User login
A 57‐year‐old man presented to an emergency department with 1 month of progressive, bilateral lower extremity pain and weakness.
The first step in evaluating weakness is to determine whether it is objective (ie, decreased muscle strength due to pathology along the neuromuscular axis) or subjective. The sensation of weakness without loss of muscle strength may result from a debilitating chronic disease (eg, congestive heart failure, anemia, or chronic obstructive pulmonary disease). In patients with true lower extremity weakness it is prudent to assess for a myelopathy with a focused history and exam that includes assessment of bowel or bladder impairment and anal reflex. The presence of pain along with weakness might suggest disease of the muscle itself. A myopathy may arise from an infectious (eg, influenza), inflammatory (eg, polymyositis), endocrine (eg, hypothyroidism), or drug‐related (eg, statin) process.
The patient described 1 month of generalized weakness and pain in his lower extremities, which had worsened progressively to the point where ambulation was difficult. He was able to rise from a seated position using his arms for assistance, but had difficulty balancing in a standing position without assistance. The pain also involved both of his knees and increased with weight bearing. He also complained of bilateral lower extremity numbness and paresthesias, which had been migrating proximally from his toes over several months. He denied any recent trauma to his legs or back.
These symmetrical, distal sensory deficits favor a peripheral neuropathy over a myopathy, with neuropathic pain and arthralgia causing his impaired ability to ambulate or remain standing. In polyneuropathy, the type of nerve involvement (sensory vs motor) and pathology (axonal vs demyelinating) helps prioritize the differential. In developed countries, the most common causes of polyneuropathy are diabetes mellitus and alcohol. However, the tempo of his disease broadens the possibilities to include acute inflammatory demyelinating polyneuropathy, paraneoplastic syndrome (eg, monoclonal gammopathy), an autoimmune process (eg, rheumatoid arthritis, vasculitis), and heavy metal toxicity such as lead poisoning.
He had no history of chronic medical illness or hospitalizations and took no medications. His social history was notable for a history of alcohol abuse. For the past several years, he had only been drinking 1 to 2 beers daily due to cost, but had a history of more significant alcohol abuse in the distant past. He smoked 1 pack of tobacco per day, and denied illicit drug use. He denied any sexual activity or recent travel. He lived in a van, and had been homeless for over 10 years.
His socioeconomic status adds a layer of complexity to the case. Human immunodeficiency virus and hepatitis C virus (HCV) are more prevalent in the homeless and are associated with polyneuropathy. His lack of funds may drive him to drink illegally distilled alcohol, which can cause polyneuropathy through lead or arsenic toxicity. Excessive smoking could be linked to a peripheral neuropathy through a paraneoplastic syndrome (eg, small cell lung cancer).
Alcohol causes polyneuropathy through toxic effects on nerves and may be playing a role in his polyneuropathy, but the rapid pace and severity suggests an additional process. Alcoholism can be associated with deficiency of various B vitamins, such as thiamine, pyridoxine, and cobalamin, which can cause polyneuropathy. In alcoholics who are hospitalized, thiamine should be administered prior to glucose to decrease risk of Wernicke encephalopathy.
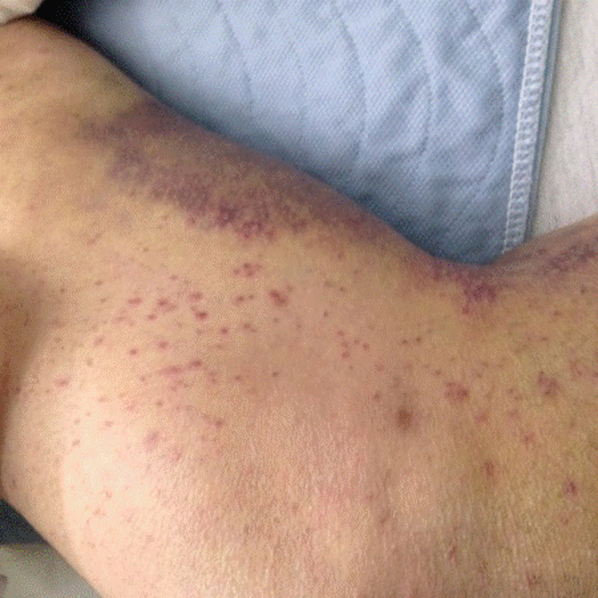
His temperature was 38.0C, heart rate 93 beats/min, blood pressure 121/60 mm Hg, respiratory rate 14/min, with an oxygen saturation of 97% on ambient air. He appeared cachectic and disheveled. He had moist mucous membranes, poor dentition with missing teeth, and no mucosal bleeding or oropharyngeal erythema. His cardiac exam revealed no murmurs, rubs, or gallops. His lungs were clear. His abdominal exam was benign, without masses or tenderness. His skin exam (Figure 1) was notable for nonpalpable petechiae on his anterior shins and thighs up to his buttocks. His extremity exam was significant for diffuse tenderness to light palpation on both lower extremities, a large indurated tender ecchymosis 15 15 cm behind the right knee, and another ecchymosis 6 8 cm behind the left knee. His dorsalis pedis and anterior tibialis pulses were appreciated by Doppler but not by palpation. He had decreased sensation to light touch of his bilateral feet to his ankles. Strength exam was challenging to assess secondary to posterior leg pain, but he demonstrated 4/5 strength of his hip flexors, quadriceps, and plantar flexors of the foot. His upper extremity strength and sensory exam were normal. Examination of the cranial nerves was normal. He had 2+ patellar and Achilles reflexes. Gait could not be adequately assessed.

Petechiae manifest as a nonblanchable rash caused by extravasated red blood cells. Common etiologies include quantitative or qualitative platelet defects, disseminated intravascular coagulopathy, trauma, and vasculitis. Cirrhosis from alcohol leading to thrombocytopenia and petechial rash is unlikely given no other stigmata of liver disease such as jaundice, spider angiomata, caput medusae, or palmar erythema. Less common causes include nutritional deficiency and light chain (AL) amyloidosis, which could explain both the neuropathy and rash.
The constellation of fever and petechial rash can represent a life‐threatening systemic process. Infectious agents that require immediate consideration with fever and petechiae include Neisseria meningitidis (meningococcemia), Rickettsia rickettsii (Rocky Mountain spotted fever), Staphylococcus, and Streptococcus. However, his normal blood pressure, dependent distribution of rash, and neuropathy make a severe bacterial infection less likely. Thrombotic thrombocytopenic purpura is possible and should prompt assessment of platelets, peripheral blood smear, and lactate dehydrogenase. Among vasculitides, the polyneuropathy, fever, and dependent distribution of petechial rash prioritize a small‐to‐medium vessel vasculitis, where the pathophysiology involves inflammation of dermal vessels and vasa nervorum (blood supply of nerves). Examples include HCV‐related cryoglobulinemic vasculitis, polyarteritis nodosa (PAN), and antineutrophilic cytoplasmic antibody (ANCA)associated vasculitis. However, ANCA‐associated vasculitis is less likely without upper or lower respiratory symptoms. Henoch‐Schonlein purpura may explain the rash but is more common in children and is not associated with neuropathy.
