User login
Comparison of Locked Plate Fixation and Nonoperative Management for Displaced Proximal Humerus Fractures in Elderly Patients
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
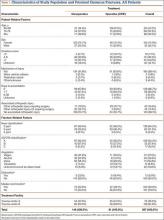
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
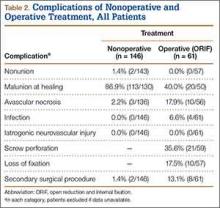
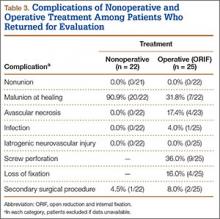
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
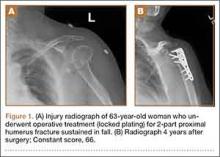
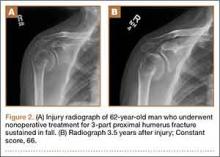
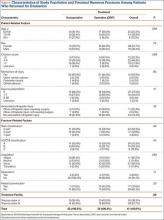
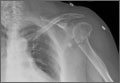
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
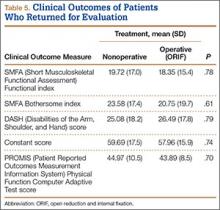
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Application of Epoxy Resin to a Solid-Foam Pelvic Model: Creating a Dry-Erase Pelvis
The value of preoperative planning and templating has been well-established in fracture surgery.1,2 Traditionally, this involves writing a surgical tactic and tracing the radiographs on paper to plan the ultimate reduction and implant placement.1 The advent of sophisticated computer programs has allowed electronic preoperative planning in trauma and arthroplasty surgery.3,4 Software for computer templating of acetabular fractures is available.5 Still, the renderings generated in these exercises are 2-dimensional, and their quality is somewhat dependent on the surgeon’s artistic ability. Ultimately, drawing a fracture is meant to help the surgeon understand its 3-dimensional (3-D) characteristics. This can be difficult working in 2 dimensions especially for bones, such as the pelvis, with complex 3-D structures. A useful alternative is to draw the fracture on a plastic-bone model.
We plan all acetabular fracture surgeries on 3-D models (plastic bones). These models are commonly provided to residents and fellows through educational courses or can be purchased online. Residents, fellows, and staff have their own models for planning, and we typically keep several models in the operating room for teaching before the surgery. Although these models are ideal for visualizing the bony anatomy, they are less than ideal for drawing fracture lines. Ink pens do not leave lines, and lines from markers and pencils cannot be easily erased. After a few planning sessions, the models typically look like a city map, making it difficult to tell the current fracture from those previously evaluated.
Here we describe a technique for turning standard plastic models into white boards so that lines can be drawn clearly with a marker and easily erased. To facilitate the correction of errors and reuse for future cases, we coat pelvic models with dry-erase epoxy resin. Although there is a commercially available product that has similar capabilities, our technique creates a significantly less expensive model that will likely be appealing to residents and fellows.
Technique
Throughout the process of creating the pelvic model, it is important to work in a well-ventilated area. Gloves should be worn at all times. The working surface should be protected with an impervious plastic sheet to avoid primer or epoxy soaking through.
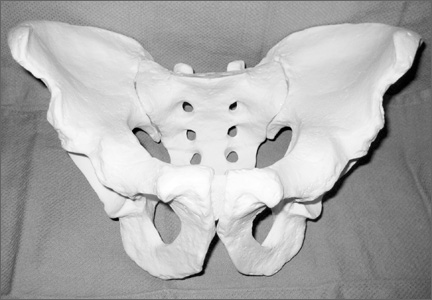
In creating our dry-erase pelvic models, we use the Sawbones large male solid-foam pelvic model (Figure 1; Model 1301, Pacific Research Laboratories, Vashon, Washington). These models are often available to residents and fellows after Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) trauma courses or resident educational sessions. Alternatively, they can be purchased online.
We sand the model to smooth out surface irregularities and to prepare it to accept primer. First, use 100-grit and then 220-grit sandpaper to create the optimum surface. We recommend suspending the pelvis from string by placing an eyelet screw into the top of the sacrum or looping a string through 1 of the sacral foramen while priming, painting, and curing. To prime the pelvic model, we have used KILZ original spray primer (Masterchem Industries, Imperial, Missouri). It is important that the entire model be well coated with primer because the epoxy resin will not adhere to unprimed plastic-foam surface. Take care to apply an even coat and to avoid drip formation.
Once the primer is completely dry, apply the dry-erase epoxy resin (Rust-Oleum, Vernon Hills, Illinois). Mix the 2 parts of the epoxy resin and apply to the pelvic model with a foam brush. It is important to cover the entire surface of the model with enough epoxy to give a smooth, even finish. This requires 2 to 3 coats applied approximately 30 minutes apart (the epoxy will remain wet but will take additional coats well).
