DIABETIC MEDICATION DOSING FOR CKD
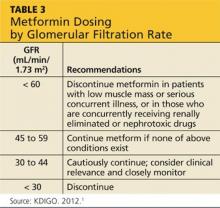
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>