Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020. 1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities. 3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV. 5,6
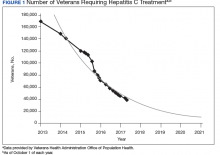
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
To date, more than 77,000 veterans have a documented sustained virologic response (SVR) with DAAs 12 weeks after treatment completion (SVR12) (VHA Population Health Service, personal communication, February 15, 2018).Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services. 5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.