User login
Guidelines for VTE Prophylaxis in Medical Patient Populations, Including Stroke
Review: VTE prophylaxis guidelines
Background: Pharmacologic interventions with heparin or related drugs and mechanical interventions have become the standard of care in the prevention of venous thromboembolism (VTE) in hospitalized patients. The studies evaluating the efficacy of these therapies in relation to each other have become more robust over the last decade. Despite these advances, however, there remains controversy over meaningful outcomes and how the results should be applied to different patient populations.
Many studies address the issue of VTE prophylaxis using surrogate outcomes, such as asymptomatic deep venous thromboembolism (DVT), given the low incidence of significant clinical outcomes, e.g., symptomatic DVT, pulmonary embolus (PE), or mortality. There have been few large, prospective, randomized trials that show a statistically significant benefit of pharmacologic or mechanical VTE prophylaxis in a purely medical population when looking for these meaningful outcomes.
Significant bleeding and thrombocytopenia are the most common risks identified in pharmacologic intervention studies against which the benefits have to be weighed. Stroke patients are one medical population in which bleeding risk has been of particular concern.
Guideline update: In November 2011, the American College of Physicians (ACP) published new guidelines for medical patients regarding VTE prophylaxis.1 These evidence-based guidelines were not based on new trial data, but rather a review of previous studies looking at only medical patients; they did not consider asymptomatic DVT as a significant outcome.
The new guidelines recommend that all hospitalized medical patients, including stroke patients, be evaluated for risk of VTE and bleeding, which is not a change from any previous standard. Routine use of VTE prophylaxis is not recommended, and prophylactic pharmacologic therapy with heparin or related drugs should only be instituted if the benefit in a decreased incidence of VTE outweighs the risk of bleeding in an individual patient. The use of mechanical prophylaxis with graduated compression stockings is not recommended, given the risk of lower extremity skin damage and a lack of clear benefit.
Last month, the American College of Chest Physicians (ACCP) followed suit in their 9th edition of clinical practice guidelines regarding VTE prevention in non-surgical patients.2 The ACC recommends the use of heparin or related drugs for VTE prophylaxis for medical patients at increased risk of thrombosis, but recommends against pharmacologic VTE prophylaxis in patients at low risk. Patients at high risk of bleeding with a concomitant high risk of VTE are recommended to use mechanical prophylaxis until the bleeding risk diminishes. These guidelines go a step further and provide parameters defining both high risk of VTE and high risk of bleeding, making them a very clinically useful tool. Neither guideline indicates a prefererence for pharmacologic prophylaxis.
Analysis: The most comprehensive and broadly accepted guidelines for VTE prevention before these updates were put forth by ACCP and published in the June 2008 issue of Chest.3
This review-based guideline, which included asymptomatic DVT as an appropriate outcome, recommended the routine use of heparin or related drugs for prophylaxis of VTE in medical patients confined to bed who have at least one risk factor for VTE. For patients with a contraindication to anticoagulant prophylaxis, they recommend mechanical thromboprophylaxis.
Most national organizations have established their standards and measures based on these clinical practice guidelines. The Joint Commission’s VTE-1 Core Measure evaluates the percentage of inpatients who received VTE prophylaxis or who had documentation as to why no prophylaxis was given.4 The measure states that “appropriately used thromboprophylaxis has a desirable risk-benefit ratio and is cost-effective,” but it does not define appropriate use and lists limited exclusion criteria. The Joint Commission has responded in a statement to the new ACP guidelines, but it has not changed its guidelines based on the 2008 Chest guidelines.5
The Centers for Medicare & Medicaid Services (CMS), through the Inpatient Prospective Payment System 2009, determined that VTE during a hospitalization for total knee or hip replacement was a hospital-acquired condition that will not be reimbursable.6 This is not the case in general medical inpatients. Also of interest, observational studies suggest that 50% of patients who develop a VTE in hospital will do so despite appropriate prophylaxis.
Surgical patient populations are not addressed in the ACP update but are covered in the 2012 Chest updated guidelines. The recommendations for surgical patients have no major changes since 2008 except for choice of anticoagulant for specific patient populations and new categories of intermediate and high risk, the specifics of which are beyond the scope of this review.
Surgical patients with risk factors for VTE undergoing major procedures should receive pharmacologic prophylaxis with the addition of mechanical prophylaxis. For patients with a high risk of bleeding, mechanical prophylaxis can be used alone until bleeding risk diminishes.
Caution is recommended for any patient undergoing neuraxial analgesia or anesthesia when considering pharmacologic prophylaxis. Routine use of pharmacologic prophylaxis is recommended in patients undergoing bariatric surgery, elective hip replacement, hip fracture surgery, major thoracic surgery, or major open urologic procedures regardless of VTE risk factors, and should be extended in patients with additional VTE risks. All other surgical populations should be evaluated for bleeding risk and VTE risk factors prior to decision for pharmacologic and/or mechanical prophylaxis.
HM takeaways: In medical populations including stroke, routine use of VTE prophylaxis is not recommended, and should only be instituted if they are at high risk of VTE and the benefit in a decreased incidence of VTE outweighs the risk of bleeding.
Dr. Pell is a hospitalist and assistant professor of medicine at the University of Colorado Denver School of Medicine.
Review: VTE prophylaxis guidelines
Background: Pharmacologic interventions with heparin or related drugs and mechanical interventions have become the standard of care in the prevention of venous thromboembolism (VTE) in hospitalized patients. The studies evaluating the efficacy of these therapies in relation to each other have become more robust over the last decade. Despite these advances, however, there remains controversy over meaningful outcomes and how the results should be applied to different patient populations.
Many studies address the issue of VTE prophylaxis using surrogate outcomes, such as asymptomatic deep venous thromboembolism (DVT), given the low incidence of significant clinical outcomes, e.g., symptomatic DVT, pulmonary embolus (PE), or mortality. There have been few large, prospective, randomized trials that show a statistically significant benefit of pharmacologic or mechanical VTE prophylaxis in a purely medical population when looking for these meaningful outcomes.
Significant bleeding and thrombocytopenia are the most common risks identified in pharmacologic intervention studies against which the benefits have to be weighed. Stroke patients are one medical population in which bleeding risk has been of particular concern.
Guideline update: In November 2011, the American College of Physicians (ACP) published new guidelines for medical patients regarding VTE prophylaxis.1 These evidence-based guidelines were not based on new trial data, but rather a review of previous studies looking at only medical patients; they did not consider asymptomatic DVT as a significant outcome.
The new guidelines recommend that all hospitalized medical patients, including stroke patients, be evaluated for risk of VTE and bleeding, which is not a change from any previous standard. Routine use of VTE prophylaxis is not recommended, and prophylactic pharmacologic therapy with heparin or related drugs should only be instituted if the benefit in a decreased incidence of VTE outweighs the risk of bleeding in an individual patient. The use of mechanical prophylaxis with graduated compression stockings is not recommended, given the risk of lower extremity skin damage and a lack of clear benefit.
Last month, the American College of Chest Physicians (ACCP) followed suit in their 9th edition of clinical practice guidelines regarding VTE prevention in non-surgical patients.2 The ACC recommends the use of heparin or related drugs for VTE prophylaxis for medical patients at increased risk of thrombosis, but recommends against pharmacologic VTE prophylaxis in patients at low risk. Patients at high risk of bleeding with a concomitant high risk of VTE are recommended to use mechanical prophylaxis until the bleeding risk diminishes. These guidelines go a step further and provide parameters defining both high risk of VTE and high risk of bleeding, making them a very clinically useful tool. Neither guideline indicates a prefererence for pharmacologic prophylaxis.
Analysis: The most comprehensive and broadly accepted guidelines for VTE prevention before these updates were put forth by ACCP and published in the June 2008 issue of Chest.3
This review-based guideline, which included asymptomatic DVT as an appropriate outcome, recommended the routine use of heparin or related drugs for prophylaxis of VTE in medical patients confined to bed who have at least one risk factor for VTE. For patients with a contraindication to anticoagulant prophylaxis, they recommend mechanical thromboprophylaxis.
Most national organizations have established their standards and measures based on these clinical practice guidelines. The Joint Commission’s VTE-1 Core Measure evaluates the percentage of inpatients who received VTE prophylaxis or who had documentation as to why no prophylaxis was given.4 The measure states that “appropriately used thromboprophylaxis has a desirable risk-benefit ratio and is cost-effective,” but it does not define appropriate use and lists limited exclusion criteria. The Joint Commission has responded in a statement to the new ACP guidelines, but it has not changed its guidelines based on the 2008 Chest guidelines.5
The Centers for Medicare & Medicaid Services (CMS), through the Inpatient Prospective Payment System 2009, determined that VTE during a hospitalization for total knee or hip replacement was a hospital-acquired condition that will not be reimbursable.6 This is not the case in general medical inpatients. Also of interest, observational studies suggest that 50% of patients who develop a VTE in hospital will do so despite appropriate prophylaxis.
Surgical patient populations are not addressed in the ACP update but are covered in the 2012 Chest updated guidelines. The recommendations for surgical patients have no major changes since 2008 except for choice of anticoagulant for specific patient populations and new categories of intermediate and high risk, the specifics of which are beyond the scope of this review.
Surgical patients with risk factors for VTE undergoing major procedures should receive pharmacologic prophylaxis with the addition of mechanical prophylaxis. For patients with a high risk of bleeding, mechanical prophylaxis can be used alone until bleeding risk diminishes.
Caution is recommended for any patient undergoing neuraxial analgesia or anesthesia when considering pharmacologic prophylaxis. Routine use of pharmacologic prophylaxis is recommended in patients undergoing bariatric surgery, elective hip replacement, hip fracture surgery, major thoracic surgery, or major open urologic procedures regardless of VTE risk factors, and should be extended in patients with additional VTE risks. All other surgical populations should be evaluated for bleeding risk and VTE risk factors prior to decision for pharmacologic and/or mechanical prophylaxis.
