User login
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
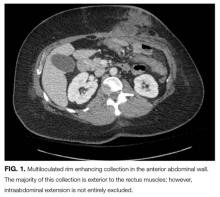
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
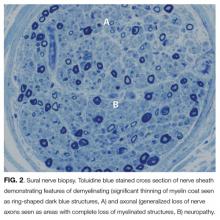
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
© 2017 Society of Hospital Medicine