User login
Vascular Ultrasonography: A Novel Method to Reduce Paracentesis Related Major Bleeding
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
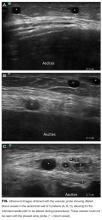
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
Ascites is the most common complication of cirrhosis and often leads to hospitalization. 1 Paracentesis is recommended for all patients admitted with ascites and cirrhosis. 1 Additionally, the Society of Hospital Medicine considers the ability to perform paracenteses a core competency for hospitalists. 2 Although considered a safe procedure, major bleeding complications occur in 0.2% to 1.7% of paracenteses. 3-7 Patients with cirrhosis form new abdominal wall vessels because of portal hypertension, and hemoperitoneum from the laceration of these vessels during paracentesis carries a high morbidity and mortality. 6,8 Ultrasound guidance using a low-frequency ultrasound probe is currently standard practice for paracentesis and has been shown to reduce bleeding complications. 9-11 However, the use of vascular ultrasound (high-frequency probe) is also recommended to identify blood vessels within the intended needle pathway to reduce bleeding, but no studies have been performed to demonstrate a benefit. 3,11 This study aimed to evaluate whether this “2-probe technique” reduces paracentesis-related bleeding complications.
METHODS
The procedure service at Cedars Sinai Medical Center (CSMC) in Los Angeles performs paracentesis regularly with ultrasound guidance. CSMC is a tertiary care, academic medical center with 861 licensed beds. We performed a pre- to postintervention study of consecutive patients (admitted and ambulatory) who underwent paracentesis done by 1 proceduralist (MJA) from the procedure service at CSMC from February 2010 through February 2016. From February 1, 2010, through August 2011, paracenteses were performed using only low-frequency, phased array ultrasound probes (preintervention group). From September 1, 2011, through February 2016, a 2-probe technique was used, whereby ultrasound interrogation of the abdomen using a low-frequency, phased array probe (to identify ascites) was supplemented with a second scan using a high-frequency, linear probe to identify vasculature within the planned needle path (postintervention group). As a standard part of quality assurance, CSMC documented all paracentesis-related complications from procedures performed by their center. Northwestern University investigators (JHB, EC, JF) independently evaluated these data to look at bleeding complications before and after the implementation of the 2-probe technique.
Procedure Protocol
Each patient’s primary team or outpatient physician requested a consultation for paracentesis from the CSMC procedure service. All patient evaluations began with an abdominal ultrasound using the low-frequency probe to determine the presence of ascites and a potential window of access to the fluid. After September 1, 2011, the CSMC procedure service implemented the 2-probe technique to also evaluate the abdominal wall for the presence of vessels. Color flow Doppler ultrasound further helped to differentiate blood vessels as necessary. The optimal window was then marked on the abdominal wall, and the paracentesis was performed. Per the routine of the CSMC procedure service, antiplatelet or anticoagulant medications were not held for paracenteses.
Measurement
All data were collected prospectively at the time of the procedure, including the volume of fluid removed, the number of needle passes required, and whether the patient was on antiplatelet or anticoagulant medications (including warfarin, direct oral anticoagulants, thrombin inhibitors, heparin, or low molecular weight heparins). Patients were followed for complications for up to 24 hours after the procedure or until a clinical question of a complication was reconciled. Minor bleeding was defined as new serosanguinous fluid on repeat paracentesis not associated with hemodynamic changes, local bruising or bleeding at the site, or abdominal wall hematoma.
A query of the electronic medical record was performed to obtain patient demographics and relevant clinical information, including age, sex, body mass index, International Normalized Ratio (INR), partial thromboplastin time (PTT), platelet counts (103/uL, hematocrit (%) and creatinine (mg/dl)
Statistical Analysis
We used a χ2 test, Student t test, or Kruskal-Wallis test to compare demographic and clinical characteristics of procedure patients between the 2 study groups (pre- and postintervention). Major and minor bleeding were compared between the 2 groups using the χ2 test.12 We used the χ2 test instead of the Fisher’s exact test for several reasons. The usual rule is that the Fisher’s exact test is necessary when 1 or more expected outcome values are less than 5. However, McDonald argues that the χ2 test should be used with large sample sizes (more than 1000) in lieu of the outcome-value-of-5 rule.12 The Fisher’s exact test also assumes that the row and column totals are fixed. However, the outcomes in our study were not fixed because any patient could have a bleeding complication during each procedure. When row and column totals are not fixed, only 5% of the time will a P value be less than 0.05, and the Fisher’s exact test is too conservative.12 We performed all statistical analyses using IBM SPSS Statistics Version 22 (IBM Corp, Armonk, NY).
RESULTS
Patient demographic and clinical information can be found in the Table. The proceduralist (MJA) performed a total of 5777 paracenteses (1000 preintervention, 4777 postintervention) on 1639 patients. Four hundred eighty-nine (10.2%) vascular anomalies were identified within the intended needle path in the postintervention group (Figure). More patients in the preintervention group were on aspirin (93 [9.3%] vs 230 [4.8%]; P < 0.001) and therapeutic intravenous anticoagulants (33 [3.3%] vs 89 [1.9%]; P = 0.004), while more patients in the postintervention group were on both an antiplatelet and oral anticoagulant (1 [0.1%] vs 38 [0.8%]; P = 0.015) and subcutaneous prophylactic anticoagulants (184 [18.4%] vs 1120 [23.4%]; P = 0.001) at the time of the procedure. There were no other differences between groups with antiplatelet or anticoagulant drugs. We found no difference in minor bleeding between pre- and postintervention groups. Major bleeding was lower after the 2-probe technique was implemented (3 [0.3%] vs 4 [0.08%]; P = 0.07). There were no between-group differences in INR, PTT, or platelet counts among major bleeders. One patient in the postintervention group had hemodynamic instability and dropped his hemoglobin by 3.8 g/dl at 7 hours after the procedure. This was unexplained, as the patient had no abdominal symptoms or findings on examination. The patient received several liters of fluid before ultimately dying, and the primary team considered sepsis as a possible cause, but no postmortem examination was performed. This was the only death attributed to a major bleeding complication. We included this patient in our analysis because the cause of his demise was not completely clear. However, excluding this patient would change the results from a trend to a statistically significant difference between groups (3 [0.3%] vs 3 [0.06%]; P = 0.03).