HM takeaways: In medical populations including stroke, routine use of VTE prophylaxis is not recommended, and should only be instituted if they are at high risk of VTE and the benefit in a decreased incidence of VTE outweighs the risk of bleeding.
Dr. Pell is a hospitalist and assistant professor of medicine at the University of Colorado Denver School of Medicine.
Review: VTE prophylaxis guidelines
Background: Pharmacologic interventions with heparin or related drugs and mechanical interventions have become the standard of care in the prevention of venous thromboembolism (VTE) in hospitalized patients. The studies evaluating the efficacy of these therapies in relation to each other have become more robust over the last decade. Despite these advances, however, there remains controversy over meaningful outcomes and how the results should be applied to different patient populations.
Many studies address the issue of VTE prophylaxis using surrogate outcomes, such as asymptomatic deep venous thromboembolism (DVT), given the low incidence of significant clinical outcomes, e.g., symptomatic DVT, pulmonary embolus (PE), or mortality. There have been few large, prospective, randomized trials that show a statistically significant benefit of pharmacologic or mechanical VTE prophylaxis in a purely medical population when looking for these meaningful outcomes.
Significant bleeding and thrombocytopenia are the most common risks identified in pharmacologic intervention studies against which the benefits have to be weighed. Stroke patients are one medical population in which bleeding risk has been of particular concern.
Guideline update: In November 2011, the American College of Physicians (ACP) published new guidelines for medical patients regarding VTE prophylaxis.1 These evidence-based guidelines were not based on new trial data, but rather a review of previous studies looking at only medical patients; they did not consider asymptomatic DVT as a significant outcome.
The new guidelines recommend that all hospitalized medical patients, including stroke patients, be evaluated for risk of VTE and bleeding, which is not a change from any previous standard. Routine use of VTE prophylaxis is not recommended, and prophylactic pharmacologic therapy with heparin or related drugs should only be instituted if the benefit in a decreased incidence of VTE outweighs the risk of bleeding in an individual patient. The use of mechanical prophylaxis with graduated compression stockings is not recommended, given the risk of lower extremity skin damage and a lack of clear benefit.
Last month, the American College of Chest Physicians (ACCP) followed suit in their 9th edition of clinical practice guidelines regarding VTE prevention in non-surgical patients.2 The ACC recommends the use of heparin or related drugs for VTE prophylaxis for medical patients at increased risk of thrombosis, but recommends against pharmacologic VTE prophylaxis in patients at low risk. Patients at high risk of bleeding with a concomitant high risk of VTE are recommended to use mechanical prophylaxis until the bleeding risk diminishes. These guidelines go a step further and provide parameters defining both high risk of VTE and high risk of bleeding, making them a very clinically useful tool. Neither guideline indicates a prefererence for pharmacologic prophylaxis.
Analysis: The most comprehensive and broadly accepted guidelines for VTE prevention before these updates were put forth by ACCP and published in the June 2008 issue of Chest.3
This review-based guideline, which included asymptomatic DVT as an appropriate outcome, recommended the routine use of heparin or related drugs for prophylaxis of VTE in medical patients confined to bed who have at least one risk factor for VTE. For patients with a contraindication to anticoagulant prophylaxis, they recommend mechanical thromboprophylaxis.
Most national organizations have established their standards and measures based on these clinical practice guidelines. The Joint Commission’s VTE-1 Core Measure evaluates the percentage of inpatients who received VTE prophylaxis or who had documentation as to why no prophylaxis was given.4 The measure states that “appropriately used thromboprophylaxis has a desirable risk-benefit ratio and is cost-effective,” but it does not define appropriate use and lists limited exclusion criteria. The Joint Commission has responded in a statement to the new ACP guidelines, but it has not changed its guidelines based on the 2008 Chest guidelines.5
The Centers for Medicare & Medicaid Services (CMS), through the Inpatient Prospective Payment System 2009, determined that VTE during a hospitalization for total knee or hip replacement was a hospital-acquired condition that will not be reimbursable.6 This is not the case in general medical inpatients. Also of interest, observational studies suggest that 50% of patients who develop a VTE in hospital will do so despite appropriate prophylaxis.
Surgical patient populations are not addressed in the ACP update but are covered in the 2012 Chest updated guidelines. The recommendations for surgical patients have no major changes since 2008 except for choice of anticoagulant for specific patient populations and new categories of intermediate and high risk, the specifics of which are beyond the scope of this review.
Surgical patients with risk factors for VTE undergoing major procedures should receive pharmacologic prophylaxis with the addition of mechanical prophylaxis. For patients with a high risk of bleeding, mechanical prophylaxis can be used alone until bleeding risk diminishes.
Caution is recommended for any patient undergoing neuraxial analgesia or anesthesia when considering pharmacologic prophylaxis. Routine use of pharmacologic prophylaxis is recommended in patients undergoing bariatric surgery, elective hip replacement, hip fracture surgery, major thoracic surgery, or major open urologic procedures regardless of VTE risk factors, and should be extended in patients with additional VTE risks. All other surgical populations should be evaluated for bleeding risk and VTE risk factors prior to decision for pharmacologic and/or mechanical prophylaxis.
HM takeaways: In medical populations including stroke, routine use of VTE prophylaxis is not recommended, and should only be instituted if they are at high risk of VTE and the benefit in a decreased incidence of VTE outweighs the risk of bleeding.
Dr. Pell is a hospitalist and assistant professor of medicine at the University of Colorado Denver School of Medicine.
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk factors for iatrogenic pneumothorax
- Residency acceptance and use of pharmaceutical industry funding
- Early cholecystectomy outcomes for gallstone pancreatitis
- Use of microbial DNA in sepsis
- Adding rifampicin to vancomycin in MRSA pneumonia
- Rate and outcomes of culture-negative severe sepsis
- Rates of surgical comanagement in U.S. hospitals
- Probiotics and rates of ventilator-associated pneumonia
Ultrasound Guidance and Operator Experience Decrease Risk of Pneumothorax Following Thoracentesis
Clinical question: How often does pneumothorax happen following thoracentesis, and what factors are associated with increased risk of this complication?
Background: Procedural complications are an important source of adverse events in the hospital. Iatrogenic pneumothorax after thoracentesis results in increased hospital length of stay, morbidity, and mortality. Large variation exists in reported pneumothorax rates, and little is known about procedure- and patient-specific factors associated with development of this complication.
Study design: Systematic review and meta-analysis.
Setting: Review of 24 MEDLINE-indexed studies from January 1966 to April 2009.
Synopsis: A total of 349 pneumothoraces were reported after 6,605 thoracenteses (overall incidence 6.0%). Chest-tube insertion was required in 34.1% of the cases. Risk for pneumothorax was significantly higher when larger needles or catheters were used compared with needles smaller than 20-gauge (odds ratio 2.5, 95% confidence interval [CI], 1.1-6.0) and after therapeutic thoracentesis compared with diagnostic procedures (OR 2.6, 95% CI, 1.8-3.8).
Procedures requiring two or more needle passes did not significantly increase pneumothorax risk (OR 2.5, 95% CI, 0.3-20.1). In contrast, pneumothorax rates were significantly lower when using ultrasound guidance (OR 0.3, 95% CI, 0.2-0.7) and with experienced operators (3.9% vs. 8.5%, P=0.04).
Examining patient risk factors, pneumothorax rates were similar regardless of effusion size and patient gender. Additionally, rates were similar among non-ICU inpatients, ICU inpatients, and outpatients. Data did show a trend toward increased risk of pneumothorax with mechanical ventilation (OR 4.0, 95% CI, 0.95-16.8), although no study directly compared rates in ICU patients with and without mechanical ventilation.
Bottom line: Ultrasound guidance is a modifiable factor that decreases the risk of post-thoracentesis pneumothorax. Pneumothorax rates are lower when performed by experienced clinicians, providing an important opportunity to reduce procedure-related complications by increasing direct trainee supervision.
Citation: Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339.
Pharmaceutical Industry Support Is Common in U.S. Internal-Medicine Residency Programs
Clinical question: What are the current attitudes of program directors regarding pharmaceutical industry support of internal-medicine residency activities? What are the potential associations between program characteristics and acceptance of industry support?
Background: Increasing evidence suggests that interactions with the pharmaceutical industry influence physician attitudes and practices. Recently, the Association of American Medical Colleges (AAMC) proposed that academic medical centers prohibit the acceptance of all gifts and restrict access by pharmaceutical industry representatives.
Study design: Survey of U.S. internal-medicine residency program directors.
Setting: Web-based survey of residency program directors in 388 U.S. internal-medicine residency programs.
Synopsis: Of the 236 program directors responding to the survey, 132 (55.9%) reported accepting some kind of support from the pharmaceutical industry. Support was most commonly provided in the form of food for conferences (90.9%), educational materials (83.3%), office supplies (68.9%), and drug samples (57.6%).
When programs reported accepting pharmaceutical industry support, 67.9% cited a lack of other funding sources as the reason for acceptance. Only 22.7% of programs with a program director who thinks pharmaceutical support is unacceptable actually accepted industry support. The likelihood of accepting support was associated with location in the Southern U.S. and was inversely associated with the three-year rolling American Board of Internal Medicine (ABIM) pass rates (each 1% decrease in the pass rate was associated with a 21% increase in the odds of accepting pharmaceutical industry support).
Bottom line: While most program directors did not find pharmaceutical industry support desirable, more than half reported acceptance of such support, with most citing lack of other funding resources as the reason for acceptance.
Citation: Loertscher LL, Halvorsen AJ, Beasley BW, Holmboe ES, Kolars JC, McDonald FS. Pharmaceutical industry support and residency education: a survey of internal medicine program directors. Arch Intern Med. 2010;170(4):356-362.