Posterior knee ecchymosis, in absence of trauma, raises suspicion for a ruptured Baker's cyst. However, the bilateral involvement and lack of calf manifestations makes this unlikely. The location raises concern for hemarthrosis, so a more likely explanation would be coagulopathy (eg, an acquired factor inhibitor) or a collagen defect. In developed countries, a commonly overlooked category of diseasenutritional deficiencywarrants serious consideration in alcoholics. Vitamin C deficiency (scurvy) may cause a petechial rash and ecchymosis from perifollicular hemorrhage and impaired collagen synthesis, respectively. Scurvy can masquerade as small vessel vasculitis because of its associated petechial rash. The neuropathy might be explained by concomitant thiamine or cobalamin deficiency. It is important to obtain a thorough dietary history and assess vibration and proprioception, which may be impaired from pathology of the dorsal column in cobalamin deficiency. The low‐grade fever may be a red herring, but if it becomes significant would be difficult to explain with nutritional deficiency.
In summary, a judicious evaluation for infection is mandatory, but the leading diagnoses are a small‐to‐medium vessel vasculitis (PAN or HCV‐related cryoglobulinemia), deficiency of multiple vitamins, and AL amyloidosis.
Initial labs showed white blood cell count 7800/L, hematocrit 39.2%, and platelet count of 251,000/L. Serum chemistry demonstrated a sodium of 131 mEq/L, potassium 4.7 mEq/L, chloride 93 mEq/L, bicarbonate 23 mEq/L, blood urea nitrogen 8 mg/dL, and creatinine 0.8 mg/dL. His aminotransferases, albumin, alkaline phosphatase, and coagulation studies were within normal limits. Urinalysis was remarkable for 2+ urobilinogen, 1+ ketones, and a bland sediment. Urine toxicology screen was negative.
His white blood cell count is normal, so with a heart rate of 93 beats/minute, he barely meets a single criterion of systemic inflammatory response syndrome (SIRS). The lack of SIRS and normal platelet, albumin, white blood cell, and red blood cell counts significantly reduces the likelihood of an infectious or inflammatory process. Without any clinical or biochemical evidence of HCV infection, HCV‐associated cryoglobulinemia is less likely. A normal creatinine might overestimate renal function in setting of decreased protein intake and muscle mass; nevertheless, the bland urine sediment further lowers probability of PAN and ANCA‐associated vasculitides. The normal platelet count and coagulation studies suggest either a qualitative platelet defect (eg, acquired von Willebrand disease) or impaired vessel integrity (eg, collagen defect) to explain the petechial rash. The urine ketones likely represent alcohol and/or starvation‐related ketosis. These data reduce the probability of infection and vasculitis, and prioritize vitamin deficiency and AL amyloidosis. Antibiotic therapy is not appropriate, given the absence of SIRS and subacute course. His presentation likely prompted a wide variety of tests, but most relevant would be a dietary history, cobalamin and vitamin C levels, serum free light chains, and skin biopsy. Biopsy of the rash would allow assessment for vasculitis and AL amyloidosis. The former is marked by inflammatory infiltrate of vessels, and the latter by perivascular invasion with amyloid fibrils. If the dietary history was consistent with ascorbic acid deficiency (scurvy), in addition to thiamine, he should be empirically treated with vitamin C. Patients with scurvy demonstrate rapid clinical improvement with treatment.
C‐reactive protein (CRP) was 47.9 mg/L and erythrocyte sedimentation rate (ESR) was 44 mm/hr. Human immunodeficiency antibody screen was negative. Anti‐nuclear antibodies and anti‐nuclear cytoplasmic antibody panel were negative. Computed tomography angiogram (CTA) of the lower extremities demonstrated severe stenosis of the left superficial femoral artery and severe stenosis of the right posterior tibial artery. Ankle‐brachial indices were 0.83 on the right side and 0.72 on the left, indicating mild to moderate arterial disease.
ESR and CRP are nonspecific markers of inflammation. Their elevation does not prioritize malignancy, autoimmunity, or infection. ANCA might be negative in commonly ANCA‐associated vasculitides such as eosinophilic granulomatosis with polyangiitis, microscopic polyangiitis, and granulomatosis with polyangiitis. However, the lack of respiratory and renal involvement in addition to the negative ANCA panel make such diagnoses unlikely. CTA of the patient's legs showed significant peripheral artery disease (PAD). This is unlikely to be the cause of his presentation; PAD should not cause petechiae, and his pain is disproportionate to the severity of the vascular disease reported. The additional information leaves the differential unchanged.
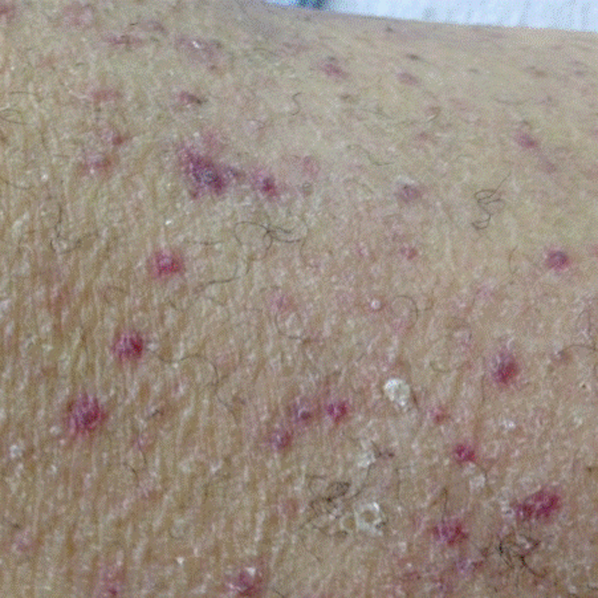
A dermatologist was consulted. She described and photographed a perifollicular distribution of the lower extremity petechiae with associated perifollicular hyperkeratosis and retained curled hairs (Figure 2).
The described rash is specific for scurvy. His homelessness and alcohol intake likely made him vulnerable to ascorbic acid deficiency from lack of access to fruits and vegetables. Measurement of vitamin C level is unnecessary as the pretest probability for scurvy is very high. More relevant than a vitamin C level or skin biopsy is empiric treatment with ascorbic acid; as mentioned, patients with scurvy respond rapidly to vitamin C therapy. Given the neuropathy, he should be assessed for concomitant thiamine and/or cobalamin deficiency. His peripheral arterial disease is unlikely to be related.