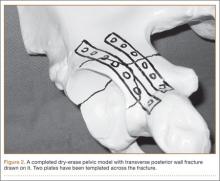
Once the final coat has been applied, the model takes about 48 hours to cure. Hang in a dry, well-ventilated location until it is fully cured. Then, use a dry-erase marker to trace fracture lines and the planned location of plates and screws (Figure 2).
An alternative to creating a dry-erase pelvis is to create a blackboard pelvis. Use Chalkboard Spray (Rust-Oleum) to create a surface that will accept white and colored chalk. The application of this product is much easier than the dry-erase epoxy, because it can be applied in a similar fashion as the spray primer. This creates a black model that can be marked with chalk. However, we have found these models to be less useful than the dry-erase versions, because chalk leaves less precise lines and is harder to remove from the model.
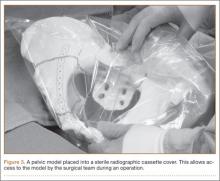
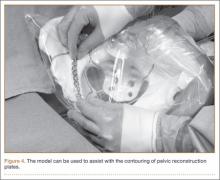
Once created, the pelvic model can be used intraoperatively to help understand fracture reduction and to facilitate the precontouring of pelvic and acetabular plates. We place the model in a sterile radiographic cassette bag (Figure 3). This gives the operating team access to the model and is useful in teaching anatomy, and particularly, screw placement. Further, the model allows an assistant to precontour plates to the model during the exposure portion of the case (Figure 4). While the precontoured plate is not always a perfect fit, it can usually be adjusted easily to fit the unique anatomy of the patient.
Discussion
Understanding the complex anatomy of pelvic and acetabular fractures can be challenging. We use models in the teaching of anatomy, in the interpretation of radiographs and computed tomography (CT) scans, and in preoperative planning. Fracture lines are traced on the pelvic model based on radiographs and/or CT scans and then compared with 3-D reconstruction images and, eventually, with operative findings. The use of our dry-erase models allows for easy correction of mistakes and reuse for further cases.
We have found dry-erase pelvic models to be an invaluable tool for resident and fellow education. While conventional 2-dimensional planning is adequate for most long-bone and periarticular fractures, the creation of these 3-D planning tools is useful in understanding the anatomy and surgical treatment of pelvic and acetabular fractures.
1. Reudi TP, Buckley R, Moran C. AO Principles of Fracture Management. New York, NY: Thieme; 2007.
2. Mast J, Jakob R, Ganz R. Planning & Reduction Techniques in Fracture Surgery. Berlin, Germany: Springer-Verlag; 2006.
3. Pilson HT, Reddix RN Jr, Mutty CE, Webb LX. The long lost art of preoperative planning—resurrected? Orthopedics. 2008;31(12):1238.
4. Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24(2):180-186.
5. Reddix RN Jr, Webb LX. Computer-assisted preoperative planning in the surgical treatment of acetabular fractures. J Surg Orthop Adv. 2007;16(3):138-143.
The value of preoperative planning and templating has been well-established in fracture surgery.1,2 Traditionally, this involves writing a surgical tactic and tracing the radiographs on paper to plan the ultimate reduction and implant placement.1 The advent of sophisticated computer programs has allowed electronic preoperative planning in trauma and arthroplasty surgery.3,4 Software for computer templating of acetabular fractures is available.5 Still, the renderings generated in these exercises are 2-dimensional, and their quality is somewhat dependent on the surgeon’s artistic ability. Ultimately, drawing a fracture is meant to help the surgeon understand its 3-dimensional (3-D) characteristics. This can be difficult working in 2 dimensions especially for bones, such as the pelvis, with complex 3-D structures. A useful alternative is to draw the fracture on a plastic-bone model.
We plan all acetabular fracture surgeries on 3-D models (plastic bones). These models are commonly provided to residents and fellows through educational courses or can be purchased online. Residents, fellows, and staff have their own models for planning, and we typically keep several models in the operating room for teaching before the surgery. Although these models are ideal for visualizing the bony anatomy, they are less than ideal for drawing fracture lines. Ink pens do not leave lines, and lines from markers and pencils cannot be easily erased. After a few planning sessions, the models typically look like a city map, making it difficult to tell the current fracture from those previously evaluated.
Here we describe a technique for turning standard plastic models into white boards so that lines can be drawn clearly with a marker and easily erased. To facilitate the correction of errors and reuse for future cases, we coat pelvic models with dry-erase epoxy resin. Although there is a commercially available product that has similar capabilities, our technique creates a significantly less expensive model that will likely be appealing to residents and fellows.
Technique
Throughout the process of creating the pelvic model, it is important to work in a well-ventilated area. Gloves should be worn at all times. The working surface should be protected with an impervious plastic sheet to avoid primer or epoxy soaking through.