Early Cholecystectomy Safely Decreases Hospital Stay in Patients with Mild Gallstone Pancreatitis
Clinical question: Can laparoscopic cholecystectomy performed within 48 hours of admission for mild gallstone pancreatitis reduce hospital length of stay without increasing perioperative complications?
Background: Although there is a clear consensus that patients who present with gallstone pancreatitis should undergo cholecystectomy to prevent recurrence, precise timing of surgery remains controversial.
Study design: Randomized prospective trial.
Setting: Harbor-UCLA Medical Center, a Los Angeles County public teaching hospital and Level I trauma center.
Synopsis: Patients were prospectively randomized to an early group and a control group. Inclusion criteria consisted of adults from the ages of 18 to 100 with mild gallstone pancreatitis and three or fewer Ranson criteria. The primary endpoint was length of hospital stay. The secondary endpoint was a composite of complications, including the need for conversion to open cholecystectomy, readmission within 30 days, bleeding requiring transfusion, bile duct injury, or wound infection.
The study was terminated after 50 patients, as there was a difference in the length of hospital stay with a predefined alpha level of 0.005. Patients in the early group were taken to the operating room at a mean of 35.1 hours after admission, compared with 77.8 hours in the control group. The overall length of hospital stay was shorter in the early group (mean 3.5 days, 95% CI, 2.7-4.3), compared with the control group (mean 5.8, 95% CI, 3.8-7.9). All cholecystectomies were completed laparoscopically, without conversion to open. No statistically significant difference existed in secondary endpoints (P=0.48, OR 1.66, 95% CI, 0.41-6.78).
Bottom line: Laparoscopic cholecystectomy performed within 48 hours of admission, irrespective of normalization of laboratory values or clinical progress, safely decreases the overall length of stay, compared with delaying laparoscopic cholecystectomy until laboratory values and clinical condition normalize.
Citation: Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg. 2010;251(4): 615-619.
Presence of Microbial DNA in Blood Correlates with Disease Severity
Clinical question: Is the presence of microbial DNA in the blood associated with disease severity in severe sepsis, and how does detection of this microbial DNA by polymerase chain reaction (PCR) compare with blood cultures (BC)?
Background: Inadequate antibiotic therapy is a strong and independent predictor of poor outcomes in sepsis. Diagnostic uncertainty regarding the causative micro-organism is compensated for by liberal use of broad-spectrum antibiotics. As a result, resistance to antibiotics is an increasing public-health problem.
Study design: Prospective multicenter controlled observational study.
Setting: Three ICUs in Germany and France.
Synopsis: From 2005 to 2007, 63 patients were enrolled in the control group and 142 patients were enrolled in the sepsis group. In control patients, blood cultures and specimens were drawn daily at a maximum of three days after admission. In the sepsis group, blood samples were obtained on the day severe sepsis was suspected. Consecutive samples for the next two days after study inclusion were taken.
Taking BC as the laboratory comparison method, the sensitivity of PCR to detect culture-positive bacteremia in sepsis was 0.80 with a specificity of 0.77. PCR detected 29 of 41 microorganisms (70.3%) found in the BC. The highest recovery rate was observed for gram-negative bacteria (78.6%), fungi (50.0%), and gram-positive bacteria (47.6%). PCR from septic patients correlated well with markers of host response (IL-6 and PCT) and disease severity (SOFA score), even when the BC remained negative.
The appropriateness of antimicrobial therapy based on culture-based methods was not recorded, so it’s impossible to conclude whether or not the PCR would have contributed to a more effective therapy.
Bottom line: Concordance between BC and PCR is moderate in septic patients. PCR-based pathogen detection correlated with disease severity even if the BC remained negative, suggesting that the presence of microbial DNA in the bloodstream is a clinically significant event.
Citation: Bloos F, Hinder F, Becker K, et al. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010;36(2):241-247.
Adding Rifampicin to Vancomycin Improves Outcomes in MRSA Pneumonia
Clinical question: Does adding rifampicin to vancomycin improve outcomes in patients with hospital-acquired MRSA pneumonia?
Background: Hospital-acquired MRSA pneumonia has a mortality of more than 20%. Vancomycin penetrates the lung tissue poorly. The value of adding rifampicin, an antibiotic with broad-spectrum coverage and good tissue penetration, was investigated.
Study design: Randomized open-label trial.
Setting: Medical ICU patients at Ulsan College of Medicine, Asan Medical Center, South Korea.
Synopsis: Patients older than 18 years of age with clinical symptoms suggestive of nosocomial pneumonia were randomized to receive vancomycin alone (V) or vancomycin plus rifampicin (VR). Clinicians could add additional antibiotics for gram-negative coverage as needed.
Of the 183 patients screened, 93 met the inclusion criteria and were randomized in a 1:1 ratio. MRSA infection was microbiologically confirmed. Clinical cure rate in VR patients was significantly greater at day 14 compared with the V group (53.7% vs. 31.0%, P=0.047) based on a modified intention-to-treat model. The overall mortality at day 28 did not significantly differ between the groups (22.0% vs. 38.1%, P=0.15), although the 60-day mortality was lower in the VR group (26.8% vs. 50.0%, P=0.042). Mortality from MRSA pneumonia had a trend toward a decrease in the VR group (14.7% vs. 28.6%, P=0.18).
The trial was limited because it was a single-site study and lacked statistical power to assess certain outcomes. Additionally, treatment protocols were not compared with other antimicrobial therapies.
Bottom line: Vancomycin plus rifampicin improves MRSA pneumonia outcomes in ICU patients.
Citation: Jung YJ, Koh Y, Hong SB, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial MRSA pneumonia. Crit Care Med. 2010;38(1):175-180.
Severe Sepsis Syndromes Are Not Always Caused by Bacteremia
Clinical question: What are the common causes of clinical sepsis?
Background: When sepsis is defined by systemic inflammatory response syndrome (SIRS) criteria, the etiology is not always infectious. Rapid initiation of antimicrobial therapy for infectious SIRS is a priority, but it could result in treating a significant number of patients who are not bacteremic.
Study design: Prospective secondary analysis of a registry of patients created to evaluate an institutional standard-of-care protocol.
Setting: Urban, 850-bed, tertiary-care teaching institution in North Carolina.
Synopsis: ED cases meeting the criteria for severe sepsis underwent a secondary review that looked at the cause of the sepsis. Only 45% of patients identified as having severe sepsis were blood-culture-positive during that episode of care. The culture-positive group was more likely to have central lines, malignancies, or reside in a nursing home.
Of the subgroup of culture-negative patients, 52% had another infectious etiology, most commonly pneumonia. Other “noninfectious mimics,” including inflammatory colitis, myocardial infarction, and pulmonary embolism, were noted in 32% of patients in the subgroup, and the cause was not identified in 16% of the patients.
In-hospital mortality was higher in the culture-positive group than in the culture-negative group (25% vs. 4%, P=0.05). There was no evidence of harm in patients with culture-negative sepsis treated for a systemic infection.
Bottom line: Many patients with a clinical picture of severe sepsis will not have positive blood cultures or an infectious etiology.
Citation: Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis. 2010;50(6):814-820.
Comanagement of Surgical Inpatients by Hospitalists Is Rapidly Expanding
Clinical question: What is the prevalence and nature of comanagement of surgical patients by medicine physicians?
Background: Comanagement of surgical patients is a common clinical role for hospitalists, but the relationship is not well characterized in the literature in terms of numbers of patients or types of physicians involved in this practice.
Study design: Retrospective cohort.
Setting: Cross-section of hospitals from a Medicare database.
Synopsis: During the study period, 35.2% of patients were comanaged by a medicine physician—23.7% by a generalist and 14% by a subspecialist. Cardiothoracic surgery patients were more likely to be comanaged by a subspecialist, whereas all other patients were more likely to be comanaged by a generalist.
Although subspecialist comanagement actually declined during the study period, overall comanagement increased from 33.3% in 1996 to 40.8% in 2006. This increase is entirely attributable to the increase in comanagement by hospitalists. Most of this growth occurred with orthopedic patients.
Patient factors associated with comanagement include advanced age, emergency admissions, and increasing comorbidities. Teaching hospitals had less comanagement, while midsize, nonteaching, and for-profit hospitals had more comanagement.
Bottom line: Comanagement of surgical patients by medicine physicians is a common and growing clinical relationship. Hospitalists are responsible for increasing numbers of comanaged surgical patients.
Citation: Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368.
Probiotics Might Decrease Risk of Ventilator-Associated Pneumonia
Clinical question: Does the administration of probiotics decrease the incidence of ventilator-associated pneumonia in critically ill patients?
Background: Ventilator-associated pneumonia (VAP) is a major nosocomial infection in ICUs. Probiotics are thought to decrease colonization and, therefore, infection with serious hospital-acquired pathogens.
Study design: Meta-analysis of five randomized controlled trials.
Setting: ICU patients on mechanical ventilation for at least 24 hours.
Synopsis: Five trials met the inclusion criteria of comparing probiotics to placebo in critically ill patients on mechanical ventilation and reporting the outcome of VAP. Administration of probiotics decreased the incidence of VAP (odds ratio 0.61, 95% CI, 0.41-0.91) and colonization of the respiratory tract with Pseudomonas aeruginosa (OR 0.35, 95% CI, 0.13-0.93).
Length of ICU stay was decreased in the probiotic arm, although this effect was not statistically significant in all analyses. Probiotics had no effect on such outcomes as ICU mortality, in-hospital mortality, or duration of mechanical ventilation.
Bottom line: Probiotics might be an effective strategy to reduce the risk of VAP, even if they do not appear to impact such outcomes as mortality.