DISCUSSION
To our knowledge, we report the largest series of paracentesis prospectively evaluated for bleeding complications, and this is the first study to evaluate whether adding a vascular ultrasound (high-frequency probe) avoids major bleeding. In our series, up to 10% of patients had abnormal vessels seen with a vascular ultrasound that were within the original intended trajectory path of the needle. These vessels were also likely present yet invisible when ultrasound-guided paracentesis using only the standard, low-frequency probe was being performed. It is unknown whether these vessels are routinely traversed with the needle, nicked, or narrowly avoided during paracenteses performed using only a low-frequency probe.
Procedure-related bleeding may not be completely avoidable, despite using the vascular probe. Some authors have suggested that the mechanism of bleeding is more related to the rapid reduction in intraperitoneal pressure, which increases the gradient across vessel walls, resulting in rupture and bleeding.6 However, in our series, using vascular ultrasound also reduced major bleeding to numbers lower than those historically reported in the literature (0.2%).3-4 Our preintervention number needed to harm was 333 procedures to cause 1 major bleed, compared to 1250 (or 1666 using the 3-patient bleeding analysis) in the postintervention group. In 2008, 150,000 Medicare beneficiaries underwent paracentesis.13 Using our study analysis, if vascular ultrasound was used on these patients, up to 360 major bleeds may have been prevented, along with a corresponding reduction in unnecessary morbidity and mortality.
Our study has several limitations. First, it was limited to 1 center with 1 very experienced proceduralist. Although it is possible that the reduction in major bleeding may have been due to the increasing experience of the proceduralist over time, we do not think that this is likely because he had already performed thousands of paracenteses over 9 years before the start of our study.
CONCLUSION
Our results suggest that using the 2-probe technique to predetermine the needle path before performing paracentesis might prevent major bleeding. Based on our findings, we believe that the addition of a vascular ultrasound during paracentesis should be considered by all hospitalists.
Acknowledgments
The authors acknowledge Drs. Douglas Vaughan and Kevin O’Leary for their support and encouragement of this work. They would also like to thank the Cedars-Sinai Enterprise Information Systems Department for assistance with their data query.
Disclosure
The authors have no relevant financial disclosures or conflicts of interest to report.
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. PubMed
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SC, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1 Suppl 1:48-56. PubMed
3. Seidler M, Sayegh K, Roy A, Mesurolle B. A fatal complication of ultrasound-guided abdominal paracentesis. J Clin Ultrasound. 2013;41:457-460. PubMed
4. McGibbon A, Chen GI, Peltekian KM, van Zanten SV. An evidence-based manual for abdominal paracentesis. Dig Dis Sci. 2007;52:3307-3315. PubMed
5. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37:946-951. PubMed
6. Kurup AN, Lekah A, Reardon ST, et al. Bleeding Rate for Ultrasound-Guided Paracentesis in Thrombocytopenic Patients. J Ultrasound Med. 2015;34:1833-1838. PubMed
7. Sharzehi K, Jain V, Naveed A, Schreibman I. Hemorrhagic complications of paracentesis: a systematic review of the literature. Gastroenterol Res Pract. 2014;2014:985141. PubMed
8. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. PubMed
9. Keil-Rios D, Terrazas-Solis H, González-Garay A, Sánchez-Ávila JF, García-Juárez I. Pocket ultrasound device as a complement to physical examination for ascites evaluation and guided paracentesis. Intern Emerg Med. 2016;11:461-466. PubMed
10. Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363-367. PubMed
11. Marcaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracenteis. Chest. 2013;143:532-538. PubMed
12. McDonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, MD: Sparky House Publishing; 2014.
13. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7:859-864. PubMed
© 2018 Society of Hospital Medicine
Thoracentesis Referral
Internal medicine (IM) residents and hospitalist physicians commonly conduct bedside thoracenteses for both diagnostic and therapeutic purposes.[1] The American Board of Internal Medicine only requires that certification candidates understand the indications, complications, and management of thoracenteses.[2] A disconnect between clinical practice patterns and board requirements may increase patient risk because poorly trained physicians are more likely to cause complications.[3] National practice patterns show that many thoracenteses are referred to interventional radiology (IR).[4] However, research links performance of bedside procedures to reduced hospital length of stay and lower costs, without increasing risk of complications.[1, 5, 6]
Simulation‐based education offers a controlled environment where trainees improve procedural knowledge and skills without patient harm.[7] Simulation‐based mastery learning (SBML) is a rigorous form of competency‐based education that improves clinical skills and reduces iatrogenic complications and healthcare costs.[5, 6, 8] SBML also is an effective method to boost thoracentesis skills among IM residents.[9] However, there are no data to show that thoracentesis skills acquired in the simulation laboratory transfer to clinical environments and affect referral patterns.
We hypothesized that a thoracentesis SBML intervention would improve skills and increase procedural self‐confidence while reducing procedure referrals. This study aimed to (1) assess the effect of thoracentesis SBML on a cohort of IM residents' simulated skills and (2) compare traditionally trained (nonSBML‐trained) residents, SBML‐trained residents, and hospitalist physicians regarding procedure referral patterns, self‐confidence, procedure experience, and reasons for referral.
METHODS AND MATERIALS
Study Design
We surveyed physicians about thoracenteses performed on patients cared for by postgraduate year (PGY)‐2 and PGY‐3 IM residents and hospitalist physicians at Northwestern Memorial Hospital (NMH) from December 2012 to May 2015. NMH is an 896‐bed, tertiary academic medical center, located in Chicago, Illinois. A random sample of IM residents participated in a thoracentesis SBML intervention, whereas hospitalist physicians did not. We compared referral patterns, self‐confidence, procedure experience, and reasons for referral between traditionally trained residents, SBML‐trained residents, and hospitalist physicians. The Northwestern University Institutional Review Board approved this study, and all study participants provided informed consent.
At NMH, resident‐staffed services include general IM and nonintensive care subspecialty medical services. There are also 2 nonteaching floors staffed by hospitalist attending physicians without residents. Thoracenteses performed on these services can either be done at the bedside or referred to pulmonary medicine or IR. The majority of thoracenteses performed by pulmonary medicine occur at the patients' bedside, and the patients also receive a clinical consultation. IR procedures are done in the IR suite without additional clinical consultation.
Procedure
One hundred sixty residents were available for training over the study period. We randomly selected 20% of the approximately 20 PGY‐2 and PGY‐3 IM residents assigned to the NMH medicine services each month to participate in SBML thoracentesis training before their rotation. Randomly selected residents were required to undergo SBML training but were not required to participate in the study. This selection process was repeated before every rotation during the study period. This randomized wait‐list control method allowed residents to serve as controls if not initially selected for training and remain eligible for SBML training in subsequent rotations.