In creating our dry-erase pelvic models, we use the Sawbones large male solid-foam pelvic model (Figure 1; Model 1301, Pacific Research Laboratories, Vashon, Washington). These models are often available to residents and fellows after Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) trauma courses or resident educational sessions. Alternatively, they can be purchased online.
We sand the model to smooth out surface irregularities and to prepare it to accept primer. First, use 100-grit and then 220-grit sandpaper to create the optimum surface. We recommend suspending the pelvis from string by placing an eyelet screw into the top of the sacrum or looping a string through 1 of the sacral foramen while priming, painting, and curing. To prime the pelvic model, we have used KILZ original spray primer (Masterchem Industries, Imperial, Missouri). It is important that the entire model be well coated with primer because the epoxy resin will not adhere to unprimed plastic-foam surface. Take care to apply an even coat and to avoid drip formation.
Once the primer is completely dry, apply the dry-erase epoxy resin (Rust-Oleum, Vernon Hills, Illinois). Mix the 2 parts of the epoxy resin and apply to the pelvic model with a foam brush. It is important to cover the entire surface of the model with enough epoxy to give a smooth, even finish. This requires 2 to 3 coats applied approximately 30 minutes apart (the epoxy will remain wet but will take additional coats well).
Once the final coat has been applied, the model takes about 48 hours to cure. Hang in a dry, well-ventilated location until it is fully cured. Then, use a dry-erase marker to trace fracture lines and the planned location of plates and screws (Figure 2).
An alternative to creating a dry-erase pelvis is to create a blackboard pelvis. Use Chalkboard Spray (Rust-Oleum) to create a surface that will accept white and colored chalk. The application of this product is much easier than the dry-erase epoxy, because it can be applied in a similar fashion as the spray primer. This creates a black model that can be marked with chalk. However, we have found these models to be less useful than the dry-erase versions, because chalk leaves less precise lines and is harder to remove from the model.
Once created, the pelvic model can be used intraoperatively to help understand fracture reduction and to facilitate the precontouring of pelvic and acetabular plates. We place the model in a sterile radiographic cassette bag (Figure 3). This gives the operating team access to the model and is useful in teaching anatomy, and particularly, screw placement. Further, the model allows an assistant to precontour plates to the model during the exposure portion of the case (Figure 4). While the precontoured plate is not always a perfect fit, it can usually be adjusted easily to fit the unique anatomy of the patient.
Discussion
Understanding the complex anatomy of pelvic and acetabular fractures can be challenging. We use models in the teaching of anatomy, in the interpretation of radiographs and computed tomography (CT) scans, and in preoperative planning. Fracture lines are traced on the pelvic model based on radiographs and/or CT scans and then compared with 3-D reconstruction images and, eventually, with operative findings. The use of our dry-erase models allows for easy correction of mistakes and reuse for further cases.
We have found dry-erase pelvic models to be an invaluable tool for resident and fellow education. While conventional 2-dimensional planning is adequate for most long-bone and periarticular fractures, the creation of these 3-D planning tools is useful in understanding the anatomy and surgical treatment of pelvic and acetabular fractures.
The value of preoperative planning and templating has been well-established in fracture surgery.1,2 Traditionally, this involves writing a surgical tactic and tracing the radiographs on paper to plan the ultimate reduction and implant placement.1 The advent of sophisticated computer programs has allowed electronic preoperative planning in trauma and arthroplasty surgery.3,4 Software for computer templating of acetabular fractures is available.5 Still, the renderings generated in these exercises are 2-dimensional, and their quality is somewhat dependent on the surgeon’s artistic ability. Ultimately, drawing a fracture is meant to help the surgeon understand its 3-dimensional (3-D) characteristics. This can be difficult working in 2 dimensions especially for bones, such as the pelvis, with complex 3-D structures. A useful alternative is to draw the fracture on a plastic-bone model.
We plan all acetabular fracture surgeries on 3-D models (plastic bones). These models are commonly provided to residents and fellows through educational courses or can be purchased online. Residents, fellows, and staff have their own models for planning, and we typically keep several models in the operating room for teaching before the surgery. Although these models are ideal for visualizing the bony anatomy, they are less than ideal for drawing fracture lines. Ink pens do not leave lines, and lines from markers and pencils cannot be easily erased. After a few planning sessions, the models typically look like a city map, making it difficult to tell the current fracture from those previously evaluated.
Here we describe a technique for turning standard plastic models into white boards so that lines can be drawn clearly with a marker and easily erased. To facilitate the correction of errors and reuse for future cases, we coat pelvic models with dry-erase epoxy resin. Although there is a commercially available product that has similar capabilities, our technique creates a significantly less expensive model that will likely be appealing to residents and fellows.