Citation: Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(3):954-962. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk factors for iatrogenic pneumothorax
- Residency acceptance and use of pharmaceutical industry funding
- Early cholecystectomy outcomes for gallstone pancreatitis
- Use of microbial DNA in sepsis
- Adding rifampicin to vancomycin in MRSA pneumonia
- Rate and outcomes of culture-negative severe sepsis
- Rates of surgical comanagement in U.S. hospitals
- Probiotics and rates of ventilator-associated pneumonia
Ultrasound Guidance and Operator Experience Decrease Risk of Pneumothorax Following Thoracentesis
Clinical question: How often does pneumothorax happen following thoracentesis, and what factors are associated with increased risk of this complication?
Background: Procedural complications are an important source of adverse events in the hospital. Iatrogenic pneumothorax after thoracentesis results in increased hospital length of stay, morbidity, and mortality. Large variation exists in reported pneumothorax rates, and little is known about procedure- and patient-specific factors associated with development of this complication.
Study design: Systematic review and meta-analysis.
Setting: Review of 24 MEDLINE-indexed studies from January 1966 to April 2009.
Synopsis: A total of 349 pneumothoraces were reported after 6,605 thoracenteses (overall incidence 6.0%). Chest-tube insertion was required in 34.1% of the cases. Risk for pneumothorax was significantly higher when larger needles or catheters were used compared with needles smaller than 20-gauge (odds ratio 2.5, 95% confidence interval [CI], 1.1-6.0) and after therapeutic thoracentesis compared with diagnostic procedures (OR 2.6, 95% CI, 1.8-3.8).
Procedures requiring two or more needle passes did not significantly increase pneumothorax risk (OR 2.5, 95% CI, 0.3-20.1). In contrast, pneumothorax rates were significantly lower when using ultrasound guidance (OR 0.3, 95% CI, 0.2-0.7) and with experienced operators (3.9% vs. 8.5%, P=0.04).
Examining patient risk factors, pneumothorax rates were similar regardless of effusion size and patient gender. Additionally, rates were similar among non-ICU inpatients, ICU inpatients, and outpatients. Data did show a trend toward increased risk of pneumothorax with mechanical ventilation (OR 4.0, 95% CI, 0.95-16.8), although no study directly compared rates in ICU patients with and without mechanical ventilation.
Bottom line: Ultrasound guidance is a modifiable factor that decreases the risk of post-thoracentesis pneumothorax. Pneumothorax rates are lower when performed by experienced clinicians, providing an important opportunity to reduce procedure-related complications by increasing direct trainee supervision.
Citation: Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339.
Pharmaceutical Industry Support Is Common in U.S. Internal-Medicine Residency Programs
Clinical question: What are the current attitudes of program directors regarding pharmaceutical industry support of internal-medicine residency activities? What are the potential associations between program characteristics and acceptance of industry support?
Background: Increasing evidence suggests that interactions with the pharmaceutical industry influence physician attitudes and practices. Recently, the Association of American Medical Colleges (AAMC) proposed that academic medical centers prohibit the acceptance of all gifts and restrict access by pharmaceutical industry representatives.
Study design: Survey of U.S. internal-medicine residency program directors.
Setting: Web-based survey of residency program directors in 388 U.S. internal-medicine residency programs.
Synopsis: Of the 236 program directors responding to the survey, 132 (55.9%) reported accepting some kind of support from the pharmaceutical industry. Support was most commonly provided in the form of food for conferences (90.9%), educational materials (83.3%), office supplies (68.9%), and drug samples (57.6%).
When programs reported accepting pharmaceutical industry support, 67.9% cited a lack of other funding sources as the reason for acceptance. Only 22.7% of programs with a program director who thinks pharmaceutical support is unacceptable actually accepted industry support. The likelihood of accepting support was associated with location in the Southern U.S. and was inversely associated with the three-year rolling American Board of Internal Medicine (ABIM) pass rates (each 1% decrease in the pass rate was associated with a 21% increase in the odds of accepting pharmaceutical industry support).
Bottom line: While most program directors did not find pharmaceutical industry support desirable, more than half reported acceptance of such support, with most citing lack of other funding resources as the reason for acceptance.
Citation: Loertscher LL, Halvorsen AJ, Beasley BW, Holmboe ES, Kolars JC, McDonald FS. Pharmaceutical industry support and residency education: a survey of internal medicine program directors. Arch Intern Med. 2010;170(4):356-362.
Early Cholecystectomy Safely Decreases Hospital Stay in Patients with Mild Gallstone Pancreatitis
Clinical question: Can laparoscopic cholecystectomy performed within 48 hours of admission for mild gallstone pancreatitis reduce hospital length of stay without increasing perioperative complications?
Background: Although there is a clear consensus that patients who present with gallstone pancreatitis should undergo cholecystectomy to prevent recurrence, precise timing of surgery remains controversial.
Study design: Randomized prospective trial.
Setting: Harbor-UCLA Medical Center, a Los Angeles County public teaching hospital and Level I trauma center.
Synopsis: Patients were prospectively randomized to an early group and a control group. Inclusion criteria consisted of adults from the ages of 18 to 100 with mild gallstone pancreatitis and three or fewer Ranson criteria. The primary endpoint was length of hospital stay. The secondary endpoint was a composite of complications, including the need for conversion to open cholecystectomy, readmission within 30 days, bleeding requiring transfusion, bile duct injury, or wound infection.
The study was terminated after 50 patients, as there was a difference in the length of hospital stay with a predefined alpha level of 0.005. Patients in the early group were taken to the operating room at a mean of 35.1 hours after admission, compared with 77.8 hours in the control group. The overall length of hospital stay was shorter in the early group (mean 3.5 days, 95% CI, 2.7-4.3), compared with the control group (mean 5.8, 95% CI, 3.8-7.9). All cholecystectomies were completed laparoscopically, without conversion to open. No statistically significant difference existed in secondary endpoints (P=0.48, OR 1.66, 95% CI, 0.41-6.78).
Bottom line: Laparoscopic cholecystectomy performed within 48 hours of admission, irrespective of normalization of laboratory values or clinical progress, safely decreases the overall length of stay, compared with delaying laparoscopic cholecystectomy until laboratory values and clinical condition normalize.
Citation: Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg. 2010;251(4): 615-619.
Presence of Microbial DNA in Blood Correlates with Disease Severity
Clinical question: Is the presence of microbial DNA in the blood associated with disease severity in severe sepsis, and how does detection of this microbial DNA by polymerase chain reaction (PCR) compare with blood cultures (BC)?
Background: Inadequate antibiotic therapy is a strong and independent predictor of poor outcomes in sepsis. Diagnostic uncertainty regarding the causative micro-organism is compensated for by liberal use of broad-spectrum antibiotics. As a result, resistance to antibiotics is an increasing public-health problem.
Study design: Prospective multicenter controlled observational study.
Setting: Three ICUs in Germany and France.
Synopsis: From 2005 to 2007, 63 patients were enrolled in the control group and 142 patients were enrolled in the sepsis group. In control patients, blood cultures and specimens were drawn daily at a maximum of three days after admission. In the sepsis group, blood samples were obtained on the day severe sepsis was suspected. Consecutive samples for the next two days after study inclusion were taken.
Taking BC as the laboratory comparison method, the sensitivity of PCR to detect culture-positive bacteremia in sepsis was 0.80 with a specificity of 0.77. PCR detected 29 of 41 microorganisms (70.3%) found in the BC. The highest recovery rate was observed for gram-negative bacteria (78.6%), fungi (50.0%), and gram-positive bacteria (47.6%). PCR from septic patients correlated well with markers of host response (IL-6 and PCT) and disease severity (SOFA score), even when the BC remained negative.
The appropriateness of antimicrobial therapy based on culture-based methods was not recorded, so it’s impossible to conclude whether or not the PCR would have contributed to a more effective therapy.
Bottom line: Concordance between BC and PCR is moderate in septic patients. PCR-based pathogen detection correlated with disease severity even if the BC remained negative, suggesting that the presence of microbial DNA in the bloodstream is a clinically significant event.
Citation: Bloos F, Hinder F, Becker K, et al. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010;36(2):241-247.
Adding Rifampicin to Vancomycin Improves Outcomes in MRSA Pneumonia
Clinical question: Does adding rifampicin to vancomycin improve outcomes in patients with hospital-acquired MRSA pneumonia?
Background: Hospital-acquired MRSA pneumonia has a mortality of more than 20%. Vancomycin penetrates the lung tissue poorly. The value of adding rifampicin, an antibiotic with broad-spectrum coverage and good tissue penetration, was investigated.
Study design: Randomized open-label trial.
Setting: Medical ICU patients at Ulsan College of Medicine, Asan Medical Center, South Korea.
Synopsis: Patients older than 18 years of age with clinical symptoms suggestive of nosocomial pneumonia were randomized to receive vancomycin alone (V) or vancomycin plus rifampicin (VR). Clinicians could add additional antibiotics for gram-negative coverage as needed.
Of the 183 patients screened, 93 met the inclusion criteria and were randomized in a 1:1 ratio. MRSA infection was microbiologically confirmed. Clinical cure rate in VR patients was significantly greater at day 14 compared with the V group (53.7% vs. 31.0%, P=0.047) based on a modified intention-to-treat model. The overall mortality at day 28 did not significantly differ between the groups (22.0% vs. 38.1%, P=0.15), although the 60-day mortality was lower in the VR group (26.8% vs. 50.0%, P=0.042). Mortality from MRSA pneumonia had a trend toward a decrease in the VR group (14.7% vs. 28.6%, P=0.18).
The trial was limited because it was a single-site study and lacked statistical power to assess certain outcomes. Additionally, treatment protocols were not compared with other antimicrobial therapies.
Bottom line: Vancomycin plus rifampicin improves MRSA pneumonia outcomes in ICU patients.
Citation: Jung YJ, Koh Y, Hong SB, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial MRSA pneumonia. Crit Care Med. 2010;38(1):175-180.
Severe Sepsis Syndromes Are Not Always Caused by Bacteremia
Clinical question: What are the common causes of clinical sepsis?
