To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
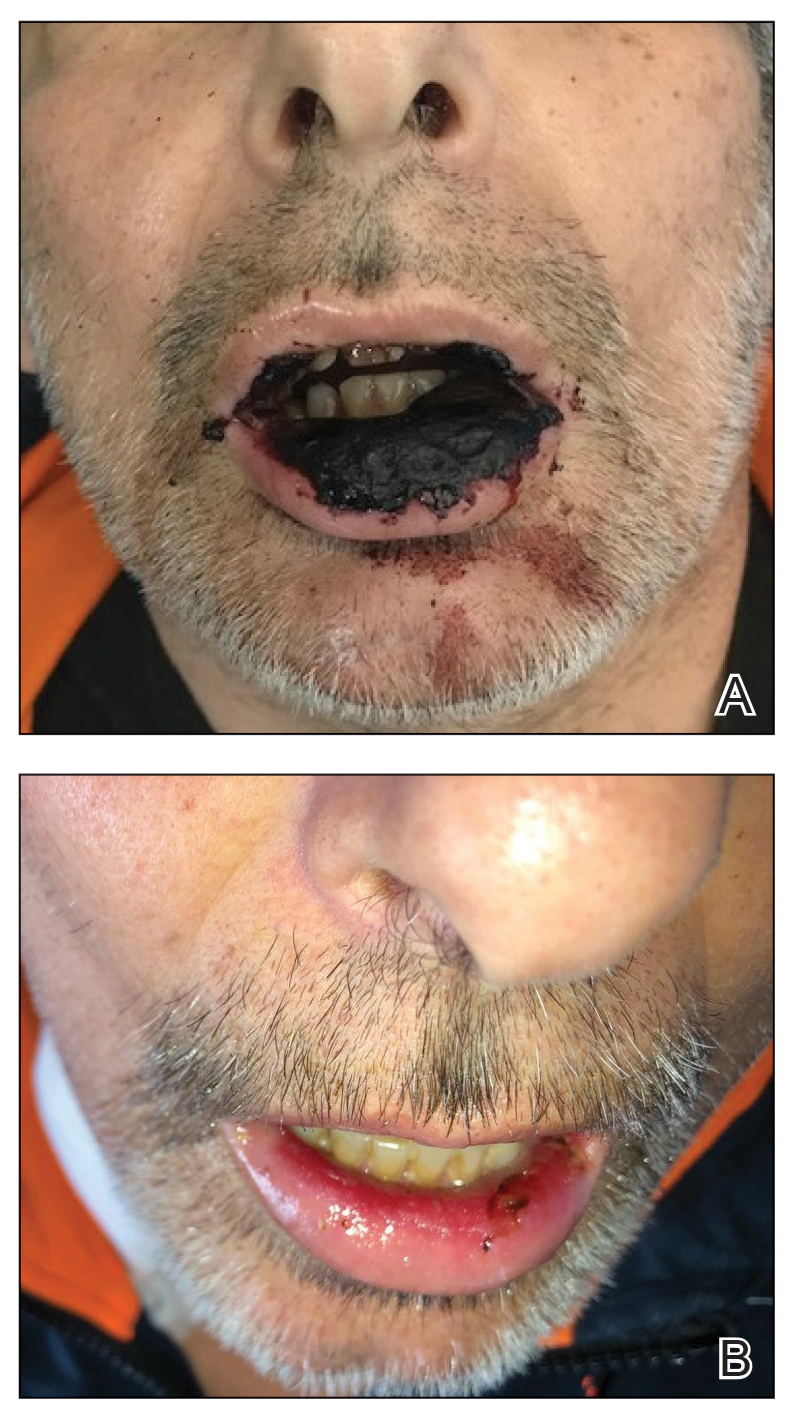
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.
Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7