User login
Case
A 68-year-old cachectic female with a history of Alzheimer’s dementia presents with a slowly progressive decline in functional status. She is bed bound, minimally verbal, and has lost interest in eating.
Her problems with decreased oral intake started when her diet was changed to nectar-thickened liquids. This change was made after the patient was hospitalized multiple times for aspiration pneumonia and she underwent a fluoroscopic swallowing evaluation that revealed aspiration of thin liquids. The patient’s husband requests that a feeding tube be placed so his wife doesn’t “die of pneumonia or starve to death.”
Overview
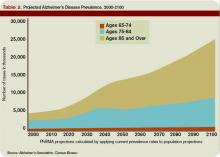
As the U.S. population ages, hospitalists are seeing a steady increase in the average patient age and the prevalence of dementia. Alzheimer’s dementia affects an estimated 4 million to 5 million Americans; this number expected to triple by the year 2050.1
As patients with dementia near the end of life, they often fail to thrive, with less oral intake and more swallowing disorders leading to aspiration. This is when physicians and patient family members must decide whether a feeding tube should be placed.
Placement of a nasogastric or percutaneous endogastric gastrostomy (PEG) feeding tube has become a relatively common medical intervention instituted to maintain or improve a patient’s nutritional status. Prior to 1980, permanent gastric or postpyloric feeding tubes were placed surgically by laparotomy, but the advent of endoscopy and computed tomography (CT) guided procedures offers a simplified procedure requiring only mild sedation and local anesthesia.2
Many patients who suffer multiple bouts of aspiration pneumonia and fail a swallowing evaluation because of an irreversible process are offered a percutaneous feeding tube to maintain nutrition. A feeding tube is also seen as a way to supply nutrition at the end of life in patients no longer able or willing to take food orally.
Although it seems logical that a feeding tube might improve the outcomes of these clinical scenarios, limited literature exists on the topic because of the legal, ethical, emotional, and religious implications a large, randomized, placebo-controlled trial would entail.
Review of the Data
Placement of a PEG has become accepted as a relatively benign procedure, although it is associated with significant morbidity and mortality. Minor complications including pain, abdominal wall ulcers, wound infections, peristomal leakage, and tube displacement occur in approximately 10% of cases.3 Major complications including hemorrhage, bowel or liver perforation, or aspiration occur in 3% of cases.4
These numbers do not account for long-term complications including peristomal infections, leakage problems, or the use of physical restraints to avoid self-extubation.
Aspiration Risk
A common indication for PEG placement is aspiration risk. PEG tubes are often placed in patients who fail swallowing evaluations in order to decrease their risk of aspiration and aspiration pneumonia.
True aspiration pneumonia is thought to originate from an inoculum of oral cavity or nasopharynx bacteria, which placement of a PEG tube would not prevent. Leibovitz, et al., showed that elderly patients with nasogastric or percutaneous feeding tubes are associated with colonization of the oropharynx with more pathogenic bacteria when compared with orally fed patients.5 Thus, the use of PEG tubes might put them at higher risk for pathogenic inoculation.
Aspiration pneumonia occurs in up to 50% of patients with feeding tubes. Studies have shown PEG tube placement decreases lower esophageal sphincter tone, potentially increasing regurgitation risk.6 It has also been shown that aspiration of gastric contents produces a pneumonitis with the resultant inflammatory response allowing for establishment of infection by smaller inoculums of or less virulent organisms.7
Small, randomized trials have shown no decrease in aspiration risk with post-pyloric versus gastric feeding tubes, nasogastric versus percutaneous feeding tubes, or continuous versus intermittent tube feeds.8 There have been no sizable randomized prospective trials to determine if feeding tube placement versus hand feeding patients with end-stage dementia alters aspiration pneumonia risk.
Pressure Ulcers
Patients with end-stage dementia often become bed bound as their disease progresses, and they commonly suffer from pressure ulcers. Pressure ulcers often coexist in patients with malnutrition, and it is well established that patients with biochemical markers of malnutrition are at higher risk for pressure ulcer formation.
Still, no studies show that improved nutrition prevents pressure ulcer formation. In a nursing home population of patients with dementia, a two-year follow-up study showed no significant improvement in pressure ulcer healing or decreased ulcer formation with nutrition by feeding tube.9 These studies are adjusted for independent risk factors for mortality and indication for PEG placement, but we can assume there are confounders that go into the decision for feeding tube placement that are not necessarily identifiable.