Background: When sepsis is defined by systemic inflammatory response syndrome (SIRS) criteria, the etiology is not always infectious. Rapid initiation of antimicrobial therapy for infectious SIRS is a priority, but it could result in treating a significant number of patients who are not bacteremic.
Study design: Prospective secondary analysis of a registry of patients created to evaluate an institutional standard-of-care protocol.
Setting: Urban, 850-bed, tertiary-care teaching institution in North Carolina.
Synopsis: ED cases meeting the criteria for severe sepsis underwent a secondary review that looked at the cause of the sepsis. Only 45% of patients identified as having severe sepsis were blood-culture-positive during that episode of care. The culture-positive group was more likely to have central lines, malignancies, or reside in a nursing home.
Of the subgroup of culture-negative patients, 52% had another infectious etiology, most commonly pneumonia. Other “noninfectious mimics,” including inflammatory colitis, myocardial infarction, and pulmonary embolism, were noted in 32% of patients in the subgroup, and the cause was not identified in 16% of the patients.
In-hospital mortality was higher in the culture-positive group than in the culture-negative group (25% vs. 4%, P=0.05). There was no evidence of harm in patients with culture-negative sepsis treated for a systemic infection.
Bottom line: Many patients with a clinical picture of severe sepsis will not have positive blood cultures or an infectious etiology.
Citation: Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis. 2010;50(6):814-820.
Comanagement of Surgical Inpatients by Hospitalists Is Rapidly Expanding
Clinical question: What is the prevalence and nature of comanagement of surgical patients by medicine physicians?
Background: Comanagement of surgical patients is a common clinical role for hospitalists, but the relationship is not well characterized in the literature in terms of numbers of patients or types of physicians involved in this practice.
Study design: Retrospective cohort.
Setting: Cross-section of hospitals from a Medicare database.
Synopsis: During the study period, 35.2% of patients were comanaged by a medicine physician—23.7% by a generalist and 14% by a subspecialist. Cardiothoracic surgery patients were more likely to be comanaged by a subspecialist, whereas all other patients were more likely to be comanaged by a generalist.
Although subspecialist comanagement actually declined during the study period, overall comanagement increased from 33.3% in 1996 to 40.8% in 2006. This increase is entirely attributable to the increase in comanagement by hospitalists. Most of this growth occurred with orthopedic patients.
Patient factors associated with comanagement include advanced age, emergency admissions, and increasing comorbidities. Teaching hospitals had less comanagement, while midsize, nonteaching, and for-profit hospitals had more comanagement.
Bottom line: Comanagement of surgical patients by medicine physicians is a common and growing clinical relationship. Hospitalists are responsible for increasing numbers of comanaged surgical patients.
Citation: Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368.
Probiotics Might Decrease Risk of Ventilator-Associated Pneumonia
Clinical question: Does the administration of probiotics decrease the incidence of ventilator-associated pneumonia in critically ill patients?
Background: Ventilator-associated pneumonia (VAP) is a major nosocomial infection in ICUs. Probiotics are thought to decrease colonization and, therefore, infection with serious hospital-acquired pathogens.
Study design: Meta-analysis of five randomized controlled trials.
Setting: ICU patients on mechanical ventilation for at least 24 hours.
Synopsis: Five trials met the inclusion criteria of comparing probiotics to placebo in critically ill patients on mechanical ventilation and reporting the outcome of VAP. Administration of probiotics decreased the incidence of VAP (odds ratio 0.61, 95% CI, 0.41-0.91) and colonization of the respiratory tract with Pseudomonas aeruginosa (OR 0.35, 95% CI, 0.13-0.93).
Length of ICU stay was decreased in the probiotic arm, although this effect was not statistically significant in all analyses. Probiotics had no effect on such outcomes as ICU mortality, in-hospital mortality, or duration of mechanical ventilation.
Bottom line: Probiotics might be an effective strategy to reduce the risk of VAP, even if they do not appear to impact such outcomes as mortality.
Citation: Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(3):954-962. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk factors for iatrogenic pneumothorax
- Residency acceptance and use of pharmaceutical industry funding
- Early cholecystectomy outcomes for gallstone pancreatitis
- Use of microbial DNA in sepsis
- Adding rifampicin to vancomycin in MRSA pneumonia
- Rate and outcomes of culture-negative severe sepsis
- Rates of surgical comanagement in U.S. hospitals
- Probiotics and rates of ventilator-associated pneumonia
Ultrasound Guidance and Operator Experience Decrease Risk of Pneumothorax Following Thoracentesis
Clinical question: How often does pneumothorax happen following thoracentesis, and what factors are associated with increased risk of this complication?
Background: Procedural complications are an important source of adverse events in the hospital. Iatrogenic pneumothorax after thoracentesis results in increased hospital length of stay, morbidity, and mortality. Large variation exists in reported pneumothorax rates, and little is known about procedure- and patient-specific factors associated with development of this complication.
Study design: Systematic review and meta-analysis.
Setting: Review of 24 MEDLINE-indexed studies from January 1966 to April 2009.
Synopsis: A total of 349 pneumothoraces were reported after 6,605 thoracenteses (overall incidence 6.0%). Chest-tube insertion was required in 34.1% of the cases. Risk for pneumothorax was significantly higher when larger needles or catheters were used compared with needles smaller than 20-gauge (odds ratio 2.5, 95% confidence interval [CI], 1.1-6.0) and after therapeutic thoracentesis compared with diagnostic procedures (OR 2.6, 95% CI, 1.8-3.8).
Procedures requiring two or more needle passes did not significantly increase pneumothorax risk (OR 2.5, 95% CI, 0.3-20.1). In contrast, pneumothorax rates were significantly lower when using ultrasound guidance (OR 0.3, 95% CI, 0.2-0.7) and with experienced operators (3.9% vs. 8.5%, P=0.04).
Examining patient risk factors, pneumothorax rates were similar regardless of effusion size and patient gender. Additionally, rates were similar among non-ICU inpatients, ICU inpatients, and outpatients. Data did show a trend toward increased risk of pneumothorax with mechanical ventilation (OR 4.0, 95% CI, 0.95-16.8), although no study directly compared rates in ICU patients with and without mechanical ventilation.
Bottom line: Ultrasound guidance is a modifiable factor that decreases the risk of post-thoracentesis pneumothorax. Pneumothorax rates are lower when performed by experienced clinicians, providing an important opportunity to reduce procedure-related complications by increasing direct trainee supervision.
Citation: Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339.
Pharmaceutical Industry Support Is Common in U.S. Internal-Medicine Residency Programs
Clinical question: What are the current attitudes of program directors regarding pharmaceutical industry support of internal-medicine residency activities? What are the potential associations between program characteristics and acceptance of industry support?
Background: Increasing evidence suggests that interactions with the pharmaceutical industry influence physician attitudes and practices. Recently, the Association of American Medical Colleges (AAMC) proposed that academic medical centers prohibit the acceptance of all gifts and restrict access by pharmaceutical industry representatives.
Study design: Survey of U.S. internal-medicine residency program directors.
Setting: Web-based survey of residency program directors in 388 U.S. internal-medicine residency programs.
Synopsis: Of the 236 program directors responding to the survey, 132 (55.9%) reported accepting some kind of support from the pharmaceutical industry. Support was most commonly provided in the form of food for conferences (90.9%), educational materials (83.3%), office supplies (68.9%), and drug samples (57.6%).
When programs reported accepting pharmaceutical industry support, 67.9% cited a lack of other funding sources as the reason for acceptance. Only 22.7% of programs with a program director who thinks pharmaceutical support is unacceptable actually accepted industry support. The likelihood of accepting support was associated with location in the Southern U.S. and was inversely associated with the three-year rolling American Board of Internal Medicine (ABIM) pass rates (each 1% decrease in the pass rate was associated with a 21% increase in the odds of accepting pharmaceutical industry support).
Bottom line: While most program directors did not find pharmaceutical industry support desirable, more than half reported acceptance of such support, with most citing lack of other funding resources as the reason for acceptance.
Citation: Loertscher LL, Halvorsen AJ, Beasley BW, Holmboe ES, Kolars JC, McDonald FS. Pharmaceutical industry support and residency education: a survey of internal medicine program directors. Arch Intern Med. 2010;170(4):356-362.
Early Cholecystectomy Safely Decreases Hospital Stay in Patients with Mild Gallstone Pancreatitis
Clinical question: Can laparoscopic cholecystectomy performed within 48 hours of admission for mild gallstone pancreatitis reduce hospital length of stay without increasing perioperative complications?
Background: Although there is a clear consensus that patients who present with gallstone pancreatitis should undergo cholecystectomy to prevent recurrence, precise timing of surgery remains controversial.
Study design: Randomized prospective trial.
Setting: Harbor-UCLA Medical Center, a Los Angeles County public teaching hospital and Level I trauma center.
Synopsis: Patients were prospectively randomized to an early group and a control group. Inclusion criteria consisted of adults from the ages of 18 to 100 with mild gallstone pancreatitis and three or fewer Ranson criteria. The primary endpoint was length of hospital stay. The secondary endpoint was a composite of complications, including the need for conversion to open cholecystectomy, readmission within 30 days, bleeding requiring transfusion, bile duct injury, or wound infection.
The study was terminated after 50 patients, as there was a difference in the length of hospital stay with a predefined alpha level of 0.005. Patients in the early group were taken to the operating room at a mean of 35.1 hours after admission, compared with 77.8 hours in the control group. The overall length of hospital stay was shorter in the early group (mean 3.5 days, 95% CI, 2.7-4.3), compared with the control group (mean 5.8, 95% CI, 3.8-7.9). All cholecystectomies were completed laparoscopically, without conversion to open. No statistically significant difference existed in secondary endpoints (P=0.48, OR 1.66, 95% CI, 0.41-6.78).
Bottom line: Laparoscopic cholecystectomy performed within 48 hours of admission, irrespective of normalization of laboratory values or clinical progress, safely decreases the overall length of stay, compared with delaying laparoscopic cholecystectomy until laboratory values and clinical condition normalize.