His ascorbic acid level was 0.0 mg/dL (reference range, 0.22.0 mg/dL). Further history was obtained, and the patient reported exclusively eating frozen hamburgers and burritos for almost 1 year. He believed he had not had a fruit or vegetable in over 10 years. He was started on 1000 mg daily of ascorbic acid. By hospital day 2, his rash had mostly resolved and he was able to stand with some support. The patient was seen by his primary care physician 3 weeks after diagnosis, with his exercise tolerance nearly back to baseline. His rash had entirely resolved.
DISCUSSION
Unlike other mammals, humans do not have the ability to convert glucose to vitamin C and thus require an exogenous source, such as fruits and vegetables. The oft‐cited observation of scurvy in sailors during long journeys in the 18th century is a classic example of clinical disease due to vitamin C deficiency.[1] Once replete, body stores of vitamin C are usually sufficient to last over 6 months of deprivation. In some patients, symptoms of deficiency may appear within 3 months.[2] The patient in this report likely suffered years of vitamin C deficiency, resulting in the significant manifestations of scurvy reported here.
Vitamin C is a water‐soluble vitamin necessary for the biosynthesis of collagen, L‐carnitine, and neurotransmitters.[3] With deficiency, the resulting impairment in the formation of collagen affects blood vessel integrity and results in perivascular edema and erythrocyte extravasation. Clinically, this leads to hemorrhagic manifestations (eg, periosteal hemorrhage and perifollicular petechiae) and poor wound healing. Corkscrew hairs result because of vitamin C's role in disulfide bonding during hair formation. Woody edema and dyspnea are thought to be a consequence of leaky capillaries.[4]
Scurvy is still a significant cause of morbidity in at‐risk populations in the United States. Several populations have been identified as high risk for vitamin C deficiency, including the elderly, persons who live alone, alcoholics, smokers, individuals of low socioeconomic status, patients on hemodialysis, and those with psychiatric disease.[5] Specifically, the high oxidative stress associated with smoking, the history of alcohol abuse, and homelessness put this patient at an especially high risk.[6] Those with oxidative stressors have been postulated to require up to 125 mg/d of vitamin C compared to 60 to 90 mg/d of those without the same risks.[7] In a national health and nutrition survey in the United States in 2004, the prevalence of vitamin C deficiency as defined by a serum level <0.2 mg/dL was noted in 7.1% of those surveyed.[8] This study also noted a significantly higher prevalence of deficiency in smokers and individuals with low socioeconomic status.
Scurvy is a clinical diagnosis based on clinical features and dietary history. Severe manifestations of scurvy may happen quickly after the initial presentation, making early diagnosis especially important.[2] These include anemia, bone pain, ocular hemorrhage, cerebral hemorrhage, and hemopericardium.[2, 4] If needed, laboratory diagnosis can be made by demonstrating a serum ascorbic acid level <0.2 mg/dL. However, the level may be normal if the patient has had recent intake of vitamin C. In that scenario, the leukocyte vitamin C concentration may be a more accurate measure of the body stores as leukocyte levels change more slowly.[4] Biopsy of skin lesions is not necessary for the diagnosis and typically show a dilated hair follicle with keratin plugging and perifollicular hemorrhage.[9] Given the lack of adverse effects, treatment with vitamin C supplementation should begin immediately, even with low suspicion of scurvy, and response can serve as further clinical evidence and render laboratory testing unnecessary.
In this patient, the diagnosis was challenging for several reasons. The presentation was concerning for vasculitis given the dependent petechiae and elevated inflammatory markers. However, in scurvy, the petechiae are perifollicular and associated with hyperkeratosis, as opposed to the palpable purpura often described in vasculitis. Further, marked elevations in ESR and CRP have also been reported in scurvy.[10] The initial concern for vasculitis and clinician discomfort with a diagnosis based solely on a rash delayed the diagnosis. The complaint of polyneuropathy also seemed inconsistent with scurvy. Very rarely, scurvy may cause a neuropathy by hemorrhage into the nerve sheath, as seen in a case of bilateral femoral neuropathy.[11] Most likely, this patient had an underlying vitamin B deficiency explaining his polyneuropathy. Unfortunately, the patient was lost to follow‐up after his postdischarge visit with his primary physician and was not tested for other concomitant vitamin deficiencies.
Scurvy is very responsive to even small doses of vitamin C supplementation. For rapid recovery, doses ranging from 100 mg 3 times daily to 1000 mg daily of oral vitamin C are recommended for at least 1 month. Resolution of symptoms will begin within 24 hours, and complete recovery should occur by 3 months.[4] Scurvy is a classic example of how nutritional deficiencies can have a myriad of presentations and may mimic other systemic diseases. Clinicians who recall these manifestations and carefully assess patients for nutritional risks may be able to quickly identify the missing element (or elements) in a patient's diet, and initiate treatment that is often rapidly effective.
KEY TEACHING POINTS
- Vitamin C deficiency initially presents with classic dermatological findings of perifollicular petechiae with associated hyperkeratosis and corkscrew hairs.
- Scurvy is a clinical diagnosis based on history and presentation. Vitamin C serum level may not accurately reflect body stores, and a leukocyte vitamin C level may be obtained. The diagnosis may also be confirmed with observed response to vitamin C supplementation.
- Scurvy should be suspected in high‐risk populations, especially the marginally housed, the elderly, alcoholics, and smokers.
- Clinicians should screen patients with scurvy for other nutritional deficiencies including thiamine, folate, B12, and vitamin D levels.
Disclosures: Nothing to report.
- . The discovery of vitamin C. Ann Nutr Metab. 2012;61(3):259–264.
- , , , , . Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24(4):432–443.
- . New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314(14):892–902.
- , . Adult scurvy. J Am Acad Dermatol. 1999;41(6):895–910.
- , . Be vigilant for scurvy in high‐risk groups. Practitioner. 2012;256(1755):23–5, 3.
- . Estimating ascorbic acid requirements for cigarette smokers. Ann N Y Acad Sci. 1993;686:335–346.
- , , . Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health. 2004;94(5):870–875.
- , , , . Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252–1263.
- , , . Scurvy: a cutaneous clinical diagnosis. Australas J Dermatol. 2003;44(1):48–51.
- , . Rheumatic manifestations of scurvy: a report of three recent cases in a major urban center and a review. Semin Arthritis Rheum. 2011;41(2):286–290.
- . Femoral neuropathy in scurvy. N Engl J Med. 1969;281(23):1292–1293.
A 57‐year‐old man presented to an emergency department with 1 month of progressive, bilateral lower extremity pain and weakness.
The first step in evaluating weakness is to determine whether it is objective (ie, decreased muscle strength due to pathology along the neuromuscular axis) or subjective. The sensation of weakness without loss of muscle strength may result from a debilitating chronic disease (eg, congestive heart failure, anemia, or chronic obstructive pulmonary disease). In patients with true lower extremity weakness it is prudent to assess for a myelopathy with a focused history and exam that includes assessment of bowel or bladder impairment and anal reflex. The presence of pain along with weakness might suggest disease of the muscle itself. A myopathy may arise from an infectious (eg, influenza), inflammatory (eg, polymyositis), endocrine (eg, hypothyroidism), or drug‐related (eg, statin) process.
