Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
In June 2017, long-acting, triple-bead mixed amphetamine salt (MAS) was FDA-approved under the brand name Mydayis for the once-daily treatment of ADHD in adolescents (age ≥13) and adults (Table 1).3 This formulation utilizes a unique triple-bead technology to extend its duration of action. When this technology was developed—nearly a decade ago—it was hoped that the duration of action of the stimulant would be significantly increased, beyond the 10- to 12-hour duration of action of available stimulants at that time. This newly approved formulation provides 16 hours of coverage, and patients may experience an onset of action within 2 to 4 hours following administration.3
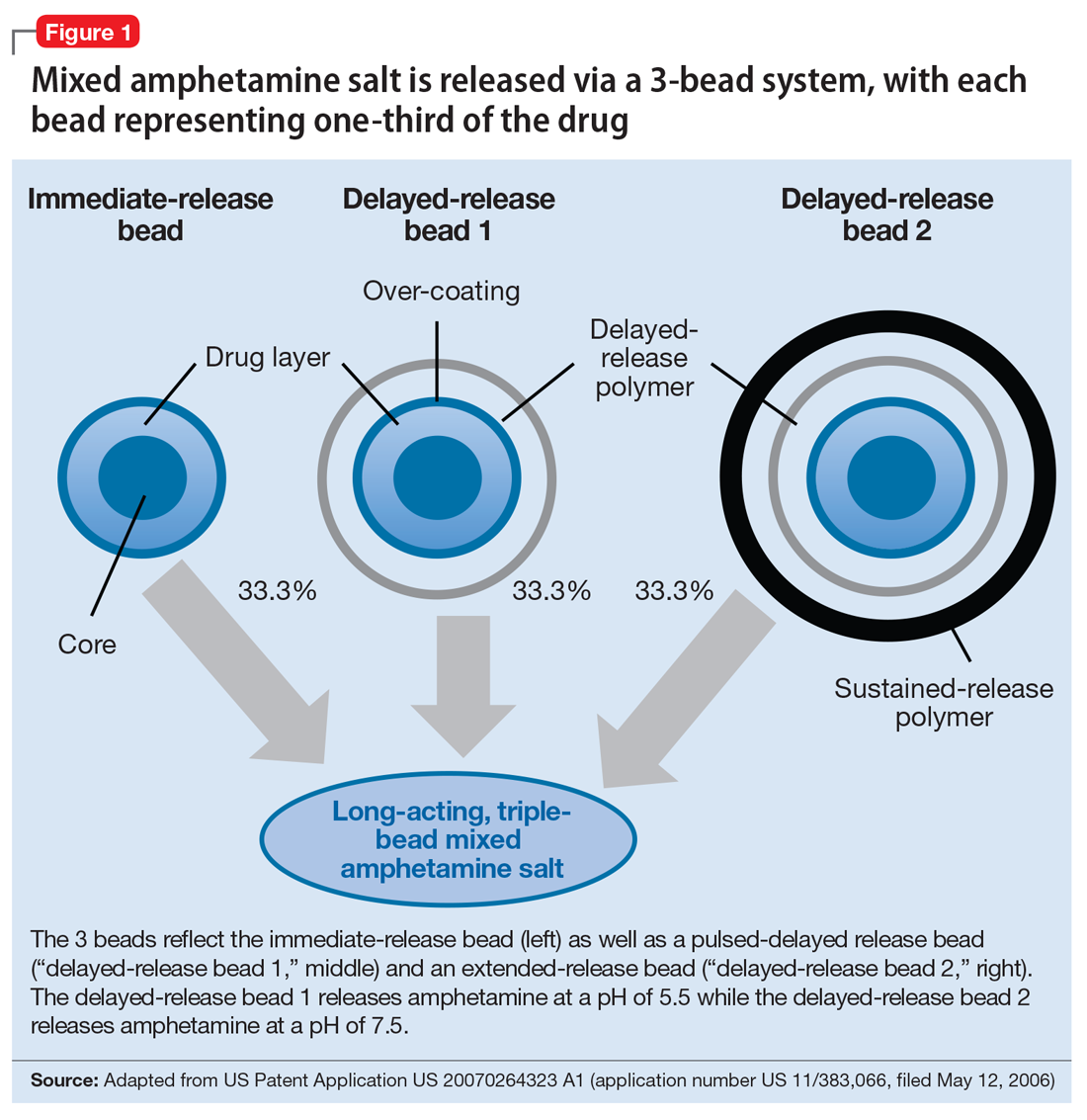
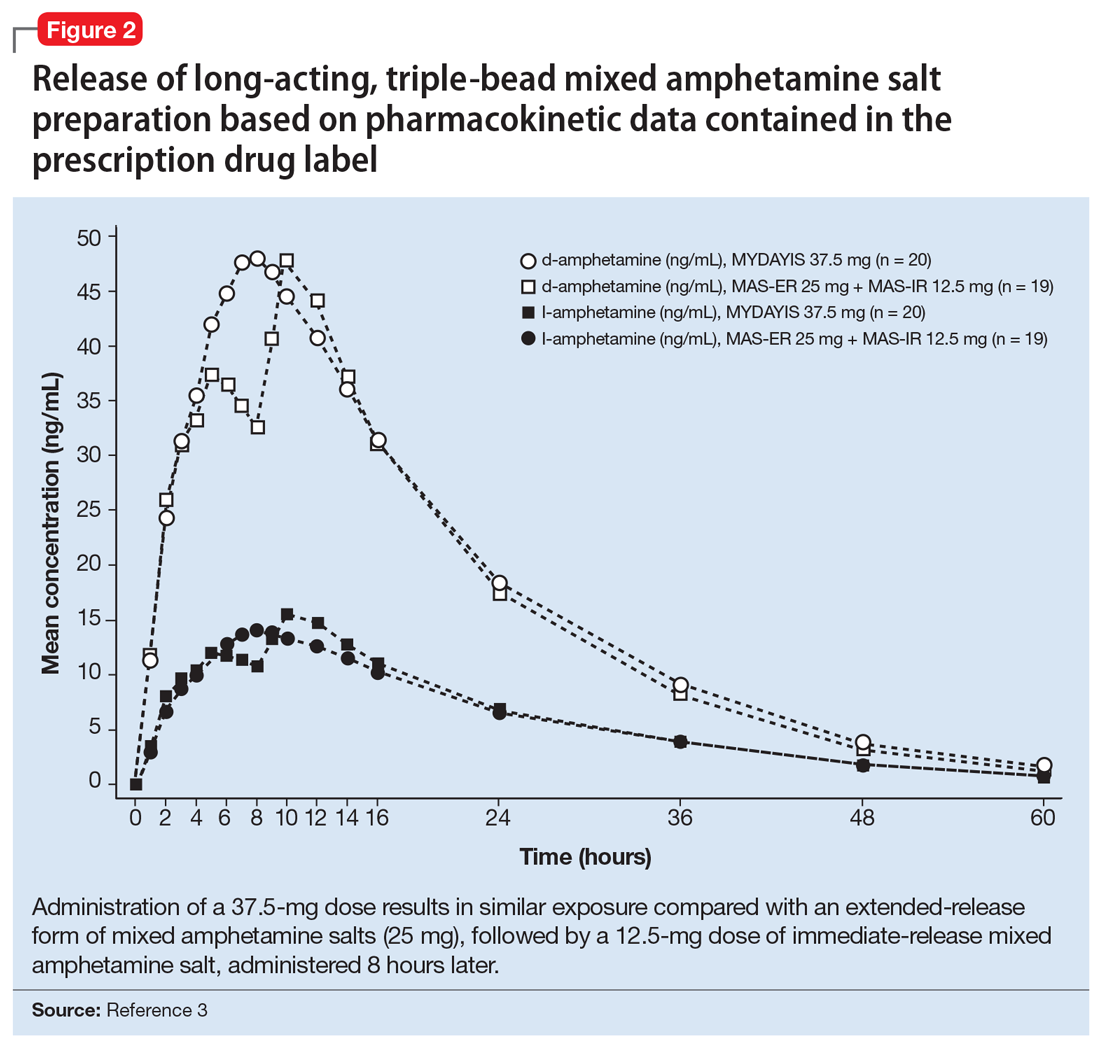
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.