User login
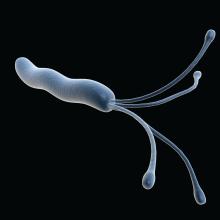
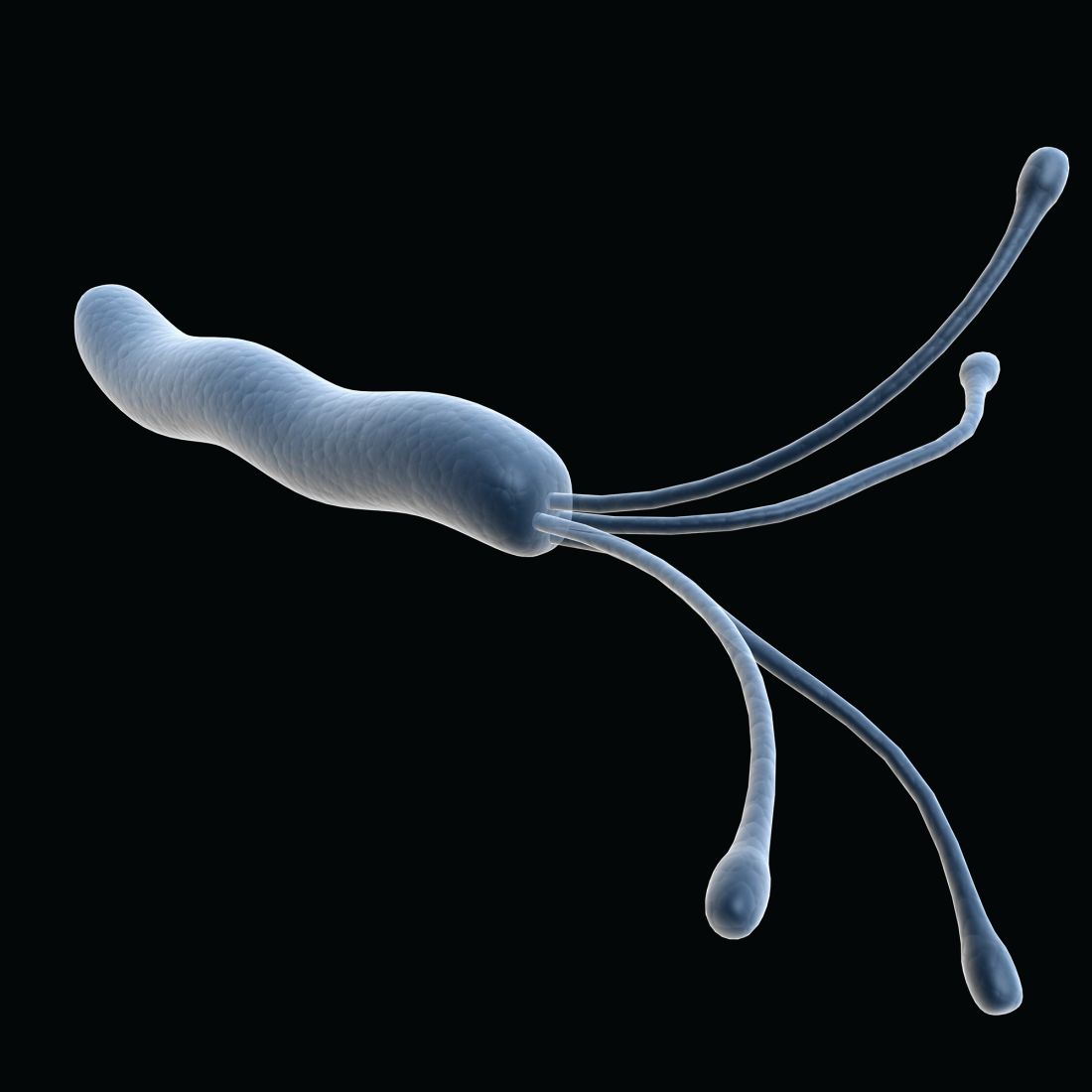
Testing for and treating Helicobacter pylori infection among individuals with a family history of gastric cancer could be a cost-effective strategy in the United States, according to a new model published in the journal Gastroenterology.
