User login
Vascular Ultrasonography: A Novel Method to Reduce Paracentesis Related Major Bleeding
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
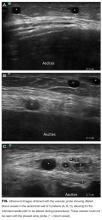
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
© 2018 Society of Hospital Medicine
Safe and effective bedside thoracentesis: A review of the evidence for practicing clinicians
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
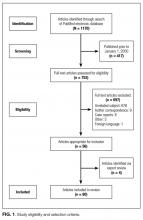
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
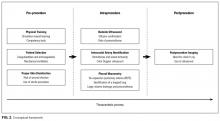
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.
PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
© 2017 Society of Hospital Medicine
Thoracentesis Referral
Internal medicine (IM) residents and hospitalist physicians commonly conduct bedside thoracenteses for both diagnostic and therapeutic purposes.[1] The American Board of Internal Medicine only requires that certification candidates understand the indications, complications, and management of thoracenteses.[2] A disconnect between clinical practice patterns and board requirements may increase patient risk because poorly trained physicians are more likely to cause complications.[3] National practice patterns show that many thoracenteses are referred to interventional radiology (IR).[4] However, research links performance of bedside procedures to reduced hospital length of stay and lower costs, without increasing risk of complications.[1, 5, 6]
Simulation‐based education offers a controlled environment where trainees improve procedural knowledge and skills without patient harm.[7] Simulation‐based mastery learning (SBML) is a rigorous form of competency‐based education that improves clinical skills and reduces iatrogenic complications and healthcare costs.[5, 6, 8] SBML also is an effective method to boost thoracentesis skills among IM residents.[9] However, there are no data to show that thoracentesis skills acquired in the simulation laboratory transfer to clinical environments and affect referral patterns.
We hypothesized that a thoracentesis SBML intervention would improve skills and increase procedural self‐confidence while reducing procedure referrals. This study aimed to (1) assess the effect of thoracentesis SBML on a cohort of IM residents' simulated skills and (2) compare traditionally trained (nonSBML‐trained) residents, SBML‐trained residents, and hospitalist physicians regarding procedure referral patterns, self‐confidence, procedure experience, and reasons for referral.
METHODS AND MATERIALS
Study Design
We surveyed physicians about thoracenteses performed on patients cared for by postgraduate year (PGY)‐2 and PGY‐3 IM residents and hospitalist physicians at Northwestern Memorial Hospital (NMH) from December 2012 to May 2015. NMH is an 896‐bed, tertiary academic medical center, located in Chicago, Illinois. A random sample of IM residents participated in a thoracentesis SBML intervention, whereas hospitalist physicians did not. We compared referral patterns, self‐confidence, procedure experience, and reasons for referral between traditionally trained residents, SBML‐trained residents, and hospitalist physicians. The Northwestern University Institutional Review Board approved this study, and all study participants provided informed consent.
At NMH, resident‐staffed services include general IM and nonintensive care subspecialty medical services. There are also 2 nonteaching floors staffed by hospitalist attending physicians without residents. Thoracenteses performed on these services can either be done at the bedside or referred to pulmonary medicine or IR. The majority of thoracenteses performed by pulmonary medicine occur at the patients' bedside, and the patients also receive a clinical consultation. IR procedures are done in the IR suite without additional clinical consultation.
Procedure
One hundred sixty residents were available for training over the study period. We randomly selected 20% of the approximately 20 PGY‐2 and PGY‐3 IM residents assigned to the NMH medicine services each month to participate in SBML thoracentesis training before their rotation. Randomly selected residents were required to undergo SBML training but were not required to participate in the study. This selection process was repeated before every rotation during the study period. This randomized wait‐list control method allowed residents to serve as controls if not initially selected for training and remain eligible for SBML training in subsequent rotations.
Intervention
The SBML intervention used a pretest/post‐test design, as described elsewhere.[9] Residents completed a clinical skills pretest on a thoracentesis simulator using a previously published 26‐item checklist.[9] Following the pretest, residents participated in 2, 1‐hour training sessions including a lecture, video, and deliberate practice on the simulator with feedback from an expert instructor. Finally, residents completed a clinical skills post‐test using the checklist within 1 week from training (but on a different day) and were required to meet or exceed an 84.3% minimum passing score (MPS). The entire training, including pre‐ and post‐tests, took approximately 3 hours to complete, and residents were given an additional 1 hour refresher training every 6 months for up to a year after original training. We compared pre‐ and post‐test checklist scores to evaluate skills improvement.
Thoracentesis Patient Identification
The NMH electronic health record (EHR) was used to identify medical service inpatients who underwent a thoracentesis during the study period. NMH clinicians must place an EHR order for procedure kits, consults, and laboratory analysis of thoracentesis fluid. We developed a real‐time query of NMH's EHR that identified all patients with electronic orders for thoracenteses and monitored this daily.
Physician Surveys
After each thoracentesis, we surveyed the PGY‐2 or PGY‐3 resident or hospitalist caring for the patient about the procedure. A research coordinator, blind to whether the resident received SBML, performed the surveys face‐to‐face on Monday to Friday during normal business hours. Residents were not considered SBML‐trained until they met or exceeded the MPS on the simulated skills checklist at post‐test. Surveys occurred on Monday for procedures performed on Friday evening through Sunday. Survey questions asked physicians about who performed the procedure, their procedural self‐confidence, and total number of thoracenteses performed in their career. For referred procedures, physicians were asked about reasons for referral including lack of confidence, work hour restrictions (residents only), and low reimbursement rates.[10] There was also an option to add other reasons.
Measurement
The thoracentesis skills checklist documented all required steps for an evidence‐based thoracentesis. Each task received equal weight (0 = done incorrectly/not done, 1 = done correctly).[9] For physician surveys, self‐confidence about performing the procedure was rated on a scale of 0 = not confident to 100 = very confident. Reasons for referral were scored on a Likert scale 1 to 5 (1 = not at all important, 5 = very important). Other reasons for referral were categorized.
Statistical Analysis
The clinical skills pre‐ and post‐test checklist scores were compared using a Wilcoxon matched pairs rank test. Physician survey data were compared between different procedure performers using the 2 test, independent t test, analysis of variance (ANOVA), or Kruskal‐Wallis test depending on data properties. Referral patterns measured by the Likert scale were averaged, and differences between physician groups were evaluated using ANOVA. Counts of other reasons for referral were compared using the 2 test. We performed all statistical analyses using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY).
RESULTS
Thoracentesis Clinical Skills
One hundred twelve (70%) residents were randomized to SBML, and all completed the protocol. Median pretest scores were 57.6% (interquartile range [IQR] 43.376.9), and final post‐test mastery scores were 96.2 (IQR 96.2100.0; P < 0.001). Twenty‐three residents (21.0%) failed to meet the MPS at initial post‐test, but met the MPS on retest after <1 hour of additional training.
Physician Surveys
The EHR query identified 474 procedures eligible for physician surveys. One hundred twenty‐two residents and 51 hospitalist physicians completed surveys for 472 procedures (99.6%); 182 patients by traditionally trained residents, 145 by SBML‐trained residents, and 145 by hospitalist physicians. As shown in Table 1, 413 (88%) of all procedures were referred to another service. Traditionally trained residents were more likely to refer to IR compared to SBML‐trained residents or hospitalist physicians. SBML‐trained residents were more likely to perform bedside procedures, whereas hospitalist physicians were most likely to refer to pulmonary medicine. SBML‐trained residents were most confident in their procedural skills, despite hospitalist physicians performing more actual procedures.
| Traditionally Trained Resident Surveys, n = 182 | SBML‐Trained Resident Surveys, n = 145 | Hospitalist Physician Surveys, n = 145 | P Value | |
|---|---|---|---|---|
| ||||
| Bedside procedures, no. (%) | 26 (14.3%) | 32 (22.1%) | 1 (0.7%) | <0.001 |
| IR procedures, no. (%) | 119 (65.4%) | 74 (51.0%) | 82 (56.6%) | 0.029 |
| Pulmonary procedures, no. (%) | 37 (20.3%) | 39 (26.9%) | 62 (42.8%) | <0.001 |
| Procedure self‐confidence, mean (SD)* | 43.6 (28.66) | 68.2 (25.17) | 55.7 (31.17) | <0.001 |
| Experience performing actual procedures, median (IQR) | 1 (13) | 2 (13.5) | 10 (425) | <0.001 |
Traditionally trained residents were most likely to rate low confidence as reasons why they referred thoracenteses (Table 2). Hospitalist physicians were more likely to cite lack of time to perform the procedure themselves. Other reasons were different across groups. SBML‐trained residents were more likely to refer because of attending preference, whereas traditionally trained residents were mostly like to refer because of high risk/technically difficult cases.
| Traditionally Trained Residents, n = 156 | SBML‐Trained Residents, n = 113 | Hospitalist Physicians, n = 144 | P Value | |
|---|---|---|---|---|
| ||||
| Lack of confidence to perform procedure, mean (SD)* | 3.46 (1.32) | 2.52 (1.45) | 2.89 (1.60) | <0.001 |
| Work hour restrictions, mean (SD) * | 2.05 (1.37) | 1.50 (1.11) | n/a | 0.001 |
| Low reimbursement, mean (SD)* | 1.02 (0.12) | 1.0 (0) | 1.22 (0.69) | <0.001 |
| Other reasons for referral, no. (%) | ||||
| Attending preference | 8 (5.1%) | 11 (9.7%) | 3 (2.1%) | 0.025 |
| Don't know how | 6 (3.8%) | 0 | 0 | 0.007 |
| Failed bedside | 0 | 2 (1.8%) | 0 | 0.07 |
| High risk/technically difficult case | 24 (15.4%) | 12 (10.6%) | 5 (3.5%) | 0.003 |
| IR or pulmonary patient | 5 (3.2%) | 2 (1.8%) | 4 (2.8%) | 0.77 |
| Other IR procedure taking place | 11 (7.1%) | 9 (8.0%) | 4 (2.8%) | 0.13 |
| Patient preference | 2 (1.3%) | 7 (6.2%) | 2 (3.5%) | 0.024 |
| Time | 9 (5.8%) | 7 (6.2%) | 29 (20.1%) | <0.001 |
DISCUSSION
This study confirms earlier research showing that thoracentesis SBML improves residents' clinical skills, but is the first to use a randomized study design.[9] Use of the mastery model in health professions education ensures that all learners are competent to provide patient care including performing invasive procedures. Such rigorous education yields downstream translational outcomes including safety profiles comparable to experts.[1, 6]
This study also shows that SBML‐trained residents displayed higher self‐confidence and performed significantly more bedside procedures than traditionally trained residents and more experienced hospitalist physicians. Although the Society of Hospital Medicine considers thoracentesis skills a core competency for hospitalist physicians,[11] we speculate that some hospitalist physicians had not performed a thoracentesis in years. A recent national survey showed that only 44% of hospitalist physicians performed at least 1 thoracentesis within the past year.[10] Research also shows a shift in medical culture to refer procedures to specialty services, such as IR, by over 900% in the past 2 decades.[4] Our results provide novel information about procedure referrals because we show that SBML provides translational outcomes by improving skills and self‐confidence that influence referral patterns. SBML‐trained residents performed almost a quarter of procedures at the bedside. Although this only represents an 8% absolute difference in bedside procedures compared to traditionally trained residents, if a large number of residents are trained using SBML this results in a meaningful number of procedures shifted to the patient bedside. According to University HealthSystem Consortium data, in US teaching hospitals, approximately 35,325 thoracenteses are performed yearly.[1] Shifting even 8% of these procedures to the bedside would result in significant clinical benefit and cost savings. Reduced referrals increase additional bedside procedures that are safe, cost‐effective, and highly satisfying to patients.[1, 12, 13] Further study is required to determine the impact on referral patterns after providing SMBL training to attending physicians.
Our study also provides information about the rationale for procedure referrals. Earlier work speculates that financial incentive, training and time may explain high procedure referral rates.[10] One report on IM residents noted an 87% IR referral rate for thoracentesis, and confirmed that both training and time were major reasons.[14] Hospitalist physicians reported lack of time as the major factor leading to procedural referrals, which is problematic because bedside procedures yield similar clinical outcomes at lower costs.[1, 12] Attending preference also prevented 11 additional bedside procedures in the SBML‐trained group. Schedule adjustments and SBML training of hospitalist physicians should be considered, because bundled payments in the Affordable Care Act may favor shifting to the higher‐value approach of bedside thoracenteses.[15]
Our study has several limitations. First, we only performed surveys at 1 institution and the results may not be generalizable. Second, we relied on an electronic query to alert us to thoracenteses. Our query may have missed procedures that were unsuccessful or did not have EHR orders entered. Third, physicians may have been surveyed more than once for different or the same patient(s), but opinions may have shifted over time. Fourth, some items such as time needed to be written in the survey and were not specifically asked. This could have resulted in under‐reporting. Finally, we did not assess the clinical outcomes of thoracenteses in this study, although earlier work shows that residents who complete SBML have safety outcomes similar to IR.[1, 6]
In summary, IM residents who complete thoracentesis SBML demonstrate improved clinical skills and are more likely to perform bedside procedures. In an era of bundled payments, rethinking current care models to promote cost‐effective care is necessary. We believe providing additional education, training, and support to hospitalist physicians to promote bedside procedures is a promising strategy that warrants further study.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Kevin O'Leary for their support and encouragement of this work. The authors also thank the internal medicine residents at Northwestern for their dedication to patient care.
Disclosures: This project was supported by grant R18HS021202‐01 from the Agency for Healthcare Research and Quality (AHRQ). AHRQ had no role in the preparation, review, or approval of the manuscript. Trial Registration:
- , , , , . Thoracentesis procedures at university hospitals: comparing outcomes by specialty. Jt Comm J Qual Patient Saf. 2015;42(1):34–40.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed March 9, 2016.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , , , et al. Cost savings of performing paracentesis procedures at the bedside after simulation‐based education. Simul Healthc. 2014;9(5):312–318.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861–866.
- , , , et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- , , , , , . Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162–168.
- , , , , , . Are we providing patient‐centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp J. 2014;1(2):94–103. Available at: http://pxjournal.org/cgi/viewcontent.cgi?article=1024
Internal medicine (IM) residents and hospitalist physicians commonly conduct bedside thoracenteses for both diagnostic and therapeutic purposes.[1] The American Board of Internal Medicine only requires that certification candidates understand the indications, complications, and management of thoracenteses.[2] A disconnect between clinical practice patterns and board requirements may increase patient risk because poorly trained physicians are more likely to cause complications.[3] National practice patterns show that many thoracenteses are referred to interventional radiology (IR).[4] However, research links performance of bedside procedures to reduced hospital length of stay and lower costs, without increasing risk of complications.[1, 5, 6]
Simulation‐based education offers a controlled environment where trainees improve procedural knowledge and skills without patient harm.[7] Simulation‐based mastery learning (SBML) is a rigorous form of competency‐based education that improves clinical skills and reduces iatrogenic complications and healthcare costs.[5, 6, 8] SBML also is an effective method to boost thoracentesis skills among IM residents.[9] However, there are no data to show that thoracentesis skills acquired in the simulation laboratory transfer to clinical environments and affect referral patterns.
We hypothesized that a thoracentesis SBML intervention would improve skills and increase procedural self‐confidence while reducing procedure referrals. This study aimed to (1) assess the effect of thoracentesis SBML on a cohort of IM residents' simulated skills and (2) compare traditionally trained (nonSBML‐trained) residents, SBML‐trained residents, and hospitalist physicians regarding procedure referral patterns, self‐confidence, procedure experience, and reasons for referral.
METHODS AND MATERIALS
Study Design
We surveyed physicians about thoracenteses performed on patients cared for by postgraduate year (PGY)‐2 and PGY‐3 IM residents and hospitalist physicians at Northwestern Memorial Hospital (NMH) from December 2012 to May 2015. NMH is an 896‐bed, tertiary academic medical center, located in Chicago, Illinois. A random sample of IM residents participated in a thoracentesis SBML intervention, whereas hospitalist physicians did not. We compared referral patterns, self‐confidence, procedure experience, and reasons for referral between traditionally trained residents, SBML‐trained residents, and hospitalist physicians. The Northwestern University Institutional Review Board approved this study, and all study participants provided informed consent.
At NMH, resident‐staffed services include general IM and nonintensive care subspecialty medical services. There are also 2 nonteaching floors staffed by hospitalist attending physicians without residents. Thoracenteses performed on these services can either be done at the bedside or referred to pulmonary medicine or IR. The majority of thoracenteses performed by pulmonary medicine occur at the patients' bedside, and the patients also receive a clinical consultation. IR procedures are done in the IR suite without additional clinical consultation.
Procedure
One hundred sixty residents were available for training over the study period. We randomly selected 20% of the approximately 20 PGY‐2 and PGY‐3 IM residents assigned to the NMH medicine services each month to participate in SBML thoracentesis training before their rotation. Randomly selected residents were required to undergo SBML training but were not required to participate in the study. This selection process was repeated before every rotation during the study period. This randomized wait‐list control method allowed residents to serve as controls if not initially selected for training and remain eligible for SBML training in subsequent rotations.
Intervention
The SBML intervention used a pretest/post‐test design, as described elsewhere.[9] Residents completed a clinical skills pretest on a thoracentesis simulator using a previously published 26‐item checklist.[9] Following the pretest, residents participated in 2, 1‐hour training sessions including a lecture, video, and deliberate practice on the simulator with feedback from an expert instructor. Finally, residents completed a clinical skills post‐test using the checklist within 1 week from training (but on a different day) and were required to meet or exceed an 84.3% minimum passing score (MPS). The entire training, including pre‐ and post‐tests, took approximately 3 hours to complete, and residents were given an additional 1 hour refresher training every 6 months for up to a year after original training. We compared pre‐ and post‐test checklist scores to evaluate skills improvement.
Thoracentesis Patient Identification
The NMH electronic health record (EHR) was used to identify medical service inpatients who underwent a thoracentesis during the study period. NMH clinicians must place an EHR order for procedure kits, consults, and laboratory analysis of thoracentesis fluid. We developed a real‐time query of NMH's EHR that identified all patients with electronic orders for thoracenteses and monitored this daily.