The patient described 1 month of generalized weakness and pain in his lower extremities, which had worsened progressively to the point where ambulation was difficult. He was able to rise from a seated position using his arms for assistance, but had difficulty balancing in a standing position without assistance. The pain also involved both of his knees and increased with weight bearing. He also complained of bilateral lower extremity numbness and paresthesias, which had been migrating proximally from his toes over several months. He denied any recent trauma to his legs or back.
These symmetrical, distal sensory deficits favor a peripheral neuropathy over a myopathy, with neuropathic pain and arthralgia causing his impaired ability to ambulate or remain standing. In polyneuropathy, the type of nerve involvement (sensory vs motor) and pathology (axonal vs demyelinating) helps prioritize the differential. In developed countries, the most common causes of polyneuropathy are diabetes mellitus and alcohol. However, the tempo of his disease broadens the possibilities to include acute inflammatory demyelinating polyneuropathy, paraneoplastic syndrome (eg, monoclonal gammopathy), an autoimmune process (eg, rheumatoid arthritis, vasculitis), and heavy metal toxicity such as lead poisoning.
He had no history of chronic medical illness or hospitalizations and took no medications. His social history was notable for a history of alcohol abuse. For the past several years, he had only been drinking 1 to 2 beers daily due to cost, but had a history of more significant alcohol abuse in the distant past. He smoked 1 pack of tobacco per day, and denied illicit drug use. He denied any sexual activity or recent travel. He lived in a van, and had been homeless for over 10 years.
His socioeconomic status adds a layer of complexity to the case. Human immunodeficiency virus and hepatitis C virus (HCV) are more prevalent in the homeless and are associated with polyneuropathy. His lack of funds may drive him to drink illegally distilled alcohol, which can cause polyneuropathy through lead or arsenic toxicity. Excessive smoking could be linked to a peripheral neuropathy through a paraneoplastic syndrome (eg, small cell lung cancer).
Alcohol causes polyneuropathy through toxic effects on nerves and may be playing a role in his polyneuropathy, but the rapid pace and severity suggests an additional process. Alcoholism can be associated with deficiency of various B vitamins, such as thiamine, pyridoxine, and cobalamin, which can cause polyneuropathy. In alcoholics who are hospitalized, thiamine should be administered prior to glucose to decrease risk of Wernicke encephalopathy.
His temperature was 38.0C, heart rate 93 beats/min, blood pressure 121/60 mm Hg, respiratory rate 14/min, with an oxygen saturation of 97% on ambient air. He appeared cachectic and disheveled. He had moist mucous membranes, poor dentition with missing teeth, and no mucosal bleeding or oropharyngeal erythema. His cardiac exam revealed no murmurs, rubs, or gallops. His lungs were clear. His abdominal exam was benign, without masses or tenderness. His skin exam (Figure 1) was notable for nonpalpable petechiae on his anterior shins and thighs up to his buttocks. His extremity exam was significant for diffuse tenderness to light palpation on both lower extremities, a large indurated tender ecchymosis 15 15 cm behind the right knee, and another ecchymosis 6 8 cm behind the left knee. His dorsalis pedis and anterior tibialis pulses were appreciated by Doppler but not by palpation. He had decreased sensation to light touch of his bilateral feet to his ankles. Strength exam was challenging to assess secondary to posterior leg pain, but he demonstrated 4/5 strength of his hip flexors, quadriceps, and plantar flexors of the foot. His upper extremity strength and sensory exam were normal. Examination of the cranial nerves was normal. He had 2+ patellar and Achilles reflexes. Gait could not be adequately assessed.

Petechiae manifest as a nonblanchable rash caused by extravasated red blood cells. Common etiologies include quantitative or qualitative platelet defects, disseminated intravascular coagulopathy, trauma, and vasculitis. Cirrhosis from alcohol leading to thrombocytopenia and petechial rash is unlikely given no other stigmata of liver disease such as jaundice, spider angiomata, caput medusae, or palmar erythema. Less common causes include nutritional deficiency and light chain (AL) amyloidosis, which could explain both the neuropathy and rash.
The constellation of fever and petechial rash can represent a life‐threatening systemic process. Infectious agents that require immediate consideration with fever and petechiae include Neisseria meningitidis (meningococcemia), Rickettsia rickettsii (Rocky Mountain spotted fever), Staphylococcus, and Streptococcus. However, his normal blood pressure, dependent distribution of rash, and neuropathy make a severe bacterial infection less likely. Thrombotic thrombocytopenic purpura is possible and should prompt assessment of platelets, peripheral blood smear, and lactate dehydrogenase. Among vasculitides, the polyneuropathy, fever, and dependent distribution of petechial rash prioritize a small‐to‐medium vessel vasculitis, where the pathophysiology involves inflammation of dermal vessels and vasa nervorum (blood supply of nerves). Examples include HCV‐related cryoglobulinemic vasculitis, polyarteritis nodosa (PAN), and antineutrophilic cytoplasmic antibody (ANCA)associated vasculitis. However, ANCA‐associated vasculitis is less likely without upper or lower respiratory symptoms. Henoch‐Schonlein purpura may explain the rash but is more common in children and is not associated with neuropathy.
Posterior knee ecchymosis, in absence of trauma, raises suspicion for a ruptured Baker's cyst. However, the bilateral involvement and lack of calf manifestations makes this unlikely. The location raises concern for hemarthrosis, so a more likely explanation would be coagulopathy (eg, an acquired factor inhibitor) or a collagen defect. In developed countries, a commonly overlooked category of diseasenutritional deficiencywarrants serious consideration in alcoholics. Vitamin C deficiency (scurvy) may cause a petechial rash and ecchymosis from perifollicular hemorrhage and impaired collagen synthesis, respectively. Scurvy can masquerade as small vessel vasculitis because of its associated petechial rash. The neuropathy might be explained by concomitant thiamine or cobalamin deficiency. It is important to obtain a thorough dietary history and assess vibration and proprioception, which may be impaired from pathology of the dorsal column in cobalamin deficiency. The low‐grade fever may be a red herring, but if it becomes significant would be difficult to explain with nutritional deficiency.
In summary, a judicious evaluation for infection is mandatory, but the leading diagnoses are a small‐to‐medium vessel vasculitis (PAN or HCV‐related cryoglobulinemia), deficiency of multiple vitamins, and AL amyloidosis.
Initial labs showed white blood cell count 7800/L, hematocrit 39.2%, and platelet count of 251,000/L. Serum chemistry demonstrated a sodium of 131 mEq/L, potassium 4.7 mEq/L, chloride 93 mEq/L, bicarbonate 23 mEq/L, blood urea nitrogen 8 mg/dL, and creatinine 0.8 mg/dL. His aminotransferases, albumin, alkaline phosphatase, and coagulation studies were within normal limits. Urinalysis was remarkable for 2+ urobilinogen, 1+ ketones, and a bland sediment. Urine toxicology screen was negative.
