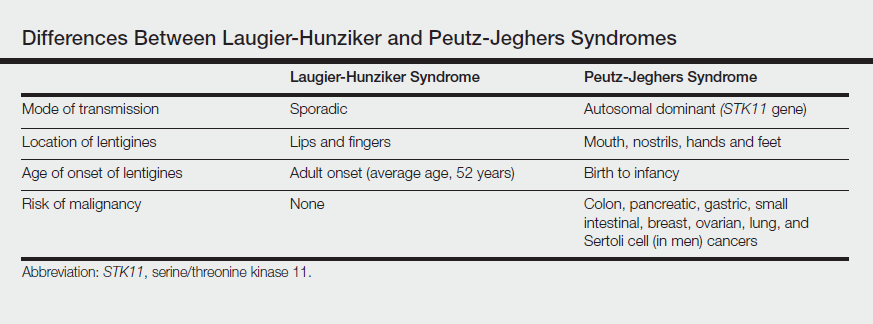
Peutz-Jeghers syndrome is characterized by mucocutaneous hyperpigmentation and intestinal hamartomatous polyposis and is associated with internal malignancies of the colon, breast, pancreas, stomach, small intestines, ovaries, lung, and Sertoli cells in men.6,7 Associated gastrointestinal tract malignancies in descending order of frequency are colon (39%), pancreatic (36%), gastric (29%), and small intestine (13%).1 It is caused by a germ line mutation of the serine/threonine kinase 11 gene, STK11. Although the appearance and distribution of the mucocutaneous lentigines is similar to individuals with LHS, by contrast the lentiginosis in individuals with Peutz-Jeghers syndrome is present from birth or develops during infancy.6 Aggressive cancer screening guidelines aid in early detection and begin at 8 years of age with a baseline colonoscopy and esophagogastroduodenoscopy; future screening is dictated by the presence or absence of polyps. If no polyps are detected at 8 years of age, a colonoscopy and esophagogastroduodenoscopy are repeated at 18 years of age and then every 3 years until 50 years of age.8
In an adult patient, the diagnosis of LHS can be made clinically and a correct diagnosis prevents frequent and unpleasant gastrointestinal tract cancer screening examinations. Lampe et al2 described a man with LHS who was incorrectly diagnosed with Peutz-Jeghers syndrome and experienced a colonic perforation as a complication of a screening colonoscopy. Their case report underscores the importance of making the correct diagnosis of LHS to avoid undertaking unnecessary aggressive cancer screening regimens.2
Although LHS is a benign condition that does not require treatment, Q-switched alexandrite or erbium:YAG laser therapy has been shown to improve the pigmentary findings associated with LHS.9,10 It has been suggested that LHS should be renamed Laugier-Hunziker pigmentation2 or mucocutaneous lentiginosis of Laugier and Hunziker1 to differentiate LHS as simply a disorder of pigmentation rather than a potentially morbid genetic defect, as in Peutz-Jeghers syndrome.
- Moore RT, Chae KA, Rhodes AR. Laugier and Hunziker pigmentation: a lentiginous proliferation of melanocytes. J Am Acad Dermatol. 2004;50(5 suppl):S70-S74.
- Lampe AK, Hampton PJ, Woodford-Richens K, et al. Laugier-Hunziker Syndrome: an important differential diagnosis for Peutz-Jeghers Syndrome. J Med Genet. 2003;40:E77.
- Baran R. Longitudinal melanotic streaks as a clue for Laugier-Hunziker syndrome. Arch Dermatol. 1979;115:1148-1149.
- Grimes P, Nordlund JJ, Pandya AG, et al. Increasing our understanding of pigmentary disorders. J Am Acad Dermatol. 2006;54(5 suppl 2):S255-S261.
- Bertherat J. Carney complex (CNC). Orphanet J Rare Dis. 2006;1:21.
- Giardiello FM, Brensinger JD, Tersemette AC, et al. Very high risk of cancer in Peutz-Jeghers Syndrome. Gastroenterology. 2000;119:1447-1453.
- Brosens LA, van Hattem WA, Jansen M, et al. Gastrointestinal polyposis syndromes. Curr Mol Med. 2007;7:29-46.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
- Zuo YG, Ma DL, Jin HZ, et al. Treatment of Laugier-Hunziker syndrome with the Q-switched alexandrite laser in 22 Chinese patients. Arch Dermatol Res. 2010;302:125-130.
- Ergun S, Saruhanog˘lu A, Migliari DA, et al. Refractory pigmentation associated with Laugier-Hunziker syndrome following Er:YAG laser treatment [published online December 3, 2013]. Case Rep Dent. 2013;2013:561040.
