CATIE phase 2 offers insights on efficacy an tolerability
After nearly 3 out of 4 phase 1 patients stopped taking their assigned antipsychotics within 18 months, researchers in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) braced themselves for phase 2.February 2006)
CATIE’s eligibility criteria are broad and include schizophrenia patients with comorbid conditions such as substance abuse and mood disorders. The primary outcome measure is all-cause treatment discontinuation, which incorporates efficacy, safety, tolerability, patient choice, and clinician choice (Table 1).
Phase 1 compared the efficacy and safety of four second-generation antipsychotics (SGA) and one first-generation antipsychotic (FGA).3 Nasrallah concluded that—despite the high discontinuation rate in that phase—there were “no winners or losers” among the five antipsychotics. The results, Nasrallah concluded:
- provide a compelling rationale for clinicians to match medication profiles to individual patients
- support the need for clinicians to have choices among medications when treating patients with schizophrenia.4
Table 1
Drug discontinuation patterns in CATIE phase 1
| Measures | Findings after 18 months | |
|---|---|---|
| % of patients who discontinued medication for any reason | Olanzapine (64%) | Ziprasidone (79%) |
| Risperidone (74%) | Quetiapine (82%) | |
| Perphenazine (75%) | ||
| Time to discontinuation for any reason | Longest (most favorable) with olanzapine, but not statistically longer with olanzapine than with ziprasidone or perphenazine | |
| No statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | ||
| Time to discontinuation for lack of efficacy* | Longer with olanzapine; no statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | |
| Time to discontinuation for intolerable side effects | No statistical difference among agents | |
| Rate of discontinuation for intolerable side effects | Highest (19%) with olanzapine (primarily because of weight gain or metabolic effects with this medication) | |
| Rate of discontinuation for extrapyramidal effects | Highest (8%) with perphenazine | |
| Rate of discontinuation for intolerability (overall) | Lowest with risperidone (10%) | |
| * Nonequivalent dosing in CATIE phase 1 is an ongoing debate. | ||
What to do next?
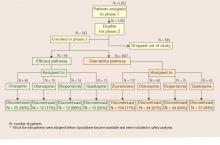
When an initial antipsychotic proves inadequate or causes intolerable side effects, how do you choose a more efficacious or tolerable medication? Phase 2 offered CATIE patients and their clinicians two choices—an efficacy and a tolerability pathway (Figure).1,2
CATIE phase 2: Distribution of patients in efficacy and tolerability pathways
Efficacy pathway. Patients who chose the efficacy pathway were randomly assigned to clozapine (50%) or olanzapine, risperidone, or quetiapine.1 Researchers selected clozapine as the major efficacy comparator because of its robust effects in treatment-refractory schizophrenia. Clozapine was given open-label because of its safety monitoring requirements; other treatments were double-blind.
As in phase 1, the primary outcome measure was time until discontinuation for any reason. Secondary outcome measures included time to discontinuation because of side effects, patient choice, or lack of efficacy.
Tolerability pathway. Patients who chose the tolerability pathway were randomly assigned to double-blind treatment with ziprasidone, olanzapine, risperidone, or quetiapine.2 Ziprasidone was the major comparator because of clinical data showing a favorable tolerability profile.
The primary outcome measure was time to discontinuation for any reason. Secondary outcomes included reason for discontinuation (as determined by the study clinician), symptomatic ratings, and evaluations of adverse effects.
Trial duration. No patients in either pathway received the same antipsychotics they had taken in phase 1. All patients could continue treatment through the 18 months of the CATIE trial or until they completed 6 months in phase 2.
Efficacy pathway results
Discontinuation. Consistent with literature about its efficacy in treatment-refractory schizophrenia, clozapine showed a robust clinical effect. Overall, more patients receiving clozapine stayed on treatment and for longer periods, compared with patients receiving olanzapine, risperidone, or quetiapine (Table 2).
On secondary measures, discontinuation for lack of efficacy was significantly lower with clozapine (11%) than with:
- olanzapine (35%)
- risperidone or quetiapine (each at 43%).
Discontinuation rates because of adverse effects or by patient choice were the same across all medications (Table 3). Patients on clozapine achieved better ratings in overall psychotic symptoms, positive symptoms, and general function, but not in negative symptoms.
Weight gain. On average, patients gained more weight while taking olanzapine (+1.1 lb/mo) than with:
- risperidone (+0.5 lb/mo)
- clozapine (+0.5 lb/mo)
- quetiapine (+0.5 lb/mo)
Differences in weight gain—or in metabolic parameters or other adverse effects—were not statistically significant, however.
Table 2
Phase 2 efficacy pathway: Discontinuation for any reason
| Measure | Clozapine | Olanzapine | Risperidone | Quetiapine |
|---|---|---|---|---|
| How many patients discontinued | 25 of 49 (56%) | 12 of 19 (71%) | 12 of 16 (86%) | 13 of 15 (93%) |
| Median time to discontinuation | 10.5 months | 2.7 months | 2.8 months | 3.3 months |
Table 3
Reasons patients stopped taking their medications in CATIE phase 2
| Reason | Efficacy pathway | Tolerability pathway |
|---|---|---|
| All cause | 69% | 74% |
| Lack of efficacy | 26% | 29% |
| Lack of tolerability | 10% | 15% |
| Patient choice | 26% | 24% |
Tolerability pathway results
Discontinuation. Patients in the tolerability pathway took olanzapine or risperidone significantly longer—median 6.3 and 7 months, respectively— compared with ziprasidone (4 months) or quetiapine (2.8 months).
- Time to discontinuation during phase 2 was the same across all drugs among patients who entered phase 2 because of intolerable side effects in phase 1.
- Time to discontinuation because of side effects also was similar whether patients discontinued phase 1 for lack of efficacy or intolerable side effects. Patients stopped treatment in the efficacy and tolerability pathways for similar reasons (Table 3).