Nutritional Status
Family members are often concerned that if the patient is unable to take food by mouth and no feeding tube is placed, then the patient will suffer from the discomfort of starvation and dehydration.
As a patient with a severe dementing illness enters the end stage of his/her clinical course, practitioners frequently make a plan with families to change the goals of care toward keeping the patient comfortable. Comfort is a difficult clinical parameter to measure, but studies in the hospice population of patients with end-stage cancer and AIDS report that the hunger and thirst are transient and improve with ice chips and mouth swabs.10
Despite the lack of evidence of PEG tubes prolonging survival in patients with dementia who are no longer able or willing to take in food orally, it is logical that withholding all hydration or nutritional support will hasten death despite the risks associated with feeding tubes. This is where the ethical argument arises regarding prolonging life of decreasing quality.
In certain medical and legal sectors, artificial nutrition, and hydration are considered a medical intervention. Therefore, the ideals of patient autonomy dictate that the patient’s proxy should decide whether or not the patient would have wanted the intervention after weighing the risks and benefits.
If hospitalists view artificial nutrition as a medical intervention, our moral obligation is to instruct patients and their families about these risks and benefits.
Often, the patient will not clinically improve with artificial nutrition. But we can maintain physiologic processes or at least slow their decline.
Emerging research indicates the standard of care in how we present this information is changing to include presentation of data instead of only using a patient’s suspected beliefs about quality of life.
A useful algorithm proposed by Rabeneck, et al., provides comprehensive guidelines for PEG placement in all patient populations based on the reason for PEG consideration.11
Back to the Case
Our patient is likely nearing the end of her life because of end-stage dementia. There is no evidence to suggest placement of a feeding tube would extend her life more than hand feeding.
We know feeding-tube placement could increase aspiration pneumonia risk and significant short- and long-term morbidity and mortality. We can keep her comfortable with small amounts of water, wetting her lips with swabs. If a feeding tube is placed, its use should be evaluated based on the patient’s clinical course. TH
Dr. Pell is an instructor of medicine in the Section of Hospital Medicine at the University of Colorado, Denver.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875.
- Hebert LE, Beckett LA, Scherr PA, and Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169-173.
- Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973-1976.
- Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13(6):520-523.
- Leibovitz A, Plotnikov G, Habot B, et al. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med. 2003;58(1):52-55.
- McCann R. Lack of evidence about tube feeding: food for thought. JAMA. 1999;282(14):1380-1381.
- Cameron JL, Caldini P, Toung J-K, et al. Aspiration pneumonia: physiologic data following experimental Aspiration. Surgery. 1972;72:238.
- Loeb MB, Becker M, Eady A, et al. Interventions to prevent aspiration pneumonia in older adults: a systematic review. JAGS. 2003;51(7):1018-1022.
- Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327-332.
- McCann RM, Hall WJ, Groth-Junker A. Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA. 1994;272:1263-1266.
- Rabeneck L, McCullough LB. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997;349(9050):496-498.
Case
A 68-year-old cachectic female with a history of Alzheimer’s dementia presents with a slowly progressive decline in functional status. She is bed bound, minimally verbal, and has lost interest in eating.
Her problems with decreased oral intake started when her diet was changed to nectar-thickened liquids. This change was made after the patient was hospitalized multiple times for aspiration pneumonia and she underwent a fluoroscopic swallowing evaluation that revealed aspiration of thin liquids. The patient’s husband requests that a feeding tube be placed so his wife doesn’t “die of pneumonia or starve to death.”
Overview
As the U.S. population ages, hospitalists are seeing a steady increase in the average patient age and the prevalence of dementia. Alzheimer’s dementia affects an estimated 4 million to 5 million Americans; this number expected to triple by the year 2050.1
As patients with dementia near the end of life, they often fail to thrive, with less oral intake and more swallowing disorders leading to aspiration. This is when physicians and patient family members must decide whether a feeding tube should be placed.
Placement of a nasogastric or percutaneous endogastric gastrostomy (PEG) feeding tube has become a relatively common medical intervention instituted to maintain or improve a patient’s nutritional status. Prior to 1980, permanent gastric or postpyloric feeding tubes were placed surgically by laparotomy, but the advent of endoscopy and computed tomography (CT) guided procedures offers a simplified procedure requiring only mild sedation and local anesthesia.2
Many patients who suffer multiple bouts of aspiration pneumonia and fail a swallowing evaluation because of an irreversible process are offered a percutaneous feeding tube to maintain nutrition. A feeding tube is also seen as a way to supply nutrition at the end of life in patients no longer able or willing to take food orally.