As many as 10% of gastric cancers aggregate within families, though just why this happens is unclear, according to Sheila D. Rustgi, MD, and colleagues. Shared environmental or genetic factors, or combinations of both, may be responsible. First-degree family history and H. pylori infection each raise gastric cancer risk by roughly 2.5-fold.
In the United States, universal screening for H. pylori infection is not currently recommended, but some studies have suggested a possible benefit in some high-risk populations. American Gastroenterological Association clinical practice guidelines suggest that a patient’s family history should be a factor when considering surveillance strategies for intestinal metaplasia.
Furthermore, a study by Il Ju Choi, MD, and colleagues in 2020 showed that H. pylori treatment with bismuth-based quadruple therapy reduced the risk of gastric cancer by 73% among individuals with a first-degree relative who had gastric cancer. The combination included a proton pump inhibitor, bismuth, metronidazole, and tetracycline for 10 days.
“We hypothesize that, given the dramatic reduction in GC demonstrated by Choi et al., that the screening strategy can be a cost-effective intervention,” Dr. Rustgi and colleagues wrote.
In the new study, the researchers used a Markov state-transition mode, employing a hypothetical cohort of 40-year-old U.S. men and women with a first-degree relative with gastric cancer. It simulated a follow-up period of 60 years or until death. The model assumed a 7-day treatment with triple therapy (proton pump inhibitor, clarithromycin, and amoxicillin) followed by a 14-day treatment period with quadruple therapy if needed. Although the model was analyzed from the U.S. perspective, the trial that informed the risk reduction was performed in a South Korean population.
No screening had a cost of $2,694.09 and resulted in 21.95 quality-adjusted life years (QALYs). 13C-Urea Breath Test screening had a cost of $2,105.28 and led to 22.37 QALYs. Stool antigen testing had a cost of $2,126.00 and yielded 22.30 QALYs.
In the no-screening group, an estimated 2.04% of patients would develop gastric cancer, and 1.82% would die of it. With screening, the frequency of gastric cancer dropped to 1.59%-1.65%, with a gastric cancer mortality rate of 1.41%-1.46%. Overall, screening was modeled to lead to a 19.1%-22.0% risk reduction.
The researchers validated their model by an assumption of an H. pylori infection rate of 100% and then compared the results of the model to the outcome of the study by Dr. Choi and colleagues. In the trial, there was a 55% reduction in gastric cancer among treated patients at a median of 9 years of follow-up. Those who had successful eradication of H. pylori had a 73% reduction. The new model estimated reductions from a testing and treatment strategy of 53.3%-64.5%.
The findings aren’t surprising, according to Joseph Jennings, MD, of the department of medicine at Georgetown University, Washington, and director of the Center for GI Bleeding at MedStar Georgetown University Hospital, who was not involved with the study. “Even eliminating one person getting gastric cancer, where they will then need major surgery, chemotherapy, all these very expensive interventions [is important],” said Dr. Jennings. “We have very efficient ways to test for these things that don’t involve endoscopy.”
One potential caveat to identifying and treating H. pylori infection is whether elimination of H. pylori may lead to some adverse effects. Some patients can experience increased acid reflux as a result, while others suffer no ill effects. “But when you’re dealing with the alternative, which is stomach cancer, those negatives would have to stack up really, really high to outweigh the positives of preventing a cancer that’s really hard to treat,” said Dr. Jennings.
Dr. Jennings pointed out that the model also projected testing and an intervention conducted in a South Korean population, and extrapolated it to a U.S. population, where the incidence of gastric cancer is lower. “There definitely are some questions about how well it would translate if applied to the general United States population,” said Dr. Jennings.
That question could prompt researchers to conduct a U.S.-based study modeled after the test and treat study in South Korea to see if the regimen produced similar results. The model should add weight to that argument, said Dr. Jennings: “This is raising the point that, at least from an intellectual level, it might be worth now designing the study to see if it works in our population,” said Dr. Jennings.
The authors and Dr. Jennings have no relevant financial disclosures.
Testing for and treating Helicobacter pylori infection among individuals with a family history of gastric cancer could be a cost-effective strategy in the United States, according to a new model published in the journal Gastroenterology.