Intervention
The SBML intervention used a pretest/post‐test design, as described elsewhere.[9] Residents completed a clinical skills pretest on a thoracentesis simulator using a previously published 26‐item checklist.[9] Following the pretest, residents participated in 2, 1‐hour training sessions including a lecture, video, and deliberate practice on the simulator with feedback from an expert instructor. Finally, residents completed a clinical skills post‐test using the checklist within 1 week from training (but on a different day) and were required to meet or exceed an 84.3% minimum passing score (MPS). The entire training, including pre‐ and post‐tests, took approximately 3 hours to complete, and residents were given an additional 1 hour refresher training every 6 months for up to a year after original training. We compared pre‐ and post‐test checklist scores to evaluate skills improvement.
Thoracentesis Patient Identification
The NMH electronic health record (EHR) was used to identify medical service inpatients who underwent a thoracentesis during the study period. NMH clinicians must place an EHR order for procedure kits, consults, and laboratory analysis of thoracentesis fluid. We developed a real‐time query of NMH's EHR that identified all patients with electronic orders for thoracenteses and monitored this daily.
Physician Surveys
After each thoracentesis, we surveyed the PGY‐2 or PGY‐3 resident or hospitalist caring for the patient about the procedure. A research coordinator, blind to whether the resident received SBML, performed the surveys face‐to‐face on Monday to Friday during normal business hours. Residents were not considered SBML‐trained until they met or exceeded the MPS on the simulated skills checklist at post‐test. Surveys occurred on Monday for procedures performed on Friday evening through Sunday. Survey questions asked physicians about who performed the procedure, their procedural self‐confidence, and total number of thoracenteses performed in their career. For referred procedures, physicians were asked about reasons for referral including lack of confidence, work hour restrictions (residents only), and low reimbursement rates.[10] There was also an option to add other reasons.
Measurement
The thoracentesis skills checklist documented all required steps for an evidence‐based thoracentesis. Each task received equal weight (0 = done incorrectly/not done, 1 = done correctly).[9] For physician surveys, self‐confidence about performing the procedure was rated on a scale of 0 = not confident to 100 = very confident. Reasons for referral were scored on a Likert scale 1 to 5 (1 = not at all important, 5 = very important). Other reasons for referral were categorized.
Statistical Analysis
The clinical skills pre‐ and post‐test checklist scores were compared using a Wilcoxon matched pairs rank test. Physician survey data were compared between different procedure performers using the 2 test, independent t test, analysis of variance (ANOVA), or Kruskal‐Wallis test depending on data properties. Referral patterns measured by the Likert scale were averaged, and differences between physician groups were evaluated using ANOVA. Counts of other reasons for referral were compared using the 2 test. We performed all statistical analyses using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY).
RESULTS
Thoracentesis Clinical Skills
One hundred twelve (70%) residents were randomized to SBML, and all completed the protocol. Median pretest scores were 57.6% (interquartile range [IQR] 43.376.9), and final post‐test mastery scores were 96.2 (IQR 96.2100.0; P < 0.001). Twenty‐three residents (21.0%) failed to meet the MPS at initial post‐test, but met the MPS on retest after <1 hour of additional training.
Physician Surveys
The EHR query identified 474 procedures eligible for physician surveys. One hundred twenty‐two residents and 51 hospitalist physicians completed surveys for 472 procedures (99.6%); 182 patients by traditionally trained residents, 145 by SBML‐trained residents, and 145 by hospitalist physicians. As shown in Table 1, 413 (88%) of all procedures were referred to another service. Traditionally trained residents were more likely to refer to IR compared to SBML‐trained residents or hospitalist physicians. SBML‐trained residents were more likely to perform bedside procedures, whereas hospitalist physicians were most likely to refer to pulmonary medicine. SBML‐trained residents were most confident in their procedural skills, despite hospitalist physicians performing more actual procedures.
| Traditionally Trained Resident Surveys, n = 182 | SBML‐Trained Resident Surveys, n = 145 | Hospitalist Physician Surveys, n = 145 | P Value | |
|---|---|---|---|---|
| ||||
| Bedside procedures, no. (%) | 26 (14.3%) | 32 (22.1%) | 1 (0.7%) | <0.001 |
| IR procedures, no. (%) | 119 (65.4%) | 74 (51.0%) | 82 (56.6%) | 0.029 |
| Pulmonary procedures, no. (%) | 37 (20.3%) | 39 (26.9%) | 62 (42.8%) | <0.001 |
| Procedure self‐confidence, mean (SD)* | 43.6 (28.66) | 68.2 (25.17) | 55.7 (31.17) | <0.001 |
| Experience performing actual procedures, median (IQR) | 1 (13) | 2 (13.5) | 10 (425) | <0.001 |
Traditionally trained residents were most likely to rate low confidence as reasons why they referred thoracenteses (Table 2). Hospitalist physicians were more likely to cite lack of time to perform the procedure themselves. Other reasons were different across groups. SBML‐trained residents were more likely to refer because of attending preference, whereas traditionally trained residents were mostly like to refer because of high risk/technically difficult cases.
