User login
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
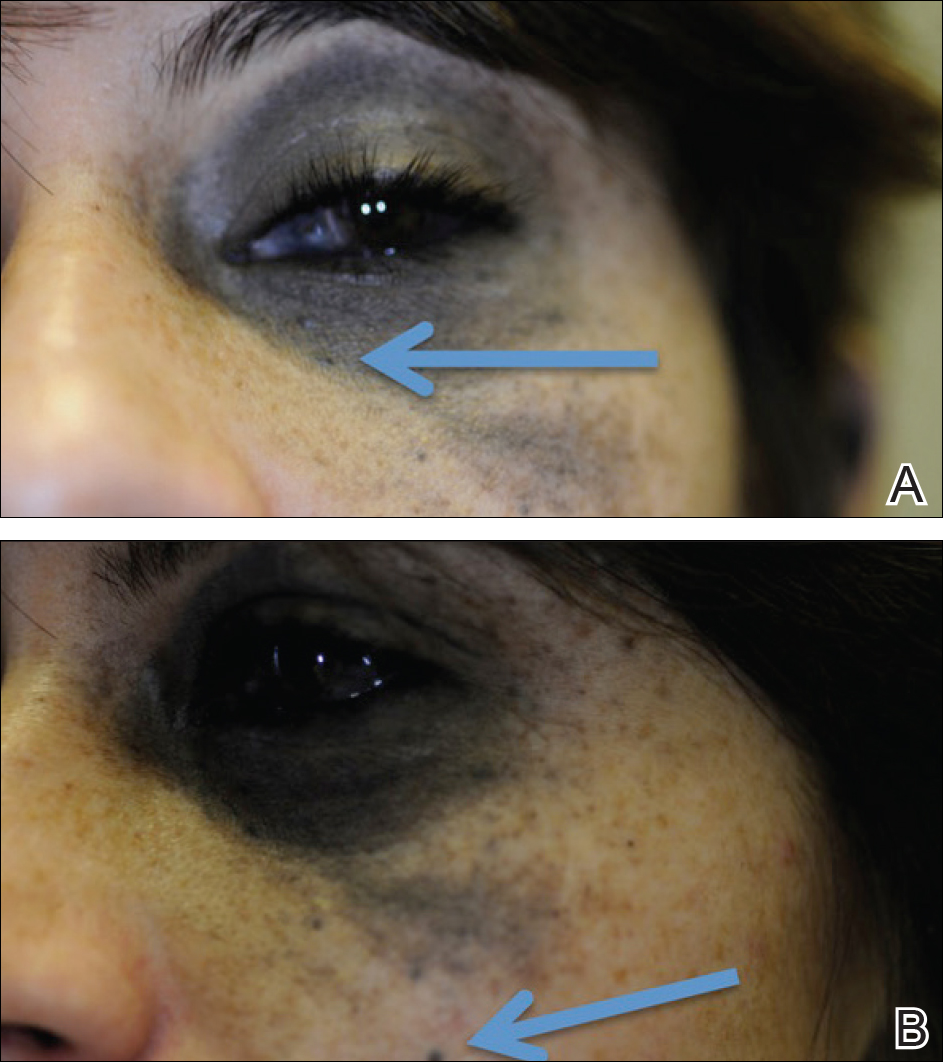
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
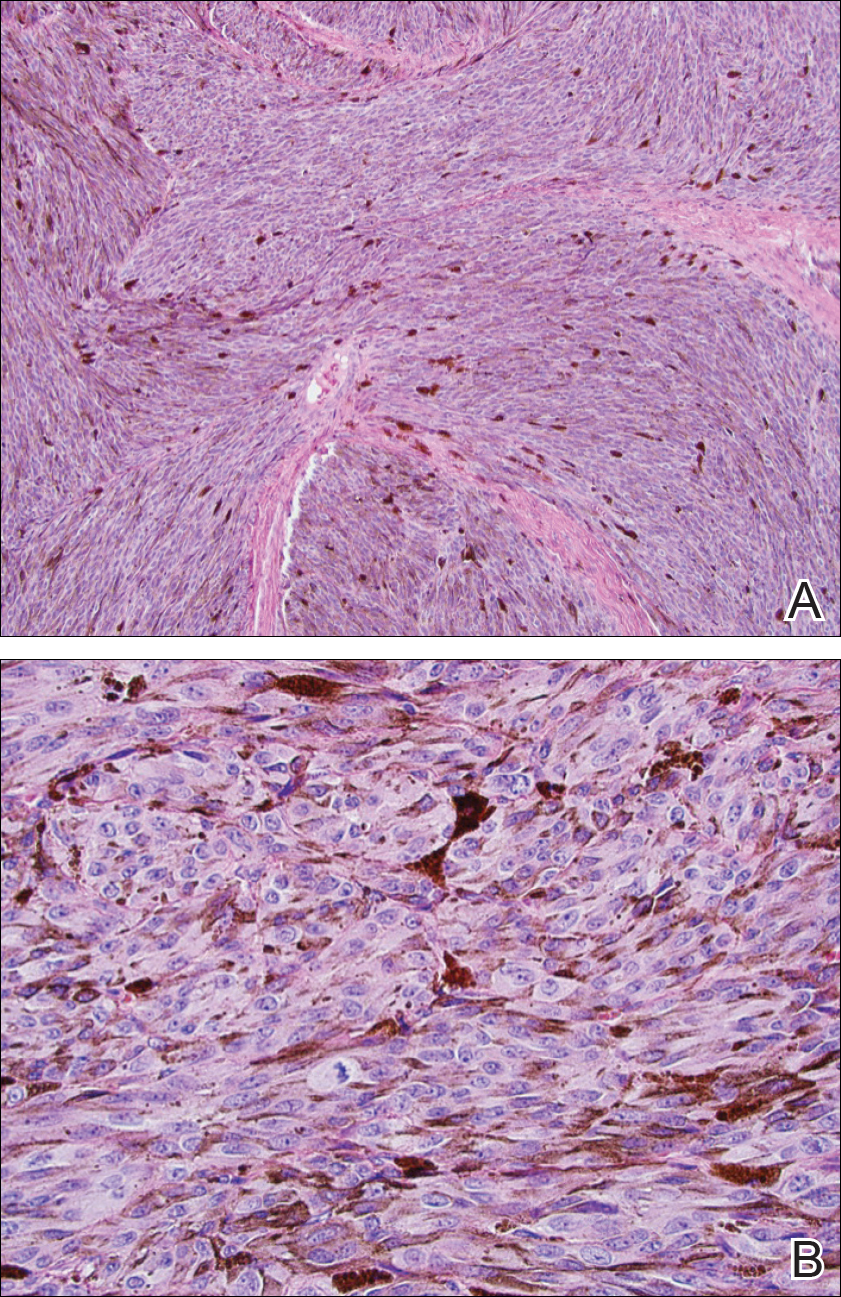
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.
The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.

The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
Nevus of Ota, originally referred to as nevus fusco-caeruleus ophthalmomaxillaris, initially was described in 1939 by Ota and Tanino.1 It is a dermal melanocytic hamartoma arising from incomplete migration of neural crest melanocytes to the epidermis during embryogenesis, resulting in nesting of subtle bands of dendritic melanocytes in the upper dermis. More common in Asians, Native Americans, and females, this hyperpigmented dermatosis most often is unilaterally distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.2 In some patients, nevus of Ota also is associated with ocular, orbital, and leptomeningeal melanocytosis. Approximately 15% of nevi of Ota have an activating guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) or G protein subunit alpha 11 (GNAQ) mutation; 85% of uveal melanomas harbor one of these mutations.3 Although uncommon, neoplastic transformation with extension or metastasis to the brain has been reported in patients with nevus of Ota.4
We report the case of a 29-year-old woman with a long-standing history of nevus of Ota who presented acutely with an intracranial melanoma as an extension of a primary uveal melanoma.
Case Report
A 29-year-old woman with a history of a nevus of Ota involving the left inner canthus, eyelids, sclera, and superior malar cheek that had been present since birth presented to the emergency department with an acute onset of severe headache, blurred vision, and vomiting. Computed tomography (CT) and magnetic resonance imaging of the brain revealed a hemorrhagic mass in the left frontal lobe. Subsequent frontal craniotomy and resection revealed an intracranial melanoma.