His white blood cell count is normal, so with a heart rate of 93 beats/minute, he barely meets a single criterion of systemic inflammatory response syndrome (SIRS). The lack of SIRS and normal platelet, albumin, white blood cell, and red blood cell counts significantly reduces the likelihood of an infectious or inflammatory process. Without any clinical or biochemical evidence of HCV infection, HCV‐associated cryoglobulinemia is less likely. A normal creatinine might overestimate renal function in setting of decreased protein intake and muscle mass; nevertheless, the bland urine sediment further lowers probability of PAN and ANCA‐associated vasculitides. The normal platelet count and coagulation studies suggest either a qualitative platelet defect (eg, acquired von Willebrand disease) or impaired vessel integrity (eg, collagen defect) to explain the petechial rash. The urine ketones likely represent alcohol and/or starvation‐related ketosis. These data reduce the probability of infection and vasculitis, and prioritize vitamin deficiency and AL amyloidosis. Antibiotic therapy is not appropriate, given the absence of SIRS and subacute course. His presentation likely prompted a wide variety of tests, but most relevant would be a dietary history, cobalamin and vitamin C levels, serum free light chains, and skin biopsy. Biopsy of the rash would allow assessment for vasculitis and AL amyloidosis. The former is marked by inflammatory infiltrate of vessels, and the latter by perivascular invasion with amyloid fibrils. If the dietary history was consistent with ascorbic acid deficiency (scurvy), in addition to thiamine, he should be empirically treated with vitamin C. Patients with scurvy demonstrate rapid clinical improvement with treatment.
C‐reactive protein (CRP) was 47.9 mg/L and erythrocyte sedimentation rate (ESR) was 44 mm/hr. Human immunodeficiency antibody screen was negative. Anti‐nuclear antibodies and anti‐nuclear cytoplasmic antibody panel were negative. Computed tomography angiogram (CTA) of the lower extremities demonstrated severe stenosis of the left superficial femoral artery and severe stenosis of the right posterior tibial artery. Ankle‐brachial indices were 0.83 on the right side and 0.72 on the left, indicating mild to moderate arterial disease.
ESR and CRP are nonspecific markers of inflammation. Their elevation does not prioritize malignancy, autoimmunity, or infection. ANCA might be negative in commonly ANCA‐associated vasculitides such as eosinophilic granulomatosis with polyangiitis, microscopic polyangiitis, and granulomatosis with polyangiitis. However, the lack of respiratory and renal involvement in addition to the negative ANCA panel make such diagnoses unlikely. CTA of the patient's legs showed significant peripheral artery disease (PAD). This is unlikely to be the cause of his presentation; PAD should not cause petechiae, and his pain is disproportionate to the severity of the vascular disease reported. The additional information leaves the differential unchanged.
A dermatologist was consulted. She described and photographed a perifollicular distribution of the lower extremity petechiae with associated perifollicular hyperkeratosis and retained curled hairs (Figure 2).

The described rash is specific for scurvy. His homelessness and alcohol intake likely made him vulnerable to ascorbic acid deficiency from lack of access to fruits and vegetables. Measurement of vitamin C level is unnecessary as the pretest probability for scurvy is very high. More relevant than a vitamin C level or skin biopsy is empiric treatment with ascorbic acid; as mentioned, patients with scurvy respond rapidly to vitamin C therapy. Given the neuropathy, he should be assessed for concomitant thiamine and/or cobalamin deficiency. His peripheral arterial disease is unlikely to be related.
His ascorbic acid level was 0.0 mg/dL (reference range, 0.22.0 mg/dL). Further history was obtained, and the patient reported exclusively eating frozen hamburgers and burritos for almost 1 year. He believed he had not had a fruit or vegetable in over 10 years. He was started on 1000 mg daily of ascorbic acid. By hospital day 2, his rash had mostly resolved and he was able to stand with some support. The patient was seen by his primary care physician 3 weeks after diagnosis, with his exercise tolerance nearly back to baseline. His rash had entirely resolved.
DISCUSSION
Unlike other mammals, humans do not have the ability to convert glucose to vitamin C and thus require an exogenous source, such as fruits and vegetables. The oft‐cited observation of scurvy in sailors during long journeys in the 18th century is a classic example of clinical disease due to vitamin C deficiency.[1] Once replete, body stores of vitamin C are usually sufficient to last over 6 months of deprivation. In some patients, symptoms of deficiency may appear within 3 months.[2] The patient in this report likely suffered years of vitamin C deficiency, resulting in the significant manifestations of scurvy reported here.
Vitamin C is a water‐soluble vitamin necessary for the biosynthesis of collagen, L‐carnitine, and neurotransmitters.[3] With deficiency, the resulting impairment in the formation of collagen affects blood vessel integrity and results in perivascular edema and erythrocyte extravasation. Clinically, this leads to hemorrhagic manifestations (eg, periosteal hemorrhage and perifollicular petechiae) and poor wound healing. Corkscrew hairs result because of vitamin C's role in disulfide bonding during hair formation. Woody edema and dyspnea are thought to be a consequence of leaky capillaries.[4]
Scurvy is still a significant cause of morbidity in at‐risk populations in the United States. Several populations have been identified as high risk for vitamin C deficiency, including the elderly, persons who live alone, alcoholics, smokers, individuals of low socioeconomic status, patients on hemodialysis, and those with psychiatric disease.[5] Specifically, the high oxidative stress associated with smoking, the history of alcohol abuse, and homelessness put this patient at an especially high risk.[6] Those with oxidative stressors have been postulated to require up to 125 mg/d of vitamin C compared to 60 to 90 mg/d of those without the same risks.[7] In a national health and nutrition survey in the United States in 2004, the prevalence of vitamin C deficiency as defined by a serum level <0.2 mg/dL was noted in 7.1% of those surveyed.[8] This study also noted a significantly higher prevalence of deficiency in smokers and individuals with low socioeconomic status.
Scurvy is a clinical diagnosis based on clinical features and dietary history. Severe manifestations of scurvy may happen quickly after the initial presentation, making early diagnosis especially important.[2] These include anemia, bone pain, ocular hemorrhage, cerebral hemorrhage, and hemopericardium.[2, 4] If needed, laboratory diagnosis can be made by demonstrating a serum ascorbic acid level <0.2 mg/dL. However, the level may be normal if the patient has had recent intake of vitamin C. In that scenario, the leukocyte vitamin C concentration may be a more accurate measure of the body stores as leukocyte levels change more slowly.[4] Biopsy of skin lesions is not necessary for the diagnosis and typically show a dilated hair follicle with keratin plugging and perifollicular hemorrhage.[9] Given the lack of adverse effects, treatment with vitamin C supplementation should begin immediately, even with low suspicion of scurvy, and response can serve as further clinical evidence and render laboratory testing unnecessary.