| Traditionally Trained Residents, n = 156 | SBML‐Trained Residents, n = 113 | Hospitalist Physicians, n = 144 | P Value | |
|---|---|---|---|---|
| ||||
| Lack of confidence to perform procedure, mean (SD)* | 3.46 (1.32) | 2.52 (1.45) | 2.89 (1.60) | <0.001 |
| Work hour restrictions, mean (SD) * | 2.05 (1.37) | 1.50 (1.11) | n/a | 0.001 |
| Low reimbursement, mean (SD)* | 1.02 (0.12) | 1.0 (0) | 1.22 (0.69) | <0.001 |
| Other reasons for referral, no. (%) | ||||
| Attending preference | 8 (5.1%) | 11 (9.7%) | 3 (2.1%) | 0.025 |
| Don't know how | 6 (3.8%) | 0 | 0 | 0.007 |
| Failed bedside | 0 | 2 (1.8%) | 0 | 0.07 |
| High risk/technically difficult case | 24 (15.4%) | 12 (10.6%) | 5 (3.5%) | 0.003 |
| IR or pulmonary patient | 5 (3.2%) | 2 (1.8%) | 4 (2.8%) | 0.77 |
| Other IR procedure taking place | 11 (7.1%) | 9 (8.0%) | 4 (2.8%) | 0.13 |
| Patient preference | 2 (1.3%) | 7 (6.2%) | 2 (3.5%) | 0.024 |
| Time | 9 (5.8%) | 7 (6.2%) | 29 (20.1%) | <0.001 |
DISCUSSION
This study confirms earlier research showing that thoracentesis SBML improves residents' clinical skills, but is the first to use a randomized study design.[9] Use of the mastery model in health professions education ensures that all learners are competent to provide patient care including performing invasive procedures. Such rigorous education yields downstream translational outcomes including safety profiles comparable to experts.[1, 6]
This study also shows that SBML‐trained residents displayed higher self‐confidence and performed significantly more bedside procedures than traditionally trained residents and more experienced hospitalist physicians. Although the Society of Hospital Medicine considers thoracentesis skills a core competency for hospitalist physicians,[11] we speculate that some hospitalist physicians had not performed a thoracentesis in years. A recent national survey showed that only 44% of hospitalist physicians performed at least 1 thoracentesis within the past year.[10] Research also shows a shift in medical culture to refer procedures to specialty services, such as IR, by over 900% in the past 2 decades.[4] Our results provide novel information about procedure referrals because we show that SBML provides translational outcomes by improving skills and self‐confidence that influence referral patterns. SBML‐trained residents performed almost a quarter of procedures at the bedside. Although this only represents an 8% absolute difference in bedside procedures compared to traditionally trained residents, if a large number of residents are trained using SBML this results in a meaningful number of procedures shifted to the patient bedside. According to University HealthSystem Consortium data, in US teaching hospitals, approximately 35,325 thoracenteses are performed yearly.[1] Shifting even 8% of these procedures to the bedside would result in significant clinical benefit and cost savings. Reduced referrals increase additional bedside procedures that are safe, cost‐effective, and highly satisfying to patients.[1, 12, 13] Further study is required to determine the impact on referral patterns after providing SMBL training to attending physicians.
Our study also provides information about the rationale for procedure referrals. Earlier work speculates that financial incentive, training and time may explain high procedure referral rates.[10] One report on IM residents noted an 87% IR referral rate for thoracentesis, and confirmed that both training and time were major reasons.[14] Hospitalist physicians reported lack of time as the major factor leading to procedural referrals, which is problematic because bedside procedures yield similar clinical outcomes at lower costs.[1, 12] Attending preference also prevented 11 additional bedside procedures in the SBML‐trained group. Schedule adjustments and SBML training of hospitalist physicians should be considered, because bundled payments in the Affordable Care Act may favor shifting to the higher‐value approach of bedside thoracenteses.[15]
Our study has several limitations. First, we only performed surveys at 1 institution and the results may not be generalizable. Second, we relied on an electronic query to alert us to thoracenteses. Our query may have missed procedures that were unsuccessful or did not have EHR orders entered. Third, physicians may have been surveyed more than once for different or the same patient(s), but opinions may have shifted over time. Fourth, some items such as time needed to be written in the survey and were not specifically asked. This could have resulted in under‐reporting. Finally, we did not assess the clinical outcomes of thoracenteses in this study, although earlier work shows that residents who complete SBML have safety outcomes similar to IR.[1, 6]
In summary, IM residents who complete thoracentesis SBML demonstrate improved clinical skills and are more likely to perform bedside procedures. In an era of bundled payments, rethinking current care models to promote cost‐effective care is necessary. We believe providing additional education, training, and support to hospitalist physicians to promote bedside procedures is a promising strategy that warrants further study.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Kevin O'Leary for their support and encouragement of this work. The authors also thank the internal medicine residents at Northwestern for their dedication to patient care.
Disclosures: This project was supported by grant R18HS021202‐01 from the Agency for Healthcare Research and Quality (AHRQ). AHRQ had no role in the preparation, review, or approval of the manuscript. Trial Registration:
- , , , , . Thoracentesis procedures at university hospitals: comparing outcomes by specialty. Jt Comm J Qual Patient Saf. 2015;42(1):34–40.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed March 9, 2016.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , , , et al. Cost savings of performing paracentesis procedures at the bedside after simulation‐based education. Simul Healthc. 2014;9(5):312–318.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861–866.
- , , , et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- , , , , , . Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162–168.
- , , , , , . Are we providing patient‐centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp J. 2014;1(2):94–103. Available at: http://pxjournal.org/cgi/viewcontent.cgi?article=1024
Internal medicine (IM) residents and hospitalist physicians commonly conduct bedside thoracenteses for both diagnostic and therapeutic purposes.[1] The American Board of Internal Medicine only requires that certification candidates understand the indications, complications, and management of thoracenteses.[2] A disconnect between clinical practice patterns and board requirements may increase patient risk because poorly trained physicians are more likely to cause complications.[3] National practice patterns show that many thoracenteses are referred to interventional radiology (IR).[4] However, research links performance of bedside procedures to reduced hospital length of stay and lower costs, without increasing risk of complications.[1, 5, 6]
Simulation‐based education offers a controlled environment where trainees improve procedural knowledge and skills without patient harm.[7] Simulation‐based mastery learning (SBML) is a rigorous form of competency‐based education that improves clinical skills and reduces iatrogenic complications and healthcare costs.[5, 6, 8] SBML also is an effective method to boost thoracentesis skills among IM residents.[9] However, there are no data to show that thoracentesis skills acquired in the simulation laboratory transfer to clinical environments and affect referral patterns.
We hypothesized that a thoracentesis SBML intervention would improve skills and increase procedural self‐confidence while reducing procedure referrals. This study aimed to (1) assess the effect of thoracentesis SBML on a cohort of IM residents' simulated skills and (2) compare traditionally trained (nonSBML‐trained) residents, SBML‐trained residents, and hospitalist physicians regarding procedure referral patterns, self‐confidence, procedure experience, and reasons for referral.
METHODS AND MATERIALS
Study Design
We surveyed physicians about thoracenteses performed on patients cared for by postgraduate year (PGY)‐2 and PGY‐3 IM residents and hospitalist physicians at Northwestern Memorial Hospital (NMH) from December 2012 to May 2015. NMH is an 896‐bed, tertiary academic medical center, located in Chicago, Illinois. A random sample of IM residents participated in a thoracentesis SBML intervention, whereas hospitalist physicians did not. We compared referral patterns, self‐confidence, procedure experience, and reasons for referral between traditionally trained residents, SBML‐trained residents, and hospitalist physicians. The Northwestern University Institutional Review Board approved this study, and all study participants provided informed consent.