Two weeks following surgery, the patient underwent magnetic resonance imaging and combined positron emission tomography and CT scans that demonstrated a fluorodeoxyglucose-avid left retro-orbital mass. Histopathology of a biopsy from the left retro-orbital mass that had been obtained intraoperatively demonstrated a pigmented, spindled to epithelioid neoplasm with areas of marked atypia and a high mitotic rate that was compatible with malignant melanoma (Figure 1). Intracranial biopsies were sent for genetic study and were found to harbor GNAQ (Q209P) and BRCA1-associated protein 1 (BAP1)(p.P324fs*11) mutations.

The patient was referred to dermatology by neurosurgery for evaluation of a suspected primary cutaneous melanoma.


The patient entered a clinical trial at an outside institution several weeks after initial presentation to our institution for treatment with a mitogen-activated protein kinase MEK1 inhibitor as well as radiation therapy. The patient was lost to follow-up.
Comment
It has been demonstrated that homozygous loss of BAP1, located on the chromosome 3p21.1 locus, allows for progression to metastatic disease in uveal melanoma. The BAP1 gene codes for ubiquitin carboxyl-terminal hydrolase 7, which is involved in the removal of ubiquitin from proteins. This enzyme binds to BRCA1 (BRCA1, DNA repair associated) via the RING (Really Interesting New Gene) finger domain and acts as a tumor suppressor.5 Biallelic BAP1 mutations allow the transition to malignancy in concert with other mutations, such as GNAQ. Identification of a BAP1 mutation may serve as a valuable diagnostic and future therapeutic target in uveal melanoma.
Currently, there are no drugs that directly target mutated GNA11 and GNAQ proteins. Because aberrant GNA11 and GNAQ proteins activate MEK1, several MEK1 inhibitors are being tested with the hope of achieving indirect suppression of GNA11/GNAQ.6
We present a rare case of BAP1 and GNAQ mutations in intracranial melanoma associated with nevus of Ota. Although the uveal melanoma was not confirmed on histopathology, the clear mention of foci within the eye by ophthalmology, positron emission tomography–CT scan showing a fluorodeoxyglucose-avid left retro-orbital mass, and genetic studies of the intracranial biopsies were highly suggestive of a primary uveal melanoma.
Our case highlights the importance of ongoing ocular screening in patients with nevus of Ota, noting the possibility of malignant transformation. Furthermore, patients with nevus of Ota with ocular involvement may benefit from testing of BAP1 protein expression by immunohistochemistry.7 Identification of BAP1 and GNAQ mutations in patients with nevus of Ota place them at markedly higher risk for malignant melanoma. Therefore, dermatologic evaluation of patients with nevus of Ota should include a thorough review of the patient’s history and skin examination as well as referral for ophthalmologic evaluation.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
- Ota M, Tanino H. A variety of nevus, frequently encountered in Japan, nevus fusco-caeruleus ophthalmomaxillaris and its relationship to pigmentary changes in the eye. Tokyo Med J. 1939;63:1243-1244.
- Swann PG, Kwong E. The naevus of Ota. Clin Exp Optom. 2010;93:264-267.
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma [published online November 17, 2010]. N Engl J Med. 2010;363:2191-2199.
- Nitta K, Kashima T, Mayuzumi H, et al. Animal-type malignancy melanoma associated with nevus of Ota in the orbit of a Japanese woman: a case report. Melanoma Res. 2014;24:286-289.
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas [published online November 4, 2010]. Science. 2010;330:1410-1413.
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724-4734.
- Kalirai H, Dodson A, Faqir S, et al. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing [published online July 24, 2010]. Br J Cancer. 2014;111:1373-1380.
Practice Points
- Nevus of Ota is a hyperpigmented dermatosis that typically is distributed along the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve.
- GNAQ and BAP1 mutations in patients with nevus of Ota confer a greater risk for malignant melanoma and metastatic progression.
- Ongoing ophthalmologic screening is paramount in patients with nevus of Ota and may prevent devastating sequelae.