User login
Is There an Association Between Hidradenitis Suppurativa and Fibromyalgia?
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).
In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).
In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).
In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
Practice Point
- Although fibromyalgia does not occur more frequently in hidradenitis suppurativa (HS) patients, it is important to recognize that HS patients can have comorbidities that should be addressed when possible to improve overall quality of life.
Clinical Characterization of Leukemia Cutis Presentation
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
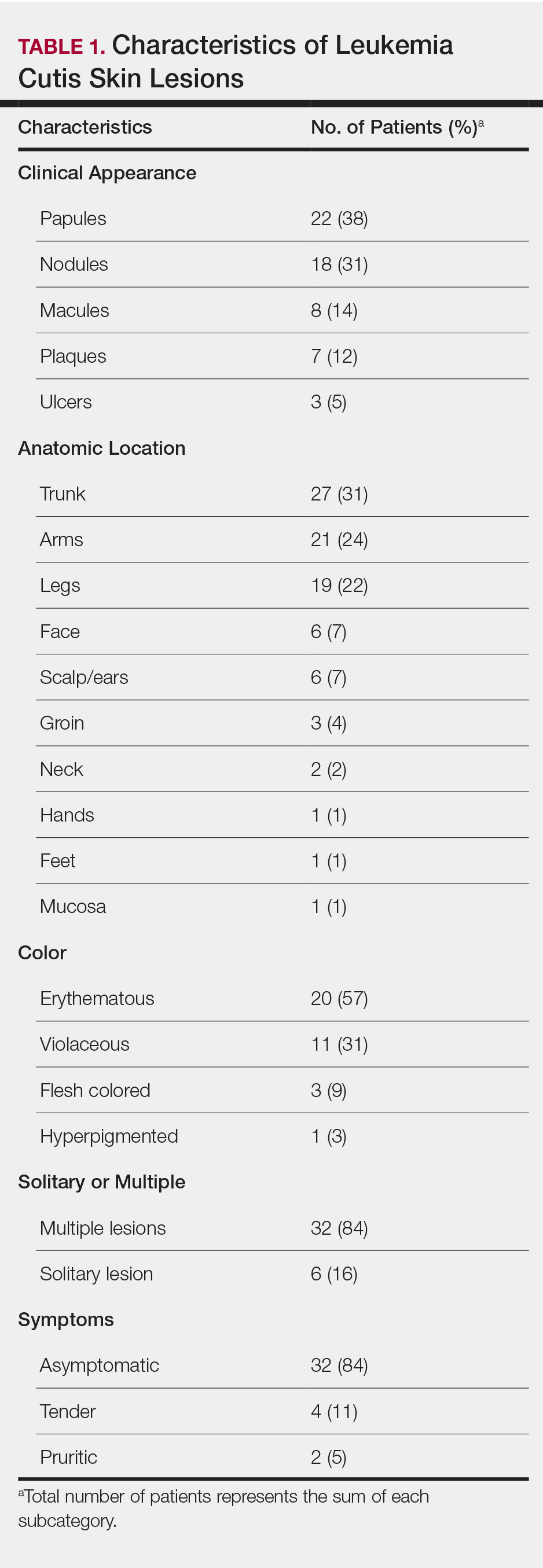
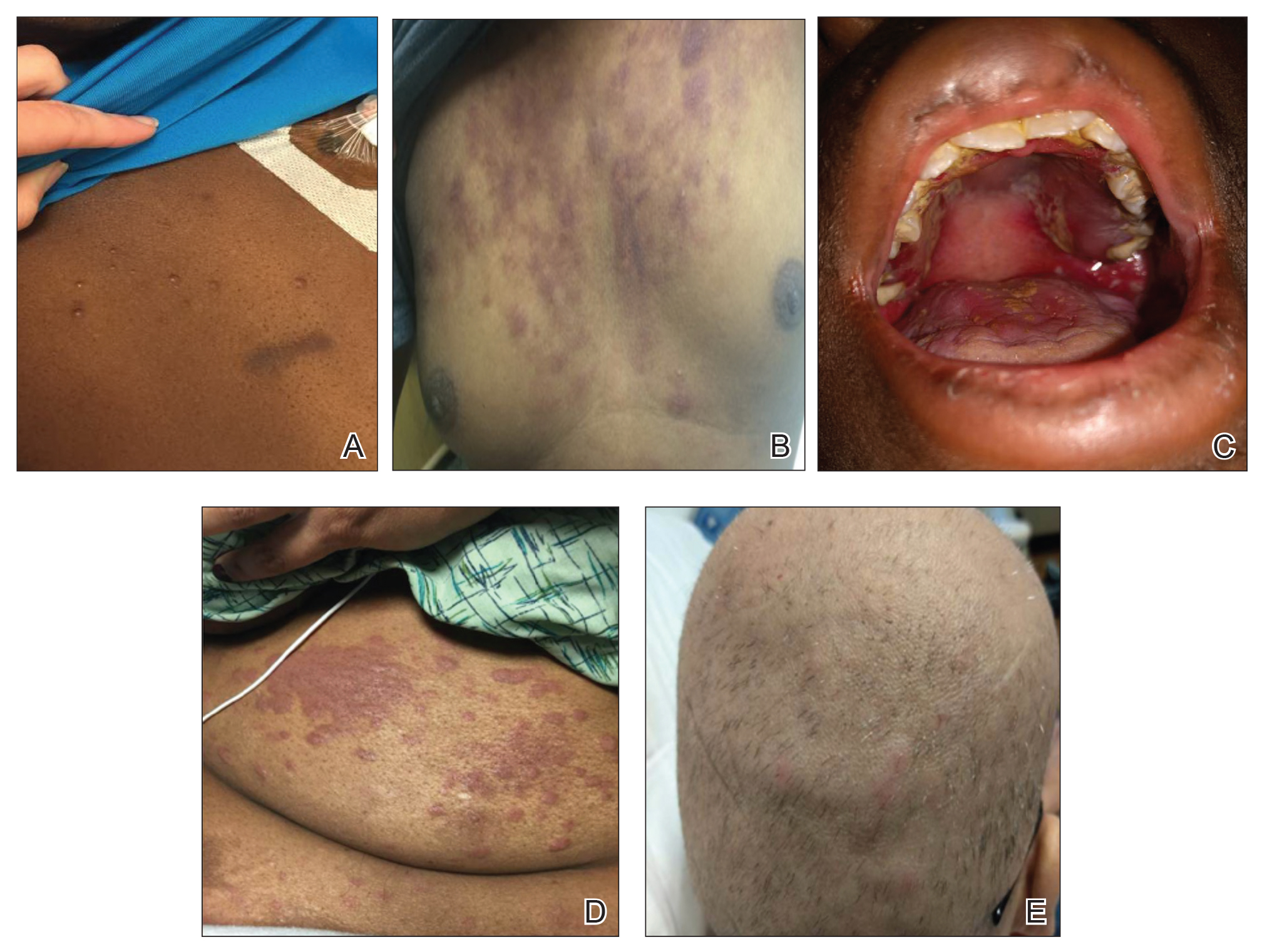
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.