Physician Surveys
After each thoracentesis, we surveyed the PGY‐2 or PGY‐3 resident or hospitalist caring for the patient about the procedure. A research coordinator, blind to whether the resident received SBML, performed the surveys face‐to‐face on Monday to Friday during normal business hours. Residents were not considered SBML‐trained until they met or exceeded the MPS on the simulated skills checklist at post‐test. Surveys occurred on Monday for procedures performed on Friday evening through Sunday. Survey questions asked physicians about who performed the procedure, their procedural self‐confidence, and total number of thoracenteses performed in their career. For referred procedures, physicians were asked about reasons for referral including lack of confidence, work hour restrictions (residents only), and low reimbursement rates.[10] There was also an option to add other reasons.
Measurement
The thoracentesis skills checklist documented all required steps for an evidence‐based thoracentesis. Each task received equal weight (0 = done incorrectly/not done, 1 = done correctly).[9] For physician surveys, self‐confidence about performing the procedure was rated on a scale of 0 = not confident to 100 = very confident. Reasons for referral were scored on a Likert scale 1 to 5 (1 = not at all important, 5 = very important). Other reasons for referral were categorized.
Statistical Analysis
The clinical skills pre‐ and post‐test checklist scores were compared using a Wilcoxon matched pairs rank test. Physician survey data were compared between different procedure performers using the 2 test, independent t test, analysis of variance (ANOVA), or Kruskal‐Wallis test depending on data properties. Referral patterns measured by the Likert scale were averaged, and differences between physician groups were evaluated using ANOVA. Counts of other reasons for referral were compared using the 2 test. We performed all statistical analyses using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY).
RESULTS
Thoracentesis Clinical Skills
One hundred twelve (70%) residents were randomized to SBML, and all completed the protocol. Median pretest scores were 57.6% (interquartile range [IQR] 43.376.9), and final post‐test mastery scores were 96.2 (IQR 96.2100.0; P < 0.001). Twenty‐three residents (21.0%) failed to meet the MPS at initial post‐test, but met the MPS on retest after <1 hour of additional training.
Physician Surveys
The EHR query identified 474 procedures eligible for physician surveys. One hundred twenty‐two residents and 51 hospitalist physicians completed surveys for 472 procedures (99.6%); 182 patients by traditionally trained residents, 145 by SBML‐trained residents, and 145 by hospitalist physicians. As shown in Table 1, 413 (88%) of all procedures were referred to another service. Traditionally trained residents were more likely to refer to IR compared to SBML‐trained residents or hospitalist physicians. SBML‐trained residents were more likely to perform bedside procedures, whereas hospitalist physicians were most likely to refer to pulmonary medicine. SBML‐trained residents were most confident in their procedural skills, despite hospitalist physicians performing more actual procedures.
| Traditionally Trained Resident Surveys, n = 182 | SBML‐Trained Resident Surveys, n = 145 | Hospitalist Physician Surveys, n = 145 | P Value | |
|---|---|---|---|---|
| ||||
| Bedside procedures, no. (%) | 26 (14.3%) | 32 (22.1%) | 1 (0.7%) | <0.001 |
| IR procedures, no. (%) | 119 (65.4%) | 74 (51.0%) | 82 (56.6%) | 0.029 |
| Pulmonary procedures, no. (%) | 37 (20.3%) | 39 (26.9%) | 62 (42.8%) | <0.001 |
| Procedure self‐confidence, mean (SD)* | 43.6 (28.66) | 68.2 (25.17) | 55.7 (31.17) | <0.001 |
| Experience performing actual procedures, median (IQR) | 1 (13) | 2 (13.5) | 10 (425) | <0.001 |
Traditionally trained residents were most likely to rate low confidence as reasons why they referred thoracenteses (Table 2). Hospitalist physicians were more likely to cite lack of time to perform the procedure themselves. Other reasons were different across groups. SBML‐trained residents were more likely to refer because of attending preference, whereas traditionally trained residents were mostly like to refer because of high risk/technically difficult cases.
| Traditionally Trained Residents, n = 156 | SBML‐Trained Residents, n = 113 | Hospitalist Physicians, n = 144 | P Value | |
|---|---|---|---|---|
| ||||
| Lack of confidence to perform procedure, mean (SD)* | 3.46 (1.32) | 2.52 (1.45) | 2.89 (1.60) | <0.001 |
| Work hour restrictions, mean (SD) * | 2.05 (1.37) | 1.50 (1.11) | n/a | 0.001 |
| Low reimbursement, mean (SD)* | 1.02 (0.12) | 1.0 (0) | 1.22 (0.69) | <0.001 |
| Other reasons for referral, no. (%) | ||||
| Attending preference | 8 (5.1%) | 11 (9.7%) | 3 (2.1%) | 0.025 |
| Don't know how | 6 (3.8%) | 0 | 0 | 0.007 |
| Failed bedside | 0 | 2 (1.8%) | 0 | 0.07 |
| High risk/technically difficult case | 24 (15.4%) | 12 (10.6%) | 5 (3.5%) | 0.003 |
| IR or pulmonary patient | 5 (3.2%) | 2 (1.8%) | 4 (2.8%) | 0.77 |
| Other IR procedure taking place | 11 (7.1%) | 9 (8.0%) | 4 (2.8%) | 0.13 |
| Patient preference | 2 (1.3%) | 7 (6.2%) | 2 (3.5%) | 0.024 |
| Time | 9 (5.8%) | 7 (6.2%) | 29 (20.1%) | <0.001 |
DISCUSSION
This study confirms earlier research showing that thoracentesis SBML improves residents' clinical skills, but is the first to use a randomized study design.[9] Use of the mastery model in health professions education ensures that all learners are competent to provide patient care including performing invasive procedures. Such rigorous education yields downstream translational outcomes including safety profiles comparable to experts.[1, 6]
This study also shows that SBML‐trained residents displayed higher self‐confidence and performed significantly more bedside procedures than traditionally trained residents and more experienced hospitalist physicians. Although the Society of Hospital Medicine considers thoracentesis skills a core competency for hospitalist physicians,[11] we speculate that some hospitalist physicians had not performed a thoracentesis in years. A recent national survey showed that only 44% of hospitalist physicians performed at least 1 thoracentesis within the past year.[10] Research also shows a shift in medical culture to refer procedures to specialty services, such as IR, by over 900% in the past 2 decades.[4] Our results provide novel information about procedure referrals because we show that SBML provides translational outcomes by improving skills and self‐confidence that influence referral patterns. SBML‐trained residents performed almost a quarter of procedures at the bedside. Although this only represents an 8% absolute difference in bedside procedures compared to traditionally trained residents, if a large number of residents are trained using SBML this results in a meaningful number of procedures shifted to the patient bedside. According to University HealthSystem Consortium data, in US teaching hospitals, approximately 35,325 thoracenteses are performed yearly.[1] Shifting even 8% of these procedures to the bedside would result in significant clinical benefit and cost savings. Reduced referrals increase additional bedside procedures that are safe, cost‐effective, and highly satisfying to patients.[1, 12, 13] Further study is required to determine the impact on referral patterns after providing SMBL training to attending physicians.
Our study also provides information about the rationale for procedure referrals. Earlier work speculates that financial incentive, training and time may explain high procedure referral rates.[10] One report on IM residents noted an 87% IR referral rate for thoracentesis, and confirmed that both training and time were major reasons.[14] Hospitalist physicians reported lack of time as the major factor leading to procedural referrals, which is problematic because bedside procedures yield similar clinical outcomes at lower costs.[1, 12] Attending preference also prevented 11 additional bedside procedures in the SBML‐trained group. Schedule adjustments and SBML training of hospitalist physicians should be considered, because bundled payments in the Affordable Care Act may favor shifting to the higher‐value approach of bedside thoracenteses.[15]
Our study has several limitations. First, we only performed surveys at 1 institution and the results may not be generalizable. Second, we relied on an electronic query to alert us to thoracenteses. Our query may have missed procedures that were unsuccessful or did not have EHR orders entered. Third, physicians may have been surveyed more than once for different or the same patient(s), but opinions may have shifted over time. Fourth, some items such as time needed to be written in the survey and were not specifically asked. This could have resulted in under‐reporting. Finally, we did not assess the clinical outcomes of thoracenteses in this study, although earlier work shows that residents who complete SBML have safety outcomes similar to IR.[1, 6]
In summary, IM residents who complete thoracentesis SBML demonstrate improved clinical skills and are more likely to perform bedside procedures. In an era of bundled payments, rethinking current care models to promote cost‐effective care is necessary. We believe providing additional education, training, and support to hospitalist physicians to promote bedside procedures is a promising strategy that warrants further study.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Kevin O'Leary for their support and encouragement of this work. The authors also thank the internal medicine residents at Northwestern for their dedication to patient care.
Disclosures: This project was supported by grant R18HS021202‐01 from the Agency for Healthcare Research and Quality (AHRQ). AHRQ had no role in the preparation, review, or approval of the manuscript. Trial Registration:
Internal medicine (IM) residents and hospitalist physicians commonly conduct bedside thoracenteses for both diagnostic and therapeutic purposes.[1] The American Board of Internal Medicine only requires that certification candidates understand the indications, complications, and management of thoracenteses.[2] A disconnect between clinical practice patterns and board requirements may increase patient risk because poorly trained physicians are more likely to cause complications.[3] National practice patterns show that many thoracenteses are referred to interventional radiology (IR).[4] However, research links performance of bedside procedures to reduced hospital length of stay and lower costs, without increasing risk of complications.[1, 5, 6]
Simulation‐based education offers a controlled environment where trainees improve procedural knowledge and skills without patient harm.[7] Simulation‐based mastery learning (SBML) is a rigorous form of competency‐based education that improves clinical skills and reduces iatrogenic complications and healthcare costs.[5, 6, 8] SBML also is an effective method to boost thoracentesis skills among IM residents.[9] However, there are no data to show that thoracentesis skills acquired in the simulation laboratory transfer to clinical environments and affect referral patterns.
We hypothesized that a thoracentesis SBML intervention would improve skills and increase procedural self‐confidence while reducing procedure referrals. This study aimed to (1) assess the effect of thoracentesis SBML on a cohort of IM residents' simulated skills and (2) compare traditionally trained (nonSBML‐trained) residents, SBML‐trained residents, and hospitalist physicians regarding procedure referral patterns, self‐confidence, procedure experience, and reasons for referral.
METHODS AND MATERIALS
Study Design
We surveyed physicians about thoracenteses performed on patients cared for by postgraduate year (PGY)‐2 and PGY‐3 IM residents and hospitalist physicians at Northwestern Memorial Hospital (NMH) from December 2012 to May 2015. NMH is an 896‐bed, tertiary academic medical center, located in Chicago, Illinois. A random sample of IM residents participated in a thoracentesis SBML intervention, whereas hospitalist physicians did not. We compared referral patterns, self‐confidence, procedure experience, and reasons for referral between traditionally trained residents, SBML‐trained residents, and hospitalist physicians. The Northwestern University Institutional Review Board approved this study, and all study participants provided informed consent.
At NMH, resident‐staffed services include general IM and nonintensive care subspecialty medical services. There are also 2 nonteaching floors staffed by hospitalist attending physicians without residents. Thoracenteses performed on these services can either be done at the bedside or referred to pulmonary medicine or IR. The majority of thoracenteses performed by pulmonary medicine occur at the patients' bedside, and the patients also receive a clinical consultation. IR procedures are done in the IR suite without additional clinical consultation.
Procedure
One hundred sixty residents were available for training over the study period. We randomly selected 20% of the approximately 20 PGY‐2 and PGY‐3 IM residents assigned to the NMH medicine services each month to participate in SBML thoracentesis training before their rotation. Randomly selected residents were required to undergo SBML training but were not required to participate in the study. This selection process was repeated before every rotation during the study period. This randomized wait‐list control method allowed residents to serve as controls if not initially selected for training and remain eligible for SBML training in subsequent rotations.
Intervention
The SBML intervention used a pretest/post‐test design, as described elsewhere.[9] Residents completed a clinical skills pretest on a thoracentesis simulator using a previously published 26‐item checklist.[9] Following the pretest, residents participated in 2, 1‐hour training sessions including a lecture, video, and deliberate practice on the simulator with feedback from an expert instructor. Finally, residents completed a clinical skills post‐test using the checklist within 1 week from training (but on a different day) and were required to meet or exceed an 84.3% minimum passing score (MPS). The entire training, including pre‐ and post‐tests, took approximately 3 hours to complete, and residents were given an additional 1 hour refresher training every 6 months for up to a year after original training. We compared pre‐ and post‐test checklist scores to evaluate skills improvement.
Thoracentesis Patient Identification
The NMH electronic health record (EHR) was used to identify medical service inpatients who underwent a thoracentesis during the study period. NMH clinicians must place an EHR order for procedure kits, consults, and laboratory analysis of thoracentesis fluid. We developed a real‐time query of NMH's EHR that identified all patients with electronic orders for thoracenteses and monitored this daily.
Physician Surveys
After each thoracentesis, we surveyed the PGY‐2 or PGY‐3 resident or hospitalist caring for the patient about the procedure. A research coordinator, blind to whether the resident received SBML, performed the surveys face‐to‐face on Monday to Friday during normal business hours. Residents were not considered SBML‐trained until they met or exceeded the MPS on the simulated skills checklist at post‐test. Surveys occurred on Monday for procedures performed on Friday evening through Sunday. Survey questions asked physicians about who performed the procedure, their procedural self‐confidence, and total number of thoracenteses performed in their career. For referred procedures, physicians were asked about reasons for referral including lack of confidence, work hour restrictions (residents only), and low reimbursement rates.[10] There was also an option to add other reasons.
Measurement
The thoracentesis skills checklist documented all required steps for an evidence‐based thoracentesis. Each task received equal weight (0 = done incorrectly/not done, 1 = done correctly).[9] For physician surveys, self‐confidence about performing the procedure was rated on a scale of 0 = not confident to 100 = very confident. Reasons for referral were scored on a Likert scale 1 to 5 (1 = not at all important, 5 = very important). Other reasons for referral were categorized.
Statistical Analysis
The clinical skills pre‐ and post‐test checklist scores were compared using a Wilcoxon matched pairs rank test. Physician survey data were compared between different procedure performers using the 2 test, independent t test, analysis of variance (ANOVA), or Kruskal‐Wallis test depending on data properties. Referral patterns measured by the Likert scale were averaged, and differences between physician groups were evaluated using ANOVA. Counts of other reasons for referral were compared using the 2 test. We performed all statistical analyses using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY).
RESULTS
Thoracentesis Clinical Skills
One hundred twelve (70%) residents were randomized to SBML, and all completed the protocol. Median pretest scores were 57.6% (interquartile range [IQR] 43.376.9), and final post‐test mastery scores were 96.2 (IQR 96.2100.0; P < 0.001). Twenty‐three residents (21.0%) failed to meet the MPS at initial post‐test, but met the MPS on retest after <1 hour of additional training.
Physician Surveys
The EHR query identified 474 procedures eligible for physician surveys. One hundred twenty‐two residents and 51 hospitalist physicians completed surveys for 472 procedures (99.6%); 182 patients by traditionally trained residents, 145 by SBML‐trained residents, and 145 by hospitalist physicians. As shown in Table 1, 413 (88%) of all procedures were referred to another service. Traditionally trained residents were more likely to refer to IR compared to SBML‐trained residents or hospitalist physicians. SBML‐trained residents were more likely to perform bedside procedures, whereas hospitalist physicians were most likely to refer to pulmonary medicine. SBML‐trained residents were most confident in their procedural skills, despite hospitalist physicians performing more actual procedures.
| Traditionally Trained Resident Surveys, n = 182 | SBML‐Trained Resident Surveys, n = 145 | Hospitalist Physician Surveys, n = 145 | P Value | |
|---|---|---|---|---|
| ||||
| Bedside procedures, no. (%) | 26 (14.3%) | 32 (22.1%) | 1 (0.7%) | <0.001 |
| IR procedures, no. (%) | 119 (65.4%) | 74 (51.0%) | 82 (56.6%) | 0.029 |
| Pulmonary procedures, no. (%) | 37 (20.3%) | 39 (26.9%) | 62 (42.8%) | <0.001 |
| Procedure self‐confidence, mean (SD)* | 43.6 (28.66) | 68.2 (25.17) | 55.7 (31.17) | <0.001 |
| Experience performing actual procedures, median (IQR) | 1 (13) | 2 (13.5) | 10 (425) | <0.001 |
Traditionally trained residents were most likely to rate low confidence as reasons why they referred thoracenteses (Table 2). Hospitalist physicians were more likely to cite lack of time to perform the procedure themselves. Other reasons were different across groups. SBML‐trained residents were more likely to refer because of attending preference, whereas traditionally trained residents were mostly like to refer because of high risk/technically difficult cases.
| Traditionally Trained Residents, n = 156 | SBML‐Trained Residents, n = 113 | Hospitalist Physicians, n = 144 | P Value | |
|---|---|---|---|---|
| ||||
| Lack of confidence to perform procedure, mean (SD)* | 3.46 (1.32) | 2.52 (1.45) | 2.89 (1.60) | <0.001 |
| Work hour restrictions, mean (SD) * | 2.05 (1.37) | 1.50 (1.11) | n/a | 0.001 |
| Low reimbursement, mean (SD)* | 1.02 (0.12) | 1.0 (0) | 1.22 (0.69) | <0.001 |
| Other reasons for referral, no. (%) | ||||
| Attending preference | 8 (5.1%) | 11 (9.7%) | 3 (2.1%) | 0.025 |
| Don't know how | 6 (3.8%) | 0 | 0 | 0.007 |
| Failed bedside | 0 | 2 (1.8%) | 0 | 0.07 |
| High risk/technically difficult case | 24 (15.4%) | 12 (10.6%) | 5 (3.5%) | 0.003 |
| IR or pulmonary patient | 5 (3.2%) | 2 (1.8%) | 4 (2.8%) | 0.77 |
| Other IR procedure taking place | 11 (7.1%) | 9 (8.0%) | 4 (2.8%) | 0.13 |
| Patient preference | 2 (1.3%) | 7 (6.2%) | 2 (3.5%) | 0.024 |
| Time | 9 (5.8%) | 7 (6.2%) | 29 (20.1%) | <0.001 |
DISCUSSION
This study confirms earlier research showing that thoracentesis SBML improves residents' clinical skills, but is the first to use a randomized study design.[9] Use of the mastery model in health professions education ensures that all learners are competent to provide patient care including performing invasive procedures. Such rigorous education yields downstream translational outcomes including safety profiles comparable to experts.[1, 6]
This study also shows that SBML‐trained residents displayed higher self‐confidence and performed significantly more bedside procedures than traditionally trained residents and more experienced hospitalist physicians. Although the Society of Hospital Medicine considers thoracentesis skills a core competency for hospitalist physicians,[11] we speculate that some hospitalist physicians had not performed a thoracentesis in years. A recent national survey showed that only 44% of hospitalist physicians performed at least 1 thoracentesis within the past year.[10] Research also shows a shift in medical culture to refer procedures to specialty services, such as IR, by over 900% in the past 2 decades.[4] Our results provide novel information about procedure referrals because we show that SBML provides translational outcomes by improving skills and self‐confidence that influence referral patterns. SBML‐trained residents performed almost a quarter of procedures at the bedside. Although this only represents an 8% absolute difference in bedside procedures compared to traditionally trained residents, if a large number of residents are trained using SBML this results in a meaningful number of procedures shifted to the patient bedside. According to University HealthSystem Consortium data, in US teaching hospitals, approximately 35,325 thoracenteses are performed yearly.[1] Shifting even 8% of these procedures to the bedside would result in significant clinical benefit and cost savings. Reduced referrals increase additional bedside procedures that are safe, cost‐effective, and highly satisfying to patients.[1, 12, 13] Further study is required to determine the impact on referral patterns after providing SMBL training to attending physicians.