To the Editor:
A 55-year-old man presented with hyperpigmented brown macules on the lips, hands, and fingertips of 6 years’ duration. The spots were persistent, asymptomatic, and had not changed in size. The patient denied a history of alopecia or dystrophic nails. He also denied a family history of similar skin findings. He had no personal history of cancer and a colonoscopy performed 5 years prior revealed no notable abnormalities. His medications included amlodipine and hydrocodone-acetaminophen. His mother died of “abdominal bleeding” at 74 years of age and his father died of a brain tumor at 64 years of age. Physical examination demonstrated numerous well-defined, dark brown macules of variable size distributed on the lower and upper mucosal lips (Figure 1A), buccal mucosa, hard palate, and gingiva, as well as the dorsal aspect of the fingers (Figure 1B) and volar aspect of the fingertips (Figure 1C).

A shave biopsy of a dark brown macule from the lower lip (Figure 2) was performed. Histopathologic examination revealed pigmentation of the basal layer of the epidermis with pigment-laden cells in the dermis immediately deep to the surface epithelium. Immunoperoxidase stains showed a normal number and distribution of melanocytes.

A diagnosis of Laugier-Hunziker syndrome (LHS) was made given the age of onset; distribution of pigmentation; and lack of pathologic colonoscopic findings, personal history of cancer, or gastrointestinal tract symptoms.
Benign hyperpigmentation of the lips and fingers has been reported.1 The average age of onset of LHS is 52 years, and it typically is diagnosed in white adults.1,2 In LHS, pigmentation is most commonly distributed on the lips, especially the lower lips and oral mucosa.2 Pigmentation of the nails in the form of longitudinal melanonychia is present in approximately half of cases.2,3 There also may be pigmentation of the neck; thorax; abdomen; and acral surfaces, especially the fingertips.1-3 Rarely, pigmented macules can occur on the genitalia or sclera.1,2 Unlike Peutz-Jeghers syndrome, the diagnosis of LHS does not result from a germline mutation and carries no risk of gastrointestinal polyposis or internal malignancy.3,4 The histopathology of a pigmented macule of LHS shows a normal number and morphology of melanocytes. Epidermal basement membrane pigmentation is common, with pigment-laden macrophages evident in the papillary dermis.3
RELATED ARTICLE: Asymptomatic Lower Lip Hyperpigmentation From Laugier-Hunziker Syndrome
The differential diagnosis of multiple lentigines is broad and includes Peutz-Jeghers syndrome; LEOPARD (lentigines, electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of genitalia, retardation of growth, deafness) syndrome; Carney complexes, including LAMB (lentigines, atrial myxoma, mucocutaneous myxoma, blue nevi) and NAME (nevi, atrial myxoma, myxoid neurofibroma, ephelide) syndromes5; primary adrenocortical insufficiency (Addison disease); and idiopathic melanoplakia.2 Peutz-Jeghers syndrome, an autosomal-dominant syndrome with mucocutaneous lentigines, has a similar clinical appearance to LHS; therefore, it is necessary to exclude this diagnosis due to its association with intestinal hamartomatous polyps and internal malignancies (Table).3,6,7

Peutz-Jeghers syndrome is characterized by mucocutaneous hyperpigmentation and intestinal hamartomatous polyposis and is associated with internal malignancies of the colon, breast, pancreas, stomach, small intestines, ovaries, lung, and Sertoli cells in men.6,7 Associated gastrointestinal tract malignancies in descending order of frequency are colon (39%), pancreatic (36%), gastric (29%), and small intestine (13%).1 It is caused by a germ line mutation of the serine/threonine kinase 11 gene, STK11. Although the appearance and distribution of the mucocutaneous lentigines is similar to individuals with LHS, by contrast the lentiginosis in individuals with Peutz-Jeghers syndrome is present from birth or develops during infancy.6 Aggressive cancer screening guidelines aid in early detection and begin at 8 years of age with a baseline colonoscopy and esophagogastroduodenoscopy; future screening is dictated by the presence or absence of polyps. If no polyps are detected at 8 years of age, a colonoscopy and esophagogastroduodenoscopy are repeated at 18 years of age and then every 3 years until 50 years of age.8
In an adult patient, the diagnosis of LHS can be made clinically and a correct diagnosis prevents frequent and unpleasant gastrointestinal tract cancer screening examinations. Lampe et al2 described a man with LHS who was incorrectly diagnosed with Peutz-Jeghers syndrome and experienced a colonic perforation as a complication of a screening colonoscopy. Their case report underscores the importance of making the correct diagnosis of LHS to avoid undertaking unnecessary aggressive cancer screening regimens.2
Although LHS is a benign condition that does not require treatment, Q-switched alexandrite or erbium:YAG laser therapy has been shown to improve the pigmentary findings associated with LHS.9,10 It has been suggested that LHS should be renamed Laugier-Hunziker pigmentation2 or mucocutaneous lentiginosis of Laugier and Hunziker1 to differentiate LHS as simply a disorder of pigmentation rather than a potentially morbid genetic defect, as in Peutz-Jeghers syndrome.