At NMH, resident‐staffed services include general IM and nonintensive care subspecialty medical services. There are also 2 nonteaching floors staffed by hospitalist attending physicians without residents. Thoracenteses performed on these services can either be done at the bedside or referred to pulmonary medicine or IR. The majority of thoracenteses performed by pulmonary medicine occur at the patients' bedside, and the patients also receive a clinical consultation. IR procedures are done in the IR suite without additional clinical consultation.
Procedure
One hundred sixty residents were available for training over the study period. We randomly selected 20% of the approximately 20 PGY‐2 and PGY‐3 IM residents assigned to the NMH medicine services each month to participate in SBML thoracentesis training before their rotation. Randomly selected residents were required to undergo SBML training but were not required to participate in the study. This selection process was repeated before every rotation during the study period. This randomized wait‐list control method allowed residents to serve as controls if not initially selected for training and remain eligible for SBML training in subsequent rotations.
Intervention
The SBML intervention used a pretest/post‐test design, as described elsewhere.[9] Residents completed a clinical skills pretest on a thoracentesis simulator using a previously published 26‐item checklist.[9] Following the pretest, residents participated in 2, 1‐hour training sessions including a lecture, video, and deliberate practice on the simulator with feedback from an expert instructor. Finally, residents completed a clinical skills post‐test using the checklist within 1 week from training (but on a different day) and were required to meet or exceed an 84.3% minimum passing score (MPS). The entire training, including pre‐ and post‐tests, took approximately 3 hours to complete, and residents were given an additional 1 hour refresher training every 6 months for up to a year after original training. We compared pre‐ and post‐test checklist scores to evaluate skills improvement.
Thoracentesis Patient Identification
The NMH electronic health record (EHR) was used to identify medical service inpatients who underwent a thoracentesis during the study period. NMH clinicians must place an EHR order for procedure kits, consults, and laboratory analysis of thoracentesis fluid. We developed a real‐time query of NMH's EHR that identified all patients with electronic orders for thoracenteses and monitored this daily.
Physician Surveys
After each thoracentesis, we surveyed the PGY‐2 or PGY‐3 resident or hospitalist caring for the patient about the procedure. A research coordinator, blind to whether the resident received SBML, performed the surveys face‐to‐face on Monday to Friday during normal business hours. Residents were not considered SBML‐trained until they met or exceeded the MPS on the simulated skills checklist at post‐test. Surveys occurred on Monday for procedures performed on Friday evening through Sunday. Survey questions asked physicians about who performed the procedure, their procedural self‐confidence, and total number of thoracenteses performed in their career. For referred procedures, physicians were asked about reasons for referral including lack of confidence, work hour restrictions (residents only), and low reimbursement rates.[10] There was also an option to add other reasons.
Measurement
The thoracentesis skills checklist documented all required steps for an evidence‐based thoracentesis. Each task received equal weight (0 = done incorrectly/not done, 1 = done correctly).[9] For physician surveys, self‐confidence about performing the procedure was rated on a scale of 0 = not confident to 100 = very confident. Reasons for referral were scored on a Likert scale 1 to 5 (1 = not at all important, 5 = very important). Other reasons for referral were categorized.
Statistical Analysis
The clinical skills pre‐ and post‐test checklist scores were compared using a Wilcoxon matched pairs rank test. Physician survey data were compared between different procedure performers using the 2 test, independent t test, analysis of variance (ANOVA), or Kruskal‐Wallis test depending on data properties. Referral patterns measured by the Likert scale were averaged, and differences between physician groups were evaluated using ANOVA. Counts of other reasons for referral were compared using the 2 test. We performed all statistical analyses using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY).
RESULTS
Thoracentesis Clinical Skills
One hundred twelve (70%) residents were randomized to SBML, and all completed the protocol. Median pretest scores were 57.6% (interquartile range [IQR] 43.376.9), and final post‐test mastery scores were 96.2 (IQR 96.2100.0; P < 0.001). Twenty‐three residents (21.0%) failed to meet the MPS at initial post‐test, but met the MPS on retest after <1 hour of additional training.
Physician Surveys
The EHR query identified 474 procedures eligible for physician surveys. One hundred twenty‐two residents and 51 hospitalist physicians completed surveys for 472 procedures (99.6%); 182 patients by traditionally trained residents, 145 by SBML‐trained residents, and 145 by hospitalist physicians. As shown in Table 1, 413 (88%) of all procedures were referred to another service. Traditionally trained residents were more likely to refer to IR compared to SBML‐trained residents or hospitalist physicians. SBML‐trained residents were more likely to perform bedside procedures, whereas hospitalist physicians were most likely to refer to pulmonary medicine. SBML‐trained residents were most confident in their procedural skills, despite hospitalist physicians performing more actual procedures.
| Traditionally Trained Resident Surveys, n = 182 | SBML‐Trained Resident Surveys, n = 145 | Hospitalist Physician Surveys, n = 145 | P Value | |
|---|---|---|---|---|
| ||||
| Bedside procedures, no. (%) | 26 (14.3%) | 32 (22.1%) | 1 (0.7%) | <0.001 |
| IR procedures, no. (%) | 119 (65.4%) | 74 (51.0%) | 82 (56.6%) | 0.029 |
| Pulmonary procedures, no. (%) | 37 (20.3%) | 39 (26.9%) | 62 (42.8%) | <0.001 |
| Procedure self‐confidence, mean (SD)* | 43.6 (28.66) | 68.2 (25.17) | 55.7 (31.17) | <0.001 |
| Experience performing actual procedures, median (IQR) | 1 (13) | 2 (13.5) | 10 (425) | <0.001 |
Traditionally trained residents were most likely to rate low confidence as reasons why they referred thoracenteses (Table 2). Hospitalist physicians were more likely to cite lack of time to perform the procedure themselves. Other reasons were different across groups. SBML‐trained residents were more likely to refer because of attending preference, whereas traditionally trained residents were mostly like to refer because of high risk/technically difficult cases.