Our study also provides information about the rationale for procedure referrals. Earlier work speculates that financial incentive, training and time may explain high procedure referral rates.[10] One report on IM residents noted an 87% IR referral rate for thoracentesis, and confirmed that both training and time were major reasons.[14] Hospitalist physicians reported lack of time as the major factor leading to procedural referrals, which is problematic because bedside procedures yield similar clinical outcomes at lower costs.[1, 12] Attending preference also prevented 11 additional bedside procedures in the SBML‐trained group. Schedule adjustments and SBML training of hospitalist physicians should be considered, because bundled payments in the Affordable Care Act may favor shifting to the higher‐value approach of bedside thoracenteses.[15]
Our study has several limitations. First, we only performed surveys at 1 institution and the results may not be generalizable. Second, we relied on an electronic query to alert us to thoracenteses. Our query may have missed procedures that were unsuccessful or did not have EHR orders entered. Third, physicians may have been surveyed more than once for different or the same patient(s), but opinions may have shifted over time. Fourth, some items such as time needed to be written in the survey and were not specifically asked. This could have resulted in under‐reporting. Finally, we did not assess the clinical outcomes of thoracenteses in this study, although earlier work shows that residents who complete SBML have safety outcomes similar to IR.[1, 6]
In summary, IM residents who complete thoracentesis SBML demonstrate improved clinical skills and are more likely to perform bedside procedures. In an era of bundled payments, rethinking current care models to promote cost‐effective care is necessary. We believe providing additional education, training, and support to hospitalist physicians to promote bedside procedures is a promising strategy that warrants further study.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Kevin O'Leary for their support and encouragement of this work. The authors also thank the internal medicine residents at Northwestern for their dedication to patient care.
Disclosures: This project was supported by grant R18HS021202‐01 from the Agency for Healthcare Research and Quality (AHRQ). AHRQ had no role in the preparation, review, or approval of the manuscript. Trial Registration:
- , , , , . Thoracentesis procedures at university hospitals: comparing outcomes by specialty. Jt Comm J Qual Patient Saf. 2015;42(1):34–40.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed March 9, 2016.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , , , et al. Cost savings of performing paracentesis procedures at the bedside after simulation‐based education. Simul Healthc. 2014;9(5):312–318.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861–866.
- , , , et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- , , , , , . Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162–168.
- , , , , , . Are we providing patient‐centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp J. 2014;1(2):94–103. Available at: http://pxjournal.org/cgi/viewcontent.cgi?article=1024
- , , , , . Thoracentesis procedures at university hospitals: comparing outcomes by specialty. Jt Comm J Qual Patient Saf. 2015;42(1):34–40.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed March 9, 2016.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , , , et al. Cost savings of performing paracentesis procedures at the bedside after simulation‐based education. Simul Healthc. 2014;9(5):312–318.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861–866.
- , , , et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- , , , , , . Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162–168.
- , , , , , . Are we providing patient‐centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp J. 2014;1(2):94–103. Available at: http://pxjournal.org/cgi/viewcontent.cgi?article=1024
Specialties Performing Paracentesis
Cirrhosis affects up to 3% of the population and is 1 of the 10 most common causes of death in the United States.[1, 2, 3, 4] Paracentesis procedures are frequently performed in patients with liver disease and ascites for diagnostic and/or therapeutic purposes. These procedures can be performed safely by trained clinicians at the bedside or referred to interventional radiology (IR).[2, 3, 4]
National practice patterns show that paracentesis procedures are increasingly referred to IR rather than performed at the bedside by internal medicine or gastroenterology clinicians.[5, 6, 7] In fact, a recent study of Medicare beneficiaries showed that inpatient and outpatient paracentesis procedures performed by radiologists increased by 964% from 1993 to 2008.[7] Reasons for the decline in bedside procedures include the increased availability of IR, lack of sufficient reimbursement, and the time required to perform paracentesis procedures.[5, 6, 7, 8] Surveys of internal medicine and family medicine residents and gastroenterology fellows show trainees often lack the confidence and experience needed to perform the procedure safely.[9, 10, 11] Additionally, many clinicians do not have expertise with ultrasound use and may not have access to necessary equipment.
Inconsistent certification requirements may also impact the competence and experience of physicians to perform paracentesis procedures. Internal medicine residents are no longer required by the American Board of Internal Medicine (ABIM) to demonstrate competency in procedures such as paracentesis for certification.[12] However, the Accreditation Council for Graduate Medical Education (ACGME) requirements state that internal medicine programs must offer residents the opportunity to demonstrate competence in the performance of procedures such as paracentesis, thoracentesis, and central venous catheter insertion.[13] The American Board of Family Medicine (ABFM) does not outline specific procedural competence for initial certification.[14] The ACGME states that family medicine residents must receive training to perform those clinical procedures required for their future practices but allows each program to determine which procedures to require.[15] Due to this uncertainty, practicing hospitalists are likely to have variable training and competence in bedside procedures such as paracentesis.
We previously showed that internal medicine residents rotating on the hepatology service of an academic medical center performed 59% of paracentesis procedures at the bedside.[16] These findings are in contrast to national data showing that 74% of paracentesis procedures performed on Medicare beneficiaries were performed by radiologists.[7] Practice patterns at university hospitals may not be reflected in this data because the study was limited to Medicare beneficiaries and included ambulatory patients.[7] In addition to uncertainty about who is performing this procedure in inpatient settings, little is known about the effect of specialty on postparacentesis clinical outcomes.[16, 17]
The current study had 3 aims: (1) evaluate which clinical specialties perform paracentesis procedures at university hospitals; (2) model patient characteristics associated with procedures performed at the bedside versus those referred to IR; and (3) among patients with a similar likelihood of IR referral, evaluate length of stay (LOS) and hospital costs of patients undergoing procedures performed by different specialties.
METHODS
We performed an observational administrative database review of patients who underwent paracentesis procedures in hospitals participating in the University HealthSystem Consortium (UHC) Clinical Database from January 2010 through December 2012. UHC is an alliance of 120 nonprofit academic medical centers and their 290 affiliated hospitals. UHC maintains databases containing clinical, operational, financial, and patient safety data from affiliated hospitals. Using the UHC database, we described the characteristics of all patients who underwent paracentesis procedures by clinical specialty performing the procedure. We then modeled the effects of patient characteristics on decision‐making about IR referral. Finally, among patients with a homogeneous predicted probability of IR referral, we compared LOS and direct costs by specialty performing the procedure. The Northwestern University institutional review board approved this study.
Procedure
We queried the UHC database for all patients over the age of 18 years who underwent paracentesis procedures (International Classification of Disease Revision 9 [ICD‐9] procedure code 54.91) and had at least 1 diagnosis code of liver disease (571.x). We excluded patients admitted to obstetrics. The query included patient and clinical characteristics such as admission, discharge, and procedure date; age, gender, procedure provider specialty, and intensive care unit (ICU) stay. We also obtained all ICD‐9 codes associated with the admission including obesity, severe liver disease, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before or during the admission, awaiting liver transplant, and complications of liver transplant. We used ICD‐9 codes to calculate patients' Charlson score[18, 19] to assess severity of illness on admission.
LOS and total direct hospital costs were compared among patients with a paracentesis performed by a single clinical group and among patients with a similar predicted probability of IR referral. UHC generates direct cost estimates by applying Medicare Cost Report ratios of cost to charges with the labor cost further adjusted by the respective area wage index. Hospital costs were not available from 8.3% of UHC hospitals. We therefore based cost estimates on nonmissing data.
Paracentesis provider specialties were divided into 6 general categories: (1) IR (interventional and diagnostic radiology); (2) medicine (family medicine, general medicine, and hospital medicine); (3) subspecialty medicine (infectious disease, cardiology, nephrology, hematology/oncology, endocrinology, pulmonary, and geriatrics); (4) gastroenterology/hepatology (gastroenterology, hepatology, and transplant medicine); (5) general surgery (general surgery and transplant surgery); and (6) all other (included unclassified specialties). We present patient characteristics categorized by these specialty groups and for admissions in which multiple specialties performed procedures.
Study Design
To analyze an individual patient's likelihood of IR referral, we needed to restrict our sample to discharges where only 1 clinical specialty performed a paracentesis. Therefore, we excluded hybrid discharges with procedures performed by more than 1 specialty in a single admission as well as discharges with procedures performed by all other specialties. To compare LOS and direct cost outcomes, and to minimize selection bias among exclusively IR‐treated patients, we excluded hospitals without procedures done by both IR and medicine.
We modeled referral to IR as a function of patients' demographic and clinical variables, which we believed would affect the probability of referral. We then examined the IR referral model predicted probabilities (propensity score).[20] Finally, we examined mean differences in LOS and direct costs among discharges with a single clinical specialty group, while using the predicted probability of referral as a filter to compare these outcomes by specialty. We further tested specialty differences in LOS and direct costs controlling for demographic and clinical variables.
Statistical Analysis
To test the significance of differences between demographic and clinical characteristics of patients across specialties, we used 2 tests for categorical variables and analysis of variance or the Kruskal‐Wallis rank test for continuous variables. Random effects logistic regression, which adjusts standard errors for clustering by hospital, was used to model the likelihood of referral to IR. Independent variables included patient age, gender, obesity, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before hospitalization, liver transplant during hospitalization, awaiting transplant, complications of liver transplant, ICU stay, Charlson score, and number of paracentesis procedures performed during the admission. Predicted probabilities derived from this IR referral model were used to investigate selection bias in our subsequent analyses of LOS and costs.[20]
We used random effects multiple linear regression to test the association of procedure specialty with hospital LOS and total direct costs, controlling for the same independent variables listed above. Analyses were conducted using both actual LOS in days and Medicare costs. We also performed a log transformation of LOS and costs to account for rightward skew. We only present actual LOS and cost results because results were virtually identical. We used SAS version 9 (SAS Institute Inc., Cary, NC) to extract data from the UHC Clinical Database. We performed all statistical analyses using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Procedure and Discharge Level Results
There were 97,577 paracentesis procedures performed during 70,862 hospital admissions in 204 UHC hospitals during the study period. Table 1 shows specific specialty groups for each procedure. The all other category consisted of 17,558 subspecialty groups including 9,434 with specialty unknown. Twenty‐nine percent of procedures were performed in IR versus 27% by medicine, 11% by gastroenterology/hepatology, and 11% by subspecialty medicine.
| Specialty Group | No. | % |
|---|---|---|
| Interventional radiology | 28,414 | 29.1 |
| Medicine | 26,031 | 26.7 |
| Family medicine | 1,026 | 1.1 |
| General medicine | 21,787 | 22.3 |
| Hospitalist | 3,218 | 3.3 |
| Subspecialty medicine | 10,558 | 10.8 |
| Infectious disease | 848 | 0.9 |
| Nephrology | 615 | 0.6 |
| Cardiology | 991 | 1.0 |
| Hematology oncology | 795 | 0.8 |
| Endocrinology | 359 | 0.4 |
| Pulmonology | 6,605 | 6.8 |
| Geriatrics | 345 | 0.4 |
| Gastroenterology/hepatology | 11,143 | 11.4 |
| Transplant medicine | 99 | 0.1 |
| Hepatology | 874 | 0.9 |
| Gastroenterology | 10,170 | 10.4 |
| General surgery | 3,873 | 4.0 |
| Transplant surgery | 2,146 | 2.2 |
| General surgery | 1,727 | 1.8 |
| All other | 17,558 | 18.0 |
| Specialty unknown | 9,434 | 9.7 |
Table 2 presents patient characteristics for 70,862 hospital discharges with paracentesis procedures grouped by whether single or multiple specialties performed procedures. Patient characteristics were significantly different across specialty groups. Medicine, subspecialty medicine, and gastroenterology/hepatology patients were younger, more likely to be male, and more likely to have severe liver disease, coagulation disorders, hypotension, and hyponatremia than IR patients.
| All Discharges, N=70,862 | Interventional Radiology, n=9,348 | Medicine, n=13,789 | Subspecialty Medicine, n=5,085 | Gastroenterology/Hepatology, n=6,664 | General Surgery, n=1,891 | All Other, n=7,912 | Discharges With Multiple Specialties, n=26,173 | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age group, y (%) | ||||||||
| 1849 | 25.4 | 22.5 | 27.6 | 24.9 | 23.5 | 20.8 | 25.5 | 26.1 |
| 5059 | 39.8 | 39.8 | 40.9 | 39.4 | 41.5 | 40.3 | 40.0 | 38.7 |
| 6069 | 24.7 | 24.9 | 21.6 | 24.7 | 26.5 | 30.0 | 23.6 | 25.8 |
| 70+ | 10.1 | 12.9 | 9.9 | 11.1 | 8.4 | 8.9 | 11.0 | 9.4 |
| Male (%) | 65.5 | 64.2 | 67.6 | 67.5 | 65.7 | 66.6 | 65.7 | 64.2 |
| Severe liver disease (%)a | 73.7 | 65.3 | 67.8 | 71.0 | 75.3 | 66.6 | 67.6 | 82.1 |
| Obesity (BMI 40+) (%) | 6.3 | 6.1 | 5.3 | 5.7 | 5.1 | 5.8 | 5.2 | 7.6 |
| Any intensive care unit stay (%) | 31.0 | 10.9 | 16.8 | 50.5 | 16.9 | 36.7 | 22.3 | 47.8 |
| Coagulation disorders (%) | 24.3 | 14.8 | 20.2 | 29.9 | 16.1 | 19.0 | 17.8 | 33.1 |
| Blood loss anemia (%) | 3.4 | 1.3 | 2.8 | 2.7 | 2.7 | 1.9 | 2.1 | 5.2 |
| Hyponatremia (%) | 29.9 | 27.1 | 29.2 | 28.9 | 28.0 | 26.6 | 27.3 | 33.1 |
| Hypotension (%) | 9.8 | 7.0 | 8.0 | 11.0 | 7.7 | 10.5 | 8.1 | 12.4 |
| Thrombocytopenia (%) | 29.6 | 24.6 | 28.3 | 32.5 | 22.1 | 21.5 | 24.0 | 35.8 |
| Complication of transplant (%) | 3.3 | 2.1 | 1.1 | 2.4 | 4.0 | 10.3 | 2.7 | 4.7 |
| Awaiting liver transplant (%) | 7.6 | 6.4 | 4.0 | 5.4 | 12.8 | 16.0 | 7.8 | 8.2 |
| Prior liver transplant (%) | 0.5 | 0.8 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 0.6 |
| Liver transplant procedure (%) | 2.7 | 0.0 | 0.0 | 0.3 | 0.4 | 15.6 | 1.6 | 5.6 |
| Mean Charlson score (SD) | 4.51 (2.17) | 4.28 (2.26) | 4.16 (2.17) | 4.72 (2.30) | 4.30 (1.98) | 4.26 (2.22) | 4.36 (2.30) | 4.84 (2.07) |
| Mean paracentesis procedures per discharge (SD) | 1.38 (0.88) | 1.21 (0.56) | 1.26 (0.66) | 1.30 (0.76) | 1.31 (0.70) | 1.28 (0.78) | 1.22 (0.61) | 1.58 (1.13) |
IR Referral Model
We first excluded 6030/70,862 discharges (8.5%) from 59 hospitals without both IR and medicine procedures. We then further excluded 24,986/70,862 (35.3%) discharges with procedures performed by multiple specialties during the same admission. Finally, we excluded 5555/70,862 (7.8%) of discharges with procedure specialty coded as all other. Therefore, 34,291 (48.4%) discharges (43,337/97,577; 44.4% procedures) from 145 UHC hospitals with paracentesis procedures performed by a single clinical specialty group remained for the IR referral analysis sample. Among admissions with multiple specialty paracentesis performed within the same admission, 3128/26,606 admissions with any IR procedure (11.8%) had a different specialty ascribed to the first, second, or third paracentesis with a subsequent IR procedure.
Model results (Table 3) indicate that patients who were obese (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10‐1.43) or had a liver transplant on a prior admission (OR: 2.03; 95% CI: 1.40‐2.95) were more likely to be referred to IR. However, male patients (OR: 0.89; 95% CI: 0.83‐0.95), or patients who required an ICU stay (OR: 0.39; 95% CI: 0.36‐0.43) were less likely to have IR procedures. Other patient factors reducing the likelihood of IR referral included characteristics associated with higher severity of illness (coagulation disorders, hyponatremia, hypotension, and thrombocytopenia).
