Clinical Review

Richard Schreckengaust, MD, Joseph P. Lang, MD, Francis L. Counselman, MD, CPE
Richard Schreckengaust is an emergency physician in the Department of Emergency Medicine at Camp Lejune, North Carolina. Joseph P. Lang is an Assistant Professor in the Department of Emergency Medicine at Eastern Virginia Medical School (EVMS) and practices at Emergency Physicians of Tidewater, both in Norfolk. Francis L. Counselman is the Distinguished Professor and Chairman of the Department of Emergency Medicine at EVMS and practices at Emergency Physicians of Tidewater. This article originally appeared in Emergency Medicine (2014;46[12]:558-561).
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]) sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, then switched to 2 L of O2 via nasal cannula. He was observed for a total of four hours after arrival; as he remained symptom-free, he was discharged home. Postdischarge follow-up information was not obtainable.
DISCUSSION
Carbon dioxide is prevalent in everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas.
Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicologic effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an eight-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Continue for other case presentations >>

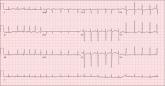
A 56-year-old man presents to the emergency department (ED) complaining of shortness of breath and a rapid heart rate. He went to bed at his...

A 78-year-old woman presents to your urgent care clinic with a four-day history of lethargy. She lives in another state but currently is visiting...