Although it seems logical that a feeding tube might improve the outcomes of these clinical scenarios, limited literature exists on the topic because of the legal, ethical, emotional, and religious implications a large, randomized, placebo-controlled trial would entail.
Review of the Data
Placement of a PEG has become accepted as a relatively benign procedure, although it is associated with significant morbidity and mortality. Minor complications including pain, abdominal wall ulcers, wound infections, peristomal leakage, and tube displacement occur in approximately 10% of cases.3 Major complications including hemorrhage, bowel or liver perforation, or aspiration occur in 3% of cases.4
These numbers do not account for long-term complications including peristomal infections, leakage problems, or the use of physical restraints to avoid self-extubation.
Aspiration Risk
A common indication for PEG placement is aspiration risk. PEG tubes are often placed in patients who fail swallowing evaluations in order to decrease their risk of aspiration and aspiration pneumonia.
True aspiration pneumonia is thought to originate from an inoculum of oral cavity or nasopharynx bacteria, which placement of a PEG tube would not prevent. Leibovitz, et al., showed that elderly patients with nasogastric or percutaneous feeding tubes are associated with colonization of the oropharynx with more pathogenic bacteria when compared with orally fed patients.5 Thus, the use of PEG tubes might put them at higher risk for pathogenic inoculation.
Aspiration pneumonia occurs in up to 50% of patients with feeding tubes. Studies have shown PEG tube placement decreases lower esophageal sphincter tone, potentially increasing regurgitation risk.6 It has also been shown that aspiration of gastric contents produces a pneumonitis with the resultant inflammatory response allowing for establishment of infection by smaller inoculums of or less virulent organisms.7
Small, randomized trials have shown no decrease in aspiration risk with post-pyloric versus gastric feeding tubes, nasogastric versus percutaneous feeding tubes, or continuous versus intermittent tube feeds.8 There have been no sizable randomized prospective trials to determine if feeding tube placement versus hand feeding patients with end-stage dementia alters aspiration pneumonia risk.
Pressure Ulcers
Patients with end-stage dementia often become bed bound as their disease progresses, and they commonly suffer from pressure ulcers. Pressure ulcers often coexist in patients with malnutrition, and it is well established that patients with biochemical markers of malnutrition are at higher risk for pressure ulcer formation.
Still, no studies show that improved nutrition prevents pressure ulcer formation. In a nursing home population of patients with dementia, a two-year follow-up study showed no significant improvement in pressure ulcer healing or decreased ulcer formation with nutrition by feeding tube.9 These studies are adjusted for independent risk factors for mortality and indication for PEG placement, but we can assume there are confounders that go into the decision for feeding tube placement that are not necessarily identifiable.
Nutritional Status
Family members are often concerned that if the patient is unable to take food by mouth and no feeding tube is placed, then the patient will suffer from the discomfort of starvation and dehydration.
As a patient with a severe dementing illness enters the end stage of his/her clinical course, practitioners frequently make a plan with families to change the goals of care toward keeping the patient comfortable. Comfort is a difficult clinical parameter to measure, but studies in the hospice population of patients with end-stage cancer and AIDS report that the hunger and thirst are transient and improve with ice chips and mouth swabs.10
Despite the lack of evidence of PEG tubes prolonging survival in patients with dementia who are no longer able or willing to take in food orally, it is logical that withholding all hydration or nutritional support will hasten death despite the risks associated with feeding tubes. This is where the ethical argument arises regarding prolonging life of decreasing quality.
In certain medical and legal sectors, artificial nutrition, and hydration are considered a medical intervention. Therefore, the ideals of patient autonomy dictate that the patient’s proxy should decide whether or not the patient would have wanted the intervention after weighing the risks and benefits.
If hospitalists view artificial nutrition as a medical intervention, our moral obligation is to instruct patients and their families about these risks and benefits.
Often, the patient will not clinically improve with artificial nutrition. But we can maintain physiologic processes or at least slow their decline.
Emerging research indicates the standard of care in how we present this information is changing to include presentation of data instead of only using a patient’s suspected beliefs about quality of life.