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Lower | Upper | ||
| |||
| Age group, y | |||
| 1849 | Reference | ||
| 5059 | 1.05 | 0.97 | 1.14 |
| 6069 | 1.12 | 1.02 | 1.22 |
| 70+ | 1.11 | 0.99 | 1.24 |
| Male | 0.89 | 0.83 | 0.95 |
| Obesity, BMI 40+ | 1.25 | 1.10 | 1.43 |
| ICU care | 0.39 | 0.36 | 0.43 |
| Coagulation disorders | 0.68 | 0.63 | 0.75 |
| Blood loss anemia | 0.52 | 0.41 | 0.66 |
| Hyponatremia | 0.85 | 0.80 | 0.92 |
| Hypotension | 0.83 | 0.74 | 0.93 |
| Thrombocytopenia | 0.94 | 0.87 | 1.01 |
| Prior liver transplant | 0.08 | 0.03 | 0.23 |
| Awaiting liver transplant | 0.86 | 0.76 | 0.98 |
| Complication of liver transplant | 1.07 | 0.88 | 1.31 |
| Liver transplant procedure | 2.03 | 1.40 | 2.95 |
| Charlson score | 1.00 | 0.99 | 1.01 |
| Number of paracentesis procedures | 0.90 | 0.85 | 0.95 |
Predicted Probabilities of IR Referral
Figure 1 presents the distribution of predicted probabilities for IR referral. Predicted probabilities were low overall, with very few patients having an equal chance of referralthe standard often used in comparative effectiveness analyses from observational data. Figure 1 indicates that IR referral probabilities were clustered in an unusual bimodal distribution. The cluster on the left, which centers around a 15% predicted probability of IR referral, consists of discharges with patient characteristics that were associated with a very low chance of an IR paracentesis. We therefore used this distribution to conduct comparative analyses of admission outcomes between clinical specialty groups, choosing to examine patients with a 20% or greater chance of IR referral.
Post hoc analysis revealed that the biggest factor driving low predicted probability of IR referral was whether patients experienced an ICU stay at any time during hospitalization. Among the discharges with a predicted probability 0.2 (n=26,615 discharges), there were only 87 discharges with ICU stays (0.3%). For the discharges with predicted probability <0.2 (n=7676), 91.9% (n=7055) had an ICU admission. We therefore used a threshold of 0.2 or greater to present the most comparable LOS and direct cost differences.
LOS and Cost Comparisons by Specialty
Mean LOS and hospital direct costs by specialty for our final analysis sample can be found in Table 4; differences between specialties were significant (P<0.0001). Patients undergoing IR procedures had equivalent LOS and costs to medicine patients, but lower LOS and costs than other clinical specialty groups. Random effects linear regression showed that neither medicine nor gastroenterology/hepatology patients had significantly different LOS from IR patients, but subspecialty medicine was associated with 0.89 additional days and general surgery with 1.47 additional days (both P<0.0001; R2=0.10). In the direct cost regression model, medicine patients were associated with $1308 lower costs and gastroenterology/hepatology patients with $803 lower costs than IR patients (both P=0.0001), whereas subspecialty medicine and general surgery had higher direct costs per discharge of $1886 and $3039, respectively (both P<0.0001, R2=0.19). Older age, obesity, coagulopathy, hyponatremia, hypotension, thrombocytopenia, liver transplant status, ICU care, higher Charlson score, and higher number of paracentesis procedures performed were all significantly associated with higher LOS and hospital costs in these linear models.
| All Admissions n=26,615 | Interventional Radiology n=7,677 | Medicine n=10,413 | Medicine Subspecialties n=2,210 | Gastroenterology/ Hepatology n=5,182 | General Surgery n=1,133 | |
|---|---|---|---|---|---|---|
| All Admissions n=24,408 | Interventional Radiology n =7,265 | Medicine n=8,965, | Medicine Subspecialties n=2,064 | Gastroenterology/Hepatology n=5,031 | General Surgery n=1,083 | |
| ||||||
| Mean length of stay, d (SD) | 5.57 (5.63) | 5.20 (4.72) | 5.59 (5.85) | 6.28 (6.47) | 5.54 (5.31) | 6.67 (8.16) |
| Mean total direct cost, $ (SD)a | 11,447 (12,247) | 10,975 (9,723) | 10,517 (10,895) | 13,705 (16,591) | 12,000 (11,712) | 15,448 (23,807) |
DISCUSSION
This study showed that internal medicine‐ and family medicine‐trained clinicians perform approximately half of the inpatient paracentesis procedures at university hospitals and their affiliates. This confirms findings from our earlier single‐institution study[16] but contrasts with previously published reports involving Medicare data. The earlier report, using Medicare claims and including ambulatory procedures, revealed that primary care physicians and gastroenterologists only performed approximately 10% of US paracentesis procedures in 2008.[7] Our findings suggest that practices are different at university hospitals, where patients with severe liver disease often seek care. Because we used the UHC database, it was not possible to determine if the clinicians who performed paracentesis procedures in this study were internal medicine or family medicine residents, fellows, or attending physicians. However, findings from our own institution show that the vast majority of bedside paracentesis procedures are performed by internal medicine residents.[16]
Our findings have implications for certification of internal medicine and family medicine trainees. In 2008, the ABIM removed the requirement that internal medicine residents demonstrate competency in paracentesis.[12] This decision was informed by a lack of standardized methods to determine procedural competency and published surveys showing that internal medicine and hospitalist physicians rarely performed bedside procedures.[5, 6] Despite this policy change, our findings show that current clinical practice at university hospitals does not reflect national practice patterns or certification requirements, because many internal medicine‐ and family medicine‐trained clinicians still perform paracentesis procedures. This is concerning because internal medicine and family medicine trainees report variable confidence, experience, expertise, and supervision regarding performance of invasive procedures.[9, 10, 21, 22, 23, 24] Furthermore, earlier research also demonstrates that graduating residents and fellows are not able to competently perform common bedside procedures such as thoracentesis, temporary hemodialysis catheter insertion, and lumbar puncture.[25, 26, 27]
The American Association for the Study of Liver Diseases (AASLD) recommends that trained clinicians perform paracentesis procedures.[3, 4] However, the AASLD provides no definition for how training should occur. Because competency in this procedure is not specifically required by the ABIM, ABFM, or ACGME, a paradoxical situation occurs in which internal medicine and family medicine residents, and internal medicine‐trained fellows and faculty continue to perform paracentesis procedures on highly complex patients, but are no longer required to be competent to do so.
In earlier research we showed that simulation‐based mastery learning (SBML) was an effective method to boost internal medicine residents' paracentesis skills.[28] In SBML, all trainees must meet or exceed a minimum passing score on a simulated procedure before performing one on an actual patient.[29] This approach improves clinical care and outcomes in procedures such as central venous catheter insertion[30, 31] and advanced cardiac life support.[32] SBML‐trained residents also performed safe paracentesis procedures with shorter hospital LOS, fewer ICU transfers, and fewer blood product transfusions than IR procedures.[16] Based on the results of this study, AASLD guidelines regarding training, and our experience with SBML, we recommend that all clinicians complete paracentesis SBML training before performing procedures on patients.
Using our propensity model we identified patient characteristics that were associated with IR referral. Patients with a liver transplant were more likely to be cared for in IR. This may be due to a belief that postoperative procedures are anatomically more complex or because surgical trainees do not commonly perform this procedure. The current study confirms findings from earlier work that obese and female patients are more likely to be referred to IR.[16] IR referral of obese patients is likely to occur because paracentesis procedures are technically more difficult. We have no explanation why female patients were more likely to be referred to IR, because most decisions appear to be discretionary. Prospective studies are needed to determine evidence‐based recommendations regarding paracentesis procedure location. Patients with more comorbidities (eg, ICU stay, awaiting liver transplant, coagulation disorders) were more likely to undergo bedside procedures. The complexity of patients undergoing bedside paracentesis procedures reinforces the need for rigorous skill assessment for clinicians who perform them because complications such as intraperitoneal bleeding can be fatal.
Finally, we showed that LOS was similar but hospital direct costs were $800 to $1300 lower for patients whose paracentesis procedure was performed by medicine or gastroenterology/hepatology compared to IR. Medical subspecialties and surgery procedures were more expensive than IR, consistent with the higher LOS seen in these groups. IR procedures add costs due to facility charges for space, personnel, and equipment.[33] At our institution, the hospital cost of an IR paracentesis in 2012 was $361. If we use this figure, and assume costs are similar across university hospitals, the resultant cost savings would be $10,257,454 (for the procedure alone) if all procedures referred to IR in this 2‐year study were instead performed at the bedside. This estimate is approximate because it does not consider factors such as cost of clinician staffing models, which may differ across UHC hospitals. As hospitals look to reduce costs, potential savings due to appropriate use of bedside and IR procedures should be considered. This is especially important because there is no evidence that the extra expense of IR procedures is justified due to improved patient outcomes.
This study has several limitations. First, this was an observational study. Although the database was large, we were limited by coding accuracy and could not control for all potential confounding factors such as Model for End‐Stage Liver Disease score,[34, 35] other specific laboratory values, amount of ascites fluid removed, or bedside procedure failures later referred to IR. However, we do know that only a small number of second, third, or fourth procedures were subsequently referred to IR after earlier ones were performed at the bedside. Additionally the UHC database does not include patient‐specific data, and therefore we could not adjust for multiple visits or procedures by the same patient. Second, we were unable to determine the level of teaching involvement at each UHC affiliated hospital. Community hospitals where attendings managed most of the patients without trainees could not be differentiated from university hospitals where trainees were involved in most patients' care. Third, we did not have specialty information for 9434 (9.7%) procedures and had to exclude these cases. We also excluded a large number of paracentesis procedures in our final outcomes analysis. However, this was necessary because we needed to perform a patient‐level analysis to ensure the propensity and outcomes models were accurate. Finally, we did not evaluate inpatient mortality or 30‐day hospital readmission rates. Mortality and readmission from complications of a paracentesis procedure are rare events.[3, 4, 36] However, mortality and hospital readmission among patients with liver disease are relatively common.[37, 38] It was impossible to link these outcomes to a paracentesis procedure without the ability to perform medical records review.
In conclusion, paracentesis procedures are performed frequently by internal medicine‐ and family medicine‐trained clinicians in university hospitals. Because of these findings regarding current practice patterns, we believe the ACGME, ABIM, and ABFM should clarify their policies to require that residents are competent to perform paracentesis procedures before performing them on patients. This may improve supervision and training for paracentesis procedures that are already occurring and possibly encourage performance of additional, less costly bedside procedures.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Mark Williams for their support and encouragement of this work.
Disclosure: Nothing to report.
- . A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol. 2011:801983.
- , , . Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2009;49:2087–2107. Available at: http://www.aasld.org/practiceguidelines/Documents/ascitesupdate2013.pdf. Accessed October 21, 2013.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , . What procedures should internists do? Ann Intern Med. 2007;146(5):392–393.
- , , , , , . Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures. Am J Med. 2006;119(1)71.e17–e24.
- , , . Perception of competency to perform procedures and future practice intent: a national survey of family practice residents. Acad Med. 2003;78(9):926–932.
- , , , . Utilization of and adherence to the gastroenterology core curriculum on hepatology training during a gastrointestinal fellowship. Clin Gastroenterol Hepatol. 2008;6(6):682–688.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed December 21, 2013.
- A CGME program requirements for graduate medical education in internal medicine. Available at: http://acgme.org/acgmeweb/portals/0/PFassets/2013‐PR‐FAQ‐PIF/140_internal_medicine_07012013.pdf. Accessed December 17, 2013.
- American Board of Family Medicine residency requirements. Available at: https://www.theabfm.org/cert/guidelines.aspx. Accessed December 17, 2013.
- ACGME program requirements for graduate medical education in family medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/120pr07012007.pdf. Accessed December 17, 2013.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- . The role of known effects in observational studies. Biometrics. 1998;45(2):557–569.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , , , et al. Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial. J Hosp Med. 2007;2(3):143–149.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , . Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol. 2008;3(4):941–947.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3:49–54.
- , , , , . Mastery learning of temporary dialysis catheter insertion skills by nephrology fellows using simulation technology and deliberate practice. Am J Kidney Dis. 2009;54:70–76.
- , , , , , . Simulation‐based education with mastery learning improves residents' lumbar puncture skills. Neurology. 2012;79:132–137.
- , , , , , . Simulation‐based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23–27.
- , , , , . Medical education featuring mastery learning with deliberate practice can lead to better health for individuals and populations. Acad Med. 2011; 86(11):e8–e9.
- , , , , . Simulation‐based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701.
- , , , , . Use of simulation‐based education to reduce catheter‐related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423.
- , , , et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. J Grad Med Educ. 2011;3(2):211–216.
- , , , et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology. 2000;216(1):269–272.
- , , , et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33(2):464–470.
- , , , et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- , , , . Videos in clinical medicine. Paracentesis. N Engl J Med. 2006;355(19):e21.
- Center for Disease Control and Prevention. Chronic liver disease or cirrhosis. National Hospital Discharge Survey: 2010 detailed diagnosis and procedure tables, number of first‐listed diagnoses (see ICD9‐CM code 571). Available at: http://www.cdc.gov/nchs/fastats/liverdis.htm. Accessed October 19, 2013.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254–259.
Cirrhosis affects up to 3% of the population and is 1 of the 10 most common causes of death in the United States.[1, 2, 3, 4] Paracentesis procedures are frequently performed in patients with liver disease and ascites for diagnostic and/or therapeutic purposes. These procedures can be performed safely by trained clinicians at the bedside or referred to interventional radiology (IR).[2, 3, 4]
National practice patterns show that paracentesis procedures are increasingly referred to IR rather than performed at the bedside by internal medicine or gastroenterology clinicians.[5, 6, 7] In fact, a recent study of Medicare beneficiaries showed that inpatient and outpatient paracentesis procedures performed by radiologists increased by 964% from 1993 to 2008.[7] Reasons for the decline in bedside procedures include the increased availability of IR, lack of sufficient reimbursement, and the time required to perform paracentesis procedures.[5, 6, 7, 8] Surveys of internal medicine and family medicine residents and gastroenterology fellows show trainees often lack the confidence and experience needed to perform the procedure safely.[9, 10, 11] Additionally, many clinicians do not have expertise with ultrasound use and may not have access to necessary equipment.
Inconsistent certification requirements may also impact the competence and experience of physicians to perform paracentesis procedures. Internal medicine residents are no longer required by the American Board of Internal Medicine (ABIM) to demonstrate competency in procedures such as paracentesis for certification.[12] However, the Accreditation Council for Graduate Medical Education (ACGME) requirements state that internal medicine programs must offer residents the opportunity to demonstrate competence in the performance of procedures such as paracentesis, thoracentesis, and central venous catheter insertion.[13] The American Board of Family Medicine (ABFM) does not outline specific procedural competence for initial certification.[14] The ACGME states that family medicine residents must receive training to perform those clinical procedures required for their future practices but allows each program to determine which procedures to require.[15] Due to this uncertainty, practicing hospitalists are likely to have variable training and competence in bedside procedures such as paracentesis.
We previously showed that internal medicine residents rotating on the hepatology service of an academic medical center performed 59% of paracentesis procedures at the bedside.[16] These findings are in contrast to national data showing that 74% of paracentesis procedures performed on Medicare beneficiaries were performed by radiologists.[7] Practice patterns at university hospitals may not be reflected in this data because the study was limited to Medicare beneficiaries and included ambulatory patients.[7] In addition to uncertainty about who is performing this procedure in inpatient settings, little is known about the effect of specialty on postparacentesis clinical outcomes.[16, 17]
The current study had 3 aims: (1) evaluate which clinical specialties perform paracentesis procedures at university hospitals; (2) model patient characteristics associated with procedures performed at the bedside versus those referred to IR; and (3) among patients with a similar likelihood of IR referral, evaluate length of stay (LOS) and hospital costs of patients undergoing procedures performed by different specialties.
METHODS
We performed an observational administrative database review of patients who underwent paracentesis procedures in hospitals participating in the University HealthSystem Consortium (UHC) Clinical Database from January 2010 through December 2012. UHC is an alliance of 120 nonprofit academic medical centers and their 290 affiliated hospitals. UHC maintains databases containing clinical, operational, financial, and patient safety data from affiliated hospitals. Using the UHC database, we described the characteristics of all patients who underwent paracentesis procedures by clinical specialty performing the procedure. We then modeled the effects of patient characteristics on decision‐making about IR referral. Finally, among patients with a homogeneous predicted probability of IR referral, we compared LOS and direct costs by specialty performing the procedure. The Northwestern University institutional review board approved this study.
Procedure
We queried the UHC database for all patients over the age of 18 years who underwent paracentesis procedures (International Classification of Disease Revision 9 [ICD‐9] procedure code 54.91) and had at least 1 diagnosis code of liver disease (571.x). We excluded patients admitted to obstetrics. The query included patient and clinical characteristics such as admission, discharge, and procedure date; age, gender, procedure provider specialty, and intensive care unit (ICU) stay. We also obtained all ICD‐9 codes associated with the admission including obesity, severe liver disease, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before or during the admission, awaiting liver transplant, and complications of liver transplant. We used ICD‐9 codes to calculate patients' Charlson score[18, 19] to assess severity of illness on admission.
LOS and total direct hospital costs were compared among patients with a paracentesis performed by a single clinical group and among patients with a similar predicted probability of IR referral. UHC generates direct cost estimates by applying Medicare Cost Report ratios of cost to charges with the labor cost further adjusted by the respective area wage index. Hospital costs were not available from 8.3% of UHC hospitals. We therefore based cost estimates on nonmissing data.
Paracentesis provider specialties were divided into 6 general categories: (1) IR (interventional and diagnostic radiology); (2) medicine (family medicine, general medicine, and hospital medicine); (3) subspecialty medicine (infectious disease, cardiology, nephrology, hematology/oncology, endocrinology, pulmonary, and geriatrics); (4) gastroenterology/hepatology (gastroenterology, hepatology, and transplant medicine); (5) general surgery (general surgery and transplant surgery); and (6) all other (included unclassified specialties). We present patient characteristics categorized by these specialty groups and for admissions in which multiple specialties performed procedures.
Study Design
To analyze an individual patient's likelihood of IR referral, we needed to restrict our sample to discharges where only 1 clinical specialty performed a paracentesis. Therefore, we excluded hybrid discharges with procedures performed by more than 1 specialty in a single admission as well as discharges with procedures performed by all other specialties. To compare LOS and direct cost outcomes, and to minimize selection bias among exclusively IR‐treated patients, we excluded hospitals without procedures done by both IR and medicine.
We modeled referral to IR as a function of patients' demographic and clinical variables, which we believed would affect the probability of referral. We then examined the IR referral model predicted probabilities (propensity score).[20] Finally, we examined mean differences in LOS and direct costs among discharges with a single clinical specialty group, while using the predicted probability of referral as a filter to compare these outcomes by specialty. We further tested specialty differences in LOS and direct costs controlling for demographic and clinical variables.