Citation: Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg. 2010;251(4): 615-619.
Presence of Microbial DNA in Blood Correlates with Disease Severity
Clinical question: Is the presence of microbial DNA in the blood associated with disease severity in severe sepsis, and how does detection of this microbial DNA by polymerase chain reaction (PCR) compare with blood cultures (BC)?
Background: Inadequate antibiotic therapy is a strong and independent predictor of poor outcomes in sepsis. Diagnostic uncertainty regarding the causative micro-organism is compensated for by liberal use of broad-spectrum antibiotics. As a result, resistance to antibiotics is an increasing public-health problem.
Study design: Prospective multicenter controlled observational study.
Setting: Three ICUs in Germany and France.
Synopsis: From 2005 to 2007, 63 patients were enrolled in the control group and 142 patients were enrolled in the sepsis group. In control patients, blood cultures and specimens were drawn daily at a maximum of three days after admission. In the sepsis group, blood samples were obtained on the day severe sepsis was suspected. Consecutive samples for the next two days after study inclusion were taken.
Taking BC as the laboratory comparison method, the sensitivity of PCR to detect culture-positive bacteremia in sepsis was 0.80 with a specificity of 0.77. PCR detected 29 of 41 microorganisms (70.3%) found in the BC. The highest recovery rate was observed for gram-negative bacteria (78.6%), fungi (50.0%), and gram-positive bacteria (47.6%). PCR from septic patients correlated well with markers of host response (IL-6 and PCT) and disease severity (SOFA score), even when the BC remained negative.
The appropriateness of antimicrobial therapy based on culture-based methods was not recorded, so it’s impossible to conclude whether or not the PCR would have contributed to a more effective therapy.
Bottom line: Concordance between BC and PCR is moderate in septic patients. PCR-based pathogen detection correlated with disease severity even if the BC remained negative, suggesting that the presence of microbial DNA in the bloodstream is a clinically significant event.
Citation: Bloos F, Hinder F, Becker K, et al. A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med. 2010;36(2):241-247.
Adding Rifampicin to Vancomycin Improves Outcomes in MRSA Pneumonia
Clinical question: Does adding rifampicin to vancomycin improve outcomes in patients with hospital-acquired MRSA pneumonia?
Background: Hospital-acquired MRSA pneumonia has a mortality of more than 20%. Vancomycin penetrates the lung tissue poorly. The value of adding rifampicin, an antibiotic with broad-spectrum coverage and good tissue penetration, was investigated.
Study design: Randomized open-label trial.
Setting: Medical ICU patients at Ulsan College of Medicine, Asan Medical Center, South Korea.
Synopsis: Patients older than 18 years of age with clinical symptoms suggestive of nosocomial pneumonia were randomized to receive vancomycin alone (V) or vancomycin plus rifampicin (VR). Clinicians could add additional antibiotics for gram-negative coverage as needed.
Of the 183 patients screened, 93 met the inclusion criteria and were randomized in a 1:1 ratio. MRSA infection was microbiologically confirmed. Clinical cure rate in VR patients was significantly greater at day 14 compared with the V group (53.7% vs. 31.0%, P=0.047) based on a modified intention-to-treat model. The overall mortality at day 28 did not significantly differ between the groups (22.0% vs. 38.1%, P=0.15), although the 60-day mortality was lower in the VR group (26.8% vs. 50.0%, P=0.042). Mortality from MRSA pneumonia had a trend toward a decrease in the VR group (14.7% vs. 28.6%, P=0.18).
The trial was limited because it was a single-site study and lacked statistical power to assess certain outcomes. Additionally, treatment protocols were not compared with other antimicrobial therapies.
Bottom line: Vancomycin plus rifampicin improves MRSA pneumonia outcomes in ICU patients.
Citation: Jung YJ, Koh Y, Hong SB, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial MRSA pneumonia. Crit Care Med. 2010;38(1):175-180.
Severe Sepsis Syndromes Are Not Always Caused by Bacteremia
Clinical question: What are the common causes of clinical sepsis?
Background: When sepsis is defined by systemic inflammatory response syndrome (SIRS) criteria, the etiology is not always infectious. Rapid initiation of antimicrobial therapy for infectious SIRS is a priority, but it could result in treating a significant number of patients who are not bacteremic.
Study design: Prospective secondary analysis of a registry of patients created to evaluate an institutional standard-of-care protocol.
Setting: Urban, 850-bed, tertiary-care teaching institution in North Carolina.
Synopsis: ED cases meeting the criteria for severe sepsis underwent a secondary review that looked at the cause of the sepsis. Only 45% of patients identified as having severe sepsis were blood-culture-positive during that episode of care. The culture-positive group was more likely to have central lines, malignancies, or reside in a nursing home.
Of the subgroup of culture-negative patients, 52% had another infectious etiology, most commonly pneumonia. Other “noninfectious mimics,” including inflammatory colitis, myocardial infarction, and pulmonary embolism, were noted in 32% of patients in the subgroup, and the cause was not identified in 16% of the patients.
In-hospital mortality was higher in the culture-positive group than in the culture-negative group (25% vs. 4%, P=0.05). There was no evidence of harm in patients with culture-negative sepsis treated for a systemic infection.
Bottom line: Many patients with a clinical picture of severe sepsis will not have positive blood cultures or an infectious etiology.
Citation: Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis. 2010;50(6):814-820.
Comanagement of Surgical Inpatients by Hospitalists Is Rapidly Expanding
Clinical question: What is the prevalence and nature of comanagement of surgical patients by medicine physicians?
Background: Comanagement of surgical patients is a common clinical role for hospitalists, but the relationship is not well characterized in the literature in terms of numbers of patients or types of physicians involved in this practice.
Study design: Retrospective cohort.
Setting: Cross-section of hospitals from a Medicare database.
Synopsis: During the study period, 35.2% of patients were comanaged by a medicine physician—23.7% by a generalist and 14% by a subspecialist. Cardiothoracic surgery patients were more likely to be comanaged by a subspecialist, whereas all other patients were more likely to be comanaged by a generalist.
Although subspecialist comanagement actually declined during the study period, overall comanagement increased from 33.3% in 1996 to 40.8% in 2006. This increase is entirely attributable to the increase in comanagement by hospitalists. Most of this growth occurred with orthopedic patients.
Patient factors associated with comanagement include advanced age, emergency admissions, and increasing comorbidities. Teaching hospitals had less comanagement, while midsize, nonteaching, and for-profit hospitals had more comanagement.
Bottom line: Comanagement of surgical patients by medicine physicians is a common and growing clinical relationship. Hospitalists are responsible for increasing numbers of comanaged surgical patients.
Citation: Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368.
Probiotics Might Decrease Risk of Ventilator-Associated Pneumonia
Clinical question: Does the administration of probiotics decrease the incidence of ventilator-associated pneumonia in critically ill patients?
Background: Ventilator-associated pneumonia (VAP) is a major nosocomial infection in ICUs. Probiotics are thought to decrease colonization and, therefore, infection with serious hospital-acquired pathogens.
Study design: Meta-analysis of five randomized controlled trials.
Setting: ICU patients on mechanical ventilation for at least 24 hours.
Synopsis: Five trials met the inclusion criteria of comparing probiotics to placebo in critically ill patients on mechanical ventilation and reporting the outcome of VAP. Administration of probiotics decreased the incidence of VAP (odds ratio 0.61, 95% CI, 0.41-0.91) and colonization of the respiratory tract with Pseudomonas aeruginosa (OR 0.35, 95% CI, 0.13-0.93).
Length of ICU stay was decreased in the probiotic arm, although this effect was not statistically significant in all analyses. Probiotics had no effect on such outcomes as ICU mortality, in-hospital mortality, or duration of mechanical ventilation.
Bottom line: Probiotics might be an effective strategy to reduce the risk of VAP, even if they do not appear to impact such outcomes as mortality.
Citation: Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(3):954-962. TH
Do feeding tubes improve outcomes in patients with dementia?
Case
A 68-year-old cachectic female with a history of Alzheimer’s dementia presents with a slowly progressive decline in functional status. She is bed bound, minimally verbal, and has lost interest in eating.
Her problems with decreased oral intake started when her diet was changed to nectar-thickened liquids. This change was made after the patient was hospitalized multiple times for aspiration pneumonia and she underwent a fluoroscopic swallowing evaluation that revealed aspiration of thin liquids. The patient’s husband requests that a feeding tube be placed so his wife doesn’t “die of pneumonia or starve to death.”
Overview
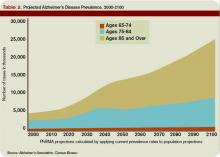
As the U.S. population ages, hospitalists are seeing a steady increase in the average patient age and the prevalence of dementia. Alzheimer’s dementia affects an estimated 4 million to 5 million Americans; this number expected to triple by the year 2050.1
As patients with dementia near the end of life, they often fail to thrive, with less oral intake and more swallowing disorders leading to aspiration. This is when physicians and patient family members must decide whether a feeding tube should be placed.
Placement of a nasogastric or percutaneous endogastric gastrostomy (PEG) feeding tube has become a relatively common medical intervention instituted to maintain or improve a patient’s nutritional status. Prior to 1980, permanent gastric or postpyloric feeding tubes were placed surgically by laparotomy, but the advent of endoscopy and computed tomography (CT) guided procedures offers a simplified procedure requiring only mild sedation and local anesthesia.2
Many patients who suffer multiple bouts of aspiration pneumonia and fail a swallowing evaluation because of an irreversible process are offered a percutaneous feeding tube to maintain nutrition. A feeding tube is also seen as a way to supply nutrition at the end of life in patients no longer able or willing to take food orally.
Although it seems logical that a feeding tube might improve the outcomes of these clinical scenarios, limited literature exists on the topic because of the legal, ethical, emotional, and religious implications a large, randomized, placebo-controlled trial would entail.