In this patient, the diagnosis was challenging for several reasons. The presentation was concerning for vasculitis given the dependent petechiae and elevated inflammatory markers. However, in scurvy, the petechiae are perifollicular and associated with hyperkeratosis, as opposed to the palpable purpura often described in vasculitis. Further, marked elevations in ESR and CRP have also been reported in scurvy.[10] The initial concern for vasculitis and clinician discomfort with a diagnosis based solely on a rash delayed the diagnosis. The complaint of polyneuropathy also seemed inconsistent with scurvy. Very rarely, scurvy may cause a neuropathy by hemorrhage into the nerve sheath, as seen in a case of bilateral femoral neuropathy.[11] Most likely, this patient had an underlying vitamin B deficiency explaining his polyneuropathy. Unfortunately, the patient was lost to follow‐up after his postdischarge visit with his primary physician and was not tested for other concomitant vitamin deficiencies.
Scurvy is very responsive to even small doses of vitamin C supplementation. For rapid recovery, doses ranging from 100 mg 3 times daily to 1000 mg daily of oral vitamin C are recommended for at least 1 month. Resolution of symptoms will begin within 24 hours, and complete recovery should occur by 3 months.[4] Scurvy is a classic example of how nutritional deficiencies can have a myriad of presentations and may mimic other systemic diseases. Clinicians who recall these manifestations and carefully assess patients for nutritional risks may be able to quickly identify the missing element (or elements) in a patient's diet, and initiate treatment that is often rapidly effective.
KEY TEACHING POINTS
- Vitamin C deficiency initially presents with classic dermatological findings of perifollicular petechiae with associated hyperkeratosis and corkscrew hairs.
- Scurvy is a clinical diagnosis based on history and presentation. Vitamin C serum level may not accurately reflect body stores, and a leukocyte vitamin C level may be obtained. The diagnosis may also be confirmed with observed response to vitamin C supplementation.
- Scurvy should be suspected in high‐risk populations, especially the marginally housed, the elderly, alcoholics, and smokers.
- Clinicians should screen patients with scurvy for other nutritional deficiencies including thiamine, folate, B12, and vitamin D levels.
Disclosures: Nothing to report.
A 57‐year‐old man presented to an emergency department with 1 month of progressive, bilateral lower extremity pain and weakness.
The first step in evaluating weakness is to determine whether it is objective (ie, decreased muscle strength due to pathology along the neuromuscular axis) or subjective. The sensation of weakness without loss of muscle strength may result from a debilitating chronic disease (eg, congestive heart failure, anemia, or chronic obstructive pulmonary disease). In patients with true lower extremity weakness it is prudent to assess for a myelopathy with a focused history and exam that includes assessment of bowel or bladder impairment and anal reflex. The presence of pain along with weakness might suggest disease of the muscle itself. A myopathy may arise from an infectious (eg, influenza), inflammatory (eg, polymyositis), endocrine (eg, hypothyroidism), or drug‐related (eg, statin) process.
The patient described 1 month of generalized weakness and pain in his lower extremities, which had worsened progressively to the point where ambulation was difficult. He was able to rise from a seated position using his arms for assistance, but had difficulty balancing in a standing position without assistance. The pain also involved both of his knees and increased with weight bearing. He also complained of bilateral lower extremity numbness and paresthesias, which had been migrating proximally from his toes over several months. He denied any recent trauma to his legs or back.
These symmetrical, distal sensory deficits favor a peripheral neuropathy over a myopathy, with neuropathic pain and arthralgia causing his impaired ability to ambulate or remain standing. In polyneuropathy, the type of nerve involvement (sensory vs motor) and pathology (axonal vs demyelinating) helps prioritize the differential. In developed countries, the most common causes of polyneuropathy are diabetes mellitus and alcohol. However, the tempo of his disease broadens the possibilities to include acute inflammatory demyelinating polyneuropathy, paraneoplastic syndrome (eg, monoclonal gammopathy), an autoimmune process (eg, rheumatoid arthritis, vasculitis), and heavy metal toxicity such as lead poisoning.
He had no history of chronic medical illness or hospitalizations and took no medications. His social history was notable for a history of alcohol abuse. For the past several years, he had only been drinking 1 to 2 beers daily due to cost, but had a history of more significant alcohol abuse in the distant past. He smoked 1 pack of tobacco per day, and denied illicit drug use. He denied any sexual activity or recent travel. He lived in a van, and had been homeless for over 10 years.
His socioeconomic status adds a layer of complexity to the case. Human immunodeficiency virus and hepatitis C virus (HCV) are more prevalent in the homeless and are associated with polyneuropathy. His lack of funds may drive him to drink illegally distilled alcohol, which can cause polyneuropathy through lead or arsenic toxicity. Excessive smoking could be linked to a peripheral neuropathy through a paraneoplastic syndrome (eg, small cell lung cancer).
Alcohol causes polyneuropathy through toxic effects on nerves and may be playing a role in his polyneuropathy, but the rapid pace and severity suggests an additional process. Alcoholism can be associated with deficiency of various B vitamins, such as thiamine, pyridoxine, and cobalamin, which can cause polyneuropathy. In alcoholics who are hospitalized, thiamine should be administered prior to glucose to decrease risk of Wernicke encephalopathy.
His temperature was 38.0C, heart rate 93 beats/min, blood pressure 121/60 mm Hg, respiratory rate 14/min, with an oxygen saturation of 97% on ambient air. He appeared cachectic and disheveled. He had moist mucous membranes, poor dentition with missing teeth, and no mucosal bleeding or oropharyngeal erythema. His cardiac exam revealed no murmurs, rubs, or gallops. His lungs were clear. His abdominal exam was benign, without masses or tenderness. His skin exam (Figure 1) was notable for nonpalpable petechiae on his anterior shins and thighs up to his buttocks. His extremity exam was significant for diffuse tenderness to light palpation on both lower extremities, a large indurated tender ecchymosis 15 15 cm behind the right knee, and another ecchymosis 6 8 cm behind the left knee. His dorsalis pedis and anterior tibialis pulses were appreciated by Doppler but not by palpation. He had decreased sensation to light touch of his bilateral feet to his ankles. Strength exam was challenging to assess secondary to posterior leg pain, but he demonstrated 4/5 strength of his hip flexors, quadriceps, and plantar flexors of the foot. His upper extremity strength and sensory exam were normal. Examination of the cranial nerves was normal. He had 2+ patellar and Achilles reflexes. Gait could not be adequately assessed.

Petechiae manifest as a nonblanchable rash caused by extravasated red blood cells. Common etiologies include quantitative or qualitative platelet defects, disseminated intravascular coagulopathy, trauma, and vasculitis. Cirrhosis from alcohol leading to thrombocytopenia and petechial rash is unlikely given no other stigmata of liver disease such as jaundice, spider angiomata, caput medusae, or palmar erythema. Less common causes include nutritional deficiency and light chain (AL) amyloidosis, which could explain both the neuropathy and rash.