Statistical Analysis
To test the significance of differences between demographic and clinical characteristics of patients across specialties, we used 2 tests for categorical variables and analysis of variance or the Kruskal‐Wallis rank test for continuous variables. Random effects logistic regression, which adjusts standard errors for clustering by hospital, was used to model the likelihood of referral to IR. Independent variables included patient age, gender, obesity, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before hospitalization, liver transplant during hospitalization, awaiting transplant, complications of liver transplant, ICU stay, Charlson score, and number of paracentesis procedures performed during the admission. Predicted probabilities derived from this IR referral model were used to investigate selection bias in our subsequent analyses of LOS and costs.[20]
We used random effects multiple linear regression to test the association of procedure specialty with hospital LOS and total direct costs, controlling for the same independent variables listed above. Analyses were conducted using both actual LOS in days and Medicare costs. We also performed a log transformation of LOS and costs to account for rightward skew. We only present actual LOS and cost results because results were virtually identical. We used SAS version 9 (SAS Institute Inc., Cary, NC) to extract data from the UHC Clinical Database. We performed all statistical analyses using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Procedure and Discharge Level Results
There were 97,577 paracentesis procedures performed during 70,862 hospital admissions in 204 UHC hospitals during the study period. Table 1 shows specific specialty groups for each procedure. The all other category consisted of 17,558 subspecialty groups including 9,434 with specialty unknown. Twenty‐nine percent of procedures were performed in IR versus 27% by medicine, 11% by gastroenterology/hepatology, and 11% by subspecialty medicine.
| Specialty Group | No. | % |
|---|---|---|
| Interventional radiology | 28,414 | 29.1 |
| Medicine | 26,031 | 26.7 |
| Family medicine | 1,026 | 1.1 |
| General medicine | 21,787 | 22.3 |
| Hospitalist | 3,218 | 3.3 |
| Subspecialty medicine | 10,558 | 10.8 |
| Infectious disease | 848 | 0.9 |
| Nephrology | 615 | 0.6 |
| Cardiology | 991 | 1.0 |
| Hematology oncology | 795 | 0.8 |
| Endocrinology | 359 | 0.4 |
| Pulmonology | 6,605 | 6.8 |
| Geriatrics | 345 | 0.4 |
| Gastroenterology/hepatology | 11,143 | 11.4 |
| Transplant medicine | 99 | 0.1 |
| Hepatology | 874 | 0.9 |
| Gastroenterology | 10,170 | 10.4 |
| General surgery | 3,873 | 4.0 |
| Transplant surgery | 2,146 | 2.2 |
| General surgery | 1,727 | 1.8 |
| All other | 17,558 | 18.0 |
| Specialty unknown | 9,434 | 9.7 |
Table 2 presents patient characteristics for 70,862 hospital discharges with paracentesis procedures grouped by whether single or multiple specialties performed procedures. Patient characteristics were significantly different across specialty groups. Medicine, subspecialty medicine, and gastroenterology/hepatology patients were younger, more likely to be male, and more likely to have severe liver disease, coagulation disorders, hypotension, and hyponatremia than IR patients.
| All Discharges, N=70,862 | Interventional Radiology, n=9,348 | Medicine, n=13,789 | Subspecialty Medicine, n=5,085 | Gastroenterology/Hepatology, n=6,664 | General Surgery, n=1,891 | All Other, n=7,912 | Discharges With Multiple Specialties, n=26,173 | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age group, y (%) | ||||||||
| 1849 | 25.4 | 22.5 | 27.6 | 24.9 | 23.5 | 20.8 | 25.5 | 26.1 |
| 5059 | 39.8 | 39.8 | 40.9 | 39.4 | 41.5 | 40.3 | 40.0 | 38.7 |
| 6069 | 24.7 | 24.9 | 21.6 | 24.7 | 26.5 | 30.0 | 23.6 | 25.8 |
| 70+ | 10.1 | 12.9 | 9.9 | 11.1 | 8.4 | 8.9 | 11.0 | 9.4 |
| Male (%) | 65.5 | 64.2 | 67.6 | 67.5 | 65.7 | 66.6 | 65.7 | 64.2 |
| Severe liver disease (%)a | 73.7 | 65.3 | 67.8 | 71.0 | 75.3 | 66.6 | 67.6 | 82.1 |
| Obesity (BMI 40+) (%) | 6.3 | 6.1 | 5.3 | 5.7 | 5.1 | 5.8 | 5.2 | 7.6 |
| Any intensive care unit stay (%) | 31.0 | 10.9 | 16.8 | 50.5 | 16.9 | 36.7 | 22.3 | 47.8 |
| Coagulation disorders (%) | 24.3 | 14.8 | 20.2 | 29.9 | 16.1 | 19.0 | 17.8 | 33.1 |
| Blood loss anemia (%) | 3.4 | 1.3 | 2.8 | 2.7 | 2.7 | 1.9 | 2.1 | 5.2 |
| Hyponatremia (%) | 29.9 | 27.1 | 29.2 | 28.9 | 28.0 | 26.6 | 27.3 | 33.1 |
| Hypotension (%) | 9.8 | 7.0 | 8.0 | 11.0 | 7.7 | 10.5 | 8.1 | 12.4 |
| Thrombocytopenia (%) | 29.6 | 24.6 | 28.3 | 32.5 | 22.1 | 21.5 | 24.0 | 35.8 |
| Complication of transplant (%) | 3.3 | 2.1 | 1.1 | 2.4 | 4.0 | 10.3 | 2.7 | 4.7 |
| Awaiting liver transplant (%) | 7.6 | 6.4 | 4.0 | 5.4 | 12.8 | 16.0 | 7.8 | 8.2 |
| Prior liver transplant (%) | 0.5 | 0.8 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 0.6 |
| Liver transplant procedure (%) | 2.7 | 0.0 | 0.0 | 0.3 | 0.4 | 15.6 | 1.6 | 5.6 |
| Mean Charlson score (SD) | 4.51 (2.17) | 4.28 (2.26) | 4.16 (2.17) | 4.72 (2.30) | 4.30 (1.98) | 4.26 (2.22) | 4.36 (2.30) | 4.84 (2.07) |
| Mean paracentesis procedures per discharge (SD) | 1.38 (0.88) | 1.21 (0.56) | 1.26 (0.66) | 1.30 (0.76) | 1.31 (0.70) | 1.28 (0.78) | 1.22 (0.61) | 1.58 (1.13) |
IR Referral Model
We first excluded 6030/70,862 discharges (8.5%) from 59 hospitals without both IR and medicine procedures. We then further excluded 24,986/70,862 (35.3%) discharges with procedures performed by multiple specialties during the same admission. Finally, we excluded 5555/70,862 (7.8%) of discharges with procedure specialty coded as all other. Therefore, 34,291 (48.4%) discharges (43,337/97,577; 44.4% procedures) from 145 UHC hospitals with paracentesis procedures performed by a single clinical specialty group remained for the IR referral analysis sample. Among admissions with multiple specialty paracentesis performed within the same admission, 3128/26,606 admissions with any IR procedure (11.8%) had a different specialty ascribed to the first, second, or third paracentesis with a subsequent IR procedure.
Model results (Table 3) indicate that patients who were obese (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10‐1.43) or had a liver transplant on a prior admission (OR: 2.03; 95% CI: 1.40‐2.95) were more likely to be referred to IR. However, male patients (OR: 0.89; 95% CI: 0.83‐0.95), or patients who required an ICU stay (OR: 0.39; 95% CI: 0.36‐0.43) were less likely to have IR procedures. Other patient factors reducing the likelihood of IR referral included characteristics associated with higher severity of illness (coagulation disorders, hyponatremia, hypotension, and thrombocytopenia).
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Lower | Upper | ||
| |||
| Age group, y | |||
| 1849 | Reference | ||
| 5059 | 1.05 | 0.97 | 1.14 |
| 6069 | 1.12 | 1.02 | 1.22 |
| 70+ | 1.11 | 0.99 | 1.24 |
| Male | 0.89 | 0.83 | 0.95 |
| Obesity, BMI 40+ | 1.25 | 1.10 | 1.43 |
| ICU care | 0.39 | 0.36 | 0.43 |
| Coagulation disorders | 0.68 | 0.63 | 0.75 |
| Blood loss anemia | 0.52 | 0.41 | 0.66 |
| Hyponatremia | 0.85 | 0.80 | 0.92 |
| Hypotension | 0.83 | 0.74 | 0.93 |
| Thrombocytopenia | 0.94 | 0.87 | 1.01 |
| Prior liver transplant | 0.08 | 0.03 | 0.23 |
| Awaiting liver transplant | 0.86 | 0.76 | 0.98 |
| Complication of liver transplant | 1.07 | 0.88 | 1.31 |
| Liver transplant procedure | 2.03 | 1.40 | 2.95 |
| Charlson score | 1.00 | 0.99 | 1.01 |
| Number of paracentesis procedures | 0.90 | 0.85 | 0.95 |
Predicted Probabilities of IR Referral
Figure 1 presents the distribution of predicted probabilities for IR referral. Predicted probabilities were low overall, with very few patients having an equal chance of referralthe standard often used in comparative effectiveness analyses from observational data. Figure 1 indicates that IR referral probabilities were clustered in an unusual bimodal distribution. The cluster on the left, which centers around a 15% predicted probability of IR referral, consists of discharges with patient characteristics that were associated with a very low chance of an IR paracentesis. We therefore used this distribution to conduct comparative analyses of admission outcomes between clinical specialty groups, choosing to examine patients with a 20% or greater chance of IR referral.

Post hoc analysis revealed that the biggest factor driving low predicted probability of IR referral was whether patients experienced an ICU stay at any time during hospitalization. Among the discharges with a predicted probability 0.2 (n=26,615 discharges), there were only 87 discharges with ICU stays (0.3%). For the discharges with predicted probability <0.2 (n=7676), 91.9% (n=7055) had an ICU admission. We therefore used a threshold of 0.2 or greater to present the most comparable LOS and direct cost differences.
LOS and Cost Comparisons by Specialty
Mean LOS and hospital direct costs by specialty for our final analysis sample can be found in Table 4; differences between specialties were significant (P<0.0001). Patients undergoing IR procedures had equivalent LOS and costs to medicine patients, but lower LOS and costs than other clinical specialty groups. Random effects linear regression showed that neither medicine nor gastroenterology/hepatology patients had significantly different LOS from IR patients, but subspecialty medicine was associated with 0.89 additional days and general surgery with 1.47 additional days (both P<0.0001; R2=0.10). In the direct cost regression model, medicine patients were associated with $1308 lower costs and gastroenterology/hepatology patients with $803 lower costs than IR patients (both P=0.0001), whereas subspecialty medicine and general surgery had higher direct costs per discharge of $1886 and $3039, respectively (both P<0.0001, R2=0.19). Older age, obesity, coagulopathy, hyponatremia, hypotension, thrombocytopenia, liver transplant status, ICU care, higher Charlson score, and higher number of paracentesis procedures performed were all significantly associated with higher LOS and hospital costs in these linear models.
| All Admissions n=26,615 | Interventional Radiology n=7,677 | Medicine n=10,413 | Medicine Subspecialties n=2,210 | Gastroenterology/ Hepatology n=5,182 | General Surgery n=1,133 | |
|---|---|---|---|---|---|---|
| All Admissions n=24,408 | Interventional Radiology n =7,265 | Medicine n=8,965, | Medicine Subspecialties n=2,064 | Gastroenterology/Hepatology n=5,031 | General Surgery n=1,083 | |
| ||||||
| Mean length of stay, d (SD) | 5.57 (5.63) | 5.20 (4.72) | 5.59 (5.85) | 6.28 (6.47) | 5.54 (5.31) | 6.67 (8.16) |
| Mean total direct cost, $ (SD)a | 11,447 (12,247) | 10,975 (9,723) | 10,517 (10,895) | 13,705 (16,591) | 12,000 (11,712) | 15,448 (23,807) |
DISCUSSION
This study showed that internal medicine‐ and family medicine‐trained clinicians perform approximately half of the inpatient paracentesis procedures at university hospitals and their affiliates. This confirms findings from our earlier single‐institution study[16] but contrasts with previously published reports involving Medicare data. The earlier report, using Medicare claims and including ambulatory procedures, revealed that primary care physicians and gastroenterologists only performed approximately 10% of US paracentesis procedures in 2008.[7] Our findings suggest that practices are different at university hospitals, where patients with severe liver disease often seek care. Because we used the UHC database, it was not possible to determine if the clinicians who performed paracentesis procedures in this study were internal medicine or family medicine residents, fellows, or attending physicians. However, findings from our own institution show that the vast majority of bedside paracentesis procedures are performed by internal medicine residents.[16]
Our findings have implications for certification of internal medicine and family medicine trainees. In 2008, the ABIM removed the requirement that internal medicine residents demonstrate competency in paracentesis.[12] This decision was informed by a lack of standardized methods to determine procedural competency and published surveys showing that internal medicine and hospitalist physicians rarely performed bedside procedures.[5, 6] Despite this policy change, our findings show that current clinical practice at university hospitals does not reflect national practice patterns or certification requirements, because many internal medicine‐ and family medicine‐trained clinicians still perform paracentesis procedures. This is concerning because internal medicine and family medicine trainees report variable confidence, experience, expertise, and supervision regarding performance of invasive procedures.[9, 10, 21, 22, 23, 24] Furthermore, earlier research also demonstrates that graduating residents and fellows are not able to competently perform common bedside procedures such as thoracentesis, temporary hemodialysis catheter insertion, and lumbar puncture.[25, 26, 27]
The American Association for the Study of Liver Diseases (AASLD) recommends that trained clinicians perform paracentesis procedures.[3, 4] However, the AASLD provides no definition for how training should occur. Because competency in this procedure is not specifically required by the ABIM, ABFM, or ACGME, a paradoxical situation occurs in which internal medicine and family medicine residents, and internal medicine‐trained fellows and faculty continue to perform paracentesis procedures on highly complex patients, but are no longer required to be competent to do so.
In earlier research we showed that simulation‐based mastery learning (SBML) was an effective method to boost internal medicine residents' paracentesis skills.[28] In SBML, all trainees must meet or exceed a minimum passing score on a simulated procedure before performing one on an actual patient.[29] This approach improves clinical care and outcomes in procedures such as central venous catheter insertion[30, 31] and advanced cardiac life support.[32] SBML‐trained residents also performed safe paracentesis procedures with shorter hospital LOS, fewer ICU transfers, and fewer blood product transfusions than IR procedures.[16] Based on the results of this study, AASLD guidelines regarding training, and our experience with SBML, we recommend that all clinicians complete paracentesis SBML training before performing procedures on patients.
Using our propensity model we identified patient characteristics that were associated with IR referral. Patients with a liver transplant were more likely to be cared for in IR. This may be due to a belief that postoperative procedures are anatomically more complex or because surgical trainees do not commonly perform this procedure. The current study confirms findings from earlier work that obese and female patients are more likely to be referred to IR.[16] IR referral of obese patients is likely to occur because paracentesis procedures are technically more difficult. We have no explanation why female patients were more likely to be referred to IR, because most decisions appear to be discretionary. Prospective studies are needed to determine evidence‐based recommendations regarding paracentesis procedure location. Patients with more comorbidities (eg, ICU stay, awaiting liver transplant, coagulation disorders) were more likely to undergo bedside procedures. The complexity of patients undergoing bedside paracentesis procedures reinforces the need for rigorous skill assessment for clinicians who perform them because complications such as intraperitoneal bleeding can be fatal.
Finally, we showed that LOS was similar but hospital direct costs were $800 to $1300 lower for patients whose paracentesis procedure was performed by medicine or gastroenterology/hepatology compared to IR. Medical subspecialties and surgery procedures were more expensive than IR, consistent with the higher LOS seen in these groups. IR procedures add costs due to facility charges for space, personnel, and equipment.[33] At our institution, the hospital cost of an IR paracentesis in 2012 was $361. If we use this figure, and assume costs are similar across university hospitals, the resultant cost savings would be $10,257,454 (for the procedure alone) if all procedures referred to IR in this 2‐year study were instead performed at the bedside. This estimate is approximate because it does not consider factors such as cost of clinician staffing models, which may differ across UHC hospitals. As hospitals look to reduce costs, potential savings due to appropriate use of bedside and IR procedures should be considered. This is especially important because there is no evidence that the extra expense of IR procedures is justified due to improved patient outcomes.
This study has several limitations. First, this was an observational study. Although the database was large, we were limited by coding accuracy and could not control for all potential confounding factors such as Model for End‐Stage Liver Disease score,[34, 35] other specific laboratory values, amount of ascites fluid removed, or bedside procedure failures later referred to IR. However, we do know that only a small number of second, third, or fourth procedures were subsequently referred to IR after earlier ones were performed at the bedside. Additionally the UHC database does not include patient‐specific data, and therefore we could not adjust for multiple visits or procedures by the same patient. Second, we were unable to determine the level of teaching involvement at each UHC affiliated hospital. Community hospitals where attendings managed most of the patients without trainees could not be differentiated from university hospitals where trainees were involved in most patients' care. Third, we did not have specialty information for 9434 (9.7%) procedures and had to exclude these cases. We also excluded a large number of paracentesis procedures in our final outcomes analysis. However, this was necessary because we needed to perform a patient‐level analysis to ensure the propensity and outcomes models were accurate. Finally, we did not evaluate inpatient mortality or 30‐day hospital readmission rates. Mortality and readmission from complications of a paracentesis procedure are rare events.[3, 4, 36] However, mortality and hospital readmission among patients with liver disease are relatively common.[37, 38] It was impossible to link these outcomes to a paracentesis procedure without the ability to perform medical records review.
In conclusion, paracentesis procedures are performed frequently by internal medicine‐ and family medicine‐trained clinicians in university hospitals. Because of these findings regarding current practice patterns, we believe the ACGME, ABIM, and ABFM should clarify their policies to require that residents are competent to perform paracentesis procedures before performing them on patients. This may improve supervision and training for paracentesis procedures that are already occurring and possibly encourage performance of additional, less costly bedside procedures.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Mark Williams for their support and encouragement of this work.
Disclosure: Nothing to report.
