User login
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
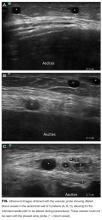
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
© 2018 Society of Hospital Medicine