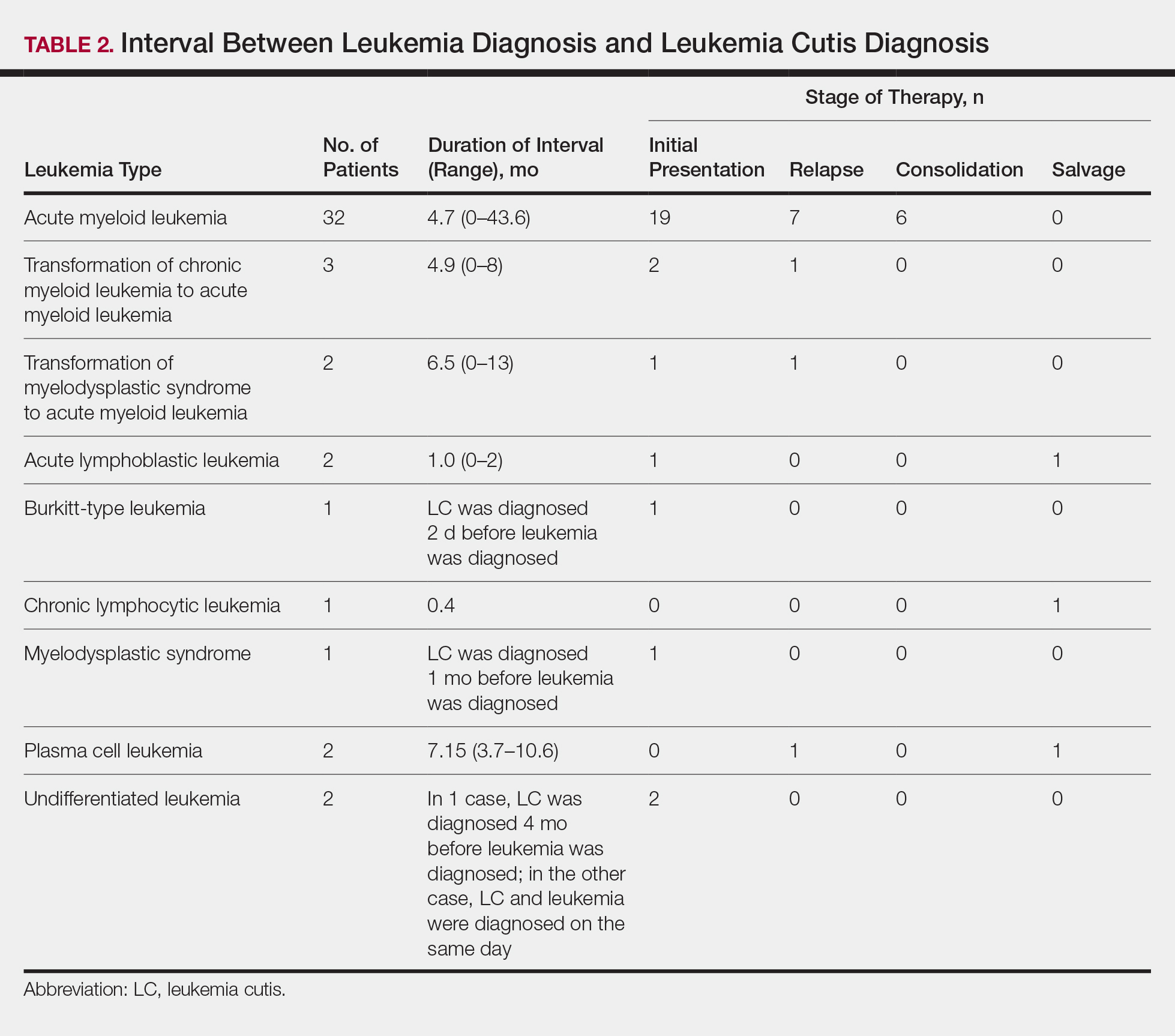
Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.
