The dermatoses of pregnancy are a poorly understood group of conditions. Their only common feature is a tendency to appear during pregnancy.
Only three of these conditions are considered unique to pregnancy, however; the others are probably exacerbations of preexisting conditions triggered by pregnancy. There isn’t even complete agreement on what to call them. To make management even more complex, two patients—mother and fetus—need to be considered in decisions about care.
Who manages these patients is another matter. These conditions fall into overlapping areas of health care, where family physicians, obstetricians, and dermatologists all might have some share in responsibility for diagnosis and treatment. You need to be sufficiently familiar with these conditions so that you can differentiate those that can be treated symptomatically and those that require referral to a specialist. This review and the handy TABLE, will help you toward that end.
TABLE
Skin disorders of pregnancy: What you’ll see, how to treat
| Disorder | Lesions | Diagnosis and sequelae | Treatment | Recurrence |
|---|---|---|---|---|
| Pemphigoid gestationis3,5 | Erythematous papules that progress to vesicles and bullae, in a periumbilical distribution that spares the face, palms, and soles |
|
| Frequent; skips a pregnancy 8% of the time |
| Pruritic urticarial papules and plaques of pregnancy8-10 | Urticarial papules and plaques on the abdomen, legs, arms, buttocks, chest, and back |
| Topical corticosteroids and antihistamines | Uncommon |
| Intrahepatic cholestasis of pregnancy14,17,19-22 | No primary lesions; secondary excoriations in any area that the patient can reach |
| Ursodeoxycholic acid, 450-1,200 mg/d | Frequent |
| Eczema of pregnancy/pruritus of pregnancy4,10,24 | Grouped, crusted erythematous papules, patches, and plaques, most often on extensor surfaces of the arms and legs or on the abdomen |
| Symptomatic treatment with topical corticosteroids or antihistamines | Frequent |
| Acute pustular psoriasis of pregnancy26-28 | Erythematous plaques and pustules that start on the inner thighs and groin and spread to the trunk and extremities |
|
| Unknown |
| Pruritic folliculitis of pregnancy24,28 | Papules and pustules concentrated around hair follicles, often beginning on the abdomen and spreading to the extremities |
| Topical corticosteroids | Unknown |
DERMATOSES UNIQUE TO PREGNANCY
1. Pemphigoid gestationis
Years ago, this disorder was referred to as herpes gestationis, because the lesions are herpetiform. Pemphigoid gestationis (PG) has an incidence of approximately 1 in 10,000 pregnancies.1,2 Time of onset is usually about the 21st week of gestation, although, in about 20% of cases, the eruption appears immediately postpartum.3
Presentation. The disease usually begins with urticarial papules and plaques around the umbilicus and extremities. Bullous lesions tend to develop as the disease progresses, and are often not present on first presentation (FIGURE 1). Lesions of PG tend to spare the face, palms, and soles. Mucosal surfaces are involved in fewer than 20% of cases. In about 75% of cases, PG flares around the time of delivery, regressing spontaneously after the baby is born.4
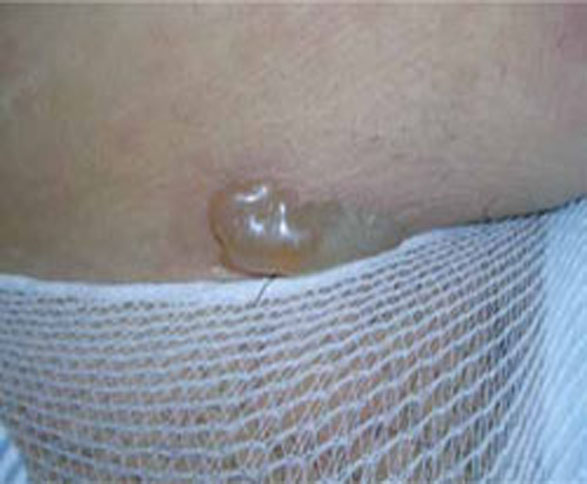
FIGURE 1 Pemphigoid gestationis
As the disease progresses, bullous lesions tend to develop.
Pathophysiology. The pathophysiology of PG is nearly identical to that of bullous pemphigoid, a blistering skin disorder seen more often in elderly patients.5 Pemphigoid disorders are immune processes, involving an immunoglobulin G (IgG) immune response directed at a 180-kDa hemidesmosome transmembrane glycoprotein. This protein is the common target in several subepidermal blistering diseases.
Differential diagnosis. Disorders that may have some of the same features as PG include pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, intrahepatic cholestasis of pregnancy (ICP), contact dermatitis, and drug reactions.
Diagnosis. A biopsy is necessary for definitive diagnosis. Direct immunofluorescence (DIF) microscopy of a sample of perilesional skin can show tissue-bound immunoreactants. Linear deposition of the complement component protein C3 along the basement membrane zone is diagnostic for PG. IgG is also deposited about 40% of the time.3