As many as 10% of gastric cancers aggregate within families, though just why this happens is unclear, according to Sheila D. Rustgi, MD, and colleagues. Shared environmental or genetic factors, or combinations of both, may be responsible. First-degree family history and H. pylori infection each raise gastric cancer risk by roughly 2.5-fold.
In the United States, universal screening for H. pylori infection is not currently recommended, but some studies have suggested a possible benefit in some high-risk populations. American Gastroenterological Association clinical practice guidelines suggest that a patient’s family history should be a factor when considering surveillance strategies for intestinal metaplasia.
Furthermore, a study by Il Ju Choi, MD, and colleagues in 2020 showed that H. pylori treatment with bismuth-based quadruple therapy reduced the risk of gastric cancer by 73% among individuals with a first-degree relative who had gastric cancer. The combination included a proton pump inhibitor, bismuth, metronidazole, and tetracycline for 10 days.
“We hypothesize that, given the dramatic reduction in GC demonstrated by Choi et al., that the screening strategy can be a cost-effective intervention,” Dr. Rustgi and colleagues wrote.
In the new study, the researchers used a Markov state-transition mode, employing a hypothetical cohort of 40-year-old U.S. men and women with a first-degree relative with gastric cancer. It simulated a follow-up period of 60 years or until death. The model assumed a 7-day treatment with triple therapy (proton pump inhibitor, clarithromycin, and amoxicillin) followed by a 14-day treatment period with quadruple therapy if needed. Although the model was analyzed from the U.S. perspective, the trial that informed the risk reduction was performed in a South Korean population.
No screening had a cost of $2,694.09 and resulted in 21.95 quality-adjusted life years (QALYs). 13C-Urea Breath Test screening had a cost of $2,105.28 and led to 22.37 QALYs. Stool antigen testing had a cost of $2,126.00 and yielded 22.30 QALYs.
In the no-screening group, an estimated 2.04% of patients would develop gastric cancer, and 1.82% would die of it. With screening, the frequency of gastric cancer dropped to 1.59%-1.65%, with a gastric cancer mortality rate of 1.41%-1.46%. Overall, screening was modeled to lead to a 19.1%-22.0% risk reduction.
The researchers validated their model by an assumption of an H. pylori infection rate of 100% and then compared the results of the model to the outcome of the study by Dr. Choi and colleagues. In the trial, there was a 55% reduction in gastric cancer among treated patients at a median of 9 years of follow-up. Those who had successful eradication of H. pylori had a 73% reduction. The new model estimated reductions from a testing and treatment strategy of 53.3%-64.5%.
The findings aren’t surprising, according to Joseph Jennings, MD, of the department of medicine at Georgetown University, Washington, and director of the Center for GI Bleeding at MedStar Georgetown University Hospital, who was not involved with the study. “Even eliminating one person getting gastric cancer, where they will then need major surgery, chemotherapy, all these very expensive interventions [is important],” said Dr. Jennings. “We have very efficient ways to test for these things that don’t involve endoscopy.”
One potential caveat to identifying and treating H. pylori infection is whether elimination of H. pylori may lead to some adverse effects. Some patients can experience increased acid reflux as a result, while others suffer no ill effects. “But when you’re dealing with the alternative, which is stomach cancer, those negatives would have to stack up really, really high to outweigh the positives of preventing a cancer that’s really hard to treat,” said Dr. Jennings.
Dr. Jennings pointed out that the model also projected testing and an intervention conducted in a South Korean population, and extrapolated it to a U.S. population, where the incidence of gastric cancer is lower. “There definitely are some questions about how well it would translate if applied to the general United States population,” said Dr. Jennings.
That question could prompt researchers to conduct a U.S.-based study modeled after the test and treat study in South Korea to see if the regimen produced similar results. The model should add weight to that argument, said Dr. Jennings: “This is raising the point that, at least from an intellectual level, it might be worth now designing the study to see if it works in our population,” said Dr. Jennings.
The authors and Dr. Jennings have no relevant financial disclosures.