| Traditionally Trained Residents, n = 156 | SBML‐Trained Residents, n = 113 | Hospitalist Physicians, n = 144 | P Value | |
|---|---|---|---|---|
| ||||
| Lack of confidence to perform procedure, mean (SD)* | 3.46 (1.32) | 2.52 (1.45) | 2.89 (1.60) | <0.001 |
| Work hour restrictions, mean (SD) * | 2.05 (1.37) | 1.50 (1.11) | n/a | 0.001 |
| Low reimbursement, mean (SD)* | 1.02 (0.12) | 1.0 (0) | 1.22 (0.69) | <0.001 |
| Other reasons for referral, no. (%) | ||||
| Attending preference | 8 (5.1%) | 11 (9.7%) | 3 (2.1%) | 0.025 |
| Don't know how | 6 (3.8%) | 0 | 0 | 0.007 |
| Failed bedside | 0 | 2 (1.8%) | 0 | 0.07 |
| High risk/technically difficult case | 24 (15.4%) | 12 (10.6%) | 5 (3.5%) | 0.003 |
| IR or pulmonary patient | 5 (3.2%) | 2 (1.8%) | 4 (2.8%) | 0.77 |
| Other IR procedure taking place | 11 (7.1%) | 9 (8.0%) | 4 (2.8%) | 0.13 |
| Patient preference | 2 (1.3%) | 7 (6.2%) | 2 (3.5%) | 0.024 |
| Time | 9 (5.8%) | 7 (6.2%) | 29 (20.1%) | <0.001 |
DISCUSSION
This study confirms earlier research showing that thoracentesis SBML improves residents' clinical skills, but is the first to use a randomized study design.[9] Use of the mastery model in health professions education ensures that all learners are competent to provide patient care including performing invasive procedures. Such rigorous education yields downstream translational outcomes including safety profiles comparable to experts.[1, 6]
This study also shows that SBML‐trained residents displayed higher self‐confidence and performed significantly more bedside procedures than traditionally trained residents and more experienced hospitalist physicians. Although the Society of Hospital Medicine considers thoracentesis skills a core competency for hospitalist physicians,[11] we speculate that some hospitalist physicians had not performed a thoracentesis in years. A recent national survey showed that only 44% of hospitalist physicians performed at least 1 thoracentesis within the past year.[10] Research also shows a shift in medical culture to refer procedures to specialty services, such as IR, by over 900% in the past 2 decades.[4] Our results provide novel information about procedure referrals because we show that SBML provides translational outcomes by improving skills and self‐confidence that influence referral patterns. SBML‐trained residents performed almost a quarter of procedures at the bedside. Although this only represents an 8% absolute difference in bedside procedures compared to traditionally trained residents, if a large number of residents are trained using SBML this results in a meaningful number of procedures shifted to the patient bedside. According to University HealthSystem Consortium data, in US teaching hospitals, approximately 35,325 thoracenteses are performed yearly.[1] Shifting even 8% of these procedures to the bedside would result in significant clinical benefit and cost savings. Reduced referrals increase additional bedside procedures that are safe, cost‐effective, and highly satisfying to patients.[1, 12, 13] Further study is required to determine the impact on referral patterns after providing SMBL training to attending physicians.
Our study also provides information about the rationale for procedure referrals. Earlier work speculates that financial incentive, training and time may explain high procedure referral rates.[10] One report on IM residents noted an 87% IR referral rate for thoracentesis, and confirmed that both training and time were major reasons.[14] Hospitalist physicians reported lack of time as the major factor leading to procedural referrals, which is problematic because bedside procedures yield similar clinical outcomes at lower costs.[1, 12] Attending preference also prevented 11 additional bedside procedures in the SBML‐trained group. Schedule adjustments and SBML training of hospitalist physicians should be considered, because bundled payments in the Affordable Care Act may favor shifting to the higher‐value approach of bedside thoracenteses.[15]
Our study has several limitations. First, we only performed surveys at 1 institution and the results may not be generalizable. Second, we relied on an electronic query to alert us to thoracenteses. Our query may have missed procedures that were unsuccessful or did not have EHR orders entered. Third, physicians may have been surveyed more than once for different or the same patient(s), but opinions may have shifted over time. Fourth, some items such as time needed to be written in the survey and were not specifically asked. This could have resulted in under‐reporting. Finally, we did not assess the clinical outcomes of thoracenteses in this study, although earlier work shows that residents who complete SBML have safety outcomes similar to IR.[1, 6]
In summary, IM residents who complete thoracentesis SBML demonstrate improved clinical skills and are more likely to perform bedside procedures. In an era of bundled payments, rethinking current care models to promote cost‐effective care is necessary. We believe providing additional education, training, and support to hospitalist physicians to promote bedside procedures is a promising strategy that warrants further study.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Kevin O'Leary for their support and encouragement of this work. The authors also thank the internal medicine residents at Northwestern for their dedication to patient care.
Disclosures: This project was supported by grant R18HS021202‐01 from the Agency for Healthcare Research and Quality (AHRQ). AHRQ had no role in the preparation, review, or approval of the manuscript. Trial Registration:
Internal medicine (IM) residents and hospitalist physicians commonly conduct bedside thoracenteses for both diagnostic and therapeutic purposes.[1] The American Board of Internal Medicine only requires that certification candidates understand the indications, complications, and management of thoracenteses.[2] A disconnect between clinical practice patterns and board requirements may increase patient risk because poorly trained physicians are more likely to cause complications.[3] National practice patterns show that many thoracenteses are referred to interventional radiology (IR).[4] However, research links performance of bedside procedures to reduced hospital length of stay and lower costs, without increasing risk of complications.[1, 5, 6]
Simulation‐based education offers a controlled environment where trainees improve procedural knowledge and skills without patient harm.[7] Simulation‐based mastery learning (SBML) is a rigorous form of competency‐based education that improves clinical skills and reduces iatrogenic complications and healthcare costs.[5, 6, 8] SBML also is an effective method to boost thoracentesis skills among IM residents.[9] However, there are no data to show that thoracentesis skills acquired in the simulation laboratory transfer to clinical environments and affect referral patterns.
We hypothesized that a thoracentesis SBML intervention would improve skills and increase procedural self‐confidence while reducing procedure referrals. This study aimed to (1) assess the effect of thoracentesis SBML on a cohort of IM residents' simulated skills and (2) compare traditionally trained (nonSBML‐trained) residents, SBML‐trained residents, and hospitalist physicians regarding procedure referral patterns, self‐confidence, procedure experience, and reasons for referral.