Technique
Throughout the process of creating the pelvic model, it is important to work in a well-ventilated area. Gloves should be worn at all times. The working surface should be protected with an impervious plastic sheet to avoid primer or epoxy soaking through.
In creating our dry-erase pelvic models, we use the Sawbones large male solid-foam pelvic model (Figure 1; Model 1301, Pacific Research Laboratories, Vashon, Washington). These models are often available to residents and fellows after Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) trauma courses or resident educational sessions. Alternatively, they can be purchased online.
We sand the model to smooth out surface irregularities and to prepare it to accept primer. First, use 100-grit and then 220-grit sandpaper to create the optimum surface. We recommend suspending the pelvis from string by placing an eyelet screw into the top of the sacrum or looping a string through 1 of the sacral foramen while priming, painting, and curing. To prime the pelvic model, we have used KILZ original spray primer (Masterchem Industries, Imperial, Missouri). It is important that the entire model be well coated with primer because the epoxy resin will not adhere to unprimed plastic-foam surface. Take care to apply an even coat and to avoid drip formation.
Once the primer is completely dry, apply the dry-erase epoxy resin (Rust-Oleum, Vernon Hills, Illinois). Mix the 2 parts of the epoxy resin and apply to the pelvic model with a foam brush. It is important to cover the entire surface of the model with enough epoxy to give a smooth, even finish. This requires 2 to 3 coats applied approximately 30 minutes apart (the epoxy will remain wet but will take additional coats well).
Once the final coat has been applied, the model takes about 48 hours to cure. Hang in a dry, well-ventilated location until it is fully cured. Then, use a dry-erase marker to trace fracture lines and the planned location of plates and screws (Figure 2).
An alternative to creating a dry-erase pelvis is to create a blackboard pelvis. Use Chalkboard Spray (Rust-Oleum) to create a surface that will accept white and colored chalk. The application of this product is much easier than the dry-erase epoxy, because it can be applied in a similar fashion as the spray primer. This creates a black model that can be marked with chalk. However, we have found these models to be less useful than the dry-erase versions, because chalk leaves less precise lines and is harder to remove from the model.
Once created, the pelvic model can be used intraoperatively to help understand fracture reduction and to facilitate the precontouring of pelvic and acetabular plates. We place the model in a sterile radiographic cassette bag (Figure 3). This gives the operating team access to the model and is useful in teaching anatomy, and particularly, screw placement. Further, the model allows an assistant to precontour plates to the model during the exposure portion of the case (Figure 4). While the precontoured plate is not always a perfect fit, it can usually be adjusted easily to fit the unique anatomy of the patient.
Discussion
Understanding the complex anatomy of pelvic and acetabular fractures can be challenging. We use models in the teaching of anatomy, in the interpretation of radiographs and computed tomography (CT) scans, and in preoperative planning. Fracture lines are traced on the pelvic model based on radiographs and/or CT scans and then compared with 3-D reconstruction images and, eventually, with operative findings. The use of our dry-erase models allows for easy correction of mistakes and reuse for further cases.
We have found dry-erase pelvic models to be an invaluable tool for resident and fellow education. While conventional 2-dimensional planning is adequate for most long-bone and periarticular fractures, the creation of these 3-D planning tools is useful in understanding the anatomy and surgical treatment of pelvic and acetabular fractures.
1. Reudi TP, Buckley R, Moran C. AO Principles of Fracture Management. New York, NY: Thieme; 2007.
2. Mast J, Jakob R, Ganz R. Planning & Reduction Techniques in Fracture Surgery. Berlin, Germany: Springer-Verlag; 2006.
3. Pilson HT, Reddix RN Jr, Mutty CE, Webb LX. The long lost art of preoperative planning—resurrected? Orthopedics. 2008;31(12):1238.
4. Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24(2):180-186.
5. Reddix RN Jr, Webb LX. Computer-assisted preoperative planning in the surgical treatment of acetabular fractures. J Surg Orthop Adv. 2007;16(3):138-143.
1. Reudi TP, Buckley R, Moran C. AO Principles of Fracture Management. New York, NY: Thieme; 2007.
2. Mast J, Jakob R, Ganz R. Planning & Reduction Techniques in Fracture Surgery. Berlin, Germany: Springer-Verlag; 2006.
3. Pilson HT, Reddix RN Jr, Mutty CE, Webb LX. The long lost art of preoperative planning—resurrected? Orthopedics. 2008;31(12):1238.
4. Unnanuntana A, Wagner D, Goodman SB. The accuracy of preoperative templating in cementless total hip arthroplasty. J Arthroplasty. 2009;24(2):180-186.
5. Reddix RN Jr, Webb LX. Computer-assisted preoperative planning in the surgical treatment of acetabular fractures. J Surg Orthop Adv. 2007;16(3):138-143.