Cirrhosis affects up to 3% of the population and is 1 of the 10 most common causes of death in the United States.[1, 2, 3, 4] Paracentesis procedures are frequently performed in patients with liver disease and ascites for diagnostic and/or therapeutic purposes. These procedures can be performed safely by trained clinicians at the bedside or referred to interventional radiology (IR).[2, 3, 4]
National practice patterns show that paracentesis procedures are increasingly referred to IR rather than performed at the bedside by internal medicine or gastroenterology clinicians.[5, 6, 7] In fact, a recent study of Medicare beneficiaries showed that inpatient and outpatient paracentesis procedures performed by radiologists increased by 964% from 1993 to 2008.[7] Reasons for the decline in bedside procedures include the increased availability of IR, lack of sufficient reimbursement, and the time required to perform paracentesis procedures.[5, 6, 7, 8] Surveys of internal medicine and family medicine residents and gastroenterology fellows show trainees often lack the confidence and experience needed to perform the procedure safely.[9, 10, 11] Additionally, many clinicians do not have expertise with ultrasound use and may not have access to necessary equipment.
Inconsistent certification requirements may also impact the competence and experience of physicians to perform paracentesis procedures. Internal medicine residents are no longer required by the American Board of Internal Medicine (ABIM) to demonstrate competency in procedures such as paracentesis for certification.[12] However, the Accreditation Council for Graduate Medical Education (ACGME) requirements state that internal medicine programs must offer residents the opportunity to demonstrate competence in the performance of procedures such as paracentesis, thoracentesis, and central venous catheter insertion.[13] The American Board of Family Medicine (ABFM) does not outline specific procedural competence for initial certification.[14] The ACGME states that family medicine residents must receive training to perform those clinical procedures required for their future practices but allows each program to determine which procedures to require.[15] Due to this uncertainty, practicing hospitalists are likely to have variable training and competence in bedside procedures such as paracentesis.
We previously showed that internal medicine residents rotating on the hepatology service of an academic medical center performed 59% of paracentesis procedures at the bedside.[16] These findings are in contrast to national data showing that 74% of paracentesis procedures performed on Medicare beneficiaries were performed by radiologists.[7] Practice patterns at university hospitals may not be reflected in this data because the study was limited to Medicare beneficiaries and included ambulatory patients.[7] In addition to uncertainty about who is performing this procedure in inpatient settings, little is known about the effect of specialty on postparacentesis clinical outcomes.[16, 17]
The current study had 3 aims: (1) evaluate which clinical specialties perform paracentesis procedures at university hospitals; (2) model patient characteristics associated with procedures performed at the bedside versus those referred to IR; and (3) among patients with a similar likelihood of IR referral, evaluate length of stay (LOS) and hospital costs of patients undergoing procedures performed by different specialties.
METHODS
We performed an observational administrative database review of patients who underwent paracentesis procedures in hospitals participating in the University HealthSystem Consortium (UHC) Clinical Database from January 2010 through December 2012. UHC is an alliance of 120 nonprofit academic medical centers and their 290 affiliated hospitals. UHC maintains databases containing clinical, operational, financial, and patient safety data from affiliated hospitals. Using the UHC database, we described the characteristics of all patients who underwent paracentesis procedures by clinical specialty performing the procedure. We then modeled the effects of patient characteristics on decision‐making about IR referral. Finally, among patients with a homogeneous predicted probability of IR referral, we compared LOS and direct costs by specialty performing the procedure. The Northwestern University institutional review board approved this study.
Procedure
We queried the UHC database for all patients over the age of 18 years who underwent paracentesis procedures (International Classification of Disease Revision 9 [ICD‐9] procedure code 54.91) and had at least 1 diagnosis code of liver disease (571.x). We excluded patients admitted to obstetrics. The query included patient and clinical characteristics such as admission, discharge, and procedure date; age, gender, procedure provider specialty, and intensive care unit (ICU) stay. We also obtained all ICD‐9 codes associated with the admission including obesity, severe liver disease, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before or during the admission, awaiting liver transplant, and complications of liver transplant. We used ICD‐9 codes to calculate patients' Charlson score[18, 19] to assess severity of illness on admission.
LOS and total direct hospital costs were compared among patients with a paracentesis performed by a single clinical group and among patients with a similar predicted probability of IR referral. UHC generates direct cost estimates by applying Medicare Cost Report ratios of cost to charges with the labor cost further adjusted by the respective area wage index. Hospital costs were not available from 8.3% of UHC hospitals. We therefore based cost estimates on nonmissing data.
Paracentesis provider specialties were divided into 6 general categories: (1) IR (interventional and diagnostic radiology); (2) medicine (family medicine, general medicine, and hospital medicine); (3) subspecialty medicine (infectious disease, cardiology, nephrology, hematology/oncology, endocrinology, pulmonary, and geriatrics); (4) gastroenterology/hepatology (gastroenterology, hepatology, and transplant medicine); (5) general surgery (general surgery and transplant surgery); and (6) all other (included unclassified specialties). We present patient characteristics categorized by these specialty groups and for admissions in which multiple specialties performed procedures.
Study Design
To analyze an individual patient's likelihood of IR referral, we needed to restrict our sample to discharges where only 1 clinical specialty performed a paracentesis. Therefore, we excluded hybrid discharges with procedures performed by more than 1 specialty in a single admission as well as discharges with procedures performed by all other specialties. To compare LOS and direct cost outcomes, and to minimize selection bias among exclusively IR‐treated patients, we excluded hospitals without procedures done by both IR and medicine.
We modeled referral to IR as a function of patients' demographic and clinical variables, which we believed would affect the probability of referral. We then examined the IR referral model predicted probabilities (propensity score).[20] Finally, we examined mean differences in LOS and direct costs among discharges with a single clinical specialty group, while using the predicted probability of referral as a filter to compare these outcomes by specialty. We further tested specialty differences in LOS and direct costs controlling for demographic and clinical variables.
Statistical Analysis
To test the significance of differences between demographic and clinical characteristics of patients across specialties, we used 2 tests for categorical variables and analysis of variance or the Kruskal‐Wallis rank test for continuous variables. Random effects logistic regression, which adjusts standard errors for clustering by hospital, was used to model the likelihood of referral to IR. Independent variables included patient age, gender, obesity, coagulation disorders, blood loss anemia, hyponatremia, hypotension, thrombocytopenia, liver transplant before hospitalization, liver transplant during hospitalization, awaiting transplant, complications of liver transplant, ICU stay, Charlson score, and number of paracentesis procedures performed during the admission. Predicted probabilities derived from this IR referral model were used to investigate selection bias in our subsequent analyses of LOS and costs.[20]
We used random effects multiple linear regression to test the association of procedure specialty with hospital LOS and total direct costs, controlling for the same independent variables listed above. Analyses were conducted using both actual LOS in days and Medicare costs. We also performed a log transformation of LOS and costs to account for rightward skew. We only present actual LOS and cost results because results were virtually identical. We used SAS version 9 (SAS Institute Inc., Cary, NC) to extract data from the UHC Clinical Database. We performed all statistical analyses using Stata version 12 (StataCorp LP, College Station, TX).
RESULTS
Procedure and Discharge Level Results
There were 97,577 paracentesis procedures performed during 70,862 hospital admissions in 204 UHC hospitals during the study period. Table 1 shows specific specialty groups for each procedure. The all other category consisted of 17,558 subspecialty groups including 9,434 with specialty unknown. Twenty‐nine percent of procedures were performed in IR versus 27% by medicine, 11% by gastroenterology/hepatology, and 11% by subspecialty medicine.
| Specialty Group | No. | % |
|---|---|---|
| Interventional radiology | 28,414 | 29.1 |
| Medicine | 26,031 | 26.7 |
| Family medicine | 1,026 | 1.1 |
| General medicine | 21,787 | 22.3 |
| Hospitalist | 3,218 | 3.3 |
| Subspecialty medicine | 10,558 | 10.8 |
| Infectious disease | 848 | 0.9 |
| Nephrology | 615 | 0.6 |
| Cardiology | 991 | 1.0 |
| Hematology oncology | 795 | 0.8 |
| Endocrinology | 359 | 0.4 |
| Pulmonology | 6,605 | 6.8 |
| Geriatrics | 345 | 0.4 |
| Gastroenterology/hepatology | 11,143 | 11.4 |
| Transplant medicine | 99 | 0.1 |
| Hepatology | 874 | 0.9 |
| Gastroenterology | 10,170 | 10.4 |
| General surgery | 3,873 | 4.0 |
| Transplant surgery | 2,146 | 2.2 |
| General surgery | 1,727 | 1.8 |
| All other | 17,558 | 18.0 |
| Specialty unknown | 9,434 | 9.7 |
Table 2 presents patient characteristics for 70,862 hospital discharges with paracentesis procedures grouped by whether single or multiple specialties performed procedures. Patient characteristics were significantly different across specialty groups. Medicine, subspecialty medicine, and gastroenterology/hepatology patients were younger, more likely to be male, and more likely to have severe liver disease, coagulation disorders, hypotension, and hyponatremia than IR patients.
| All Discharges, N=70,862 | Interventional Radiology, n=9,348 | Medicine, n=13,789 | Subspecialty Medicine, n=5,085 | Gastroenterology/Hepatology, n=6,664 | General Surgery, n=1,891 | All Other, n=7,912 | Discharges With Multiple Specialties, n=26,173 | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age group, y (%) | ||||||||
| 1849 | 25.4 | 22.5 | 27.6 | 24.9 | 23.5 | 20.8 | 25.5 | 26.1 |
| 5059 | 39.8 | 39.8 | 40.9 | 39.4 | 41.5 | 40.3 | 40.0 | 38.7 |
| 6069 | 24.7 | 24.9 | 21.6 | 24.7 | 26.5 | 30.0 | 23.6 | 25.8 |
| 70+ | 10.1 | 12.9 | 9.9 | 11.1 | 8.4 | 8.9 | 11.0 | 9.4 |
| Male (%) | 65.5 | 64.2 | 67.6 | 67.5 | 65.7 | 66.6 | 65.7 | 64.2 |
| Severe liver disease (%)a | 73.7 | 65.3 | 67.8 | 71.0 | 75.3 | 66.6 | 67.6 | 82.1 |
| Obesity (BMI 40+) (%) | 6.3 | 6.1 | 5.3 | 5.7 | 5.1 | 5.8 | 5.2 | 7.6 |
| Any intensive care unit stay (%) | 31.0 | 10.9 | 16.8 | 50.5 | 16.9 | 36.7 | 22.3 | 47.8 |
| Coagulation disorders (%) | 24.3 | 14.8 | 20.2 | 29.9 | 16.1 | 19.0 | 17.8 | 33.1 |
| Blood loss anemia (%) | 3.4 | 1.3 | 2.8 | 2.7 | 2.7 | 1.9 | 2.1 | 5.2 |
| Hyponatremia (%) | 29.9 | 27.1 | 29.2 | 28.9 | 28.0 | 26.6 | 27.3 | 33.1 |
| Hypotension (%) | 9.8 | 7.0 | 8.0 | 11.0 | 7.7 | 10.5 | 8.1 | 12.4 |
| Thrombocytopenia (%) | 29.6 | 24.6 | 28.3 | 32.5 | 22.1 | 21.5 | 24.0 | 35.8 |
| Complication of transplant (%) | 3.3 | 2.1 | 1.1 | 2.4 | 4.0 | 10.3 | 2.7 | 4.7 |
| Awaiting liver transplant (%) | 7.6 | 6.4 | 4.0 | 5.4 | 12.8 | 16.0 | 7.8 | 8.2 |
| Prior liver transplant (%) | 0.5 | 0.8 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 0.6 |
| Liver transplant procedure (%) | 2.7 | 0.0 | 0.0 | 0.3 | 0.4 | 15.6 | 1.6 | 5.6 |
| Mean Charlson score (SD) | 4.51 (2.17) | 4.28 (2.26) | 4.16 (2.17) | 4.72 (2.30) | 4.30 (1.98) | 4.26 (2.22) | 4.36 (2.30) | 4.84 (2.07) |
| Mean paracentesis procedures per discharge (SD) | 1.38 (0.88) | 1.21 (0.56) | 1.26 (0.66) | 1.30 (0.76) | 1.31 (0.70) | 1.28 (0.78) | 1.22 (0.61) | 1.58 (1.13) |
IR Referral Model
We first excluded 6030/70,862 discharges (8.5%) from 59 hospitals without both IR and medicine procedures. We then further excluded 24,986/70,862 (35.3%) discharges with procedures performed by multiple specialties during the same admission. Finally, we excluded 5555/70,862 (7.8%) of discharges with procedure specialty coded as all other. Therefore, 34,291 (48.4%) discharges (43,337/97,577; 44.4% procedures) from 145 UHC hospitals with paracentesis procedures performed by a single clinical specialty group remained for the IR referral analysis sample. Among admissions with multiple specialty paracentesis performed within the same admission, 3128/26,606 admissions with any IR procedure (11.8%) had a different specialty ascribed to the first, second, or third paracentesis with a subsequent IR procedure.
Model results (Table 3) indicate that patients who were obese (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10‐1.43) or had a liver transplant on a prior admission (OR: 2.03; 95% CI: 1.40‐2.95) were more likely to be referred to IR. However, male patients (OR: 0.89; 95% CI: 0.83‐0.95), or patients who required an ICU stay (OR: 0.39; 95% CI: 0.36‐0.43) were less likely to have IR procedures. Other patient factors reducing the likelihood of IR referral included characteristics associated with higher severity of illness (coagulation disorders, hyponatremia, hypotension, and thrombocytopenia).
| Odds Ratio | 95% CI | ||
|---|---|---|---|
| Lower | Upper | ||
| |||
| Age group, y | |||
| 1849 | Reference | ||
| 5059 | 1.05 | 0.97 | 1.14 |
| 6069 | 1.12 | 1.02 | 1.22 |
| 70+ | 1.11 | 0.99 | 1.24 |
| Male | 0.89 | 0.83 | 0.95 |
| Obesity, BMI 40+ | 1.25 | 1.10 | 1.43 |
| ICU care | 0.39 | 0.36 | 0.43 |
| Coagulation disorders | 0.68 | 0.63 | 0.75 |
| Blood loss anemia | 0.52 | 0.41 | 0.66 |
| Hyponatremia | 0.85 | 0.80 | 0.92 |
| Hypotension | 0.83 | 0.74 | 0.93 |
| Thrombocytopenia | 0.94 | 0.87 | 1.01 |
| Prior liver transplant | 0.08 | 0.03 | 0.23 |
| Awaiting liver transplant | 0.86 | 0.76 | 0.98 |
| Complication of liver transplant | 1.07 | 0.88 | 1.31 |
| Liver transplant procedure | 2.03 | 1.40 | 2.95 |
| Charlson score | 1.00 | 0.99 | 1.01 |
| Number of paracentesis procedures | 0.90 | 0.85 | 0.95 |
Predicted Probabilities of IR Referral
Figure 1 presents the distribution of predicted probabilities for IR referral. Predicted probabilities were low overall, with very few patients having an equal chance of referralthe standard often used in comparative effectiveness analyses from observational data. Figure 1 indicates that IR referral probabilities were clustered in an unusual bimodal distribution. The cluster on the left, which centers around a 15% predicted probability of IR referral, consists of discharges with patient characteristics that were associated with a very low chance of an IR paracentesis. We therefore used this distribution to conduct comparative analyses of admission outcomes between clinical specialty groups, choosing to examine patients with a 20% or greater chance of IR referral.

Post hoc analysis revealed that the biggest factor driving low predicted probability of IR referral was whether patients experienced an ICU stay at any time during hospitalization. Among the discharges with a predicted probability 0.2 (n=26,615 discharges), there were only 87 discharges with ICU stays (0.3%). For the discharges with predicted probability <0.2 (n=7676), 91.9% (n=7055) had an ICU admission. We therefore used a threshold of 0.2 or greater to present the most comparable LOS and direct cost differences.
LOS and Cost Comparisons by Specialty
Mean LOS and hospital direct costs by specialty for our final analysis sample can be found in Table 4; differences between specialties were significant (P<0.0001). Patients undergoing IR procedures had equivalent LOS and costs to medicine patients, but lower LOS and costs than other clinical specialty groups. Random effects linear regression showed that neither medicine nor gastroenterology/hepatology patients had significantly different LOS from IR patients, but subspecialty medicine was associated with 0.89 additional days and general surgery with 1.47 additional days (both P<0.0001; R2=0.10). In the direct cost regression model, medicine patients were associated with $1308 lower costs and gastroenterology/hepatology patients with $803 lower costs than IR patients (both P=0.0001), whereas subspecialty medicine and general surgery had higher direct costs per discharge of $1886 and $3039, respectively (both P<0.0001, R2=0.19). Older age, obesity, coagulopathy, hyponatremia, hypotension, thrombocytopenia, liver transplant status, ICU care, higher Charlson score, and higher number of paracentesis procedures performed were all significantly associated with higher LOS and hospital costs in these linear models.
| All Admissions n=26,615 | Interventional Radiology n=7,677 | Medicine n=10,413 | Medicine Subspecialties n=2,210 | Gastroenterology/ Hepatology n=5,182 | General Surgery n=1,133 | |
|---|---|---|---|---|---|---|
| All Admissions n=24,408 | Interventional Radiology n =7,265 | Medicine n=8,965, | Medicine Subspecialties n=2,064 | Gastroenterology/Hepatology n=5,031 | General Surgery n=1,083 | |
| ||||||
| Mean length of stay, d (SD) | 5.57 (5.63) | 5.20 (4.72) | 5.59 (5.85) | 6.28 (6.47) | 5.54 (5.31) | 6.67 (8.16) |
| Mean total direct cost, $ (SD)a | 11,447 (12,247) | 10,975 (9,723) | 10,517 (10,895) | 13,705 (16,591) | 12,000 (11,712) | 15,448 (23,807) |
DISCUSSION
This study showed that internal medicine‐ and family medicine‐trained clinicians perform approximately half of the inpatient paracentesis procedures at university hospitals and their affiliates. This confirms findings from our earlier single‐institution study[16] but contrasts with previously published reports involving Medicare data. The earlier report, using Medicare claims and including ambulatory procedures, revealed that primary care physicians and gastroenterologists only performed approximately 10% of US paracentesis procedures in 2008.[7] Our findings suggest that practices are different at university hospitals, where patients with severe liver disease often seek care. Because we used the UHC database, it was not possible to determine if the clinicians who performed paracentesis procedures in this study were internal medicine or family medicine residents, fellows, or attending physicians. However, findings from our own institution show that the vast majority of bedside paracentesis procedures are performed by internal medicine residents.[16]
Our findings have implications for certification of internal medicine and family medicine trainees. In 2008, the ABIM removed the requirement that internal medicine residents demonstrate competency in paracentesis.[12] This decision was informed by a lack of standardized methods to determine procedural competency and published surveys showing that internal medicine and hospitalist physicians rarely performed bedside procedures.[5, 6] Despite this policy change, our findings show that current clinical practice at university hospitals does not reflect national practice patterns or certification requirements, because many internal medicine‐ and family medicine‐trained clinicians still perform paracentesis procedures. This is concerning because internal medicine and family medicine trainees report variable confidence, experience, expertise, and supervision regarding performance of invasive procedures.[9, 10, 21, 22, 23, 24] Furthermore, earlier research also demonstrates that graduating residents and fellows are not able to competently perform common bedside procedures such as thoracentesis, temporary hemodialysis catheter insertion, and lumbar puncture.[25, 26, 27]
The American Association for the Study of Liver Diseases (AASLD) recommends that trained clinicians perform paracentesis procedures.[3, 4] However, the AASLD provides no definition for how training should occur. Because competency in this procedure is not specifically required by the ABIM, ABFM, or ACGME, a paradoxical situation occurs in which internal medicine and family medicine residents, and internal medicine‐trained fellows and faculty continue to perform paracentesis procedures on highly complex patients, but are no longer required to be competent to do so.