METHODS AND MATERIALS
Study Design
We surveyed physicians about thoracenteses performed on patients cared for by postgraduate year (PGY)‐2 and PGY‐3 IM residents and hospitalist physicians at Northwestern Memorial Hospital (NMH) from December 2012 to May 2015. NMH is an 896‐bed, tertiary academic medical center, located in Chicago, Illinois. A random sample of IM residents participated in a thoracentesis SBML intervention, whereas hospitalist physicians did not. We compared referral patterns, self‐confidence, procedure experience, and reasons for referral between traditionally trained residents, SBML‐trained residents, and hospitalist physicians. The Northwestern University Institutional Review Board approved this study, and all study participants provided informed consent.
At NMH, resident‐staffed services include general IM and nonintensive care subspecialty medical services. There are also 2 nonteaching floors staffed by hospitalist attending physicians without residents. Thoracenteses performed on these services can either be done at the bedside or referred to pulmonary medicine or IR. The majority of thoracenteses performed by pulmonary medicine occur at the patients' bedside, and the patients also receive a clinical consultation. IR procedures are done in the IR suite without additional clinical consultation.
Procedure
One hundred sixty residents were available for training over the study period. We randomly selected 20% of the approximately 20 PGY‐2 and PGY‐3 IM residents assigned to the NMH medicine services each month to participate in SBML thoracentesis training before their rotation. Randomly selected residents were required to undergo SBML training but were not required to participate in the study. This selection process was repeated before every rotation during the study period. This randomized wait‐list control method allowed residents to serve as controls if not initially selected for training and remain eligible for SBML training in subsequent rotations.
Intervention
The SBML intervention used a pretest/post‐test design, as described elsewhere.[9] Residents completed a clinical skills pretest on a thoracentesis simulator using a previously published 26‐item checklist.[9] Following the pretest, residents participated in 2, 1‐hour training sessions including a lecture, video, and deliberate practice on the simulator with feedback from an expert instructor. Finally, residents completed a clinical skills post‐test using the checklist within 1 week from training (but on a different day) and were required to meet or exceed an 84.3% minimum passing score (MPS). The entire training, including pre‐ and post‐tests, took approximately 3 hours to complete, and residents were given an additional 1 hour refresher training every 6 months for up to a year after original training. We compared pre‐ and post‐test checklist scores to evaluate skills improvement.
Thoracentesis Patient Identification
The NMH electronic health record (EHR) was used to identify medical service inpatients who underwent a thoracentesis during the study period. NMH clinicians must place an EHR order for procedure kits, consults, and laboratory analysis of thoracentesis fluid. We developed a real‐time query of NMH's EHR that identified all patients with electronic orders for thoracenteses and monitored this daily.
Physician Surveys
After each thoracentesis, we surveyed the PGY‐2 or PGY‐3 resident or hospitalist caring for the patient about the procedure. A research coordinator, blind to whether the resident received SBML, performed the surveys face‐to‐face on Monday to Friday during normal business hours. Residents were not considered SBML‐trained until they met or exceeded the MPS on the simulated skills checklist at post‐test. Surveys occurred on Monday for procedures performed on Friday evening through Sunday. Survey questions asked physicians about who performed the procedure, their procedural self‐confidence, and total number of thoracenteses performed in their career. For referred procedures, physicians were asked about reasons for referral including lack of confidence, work hour restrictions (residents only), and low reimbursement rates.[10] There was also an option to add other reasons.
Measurement
The thoracentesis skills checklist documented all required steps for an evidence‐based thoracentesis. Each task received equal weight (0 = done incorrectly/not done, 1 = done correctly).[9] For physician surveys, self‐confidence about performing the procedure was rated on a scale of 0 = not confident to 100 = very confident. Reasons for referral were scored on a Likert scale 1 to 5 (1 = not at all important, 5 = very important). Other reasons for referral were categorized.
Statistical Analysis
The clinical skills pre‐ and post‐test checklist scores were compared using a Wilcoxon matched pairs rank test. Physician survey data were compared between different procedure performers using the 2 test, independent t test, analysis of variance (ANOVA), or Kruskal‐Wallis test depending on data properties. Referral patterns measured by the Likert scale were averaged, and differences between physician groups were evaluated using ANOVA. Counts of other reasons for referral were compared using the 2 test. We performed all statistical analyses using IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY).
RESULTS
Thoracentesis Clinical Skills
One hundred twelve (70%) residents were randomized to SBML, and all completed the protocol. Median pretest scores were 57.6% (interquartile range [IQR] 43.376.9), and final post‐test mastery scores were 96.2 (IQR 96.2100.0; P < 0.001). Twenty‐three residents (21.0%) failed to meet the MPS at initial post‐test, but met the MPS on retest after <1 hour of additional training.
Physician Surveys
The EHR query identified 474 procedures eligible for physician surveys. One hundred twenty‐two residents and 51 hospitalist physicians completed surveys for 472 procedures (99.6%); 182 patients by traditionally trained residents, 145 by SBML‐trained residents, and 145 by hospitalist physicians. As shown in Table 1, 413 (88%) of all procedures were referred to another service. Traditionally trained residents were more likely to refer to IR compared to SBML‐trained residents or hospitalist physicians. SBML‐trained residents were more likely to perform bedside procedures, whereas hospitalist physicians were most likely to refer to pulmonary medicine. SBML‐trained residents were most confident in their procedural skills, despite hospitalist physicians performing more actual procedures.
| Traditionally Trained Resident Surveys, n = 182 | SBML‐Trained Resident Surveys, n = 145 | Hospitalist Physician Surveys, n = 145 | P Value | |
|---|---|---|---|---|
| ||||
| Bedside procedures, no. (%) | 26 (14.3%) | 32 (22.1%) | 1 (0.7%) | <0.001 |
| IR procedures, no. (%) | 119 (65.4%) | 74 (51.0%) | 82 (56.6%) | 0.029 |
| Pulmonary procedures, no. (%) | 37 (20.3%) | 39 (26.9%) | 62 (42.8%) | <0.001 |
| Procedure self‐confidence, mean (SD)* | 43.6 (28.66) | 68.2 (25.17) | 55.7 (31.17) | <0.001 |
| Experience performing actual procedures, median (IQR) | 1 (13) | 2 (13.5) | 10 (425) | <0.001 |
Traditionally trained residents were most likely to rate low confidence as reasons why they referred thoracenteses (Table 2). Hospitalist physicians were more likely to cite lack of time to perform the procedure themselves. Other reasons were different across groups. SBML‐trained residents were more likely to refer because of attending preference, whereas traditionally trained residents were mostly like to refer because of high risk/technically difficult cases.