Review of the Data
Placement of a PEG has become accepted as a relatively benign procedure, although it is associated with significant morbidity and mortality. Minor complications including pain, abdominal wall ulcers, wound infections, peristomal leakage, and tube displacement occur in approximately 10% of cases.3 Major complications including hemorrhage, bowel or liver perforation, or aspiration occur in 3% of cases.4
These numbers do not account for long-term complications including peristomal infections, leakage problems, or the use of physical restraints to avoid self-extubation.
Aspiration Risk
A common indication for PEG placement is aspiration risk. PEG tubes are often placed in patients who fail swallowing evaluations in order to decrease their risk of aspiration and aspiration pneumonia.
True aspiration pneumonia is thought to originate from an inoculum of oral cavity or nasopharynx bacteria, which placement of a PEG tube would not prevent. Leibovitz, et al., showed that elderly patients with nasogastric or percutaneous feeding tubes are associated with colonization of the oropharynx with more pathogenic bacteria when compared with orally fed patients.5 Thus, the use of PEG tubes might put them at higher risk for pathogenic inoculation.
Aspiration pneumonia occurs in up to 50% of patients with feeding tubes. Studies have shown PEG tube placement decreases lower esophageal sphincter tone, potentially increasing regurgitation risk.6 It has also been shown that aspiration of gastric contents produces a pneumonitis with the resultant inflammatory response allowing for establishment of infection by smaller inoculums of or less virulent organisms.7
Small, randomized trials have shown no decrease in aspiration risk with post-pyloric versus gastric feeding tubes, nasogastric versus percutaneous feeding tubes, or continuous versus intermittent tube feeds.8 There have been no sizable randomized prospective trials to determine if feeding tube placement versus hand feeding patients with end-stage dementia alters aspiration pneumonia risk.
Pressure Ulcers
Patients with end-stage dementia often become bed bound as their disease progresses, and they commonly suffer from pressure ulcers. Pressure ulcers often coexist in patients with malnutrition, and it is well established that patients with biochemical markers of malnutrition are at higher risk for pressure ulcer formation.
Still, no studies show that improved nutrition prevents pressure ulcer formation. In a nursing home population of patients with dementia, a two-year follow-up study showed no significant improvement in pressure ulcer healing or decreased ulcer formation with nutrition by feeding tube.9 These studies are adjusted for independent risk factors for mortality and indication for PEG placement, but we can assume there are confounders that go into the decision for feeding tube placement that are not necessarily identifiable.
Nutritional Status
Family members are often concerned that if the patient is unable to take food by mouth and no feeding tube is placed, then the patient will suffer from the discomfort of starvation and dehydration.
As a patient with a severe dementing illness enters the end stage of his/her clinical course, practitioners frequently make a plan with families to change the goals of care toward keeping the patient comfortable. Comfort is a difficult clinical parameter to measure, but studies in the hospice population of patients with end-stage cancer and AIDS report that the hunger and thirst are transient and improve with ice chips and mouth swabs.10
Despite the lack of evidence of PEG tubes prolonging survival in patients with dementia who are no longer able or willing to take in food orally, it is logical that withholding all hydration or nutritional support will hasten death despite the risks associated with feeding tubes. This is where the ethical argument arises regarding prolonging life of decreasing quality.
In certain medical and legal sectors, artificial nutrition, and hydration are considered a medical intervention. Therefore, the ideals of patient autonomy dictate that the patient’s proxy should decide whether or not the patient would have wanted the intervention after weighing the risks and benefits.
If hospitalists view artificial nutrition as a medical intervention, our moral obligation is to instruct patients and their families about these risks and benefits.
Often, the patient will not clinically improve with artificial nutrition. But we can maintain physiologic processes or at least slow their decline.
Emerging research indicates the standard of care in how we present this information is changing to include presentation of data instead of only using a patient’s suspected beliefs about quality of life.
A useful algorithm proposed by Rabeneck, et al., provides comprehensive guidelines for PEG placement in all patient populations based on the reason for PEG consideration.11
Back to the Case
Our patient is likely nearing the end of her life because of end-stage dementia. There is no evidence to suggest placement of a feeding tube would extend her life more than hand feeding.
We know feeding-tube placement could increase aspiration pneumonia risk and significant short- and long-term morbidity and mortality. We can keep her comfortable with small amounts of water, wetting her lips with swabs. If a feeding tube is placed, its use should be evaluated based on the patient’s clinical course. TH
Dr. Pell is an instructor of medicine in the Section of Hospital Medicine at the University of Colorado, Denver.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875.
- Hebert LE, Beckett LA, Scherr PA, and Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169-173.
- Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973-1976.
- Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13(6):520-523.
- Leibovitz A, Plotnikov G, Habot B, et al. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med. 2003;58(1):52-55.
- McCann R. Lack of evidence about tube feeding: food for thought. JAMA. 1999;282(14):1380-1381.
- Cameron JL, Caldini P, Toung J-K, et al. Aspiration pneumonia: physiologic data following experimental Aspiration. Surgery. 1972;72:238.
- Loeb MB, Becker M, Eady A, et al. Interventions to prevent aspiration pneumonia in older adults: a systematic review. JAGS. 2003;51(7):1018-1022.
- Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327-332.
- McCann RM, Hall WJ, Groth-Junker A. Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA. 1994;272:1263-1266.
- Rabeneck L, McCullough LB. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997;349(9050):496-498.
Case
A 68-year-old cachectic female with a history of Alzheimer’s dementia presents with a slowly progressive decline in functional status. She is bed bound, minimally verbal, and has lost interest in eating.
Her problems with decreased oral intake started when her diet was changed to nectar-thickened liquids. This change was made after the patient was hospitalized multiple times for aspiration pneumonia and she underwent a fluoroscopic swallowing evaluation that revealed aspiration of thin liquids. The patient’s husband requests that a feeding tube be placed so his wife doesn’t “die of pneumonia or starve to death.”
Overview
As the U.S. population ages, hospitalists are seeing a steady increase in the average patient age and the prevalence of dementia. Alzheimer’s dementia affects an estimated 4 million to 5 million Americans; this number expected to triple by the year 2050.1
As patients with dementia near the end of life, they often fail to thrive, with less oral intake and more swallowing disorders leading to aspiration. This is when physicians and patient family members must decide whether a feeding tube should be placed.
Placement of a nasogastric or percutaneous endogastric gastrostomy (PEG) feeding tube has become a relatively common medical intervention instituted to maintain or improve a patient’s nutritional status. Prior to 1980, permanent gastric or postpyloric feeding tubes were placed surgically by laparotomy, but the advent of endoscopy and computed tomography (CT) guided procedures offers a simplified procedure requiring only mild sedation and local anesthesia.2
Many patients who suffer multiple bouts of aspiration pneumonia and fail a swallowing evaluation because of an irreversible process are offered a percutaneous feeding tube to maintain nutrition. A feeding tube is also seen as a way to supply nutrition at the end of life in patients no longer able or willing to take food orally.
Although it seems logical that a feeding tube might improve the outcomes of these clinical scenarios, limited literature exists on the topic because of the legal, ethical, emotional, and religious implications a large, randomized, placebo-controlled trial would entail.
Review of the Data
Placement of a PEG has become accepted as a relatively benign procedure, although it is associated with significant morbidity and mortality. Minor complications including pain, abdominal wall ulcers, wound infections, peristomal leakage, and tube displacement occur in approximately 10% of cases.3 Major complications including hemorrhage, bowel or liver perforation, or aspiration occur in 3% of cases.4
These numbers do not account for long-term complications including peristomal infections, leakage problems, or the use of physical restraints to avoid self-extubation.
Aspiration Risk
A common indication for PEG placement is aspiration risk. PEG tubes are often placed in patients who fail swallowing evaluations in order to decrease their risk of aspiration and aspiration pneumonia.
True aspiration pneumonia is thought to originate from an inoculum of oral cavity or nasopharynx bacteria, which placement of a PEG tube would not prevent. Leibovitz, et al., showed that elderly patients with nasogastric or percutaneous feeding tubes are associated with colonization of the oropharynx with more pathogenic bacteria when compared with orally fed patients.5 Thus, the use of PEG tubes might put them at higher risk for pathogenic inoculation.
Aspiration pneumonia occurs in up to 50% of patients with feeding tubes. Studies have shown PEG tube placement decreases lower esophageal sphincter tone, potentially increasing regurgitation risk.6 It has also been shown that aspiration of gastric contents produces a pneumonitis with the resultant inflammatory response allowing for establishment of infection by smaller inoculums of or less virulent organisms.7
Small, randomized trials have shown no decrease in aspiration risk with post-pyloric versus gastric feeding tubes, nasogastric versus percutaneous feeding tubes, or continuous versus intermittent tube feeds.8 There have been no sizable randomized prospective trials to determine if feeding tube placement versus hand feeding patients with end-stage dementia alters aspiration pneumonia risk.
Pressure Ulcers
Patients with end-stage dementia often become bed bound as their disease progresses, and they commonly suffer from pressure ulcers. Pressure ulcers often coexist in patients with malnutrition, and it is well established that patients with biochemical markers of malnutrition are at higher risk for pressure ulcer formation.
Still, no studies show that improved nutrition prevents pressure ulcer formation. In a nursing home population of patients with dementia, a two-year follow-up study showed no significant improvement in pressure ulcer healing or decreased ulcer formation with nutrition by feeding tube.9 These studies are adjusted for independent risk factors for mortality and indication for PEG placement, but we can assume there are confounders that go into the decision for feeding tube placement that are not necessarily identifiable.
Nutritional Status
Family members are often concerned that if the patient is unable to take food by mouth and no feeding tube is placed, then the patient will suffer from the discomfort of starvation and dehydration.
