Article
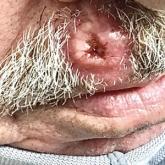
Ulcerated Nodule on the Lip
- Author:
- Georgeanne Cornell, DO
- Wei Su, MD
- John Moesch, DO
A 79-year-old man with a medical history of type 2 diabetes mellitus, hypothyroidism, and atrial fibrillation presented with an enlarging lesion...
Article

Granulomatous Pigmented Purpuric Dermatosis
- Author:
- Adam Allan, DO
- David A. Altman, MD
- Wei Su, MD
Granulomatous pigmented purpuric dermatosis (GPPD) is a rare histologic variant of pigmented purpuric dermatosis (PPD). It includes classic...