The constellation of fever and petechial rash can represent a life‐threatening systemic process. Infectious agents that require immediate consideration with fever and petechiae include Neisseria meningitidis (meningococcemia), Rickettsia rickettsii (Rocky Mountain spotted fever), Staphylococcus, and Streptococcus. However, his normal blood pressure, dependent distribution of rash, and neuropathy make a severe bacterial infection less likely. Thrombotic thrombocytopenic purpura is possible and should prompt assessment of platelets, peripheral blood smear, and lactate dehydrogenase. Among vasculitides, the polyneuropathy, fever, and dependent distribution of petechial rash prioritize a small‐to‐medium vessel vasculitis, where the pathophysiology involves inflammation of dermal vessels and vasa nervorum (blood supply of nerves). Examples include HCV‐related cryoglobulinemic vasculitis, polyarteritis nodosa (PAN), and antineutrophilic cytoplasmic antibody (ANCA)associated vasculitis. However, ANCA‐associated vasculitis is less likely without upper or lower respiratory symptoms. Henoch‐Schonlein purpura may explain the rash but is more common in children and is not associated with neuropathy.
Posterior knee ecchymosis, in absence of trauma, raises suspicion for a ruptured Baker's cyst. However, the bilateral involvement and lack of calf manifestations makes this unlikely. The location raises concern for hemarthrosis, so a more likely explanation would be coagulopathy (eg, an acquired factor inhibitor) or a collagen defect. In developed countries, a commonly overlooked category of diseasenutritional deficiencywarrants serious consideration in alcoholics. Vitamin C deficiency (scurvy) may cause a petechial rash and ecchymosis from perifollicular hemorrhage and impaired collagen synthesis, respectively. Scurvy can masquerade as small vessel vasculitis because of its associated petechial rash. The neuropathy might be explained by concomitant thiamine or cobalamin deficiency. It is important to obtain a thorough dietary history and assess vibration and proprioception, which may be impaired from pathology of the dorsal column in cobalamin deficiency. The low‐grade fever may be a red herring, but if it becomes significant would be difficult to explain with nutritional deficiency.
In summary, a judicious evaluation for infection is mandatory, but the leading diagnoses are a small‐to‐medium vessel vasculitis (PAN or HCV‐related cryoglobulinemia), deficiency of multiple vitamins, and AL amyloidosis.
Initial labs showed white blood cell count 7800/L, hematocrit 39.2%, and platelet count of 251,000/L. Serum chemistry demonstrated a sodium of 131 mEq/L, potassium 4.7 mEq/L, chloride 93 mEq/L, bicarbonate 23 mEq/L, blood urea nitrogen 8 mg/dL, and creatinine 0.8 mg/dL. His aminotransferases, albumin, alkaline phosphatase, and coagulation studies were within normal limits. Urinalysis was remarkable for 2+ urobilinogen, 1+ ketones, and a bland sediment. Urine toxicology screen was negative.
His white blood cell count is normal, so with a heart rate of 93 beats/minute, he barely meets a single criterion of systemic inflammatory response syndrome (SIRS). The lack of SIRS and normal platelet, albumin, white blood cell, and red blood cell counts significantly reduces the likelihood of an infectious or inflammatory process. Without any clinical or biochemical evidence of HCV infection, HCV‐associated cryoglobulinemia is less likely. A normal creatinine might overestimate renal function in setting of decreased protein intake and muscle mass; nevertheless, the bland urine sediment further lowers probability of PAN and ANCA‐associated vasculitides. The normal platelet count and coagulation studies suggest either a qualitative platelet defect (eg, acquired von Willebrand disease) or impaired vessel integrity (eg, collagen defect) to explain the petechial rash. The urine ketones likely represent alcohol and/or starvation‐related ketosis. These data reduce the probability of infection and vasculitis, and prioritize vitamin deficiency and AL amyloidosis. Antibiotic therapy is not appropriate, given the absence of SIRS and subacute course. His presentation likely prompted a wide variety of tests, but most relevant would be a dietary history, cobalamin and vitamin C levels, serum free light chains, and skin biopsy. Biopsy of the rash would allow assessment for vasculitis and AL amyloidosis. The former is marked by inflammatory infiltrate of vessels, and the latter by perivascular invasion with amyloid fibrils. If the dietary history was consistent with ascorbic acid deficiency (scurvy), in addition to thiamine, he should be empirically treated with vitamin C. Patients with scurvy demonstrate rapid clinical improvement with treatment.
C‐reactive protein (CRP) was 47.9 mg/L and erythrocyte sedimentation rate (ESR) was 44 mm/hr. Human immunodeficiency antibody screen was negative. Anti‐nuclear antibodies and anti‐nuclear cytoplasmic antibody panel were negative. Computed tomography angiogram (CTA) of the lower extremities demonstrated severe stenosis of the left superficial femoral artery and severe stenosis of the right posterior tibial artery. Ankle‐brachial indices were 0.83 on the right side and 0.72 on the left, indicating mild to moderate arterial disease.
ESR and CRP are nonspecific markers of inflammation. Their elevation does not prioritize malignancy, autoimmunity, or infection. ANCA might be negative in commonly ANCA‐associated vasculitides such as eosinophilic granulomatosis with polyangiitis, microscopic polyangiitis, and granulomatosis with polyangiitis. However, the lack of respiratory and renal involvement in addition to the negative ANCA panel make such diagnoses unlikely. CTA of the patient's legs showed significant peripheral artery disease (PAD). This is unlikely to be the cause of his presentation; PAD should not cause petechiae, and his pain is disproportionate to the severity of the vascular disease reported. The additional information leaves the differential unchanged.
A dermatologist was consulted. She described and photographed a perifollicular distribution of the lower extremity petechiae with associated perifollicular hyperkeratosis and retained curled hairs (Figure 2).

The described rash is specific for scurvy. His homelessness and alcohol intake likely made him vulnerable to ascorbic acid deficiency from lack of access to fruits and vegetables. Measurement of vitamin C level is unnecessary as the pretest probability for scurvy is very high. More relevant than a vitamin C level or skin biopsy is empiric treatment with ascorbic acid; as mentioned, patients with scurvy respond rapidly to vitamin C therapy. Given the neuropathy, he should be assessed for concomitant thiamine and/or cobalamin deficiency. His peripheral arterial disease is unlikely to be related.
His ascorbic acid level was 0.0 mg/dL (reference range, 0.22.0 mg/dL). Further history was obtained, and the patient reported exclusively eating frozen hamburgers and burritos for almost 1 year. He believed he had not had a fruit or vegetable in over 10 years. He was started on 1000 mg daily of ascorbic acid. By hospital day 2, his rash had mostly resolved and he was able to stand with some support. The patient was seen by his primary care physician 3 weeks after diagnosis, with his exercise tolerance nearly back to baseline. His rash had entirely resolved.
