Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
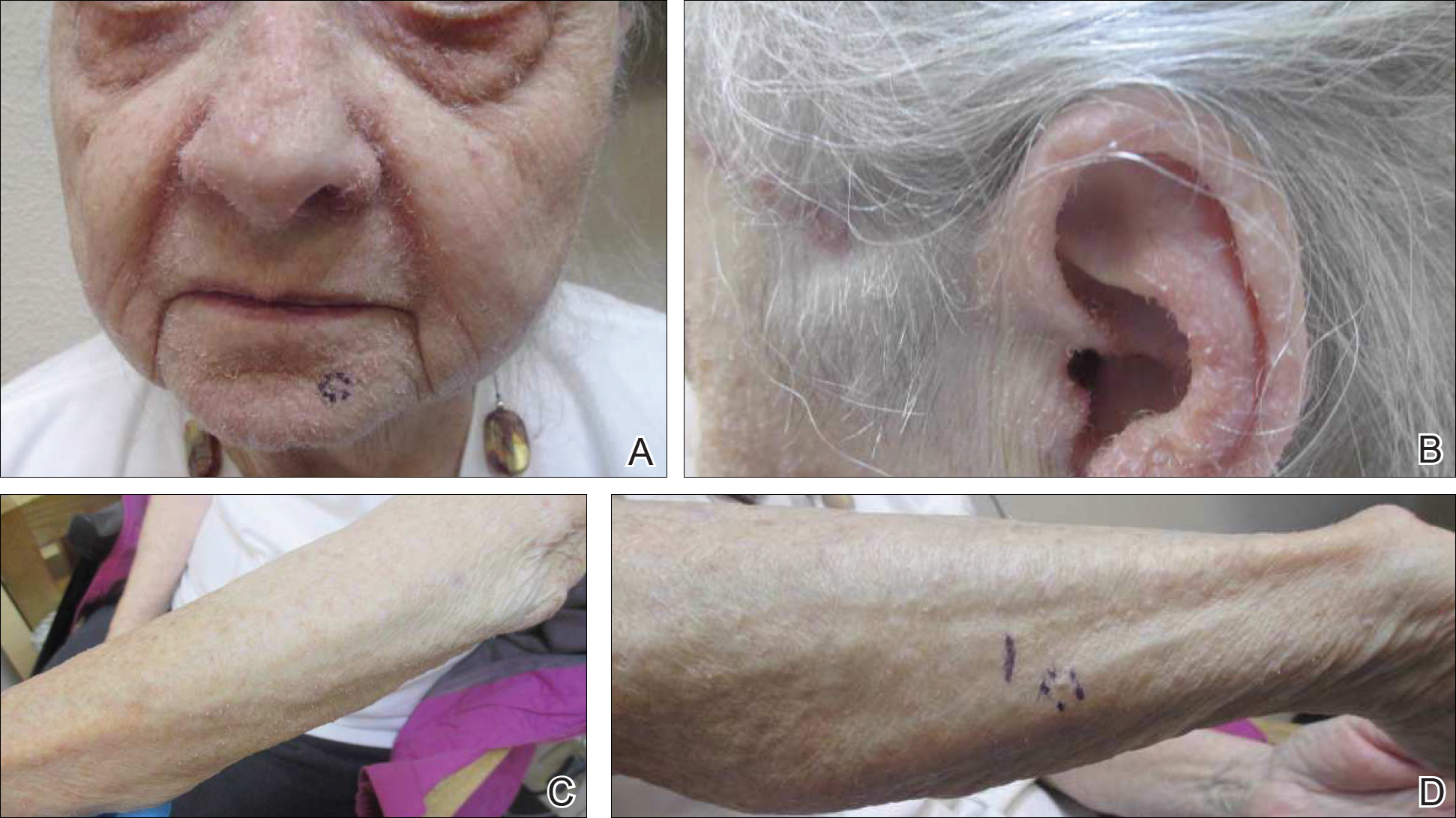
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).
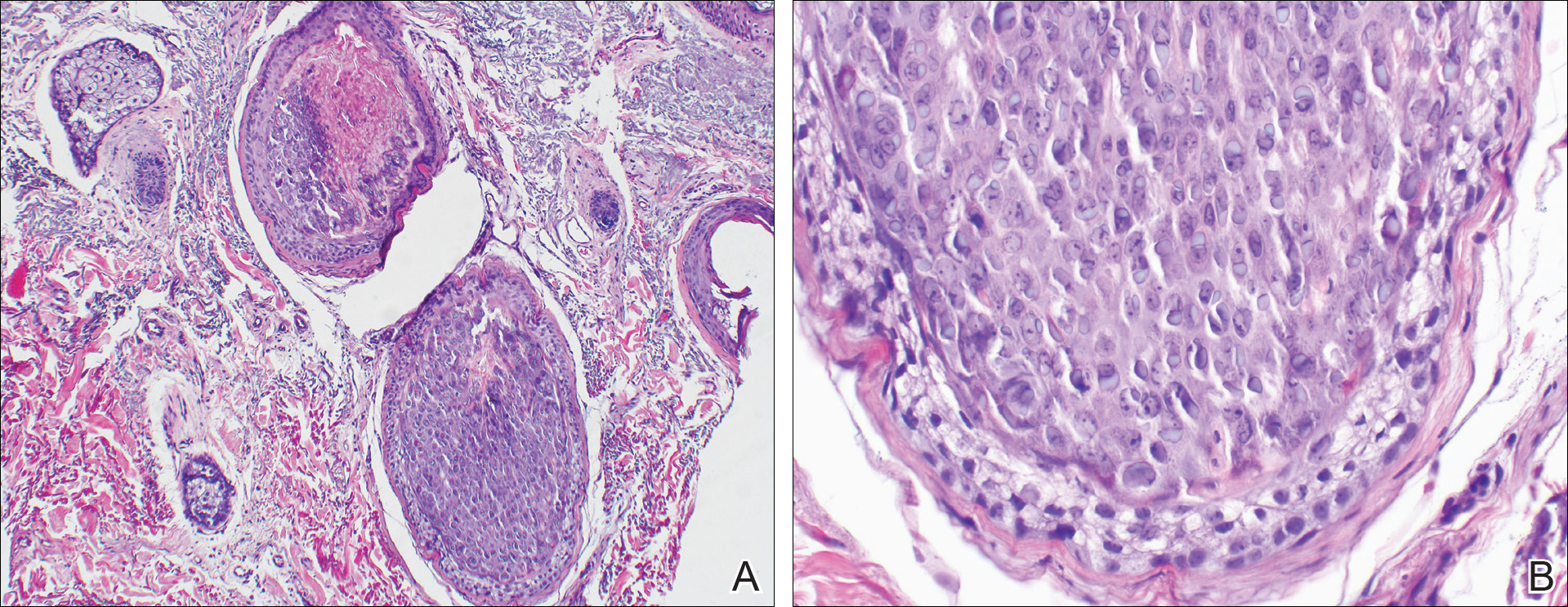
A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.
The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.