Testing for and treating Helicobacter pylori infection among individuals with a family history of gastric cancer could be a cost-effective strategy in the United States, according to a new model published in the journal Gastroenterology.
As many as 10% of gastric cancers aggregate within families, though just why this happens is unclear, according to Sheila D. Rustgi, MD, and colleagues. Shared environmental or genetic factors, or combinations of both, may be responsible. First-degree family history and H. pylori infection each raise gastric cancer risk by roughly 2.5-fold.
In the United States, universal screening for H. pylori infection is not currently recommended, but some studies have suggested a possible benefit in some high-risk populations. American Gastroenterological Association clinical practice guidelines suggest that a patient’s family history should be a factor when considering surveillance strategies for intestinal metaplasia.
Furthermore, a study by Il Ju Choi, MD, and colleagues in 2020 showed that H. pylori treatment with bismuth-based quadruple therapy reduced the risk of gastric cancer by 73% among individuals with a first-degree relative who had gastric cancer. The combination included a proton pump inhibitor, bismuth, metronidazole, and tetracycline for 10 days.
“We hypothesize that, given the dramatic reduction in GC demonstrated by Choi et al., that the screening strategy can be a cost-effective intervention,” Dr. Rustgi and colleagues wrote.
In the new study, the researchers used a Markov state-transition mode, employing a hypothetical cohort of 40-year-old U.S. men and women with a first-degree relative with gastric cancer. It simulated a follow-up period of 60 years or until death. The model assumed a 7-day treatment with triple therapy (proton pump inhibitor, clarithromycin, and amoxicillin) followed by a 14-day treatment period with quadruple therapy if needed. Although the model was analyzed from the U.S. perspective, the trial that informed the risk reduction was performed in a South Korean population.
No screening had a cost of $2,694.09 and resulted in 21.95 quality-adjusted life years (QALYs). 13C-Urea Breath Test screening had a cost of $2,105.28 and led to 22.37 QALYs. Stool antigen testing had a cost of $2,126.00 and yielded 22.30 QALYs.
In the no-screening group, an estimated 2.04% of patients would develop gastric cancer, and 1.82% would die of it. With screening, the frequency of gastric cancer dropped to 1.59%-1.65%, with a gastric cancer mortality rate of 1.41%-1.46%. Overall, screening was modeled to lead to a 19.1%-22.0% risk reduction.
The researchers validated their model by an assumption of an H. pylori infection rate of 100% and then compared the results of the model to the outcome of the study by Dr. Choi and colleagues. In the trial, there was a 55% reduction in gastric cancer among treated patients at a median of 9 years of follow-up. Those who had successful eradication of H. pylori had a 73% reduction. The new model estimated reductions from a testing and treatment strategy of 53.3%-64.5%.
The findings aren’t surprising, according to Joseph Jennings, MD, of the department of medicine at Georgetown University, Washington, and director of the Center for GI Bleeding at MedStar Georgetown University Hospital, who was not involved with the study. “Even eliminating one person getting gastric cancer, where they will then need major surgery, chemotherapy, all these very expensive interventions [is important],” said Dr. Jennings. “We have very efficient ways to test for these things that don’t involve endoscopy.”
One potential caveat to identifying and treating H. pylori infection is whether elimination of H. pylori may lead to some adverse effects. Some patients can experience increased acid reflux as a result, while others suffer no ill effects. “But when you’re dealing with the alternative, which is stomach cancer, those negatives would have to stack up really, really high to outweigh the positives of preventing a cancer that’s really hard to treat,” said Dr. Jennings.
Dr. Jennings pointed out that the model also projected testing and an intervention conducted in a South Korean population, and extrapolated it to a U.S. population, where the incidence of gastric cancer is lower. “There definitely are some questions about how well it would translate if applied to the general United States population,” said Dr. Jennings.
That question could prompt researchers to conduct a U.S.-based study modeled after the test and treat study in South Korea to see if the regimen produced similar results. The model should add weight to that argument, said Dr. Jennings: “This is raising the point that, at least from an intellectual level, it might be worth now designing the study to see if it works in our population,” said Dr. Jennings.
The authors and Dr. Jennings have no relevant financial disclosures.
FROM GASTROENTEROLOGY