To the Editor:
A 55-year-old man presented with hyperpigmented brown macules on the lips, hands, and fingertips of 6 years’ duration. The spots were persistent, asymptomatic, and had not changed in size. The patient denied a history of alopecia or dystrophic nails. He also denied a family history of similar skin findings. He had no personal history of cancer and a colonoscopy performed 5 years prior revealed no notable abnormalities. His medications included amlodipine and hydrocodone-acetaminophen. His mother died of “abdominal bleeding” at 74 years of age and his father died of a brain tumor at 64 years of age. Physical examination demonstrated numerous well-defined, dark brown macules of variable size distributed on the lower and upper mucosal lips (Figure 1A), buccal mucosa, hard palate, and gingiva, as well as the dorsal aspect of the fingers (Figure 1B) and volar aspect of the fingertips (Figure 1C).

A shave biopsy of a dark brown macule from the lower lip (Figure 2) was performed. Histopathologic examination revealed pigmentation of the basal layer of the epidermis with pigment-laden cells in the dermis immediately deep to the surface epithelium. Immunoperoxidase stains showed a normal number and distribution of melanocytes.

A diagnosis of Laugier-Hunziker syndrome (LHS) was made given the age of onset; distribution of pigmentation; and lack of pathologic colonoscopic findings, personal history of cancer, or gastrointestinal tract symptoms.
Benign hyperpigmentation of the lips and fingers has been reported.1 The average age of onset of LHS is 52 years, and it typically is diagnosed in white adults.1,2 In LHS, pigmentation is most commonly distributed on the lips, especially the lower lips and oral mucosa.2 Pigmentation of the nails in the form of longitudinal melanonychia is present in approximately half of cases.2,3 There also may be pigmentation of the neck; thorax; abdomen; and acral surfaces, especially the fingertips.1-3 Rarely, pigmented macules can occur on the genitalia or sclera.1,2 Unlike Peutz-Jeghers syndrome, the diagnosis of LHS does not result from a germline mutation and carries no risk of gastrointestinal polyposis or internal malignancy.3,4 The histopathology of a pigmented macule of LHS shows a normal number and morphology of melanocytes. Epidermal basement membrane pigmentation is common, with pigment-laden macrophages evident in the papillary dermis.3
RELATED ARTICLE: Asymptomatic Lower Lip Hyperpigmentation From Laugier-Hunziker Syndrome
The differential diagnosis of multiple lentigines is broad and includes Peutz-Jeghers syndrome; LEOPARD (lentigines, electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of genitalia, retardation of growth, deafness) syndrome; Carney complexes, including LAMB (lentigines, atrial myxoma, mucocutaneous myxoma, blue nevi) and NAME (nevi, atrial myxoma, myxoid neurofibroma, ephelide) syndromes5; primary adrenocortical insufficiency (Addison disease); and idiopathic melanoplakia.2 Peutz-Jeghers syndrome, an autosomal-dominant syndrome with mucocutaneous lentigines, has a similar clinical appearance to LHS; therefore, it is necessary to exclude this diagnosis due to its association with intestinal hamartomatous polyps and internal malignancies (Table).3,6,7

Peutz-Jeghers syndrome is characterized by mucocutaneous hyperpigmentation and intestinal hamartomatous polyposis and is associated with internal malignancies of the colon, breast, pancreas, stomach, small intestines, ovaries, lung, and Sertoli cells in men.6,7 Associated gastrointestinal tract malignancies in descending order of frequency are colon (39%), pancreatic (36%), gastric (29%), and small intestine (13%).1 It is caused by a germ line mutation of the serine/threonine kinase 11 gene, STK11. Although the appearance and distribution of the mucocutaneous lentigines is similar to individuals with LHS, by contrast the lentiginosis in individuals with Peutz-Jeghers syndrome is present from birth or develops during infancy.6 Aggressive cancer screening guidelines aid in early detection and begin at 8 years of age with a baseline colonoscopy and esophagogastroduodenoscopy; future screening is dictated by the presence or absence of polyps. If no polyps are detected at 8 years of age, a colonoscopy and esophagogastroduodenoscopy are repeated at 18 years of age and then every 3 years until 50 years of age.8
In an adult patient, the diagnosis of LHS can be made clinically and a correct diagnosis prevents frequent and unpleasant gastrointestinal tract cancer screening examinations. Lampe et al2 described a man with LHS who was incorrectly diagnosed with Peutz-Jeghers syndrome and experienced a colonic perforation as a complication of a screening colonoscopy. Their case report underscores the importance of making the correct diagnosis of LHS to avoid undertaking unnecessary aggressive cancer screening regimens.2
Although LHS is a benign condition that does not require treatment, Q-switched alexandrite or erbium:YAG laser therapy has been shown to improve the pigmentary findings associated with LHS.9,10 It has been suggested that LHS should be renamed Laugier-Hunziker pigmentation2 or mucocutaneous lentiginosis of Laugier and Hunziker1 to differentiate LHS as simply a disorder of pigmentation rather than a potentially morbid genetic defect, as in Peutz-Jeghers syndrome.