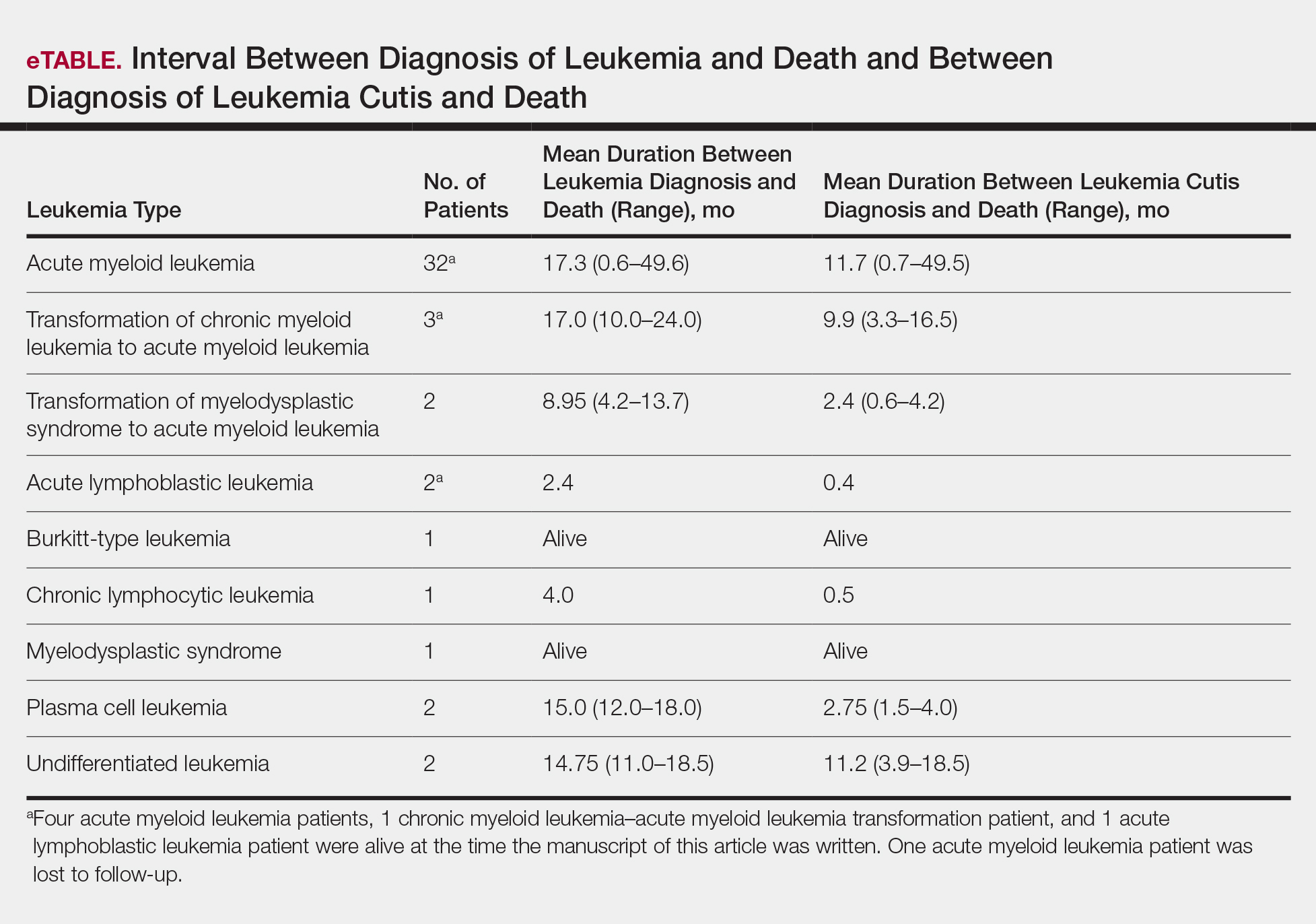
Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).
Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.
Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.
Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).
Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
Leukemia is a malignant, life-threatening neoplasm affecting the hematopoietic system. Extramedullary manifestations can occur in various organs, including skin.1 Skin findings in leukemia patients are common and varied, including pallor secondary to anemia, petechiae or ecchymoses due to thrombocytopenia, and skin manifestations of neutropenia and chemotherapy.2 When patients with leukemia develop skin lesions without leukemic infiltration, the resulting nonspecific cutaneous manifestations are known as leukemids.3 Specific cutaneous manifestations of leukemia resulting from direct invasion of leukemic cells into the epidermis, dermis, or subcutis are referred to as leukemia cutis (LC).2,3
Acute myeloid leukemia (AML) is the most common type of leukemia associated with LC, but LC also is seen in other leukemias with various frequencies.1 The lesions of LC can present anywhere on skin, though it has been reported that LC has a tendency to occur at sites of prior ongoing inflammation,2,4 most commonly the extremities, trunk, and face.2,5,6 LC lesions have a range of morphological findings and most commonly present as nodules, papules, and plaques.1,7
Most reports of LC in the literature are case reports or case series with small numbers of subjects.3,6,8 A study of LC patients (N=75) in Korea by Kang et al7 has been the only one to analyze clinical characteristics of LC since 2000.
