Article
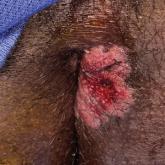
Painful Fungating Perianal Mass
A 21-year-old man presented to our clinic with rectal pain of 2 months’ duration that occurred in association with bowel movements and rectal...
From the Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
The authors report no conflict of interest.
Correspondence: Mark C. Marchitto, MD, Johns Hopkins University School of Medicine, Department of Dermatology, Baltimore, MD 21287 (mmarchi3@jhmi.edu).
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9

A 21-year-old man presented to our clinic with rectal pain of 2 months’ duration that occurred in association with bowel movements and rectal...
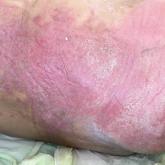
A 68-year-old man presented with an extensive erythematous plaque of 3 weeks’ duration that started in the groin and spread to the buttocks. He...
