User login
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
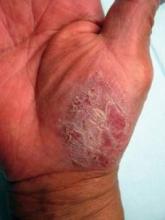
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
RALEIGH, N.C. – Ustekinumab proved effective for the clearance of palmoplantar psoriasis in an open-label pilot study, but only at the higher 90-mg dose reserved under current labeling for psoriasis patients weighing at least 100 kg.
"Higher or more frequent dosing may be required to achieve clinical clearance in patients with palmoplantar psoriasis compared to current recommendations for plaque-type psoriasis," said Dr. Shiu-Chung Au of Tufts Medical Center, Boston.
Dr. Au presented an open-label, 24-week study of 20 patients with moderate to severe palmoplantar psoriasis that was refractory to topical corticosteroids. Dosing of ustekinumab (Stelara) was as for plaque-type psoriasis: The 11 patients weighing less than 100 kg received 45 mg subcutaneously at weeks 0, 4, and 16, whereas the 9 patients weighing 100 kg or more received 90 mg on the same schedule.
The primary study end point was a Palm-Sole PGA (Physician’s Global Assessment) score of 0 or 1 at week 16. The average baseline score on this 0-5 measure of induration, erythema, and scaling was 4. The primary end point was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose (J. Dermatolog. Treat. 2012 May 8. [Epub ahead of print]). Of 20 patients, 12 improved at least 2 points on the Palm-Sole PGA at week 16.
In addition, significant improvements were documented with respect to the secondary end points for pain and quality of life. The mean pain score on a 100-point visual analog scale improved from 51 at week 0 to 29 at week 16, and held steady with a score of 30 at 24 weeks. Moreover, the mean score on the Dermatology Life Quality Index improved from 13.9 at baseline to 6.05 at week 16 and to 6.18 at week 24.
Ustekinumab was well tolerated with no serious adverse events.
Palmoplantar psoriasis is an uncommon variant of psoriasis that constitutes a therapeutic challenge, noted Dr. Au. It can be disfiguring and disabling, even though affected patients don’t necessarily have a large area of body surface involvement. The condition is characterized by fissures and sterile inflammatory pustules, in addition to classic well-demarcated psoriasis plaques. Affected patients experience considerable skin pain, often accompanied by a burning sensation. Some patients are unable to walk.
Clinically, palmoplantar psoriasis can look like other diseases, including palmoplantar pustulosis, dyshidrotic eczema, tinea pedis, and contact dermatitis, he explained. To help eliminate other possible diagnoses in the differential diagnosis, participants in the ustekinumab study had to have at least one classic psoriasis plaque somewhere other than the palms and soles.
Current treatment of palmoplantar psoriasis has been unsatisfactory, noted Dr. Au. No standard therapy is recognized, which was the impetus for studying ustekinumab. Future studies will look at employing the 90-mg dose in patients who weigh less than 100 kg and/or administering the 45-mg dose more frequently than the current standard every 3 months, he added.
In response to an audience question, he noted that responsiveness to the 90-mg dose was not weight related. The average weight in the high-dose group was 250 pounds, and the three nonresponders weighed in similarly to the six responders.
This investigator-initiated ustekinumab study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator Dr. Alice B. Gottlieb reported having numerous industry relationships.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Major Finding: The primary endpoint of a palm-sole PGA score of 0 or 1 was achieved in 1 of 11 patients on the 45-mg dose, compared with 6 of 9 on the 90-mg dose
Data Source: An open-label, 24-week study of 20 patients treated with ustekinumab for moderate-to-severe palmoplantar psoriasis refractory to topical corticosteroids.
Disclosures: This investigator-initiated study was funded by a grant from Janssen Pharmaceuticals. Dr. Au reported having no financial conflicts. Senior investigator, Dr. Alice B. Gottlieb, reported having numerous industry relationships.