The aim of this study was to further contribute to the knowledge of clinical characteristics of LC. Clinical patterns of 46 patients were analyzed to further characterize the presentation of LC and to compare our results with those in the literature.
Methods
We conducted a single-institution retrospective review of medical records of patients with LC diagnosed in the Department of Dermatology at Wake Forest School of Medicine (Winston-Salem, North Carolina) over a 17-year period (2001-2017). The study protocol was approved by the institutional review board of Wake Forest University School of Medicine (IRB No. 00054474). Patients had a leukemia diagnosis established by bone marrow biopsy. Patients were included in this analysis if they had ongoing active leukemia and a skin biopsy consistent with LC. Patients of all sexes and ages were included in the cohort. Patients were excluded if they presented only with nonspecific cutaneous lesions associated with leukemia (leukemids). After removing duplicate records from a total of 60 patients initially identified, 46 unique patients were included in this study.
Results
Demographics
Fifty-six percent (26/46) of patients were male. The average age at diagnosis of leukemia was 58 years (range, 8.5 months–84 years). Eighty-five percent of patients were white (39/46), 11% were black (5/46), 2% were Hispanic (1/46), and 2% were of unknown ethnicity (1/46).
Eighty percent (37/46) of patients with LC had AML; 3 of these patients had a prior diagnosis of chronic myeloid leukemia (CML) and 2 had myelodysplastic syndrome (MDS) that did not develop LC until after they had transitioned to AML. Other subtypes of leukemia in this patient population included acute lymphoblastic leukemia (ALL)(n=2), plasma cell leukemia (PCL)(n=2), undifferentiated leukemia (n=2), chronic lymphocytic leukemia (CLL)(n=1), myelodysplastic syndrome (n=1), and Burkitt-type leukemia (n=1).
Distribution and Morphology of LC Lesions
The clinical appearance of LC was widely variable in morphology and anatomic location (Table 1 and Figure). Eighty-four percent of LC occurrences involved more than one lesion (n=32); 14% were a solitary lesion (n=6). For the 2 patients who had 2 separate episodes of LC, the initial presentation of LC was multiple lesions; recurrent LC at relapse presented as a solitary lesion in both cases. Most LC lesions (77% [67/87]) occurred on the trunk or extremities; 23% (20/87) of LC lesions occurred on less common sites, such as the groin, face, hands, feet, and mucosa. Papules (38% [22/58]) and nodules (31% [18/58]) were the most common morphology; macules, plaques, and ulcers were observed less frequently. Clinical descriptions of LC lesions varied widely, with the most common descriptive characteristics being erythematous (57% [20/35]), violaceous (31% [11/35]), and asymptomatic (84% [32/38]). Rare descriptors included flesh colored, hyperpigmented, tender, pruritic, edema, crusting, and confluent erythematous.
Interval Between Leukemia Diagnosis and LC Diagnosis
Approximately 59% (n=27) of patients had LC as a presenting finding of their leukemia (Table 2). Twenty-two percent (n=10) developed LC at the time of leukemia relapse; 20% (n=9) developed LC during consolidation or salvage chemotherapy. Two AML patients had recurrent episodes of LC both at initial presentation of leukemia and when AML relapsed. Two other AML patients received a diagnosis of LC at the same time as a negative concurrent bone marrow biopsy (ie, aleukemic LC). Mean duration between diagnosis of leukemia and diagnosis of LC was 0.4 months (CLL), 1.0 month (ALL), 4.7 months (AML), and 7.15 months (PCL). In cases of MDS and CML transformation to AML, the interval was 6.5 and 4.9 months, respectively.