| Traditionally Trained Residents, n = 156 | SBML‐Trained Residents, n = 113 | Hospitalist Physicians, n = 144 | P Value | |
|---|---|---|---|---|
| ||||
| Lack of confidence to perform procedure, mean (SD)* | 3.46 (1.32) | 2.52 (1.45) | 2.89 (1.60) | <0.001 |
| Work hour restrictions, mean (SD) * | 2.05 (1.37) | 1.50 (1.11) | n/a | 0.001 |
| Low reimbursement, mean (SD)* | 1.02 (0.12) | 1.0 (0) | 1.22 (0.69) | <0.001 |
| Other reasons for referral, no. (%) | ||||
| Attending preference | 8 (5.1%) | 11 (9.7%) | 3 (2.1%) | 0.025 |
| Don't know how | 6 (3.8%) | 0 | 0 | 0.007 |
| Failed bedside | 0 | 2 (1.8%) | 0 | 0.07 |
| High risk/technically difficult case | 24 (15.4%) | 12 (10.6%) | 5 (3.5%) | 0.003 |
| IR or pulmonary patient | 5 (3.2%) | 2 (1.8%) | 4 (2.8%) | 0.77 |
| Other IR procedure taking place | 11 (7.1%) | 9 (8.0%) | 4 (2.8%) | 0.13 |
| Patient preference | 2 (1.3%) | 7 (6.2%) | 2 (3.5%) | 0.024 |
| Time | 9 (5.8%) | 7 (6.2%) | 29 (20.1%) | <0.001 |
DISCUSSION
This study confirms earlier research showing that thoracentesis SBML improves residents' clinical skills, but is the first to use a randomized study design.[9] Use of the mastery model in health professions education ensures that all learners are competent to provide patient care including performing invasive procedures. Such rigorous education yields downstream translational outcomes including safety profiles comparable to experts.[1, 6]
This study also shows that SBML‐trained residents displayed higher self‐confidence and performed significantly more bedside procedures than traditionally trained residents and more experienced hospitalist physicians. Although the Society of Hospital Medicine considers thoracentesis skills a core competency for hospitalist physicians,[11] we speculate that some hospitalist physicians had not performed a thoracentesis in years. A recent national survey showed that only 44% of hospitalist physicians performed at least 1 thoracentesis within the past year.[10] Research also shows a shift in medical culture to refer procedures to specialty services, such as IR, by over 900% in the past 2 decades.[4] Our results provide novel information about procedure referrals because we show that SBML provides translational outcomes by improving skills and self‐confidence that influence referral patterns. SBML‐trained residents performed almost a quarter of procedures at the bedside. Although this only represents an 8% absolute difference in bedside procedures compared to traditionally trained residents, if a large number of residents are trained using SBML this results in a meaningful number of procedures shifted to the patient bedside. According to University HealthSystem Consortium data, in US teaching hospitals, approximately 35,325 thoracenteses are performed yearly.[1] Shifting even 8% of these procedures to the bedside would result in significant clinical benefit and cost savings. Reduced referrals increase additional bedside procedures that are safe, cost‐effective, and highly satisfying to patients.[1, 12, 13] Further study is required to determine the impact on referral patterns after providing SMBL training to attending physicians.
Our study also provides information about the rationale for procedure referrals. Earlier work speculates that financial incentive, training and time may explain high procedure referral rates.[10] One report on IM residents noted an 87% IR referral rate for thoracentesis, and confirmed that both training and time were major reasons.[14] Hospitalist physicians reported lack of time as the major factor leading to procedural referrals, which is problematic because bedside procedures yield similar clinical outcomes at lower costs.[1, 12] Attending preference also prevented 11 additional bedside procedures in the SBML‐trained group. Schedule adjustments and SBML training of hospitalist physicians should be considered, because bundled payments in the Affordable Care Act may favor shifting to the higher‐value approach of bedside thoracenteses.[15]
Our study has several limitations. First, we only performed surveys at 1 institution and the results may not be generalizable. Second, we relied on an electronic query to alert us to thoracenteses. Our query may have missed procedures that were unsuccessful or did not have EHR orders entered. Third, physicians may have been surveyed more than once for different or the same patient(s), but opinions may have shifted over time. Fourth, some items such as time needed to be written in the survey and were not specifically asked. This could have resulted in under‐reporting. Finally, we did not assess the clinical outcomes of thoracenteses in this study, although earlier work shows that residents who complete SBML have safety outcomes similar to IR.[1, 6]
In summary, IM residents who complete thoracentesis SBML demonstrate improved clinical skills and are more likely to perform bedside procedures. In an era of bundled payments, rethinking current care models to promote cost‐effective care is necessary. We believe providing additional education, training, and support to hospitalist physicians to promote bedside procedures is a promising strategy that warrants further study.
Acknowledgements
The authors acknowledge Drs. Douglas Vaughan and Kevin O'Leary for their support and encouragement of this work. The authors also thank the internal medicine residents at Northwestern for their dedication to patient care.
Disclosures: This project was supported by grant R18HS021202‐01 from the Agency for Healthcare Research and Quality (AHRQ). AHRQ had no role in the preparation, review, or approval of the manuscript. Trial Registration:
- , , , , . Thoracentesis procedures at university hospitals: comparing outcomes by specialty. Jt Comm J Qual Patient Saf. 2015;42(1):34–40.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed March 9, 2016.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , , , et al. Cost savings of performing paracentesis procedures at the bedside after simulation‐based education. Simul Healthc. 2014;9(5):312–318.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861–866.
- , , , et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- , , , , , . Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162–168.
- , , , , , . Are we providing patient‐centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp J. 2014;1(2):94–103. Available at: http://pxjournal.org/cgi/viewcontent.cgi?article=1024
- , , , , . Thoracentesis procedures at university hospitals: comparing outcomes by specialty. Jt Comm J Qual Patient Saf. 2015;42(1):34–40.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed March 9, 2016.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , . National fluid shifts: fifteen‐year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859–864.
- , , , et al. Cost savings of performing paracentesis procedures at the bedside after simulation‐based education. Simul Healthc. 2014;9(5):312–318.
- , , , , . Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349–356.
- , , , et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861–866.
- , , , et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Procedures performed by hospitalist and non‐hospitalist general internists. J Gen Intern Med. 2010;25(5):448–452.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- , , , , , . Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162–168.
- , , , , , . Are we providing patient‐centered care? Preferences about paracentesis and thoracentesis procedures. Patient Exp J. 2014;1(2):94–103. Available at: http://pxjournal.org/cgi/viewcontent.cgi?article=1024