In earlier research we showed that simulation‐based mastery learning (SBML) was an effective method to boost internal medicine residents' paracentesis skills.[28] In SBML, all trainees must meet or exceed a minimum passing score on a simulated procedure before performing one on an actual patient.[29] This approach improves clinical care and outcomes in procedures such as central venous catheter insertion[30, 31] and advanced cardiac life support.[32] SBML‐trained residents also performed safe paracentesis procedures with shorter hospital LOS, fewer ICU transfers, and fewer blood product transfusions than IR procedures.[16] Based on the results of this study, AASLD guidelines regarding training, and our experience with SBML, we recommend that all clinicians complete paracentesis SBML training before performing procedures on patients.
Using our propensity model we identified patient characteristics that were associated with IR referral. Patients with a liver transplant were more likely to be cared for in IR. This may be due to a belief that postoperative procedures are anatomically more complex or because surgical trainees do not commonly perform this procedure. The current study confirms findings from earlier work that obese and female patients are more likely to be referred to IR.[16] IR referral of obese patients is likely to occur because paracentesis procedures are technically more difficult. We have no explanation why female patients were more likely to be referred to IR, because most decisions appear to be discretionary. Prospective studies are needed to determine evidence‐based recommendations regarding paracentesis procedure location. Patients with more comorbidities (eg, ICU stay, awaiting liver transplant, coagulation disorders) were more likely to undergo bedside procedures. The complexity of patients undergoing bedside paracentesis procedures reinforces the need for rigorous skill assessment for clinicians who perform them because complications such as intraperitoneal bleeding can be fatal.
Finally, we showed that LOS was similar but hospital direct costs were $800 to $1300 lower for patients whose paracentesis procedure was performed by medicine or gastroenterology/hepatology compared to IR. Medical subspecialties and surgery procedures were more expensive than IR, consistent with the higher LOS seen in these groups. IR procedures add costs due to facility charges for space, personnel, and equipment.[33] At our institution, the hospital cost of an IR paracentesis in 2012 was $361. If we use this figure, and assume costs are similar across university hospitals, the resultant cost savings would be $10,257,454 (for the procedure alone) if all procedures referred to IR in this 2‐year study were instead performed at the bedside. This estimate is approximate because it does not consider factors such as cost of clinician staffing models, which may differ across UHC hospitals. As hospitals look to reduce costs, potential savings due to appropriate use of bedside and IR procedures should be considered. This is especially important because there is no evidence that the extra expense of IR procedures is justified due to improved patient outcomes.
This study has several limitations. First, this was an observational study. Although the database was large, we were limited by coding accuracy and could not control for all potential confounding factors such as Model for End‐Stage Liver Disease score,[34, 35] other specific laboratory values, amount of ascites fluid removed, or bedside procedure failures later referred to IR. However, we do know that only a small number of second, third, or fourth procedures were subsequently referred to IR after earlier ones were performed at the bedside. Additionally the UHC database does not include patient‐specific data, and therefore we could not adjust for multiple visits or procedures by the same patient. Second, we were unable to determine the level of teaching involvement at each UHC affiliated hospital. Community hospitals where attendings managed most of the patients without trainees could not be differentiated from university hospitals where trainees were involved in most patients' care. Third, we did not have specialty information for 9434 (9.7%) procedures and had to exclude these cases. We also excluded a large number of paracentesis procedures in our final outcomes analysis. However, this was necessary because we needed to perform a patient‐level analysis to ensure the propensity and outcomes models were accurate. Finally, we did not evaluate inpatient mortality or 30‐day hospital readmission rates. Mortality and readmission from complications of a paracentesis procedure are rare events.[3, 4, 36] However, mortality and hospital readmission among patients with liver disease are relatively common.[37, 38] It was impossible to link these outcomes to a paracentesis procedure without the ability to perform medical records review.
In conclusion, paracentesis procedures are performed frequently by internal medicine‐ and family medicine‐trained clinicians in university hospitals. Because of these findings regarding current practice patterns, we believe the ACGME, ABIM, and ABFM should clarify their policies to require that residents are competent to perform paracentesis procedures before performing them on patients. This may improve supervision and training for paracentesis procedures that are already occurring and possibly encourage performance of additional, less costly bedside procedures.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Mark Williams for their support and encouragement of this work.
Disclosure: Nothing to report.
- . A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol. 2011:801983.
- , , . Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2009;49:2087–2107. Available at: http://www.aasld.org/practiceguidelines/Documents/ascitesupdate2013.pdf. Accessed October 21, 2013.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , . What procedures should internists do? Ann Intern Med. 2007;146(5):392–393.
- , , , , , . Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures. Am J Med. 2006;119(1)71.e17–e24.
- , , . Perception of competency to perform procedures and future practice intent: a national survey of family practice residents. Acad Med. 2003;78(9):926–932.
- , , , . Utilization of and adherence to the gastroenterology core curriculum on hepatology training during a gastrointestinal fellowship. Clin Gastroenterol Hepatol. 2008;6(6):682–688.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed December 21, 2013.
- A CGME program requirements for graduate medical education in internal medicine. Available at: http://acgme.org/acgmeweb/portals/0/PFassets/2013‐PR‐FAQ‐PIF/140_internal_medicine_07012013.pdf. Accessed December 17, 2013.
- American Board of Family Medicine residency requirements. Available at: https://www.theabfm.org/cert/guidelines.aspx. Accessed December 17, 2013.
- ACGME program requirements for graduate medical education in family medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/120pr07012007.pdf. Accessed December 17, 2013.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- . The role of known effects in observational studies. Biometrics. 1998;45(2):557–569.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , , , et al. Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial. J Hosp Med. 2007;2(3):143–149.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , . Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol. 2008;3(4):941–947.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3:49–54.
- , , , , . Mastery learning of temporary dialysis catheter insertion skills by nephrology fellows using simulation technology and deliberate practice. Am J Kidney Dis. 2009;54:70–76.
- , , , , , . Simulation‐based education with mastery learning improves residents' lumbar puncture skills. Neurology. 2012;79:132–137.
- , , , , , . Simulation‐based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23–27.
- , , , , . Medical education featuring mastery learning with deliberate practice can lead to better health for individuals and populations. Acad Med. 2011; 86(11):e8–e9.
- , , , , . Simulation‐based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701.
- , , , , . Use of simulation‐based education to reduce catheter‐related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423.
- , , , et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. J Grad Med Educ. 2011;3(2):211–216.
- , , , et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology. 2000;216(1):269–272.
- , , , et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33(2):464–470.
- , , , et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- , , , . Videos in clinical medicine. Paracentesis. N Engl J Med. 2006;355(19):e21.
- Center for Disease Control and Prevention. Chronic liver disease or cirrhosis. National Hospital Discharge Survey: 2010 detailed diagnosis and procedure tables, number of first‐listed diagnoses (see ICD9‐CM code 571). Available at: http://www.cdc.gov/nchs/fastats/liverdis.htm. Accessed October 19, 2013.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254–259.
- . A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol. 2011:801983.
- , , . Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
- ; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2009;49:2087–2107. Available at: http://www.aasld.org/practiceguidelines/Documents/ascitesupdate2013.pdf. Accessed October 21, 2013.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , . What procedures should internists do? Ann Intern Med. 2007;146(5):392–393.
- , , , , , . Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures. Am J Med. 2006;119(1)71.e17–e24.
- , , . Perception of competency to perform procedures and future practice intent: a national survey of family practice residents. Acad Med. 2003;78(9):926–932.
- , , , . Utilization of and adherence to the gastroenterology core curriculum on hepatology training during a gastrointestinal fellowship. Clin Gastroenterol Hepatol. 2008;6(6):682–688.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed December 21, 2013.
- A CGME program requirements for graduate medical education in internal medicine. Available at: http://acgme.org/acgmeweb/portals/0/PFassets/2013‐PR‐FAQ‐PIF/140_internal_medicine_07012013.pdf. Accessed December 17, 2013.
- American Board of Family Medicine residency requirements. Available at: https://www.theabfm.org/cert/guidelines.aspx. Accessed December 17, 2013.
- ACGME program requirements for graduate medical education in family medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/120pr07012007.pdf. Accessed December 17, 2013.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- , , . Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079; discussion 1081–1090.
- . The role of known effects in observational studies. Biometrics. 1998;45(2):557–569.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , , , et al. Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial. J Hosp Med. 2007;2(3):143–149.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- , . Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol. 2008;3(4):941–947.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3:49–54.
- , , , , . Mastery learning of temporary dialysis catheter insertion skills by nephrology fellows using simulation technology and deliberate practice. Am J Kidney Dis. 2009;54:70–76.
- , , , , , . Simulation‐based education with mastery learning improves residents' lumbar puncture skills. Neurology. 2012;79:132–137.
- , , , , , . Simulation‐based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23–27.
- , , , , . Medical education featuring mastery learning with deliberate practice can lead to better health for individuals and populations. Acad Med. 2011; 86(11):e8–e9.
- , , , , . Simulation‐based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701.
- , , , , . Use of simulation‐based education to reduce catheter‐related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423.
- , , , et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. J Grad Med Educ. 2011;3(2):211–216.
- , , , et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology. 2000;216(1):269–272.
- , , , et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33(2):464–470.
- , , , et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- , , , . Videos in clinical medicine. Paracentesis. N Engl J Med. 2006;355(19):e21.
- Center for Disease Control and Prevention. Chronic liver disease or cirrhosis. National Hospital Discharge Survey: 2010 detailed diagnosis and procedure tables, number of first‐listed diagnoses (see ICD9‐CM code 571). Available at: http://www.cdc.gov/nchs/fastats/liverdis.htm. Accessed October 19, 2013.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9(3):254–259.
© 2014 Society of Hospital Medicine
Appropriate Loop Diuretic Dosing
Injectable furosemide was first approved for use by the US Food and Drug Administration in 1968.1 For more than 40 years, loop diuretics have been the mainstay of therapy for relief of congestion and fluid removal in patients admitted with acute decompensated heart failure (ADHF). Despite the widespread use of loop diuretics in clinical practice, robust data supporting their role is scarce. Furthermore, the optimal approach to the management of the patient with acute volume overload has not been well defined.
In this issue of the Journal of Hospital Medicine, Amer et al.2 present a meta‐analysis of randomized controlled trials comparing continuous infusion to bolus doses of furosemide in hospitalized patients with ADHF. The study demonstrates that continuous infusion is superior to bolus in terms of weight loss and urine output over 24 hours. Specifically, patients receiving a continuous infusion of furosemide had 240 mL/day (95% CI, 462.42 to 18.66) more urine and lost an additional 0.78 kg (95% CI, 1.54 to 0.03) in their hospital stay compared with patients receiving a bolus infusion. The heterogeneity in study designs for urine output and wide confidence intervals for urine output and weight loss create uncertainty about the superiority of continuous infusion. The small difference in daily urine output questions the clinical significance of the results. Many of the studies evaluated in the meta‐analysis lacked rigorous design and/or appropriately dosed furosemide.
Despite the shortcomings of the available studies, the authors have published a sound and reasonable meta‐analysis. This is the first meta‐analysis comparing the use of furosemide alone as a continuous infusion versus bolus dose in patients with ADHF. Additionally, Amer et al. are the first to include recent data from the DOSE trial,3 which showed no difference in volume loss between heart failure patients receiving bolus versus continuous infusions dosing of loop diuretics. Although the benefits of continuous infusion in the meta‐analysis by Amer et al. represent only a modest clinical advantage over bolus infusions, the authors should be commended for addressing an important controversy in the management of patients with volume overload.
Although the method of dose delivery is an important issue in the management of such patients, we believe that a number of critical factors must be taken into consideration to assure sufficient fluid removal and quick relief of congestion. Ensuring the delivery of an adequate loop diuretic dose is critical. Additionally, the dose response must be assessed at an appropriate interval so adjustments can be made in a timely manner. Using this method, diuretic dosing can be individualized based on response.
Current guidelines jointly published by the American Heart Association (AHA) and American College of Cardiology (ACC) do not provide clinicians with specific details about the optimal approach to volume‐overloaded patients.4 In a 2009 update, the ACC and AHA recommend diuretic use to optimize volume status and relieve signs and symptoms of congestion without inducing excessively rapid reduction in intravascular volume.4 They further recommend that patients already receiving a loop diuretic who present with volume overload should receive a dose of diuretic equal to or higher than the outpatient dose. Urine output and congestion should be reassessed serially, and diuretics should be titrated accordingly. Current guidelines do not adequately address several topics, including: (1) appropriate urine output in 24 hours, and how frequently urine output should be assessed; (2) optimal frequency of diuretic dosing; and (3) appropriate choice of diuretic.
An understanding of the pharmacokinetics of loop diuretics helps answer these questions. Intravenous furosemide and bumetanide have similar elimination half‐lives of 1 to 2 hours and peak intravenous action at 30 minutes.5, 6 Intravenous torsemide has not been widely available, but has a longer half‐life of 3 to 4 hours, with peak action in 1 to 2 hours.5, 6 The magnitude of a patient's diuretic response compared with the amount of drug administered is best represented by a sigmoid curve.5 Therefore, after a specific dose threshold, further natriuresis is not achieved. Based on the elimination half‐life, proper bolus dosing of furosemide or bumetanide should be every 4 to 8 hours in patients with volume overload and adequate blood pressure.6 The administration of a loading dose of loop diuretic is of paramount importance to rapidly achieve therapeutic levels immediately before initiating a continuous infusion. Without a proper loading dose, it can take up to 20 hours to achieve steady state serum levels of diuretic during continuous infusion.5 The ACC and AHA acknowledge this point in their guidelines for chronic heart failure by recommending a bolus dose before initiation of continuous infusion.7 The negative results of the DOSE trial may have been due to lack of a loading dose before infusion initiation.3 Additionally, the total volume loss during continuous infusion compared with bolus dosing might be greater if loading doses were consistently given before starting infusions in published studies. Overall, individual patient response to a diuretic dose is variable and dependent on several factors, including serum albumin level, renal and liver function, and diuretic resistance.5
Teamwork and collaboration are essential to overcome barriers to proper diuretic dosing and provide patients with safe and effective care. Closed loop communication between nurses, physicians, and pharmacists in structured daily interdisciplinary rounds appears to reduce adverse drug events in hospitalized patients.8 The increased mortality9, 10 associated with high doses of diuretic, as well as registry data suggesting that over 50% of patients are discharged with significant heart failure symptoms and minimal weight loss,11 call for a more structured approach toward fluid removal. A team‐based protocol that directs titration of medication, monitors response, and clearly outlines communication channels to adjust doses allows for more efficacious medication administration with lower rates of serious events. This method was used with a dosing algorithm for the administration of opioids for patients with acute pain syndromes.12 Serious or fatal opioid‐related adverse drug events were reduced to zero using this communication‐enhancing approach.12 A similar approach should be used for diuretic dosing in patients who are admitted with ADHF.
We believe frequent follow‐up of diuretic response is critical in the successful treatment of the volume‐overloaded patient. Many clinicians who treat hospitalized patients with ADHF prescribe a fixed daily diuretic dose and evaluate the natriuretic response based on 24‐hour urine output and weight loss. This can lead to unnecessary increases in length of hospital stay. We recommend using a protocol for diuretic administration that includes more frequent assessment and follow‐up of dose response. After a diuretic dose is given, nurses communicate with the physician about the amount of urine output after a prespecified time based on an understanding of the pharmacokinetics of the medication administered. If the urine output is not within the desired range, then the diuretic dose can be increased and immediately administered. If the urine output is above a desired range, doses can be decreased, delayed, or held. With optimal protocol dosing for loop diuretics, continuous infusion may be superfluous. In one study, Peacock et al.13 evaluated a diuretic protocol used to treat patients with ADHF who were admitted to an observation unit. This protocol set 2‐hour urine output goals after loop diuretic bolus doses were administered. If the urine output goals were not met, the diuretic dose was doubled and 2‐hour urine measurements were repeated.13 Limits were set on maximum dosing to ensure patient safety, and electrolytes and renal function were monitored. Using this protocol with other ADHF multidisciplinary interventions, 90‐day heart failure readmission rates decreased by 64% (P = 0.007) with a trend toward decreased 90‐day mortality.13 Although the multidisciplinary approach may have been the major contributor to these outcomes, the diuretic protocol allowed rapid achievement of euvolemia in an observation unit patient population with ADHF. Future investigation needs to specifically evaluate dosing protocols and patient safety because of the association between high doses of diuretics and increased mortality. However, studies showing that high diuretic doses are harmful may simply reflect the fact that patients who require high doses of diuretic have more advanced cardiac or renal disease. In such situations, the clinician needs to be aware of the possibility of decreased cardiac output, hypotension, and intrinsic renal disease as potential barriers to diuresis.