As a patient with a severe dementing illness enters the end stage of his/her clinical course, practitioners frequently make a plan with families to change the goals of care toward keeping the patient comfortable. Comfort is a difficult clinical parameter to measure, but studies in the hospice population of patients with end-stage cancer and AIDS report that the hunger and thirst are transient and improve with ice chips and mouth swabs.10
Despite the lack of evidence of PEG tubes prolonging survival in patients with dementia who are no longer able or willing to take in food orally, it is logical that withholding all hydration or nutritional support will hasten death despite the risks associated with feeding tubes. This is where the ethical argument arises regarding prolonging life of decreasing quality.
In certain medical and legal sectors, artificial nutrition, and hydration are considered a medical intervention. Therefore, the ideals of patient autonomy dictate that the patient’s proxy should decide whether or not the patient would have wanted the intervention after weighing the risks and benefits.
If hospitalists view artificial nutrition as a medical intervention, our moral obligation is to instruct patients and their families about these risks and benefits.
Often, the patient will not clinically improve with artificial nutrition. But we can maintain physiologic processes or at least slow their decline.
Emerging research indicates the standard of care in how we present this information is changing to include presentation of data instead of only using a patient’s suspected beliefs about quality of life.
A useful algorithm proposed by Rabeneck, et al., provides comprehensive guidelines for PEG placement in all patient populations based on the reason for PEG consideration.11
Back to the Case
Our patient is likely nearing the end of her life because of end-stage dementia. There is no evidence to suggest placement of a feeding tube would extend her life more than hand feeding.
We know feeding-tube placement could increase aspiration pneumonia risk and significant short- and long-term morbidity and mortality. We can keep her comfortable with small amounts of water, wetting her lips with swabs. If a feeding tube is placed, its use should be evaluated based on the patient’s clinical course. TH
Dr. Pell is an instructor of medicine in the Section of Hospital Medicine at the University of Colorado, Denver.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875.
- Hebert LE, Beckett LA, Scherr PA, and Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169-173.
- Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973-1976.
- Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13(6):520-523.
- Leibovitz A, Plotnikov G, Habot B, et al. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med. 2003;58(1):52-55.
- McCann R. Lack of evidence about tube feeding: food for thought. JAMA. 1999;282(14):1380-1381.
- Cameron JL, Caldini P, Toung J-K, et al. Aspiration pneumonia: physiologic data following experimental Aspiration. Surgery. 1972;72:238.
- Loeb MB, Becker M, Eady A, et al. Interventions to prevent aspiration pneumonia in older adults: a systematic review. JAGS. 2003;51(7):1018-1022.
- Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327-332.
- McCann RM, Hall WJ, Groth-Junker A. Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA. 1994;272:1263-1266.
- Rabeneck L, McCullough LB. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997;349(9050):496-498.
Case
A 68-year-old cachectic female with a history of Alzheimer’s dementia presents with a slowly progressive decline in functional status. She is bed bound, minimally verbal, and has lost interest in eating.
Her problems with decreased oral intake started when her diet was changed to nectar-thickened liquids. This change was made after the patient was hospitalized multiple times for aspiration pneumonia and she underwent a fluoroscopic swallowing evaluation that revealed aspiration of thin liquids. The patient’s husband requests that a feeding tube be placed so his wife doesn’t “die of pneumonia or starve to death.”
Overview
As the U.S. population ages, hospitalists are seeing a steady increase in the average patient age and the prevalence of dementia. Alzheimer’s dementia affects an estimated 4 million to 5 million Americans; this number expected to triple by the year 2050.1
As patients with dementia near the end of life, they often fail to thrive, with less oral intake and more swallowing disorders leading to aspiration. This is when physicians and patient family members must decide whether a feeding tube should be placed.
Placement of a nasogastric or percutaneous endogastric gastrostomy (PEG) feeding tube has become a relatively common medical intervention instituted to maintain or improve a patient’s nutritional status. Prior to 1980, permanent gastric or postpyloric feeding tubes were placed surgically by laparotomy, but the advent of endoscopy and computed tomography (CT) guided procedures offers a simplified procedure requiring only mild sedation and local anesthesia.2
Many patients who suffer multiple bouts of aspiration pneumonia and fail a swallowing evaluation because of an irreversible process are offered a percutaneous feeding tube to maintain nutrition. A feeding tube is also seen as a way to supply nutrition at the end of life in patients no longer able or willing to take food orally.
Although it seems logical that a feeding tube might improve the outcomes of these clinical scenarios, limited literature exists on the topic because of the legal, ethical, emotional, and religious implications a large, randomized, placebo-controlled trial would entail.
Review of the Data
Placement of a PEG has become accepted as a relatively benign procedure, although it is associated with significant morbidity and mortality. Minor complications including pain, abdominal wall ulcers, wound infections, peristomal leakage, and tube displacement occur in approximately 10% of cases.3 Major complications including hemorrhage, bowel or liver perforation, or aspiration occur in 3% of cases.4
These numbers do not account for long-term complications including peristomal infections, leakage problems, or the use of physical restraints to avoid self-extubation.
Aspiration Risk
A common indication for PEG placement is aspiration risk. PEG tubes are often placed in patients who fail swallowing evaluations in order to decrease their risk of aspiration and aspiration pneumonia.
True aspiration pneumonia is thought to originate from an inoculum of oral cavity or nasopharynx bacteria, which placement of a PEG tube would not prevent. Leibovitz, et al., showed that elderly patients with nasogastric or percutaneous feeding tubes are associated with colonization of the oropharynx with more pathogenic bacteria when compared with orally fed patients.5 Thus, the use of PEG tubes might put them at higher risk for pathogenic inoculation.
Aspiration pneumonia occurs in up to 50% of patients with feeding tubes. Studies have shown PEG tube placement decreases lower esophageal sphincter tone, potentially increasing regurgitation risk.6 It has also been shown that aspiration of gastric contents produces a pneumonitis with the resultant inflammatory response allowing for establishment of infection by smaller inoculums of or less virulent organisms.7
Small, randomized trials have shown no decrease in aspiration risk with post-pyloric versus gastric feeding tubes, nasogastric versus percutaneous feeding tubes, or continuous versus intermittent tube feeds.8 There have been no sizable randomized prospective trials to determine if feeding tube placement versus hand feeding patients with end-stage dementia alters aspiration pneumonia risk.
Pressure Ulcers
Patients with end-stage dementia often become bed bound as their disease progresses, and they commonly suffer from pressure ulcers. Pressure ulcers often coexist in patients with malnutrition, and it is well established that patients with biochemical markers of malnutrition are at higher risk for pressure ulcer formation.
Still, no studies show that improved nutrition prevents pressure ulcer formation. In a nursing home population of patients with dementia, a two-year follow-up study showed no significant improvement in pressure ulcer healing or decreased ulcer formation with nutrition by feeding tube.9 These studies are adjusted for independent risk factors for mortality and indication for PEG placement, but we can assume there are confounders that go into the decision for feeding tube placement that are not necessarily identifiable.
Nutritional Status
Family members are often concerned that if the patient is unable to take food by mouth and no feeding tube is placed, then the patient will suffer from the discomfort of starvation and dehydration.
As a patient with a severe dementing illness enters the end stage of his/her clinical course, practitioners frequently make a plan with families to change the goals of care toward keeping the patient comfortable. Comfort is a difficult clinical parameter to measure, but studies in the hospice population of patients with end-stage cancer and AIDS report that the hunger and thirst are transient and improve with ice chips and mouth swabs.10
Despite the lack of evidence of PEG tubes prolonging survival in patients with dementia who are no longer able or willing to take in food orally, it is logical that withholding all hydration or nutritional support will hasten death despite the risks associated with feeding tubes. This is where the ethical argument arises regarding prolonging life of decreasing quality.
In certain medical and legal sectors, artificial nutrition, and hydration are considered a medical intervention. Therefore, the ideals of patient autonomy dictate that the patient’s proxy should decide whether or not the patient would have wanted the intervention after weighing the risks and benefits.
If hospitalists view artificial nutrition as a medical intervention, our moral obligation is to instruct patients and their families about these risks and benefits.
Often, the patient will not clinically improve with artificial nutrition. But we can maintain physiologic processes or at least slow their decline.
Emerging research indicates the standard of care in how we present this information is changing to include presentation of data instead of only using a patient’s suspected beliefs about quality of life.
A useful algorithm proposed by Rabeneck, et al., provides comprehensive guidelines for PEG placement in all patient populations based on the reason for PEG consideration.11
Back to the Case
Our patient is likely nearing the end of her life because of end-stage dementia. There is no evidence to suggest placement of a feeding tube would extend her life more than hand feeding.
We know feeding-tube placement could increase aspiration pneumonia risk and significant short- and long-term morbidity and mortality. We can keep her comfortable with small amounts of water, wetting her lips with swabs. If a feeding tube is placed, its use should be evaluated based on the patient’s clinical course. TH
Dr. Pell is an instructor of medicine in the Section of Hospital Medicine at the University of Colorado, Denver.
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875.
- Hebert LE, Beckett LA, Scherr PA, and Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169-173.
- Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973-1976.
- Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13(6):520-523.
- Leibovitz A, Plotnikov G, Habot B, et al. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J Gerontol A Biol Sci Med. 2003;58(1):52-55.
- McCann R. Lack of evidence about tube feeding: food for thought. JAMA. 1999;282(14):1380-1381.
- Cameron JL, Caldini P, Toung J-K, et al. Aspiration pneumonia: physiologic data following experimental Aspiration. Surgery. 1972;72:238.
- Loeb MB, Becker M, Eady A, et al. Interventions to prevent aspiration pneumonia in older adults: a systematic review. JAGS. 2003;51(7):1018-1022.
- Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327-332.
- McCann RM, Hall WJ, Groth-Junker A. Comfort care for terminally ill patients: the appropriate use of nutrition and hydration. JAMA. 1994;272:1263-1266.
- Rabeneck L, McCullough LB. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997;349(9050):496-498.