Interval Between LC Diagnosis and Death
As a whole, 17% (n=8) of patients were living at the time this article was written (eTable). Of patients who are still living, 10.9% (n=5) have AML. Looking at the cohort of patients with AML and LC, average age at AML diagnosis was 59.8 years. Average time from diagnosis of leukemia to death was 17.3 months (range, 0.6–49.6 months) for AML; 17.0 months (range, 10.0–24.0 months) for CML transformation to AML; 15.0 months (range, 12.0–18.0 months) for PCL; 14.75 months (range, 11.0–18.5 months) for undifferentiated leukemia; and 8.95 months (range, 4.2–13.7 months) for MDS transformation to AML. The interval between leukemia diagnosis and death was notably shorter for the CLL patient (4.0 months) and the deceased ALL patient (2.4 months). Mean duration between LC diagnosis and death was 11.7 months (AML), 11.2 months (undifferentiated leukemia), 9.9 months (CML transformation to AML), 2.75 months (PCL), and 2.4 months (MDS transformation to AML). The shortest intervals between LC diagnosis and death were seen in CLL (0.5 months) and ALL (0.4 months).
Discussion
Cutaneous manifestations are not uncommon in leukemia patients and can have a number of causes, including paraneoplastic cutaneous manifestations, such as pyoderma gangrenosum and Sweet syndrome; infection; cutaneous toxicities from antineoplastic agents; and LC.2 Leukemia cutis can be confused with other skin lesions in leukemia patients; diagnosis requires biopsy.2,9
We analyzed clinical characteristics and prognosis of 46 patients with LC over a 17-year period. To the best of our knowledge, this is the largest study of LC patients published in the United States. A similar study by Kang et al7 analyzed 75 patients in Korea; however, the incidence of LC among different types of leukemia in the Korean population cannot be applied to Western countries. We did compare the clinical characteristics of our cohort of patients to those reported by Kang et al7 and other studies including a smaller number of patients.
In this study, the male to female ratio was 1.3 to 1 compared to the 2:1 ratio reported by Kang et al.7 The mean age of leukemia diagnosis among our patients was 58 years, which is notably older than the mean age previously reported.7 In this cohort, 4 patients (8.7%) were 34 years or younger, including 1 infant aged 8.5 months; 24 (52.2%) were aged 35 to 64 years; and 18 (39.1%) were 65 years and older.
Consistent with other studies,2,5,7 the most common type of leukemia in patients who developed LC was AML (80%). Among AML patients, the mean age at AML diagnosis (59.8 years) was notably younger than the reported US average age of patients who had a diagnosis of AML (68 years).10 Gender breakdown was slightly different than US statistics: 63% of AML patients in our group were male, whereas AML is only slightly more common among men in the United States.10
Clinically, skin lesions observed most commonly were (in decreasing order) papules, nodules, macules, plaques, and ulcers. Papules (38%) were the most common lesion overall in our study, which differed from the Kang et al7 report in which nodules were the most common. Nodules (31%) were the second most common LC morphology among our patients. Among AML patients, papules were seen in 56% of patients (18/32); nodules were seen in 44% (14/32). The extremities (when combined together) were the most common location of LC lesions (46% [arms, 24%; legs, 22%]); the trunk was the second most common body region (31%). Our study did not find a difference among most common LC anatomic sites compared to other studies.5,7 Less common sites in our cohort included the head, scalp/ears, neck, hands, mucosa, and feet. All body sites were represented, including ocular and oral mucosa and groin, a finding that underscores the importance of complete and comprehensive skin examinations in this patient population. The terms erythematous and violaceous were used to describe the color of most lesions (88%), which commonly presented as multiple lesions (84%) and often were asymptomatic (84%).