Currently, clinicians have no clear evidence‐based strategies for using diuretics to safely reduce congestion in patients with ADHF. As shown by Amer et al.,2 continuous furosemide infusion may provide more effective weight and volume loss than bolus injections. More rigorous studies comparing effectively dosed diuretics regimens are needed. These studies should optimize diuretic use by accounting for individual patient characteristics and drug pharmacokinetics, using a protocol that monitors response in an appropriate interval, and facilitates care team communication. Ultimately, the mode of diuretic administration is only 1 part of developing a process to remove fluid in patients with ADHF.
Acknowledgements
Disclosure: Nothing to report.
- US Food and Drug Administration. FDA Approved Drug Products. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed May 2, 2011.
- , , . Continuous infusion with intermittent bolus injections of furosemide in patients hospitalized with acute decompensated heart failure: a metaanalysis of randomized control trials. J Hosp Med. 2011;7:270–275.
- , , , et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805.
- , , , et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977–2016.
- . Diuretic therapy. N Engl J Med. 1998;339:387– 395.
- Elsevier. Clinical Pharmacology. Available at: http://clinicalpharmacology‐ip.com/Default.aspx. Accessed April 18, 2011.
- . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–e82.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–1069.
- , , , et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84.
- , , , et al. Application of software design principles and debugging methods to an analgesia prescription reduces risk of severe injury from medical use of opioids. Clin Pharmacol Ther. 2008;84:385–392.
- , , , , , . Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8:68–73.
Injectable furosemide was first approved for use by the US Food and Drug Administration in 1968.1 For more than 40 years, loop diuretics have been the mainstay of therapy for relief of congestion and fluid removal in patients admitted with acute decompensated heart failure (ADHF). Despite the widespread use of loop diuretics in clinical practice, robust data supporting their role is scarce. Furthermore, the optimal approach to the management of the patient with acute volume overload has not been well defined.
In this issue of the Journal of Hospital Medicine, Amer et al.2 present a meta‐analysis of randomized controlled trials comparing continuous infusion to bolus doses of furosemide in hospitalized patients with ADHF. The study demonstrates that continuous infusion is superior to bolus in terms of weight loss and urine output over 24 hours. Specifically, patients receiving a continuous infusion of furosemide had 240 mL/day (95% CI, 462.42 to 18.66) more urine and lost an additional 0.78 kg (95% CI, 1.54 to 0.03) in their hospital stay compared with patients receiving a bolus infusion. The heterogeneity in study designs for urine output and wide confidence intervals for urine output and weight loss create uncertainty about the superiority of continuous infusion. The small difference in daily urine output questions the clinical significance of the results. Many of the studies evaluated in the meta‐analysis lacked rigorous design and/or appropriately dosed furosemide.
Despite the shortcomings of the available studies, the authors have published a sound and reasonable meta‐analysis. This is the first meta‐analysis comparing the use of furosemide alone as a continuous infusion versus bolus dose in patients with ADHF. Additionally, Amer et al. are the first to include recent data from the DOSE trial,3 which showed no difference in volume loss between heart failure patients receiving bolus versus continuous infusions dosing of loop diuretics. Although the benefits of continuous infusion in the meta‐analysis by Amer et al. represent only a modest clinical advantage over bolus infusions, the authors should be commended for addressing an important controversy in the management of patients with volume overload.
Although the method of dose delivery is an important issue in the management of such patients, we believe that a number of critical factors must be taken into consideration to assure sufficient fluid removal and quick relief of congestion. Ensuring the delivery of an adequate loop diuretic dose is critical. Additionally, the dose response must be assessed at an appropriate interval so adjustments can be made in a timely manner. Using this method, diuretic dosing can be individualized based on response.
Current guidelines jointly published by the American Heart Association (AHA) and American College of Cardiology (ACC) do not provide clinicians with specific details about the optimal approach to volume‐overloaded patients.4 In a 2009 update, the ACC and AHA recommend diuretic use to optimize volume status and relieve signs and symptoms of congestion without inducing excessively rapid reduction in intravascular volume.4 They further recommend that patients already receiving a loop diuretic who present with volume overload should receive a dose of diuretic equal to or higher than the outpatient dose. Urine output and congestion should be reassessed serially, and diuretics should be titrated accordingly. Current guidelines do not adequately address several topics, including: (1) appropriate urine output in 24 hours, and how frequently urine output should be assessed; (2) optimal frequency of diuretic dosing; and (3) appropriate choice of diuretic.
An understanding of the pharmacokinetics of loop diuretics helps answer these questions. Intravenous furosemide and bumetanide have similar elimination half‐lives of 1 to 2 hours and peak intravenous action at 30 minutes.5, 6 Intravenous torsemide has not been widely available, but has a longer half‐life of 3 to 4 hours, with peak action in 1 to 2 hours.5, 6 The magnitude of a patient's diuretic response compared with the amount of drug administered is best represented by a sigmoid curve.5 Therefore, after a specific dose threshold, further natriuresis is not achieved. Based on the elimination half‐life, proper bolus dosing of furosemide or bumetanide should be every 4 to 8 hours in patients with volume overload and adequate blood pressure.6 The administration of a loading dose of loop diuretic is of paramount importance to rapidly achieve therapeutic levels immediately before initiating a continuous infusion. Without a proper loading dose, it can take up to 20 hours to achieve steady state serum levels of diuretic during continuous infusion.5 The ACC and AHA acknowledge this point in their guidelines for chronic heart failure by recommending a bolus dose before initiation of continuous infusion.7 The negative results of the DOSE trial may have been due to lack of a loading dose before infusion initiation.3 Additionally, the total volume loss during continuous infusion compared with bolus dosing might be greater if loading doses were consistently given before starting infusions in published studies. Overall, individual patient response to a diuretic dose is variable and dependent on several factors, including serum albumin level, renal and liver function, and diuretic resistance.5
Teamwork and collaboration are essential to overcome barriers to proper diuretic dosing and provide patients with safe and effective care. Closed loop communication between nurses, physicians, and pharmacists in structured daily interdisciplinary rounds appears to reduce adverse drug events in hospitalized patients.8 The increased mortality9, 10 associated with high doses of diuretic, as well as registry data suggesting that over 50% of patients are discharged with significant heart failure symptoms and minimal weight loss,11 call for a more structured approach toward fluid removal. A team‐based protocol that directs titration of medication, monitors response, and clearly outlines communication channels to adjust doses allows for more efficacious medication administration with lower rates of serious events. This method was used with a dosing algorithm for the administration of opioids for patients with acute pain syndromes.12 Serious or fatal opioid‐related adverse drug events were reduced to zero using this communication‐enhancing approach.12 A similar approach should be used for diuretic dosing in patients who are admitted with ADHF.
We believe frequent follow‐up of diuretic response is critical in the successful treatment of the volume‐overloaded patient. Many clinicians who treat hospitalized patients with ADHF prescribe a fixed daily diuretic dose and evaluate the natriuretic response based on 24‐hour urine output and weight loss. This can lead to unnecessary increases in length of hospital stay. We recommend using a protocol for diuretic administration that includes more frequent assessment and follow‐up of dose response. After a diuretic dose is given, nurses communicate with the physician about the amount of urine output after a prespecified time based on an understanding of the pharmacokinetics of the medication administered. If the urine output is not within the desired range, then the diuretic dose can be increased and immediately administered. If the urine output is above a desired range, doses can be decreased, delayed, or held. With optimal protocol dosing for loop diuretics, continuous infusion may be superfluous. In one study, Peacock et al.13 evaluated a diuretic protocol used to treat patients with ADHF who were admitted to an observation unit. This protocol set 2‐hour urine output goals after loop diuretic bolus doses were administered. If the urine output goals were not met, the diuretic dose was doubled and 2‐hour urine measurements were repeated.13 Limits were set on maximum dosing to ensure patient safety, and electrolytes and renal function were monitored. Using this protocol with other ADHF multidisciplinary interventions, 90‐day heart failure readmission rates decreased by 64% (P = 0.007) with a trend toward decreased 90‐day mortality.13 Although the multidisciplinary approach may have been the major contributor to these outcomes, the diuretic protocol allowed rapid achievement of euvolemia in an observation unit patient population with ADHF. Future investigation needs to specifically evaluate dosing protocols and patient safety because of the association between high doses of diuretics and increased mortality. However, studies showing that high diuretic doses are harmful may simply reflect the fact that patients who require high doses of diuretic have more advanced cardiac or renal disease. In such situations, the clinician needs to be aware of the possibility of decreased cardiac output, hypotension, and intrinsic renal disease as potential barriers to diuresis.
Currently, clinicians have no clear evidence‐based strategies for using diuretics to safely reduce congestion in patients with ADHF. As shown by Amer et al.,2 continuous furosemide infusion may provide more effective weight and volume loss than bolus injections. More rigorous studies comparing effectively dosed diuretics regimens are needed. These studies should optimize diuretic use by accounting for individual patient characteristics and drug pharmacokinetics, using a protocol that monitors response in an appropriate interval, and facilitates care team communication. Ultimately, the mode of diuretic administration is only 1 part of developing a process to remove fluid in patients with ADHF.
Acknowledgements
Disclosure: Nothing to report.
Injectable furosemide was first approved for use by the US Food and Drug Administration in 1968.1 For more than 40 years, loop diuretics have been the mainstay of therapy for relief of congestion and fluid removal in patients admitted with acute decompensated heart failure (ADHF). Despite the widespread use of loop diuretics in clinical practice, robust data supporting their role is scarce. Furthermore, the optimal approach to the management of the patient with acute volume overload has not been well defined.
In this issue of the Journal of Hospital Medicine, Amer et al.2 present a meta‐analysis of randomized controlled trials comparing continuous infusion to bolus doses of furosemide in hospitalized patients with ADHF. The study demonstrates that continuous infusion is superior to bolus in terms of weight loss and urine output over 24 hours. Specifically, patients receiving a continuous infusion of furosemide had 240 mL/day (95% CI, 462.42 to 18.66) more urine and lost an additional 0.78 kg (95% CI, 1.54 to 0.03) in their hospital stay compared with patients receiving a bolus infusion. The heterogeneity in study designs for urine output and wide confidence intervals for urine output and weight loss create uncertainty about the superiority of continuous infusion. The small difference in daily urine output questions the clinical significance of the results. Many of the studies evaluated in the meta‐analysis lacked rigorous design and/or appropriately dosed furosemide.
Despite the shortcomings of the available studies, the authors have published a sound and reasonable meta‐analysis. This is the first meta‐analysis comparing the use of furosemide alone as a continuous infusion versus bolus dose in patients with ADHF. Additionally, Amer et al. are the first to include recent data from the DOSE trial,3 which showed no difference in volume loss between heart failure patients receiving bolus versus continuous infusions dosing of loop diuretics. Although the benefits of continuous infusion in the meta‐analysis by Amer et al. represent only a modest clinical advantage over bolus infusions, the authors should be commended for addressing an important controversy in the management of patients with volume overload.
Although the method of dose delivery is an important issue in the management of such patients, we believe that a number of critical factors must be taken into consideration to assure sufficient fluid removal and quick relief of congestion. Ensuring the delivery of an adequate loop diuretic dose is critical. Additionally, the dose response must be assessed at an appropriate interval so adjustments can be made in a timely manner. Using this method, diuretic dosing can be individualized based on response.
Current guidelines jointly published by the American Heart Association (AHA) and American College of Cardiology (ACC) do not provide clinicians with specific details about the optimal approach to volume‐overloaded patients.4 In a 2009 update, the ACC and AHA recommend diuretic use to optimize volume status and relieve signs and symptoms of congestion without inducing excessively rapid reduction in intravascular volume.4 They further recommend that patients already receiving a loop diuretic who present with volume overload should receive a dose of diuretic equal to or higher than the outpatient dose. Urine output and congestion should be reassessed serially, and diuretics should be titrated accordingly. Current guidelines do not adequately address several topics, including: (1) appropriate urine output in 24 hours, and how frequently urine output should be assessed; (2) optimal frequency of diuretic dosing; and (3) appropriate choice of diuretic.
An understanding of the pharmacokinetics of loop diuretics helps answer these questions. Intravenous furosemide and bumetanide have similar elimination half‐lives of 1 to 2 hours and peak intravenous action at 30 minutes.5, 6 Intravenous torsemide has not been widely available, but has a longer half‐life of 3 to 4 hours, with peak action in 1 to 2 hours.5, 6 The magnitude of a patient's diuretic response compared with the amount of drug administered is best represented by a sigmoid curve.5 Therefore, after a specific dose threshold, further natriuresis is not achieved. Based on the elimination half‐life, proper bolus dosing of furosemide or bumetanide should be every 4 to 8 hours in patients with volume overload and adequate blood pressure.6 The administration of a loading dose of loop diuretic is of paramount importance to rapidly achieve therapeutic levels immediately before initiating a continuous infusion. Without a proper loading dose, it can take up to 20 hours to achieve steady state serum levels of diuretic during continuous infusion.5 The ACC and AHA acknowledge this point in their guidelines for chronic heart failure by recommending a bolus dose before initiation of continuous infusion.7 The negative results of the DOSE trial may have been due to lack of a loading dose before infusion initiation.3 Additionally, the total volume loss during continuous infusion compared with bolus dosing might be greater if loading doses were consistently given before starting infusions in published studies. Overall, individual patient response to a diuretic dose is variable and dependent on several factors, including serum albumin level, renal and liver function, and diuretic resistance.5
Teamwork and collaboration are essential to overcome barriers to proper diuretic dosing and provide patients with safe and effective care. Closed loop communication between nurses, physicians, and pharmacists in structured daily interdisciplinary rounds appears to reduce adverse drug events in hospitalized patients.8 The increased mortality9, 10 associated with high doses of diuretic, as well as registry data suggesting that over 50% of patients are discharged with significant heart failure symptoms and minimal weight loss,11 call for a more structured approach toward fluid removal. A team‐based protocol that directs titration of medication, monitors response, and clearly outlines communication channels to adjust doses allows for more efficacious medication administration with lower rates of serious events. This method was used with a dosing algorithm for the administration of opioids for patients with acute pain syndromes.12 Serious or fatal opioid‐related adverse drug events were reduced to zero using this communication‐enhancing approach.12 A similar approach should be used for diuretic dosing in patients who are admitted with ADHF.
We believe frequent follow‐up of diuretic response is critical in the successful treatment of the volume‐overloaded patient. Many clinicians who treat hospitalized patients with ADHF prescribe a fixed daily diuretic dose and evaluate the natriuretic response based on 24‐hour urine output and weight loss. This can lead to unnecessary increases in length of hospital stay. We recommend using a protocol for diuretic administration that includes more frequent assessment and follow‐up of dose response. After a diuretic dose is given, nurses communicate with the physician about the amount of urine output after a prespecified time based on an understanding of the pharmacokinetics of the medication administered. If the urine output is not within the desired range, then the diuretic dose can be increased and immediately administered. If the urine output is above a desired range, doses can be decreased, delayed, or held. With optimal protocol dosing for loop diuretics, continuous infusion may be superfluous. In one study, Peacock et al.13 evaluated a diuretic protocol used to treat patients with ADHF who were admitted to an observation unit. This protocol set 2‐hour urine output goals after loop diuretic bolus doses were administered. If the urine output goals were not met, the diuretic dose was doubled and 2‐hour urine measurements were repeated.13 Limits were set on maximum dosing to ensure patient safety, and electrolytes and renal function were monitored. Using this protocol with other ADHF multidisciplinary interventions, 90‐day heart failure readmission rates decreased by 64% (P = 0.007) with a trend toward decreased 90‐day mortality.13 Although the multidisciplinary approach may have been the major contributor to these outcomes, the diuretic protocol allowed rapid achievement of euvolemia in an observation unit patient population with ADHF. Future investigation needs to specifically evaluate dosing protocols and patient safety because of the association between high doses of diuretics and increased mortality. However, studies showing that high diuretic doses are harmful may simply reflect the fact that patients who require high doses of diuretic have more advanced cardiac or renal disease. In such situations, the clinician needs to be aware of the possibility of decreased cardiac output, hypotension, and intrinsic renal disease as potential barriers to diuresis.
Currently, clinicians have no clear evidence‐based strategies for using diuretics to safely reduce congestion in patients with ADHF. As shown by Amer et al.,2 continuous furosemide infusion may provide more effective weight and volume loss than bolus injections. More rigorous studies comparing effectively dosed diuretics regimens are needed. These studies should optimize diuretic use by accounting for individual patient characteristics and drug pharmacokinetics, using a protocol that monitors response in an appropriate interval, and facilitates care team communication. Ultimately, the mode of diuretic administration is only 1 part of developing a process to remove fluid in patients with ADHF.
Acknowledgements
Disclosure: Nothing to report.
- US Food and Drug Administration. FDA Approved Drug Products. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed May 2, 2011.
- , , . Continuous infusion with intermittent bolus injections of furosemide in patients hospitalized with acute decompensated heart failure: a metaanalysis of randomized control trials. J Hosp Med. 2011;7:270–275.
- , , , et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805.
- , , , et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977–2016.
- . Diuretic therapy. N Engl J Med. 1998;339:387– 395.
- Elsevier. Clinical Pharmacology. Available at: http://clinicalpharmacology‐ip.com/Default.aspx. Accessed April 18, 2011.
- . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–e82.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–1069.
- , , , et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84.
- , , , et al. Application of software design principles and debugging methods to an analgesia prescription reduces risk of severe injury from medical use of opioids. Clin Pharmacol Ther. 2008;84:385–392.
- , , , , , . Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8:68–73.
- US Food and Drug Administration. FDA Approved Drug Products. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed May 2, 2011.
- , , . Continuous infusion with intermittent bolus injections of furosemide in patients hospitalized with acute decompensated heart failure: a metaanalysis of randomized control trials. J Hosp Med. 2011;7:270–275.
- , , , et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805.
- , , , et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977–2016.
- . Diuretic therapy. N Engl J Med. 1998;339:387– 395.
- Elsevier. Clinical Pharmacology. Available at: http://clinicalpharmacology‐ip.com/Default.aspx. Accessed April 18, 2011.
- . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–e82.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–1069.
- , , , et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84.
- , , , et al. Application of software design principles and debugging methods to an analgesia prescription reduces risk of severe injury from medical use of opioids. Clin Pharmacol Ther. 2008;84:385–392.
- , , , , , . Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8:68–73.