- Moore RT, Chae KA, Rhodes AR. Laugier and Hunziker pigmentation: a lentiginous proliferation of melanocytes. J Am Acad Dermatol. 2004;50(5 suppl):S70-S74.
- Lampe AK, Hampton PJ, Woodford-Richens K, et al. Laugier-Hunziker Syndrome: an important differential diagnosis for Peutz-Jeghers Syndrome. J Med Genet. 2003;40:E77.
- Baran R. Longitudinal melanotic streaks as a clue for Laugier-Hunziker syndrome. Arch Dermatol. 1979;115:1148-1149.
- Grimes P, Nordlund JJ, Pandya AG, et al. Increasing our understanding of pigmentary disorders. J Am Acad Dermatol. 2006;54(5 suppl 2):S255-S261.
- Bertherat J. Carney complex (CNC). Orphanet J Rare Dis. 2006;1:21.
- Giardiello FM, Brensinger JD, Tersemette AC, et al. Very high risk of cancer in Peutz-Jeghers Syndrome. Gastroenterology. 2000;119:1447-1453.
- Brosens LA, van Hattem WA, Jansen M, et al. Gastrointestinal polyposis syndromes. Curr Mol Med. 2007;7:29-46.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
- Zuo YG, Ma DL, Jin HZ, et al. Treatment of Laugier-Hunziker syndrome with the Q-switched alexandrite laser in 22 Chinese patients. Arch Dermatol Res. 2010;302:125-130.
- Ergun S, Saruhanog˘lu A, Migliari DA, et al. Refractory pigmentation associated with Laugier-Hunziker syndrome following Er:YAG laser treatment [published online December 3, 2013]. Case Rep Dent. 2013;2013:561040.
- Moore RT, Chae KA, Rhodes AR. Laugier and Hunziker pigmentation: a lentiginous proliferation of melanocytes. J Am Acad Dermatol. 2004;50(5 suppl):S70-S74.
- Lampe AK, Hampton PJ, Woodford-Richens K, et al. Laugier-Hunziker Syndrome: an important differential diagnosis for Peutz-Jeghers Syndrome. J Med Genet. 2003;40:E77.
- Baran R. Longitudinal melanotic streaks as a clue for Laugier-Hunziker syndrome. Arch Dermatol. 1979;115:1148-1149.
- Grimes P, Nordlund JJ, Pandya AG, et al. Increasing our understanding of pigmentary disorders. J Am Acad Dermatol. 2006;54(5 suppl 2):S255-S261.
- Bertherat J. Carney complex (CNC). Orphanet J Rare Dis. 2006;1:21.
- Giardiello FM, Brensinger JD, Tersemette AC, et al. Very high risk of cancer in Peutz-Jeghers Syndrome. Gastroenterology. 2000;119:1447-1453.
- Brosens LA, van Hattem WA, Jansen M, et al. Gastrointestinal polyposis syndromes. Curr Mol Med. 2007;7:29-46.
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
- Zuo YG, Ma DL, Jin HZ, et al. Treatment of Laugier-Hunziker syndrome with the Q-switched alexandrite laser in 22 Chinese patients. Arch Dermatol Res. 2010;302:125-130.
- Ergun S, Saruhanog˘lu A, Migliari DA, et al. Refractory pigmentation associated with Laugier-Hunziker syndrome following Er:YAG laser treatment [published online December 3, 2013]. Case Rep Dent. 2013;2013:561040.
Practice Points
- Laugier-Hunziker syndrome (LHS) comprises benign mucosal pigmentation in the absence of gastrointestinal pathology.
- Differentiating LHS from Peutz-Jeghers syndrome can prevent unnecessary aggressive cancer screening protocols.
- The average age of onset of LHS is 52 years and typically occurs in white adults.
- Pigmentation in LHS is most commonly distributed on the lower lips and oral mucosa.