A useful algorithm proposed by Rabeneck, et al., provides comprehensive guidelines for PEG placement in all patient populations based on the reason for PEG consideration.11
Back to the Case
Our patient is likely nearing the end of her life because of end-stage dementia. There is no evidence to suggest placement of a feeding tube would extend her life more than hand feeding.
We know feeding-tube placement could increase aspiration pneumonia risk and significant short- and long-term morbidity and mortality. We can keep her comfortable with small amounts of water, wetting her lips with swabs. If a feeding tube is placed, its use should be evaluated based on the patient’s clinical course. TH
Dr. Pell is an instructor of medicine in the Section of Hospital Medicine at the University of Colorado, Denver.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875.
- Hebert LE, Beckett LA, Scherr PA, and Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169-173.
- Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973-1976.
- Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13(6):520-523.
- Leibovitz A, Plotnikov G, Habot B, et al. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med. 2003;58(1):52-55.
- McCann R. Lack of evidence about tube feeding: food for thought. JAMA. 1999;282(14):1380-1381.
- Cameron JL, Caldini P, Toung J-K, et al. Aspiration pneumonia: physiologic data following experimental Aspiration. Surgery. 1972;72:238.
- Loeb MB, Becker M, Eady A, et al. Interventions to prevent aspiration pneumonia in older adults: a systematic review. JAGS. 2003;51(7):1018-1022.
- Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327-332.
- McCann RM, Hall WJ, Groth-Junker A. Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA. 1994;272:1263-1266.
- Rabeneck L, McCullough LB. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997;349(9050):496-498.
Case
A 68-year-old cachectic female with a history of Alzheimer’s dementia presents with a slowly progressive decline in functional status. She is bed bound, minimally verbal, and has lost interest in eating.
Her problems with decreased oral intake started when her diet was changed to nectar-thickened liquids. This change was made after the patient was hospitalized multiple times for aspiration pneumonia and she underwent a fluoroscopic swallowing evaluation that revealed aspiration of thin liquids. The patient’s husband requests that a feeding tube be placed so his wife doesn’t “die of pneumonia or starve to death.”
Overview
As the U.S. population ages, hospitalists are seeing a steady increase in the average patient age and the prevalence of dementia. Alzheimer’s dementia affects an estimated 4 million to 5 million Americans; this number expected to triple by the year 2050.1
As patients with dementia near the end of life, they often fail to thrive, with less oral intake and more swallowing disorders leading to aspiration. This is when physicians and patient family members must decide whether a feeding tube should be placed.
Placement of a nasogastric or percutaneous endogastric gastrostomy (PEG) feeding tube has become a relatively common medical intervention instituted to maintain or improve a patient’s nutritional status. Prior to 1980, permanent gastric or postpyloric feeding tubes were placed surgically by laparotomy, but the advent of endoscopy and computed tomography (CT) guided procedures offers a simplified procedure requiring only mild sedation and local anesthesia.2
Many patients who suffer multiple bouts of aspiration pneumonia and fail a swallowing evaluation because of an irreversible process are offered a percutaneous feeding tube to maintain nutrition. A feeding tube is also seen as a way to supply nutrition at the end of life in patients no longer able or willing to take food orally.
Although it seems logical that a feeding tube might improve the outcomes of these clinical scenarios, limited literature exists on the topic because of the legal, ethical, emotional, and religious implications a large, randomized, placebo-controlled trial would entail.
Review of the Data
Placement of a PEG has become accepted as a relatively benign procedure, although it is associated with significant morbidity and mortality. Minor complications including pain, abdominal wall ulcers, wound infections, peristomal leakage, and tube displacement occur in approximately 10% of cases.3 Major complications including hemorrhage, bowel or liver perforation, or aspiration occur in 3% of cases.4
These numbers do not account for long-term complications including peristomal infections, leakage problems, or the use of physical restraints to avoid self-extubation.
Aspiration Risk
A common indication for PEG placement is aspiration risk. PEG tubes are often placed in patients who fail swallowing evaluations in order to decrease their risk of aspiration and aspiration pneumonia.
True aspiration pneumonia is thought to originate from an inoculum of oral cavity or nasopharynx bacteria, which placement of a PEG tube would not prevent. Leibovitz, et al., showed that elderly patients with nasogastric or percutaneous feeding tubes are associated with colonization of the oropharynx with more pathogenic bacteria when compared with orally fed patients.5 Thus, the use of PEG tubes might put them at higher risk for pathogenic inoculation.