It has been reported that, first, in most cases of LC, the condition develops in patients who have already been given a diagnosis of leukemia and, second, simultaneous manifestation of systemic leukemia and LC is less common.11,12 Leukemia cutis also can precede peripheral or bone marrow leukemia (known as aleukemic LC).1,13 Two AML patients (4.3% [2/46]) in this study met criteria for aleukemic LC because they had LC at the same time as negative bone marrow biopsy, which is consistent with a prior report that aleukemic LC can affect as many as 7% of patients.1 Our results differed slightly from prior studies in that most of our patients had LC as one of the presenting manifestations of their leukemia.3,7
Regardless of leukemia type, patients were likely to die within 1 year of LC diagnosis, on average, which is consistent with prior reports.7,11,12 However, the time between diagnosis of LC and death varied greatly among our patients (range, 12 days to 4.1 years). From 2007 to 2013, the 5-year relative survival rate overall for leukemia patients in the US population (by type) was 86.2% (CLL), 71.0% (ALL), 68.0% (CML), and 27.4% (AML).14 Compared to these national statistics, the relative survival rate in LC is poor, with patients who have AML surviving, on average, less than 8 months from time of leukemia diagnosis, whereas ALL and CLL patients survive less than 6 months.
When LC is a late presentation of B-cell CLL or when it presents as myeloid leukemia, blastic transformation (Richter syndrome), or T-cell CLL, it is occasionally associated with poor prognosis, though LC does not affect survival.15-17 In a study of the association of LC with survival in AML, 5-year survival among 62 AML patients with LC was 8.6%, shorter than 28.3% among the 186 matched patients with AML without LC.18 Similarly, the estimated 5-year survival for all patients with AML, according to Surveillance, Epidemiology, and End Results Program data (2007-2013), was 27.4%.14 Based on those results, LC might be a good prognostic indicator in patients with AML.
Conclusion
This study characterized the clinical presentation of LC, which is highly variable in appearance, symptoms, distribution, and stage of leukemia at presentation. In our study cohort, LC most commonly presented as asymptomatic erythematous or violaceous papules or nodules in older male AML patients at leukemia diagnosis. Given such wide variability, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy. Complete and thorough skin examinations should be performed on leukemia patients throughout the course of their disease to identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359-365.
- Martínez-Leboráns L, Victoria-Martínez A, Torregrosa-Calatayu JL, et al. Leukemia cutis: a report of 17 cases and a review of literature. Actas Dermosifiliogr. 2016;107:e65-e69.
- Li L, Wang Y, Lian CG, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216-221.
- Paydas¸ S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193-196.
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614-619.
- Stern M, Halter J, Buser A, et al. Leukemia cutis preceding systemic relapse of acute myeloid leukemia. Int J Hematol. 2008;87:108-109.
- Patel LM, Maghari A, Schwartz RA, et al. Myeloid leukemia cutis in the setting of myelodysplastic syndrome: a crucial dermatological diagnosis. Int J Dermatol. 2012;51:383-388.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed November 21, 2019.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Barzilai A, Lyakhovitsky A, Goldberg I, et al. Aleukemic monocytic leukemia cutis. Cutis. 2002;69:301-304
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review (CSR) 1975-2014. Bethesda, MD: National Cancer Institute; April 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed November 21, 2019.
- Cerroni L, Zenahlik P, Höfler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187-188.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419-431.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826-832.
Practice Points
- Complete and comprehensive skin examination is important in leukemia patients, as leukemia cutis (LC) lesions can present in all body sites including ocular and oral mucosa as well as the groin.
- Given the wide variability in appearance, symptoms, distribution, and stage of leukemia at presentation, dermatologists and oncologists need to keep LC in the differential diagnosis for any new skin lesion and to have a low threshold for performing skin biopsy.
- Performing thorough skin examination on leukemia patients throughout the course of their disease may help identify LC early so that treatment can be implemented in a timely fashion at initial diagnosis, first sign of relapse, or change in disease state.
How Do Drug Shortages Affect Dermatologists?
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.