DISCUSSION
Unlike other mammals, humans do not have the ability to convert glucose to vitamin C and thus require an exogenous source, such as fruits and vegetables. The oft‐cited observation of scurvy in sailors during long journeys in the 18th century is a classic example of clinical disease due to vitamin C deficiency.[1] Once replete, body stores of vitamin C are usually sufficient to last over 6 months of deprivation. In some patients, symptoms of deficiency may appear within 3 months.[2] The patient in this report likely suffered years of vitamin C deficiency, resulting in the significant manifestations of scurvy reported here.
Vitamin C is a water‐soluble vitamin necessary for the biosynthesis of collagen, L‐carnitine, and neurotransmitters.[3] With deficiency, the resulting impairment in the formation of collagen affects blood vessel integrity and results in perivascular edema and erythrocyte extravasation. Clinically, this leads to hemorrhagic manifestations (eg, periosteal hemorrhage and perifollicular petechiae) and poor wound healing. Corkscrew hairs result because of vitamin C's role in disulfide bonding during hair formation. Woody edema and dyspnea are thought to be a consequence of leaky capillaries.[4]
Scurvy is still a significant cause of morbidity in at‐risk populations in the United States. Several populations have been identified as high risk for vitamin C deficiency, including the elderly, persons who live alone, alcoholics, smokers, individuals of low socioeconomic status, patients on hemodialysis, and those with psychiatric disease.[5] Specifically, the high oxidative stress associated with smoking, the history of alcohol abuse, and homelessness put this patient at an especially high risk.[6] Those with oxidative stressors have been postulated to require up to 125 mg/d of vitamin C compared to 60 to 90 mg/d of those without the same risks.[7] In a national health and nutrition survey in the United States in 2004, the prevalence of vitamin C deficiency as defined by a serum level <0.2 mg/dL was noted in 7.1% of those surveyed.[8] This study also noted a significantly higher prevalence of deficiency in smokers and individuals with low socioeconomic status.
Scurvy is a clinical diagnosis based on clinical features and dietary history. Severe manifestations of scurvy may happen quickly after the initial presentation, making early diagnosis especially important.[2] These include anemia, bone pain, ocular hemorrhage, cerebral hemorrhage, and hemopericardium.[2, 4] If needed, laboratory diagnosis can be made by demonstrating a serum ascorbic acid level <0.2 mg/dL. However, the level may be normal if the patient has had recent intake of vitamin C. In that scenario, the leukocyte vitamin C concentration may be a more accurate measure of the body stores as leukocyte levels change more slowly.[4] Biopsy of skin lesions is not necessary for the diagnosis and typically show a dilated hair follicle with keratin plugging and perifollicular hemorrhage.[9] Given the lack of adverse effects, treatment with vitamin C supplementation should begin immediately, even with low suspicion of scurvy, and response can serve as further clinical evidence and render laboratory testing unnecessary.
In this patient, the diagnosis was challenging for several reasons. The presentation was concerning for vasculitis given the dependent petechiae and elevated inflammatory markers. However, in scurvy, the petechiae are perifollicular and associated with hyperkeratosis, as opposed to the palpable purpura often described in vasculitis. Further, marked elevations in ESR and CRP have also been reported in scurvy.[10] The initial concern for vasculitis and clinician discomfort with a diagnosis based solely on a rash delayed the diagnosis. The complaint of polyneuropathy also seemed inconsistent with scurvy. Very rarely, scurvy may cause a neuropathy by hemorrhage into the nerve sheath, as seen in a case of bilateral femoral neuropathy.[11] Most likely, this patient had an underlying vitamin B deficiency explaining his polyneuropathy. Unfortunately, the patient was lost to follow‐up after his postdischarge visit with his primary physician and was not tested for other concomitant vitamin deficiencies.
Scurvy is very responsive to even small doses of vitamin C supplementation. For rapid recovery, doses ranging from 100 mg 3 times daily to 1000 mg daily of oral vitamin C are recommended for at least 1 month. Resolution of symptoms will begin within 24 hours, and complete recovery should occur by 3 months.[4] Scurvy is a classic example of how nutritional deficiencies can have a myriad of presentations and may mimic other systemic diseases. Clinicians who recall these manifestations and carefully assess patients for nutritional risks may be able to quickly identify the missing element (or elements) in a patient's diet, and initiate treatment that is often rapidly effective.
KEY TEACHING POINTS
- Vitamin C deficiency initially presents with classic dermatological findings of perifollicular petechiae with associated hyperkeratosis and corkscrew hairs.
- Scurvy is a clinical diagnosis based on history and presentation. Vitamin C serum level may not accurately reflect body stores, and a leukocyte vitamin C level may be obtained. The diagnosis may also be confirmed with observed response to vitamin C supplementation.
- Scurvy should be suspected in high‐risk populations, especially the marginally housed, the elderly, alcoholics, and smokers.
- Clinicians should screen patients with scurvy for other nutritional deficiencies including thiamine, folate, B12, and vitamin D levels.
Disclosures: Nothing to report.
- . The discovery of vitamin C. Ann Nutr Metab. 2012;61(3):259–264.
- , , , , . Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24(4):432–443.
- . New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314(14):892–902.
- , . Adult scurvy. J Am Acad Dermatol. 1999;41(6):895–910.
- , . Be vigilant for scurvy in high‐risk groups. Practitioner. 2012;256(1755):23–5, 3.
- . Estimating ascorbic acid requirements for cigarette smokers. Ann N Y Acad Sci. 1993;686:335–346.
- , , . Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health. 2004;94(5):870–875.
- , , , . Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252–1263.
- , , . Scurvy: a cutaneous clinical diagnosis. Australas J Dermatol. 2003;44(1):48–51.
- , . Rheumatic manifestations of scurvy: a report of three recent cases in a major urban center and a review. Semin Arthritis Rheum. 2011;41(2):286–290.
- . Femoral neuropathy in scurvy. N Engl J Med. 1969;281(23):1292–1293.
- . The discovery of vitamin C. Ann Nutr Metab. 2012;61(3):259–264.
- , , , , . Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24(4):432–443.
- . New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314(14):892–902.
- , . Adult scurvy. J Am Acad Dermatol. 1999;41(6):895–910.
- , . Be vigilant for scurvy in high‐risk groups. Practitioner. 2012;256(1755):23–5, 3.
- . Estimating ascorbic acid requirements for cigarette smokers. Ann N Y Acad Sci. 1993;686:335–346.
- , , . Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health. 2004;94(5):870–875.
- , , , . Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252–1263.
- , , . Scurvy: a cutaneous clinical diagnosis. Australas J Dermatol. 2003;44(1):48–51.
- , . Rheumatic manifestations of scurvy: a report of three recent cases in a major urban center and a review. Semin Arthritis Rheum. 2011;41(2):286–290.
- . Femoral neuropathy in scurvy. N Engl J Med. 1969;281(23):1292–1293.