Aspiration pneumonia occurs in up to 50% of patients with feeding tubes. Studies have shown PEG tube placement decreases lower esophageal sphincter tone, potentially increasing regurgitation risk.6 It has also been shown that aspiration of gastric contents produces a pneumonitis with the resultant inflammatory response allowing for establishment of infection by smaller inoculums of or less virulent organisms.7
Small, randomized trials have shown no decrease in aspiration risk with post-pyloric versus gastric feeding tubes, nasogastric versus percutaneous feeding tubes, or continuous versus intermittent tube feeds.8 There have been no sizable randomized prospective trials to determine if feeding tube placement versus hand feeding patients with end-stage dementia alters aspiration pneumonia risk.
Pressure Ulcers
Patients with end-stage dementia often become bed bound as their disease progresses, and they commonly suffer from pressure ulcers. Pressure ulcers often coexist in patients with malnutrition, and it is well established that patients with biochemical markers of malnutrition are at higher risk for pressure ulcer formation.
Still, no studies show that improved nutrition prevents pressure ulcer formation. In a nursing home population of patients with dementia, a two-year follow-up study showed no significant improvement in pressure ulcer healing or decreased ulcer formation with nutrition by feeding tube.9 These studies are adjusted for independent risk factors for mortality and indication for PEG placement, but we can assume there are confounders that go into the decision for feeding tube placement that are not necessarily identifiable.
Nutritional Status
Family members are often concerned that if the patient is unable to take food by mouth and no feeding tube is placed, then the patient will suffer from the discomfort of starvation and dehydration.
As a patient with a severe dementing illness enters the end stage of his/her clinical course, practitioners frequently make a plan with families to change the goals of care toward keeping the patient comfortable. Comfort is a difficult clinical parameter to measure, but studies in the hospice population of patients with end-stage cancer and AIDS report that the hunger and thirst are transient and improve with ice chips and mouth swabs.10
Despite the lack of evidence of PEG tubes prolonging survival in patients with dementia who are no longer able or willing to take in food orally, it is logical that withholding all hydration or nutritional support will hasten death despite the risks associated with feeding tubes. This is where the ethical argument arises regarding prolonging life of decreasing quality.
In certain medical and legal sectors, artificial nutrition, and hydration are considered a medical intervention. Therefore, the ideals of patient autonomy dictate that the patient’s proxy should decide whether or not the patient would have wanted the intervention after weighing the risks and benefits.
If hospitalists view artificial nutrition as a medical intervention, our moral obligation is to instruct patients and their families about these risks and benefits.
Often, the patient will not clinically improve with artificial nutrition. But we can maintain physiologic processes or at least slow their decline.
Emerging research indicates the standard of care in how we present this information is changing to include presentation of data instead of only using a patient’s suspected beliefs about quality of life.
A useful algorithm proposed by Rabeneck, et al., provides comprehensive guidelines for PEG placement in all patient populations based on the reason for PEG consideration.11
Back to the Case
Our patient is likely nearing the end of her life because of end-stage dementia. There is no evidence to suggest placement of a feeding tube would extend her life more than hand feeding.
We know feeding-tube placement could increase aspiration pneumonia risk and significant short- and long-term morbidity and mortality. We can keep her comfortable with small amounts of water, wetting her lips with swabs. If a feeding tube is placed, its use should be evaluated based on the patient’s clinical course. TH
Dr. Pell is an instructor of medicine in the Section of Hospital Medicine at the University of Colorado, Denver.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875.
- Hebert LE, Beckett LA, Scherr PA, and Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169-173.
- Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973-1976.
- Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13(6):520-523.
- Leibovitz A, Plotnikov G, Habot B, et al. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med. 2003;58(1):52-55.
- McCann R. Lack of evidence about tube feeding: food for thought. JAMA. 1999;282(14):1380-1381.
- Cameron JL, Caldini P, Toung J-K, et al. Aspiration pneumonia: physiologic data following experimental Aspiration. Surgery. 1972;72:238.
- Loeb MB, Becker M, Eady A, et al. Interventions to prevent aspiration pneumonia in older adults: a systematic review. JAGS. 2003;51(7):1018-1022.
- Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327-332.
- McCann RM, Hall WJ, Groth-Junker A. Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA. 1994;272:1263-1266.
- Rabeneck L, McCullough LB. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997;349(9050):496-498.